Introduction
There is an unmet need for new treatments for idiopathic pulmonary fibrosis (IPF). The oral preferential phosphodiesterase 4B inhibitor, BI 1015550, prevented a decline in forced vital capacity (FVC) in a phase II study in patients with IPF. This study design describes the subsequent pivotal phase III study of BI 1015550 in patients with IPF (FIBRONEER-IPF).
Methods and analysis
In this placebo-controlled, double-blind, phase III trial, patients are being randomised in a 1:1:1 ratio to receive 9 mg or 18 mg of BI 1015550 or placebo two times per day over at least 52 weeks, stratified by use of background antifibrotics (nintedanib/pirfenidone vs neither). The primary endpoint is the absolute change in FVC at week 52. The key secondary endpoint is a composite of time to first acute IPF exacerbation, hospitalisation due to respiratory cause or death over the duration of the trial.
Ethics and dissemination
The trial is being carried out in compliance with the ethical principles of the Declaration of Helsinki, in accordance with the International Council on Harmonisation Guideline for Good Clinical Practice and other local ethics committees. The results of the study will be disseminated at scientific congresses and in peer-reviewed publications.
Trial registration number
Keywords: Lung Transplantation
WHAT IS ALREADY KNOWN ON THIS TOPIC
Idiopathic pulmonary fibrosis (IPF) is a chronic, progressive disease associated with a decline in lung function and early mortality. In a phase II trial, treatment with the oral preferential phosphodiesterase 4B inhibitor, BI 1015550, prevented a decline in lung function over 12 weeks and had an acceptable safety profile in patients with IPF, regardless of background use of antifibrotics, supporting phase III clinical development.
WHAT THIS STUDY ADDS
This article describes the study design of FIBRONEER-IPF, a phase III, double-blind, randomised, placebo-controlled trial of BI 1015550 in patients with IPF, either alone or in addition to background antifibrotic treatment.
HOW THIS STUDY MIGHT AFFECT RESEARCH, PRACTICE OR POLICY
The FIBRONEER-IPF trial will provide further evidence on the efficacy and safety of BI 1015550 in patients with IPF, potentially leading to a new treatment for what remains a life-shortening disease.
Introduction
Idiopathic pulmonary fibrosis (IPF) is a chronic and progressive fibrotic interstitial lung disease (ILD) of unknown cause that is characterised by progressive lung function decline, increased symptom burden and early mortality.1–3 Treatment options for IPF are currently limited to two antifibrotic treatments, nintedanib and pirfenidone, which have been shown to reduce the decline in forced vital capacity (FVC).4 5 Management of IPF may also include pulmonary rehabilitation, which has been shown to improve exercise capacity, dyspnoea and health-related quality of life (QoL).6 7 Lung transplantation has been shown to increase life expectancy and may be considered for selected patients.8
Nintedanib is a tyrosine kinase inhibitor that has been approved for the treatment of IPF, non-IPF progressive pulmonary fibrosis (PPF) and systemic sclerosis-associated ILD.9–11 Pirfenidone is a pyridine with an unknown mechanism of action and has been approved for the treatment of IPF.12–14 Although these antifibrotic treatments slow down the progressive decline in lung function over time, prognosis remains poor in most patients with IPF, as their lung function continues to decline, leading to premature death due to respiratory failure.15 In addition, nintedanib and pirfenidone are both associated with adverse events, which can result in treatment discontinuation.4 5 16–20 Therefore, there is an unmet need for more effective treatments with acceptable tolerability that can modify the disease course and preserve QoL in patients with IPF.
Phosphodiesterase 4 (PDE4) enzymes mediate the breakdown of the intracellular secondary messenger cyclic adenosine monophosphate, and are implicated in a range of inflammatory cell functions.21 There are four PDE4 subtypes (PDE4A, B, C, D) with varied roles and distributions in the body.22 23 PDE4 inhibition has been investigated as a treatment in inflammatory respiratory diseases, such as chronic obstructive pulmonary disease, and may also have potential therapeutic effects in pulmonary fibrosis through its regulation of inflammation and the modulation of immunocompetent cells.24–28 Common adverse events associated with marketed oral pan-PDE4 inhibitors include diarrhoea, nausea and headache,26 with other adverse events including depression, suicidal ideation and behaviour.29 30 PDE4 inhibitors have also been associated with vasculitis in preclinical toxicology studies.31 The emetic side effects associated with pan-PDE4 inhibition may be linked to the inhibition of the PDE4D subtype.32 It has been suggested that preferential inhibition of the PDE4B subtype may lead to anti-inflammatory and antifibrotic effects but with a reduced risk of side effects,25 33 making it a potential promising target for treating pulmonary fibrosis.23
BI 1015550 is an oral preferential PDE4B inhibitor with approximately 10-fold selectivity for inhibition of PDE4B compared with PDE4D.33 In preclinical studies, BI 1015550 was associated with anti-inflammatory and antifibrotic effects in in vitro and in vivo models of pulmonary fibrosis, and demonstrated potential synergistic effects with nintedanib on fibroblast proliferation.33 In phase I trials, treatment with BI 1015550 showed acceptable safety and tolerability in healthy male adults and in male and female patients with IPF.34 In a phase II trial, the efficacy and safety of BI 1015550 were investigated in 147 patients with IPF stratified by use of background antifibrotics (nintedanib/pirfenidone vs neither).35 Treatment with BI 1015550 18 mg two times per day prevented a decline in FVC over 12 weeks and had an acceptable safety profile both as a monotherapy and in addition to background antifibrotic treatment.35
This manuscript describes the design of the subsequent study, FIBRONEER-IPF, a randomised, placebo-controlled, pivotal phase III trial investigating the safety and efficacy of BI 1015550 9 mg and 18 mg two times per day compared with placebo in adult patients with IPF.
Methods and analysis
Trial design
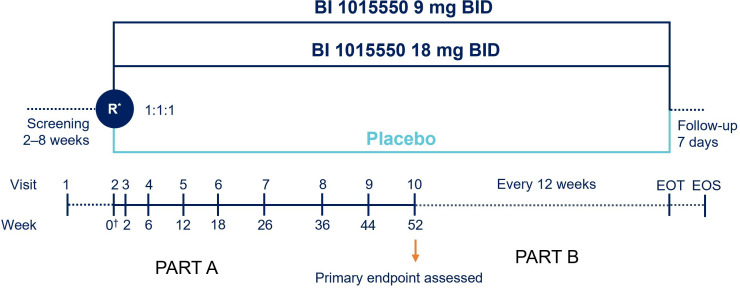
This multicentre, double-blind, randomised, placebo-controlled trial is investigating the efficacy and safety of BI 1015550 in patients with IPF stratified by use of background antifibrotics over at least 52 weeks (EudraCT: 1305-0014; ClinicalTrials.gov: NCT05321069). The trial began in September 2022 and is estimated to complete in November 2024. Patients are being recruited globally from approximately 400 sites in 45 countries across the Americas, Europe, Africa, Australia and Asia (online supplemental figure 1). Patients will be enrolled at both urban and rural centres, ILD academic and expert centres, as well as community pulmonary centres, all with pulmonologists as principal investigators. It is planned that 963 patients will be randomised in a 1:1:1 ratio to receive 9 mg or 18 mg of BI 1015550 or placebo two times per day. Randomisation will be performed with the use of an interactive voice-response system and stratified according to background use of an antifibrotic treatment at screening. The trial is composed of two parts (see figure 1). In part A, patients will attend study visits over a 52-week treatment period. This will be followed by part B, during which patients will remain in the study and continue treatment with blinded trial medication over a variable period until the end of the trial, which will be defined as the point when the last patient randomised is expected to reach 52 weeks of treatment. A follow-up visit will be performed 7 days after the end of treatment. Patients who discontinue early from their treatment will be asked to continue study visits. Temporary treatment interruption of BI 1015550 will be considered to manage adverse events, and reintroduction of treatment will be encouraged if considered safe by the investigator. Dose reductions and treatment interruption of background antifibrotics are allowed to manage adverse events, as per the prescribing information for each drug.9 13
Figure 1.
FIBRONEER-IPF trial design. *Randomisation will be stratified by use of background antifibrotic (nintedanib/pirfenidone vs neither). †Day 1. Part B will begin from visit 10 to EOT over a variable period; patients will continue treatment with blinded trial medication and have trial visits every 12 weeks. EOS is expected to be up to 130 weeks with an assumed recruitment period of 18 months. BID, two times per day; EOS, end of study; EOT, end of trial (ie, last randomised patient reaches 52 weeks of treatment); R, randomisation.
bmjresp-2022-001563supp001.pdf (919.8KB, pdf)
Patient population
Eligible patients are aged ≥40 years, have received a diagnosis of IPF, and have an FVC≥45% of predicted and a diffusing capacity of the lung for carbon monoxide (DLco) ≥25% and <90% of predicted. A diagnosis of IPF is based on: (1) 2022 international guidelines,3 as confirmed by the investigator based on a chest high-resolution CT (HRCT) scan taken within 12 months of visit 1; and (2) usual interstitial pneumonia (UIP) or probable UIP at HRCT, as confirmed by central review before randomisation. Patients with an HRCT finding indeterminate for UIP are eligible if a clinical diagnosis of IPF can be confirmed locally, based on historical surgical lung biopsy or cryobiopsy demonstrating a histopathology pattern consistent with UIP or probable UIP.
As patients are stratified by background antifibrotic use, patients with background antifibrotic treatment are defined as patients on stable treatment with an approved antifibrotic drug (nintedanib or pirfenidone) for at least 12 weeks prior to study entry and planning to continue this background treatment during the trial. Patients without background antifibrotic treatment must not have been on approved antifibrotic treatment for at least 8 weeks before and throughout the duration of the trial, but antifibrotic treatment may be used as rescue medication after the first 12 weeks as deemed appropriate by the investigator.
Patients who have relevant acute or chronic infections, an acute IPF exacerbation 3 months before visit 1 and/or during the screening period, active vasculitis within the past 8 weeks, suicidal behaviour in the past 2 years or suicidal ideation of type 4 or 5 on the Columbia-Suicide Severity Rating Scale (C-SSRS) (past 3 months) are excluded. Patients who are taking immunosuppressants other than prednisone ≤15 mg/day or equivalent for respiratory or pulmonary indications are excluded. The main inclusion and exclusion criteria are shown in box 1 and online supplemental table 1.
Box 1. Main inclusion and exclusion criteria.
Inclusion criteria
An IPF diagnosis based on 2022 international guidelines.3
Chest HRCT scans must be performed within 12 months of visit 1 and confirmed by central review prior to visit 2.
Patients on stable treatment with background antifibrotic treatment need to have received antifibrotic treatment for at least 12 weeks prior to visit 1 and during screening, and plan to stay on this background treatment after randomisation. A combination of nintedanib plus pirfenidone is not allowed. Patients not on stable treatment with background antifibrotics need to have not received antifibrotic treatment for at least 8 weeks prior to visit 1 and during the screening period (eg, either antifibrotic treatment naïve or previously discontinued), and must not be planning to start or restart antifibrotic treatment.*
-
To meet the following criteria at visit 1:
FVC≥45% predicted.
DLco corrected for Hb≥25% and <90% predicted.
Exclusion criteria
Relevant airways obstruction (prebronchodilator FEV1/FVC ratio <0.7) at visit 1.
Experienced an acute IPF exacerbation within 3 months and/or during the screening period.
Confirmed infection with SARS-CoV-2 and not fully recovered according to investigator judgement within the 4 weeks prior to randomisation.
AST or ALT>2.5×ULN or total bilirubin >1.5×ULN at visit 1.
eGFR≤30 mL/min/1.73 m2 at visit 1 (CKD-EPI formula or Japanese version of CKD-EPI for Japanese patients).
Active, unstable or uncontrolled vasculitis within the past 8 weeks prior to visit 1 or during the screening period.
Any suicidal behaviour (past 2 years) or any suicidal ideation of type 4 or 5 on the C-SSRS in the past 3 months.
Acute or chronic severe depression defined as HADS subscore >14 at visit 1 and/or visit 2.
Patients treated with PDE1, PDE3, PDE4 or PDE10 inhibitors, or non-selective PDE inhibitors, within 30 days before visit 1.
ALT, alanine aminotransferase; AST, aspartate aminotransferase; CKD-EPI, Chronic Kidney Disease Epidemiology Collaboration; C-SSRS, Columbia-Suicide Severity Rating Scale; DLco, diffusing capacity of the lung for carbon monoxide; eGFR, estimated glomerular filtration rate; FEV1, forced expiratory volume in 1 s; FVC, forced vital capacity; IPF, idiopathic pulmonary fibrosis; HADS, Hospital Anxiety and Depression Scale; Hb, haemoglobin; HRCT, high-resolution computed tomography; PDE, phosphodiesterase; SARS, severe acute respiratory syndrome; ULN, upper limit of normal.
*Stable therapy is defined as the individually and generally tolerated regimen of either nintedanib or pirfenidone (no dose changes) for at least 12 weeks.
Endpoints
The primary endpoint is absolute change from baseline in FVC (mL) at week 52. This will be assessed using spirometry according to American Thoracic Society/European Respiratory Society 2019 guidelines.36 The key secondary endpoint is a composite of the time to first acute IPF exacerbation, first hospitalisation for respiratory cause or death (whichever occurs first) over the duration of the trial. Other endpoints include change in QoL, as measured by the patient-reported outcome measure (PROM), the Living with Pulmonary Fibrosis Questionnaire (L-PF).37 Time-to-event endpoints will also be assessed separately over the duration of the trial, including time to first acute IPF exacerbation or death, and time to first hospitalisation due to respiratory cause or death. Absolute change from baseline in DLco% predicted and FVC% predicted will also be assessed at week 52 (box 2 and online supplemental table 2).
Box 2. Endpoints to be assessed.
Primary endpoint
Absolute change from baseline in FVC (mL) at week 52.
Key secondary endpoint
Time to first IPF acute exacerbation, first hospitalisation for respiratory cause or death, over the duration of the trial.
Other secondary endpoints
Time to first acute IPF exacerbation or death over the duration of the trial.
Time to hospitalisation for respiratory cause or death over the duration of the trial.
Time to absolute decline from baseline in FVC% predicted >10% from baseline or death over the duration of the trial.
Time to absolute decline in DLco% predicted >15% from baseline or death over the duration of the trial.
Time to death over the duration of the trial.
Absolute change from baseline in L-PF Symptoms dyspnoea domain score at week 52.
Absolute change from baseline in L-PF Symptoms cough domain score at week 52.
Absolute change from baseline in L-PF Symptoms fatigue domain score at week 52.
Absolute change from baseline in FVC% predicted at week 52.
Absolute change from baseline in DLco% predicted at week 52.
DLco, diffusing capacity of the lung for carbon monoxide; FVC, forced vital capacity; IPF, idiopathic pulmonary fibrosis; L-PF, living with pulmonary fibrosis.
Pharmacokinetic endpoints include predose plasma concentrations of BI 1015550. Exploratory blood biomarkers of inflammation, epithelial injury, tissue degradation and fibrosis will be assessed in blood over time, including protein biomarkers. Collection of blood samples for DNA banking is optional and not a prerequisite for participation (only for patients who have signed a separate informed consent form). Samples may be analysed retrospectively.
Safety
For safety analyses, physical examination, vital signs, safety laboratory parameters and ECG will be performed. Depression, suicidal ideation and behaviour are listed as side effects associated with marketed oral pan-PDE4 inhibitors.38–42 Suicide risk monitoring is required during the clinical development of PDE4 inhibitors, and this will be assessed using the C-SSRS in this study. Depression and anxiety will be measured using the Hospital Anxiety and Depression Scale (HADS).
Adverse events will be coded using the Medical Dictionary for Drug Regulatory Activities. Adverse events of special interest, which include potential severe drug-induced liver injury, vasculitis, severe infections, new onset of severe depression or new onset of severe anxiety, will be monitored throughout the study.
Statistical analysis
The primary endpoint of absolute change from baseline in FVC (mL) at week 52 will be estimated using a mixed-effect model for repeated measures analysis, including treatment at each visit, baseline background antifibrotic treatment and continuous fixed effects for baseline FVC (mL) at each visit as covariates. The key secondary endpoint of time to first acute IPF exacerbation, first hospitalisation for respiratory cause or death will be analysed using a Cox proportional hazards model using data over the whole trial (ie, including data beyond 52 weeks). The model will include treatment, background antifibrotic treatment, age and FVC% predicted and DLco% predicted (corrected for haemoglobin) at baseline as covariates. Kaplan-Meier plots will be presented for each treatment arm. The percentage of patients with an event at week 52 will be presented descriptively and by Kaplan-Meier estimates.
To account for multiple testing associated with two doses of BI 1015550 being compared with placebo for both the primary and key secondary endpoints, a graphical testing procedure43 will be applied, with a focus on the 18 mg dose and primary endpoint. A sample size of 321 patients per group will yield 90% power to detect an effect of a 74 mL change in FVC at week 52 for a BI 1015550 dose group over placebo, with an overall type I error rate of 0.05. For the primary endpoint, statistical significance will be declared if the analyses in either dose group are statistically significant at the designated alpha level according to the graphical testing procedure (online supplemental figure 2).
In the primary analysis, intercurrent events except for death will be handled via a treatment policy strategy. Missing data will not be imputed except for death, for which a poor outcome will be assigned. The intercurrent event of lung transplantation will be treated with a hypothetical strategy approach (which considers the effect of what would have happened if the intercurrent event did not occur) with the data set as missing and handled by the model via the missing at random (MAR) assumption (primary endpoint) or censoring (key secondary endpoint). The mixed-effect model will handle missing data based on a likelihood method under the ‘MAR’ assumption. A tipping-point analysis is planned as a sensitivity analysis to assess how robust the primary analysis is against deviations from the MAR assumption. The effect of missing data on the primary endpoint will be further investigated using other multiple imputation techniques. In addition, sensitivity analysis including additional covariates in the primary analysis model will be performed.
Trial governance and oversight
An independent data monitoring committee (DMC) that may be unblinded to the treatment allocation will evaluate safety data on a regular basis. An interim analysis to assess the potential futility of the BI 1015550 9 mg two times per day arm will be conducted by the independent DMC after approximately 300 patients (100 patients per arm) have completed 12 weeks of treatment. The independent DMC will make a recommendation to the sponsor on the continuation or stoppage of the BI 1015550 9 mg two times per day dose arm.
Two independent adjudication committees will conduct regular reviews of the trial safety data. An independent adjudication committee involving cardiologists and neurologists will review all fatal cases for the primary cause of death and will review all adverse events categorised as major adverse cardiovascular events. A separate independent adjudication committee of rheumatologists and vasculitis experts will review all events of suspected or possible vasculitis.
A contingency plan has been developed for this trial in case patients are not able to perform an on-site visit due to their health status or COVID-19, which includes the direct shipment of investigational products to patients, remote check-ups and home spirometry.
Patient and public involvement
Feedback on the trial design was obtained from a trial simulation involving patients, caregivers and healthcare professionals, and from advisory board meetings with members of patient organisations and expert investigators.
Discussion
Rationale for conducting the study
Real-world data from registries suggest that antifibrotics improve survival in patients with IPF; however, these treatments do not stop disease progression and are associated with adverse events, such as diarrhoea and nausea, leading to a proportion of patients discontinuing treatment.18–20 Therefore, there is a need for additional treatments that can be used as monotherapy or in addition to existing antifibrotic treatments. Preferential PDE4B inhibition demonstrates anti-inflammatory and antifibrotic effects, suggesting that it may have potential as a treatment for patients with IPF and other forms of PPF.33 A phase II trial of BI 1015550 in patients with IPF showed promising results in preventing a decline in lung function combined with an acceptable safety profile, with the most frequently reported adverse event being diarrhoea.35 However, this proof-of-concept study was limited by its 12-week duration and relatively small sample size.35
BI 1015550 is the first oral preferential PDE4B inhibitor advancing to phase III clinical development in IPF. The FIBRONEER-IPF trial is investigating the efficacy and safety of BI 1015550, alone or in combination with background antifibrotic use, in patients with IPF. As IPF and PPF may share common pathobiological pathways for pulmonary fibrosis,44 45 a sister trial is underway that is evaluating the efficacy and safety of BI 1015550 in patients with PPF (FIBRONEER-ILD; NCT05321082). Collectively, these studies add to the body of evidence for the safety and efficacy of BI 1015550 in pulmonary fibrosis.
FIBRONEER-IPF is also investigating BI 1015550 as an add-on therapy to background antifibrotics, which are the standard of care in IPF.46 Preclinical data show that BI 1015550 has complementary activity to nintedanib on human myofibroblast transformation and a synergistic effect in combination with nintedanib on fibroblast proliferation.33 A post hoc analysis of the phase II trial also suggests that BI 1015550 may have an additive effect with nintedanib.47 Given that there are multiple fibrotic pathways involved in IPF48 49 and preclinical evidence suggesting synergistic effects with nintedanib,33 data from this trial on the use of BI 1015550 in patients receiving background antifibrotics may lead to a new frontier for IPF combination treatments.
Rationale for trial design
The age and lung function inclusion criteria of this study are inclusive of a broader population of patients compared with previous IPF clinical trials.4 5 There is no upper limit for age or FVC, and patients will be included if they have FVC% predicted ≥45%, which may be more representative of patients with IPF in the real world.
Due to the COVID-19 pandemic, there may be unprecedented challenges involved with setting up a multicentre and international phase III trial.50 Several contingency measures will be implemented if required to mitigate potential disruptions from COVID-19 when initiating and conducting the trial or if a patient with a poor health status cannot attend on-site visits.
Rationale for dose selection
The selection of the BI 1015550 18 mg two times per day dose is supported by the results from the phase II study, in which treatment with this dose was associated with preserving lung function in patients with IPF.35 In this phase III trial, we have an additional 9 mg two times per day dose to evaluate the benefit–risk profile at a lower dose, as well as to provide further dose–response and exposure–response data. An interim analysis is planned to assess the efficacy and safety profile of the 9 mg two times per day dose, ensuring patients are exposed to the lower dose for the shortest period possible in case of a non-favourable benefit–risk profile.
Rationale for endpoints
Change in FVC is a commonly used primary endpoint in IPF trials and has been associated with mortality.51 52 Patients in this study have a treatment period of at least 52 weeks and remain on blinded treatment for a variable period beyond week 52 depending on the recruitment rate. This will allow more time to assess time-to-event efficacy endpoints, since changes in these clinically meaningful outcomes may vary in occurrence and may only become apparent over a longer time period than 52 weeks.53 In addition, for the assessment of the key secondary endpoint (time to first exacerbation, hospitalisation due to respiratory cause or death), a longer follow-up should allow more events to be captured and consequently increase the power of the analysis. An issue in IPF clinical trials is determining appropriate PROMs with good sensitivity to capture change in QoL over time.54 The L-PF questionnaire is a validated PROM that can be used in patients with IPF35 37 and was found to best capture the signs/symptoms and impacts on patients in a consensus study on PROMs for IPF and PPF.55
Depression, suicidal ideation and behaviour have also been reported in previous studies of pan-PDE4 inhibitors.38 56–59 New onset of severe depression, defined as HADS subscore >14, and new onset of severe anxiety, defined as HADS subscore >14, are investigated as adverse events of special interest as part of the safety assessment in this trial. In preclinical toxicology studies, vasculitis has been associated with PDE4 inhibitor treatment.31 In the phase I trials with BI 1015550, there were no confirmed adverse events of vasculitis, depression or suicidal ideation or behaviour.34 In the phase II study, there was one patient with suspected vasculitis, which was not confirmed by the independent DMC, and one report of suicidal ideation type 1 that had occurred after the residual effect period of BI 1015550, 9 days after the completion of treatment.35 Patients are monitored for vasculitis during this trial and suspected cases will be assessed by independent adjudication and DMCs.
Conclusions
FIBRONEER-IPF is the first phase III trial of a preferential PDE4B inhibitor in patients with IPF. The results of this trial will increase our understanding of the safety and efficacy of BI 1015550 as a monotherapy or in combination with current antifibrotic standard of care in a larger and broader population of patients with IPF. These data will help to address an unmet need for new treatments for patients with IPF and potentially provide evidence for combination treatment in IPF.
Acknowledgments
The authors meet criteria for authorship as recommended by the International Committee of Medical Journal Editors (ICMJE). The authors did not receive payment related to the development of the manuscript. Darren Chow, MSc, of Meditech Media provided writing, editorial support and formatting assistance, which was contracted and funded by Boehringer Ingelheim International (BI). BI was given the opportunity to review the manuscript for medical and scientific accuracy as well as intellectual property considerations.
Footnotes
Contributors: All authors were involved in the study design, reviewed and revised drafts of the manuscript and approved the final draft.
Funding: This trial is supported and funded by Boehringer Ingelheim International GmbH.
Map disclaimer: The inclusion of any map (including the depiction of any boundaries therein), or of any geographic or locational reference, does not imply the expression of any opinion whatsoever on the part of BMJ concerning the legal status of any country, territory, jurisdiction or area or of its authorities. Any such expression remains solely that of the relevant source and is not endorsed by BMJ. Maps are provided without any warranty of any kind, either express or implied.
Competing interests: LR has received research grants from Boehringer Ingelheim and the Italian Medicine Agency; has been an advisory board member for Roche, Boehringer Ingelheim, FibroGen, Biogen and Promedior; has been involved in consulting activity for Biogen, Celgene, Nitto, Pliant Therapeutics, Toray, BMS, RespiVant, Zambon and CSL Behring; has received payment for lectures from Boehringer Ingelheim, Zambon and Cipla; has received support for attending meetings from Boehringer Ingelheim and Roche; and been a steering committee member for Boehringer Ingelheim and Roche. AA has received research grants and speaker fees from Boehringer Ingelheim and Taiho Pharm. Co.; and has been an advisory committee member for Boehringer Ingelheim, Taiho Pharm. Co., Toray Medical Co. and Kyorin Pharm. Co. VC has received unrestricted grants from Boehringer Ingelheim; consulting fees from AstraZeneca, Boehringer Ingelheim, Celgene/BMS, CSL Behring, Ferrer, Galapagos, Pliant Therapeutics, PureTech, RedX, Roche, Sanofi and Shionogi; lecture fees from Boehringer Ingelheim and Roche; support for attending meetings from Boehringer Ingelheim and F. Hoffmann-La Roche; has participated in data and safety monitoring boards for Galapagos, Galecto and Roche; and has been on an adjudication committee for FibroGen. MK is an advisor or review panel member for Boehringer Ingelheim, Galapagos and Roche; and has received consultancy fees, grants and speaker fees from Boehringer Ingelheim and Roche. TMM has received consulting fees from Boehringer Ingelheim, Roche/Genentech, AstraZeneca, Bayer, Blade Therapeutics, Bristol Myers Squibb, Galapagos, Galecto, GSK, IQVIA, Pliant Therapeutics, Respivant, Theravance Biopharma and Veracyte. He has also received speaker fees from Boehringer Ingelheim and Roche/Genentech. FJM has served on a steering committee, advisory board, data safety monitoring board or adjudication committee for Afferent/Merck, Bayer, Biogen, Boehringer Ingelheim, DevPro, Nitto, Novartis, Respivant, Roche and Veracyte; and has received consulting fees or payment for presentations from AbbVie, Boehringer Ingelheim, Bristol Myers Squibb, Bridge Biotherapeutics, CSL Behring, DevPro, IQVIA, Lung Therapeutics, Roche/Genentech, Sanofi, Shionogi, twoXAR, United Therapeutics and Veracyte. JMO has received consultancy fees and grants from Boehringer Ingelheim and consultancy fees from Roche/Genentech and Lupin Pharmaceuticals. MG, YL, SS and DFZ are employees of Boehringer Ingelheim. CV has received personal fees from Boehringer Ingelheim, F. Hoffmann-La Roche and Bristol Myers Squibb; and support for attending meetings from Boehringer Ingelheim and F. Hoffmann-La Roche. MSW has received grants from AstraZeneca-Daiichi, Boehringer Ingelheim, F. Hoffmann-La Roche, The Netherlands Organisation for Health Research and Development, The Dutch Lung Foundation and The Dutch Pulmonary Society; consulting fees from Boehringer Ingelheim, Galapagos, Bristol Myers Squibb, Galecto, Respivant and NeRRe Therapeutics, Horizon Therapeutics, PureTech Health, Kinevant Sciences, Molecure, CSL Behring, Thyron and Vicore; speaker fees from Boehringer Ingelheim, Galapogas, F. Hoffmann-La Roche, Novartis and CSL Behring; support for attending meetings from Boehringer Ingelheim, Galapagos and F. Hoffmann-La Roche; has participated in advisory boards for Savara, Galapagos and Dutch lung fibrosis and sarcoidosis patient associations (unpaid); and has held leadership roles as Chair of the Idiopathic Interstitial Pneumonia group of the European Respiratory Society, Member of the board of the Netherlands Respiratory Society, Member of the scientific advisory board of the European Idiopathic Pulmonary Fibrosis and Related Disorders Federation and Chair of the educational committee of the European Reference Network for Rare Lung Diseases. All grants and fees were paid to her institution.
Patient and public involvement: Patients and/or the public were involved in the design, or conduct, or reporting, or dissemination plans of this research. Refer to the Methods section for further details.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Data availability statement
Data are available on reasonable request. To ensure independent interpretation of clinical study results and enable authors to fulfill their role and obligations under the ICMJE criteria, Boehringer Ingelheim grants all external authors access to clinical study data pertinent to the development of the publication. In adherence with the Boehringer Ingelheim Policy on Transparency and Publication of Clinical Study Data, scientific and medical researchers can request access to clinical study data when it becomes available on https://vivli.org/, and earliest after publication of the primary manuscript in a peer-reviewed journal, regulatory activities are complete, and other criteria are met. Please visit https://www.mystudywindow.com/msw/datasharing for further information.
Ethics statements
Patient consent for publication
Not applicable.
Ethics approval
The trial is being carried out in compliance with the ethical principles of the Declaration of Helsinki, in accordance with the International Council on Harmonisation Guideline for Good Clinical Practice, the EU directive 2001/20/EC and the Japanese Good Clinical Practice regulations (Ministry of Health and Welfare Ordinance No. 28, 27 March 1997). Written informed consent was obtained from each patient before entry into the study. The results of the study will be disseminated at scientific congresses and in peer-reviewed publications.
References
- 1.Raghu G, Collard HR, Egan JJ, et al. An official ATS/ERS/JRS/ALAT statement: idiopathic pulmonary fibrosis: evidence-based guidelines for diagnosis and management. Am J Respir Crit Care Med 2011;183:788–824. 10.1164/rccm.2009-040GL [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lederer DJ, Martinez FJ. Idiopathic pulmonary fibrosis. N Engl J Med 2018;379:797–8. 10.1056/NEJMc1807508 [DOI] [PubMed] [Google Scholar]
- 3.Raghu G, Remy-Jardin M, Richeldi L, et al. Idiopathic pulmonary fibrosis (an update) and progressive pulmonary fibrosis in adults: an official ATS/ERS/JRS/ALAT clinical practice guideline. Am J Respir Crit Care Med 2022;205:e18–47. 10.1164/rccm.202202-0399ST [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Richeldi L, du Bois RM, Raghu G, et al. Efficacy and safety of nintedanib in idiopathic pulmonary fibrosis. N Engl J Med 2014;370:2071–82. 10.1056/NEJMoa1402584 [DOI] [PubMed] [Google Scholar]
- 5.King TE, Bradford WZ, Castro-Bernardini S, et al. A phase 3 trial of pirfenidone in patients with idiopathic pulmonary fibrosis. N Engl J Med 2014;370:2083–92. 10.1056/NEJMoa1402582 [DOI] [PubMed] [Google Scholar]
- 6.Dowman L, Hill CJ, May A, et al. Pulmonary rehabilitation for interstitial lung disease. Cochrane Database Syst Rev 2021;2:CD006322. 10.1002/14651858.CD006322.pub4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nolan CM, Polgar O, Schofield SJ, et al. Pulmonary rehabilitation in idiopathic pulmonary fibrosis and COPD: a propensity-matched real-world study. Chest 2022;161:728–37. 10.1016/j.chest.2021.10.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.George PM, Patterson CM, Reed AK, et al. Lung transplantation for idiopathic pulmonary fibrosis. Lancet Respir Med 2019;7:271–82. 10.1016/S2213-2600(18)30502-2 [DOI] [PubMed] [Google Scholar]
- 9.Boehringer Ingelheim International GmbH . OFEV® (nintedanib) capsules, for oral use. 2022. Available: https://docs.boehringer-ingelheim.com/Prescribing%20Information/PIs/Ofev/ofev.pdf [Accessed 24 Nov 2022].
- 10.US Food and Drug Administration . OFEV® (nintedanib): prescribing information. 2020. Available: https://www.accessdata.fda.gov/drugsatfda_docs/label/2020/205832s014lbl.pdf [Accessed 17 Aug 2022].
- 11.European Medicines Agency . OFEV® (nintedanib): summary of product characteristics. 2021. Available: https://www.ema.europa.eu/en/documents/product-information/ofev-epar-product-information_en.pdf [Accessed 12 Aug 2022].
- 12.US Food and Drug Administration . ESBRIET® (pirfenidone): prescribing information. 2019. Available: https://www.accessdata.fda.gov/drugsatfda_docs/label/2019/022535s012,208780s002lbl.pdf [Accessed 12 Aug 2022].
- 13.Genentech . ESBRIET® (pirfenidone) capsules and film-coated tablets, for oral use. 2022. Available: https://www.gene.com/download/pdf/esbriet_prescribing.pdf [Accessed 17 Aug 2022].
- 14.European Medicines Agency . Esbriet® (pirfenidone): summary of product characteristics. 2022. Available: http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/002154/WC500103049.pdf [Accessed 17 Aug 2022].
- 15.Wijsenbeek M, Cottin V. Spectrum of fibrotic lung diseases. N Engl J Med 2020;383:958–68. 10.1056/NEJMra2005230 [DOI] [PubMed] [Google Scholar]
- 16.Maher TM, Strek ME. Antifibrotic therapy for idiopathic pulmonary fibrosis: time to treat. Respir Res 2019;20:205. 10.1186/s12931-019-1161-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jouneau S, Gamez A-S, Traclet J, et al. A 2-year observational study in patients suffering from idiopathic pulmonary fibrosis and treated with Pirfenidone: a French ancillary study of PASSPORT. Respiration 2019;98:19–28. 10.1159/000496735 [DOI] [PubMed] [Google Scholar]
- 18.Lancaster L, Crestani B, Hernandez P, et al. Safety and survival data in patients with idiopathic pulmonary fibrosis treated with nintedanib: pooled data from six clinical trials. BMJ Open Respir Res 2019;6:e000397. 10.1136/bmjresp-2018-000397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Behr J, Prasse A, Wirtz H, et al. Survival and course of lung function in the presence or absence of antifibrotic treatment in patients with idiopathic pulmonary fibrosis: long-term results of the INSIGHTS-IPF registry. Eur Respir J 2020;56:1902279. 10.1183/13993003.02279-2019 [DOI] [PubMed] [Google Scholar]
- 20.Wright WA, Crowley LE, Parekh D, et al. Real-world retrospective observational study exploring the effectiveness and safety of antifibrotics in idiopathic pulmonary fibrosis. BMJ Open Respir Res 2021;8:e000782. 10.1136/bmjresp-2020-000782 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zuo H, Cattani-Cavalieri I, Musheshe N, et al. Phosphodiesterases as therapeutic targets for respiratory diseases. Pharmacol Ther 2019;197:225–42. 10.1016/j.pharmthera.2019.02.002 [DOI] [PubMed] [Google Scholar]
- 22.Azevedo MF, Faucz FR, Bimpaki E, et al. Clinical and molecular genetics of the phosphodiesterases (PDEs). Endocr Rev 2014;35:195–233. 10.1210/er.2013-1053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kolb M, Crestani B, Maher TM. Phosphodiesterase 4B inhibition: a potential novel strategy for treating pulmonary fibrosis. Eur Respir Rev 2023;32:220206. 10.1183/16000617.0206-2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Matsuhira T, Nishiyama O, Tabata Y, et al. A novel phosphodiesterase 4 inhibitor, Aa6216, reduces macrophage activity and fibrosis in the lung. Eur J Pharmacol 2020;885:173508. 10.1016/j.ejphar.2020.173508 [DOI] [PubMed] [Google Scholar]
- 25.Phillips JE. Inhaled phosphodiesterase 4 (PDE4) inhibitors for inflammatory respiratory diseases. Front Pharmacol 2020;11:259. 10.3389/fphar.2020.00259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Li H, Zuo J, Tang W. Phosphodiesterase-4 inhibitors for the treatment of inflammatory diseases. Front Pharmacol 2018;9:1048. 10.3389/fphar.2018.01048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sisson TH, Christensen PJ, Muraki Y, et al. Phosphodiesterase 4 inhibition reduces lung fibrosis following targeted type II alveolar epithelial cell injury. Physiol Rep 2018;6:e13753. 10.14814/phy2.13753 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Selige J, Hatzelmann A, Dunkern T. The differential impact of PDE4 subtypes in human lung fibroblasts on cytokine-induced proliferation and myofibroblast conversion. J Cell Physiol 2011;226:1970–80. 10.1002/jcp.22529 [DOI] [PubMed] [Google Scholar]
- 29.Cazzola M, Calzetta L, Rogliani P, et al. The discovery of Roflumilast for the treatment of chronic obstructive pulmonary disease. Expert Opin Drug Discov 2016;11:733–44. 10.1080/17460441.2016.1184642 [DOI] [PubMed] [Google Scholar]
- 30.Wells AF, Edwards CJ, Kivitz AJ, et al. Apremilast monotherapy in DMARD-naive psoriatic arthritis patients: results of the randomized, placebo-controlled PALACE 4 trial. Rheumatology (Oxford) 2018;57:1253–63. 10.1093/rheumatology/key032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dietsch GN, Dipalma CR, Eyre RJ, et al. Characterization of the inflammatory response to a highly selective PDE4 inhibitor in the rat and the identification of biomarkers that correlate with toxicity. Toxicol Pathol 2006;34:39–51. 10.1080/01926230500385549 [DOI] [PubMed] [Google Scholar]
- 32.Robichaud A, Stamatiou PB, Jin S-LC, et al. Deletion of phosphodiesterase 4D in mice shortens alpha(2)-adrenoceptor-mediated anesthesia, a behavioral correlate of emesis. J Clin Invest 2002;110:1045–52. 10.1172/JCI15506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Herrmann FE, Hesslinger C, Wollin L, et al. BI 1015550 is a PDE4B inhibitor and a clinical drug candidate for the oral treatment of idiopathic pulmonary fibrosis. Front Pharmacol 2022;13:838449. 10.3389/fphar.2022.838449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Maher TM, Schlecker C, Luedtke D, et al. Phase I studies of BI 1015550, a preferential Pde4B inhibitor, in healthy males and patients with idiopathic pulmonary fibrosis. ERJ Open Res 2022;8:00240-2022. 10.1183/23120541.00240-2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Richeldi L, Azuma A, Cottin V. Trial of a preferential phosphodiesterase 4B inhibitor for idiopathic pulmonary fibrosis. N Engl J Med 2022;386:2178. 10.1056/NEJMc2209529 [DOI] [PubMed] [Google Scholar]
- 36.Graham BL, Steenbruggen I, Miller MR, et al. Standardization of spirometry 2019 update. an official American Thoracic Society and European Respiratory Society technical statement. Am J Respir Crit Care Med 2019;200:e70–88. 10.1164/rccm.201908-1590ST [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Swigris J, Cutts K, Male N, et al. The living with pulmonary fibrosis questionnaire in progressive fibrosing interstitial lung disease. ERJ Open Res 2021;7:00145–2020. 10.1183/23120541.00145-2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cazzola M, Calzetta L, Rogliani P, et al. The discovery of roflumilast for the treatment of chronic obstructive pulmonary disease. Expert Opin Drug Discov 2016;11:733–44. 10.1080/17460441.2016.1184642 [DOI] [PubMed] [Google Scholar]
- 39.European Medicines Agency . Otezla® (apremilast): summary of product characteristics. 2022. Available: https://www.ema.europa.eu/en/documents/product-information/otezla-epar-product-information_en.pdf [Accessed 2 Aug 2022].
- 40.US Food and Drug Administration . OTEZLA® (apremilast): prescribing information. 2021. Available: https://www.accessdata.fda.gov/drugsatfda_docs/label/2021/205437s011lbl.pdf [Accessed 23 Feb 2022].
- 41.US Food and Drug Administration . DALIRESP® (roflumilast): prescribing information. 2018. Available: https://www.accessdata.fda.gov/drugsatfda_docs/label/2018/022522s009lbl.pdf [Accessed 28 Jan 2022].
- 42.European Medicines Agency . Daxas® (roflumilast): summary of product characteristics. 2022. Available: https://www.ema.europa.eu/en/documents/product-information/daxas-epar-product-information_en.pdf [Accessed 2 Aug 2022].
- 43.Bretz F, Maurer W, Brannath W, et al. A graphical approach to sequentially rejective multiple test procedures. Stat Med 2009;28:586–604. 10.1002/sim.3495 [DOI] [PubMed] [Google Scholar]
- 44.Kolb M, Vašáková M. The natural history of progressive fibrosing interstitial lung diseases. Respir Res 2019;20:57. 10.1186/s12931-019-1022-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Selman M, Pardo A. From pulmonary fibrosis to progressive pulmonary fibrosis: a lethal pathobiological jump. Am J Physiol Lung Cell Mol Physiol 2021;321:L600–7. 10.1152/ajplung.00310.2021 [DOI] [PubMed] [Google Scholar]
- 46.Kaner RJ, Bajwa EK, El-Amine M, et al. Design of idiopathic pulmonary fibrosis clinical trials in the era of approved therapies. Am J Respir Crit Care Med 2019;200:133–9. 10.1164/rccm.201903-0592PP [DOI] [PubMed] [Google Scholar]
- 47.Richeldi L, Azuma A, Cottin V, et al. Additive effect of BI 1015550 and nintedanib in patients with IPF (OA1396). ERS International Congress 2022 abstracts; Barcelona, Spain: European Respiratory Society, September 4, 2022. 10.1183/13993003.congress-2022.4606 [DOI] [Google Scholar]
- 48.Sontake V, Gajjala PR, Kasam RK, et al. New Therapeutics based on emerging concepts in pulmonary fibrosis. Expert Opin Ther Targets 2019;23:69–81. 10.1080/14728222.2019.1552262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mei Q, Liu Z, Zuo H, et al. Idiopathic pulmonary fibrosis: an update on pathogenesis [published Online First]. Front Pharmacol 2021;12:797292. 10.3389/fphar.2021.797292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Shiely F, Foley J, Stone A, et al. Managing clinical trials during COVID-19: experience from a clinical research facility. Trials 2021;22:62. 10.1186/s13063-020-05004-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Paterniti MO, Bi Y, Rekić D, et al. Acute exacerbation and decline in forced vital capacity are associated with increased mortality in idiopathic pulmonary fibrosis. Ann Am Thorac Soc 2017;14:1395–402. 10.1513/AnnalsATS.201606-458OC [DOI] [PubMed] [Google Scholar]
- 52.du Bois RM, Weycker D, Albera C, et al. Forced vital capacity in patients with idiopathic pulmonary fibrosis: test properties and minimal clinically important difference. Am J Respir Crit Care Med 2011;184:1382–9. 10.1164/rccm.201105-0840OC [DOI] [PubMed] [Google Scholar]
- 53.Nathan SD, Meyer KC. IPF clinical trial design and endpoints. Curr Opin Pulm Med 2014;20:463–71. 10.1097/MCP.0000000000000091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Swigris JJ, Brown KK, Abdulqawi R, et al. Patients' perceptions and patient-reported outcomes in progressive-fibrosing interstitial lung diseases. Eur Respir Rev 2018;27:180075. 10.1183/16000617.0075-2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wijsenbeek M, Molina-Molina M, Chassany O, et al. Developing a conceptual model of symptoms and impacts in progressive fibrosing interstitial lung disease to evaluate patient-reported outcome measures. ERJ Open Res 2022;8:00681-2021. 10.1183/23120541.00681-2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kavanaugh A, Gladman DD, Edwards CJ, et al. Long-term experience with apremilast in patients with psoriatic arthritis: 5-year results from a PALACE 1-3 pooled analysis. Arthritis Res Ther 2019;21:118. 10.1186/s13075-019-1901-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.US Food and Drug Administration . Roflumilast: tertiary pharmacology review. 2009. Available: https://www.accessdata.fda.gov/drugsatfda_docs/nda/2011/022522Orig1s000PharmR.pdf [Accessed 2 Aug 2022].
- 58.European Medicines Agency . Summary of risk management plan for Otezla® (Apremilast). 2022. Available: https://www.ema.europa.eu/en/documents/rmp-summary/otezla-epar-risk-management-plan-summary_en.pdf [Accessed 2 Aug 2022].
- 59.US Food and Drug Administration . Apremilast: tertiary pharmacology review. 2014. Available: https://www.accessdata.fda.gov/drugsatfda_docs/nda/2014/205437Orig1s000PharmR.pdf [Accessed 2 Aug 2022].
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjresp-2022-001563supp001.pdf (919.8KB, pdf)
Data Availability Statement
Data are available on reasonable request. To ensure independent interpretation of clinical study results and enable authors to fulfill their role and obligations under the ICMJE criteria, Boehringer Ingelheim grants all external authors access to clinical study data pertinent to the development of the publication. In adherence with the Boehringer Ingelheim Policy on Transparency and Publication of Clinical Study Data, scientific and medical researchers can request access to clinical study data when it becomes available on https://vivli.org/, and earliest after publication of the primary manuscript in a peer-reviewed journal, regulatory activities are complete, and other criteria are met. Please visit https://www.mystudywindow.com/msw/datasharing for further information.