Abstract
Background
Accurate senses depend on high fidelity encoding by sensory receptors and error-free central processing in the brain. Progress has been made towards restoring damaged sensory receptors. However, methods for providing on demand treatment of impaired central sensory processing arising from factors including aging, neurological dysfunction, inattention, and fatigue are scarce. Recent studies have demonstrated that tonic vagus nerve stimulation in rodents can activate the locus coeruleus-norepinephrine system in the brain to improve sensory processing rapidly and continuously.
Hypothesis
We hypothesized that non-invasive neuromodulation via tonic transcutaneous vagus nerve stimulation (tVNS) improves sensory performance in humans.
Methods
Twenty-nine adults with no reported neurological dysfunction completed three sham-controlled experiments that measured effects of tVNS on sensory performance metrics (auditory gap detection, visual letter discrimination) and heart rate variability. Tonic tVNS was delivered continuously to cervical (neck) or auricular (ear) branches of the vagus nerve while participants performed psychophysics tasks or passively viewed a display without an accompanying task.
Results
Cervical tVNS improved auditory gap detection by 35% and visual letter discrimination by 20%, on average, relative to sham stimulation. Notably, participants with lower sensory performance during control conditions experienced larger tVNS-mediated improvements. Lastly, tVNS increased heart rate variability relative to sham stimulation during passive viewing, corroborating vagal engagement.
Conclusion
We demonstrate that non-invasive vagus nerve stimulation improves sensory processing in neurotypical human adults. These findings substantiate foundational studies in rodents and position tVNS as a neuromodulation method for targeted and on-demand interventions of impairments associated with central sensory processing dysfunction.
Keywords: vagus nerve stimulation, sensory processing, heart rate variability, neuromodulation, locus coeruleus, norepinephrine
Introduction
Accurate and detailed sensory perception is vital for navigating daily life. But cognitive constraints – including aging, inattention, and fatigue – reduce the precision with which the brain processes sensory stimuli and lead to consequential misperceptions [1–3]. Recent research in rodents showed that activation of the locus coeruleus-norepinephrine system (LC-NE), a major subcortical noradrenergic nucleus, can increase sensory processing fidelity when stimulated directly or via the vagus nerve [4–6]. In humans, branches of the vagus nerve traverse the neck and ear and project to the brain where it modulates the LC-NE via the nucleus tractus solitarius pathway [6–9]. Non-invasive stimulation of these vagal branches is tolerated and safe [10]. In this study, we investigated the potential of transcutaneous vagus nerve stimulation (tVNS) for improving sensory performance in humans.
Neuromodulatory systems, such as the LC-NE, are well-known to modulate the functioning of subcortical and cortical sensory processing pathways [11–17]. Noradrenergic and cholinergic systems play influential roles in inducing behavioral states, such as attention and arousal, that promote heightened sensory performance [14–20]. Recent work has shown that continuous (tonic) activation of the LC-NE can rapidly enhance somatosensory processing via NE-mediated suppression of thalamic burst spiking that would otherwise degrade detailed sensory transmission [4]. Continuous vagus nerve stimulation can similarly activate the LC-NE to generate steady sensory enhancements that parallel direct LC activation [5]. Collectively, these findings demonstrate that direct activation of neuromodulatory systems or indirect activation via vagus nerve stimulation generate on demand improvements to central sensory processing.
By coupling these recent neuroscientific advances with contemporary neuromodulation techniques, it may be possible to enhance human sensory performance safely and on demand. tVNS emerges as a promising approach due to its ability to safely activate the vagus nerve in humans [10], which can consequently activate the LC-NE system [21] via the nucleus tractus solitarius pathway. Neuroimaging studies support this potential by demonstrating that tVNS can activate the vagus nerve and its afferent vagal projections, including the LC [22–25]. Transcutaneous auricular VNS (taVNS) activates the auricular branch of the vagus nerve in the ear [22, 24] whereas transcutaneous cervical VNS (tcVNS) activates the cervical branch of the vagus nerve in the neck [23, 25]. Furthermore, stimulation of terminal vagal fibers in muscles of the larynx and pharynx [26], which are in close proximity to the tcVNS site, may provide antidromic vagal activation during tcVNS.
Here, we investigated the hypothesis that tonic tVNS improves metrics of sensory performance in neurotypical human adults. We further examined cardiac markers that indicate vagus nerve engagement. Using a crossover design, participants underwent three experiments that included control conditions (no stimulation or continuous sham stimulation at the forearm, ear, or neck), and active taVNS or tcVNS conditions. While receiving stimulation, participants performed auditory gap discrimination and visual letter discrimination psychophysics tasks, or passively viewed a computer display.
Briefly, our findings reveal that tcVNS improved auditory performance by 35% and visual performance by 20%, on average, compared to control conditions. Notably, both tcVNS and taVNS led to greater improvements in individuals with poorer sensory performance during control conditions. Furthermore, tcVNS and taVNS increased heart rate variability (HRV) during passive viewing, corroborating vagal engagement. However, tVNS-mediated changes in HRV were absent during active task engagement. These results provide evidence that continuous tVNS can enhance sensory performance in humans.
Methods
Ethics
Participants were passively recruited, provided written informed consent, and compensated $30/hour. All experimental protocols complied with the Declaration of Helsinki and were approved by Cornell University’s Institutional Review Board for Human Participant Research.
Sample
Twenty-nine individuals participated (18 females; age: 28±4.9 (standard deviation); range: 18–39). Most participated in a single experiment (ten in Exp.1, seven in Exp.2, seven in Exp.3), three completed Experiments 1–2, and two completed all three experiments. Each experiment had a sample of 12, determined a priori using G*Power 3.1.9.4 [27] based on published data [4]. For further information, see Supplementary Materials – Sample.
Participants were asked to refrain from any stimulants (e.g., caffeine, nicotine) for three hours prior to participating, and all passed the inclusion criteria: no substance abuse history, no neurological or psychiatric disorders, no cardiac disorders, no medical implants, no prior abnormalities or surgeries involving the neck or vagus nerve, and normal or corrected-to-normal hearing and vision. All participants were naïve to the study’s purpose.
Stimulation
A Mettler Sys*Stim 240 neuromuscular stimulator (Mettler Electronics Corporation) delivered current through a pair of gel electrodes (diameter: 1”; PALS, Axelgaard Manufacturing) placed at one of 5 sites (Figure 1A). Biphasic square pulses were delivered (100 μs/phase; 200 μs total) at one of two frequencies (30 Hz, 3 Hz) or in bursts of 3 sequential pulses (100 μs/phase; 600 μs total) at 30 Hz (Figure 1B). To determine current amplitude (Table 1), the experimenter gradually ramped up current intensity until participants reported muscle contractions or pain, after which it was reduced to the maximum level that was perceptible, tolerable, and did not induce muscle activation.
Figure 1. Stimulation protocols and psychophysics procedures.
(A) Stimulation sites. Electrode placement is shown on an author (M.J.). Colored boxes group the stimulation sites assessed in each experiment. (B) Stimulation waveforms and frequencies. (C) Trial sequences for the auditory and visual sensory tasks. (top) Trials of the auditory task consisted of two tone stimuli – one tone contained a gap and participants reported whether it occurred in Tone 1 or 2. (bottom) Trials of the visual task comprised a single letter display and participants reported its identity using a keyboard. (D) Adaptive psychophysical procedures for measuring auditory (top) and visual sensory performance (bottom). The panels depict data from representative participants. In Experiment 1, the adaptive procedure began by presenting gap durations that could be easily judged correctly but became progressively more difficult until it converged on a duration that maintained 75% accuracy. In Experiments 2 and 3, the adaptive procedure dynamically presented stimuli that yielded performance between chance-level and near-perfect accuracy. Logistic functions were fitted to trial-wise data, which related task accuracy to changes in the sensory stimulus; the size of individual dots on the logistic function depict the number of trials at a given letter size. An adaptive procedure for the visual task is displayed, and a similar procedure was followed for the auditory task. The gray highlighted regions depict the initial sweep that calibrated the adaptive procedures prior to converging at the desired performance level. (E) Stimulation site order in each experiment. Brackets indicate the randomization procedure. The inset in Experiment 2 labeled “Block structure” depicts the order in which participants completed sensory tasks and passive viewing within each stimulation block.
Table 1.
Current amplitude delivered to each stimulation site in each experiment.
| Experiment | Site | Current (average ± SD) |
|---|---|---|
| 1 | Cervical Sham | 7.8 ±1.7 mA |
| tcVNS 30 Hz | 6.5 ± 1.5 mA | |
| 2 | Arm | 5.1 ± 1.6 mA |
| Cervical Sham | 5.4 ± 2.0 mA | |
| Auricular Sham | 2.5 ± 2.0 mA | |
| tcVNS 30 Hz | 5.5 ± 1.6 mA | |
| tcVNS Burst | 5.1 ± 1.9 mA | |
| taVNS 30 Hz | 2.3 ± 1.6 mA | |
| taVNS 3 Hz | 2.5 ± 1.8 mA | |
| 3 | Arm | 6.9 ± 1.2 mA |
| tcVNS 30 Hz | 7.1 ± 1.2 mA | |
| taVNS 30 Hz | 2.0 ± 0.9 mA |
Ten stimulation conditions were tested:
Active
-
1
tcVNS 30 Hz. Electrodes were placed within the left carotid triangle, lateral to the larynx, medial to the sternocleidomastoid muscle (SCM), and oriented parallel to the SCM (3–5 cm center-to-center) to target the cervical branch of the vagus nerve [8, 9]. Pulses were delivered at 30 Hz.
-
2
tcVNS Burst. Electrodes were positioned identically to tcVNS 30 Hz, and burst waveforms were delivered at 30 Hz.
-
3
taVNS 30 Hz. Electrodes were placed on the lateral and medial surfaces of the left ear to direct current flow through the ear and into the auricular branch of the vagus nerve (ABVN). Based on cadaveric dissections [7], one electrode was placed on the lateral surface overlapping the cymba concha and cavum concha, and the second was placed on the middle-third of the dorsal medial surface. Pulses were delivered at 30 Hz.
-
4
taVNS 3 Hz. Electrodes were positioned identically to taVNS 30 Hz, but pulses were delivered at 3 Hz.
Control
-
5
Baseline. No electrodes were placed on the participant; accordingly, no stimulation was delivered.
-
6
Arm. Electrodes were positioned on the left forearm (3–4 cm center-to-center), approximately over the brachioradialis muscle. Pulses were delivered at 30 Hz.
-
7
Cervical Sham. Electrodes were placed near the junction between the rear of the neck and the shoulder (3–4 cm center-to-center), overlapping the trapezius muscle, which is primarily innervated by efferent fibers of cranial nerves II, III, IV, and XI, but not by the vagus nerve [28]. Pulses were delivered at 30 Hz. Because stimulation of cranial nerve II afferents can improve cognition [29], any tVNS-mediated improvements we observe relative to this sham site are potentially underestimated.
-
8
Auricular Sham. Electrodes were placed on the superior crus of antihelix, with one electrode on the ear’s lateral surface, and another on the medial surface. The great auricular nerve, innervates the lateral location, and the ABVN does not run through the medial location in cadaveric dissections [7]. Pulses were delivered at 30 Hz.
-
9
tcVNS Sham. Electrodes were positioned identically to tcVNS 30 Hz, but current was not delivered. To maintain participant blinding, current was initially ramped up to a low but perceptible level for 30–60 seconds, then ramped down to 0 mA prior to the start of sensory tests.
-
10
taVNS Sham. Electrodes were positioned identically to taVNS 30 Hz and followed the ramp-up, ramp-down procedure of tcVNS no-stim.
Procedure
Stimulation was delivered continuously, except during control conditions without stimulation (Baseline, tcVNS Sham, taVNS Sham). Across experiments, participants completed auditory (Experiments 1–3) and visual tasks (Experiments 2–3; Figure 1C), or passively viewed a blank computer display with no associated task for five minutes (Experiment 2). During sensory tasks, adaptive procedures determined auditory and visual thresholds that indexed sensory performance (Figure 1D). Then, participants completed a brief survey (Experiments 2–3). All experiments followed crossover, sham-controlled, and single-blind designs, but differed in the number of stimulation and sham conditions, each separated by 10–30 minute washout periods (Figure 1E). For further information, see Supplementary Materials – Procedure.
Apparatus
Psychtoolbox-3 [30] generated sensory stimuli in MATLAB (MathWorks) on a Lenovo ThinkStation running Windows 10. An Empatica E4 wristband (Empatica Inc.) equipped with photoplethysmography was positioned on participants’ left wrist during Experiment 2 to monitor HRV. Auditory stimuli were delivered via factory-calibrated ER-2 insert earphones (Etymotic Research Inc) and visual stimuli were displayed on gamma-calibrated LCD displays. For further information, see Supplementary Materials – Apparatus.
Psychophysics
Auditory.
Participants performed an auditory gap discrimination task commonly used to assess central auditory temporal processing [31–34], and similar to the commercially available Random Gap Detection Test [35]. To control eye movements, participants maintained visual fixation on a dot (luminance: 0.23 cd/m2) presented on a mid-gray background (55 cd/m2). Participants reported which of two temporal intervals contained an auditory tone with a gap; 500 ms of silence separate intervals. Auditory stimuli were pairs of 17 ms pure tones at a given frequency (Experiment 1 used 1000 or 2000 Hz presented in separate blocks; Experiments 2–3 used 10 frequencies logarithmically spaced between 1000–4000 Hz, interleaved within blocks). One tone-pair had no gap while another was separated by a given duration. The tone-pair containing a gap was randomly determined on each trial. Participants reported whether the gap occurred in the first or second tone-pair, respectively. An inter-trial interval followed responses (3000 ms for Experiment 1; 500 ms for Experiments 2–3).
Visual.
Participants performed a letter discrimination task adapted from clinical measures of visual acuity – Bailey-Lovie and ETDRS charts [36]. On each trial, participants maintained visual fixation on a central dot (0.23 cd/m2) presented on a white background (228 cd/m2) and reported which letter appeared on-screen for 500 ms. The letter was displayed in Sloan font, as used in commercially available eye charts [36]. Participants responded by pressing the corresponding letter on a keyboard after stimulus offset. One of ten standardized optotypes appeared on each trial – C, D, H, K, N, O, R, S, V, Z. Participants were not informed that only these letters would be displayed and could respond with any letter of the alphabet. A 500 ms inter-trial interval followed responses.
For all sensory tasks, participants first completed practice trials during which they received feedback on their accuracy. For more details see Supplementary Materials – Practice. Then, they completed 30 trials (Experiment 1) or 100 trials (Experiments 2–3) without feedback during which gap duration or letter size was adaptively controlled (see “Thresholding”). This procedure of providing then withholding feedback follows prior studies [37].
Thresholding
We used QUEST procedures [38], an adaptive procedure implemented in Palamedes toolbox [39], commonly used in psychophysics protocols [40–44] to adjust task difficulty on each trial based on participants’ response accuracy. Indices of task difficulty were gap duration and letter size for auditory and visual tasks, respectively. Each procedure began with a calibration sweep that presented 10 (Experiment 1) or 5 (Experiment 2) pre-determined stimulus levels. These levels spanned a large range of possible stimuli and initialized each procedure near the participant’s threshold. Then, after correct responses, progressively harder stimuli were presented. After incorrect responses, slightly easier trials were presented. QUEST procedures targeted sensory stimuli that maintained 75% task accuracy (Figure 1D). We refer to this stimulus level as threshold, and its inverse indexes sensory performance; lower thresholds indicate better performance. For further details, see Supplementary Materials – Thresholding.
Behavior
Linear mixed models (LMM) and robust linear regression examined study-wide trends whereas paired t-tests examined within-experiment comparisons. For LMMs and robust linear regression, data were merged across experiments. LMMs evaluated whether tcVNS and taVNS improved sensory performance, relative to all control conditions. Robust linear regression evaluated whether sensory performance during control conditions, z-scored within each task, predicted the magnitude of tcVNS or taVNS effects, which was quantified as the percent change in sensory thresholds between each active and control condition. Within each experiment, Shapiro-Wilk tests assessed normality. If normality was established, paired t-tests were used (Experiment 1). If not, permutation paired t-tests were used (100,000 permutations; Experiments 2–3). Multiple comparisons were corrected via the Max T method [45]. For additional details, see Supplementary Materials – Behavior.
HRV
Interbeat intervals were measured during passive viewing and task engagement in Experiment 2. Sensory tasks lasted 3.6±0.6 minutes, which is sufficient for estimating HRV metrics [46]. Three HRV metrics were computed: standard deviation of normal-to-normal intervals (SDNN), root mean square of successive differences between heartbeats (RMSSD), and total spectral power within low (LF: 0.04–0.15 Hz) and high frequency (HF: 0.15–0.4 Hz) ranges. Lomb-Scargle periodograms computed power spectra. HRV metrics were averaged across sensory tasks then compared to those during passive viewing. One participant’s HRV data were discarded because they closed their eyes during passive viewing.
Repeated measures ANOVAs assessed the impact of Site and State (passive vs active). Then, permutation t-tests assessed differences among sites within each state. Pearson correlation quantified linear relations among HRV metrics.
Survey
We surveyed participants’ subjective experience upon completing sensory tasks (Experiments 2–3). The survey asked “Did stimulation cause you any discomfort?” and provided two response options: “Yes” and “No”. If “Yes”, participants rated their discomfort using a Likert scale: 1=Slight, 2=Moderate, 3=Very High, and 4=Extreme.
Results
tcVNS improved auditory performance
Participants detected gaps between auditory tones while receiving continuous sham or active tcVNS. Adaptive procedures adjusted the gap’s duration to a level that maintained 75% accuracy – shorter gaps indicated improved performance. We examined study-wide trends that jointly assessed the efficacy of 30 Hz and Burst tcVNS (Figure 2A). Across all experiments, tcVNS improved auditory performance relative to controls by 35% on average (p=0.0026, Supplementary Table 1).
Figure 2. tcVNS improved sensory performance.
(A) Gap duration thresholds during control conditions (Baseline, Arm, Cervical Sham) and tcVNS conditions across all experiments and stimulation protocols (30 Hz and Burst). Each colored point depicts a participant in each experiment or stimulation protocol. The black point and error bars show the study-wide average and ±1 standard error of the mean (SEM) for tcVNS (horizontal line) and control conditions (vertical line). Points within the gray shaded region above the diagonal line indicate tcVNS-mediated sensory improvements. The p-value denotes the result of a linear mixed model that used the full dataset. (B) Gap duration thresholds in each stimulation condition of Experiment 1. Each point depicts an individual participant, bars depict the group-average, and error bars denote ±1 within-subject SEM [47]. The p-values denote results of a paired t-test; p-values for non-significant comparisons are not displayed. Subsequent bar plots follow these conventions. (C) Gap duration thresholds in each stimulation condition of Experiment 2 and (D) Experiment 3. The p-values denote results of permutation paired t-tests. (E) Letter size thresholds during control conditions and tcVNS conditions across experiments and stimulation protocols. Plotting conventions follow those in A. (F) Letter size thresholds in each stimulation condition of Experiment 2 and (G) Experiment 3.
tcVNS-mediated improvements were consistent across multiple experiments and controls (Figure 2B–D). In Experiment 1, tcVNS 30 Hz significantly improved performance relative to Cervical Sham (t(11)=2.54, p=0.027). Likewise, in Experiment 2, there were significant differences between tcVNS 30 Hz and Cervical Sham (t(11)=2.32, p=0.046), Baseline (t(11)=2.64, p=0.027), and Arm controls (t(11)=3.68, p=0.006). In Experiment 3, tcVNS 30 Hz significantly improved performance relative to tcVNS Sham (t(11)=2.36, p=0.041). However, tcVNS Burst did not significantly improve performance in Experiment 2 despite inducing statistically similar performance to tcVNS 30 Hz (p>0.05). We attribute these differences among tcVNS protocols to the small sample size used in individual experiments, which was potentially insufficient for reliably observing improvements by each protocol. Study-wide analyses, which benefited from higher statistical power, found tcVNS-mediated effects despite differences among 30 Hz and Burst stimulation (Figure 2A). Together, these findings demonstrate that tcVNS-mediated improvements were robust, occurring in both study-wide analyses and reliably across multiple controls (tcVNS Sham and Cervical Sham).
Improvements to auditory performance were not mediated by speed-accuracy tradeoffs – the propensity to make quick but error-prone or slow but accurate responses. Participants’ reaction times (RTs) did not differ among conditions in which tcVNS improved performance, namely between tcVNS 30 Hz, Cervical Sham, and tcVNS Sham (Supplementary Table 2). The only exceptions were differences among tcVNS 30 Hz, Baseline and Arm stimulation in Experiment 2 (Figure 2C), which we cannot disentangle from practice effects as Baseline/Arm were always completed first when participants were likely less familiar with the task.
tcVNS improved visual performance
Participants reported a letter’s identity while receiving continuous sham or active tcVNS. Adaptive procedures identified letter size thresholds – the size that maintained 75% accuracy; smaller letters indicated improved performance. We examined study-wide trends that jointly assessed the efficacy of both tcVNS protocols (Figure 2E). Across all experiments, tcVNS reduced size thresholds relative to controls by 3% on average (p=0.023, Supplementary Table 3). This letter size reduction corresponds to a 20% improvement in logMAR scores – a logarithmic transformation of letter size that is an accepted clinical measure of visual acuity [36] (Supplementary Table 4).
Visual improvements were found compared to two different controls, Cervical Sham and tcVNS Sham (Figure 2F–G). In Experiment 2, tcVNS Burst significantly improved performance relative to Cervical Sham (t(11)=2.81, p=0.020). In Experiment 3, performance was significantly better with tcVNS 30 Hz than tcVNS Sham (t(11)=2.53, p=0.031). However, tcVNS 30 Hz did not significantly improve performance in Experiment 2 despite inducing statistically similar performance to tcVNS Burst (p>0.3). Like the auditory results, we attribute these differences among tcVNS protocols to the small sample size used in individual experiments. Study-wide analyses found robust tcVNS-mediated effects despite differences among 30 Hz and Burst stimulation.
Visual improvements were not mediated by speed-accuracy tradeoffs. RTs did not differ among conditions that exhibited tcVNS-mediated improvements, namely between tcVNS Burst and Cervical Sham in Experiment 2 and tcVNS 30 Hz and tcVNS Sham in Experiment 3 (Supplementary Table 5).
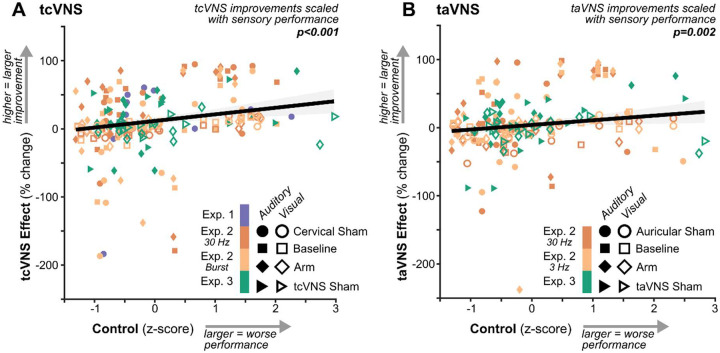
tcVNS and taVNS-mediated improvements scaled with sensory performance
We leveraged the natural variation in participants’ sensory performance during control conditions to examine whether it predicteds the magnitude of tVNS-mediated sensory improvements (Figure 3). The high variability associated with high-performing individuals (i.e., those with negative z-scores) was minimized using robust linear regression. These outlying values were not specific to tVNS but occurred by chance across performance metrics (Supplementary Figure 1).
Figure 3. tcVNS and taVNS-mediated improvements scaled with sensory performance.
(A) Relation between sensory performance during control conditions (Sham, Baseline, and Arm) and the tcVNS effect – the percent change in sensory performance measured during tcVNS relative to the corresponding control condition. Each point depicts an individual participant in each experiment, sensory modality, and stimulation condition. The solid line depicts the best-fitting robust linear regression and the shaded region depicts its 95% confidence interval. The p-value represents the result of robust linear regression using the full dataset. (B) Follows the same conventions as A but depicts data for auricular tVNS.
Regression revealed positive slopes between sensory performance during controls and the percent change in performance induced by tcVNS (p=0.000030; Figure 3A, Supplementary Table 6) and taVNS (p=0.0023; Figure 3B, Supplementary Table 7). Thus, tVNS induced larger improvements for individuals with lower-than-average sensory performance. Similar patterns were observed within individual experiments (Supplementary Figures 2–3).
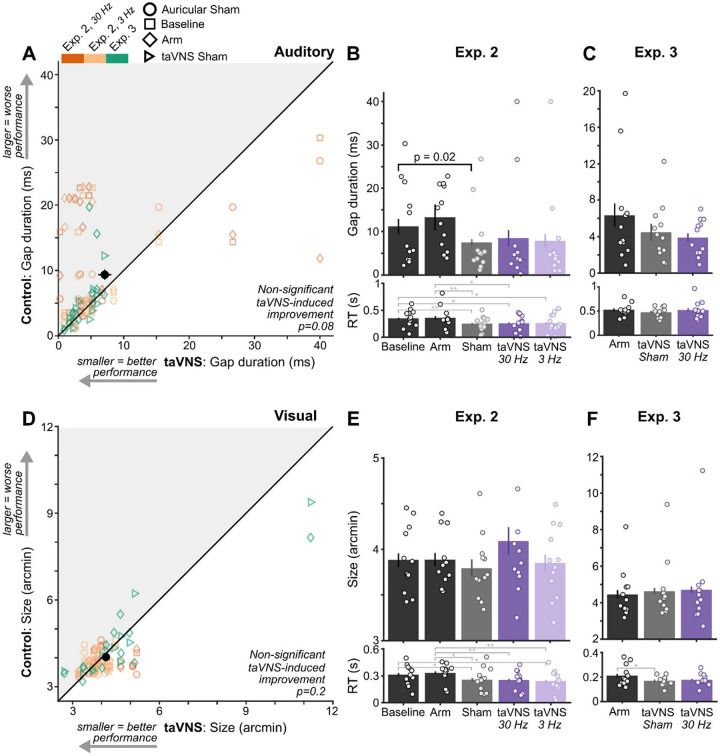
taVNS generated weaker group-level effects
Although taVNS appears to have beneficial effects on study-wide auditory performance, this difference did not reach statistical significance (p=0.079, Figure 4A; Supplementary Table 8). Within Experiment 2, neither 30 Hz nor 3 Hz taVNS significantly improved performance (p>0.6; Figure 4B). Instead, performance only differed among control conditions (Auricular Sham vs Baseline, t(11)=2.91, p=0.017). Likewise, within Experiment 3, performance during taVNS 30 Hz did not differ significantly between Arm (p=0.054) or taVNS Sham (p=0.67; Figure 4C). Similarly, taVNS did not induce significant study-wide visual improvements (p=0.21, Supplementary Table 9; Figure 4D), which coincided with the lack of differences within experiments (Figure 4E–F).
Figure 4. taVNS generated weaker group-level effects.
(A) Gap duration thresholds during control conditions (Auricular Sham, Baseline, and Arm) and taVNS conditions across all experiments and stimulation protocols (30 Hz and 3 Hz). Plotting conventions follow those in Figure 2A. (B) Gap duration thresholds in each stimulation condition of Experiment 2 and (C) Experiment 3. Plotting conventions follow those in Figure 2B. (D) Letter size thresholds during control conditions and taVNS conditions across experiments and stimulation protocols. Plotting conventions follow those in A. (E) Letter size thresholds in each stimulation condition of Experiment 2 and (F) Experiment 3.
taVNS improved performance in participants that were comfortable during stimulation
We investigated potential factors that could have impacted tVNS effects – practice effects and discomfort during stimulation. Repeated practice of sensory tasks did not influence tVNS effects (Supplementary Figures 4–5). Discomfort was reported using surveys (Figure 5A). In Experiment 2, 2% of participants felt slight discomfort with Arm stimulation (Figure 5B). No discomfort was reported during Arm, tcVNS no-stim, or taVNS no-stim conditions in Experiment 3 (Figure 5C). A maximum of 7% reported slight discomfort across Cervical Sham (4%), tcVNS 30 Hz (4%), and tcVNS Burst (7%) conditions in Experiment 2 (Figure 5D). In contrast, a maximum of 13% reported that taVNS or Auricular Sham produced Very High (8%), Moderate (13%), and Slight (13%) levels of discomfort (Figure 5E). Similarly, 33% reported taVNS as slightly (8%) or moderately (25%) uncomfortable in Experiment 3 (Figure 5F).
Figure 5. Participant discomfort diminished taVNS-mediated improvements.
(A) Flowchart of the survey question and corresponding response options. (B-F) Histograms of participant responses for each experiment and stimulation protocol.
To explore whether discomfort levels influenced taVNS-mediated improvements, we excluded sensory thresholds associated with discomfort levels uniquely experienced during taVNS (Moderate or higher, n=24). After exclusion, we found significant taVNS-mediated improvements relative to controls across sensory tasks (p=0.026, Supplementary Figure 6 & Table 11), suggesting that individuals who find taVNS comfortable may experience sensory benefits.
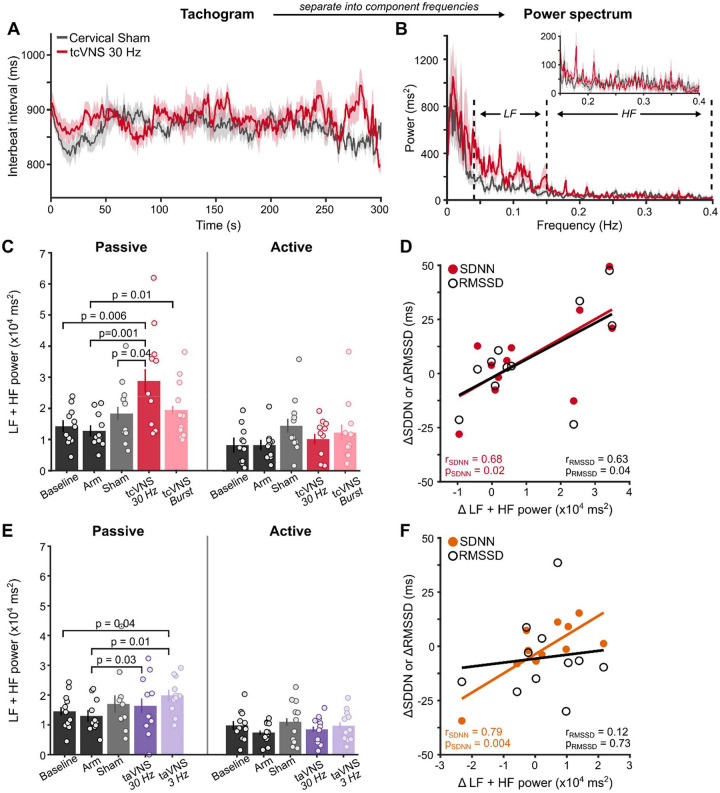
Behavioral state-dependent tVNS effects on heart rate variability
HRV, a cardiac indicator of vagal engagement, increased during tVNS relative to control stimulation, but only during passive behavioral states (Figure 6). Given that high cognitive demands, including performing sensory tasks, reduce HRV [48–51], we measured during two behavioral states – active task engagement and passive viewing. Interbeat intervals oscillated throughout each state (Figure 6A) and total spectral power at low (LF) and high frequencies (HF) indexed modulations of vagal activity [52–54] (Figure 6B).
Figure 6. Behavioral state-dependent VNS effects on heart rate variability.
(A) Interbeat interval tachogram during passive viewing. Solid lines show group-average and shaded regions depict ±1 within-subject SEM [47]. (B) Group-average power spectrum of the tachogram. Dashed lines demarcate boundaries for low (LF) and high frequency (HF) ranges. The inset shows a zoomed-in view of the HF range. (C) Summed LF and HF power for tcVNS during passive and active behavioral states. P-values denote results of permutation t-tests among sites. (D) Correlation among spectral and temporal HRV metrics. Points depict the difference between Cervical Sham and tcVNS 30 Hz in the frequency and temporal domain. Solid lines represent best-fitting linear regression to the data. (E) Summed LF and HF power for taVNS. (F) Correlation among spectral and temporal HRV metrics. Points depict the difference between Auricular Sham and taVNS 3 Hz.
Spectral power was significantly higher when participants exerted less cognitive effort during passive viewing (Figure 6C; F(1, 10)=6.50, p=0.0072) [48–51], but differed among stimulation sites (F(3, 30)=19.11, p=0.0014). Differences among sites interacted with behavioral state (F(3, 30)=4.54, p=0.028). tcVNS did not alter HRV during active task engagement. During passive viewing, tcVNS 30 Hz significantly increased power relative to Baseline (t(10)=3.70, p=0.0055), Arm (t(10)=4.83, p=0.001), and Cervical Sham (t(10)=2.48, p=0.044). Although tcVNS Burst increased power relative to Cervical Sham and Arm stimulation, only the latter was significant (t(10)=3.36, p=0.012). Temporal domain metrics (SDNN, RMSSD) corroborated these tcVNS-mediated increases in HRV (Figure 6D). Differences between Cervical Sham and tcVNS 30 Hz for each HRV metric indexed stimulation-mediated effects. Increases in power were significantly correlated with increases in SDNN (r(9)=0.68, p=0.022) and RMSSD (r(9)=0.63, p=0.040). These findings suggest that HRV during passive states provides a clearer indication of vagal engagement by tcVNS.
taVNS generated similar state-dependent HRV changes (Figure 6E). Spectral power was significantly higher during passive than active behavioral states (F(1, 10)=27.2, p=0.0004). During passive viewing, power during taVNS 3 Hz was higher than Baseline (t(10)=2.44, p=0.043) and Arm conditions (t(10)=3.63, p=0.0098). Additionally, taVNS 30 Hz increased power relative to Arm stimulation (t(10)=2.65, p=0.032). However, no differences were observed between taVNS and Auricular Sham (p>0.2). This lack of difference possibly stems from the discomfort experienced, which is associated with lowered HRV [55]. Yet, temporal domain metrics bolstered taVNS-mediated increases in HRV (Figure 6F). Differences between Auricular Sham and taVNS 3 Hz indexed stimulation-mediated changes. Increases in power were significantly correlated with increases in SDNN (r(9)=0.79, p=0.0041), but not RMSSD (r(9)=0.12, p=0.073). Thus, taVNS and tcVNS increased HRV during a passive state, but tcVNS did so reliably across HRV metrics.
Discussion
We measured the effects of continuous tVNS on sensory performance in neurotypical adults. tcVNS improved auditory gap discrimination by 35% and visual letter discrimination by 20%, on average, without altering reaction time. Individuals with lower sensory performance experienced larger improvements during tcVNS and taVNS. However, taVNS did not significantly improve group-level performance, potentially because participants felt discomfort during stimulation. Lastly, both tcVNS and taVNS increased HRV relative to controls during passive viewing, corroborating vagal engagement. These results demonstrate that continuous tVNS can enhance sensory performance in humans, particularly those with relatively poor sensory capabilities.
Our findings corroborate foundational studies in rodents. Steady activation of the LC-NE system through continuous LC stimulation [4] or indirectly via VNS [5] produced rapid and sustained improvements in sensory processing without affecting neural or behavioral response latencies. Improvements were driven by NE-mediated suppression of calcium T-type channels responsible for bursting activity by sensory relay neurons in the thalamus that reduces accuracy and efficiency of sensory transmission [4–6]. Here, we show that non-invasive tVNS can induce similar sensory enhancements in humans, suggesting that peripheral nerve activation induces NE-mediated improvements in both species.
Notably, in both rodents [4, 5] and humans, continuous stimulation generated rapid and transient sensory benefits that generalized across different experiments, sensory tasks, and stimulus properties. Improvements occurred even when sensory stimuli were randomized across trials. In contrast, phasic VNS protocols, in which stimuli are repeatedly paired with short VNS bursts over several days or weeks, induce relatively delayed and lasting sensory improvements specific to the paired stimulus [56–66]. Thus, our findings position tonic tVNS as a potential tool for promoting general, on-demand improvements in sensory processing.
Our behavioral findings were bolstered by tVNS-mediated increases in HRV supportive of vagal engagement [46, 52–54]. Cardiac activity is known to be regulated by the vagus nerve [67] and invasive stimulation of the vagus nerve modulates HRV [68]. However, effects of non-invasive stimulation on cardiac activity have been conflicting [69–71]. Here, tVNS-mediated HRV effects depended on behavioral state – tVNS increased HRV during passive, but not active states. Consistent with earlier work [48–51], heightened cognitive demands during task engagement diminished HRV, which potentially reduced the likelihood of observing tVNS-mediated effects. Thus, behavioral state exerts a strong influence on HRV and should be considered when measuring tVNS-related effects, alongside prior recommendations [71]. The observed tVNS-mediated behavioral and cardiac effects correspond with animal work that established their correlation with non-invasive readouts of LC activity (i.e., pupil size) [4, 6, 72–75]. Therefore, our findings suggest that tVNS altered vagal activity and subsequently modulated brain function to improve human sensory processing.
To bolster our findings’ validity, several controls were assessed. tcVNS-mediated improvements were significant relative to two controls – stimulation to the upper trapezius (Cervical Sham) or withholding current from electrodes placed on the tcVNS site (tcVNS Sham). Although forearm and electrode-free conditions produced similar levels of sensory performance, they were more variable across individuals, which resulted in non-significant results. Larger variability necessitates larger sample sizes to observe significant tcVNS-mediated improvements relative to these controls. Therefore, the reliability of Cervical Sham and tcVNS Sham position them as effective controls for assessing tcVNS-mediated effects, particularly in small samples.
Surprisingly, taVNS-mediated improvements were only observed in individuals that found stimulation comfortable. Although taVNS can activate the LC [22, 24] and presumably increase sensory processing fidelity, participants’ discomfort influenced its efficacy. Discomfort and pain are known to decrease vagal activity [55], which may have diminished the ability for taVNS to modulate sensory performance and similarly contributed to the weaker, but significant, taVNS-mediated effects on HRV. Despite these potential obstacles hindering taVNS, it generated improvements for individuals with lower sensory capabilities and induced group-level sensory improvements after excluding participants that experienced moderate or higher discomfort levels. Together, these outcomes substantiate the effectiveness of taVNS and demonstrate the utility of considering individuals’ subjective experience.
We additionally assessed two tcVNS (30 Hz and Burst) and taVNS protocols (30 Hz and 3 Hz) guided by prior studies with animal models that demonstrated VNS-induced LC activity [21]. Whereas both taVNS protocols produced similar behavioral and HRV effects, tcVNS protocols yielded numerically similar but statistically different results. tcVNS 30 Hz significantly increased HRV, auditory, and visual performance relative to controls whereas tcVNS Burst significantly improved visual performance and HRV relative to a subset of controls. The sample size within each experiment was determined via a priori power analyses and may have been insufficient to measure statistically significant differences among all stimulation protocols. Therefore, it is possible that both tcVNS protocols yield similar statistical results at larger sample sizes. The use of finite element modeling of tcVNS [76] may provide further insight into whether each protocol differentially drives vagal activity.
Collectively, we demonstrated that continuous tVNS can enhance sensory performance in humans. These findings provide evidence that peripheral neuromodulation may be a useful tool for augmenting hearing and vision, particularly for individuals with relatively impaired sensory capabilities. This study bridges the gap between animal and human research and offers promising implications for the development of new therapies for sensory disorders.
Supplementary Material
Highlights.
Methods for on demand treatment of impaired central sensory processing are scarce.
Non-invasive tVNS on the neck improved auditory gap detection by 35% on average.
tVNS on the neck improved visual discrimination performance by 20% on average.
Neck and ear tVNS increased heart rate variability, corroborating vagal engagement.
Neuromodulation via tVNS is a promising tool for rapidly treating impaired senses.
Acknowledgements
The authors acknowledge the support of the NSF I-Corps program and the input of Dr. Frank Lin on an initial version of this work.
Funding
This work was funded by Sharper Sense, Inc. Funding for publication of this manuscript is provided by the Jacobs Technion-Cornell Institute at Cornell Tech. Qi Wang was supported by NIH R01NS119813, R01AG075114, R21MH125107, NSF CBET 1847315, NSF TI 2232149, and the Air Force Office of Scientific Research under award number FA9550-22-1-0337. Any opinions, findings, and conclusions or recommendations expressed in this material are those of the authors and do not necessarily reflect the views of the United States Air Force.
Funding Statement
This work was funded by Sharper Sense, Inc. Funding for publication of this manuscript is provided by the Jacobs Technion-Cornell Institute at Cornell Tech. Qi Wang was supported by NIH R01NS119813, R01AG075114, R21MH125107, NSF CBET 1847315, NSF TI 2232149, and the Air Force Office of Scientific Research under award number FA9550-22-1-0337. Any opinions, findings, and conclusions or recommendations expressed in this material are those of the authors and do not necessarily reflect the views of the United States Air Force.
Footnotes
CRediT authorship contribution statement:
Michael Jigo: Conceptualization, Methodology, Software, Validation, Formal analysis, Investigation, Data Curation, Visualization, Writing – Original Draft, Review & Editing,
Jason B. Carmel: Conceptualization, Supervision, Writing – Review & Editing
Qi Wang: Conceptualization, Supervision, Writing – Review & Editing
Charles Rodenkirch: Conceptualization, Methodology, Supervision, Project administration, Resources, Funding acquisition, Writing – Review & Editing.
Declaration of competing interest
All authors have financial interest in Sharper Sense, a company developing methods for enhancing sensory processing with vagus nerve stimulation. Jason B. Carmel is a Founder and stockholder in BackStop Neural and has received honoraria from Pacira, Motric Bio, and Restorative Therapeutics.
References
- [1].Boksem MA, Tops M. Mental fatigue: costs and benefits. Brain research reviews 2008;59(1):125–39. [DOI] [PubMed] [Google Scholar]
- [2].Humes LE. Age-Related Changes in Cognitive and Sensory Processing: Focus on Middle-Aged Adults. American journal of audiology 2015;24(2):94–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].McGinley MJ, Vinck M, Reimer J, Batista-Brito R, Zagha E, Cadwell CR, et al. Waking State: Rapid Variations Modulate Neural and Behavioral Responses. Neuron 2015;87(6):1143–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Rodenkirch C, Liu Y, Schriver BJ, Wang Q. Locus coeruleus activation enhances thalamic feature selectivity via norepinephrine regulation of intrathalamic circuit dynamics. Nat Neurosci 2019;22(1):120–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Rodenkirch C, Wang Q. Rapid and transient enhancement of thalamic information transmission induced by vagus nerve stimulation. J Neural Eng 2020;17(2):026027. [DOI] [PubMed] [Google Scholar]
- [6].Rodenkirch C, Carmel JB, Wang Q. Rapid Effects of Vagus Nerve Stimulation on Sensory Processing Through Activation of Neuromodulatory Systems. Frontiers in neuroscience 2022;16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Peuker ET, Filler TJ. The nerve supply of the human auricle. Clinical Anatomy 2002;15(1):35–7. [DOI] [PubMed] [Google Scholar]
- [8].Yuan H, Silberstein SD. Vagus Nerve and Vagus Nerve Stimulation, a Comprehensive Review: Part II. Headache 2016;56(2):259–66. [DOI] [PubMed] [Google Scholar]
- [9].Yap JYY, Keatch C, Lambert E, Woods W, Stoddart PR, Kameneva T. Critical Review of Transcutaneous Vagus Nerve Stimulation: Challenges for Translation to Clinical Practice. Frontiers in neuroscience 2020;14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Redgrave J, Day D, Leung H, Laud P, Ali A, Lindert R, et al. Safety and tolerability of transcutaneous vagus nerve stimulation in humans; a systematic review. Brain stimulation 2018;11(6):1225–38. [DOI] [PubMed] [Google Scholar]
- [11].Rodieck R. Visual pathways. Annual Review of neuroscience 1979;2(1):193–225. [DOI] [PubMed] [Google Scholar]
- [12].Chechik G, Anderson MJ, Bar-Yosef O, Young ED, Tishby N, Nelken I. Reduction of information redundancy in the ascending auditory pathway. Neuron 2006;51(3):359–68. [DOI] [PubMed] [Google Scholar]
- [13].Wang Q, Webber RM, Stanley GB. Thalamic synchrony and the adaptive gating of information flow to cortex. Nat Neurosci 2010;13(12):1534–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Berridge CW, Waterhouse BD. The locus coeruleus-noradrenergic system: modulation of behavioral state and state-dependent cognitive processes. Brain Res Brain Res Rev 2003;42(1):33–84. [DOI] [PubMed] [Google Scholar]
- [15].Aston-Jones G, Waterhouse B. Locus coeruleus: From global projection system to adaptive regulation of behavior. Brain research 2016;1645:75–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Chandler DJ, Jensen P, McCall JG, Pickering AE, Schwarz LA, Totah NK. Redefining noradrenergic neuromodulation of behavior: impacts of a modular locus coeruleus architecture. Journal of Neuroscience 2019;39(42):8239–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Pinto L, Goard MJ, Estandian D, Xu M, Kwan AC, Lee S-H, et al. Fast modulation of visual perception by basal forebrain cholinergic neurons. Nature Neuroscience 2013;16(12):1857–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Hulsey DR, Shedd CM, Sarker SF, Kilgard MP, Hays SA. Norepinephrine and serotonin are required for vagus nerve stimulation directed cortical plasticity. Exp Neurol 2019;320:112975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Slater C, Liu Y, Weiss E, Yu K, Wang Q. The Neuromodulatory Role of the Noradrenergic and Cholinergic Systems and Their Interplay in Cognitive Functions: A Focused Review. Brain Sciences 2022;12(7):890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Weiss E, Kann M, Wang Q. Neuromodulation of Neural Oscillations in Health and Disease. Biology 2023;12(3):371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Hulsey DR, Riley JR, Loerwald KW, Rennaker RL 2nd, Kilgard MP, Hays SA. Parametric characterization of neural activity in the locus coeruleus in response to vagus nerve stimulation. Exp Neurol 2017;289:21–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Frangos E, Ellrich J, Komisaruk BR. Non-invasive access to the vagus nerve central projections via electrical stimulation of the external ear: fMRI evidence in humans. Brain stimulation 2015;8(3):624–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Frangos E, Komisaruk BR. Access to vagal projections via cutaneous electrical stimulation of the neck: fMRI evidence in healthy humans. Brain stimulation 2017;10(1):19–27. [DOI] [PubMed] [Google Scholar]
- [24].Yakunina N, Kim SS, Nam E-C. Optimization of transcutaneous vagus nerve stimulation using functional MRI. Neuromodulation: technology at the neural interface 2017;20(3):290–300. [DOI] [PubMed] [Google Scholar]
- [25].Nonis R, D’Ostilio K, Schoenen J, Magis D. Evidence of activation of vagal afferents by non-invasive vagus nerve stimulation: an electrophysiological study in healthy volunteers. Cephalalgia 2017;37(13):1285–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Erman AB, Kejner AE, Hogikyan ND, Feldman EL. Disorders of cranial nerves IX and X. Seminars in neurology. 29. © Thieme Medical Publishers; 2009:085–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Faul F, Erdfelder E, Buchner A, Lang A-G. Statistical power analyses using G* Power 3.1: Tests for correlation and regression analyses. Behavior research methods 2009;41(4):1149–60. [DOI] [PubMed] [Google Scholar]
- [28].Tubbs RS, Shoja MM, Loukas M, Lancaster J, Mortazavi MM, Hattab EM, et al. Study of the cervical plexus innervation of the trapezius muscle. Journal of Neurosurgery: Spine 2011;14(5):626–9. [DOI] [PubMed] [Google Scholar]
- [29].Adair D, Truong D, Esmaeilpour Z, Gebodh N, Borges H, Ho L, et al. Electrical Stimulation of Cranial Nerves in Cognition and Disease. Brain stimulation 2020;13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Kleiner M, Brainard D, Pelli D. What’s new in Psychtoolbox-3? 2007.
- [31].Strouse A, Ashmead DH, Ohde RN, Grantham DW. Temporal processing in the aging auditory system. The Journal of the Acoustical Society of America 1998;104(4):2385–99. [DOI] [PubMed] [Google Scholar]
- [32].Dias KZ, Jutras B, Acrani IO, Pereira LD. Random Gap Detection Test (RGDT) performance of individuals with central auditory processing disorders from 5 to 25 years of age. International journal of pediatric otorhinolaryngology 2012;76(2):174–8. [DOI] [PubMed] [Google Scholar]
- [33].Hoover E, Pasquesi L, Souza P. Comparison of clinical and traditional gap detection tests. Journal of the American Academy of Audiology 2015;26(06):540–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Babkoff H, Fostick L. Age-related changes in auditory processing and speech perception: cross-sectional and longitudinal analyses. European journal of ageing 2017;14(3):269–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Keith RW. Random gap detection test. St Louis, MO: Auditec; 2000;13. [Google Scholar]
- [36].Bailey IL, Lovie-Kitchin JE. Visual acuity testing. From the laboratory to the clinic. Vision research 2013;90:2–9. [DOI] [PubMed] [Google Scholar]
- [37].Petrov AA, Dosher BA, Lu Z-L. Perceptual learning without feedback in non-stationary contexts: Data and model. Vision research 2006;46(19):3177–97. [DOI] [PubMed] [Google Scholar]
- [38].Watson AB, Pelli DG. QUEST: A Bayesian adaptive psychometric method. Perception & psychophysics 1983;33(2):113–20. [DOI] [PubMed] [Google Scholar]
- [39].Prins N, Kingdom FA. Applying the model-comparison approach to test specific research hypotheses in psychophysical research using the Palamedes toolbox. Frontiers in psychology 2018;9:1250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Burr DC, Morrone MC, Ross J. Selective suppression of the magnocellular visual pathway during saccadic eye movements. Nature 1994;371(6497):511–3. [DOI] [PubMed] [Google Scholar]
- [41].Pelli DG, Palomares M, Majaj NJ. Crowding is unlike ordinary masking: Distinguishing feature integration from detection. Journal of vision 2004;4(12):12-. [DOI] [PubMed] [Google Scholar]
- [42].Kamitani Y, Tong F. Decoding seen and attended motion directions from activity in the human visual cortex. Current biology 2006;16(11):1096–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Landau AN, Fries P. Attention samples stimuli rhythmically. Current biology 2012;22(11):1000–4. [DOI] [PubMed] [Google Scholar]
- [44].Jigo M, Tavdy D, Himmelberg MM, Carrasco M. Cortical magnification eliminates differences in contrast sensitivity across but not around the visual field. Elife 2023;12:e84205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Westfall PH, Young SS. Resampling-based multiple testing: Examples and methods for p-value adjustment. John Wiley & Sons; 1993. [Google Scholar]
- [46].Shaffer F, Ginsberg JP. An overview of heart rate variability metrics and norms. Frontiers in public health 2017:258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Morey RD. Confidence intervals from normalized data: A correction to Cousineau (2005). Tutorials in Quantitative Methods for Psychology 2008;4(2):61–4. [Google Scholar]
- [48].Mulder G, Mulder LJ. Information processing and cardiovascular control. Psychophysiology 1981;18(4):392–402. [DOI] [PubMed] [Google Scholar]
- [49].Duschek S, Muckenthaler M, Werner N, Del Paso GAR. Relationships between features of autonomic cardiovascular control and cognitive performance. Biological psychology 2009;81(2):110–7. [DOI] [PubMed] [Google Scholar]
- [50].Luft CDB, Takase E, Darby D. Heart rate variability and cognitive function: Effects of physical effort. Biological psychology 2009;82(2):186–91. [DOI] [PubMed] [Google Scholar]
- [51].Luque-Casado A, Perales JC, Cárdenas D, Sanabria D. Heart rate variability and cognitive processing: The autonomic response to task demands. Biological psychology 2016;113:83–90. [DOI] [PubMed] [Google Scholar]
- [52].Rahman F, Pechnik S, Gross D, Sewell L, Goldstein DS. Low frequency power of heart rate variability reflects baroreflex function, not cardiac sympathetic innervation. Clinical Autonomic Research 2011;21:133–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Reyes del Paso GA, Langewitz W, Mulder LJ, Van Roon A, Duschek S. The utility of low frequency heart rate variability as an index of sympathetic cardiac tone: a review with emphasis on a reanalysis of previous studies. Psychophysiology 2013;50(5):477–87. [DOI] [PubMed] [Google Scholar]
- [54].Thomas BL, Claassen N, Becker P, Viljoen M. Validity of commonly used heart rate variability markers of autonomic nervous system function. Neuropsychobiology 2019;78(1):14–26. [DOI] [PubMed] [Google Scholar]
- [55].Koenig J, Jarczok M, Ellis R, Hillecke T, Thayer JF. Heart rate variability and experimentally induced pain in healthy adults: a systematic review. European Journal of Pain 2014;18(3):301–14. [DOI] [PubMed] [Google Scholar]
- [56].Neuhaus AH, Luborzewski A, Rentzsch J, Brakemeier EL, Opgen-Rhein C, Gallinat J, et al. P300 is enhanced in responders to vagus nerve stimulation for treatment of major depressive disorder. Journal of affective disorders 2007;100(1–3):123–8. [DOI] [PubMed] [Google Scholar]
- [57].Engineer CT, Engineer ND, Riley JR, Seale JD, Kilgard MP. Pairing Speech Sounds With Vagus Nerve Stimulation Drives Stimulus-specific Cortical Plasticity. Brain stimulation 2015;8(3):637–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Tyler R, Cacace A, Stocking C, Tarver B, Engineer N, Martin J, et al. Vagus Nerve Stimulation Paired with Tones for the Treatment of Tinnitus: A Prospective Randomized Double-blind Controlled Pilot Study in Humans. Sci Rep 2017;7(1):11960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Vanneste S, Martin J, Rennaker RL 2nd, Kilgard MP. Pairing sound with vagus nerve stimulation modulates cortical synchrony and phase coherence in tinnitus: An exploratory retrospective study. Sci Rep 2017;7(1):17345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Kilgard MP, Rennaker RL, Alexander J, Dawson J. Vagus nerve stimulation paired with tactile training improved sensory function in a chronic stroke patient. NeuroRehabilitation 2018;42(2):159–65. [DOI] [PubMed] [Google Scholar]
- [61].Adcock KS, Chandler C, Buell EP, Solorzano BR, Loerwald KW, Borland MS, et al. Vagus nerve stimulation paired with tones restores auditory processing in a rat model of Rett syndrome. Brain stimulation 2020;13(6):1494–503. [DOI] [PubMed] [Google Scholar]
- [62].Llanos F, McHaney JR, Schuerman WL, Yi HG, Leonard MK, Chandrasekaran B. Non-invasive peripheral nerve stimulation selectively enhances speech category learning in adults. NPJ Sci Learn 2020;5(1):12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Thakkar VJ, Engelhart AS, Khodaparast N, Abadzi H, Centanni TM. Transcutaneous auricular vagus nerve stimulation enhances learning of novel letter-sound relationships in adults. Brain stimulation 2020;13(6):1813–20. [DOI] [PubMed] [Google Scholar]
- [64].Altidor LK, Bruner MM, Deslauriers JF, Garman TS, Ramirez S, Dirr EW, et al. Acute vagus nerve stimulation enhances reversal learning in rats. Neurobiology of learning and memory 2021;184:107498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Phillips I, Calloway RC, Karuzis VP, Pandza NB, O’Rourke P, Kuchinsky SE. Transcutaneous Auricular Vagus Nerve Stimulation Strengthens Semantic Representations of Foreign Language Tone Words during Initial Stages of Learning. Journal of cognitive neuroscience 2021;34(1):127–52. [DOI] [PubMed] [Google Scholar]
- [66].Bowles S, Hickman J, Peng X, Williamson WR, Huang R, Washington K, et al. Vagus nerve stimulation drives selective circuit modulation through cholinergic reinforcement. Neuron 2022;110(17):2867–85. e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Capilupi MJ, Kerath SM, Becker LB. Vagus nerve stimulation and the cardiovascular system. Cold Spring Harbor perspectives in medicine 2020;10(2). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Stemper B, Devinsky O, Haendl T, Welsch G, Hilz M. Effects of vagus nerve stimulation on cardiovascular regulation in patients with epilepsy. Acta neurologica scandinavica 2008;117(4):231–6. [DOI] [PubMed] [Google Scholar]
- [69].Clancy JA, Mary DA, Witte KK, Greenwood JP, Deuchars SA, Deuchars J. Non-invasive vagus nerve stimulation in healthy humans reduces sympathetic nerve activity. Brain stimulation 2014;7(6):871–7. [DOI] [PubMed] [Google Scholar]
- [70].Borges U, Laborde S, Raab M. Influence of transcutaneous vagus nerve stimulation on cardiac vagal activity: Not different from sham stimulation and no effect of stimulation intensity. PLoS One 2019;14(10):e0223848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Burger AM, D’Agostini M, Verkuil B, Van Diest I. Moving beyond belief: A narrative review of potential biomarkers for transcutaneous vagus nerve stimulation. Psychophysiology 2020;57(6):e13571. [DOI] [PubMed] [Google Scholar]
- [72].Reimer J, McGinley MJ, Liu Y, Rodenkirch C, Wang Q, McCormick DA, et al. Pupil fluctuations track rapid changes in adrenergic and cholinergic activity in cortex. Nat Commun 2016;7:13289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Liu Y, Rodenkirch C, Moskowitz N, Schriver B, Wang Q. Dynamic Lateralization of Pupil Dilation Evoked by Locus Coeruleus Activation Results from Sympathetic, Not Parasympathetic, Contributions. Cell Reports 2017;20:3099–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Schriver BJ, Bagdasarov S, Wang Q. Pupil-linked arousal modulates behavior in rats performing a whisker deflection direction discrimination task. J Neurophysiol 2018;120(4):1655–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Liu Y, Narasimhan S, Schriver BJ, Wang Q. Perceptual behavior depends differently on pupil-linked arousal and heartbeat dynamics-linked arousal in rats performing tactile discrimination tasks. Frontiers in systems neuroscience 2021;14:614248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Mourdoukoutas AP, Truong DQ, Adair DK, Simon BJ, Bikson M. High-resolution multi-scale computational model for non-invasive cervical vagus nerve stimulation. Neuromodulation: Technology at the Neural Interface 2018;21(3):261–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.