Abstract
Objectives
This multicentre retrospective study in Japan aimed to assess the retention of biological disease-modifying antirheumatic drugs and Janus kinase inhibitors (JAKi), and to clarify the factors affecting their retention in a real-world cohort of patients with rheumatoid arthritis.
Methods
The study included 6666 treatment courses (bDMARD-naïve or JAKi-naïve cases, 55.4%; tumour necrosis factor inhibitors (TNFi) = 3577; anti-interleukin-6 receptor antibodies (aIL-6R) = 1497; cytotoxic T lymphocyte-associated antigen-4-Ig (CTLA4-Ig) = 1139; JAKi=453 cases). The reasons for discontinuation were divided into four categories (ineffectiveness, toxic adverse events, non-toxic reasons and remission); multivariate Cox proportional hazards modelling by potential confounders was used to analyse the HRs of treatment discontinuation.
Results
TNFi (HR=1.93, 95% CI: 1.69 to 2.19), CTLA4-Ig (HR=1.42, 95% CI: 1.20 to 1.67) and JAKi (HR=1.29, 95% CI: 1.03 to 1.63) showed a higher discontinuation rate due to ineffectiveness than aIL-6R. TNFi (HR=1.28, 95% CI: 1.05 to 1.56) and aIL-6R (HR=1.27, 95% CI: 1.03 to 1.57) showed a higher discontinuation rate due to toxic adverse events than CTLA4-Ig. Concomitant use of oral glucocorticoids (GCs) at baseline was associated with higher discontinuation rate due to ineffectiveness in TNFi (HR=1.24, 95% CI: 1.09 to 1.41), as well as toxic adverse events in JAKi (HR=2.30, 95% CI: 1.23 to 4.28) and TNFi (HR=1.29, 95%CI: 1.07 to 1.55).
Conclusions
TNFi (HR=1.52, 95% CI: 1.37 to 1.68) and CTLA4-Ig (HR=1.14, 95% CI: 1.00 to 1.30) showed a higher overall drug discontinuation rate, excluding non-toxicity and remission, than aIL-6R.
Keywords: antirheumatic agents, glucocorticoids, rheumatoid arthritis, tumor necrosis factor inhibitors
WHAT IS ALREADY KNOWN ON THIS TOPIC
Only a few studies have demonstrated drug retention of targeted synthetic disease-modifying antirheumatic drugs (tsDMARDs) and biological DMARDs in a real-world setting.
A recent large registry study demonstrated that anti-interleukin-6 receptor antibodies (aIL-6R) and Janus kinase inhibitors (JAKi) had higher drug retention due to effectiveness, despite having lower drug retention due to safety when compared with tumour necrosis factor inhibitors (TNFi).
WHAT THIS STUDY ADDS
This multicentre, retrospective cohort study revealed that aIL-6R showed higher retention than TNFi, cytotoxic T lymphocyte-associated antigen-4-Ig (CTLA4-Ig) and JAKi due to their ineffectiveness.
CTLA4-Ig showed higher retention than TNFi and aIL-6R due to safety, as well as lower discontinuation than TNFi due to remission.
The adjusted overall drug retention, excluding non-toxicity and remission, was higher in aIL-6R compared with CTLA4-Ig and TNFi.
Concomitant use of oral glucocorticoids at baseline was significantly associated with increased risk of treatment discontinuation due to ineffectiveness in TNFi, as well as toxic adverse events in JAKi and TNFi.
HOW THIS STUDY MIGHT AFFECT RESEARCH, PRACTICE OR POLICY
Considering the drug retention differences due to effectiveness and safety, this study might affect the initial selection of biological disease-modifying antirheumatic drugs and JAKi as well as the concomitant use of oral glucocorticoids in clinical practice.
Introduction
EULAR 2019 recommendations deemed targeted synthetic disease-modifying antirheumatic drugs (tsDMARDs) such as Janus kinase inhibitors (JAKi) equivalent in effectiveness and safety to biological disease-modifying antirheumatic drugs (bDMARDs).1 However, the results of the ORAL-Surveillance Trial2 prompted some modifications to these recommendations, because among patients over 50 years of age with cardiovascular risk factors, higher rates of major adverse cardiovascular events and malignancy were observed with tofacitinib (TOF) compared with tumour necrosis factor inhibitors (TNFi). The EULAR 2022 recommendations stated that tsDMARDs may be considered in phase II treatments if relevant risk factors are considered.3 In addition, bDMARDs and tsDMARDs should be used in conjunction with a conventional synthetic (cs) DMARD, and in patients who are unable to use csDMARDs as comedication, anti-interleukin-6 receptor antibodies (aIL-6R) and tsDMARDs are recommended and considered superior to other bDMARDs. These recommendations emphasise the importance of short-term glucocorticoids (GCs) when initiating or modifying csDMARDs therapy, with the necessary rapid taper and discontinuation.3 Thus, the 2022 recommendation prioritised safety, the immediate discontinuation of GCs and the use of csDMARDs in the selection of bDMARDs and JAKi. However, the ultimate choice of these drugs by clinicians may depend on various factors such as patients’ age, comorbidities, prior bDMARDs or JAKi use and economic burden. Thus, current clinical practice lacks robust criteria for reliable treatment selection.
Randomised controlled trials (RCTs) have been criticised for sometimes recruiting patients dissimilar to those patients frequently seen in real-world settings, characterised by factors such as younger age and fewer comorbidities.4 In response, observational studies, particularly those based on cohorts, have gained popularity for assessing the efficacy of bDMARDs.5 Drug retention is considered an good index of drugs’ safety, effectiveness and tolerability in observational studies,6 even when treatment selection and discontinuation may be influenced by variables such as differences in physician care and patient characteristics.7 However, multicentre studies and the national health insurance system in our country may mitigate these potential deviations.
Recently published studies documented the retention and discontinuation reasons for bDMARDs8 and JAKi9 as well as factors related to the achievement of bDMARD-free remission10 in our retrospective cohort of patients with rheumatoid arthritis (RA) across multiple centres. Nonetheless, the studies were limited by their small sample size and the lack of a direct comparison between bDMARDs and JAKi. Hence, the primary endpoint of this multicentre, retrospective study was to determine the retention and reasons for discontinuation of bDMARDs and JAKi, while the secondary endpoint was to investigate the factors influencing each reason for discontinuation in real-world settings, using a larger sample of treatment courses.
Materials and methods
Patients
The Kansai Consortium for Well-being of Rheumatic Disease Patients (ANSWER) cohort is a multicentre, observational registry of patients with rheumatic disease in the Kansai region of Japan.11–14 The data were retrospectively obtained from patients treated at eight prominent university-affiliated hospitals, including Osaka University, Kyoto University, Osaka Metropolitan University, Osaka Medical and Pharmaceutical University, Kansai Medical University, Kobe University, Nara Medical University and Osaka Red Cross Hospital. RA diagnoses were made in accordance with either the American College of Rheumatology (ACR) 1987 RA classification criteria15 or the 2010 ACR/EULAR RA classification criteria.16 The administration of bDMARDs and JAKi was at the discretion of the attending rheumatologists, consistent with the Japan College of Rheumatology (JCR) guidelines.17 18 In the JCR guideline of 2014, if patients failed to achieve low disease activity during phase I treatment with csDMARDs, it is recommended to augment therapy with additional csDMARDs or bDMARDs during phase II. If patients experience treatment failure during phase II, transitioning to alternative bDMARDs or TOF is considered. In the JCR guideline of 2020, patients who failed to achieve low disease activity with csDMARDs in phase I are advised to introduce bDMARDs or JAKi during phase II. However, from a long-term safety and cost-effectiveness standpoint, bDMARDs are generally preferred. Non- TNFi, specifically aIL-6R, are recommended when a bDMARD is used without MTX. If an inadequate response to a TNFi occurs, priority should be given to switching to a non-TNFi agent. The dosing of each agent was determined according to the manufacturer’s recommendations.
Patients treated with either bDMARDs (including both intravenous and subcutaneous forms and biosimilar agents) or JAKi between 2003 and 2022 and with complete data on initiation and discontinuation dates, as well as the reasons for discontinuation, were included in this study. Patients who lacked the data of age; sex; prior use and the number of switched bDMARDs or JAKi; initiation and discontinuation dates, as well as the reasons for discontinuation of bDMARDs or JAKi were all excluded.
Additional data were collected, including baseline demographic information such as disease duration, the disease activity score in 28 joints using the erythrocyte sedimentation rate (DAS28-ESR), the Clinical Disease Activity Index (CDAI) score, concomitant dosages (represented as blank if not combined) and ratios of methotrexate (MTX) and GCs (prednisolone (PSL) equivalent), concomitant ratios of other csDMARDs (including salazosulfapyridine, bucillamine, iguratimod, tacrolimus and leflunomide), positivity for rheumatoid factor (RF) and anticyclic citrullinated peptide antibody and Health Assessment Questionnaire Disability Index score.9
Drug retention was retrospectively assessed based on the time until definitive treatment cessation. The reasons for termination were categorised into four main categories, and physicians were restricted to citing a single rationale for termination as follows: (1) ineffectiveness (comprising both primary and secondary); (2) toxic adverse events (infection, skin reaction, systemic reaction and other toxic events, such as haematologic, pulmonary, renal, cardiovascular complications and malignancies); (3) non-toxic reasons (patient preference, hospital transfer, desire for pregnancy, etc) and (4) remission.19 20
Statistical analysis
The baseline characteristics of patients taking bDMARDs and JAKi were compared using the Kruskal–Wallis non-parametric test for continuous variables and Pearson’s χ2 test for categorical variables.
The Kaplan–Meier method was used to examine the survival curves of each agent as explained by specific causes.8
Multivariate Cox proportional hazards modelling was used to analyse the HRs and Cox p values for each reason for treatment discontinuation in the adjusted model21 using previously reported potential confounders such as baseline age; sex; disease duration; CDAI; concomitant use of GCs, MTX and other csDMARDs; number of switched bDMARDs or JAKi and prior or current use of TNFi, aIL-6R, cytotoxic T lymphocyte-associated antigen-4-Ig (CTLA4-Ig/abatacept; ABT) or JAKi.22–24 In order to handle the presumably missing values pertaining to disease duration and baseline disease activity, multiple imputations by chained equations were performed. Consequently, 20 imputed data sets were generated, encompassing all covariates and outcomes.25 Subsequently, the imputation estimates and SEs were amalgamated according to Rubin’s rule.26
All statistical analyses were performed using EZR (Saitama Medical Centre, Jichi Medical University, Saitama, Japan), a graphical user interface for R software (R Foundation for Statistical Computing, Vienna, Austria).27 A p value<0.05 in a two-sided test was considered statistically significant.
Results
Baseline characteristics
The study population was selected among patients with RA in the ANSWER cohort. As a result, 11 039 patients were recruited from the cohort, and 6666 bDMARD or JAKi treatment courses of 3698 patients met the inclusion criteria. Table 1 presents the baseline demographic and clinical characteristics of the enrolled patients (treatment courses: TNFi=3577 (ratio 53.7%), aIL-6R=1497 (ratio 22.5%), CTLA4-Ig=1139 (ratio 17.1%), JAKi=453 (ratio 6.8%); 55.4% were bio/JAKi-naïve; average age was 58.8 years, 82.6% were female; 76.2% were positive for RF, the DAS28-ESR was 4.3, the combined MTX dose was 8.3 mg/week (ratio 58.5%) and the GCs dose was 5.7 mg/day (ratio 36.4%). Compared with other groups, the TNFi group had the youngest average age, shortest disease duration and highest rate of bDMARD/JAKi naivety. In contrast, the CTLA4-Ig group had the highest average age, the lowest combined MTX dose and ratio and the highest ratio of hypertension, whereas the JAKi group had the longest disease duration, the highest CDAI score and the highest ratio of previous treatment with bDMARDs/JAKi.
Table 1.
Patients’ clinical characteristics at the beginning of treatment with each agent
| Variable | TNFi (n=3577) |
aIL-6R (n=1497) |
CTLA4-Ig (n=1139) |
JAKi (n=453) |
P value |
| Agents (number of treatment courses) | ETN=959 GLM=743 ADA=725 IFX=656 CZP=314 ETN-BS=156 IFX-BS=24 |
TCZ=1410 SAR=87 |
ABT=1139 | BAR=217 TOF=203 PEF=27 UPA=6 |
N.A. |
| Age (years) | 56.3±15.1 | 58.8±14.5 | 65.4±12.8 | 61.5±13.3 | <0.001 |
| Female sex (%) | 82.8 | 82.5 | 82.4 | 82.1 | 0.96 |
| Disease duration (years) | 8.9±9.7 | 9.9±9.8 | 10.6±10.7 | 11.9±10.4 | <0.001 |
| RF positivity (%) | 74.2 | 77.9 | 78.7 | 79.4 | 0.0045 |
| ACPA positivity (%) | 78.9 | 79.0 | 82.7 | 79.6 | 0.14 |
| DAS28-ESR | 4.2±0.9 | 4.2±1.4 | 4.2±1.1 | 4.2±1.1 | 0.49 |
| CDAI | 14.6±6.5 | 15.0±8.4 | 15.0±7.7 | 15.7±8.9 | 0.020 |
| HAQ-DI | 0.9±0.9 | 0.9±0.8 | 1.0±0.8 | 0.9±0.8 | 0.33 |
| eGFR (mL/min/1.73 m2) | 80.3±23.2 | 79.8±25.8 | 73.0±23.1 | 72.5±20.7 | <0.001 |
| Oral GCs use (%) | 31.7 | 41.3 | 41.6 | 44.4 | <0.001 |
| GCs dose (mg/day; PSL equivalent) | 5.5±3.6 | 6.2±4.1 | 6.2±6.5 | 5.2±3.4 | <0.001 |
| MTX use (%) | 66.3 | 51.5 | 43.5 | 57.0 | <0.001 |
| MTX dose (mg/week) | 8.4±3.2 | 8.1±3.2 | 7.8±3.1 | 8.6±3.1 | <0.001 |
| Other csDMARDs use (%) | 20.6 | 28.7 | 34.8 | 37.3 | <0.001 |
| bDMARD-naïve or JAKi-naïve (%) | 63.4 | 43.5 | 57.9 | 24.7 | <0.001 |
| Second bDMARDs or JAKi (%) | 22.5 | 30.2 | 21.9 | 23.4 | |
| ≥Third bDMARDs or JAKi (%) | 14.1 | 26.3 | 20.1 | 51.9 | |
| Prior TNFi use (%) | 28.6 | 45.4 | 31.9 | 57.6 | <0.001 |
| Prior anti-IL-6R use (%) | 10.3 | 13.6 | 18.0 | 36.9 | <0.001 |
| Prior CTLA4-Ig use (%) | 8.2 | 12.7 | 6.1 | 30.0 | <0.001 |
| Prior JAKi use (%) | 1.7 | 2.6 | 1.8 | 16.1 | <0.001 |
| Hypertension (%) | 28.1 | 30.4 | 38.4 | 30.1 | <0.001 |
| Dyslipidaemia (%) | 24.9 | 32.5 | 23.9 | 33.4 | <0.001 |
| Diabetes (%) | 33.0 | 35.7 | 34.8 | 36.8 | 0.32 |
Values are presented as the mean±SD or percentage. Differences between the groups were assessed using the Kruskal–Wallis non-parametric test or Pearson’s χ2 test.
ABT, abatacept; ACPA, anticyclic citrullinated peptide antibody; ADA, adalimumab; aIL-6R, anti-interleukin-6 receptor antibodies; BAR, baricitinib; bDMARDs, biological disease-modifying antirheumatic drugs; BS, biosimilar; CDAI, Clinical Disease Activity Index; csDMARDs, conventional synthetic disease-modifying antirheumatic drugs; CTLA4-Ig, cytotoxic T lymphocyte-associated antigen-4-Ig; CZP, certolizumab pegol; DAS28-ESR, Disease Activity Score in 28 joints using erythrocyte sedimentation rate; eGFR, estimated glomerular filtration rate; ETN, etanercept; GCs, glucocorticoids; GLM, golimumab; HAQ-DI, Health Assessment Questionnaire Disability Index; IFX, infliximab; JAKi, Janus kinase inhibitors; MTX, methotrexate; NA, not applicable; PEF, peficitinib; PSL, prednisolone; RF, rheumatoid factor; SAR, salirumab; TCZ, tocilizumab; TNFi, tumour necrosis factor inhibitors; TOF, tofacitinib; UPA, upadacitinib.
Treatment retention
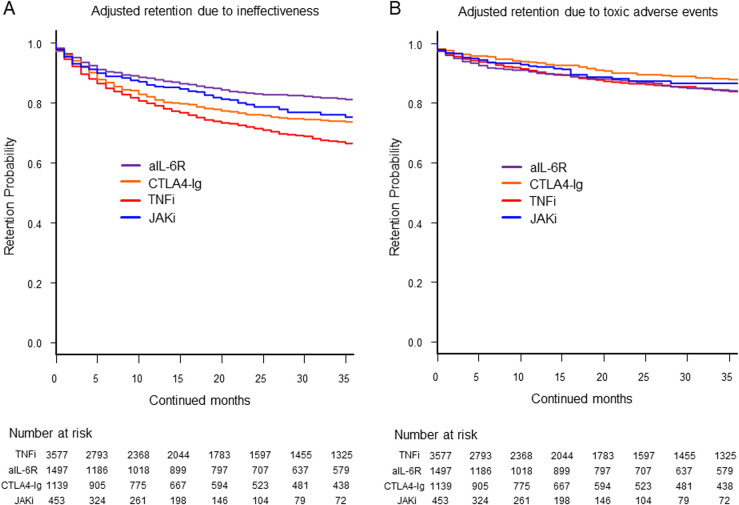
Overall, 4122 treatment courses (61.8%) were discontinued. Among the reasons of treatment discontinuation, 1878 treatment courses (45.6%) were due to ineffectiveness, 970 treatment courses (23.5%) were due to toxic adverse events, 945 treatment courses (22.9%) were due to non-toxic reasons and 329 treatment courses (8.0%) were due to remission. The HRs for treatment discontinuation with each agent due to specific causes were calculated in comparison to TNFi, via multivariate Cox proportional hazards modelling adjusted for potential confounders (baseline age; sex; disease duration; CDAI; concomitant use of GCs, MTX and other csDMARDs; number of switched bDMARDs or JAKi; prior use of TNFi, aIL-6R, CTLA4-Ig or other JAKi) (table 2). The HRs for ineffectiveness were significantly lower for aIL-6R (HR=0.52, 95% CI: 0.46 to 0.61), CTLA4-Ig (HR=0.74, 95% CI: 0.64 to 0.84) and JAKi (HR=0.67, 95% CI: 0.54 to 0.83) than those for TNFi. When compared with aIL-6R, TNFi (HR=1.93, 95% CI: 1.69 to 2.19), CTLA4-Ig (HR=1.42, 95% CI: 1.20 to 1.67) and JAKi (HR=1.29, 95% CI: 1.03 to 1.63) showed higher HR of treatment discontinuation due to ineffectiveness (figure 1A).
Table 2.
Adjusted HRs for treatment discontinuation due to specific reasons with each agent, in comparison to TNFi
| Variable | Reference | HR (95% CI) | ||
| TNFi (n=3577) | aIL-6R (n=1497) | CTLA4-Ig (n=1139) | JAKi (n=453) | |
| Ineffectiveness | 1 | 0.52 (0.46 to 0.61)*** | 0.74 (0.64 to 0.84)*** | 0.67 (0.54 to 0.83)*** |
| Toxic adverse events | 1 | 0.99 (0.85 to 1.17) | 0.78 (0.64 to 0.95)* | 0.88 (0.65 to 1.20) |
| Non-toxic reasons | 1 | 0.97 (0.82 to 1.18) | 1.20 (1.00 to 1.44)* | 0.93 (0.66 to 1.32) |
| Remission | 1 | 0.87 (0.65 to 1.17) | 0.66 (0.45 to 0.98)* | 1.12 (0.58 to 2.16) |
| Total discontinuation (excluding non-toxic reasons and remission) | 1 | 0.66 (0.60 to 0.73)*** | 0.75 (0.67 to 0.84)*** | 0.73 (0.62 to 0.87)*** |
Differences between drugs were assessed using the Cox p value.
*p<0.05, ***p<0.001.
aIL-6R, anti-interleukin-6 receptor antibodies; CTLA4-Ig, cytotoxic T lymphocyte-associated antigen-4-Ig; JAKi, Janus kinase inhibitors; TNFi, tumour necrosis factor inhibitors.
Figure 1.
Adjusted drug retention due to (A) ineffectiveness and (B) toxic adverse events. The adjusted confounders included baseline age, sex, disease duration, Clinical Disease Activity Index (CDAI), concomitant use of glucocorticoids, methotrexate and other conventional synthetic disease-modifying antirheumatic drugs (csDMARDs), number of switched biological DMARDs or JAKis and prior use of TNFi, aIL-6R, CTLA4-Ig and JAKi. aIL-6R, anti-IL-6 receptor antibodies; CTLA4-Ig, cytotoxic T lymphocyte-associated antigen-4-Ig; JAKi, Janus kinase inhibitors; TNFi, tumour necrosis factor inhibitors.
In terms of the HRs for discontinuation due to toxic adverse events, TNFi (HR=1.28, 95% CI: 1.05 to 1.56) and aIL-6R (HR=1.27, 95% CI: 1.03 to 1.57) showed a higher discontinuation rate than CTLA4-Ig (figure 1B).
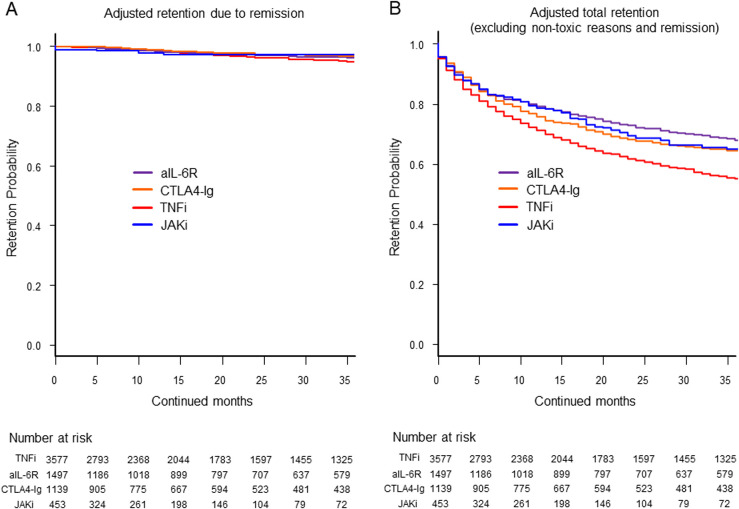
In terms of HRs for discontinuation due to non-toxic reasons, CTLA4-Ig demonstrated a higher rate (HR=1.20, 95% CI: 1.00 to 1.44) than TNFi. In terms of HRs for discontinuation due to remission, CTLA4-Ig showed a significantly lower rate (HR=0.66, 95% CI: 0.45 to 0.98) than TNFi (figure 2A). Finally, the HRs for total discontinuation (excluding non-toxic reasons and remission) were significantly lower for aIL-6R (HR=0.66, 95% CI: 0.60 to 0.73), CTLA4-Ig (HR=0.75, 95% CI: 0.67 to 0.84) and JAKi (HR=0.73, 95% CI: 0.62 to 0.87) than those for TNFi. When compared with the HRs of aIL-6R, the HRs were significantly higher for CTLA4-Ig (HR=1.14, 95% CI: 1.00 to 1.30) and TNFi (HR=1.52, 95% CI: 1.37 to 1.68), while no significant difference was observed between JAKi (HR=1.11, 95% CI: 0.93 to 1.34) (figure 2B).
Figure 2.
Adjusted drug retention due to (A) remission and (B) total drug retention (excluding non-toxic reasons and remission). The adjusted confounders included baseline age; sex; disease duration; Clinical Disease Activity Index (CDAI); concomitant use of glucocorticoids; methotrexate and other conventional synthetic disease-modifying antirheumatic drugs (csDMARDs); number of switched biological DMARDs or JAKi; and prior use of TNFi, aIL-6R, CTLA4-Ig and JAKi. aIL-6R, anti-IL-6 receptor antibodies; CTLA4-Ig, cytotoxic T lymphocyte-associated antigen-4-Ig; JAKi, Janus kinase inhibitors; TNFi, tumour necrosis factor inhibitors.
Factors affecting treatment retention
To investigate the secondary endpoint, we further examined the factors that impact drug discontinuation due to ineffectiveness using multivariate Cox proportional hazards modelling (table 3).
Table 3.
Cox proportional hazard analysis for the risk factors of treatment discontinuation due to ineffectiveness
| Variable | HR (95% CI) | ||||
| Total | TNFi | aIL-6R | CTLA4-Ig | JAKi | |
| Switched number of bDMARDs or JAKi (naïve, second and ≥third) | 1.45 (1.26 to 1.66)*** | 1.61 (1.34 to 1.95)*** | 1.40 (1.06 to 1.87)* | 1.35 (0.94 to 1.94) | 0.87 (0.53 to 1.44) |
| Current aIL-6R treatment (vs TNFi) | 0.52 (0.46 to 0.59)*** | N.A. | N.A. | N.A. | N.A. |
| Current JAKi treatment (vs TNFi) | 0.67 (0.54 to 0.83)*** | N.A. | N.A. | N.A. | N.A. |
| Current CTLA4-Ig treatment (vs TNFi) | 0.74 (0.64 to 0.84)*** | N.A. | N.A. | N.A. | N.A. |
| CDAI | 1.01 (1.00 to 1.02)*** | 1.01 (1.01 to 1.02)** | 1.01 (1.00 to 1.02) | 1.02 (1.01 to 1.04)** | 0.99 (0.97 to 1.02) |
| Disease duration (years) | 0.99 (0.99 to 1.00)*** | 0.99 (0.98 to 1.00)** | 0.99 (0.98 to 1.01) | 0.99 (0.98 to 1.01) | 0.98 (0.95 to 1.00)* |
| Concomitant oral GCs use (%) | 1.15 (1.04 to 1.27)** | 1.24 (1.09 to 1.41)*** | 1.17 (0.93 to 1.48) | 0.89 (0.69 to 1.14) | 0.82 (0.55 to 1.24) |
| Prior aIL-6R use (%) | 1.25 (1.06 to 1.47)** | 1.14 (0.91 to 1.44) | 1.03 (0.71 to 1.50) | 1.37 (0.90 to 2.09) | 1.95 (1.17 to 3.25)* |
| Concomitant MTX use (%) | 0.89 (0.81 to 0.97)* | 0.79 (0.70 to 0.89)*** | 0.91 (0.72 to 1.15) | 1.23 (0.97 to 1.55) | 0.98 (0.64 to 1.51) |
| Prior JAKi use (%) | 1.14 (0.86 to 1.51) | 0.88 (0.56 to 1.40) | 1.43 (0.74 to 2.77) | 1.67 (0.80 to 3.49) | 1.33 (0.76 to 2.33) |
| Concomitant other csDMARDs use (%) | 1.05 (0.94 to 1.17) | 1.18 (1.02 to 1.37)* | 0.82 (0.62 to 1.08) | 0.86 (0.67 to 1.12) | 1.35 (0.89 to 2.04) |
| Prior TNFi use (%) | 0.93 (0.78 to 1.12) | 0.82 (0.64 to 1.04) | 0.91 (0.60 to 1.36) | 1.01 (0.63 to 1.62) | 1.72 (0.92 to 3.24) |
| Sex (male) | 1.00 (0.89 to 1.13) | 1.04 (0.89 to 1.21) | 1.04 (0.76 to 1.42) | 1.02 (0.74 to 1.39) | 0.60 (0.35 to 1.04) |
| Age (years) | 1.00 (1.00 to 1.00) | 1.00 (1.00 to 1.01) | 1.00 (0.99 to 1.00) | 1.00 (0.99 to 1.01) | 0.99 (0.98 to 1.01) |
| Prior CTLA4-Ig use (%) | 0.98 (0.82 to 1.17) | 0.95 (0.74 to 1.21) | 1.10 (0.76 to 1.60) | 0.61 (0.34 to 1.17) | 1.16 (0.73 to 1.85) |
*p<0.05, **p<0.01, ***p<0.001.
aIL-6R, anti-interleukin-6 receptor antibodies; bDMARDs, biological disease-modifying antirheumatic drugs; CDAI, Clinical Disease Activity Index; csDMARDs, conventional synthetic disease-modifying antirheumatic drugs; CTLA4-Ig, cytotoxic T lymphocyte-associated antigen-4-Ig; GCs, glucocorticoids; JAKi, Janus kinase inhibitors; MTX, methotrexate; N.A., not applicable; TNFi, tumour necrosis factor inhibitors.
The results indicated that a high number of switched bDMARDs or JAKi (naïve, second, ≥third) was significantly associated with the HR of treatment discontinuation in TNFi (HR=1.61, 95% CI: 1.34 to 1.95) and aIL-6R (HR=1.40, 95% CI: 1.06 to 1.87). Concomitant oral GCs use was significantly associated with the HR of treatment discontinuation in TNFi (HR=1.24, 95% CI: 1.09 to 1.41). Prior aIL-6R treatment was significantly associated with the HR of treatment discontinuation in JAKi (HR=1.95, 95% CI: 1.17 to 3.25). Concomitant MTX use was significantly associated with the lower HR of treatment discontinuation in TNFi (HR=0.79, 95% CI: 0.70 to 0.89).
With respect to the factors that contribute to drug discontinuation due to toxic adverse events, in multivariate Cox proportional hazards modelling (table 4), concomitant oral GCs use was significantly associated with the HR of treatment discontinuation in TNFi (HR=1.29, 95% CI: 1.07 to 1.55) and JAKi (HR=2.30, 95% CI: 1.23 to 4.28). In addition, higher age was significantly associated with the HR of treatment discontinuation in TNFi (HR=1.01, 95% CI: 1.01 to 1.02), aIL-6R (HR=1.01, 95% CI: 1.00 to 1.02) and JAKi (HR=1.04, 95% CI: 1.01 to 1.07). Male gender was significantly associated with the HR of treatment discontinuation in CTLA4-Ig (HR=1.86, 95% CI: 1.27 to 2.72).
Table 4.
Cox proportional hazard analysis for the risk factors of treatment discontinuation due to toxic adverse events
| Variable | HR (95% CI) | ||||
| Total | TNFi | aIL-6R | CTLA4-Ig | JAKi | |
| Concomitant oral GCs use (%) | 1.27 (1.11 to 1.45)*** | 1.29 (1.07 to 1.55)** | 1.08 (0.83 to 1.41) | 1.34 (0.94 to 1.91) | 2.30 (1.23 to 4.28)** |
| Age (years) | 1.01 (1.00 to 1.02)*** | 1.01 (1.01 to 1.02)*** | 1.01 (1.00 to 1.02)* | 1.01 (0.99 to 1.02) | 1.04 (1.01 to 1.07)** |
| Current CTLA4-Ig treatment (vs TNFi) | 0.78 (0.64 to 0.93)* | N.A. | N.A. | N.A. | N.A. |
| CDAI | 1.00 (0.99 to 1.01) | 1.00 (0.99 to 1.02) | 1.00 (0.98 to 1.01) | 0.99 (0.97 to 1.02) | 1.03 (1.00 to 1.06) |
| Disease duration (years) | 1.00 (0.99 to 1.01) | 0.99 (0.98 to 1.00)* | 1.02 (1.00 to 1.03)* | 1.00 (0.98 to 1.02) | 0.99 (0.97 to 1.02) |
| Prior CTLA4-Ig use (%) | 1.26 (0.97 to 1.63) | 1.20 (0.78 to 1.84) | 1.54 (0.99 to 2.42) | 1.19 (0.52 to 2.75) | 1.10 (0.53 to 2.29) |
| Sex (male) | 1.14 (0.97 to 1.35) | 1.01 (0.81 to 1.27) | 1.15 (0.82 to 1.61) | 1.86 (1.27 to 2.72)** | 0.76 (0.35 to 1.66) |
| Prior JAKi use (%) | 1.39 (0.91 to 2.13) | 1.71 (0.88 to 3.34) | 2.04 (0.96 to 4.31) | 1.14 (0.27 to 4.79) | 0.76 (0.28 to 2.05) |
| Current JAKi treatment (vs TNFi) | 0.88 (0.65 to 1.20) | N.A. | N.A. | N.A. | N.A. |
| Concomitant other csDMARDs use (%) | 1.04 (0.90 to 1.22) | 0.95 (0.76 to 1.19) | 1.17 (0.88 to 1.56) | 1.14 (0.80 to 1.62) | 0.98 (0.53 to 1.82) |
| Prior TNFi use (%) | 0.95 (0.72 to 1.25) | 0.85 (0.55 to 1.31) | 1.19 (0.72 to 1.96) | 1.23 (0.57 to 2.67) | 0.86 (0.35 to 2.15) |
| Prior aIL-6R use (%) | 1.04 (0.80 to 1.35) | 1.31 (0.85 to 2.01) | 0.83 (0.51 to 1.35) | 1.18 (0.58 to 2.39) | 0.56 (0.26 to 1.18) |
| Current aIL-6R treatment (vs TNFi) | 0.99 (0.85 to 1.17) | N.A. | N.A. | N.A. | N.A. |
| Switched number of bDMARDs or JAKi (naïve, second, ≥third) | 1.02 (0.83 to 1.27) | 0.98 (0.68 to 1.41) | 0.89 (0.61 to 1.28) | 1.01 (0.55 to 1.87) | 1.43 (0.71 to 2.86) |
| Concomitant MTX use (%) | 1.03 (0.90 to 1.17) | 1.05 (0.87 to 1.26) | 0.95 (0.73 to 1.25) | 1.00 (0.71 to 1.40) | 1.45 (0.77 to 2.73) |
*p<0.05, **p<0.01, ***p<0.001.
aIL-6R, anti-interleukin-6 receptor antibodies; bDMARDs, biological disease-modifying antirheumatic drugs; CDAI, Clinical Disease Activity Index; csDMARDs, conventional synthetic disease-modifying antirheumatic drugs; CTLA4-Ig, cytotoxic T lymphocyte-associated antigen-4-Ig; GCs, glucocorticoids; JAKi, Janus kinase inhibitors; MTX, methotrexate; N.A., not applicable; TNFi, tumour necrosis factor inhibitors.
Discussion
In this investigation, aIL-6R showed higher retention than TNFi, CTLA4-Ig and JAKi due to ineffectiveness. CTLA4-Ig demonstrated higher retention than TNFi and aIL-6R due to safety, as well as lower discontinuation than TNFi due to remission. The overall drug retention was higher in aIL-6R, compared with CTLA4-Ig and TNFi. Furthermore, concomitant use of oral GCs at baseline was significantly associated with increased risk of treatment discontinuation due to ineffectiveness in TNFi, as well as due to safety in JAKi and TNFi
Our previous research indicated that among switch-bDMARD cases, aIL-6R (tocilizumab; TCZ) demonstrated a higher treatment retention rate than TNFi and CTLA4-Ig (ABT).28 Regarding JAKi, the Swiss RA Registry cohort showed that non-TNFi bDMARDs and TOF had a higher retention rate than TNFi,29 and another large cohort revealed that aIL-6R and JAKi had similar or higher retention due to their higher effectiveness than TNFi.5
However, a further interesting aspect of this study is that aIL-6R demonstrated a lower discontinuation than JAKi due to its effectiveness. Among patients with RA, around a 150-fold variation in serum IL-6 levels and a fivefold variation in serum soluble IL-6R levels have been reported.30 Previous in vitro studies revealed that a simulation of weekly subcutaneous TCZ injection achieved over 99% IL-6R occupancy,31 although JAKi simulation demonstrated 43%–55% signaling inhibition of IL-6/pSTAT1 in monocytes.32 Taken together, in patients with a stronger involvement of IL-6, aIL-6R may have a greater potential than JAKi to improve the condition, although further research is required to confirm this assumption. In addition, prior treatment with aIL-6R in the JAKi group may have a significant negative impact on drug discontinuation. Nevertheless, considering real-world settings, JAKi are frequently used in such ‘difficult-to-treat’ cases, so the findings may be more relevant in actual clinical settings.
In terms of toxic adverse events, a previous report demonstrated that among all bDMARDs used for treating patients with RA, ABT exhibited the lowest risk of hospitalised infection than TNFi and aIL-6R.33 Furthermore, a meta-analysis demonstrated that ABT was associated with a significantly lower risk of cardiovascular events than TNFi (risk ratio, RR=0.37; 95% CI: 0.24 to 0.55).34 Conversely, the results from a large international registry revealed that JAKi were associated with a higher incidence of adverse event-related discontinuation than TNFi (adjusted HR=1.16, 95% CI: 1.03 to 1.33).5 However, a recent systematic review and meta-analysis indicated only tendencies with relatively large variances and no significant differences in the safety profile between TNFi and JAKi (RR=3.54, 95% CI: 0.30 to 42.09).34 Another meta-analysis showed that while ABT exhibited a slightly increased risk of malignancy, no such increased risk was observed with TOF and TCZ compared with TNFi.35 Altogether, this study suggests that the higher retention rate of CTLA4-Ig was a result of its comparatively superior safety profile compared with TNFi and aIL-6R.
With respect to the impact of concomitant GCs administration, patients in clinical remission with bDMARDs experienced significantly longer survival of their bDMARDs with discontinued GCs use.36 Further, a recent Japanese registry report revealed that daily GCs doses of over 3 mg were predictive of poor responsiveness to newly administered bDMARDs treatment.37 In addition, the concomitant use of GCs (PSL over 5 mg/day) with bDMARDs38 39 and JAKi treatments9 was associated with an increased risk of toxic adverse events. We postulated that patients requiring oral GCs might be influenced by various cytokines, such as IL-6, IL-17, IL-1β and IFN-γ,40 which could be challenging to counteract solely through TNF inhibition, as it acts relatively downstream in the cytokine cascade, unlike aIL-6R,41 CTLA4-Ig42 and JAKi. In addition, broad inhibition of cytokines by both GCs and JAKi could result in compromised safety. However, the underlying reasons for the diminished retention due to safety in the combination of GCs and TNFi should be elucidated through further studies.
With respect to ageing, the incidence of serious infections in elderly patients undergoing treatment with TNFi was approximately three times higher than in younger patients.43 Additionally, the increased age correlated with an elevated risk of herpes zoster,44 major adverse cardiovascular events45 and gastrointestinal perforation during TOF treatment.46
In terms of treatment discontinuation due to remission with bDMARDs, previous studies demonstrated that infliximab (IFX) and adalimumab (ADA) have a greater potential for discontinuation, as evidenced by the BeSt and HIT HARD studies in early RA and by the HONOR studies in established RA.47–49 Moreover, our prior research indicated that TNF monoclonal antibodies (IFX, ADA and golimumab) are more effective in achieving sustained bDMARDs-free remission than TCZ and ABT.10 Nevertheless, these previous findings may have influenced the discontinuation decisions reached by individual physicians.
The reason for the higher discontinuation rate of CTLA4-Ig due to its non-toxicity compared with TNFi is still elusive. The higher median age of patients receiving ABT (65.4 years) compared with those receiving TNFi (56.3 years) may have contributed to a higher rate of hospital transitions or a greater reluctance toward self-injection. Further research is necessary to validate these hypotheses.
In addition, the efficacy of low-dose MTX in Japanese populations merits consideration. The intraerythrocyte concentration of MTX-polyglutamate, a valuable biomarker of MTX efficacy, was 65 nmol/L with a weekly dose of 13.4 mg in patients from the USA; however, it reached 94 nmol/L with a weekly dose of 10.3 mg in Japanese patients.50
This study has several limitations. First, the reasons for discontinuation of treatment were based on the decisions of individual physicians without standardised criteria, despite the patients being monitored by experienced senior rheumatologists in university-affiliated hospitals. Second, the initial dosages of each agent were determined based on manufacturer recommendations, although difficult to monitor minor dosage adjustments over the course of the study. Third, this study could not determine the difference between intravenous and subcutaneous, or original and biosimilar bDMARDs. Fourth, comorbidities that could potentially impact drug retention, such as lung diseases and history of malignancies and major adverse cardiovascular events, were not fully assessed. Fifth, some patients lacked the data of baseline disease duration and disease activity, and the missing value was completed using multiple imputations. Finally, the results may have been influenced by the smaller number of patients treated with JAKi. However, the study’s strength was its examination of the factors affecting bDMARD and JAKi retention, considering relevant clinical backgrounds such as the prior histories of each bDMARD and JAKi, particularly in ‘difficult-to-treat’ patients with RA who may not have been included in RCTs.
Conclusions
In this investigation, aIL-6R showed higher retention than TNFi, CTLA4-Ig and JAKi due to ineffectiveness. CTLA4-Ig demonstrated higher retention than TNFi and aIL-6R due to safety, as well as lower discontinuation than TNFi due to remission. The adjusted overall drug retention, excluding non-toxicity and remission, was higher in aIL-6R, compared with CTLA4-Ig and TNFi. Furthermore, concomitant use of oral GCs at baseline was significantly associated with increased risk of treatment discontinuation due to ineffectiveness in TNFi, as well as due to safety in JAKi and TNFi.
Acknowledgments
We thank all the medical staff at all the institutions who participated in the ANSWER cohort for providing the data.
Footnotes
Contributors: KE is the guarantor and responsible for the study. KE, YE, YM, YO, KM, RH, KN, YH, YS, HA, TF, TakO, YU and MK contributed to data extraction and interpretation. KE, YE, ST, SH and WY contributed to the design and conduction of statistical analysis. KE and YE prepared the manuscript. MaH, MoH, TadO, AK, SO and KN supervised the manuscript. All the authors read and approved the final manuscript.
Funding: The study reported in this publication uses the ANSWER Cohort, was supported by grants from 12 pharmaceutical companies (AbbVie GK, Asahi-Kasei, Ayumi, Chugai, Eisai, Eli Lilly, Janssen KK, Ono, Sanofi KK, Taisho, Teijin Healthcare and UCB Japan) and an information technology service company (CAC). This study was conducted as an investigator-initiated study. These companies had no roles in the study design, data collection, data analysis, data interpretation or writing of the report.
Competing interests: KE is affiliated with the Department of Musculoskeletal Regenerative Medicine, Osaka University, Graduate School of Medicine, which is supported by Taisho. KE has received research grants from AbbVie, Asahi-Kasei, Eisai, Mitsubishi-Tanabe and Teijin Pharma. KE has received a speaker fee from AbbVie, Amgen, Asahi-Kasei, Astellas, Ayumi, Bristol-Myers Squibb, Chugai, Daiichi-Sankyo, Eisai, Eli Lilly, Janssen, Mitsubishi-Tanabe, Ono Pharmaceutical, Pfizer, Sanofi, Taisho and UCB Japan. YE received a research grant from Eli Lilly. YM received a research grant from Eli Lilly, and speaker fee from Eli Lilly, Chugai, Pfizer, Bristol-Myers Squibb and Mitsubishi-Tanabe. YO received a speaker fee from Chugai, Ono Pharmaceutical and Pfizer. MaH received a speaker fee from Astellas, Ayumi, Eli Lilly, Mitsubishi-Tanabe, Ono Pharmaceutical, Pfizer and Takeda. MoH received a research grant from Mitsubishi-Tanabe, Eisai, Eli Lilly, Bristol-Myers Squibb and Novartis Pharma, and a speaker fee from Mitsubishi-Tanabe, Eisai, Eli Lilly, Bristol-Myers Squibb and Novartis Pharma. KM is affiliated with a department that is financially supported by four pharmaceutical companies (Asahi-Kasei, Mitsubishi-Tanabe, Chugai, Ayumi and UCB Japan) and the city government (Nagahama City), and received a research grant from Daiichi-Sankyo and speaker fee from AbbVie, Asahi-Kasei, Chugai, Daiichi-Sankyo, Eisai, Mitsubishi-Tanabe, Bristol-Myers Squibb and Pfizer. RH received a speaker fee from AbbVie and Eisai. TF is affiliated with a department that is financially supported by four pharmaceutical companies (Asahi-Kasei, Mitsubishi-Tanabe, Chugai, Ayumi and UCB Japan) and the city government (Nagahama City), and received a speaker fee from Asahi-Kasei, AbbVie, Janssen, Mitsubishi-Tanabe and Eisai. TadO received a research grant from AbbVie, Asahi-Kasei, Chugai, Eisai, Eli Lilly and Mitsubishi-Tanabe, and a speaker fee from AbbVie, Chugai, Eli Lilly, Janssen and Novartis Pharma. AK received a research grant from Chugai, and a speaker fee from Chugai, Eisai, Mitsubishi-Tanabe, Abbvie and Ono Pharmaceutical. KN has received a research grant from Astellas, and supervises the Department of Musculoskeletal Regenerative Medicine, Osaka University, Graduate School of Medicine, which is supported by Taisho. WY, KN, YH, YS, HA, TakO, YU, MK, ST, SH and SO have no financial conflicts of interest to disclose concerning this manuscript. These companies had no role in the study design, data collection, data analysis, data interpretation and preparation of the manuscript.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data availability statement
Data are available upon reasonable request.
Ethics statements
Patient consent for publication
Not required.
Ethics approval
This study involves human participants. The representative facility for this registry was Kyoto University. This observational study was conducted in accordance with the Declaration of Helsinki and approved by the ethics committees of the following seven institutes: Kyoto University (granted on 24 March 2016/approval no. R053), Osaka University (granted on 4 November 2015/approval no. 15300), Osaka Metropolitan University (granted on 9 June 2021/approval no. 2021-074), Osaka Medical and Pharmaceutical University (granted on 14 July 2014/approval no. 1529), Kansai Medical University (granted on 21 November 2017/approval no. 2014625), Kobe University (granted on 20 March 2015/approval no. 1738), Nara Medial University (granted on 23 January 2018/approval no. 1692) and Osaka Red Cross Hospital (granted on 1 September 2015/approval no. 644). Written informed consent was obtained from the participants. The ethics committee at Osaka University Hospital waived the requirement for patient informed consent by posting the opt-out information in the hospital’s homepage. Participants gave informed consent to participate in the study before taking part.
References
- 1.Smolen JS, Landewé RBM, Bijlsma JWJ, et al. EULAR recommendations for the management of rheumatoid arthritis with synthetic and biological disease-modifying Antirheumatic drugs: 2019 update. Ann Rheum Dis 2020;79:685–99. 10.1136/annrheumdis-2019-216655 [DOI] [PubMed] [Google Scholar]
- 2.Curtis JR, Yamaoka K, Chen Y-H, et al. Malignancy risk with tofacitinib versus TNF inhibitors in rheumatoid arthritis: results from the open-label, randomised controlled ORAL surveillance trial. Ann Rheum Dis 2023;82:331–43. 10.1136/ard-2022-222543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Smolen JS, Landewé RBM, Bergstra SA, et al. EULAR recommendations for the management of rheumatoid arthritis with synthetic and biological disease-modifying Antirheumatic drugs: 2022 update. Ann Rheum Dis 2023;82:3–18. 10.1136/ard-2022-223356 [DOI] [PubMed] [Google Scholar]
- 4.Wolfe F, Michaud K, Dewitt EM. Why results of clinical trials and observational studies of Antitumour necrosis factor (anti-TNF) therapy differ: methodological and interpretive issues. Ann Rheum Dis 2004;63:ii13–7. 10.1136/ard.2004.028530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lauper K, Iudici M, Mongin D, et al. “Effectiveness of TNF-inhibitors, Abatacept, Il6-inhibitors and JAK-inhibitors in 31 846 patients with rheumatoid arthritis in 19 registers from the 'JAK-pot' collaboration”. Ann Rheum Dis 2022;81:1358–66. 10.1136/annrheumdis-2022-222586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Neovius M, Arkema EV, Olsson H, et al. Drug survival on TNF inhibitors in patients with rheumatoid arthritis comparison of Adalimumab, Etanercept and Infliximab. Ann Rheum Dis 2015;74:354–60. 10.1136/annrheumdis-2013-204128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hyrich KL, Watson KD, Lunt M, et al. Changes in disease characteristics and response rates among patients in the United Kingdom starting anti-tumour necrosis factor therapy for rheumatoid arthritis between 2001 and 2008. Rheumatology (Oxford) 2011;50:117–23. 10.1093/rheumatology/keq209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ebina K, Hashimoto M, Yamamoto W, et al. Correction to: drug tolerability and reasons for discontinuation of seven Biologics in 4466 treatment courses of rheumatoid arthritis-the ANSWER cohort study. Arthritis Res Ther 2019;21:114. 10.1186/s13075-019-1897-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ebina K, Hirano T, Maeda Y, et al. Factors affecting drug retention of Janus kinase inhibitors in patients with rheumatoid arthritis: the ANSWER cohort study. Sci Rep 2022;12:134. 10.1038/s41598-021-04075-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hashimoto M, Furu M, Yamamoto W, et al. Factors associated with the achievement of biological disease-modifying Antirheumatic drug-free remission in rheumatoid arthritis: the ANSWER cohort study. Arthritis Res Ther 2018;20:165. 10.1186/s13075-018-1673-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jinno S, Onishi A, Dubreuil M, et al. Comparison of the drug retention and reasons for discontinuation of tumor necrosis factor inhibitors and Interleukin-6 inhibitors in Japanese patients with elderly-onset rheumatoid arthritis-the ANSWER cohort study. Arthritis Res Ther 2021;23:116. 10.1186/s13075-021-02496-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Maeda Y, Hirano T, Ebina K, et al. Comparison of efficacy between anti-IL-6 receptor antibody and other biological disease-modifying Antirheumatic drugs in the patients with rheumatoid arthritis who have knee joint involvement: the ANSWER cohort, retrospective study. Rheumatol Int 2021;41:1233–41. 10.1007/s00296-021-04862-y [DOI] [PubMed] [Google Scholar]
- 13.Nakayama Y, Hashimoto M, Watanabe R, et al. Favorable clinical response and drug retention of anti-IL-6 receptor inhibitor in rheumatoid arthritis with high CRP levels: the ANSWER cohort study. Scand J Rheumatol 2022;51:431–40. 10.1080/03009742.2021.1947005 [DOI] [PubMed] [Google Scholar]
- 14.Onishi A, Akashi K, Yamamoto W, et al. The Association of disease activity and estimated GFR in patients with rheumatoid arthritis: findings from the ANSWER study. Am J Kidney Dis 2021;78:761–4. 10.1053/j.ajkd.2021.02.338 [DOI] [PubMed] [Google Scholar]
- 15.Arnett FC, Edworthy SM, Bloch DA, et al. The American rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum 1988;31:315–24. 10.1002/art.1780310302 [DOI] [PubMed] [Google Scholar]
- 16.Aletaha D, Neogi T, Silman AJ, et al. Rheumatoid arthritis classification criteria: an American college of rheumatology/European League against rheumatism collaborative initiative. Ann Rheum Dis 2010;69:1580–8. 10.1136/ard.2010.138461 [DOI] [PubMed] [Google Scholar]
- 17.Kawahito Y. Guidelines for the management of rheumatoid arthritis. Nihon Rinsho 2016;74:939–43. [PubMed] [Google Scholar]
- 18.Koike R, Harigai M, Atsumi T, et al. Japan college of rheumatology 2009 guidelines for the use of Tocilizumab, a Humanized anti-Interleukin-6 receptor Monoclonal antibody, in rheumatoid arthritis. Mod Rheumatol 2009;19:351–7. 10.1007/s10165-009-0197-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ebina K, Hashimoto M, Yamamoto W, et al. Drug tolerability and reasons for discontinuation of seven Biologics in elderly patients with rheumatoid arthritis -The ANSWER cohort study. PLoS One 2019;14:e0216624. 10.1371/journal.pone.0216624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ebina K, Hashimoto M, Yamamoto W, et al. Drug retention and discontinuation reasons between seven Biologics in patients with rheumatoid arthritis -The ANSWER cohort study. PLoS One 2018;13:e0194130. 10.1371/journal.pone.0194130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Du Pan SM, Dehler S, Ciurea A, et al. Comparison of drug retention rates and causes of drug discontinuation between anti-tumor necrosis factor agents in rheumatoid arthritis. Arthritis Rheum 2009;61:560–8. 10.1002/art.24463 [DOI] [PubMed] [Google Scholar]
- 22.Favalli EG, Biggioggero M, Marchesoni A, et al. Survival on treatment with second-line biologic therapy: a cohort study comparing Cycling and swap strategies. Rheumatology (Oxford) 2014;53:1664–8. 10.1093/rheumatology/keu158 [DOI] [PubMed] [Google Scholar]
- 23.Gabay C, Riek M, Scherer A, et al. Effectiveness of biologic Dmards in monotherapy versus in combination with synthetic Dmards in rheumatoid arthritis: data from the Swiss clinical quality management Registry. Rheumatology (Oxford) 2015;54:1664–72. 10.1093/rheumatology/kev019 [DOI] [PubMed] [Google Scholar]
- 24.Jørgensen TS, Kristensen LE, Christensen R, et al. Effectiveness and drug adherence of biologic monotherapy in routine care of patients with rheumatoid arthritis: a cohort study of patients registered in the Danish Biologics Registry. Rheumatology (Oxford) 2015;54:2156–65. 10.1093/rheumatology/kev216 [DOI] [PubMed] [Google Scholar]
- 25.Kobayashi M, Kudo D, Ohbe H, et al. Antiplatelet pretreatment and mortality in patients with severe sepsis: A secondary analysis from a multicenter, prospective survey of severe sepsis in Japan. J Crit Care 2022;69:S0883-9441(22)00041-7. 10.1016/j.jcrc.2022.154015 [DOI] [PubMed] [Google Scholar]
- 26.Rubin DB, Schenker N. Multiple imputation in health-care databases: an overview and some applications. Stat Med 1991;10:585–98. 10.1002/sim.4780100410 [DOI] [PubMed] [Google Scholar]
- 27.Kanda Y. “Investigation of the freely available easy-to-use software 'EZR' for medical Statistics”. Bone Marrow Transplant 2013;48:452–8. 10.1038/bmt.2012.244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ebina K, Hirano T, Maeda Y, et al. Drug retention of 7 Biologics and tofacitinib in Biologics-naive and Biologics-switched patients with rheumatoid arthritis: the ANSWER cohort study. Arthritis Res Ther 2020;22:142. 10.1186/s13075-020-02232-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Finckh A, Tellenbach C, Herzog L, et al. Comparative effectiveness of Antitumour necrosis factor agents, Biologics with an alternative mode of action and tofacitinib in an observational cohort of patients with rheumatoid arthritis in Switzerland. RMD Open 2020;6:e001174. 10.1136/rmdopen-2020-001174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Robak T, Gladalska A, Stepień H, et al. Serum levels of Interleukin-6 type Cytokines and soluble Interleukin-6 receptor in patients with rheumatoid arthritis. Mediators Inflamm 1998;7:347–53. 10.1080/09629359890875 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Xu C, Rafique A, Potocky T, et al. Differential binding of Sarilumab and Tocilizumab to IL-6Ralpha and effects of receptor occupancy on clinical parameters. J Clin Pharmacol 2021;61:714–24. 10.1002/jcph.1795 [DOI] [PubMed] [Google Scholar]
- 32.Traves PG, Murray B, Campigotto F, et al. JAK selectivity and the implications for clinical inhibition of pharmacodynamic cytokine signalling by Filgotinib, Upadacitinib, tofacitinib and Baricitinib. Ann Rheum Dis 2021;80:865–75. 10.1136/annrheumdis-2020-219012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yun H, Xie F, Delzell E, et al. Comparative risk of hospitalized infection associated with biologic agents in rheumatoid arthritis patients enrolled in Medicare. Arthritis Rheumatol 2016;68:56–66. 10.1002/art.39399 [DOI] [PubMed] [Google Scholar]
- 34.de Queiroz MJ, de Castro CT, Albuquerque FC, et al. Safety of biological therapy in patients with rheumatoid arthritis in administrative health databases: A systematic review and meta-analysis. Front Pharmacol 2022;13:928471. 10.3389/fphar.2022.928471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Xie W, Yang X, Huang H, et al. Risk of malignancy with non-Tnfi biologic or tofacitinib therapy in rheumatoid arthritis: A meta-analysis of observational studies. Semin Arthritis Rheum 2020;50:930–7. 10.1016/j.semarthrit.2020.08.007 [DOI] [PubMed] [Google Scholar]
- 36.Fornaro M, Cacciapaglia F, Lopalco G, et al. Predictors of long-term clinical remission in rheumatoid arthritis. Eur J Clin Invest 2021;51:e13363. 10.1111/eci.13363 [DOI] [PubMed] [Google Scholar]
- 37.Ochi S, Mizoguchi F, Nakano K, et al. Difficult-to-treat rheumatoid arthritis with respect to responsiveness to biologic/targeted synthetic Dmards: a retrospective cohort study from the FIRST Registry. Clin Exp Rheumatol 2022;40:86–96. 10.55563/clinexprheumatol/g33ia5 [DOI] [PubMed] [Google Scholar]
- 38.Koike T, Harigai M, Inokuma S, et al. Effectiveness and safety of Tocilizumab: Postmarketing surveillance of 7901 patients with rheumatoid arthritis in Japan. J Rheumatol 2014;41:15–23. 10.3899/jrheum.130466 [DOI] [PubMed] [Google Scholar]
- 39.Mori S, Yoshitama T, Hidaka T, et al. Comparative risk of hospitalized infection between biological agents in rheumatoid arthritis patients: A multicenter retrospective cohort study in Japan. PLoS One 2017;12:e0179179. 10.1371/journal.pone.0179179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sardana K, Sachdeva S. Update on pharmacology, actions, Dosimetry and regimens of oral glucocorticoids in Dermatology. J Cosmetic Dermatol 2022;21:5370–85. 10.1111/jocd.15108 Available: https://onlinelibrary.wiley.com/toc/14732165/21/11 [DOI] [PubMed] [Google Scholar]
- 41.Ogura H, Murakami M, Okuyama Y, et al. Interleukin-17 promotes Autoimmunity by triggering a positive-feedback loop via Interleukin-6 induction. Immunity 2008;29:628–36. 10.1016/j.immuni.2008.07.018 [DOI] [PubMed] [Google Scholar]
- 42.Wunderlich C, Oliviera I, Figueiredo CP, et al. Effects of Dmards on Citrullinated peptide autoantibody levels in RA patients-A longitudinal analysis. Semin Arthritis Rheum 2017;46:709–14. 10.1016/j.semarthrit.2016.09.011 [DOI] [PubMed] [Google Scholar]
- 43.Toh S, Li L, Harrold LR, et al. Comparative safety of Infliximab and Etanercept on the risk of serious infections: does the Association vary by patient characteristics Pharmacoepidemiol Drug Saf 2012;21:524–34. 10.1002/pds.3238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cohen S, Radominski SC, Gomez-Reino JJ, et al. Analysis of infections and all-cause mortality in phase II, phase III, and long-term extension studies of tofacitinib in patients with rheumatoid arthritis. Arthritis Rheumatol 2014;66:2924–37. 10.1002/art.38779 [DOI] [PubMed] [Google Scholar]
- 45.Charles-Schoeman C, DeMasi R, Valdez H, et al. Risk factors for major adverse cardiovascular events in phase III and long-term extension studies of tofacitinib in patients with rheumatoid arthritis. Arthritis Rheumatol 2019;71:1450–9. 10.1002/art.40911 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Xie F, Yun H, Bernatsky S, et al. Brief report: risk of gastrointestinal Perforation among rheumatoid arthritis patients receiving tofacitinib, Tocilizumab, or other biologic treatments. Arthritis Rheumatol 2016;68:2612–7. 10.1002/art.39761 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Detert J, Bastian H, Listing J, et al. Induction therapy with Adalimumab plus methotrexate for 24 weeks followed by methotrexate monotherapy up to week 48 versus methotrexate therapy alone for DMARD-naive patients with early rheumatoid arthritis: HIT HARD, an investigator-initiated study. Ann Rheum Dis 2013;72:844–50. 10.1136/annrheumdis-2012-201612 [DOI] [PubMed] [Google Scholar]
- 48.Hirata S, Saito K, Kubo S, et al. Discontinuation of Adalimumab after attaining disease activity score 28-Erythrocyte sedimentation rate remission in patients with rheumatoid arthritis (HONOR study): an observational study. Arthritis Res Ther 2013;15:R135. 10.1186/ar4315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tanaka Y, Hirata S, Saleem B, et al. Discontinuation of Biologics in patients with rheumatoid arthritis. Clin Exp Rheumatol 2013;31:S22–7. [PubMed] [Google Scholar]
- 50.Takahashi C, Kaneko Y, Okano Y, et al. Association of Erythrocyte methotrexate-Polyglutamate levels with the efficacy and hepatotoxicity of methotrexate in patients with rheumatoid arthritis: a 76-week prospective study. RMD Open 2017;3:e000363. 10.1136/rmdopen-2016-000363 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data are available upon reasonable request.