Abstract
Patients diagnosed with soft tissue sarcoma (STS) present a number of challenges for physicians, due to the vast array of subtypes and aggressive tumor biology.
There is currently no agreed-upon management strategy for these tumors, which has led to the ongoing debate surrounding how frequently surveillance scans should be performed following surgery. However, advances in multidisciplinary care have improved patient outcomes over recent years.
The early detection of local recurrence reflects a more aggressive tumor, even in association with the same histopathologic entity.
Treating the local recurrence of extremity STS is a difficult clinical challenge. The goal should be to salvage limbs when possible, with treatments such as resection and irradiation, although amputation may be necessary in some cases. Regional therapies such as high-intensity, low-dose or interleukin-1 receptor antagonist treatment are appealing options for either definitive or adjuvant therapy, depending on the location of the disease’s recurrence.
The higher survival rate following late recurrence may be explained by variations in tumor biology. Since long-term survival is, in fact, inferior in patients with high-grade STS, this necessitates the implementation of an active surveillance approach.
Keywords: soft tissue sarcoma, local recurrence, management of recurrence, surgical resection
Introduction
Soft tissue sarcomas (STS) represent a heterogeneous group of malignancies that are all distinguished by mesodermal differentiation, with unique clinical and pathologic characteristics. Only 1% of adult malignancies are STS, which makes them comparatively uncommon. This low incidence is further complicated by their variable presentation, behavior, and long-term outcomes. There are more than 100 distinct histologic subtypes of soft tissue tumors, the majority of which are STS, according to the fifth edition of the WHO Classification of Tumors of Soft Tissue and Bone (1). The rarity and heterogeneity of these tumors, together with the difficult management paradigm, necessitate a multidisciplinary approach involving a skilled group of radiologists, pathologists, radiation and medical oncologists, and orthopedic oncologists.
Local recurrence, metastasis, and death are examples of treatment failures related to STS. The metastatic disease accounts for one-third of STS patients’ deaths, and it is more common in patients with local recurrence. An assessment of the relationship between local recurrence and metastasis suggests several primary tumor treatment modalities. One method involves narrow surgical margins and no radiation; this results in less loss of function but leads to numerous local recurrences that would not kill the patient but would necessitate further local treatment.
Broader margins and more liberal radiation usage are advised if local recurrences can result in metastatic disease. This second approach is initially more costly and causes a greater loss of function, but the probability of local recurrence is lower. Even though local recurrences do not increase the risk of metastasis, they do require further, frequently costly, surgeries and can eventually result in limb loss.
Malignancy grade, size, tumor depth, surgical margins, specific histological subtype, and tumor features are some of the prognostic variables associated with a higher risk of local recurrence and distant metastasis. The use of radiation, low-grade histology tumors, and negative resection margins are crucial elements in attaining local disease control.
A comprehensive identification of patients at higher risk can aid in the creation of personalized monitoring programs. Early diagnosis of STS recurrence is crucial to provide the patient with a realistic second treatment opportunity. To lower the probability of recurrence, these patients might need more extensive resection, closer postoperative surveillance, and reconsideration of extra preoperative treatment or more aggressive adjuvant chemotherapy.
History and clinical examination
The local recurrence rates for STS are reported to range from 80 to 90% when a basic resection is carried out with insufficient or positive margins (2, 3).
STS are frequently locally aggressive. However, postoperative local recurrence rates of STS have decreased to between 7 and 15% because of advanced imaging modalities, neoadjuvant therapy, adjuvant radiation, and effective limb salvage procedures (4). Limb salvage procedures can lead to higher recurrences rates, but nevertheless lower than in the decades before because of the therapies mentioned earlier.
Local recurrence is defined as tumor relapse in the operative field following resection according to follow-up radiographic evidence, a physical exam, or self-reported symptoms. Recurrence can occur after R0 resection, R1 (marginal) resection followed by radiotherapy and also after R2 (intralesional) resection.
When a patient feels a new lump in the previously treated area, local STS recurrence is frequently identified (Fig. 1A and B). The diagnosis should be verified by radiographic or cytological/histological tests, even if there may appear to be no recurrence.
Figure 1.
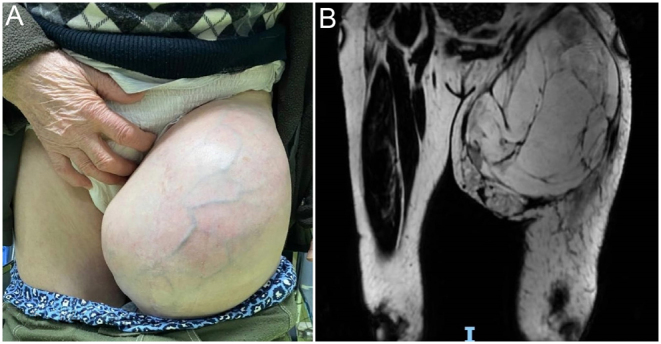
A 77-year-old patient diagnosed approximately 2 years ago with liposarcoma is referred to our department with a rapidly increasing mass located at the root of the thigh (A). The MRI (coronal T1) revealed a tumor mass with a predominantly fatty structure, but inhomogeneous through gadolinophilic and cystic areas with contrast uptake at the level of walls and some septa, as well as left inguinal-femoral adenopathies (B).
Imaging surveillance issues and diagnosis
Early diagnosis is key to managing local recurrence, and imaging techniques have been suggested as a way to identify high-risk patients at an earlier stage. Confirming the diagnosis of a clinically suspicious lesion is the main goal of diagnosis. The goal of staging is then to offer information so that decisions may be made and an early and appropriate treatment protocol can be applied.
In extremity STS, local recurrence has been linked to an increased risk of distant metastasis and a lower survival rate. The best oncologic results and lowest amounts of functional impairment are often obtained with early identification and surgical excision. As a result, in extremity STS, an efficient post-surgery monitoring plan is required. The current methods of STS screening are based on an understanding of the condition, available treatments, and worldwide guidelines according to the updated results of the randomized TOSS study and SAFETY trial (5, 6); nevertheless, even these guidelines lack precise recommendations and are frequently based on consensus rather than randomized controlled studies.
Because of their limited use for STS evaluation, radiographs are frequently used in conjunction with ultrasonography or magnetic resonance imaging (MRI) to confirm a suspected diagnosis.
Ultrasound is a valuable and affordable imaging tool that can be used to guide biopsies and detect early local STS recurrence. Ultrasound-guided biopsies make it possible to directly visualize the lesion and avoid nearby neurovascular systems. Additionally, ultrasound can be used to identify cystic regions, reducing the need for biopsy. While no studies have proven that ultrasound is preferable to MRI for local recurrence surveillance, the diagnostic accuracy of ultrasound in the identification of tumor recurrence has been reported with an overall sensitivity and specificity of 83–84% and 93–94%, respectively. If an orthopedic implant is present and recurrence is clinically suspected, ultrasound may be helpful. Although hypovascular tumor recurrence may resemble benign manifestations, Doppler testing may help to identify recurring tumors from avascular fibrous tissue in the postoperative resection bed. Ultrasound has its drawbacks, including difficulties in assessing serial examinations because of between-operator differences, and inadequacy when planning the surgical re-excision of a recurring sarcoma (7, 8).
The applicability of computed tomography (CT) scanning in STS imaging is quite constrained. Invasion into nearby organs, margin irregularity, calcification, necrosis, and hypervascularity are some characteristics visible on a CT scan that are thought to be indicators of malignancy (Fig. 1 and 2). Particularly for certain kinds of STS, CT is important in TNM staging. In order to stage STS and determine the course of the illness, a chest CT scan is crucial, since STS metastasizes mostly to the lung; thus, the preferred modality for determining the presence and monitoring of distant metastases is CT . When an essential structure cannot be clearly seen by ultrasonography, or when the lesion is too deep to reach, CT can also be used for percutaneous biopsy guidance
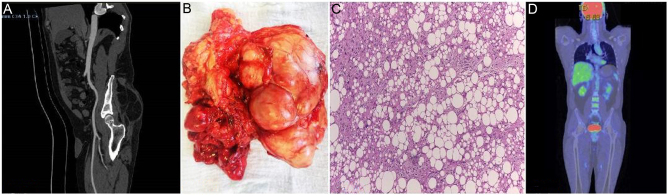
Figure 2.
A 46-year-old patient with a history of recurrent liposarcoma is referred to our department with a painless mass in the gluteal area. Sagittal view CT scan revealed a soft tissue lump with a high probability of relapse (A). We performed an en-bloc resection with negative margins (R0) (B). The HP finding revealed multiple groups of atypical adipoblasts ob 10 X with immature lipoblasts (signet ring-type cells) and mature adipocytes pledging for a well-differentiated liposarcoma (C). At 6 months postoperatively, the PET-CT scan revealed no local recurrence or distant metastases (D).
A combination of focal MRI and a chest CT scan of the lungs may be used to detect local recurrence and lung metastases, according to the most recent clinical guidelines from the European Society for Medical Oncology (9). However, the advantages and cost-effectiveness of such a program have not yet been determined.
Because of its built-in soft tissue contrast resolution, MRI is the traditional imaging technique of choice for assessing soft tissue masses. For local regional staging, MRI is preferable to CT because it offers significantly better soft tissue resolution and may provide images in any plane or multiple planes. Conventional MRI techniques include T1-weighted (T1W), T1-fat suppression (T1FS), T2-weighted, diffusion-weighted imaging, and magnetic resonance spectroscopy, each of which characterizes various tissue foci. For identifying lipomas and increasing the index of suspicion for STS, T1W and T1FS sequences are very useful. Contrast-enhanced MRI using intravenous gadolinium-based contrast can be helpful for determining whether a lesion is solid or cystic, evaluating the vascularity of the lesion’s tissue planes, and determining its vascular extension. Post-operative imaging of STS requires the extensive use of MRI. The risk of a false-positive MRI when using MRI for routine surveillance with its attendant ramifications it is an important aspect that should be considered when using MRI as a routine surveillance.
MRI is a crucial part of regular surveillance since it can detect more than one-third of local recurrences before signs and symptoms manifest (10). MRI can be used to distinguish between recurring tumors and post-surgical seroma, inflammation, scarring, and hemorrhage. Traditional T1W, T2W, and static post-contrast imaging sequences frequently fail to distinguish between post-operative alterations and disease recurrence. A significant, if seldom observed, indicator of no local recurrence is a total lack of any fluid signal in the surgical bed. On T1W images, a recurrent tumor may occasionally show areas of architectural distortion, and intravenous contrast can further disclose disease recurrence by revealing a mass-like or nodular area of enhancement.
In the first 2 post-operative years of our early follow-up program, due to its excellent soft tissue contrast and capacity to image both superficial and deep soft tissues, we performed a local MRI every 3 months, because this imaging method is the most efficient modality for the assessment of local recurrence in postoperative surveillance. Nevertheless, distinguishing between post-treatment changes and recurrent STS is quite difficult and can lead to false-positive results, as previous studies have repeatedly demonstrated how scar tissue and post-treatment alterations might mask recurrent STS, which prompts pointless biopsies (11). At the same time, all patients are evaluated by CT of the chest, abdomen, and pelvis once every 6 months, to screen for secondary occurrences.
Rarely, STS will spread through the lymphatic system and manifest as lymph node metastases (LNM). The reported incidence of regional LNM from extremity STS ranges from 0.9 to 6% (12, 13). Several histological forms, including epithelioid sarcoma, synovial sarcoma, angiosarcoma, rhabdomyosarcoma, and clear cell sarcoma, have been documented to have a preference for regional LNM14. These entities are sometimes shown together under the acronym ‘SCARE’ (Synovial, Clear cell, Angiosarcoma, Rhabdomyosarcoma and Epithelioid). The majority of LNM patients were identified by the presence of a palpable mass in an anatomic region that was indicative of a lymphatic cluster, such as the groin or axilla. The diagnosis was then confirmed by advanced cross-sectional imaging using either CT or MRI
When MRI is not an option or when the results from other screening procedures are unclear or nondiagnostic, such as when metal artifacts are present, positron emission tomography (PET) scanning can be valuable for local monitoring. Additionally, when CT results are uncertain, PET-CT can assist in identifying the target locations for biopsy. Fluorodeoxyglucose-PET has been explored as an imaging technique for local recurrence and/or lung metastasis, but its use as a primary method in this regard is not advised. In certain cases, PET-CT has proven its usefulness in separating fibrosis-affected regions in the affected limb from areas that, on examination via traditional imaging techniques, seem to be local recurrences.
Current state of histotyping: is rebiopsy necessary?
Incisional biopsy was considered the gold standard for the anatomical–pathological diagnosis of soft tissue tumors. Recent data favor the use of imaging-guided biopsies and core needle biopsy for soft tissue tumors (14, 15). The local recurrence of a STS is most often diagnosed on the basis of a palpable change in the primary resection area. Although the recurrence seems obvious most of the time, it must be confirmed by imaging and anatomopathology, especially in cases where major resections or adjuvant treatments are to be proposed. Although most sarcoma treatment centers use MRI to detect local recurrences, it is necessary to confirm the histopathological type before performing surgical resection.The use of fine needle aspiration cytology as an alternative to conventional biopsy results in high-accuracy diagnosis.
Recurrent genetic alterations are present in nearly half of all tumor subtypes; fusion between the SS18 gene and one of the SSx genes is pathognomonic for synovial sarcoma, while a PAx3-FOxO1A fusion gene is found in 80% of alveolar rhabdomyosarcomas (16, 17). Fluorescence in situ hybridization analysis, rtPCR, and NGS are therefore indispensable (18). Targeted therapies can be administered to patients based on specific genetic alterations (19, 20). However, the amount of biopsy material can limit the ability to achieve a complete diagnosis.
The need to biopsy recurrent STS derives from the fact that diagnosis cannot be made with complete certainty following only imaging investigations or clinical examination; the locoregional changes after primary resection are significant, which creates a risk of false positive suspicion. The implications of a possible recurrence are important in terms of treatment and local and general spread; therefore, rebiopsy is essential. The identification of genetic mutations with the help of genomic sequencing is equally important for the predictability of spread as well as for the identification of systemic therapies.
Management of recurrence
Local recurrence following prior resection, with or without prior radiotherapy, can be fatal for patients diagnosed with STS. Previous treatments and the development of local recurrence inside a previously irradiated region make management of the local recurrence challenging. Even in the presence of recurring illness, limb salvage therapy is becoming increasingly popular, with an emphasis on maintaining extremity function and quality of life. When treating a local recurrence of sarcoma, wide resection is crucial for preventing additional local relapse. Unfortunately, positive margins after several resections for recurrence are frequent, occurring in up to 30% of cases. Furthermore, there is a considerable chance (up to 21%) of a further relapse following limb salvage for local recurrence.
The therapy for recurrent STS is consequently linked with increased morbidity since many patients underwent multimodal treatment before the emergence of relapse. As a result, treatment strategies that are otherwise viable for the management of recurring illnesses may not be used as frequently. The management of the local recurrence must take into account these elements from the initial treatment and others, such as anatomical restrictions, synchronous distant metastasis, and aggressive tumor biology.
Surgical resection
For all patients diagnosed with adult-type, localized STS recurrence, surgery remains the conventional course of treatment and should be carried out by a surgeon with relevant experience in the treatment of sarcoma. The surgeon evaluates a tumor’s resectability based on the tumor’s stage, the tumor’s anatomical location, and the comorbidities of the patient. The main goal of surgery is to completely remove the tumor while leaving a margin of normal tissue.
Although there is disagreement as to what constitutes an acceptable margin of normal tissue, it is commonly believed that 1 cm of soft tissue, or similar, is sufficient (e.g. a layer of fascia). However, there are circumstances in which anatomical restrictions prevent a true wide resection from being conducted without sacrificing important anatomical structures (such as major blood vessels or nerves). In these conditions, it may be acceptable to leave a planned microscopic positive surgical margin after carefully considering the risks of recurrence and morbidity associated with more aggressive surgery and having fully discussed these with the patient (21).
Wide excision (also known as ‘en bloc resection’) with negative margins (R0) and limb salvage surgery when possible are the usual surgical techniques. Plastic surgery can help with the reconstruction of extensive STS procedures in some specific circumstances, and reconstructive surgery should be considered in other cases.
To properly design a re-excision, local re-staging must be completed. A postoperative hematoma is regarded as tumor contamination and must be included in the surgical tumor bed of re-excision. Reintervention is required in cases of R2 surgery (macroscopic residual tumor after primary excision) and, depending on the histological subtype, preoperative therapies should be considered when acceptable oncology margins cannot be reached. If, despite planned procedures, the oncological safety margins are unsatisfactory, re-excision should be considered. However, radiotherapy may be appropriate if a larger or wider margin is unattainable due to the anatomical positioning. For extracompartmental atypical lipomatous tumors, resections with microscopically positive margins (R1) may be necessary.
Wide surgical margins remain the most important factor in preventing further local relapse from sarcoma. Unfortunately, a high incidence rate of positive margins is reported, ranging from 15% to 36% after surgery for local recurrence (22).
Local recurrence of STS is often first detected by the patient, who feels a new lump in the previously treated region. It is crucial to begin with the presumption that local recurrence must be treated as a de novo STS, and follow the same therapeutic guidelines.
The management of recurrence after the first treatment of primary extremity STS is important. Increased morbidity may result from greater cumulative radiation. Additionally, achieving negative margins may be challenging, and inadequate resection of isolated recurrences is linked to a high relapse incidence.
Resectable soft tissue sarcoma recurrence
Irradiation
Neoadjuvant radiotherapy would be appropriate in a situation where, following primary resection of the recurrence, the limb would be non-functional post-operatively, or in cases where only amputation is considered. Limb-sparing surgery may be possible if neoadjuvant treatments such as radiotherapy, radiochemotherapy, systemic chemotherapy with local hyperthermia, or regional chemotherapy are used (23).
Without prior radiation – a decision on radiotherapy
In the case of local recurrence, as in the case of initial tumors, resection with wide margins is necessary to prevent recurrence. It is recommended that these margins are 2 to 3 mm if the tumor affects only the fascia, or up to 1 cm when the tumor penetrates striated muscle or adipose tissue. If these cannot be obtained, local treatment is indicated, usually radiotherapy, which can be administered pre- or post-operatively. The neoadjuvant approach is preferred, although the oncological outcome is similar between pre- and post-operative treatment. Long-term toxicity, however, is more important in the case of patients treated adjuvantly, especially grade 3–4 local fibrosis. Immediate toxicity is more important in neoadjuvant treated patients (especially in terms of more difficult post-operative healing) (24).
However, it should be noted that studies comparing adjuvant and neoadjuvant radiotherapy in limb STS do not include many patients with local recurrences. In addition, the main advantages of neoadjuvant radiotherapy derive from the use of a narrower field, but in the case of irradiating preoperative tissue, where the entire area is at risk of dissemination, this advantage no longer exists (25). Current guidelines recommend customizing the radiotherapy decision for patients with recurrence (26).
Neoadjuvant chemoradiotherapy
The decision on concomitant chemotherapy, especially in the case of neoadjuvant treatment, is not standardized and is made according to the preferences of each center and according to histology. The proposal of the current guidelines in this case is enrollment in a clinical trial, or the use of regimens with proven efficiency in histologies such as Ewing’s sarcoma or rhabdomyosarcoma (27, 28).
The skepticism with which concurrent chemotherapy is viewed in these cases is due to concern over the higher rates of toxicity related to the treatment, as well as secondary myeloid neoplasms (29). However, the rates at which these occur are low, suggesting that they may arise due to genetic predisposition (30).
Reirradiation
Surgery is the most effective option for patients with local recurrence and those who have previously been treated by irradiation. However, resection with appropriate margins in the case of relapse in the limbs on a previously irradiated area is an even greater challenge. In these cases, limb-sparing surgery and adjuvant reirradiation using techniques such as brachytherapy, intensity-modulated radiotherapy, or proton therapy can be considered. There is also the option of regional chemotherapy or systemic chemotherapy with regional hyperthermia.
Evidence in the literature regarding the effectiveness of adjuvant reirradiation is limited. There is a case series that included 14 patients reirradiated for recurrent STS, in which severe local adverse effects were reported that negatively impacted the function of the limb and exposed the patient to severe infections. For the vast majority of these patients, external beam radiotherapy was used (31).
The utility of reirradiation with protons was explored in a prospective trial that included 23 patients. Better tolerability was reported, with no grade 4 or 5 toxicities. Adverse effects related to wound healing predominated. The post-reirradiation local recurrence rate was 41% (32).
Unresectable soft tissue sarcoma recurrence
Regional therapy
There are two therapeutic options for regional chemotherapy that can be indicated for recurrent unresectable tumors in an attempt to avoid amputation: isolated limb perfusion (ILP) and isolated limb infusion (ILI). These options are associated with excellent local results: a response rate of 73%, with a complete response of 23%, and a greater possibility of avoiding amputation (33).
The ILP technique involves the vascular isolation of the extremity and the injection of chemotherapy only locally, in order to avoid exposing the whole organism to high doses of chemotherapy (the kidneys and liver are excluded from the infusion circuit). The extremity is also exposed to moderate hyperthermia (up to 40°C) for better local results. ILP consists of the local administration of tumor necrosis factor (TNF)-based or melphalan therapies and has usually been studied in patients with a history of radiotherapy who would only be candidates for amputation. It should be mentioned that access to TNF therapies is only possible in Europe; in the USA, it is granted only within clinical trials. TNF toxicity can result in a shock-like syndrome that can be avoided by the local administration. Even when administered regionally, it can induce fever, chills, and arterial hypotension (34, 35). The publication that included the greatest number of patients receiving ILP (n = 208), of whom n = 97 had locally recurrent disease, followed for an average of 12 years, a limb salvage rate of 81%, overall survival rates of 42% at 5 years and 33% at 10 years, and a local recurrence rate of 30% were reported (36).
The quality of the data related to this subject is limited, however, as there was no comparison condition with an alternative local treatment, and the local administration method is very different for each individual patient. Data regarding the functionality of the limb after this procedure are also limited (37).
ILI is another possible procedure in cases of locally relapsed limb sarcoma, having been adapted from the treatment options for malignant melanoma. It technically differs from ILP in that it creates much slower blood circulation in the limb, and the limb is intensely hypoxic during the procedure. In addition, standard chemotherapy is used in this technique. The publication that included the most patients (n = 40) was a retrospective study that verified the effectiveness of ILI preoperatively in patients for whom only amputation was considered. The procedure was performed using doxorubicin at a dose of 0.7 mg/kg for the upper limbs and 1.4 mg/kg for the lower limbs. Later, the patients received external beam RT at a total dose of 35 Gy, followed by surgery performed up to 7 weeks later. The procedure could be considered effective, with a response rate of 85%, that is, the conversion of patients to resectability without requiring amputation. Regarding toxicity, the procedure was relatively well tolerated; only skin reactions and reduced mobility were reported. ILI, therefore, represents a local treatment option for very well-selected patients and is ideal for use in a clinical trial.
Palliative chemotherapy
For cases of relapse that are not candidates for local treatment, palliative systemic chemotherapy can be considered. Enrollment in a clinical trial is always preferred. If this is not an option, conventional options can be considered. In patients with histologies sensitive to anthracyclines, such as leiomyosarcoma, epithelioid sarcoma, angiosarcoma, liposarcoma, pleomorphic sarcoma, or a malignant peripheral nerve tumor, doxorubicin, with or without ifosfamide, is administered as the first line of standard treatment. The combination is preferred if the patient’s symptoms are of significant intensity and if there is an absolute need for a quick response. Angiosarcoma may represent an exception to this rule, with some clinicians preferring taxanes as the first line in this type of tumor (38).
Personalized medicine: the role of immunotherapy
It is obvious that, in sarcomas, the tumor microenvironment has a variable appearance, but certain histologies have a representative immune infiltrate (leiomyosarcomas, chondrosarcomas, liposarcomas, and undifferentiated pleomorphic sarcomas) (27, 39). Immune checkpoint inhibitors have been demonstrated to be useful in STS in a very small number of patients who are not given the standard treatments for these histologies.
Monotherapy with CTLA-4 inhibitors is the only immunotherapy-based approach that has been used, but it did not demonstrate effectiveness in synovial sarcoma (at a dose of 3 mg/kg administered every 3 weeks). The study was closed before the expected date due to the very low response rate (40).
Later, axitinib and pembrolizumab were tested for their effectiveness in patients with alveolar soft part sarcoma (ASPS) and other histopathological subtypes of STS (n = 33 patients). The survival rate at 12 months was 28%, with overall response rates of 25% and 50.4% in ASPS patients. The reason for mainly enrolling patients with ASPS is that these tumors have a high incidence of mismatch repair deficiency (41, 42).
The utility of anti-PD-1 was verified in a phase II trial that enrolled 42 patients with STS, along with bone sarcomas. The response rate was lower in this trial, at only 18%, but a complete response was recorded. After the completion of the proposed follow-up period, it was demonstrated that patients with undifferentiated pleomorphic sarcoma benefitted the most (43).
Amputation
While limb salvage procedures can provide local control for 85–90% of patients, the emergence of local recurrence might complicate conservative care. If limited resection is performed on tumors that invade nerves or arteries, it may not be viable to save the limb. Additionally, toxicities linked to therapy, such as nonhealing wounds or difficulties with wound healing, can cause excruciating pain and impair the function of the affected extremity. The patient’s quality of life and management of recurrent STS tumors may be complicated by tumors that fungate, that bleed, or are unbearably painful. In this uncommon situation, amputation could be the most suitable palliative surgery and may provide pain relief for treatment-related problems. Amputation is still recommended if infection and/or ischemia following limb salvage surgery cannot be addressed in any other way.
The options for distal extremity amputation range from individual digit amputations to above-elbow or above-knee amputations. Amputations of the forequarter or hindquarter may be indicated in cases with proximal vascular or neurological involvement. Regarding the complications, morbidity after radical amputations, particularly of the hindquarter, can be substantial. Wound healing problems and flap necrosis have been documented most often. Long-term hernia development is also possible. Phantom pain following amputation can be an issue, especially for patients who had substantial pain prior to the amputation. Radical amputation, however, may alleviate symptoms, with up to 78% relief of pain for patients with symptomatic, debilitating illness. Modern limb prosthetics can enable patients to maintain their independence while restoring a limited amount of function and aesthetic appearance.
Outcomes
The management of the local recurrence of a STS is a challenging therapeutic issue. Where feasible, therapy should focus on limb salvage techniques. Excision and irradiation of the recurrence are related to potentially significant morbidity that may negatively impact function. Amputation may be necessary to address the complications brought on by the treatment of a recurrence, although it has no significant effect on survival and may have a detrimental effect on the patient’s function and quality of life (44, 45).
Although there is no consensus, the long-term prognosis after the development of local recurrence is cautious. For most primary extremity STS, factors affecting survival, such as grade, size, location, and histologic type, have all been thoroughly documented. Several of these initial tumor features also come into play when dealing with relapse. When diagnosed, recurrence is a sign of high-grade tumor biology and is attributed to the emergence of subsequent recurrences as well as distant metastasis, and it is the most significant factor associated with decreased survival, according to data from the UCLA Sarcoma Research Group (46).
Conclusions
Early detection of local recurrence and distant metastases may allow for more effective therapy and may improve the chances of long-term survival. Studies have shown that approximately one-half of local recurrences are detected by the patient prior to the detection of clinical symptoms (47). In order to improve surveillance as a complement to standard follow-up, patient education and awareness of self-examination are, therefore, crucial.
The early detection of local recurrence reflects a more aggressive tumor, even in association with the same histopathologic entity. The higher survival rate following late recurrence may be explained by variations in tumor biology. Since long-term survival is, in fact, better in patients with high-grade STS, this necessitates the implementation of an active surveillance approach.
ICMJE conflict of interest statement
The authors declare that there is no conflict of interest that could be perceived as prejudicing the impartiality of the research reported.
Funding statement
This work did not receive any specific grant from any funding agency in thepublic, commercial or not-for-profit sector.
Institutional review board statement
This study was conducted according to the guidelines of the Declaration of Helsinki and was approved by the Ethics Committee of University Emergency Hospital Bucharest (no. 40305/12 February 2023).
Informed consent statement
Informed consent was obtained from all subjects involved in the study.
Data availability statement
Further data concerning the study can be obtained by contacting the corresponding author.
References
- 1.Choi JH & Ro JY. The 2020 WHO classification of tumors of soft tissue: selected changes and new entities. Advances in Anatomic Pathology 20212844–58. ( 10.1097/PAP.0000000000000284) [DOI] [PubMed] [Google Scholar]
- 2.Karakousis CP Proimakis C Rao U Velez AF & Driscoll DL. Local recurrence and survival in soft-tissue sarcomas. Annals of Surgical Oncology 19963255–260. ( 10.1007/BF02306280) [DOI] [PubMed] [Google Scholar]
- 3.Ramanathan RC A’Hern R Fisher C & Thomas JM. Prognostic index for extremity soft tissue sarcomas with isolated local recurrence. Annals of Surgical Oncology 20018278–289. ( 10.1007/s10434-001-0278-z) [DOI] [PubMed] [Google Scholar]
- 4.Eilber FC Brennan MF Riedel E Alektiar KM Antonescu CR & Singer S. Prognostic factors for survival in patients with locally recurrent extremity soft tissue sarcomas. Annals of Surgical Oncology 200512228–236. ( 10.1245/ASO.2005.03.045) [DOI] [PubMed] [Google Scholar]
- 5.Puri A Ranganathan P Gulia A Crasto S Hawaldar R & Badwe RA. Does a less intensive surveillance protocol affect the survival of patients after treatment of a sarcoma of the limb? updated results of the randomized TOSS study. Bone and Joint Journal 2018100–B262–268. ( 10.1302/0301-620X.100B2.BJJ-2017-0789.R1) [DOI] [PubMed] [Google Scholar]
- 6.SAFETY Investigators. The Surveillance after Extremity Tumor Surgery (SAFETY) trial: protocol for a pilot study to determine the feasibility of a multi-centre randomised controlled trial. BMJ Open 20199 e029054. ( 10.1136/bmjopen-2019-029054) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Arya S Nagarkatti DG Dudhat SB Nadkarni KS Joshi MS & Shinde SR. Soft tissue sarcomas: ultrasonographic evaluation of local recurrences. Clinical Radiology 200055193–197. ( 10.1053/crad.1999.0343) [DOI] [PubMed] [Google Scholar]
- 8.Ezuddin NS Pretell-Mazzini J Yechieli RL Kerr DA Wilky BA & Subhawong TK. Local recurrence of soft-tissue sarcoma: issues in imaging surveillance strategy. Skeletal Radiology 2018471595–1606. ( 10.1007/s00256-018-2965-x) [DOI] [PubMed] [Google Scholar]
- 9.ESMO/European Sarcoma Network Working Group. Soft tissue and visceral sarcomas: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Annals of Oncology 201425(Supplement 3) iii102–iii112. ( 10.1093/annonc/mdu254) [DOI] [PubMed] [Google Scholar]
- 10.England P Hong Z Rhea L Hirbe A McDonald D & Cipriano C. Does advanced imaging have a role in detecting local recurrence of soft-tissue sarcoma? Clinical Orthopaedics and Related Research 20204782812–2820. ( 10.1097/CORR.0000000000001351) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Garner HW Kransdorf MJ Bancroft LW Peterson JJ Berquist TH & Murphey MD. Benign and malignant soft-tissue tumors: posttreatment MR imaging. RadioGraphics 200929119–134. ( 10.1148/rg.291085131) [DOI] [PubMed] [Google Scholar]
- 12.Johannesmeyer D Smith V Cole DJ Esnaola NF & Camp ER. The impact of lymph node disease in extremity soft-tissue sarcomas: a population-based analysis. American Journal of Surgery 2013206289–295. ( 10.1016/j.amjsurg.2012.10.043) [DOI] [PubMed] [Google Scholar]
- 13.Riad S, Griffin AM, Liberman B, Blackstein ME, Catton CN, Kandel RA, O’Sullivan B, White LM, Bell RS, Ferguson PC, et al. Lymph node metastasis in soft tissue sarcoma in an extremity. Clinical Orthopaedics and Related Research 2004. (426) 129–134. ( 10.1097/01.blo.0000141660.05125.46) [DOI] [PubMed] [Google Scholar]
- 14.Casali PG, Abecassis N, Bauer S, Biagini R, Bielack S, Bonvalot S, Boukovinas I, Bovee JVMG, Brodowicz T, Broto JM, et al. Soft tissue and visceral sarcomas: ESMO-EURACAN Clinical Practice Guidelines for diagnosis, treatment and follow-up. Annals of Oncology 201829(Supplement 4) iv51–iv67. ( 10.1093/annonc/mdy096) [DOI] [PubMed] [Google Scholar]
- 15.Birgin E Yang C Hetjens S Reissfelder C Hohenberger P & Rahbari NN. Core needle biopsy versus incisional biopsy for differentiation of soft-tissue sarcomas: a systematic review and meta-analysis. Cancer 20201261917–1928. ( 10.1002/cncr.32735) [DOI] [PubMed] [Google Scholar]
- 16.Mehra S de la Roza G Tull J Shrimpton A Valente A & Zhang S. Detection of FOXO1 (FKHR) gene break- apart by fluorescence in situ hybridization in formalin-fixed, paraffin-embedded alveolar rhabdomyosarcomas and its clinicopathologic correlation. Diagnostic Molecular Pathology 20081714–20. ( 10.1097/PDM.0b013e3181255e62) [DOI] [PubMed] [Google Scholar]
- 17.Oda Y Sakamoto A Saito T Kinukawa N Iwamoto Y & Tsuneyoshi M. Expression of hepatocyte growth factor (HGF)/scatter factor and its receptor c-MET correlates with poor prognosis in synovial sarcoma. Human Pathology 200031185–192. ( 10.1016/s0046-8177(0080218-x) [DOI] [PubMed] [Google Scholar]
- 18.Iwasaki H Nabeshima K Nishio J Jimi S Aoki M Koga K Hamasaki M Hayashi H & Mogi A. Pathology of soft-tissue tumors: daily diagnosis, molecular cytogenetics and experimental approach. Pathology International 200959501–521. ( 10.1111/j.1440-1827.2009.02401.x) [DOI] [PubMed] [Google Scholar]
- 19.Reichardt P. Soft tissue sarcomas, a look into the future: different treatments for different subtypes. Future Oncology 201410(Supplement) s19–s27. ( 10.2217/fon.14.116) [DOI] [PubMed] [Google Scholar]
- 20.Wardelmann E Schildhaus HU Merkelbach-Bruse S Hartmann W Reichardt P Hohenberger P & Büttner R. Soft tissue sarcoma: from molecular diagnosis to selection of treatment. Pathological diagnosis of soft tissue sarcoma amid molecular biology and targeted therapies. Annals of Oncology 201021(Supplement 7) vii265–vii269. ( 10.1093/annonc/mdq381) [DOI] [PubMed] [Google Scholar]
- 21.Gerrand CH Wunder JS Kandel RA O’Sullivan B Catton CN Bell RS Griffin AM & Davis AM. Classification of positive margins after resection of soft-tissue sarcoma of the limb predicts the risk of local recurrence. Journal of Bone and Joint Surgery. British Volume 2001831149–1155. ( 10.1302/0301-620x.83b8.12028) [DOI] [PubMed] [Google Scholar]
- 22.Catton C Davis A Bell R O’Sullivan B Fornasier V Wunder J & McLean M. Soft tissue sarcoma of the extremity. Limb salvage after failure of combined conservative therapy. Radiotherapy and Oncology 199641209–214. ( 10.1016/s0167-8140(9601856-7) [DOI] [PubMed] [Google Scholar]
- 23.Braschi EL Kharod SM Morris CG Spiguel AR Gibbs CP Scarborough MT & Zlotecki RA. Reirradiation in conservative salvage of recurrent soft-tissue sarcoma: an analysis of treatment efficacy and toxicities. American Journal of Clinical Oncology 202144624–628. ( 10.1097/COC.0000000000000874) [DOI] [PubMed] [Google Scholar]
- 24.O’Sullivan B, Davis AM, Turcotte R, Bell R, Catton C, Chabot P, Wunder J, Kandel R, Goddard K, Sadura A, et al. Preoperative versus postoperative radiotherapy in soft-tissue sarcoma of the limbs: a randomised trial. Lancet 2002359 2235–2241. ( 10.1016/S0140-6736(0209292-9) [DOI] [PubMed] [Google Scholar]
- 25.Lyu HG Saadat LV Bertagnolli MM Wang J Baldini EH Stopfkuchen-Evans M Bleday R & Raut CP. Enhanced recovery after surgery pathway in patients with soft tissue sarcoma. British Journal of Surgery 2020107 1667–1672. ( 10.1002/bjs.11758) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Palassini E, Ferrari S, Verderio P, De Paoli A, Martin Broto J, Quagliuolo V, Comandone A, Sangalli C, Palmerini E, Lopez-Pousa A, et al. Feasibility of preoperative chemotherapy with or without radiation therapy in localized soft tissue sarcomas of limbs and superficial trunk in the Italian sarcoma group/Grupo Español de investigación en sarcomas randomized clinical trial: three versus five cycles of full-dose epirubicin plus ifosfamide. Journal of Clinical Oncology 201533 3628–3634. ( 10.1200/JCO.2015.62.9394) [DOI] [PubMed] [Google Scholar]
- 27.Gebhardt MC.Overview of Multimodality Treatment for Primary Soft Tissue Sarcoma of the Extremities and In UpToDate, Update 29. Waltham, MA, USA: 2022. [Google Scholar]
- 28.Kraybill WG, Harris J, Spiro IJ, Ettinger DS, DeLaney TF, Blum RH, Lucas DR, Harmon DC, Letson GD, Eisenberg B, et al. Phase II study of neoadjuvant chemotherapy and radiation therapy in the management of high-risk, high-grade, soft tissue sarcomas of the extremities and body wall: Radiation Therapy Oncology Group Trial 9514. Journal of Clinical Oncology 200624 619–625. ( 10.1200/JCO.2005.02.5577) [DOI] [PubMed] [Google Scholar]
- 29.Hong NJL, Hornicek FJ, Harmon DC, Choy E, Chen YL, Yoon SS, Nielsen GP, Szymonifka J, Yeap BY, DeLaney TF, et al. Neoadjuvant chemoradiotherapy for patients with high-risk extremity and truncal sarcomas: a 10-year single institution retrospective study. European Journal of Cancer 201349 875–883. ( 10.1016/j.ejca.2012.10.002) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fianchi L, Pagano L, Piciocchi A, Candoni A, Gaidano G, Breccia M, Criscuolo M, Specchia G, Maria Pogliani E, Maurillo L, et al. Characteristics and outcome of therapy-related myeloid neoplasms: report from the Italian network on secondary leukemias. American Journal of Hematology 201590E80–E85. ( 10.1002/ajh.23966) [DOI] [PubMed] [Google Scholar]
- 31.Indelicato DJ Meadows K Gibbs CP Morris CG Scarborough MT & Zlotecki RA. Effectiveness and morbidity associated with reirradiation in conservative salvage management of recurrent soft tissue sarcoma. International Journal of Radiation Oncology, Biology, Physics 200973267–272. ( 10.1016/j.ijrobp.2008.04.032) [DOI] [PubMed] [Google Scholar]
- 32.Lindsley RC, Mar BG, Mazzola E, Grauman PV, Shareef S, Allen SL, Pigneux A, Wetzler M, Stuart RK, Erba HP, et al. Acute myeloid leukemia ontogeny is defined by distinct somatic mutations. Blood 2015125 1367–1376. ( 10.1182/blood-2014-11-610543) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Guttmann DM Frick MA Carmona R Deville C Levin WP Berman AT Chinniah C Hahn SM Plastaras JP & Simone CB. A prospective study of proton reirradiation for recurrent and secondary soft tissue sarcoma. Radiotherapy and Oncology 2017124 271–276. ( 10.1016/j.radonc.2017.06.024) [DOI] [PubMed] [Google Scholar]
- 34.Neuwirth MG Song Y Sinnamon AJ Fraker DL Zager JS & Karakousis GC. Isolated limb perfusion and infusion for extremity soft tissue sarcoma: a contemporary systematic review and meta-analysis. Annals of Surgical Oncology 201724 3803–3810. ( 10.1245/s10434-017-6109-7) [DOI] [PubMed] [Google Scholar]
- 35.Lans TE Grünhagen DJ de Wilt JHW Van Geel AN & Eggermont AMM. Isolated limb perfusions with tumor necrosis factor and melphalan for locally recurrent soft tissue sarcoma in previously irradiated limbs. Annals of Surgical Oncology 200512 406–411. ( 10.1245/ASO.2005.03.093) [DOI] [PubMed] [Google Scholar]
- 36.Grünhagen DJ de Wilt JH Van Geel AN Graveland WJ Verhoef C & Eggermont AMM. TNF dose reduction in isolated limb perfusion. European Journal of Surgical Oncology 200531 1011–1019. ( 10.1016/j.ejso.2005.07.003) [DOI] [PubMed] [Google Scholar]
- 37.Deroose JP Eggermont AM van Geel AN Burger JW den Bakker MA de Wilt JH & Verhoef C. Long-term results of tumor necrosis factor alpha- and melphalan-based isolated limb perfusion in locally advanced extremity soft tissue sarcomas. Journal of Clinical Oncology 201129 4036–4044. ( 10.1200/JCO.2011.35.6618) [DOI] [PubMed] [Google Scholar]
- 38.Trabulsi NH Patakfalvi L Nassif MO Turcotte RE Nichols A & Meguerditchian AN. Hyperthermic isolated limb perfusion for extremity soft tissue sarcomas: systematic review of clinical efficacy and quality assessment of reported trials. Journal of Surgical Oncology 2012106 921–928. ( 10.1002/jso.23200) [DOI] [PubMed] [Google Scholar]
- 39.Movva S Wen W Chen W Millis SZ Gatalica Z Reddy S von Mehren M & Van Tine BA. Multi-platform profiling of over 2000 sarcomas: identification of biomarkers and novel therapeutic targets. Oncotarget 2015612234–12247. ( 10.18632/oncotarget.3498) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Feng Y Shen J Gao Y Liao Y Cote G Choy E Chebib I Mankin H Hornicek F & Duan Z. Expression of programmed cell death ligand 1 (PD-L1) and prevalence of tumour-infiltrating lymphocytes (tils) in chordoma. Oncotarget 2015611139–11149. ( 10.18632/oncotarget.3576) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Maki RG, Jungbluth AA, Gnjatic S, Schwartz GK, D’Adamo DR, Keohan ML, Wagner MJ, Scheu K, Chiu R, Ritter E, et al. A pilot study of antictla4 antibody ipilimumab in patients with synovial sarcoma. Sarcoma 20132013 168145. ( 10.1155/2013/168145) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wilky BA, Trucco MM, Subhawong TK, Florou V, Park W, Kwon D, Wieder ED, Kolonias D, Rosenberg AE, Kerr DA, et al. Axitinib plus pembrolizumab in patients with advanced sarcomas including alveolar soft-part sarcoma: a single-centre, single-arm, phase 2 trial. Lancet. Oncology 201920837–848. ( 10.1016/S1470-2045(1930153-6) [DOI] [PubMed] [Google Scholar]
- 43.Lewin J, Davidson S, Anderson ND, Lau BY, Kelly J, Tabori U, Salah S, Butler MO, Aung KL, Shlien A, et al. Response to immune checkpoint inhibition in two patients with alveolar soft-part sarcoma. Cancer Immunology Research 201861001–1007. ( 10.1158/2326-6066.CIR-18-0037) [DOI] [PubMed] [Google Scholar]
- 44.Tawbi HA, Burgess M, Bolejack V, Van Tine BA, Schuetze SM, Hu J, D'Angelo S, Attia S, Riedel RF, Priebat DA, et al. Pembrolizumab in advanced soft-tissue sarcoma and bone sarcoma (sarc028): a multicentre, two-cohort, single-arm, open-label, phase 2 trial. Lancet. Oncology 2017181493–1501. ( 10.1016/S1470-2045(1730624-1) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rosenberg SA, Tepper J, Glatstein E, Costa J, Baker A, Brennan M, Demoss EV, Seipp C, Sindelar WF, Sugarbaker P, et al. The treatment of soft-tissue sarcomasof the extremities: prospective randomized evaluations of (1). Annals of Surgery 1982196305–315. ( 10.1097/00000658-198209000-00009) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lewis JJ Leung D Heslin M Woodruff JM & Brennan MF. Association of local recurrence with subsequent survival in extremity soft tissue sarcoma. Journal of Clinical Oncology 199715646–652. ( 10.1200/JCO.1997.15.2.646) [DOI] [PubMed] [Google Scholar]
- 47.Eilber FC Rosen G Nelson SD Selch M Dorey F Eckardt J & Eilber FR. High-grade extremity soft tissue sarcomas: factors predictive of local recurrence and its effect on morbidity and mortality. Annals of Surgery 2003237218–226. ( 10.1097/01.SLA.0000048448.56448.70) [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Further data concerning the study can be obtained by contacting the corresponding author.



 This work is licensed under a
This work is licensed under a