Abstract
Purpose
To systematically review and analyze the data available in the literature to evaluate the role of patellofemoral overstuffing in affecting clinical outcomes following primary total knee arthroplasty.
Methods
A systematic literature review was conducted following the PRISMA guidelines. Only studies including primary total knee arthroplasty in the setting of osteoarthritis with a quantifiable method of measuring patellofemoral overstuffing using pre- and post-operative x-rays or advanced imaging, as well as reported subjective and/or objective patient outcomes in relation to patellofemoral overstuffing were included. Extracted data included patellofemoral overstuffing quantitative measurement method, outcome measurements, follow-up, patient demographics, author, and publication details. Descriptive analysis was provided for the available literature.
Results
There were six included articles with a total of 2325 TKAs assessed. All papers found no significant effect on clinical outcomes when the amount of PFJ overstuffing was within reason.
Conclusion
The amount of overstuffing that routinely takes place seems to be within tolerable limits and does not create a significant difference in clinical outcomes. Nevertheless, it is recommended to recreate the anatomic dimensions of the PFJ in order to best obtain a joint that is within this safe margin of error.
Keywords: patellofemoral overstuffing, patellofemoral compartment overstuffing, patellofemoral joint stuffing, anterior knee stuffing
Introduction
Total knee arthroplasty (TKA) is generally considered a successful surgery, although 5–20% of individuals are unhappy with their outcome following surgery (1, 2), and approximately 5–10% will eventually go on to revision surgery within 10 years (3, 4). There are reports of up to 23% of patients experiencing of anterior knee pain (AKP) postoperatively (5, 6, 7). Loss of motion, decreased function, and pain are three causes of post-TKA AKP that are believed to be attributable to the anatomy and function of the patellofemoral joint (PFJ) (8, 9, 10, 11, 12, 13, 14, 15, 16). The dimensions of the PFJ are affected by the femoral implant position and size, as well as the position and size of the patellar prosthesis after resurfacing is performed (15, 17). PFJ overstuffing occurs as the amount of bone and cartilage resected is less than the thickness of the prosthesis replacing it (11, 12, 18). The anteroposterior PFJ size is altered by a combination of changes in the anterior patellar displacement (APD), anterior-posterior femur diameter (APFD), anterior femoral offset (AFO), and posterior femoral offset (PFO) (11, 12, 17, 18). There have been several biomechanical (19, 20, 21, 22, 23, 24, 25) and clinical papers suggesting the negative effects of PFJ overstuffing (11, 26, 27, 28, 29, 30, 31). In contrast, there have also been a number of biomechanical (20, 32) and clinical studies suggesting no effect on clinical outcomes when the amount of overstuffing is within reason (7, 15, 16, 18, 33, 34, 35). This study aims to systematically review all of the available literature to determine if PFJ overstuffing in TKA affects clinical outcomes.
Methods
This systematic review was registered with PROSPERO International prospective register of systematic reviews. The Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines (www.prisma-statement.org) were adhered to in the production of this systematic review. PubMed, Scopus, and Cochrane databases were reviewed for English language, human studies published between inception of the databases and June 2020. The following search terms and variations were used: ‘Patellofemoral Overstuffing’ OR ‘Total Knee Arthroplasty Patellofemoral Overstuffing’ OR ‘Patellofemoral Stuffing’ OR ‘Overstuffing in Total Knee Arthroplasty’ OR ‘Patellofemoral Joint Offset” OR ‘Overstuffing the Patellofemoral Compartment’ OR ‘Patellofemoral Joint Overstuffing’.
Inclusion criteria consisted of the following: (i) studies published in the English language; (ii) a quantifiable method of measuring PFJ overstuffing that includes APD or patellar thickness (PT), if resurfaced, and/or AFO or APFD using pre- and postoperative x-rays or advanced imaging; (iii) TKA performed for osteoarthritis; (iv) reported subjective and/or objective patient outcomes in relation to PFJ overstuffing; (v) patient follow-up of at least 1 year; (vi) prosthetic implant that is cruciate retaining (CR) or posterior stabilized (PS); and (vii) peer-reviewed full-text publications. Exclusion criteria included the following: (i) TKA performed for inflammatory arthropathy or trauma; (ii) revision TKA; and (iii) hinged prostheses, mega prostheses, or other implants not routinely used in primary TKA across multiple populations. Case reports, biomechanical studies, cadaveric studies, and review articles were excluded from data collection but were reviewed as part of the literature. All abstracts were reviewed by two of the included authors and evaluated with these criteria in mind. The same authors then reviewed the full text of eligible studies to determine final inclusion. Reference lists and citations were cross-referenced for included studies that met inclusion criteria but were not found through a direct search of the databases. Data was extracted by the same two authors from all the included studies using a standardized data form created by the authors at the onset of the study. Inconsistencies between authors were resolved by joint review of the content in question. Data from individual studies is listed in Table 1.
Table 1.
Data from included papers.
| Study | Country of study | Surgeons, n | COI | Study type | LOE | Quality (Minors) | TKA, n | F vs M | Age, years | BMI | CR vs PR | Femoral reference | F/U, years |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Beldman et al. (33) | the Netherlands | 5 | No | PC | II | 14 | 193 | 128 vs 65 | 71 | 29.9 | 0 vs 193 | Anterior | 1 |
| Kemp et al. (34) | UK | NR | Yes | PC | II | 14 | 107 | 55 vs 45 | 68.9 | 30.8 | 96 vs 11 | Posterior | 1 |
| Matz et al. (35) | Canada | 6 | Yes | RC | III | 12 | 970 | 607 vs 363 | 76 | 33 | 0 vs 970 | Both | 2 |
| Pierson et al. (18) | USA | 6 | Yes | RC | III | 15 | 830 | 348 vs 208 | 68.9 | NR | 715 vs 115 | NR | 4.4 |
| White et al. (7) | USA | 1 | Yes | PC | II | 14 | 90 | 64 vs 26 | 70.3 | 29.1 | 0 vs 90 | Posterior | 2 |
| Daluga et al. (26) | USA | NR | NR | RC | III | 21 | 135 | 67 vs 21 | 67.3 | NR | 0 vs 135 | NR | 2.9 |
BMI, body mass index; COI, conflict of interest; CR, cruciate retaining; F vs m, female vs male; F/U, follow-up; LOE, level of evidence; PC, prospective cohort; PS, posterior stabilized; RC, retrospective cohort.
SS, subscale.
The methodological quality of the included studies was assessed using the methodological index for non-randomized studies (MINORS) tool, which is a validated instrument to determine the methodological quality of such studies for the purpose of meta-analysis (36). These results are included in Table 1, with each paper's detailed score in Appendix A (see section on supplementary materials given at the end of this article). The level of evidence of each article was graded using the classification published by Marx et al. (37) and is included in Table 1.
The included studies were required in our analysis to have preoperative and postoperative imaging to quantify overstuffing. As there is no standardized way of measuring PFJ overstuffing, the included papers were heterogeneous in this respect. All included papers employed protocols that incorporated PT or APD if resurfacing was performed and/or AFO or APFD. Different terminology is utilized throughout the papers by the various authors to describe similar measurements. All their terms will be defined and aggregated here.
Figure 1 displays AFO, PFO, APFD, and APD. Based on the data produced by Ghosh et al. (38), medial patellofemoral ligament (MPFL) length increases with PFJ overstuffing so Kemp et al. (34) included this in their PFJ measurement methods. They demonstrated the MPFL distance (Fig. 2D) and teardrop distance (Fig. 2C) are ways of combining APD and AFO. They also measured the anterior trochlear offset, which is the same as AFO. They divided these measurements by femoral diameter to obtain ratios to mitigate magnification errors. This method is displayed in Fig. 2.
Figure 1.
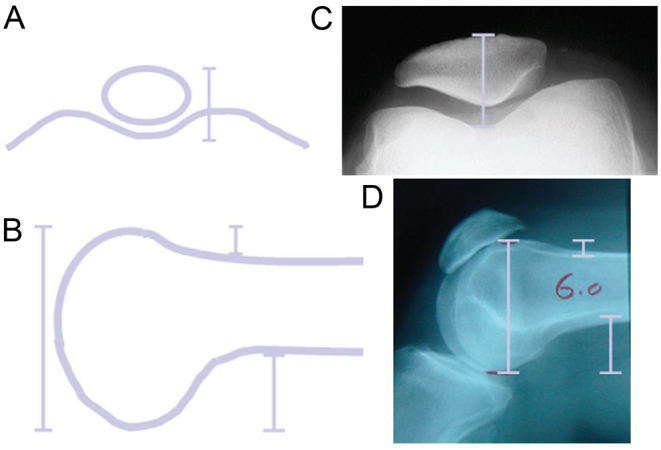
Methods of quantifying PFJ overstuffing produced by Pierson et al. (18) (A) Anterior patellar displacement (APD), (B) Anterior posterior femoral diameter (APFD), (C) Anterior femoral offset (AFO), (D) Posterior femoral offset (PFO).
Figure 2.
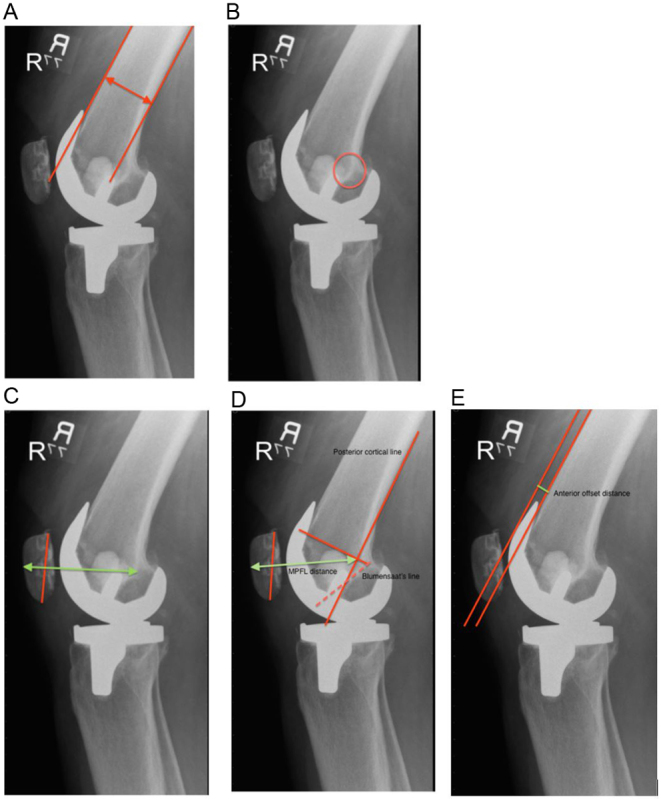
Methods of quantifying PFJ overstuffing produced by Kemp et al. (34) The teardrop distance (A) is measured from the most posterior aspect of teardrop (B) to the anterior surface of the patella. The teardrop distance ratio (TDR) is composed of the teardrop distance divided by the shaft diameter (A). The medial patellofemoral ligament (MPFL) distance (D) is measured from the MPFL origin to the anterior surface of the patella. The MPFL distance ratio (MPFLDR) is composed of MPFL distance divided by shaft diameter (A). The anterior trochlear offset ratio (ATOR) is composed of the anterior offset distance (E) divided by the shaft diameter ().
Results
Our initial literature search yielded 1313 articles. Following the elimination of duplicates and unrelated articles based on the evaluation of titles, 90 full-text papers were evaluated. After applying our exclusion and inclusion criteria, outlined above, six articles were deemed appropriate for inclusion in this analysis. A flowchart of study selection is shown in Fig. 3.
Figure 3.
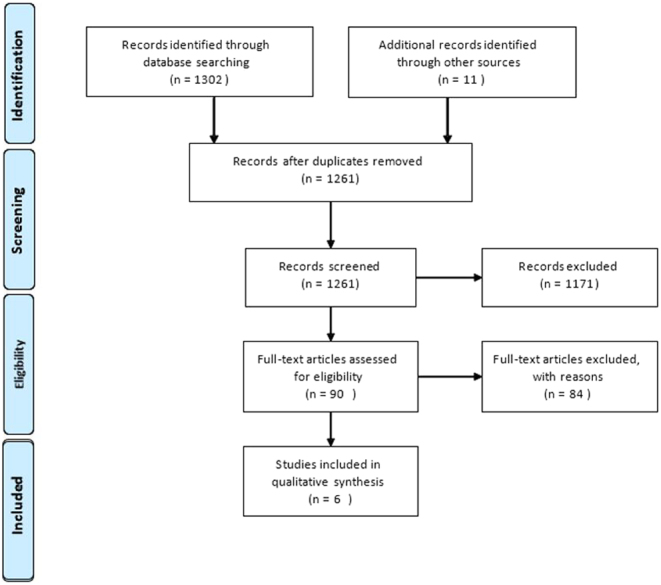
PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) flowchart of study selection.
The six included articles consisted of three retrospective cohorts (18, 26, 35) and three prospective cohorts (7, 33, 34) published in four different countries from 1991 to 2019. The retrospective studies were classified as level III evidence and the prospective studies as level II evidence, according to Marx et al. (37). All papers used a combination of outcomes instruments such as the Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC), Knee Society Score (KSS), Knee Society Function Scale (KSFS), Knee Society Pain Scale (KSPS), Hospital for Special Surgery Score (HSS), range of motion (ROM), and AKP. In total, there were 2325 TKAs assessed from patients with mean ages ranging from 67.3–76 years. Of the TKAs, 811 were CR knees and 1514 were PS knees. There were 193 anterior referenced knees and 197 posterior referenced knees. Participant makeup was 728 males and 1269 females. Follow-up averages ranged from 1 to 4.4 years. Based on the MINORS tool the methodological quality of papers ranged from 12 to 21 (36). Individual paper data are listed in Table 1.
Matz et al. (35) evaluated 970 TKAs. The authors found that if overstuffing is defined as 1 mm increase in AFO from preoperative to postoperative radiographs, 50.8% were considered overstuffed, with an average 2.8 mm increase in AFO for the overstuffed TKAs. About 43.6% were considered overstuffed when defined as a 1 mm increase in APFD, with an average 4.7 mm increase in the APFD in these overstuffed TKAs. About 11.8% were considered overstuffed when defined as a 1 mm increase in APD, with an average 3.1 mm increase for this group. On average, for all TKAs in this study, AFO was unchanged, APFD was decreased by 0.7 mm, and APD was decreased 4.2 mm. Increased, maintained, or decreased AFO was not significantly associated with changes in postoperative WOMAC and KSS scores or subscale scores. Similarly, APFD and APD were not significantly associated with changes in postoperative WOMAC and KSS scores or subscale scores. Range of motion was not affected by changes in AFO, APFD, and APD. These P-values are listed in Table 2. They identified the mean average of patellar tilt postoperatively as 6.7 degrees, which did correlate with an increase in APD but did not correlate with decreased outcome scores or total motion. They concluded that PFJ dimensions are extremely hard to recreate anatomically and small changes in dimensions likely do not adversely affect clinical outcomes.
Table 2.
Matz et al. (35) showed that increased, maintained, or decreased AFO, APFD, and APD were not significantly associated with changes in postoperative WOMAC or KSS scores. P-values are presented in the table.
| AFO* | APD† | APFD‡ | |
|---|---|---|---|
| ROM | 0.690 | 0.060 | 0.883 |
| KSS | 0.885 | 0.619 | 0.620 |
| KSFS | 0.519 | NR | 0.440 |
| KSPS | 0.735 | NR | 0.300 |
| WOMAC | 0.681 | 0.490 | 0.589 |
| Pain SS | 0.904 | 0.236 | 0.259 |
| Function SS | 0.647 | 0.703 | 0.316 |
| Stiffness | 0.098 | NR | 0.316 |
*Stuffing defined as AFO increased by at least 1mm (2.8 average); †Stuffing defined as APD increased by at least 1mm (3.1 average); ‡Stuffing defined as APFD increased by at least 1mm (4.7 average).
SS, subscale.
Pierson et al. (18) studied 830 knees. A total of 19 knees were considered overstuffed when the term was defined as a 15% increase in combined APD and APFD. The mean outcome scores and ROM when comparing overstuffed vs unstuffed TKAs are listed in Table 3. The authors found that an increase in APD was correlated with a significant decrease in ROM (P = 0.0079, 0.18 degrees/1 mm), increase in the KSFS (P < 0.0001, 0.52/1 mm), and a lower probability for the need for a lateral retinacular release (P = 0.0022, odds ratio = 0.50/1 cm). APD had no significant association with KSS (P = 0.1824) and KSPS (P = 0.4838). Increased APFD demonstrated a significant increase in ROM (P = 0.0182, 0.14 degrees/1 mm), decrease in the KSFS (P = 0.0094, 0.23/1 mm), and higher odds of lateral retinacular release (P = 0.0010, odds ratio = 1.9/1 cm). APFD had no significant association with KSS (P = 0.8030) and KSPS (P = 0.2688). The combined APD and APFD were found to have no significant effects on ROM (P = 0.8869), KSS (P = 0.3127), KSFS (P = 0.3191), and KSPS (P = 0.5372). An increase AFO increased the odds of lateral release (P = 0.0006, odds ratio = 2.2/1 cm). An increase in PFO resulted in a significant increase in ROM (P = 0.0492, 0.12 degrees/mm), but AFO did not correlate with ROM. Increases in AFO (P = 0.0092, .27/1 mm) and PFO (P = 0.0385, 0.20/1 mm) produced significant decreases in the KSFS. AFO and PFO did not correlate with KSS or KSPS. They found no clinically significant difference in KSS (P = 0.9949) , KSFS (P= .7898), KSPS (P = 0.0851), or need for lateral release (P = 0.0612) related to PFJ overstuffing between PS and CR knees. There was a significant increase in ROM of 4.4 degrees in PS vs CR implants (P < 0.0001) independent of patellar stuffing. They concluded that PFJ overstuffing is not significantly associated with adverse outcomes, caution should be exercised when attributing unexplained pain to PFJ overstuffing, and revision should not be performed for this alone.
Table 3.
Comparison of overstuffed and unstuffed knees by Pierson et al. (18).
| ROM | KSS | KSFS | KSPS | |||||
|---|---|---|---|---|---|---|---|---|
| Value | n | Value | n | Value | n | Value | n | |
| APD* | ||||||||
| Overstuffed | 111.8 | 41 | 92.1 | 41 | 84.3 | 41 | 44.8 | 41 |
| Unstuffed | 114.3 | 750 | 94.3 | 757 | 82.4 | 723 | 47.5 | 757 |
| P-value | 0.2019 | 0.2594 | 0.5168 | 0.1457 | ||||
| APD + APFD† | ||||||||
| Overstuffed | 113.1 | 19 | 97.5 | 19 | 79.5 | 19 | 49.7 | 19 |
| Unstuffed | 114.2 | 762 | 94.3 | 769 | 82.5 | 723 | 47.5 | 757 |
| P-value | 0.6917 | 0.0001 | 0.4547 | 0.0001 | ||||
*Stuffing being defined as at least a 15% increase in APD; †Overstuffing being defined as at least a 15% increase in combined APD and APFD.
Kemp et al. (34) collected data on 107 patients and reported a significant association between an increase in anterior trochlear offset ratio (ATOR) and a reduction in WOMAC pain (P = 0.039) and WOMAC function (P = 0.036) scores one year postoperatively. No association was found between increased ATOR and WOMAC stiffness score and no significant associations were identified between changes in teardrop distance ratio (TDR) or MPFL distance ratio (MPFLDR) and any of the postoperative WOMAC scores. It is noted that pain, function, and stiffness scores were transformed to 0–100 scales, with 100 indicating no pain/functional difficulty/stiffness and 0 indicating extreme pain/functional difficulty/stiffness. They concluded that there was no association between PFJ overstuffing and negative clinical outcomes, although there is a small, statistically significant association between increased AFO and worse pain and function. Results from this study are listed in Table 4.
Table 4.
Linear regressions between WOMAC pain (a) and stiffness (b) scores at 12 months and change in TDR, MPFLR, and ATOR adjusted for age, sex, and baseline WOMAC scores by Kemp et al. (34).
| Regression coefficient | 95% CI | P-value | |
|---|---|---|---|
| A: Stiffness | |||
| Change in TDR | −11.89 | −69.21 to 45.44 | 0.681 |
| Change in MPFLR | −48.32 | −122.36 to 25.71 | 0.198 |
| Change in ATOR | −8.14 | −15.86 to –0.42 | 0.039 |
| B: Scores at 12 months | |||
| Change in TDR | 7.35 | −55.52 to 70.22 | 0.817 |
| Change in MPFLR | −8.54 | −90.25 to 73.17 | 0.836 |
| Change in ATOR | −5.75 | −14.53 to 3.04 | 0.197 |
Beldman et al. (33) reported on 197 knees and defined overstuffing as any increase in measurements. Their results demonstrated 84 knees with increased AFO, 168 with increased PFO, and 155 with increased APFD. The WOMAC scores for all patients were normalized on a scale from 0 to 100. Patients with increased AFO demonstrated a statistically significant decrease in WOMAC stiffness subscale, but there was no significant difference when comparing the total WOMAC score or other subscales. Patients with increased APFD demonstrated a statistically significant decrease in the WOMAC pain subscale, but no significant difference when comparing the total WOMAC score or other subscales. Increased PFO had no significant effect on outcome scores. The authors found no statistically significant increase in AKP with an increase in AFO, PFO, and APFD. They conclude no relation between PFJ overstuffing and AKP or patient-reported outcome. Their results are shown in Table 5.
Table 5.
Comparison of overstuffed and unstuffed knees by Beldman et al. (33)
| n | Anterior knee pain | WOMAC | Pain SS | Function SS | Stiffness SS | ||
|---|---|---|---|---|---|---|---|
| No | Yes | ||||||
| Anterior overstuffing – ACO* | |||||||
| Overstuffed | 84 | 57 | 27 | 20.8 | 14.8 | 19.3 | 28.6 |
| Unstuffed | 109 | 85 | 24 | 19.1 | 13.4 | 18.4 | 24.8 |
| P-value | 0.14 | 0.11 | 0.36 | 0.54 | 0.02 | ||
| Posterior overstuffing – PCO† | |||||||
| Overstuffed | 168 | 121 | 47 | 20.1 | 14.5 | 19.2 | 26.4 |
| Unstuffed | 25 | 21 | 4 | 18.2 | 11.0 | 15.5 | 26.5 |
| P-value | 0.24 | 0.65 | 0.05 | 0.93 | 0.38 | ||
| Anterior stuffing– APFD‡ | |||||||
| Overstuffed | 155 | 112 | 43 | 20.4 | 14.4 | 19.5 | 26.8 |
| Unstuffed | 38 | 30 | 8 | 17.7 | 12.5 | 15.7 | 25.0 |
| P-value | 0.54 | 0.06 | 0.02 | 0.10 | 0.55 | ||
*Anterior overstuffing defined as an increase in ACO; †Posterior overstuffing defined as an increase in PCO; ‡Anterior stuffing defined as an increase in APFD.
White et al. (7) evaluated 90 patients and defined overstuffing as a 6 mm increase in APD post-TKA. In their study, 2 of 90 knees were overstuffed. Neither of these patients had a statistically significant change in ROM, KSS, KSFS, or AKP. The mean APD preoperatively was 31.2 mm and 29.0 mm. Results are listed below. They report that PFJ overstuffing does not negatively affect clinical outcomes. Their results are listed in Table 6.
Table 6.
Data presented as an odds ratio of change in clinical outcome by White et al. (7). Overstuffed (n=2) defined as a 6mm increase in APD.
| Odds ratio | P-value | |
|---|---|---|
| ROM | −0.73 (−0.21 to 1.78) | 0.121 |
| KSS | −0.011 (−1.38 to 1.15) | 0.861 |
| KSFS | −0.010 (−1.21 to 1.0) | 0.056 |
| AKP | 1.01 (0.79 to 1.10) | 0.901 |
Daluga et al. (26) studied 135 TKAs and when defining TKA overstuffing as a 12% increase in APFD, 10 were overstuffed. All 10 of these joints required manipulation (P = .026), compared to 84/125 unstuffed knees that also required manipulation in this series. The TKAs requiring manipulation had a 6.6% +/− 6% increase in APFD, compared to 4.6% +/− 4.7% in those not requiring manipulation, but this was statistically insignificant (P = −0.15). Two of 10 in the overstuffed group, compared to 5/125 in the unstuffed group, required revision surgery. An increase in APFD did not correlate with a decrease in ROM or HSS scores. They concluded that a 12% increase in APFD is an independent variable for manipulation and may increase the chance of implant failure. Their data is shown in Table 7.
Table 7.
Comparison of overstuffed and unstuffed knees by Daluga et al. (26). Overstuffed defined as a 12% increase in APFD, all manipulations within 3 months.
| Overstuffed | Unstuffed | |
|---|---|---|
| n | 10 | 125 |
| HSS | 84 | 87 |
| ROM | 102 | 106 |
| Manipulation | 10 (100%) | 84 (67.2%) |
| Revisions | 2 (20%) | 5 (4%) |
Discussion
Some studies report that 10% of TKA revisions are due to PFJ pain and the exact generator of this pain often remains unknown. (7, 39, 40, 41) The etiology of changes in the PFJ dimensions and kinematics is multifactorial, including: AFO, PFO, APFD, APD, component position, component design, component material, surgical technique, and more (7, 14, 15, 41, 42, 43). PFJ overstuffing has been shown to have the potential to affect the lever arm provided by the quadriceps mechanism in the knee, alter the contact forces between the implants, and potentially lead to decreased strength, motion, and an increase in loosening and pain (15, 19, 20, 21, 22, 23, 24). The degree of these alterations required to produce a clinically important difference has not been established in the literature. Some have stated that the amount of overstuffing that routinely occurs across a wide array of surgeon skill levels and techniques does not significantly alter patient outcomes (15, 18, 33, 34).
Pierson et al. (10) reported for every millimeter of increase in APD, there is a corresponding 0.18° decrease in ROM and a 0.52-point increase in the KSFS. Clinically, this indicates that a 1 cm change in APD, which is well outside the norm, could result in a 1.8° change in the ROM and a 5.2-point change in the KSFS. Similarly, they showed for every millimeter of increase in APFD, the ROM increased by 0.14° and the KSFS decreased by 0.23 points. For every millimeter increase in AFO and PFO, the KSFS decreased by .27 and .20. Matz et al. (35) analyzed their results by adding 1–4 mm to AFO and APFD to account for lack of cartilage appreciation on x-ray and they found no significant effect on ROM or outcome scores, demonstrating that adjustments of up to 4 mm do not change the outcome. They concluded that small changes in dimensions likely do not adversely affect clinical outcomes.
An excluded study produced by Alcerro et al. (15) examined PT pre- and post-TKA and found that a range of −1.06 mm to 2.58 mm within native PT did not significantly affect ROM or other outcome scores. Beldman et al. (33) showed no statistical difference in WOMAC score, but analysis of the component items within the WOMAC questionnaire revealed a statistically significant improvement in mean stiffness and pain subscale for the patients without PFJ overstuffing. However, these two subscales only showed an average difference of 3 points between patients with and without overstuffing. They note that the minimum clinically important difference (MCID) estimate for the WOMAC is 15 points (44). The authors concluded that patients with overstuffing had similar patient-reported outcomes compared to those without overstuffing and observed no correlation between PFJ overstuffing and AKP. The results of Kemp et al. (34) suggest that doubling the preoperative ATOR would cause an approximate eight-point reduction in both the WOMAC pain and function scores which falls under the MCID of 15 for the WOMAC score. Therefore, this study would suggest that AFO would have to triple the preoperative value to cause MCID. The authors did identify a small, statistically significant association between the AFO and worse postoperative knee pain and function scores, though this was well under MCID for the WOMAC scores.
Pierson et al. (18) found the probability of lateral retinacular release decreased with increased APD. Probability of retinacular release increased with increased APFD and AFO, but only 10% of releases could be associated with these increased measurements. The probability of requiring release was more associated with factors such as gender, absolute femoral component size, and patellar button size. Theoretically, increases in all three measurements should increase tension on the lateral retinaculum, but APD decreased the odds of needing a release in this study. Some studies have stated the negative effect of an uneven patellar cut leading to non-uniform distribution of forces, increased patellar tilt, and patellar maltracking (7, 45, 46, 47). White et al. (7) found that a patellar resection angle greater than 5° was associated with an increased incidence of AKP (7). These findings confirm that PFJ overstuffing does affect the kinematics of the PFJ, but multiple other variables are involved and maybe more influential in post-TKA AKP and dysfunction. While a comprehensive examination of these variables exceeds the scope of this paper, trochlear design serves as a prime example. Typically, TKA implants feature a fixed trochlear design that remains unchanged for each patient. However, a study by Dejour et al. (43) demonstrated the variation of trochlear design in 14 commonly employed implants from the normal trochlear anatomy.
Overall, the variability in measured parameters for overstuffing was relatively small. Matz et al. (35) reported a tendency toward decreased PFJ stuffing with an overall average increase in AFO of 0 mm (SD, 2.9), APFD of 0.7 mm (5.9), and APD decrease of 4.2 mm (3.8). Kemp et al. (34) reported the magnitude of overstuffing seen as relatively small when TDR and MPFLD are used with an upper limit of 112.9% and 118.1% (expressed as a percentage of preoperative values). However, when ATOR was used, larger magnitudes were identified with a maximum of 299%. White et al. (7) reported 5/90 with >2 mm increase in APD and 2/90 with a 6 mm increase in APD. The mean APD decreased by 2 mm postoperatively. Alcerro et al. (15) showed that 80% of patellae in their study were thicker following TKA by an average of 2.58 mm. Daluga et al. (26) found 10/135 with a 12% or more increase in APFD.
Multiple studies have shown that PFJ overstuffing may lead to decreased ROM but this does not necessarily correlate with a decrease in outcome scores (16, 26, 31, 48). Pierson et al. (18) noticed that as ROM decreased, there was an increase in KSFS. They proposed that this was due to an anterior displacement of the extensor mechanism which limited excursion but improved efficiency by increasing the moment arm of the quadriceps. In their cohort of 135, Daluga et al. (26) showed that all 10 overstuffed TKAs, defined as a 12% increase in APFD, required manipulation due to various limitations in ROM at variable time points within 3 months of the initial surgery. Compared to 84/125 unstuffed knees that also required manipulation within 3 months, the overstuffed group achieved a very similar HSS score (84 vs87), and ROM (102 vs 106) at the final follow-up. This study concluded that there was a significantly increased risk of manipulation in patients with PFJ overstuffing, but no statistical difference in outcome score or ROM at 2.9 years.
In terms of surgical technique, overstuffing is determined by the size, position, and design of the implant and the amount of bone removed. Bone removal is determined by the positioning of the cutting jig using a posterior or anterior referencing system. It is plausible that posterior referencing allows greater potential to create overstuffing of the PFJ because this provides constant posterior cut and variable anterior cut, but both methods pose a potential to change PFJ dimensions (34). This was shown by Beldman et al. (33) who used anterior referencing and demonstrated an increase in PFO in 87% of subjects compared to 43.5% of subjects with an increase in AFO. None of the papers directly compared the amount of overstuffing in anterior-referenced systems vs posterior- referenced systems, but there were 193 anterior-referenced and 197 posterior-referenced knees with no findings to suggest PFJ overstuffing significantly impacts relative outcomes. Similarly, only one of the six papers directly compared CR to PS. Pierson et al. (18) found no clinically significant difference in KSS, KSFS, KSPS, or need for lateral release related to PFJ overstuffing between PS and CR knees. There was a significant increase in ROM of 4.4 degrees in PS versus CR implants.
Another variable that differed in each paper was the quantification method. Prosthetic designs may provide different trochlear depths, so methods that measure APD (18) versus AFO (33) may be better. Additionally, methods that incorporate APFD or PCO (18) may be more accurate because an increased PFO may anteriorly displace the patella. Although anteriorization of the femur may not impact APFD or APD, it does affect AFO and PFO, which, in turn, influences the degree of overstuffing. Consequently, a comprehensive evaluation may require a combination of all four measurements we have discussed in our paper or other relevant variations. Methods that used ratios to help eliminate magnification variables may be superior, as well (34). Nevertheless, there is no gold standard for quantification method nor the parameters that define PFJ overstuffing, and this will need to be established before more definitive research on the effects of PFJ overstuffing can be produced.
Limitations
As with many surgical treatments, numerous unrecognized variables may have affected outcomes, including, but not limited to: multiple surgeons, multiple implants, ceiling effect of patient-reported outcomes, PFJ overstuffing quantification methods, statistical analysis, rehabilitation protocol, observer bias, and patient comorbidities. All the studies included had at least 1 year of follow up, but this is relatively short in the expected lifetime of a TKA. Variables such as increased contact forces that could lead to early implant failure would need to be followed for a much longer time. Included papers were published from 1991 to 2019 in 4 different countries, so techniques, implants, and patient population likely differed greatly between the studies. In terms of quality, the majority of included studies are considered lower quality by the MINORS tool (36). There is a large amount of heterogeneity within the included studies and this is generally considered a limitation. In this series, all papers arrive at similar conclusions, which may indicate generalizable application of the findings to the population of patients and surgeons.
Conclusion
PFJ overstuffing can be defined as any amount of increase in APD, APFD, AFO, and PFO with no gold standard measurement technique identified to quantify this. On average, the amount of overstuffing that takes place seems to be relatively small and well beneath the amount required to manifest as significant differences in clinical outcome. Nevertheless, it is recommended to recreate the anatomic dimensions of the PFJ, as this will likely keep the surgeon within a safe margin of error. The kinematics of the PFJ are complex and various other factors may contribute to PFJ dysfunction and pain. Caution should be exercised when attributing AKP and dysfunction to PFJ overstuffing alone, or at all.
Supplementary Materials
ICMJE conflict of interest statement
The authors declare that there is no conflict of interest that could be perceived as prejudicing the impartiality of the research reported.
Funding statement
This work did not receive any specific grant from any funding agency in the public, commercial or not-for-profit sector.
Acknowledgements
The authors would like to acknowledge MountainView Regional Medical Center Orthopedic Surgery Residency program for use of their facilities during production of this paper.
References
- 1.Boyd AD Ewald FC Thomas WH Poss R & Sledge CB. Long-term complications after total knee arthroplasty with or without resurfacing of the patella. Journal of Bone and Joint Surgery. American Volume 199375674–681. ( 10.2106/00004623-199305000-00006) [DOI] [PubMed] [Google Scholar]
- 2.Kahlenberg CA Nwachukwu BU McLawhorn AS Cross MB Cornell CN & Padgett DE. Patient satisfaction after total knee replacement: a systematic review. HSS Journal 201814192–201. ( 10.1007/s11420-018-9614-8) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Delanois RE Mistry JB Gwam CU Mohamed NS Choksi US & Mont MA. Current epidemiology of revision total knee arthroplasty in the United States. Journal of Arthroplasty 2017322663–2668. 10.1016/j.arth.2017.03.066:S0883-5403(17)30303-0) [DOI] [PubMed] [Google Scholar]
- 4.Bass AR McHugh K Fields K Goto R Parks ML & Goodman SM. Higher total knee arthroplasty revision rates among United States blacks than whites: a systematic literature review and meta-analysis. Journal of Bone and Joint Surgery. American Volume 2016982103–2108. ( 10.2106/JBJS.15.00976) [DOI] [PubMed] [Google Scholar]
- 5.Elson DW & Brenkel IJ. Predicting pain after total knee arthroplasty. Journal of Arthroplasty 2006211047–1053. ( 10.1016/j.arth.2005.12.010) [DOI] [PubMed] [Google Scholar]
- 6.Pilling RWD Moulder E Allgar V Messner J Sun Z & Mohsen A. Patellar resurfacing in primary total knee replacement: a meta-analysis. Journal of Bone and Joint Surgery. American Volume 2012942270–2278. ( 10.2106/JBJS.K.01257) [DOI] [PubMed] [Google Scholar]
- 7.White PB Sharma M Siddiqi A Satalich JR Ranawat AS & Ranawat CS. Role of anatomical patella replacement on anterior knee pain. Journal of Arthroplasty 201934887–892. ( 10.1016/j.arth.2019.01.011) [DOI] [PubMed] [Google Scholar]
- 8.Leopold SS Silverton CD Barden RM & Rosenberg AG. Isolated revision of the patellar component in total knee arthroplasty. Journal of Bone and Joint Surgery. American Volume 20038541–47. ( 10.2106/00004623-200301000-00007) [DOI] [PubMed] [Google Scholar]
- 9.Armstrong AD Brien HJ Dunning CE King GJ Johnson JA & Chess DG. Patellar position after total knee arthroplasty: influence of femoral component malposition. Journal of Arthroplasty 200318458–465. 10.1016/s0883-5403(0300145-1:S0883540303001451) [DOI] [PubMed] [Google Scholar]
- 10.Campbell DG Duncan WW Ashworth M Mintz A Stirling J Wakefield L & Stevenson TM. Patellar resurfacing in total knee replacement: a ten-year randomised prospective trial. Journal of Bone and Joint Surgery. British Volume 200688734–739. 10.1302/0301-620X.88B6.16822:88-B/6/734) [DOI] [PubMed] [Google Scholar]
- 11.Scranton PE. Management of knee pain and stiffness after total knee arthroplasty. Journal of Arthroplasty 200116428–435. 10.1054/arth.2001.22250:S0883-5403(01)72857-4) [DOI] [PubMed] [Google Scholar]
- 12.Bong MR & Di Cesare PE. Stiffness after total knee arthroplasty. Journal of the American Academy of Orthopaedic Surgeons 200412164–171. ( 10.5435/00124635-200405000-00004) [DOI] [PubMed] [Google Scholar]
- 13.Schiavone Panni A Cerciello S Del Regno C Felici A & Vasso M. Patellar resurfacing complications in total knee arthroplasty. International Orthopaedics 201438313–317. ( 10.1007/s00264-013-2244-3) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Russell RD Huo MH & Jones RE. Avoiding patellar complications in total knee replacement. Bone and Joint Journal 201496–B(Supplement A) 84–86. ( 10.1302/0301-620X.96B11.34305) [DOI] [PubMed] [Google Scholar]
- 15.Alcerro JC Rossi MD & Lavernia CJ. Primary total knee arthroplasty: how does residual patellar thickness affect patient-oriented outcomes? Journal of Arthroplasty 2017323621–3625. 10.1016/j.arth.2017.06.046:S0883-5403(17)30575-2) [DOI] [PubMed] [Google Scholar]
- 16.Koh JS Yeo SJ Lee BP Lo NN Seow KH & Tan SK. Influence of patellar thickness on results of total knee arthroplasty: does a residual bony patellar thickness of <or=12 mm lead to poorer clinical outcome and increased complication rates? Journal of Arthroplasty 20021756–61. 10.1054/arth.2002.29320:S0883540302724479) [DOI] [PubMed] [Google Scholar]
- 17.Matz J Lanting BA & Howard JL. Understanding the patellofemoral joint in total knee arthroplasty. Canadian Journal of Surgery. Journal Canadien de Chirurgie 20196257–65. ( 10.1503/cjs.001617) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pierson JL Ritter MA Keating EM Faris PM Meding JB Berend ME & Davis KE. The effect of stuffing the patellofemoral compartment on the outcome of total knee arthroplasty. Journal of Bone and Joint Surgery. American Volume 2007892195–2203. ( 10.2106/JBJS.E.01223) [DOI] [PubMed] [Google Scholar]
- 19.Abolghasemian M Samiezadeh S Sternheim A Bougherara H Barnes CL & Backstein DJ. Effect of patellar thickness on knee flexion in total knee arthroplasty: a biomechanical and experimental study. Journal of Arthroplasty 20142980–84. ( 10.1016/j.arth.2013.04.026) [DOI] [PubMed] [Google Scholar]
- 20.Hsu HC Luo ZP Rand JA & An KN. Influence of patellar thickness on patellar tracking and patellofemoral contact characteristics after total knee arthroplasty. Journal of Arthroplasty 19961169–80. 10.1016/s0883-5403(9680163-x:S0883-5403(96)80163-X) [DOI] [PubMed] [Google Scholar]
- 21.Kawahara S Matsuda S Fukagawa S Mitsuyasu H Nakahara H Higaki H Shimoto T & Iwamoto Y. Upsizing the femoral component increases patellofemoral contact force in total knee replacement. Journal of Bone and Joint Surgery. British Volume 20129456–61. ( 10.1302/0301-620X.94B1.27514) [DOI] [PubMed] [Google Scholar]
- 22.Bracey DN Brown ML Beard HR Mannava S Nazir OF Seyler TM & Lang JE. Effects of patellofemoral overstuffing on knee flexion and patellar kinematics following total knee arthroplasty: a cadaveric study. International Orthopaedics 2015391715–1722. ( 10.1007/s00264-015-2715-9) [DOI] [PubMed] [Google Scholar]
- 23.Merican AM Ghosh KM Baena FRY Deehan DJ & Amis AA. Patellar thickness and lateral retinacular release affects patellofemoral kinematics in total knee arthroplasty. Knee Surgery, Sports Traumatology, Arthroscopy 201422526–533. ( 10.1007/s00167-012-2312-z) [DOI] [PubMed] [Google Scholar]
- 24.Oishi CS Kaufman KR Irby SE & Colwell CW. Effects of patellar thickness on compression and shear forces in total knee arthroplasty. Clinical Orthopaedics and Related Research 1996331 283–290. ( 10.1097/00003086-199610000-00040) [DOI] [PubMed] [Google Scholar]
- 25.Star MJ Kaufman KR Irby SE & Colwell CW. The effects of patellar thickness on patellofemoral forces after resurfacing. Clinical Orthopaedics and Related Research 1996322279–284. ( 10.1097/00003086-199601000-00033) [DOI] [PubMed] [Google Scholar]
- 26.Daluga D Lombardi AV Mallory TH & Vaughn BK. Knee manipulation following total knee arthroplasty. Analysis of prognostic variables. Journal of Arthroplasty 19916119–128. ( 10.1016/s0883-5403(1180006-9) [DOI] [PubMed] [Google Scholar]
- 27.Kandhari VK Desai MM Bava SS & Wade RN. Digging deeper into the patello – femoral joint: patello – femoral composite - a new dimension for overstuffing of patello – femoral joint. Journal of Clinical and Diagnostic Research 201711RC04–RC07. ( 10.7860/JCDR/2017/23192.9546) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Katagiri H Nakamura K Watanabe T Koga H Yagishita K Sekiya I & Muneta T. Increase of patellofemoral height has decreased maximum knee flexion after total knee arthroplasty of posterior cruciate-substituting prosthesis in a clinical series. Journal of Orthopaedic Science 201621458–462. ( 10.1016/j.jos.2016.02.011) [DOI] [PubMed] [Google Scholar]
- 29.Moya-Angeler J Bas MA Cooper HJ Hepinstall MS Rodriguez JA & Scuderi GR. Stiffness after primary total knee arthroplasty: a radiographic analysis with a matched-control population. Current Orthopaedic Practice 201728383–387. ( 10.1097/BCO.0000000000000522) [DOI] [Google Scholar]
- 30.Kelly MA. Patellofemoral complications following total knee arthroplasty. Instructional Course Lectures 200150403–407. [PubMed] [Google Scholar]
- 31.Ryu J Saito S Yamamoto K & Sano S. Factors influencing the postoperative range of motion in total knee arthroplasty. Bulletin 19935335–40. [PubMed] [Google Scholar]
- 32.Mihalko W Fishkin Z & Krackow K. Patellofemoral overstuff and its relationship to flexion after total knee arthroplasty. Clinical Orthopaedics and Related Research 2006449283–287. 10.1097/01.blo.0000218756.89439.06:00003086-200608000-00051) [DOI] [PubMed] [Google Scholar]
- 33.Beldman M Breugem SJM & van Jonbergen HPW. Overstuffing in total knee replacement: no effect on clinical outcomes or anterior knee pain. International Orthopaedics 201539887–891. ( 10.1007/s00264-014-2548-y) [DOI] [PubMed] [Google Scholar]
- 34.Kemp MA Metcalfe AJ Sayers A Wylde V Eldridge JD & Blom AW. Does overstuffing of the patellofemoral joint in total knee arthroplasty have a significant effect on postoperative outcomes?; 29933936. Knee 201825874–881. ( 10.1016/j.knee.2018.05.007) [DOI] [PubMed] [Google Scholar]
- 35.Matz J Howard JL Morden DJ MacDonald SJ Teeter MG & Lanting BA. Do changes in patellofemoral joint offset lead to adverse outcomes in total knee arthroplasty with patellar resurfacing? A radiographic review. Journal of Arthroplasty 201732783–787.e1. ( 10.1016/j.arth.2016.08.032) [DOI] [PubMed] [Google Scholar]
- 36.Slim K Nini E Forestier D Kwiatkowski F Panis Y & Chipponi J. Methodological index for non-randomized studies (minors): development and validation of a new instrument. ANZ Journal of Surgery 200373712–716. ( 10.1046/j.1445-2197.2003.02748.x:2748) [DOI] [PubMed] [Google Scholar]
- 37.Marx RG Wilson SM & Swiontkowski MF. Updating the assignment of levels of evidence. Journal of Bone and Joint Surgery. American Volume 2015971–2. ( 10.2106/JBJS.N.01112) [DOI] [PubMed] [Google Scholar]
- 38.Ghosh KM Merican AM Iranpour F Deehan DJ & Amis AA. The effect of overstuffing the patellofemoral joint on the extensor retinaculum of the knee. Knee Surgery, Sports Traumatology, Arthroscopy 2009171211–1216. ( 10.1007/s00167-009-0830-0) [DOI] [PubMed] [Google Scholar]
- 39.Australian Orthopaedic Association National Joint Replacement Registry. Annual Report 2011. AOA: Adelaide, Australia; 2011. Available at: https://aoanjrr.sahmri.com/en-GB/annual-reports-2011 [Google Scholar]
- 40.Sensi L Buzzi R Giron F De Luca L & Aglietti P. Patellofemoral function after total knee arthroplasty: gender-related differences. Journal of Arthroplasty 2011261475–1480. ( 10.1016/J.ARTH.2011.01.016) [DOI] [PubMed] [Google Scholar]
- 41.Petersen W Rembitzki IV Brüggemann GP Ellermann A Best R Koppenburg AG & Liebau C. Anterior knee pain after total knee arthroplasty: a narrative review. International Orthopaedics 201438 319–328. ( 10.1007/S00264-013-2081-4) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Roussot MA & Haddad FS. The evolution of patellofemoral prosthetic design in total knee arthroplasty: how far have we come? EFORT Open Reviews 20194 503–512. ( 10.1302/2058-5241.4.180094) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dejour D Ntagiopoulos PG & Saffarini M. Evidence of trochlear dysplasia in femoral component designs. Knee Surgery, Sports Traumatology, Arthroscopy 2014222599–2607. ( 10.1007/S00167-012-2268-Z) [DOI] [PubMed] [Google Scholar]
- 44.Escobar A Quintana JM Bilbao A Aróstegui I Lafuente I & Vidaurreta I. Responsiveness and clinically important differences for the WOMAC and SF-36 after total knee replacement. Osteoarthritis and Cartilage 200715273–280. 10.1016/j.joca.2006.09.001:S1063-4584(06)00266-4) [DOI] [PubMed] [Google Scholar]
- 45.Kawano T Miura H Nagamine R Urabe K Matsuda S Mawatari T Moro-Oka T & Iwamoto Y. Factors affecting patellar tracking after total knee arthroplasty. Journal of Arthroplasty 200217942–947. ( 10.1054/ARTH.2002.34826) [DOI] [PubMed] [Google Scholar]
- 46.Fukagawa S Matsuda S Mizu-uchi H Miura H Okazaki K & Iwamoto Y. Changes in patellar alignment after total knee arthroplasty. Knee Surgery, Sports Traumatology, Arthroscopy 20111999–104. ( 10.1007/S00167-010-1164-7) [DOI] [PubMed] [Google Scholar]
- 47.Chan KC & Gill GS. Postoperative patellar tilt in total knee arthroplasty. Journal of Arthroplasty 199914300–304. ( 10.1016/S0883-5403(9990055-4) [DOI] [PubMed] [Google Scholar]
- 48.Shoji H Solomonow M Yoshino S D’Ambrosia R & Dabezies E. Factors affecting postoperative flexion in total knee arthroplasty. Orthopedics 199013643–649. ( 10.3928/0147-7447-19900601-08) [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.



 This work is licensed under a
This work is licensed under a