Foamy viruses (FVs; also known as spumaviruses or spumaretroviruses) are classified as one of the genera of the Retroviridae. The FVs, first described in 1954 (18) and isolated in 1971 (1), have been rather neglected relative to their better known retroviral cousins, the type C oncoviruses and lentiviruses. Nonetheless, detailed analyses of this group during the past few years have revealed surprising, and in some cases remarkable, differences from all other retroviruses. In fact, in some respects their replication bridges the gap between retroviruses and the only other family of vertebrate reverse transcriptase (RT)-encoding viruses, the Hepadnaviridae.
FVs are widespread, and viruses have been readily isolated from a variety of primate species, including gorillas (7), chimpanzees (33), baboons (13), African green monkeys (64), and numerous others. Recent phylogenetic analysis indicates that FVs have coevolved with their hosts (13). Feline (54, 57) and bovine species (40) are also infected by FV. There are several reports that FVs were detected in other species, such as sea lions (43) and hamsters (38), but no viral isolates are currently available. Studies of both simian foamy virus (SFV) and bovine foamy virus (BFV) in their natural hosts indicate that antiviral antibodies, but not viral infections, are acquired maternally. Animals become infected as young adults, presumably via saliva through biting or licking (13, 44).
These viruses are highly cytopathic in many types of cells in tissue culture, leading to rapid syncytium formation, vacuolization of cells (hence the name foamy), and cell death (the biology of FVs is reviewed in reference 37). Human diploid fibroblast cells and baby hamster kidney (BHK) cells are particularly sensitive to FV-induced cytopathic effects. Paradoxically, it is also possible to infect some cell lines, such as cell lines derived from human hematopoietic cells, and obtain persistently infected cultures which produce fairly high titers of replication-competent virus in the absence of cell death (91). The difference in host cell response that potentiates a lytic versus a persistent infection is not yet understood. It is rather curious that a virus which can be exceedingly cytopathic in vitro has not been unequivocally shown to cause disease. A major difference between FVs and the other retroviruses, as well as the hepadnaviruses, is that there is no evidence that FVs are pathogenic in either naturally or accidentally infected hosts. This has kindled interest in the use of FV as a vector for gene transfer (11, 62, 72).
The prototype FV, human FV (HFV) was isolated from tissue culture cells of a human nasopharyngeal cell line of African origin (1). This isolate was the first FV to be cloned and sequenced (24, 55), and an infectious molecular clone was derived (53, 70). Recent work indicates that this virus is virtually identical to SFV isolated from chimpanzees, SFV type 6 (SVF-6), SFV-7, and SFV-cpz (34) (Fig. 1). Humans do not appear to harbor an FV homologous to the chimpanzee virus (2, 80); thus, the origin of HFV remains obscure. The HFV designation is mainly for historical reasons. The use of the term human for this virus is admittedly misleading, and a more reasonable nomenclature would be SFV-hu, indicative of its isolation from a human cell line.
FIG. 1.
Unrooted tree of pol sequences from retroviral genomes. Alignment was done using the Clustal-X algorithm. The tree was derived from ungapped portions of the Pol protein alignment. Bootstrap analysis shows that HERV-L and MERV cluster with the foamy viruses 94 of 100 times. The viral designations not given in the text, are as follows: SFV-1 (rhesus macaques), SFV-3 (African green monkey), FFV (feline FV), SHRV (snakehead retrovirus), ALV (avian leukosis virus), MMTV (murine mammary tumor virus), HTLV1 and HTLV2 (human T-cell leukemia virus types 1 and 2), EIAV (equine infectious anemia virus), FIV (feline immunodeficiency virus), SIV (simian immunodeficiency virus type 1). The accession numbers used for endogenous element sequences are: MERV, EMBO no. Y12713, MERVLPOLY; HERV-L, GenBank no. AF070718 and AF070718 (reverse compliment of nucleotides 46480 to 44147, with frameshifts corrected).
Most of the molecular analyses of FVs have utilized strains of HFV which have been passaged extensively in tissue culture, although in many cases findings in HFV have been confirmed using simian, bovine, and feline strains. Sequence comparisons of the polymerase (pol) genes from FVs and other retroviruses show that FV pol genes comprise a distinct grouping, distantly related to other retroviruses (Fig. 1). The closest non-FV relatives are endogenous viral elements from mice (MERV) and humans (HERV-L) (17), as well as endogenous pol-like sequences from an unusual assortment of vertebrates, including the common possum and the tuatara (35).
The organization of the DNA proviral genome of HFV (clone 13) (53) is shown at the top of Fig. 2. FVs are complex retroviruses; they encode several open reading frames (ORFs) at the 3′ end of the genome, in addition to canonical gag, pol, and env genes. Only two of these ORFs are known to encode proteins (discussed further below). The arrangement of the genome is similar to that of other complex retroviruses, such as human immunodeficiency virus (HIV). HFV utilizes a tRNA1,2Lys primer for reverse transcription, and all indications are that reverse transcription proceeds by the mechanism used by other well-studied conventional retroviruses. FVs contain many of the cis-acting sequences utilized by other retroviruses, such as a polypurine tract for positive-strand priming, as well as packaging sequences located near the 5′ end of the genome (21, 32). Additional cis-acting sequences required for transfer of HFV vectors have been mapped to the pol gene (21, 32). Such sequences may be required for packaging or for other posttranscriptional processes, such as RNA stability or efficient export. If indeed these pol sequences are part of the packaging signal, as has been suggested (32), this would be another unique feature of the FV genome. FVs have large long terminal repeat (LTR) regions; the full-length HFV LTR is 1,769 bp. When virus is passaged in culture, specific sequences of the LTR U3 region are deleted and such deletions appear to confer a selective advantage to virus replication in some tissue culture cells but not in lymphoid cells or in vivo in chimpanzees (77). Regions which are deleted appear to contain negative regulatory elements, but their exact nature has not been determined. The U5 region of the LTR also contains a negative element (87). Transcription from the LTR promoter-enhancer requires the Tas protein. This requirement is absolute, and in the absence of Tas, LTR-mediated transcription cannot be detected (5, 53, 90). Tas is not found in viral particles, so how is infection initiated? FVs utilize a mechanism seen in complex DNA viruses but not other retroviruses: multiple promoters. There is an internal promoter (IP) located near the 3′ end of the env gene, which was first found in HFV (51) and later in SFV and feline FV (15, 56, 86). The IP is required for viral infectivity in tissue culture (52). This promoter has a higher basal transcription level than the LTR promoter (87), and its use leads to transcripts encoding Tas and Bet (Fig. 2). Once levels of Tas accumulate, there is a switch to use of the LTR promoter, which binds Tas with lower affinity than the IP (42) and leads to accumulation of gag, pol, and env transcripts. Presumably, transcription also continues from the IP even at late times in infection. Among the retroid elements, only FVs exhibit this temporal regulation of transcription.
FIG. 2.
Genome of HFV. Depicted is the molecular clone HFV-13 (Genbank accession no. U21247; 11,954 bp). This infectious clone has a deletion of 648 bp in the U3 region of each LTR relative to that of clone HFV-2 (Genbank accession no. Y07725; 13,242 bp). Otherwise, the two clones are essentially identical. The locations of the ORFs (boxes) indicate the reading frames of the deduced proteins. The thin lines indicate the mRNAs for which proteins have been identified. The shaded box depicts the HFV provirus and gives the locations of the LTR promoter and IP indicated by P and also shows the location of the major 5′ splice site (ss) used as the donor for all of the mRNAs originating from the LTR promoter. The shaded boxes at the bottom show the ΔHFV provirus lacking a functional Tas gene (see text). RNAs for Tas and Bet are encoded by both the LTR promoter and the IP, but only those originating from the IP are indicated. The ORFs are indicated in italics. The ATGs indicate translational start sites. GR, glycine-arginine-rich boxes; PR, protease domain; RT, RT domain; IN, integrase domain; SU, surface domain; TM, transmembrane domain. The tas gene (transactivator of spumavirus) was formerly called bel-1. bel-2 and bel-3 are ORFs for which a protein has not conclusively been demonstrated. The Bet protein is translated from a spliced RNA and contains residues from part of tas and from bel-2 (as also indicated by the diagonal arrow). The Env-Bet mRNA is a multiply spliced RNA encoding a protein of unknown function.
Gag protein.
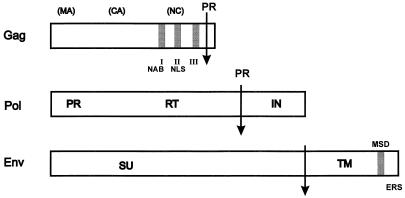
Completion of the HFV sequence made it apparent that the two distinguishing hallmarks of retroviral Gag proteins are lacking. These are the major homology region (MHR) in the capsid domain and the Cys-His box(es) in the nucleocapsid domain. While the function of the MHR is unknown, the Cys-His boxes and surrounding basic residues are important for RNA binding (reviewed in reference 6). In addition, FV proteins do not appear to be efficiently cleaved and assays of extracellular proteins show two predominant forms of Gag, of 78 and 74 kDa. Thus, there is no compelling evidence that cleavage of Gag protein by the viral protease leads to the matrix, capsid, and nucleocapsid polypeptides that comprise mature, infectious conventional retroviral particles. There are data that suggest that some cleavage of Gag into smaller proteins occurs during the early steps of infection, but this is far from complete (27). The cleavage of P78 to P74 does require viral protease (46) and results in approximately equimolar amounts of the two proteins in infectious virions. This cleavage appears to be necessary for viral infectivity, and deletion of the gag sequences distal to the cleavage site leads to virus with very low infectivity (19, 93). Mutants lacking the terminal 3 kDa of Gag protein assemble and bud normally, and it is suggested that this part of Gag could have a critical role in infection of new host cells (93).
Retroviruses, and a majority of RNA viruses, encode nucleocapsid (NC) proteins which bind nonspecifically along the viral genome and presumably protect the RNA from degradation. The absence of such a protein in FVs is therefore remarkable. The carboxyl-terminal region of HFV Gag contains three glycine-arginine-rich domains called GR boxes (76) (shaded boxes in Fig. 3). The carboxyl-terminal one-third of HFV Gag binds to RNA and DNA with equal affinity, and this nucleic acid binding domain (NAB) maps to GR box 1 (89). The FV Gag is similar to the single core protein of hepadnaviruses such as human hepatitis B virus (HBV), which is also a multifunctional protein whose carboxyl terminus contains specific arginine residues which bind either to RNA or DNA (30, 61). This type of viral structural protein makes sense for a DNA virus but is rather unusual for an RNA virus. The NAB of FV Gag is present on a multifunctional protein rather than a free NC protein and is thus unlikely to bind the viral RNA genome in a histone-like fashion as does the free NC of other retroviruses. Additionally, the high-affinity DNA binding of FV Gag is a puzzle if the FV genome is RNA. In fact, these features of FV Gag are much more similar to those of the core protein of HBV, which interacts with both RNA in the early stages of reverse transcription and assembly and with the encapsidated DNA genome in the mature particle.
FIG. 3.
Details of the viral structural proteins. (Top) Gag protein. The locations of the three glycine-arginine-rich GR boxes (I, II, and III) are indicated by shaded boxes. The NAB and NLS are indicated below boxes I and II. The arrow indicates the cleavage site at which viral protease (PR) (encoded in Pol) cleaves about 3 kDa from the 78 kDa Gag precursor. The locations of the expected sites for the retroviral matrix (MA), capsid (CA), and nucleocapsid (NC) polypeptides are shown. These cleaved proteins, however, have not been found in mature, infectious virions. (Middle) Pol protein. The PR, RT, and integrase (IN) domains are shown. The PR cleavage site is indicated by the arrow labeled PR. (Bottom) Env protein. The surface (SU) and transmembrane (TM) domains are shown as well as the proteolytic cleavage site (arrow). MSD, membrane spanning domain in TM; ERS, endoplasmic reticulum sorting signal in the cytoplasmic tail.
An interesting and characteristic feature of FV infection is the localization of newly synthesized Gag proteins to the nucleus of the infected cell. It has long been known that a characteristic feature of FV infection is the detection of strong nuclear staining by anti-FV antibodies; this is a diagnostic feature of FV in cell culture. The nuclear fluorescence is caused by Gag accumulation. It was shown that the Gag GR box 2 contains a nuclear localization signal (NLS) (Fig. 3, Top) and that this sequence results in nascent Gag localization to the nucleus (76, 89). Deletion of the NLS does not abrogate replication in vitro (89), so the role of nuclear localization in the viral life cycle is unknown.
Pol protein.
RTs have the potential to copy any RNA into DNA, even in the absence of specific tRNA primers, a feature that has made them indispensable to molecular biology. Thus, a key aspect of retroviral adaptation to host cells is a mechanism for sequestering RT in an inactive form until assembly into virions, where the enzyme is coupled with the viral genome rather than random cellular RNAs. Conventional retroviruses have evolved a mechanism wherein Pol is synthesized as a Gag-Pol fusion protein by a fairly rare frameshift or nonsense codon suppression event (reviewed in reference 39). The Gag protein contains assembly domains which lead to the formation of immature capsids, most commonly at the cell membrane (85). The Gag-Pol protein is also rapidly incorporated into nascent particles via the Gag assembly domains, and Gag-Pol has much lower RT activity than cleaved Pol (reviewed in reference 82). The virion protease (PR) is usually encoded within Pol, and virion protease needs to dimerize to be an active enzyme. Therefore, only within particles is the concentration of PR high enough for efficient enzymatic activity. Consequently, high levels of reverse transcription occur only in particles and then the major and preferred template is the encapsidated RNA. For conventional retroviruses, a majority of reverse transcription of the viral genome does not occur until viruses infect new cells and are partially uncoated, although small amounts of strong-stop and longer DNAs can be detected in released particles (83).
The situation is quite different for FVs. The HFV Pol ORF is not in the −1 reading frame relative to Gag but rather in the +1 reading frame, and there is no stop codon separating the two reading frames. Essentially, all eukaryotic ribosomal frameshifting occurs only when the second gene is in the −1 reading frame, which makes this mechanism of Pol synthesis unlikely. It was also shown that a protein containing both Gag and Pol determinants could not be detected even when the active site of the HFV viral protease was mutated (46). Thus, it was highly unlikely that HFV Pol is synthesized as a Gag-Pol fusion protein. It was shown that either the natural ATG at the beginning of the pol coding sequence or the removal of this ATG and the introduction of a new ATG distal to the normal signal was necessary for viral replication (20). This left three possibilities: an internal ribosomal initiation site for Pol expression as seen for some other RNA viruses, an IP at the end of Gag allowing a Pol-specific transcript to be synthesized, or a spliced mRNA encoding the Pol AUG. In fact, it was shown that HFV Pol is expressed from a unique mRNA using the major 5′ splice site (ss) and a 3′ ss in the gag gene upstream from the ATG at the start of Pol (88). This was quickly confirmed for HFV, as well as for SFV and BFV (10, 36, 41, 50) (Fig. 2). Synthesis of Pol without Gag domains poses interesting questions regarding its activation and sequestration into particles. All RT-encoding viruses have evolved mechanisms to sequester RT with the viral genome and thus prevent nonspecific reverse transcription of cellular RNAs, and it is highly likely that FVs have also evolved a mechanism to prevent RT activation in cells. Two possible mechanisms for the specific assembly of Pol into particles are the high-affinity binding of Pol to Gag or the specific interaction of Pol with genomic RNA. The latter mechanism is used by hepadnaviruses to ensure encapsidation of P protein into particles. P is synthesized after a rare internal initiation from genomic-length RNA and is thought to bind in cis to its own mRNA at a specific 5′ sequence at which reverse transcription commences immediately. P protein provides both the RT enzyme and the primer for DNA synthesis and becomes covalently linked to the cDNA product. cDNA elongation occurs only in the presence of core, so that assembly and DNA synthesis are temporally and physically linked (reviewed in reference 81). Such a mechanism is unlikely for HFV, as it is synthesized from a subgenomic mRNA and encapsidation of that RNA rather than genomic RNA would lead to deletion of the genome and viral “suicide.” It is possible that there are specific high-affinity FV Gag-Pol interactions, but these have not been identified as yet.
Another interesting feature of the HFV Pol protein is that the only well-documented cleavage by the viral protease encoded within the pol gene is between RT and integrase (IN) (Fig. 3, Middle). The major cleavage products of the 127-kDa Pol precursor are a ca. 85-kDa PR-RT protein and a ca. 40-kDa IN protein (45, 63). This is in contrast to other retroviruses such as HIV, in which Pol is cleaved into three proteins, PR, RT, and IN. Expression in vitro of either HFV RT or PR without the other domain leads to enzymatically active protein (45, 68). However, in infected cells, proteins corresponding to the individual RT or PR domains have not been detected. In contrast, in vitro, using bacterially expressed proteins, cleavage between PR and RT is observed but very inefficiently (67). These authors also conclude that cleavage of PR-RT is not required for viral infection. It is difficult to detect Pol proteins in extracellular virions with currently available antibodies, presumably because of low abundance, so the composition of Pol protein in extracellular virions has not yet been analyzed.
The FVs do encode a bona fide IN protein with all the characteristics of other retroviral integrases, including an amino-terminal HHCC zinc finger, a D,D35,E active site, and a DNA binding domain. Purified protein carries out endonucleolytic activity and integrase and disintegrase activities (66). A DDE active site mutant is not replication competent, showing that integration is required for viral infection (16a). It has been difficult to study FV integration, because a majority of cells infected with the virus die rapidly. However, the use of persistently infected H92 erythroblastoid cell lines (91) has allowed such an analysis. These cells contain 10 to 50 integrated copies of provirus. This high copy number could possibly be due to an intracellular recycling mechanism involving the NLS in Gag, since the same cells infected with an NLS mutant contain only 1 to 3 integrated proviruses (16a). Such a recycling mechanism is somewhat reminiscent of hepadnavirus infection, in which an internal recycling mechanism leads to the accumulation of additional copies of covalently closed circular DNA in the nucleus of the infected cell (Fig. 4C).
FIG. 4.
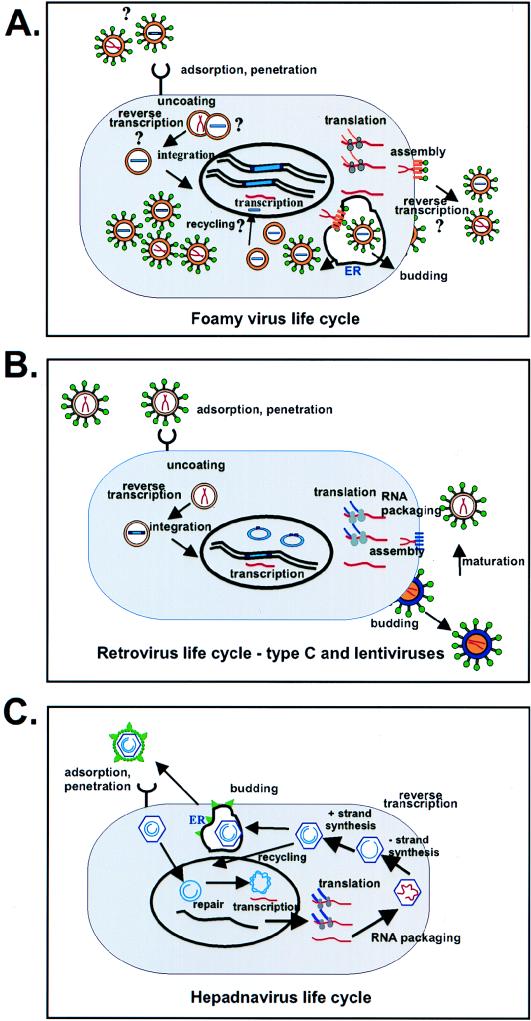
Replication pathway of FV compared to that of the conventional retroviruses and hepadnaviruses. (A) Proposed replication pathway of FVs. Viral RNA is indicated by red lines, and DNA is indicated by blue bars. Gold indicates the viral Gag protein, and green circles are the glycoproteins. Grey circles indicate polysomes. ER denotes endoplasmic reticulum. Several intracellular particles are shown, which represent the large numbers of intracellular particles in infected tissue culture cells which have been detected by both electron microscopy and viral assays. It is not known whether such particles contain RNA or DNA or both. The uncertain steps in the life cycle are indicated by question marks. (B) Retroviral life cycle. Colors used are the same as in panel A. Immature particles are shown with gold centers; after protease cleavage, the mature extracellular particles are shown with white central cores. Details of the replication pathway can be found in reference 16. (C) Hepadnavirus life cycle. Abbreviations are as in panels A and B. Viral core proteins are indicated by purple, RNA by red, DNA by blue, and S glycoproteins by green. Details of the replication pathway can be found in reference 25.
Env protein.
The only viral protein required for conventional retroviruses to bud from infected cells is Gag. Mutants with complete deletions of the env gene produce wild-type levels of extracellular virions which are infectious when fused into cells by artificial means, such as inactivated Sendai virus or polyethylene glycol. In the case of HIV, the envelope glycoproteins do enhance budding of Env particles, but only about 10-fold (12, 71). Again, FVs do not follow this replication paradigm. The HFV glycoprotein appears to be similar in structure to that of other retroviruses containing SU and TM moieties (Fig. 3, bottom). In the absence of Env proteins, HFV particles are synthesized intracellularly but do not appear in the culture supernatant (4, 22). Additionally, it is not possible to create viral pseudotypes of HFV with glycoproteins from murine retroviruses such as murine leukemia virus (MLV) (49) or vesicular stomatitis virus (VSV) G protein (69). The latter finding is quite remarkable, since VSV G is able to complement the envelope glycoprotein-deficient mutants of a large range of viruses. MLV can be pseudotypes with HFV glycoproteins but only if the cytoplasmic tail of HFV TM is removed and replaced with that of MLV (49). Taken together, these findings indicate that FV Gag and Env interact in a unique way and that other retroviral Env proteins are lacking the domains present in FV Env which are required for assembly of retroviral particles. One such domain in HFV Env is an endoplasmic reticulum sorting signal (ERS; Fig. 3) in the cytoplasmic tail of the TM domain (28). This is similar to the case of HBV in which the surface (S) protein, which is the viral glycoprotein, also contains an ERS. HBV does not bud from infected cells unless S protein is present. Early in infection, prior to synthesis of S protein, nascent HBV nucleocapsids containing gapped circular DNA and core and P proteins are thought to recycle into the nucleus of the infected cell where reverse transcription is completed. This leads to accumulation in the nucleus of multiple copies of active viral genomes.
In HFV-infected cells, the majority of virus is seen associated with intracellular membranes, intracytoplasmic vesicles, or the cell cytoplasm, rather than budding from the plasma membrane (93). Only a small fraction of virus is extracellular, most of the infectious virions are tightly cell associated but can be released by multiple rounds of freezing and thawing (90). It is likely that the major site of association of Gag and Env leading to viral assembly is at the ER. It is not clear why some virions bud from the plasma membrane or what proportion of virus which buds through the ER is released from the cell rather than back into the cytoplasm. If the ERS is mutated, virus buds exclusively from the plasma membrane and many more extracellular particles are detected. However, the amount of infectivity in the culture supernatants is not increased, indicating that virus that buds from the plasma membrane is intrinsically less infectious than that which buds from the ER (27a, 28). The HFV Gag protein is not myristylated (59), and if a myristylation signal is engineered into the amino terminus of Gag the virus is totally noninfectious (17a). This again suggests that Gag and Env must interact for the assembly of infectious virus and additionally that this interaction is likely to occur at the ER.
Viral genome.
The FV genomic organization and mechanism of reverse transcription are highly similar to those of other retroviruses. However, unlike the case with conventional retroviruses, there are no published data, such as Northern blots, indicating that particles contain RNA molecules of about 11 kb in length. In fact, many of the characteristics of FVs are more consistent with those of RT-encoding viruses which have DNA genomes (hepadnaviruses) than RNA genomes (retroviruses). These include the lack of a nucleocapsid protein and equal affinity in binding of the carboxyl-terminal portions of Gag to RNA and DNA, possible through arginine residues. When FV particles were analyzed for their DNA content using Southern blots, it was found that DNAs of about 12 kb could easily be detected in sucrose gradient particles (88). A majority of the particles did not contain large DNA molecules, but in this analysis, it was concluded that enough of the particles contained DNA to account for the infectivity of the viral preparation. The initial nucleic acid packaged into virions is RNA (4), which indicates that RT is activated very early in viral assembly/budding. Further experiments with the inhibitor of reverse transcription 3′-azido-3′-deoxythymidine indicated that the genome was likely to be DNA rather than RNA (58, 92). In addition, DNA extracted from extracellular virions is infectious (92). FV-infected cells contain a large amount of unintegrated DNA: hundreds to thousands of copies per cell (58, 79, 88). This DNA is likely to be in intracellular viral particles which are also abundant. The intracellular DNA is a linear duplex of proviral length; many of the molecules contain a single-stranded region (47, 65). It has been suggested that the single-stranded gap arises from discontinuous plus-strand DNA synthesis (47). The large amount of intracellular DNA is not dependent on superinfection and appears to be a late step in the viral life cycle (58).
Tas (Bel-1) protein.
As in the case of other complex retroviruses, the FV genome encodes a transactivator protein required for transcription from both the LTR promoter and the internal promoter IP. The Tas proteins of the FVs do not share sequence identity with other known transcriptional transactivators and presumably activate transcription by a unique mechanism. The Tas protein (originally called Bel-1) is a 36-kDa phosphoprotein which contains an acidic transcription activation domain at its carboxyl terminus and a centrally located DNA binding domain (8, 31, 84). As Tas binds to both the LTR promoter (at several sites called BREs for Bel-1-responsive elements) and to the IP and these regions do not contain similar DNA sequences, the Tas-specific DNA binding sequence is predicted to be degenerate. A 25-bp binding site was found in both the LTR and IP, and sequence alignment shows that there are conserved critical purine residues, as detected by methylation interference, but little sequence identity (42). In the case of SFV-1, an additional Tas binding site has been mapped to the end of the gag gene (14), but its functional significance is as yet unknown as Pol is not synthesized from an internally initiated RNA. As discussed above, the higher affinity of Tas for the IP site and the higher level of basal transcription from the IP can explain the temporal regulation of transcription starting from the IP and later switching to the LTR when the Tas concentration increases. The Tas protein differs from the Tat transcriptional activator of HIV and the Tax transcriptional activator of human T-cell lymphotropic virus in that only Tas is a sequence-specific DNA binding protein. More work is required to work out the details of the protein-DNA interaction. It is also interesting to note that thus far, FVs are the only complex retroviruses which do not encode posttranscriptional regulatory genes (5, 48).
Bet protein.
The Bet protein is synthesized from a doubly spliced mRNA and is translated from the Tas and Bel-2 reading frames (60) (Fig. 2). The protein has 88 amino acids of Tas fused to the entire Bel-2 ORF. Bet is a major protein synthesized by infected cells both in vitro and in vivo (29), but its function is unknown. Sequence analysis yields no clues as to its function. While Bet is conserved among the FVs, it does not share significant sequence identity with any known proteins and contains no known protein motifs. It has been suggested that Bet may play a role analogous to that of HIV-1 Vif because of conserved histidine and cysteine residues (23). When the Bel-2 ORF portion of Bet was deleted, infectious virus was produced, albeit at a titer of about 10% that of wild type. This decrease in titer was only seen when cell-free virus was used: cell lysates from Bet+ and Bet− viruses yielded the same titer (90). However, the small effect makes it difficult to study further, and other explanations are possible. It is very likely that Bet may play a significant role in in vivo infections. However, there is no evidence for this, and one can only speculate as to whether Bet down modulates infection or enhances infectivity.
Recently, it has been observed that overexpression of Bet in tissue culture cells prevents infection by wild-type FV. The mechanism is not known, but the block appears to be downstream of viral entry and upstream of viral gene expression, possibly at the viral integration site (9). Bet has been shown to be secreted from tissue culture cells and can be taken up by other cells (26). If Bet uptake also occurs in infected animals and Bet inhibits new infection, this could be a factor contributing to limitation of viral spread in vivo and could perhap help prevent pathogenicity. FV infection in vivo is apparently at a low level, as it is difficult to culture virus from infected animals and the virus appears to have evolved to maintain itself at low levels without compromising transmission.
A unique Env-Bet fusion protein of 160 to 170 kDa has also been detected after infection of cell cultures (26, 49a). Analysis of the RNA encoding this protein indicates that the splice donor site in env is upstream of both the ERS and the TM domain (Fig. 2). It was predicted that this protein would also be secreted from cells and not be found in particles. Both of these predictions have been confirmed. Thus far, no role in replication has been ascribed to this Env-Bet protein, as at least in tissue culture, a mutant lacking the env 5′ ss replicates as well as the wild type. Additionally, no evidence was found that the ss mutant is neutralized differently by antiserum than the wild type, suggesting that there may be no role for Env-Bet in altering the humoral immune response (49a). However, these experiments do not necessarily reflect the in vivo situation.
Another interesting finding is the rapid accumulation in tissue culture and in infected rabbits of viral DNA which lacks the coding capacity for Tas (73–75, 91) (Fig. 2, bottom). This occurs because the mRNA encoding Bet lacks the Tas reading frame (Fig. 2). However, as this mRNA apparently contains all the cis-acting sequences required for stability, export, and packaging (21, 32), it can be reverse transcribed into cDNAs. The Tas-defective form of HFV has been called ΔHFV. In one study, it was found that high copy numbers of ΔHFV proviruses can prevent cell lysis after infection with HFV and lead to persistent infection (73). The authors suggest that the ΔHFV behaves like a defective interfering (DI) virus and that Bet is required for the DI effect because a ΔHFV Bet− mutant does not prevent lysis. This idea is consistent with the more recent finding that Bet protein itself can prevent HFV infection. However, nothing is known about the status of Bet or Tas expression in naturally infected animals, and further experiments will be required to determine whether Bet is an important player in the maintenance of persistent low-level infections in vivo.
Taken together, these data suggest that the unique FV Bet protein is a key player in the vivo response to viral infection and perhaps explain why this group of viruses does not induce disease in infected animals or people.
HFV replication pathway.
The key points discussed in this review are summarized in Fig. 4A, where the life cycle of HFV is compared to that of conventional retroviruses (Fig. 4B) and hepadnaviruses (Fig. 4C). The FV replication pathway differs from that of retroviruses in the following ways. (i) Reverse transcription appears to be a late event in viral morphogenesis, and the infectious particles probably have DNA genomes. (ii) Mature virions do not contain matrix, capsid, or nucleocapsid proteins but instead are composed of two large Gag proteins which differ at the carboxyl terminus by the removal of 3 kDa. (iii) Viral budding requires both Gag and Env proteins. A majority of virus buds through the ER. (iv) Most virus is intracellular and probably accounts for the large amount of unintegrated DNA seen in infected cells. (v) Persistently infected cells contain large amounts of integrated DNA. This could occur through an intracellular recycling pathway, although there is as yet no firm evidence that such a pathway occurs during FV infection either in vitro or in vivo. In each of these steps, FVs have characteristics similar to those of the hepadnaviruses. In the case of HBV, reverse transcription is completed prior to budding, the viral core is not cleaved into smaller polypeptides, viral budding requires core and S proteins, and a recycling pathway allows the buildup of many copies of covalently closed circular viral DNA in the nucleus. Clearly FVs and other retroviruses are distinct from the hepadnaviruses in that they encode proteases and integrases and integration is a obligate event in the viral life cycle. Given the greater differences between FVs and the other retroviral genera, their classification into a subfamily distinct from the other conventional retroviruses appears to make sense.
Future directions.
There remain many unknowns about the FVs which probably contribute to the unique aspects of their interactions with host cells. Because most portions of the viral life cycle do not conform to the conventional retroviral paradigm, understanding each step in viral replication is a challenge. The domains in Gag which interact with the Env protein are likely to be very different from those in HIV or other retroviruses, and the requirement of Env for budding remains to be explained. The mechanism of Pol assembly into particles is clearly unique. The role of the localization of Gag to the nucleus is also a striking characteristic of FVs and is not well understood. Confirmation of an intracellular recycling mechanism and elucidation of its role in viral replication, if any, also remain to be shown.
However, the biggest challenges lie in understanding the interactions of these viruses with their hosts. Clearly the situation in vivo does not mirror what is seen in tissue culture cells, where viruses are generally cytopathic. More work needs to be done to define the target cells that are infected in vivo and the host immune response to infection. The possible role of the Bet protein in maintaining persistent low levels of infection is an area of interest as is the function of the Env-Bet fusion protein. Such studies should be feasible because of the availability of small animal models, such as rabbits and mice, which can be infected with FV (74, 78). Such questions are of more than academic interest because of the proposed use of FV vectors for gene therapy and the widespread presence of FV in animal models used for other viral diseases. Most importantly perhaps, FV is present in some species, such as baboons, used for xenotransplantation, and FV sequences have been found at distal sites in human recipients of baboon liver transplants (3).
ACKNOWLEDGMENTS
I thank Michael Emerman, David Baldwin and Jen Banks for thoughtful critiques and Elizabeth Greene of the Biocomputing Shared Resource at Fred Hutchinson Cancer Research Center for compiling the data used in Fig. 1.
REFERENCES
- 1.Achong B G, Mansell W A, Epstein M A, Clifford P. An unusual virus in cultures from a human nasopharyngeal carcinoma. J Natl Cancer Inst. 1971;46:299–307. [PubMed] [Google Scholar]
- 2.Ali M, Taylor G P, Pitman R J, Parker D, Rethwilm A, Cheingsongpopov R, Weber J N, Bieniasz P D, Bradley J, McClure M O. No evidence of antibody to human foamy virus in widespread human populations. AIDS Res Hum Retroviruses. 1996;12:1473–1483. doi: 10.1089/aid.1996.12.1473. [DOI] [PubMed] [Google Scholar]
- 3.Allan J S, Broussard S R, Michaels M G, Starzl T E, Leighton K L, Whitehead E M, Comuzzie A G, Lanford R E, Leland M M, Switzer W M, Heneine W. Amplification of simian retroviral sequences from human recipients of baboon liver transplants. AIDS Res Hum Retroviruses. 1998;14:821–824. doi: 10.1089/aid.1998.14.821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Baldwin D N, Linial M L. The roles of Pol and Env in the assembly pathway of human foamy virus. J Virol. 1998;72:3658–3665. doi: 10.1128/jvi.72.5.3658-3665.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Baunach G, Maurer B, Hahn H, Kranz M, Rethwilm A. Functional analysis of human foamy virus accessory reading frames. J Virol. 1993;67:5411–5418. doi: 10.1128/jvi.67.9.5411-5418.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Berkowitz R, Fisher J, Goff S P. RNA packaging. Curr Top Microbiol Immunol. 1996;214:177–218. doi: 10.1007/978-3-642-80145-7_6. [DOI] [PubMed] [Google Scholar]
- 7.Bieniasz P D, Rethwilm A, Pitman R, Daniel M D, Chrystie I, McClure M O. A comparative study of higher primate foamy viruses, including a new virus from a gorilla. Virology. 1995;207:217–228. doi: 10.1006/viro.1995.1068. [DOI] [PubMed] [Google Scholar]
- 8.Blair W S, Bogerd H, Cullen B R. Genetic analysis indicates that the human foamy virus Bel-1 protein contains a transcription activation domain of the acidic class. J Virol. 1994;68:3803–3808. doi: 10.1128/jvi.68.6.3803-3808.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bock M, Heinkelein M, Lindemann D, Rethwilm A. Cells expressing the human foamy virus (HFV) accessory Bet protein are resistant to productive HFV superinfection. Virology. 1998;250:194–204. doi: 10.1006/viro.1998.9362. [DOI] [PubMed] [Google Scholar]
- 10.Bodem J, Lochelt M, Winkler I, Flower R P, Delius H, Flugel R M. Characterization of the spliced pol transcript of feline foamy virus—the splice acceptor site of the pol transcript is located in gag of foamy viruses. J Virol. 1996;70:9024–9027. doi: 10.1128/jvi.70.12.9024-9027.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bodem J, Lochelt M, Yang P, Flugel R M. Regulation of gene expression by human foamy virus and potentials of foamy viral vectors. Stem Cells. 1997;15:141–147. doi: 10.1002/stem.5530150818. [DOI] [PubMed] [Google Scholar]
- 12.Bour S, Schubert U, Peden K, Strebel K. The envelope glycoprotein of human immunodeficiency virus type 2 enhances viral particle release: a Vpu-like factor? J Virol. 1996;70:820–829. doi: 10.1128/jvi.70.2.820-829.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Broussard S R, Comuzzie A G, Leighton K L, Leland M M, Whitehead E M, Allan J S. Characterization of new simian foamy viruses from African nonhuman primates. Virology. 1997;237:349–359. doi: 10.1006/viro.1997.8797. [DOI] [PubMed] [Google Scholar]
- 14.Campbell M, Eng C, Luciw P A. The simian foamy virus type 1 transcriptional transactivator (Tas) binds and activates an enhancer element in the gag gene. J Virol. 1996;70:6847–6855. doi: 10.1128/jvi.70.10.6847-6855.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Campbell M, Renshaw-Gegg L, Renne R, Luciw P A. Characterization of the internal promoter of simian foamy viruses. J Virol. 1994;68:4811–4820. doi: 10.1128/jvi.68.8.4811-4820.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Coffin J M, Hughes S H, Varmus H E. Retroviruses. Plainview, N.Y: Cold Spring Harbor Press; 1997. [PubMed] [Google Scholar]
- 16a.Comstock, K., C. Meiering, and M. Linial. Unpublished data.
- 17.Cordonnier A, Casella J F, Heidmann T. Isolation of novel human endogenous retrovirus-like elements with foamy virus-related pol sequence. J Virol. 1995;69:5890–5897. doi: 10.1128/jvi.69.9.5890-5897.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17a.Eastman, S., and M. Linial. Unpublished data.
- 18.Enders J, Peebles T. Propagation in tissue culture of cytopathogenic agents from patients with measles. Proc Soc Biol Med. 1954;86:277–287. doi: 10.3181/00379727-86-21073. [DOI] [PubMed] [Google Scholar]
- 19.Enssle J, Fischer N, Moebes A, Mauer B, Smola U, Rethwilm A. Carboxy-terminal cleavage of the human foamy virus gag precursor molecule is an essential step in the viral life cycle. J Virol. 1997;71:7312–7317. doi: 10.1128/jvi.71.10.7312-7317.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Enssle J, Jordan I, Mauer B, Rethwilm A. Foamy virus reverse transcriptase is expressed independently from the Gag protein. Proc Natl Acad Sci USA. 1996;93:4137–4141. doi: 10.1073/pnas.93.9.4137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Erlwein O, Bieniasz P D, McClure M O. Sequences in pol are required for transfer of human foamy virus-based vectors. J Virol. 1998;72:5510–5516. doi: 10.1128/jvi.72.7.5510-5516.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fischer N, Heinkelein M, Lindemann D, Enssle J, Baum C, Werder E, Zentgraf H, Muller J G, Rethwilm A. Foamy virus particle formation. J Virol. 1998;72:1610–1615. doi: 10.1128/jvi.72.2.1610-1615.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Flugel R M. Spumaviruses: a group of complex retroviruses. J Acquired Immune Defic Syndr. 1992;4:739–759. [PubMed] [Google Scholar]
- 24.Flugel R M, Rethwilm A, Maurer B, Darai G. Nucleotide sequence analysis of the env gene and its flanking regions of the human spumaretrovirus reveals two novel genes. EMBO J. 1987;6:2077–2084. doi: 10.1002/j.1460-2075.1987.tb02473.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ganem D. Hepadnaviridae: the viruses and their replication. In: Fields B N, Knipe D M, Howley P M, Chanock R M, Melnick J L, editors. Fields virology. Philadelphia, Pa: Lippincott-Raven; 1996. pp. 2703–2737. [Google Scholar]
- 26.Giron M-L, de The H, Saib A. An evolutionarily conserved splice generates a secreted Env-Bet fusion protein during human foamy virus infection. J Virol. 1998;72:4906–4910. doi: 10.1128/jvi.72.6.4906-4910.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Giron M-L, Colas S, Wybier J, Rozain F, Emanoil-Ravier R. Expression and maturation of human foamy virus gag precursor polypeptides. J Virol. 1997;71:1635–1639. doi: 10.1128/jvi.71.2.1635-1639.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27a.Goepfert, P., and M. Mulligan. Personal communication.
- 28.Goepfert P A, Shaw K L, Ritter G D, Mulligan M J. A sorting motif localizes the foamy virus glycoprotein to the endoplasmic reticulum. J Virol. 1997;71:778–784. doi: 10.1128/jvi.71.1.778-784.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hahn H, Baunach G, Brautigam S, Mergia A, Neumann-Haefelin D, Daniel M D, McClure M O, Rethwilm A. Reactivity of primate sera to foamy virus Gag and Bet proteins. J Gen Virol. 1994;75:2635–2644. doi: 10.1099/0022-1317-75-10-2635. [DOI] [PubMed] [Google Scholar]
- 30.Hatton T, Zhou S, Standring D N. RNA- and DNA-binding activities in hepatitis B virus capsid protein: a model for their roles in virus replication. J Virol. 1992;66:5232–5241. doi: 10.1128/jvi.66.9.5232-5241.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.He F, Blair W S, Fukushima J, Cullen B R. The human foamy virus Bel-1 transcription factor is a sequence-specific DNA binding protein. J Virol. 1996;70:3902–3908. doi: 10.1128/jvi.70.6.3902-3908.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Heinkelein M, Schmidt M, Fischer N, Moebes A, Lindemann D, Enssle J, Rethwilm A. Characterization of a cis-acting sequence in the pol region required to transfer human foamy virus vectors. J Virol. 1998;72:6307–6314. doi: 10.1128/jvi.72.8.6307-6314.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Herchenroder O, Renne R, Loncar D, Cobb E K, Murthy K K, Schneider J, Mergia A, Luciw P A. Isolation, cloning, and sequencing of simian foamy viruses from chimpanzees (SFVcpz): high homology to human foamy virus (HFV) Virology. 1994;201:187–199. doi: 10.1006/viro.1994.1285. [DOI] [PubMed] [Google Scholar]
- 34.Herchenroder O, Turek R, Neumann-Haefelin D, Rethwilm A, Schneider J. Infectious proviral clones of chimpanzee foamy virus (SFVcpz) generated by long PCR reveal close functional relatedness to human foamy virus. Virology. 1995;214:685–689. doi: 10.1006/viro.1995.0086. [DOI] [PubMed] [Google Scholar]
- 35.Herniou E, Martin J, Miller K, Cook J, Wilkinson M, Tristem M. Retroviral diversity and distribution in vertebrates. J Virol. 1998;72:5955–5966. doi: 10.1128/jvi.72.7.5955-5966.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Holzschu D L, Delaney M A, Renshaw R W, Casey J W. The nucleotide sequence and spliced pol mRNA levels of the nonprimate spumavirus bovine foamy virus. J Virol. 1998;72:2177–2182. doi: 10.1128/jvi.72.3.2177-2182.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hooks J J, Gibbs C J. The foamy viruses. Bacteriol Rev. 1975;39:169–185. doi: 10.1128/br.39.3.169-185.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hruska J F, Takemoto K K. Biochemical properties of a hamster syncytium-forming (“foamy”) virus. J Natl Cancer Inst. 1975;54:601–605. [PubMed] [Google Scholar]
- 39.Jacks T. Translational suppression in gene expression in retroviruses and retrotransposons. In: Swanstrom R, Vogt P K, editors. Retroviruses: strategies of replication. Berlin, Germany: Springer-Verlag; 1990. pp. 93–124. [DOI] [PubMed] [Google Scholar]
- 40.Johnson R H, Oginnusi A A, Ladds P W. Isolations and serology of bovine spumavirus. Aust Vet J. 1983;60:147. doi: 10.1111/j.1751-0813.1983.tb05928.x. [DOI] [PubMed] [Google Scholar]
- 41.Jordan I, Enssle J, Guttler E, Mauer B, Rethwilm A. Expression of human foamy virus reverse transcriptase involves a spliced pol mRNA. Virology. 1996;224:314–319. doi: 10.1006/viro.1996.0534. [DOI] [PubMed] [Google Scholar]
- 42.Kang Y B, Blair W S, Cullen B R. Identification and functional characterization of a high-affinity Bel-1 DNA binding site located in the human foamy virus internal promoter. J Virol. 1998;72:504–511. doi: 10.1128/jvi.72.1.504-511.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kennedy-Stoskopf S, Stoskopf M K, Eckhaus M A, Strandberg J D. Isolation of a retrovirus and a herpesvirus from a captive California sea lion. J Wild Dis. 1986;22:156–164. doi: 10.7589/0090-3558-22.2.156. [DOI] [PubMed] [Google Scholar]
- 44.Kertayadnya I G, Johnson R H, Abher I, Burgess G W. Detection of immunological tolerance to bovine spumavirus (BSV) with evidence for salivary excretion and spread of BSV from the tolerant animal. Vet Microbiol. 1988;16:35–39. doi: 10.1016/0378-1135(88)90125-3. [DOI] [PubMed] [Google Scholar]
- 45.Kogel D, Aboud M, Flugel R M. Molecular biological characterization of the human foamy virus reverse transcriptase and ribonuclease H domains. Virology. 1995;213:97–108. doi: 10.1006/viro.1995.1550. [DOI] [PubMed] [Google Scholar]
- 46.Konvalinka J, Lochelt M, Zentgraf H, Flugel R M, Krausslich H-G. Active spumavirus proteinase is essential for virus infectivity but not for formation of the Pol polyprotein. J Virol. 1995;69:7264–7268. doi: 10.1128/jvi.69.11.7264-7268.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kupiec J J, Tobaly-Tapiero J, Canivet M, Santillana-Hayat M, Flugel R M, Peries J, Emanoil-Ravier R. Evidence for a gapped linear duplex DNA intermediate in the replicative cycle of human and simian spumaviruses. Nucleic Acids Res. 1988;16:9557–9565. doi: 10.1093/nar/16.20.9557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lee A H, Lee H Y, Sung Y C. The gene expression of human foamy virus does not require a post-transcriptional transactivator. Virology. 1994;204:409–413. doi: 10.1006/viro.1994.1545. [DOI] [PubMed] [Google Scholar]
- 49.Lindemann D, Bock M, Schweizer M, Rethwilm A. Efficient pseudotyping of murine leukemia virus particles with chimeric human foamy virus envelope proteins. J Virol. 1997;71:4815–4820. doi: 10.1128/jvi.71.6.4815-4820.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49a.Lindemann D, Rethwilm A. Characterization of a human foamy virus 170-kilodalton Env-Bet fusion protein generated by alternative splicing. J Virol. 1998;72:4088–4094. doi: 10.1128/jvi.72.5.4088-4094.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lochelt M, Flugel R M. The human foamy virus pol gene is expressed as a Pro-Pol polyprotein and not as a Gag-Pol fusion protein. J Virol. 1996;70:1033–1040. doi: 10.1128/jvi.70.2.1033-1040.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lochelt M, Muranyi W, Flugel R M. Human foamy virus genome possesses an internal, bel-1 dependent and functional promoter. Proc Natl Acad Sci USA. 1993;90:7317–7321. doi: 10.1073/pnas.90.15.7317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lochelt M, Yu S F, Linial M L, Flugel R M. The human foamy virus internal promoter is required for efficient gene expression and infectivity. Virology. 1995;206:601–610. doi: 10.1016/s0042-6822(95)80077-8. [DOI] [PubMed] [Google Scholar]
- 53.Lochelt M, Zentgraf H, Flugel R M. Construction of an infectious DNA clone of the full-length human spumaretrovirus genome and mutagenesis of the bel 1 gene. Virology. 1991;184:43–54. doi: 10.1016/0042-6822(91)90820-2. [DOI] [PubMed] [Google Scholar]
- 54.Lutz H. Feline retroviruses: a brief review. Vet Microbiol. 1990;23:131–146. doi: 10.1016/0378-1135(90)90143-j. [DOI] [PubMed] [Google Scholar]
- 55.Maurer B, Bannert H, Darai G, Flugel R M. Analysis of the primary structure of the long terminal repeat and the gag and pol genes of the human spumaretrovirus. J Virol. 1988;62:1590–1597. doi: 10.1128/jvi.62.5.1590-1597.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Mergia A. Simian foamy virus type 1 contains a second promoter located at the 3′ end of the env gene. Virology. 1994;199:219–222. doi: 10.1006/viro.1994.1114. [DOI] [PubMed] [Google Scholar]
- 57.Mochizuki M, Akuzawa M, Nagatomo H. Serological survey of the Iriomote cat (Felis iriomotensis) in Japan. J Wild Dis. 1990;26:236–245. doi: 10.7589/0090-3558-26.2.236. [DOI] [PubMed] [Google Scholar]
- 58.Moebes A, Enssle J, Bieniasz P D, Heinkelein M, Lindemann D, Bock D, McClure M O, Rethwilm A. Human foamy virus reverse transcription that occurs late in the viral replication cycle. J Virol. 1997;71:7305–7311. doi: 10.1128/jvi.71.10.7305-7311.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Morozov V A, Copeland T D, Nagashima K, Gonda M A, Oroszlan S. Protein composition and morphology of human foamy virus intracellular cores and extracellular particles. Virology. 1997;228:307–317. doi: 10.1006/viro.1996.8379. [DOI] [PubMed] [Google Scholar]
- 60.Muranyi W, Flugel R M. Analysis of splicing patterns of human spumaretrovirus by polymerase chain reaction reveals complex RNA structures. J Virol. 1991;65:727–735. doi: 10.1128/jvi.65.2.727-735.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Nassal M. The arginine-rich domain of the hepatitis B virus core protein is required for pregenome encapsidation and productive viral positive-strand DNA synthesis but not for virus assembly. J Virol. 1992;66:4107–4116. doi: 10.1128/jvi.66.7.4107-4116.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Nestler U, Heinkelein M, Lucke M, Meixensberger J, Scheurlen W, Kretschmer A, Rethwilm A. Foamy virus vectors for suicide gene therapy. Gene Ther. 1997;4:1270–1277. doi: 10.1038/sj.gt.3300561. [DOI] [PubMed] [Google Scholar]
- 63.Netzer K O, Rethwilm A, Maurer B, ter Meulen V. Identification of the major immunogenic structural proteins of human foamy virus. J Gen Virol. 1990;71:1237–1241. doi: 10.1099/0022-1317-71-5-1237. [DOI] [PubMed] [Google Scholar]
- 64.Neumann-Haefelin D, Fleps U, Renne R, Schweizer M. Foamy viruses. Intervirology. 1993;35:196–207. doi: 10.1159/000150310. [DOI] [PubMed] [Google Scholar]
- 65.Neumann-Haefelin D, Schweizer M, Corsten B, Matz B. Detection and characterization of infectious DNA intermediates of a primary foamy virus. J Gen Virol. 1986;67:1993–1999. doi: 10.1099/0022-1317-67-9-1993. [DOI] [PubMed] [Google Scholar]
- 66.Pahl A, Flugel R M. Characterization of the human spumaretrovirus integrase by site-directed mutagenesis, by complementation analysis, and by swapping the zinc finger domain of HIV-1. J Biol Chem. 1995;270:2957–2966. doi: 10.1074/jbc.270.7.2957. [DOI] [PubMed] [Google Scholar]
- 67.Pfrepper K-I, Rackwitz H-R, Schnolzer M, Heid H, Lochelt M, Flugel R M. Molecular characterization of proteolytic processing of the Pol proteins of human foamy virus reveals novel features of the viral protease. J Virol. 1998;72:7648–7652. doi: 10.1128/jvi.72.9.7648-7652.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Pfrepper K I, Lochelt M, Schnolzer M, Flugel R M. Expression and molecular characterization of an enzymatically active recombinant human spumaretrovirus protease. Biochem Biophys Res Commun. 1997;237:548–553. doi: 10.1006/bbrc.1997.7187. [DOI] [PubMed] [Google Scholar]
- 69.Pietschmann, T., M. Heinkelein, M. Heldmann, H. Zentgraf, A. Rethwilm, and D. Lindemann. Foamy virus capsids require the cognate envelope proteins for particle export. Submitted for publication. [DOI] [PMC free article] [PubMed]
- 70.Rethwilm A, Baunach G, Netzer K O, Maurer B, Borisch B, ter Meulen V. Infectious DNA of the human spumaretrovirus. Nucleic Acids Res. 1990;18:733–738. doi: 10.1093/nar/18.4.733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ritter G D, Jr, Yamshchikov G, Cohen S J, Mulligan M J. Human immunodeficiency virus type 2 glycoprotein enhancement of particle budding: role of the cytoplasmic domain. J Virol. 1996;70:2669–2673. doi: 10.1128/jvi.70.4.2669-2673.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Russell D W, Miller A D. Foamy virus vectors. J Virol. 1996;70:217–222. doi: 10.1128/jvi.70.1.217-222.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Saib A, Koken M H, van der Spek P, Peries J, de The H. Involvement of a spliced and defective human foamy virus in the establishment of chronic infection. J Virol. 1995;69:5261–5268. doi: 10.1128/jvi.69.9.5261-5268.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Saib A, Neves M, Giron M L, Guillemin M C, Valla J, Peries J, Canivet M. Long-term persistent infection of domestic rabbits by the human foamy virus. Virology. 1997;228:263–268. doi: 10.1006/viro.1996.8383. [DOI] [PubMed] [Google Scholar]
- 75.Saib A, Peries J, Dethe H. A defective human foamy provirus generated by pregenome splicing. EMBO J. 1993;12:4439–4444. doi: 10.1002/j.1460-2075.1993.tb06129.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Schliephake A W, Rethwilm A. Nuclear localization of foamy virus Gag precursor protein. J Virol. 1994;68:4946–4954. doi: 10.1128/jvi.68.8.4946-4954.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Schmidt M, Herchenroder O, Heeney J, Rethwilm A. Long terminal repeat U3 length polymorphism of human foamy virus. Virology. 1997;230:167–178. doi: 10.1006/viro.1997.8463. [DOI] [PubMed] [Google Scholar]
- 78.Schmidt M, Niewiesk S, Heeney J, Aguzzi A, Rethwilm A. Mouse model to study the replication of primate foamy viruses. J Gen Virol. 1997;78:1929–1933. doi: 10.1099/0022-1317-78-8-1929. [DOI] [PubMed] [Google Scholar]
- 79.Schweizer M, Renne R, Neumann-Haefelin D. Structural analysis of proviral DNA in simian foamy virus (LK-3)-infected cells. Arch Virol. 1989;109:103–114. doi: 10.1007/BF01310521. [DOI] [PubMed] [Google Scholar]
- 80.Schweizer M, Turek R, Hahn H, Schliephake A, Netzer K O, Eder G, Reinhardt M, Rethwilm A, Neumann-Haefelin D. Markers of foamy virus infections in monkeys, apes, and accidentally infected humans—appropriate testing fails to confirm suspected foamy virus prevalence in humans. AIDS Res Hum Retroviruses. 1995;11:161–170. doi: 10.1089/aid.1995.11.161. [DOI] [PubMed] [Google Scholar]
- 81.Seeger C, Mason W S. Replication of the hepatitis virus genome. In: dePamphilis M, editor. DNA replication in eukaryotic cells. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1996. pp. 815–831. [Google Scholar]
- 82.Telesnitsky A, Goff S. Reverse transcription and the generation of retroviral DNA. In: Coffin J M, Hughes S H, Varmus H E, editors. Retroviruses. Plainview, N.Y: Cold Spring Harbor Laboratory Press; 1997. pp. 121–161. [PubMed] [Google Scholar]
- 83.Trono D. Partial reverse transcripts in virions from human immunodeficiency and murine leukemia viruses. J Virol. 1992;66:4893–4900. doi: 10.1128/jvi.66.8.4893-4900.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Venkatesh L K, Theodorakis P A, Chinnadurai G. Functional dissection of the human spumaretrovirus transactivator identifies distinct classes of dominant-negative mutants. J Virol. 1992;67:161–169. doi: 10.1128/jvi.67.1.161-169.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Wills J W, Craven R C. Form, function, and use of retroviral Gag proteins. AIDS. 1991;5:639–654. doi: 10.1097/00002030-199106000-00002. [DOI] [PubMed] [Google Scholar]
- 86.Winkler I, Bodem J, Haas L, Zemba M, Delius H, Flower R, Flugel R M, Lochelt M. Characterization of the genome of feline foamy virus and its proteins shows distinct features different from those of primate spumaviruses. J Virol. 1997;71:6727–6741. doi: 10.1128/jvi.71.9.6727-6741.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Yang P, Zemba M, Aboud M, Flugel R M, Lochelt M. Deletion analysis of both the long terminal repeat and the internal promoters of the human foamy virus. Virus Genes. 1997;15:17–23. doi: 10.1023/a:1007994527345. [DOI] [PubMed] [Google Scholar]
- 88.Yu S F, Baldwin D N, Gwynn S R, Yendapalli S, Linial M L. Human foamy virus replication—a pathway distinct from that of retroviruses and hepadnaviruses. Science. 1996;271:1579–1582. doi: 10.1126/science.271.5255.1579. [DOI] [PubMed] [Google Scholar]
- 89.Yu S F, Edelmann K, Strong R K, Moebes A, Rethwilm A, Linial M L. The carboxyl terminus of the human foamy virus Gag protein contains separable nucleic acid binding and nuclear transport domains. J Virol. 1996;70:8255–8262. doi: 10.1128/jvi.70.12.8255-8262.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Yu S F, Linial M L. Analysis of the role of the bel and bet open reading frames of human foamy virus by using a new quantitative assay. J Virol. 1993;67:6618–6624. doi: 10.1128/jvi.67.11.6618-6624.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Yu S F, Stone J, Linial M L. Productive persistent infection of hematopoietic cells by human foamy virus. J Virol. 1996;70:1250–1254. doi: 10.1128/jvi.70.2.1250-1254.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Yu S F, Sullivan M D, Linial M L. Evidence that the human foamy virus genome is DNA. J Virol. 1999;73:1565–1572. doi: 10.1128/jvi.73.2.1565-1572.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Zemba M, Wilk T, Rutten T, Wagner A, Flugel R M, Lochelt M. The carboxy-terminal p3Gag domain of the human foamy virus Gag precursor is required for efficient virus infectivity. Virology. 1998;247:7–13. doi: 10.1006/viro.1998.9234. [DOI] [PubMed] [Google Scholar]