Abstract
Objective:
Management of patients with phenylketonuria mainly includes limiting the content of phenylalanine in the diet. Therefore, the purpose of this study was to investigate oral problems in children with phenylketonuria compared to the healthy population as a case–control study.
Materials and Methods:
The subjects of the case and control groups were selected according to the inclusion criteria. First, the oral cavity and tooth were examined by a specialist dentist to indicate the decayed, missing due to caries, and filled teeth index in both groups. To investigate the level of phenylalanine and evaluate other laboratory examinations, 2 mL of blood and saliva samples was taken from the subjects. Blood and saliva phenylalanine levels were measured by high-performance liquid chromatography. Phosphorus, calcium, and pH levels were investigated through calorimetric measurement. The data were analyzed using Statistical Package for Social Sciences software.
Results:
There was no significant difference between the case and control groups in terms of age and sex. The average level of calcium and phosphorus in the case group was higher than in the control group. Also, the average decayed, missing due to caries, and filled teeth index in the case group was not significantly different compared to the control group. None of the above-investigated indicators had a significant relationship with each other. On the other hand, it was found that there was a positive and significant relationship between phenylalanine in blood, saliva, and pH as well as between saliva phenylalanine with decayed, missing due to caries, and filled teeth.
Conclusion:
The results of this study indicate a significant effect of phenylketonuria disease on calcium, phosphorus, and oral pH levels in children.
Keywords: Phenylalanine, DMF index, dental decay
What is already known on this topic?
According to the studies that have been conducted, we know that the diet of patients with phenylketonuria is rich in carbohydrates and is carious, and also the decayed, missing due to caries, and filled teeth index is a measure to evaluate the health of the mouth and teeth. Also, oral and dental hygiene in children has a great impact on the lifestyle and general health of the body.
What this study adds on this topic?
According to the results of the study, there was a relation between blood phenylalanine and saliva phenylalanine, based on which saliva phenylalanine can be measured instead of blood phenylalanine in the future.
Introduction
Phenylketonuria (PKU) is a consequence of phenylalanine (Phe) hydroxylase (PAH) gene mutation, attributed to an inborn metabolism error. Due to the PAH activity disruption, the blood level of Phe increased, and its concentration turned to a toxic level in the brain. It has been well demonstrated that multiple mental disorders, including eczematous rashes, autism, seizures, developmental problems, abnormal behavior, psychiatric symptoms, and motor deficits, are associated with untreated PKU.1 The prevalence of PKU varies significantly among ethnicities and between different geographic regions around the world, such that the prevalence of PKU is generally higher in Caucasian or East Asian populations. Hence, dietary Phe restriction is the primary approach to managing PKU, which is usually initiated immediately after confirmation of hyperphenylalaninemia in the infant.1,2
Patients with PKU, especially children, follow a special low-protein diet to minimize Phe intake. A specialist physician prescribes this special diet right after PKU diagnosis, which contains no or a meager amount of Phe. It is very critical that patients adhere to the prescription, as the physician noted, which may need to take by patients 3 to 4 times a day and sometimes up to 6 times a day, depending on individual needs. Hence, the children with PKU consume more carbohydrates and fat for energy supplements to compensate for their low protein intake. As such, these patients turn to take more snacks throughout the day. Besides the high carbohydrates level in their males, they are also exposed to Phe-free low pH drinks. All these together can enhance the chance of children with PKU developing caries more likely than healthy ones. Furthermore, high consumption of Phe-free medical formulas, rich in sweet and acidic ingredients, is considered harmful to oral health.3-6
As mentioned, the daily diet plays a crucial role in maintaining oral health.7 Oral and dental health significantly impacts the quality of life representing the critical health aspect.8 However, PKU is still considered a global problem even in developed societies.9 For over 70 years, the index of decayed, missing, and filled teeth (DMFT index) has been used globally to evaluate oral and dental health.10 This index determines the number of decayed teeth, teeth under treatment, and teeth lost due to decay.11 As a result, by examining the DMFT index, it could be concluded that children with PKU are at high risk for caries progression or not.3 Given the critical impact of PKU on oral health and considering limited and contradictory studies in this field3,7,12 here, we have investigated the oral and dental complications in patients with PKU, focusing on the DMFT index and its association with Phe, pH, calcium, and phosphorus. Since the consumption of fat and carbohydrates in PKU patients is higher and they consume less protein, the amount of calcium and phosphorus was evaluated to indicate the amount of their absorption in the bone tissues which can resemble osteoporosis.
Materials and Methods
Population
The present case–control study was conducted to determine the effect of the PKU special diet on oral health in children aged 2-6 years. The present study has been confirmed by the code of IR.ZUMS.REC.1400.366 at the Iran National Committee for Ethics in Biomedical Research. In this study, the selection of samples was based on easy (accessible) sampling. Children’s parents who agreed to participate in the research were included according to the sample size. Subjects have been allocated to the following groups:
The control group, children with no detected PKU described as the health participants with regular diet.
The case group, children with PKU, used a special diet lacking proteins and riches in fatty acids and carbohydrates.
Children were selected based on the following criteria: the consent of the child and their parents, having confirmed PKU disease in the case group, without physical and mental problems, without chronic diseases such as diabetes, cancer, and other oral and dental diseases, as well as imported children. In the study, the case group had a severe restriction of protein in the diet. Children in both case and control groups were selected in such a way that they did not differ significantly in terms of age and gender.
It should be noted that the diagnosis of PKU patients was confirmed by other institutions and they had already received nutritional treatments. In this study, no attempt was made to diagnose PKU patients, and their diagnosis was already confirmed and included in their medical records.
Clinical Examination
All children included in this study in both groups were examined by dental specialists. Patients were examined in terms of dental–oral complications regardless of grouping. The DMFT index was investigated in patients. For this purpose, the dentist examined the patients’ mouths with a dental mirror probe, and the DMFT index score was determined for each patient. In permanent teeth, DMFT is calculated by adding up how many teeth are decayed, missing because of dental caries, and filled. The mean number of DMFT is the sum of individual DMFT values divided by the sum of the population.
Laboratory Evaluation
We conducted 2 phases of laboratory tests in order to obtain results. In the first phase, 2 mm of blood and saliva were taken from each patient. Both samples obtained were analyzed in order to determine the level of Phe. The amount of Phe was measured by high-performance liquid chromatography.13 Samples were deproteinized by adding an equal volume of 5% sulfosalicylic acid and the amino acids were separated by ion exchange column chromatography.
In the second phase of the study, the amount of calcium and phosphorus in the blood of the patients was determined. Using an auto-analyzer (Hitachi), calcium and phosphorus were detected by the colorimetric method.14
A calibrated pH meter device was used to measure the acidity of saliva.
Statistical Analysis
The obtained data were analyzed using Statistical Package for Social Sciences version 26.0 software (IBM Corp., Armonk, NY, USA). Qualitative data were reported as percentage and frequency. The age, phosphorus level, calcium level, and pH were reported as the mean, considering that they were characterized by parametric distribution, and the t-test was used to compare the groups. The Mann–Whitney U-test was used to compare DMFT between 2 groups since the data distribution was non-parametric. Spearman’s test was used to investigate the relationship between DMFT, calcium, and phosphorus parameters, and Pearson’s test was used to analyze blood Phe, saliva Phe, and pH.
Results
Demographic Information of the Participants
One hundred twenty participants were finally included in the present study, of which 30 (25%) were cases, and 90 (75%) were control subjects. As high as 60% of the cases and 53.3% of the control individual were men, with no statistically significant difference (P = .525). The mean (SD) age of the children in the case and control groups did not have a significant difference (P = .619) (Table 1).
Table 1.
Demographic Information
| n (%) | Case Samples | Control Samples | P | |
|---|---|---|---|---|
| 30 (25.0) | 90 (75.0) | — | ||
| Gender—n (%)* | Male | 18 (60.0) | 48 (53.3) | .525 |
| Female | 12 (40.0) | 42 (46.7) | ||
| Age—year (SD)# | 4.00 (1.23) | 4.16 (1.26) | .619 | |
*Chi-square analysis.
#Independent t-test.
Evaluated Decayed, Missing due to Caries, and Filled Teeth
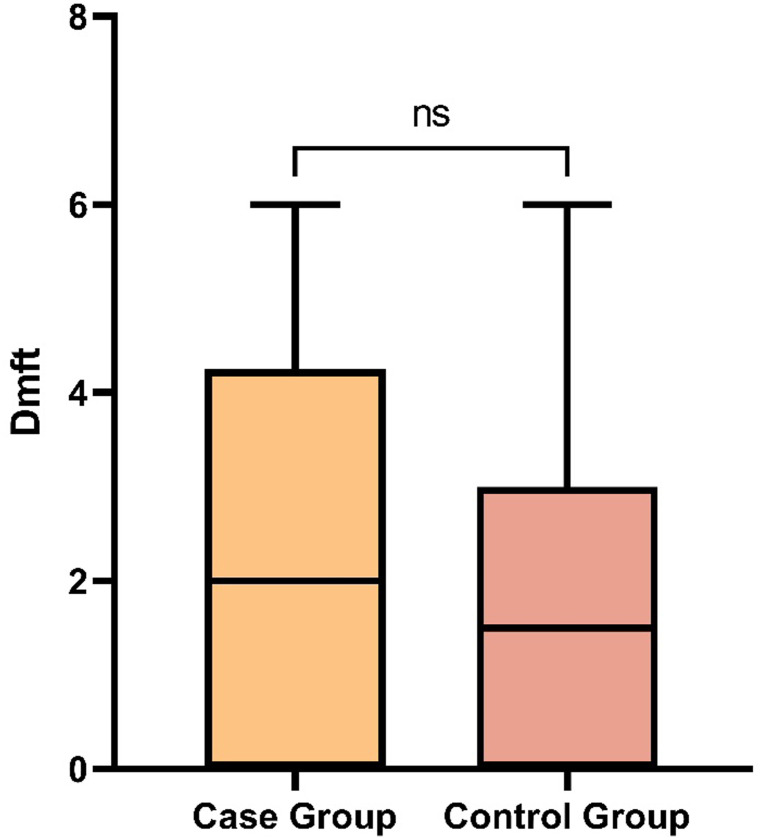
The DMFT was investigated according to dentists’ examination. The Kolmogorov–Smirnov represented the non-parametric distribution of DMFT data. The Mann–Whitney analysis showed that the DMFT index in the case group was not significantly compared to the control group (P = .142; Figure 1).
Figure 1.
The median (minimum–maximum) of DMFT of the case and control groups were 2.00 (0-6) and 1.50 (0-6), respectively. DMFT, decayed, missing due to caries, and filled teeth.
Calcium and Phosphorus Evaluation
The calcium and phosphorus level of the subjects has been measured in both groups. Based on these findings, the mean ± SD of calcium and phosphorus in the case group measured as 9.88 ± 0.44 mg/dL and 3.86 ± 0.59 mg/dL, respectively, represented significantly lower than the control subjects (P < .05) (Table 2).
Table 2.
Investigated Factors in Subjects, Including DMFT, Calcium, Phosphorus, pH, Salivary Phe, and Blood Phe
| Investigated Parameters | Case Samples (n = 30) | Control Samples (n = 90) | P |
|---|---|---|---|
| Mean (SD) | Mean (SD) | ||
| Calcium# | 9.88 (0.44) | 9.54 (0.56) | .004 |
| Phosphorus# | 3.86 (0.59) | 3.50 (0.63) | .007 |
DMFT, decayed, missing due to caries, and filled teeth; Phe, phenylalanine.
Bold P values are statistically significant.
#Independent t-test.
Moreover, the correlation between DMFT and calcium and phosphorus level was carried out by 2-tailed Spearman’s correlation coefficient test. Our findings revealed no significant relationship between DMFT and calcium & phosphorus in both groups (P > .05) (Table 3).
Table 3.
Investigated the Correlation Between Calcium/Phosphorus and DMFT Indexes
| Investigated Parameters | 2-Tailed Spearman’s Correlation | P | |
|---|---|---|---|
| r | |||
| DMFT and Calcium | Case (n = 30) | 0.129 | .496 |
| Control (n = 90) | 0.068 | .527 | |
| DMFT and phosphorus | Case (n = 30) | −0.065 | .732 |
| Control (n = 90) | −0.047 | .662 | |
DMFT, decayed, missing due to caries, and filled teeth.
Phenylalanine and pH Evaluation
The Phe of pH, blood, and saliva Phe in case subjects have been measured and the results are indicated in Figure 2.
Figure 2.
The mean ± SD of pH, blood Phe, and saliva Phe of case subjects were 7.30 ± 0.63, 12.13 ± 4.12 and 53.83 ± 24.48, respectively. Phe, phenylalanine.
The 2-tailed Spearman’s correlation analysis has revealed that DMFT did not have a significant correlation with blood Phe (P = .963), while representing a significant direct correlation with salivary Phe (P = .043). Moreover, Spearman’s correlation test indicated a direct and significant correlation between saliva Phe and blood Phe (P < .001), pH and saliva Phe (P < .001), and pH and blood Phe (P = .002) (Table 4).
Table 4.
Investigated the Correlation Between Phe, DMFT indexes, and pH in case subjects
| Investigated Parameters | 2-Tailed Spearman’s rho | P |
|---|---|---|
| r | ||
| DMFT and blood Phe | −0.009 | .963 |
| DMFT and salivary Phe | 0.372* | .043 |
| Salivary Phe and blood Phe | 0.665** | <.001 |
| pH and salivary Phe | 0.787** | <.001 |
| pH and blood Phe | 0.539** | .002 |
DMFT, decayed, missing due to caries, and filled teeth; Phe, phenylalanine.
*Correlation is significant at the .05 level (2-tailed).
**Correlation is significant at the .01 level (2-tailed).
As such, it could be noted that there was a medium relationship between salivary Phe with blood Phe and between pH with blood Phe, while a high relationship was detected between pH with salivary Phe.
Discussion
The management of patients with PKU mainly includes limiting the content of Phe in the diet. Therefore, patients have been prescribed an extremely low-protein diet and Phe-free amino acid supplements (medical foods) to meet protein requirements. Diet plays an essential role in maintaining oral health, but diet modification due to medical problems such as PKU can have adverse effects on oral health. Therefore, this study aimed to investigate oral issues in patients with PKU compared to the healthy population.
Studies that evaluate the oral and dental health status of children with PKU are limited and contradictory.5 Kilpatrick et al12 compared 40 PKU patients with healthy children and reported a 17% prevalence of dental caries in PKU patients, which was similar to healthy individuals. Although in our study, the incidence and prevalence of caries were not investigated, we showed that the DMFT in PKU patients was not substantially higher than in the control group, so it can be concluded that the results of our study are not different from the study of Kilpatrick et al.12 In another study, Winter et al15 concluded that the presence of caries in 105 children with PKU was similar to healthy children, which also confirms our results. In contrast to these results, a high-fat and carbohydrate-rich diet is expected to exacerbate tooth decay in PKU patients. According to Winter et al15 1 factor that can explain the similar caries between the control and PKU groups is the increased level of Phe, which may lead to limiting the growth of plaque bacteria.
Turkey’s researchers have demonstrated that children with 5-year-olds have an average DMFT of 3.7 ± 3.9.16 This result was higher than ours, since the median (95% CI) of the DMFT for the case and control groups was 2.00 [95% CI: 1.50-3.23] and 1.50 [95% CI: 1.24-1.89] respectively. Although these differences in habits can be attributed to demographic differences in the 2 countries and different dietary, as Gökalp et al16 showed in their study, the subject’s age can have a significant effect on DMFT. Since the average age of the examined children in our study was about 4 years old and in the mentioned study the age of the examined children was 5 years old, it might be the underlying reason for the DMFT differences between studies.
However, there are various studies that show that patients with PKU have more oral complications than normal ones. For example, Ābola et al17 reported that the dental status and periodontal health of PKU patients were found to be significantly inferior compared to healthy controls associated with the regular consumption of PKU formula and the difficulties which mentally and/or physically disabled PKU patients experience with their oral hygiene. But there is a big difference with our study, we have performed a study on children with ages around 4 years while the median age of people in Ābola et al17 study was 22 years. On the other hand, Ballikaya et al7 reported that 67% of PKU patients have dental caries, but they also mentioned that of the patients, 85.3% did not brush their teeth regularly and 90.4% had never visited a dentist before, which makes the conclusion ambiguous.
Another finding of our study was the higher level of calcium and phosphorus in patients compared to the control subjects. Wang et al14 reported that the blood calcium was higher in 4-year-old children with PKU compared to the control group (P = .029), which is consistent with our study, so it can be concluded that PKU disease can affect the level of calcium and increase its amount in the blood that indicated a decrease in its absorption by cells. As a result of low calcium absorption, it has been reported that more than 40% of young PKU patients have a low bone density.18 Wang et al14 also noted in their study that there was no difference in blood phosphorus levels between patients and the control group, which was contrary to the results of our research. This difference can be attributed to the difference in the study population; however, we strongly recommended it for future studies.
Our study also indicated a positive and significant correlation between blood Phe and saliva Phe. In this regard, Hall et al19 investigated whether saliva Phe can replace blood Phe or not. Their results implied a positive correlation between blood Phe and saliva Phe (r: 0.69; P < .02), but despite the significant correlation, they claimed that the correlation coefficient was very low to conclude an established correlation between salivary and blood Phe concentration in patients with PKU. It cannot be of practical value as an alternative measure of blood concentration for routine monitoring. Our study also showed a correlation coefficient of 0.64, which was statistically significant and similar to Hall et al’s19 study. Therefore, due to the closeness of the correlation coefficients of the 2 studies, we suggest that these data be interpreted with caution, and more comprehensive studies should be conducted to determine the relationship between Phe in blood and saliva.
We also showed in this study that the level of Phe in blood and saliva had a positive and significant correlation with increasing pH. Due to the frequent consumption of a carbohydrate-rich diet which results in a very acidic pH (lowered pH) in their oral environment, we expected to have a negative correlation between pH and PKU, but obtained results showed the opposite.
One of the most important limitations of the present study was the limited cooperation of children and the problems of working with children, which were tried to be passed by using a detailed examination and obtaining informed consent. In addition, since 2 mL of the patients’ blood was sampled during the study, it was not possible to evaluate other parameters such as vitamin D and alkaline phosphatase due to the limited blood sample. On the other hand, due to the dispersion of the patients and the lack of access to them again, it was not possible to take samples again to measure these parameters.
Conclusion
According to the presented results, it can be concluded that the daily diet of patients with PKU has significantly increased calcium and phosphorus, which may represent lower absorption by bone tissue. However, our findings did not demonstrate the destructive effect of the diet of patients with PKU on teeth, as indicated by the similar DMFT indices between case and control groups. While there is a strong need to evaluate the underlying reasons, it has been attributed to the high level of Phe which may hindered the plaque formation. Finally, we have shown the positive correlation between saliva-Phe, blood-Phe, and pH as well as between salvia-Phe with DMFT which requires more detailed investigations to interpret.
Footnotes
Ethics Committee Approval: The present study has been confirmed by the code of IR.ZUMS.REC.1400.366 at the Iran National Committee for Ethics in Biomedical Research.
Informed Consent: Verbal/written informed consent was obtained from the patients/patient who agreed to take part in the study.
Peer-review: Externally peer-reviewed.
Author Contributions: Concept – M.A.N.; Design – M.A.N., K.M., N.G.; Supervision – M.A.N., K.M., N.G.; Resources – M.A.N., K.M., N.G.; Materials – M.A.N., K.M., N.G.; Data Collection and/or Processing – M.A.N., K.M., N.G.; Analysis and/or Interpretation – M.A.N., K.M., N.G.; Literature Search – M.A.N., K.M., N.G.; Writing – N.G.; Critical Review – M.A.N., K.M.
Declaration of Interests: The authors have no conflict of interest to declare.
Funding: This study received no funding.
References
- 1. Blau N, van Spronsen FJ, Levy HL. Phenylketonuria. Lancet. 2010;376(9750):1417 1427. ( 10.1016/S0140-6736(10)60961-0) [DOI] [PubMed] [Google Scholar]
- 2. van Spronsen FJ, Blau N, Harding C, Burlina A, Longo N, Bosch AM. Phenylketonuria. Nat Rev Dis Primers. 2021;7(1):36. ( 10.1038/s41572-021-00267-0) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Singh-Hüsgen P, Meissner T, Bizhang M, Henrich B, Raab WH. Investigation of the oral status and microorganisms in children with phenylketonuria and type 1 diabetes. Clin Oral Investig. 2016;20(4):841 847. ( 10.1007/s00784-015-1564-7) [DOI] [PubMed] [Google Scholar]
- 4. MacDonald A, Chakrapani A, Hendriksz C, et al. Protein substitute dosage in PKU: how much do young patients need? Arch Dis Child. 2006;91(7):588 593. ( 10.1136/adc.2005.084285) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Lucas VS, Contreras A, Loukissa M, Roberts GJ. Dental disease indices and caries related oral microflora in children with phenylketonuria. ASDC J Dent Child. 2001;68(4):263 267, 229. [PubMed] [Google Scholar]
- 6. Johnson R, Gardner R, Kozlowski R. Phenylketonuria--oral manifestations. ASDC J Dent Child. 1970;37(6):515 518. [PubMed] [Google Scholar]
- 7. Ballikaya E, Yildiz Y, Sivri HS, et al. Oral health status of children with phenylketonuria. J Pediatr Endocrinol Metab. 2020;33(3):361 365. ( 10.1515/jpem-2019-0439) [DOI] [PubMed] [Google Scholar]
- 8. Moradi G, Mohamadi Bolbanabad A, Moinafshar A, Adabi H, Sharafi M, Zareie B. Evaluation of oral health status based on the decayed, missing and filled teeth (DMFT) index. Iran J Public Health. 2019;48(11):2050 2057. ( 10.18502/ijph.v48i11.3524) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Petersen PE. The World Oral Health Report 2003: continuous improvement of oral health in the 21st century--the approach of the WHO Global Oral Health Programme. Community Dent Oral Epidemiol. 2003;31(suppl 1):3 23. ( 10.1046/j..2003.com122.x) [DOI] [PubMed] [Google Scholar]
- 10. Broadbent JM, Thomson WM. For debate: problems with the DMF index pertinent to dental caries data analysis. Community Dent Oral Epidemiol. 2005;33(6):400 409. ( 10.1111/j.1600-0528.2005.00259.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Roland E, Gueguen G, Longis MJ, Boiselle J. Validation of the reproducibility of the DMF Index used in bucco-dental epidemiology and evaluation of its 2 clinical forms. World Health Stat Q. 1994;47(2):44 61. [PubMed] [Google Scholar]
- 12. Kilpatrick NM, Awang H, Wilcken B, Christodoulou J. The implication of phenylketonuria on oral health. Pediatr Dent. 1999;21(7):433 437. [PubMed] [Google Scholar]
- 13. Liappis N, Pohl B, Weber HP, el-Karkani H. Free amino acids in the saliva of children with phenylketonuria. Klin Padiatr. 1986;198(1):25 28. ( 10.1055/s-2008-1026847) [DOI] [PubMed] [Google Scholar]
- 14. Wang K, Shen M, Li H, Li X, He C. Reduced bone mineral density in Chinese children with phenylketonuria. J Pediatr Endocrinol Metab. 2017;30(6):651 656. ( 10.1515/jpem-2016-0308) [DOI] [PubMed] [Google Scholar]
- 15. Winter GB, Murray JJ, Goose DHJCr. Prevalence of dental caries in phenylketonuric children. Caries Res. 1974;8(3):256 266. ( 10.1159/000260114) [DOI] [PubMed] [Google Scholar]
- 16. Gökalp SG, Doğan BG, Tekçiçek MT, Berberoğlu A, Unlüer S. National survey of oral health status of children and adults in Turkey. Community Dent Health. 2010;27(1):12 17. [PubMed] [Google Scholar]
- 17. Ābola I, Emuliņa DE, Skadiņš I, Brinkmane A, Gailīte L, Auzenbaha M. Dental status and periodontal health of patients with phenylketonuria in Latvia. Acta Stomatol Croat. 2022;56(2):109 119. ( 10.15644/asc56/2/2) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kumar Dalei S, Adlakha N. Food regime for phenylketonuria: presenting complications and possible solutions. J Multidiscip Healthc. 2022;15:125 136. ( 10.2147/JMDH.S330845) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Hall SK, Robinson P, Green A. Could salivary phenylalanine concentrations replace blood concentrations? Ann Clin Biochem. 2000;37(2):222 223. ( 10.1258/0004563001899050) [DOI] [PubMed] [Google Scholar]



 Content of this journal is licensed under a
Content of this journal is licensed under a