Abstract
Alterations in the tumor suppressor ATRX are recurrently observed in several cancer types including sarcomas, which are mesenchymal neoplasms. ATRX has multiple epigenetic functions including heterochromatin formation and maintenance and regulation of transcription through modulation of chromatin accessibility. Here, we show in murine mesenchymal progenitor cells (MPCs) that Atrx deficiency aberrantly activated mesenchymal differentiation programs. This includes adipogenic pathways where ATRX loss induced expression of adipogenic transcription factors (Pparγ and Cebpα) and enhanced adipogenic differentiation in response to differentiation stimuli. These changes are linked to loss of heterochromatin near mesenchymal lineage genes together with increased chromatin accessibility and gains of active chromatin marks at putative enhancer elements and promoters. Finally, we observed depletion of H3K9me3 at transposable elements, which are derepressed including near mesenchymal genes where they could serve as regulatory elements. Our results demonstrate that ATRX functions to buffer against differentiation in mesenchymal progenitor cells, which has implications for understanding ATRX loss of function in sarcomas.
Introduction
Alpha Thalassemia/Mental Retardation Syndrome X-Linked (ATRX) belongs to the SWI/SNF family of ATP-dependent chromatin remodeling proteins [1]. Germline mutations in ATRX cause cognitive impairment as part of the alpha-thalassemia (ATR-X) syndrome, which is accompanied by disturbances of DNA methylation [2], highlighting the function of ATRX in development [3]. Somatic alterations in ATRX occur in cancers, such as sarcomas [4].
ATRX is an important epigenetic regulator, functioning as a chromatin remodeler and promoting the formation and maintenance of heterochromatin [5]. For example, ATRX cooperates with the H3K9 methyltransferase SETDB1 to establish and maintain heterochromatin, including at retrotransposons [5]. ATRX also binds to H3K9me3 via its ADD domain to directly target it to heterochromatin, which may be important for its role in maintaining H3K9me3 domains [5]. ATRX also binds to DAXX, functioning as a histone chaperone depositing H3.3 into telomeric regions [6].
Loss of function genetic alterations in ATRX are highly recurrent in several cancers including gliomas, pancreatic neuroendocrine tumors, and multiple sarcoma subtypes [7, 8]. Within sarcomas, ATRX is altered in more than 10 percent of undifferentiated pleomorphic sarcoma, leiomyosarcoma, myxofibrosarcoma, perivascular epithelioid tumors, pleomorphic liposarcoma and angiosarcoma. In uterine leiomyosarcoma (ULMS), the frequency of ATRX alterations is approximately one third of cases [7]. Consistent with these genetic studies, loss of ATRX expression occurs in 20–30% of undifferentiated pleomorphic sarcoma (UPS) and leiomyosarcomas [9, 10]. While the role of ATRX deficiency in the alternative lengthening of telomeres pathway is well described in sarcomas and other ATRX deficient cancers, the chromatin-specific consequences of ATRX loss are not fully understood in the context of disease mechanisms [11, 12].
Sarcomas are mesenchymal neoplasms occurring in connective tissue such as fat, bone, cartilage and muscle [13]. It has been proposed that sarcomagenesis occurs, at least in part, through aberrant differentiation of mesenchymal progenitor cells (MPCs), which has been modeled by introducing sarcoma-relevant driver alterations into MPCs [14–18]. MPCs are multipotent cells that are able to self-renew and undergo differentiation [19] making them well-suited to study perturbations in epigenetic states, which are a key determinants of cell fate and lineage commitment [20–25]. For example, sarcoma-associated mutations in histone genes, which alter histone posttranslational modifications, are sufficient to induce sarcomagenesis when expressed in MPCs [26, 27]. Given the high frequency of ATRX alterations in soft tissue sarcoma, we sought to investigate the effect of ATRX deficiency on chromatin and chromatin-dependent processes in the mesenchymal context.
Our findings demonstrate that deletion of ATRX leads to abnormal differentiation in MPCs, which is associated with perturbations in the profiles of histone post-translational modifications and chromatin accessibility in specific regions, which are accompanied by the activation of transposable elements. Our results suggest an important role for the epigenetic regulatory functions of ATRX in the mesenchymal lineage.
Results
Loss of ATRX dysregulates transcriptional programs in mesenchymal progenitors
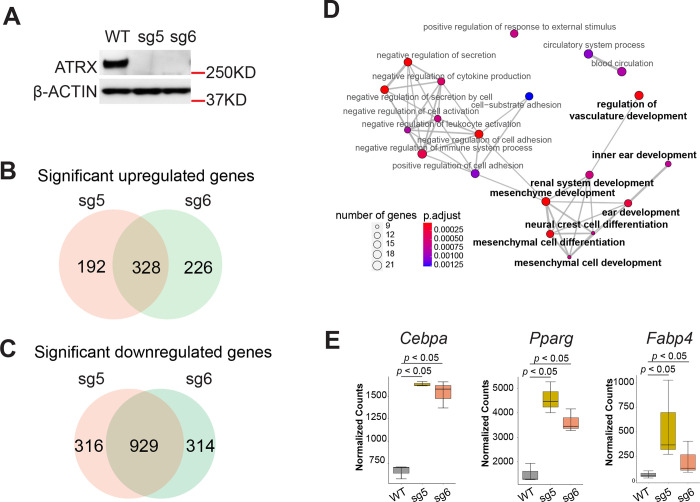
It is well established that the chromatin landscape has a key role in establishing patterns of gene expression, and since ATRX acts as chromatin regulator we hypothesized that ATRX deficiency would lead to changes in transcription. Given that ATRX loss frequently occurs in mesenchymal malignancies, we established Atrx KO lines in the C3H/10T1/2 cells, a mesenchymal progenitor cell line, using CRISPR-cas9 with two independent sgRNAs targeting the Atrx gene (Fig. S1A). One clone from each guide and an isogenic WT control were selected for further study (Fig. 1A, Fig. S1B). ATRX deletion was confirmed by immunofluorescence and immunoblotting (Fig. 1A, Fig. S1C). Under normal culture conditions without differentiation cues, transcriptomic profiling of Atrx KO vs WT cells was performed using bulk RNA-seq. Compared to Atrx WT, the Atrx KO MPC lines displayed a marked transcriptional dysregulation, with significant gains and losses of gene expression (Fig. S2A, S2B). Focusing on genes that significantly change expression (padj < 0.05) by an absolute magnitude of at least 2-fold in both Atrx KO clones when compared to WT, we identified 328 upregulated and 929 downregulated genes (Fig. 1B, 1C, Supplementary Table 1, 2). Given the role of ATRX in establishing and maintaining transcriptionally silenced regions of heterochromatin, we focused on the upregulated gene set. Gene ontology (GO) analysis of the upregulated genes showed an enrichment of gene sets associated with development and differentiation programs, including mesenchymal programs (Fig. 1D, Supplementary Table 3).
Figure 1: ATRX deficiency alters the transcriptome.
(A) Immunoblot demonstrating ATRX protein loss in MPC lines. β-ACTIN acts as loading control. (B) The intersection of significantly upregulated genes (log2foldchange > 1, padj < 0.05) in both Atrx KO clones based on polyA-RNA-seq datasets. (C) The intersection of significantly downregulated genes (log2foldchange < −1, padj value < 0.05) in both Atrx KO clones based on polyA-RNA seq datasets. (D) Dot plot of gene ontology (GO) (biological process) analysis for significant upregulated genes from (B). The size of nodes indicates the numbers of genes. The color gradient indicates the padj value. Bold type indicates gene ontology terms which are associated with development. (E) Boxplot depicting mRNA levels from the RNA-seq dataset. The y-axis indicates RNA-seq read counts normalized by DESeq2. The p value (p<0.05) was determined by DESeq2.
To explore the impact of Atrx KO on specific mesenchymal lineages, we examined the expression of key adipogenic pathway regulators including the adipogenic transcription factors Pparγ and Cebpa and the lineage-specific marker Fabp4. All three were significantly (log2foldchange >1 & padj < 0.05) upregulated in Atrx KO MPCs (Fig. 1E, Supplementary Table 4), even in the absence of adipogenic differentiation factors. Under the same conditions, we observed significantly decreased expression of mesenchymal stemness markers, Etv1 [26] and Cd34 [28], in both Atrx KO lines (Fig. S2C). The GO terms associated with downregulated genes were not enriched for pathways relevant for cell lineage differentiation and development (Fig. S2D, Supplementary Table 5).
ATRX deficiency promotes mesenchymal lineage differentiation and attenuates progenitor properties
The de-repression of developmental and differentiation pathways in Atrx KO cells suggests that ATRX may have an important role in restricting differentiation in MPCs. Given the marked upregulation of adipogenic transcription factors and lineage markers in Atrx KO lines, we hypothesized that ATRX loss would sensitize MPCs to adipogenic differentiation cues. Atrx WT and KO MPCs were treated with adipogenic media to induce differentiation and the degree of differentiation was quantified by staining by Oil Red O (ORO) [29]. Compared to WT MPCs, the Atrx KO lines nearly doubled ORO staining intensity after 7 days (p<0.05), indicating that ATRX loss promotes adipogenic differentiation (Fig. 2A). Over the course of the 7-day differentiation treatment, we measured protein expression of PPARγ, C/EBPα, and FABP4 (Fig. 2B). The levels of C/EBP and PPARγ were more highly induced beginning on day 1 of treatment in Atrx KO lines compared to WT control whereas the FABP4, a marker for late differentiation, was equally and strongly induced in both settings beginning on day 3. These data suggest that ATRX deletion disturbs the lineage commitment of mesenchymal progenitor cells and promotes differentiation to adipocyte lineage.
Figure 2: ATRX deficiency promotes differentiation.
(A) Oil Red O (ORO) staining in adipogenic differentiation experiments. adipo diff: adipogenic media treatment. normal: normal culture media treatment. The y-axis shows the optical density (OD) of the solubilized ORO after adipocyte-specific staining minus the OD value from the normal media control (clt). Data from three biological replicates are plotted with each point representing individual value from each replicate. p value was calculated by unpaired t-test (two-tailed). (B) Western blot of adipocyte markers. The “control” indicates cells grown in the normal media culture at the end of the experiment (Day 7). Day 1, 3, and 7 indicate the duration of adipogenic media treatment. The red arrows indicate the protein bands of interest. Two isoforms of C/EBPα are shown. (C) Colony formation assays. The bar plot shows the colony formation from three biological replicates normalized to WT. Cell groups with at least 50 cells were classified as a colony. For each replicate, the percentage of colony formation was normalized to the WT group. The error bars were calculated from three biological replicates. The p value was determined by paired one-way ANOVA (WT vs. sg5, WT vs. sg6). (D) MPC proliferation assay. Relative numbers of viable cells were determined using an ATPase assay on day 1, day 3 and day 5 after seeding cells. The OD value of luminescence was normalized with day 1 for each group. Three biological replicates were performed. p value was calculated by paired t-test (two-tailed) for day 5 data. (E) and (F) RT-qPCR experiments for Etv1 gene expression in two knockout lines Corresponding to the data in panel (B). p value was calculated using one-sample, two-sided t-test to compare the mean values for sgRNA samples and WT. The error bars indicate the standard variance.
To assess if Atrx KO reduced the stemness properties of mesenchymal progenitors, we performed assays for colony formation (Fig. 2C) and proliferation (Fig. 2D). The two Atrx KO lines had reduced colony formation capacity and proliferation compared to Atrx WT (p<0.05). We also compared mRNA levels of Etv1, a mesenchymal stemness marker, between Atrx KO and WT MPCs (Fig. 2E, 2F). The two knock out lines expressed lower levels of Etv1 during the differentiation time course, including at Day 0, which is consistent with the RNA-seq findings, suggesting that Etv1 is suppressed at baseline in Atrx KO MPCs. These results demonstrate that Atrx KO reduces the stem-like properties of mesenchymal progenitors.
Atrx KO reduces H3K9me3-marked heterochromatin associated with transcriptionally repressed linage commitment genes
Histone post-translational modifications contribute to defining the chromatin states, which in turn impact gene expression [30, 31]. Given that histone post-translational modifications are regulated in part by ATRX, we investigated how ATRX deficiency impacted chromatin states in the MPC model. ATRX, together with DAXX, deposits the histone variant H3.3 at specific genomic regions including telomeres [6, 32, 33]. To confirm loss of ATRX epigenetic function in the knockout cell lines, we performed CUT&RUN (Cleavage Under Targets & Release Using Nuclease) [34] for the histone variant H3.3 in order to compare H3.3 deposition in Atrx KO vs WT MPCs. Analysis of three biologic replicates indicated that there were consistent changes in H3.3 localization with gains at some genomic loci and losses at others (Fig. S3A) including significant depletion at telomeres (Fig. S3B), which is consistent with the previous reports [35].
Another known function of ATRX is to establish and maintain locus-specific heterochromatin though recruitment of H3K9 methyltransferases [5]. Using CUT&RUN, we next analyzed how the heterochromatin-associated histone mark, H3K9me3, was altered in Atrx KO MPCs. H3K9me3 was significantly changed at specific regions with 4272 gained peaks and 4738 loss peaks (p value as 0.05), demonstrating that the ATRX deficiency has effects on heterochromatin in our model (Fig. S3C). Applying GO analysis to genes that are near H3K9me3 differential peaks, we found that many of the top enriched gene sets for regions that had increased or decreased H3K9me3 signal in both Atrx KO lines were related to development (Fig. S3D, S3E; Supplementary Table 6, 7).
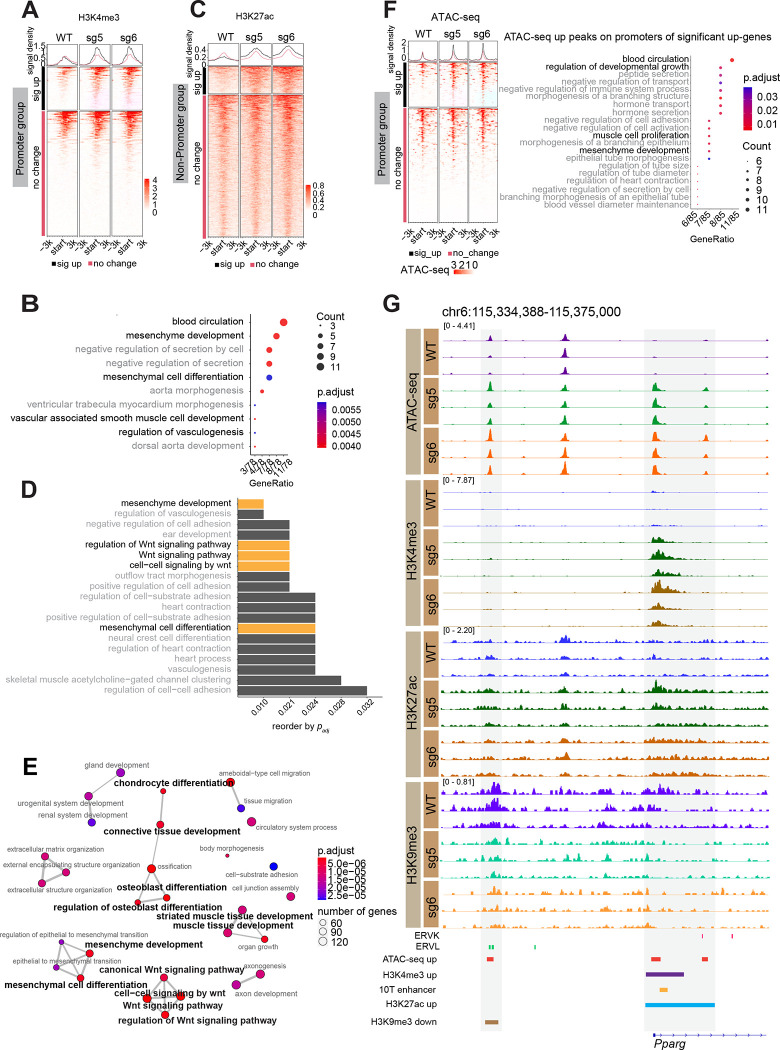
To further characterize the regions in which H3K9me3 was lost, we first used ChromHMM to annotate chromatin states across the whole genome using CUT&RUN-derived data for H3K4me3, H3K27ac, H3K9me3, and H3K27me3 in Atrx WT MPCs (Fig. 3A, S3F, S4) [36]. We found that H3K9me3 was enriched in a state marked by H3K9me3 alone (State 4, Supplementary Table 9), annotated as heterochromatin, as well as in a small subset of regions marked by H3K27me3 and H3K9me3 (State 3, Supplementary Table 8), annotated as repressed chromatin (Fig. 3A). We then compared H3K9me3 CUT&RUN signal within the regions that make up the states containing H3K9me3 in Atrx KO and WT MPCs and found that H3K9me3 was reduced in the Atrx KO MPCs at these sites (Fig. 3B, 3C). Genes associated with lost H3K9me3 are notably related to cell development and differentiation.
Figure 3: ATRX deficiency reduces H3K9me3 near developmental genes and ERVs in mesenchymal progenitor cells.
(A) Chromatin states defined by enrichment of histone modifications (H3K4me3, H3K9me3, H3K27ac, and H3K27me3) using ChromHMM. The heatmap shows the emission probabilities of histone modifications in chromatin states. The ten different states. (B) The range-based heatmap shows the H3K9me3 signal in repressed regions (State 3) (left) and (C) heterochromatin regions (State 4) (left). All peaks are similarly scaled, where “start” and “end” indicate the start and end of the scaled peak. The dot plot shows the GO analysis (biological process) of genes associated with H3K9me3 in each chromatin state (right). (D) Percentage of H3K9me3 lost peaks (p<0.05) associated genes (closest gene to peak) that do (“yes”) or do not (“no”) overlap with up-regulated genes (log2foldchange > 1 & padj < 0.05). (E) Heatmap of H3K9me3 signal at repetitive regions in Atrx WT vs KO MPCs derived by SQuIRE analysis of three biologic replicates. Bold indicates elements of interest (see main text). (F) IGV tracks show a representative H3K9me3 differential peak at an IAPEzint ERV element. The yellow bar indicates a differential H3K9me3 peak. The green bar shows the mouse IAPEz-int element. Within each sample genotype, each track represents an independent biologic replicate. (G) Percentages of each family of TE up-regulated in Atrx KO vs WT MPCs based on an rRNA-depletion sequencing dataset. The up-regulated transposable elements were mapped at an individual locus level. (H) Percentage of up-regulated genes in polyA-RNA-seq data that annotated by up-regulated TEs (TE method by locus).
Next, to better understand the consequences of this observation on gene regulation, we examined the connection between H3K9me3 loss and changes in gene expression. Of the 328 significantly upregulated genes in Atrx KO MPCs, 19.5% (64/328) were associated with peaks that had significantly less H3K9me3 signal in the ATRX deficient line (Fig. 3D). GO pathway analysis of these genes revealed enrichment of gene sets related to adipocyte differentiation, including lipid localization, fat cell differentiation, and brown fat cell differentiation (Supplementary Table 10). These genes included key adipogenic regulators and markers such as Fabp4 and Pparγ, which are part of the fat cell differentiation GO gene set (Supplementary Table 10). In addition, another gene in the same gene set, Lpl, has roles in adipogenic metabolism by mediating hydrolysis of circulating lipoprotein particles [37]. Examination of integrated genomic viewer (IGV) tracks in a putative Fabp4 regulatory element demonstrates H3K9me3 depletion in Atrx KO cells (Fig. S3G).
These observations indicate that ATRX deficiency reduced the heterochromatic histone mark H3K9me3 at genomic regions that were associated with genes relevant to linage-specific differentiation and development. These data suggest that H3K9me3 loss may contribute to gene expression changes in ATRX deficient MPCs, leading to their enhanced capacity for adipogenic differentiation.
Atrx KO reduces H3K9me3 at ERV repetitive regions
ATRX and the ATRX-associated H3K9 methyltransferase, SETDB1, promote H3K9me3 deposition and silencing at repetitive elements [5]. These sequences include transposable elements (TEs) such as endogenous retroviral elements (ERVs), which are a type of long terminal repeat derived from integrated retroviral elements [38]. De-repression of ERVs can lead to formation of dsRNA, which is detected by sensors that in turn stimulate innate immune signaling, which has been implicated as a mediator of antitumor immune response [39–42]. Separately, ERVs can act as gene regulatory elements with enhancer-like features and as transcription factors binding sites to regulate gene expression [43–45]. Given that H3K9me3 is depleted in heterochromatin regions in Atrx KO MPCs, we sought to determine how this affected ERV expression and gene regulation in this system.
We compared H3K9me3 enrichment at repetitive elements between Atrx KO and WT MPCs. We found that H3K9me3 signal was reduced by deletion of ATRX on multiple types of TEs, including ERV family members (ERV1, ERVK), Alu, ID and B2 (Fig. 3E). To further define the relationship between H3K9me3 loss at repetitive elements and differentiation phenotypes in Atrx KO lines, we mapped the repetitive elements onto the previously defined chromatin states (Fig. 3A). While most of repetitive elements were in quiescent (State 6) regions devoid of detectable chromatin marks (Fig. S5A), outside of these regions ERV1 and ERVK elements preferentially mapped to heterochromatin regions where H3K9me3 was reduced in Atrx KO vs WT MPCs (State 4) (Fig. S5B).
To identify specific subfamilies of ERVs where H3K9me3 is depleted in Atrx KO MPCs, we analyzed the H3K9me3 differential peaks by adapting SQuIRE analysis, a RNA-seq analysis pipeline that provides a quantitative and locus-specific information on TE expression [46]. The top reduced regions of H3K9me3 in both Atrx KO lines is an IAP element (Fig. S6A, S6B, Supplementary Table 11,12), which is a member of the ERVK family. Loss of H3K9me3 in Atrx KO vs. WT at a representative region containing an ERVK (IAPEzint) element can be appreciated by review of IGV tracks (Fig. 3F).
To determine if TEs are transcriptionally de-repressed in the setting of ATRX-dependent H3K9me3 reduction, we performed RNA-sequencing following rRNA-depletion and analyzed locus-specific repetitive element differential expression between Atrx KO and WT MPCs using SQuIRE. We identified 116 significant (padj <0.05) upregulated TEs in common between both Atrx KO clones (Supplementary Table 13). Among these upregulated TEs, 42 (36.2%) belong to the ERV superfamily (Fig. 3G), which is the largest subset of upregulated TEs.
To investigate whether de-repressed TEs correlated with nearby gene expression, we analyzed the intersection of upregulated TEs with significantly upregulated genes. We found that the 116 significant upregulated TEs were annotated by 136 genes (using the TE transcript by locus method, Supplementary Table 14). Among those genes, 29 genes (21.3%) were significantly upregulated as measured by RNA-seq (Fig. 3H). The adipocytic lineage transcription factor, Pparγ, was observed in this gene set, raising the possibility that the associated TE could serve as a regulatory element for Pparγ. In addition, we observed 445 significantly downregulated TEs near 163 genes, of which 30 genes were also downregulated (Fig. S6C, Supplementary Table 15). These results demonstrate that Atrx KO leads to reduction of H3K9me3 on specific TEs including those in regions, which potentially regulate an adipogenesis master regulator.
Loss of ATRX promotes chromatin accessibility and enrichment of active histone modifications at developmental genes
ATRX regulates euchromatin as well as heterochromatin [47], leading us to investigate whether the dysregulation of developmental transcriptional programs in MPCs that we see in Atrx KO was connected to changes in active histone modifications, such as H3K4me3 and H3K27ac. We hypothesized that loss of ATRX leads to gain of H3K4me3 in specific promoters and H3K27ac in enhancer-like regions. First, we investigated the distribution of H3K4me3 and H3K27ac in Atrx WT and KO MPCs and identified genomic features of differential peaks of H3K4me3 and H3K27ac in Atrx KO. As expected, H3K4me3 gained peaks in Atrx KO clones are highly represented at promoters (Fig. S7A, Supplementary Table 16). Enrichment of H3K27ac was mainly in introns and distal intergenic regions in Atrx KO lines (Fig. S7B, S7C, Supplementary Table 17).
Next, we examined the relationship between distribution of H3K4me3 at promoter regions and transcriptional profiles of those genes. Interestingly, less than half of genes upregulated in Atrx KO cells gain H3K4me3 at their promoters (Fig. 4A) suggesting that other factors may mediate the increased expression in the group where H3K4me3 does not change. Focusing specifically on genes that gain H3K4me3 and are upregulated in an ATRX-dependent fashion, GO pathway analysis revealed genes related to mesenchymal differentiation and which included the adipogenesis master regulator, Pparγ (Fig. 4B, Supplementary Table 18).
Figure 4: Loss of ATRX alters active chromatin marks and perturbs chromatin accessibility to induce expression of a key adipogenic regulator.
(A) H3K4me3 signal around promoters of significantly upregulated genes grouped by whether H3K4me3 is gained (black bar) or unchanged (red bar) (B) GO terms (biological process) associated with H3K4me3 gained regions. (C) H3K27ac signal +/− 3 kb around the center of H3K27ac peaks that do not overlap promoters grouped by whether H3K27ac is gained (black bar) or unchanged (red bar) (D) GO analysis (biological process) of H3K27ac significantly increased regions. (E) Network plot of gene programs associated with increased accessibility in Atrx KO lines. The terms labeled with bold black font indicate those related to the mesenchymal lineage. (F) Signal of ATAC-seq +/− 3 kb around the transcription start site (TSS) of genes that have significantly increased expression in Atrx KO MPCs and have an ATAC-seq peak in the TSS region. The regions are grouped based on whether the overlapping ATAC peak has significantly increased signal (black bar) or is unchanged (red bar). The dot plot shows the GO terms associated with upregulated genes. (G) IGV tracks of CUT&RUN and ATAC-seq replicates showing a region near the promoter of the Pparγ gene. Enhancer annotation is from EnhancerAtlas 2.0 database (http://www.enhanceratlas.org/) [52] and converted to the mouse mm10 genome.
We next examined the distribution of H3K27ac, focusing on non-promoter regions near upregulated genes since in these regions, H3K27ac is commonly attributed to active enhancers. Similar to our findings with H3K4me3 in ATRX deficient cells, H3K27ac gain was seen in a minor subset of upregulated genes (Fig. 4C). However, these genes again were enriched in GO terms related to mesenchymal development, including Wnt signaling, which is important for mesenchymal differentiation [48–50] (Fig. 4D, Supplementary Table 19). Thus, aberrant enhancer activation following ATRX loss could contribute to the pro-differentiation phenotype in Atrx KO MPCs.
Our data suggests that Atrx KO in MPCs leads to changes in both the heterochromatic histone mark H3K9me3 and the euchromatic marks H3K4me3 and H43K27ac. Given the connection of these posttranslational modifications with chromatin compaction, we speculated that ATRX deficiency would lead to a shift in chromatin accessibility. To test this, we performed ATAC-seq, which demonstrated increased accessibility in Atrx KO cells at genes associated with mesenchymal development and differentiation (Fig. 4E, Supplementary Table 20). To investigate the connection of increased chromatin accessibility driven by ATRX deficiency and gene expression changes in Atrx KO clones, we separated ATAC-seq peaks at promoters of significantly upregulated genes (Supplementary Table 21) from those at non-promoter regions near or in the same genes (Supplementary Table 22) (Fig. 4F). Similar to H3K4me3, while a minority of the upregulated genes showed increased accessibility at their promoters (Fig. 4F), these genes were associated with development. The observation that a group of genes increased expression but did not gain chromatin accessibility suggests, as is in the analogous case of upregulated genes that did not gain H3K4me3, that non-chromatin mechanisms may account for transcriptional changes upon deletion of ATRX.
To understand the relationship between heterochromatin changes and increased chromatin accessibility in ATRX deficient MPCs, we intersected the ATAC-seq gained peaks (8742 peaks) that overlapped with regions showing decreased H3K9me3 (4568 peaks) and identified 170 overlapping regions (Fig. S8A). Despite this small number of overlapping peaks, GO analysis suggested that this subset was highly enriched for programs related to development including in along the mesenchymal lineage (Fig. S8B, Supplementary Table 23). These results demonstrate that the increased chromatin accessibility coupled with a reduction in H3K9me3 at specific genes in Atrx KO MPCs is associated with altered gene expression and a pro-differentiation phenotype in Atrx KO MPCs.
ATRX deficiency induces an active chromatin state at the promoter and putative regulatory element of the adipogenic transcription factor Pparγ
ATRX loss leads to an aberrant increase in expression of Pparγ (Fig. 1E), which is an important adipogenic transcription factor [51]. In addition, in functional assays for adipocytic differentiation, PPARγ protein levels are markedly induced in Atrx KO MPCs immediately after induction of adipogenic differentiation (Fig. 2B). In order to understand how these phenotypes are functionally linked to the epigenetic changes driven by ATRX deficiency, we investigated ATRX-dependent changes in the chromatin state near the Pparγ gene. In Atrx KO MPCs the promoter of Pparγ showed increased accessibility and increased H3K4me3 and H3K27ac, suggesting a more active chromatin state (Fig. 4G). Notably, previous work has identified a Pparγ enhancer element in this same region [52] (Fig. 4G, Supplementary Table 24), suggesting multiple mechanisms by which these chromatin changes could enhance Pparγ expression. In addition, we observed a loss of H3K9me3 and increased accessibility at an ERVL element 20 kb upstream from the Pparγ promoter, raising the possibility that it functions as an ATRX-dependent gene regulatory element for Pparγ.
ATRX associates with accessible and active chromatin in MPCs.
To understand whether direct ATRX binding could influence the gene expression and chromatin changes observed in Atrx KO MPCs, we mapped ATRX binding sites using CUT&RUN. At telomeric regions, where ATRX is known to function with DAXX to deposit H3.3 and bind to G-quadruplex structures [6], ATRX signal was decreased in both knockout clones compared to WT (p < 0.05) (Fig. S9A). As an additional control, we detected ATRX peaks at zinc finger gene clusters (Fig. S9B), where ATRX is known to bind [53] that were lost in the ATRX KO MPC.
ATRX enrichment at the sites of active transcription start sites (TSS) was recently reported in lymphoblastoid cell lines, suggesting that ATRX has an important role in euchromatin where it regulates gene expression in addition to its more established role at heterochromatin [47]. This is consistent with MPC ATRX peaks identified here (n=114; padj < 0.05) (Fig S9C, Supplementary Table 25, 26), where we observed that of the 114 ATRX binding sites, 33.3% were located at promoter regions, and 38.6% were in distal intergenic regions (Fig. 5A). The remainder associated with 5’UTRs (5.3%), exons (7.9%), 3’UTRs (2.6%), and introns (12.3%). Mapping ATRX binding sites onto the ChromHMM-defined chromatin states revealed an association with active transcription start sites (TSS, State 8) (Fig. 5B). Comparing the ATRX binding sites to Atrx WT ATAC-seq peaks, we found that 87.7% (100/114) of ATRX binding sites overlapped with chromatin accessible regions (Fig. 5C, Supplementary Table 27). ATRX binding corresponded to H3K4me3 enriched regions in 70.1% of sites (80/114) (Fig. 5D, Supplementary Table 28) and with H3K27ac in 81.5% (93/114) (Fig. 5E, Supplementary Table 29). Interestingly, in the intersection of H3K27ac occupied regions with ATRX binding sites, 66.7% (62/93) were on non-promoter regions, suggesting a potential role for a subset of ATRX at active enhancers in MPCs (Fig. S9D, Supplementary Table 30).
Figure 5: ATRX binding to active chromatin.
(A) ATRX binding sites by genomic feature. (B) Overlap of ATRX binding sites with chromatin states excluding state 5 (artifact/blacklist). x-axis indicates the level of ATRX binding calculated by: (number of ATRX peaks that overlap state bins)/(total bins with that state) (C) The percentage of ATRX peaks that overlap ATAC-seq peaks. (D) The numbers of ATRX peaks that overlap H3K4me3 peaks in Atrx WT MPCs. (E) The numbers of ATRX peaks that overlap H3K27ac peaks in Atrx WT MPCs (F) ATRX signal near TSS in gene groups binned based on expression levels. The genes were grouped as follows: “no expression” indicates that TPM (Transcripts Per Million) = 0, “very low expression” indicates the quintile of TPM between 0–20%, “low expression” indicates the quintile of TPM between 20–40%, “moderate expression” indicates the quintile of TPM between 40–60%, “high expression” indicates the quintile of TPM between 60–80%, and “very high expression” indicates the quintile of TPM between 80–100%. (G) IGV tracks at the Fam46a gene region. (H) Heatmap of ATRX signal over repetitive elements.
Given that ATRX peaks were often localized to open chromatin and active promoters, we explored the relationship between ATRX binding and gene expression. Genes were binned based on expression and ATRX signal was quantified around the TSS of genes in each group (Fig. 5F). The most highly expressed genes had the greatest ATRX signal, whereas non-expressed genes had no ATRX enrichment at the TSS. For example, Fam46a, which is known to downregulated in differentiating adipocytes [54] and was significantly downregulated in Atrx KO MPCs (Fig. S9E) has an ATRX binding site at its promoter region (Fig. 5G). This association of ATRX with active genes is similar to a recent report in a hematopoietic lineage [47] and suggests that ATRX may play a role in maintaining and potentially establishing gene expression in the mesenchymal cells. However, given that only a subset of ATRX binding sites correlate with changes in gene expression in Atrx KO MPCs (Supplementary Table 28), there are likely other mechanisms by which ATRX regulates transcription. One possibility may be an indirect mechanism via binding and chromatin-mediated regulation of transposable elements which in turn influence transcription. In support of this possibility, we observed that ATRX binds several families of repetitive elements, including SINEs (Alu, ID, B2, MIR) and Satellite DNAs (Fig. 5H), a finding that was confirmed by a second analysis method (Fig. S9F).
Discussion
The mesenchymal lineage gives rise to connective tissues. Sarcomas, which are cancers of connective tissues, have recurrent loss of ATRX in up to 30% of specific subtypes, suggesting the potential importance of ATRX deficiency in sarcoma biology and possibilities for precision medicine approaches to treatment [7]. However, while ATRX loss is known to contribute to the alternative lengthening of telomere phenotype in sarcomas, the functions of ATRX in the epigenetic regulation of gene expression and downstream processes in the mesenchymal context is less understood.
Given the important role for ATRX regulating epigenetic states and the intersection of epigenetics with development, we hypothesized that loss of ATRX would affect cell commitment in mesenchymal progenitor cells. We demonstrated that the Atrx KO MPCs were sensitive to differentiation induction and exhibited reduced progenitor properties. At the transcriptional level, ATRX loss increased expression of adipogenesis regulators and markers, including Pparγ, which is an early and direct master regulator of adipogenesis [51]. We then investigated ATRX mediation of these phenotypes through epigenetic changes, with a focus on several potential and non-exclusive mechanisms (Fig. 6).
Figure 6: Model for ATRX-dependent chromatin and gene regulation in MPCs.
Atrx KO MPCs demonstrate reduced stem-like properties, such as slower proliferation, reduced colony formation and downregulated mesenchymal stemness gene expression. In adipogenic media, Atrx KO MPCs exhibit accelerated adipocytic differentiation and expression of adipogenic transcription factors. We propose a potential model in which these phenotypes are regulated by multiple epigenetic mechanisms at developmental genes including loss of H3K9me3 near promoters and at ERVs with putative cis-regulatory functions, gain of chromatin accessibility, and enrichment of active histone marks. In addition, loss of direct binding of ATRX, which is associated with high levels of genes expression, may contribute. Together, these mechanisms could explain gene expression changes in ATRX deficient MPCs, which in turn mediate cell differentiation in the mesenchymal lineage context.
First, ATRX deficiency promotes chromatin accessibility and increased transcription at genes associated with mesenchymal differentiation including Pparγ and Fabp4. Whether ATRX regulates chromatin accessibility directly or indirectly remains an interesting question to be addressed in future work. Second, the loss of ATRX leads to changes in histone posttranslational modifications at specific loci, including the heterochromatin mark H3K9me3 and active chromatin marks, H3K4me3 and H3K27ac. The genes associated with H3K9me3-depleted heterochromatin regions in ATRX-deficient cells are relevant for differentiation and development. This suggests that loss of this repressive mark may create a chromatin environment permissive to signals that activate these programs, which is consistent with the accelerated and increased differentiation phenotype observed in Atrx KO MPCs following simulation with adipogenic media. In addition, ATRX deficiency leads to the loss of H3K9me3 at ERVs, which could represent another potential mechanism for the transcriptional changes induced by ATRX loss given that ERVs can serve as cis-regulatory elements. Finally, histone marks associated with active transcription were also altered upon ATRX loss. We observed increased H3K4me3 at promoters and H3K27ac at putative enhancer (i.e., non-promoter) regions near significantly upregulated genes. This suggests that increased H3K4me3 and H3K27ac may also contribute to the induction of mesenchymal development programs, such as Wnt-associated signaling [48–50].
Third, ATRX may regulate gene expression through direct interactions with active chromatin. Recent reports in lymphoblastoid cell lines shows that 38% of ATRX binding sites are localized to promoters [47], which is consistent with our results demonstrating that approximately one-third of ATRX binding sites in MPCs occur at promoters. In contrast, ATRX binding sites in murine embryonic stem cells are mainly restricted to distal intergenic regions and gene bodies, with only 1% on promoters [55]. While in murine neuroepithelial progenitors, 17% of ATRX binding sites were located in promoters [55]. Therefore, ATRX binding sites differ depending on the cell and developmental contexts with a trend towards increased association with promoters in lineage specific progenitors compared to less differentiated stem cells. Our findings demonstrate that ATRX is not only required for maintaining heterochromatin, but that it may also regulate gene expression through binding to active chromatin regions, consistent with this recently discovered role for ATRX. In our system, ATRX binding was associated with increased gene expression. However, whether ATRX occupancy directly drives transcriptional upregulation is unclear since only a subset of genes near ATRX binding sites are downregulated following ATRX loss. We speculate that ATRX binding might regulate gene expression changes through a distal enhancer mechanism in the case of ATRX binding sites that do not influence expression of nearby genes. In support of this, ATRX signal was decreased at several types of SINEs in Atrx KO MPCs, including Alu, B2, B4, ID, and MIR, which act as transcription factor binding sites (Alu[56], MIR[57]) or harbor promoter-like (B2) [58] or enhancer-like features (B4) [59], respectively.
Our findings demonstrate that ATRX restricts differentiation in mesenchymal progenitor cells. Deletion of ATRX leads to aberrant de-repression of the lineage-restricted transcriptome, accompanied by changes in chromatin accessibility and histone post-translation modifications near transposable elements and at specific genes associated with differentiation. These findings raise several questions including whether the changes observed in active histone marks and chromatin accessibility after Atrx KO is a direct or indirect effect and how these findings are relevant to mesenchymal malignancies where ATRX loss is common (e.g., undifferentiated pleomorphic sarcoma and leiomyosarcoma). One intriguing possibility is that ATRX loss creates a permissive chromatin and transcriptional context in which mesenchymal progenitors are susceptible to subsequent sarcomagenic events such as loss of TP53 and RB1 [7]. Overall, our results expand our understanding of ATRX function in in the mesenchymal context.
Methods
Cell culture.
C3H/10T1/2 (CCL-226) cells (MPCs) were obtained from ATCC. Except where otherwise stated for specific experiments, cells were cultured in DMEM (Corning, 10-013-CV) plus 10% FBS (ATLANTA biologicals, S11150, heat inactive) with 1% penicillin/streptomycin (Gibco, 15140-122) at 37-degree Celsius culture condition with 5% CO2. The parental cell line was tested for mycoplasma and authenticated by ATCC.
CRISPR-Cas9 cloning.
The vector for CRISPR-cas9 pSpCas9(BB)-2A-GFP (PX458) (Addgene, #48138) was obtained from Addgene. Mouse Atrx sgRNAs were designed using Benchling (https://www.benchling.com/). Target sequences: sg5: 5’ TGGCCGTAAAAGTTCTGGGG-3’, sg6: 5’-CTACTGGACTTGGTGACTGC-3’. The control group was generated using an empty vector without sgRNA but carrying Cas9. The vector was digested by Bbsl (NEB, R0539S) and dephosphorylated by calf intestinal alkaline phosphatase (NEB, M0290). The oligos were annealed with T4 ligation buffer (NEB, B0202S) with T4 PNK (NEB, M0201L) enzyme. The ligation step was performed using Quickligation buffer (NEB, B2200S) and Quick Ligase (NEB, M2200L). The ligation products were expressed in Stbl3 competent cells (Thermofisher, C737303).
Transient transfection.
Two micrograms of plasmid products carrying sgRNAs with Cas9 were transfected using Lipo2000 (Thermofisher, 11668019) into 0.7 × 104 MPCs cells. The transfection procedure was according to the manufacturer’s instructions. Cells were harvested 48 h after transfection for sorting and sorted into 96 well plates using BD FACSAria II Cell Sorter based on GFP signal.
Proliferation assay.
50 Atrx WT and Atrx KO cells were grown in 96-well plates (Costar, 3917) for 5 days. Proliferation was evaluated using the CellTiter-Glo Luminescent Cell Viability Assay kit (Promega, G7572), following the manufacturer’s instruction. The luminescence was read immediately in 96-well plate reader (BioTek, Synergy hybrid H4 Reader).
Colony formation.
100 C3H/10T1/2 cells (WT and KO clones) were seeded in 10 cm dishes, cultured in α-MEM (Corning, 10-009-CV) with 20% FBS (ATLANTA biologicals, S11150), with 1% penicillin/streptomycin (Gibco, 15140-122). Cultures were incubated at 37-degree Celsius with 5% CO2 for 10–14 days. When visible colonies formed, colonies were washed once with PBS. Cells were stained with 0.5% crystal violet (SIGMA-ALDRICH, C3886) in 4% formaldehyde (Fisher chemical, F79-500) for 1 h at RT. After removing the staining buffer, cells were washed with ddH2O for 3–5 minutes to remove the background staining and then digitally imaged (Epson Perfection V800 photo). Colonies were manually counted. A group of cells had to contain at least 50 cells to be considered a colony.
Adipogenic differentiation.
C3H10T1/2 were differentiated into mature adipocytes following treatment with insulin, dexamethasone, troglitazone, and methylisobutylxanthine per an established protocol [29]. 1 × 105 C3H/10T1/2 were seeded in 24-well plates such they were fully confluent. Adipocyte differentiation medium [29] consisting of DMEM (Corning, 10-013-CV) with 10% heat inactive FBS (ATLANTA biologicals, S11150), 0.5 mM isobutyl methylxanthine (Sigma, I7018), 1 μM dexamethasone (sigma, D4902), 5 μg/mL insulin (Sigma, I9278), 5 μM troglitazone (Sigma, T2573) was used to replace the media the following day. After 2 days, the media was changed every 2 days with insulin and FBS the only additive to the DMEM. Differentiation was assessed by Oil Red O after day 7.
Oil Red O staining.
The Oil Red O staining was performed as previously described [29]. 0.5% w/v Oil Red O stock (Sigma, O0625) in 100% isopropanol (Fisher chemical, A416SK-4) was prepared fresh. A working solution was prepared by mixing contained 6 mL of stock solution and 4 mL of H2O, followed by filtering (0.45 μM, Pall Corporation syringe filter, PN-4614) to remove precipitate. Cells were washed by PBS. Cells were fixed with 4% formaldehyde in PBS for 2 minutes at room temperature, followed by washing with H2O. Next, 220 μL Oil Red O working solution was added to the wells and incubated cells for 1 h at room temperature. Then cells were washed twice with 0.5% isopropanol to remove background staining. Plates were digitally imaged (Epson Perfection V800 photo) before the Oil Red O was solubilized by adding 650 μL of 100% isopropanol to the stained cells, which were incubated for 20 minutes on a shaker. The eluate was transferred into a 96 well plate (Corning, 3361) and the absorbance measured at 500 nm with 96-well plate reader (BioTek, Synergy hybrid H4 Reader).
Immunofluorescence.
800 C3H/10T1/2 cells were seeded in a 96 well plate (glass bottom culture plates, MatTek, PBK96G-1.5-5-F) and keep cells in 37-degree for 24 h. Cells were fixed with 4% formaldehyde in PBS for 10 minutes at room temperature. Cells were permeabilized with 0.2% digitonin (EMD Millipore, 300410) in PBS (Corning, 21-040-CV) for 10 minutes at room temperature. Next, the cells were incubated in 3% BSA (in PBS, Sigma, #9418) for blocking for 1 h at room temperature followed by incubation in primary antibody (ATRX, Santa Cruz, sc-15408, 1:200) at 4-degree overnight, followed by secondary antibody (1:600, Invitrogen, A32754) incubation at room temperature for 1 h. Cells were washed three times with PBS for 5 minutes followed by DAPI staining (2 μg/ml in PBS, Sigma, D9564) for 5 minutes at room temperature. Mounting media (Vectashield, H-1000) was added immediately after DAPI staining. Cells were imaged using Widefield Microscope CellDiscoverer7 (CD7) automated widefield high-throughput system (Zeiss). Images were processed with ImageJ software (http://rsb.info.nih.gov/ij/). For the ATRX antibody, the ImageJ Brightness/Contrast was set as 30/112. For DAPI, the Brightness/Contrast was set as 37/123. All images were processed with the same parameter settings for each antibody.
Protein isolation and Western Blot.
Cell pellets were lysed in lysis buffer NETN (20 mM Tris (pH 7.5), 1 mM EDTA, 150 mM NaCl, 0.5% NP-40, protease inhibitor tablet (Roche), 0.5 mM DTT). Samples were incubated in the cold room for 30 minutes followed centrifugation at 4-degree Celsius with maximum speed for 10 minutes. The supernatant was collected and the concentration determined by BCA quantification (Pierce BCA protein Assay kit, Cat.23225, Thermo scientific) to allow normalization between samples for Western blotting. Samples were mixed with Laemmli Sample Buffer (4X) (containing 1.0 M Tris-pH6.8, 8% SDS, Glycerol, β-Mercaptoethanol (10%), Bromophenol blue) for boiling at 100-degree Celsius for 10 minutes. The samples were then separated by SDS-PAGE and analyzed by standard immunoblotting using running buffer from Invitrogen (NuPAGE™ Tris-Acetate SDS Running Buffer (20X), LA0041) and transfer buffer from Thermofisher (NUPAGE transfer buffer, NP00061). The blotting processes was performed as previous described [26]. Antibodies used for Western blot are the following: ATRX (Santa Cruz, sc-15408, 1:800), FABP4 (R&D, AF1443, 1:5000), C/EBPα (CST, #2295, 1:1000), PPARγ (CST, #2430), β-actin (Abcam, ab8224), GAPDH (Abcam, ab8245, 1:1000).
Reverse Transcriptase Quantitative PCR (RT-qPCR).
For RT-qPCR, RNA was prepared with RNeasy Mini kits (Qiagen, 74104). The RNAs concentration was determined using a Nanodrop (Spectrophotometer, ND-1000). cDNA was prepared published procedures [26]. qPCRs were performed using SYBR green PCR master mix (Applied Biosystems, 4367659). The detailed steps are following previous described [26]. The endogenous control gene was 18S. The sequences of primers are as following: mouse-Etv1: F:5’-GTTTGTTCCAGACTATCAGGCTG-3’, R: 5’-GGGCTGTGGGGTTCTTTCTT-3’. mouse-18s: F:5’-GTAACCCGTTGAACCCCATT-3’, R: 5’-CCATCCAATCGGTAGTAGCG-3’. The statistical analysis was performed using a one-sample, two-sided t-test.
PolyA-RNA-seq and data analysis.
Approximately 1 million cells were collected from 10 cm dishes. RNA extraction, polyA selection, library preparation, and RNA sequencing were performed at the MSKCC integrated Genomics Operation. An average of 30–40 million paired reads was generated per sample. We used the log2foldchange > 1 or < −1 with padj<0.05 as the threshold for significant changed genes. Quality control of FASTQ files was performed using the Rfastp R Bioconductor package (v0.1.2). Full genome sequence and transcript coordinates for the mm10 UCSC genomes and gene models were retrieved from the R Bioconductor packages BSgenome.Mmusculus.UCSC.mm10 (v1.4.0) and TxDb.Mmusculus.UCSC.mm10.knownGene (v3.4.0). Transcript abundance was determined from FASTQ files using Salmon (v0.8.1) [60], and transcript counts and TPM values were imported into R with the tximport R Bioconductor package (v1.8.0) [61]. Differential gene expression was performed with the DESeq2 R Bioconductor package (v1.20.0) [62]. For plots comparing the read counts of genes between samples, counts were normalized by dividing the raw read counts by the size factors for each sample as determined by DESeq2.
rRNA-depletion RNA-seq and data analysis.
Approximately 1 million cells were collected from 10 cm dishes. RNA extraction, rRNA depletion, library preparation, and RNA sequencing were performed by Novogene. An average of 30 million paired reads was generated per sample. Quantification of rRNA-depleted RNAseq reads over individual TE loci was performed using either the SQuIRE pipeline (v0.9.9.9a-beta - https://github.com/wyang17/SQuIRE) [46] or TE local (v1.1.1 - https://github.com/mhammell-laboratory/TElocal). The SQuIRE pipeline was used for alignment, counting, and calling of differential TEs. When using TElocal, reads were aligned with STAR (v2.27.10a) setting the ‘—winAnchorMultimapNmax’ and ‘—outFilterMultimapNmax’ arguments to 100[63], followed by counting with the TElocal function and differential abundance calculated with DESeq2 (v1.32.0). Reads were aligned to the mm10 genome sequence from the BSgenome.Mmusculus.UCSC.mm10 R Bioconductor package (v1.4.0).
CUT&RUN.
0.5 million cells from each line were collected. The detailed protocol for CUT&RUN for histone marks (H3K4me3, H3K27ac, H3K9me3, H3K27me3) and H3.3 are following previous described [34] with following modifications: final concentration of digitonin: 0.05%. Antibodies for CUT&RUN are following: H3.3 (Active Motif, 91191), H3K9me3 (Abcam, ab8898), H3K4me3 (Active Motif, 39159), H3K27me3 (Cell Signaling Technologies, #9733), H3K27ac (Active Motif, 39133), Rabbit IgG (Diagenode, C15410206). For ATRX CUT&RUN experiment, cells were fixed using 0.1% PFA in PBS for 1 minute at room temperature, followed by nuclei extraction with NE buffer (20 mM HEPES (pH7.9), 10 mM KCl, 0.1% Triton X-100, 20% Glycerol, 1 mM MnCl2, 1×cOmplete Mini-Tablet (1 tablet), 0.5 mM Spermidine) according to the protocol from EpiCypher (v2.0, https://www.epicypher.com/resources/protocols/cutana-cut-and-run-protocol/). The final concentration of digitonin buffer for ATRX CUT&RUN was 0.01%. For each sample, 2 μg ATRX antibody (Abcam, ab97508) was added. Libraries were prepared using the NEB Ultra II DNA library prep kit. Samples were PCR amplified for 14 cycles library pools was sequenced in the genomics core at the Rockefeller university.
CUT&RUN alignment and differential peak analysis.
Quality control of FASTQ files was performed using the Rfastp R Bioconductor package (v0.1.2). CUT&RUN reads were aligned using the Rsubread R Bioconductor package (v1.30.6), and predicted fragment lengths were calculated by the ChIPQC R Bioconductor package (v1.16.2) [64, 65]. The full mm10 genome sequence was retrieved from the R Bioconductor package BSgenome.Mmusculus.UCSC.mm10 (v1.4.0). Normalized, fragment-extended signal bigWigs were created using the rtracklayer R Bioconductor package (v1.40.6) [66]. Range-based heatmaps showing signal over genomic regions were generated using the profileplyr R Bioconductor package (v1.8.1) [67]. Any regions included in the ENCODE blacklisted regions of the genome were excluded from all region-specific analyses [68]. Bedgraph files generated with the deepTools package (v3.5.1) [69] were used for peak calling with SEACR in ‘stringent’ mode (v1.3) [70]. For H3K4me3, H3K27ac, H3K9me3, and H3.3, peaks were ranked based on the signal within peaks, and only the top 1% of peaks were used for downstream analysis. For ATRX, peaks were called compared to an IgG control sample.
Differential enrichment of signal within peaks was performed by counting the overlap of reads over a high confidence consensus peak set. This was obtained by reducing all replicates within all conditions to one peak set, and then keeping the peaks that overlap at least two out of the three replicates for any condition. Reads overlapping these peaks were counted for each replicate using the summarizeOverlaps function from the GenomicAlignments R Bioconductor package (v1.28.0) [71]. Differential peak enrichment between Atrx WT and Atrx KO replicates was then calculated using the DESeq2 R Bioconductor package (v1.32.0). To find differential enrichment in broad genomic regions for the H3K9me3 samples, reads were counted in 20 kb bins across the entire genome, and DESeq2 was used to find bins with enriched signal in the Atrx KO clones. Only bins in the top 10 percent in terms of read count were used for this analysis. The union of these differential bins and the differential SEACR peak regions was determined and used for downstream analysis of regions with enriched H3K9me3 signal in the Atrx KO clones.
Quantification of CUT&RUN signal over repeats at the individual locus and sub family level was performed with SQuIRE. The counts were transformed with rlog from the DESeq2 R Bioconductor package (v1.32.0), and these values were plotted on a heatmap using the ComplexHeatmap R Bioconductor package (v2.6.2) [72]. To assess ARTX CUT&RUN signal in telomeres, FASTQ files were aligned to a DNA sequence of 150 conserved telomere repeats (TTAGGG) using the R Bioconductor package Rbowtie2 (v1.12.0) [73, 74]. The z-scores of the log2(reads per million) were plotted on a heatmap using the ComplexHeatmap R Bioconductor package.
For quantification of ATRX CUT&RUN signal over genes, genes were divided into groups based on their expression levels. TPM values from the Atrx WT RNAseq dataset were calculated by Salmon and were split into five quantiles. The genomic location of each gene was then retrieved from the R Bioconductor package TxDb.Mmusculus.UCSC.mm10.knownGene (v3.10.0). IgG normalized bigwig signal for the ATRX CUT&RUN samples was obtained using the deepTools (v3.5.1) ‘bigwigCompare’ function, with the ‘operation’ argument set to ‘log2’. Signal over the gene regions (separated by RNAseq quantile) was computed with the deepTools computeMatrix function. The signal was visualized with the ggplot2 R package (v3.3.6).
Omni-ATAC-seq.
The samples are prepared according to the methods previous described [75–77] with minor modifications. 50,000 C3H/10T1/2 cells were collected for Omni-ATAC-seq. Amplification was performed with NEBNext High-Fidelity 2× PCR Master Mix (NEB, M0541s) and Nextera PCR Primers (8 cycles). The sequences of PCR primers with index adapters are listed in the Supplementary Table 31. The library pool was sequenced by Rockefeller University genomic core using a NextSeq High Output platform with 75bp paired-end reads in duplicates. An average of 30–40 million paired reads was generated per sample.
ATAC-seq alignment and differential peak analysis.
Quality control of FASTQ files was performed using the Rfastp R Bioconductor package (v0.1.2). ATAC-seq FASTQs were aligned to the mm10 genome from the Bsgenome.Mmusculus.UCSC.mm10 Bioconductor package (version 1.4.0) using Rsubread’s (v1.30.6) align method in paired-end mode with fragments between 1 to 5000 base-pairs considered properly paired [65]. Normalized, fragment signal bigWigs were created using the rtracklayer R Bioconductor package (v1.40.6) [66]. Peak calls for each replicate were made with MACS2 software in BAMPE mode [78].
Differential enrichment of signal within peaks was performed by counting the overlap of reads over a high confidence consensus peak set. This was obtained by reducing all replicates in all conditions to one peak set, and then keeping all peaks that overlap at least two out of the three replicates for any condition. Reads overlapping these peaks were counted for each replicate using the summarizeOverlaps function from the GenomicAlignments R Bioconductor package (v1.28.0) [71]. Differential peak enrichment between Atrx WT and Atrx KO replicates was then calculated using the DESeq2 R Bioconductor package (v1.28.1).
Downstream peak analysis (CUT&RUN and ATAC-seq).
Peaks for both CUT&RUN and ATAC-seq were annotated with nearby genes using the rGREAT R Bioconductor package (v1.24.0) or with the closest gene and type of genomic region (e.g. promoter, intergenic, etc.) using the ChIPseeker R Bioconductor package (v1.28.3) [79–81]. The closest gene is defined as the gene for which the transcription start site is closest to the peak, and rGREAT defines a regulatory region around each gene and then annotates any peak in that region to that gene. For rGREAT annotation, the default settings were used to define regulatory regions of genes, which was 5Kb upstream and 1 kb downstream of each gene, and then extended a maximum of 1 Mb to the regulatory region of the next gene. Gene ontology analysis of genes associated with differential peaks (using the Biological Processes gene lists) was performed with the clusterProfiler R Bioconductor package (v4.0.5) [82]. Overlaps of differential peaks with other peak sets was done with the findOverlapsOfPeaks function from the ChIPpeakAnno R Bioconductor package (v3.26.4) [83]. Range heatmaps of CUT&RUN and ATAC-seq signal were made with the profileplyr R Bioconductor package [84]. For overlap of signal and peaks with enhancers, a peak file with enhancers from 10T cells was downloaded from the EnhancerAtlas 2.0 (http://www.enhanceratlas.org/). The downloaded peaks were converted from mm9 to mm10 using the ‘liftOver’ function from rtracklayer R Bioconductor package and the ‘mm9ToMm10.over.chain’ file downloaded from UCSC.
ChromHMM analysis.
Genome-wide ChromHMM (v1.23) models were generated using CUT&RUN signal for H3K4me3, H3K27ac, H3K27me3, H3K9me3, with IgG samples used as controls. Only the CUT&RUN samples from the Atrx WT cells were used to generate the models.
Specifically, the BinarizeBam function was performed with default settings (mm10 genome), and this output was used in the LearnModel function. To determine the optimal number of states, we employed a previously published method to calculate the ratio of the ‘between sum of squares’ over the ‘total sum of squares’ of kmeans clustered emissions probabilities from all states of all models, considering 2–16 states [85]. The value of K at which the sum of squares ratio crossed 95% of the maximum was the 10 State model. To determine overlap of ChromHMM states with repeats, repeats from RepeatMasker were downloaded from UCSC (downloaded on Feb 4, 2022). To compare levels of overlap between states, the number of repeats that overlap a state was divided by the total number of regions in that state. A z-score of this value across all states was then plotted in a heatmap using the ComplexHeatmap R Bioconductor package (v2.6.2). A similar strategy was employed to measure ATRX peak overlap with ChromHMM states. H3K9me3 signal in specific states was quantified and visualized with a ranged heatmap using the profileplyr R Bioconductor package.
Statistics.
Statistical analysis for proliferation, colony formation and qPCR were performed as indicated in the figure legends using paired or unpaired, two-tailed t-test, or one-way ANOVA. The analysis was not blinded.
Supplementary Material
Acknowledgments
We are very grateful to Prof. C. David Allis (CDA), who unfortunately passed away in January 2023, for his support, invaluable suggestions, and insight. We also thank Allis lab members for suggestions and advice. We also acknowledge Samer Shalaby from the Flow Cytometry Resource Center at the Rockefeller University for the cell sorting assistance. The CUT&RUN sequencing and Omni-ATAC sequencing were performed by Rockefeller University genomic core. This work was supported by a Connective Tissue Oncology Society Basic Science Research Award (BAN, CDA, WDT), NCI K08 CA245212 (BAN), the Memorial Sloan Kettering Cancer Center Support Grant (P30 CA008748), and the University of Pittsburgh Hillman Cancer Center Support Grant (P30CA047904).
Footnotes
Conflict of interest
The authors declare no competing interests.
Data availability
All genomic and transcriptional data will be deposited in the Gene Expression Omnibus (GEO) at the time of publication.
References
- 1.Xue Y.T., et al. , The ATRX syndrome protein forms a chromatin-remodeling complex with Daxx and localizes in promyelocytic leukemia nuclear bodies. Proceedings of the National Academy of Sciences of the United States of America, 2003. 100(19): p. 10635–10640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gibbons R.J., et al. , Mutations in the chromatin-associated protein ATRX. Human Mutation, 2008. 29(6): p. 796–802. [DOI] [PubMed] [Google Scholar]
- 3.Gibbons R.J., et al. , Identification of acquired somatic mutations in the gene encoding chromatin-remodeling factor ATRX in the alpha-thalassemia myelodysplasia syndrome (ATMDS). Nature Genetics, 2003. 34(4): p. 446–449. [DOI] [PubMed] [Google Scholar]
- 4.Nacev B.A., et al. , The epigenomics of sarcoma. Nature Reviews Cancer, 2020. 20(10): p. 608–623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sadic D., et al. , Atrx promotes heterochromatin formation at retrotransposons. EMBO Rep, 2015. 16(7): p. 836–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lewis P.W., et al. , Daxx is an H3.3-specific histone chaperone and cooperates with ATRX in replication-independent chromatin assembly at telomeres. Proceedings of the National Academy of Sciences of the United States of America, 2010. 107(32): p. 14075–14080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nacev B.A., et al. , Clinical sequencing of soft tissue and bone sarcomas delineates diverse genomic landscapes and potential therapeutic targets. Nature Communications, 2022. 13(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cancer Genome Atlas Research Network. Electronic address, e.d.s.c. and N. Cancer Genome Atlas Research, Comprehensive and Integrated Genomic Characterization of Adult Soft Tissue Sarcomas. Cell, 2017. 171(4): p. 950–965 e28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liau J.Y., et al. , Comprehensive screening of alternative lengthening of telomeres phenotype and loss of ATRX expression in sarcomas. Mod Pathol, 2015. 28(12): p. 1545–54. [DOI] [PubMed] [Google Scholar]
- 10.Liau J.-Y., et al. , Leiomyosarcoma With Alternative Lengthening of Telomeres Is Associated With Aggressive Histologic Features, Loss of ATRX Expression, and Poor Clinical Outcome. The American Journal of Surgical Pathology, 2015. 39(2): p. 236–244. [DOI] [PubMed] [Google Scholar]
- 11.Li F., et al. , ATRX loss induces telomere dysfunction and necessitates induction of alternative lengthening of telomeres during human cell immortalization. Embo Journal, 2019. 38(19). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Heaphy C.M., et al. , Altered telomeres in tumors with ATRX and DAXX mutations. Science, 2011. 333(6041): p. 425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Morozov A., et al. , Benign Mesenchymal Stromal Cells in Human Sarcomas. Clinical Cancer Research, 2010. 16(23): p. 5630–5640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Damerell V., Pepper M.S., and Prince S., Molecular mechanisms underpinning sarcomas and implications for current and future therapy. Signal Transduct Target Ther, 2021. 6(1): p. 246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sole A., et al. , Unraveling Ewing Sarcoma Tumorigenesis Originating from Patient-Derived Mesenchymal Stem Cells. Cancer Res, 2021. 81(19): p. 4994–5006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li Y., et al. , Evidence for Kaposi Sarcoma Originating from Mesenchymal Stem Cell through KSHV-induced Mesenchymal-to-Endothelial Transition. Cancer Res, 2018. 78(1): p. 230–245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rodriguez R., et al. , Expression of FUS-CHOP fusion protein in immortalized/transformed human mesenchymal stem cells drives mixoid liposarcoma formation. Stem Cells, 2013. 31(10): p. 2061–2072. [DOI] [PubMed] [Google Scholar]
- 18.Wang J.Y., et al. , Generation of Osteosarcomas from a Combination of Rb Silencing and c-Myc Overexpression in Human Mesenchymal Stem Cells. Stem Cells Translational Medicine, 2017. 6(2): p. 512–526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cancedda R., et al. , Developmental control of chondrogenesis and osteogenesis. International Journal of Developmental Biology, 2000. 44(6): p. 707–714. [PubMed] [Google Scholar]
- 20.Sun L., et al. , Chromatin and Epigenetic Rearrangements in Embryonic Stem Cell Fate Transitions. Front Cell Dev Biol, 2021. 9: p. 637309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hu X.L., Wang Y., and Shen Q., Epigenetic control on cell fate choice in neural stem cells. Protein Cell, 2012. 3(4): p. 278–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kim M. and Costello J., DNA methylation: an epigenetic mark of cellular memory. Exp Mol Med, 2017. 49(4): p. e322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Deng P., et al. , Histone methyltransferases and demethylases: regulators in balancing osteogenic and adipogenic differentiation of mesenchymal stem cells. Int J Oral Sci, 2015. 7(4): p. 197–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lee J.E. and Ge K., Transcriptional and epigenetic regulation of PPARgamma expression during adipogenesis. Cell Biosci, 2014. 4: p. 29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yin B., et al. , Epigenetic Control of Mesenchymal Stem Cell Fate Decision via Histone Methyltransferase Ash1l. Stem Cells, 2019. 37(1): p. 115–127. [DOI] [PubMed] [Google Scholar]
- 26.Lu C., et al. , Histone H3K36 mutations promote sarcomagenesis through altered histone methylation landscape. Science, 2016. 352(6287): p. 844–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sankaran S.M. and Gozani O., Characterization of H3.3K36M as a tool to study H3K36 methylation in cancer cells. Epigenetics, 2017. 12(11): p. 917–922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Stzepourginski I., et al. , CD34+ mesenchymal cells are a major component of the intestinal stem cells niche at homeostasis and after injury. Proc Natl Acad Sci U S A, 2017. 114(4): p. E506–E513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ramirez-Zacarias J.L., Castro-Munozledo F., and Kuri-Harcuch W., Quantitation of adipose conversion and triglycerides by staining intracytoplasmic lipids with Oil red O. Histochemistry, 1992. 97(6): p. 493–7. [DOI] [PubMed] [Google Scholar]
- 30.Wang Y.C., Peterson S.E., and Loring J.F., Protein post-translational modifications and regulation of pluripotency in human stem cells. Cell Res, 2014. 24(2): p. 143–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Allis C.D. and Jenuwein T., The molecular hallmarks of epigenetic control. Nat Rev Genet, 2016. 17(8): p. 487–500. [DOI] [PubMed] [Google Scholar]
- 32.Goldberg A.D., et al. , Distinct factors control histone variant H3.3 localization at specific genomic regions. Cell, 2010. 140(5): p. 678–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wong L.H., et al. , ATRX interacts with H3.3 in maintaining telomere structural integrity in pluripotent embryonic stem cells. Genome Research, 2010. 20(3): p. 351–360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Skene P.J., Henikoff J.G., and Henikoff S., Targeted in situ genome-wide profiling with high efficiency for low cell numbers. Nat Protoc, 2018. 13(5): p. 1006–1019. [DOI] [PubMed] [Google Scholar]
- 35.Elsasser S.J., et al. , Histone H3.3 is required for endogenous retroviral element silencing in embryonic stem cells. Nature, 2015. 522(7555): p. 240–244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ernst J. and Kellis M., Chromatin-state discovery and genome annotation with ChromHMM. Nat Protoc, 2017. 12(12): p. 2478–2492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gonzales A.M. and Orlando R.A., Role of adipocyte-derived lipoprotein lipase in adipocyte hypertrophy. Nutr Metab (Lond), 2007. 4: p. 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bannert N. and Kurth R., The evolutionary dynamics of human endogenous retroviral families. Annual Review of Genomics and Human Genetics, 2006. 7: p. 149–173. [DOI] [PubMed] [Google Scholar]
- 39.Chiappinelli K.B., et al. , Inhibiting DNA Methylation Causes an Interferon Response in Cancer via dsRNA Including Endogenous Retroviruses (vol 152, pg 974, 2015). Cell, 2016. 164(5): p. 1073–1073. [DOI] [PubMed] [Google Scholar]
- 40.Chiappinelli K.B., et al. , Combining Epigenetic and Immunotherapy to Combat Cancer. Cancer Res, 2016. 76(7): p. 1683–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kassiotis G. and Stoye J.P., Immune responses to endogenous retroelements: taking the bad with the good. Nature Reviews Immunology, 2016. 16(4): p. 207–219. [DOI] [PubMed] [Google Scholar]
- 42.Pan D., et al. , SETDB1 Restrains Endogenous Retrovirus Expression and Antitumor Immunity during Radiotherapy. Cancer Res, 2022. 82(15): p. 2748–2760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chuong E.B., Elde N.C., and Feschotte C., Regulatory evolution of innate immunity through co-option of endogenous retroviruses. Science, 2016. 351(6277): p. 1083–1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sakashita A., et al. , Endogenous retroviruses drive species-specific germline transcriptomes in mammals. Nature Structural & Molecular Biology, 2020. 27(10): p. 967–+. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Shikata D., et al. , Suppression of endogenous retroviral enhancers in mouse embryos derived from somatic cell nuclear transfer. Front Genet, 2022. 13: p. 1032760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yang W.R., et al. , SQuIRE reveals locus-specific regulation of interspersed repeat expression. Nucleic Acids Res, 2019. 47(5): p. e27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Truch J., et al. , The chromatin remodeller ATRX facilitates diverse nuclear processes, in a stochastic manner, in both heterochromatin and euchromatin. Nature Communications, 2022. 13(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.D’Alimonte I., et al. , Wnt signaling behaves as a “master regulator” in the osteogenic and adipogenic commitment of human amniotic fluid mesenchymal stem cells. Stem Cell Rev Rep, 2013. 9(5): p. 642–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Schizas N.P., et al. , Inhibition versus activation of canonical Wnt-signaling, to promote chondrogenic differentiation of Mesenchymal Stem Cells. A review. Orthop Rev (Pavia), 2021. 13(2): p. 27098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Jing H., et al. , Epigenetic inhibition of Wnt pathway suppresses osteogenic differentiation of BMSCs during osteoporosis. Cell Death Dis, 2018. 9(2): p. 176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rosen E.D., et al. , C/EBPalpha induces adipogenesis through PPARgamma: a unified pathway. Genes Dev, 2002. 16(1): p. 22–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gao T. and Qian J., EnhancerAtlas 2.0: an updated resource with enhancer annotation in 586 tissue/cell types across nine species. Nucleic Acids Res, 2020. 48(D1): p. D58–D64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Valle-Garcia D., et al. , ATRX binds to atypical chromatin domains at the 3 ‘ exons of zinc finger genes to preserve H3K9me3 enrichment. Epigenetics, 2016. 11(6): p. 398–414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Carayol J., et al. , Protein quantitative trait locus study in obesity during weight-loss identifies a leptin regulator. Nature Communications, 2017. 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Danussi C., et al. , Atrx inactivation drives disease-defining phenotypes in glioma cells of origin through global epigenomic remodeling. Nature Communications, 2018. 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Polak P. and Domany E., Alu elements contain many binding sites for transcription factors and may play a role in regulation of developmental processes. Bmc Genomics, 2006. 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Jjingo D., et al. , Mammalian-wide interspersed repeat (MIR)-derived enhancers and the regulation of human gene expression. Mobile DNA, 2014. 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Horton I., et al. , Mouse B2 SINE elements function as IFN-inducible enhancers. bioRxiv, 2022: p. 2022.08.02.502523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Azazi D., et al. , Functional signatures of evolutionarily young CTCF binding sites. Bmc Biology, 2020. 18(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Patro R., et al. , Salmon provides fast and bias-aware quantification of transcript expression. Nat Methods, 2017. 14(4): p. 417–419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Soneson C., Love M.I., and Robinson M.D., Differential analyses for RNA-seq: transcript-level estimates improve gene-level inferences. F1000Res, 2015. 4: p. 1521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Love M.I., Huber W., and Anders S., Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol, 2014. 15(12): p. 550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Dobin A., et al. , STAR: ultrafast universal RNA-seq aligner. Bioinformatics, 2013. 29(1): p. 15–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Carroll T.S., et al. , Impact of artifact removal on ChIP quality metrics in ChIP-seq and ChIP-exo data. Front Genet, 2014. 5: p. 75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Liao Y., Smyth G.K., and Shi W., The R package Rsubread is easier, faster, cheaper and better for alignment and quantification of RNA sequencing reads. Nucleic Acids Res, 2019. 47(8): p. e47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Lawrence M., Gentleman R., and Carey V., rtracklayer: an R package for interfacing with genome browsers. Bioinformatics, 2009. 25(14): p. 1841–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Carroll T. and Barrows D., profileplyr: Visualization and annotation of read signal over genomic ranges with profileplyr. R package version 1.0. 1. 2019. [Google Scholar]
- 68.Amemiya H.M., Kundaje A., and Boyle A.P., The ENCODE Blacklist: Identification of Problematic Regions of the Genome. Sci Rep, 2019. 9(1): p. 9354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ramirez F., et al. , deepTools2: a next generation web server for deep-sequencing data analysis. Nucleic Acids Res, 2016. 44(W1): p. W160–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Meers M.P., Tenenbaum D., and Henikoff S., Peak calling by Sparse Enrichment Analysis for CUT&RUN chromatin profiling. Epigenetics Chromatin, 2019. 12(1): p. 42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Lawrence M., et al. , Software for computing and annotating genomic ranges. PLoS Comput Biol, 2013. 9(8): p. e1003118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Gu Z., Eils R., and Schlesner M., Complex heatmaps reveal patterns and correlations in multidimensional genomic data. Bioinformatics, 2016. 32(18): p. 2847–9. [DOI] [PubMed] [Google Scholar]
- 73.Langmead B., et al. , Ultrafast and memory-efficient alignment of short DNA sequences to the human genome. Genome Biol, 2009. 10(3): p. R25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Au K.F., et al. , Detection of splice junctions from paired-end RNA-seq data by SpliceMap. Nucleic Acids Res, 2010. 38(14): p. 4570–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Buenrostro J.D., et al. , Transposition of native chromatin for fast and sensitive epigenomic profiling of open chromatin, DNA-binding proteins and nucleosome position. Nature Methods, 2013. 10(12): p. 1213–+. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Buenrostro J.D., et al. , ATAC-seq: A Method for Assaying Chromatin Accessibility Genome-Wide. Curr Protoc Mol Biol, 2015. 109: p. 21 29 1–21 29 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Corces M.R., et al. , An improved ATAC-seq protocol reduces background and enables interrogation of frozen tissues. Nat Methods, 2017. 14(10): p. 959–962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Zhang Y., et al. , Model-based analysis of ChIP-Seq (MACS). Genome Biol, 2008. 9(9): p. R137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.McLean C.Y., et al. , GREAT improves functional interpretation of cis-regulatory regions. Nat Biotechnol, 2010. 28(5): p. 495–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Gu Z. and Hubschmann D., rGREAT: an R/bioconductor package for functional enrichment on genomic regions. Bioinformatics, 2023. 39(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Yu G., Wang L.G., and He Q.Y., ChIPseeker: an R/Bioconductor package for ChIP peak annotation, comparison and visualization. Bioinformatics, 2015. 31(14): p. 2382–3. [DOI] [PubMed] [Google Scholar]
- 82.Yu G., et al. , clusterProfiler: an R package for comparing biological themes among gene clusters. OMICS, 2012. 16(5): p. 284–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Zhu L.J., et al. , ChIPpeakAnno: a Bioconductor package to annotate ChIP-seq and ChIP-chip data. BMC Bioinformatics, 2010. 11: p. 237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Barrows D. and Carroll T., profileplyr: Visualization and annotation of read signal over genomic ranges with profileplyr. Bioconductor (R package version 1.2. 0, 2019). [Google Scholar]
- 85.Gorkin D.U., et al. , An atlas of dynamic chromatin landscapes in mouse fetal development. Nature, 2020. 583(7818): p. 744–751. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All genomic and transcriptional data will be deposited in the Gene Expression Omnibus (GEO) at the time of publication.