Abstract
The importance of memory in bacterial decision-making is relatively unexplored. We show here that a prior experience of swarming is remembered when E. coli encounters a new surface, improving its future swarming efficiency. We conducted >10,000 single-cell swarm assays to discover that cells store memory in the form of cellular iron levels. This memory pre-exists in planktonic cells, but the act of swarming reinforces it. A cell with low iron initiates swarming early and is a better swarmer, while the opposite is true for a cell with high iron. The swarming potential of a mother cell, whether low or high, is passed down to its fourth-generation daughter cells. This memory is naturally lost by the seventh generation, but artificially manipulating iron levels allows it to persist much longer. A mathematical model with a time-delay component faithfully recreates the observed dynamic interconversions between different swarming potentials. We also demonstrate that iron memory can integrate multiple stimuli, impacting other bacterial behaviors such as biofilm formation and antibiotic tolerance.
Keywords: Swarming, Memory, Iron, E. coli, Antibiotics, Biofilms, Time-delay model
Introduction
Constantly changing environments present a challenge to the survival of all organisms1. They can adapt to change either by behavioral or by phenotypic plasticity2, but must decide among available phenotype choices3. Response times during decision making can be critical for survival3. Vertebrates use their nervous system for faster decision-making by storing information about their prior experiences4. The process of storage and retrieval of information is called memory4,5, which can be stored for a few seconds to several years6. A stronger memory can be brought to bear on the system by ‘conditioning’7 – a process where repeated encounters to a stimulus can be stably linked to a specific response8,9, drastically reducing decision-making time10.
Bacteria experience large environmental fluctuations to which they can readily adapt11. They commonly do this by transducing environmental signals to elicit appropriate transcriptional responses12 that occur on the time scale of minutes, dissipating in the absence of the signal. Keeping these systems always ‘on’ is costly12, so bacteria also employ less-costly bet-hedging strategies that stochastically switch among many alternative phenotypic states in similar time frames 13,14. Alternatively, different cell types can pre-exist in an isogenic population and respond heterogeneously to antibiotic stress15 or to starvation16. All of these strategies come under the umbrella of bacterial memory17.
Whether bacteria can store memory has been explored both theoretically17,18 and experimentally19–22. Memory can influence the fitness of individuals18 or of a community19, as well as bacterial interaction with their host20 and defense against phages23. The mechanism of memory storage in bacteria involves genetic5 or epigenetic factors24,25 such as type 1 fimbriae phase variation5, or motile-sessile transition26 or chemotaxis27–29. These varied molecular mechanisms of bacterial memory storage are more stimulus-specific in nature, in contrast to the nervous system where no matter the type of stimulus, the same molecular principle of storage is employed4. The existence of the latter kind of memory mechanism in bacteria can only be conjectured from some recent reports21 22.
In this study, using E. coli swarms as our experimental system, we asked a question not considered before: is there a heritable memory in bacteria that can integrate multiple stimuli? The choice of this system was based on observations that a prior experience of swarming hastens the onset of swarming. Swarming motility is a collective, flagella-driven, adaptation to colonizing the surface of semi-solid media by population expansion30–34. E. coli will typically swarm on semi-solid media (~0.5% agar), but are non-motile on solid or hard media (~1.5% agar). The ‘softer’ agar is more conducive to swarming because among other properties, it also holds more water, allowing flagella to work on a surface terrain. Surface colonization presents varied challenges, both physical and nutritional33. Nutritional challenges include acquisition of iron35 leading to upregulation of efflux pumps essential for the secretion of iron-binding siderophores into the extracellular matrix, as well as upregulation of iron import systems36–40. When bacteria cultured in liquid media are transferred to swarm media, a long lag ensues before swarming begins32. Widespread transcriptome changes accompany the onset of swarming38,41–44, revealing that the bacteria are adapting to a distinctly different environment during the lag phase. If, however, bacteria from a swarm are transferred to a fresh swarm media, the lag is considerably shortened45, suggesting that the bacteria may be already ‘conditioned’ to swarm.
To interrogate whether swarming bacteria ‘remember’ the adaptation mechanisms employed while swarming, we designed an experimental set-up that allowed monitoring of swarms initiated from >10,000 single cells. We report here that the bacteria indeed possess a heritable memory of the swarming state that is retained on the time scale of hours over at least four generations. We show that the mechanism of this type of memory storage is primarily related to how much iron is stored intracellularly. Other cues that promote biofilm formation and antibiotic tolerance are also routed through iron memory, reflecting the essential role iron plays in central metabolism. Our findings highlight a more general physiological mechanism of memory storage in E. coli, which we predict will be widely present in other bacteria.
Results
Swarming proficiency pre-exists in planktonic cells
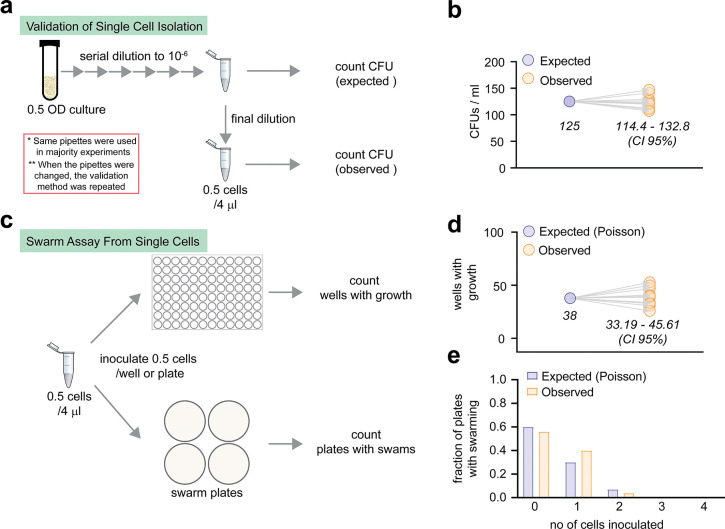
The impetus for experiments performed is this study originated from a serendipitous observation that swarm cells plated for determining CFU counts (colony forming units) on freshly-made (hence moist) hard agar, produced colonies whose phenotype was different from those made by planktonic cells (growing in liquid culture) plated on similar agar. Compared to planktonic (P) colonies, swarm-derived (S) colonies had more irregular margins, were larger, and flatter (when viewed from the side) (Fig. 1, a–b). We posited that these differences stemmed from prior ‘conditioning’ of S cells, so they were attempting to swarm even on hard agar because the plates were still moist. We quantified these morphologies by measuring colony circularity (using both area and perimeter), and size (diameter) (Fig. 1c); S colonies were significantly less circular and larger. On closer inspection, a fraction (~20%) of P-derived colonies also displayed S-colony characteristics, although less prominently (Fig. 1b, top; compare #9–10 with #1–8), suggesting that P-cells were also heterogeneous for swarming proficiency, with some inherently primed for swarming. To test the latter deduction, we performed Single Cell Inoculum (SCI) swarm assays, where P cell-derived swarms were initiated from single P cells isolated using the dilution-to-extinction method46 targeted to yield an average of 0.5 cells per unit volume (Fig. 1d and Extended Data Fig. 1, a–b). The sampling events would follow a Poisson distribution, so ~40% of seeded swarm plates would have received a cell. This was confirmed by parallel growth in microtiter plates (Extended Data Fig. 1c, top), which matched the expected positive sampling events with a high degree of precision (Extended Data Fig. 1d). Therefore, in all SCI assays, at least 120 swarm plates were inoculated to gather data for 50 swarms (Extended Data Fig. 1c, bottom), again with high precision (Extended Data Fig. 1e). The sample size was kept sufficiently large to increase confidence in the statistical analysis. Additionally, such Poisson distributions would sample multiple cells in ~5% of events. We could identify those events on a swarm plate by just counting the number of swarm ‘nucleation’ centers and exclude them from our analysis (Fig. 1e). In parallel, we compared data from SCIs versus 100- or 10000-cell inoculums, where any heterogeneity present in single cells is expected to be subsumed within the large cohort. Swarming proficiency was calculated as distance covered over time (Fig. 1f). SCIs showed a large variability in swarming, in contrast to the larger cell inoculums (see the endpoints of lines in each group). The comparison of this variability between the three data sets was complicated by the fact that each cohort had a different lag period, as expected from the dependence of lag on cell-density33,47. A zone-adjustment was therefore performed by arbitrarily dividing the plate into 3 zones (C/M/O) (Fig. 1g) and comparing swarm diameters of every cohort within each zone (Fig. 1h; see Methods). The zone-wise variability in frequency distributions (FD) of swarm diameters (5 mm bins) were then compared (Fig. 1, i–k), and the noise calculated (Fig. 1, l–n). In every zone, SCIs had significantly higher levels of noise (a reflection of heterogeneity) compared to other cohorts, the maximal noise evident in the outermost zone O (Fig. 1k and n). The large heterogeneity observed within the P cell-derived SCIs reveals that swarming proficiency pre-exists even in planktonic cells.
Fig. 1 |. Swarming proficiency pre-exists in planktonic cells.
a, Representative hard-agar plates showing CFUs of planktonic (P) cells from a mid-log phase liquid culture, and swarm (S) cells collected after growth for 16h. In both cases, a 10−6 dilution of 0.5 OD cells was plated on hard agar plates and incubated for 16h at 37°C. b, Blown-up images of 10 randomly selected colonies from A (indicated by colored circles). c, Distribution of physical parameters of P and S colonies (). See Methods for circularity calculations. [****p <0.0001, Mann-Whitney test] d, Flow chart of single P-cell swarm assays, where the dilution was estimated to yield a single cell in a unit volume of 4ul (see Extended Data Fig. 1 and Methods for details). e, Swarms with varying number (1–4) of swarm nucleation centers (red arrow) dictated by the initial number of cells spotted on the plate. f, a spaghetti plot showing swarm diameters (Y-axis) measured over time (X-axis). Each line represents a replicate. g, Three arbitrary zones considered for adjustment: C, central, M, medial; O, outer. h, zone-adjusted comparisons (see Methods and text). i-k, Frequency distribution of swarm diameters (5 mm bins), color-coded as in (f). l-n, Comparison of noise (SD/mean) within cohorts. p values were calculated from F-tests (see Methods).
Swarming-related memory is present in both P and S cells
To explore whether the heterogeneity of swarming proficiency was random or inherited, we evaluated the swarming capability of offspring from single cells over several generations (Fig. 2a). The starting cells represent our G0 generation ‘mothers’, and were derived from both P and S conditions. We first standardized the established dilution-based protocol46 to precisely collect daughter cells at different generations (Extended Data Fig. 2, a–c). Some G0 mothers () were directly spotted on swarm plates (Fig. 2, b–c and Extended Data Fig. 2d). Others were grown in 96-well plates for either 4 (Fig. 2, d–e) or 7 (Fig. 2, f–g) generations (, x axis) before plating single daughters of each mother (16 expected, Y axis in Fig. 2, d & f) on swarm media (see Extended Data Fig. 2, d & f). We initially probed other generations as well; but to reduce time and effort in these labor-intensive experiments, we confined our observations to these generations, which gave conclusive results during our initial screening. In the bubble plots (Fig. 2, b–g), the size of each bubble represents the swarm diameter and the color represents the noise within 16 daughters of the same mother. The behavioral patterns of P mothers (P→S data, b-d-f) was distinctly different from the S mothers (S→S data, c-e-g). P mothers (G0) showed the expected heterogeneity (see 1-cell data in Fig. 1f) in swarming (Fig. 2b, Supplementary Fig. 1). In contrast, all the G4 daughters descended from a single P mother were more homogeneous (Fig. 2d, follow the data vertically or column-wise for the 16 siblings). Some mothers (#11, #13, #14) showed variability, which could have come from experimental noise. A K-means clustering of the means derived from each column resulted in three distinct groupings labeled XS (poor), M (moderate) and L (efficient) swarmers. The majority patterns, i.e., consistent behavior of each sibling group, suggested that some information stored in the mother was passed down to the daughters. This information was lost by G7 as the variability returned (Fig. 2f, Supplementary Fig. 1). In summary, P daughters inherit some form of memory that is intact up to four generations, but is lost by the seventh generation, restoring the original heterogeneity.
Fig. 2 |. Swarming-related Memory is present in both P and S cells.
a, Flowchart summarizing the operations involved. P and S cells were collected and diluted to get single cells; these are the starter G0 mother cells which were directly spotted on swarm plates (P→S), as well as seeded in 96-well plates and grown for either 4 or 7 generations (see Extended Data Fig. 2 for the standardization process of the generations and statistics). All the resulting daughter cells (16 from each mother) were further similarly diluted and spotted separately on swarm plates. b-g, Bubble-array plots where each bubble represents swarm diameters from SCIs (~1700 total plates with swarms). The size of the bubble represents the swarm diameter and the color represents the noise within a group of daughters born from the same mother (b-c: G0; d-e: G4; f-g: G7; b, c, d: P mother; e, f, g: S mother). See Supplementary Fig. 1 for the noise data of each mother. Based on the daughters’ ability to swarm in G4 and G7, a K-means clustering of mother cells resulted in three distinct groups; XS – poor, M – moderate, and L – efficient swarmers.
The same set of experiments performed on S mothers (S→S data, c-e-g) showed a very different outcome at G0 and G4. In contrast to P mothers, swarm diameters of S mothers at G0 were uniform (Fig. 2c, Supplementary Fig. 1), suggesting that they retained ‘swarm’ memory or were ‘conditioned’ in some way while swarming. Similar to P cells, this memory was maintained in S daughters until G4, but with even lower noise (Fig. 2e). In addition, the K-means clustering resulted in only in one group (L), in contrast to 3 groups in P daughters. By G7, the S daughters had lost this memory, similar to P cells (Fig. 2g, Supplementary Fig. 1), but even here the noise was lower than in P cells. In summary, the uniform behavior of G0 S mothers, as well as the single cluster of G4 daughters indicates that S cells are conditioned, and that this swarm memory is retained at least for 4 generations but lost by the 7th generation.
In conclusion both P and S cells have a heritable multigenerational memory of swarming, henceforth referred to simply as memory. While this memory is heterogeneously distributed in P cells, it is homogenously present in S cells.
Cells have an iron memory
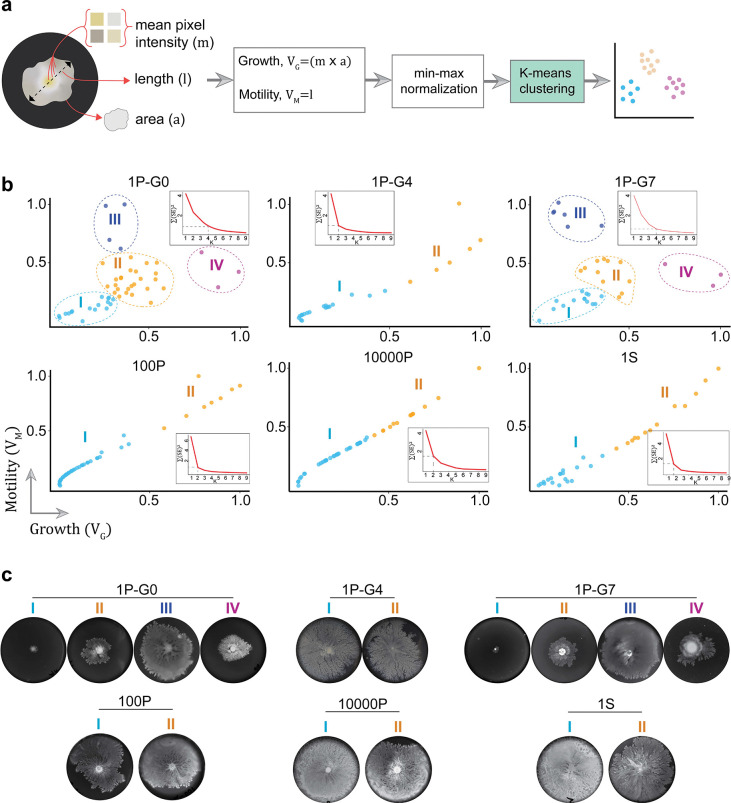
What is the nature of this multigenerational memory? Given the significant variation in the swarming proficiency of SCIs, we reasoned that any factor(s) that alters this heterogeneity would provide insights into its nature. Since swarming involves both growth and motility, we first looked at the SCI variation in these two factors. We measured the total pixel intensity of each swarm as the growth variable (VG) and the maximum swarm diameter as the motility variable (VM) (Extended Data Fig. 3a). A K-means clustering (Extended Data Fig. 3b) of the VG-VM data from P mothers at G0 resulted in four distinct groups – poor growth & motility (I), moderate growth & motility (II), moderate growth & good motility (III), and good growth & moderate motility (IV). The actual plate images from these clusters revealed that the clustering was real and could be visually correlated (Extended Data Fig. 3c). Cohorts that showed less variation (G4 P, G0 S, 100-cell,10000-cell) exhibited only two clusters (Extended Data Fig. 3b) but could not be visually correlated (Extended Data Fig. 3c), suggesting the presence of only a single cluster in these samples. Interestingly, the four real clusters of G0 P cells that disappeared at G4, reappeared in G7 (Extended Data Fig. 3c, 1P-G7), consistent with memory retention for 4 generations but not for 7. Together, the clustering analysis confirmed that VG and VM are both important for swarming heterogeneity.
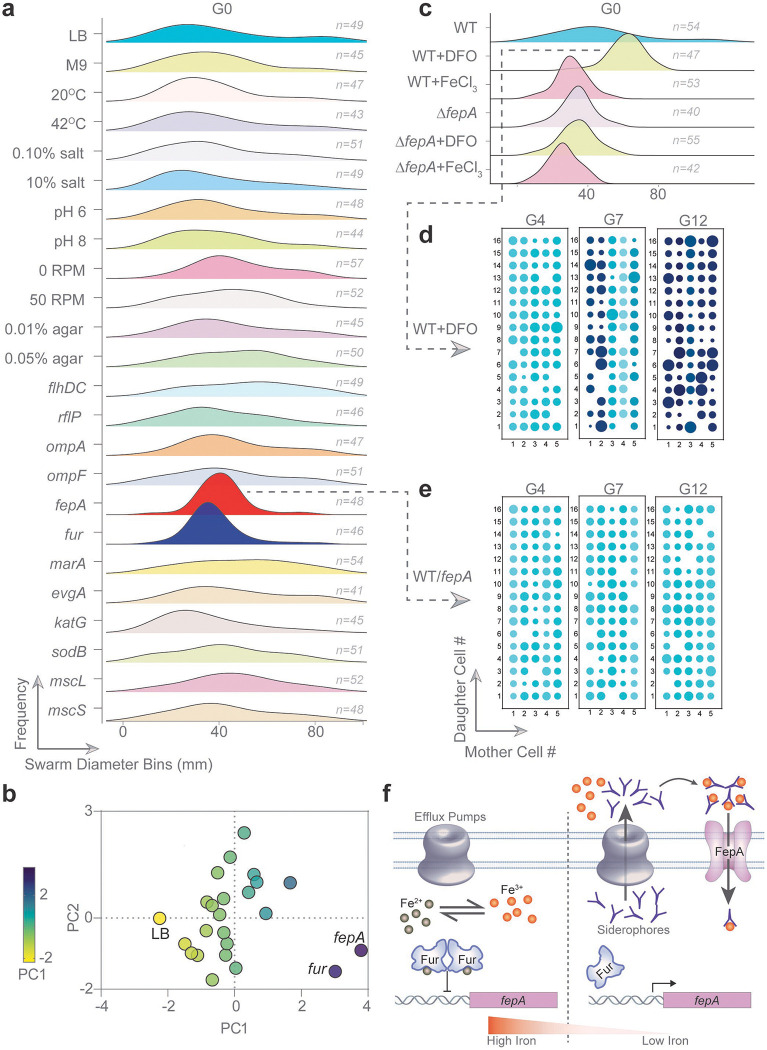
To explore environmental or genetic factors that might reduce the swarming heterogeneity of the P mother cells (measured as swarm diameters), we perturbed several factors prior to inoculation of these G0 cells on swarm plates. The environment was perturbed by changing several parameters such as media, temperature, pH etc. (Fig. 3a, Environmental; see Supplementary Fig. 2a for noise data), but no apparent decrease in swarming heterogeneity was observed. These observations were further quantified by performing a Principal Component Analysis (PCA). Since the mean and the noise are major descriptors of sample data, these values were taken as variables for PCA48. None of the environmental perturbations changed principal components significantly (Fig. 3b, Supplementary Fig. 2b). Genetic factors were assessed by introducing plasmids carrying representative genes that had been identified as contributing to E. coli swarm physiology38,44,49,50. These genes encoded for: porins (ompA, ompF), iron regulation (fepA, fur), efflux (evgA, marA), redox (katG, sodB), and mechanosensing (mscL, mscS). Of these, perturbation of iron homeostasis significantly reduced noise and clustered the data very differently in PCA when compared to LB control (Fig. 3b, fepA and fur; 3b; Supplementary Fig. 2b). Iron starvation is known to be crucial signal for swarming37,44. FepA is an outer membrane transporter for ferric enterobactin, while Fur represses iron uptake genes51. Compared to WT, ΔfepA and Δfur strains showed no significant differences in growth or swimming motility as assayed in soft agar (0.3% w/v) (Extended data Fig. 4, a–b), yet had different swarming phenotypes: the ΔfepA strain was a poor swarmer, whereas Δfur did not swarm (Extended data Fig. 4, c–d). The importance of iron in maintaining swarming heterogeneity was confirmed by growing P mothers in iron-rich (FeCl3) or iron-starved conditions (deferoxamine mesylate or DFO, an iron chelator) before conducting SCI assays (Fig. 3c,). These treatments severely reduced the heterogeneity, creating defined peaks (Fig. 3c). A shift to the right on the graph indicates better swarming (+DFO), while that to the left indicates the opposite (+FeCl3), validating studies showing that iron starvation is a swarming signal37,50. Interestingly, neither DFO nor FeCl3 had any effect on ΔfepA mothers (Fig. 3c), consistent with the fact that iron uptake is impaired in this mutant.
Fig. 3 |. Cells have an iron memory.
a, Ridgeline plots showing frequency distribution (0 to 1 in each plot) of swarm diameters of G0 P cell-SCIs (~2400 total), where prior to plating, P mothers were either subjected to indicated growth environments (in liquid), or transformed with plasmids expressing genes known to influence swarming. See Supplementary Fig. 2a for the noise data. b, Principal Component Analysis of data from a. The mean, SD, and noise were considered as variables of each condition. Eigenvalues of PC1 (60.06%) and PC2(39.87%) are expressed on the X- and Y-axis, respectively. Each dot represents a condition with relevant ones labeled. See Supplementary Fig. 2b for the biplot. c, Ridgeline plots specific to WT or ΔfepA P cells experiencing iron-poor (DFO) or iron-rich (FeCl3) conditions. d-e, bubble-array plot of G4, G7, and G12 daughter P cell swarm assays (as in Fig. 2) of WT strains either starved for iron (DFO) or had abundant iron. f, Schematic of Iron homeostasis as controlled by Fur, the global negative regulator of intracellular iron. See text for details.
To investigate the effect of iron starvation on memory, we followed memory inheritance for G4, G7 and G12 daughters in WT P cells treated with DFO. Memory was now largely maintained till G7 (Fig. 3d), but was lost by G12, showing that experimentally induced iron starvation extends memory beyond G4. This suggests that memory might persist for longer durations by increasing intracellular iron. Overexpression of fepA (expected to import iron) indeed retained memory till G12, the last generation we tested (Fig. 3e).
In summary, intracellular levels of iron are an agent of memory that can be either environmentally or genetically conditioned.
Swarming iron memory tracks from mother to daughters
Most methods for measuring intracellular iron require complex endpoint chemistry52 and those that measure iron in live cells can impact cellular physiologies53. To avoid such experimentally-induced perturbations while quantifying iron in our setup, we designed a fluorescent reporter-based iron biosensor (Supplementary Fig. 3). The reporter is sfGFP, transcribed from the Fur-regulated E. coli fepA promoter. Fur is the global repressor of iron uptake35 (Fig. 3f). The fepA promoter was used to drive sfGFP because this gene has a strong Fur binding site (see Table I in54), is crucial for swarming55, and is highly expressed during swarming44. When intracellular iron levels are high, Fur stops further iron intake by complexing with iron and binding to a ‘Fur box’ adjacent to promoters of hundreds of iron homeostasis genes56 to repress transcription (Fig. 3f). When iron levels drop below a threshold, the system is depressed and genes involved in siderophore production, secretion (efflux pumps), and uptake (siderophore receptors) are expressed (Fig. 3f). Thus, high intracellular iron should cause strong suppression by Fur yielding a low fluorescence in our setup and vice versa. The sfGFP fluorophore was expected to yield a signal strong enough57 to be detected even under strong Fur repression. A control plasmid expressing sfGFP from a Ptrc promoter in WT cells gave a homogeneous background signal (Fig. 4a–b, top), whereas sfGFP driven by the fepA promoter (PfepA) produced a heterogeneous signal, reflecting heterogeneity of iron levels (Fig. 4a–b, middle). The ability of the biosensor to report on cellular iron levels was further tested by subjecting WT/PfepAsfGFP cells to FeCl3 and DFO treatment. Not only did fluorescence decrease and increase, respectively, the heterogeneity in signals also reduced, as expected (Extended Data Figure 4e; heterogeneity due to different plasmid copy numbers is controlled for by the IPTG-induced, continuously ‘ON’ sfGFP plasmid). When PfepAsfGFP was introduced into the ΔfepA strain, which cannot import iron, the fluorescence signal became homogeneous, as expected if all cells had low iron (Fig. 4a–b, bottom). FACS analysis confirmed results from microscopy (Fig. 4c, Extended data Fig. 5): only WT/ PfepA-sfGFP showed heterogeneity (Fig. 4c, middle, green singlets). Having validated this construct as an iron biosensor, we used it next to validate iron memory in swarming, as well as other phenotypes expected to be impacted by iron levels (Fig. 4d).
Fig. 4 |. Swarming iron memory tracks from mother to daughters.
a. WT or ΔfepA cells harboring Ptrc-sfGFP or PfepA-sfGFP plasmids. See Supplementary Fig. 3 for cloning details. b, Brightfield (left) and fluorescence (right) microscopic images of strains depicted in a. c, Results of FACS analysis of strains indicated in a. Singlets were gated based on SSC-A (side scatter channel - area) and GFP intensity. A sort gate was defined to collect singlets where the background fluorescence of WT cells without GFP was defined as the minimum threshold See Extended Data Fig. 5 and Methods for details). Gray, cells below threshold. Green, cells within the defined gate. d, Flow chart of how sorted cells were used. e, Correlation of single-cell GFP intensities (from FACS, n=96) with swarming of G0 mother cells collected from the different strains indicated. r, spearman correlation coefficient, p value as indicated. The blue line represents the exponential regression line with indicated R2 values. The blue error band represents 95% confidence interval (CI). f, Fluorescence intensities of sorted G0 mothers that were dropped into one well of a 96-well plate, indicated as bars. g, The G4 or G7 daughters of the mothers with known GFP intensities (indicated as bars in f) were assayed for their swarming ability and the results are shown as bubble-matrix plots. See Supplementary Fig. 4 for the FACS data of each experiment.
FACS was used to first sort P cells harboring all the biosensor-harboring constructs through a pre-defined sort gate that included a range of signals covering low to high iron levels (Extended Data Fig. 5 and Supplementary Fig. 4). Single cells covering this range were then dropped on swarm plates to measure swarming proficiency (Fig. 4d). Although cells with the control Ptrc plasmid showed heterogeneity in swarming (Fig. 4e, left, follow x-axis), no correlation between the sfGFP signal and swarming diameter was observed (Fig. 4e, left). As expected, however, a positive correlation between these two measurements was observed in WT/ PfepA sfGFP G0 cells, i.e., cells with high sfGFP (low iron) were better swarmers (Fig. 4e, middle). In ΔfepA/ PfepA-sfGFP strain, where iron import is impaired, we observed a clustering of swarm diameters (Fig. 4e, right, follow x-axis), consistent with the reduction of heterogeneity (see Fig. 3b, ΔfepA). Next, for each of the strains shown in Figure 4e, we performed a multigeneration analysis with randomly sorted G0 cells (see Supplementary Fig. 4 for sort results). Five such random P mothers from each strain were propagated until G4 or G7 (Fig. 4f, g). Unlike in Fig. 2a, where the physiological state of the seeded mothers was unknown, this time we had knowledge of their iron levels based on their fluorescence levels, shown as bars (Fig 4f, y axis - log scales; high bars = low iron). The swarming proficiencies of each sibling set is plotted as before (follow the columns below each bar for daughter cell data). The swarm diameters of daughters correlated closely with iron levels of the mother. For example, in the G4 cohort of WT/ PfepAsfGFP mothers (Fig 4g, third panel from the left), mother #1 (first column, short bar=high iron) birthed daughters that were all poor swarmers, while mother #3 (third column, higher bar=low iron) birthed daughters that were efficient swarmers. The G7 data from these mothers mirrored previous observations that iron memory is lost by G7 (Fig. 4g, fourth panel). In contrast, no such correlation between iron and swarm diameters was observed in the other two strains shown in Fig. 4e (left, right). In both strains, memory was maintained till G4 (Fig. 4g, first and fifth panel from the left). The ΔfepA strain exhibited high sfGFP signals due to their low intracellular iron (Fig 4e, right, follow y-axis). Interestingly, memory of low iron in this ΔfepA strain was maintained at least till G7, which is an improvement upon the G4 memory of WT cells (Fig. 4g, second and sixth panels from the left). This observation, along with the data in Fig. 3e, revealed that both decreases or increases in iron levels can extend the span of memory beyond WT levels, although the phenotypes in both cases are at the opposite ends of the spectrum.
In summary, we constructed and validated an iron biosensor that can be used to quantify the iron memory and sort cells based on the quantification. Results from this biosensor showed a strong correlation of iron memory with swarming, which is propagated from mothers to daughters till G4 in WT cells.
Iron memory impacts biofilm formation and antibiotic survival
Besides swarming, iron is also crucial for several physiologies58. We tested a few such phenotypes for their correlations with intracellular iron levels (Fig. 5, a–d, Supplementary Fig. 4). Recent studies have shown that iron is crucial for the decision to enter the biofilm state59,60, which is a contrasting sedentary lifestyle choice when compared to swarming34. To test if iron memory is a predictor of biofilm formation, we measured this ability in G0 P cell SCIs using a standard crystal violet staining assay61 (Fig. 5a). As expected, we observed no correlation between iron and biofilm formation in the control WT/Ptrc-sfGFP strain (Fig 5a, left; see 5d for correlation data), but a strong anticorrelation between low iron and biofilms in WT/ PfepA sfGFP cells (Fig. 5a, middle; 5d), i.e., more iron is a signal for biofilms. This anticorrelation was absent in strains lacking FepA (Fig. 5a, right; 5d). These data are consistent with the opposing ‘move vs stay’ decisions associated with the two behaviors.
Fig. 5 |. Iron memory impacts biofilm formation and antibiotic survival.
a-c, Correlation of single-cell GFP intensities of G0 mother cells from FACS (, for each sample) with: a, biofilm formation; b-c, antibiotic survival (2 μg/ml Kan & 1.5 μg/ml Cam). Blue lines represent exponential regression lines with indicated R2 values. The blue error bands represent 95% CI. d, Distribution of r, the spearman correlation coefficients of GFP intensity and growth under the various conditions tested. The error bars represent 95% CI. p values are given on the right. See Supplementary Fig. 4 for the FACS data of each experiment.
Iron is critical for redox homeostasis35,62, which is upended by antibiotics63,64. To test if iron levels can be a predictor for antibiotic survival, sorted cells of the same three strains shown in Fig. 5a were treated with ~½ MIC of the antibiotics Kanamycin (Kan) and Chloramphenicol (Cam) for 4h. A positive correlation was observed between low iron and increased survival with both antibiotics (Fig. 5b–d, Supplementary Fig. 4). This correlation was absent in ΔfepA/ PfepA-sfGFP or Ptrc-sfGFP strains.
In summary, iron memory is not restricted to swarming decisions alone, but plays a crucial role in several bacterial physiologies.
A mathematical model that explains the propagation and switching of iron memory states
To understand how iron memory can be propagated from mother to daughter, we thought it prudent to first understand the phenomenon mathematically, before pursuing detailed future investigations into underlying molecular mechanisms.
Given that cells switched dynamically over multiple generations among three different states (XS, M, L; Fig. 2), we used an ordinary differential equation model as our mathematical framework (see Methods). The model assumes different rates of switching between the states (Fig. 6a). Starting from any state, cells could stochastically switch to other states over time, ultimately recreating the original phenotypic heterogeneity (Fig. 6b–d). In order to satisfy the curious observation that the S data had lower noise, i.e. S daughters took longer to switch compared to P daughters (compare Fig. 2d to 2e and 2f to 2g), a time delay component had to be incorporated. Model parameters such as the delay duration and kinetic rates were inferred by fitting the model to data at G4 (Fig. 2d–e), and these rates were further constrained so as to recreate the intercellular variation seen in single G0 mothers by G7 (see Methods). Our modeling results reveal that in the case of high to moderate iron states (XS and M), all three states started to appear during G3-G4 transition (Fig. 6b–c). In contrast, the low iron state cells (L) initiated this process at G4-G5 transition suggesting that conditioning (prior swarming experience) can enforce iron memory further than stochastic switching (Fig. 6d). Our model could capture the real data efficiently as seen when comparing fractions of different cell states in Figs. 6b–d (dashed lines for G0 data) to the experimental data in Fig. 2 (see Supplementary Fig. 5). In other words, no matter the starting cell state, the emerging population eventually converges to the original ratio of cell states. Using the model data, a cell lineage dendrogram was generated, which visually illustrates how these cell states can reappear (Figs. 6–e–g).
Fig. 6 |. A mathematical model that explains the propagation and switching of iron memory states.
a, Depiction of the mathematical framework used to model the data in Figure 2. Briefly, XS, M, and L cells with poor, moderate, and efficient swarming capabilities respectively can switch to other states by the given rates with a time delay. See text and Methods for details. b-d, Results from the time delay model showing how all three swarming states can be spawned starting from any cell type (as indicated on top). The XS and M cells can maintain the memory till G3 and then start to switch to other cell types, whereas L cells take until G4 before switching. The dashed lines show the fractions of cell types obtained from G0 mothers data of Fig. 2b (also see Supplementary Fig 5). Irrespective of the initial cell type, the resulting population converges to the original ratio of the cell types. e-g, A cell lineage illustrating the memory model. Starting from a specific cell type, the fractions of all three cell types in each of the G1-G5 generations (1–5) were obtained from the model in b-d to get the approximate number of each cell type. That information was used to create the circular dendrograms.
In summary, our mathematical model shows that specific rates of dissipation and acquisition of memory can be correlated with the experimentally obtained fractions of cells in different iron states – thus linking the two together.
Discussion
This study has uncovered a new role of iron in bacterial behavior by demonstrating the existence of physiologically stored ‘iron’ memory in E. coli that is retrieved over multiple generations. In addition, this memory can integrate multiple stimuli, and thus influence multiple physiologies. To our knowledge, this kind of memory has not been unearthed before, but there is no reason to think that it is limited to the iron pathway monitored in this study.
Iron memory in swarming
The original observation that led to the present study was a particular colony phenotype present uniformly in swarm (S) cells plated on hard agar, but present non-uniformly in planktonic (P) cells (Fig. 1, a–c), suggesting that some attribute of S cells was pre-present in P cells. The latter deduction was confirmed by assaying the swarming potential of a large number of single P cells (Fig. 1, f–n). To test whether the heterogeneity in swarming potential of these cells was random or inherited, the offspring of G0 P cells tracked over several generations were found to inherit their starting swarming potential until G4 but not up to G7 (Fig. 2, b–d–f). The swarming proficiency of S mothers was homogeneous, and also inherited until G4 and lost by G7 (Fig. 2, c–e–g). Thus, both P and S cells have a heritable multigenerational memory of swarming.
Based on prior knowledge of gene regulation during swarming, we tested various candidates that might contribute to swarming memory (Fig. 3a, Genetic). Of these, only the over-expression of critical players in the iron acquisition pathway - fepA and fur - reduced the noise in G0 mothers significantly. Efflux pumps also play a role in this pathway because they are responsible for siderophore secretion65. However, these pumps were not observed to contribute to memory (Fig. 3a, marA and evgA), ruling out the possibility that the heterogeneity of swarming is caused by the known heterogeneity in efflux pumps66. The role of intracellular iron in encoding swarming memory was ascertained using an iron biosensor (Fig. 4). Swarming proficiency tracked with intracellular iron levels, i.e. cells with low iron were better swarmers and vice versa. Memory retention could be manipulated by decreases or increasing iron levels (Fig. 3, d–e), establishing a strong correlation of iron memory with swarming.
A time delay model for switching of iron memory states
What explains the mixed nature of swarm phenotypes and their heritability (XS, M, and L in Fig. 2)? The short duration of this heritability excludes epigenetic mechanisms67,68, or bistable69 and toggle switches70. Although epigenetics might play a role in fur regulon71, a stochastic fluctuation model72,73 could explain the observed fluctuations within the population (Figs. 6a–d). Stochasticity may arise due to intrinsic or extrinsic noise in gene expression74,75. The stochastic fluctuations in transcription and translation of a given gene constitute intrinsic noise (e.g., Fig. 3a, genetic), whereas the effect of other cellular components on the expression of that gene constitute extrinsic noise (e.g., iron manipulation in Fig. 3c). That memory could be prolonged beyond G4 by tweaking intrinsic or extrinsic components, possibly due to an increase in signal-to-noise ratio76, can be interpreted as a form of conditioning77–79. Additionally, a continuous loss of the Fe-S proteins due to noisy dilution of these proteins during cell division80 and damage during aerobic respiration81,82 may further contribute to phenotype switching.
The incorporation of a delay in the time of transition to another state was necessary to reproduce the experimentally obtained fractions of different cell states (Figs. 6b–d). Time delay often arises from operation of a feedback83, or a slow incorporation of the input signal84,85, or a slow response time84,86. The differences in the delays specific to different states (for example, it took 3 generations for XS/M cells to switch vs 4 for L cells; Fig. 6b–d) can be due to differences in feedback time or the number of steps involved in switching. Although the fur regulon operates on a negative feedback loop (Fig. 3f)87, the expression of fur itself is under positive regulation of the upstream regulators Crp88, OxyR89, and SoxS90. This complex genetic circuit could have different feedback times depending on the cell states. For example, XS G0 mothers and their daughters would be in a Fur-repressed state till G3–4 when their iron pool goes below the threshold, so they switch to the Fur-derepressed M state. This would initiate synthesis of iron import proteins. M cells could stay derepressed for another 3–4 generations, where they accumulate enough iron to switch to the L state. This timescale is consistent with previous calculations for fur regulon87. In contrast, the switch of L back to M would require active degradation of the newly synthesized iron import proteins and mRNAs, which could take longer.
Evolutionary significance of using iron as memory
Iron metabolism is critical for life91 and for its evolution92,93. Both iron-limiting and iron-rich conditions can be beneficial35,94 or harmful95,96. So, cells should not prolong their stay in either environment. This constant switching between different iron memory states can act as a bet-hedging strategy, common all organisms97. Changes in iron metabolism have been reported to affect mutation rates62, which can directly affect natural selection98. Iron levels can also influence the rate of antibiotic resistance99, host-pathogen interaction100–102, composition of the gut microbiome19, and various other stresses in both clinical and natural settings103. Given the importance of iron in diverse environments51, iron memory is expected to be widespread in nature and to impact other physiologies. In this study we tested two other pathways influenced by iron: entering the biofilm state and resistance to antibiotics. Swarming and biofilm formation are two opposite lifestyles. While low iron is a signal for swarming, high iron was observed to be a signal for biofilms (Fig. 5a). Low iron was also positively correlated with increased survival to antibiotics (Fig. 5b, c; red circles), likely because less cellular damage is expected when iron levels are low. Antibiotics trigger production of ROS64, and high iron levels would increase ROS, increasing lethality35. A multigenerational iron memory would improve the survival chances of at least some individuals within the population under antibiotic stress. Thus, iron memory is not restricted to swarming decisions alone, and may impact additional physiologies not tested here.
Why might iron pools encode memory? Given the central role of iron in cellular metabolism, an iron-based memory offers the advantage of providing a hub connecting various stress responses. For example, swarm cells are subject to surface stress30 while at the same time exhibiting adaptation to antibiotic stress44. Changes in iron outside are not necessary to change iron inside, given that multiple other stimuli can enforce this memory.
Limitations
1) Although we have provided a molecular framework by which iron memory operates, investigating the exact molecular basis of this memory is beyond the scope of this study. 2) An agent-based simulation is necessary to recapture the details of switching events with a feedback loop. 3) Data for the in-between generations (G2-G3, G5-G6) would have been ideal to collect, but lack of this information does not detract from the findings.
Methods
Strains, plasmids, media, and genetics
All bacterial strains, plasmids, and oligonucleotides are listed in Supplementary Table 1. The strains were propagated in LB broth (10 g/L tryptone, 5 g/L yeast extract, 5 g/L NaCl) or in 1.6% Bacto agar plates with the following antibiotics for marker selection as applicable – Kan (Kanamycin) 25 μg/ml, Amp (Ampicillin) 100 μg/ml, Cam (Chloramphenicol) 30 μg/ml.
fepA and fur genes were deleted in E. coli MG1655 by transferring the KanR deletion marker from appropriate donors strains in the Keio collection104 using P1 transduction (P1vir)105, and the deletions were confirmed by fepA-F/fepA-R and fur-F/furR oligo combinations respectively.
Collection of S cells
S cells were collected by flushing the actively moving edge of a 12h old swarm colony with 2 ml LB broth. After a quick spin of 1000g for 2 sec to remove any agar lumps that may have been flushed as well, the supernatant cell suspension was diluted to 0.5 OD600 for further assays.
Imaging of plates
Swarm plates were imaged using a Canon EOS M6 Mark II camera and a Canon EF-M 28mm Macro IS STM lens with the following manual settings – ISO100, f/8, 1/160. The camera-generated jpg files were then loaded into Adobe Photoshop to perform equal amount of tone curve, exposure, and shadow adjustments, which was achieved by using a pre-recorded action sequence as a macro. The processed images were then exported as B&W images for further analysis.
Colony Morphology
Hard agar LB plates (1.6% agar w/v) were dried for 8h at room temperature (RT), which is different from the normal practice of drying these plates for 24–48h before use. 100 μl of P and S cells from a 10−5 dilution of 0.5 OD600 were spread on these partially dried plates, which were incubated at 37°C for 16h and imaged. These images were processed in Adobe Photoshop as described above, then loaded in ImageJ106 and adjusted with a defined amount of thresholding. The circularity and diameter of the colonies was measured with the following settings of the particles: 200-infinity pixels, 0.3–1.00 circularity. ImageJ calculates circularity from the following equation: circularity = 4π(area/perimeter2); a perfect circle has a circularity of 1. The diameter was measured as the maximum distance between any two given points on the perimeter of the colony.
Dilution method
The overall dilution-to-extinction method46 used in this study is summarized in Extended Data Fig. 1a. Instead of FACS-based sorting, dilution was chosen as the primary method of single cell isolation because of its low cost and the relative ease of inoculating a large number of swarm plates immediately after final dilution. First, the OD600 of the starting cell suspension – either P or S – was adjusted to 0.5. Then, 100 μl of cell suspension was serially diluted by adding 900 μl of LB medium in each step. Any recalibration or change in pipettes (or change in personnel) was accompanied by redoing the standardization steps. The accuracy of dilution was always confirmed by CFU counts. Ascertaining that the fraction of wells with growth in a 96-well plate followed a Poisson distribution, was used as an additional confirmation (Extended Data Figure 1b).
SCI swarm assays
SCI swarm plates were prepared with 0.45% Eiken agar (Eiken Chem. Co. Japan) and 0.5% glucose. Swarm plates were dried for 8h at RT before use. 4 μL of the cell suspension was inoculated at the center of each plate and incubated at 30°C for 30h. The maximum diameter of each swarm was physically measured with a ruler. For those swarms with low radial symmetry, i.e., with no apparent max diameter upon visual inspection, three measurements with a ruler were made at different degrees of rotation to the center of the plate, and the highest value chosen as the max diameter. The highest value obtained was recorded to be the diameter.
Zone Adjustment
Since different inoculum sizes showed large variability in the lag period before initiating swarming, a time-point based comparison of diameters across groups would not be ideal because the mean values will vary greatly. To alleviate this problem, a zone-adjustment was performed to classify data from each group into specific zones. Three arbitrary zones were assigned – central (C, 0–20 mm), medial (M, 21–60 mm), outer (O, 61–85 mm). When at least 5 members of a given group entered in a zone, swarming diameters of all the members within that same group were classified as belonging to that zone. Taking different lag durations in each group into account, 18h, 12h, and 10h data of SCIs ,100, and 10000 groups, respectively, were classified as C. For ‘M’, these values were 24h, 18h, and 12h, and for ‘O’ they were 30h, 24h, and 24h. The frequency distributions of swarm diameters for each group were then calculated keeping a swarm diameter bin size of 5 mm.
‘Generation’ Swarm Assay
The ideal time points for daughter cell isolation from a specific generation was first standardized as summarized in Extended Data Fig. 2a–c. A suspension of 0.5 cell/ 4 μl was prepared as described in the ‘Dilution method’ section and used to inoculate 96-well plates pre-filled with 196 μl of LB medium. The plates were covered with lids, taped on the sides with parafilm, and then incubated at 37°C with shaking at 200 RPM. The number of cells per well were estimated by CFU counts at different times (see Extended Data Fig. 2a). Expectedly, some wells did not receive any cells (Extended Data Fig. 2b). Once the timepoints for specific generations were identified, the 96-well plates were inoculated and incubated as before, except, instead of CFU counts, the cells from G0, G4, and G7 were spotted on swarm plates (0.5 cell per plate) as summarized in Extended Data Fig. 2d–f.
Environmental and Genetic Perturbations
P cells were grown till 0.5 OD600 in 2 ml media under various environmental conditions. M9 medium (1x M9 salts, 2mM MgSO4, 0.1mM CaCl2, 0.4% glucose, 0.2% casamino acid) was used as non-rich medium, 0.1% or 10% NaCl was used for salt stress, acidic conditions were created by adjusting the pH of LB media to 6.0 or 8.0 by using 0.1 M HCl or NaOH respectively, oxygen availability was reduced by either maintaining culture tubes on bench (0 RPM) or with low shaking (50 RPM), and 0.01% or 0.05% Eiken agar was added to the LB liquid media to mimic the presence of agar in swarm plates. Cloned genes from the pCA24N plasmid-based ASKA library collection107, were induced with 0.1 mM IPTG. The flhDC clone was overexpressed from pBAD33 with 0.1% arabinose induction. To create iron-starved or iron-replete conditions, P cells were grown till 0.5 OD600 in 2 ml LB with 1 mM deferoxamine mesylate (DFO, Sigma) or 1 mM FeCl3 respectively. G0, G4, and G7 cells were collected as described in the “Generation’ Swarm Assay’ section For G12, cells were collected at 300 minutes after inoculation in wells.
Iron Biosensor
A Gibson assembly protocol108 was used to create the iron biosensor. First, the PfepA from WT genome, sfGFP from pMAZ plasmid, and the pTrc backbone excluding Ptrc promoter were PCR-amplified using primer combinations fepA-ins-F/ fepA-ins-R, sfGFP-ins-F/ sfGFP-ins-R, and pTrc-F/pTrc-R, respectively. For Gibson assembly of PfepAsfGFP, all three amplicons were incubated with 2.67 μl 5X ISO buffer, 0.05 of U T5 exonuclease (NEB), 0.34 U of Phusion polymerase (NEB), 53.34 U of Taq ligase (NEB) in a 20 μl reaction. The overlapping regions at the junction of PfepA and sfGFP fragments, and complete sequence of the clones were checked using primers fepA-check-F/sfGFP-check-R and Seq-check-F/Seq-check-R, respectively. Separately, control plasmid sfGFP, driven by Ptrc, was also constructed using Gibson assembly with fragments of sfGFP and pTrc plasmid backbone including Ptrc.
Microscopy
All cells were visualized using a light microscope with fluorescent channels (BX53F; Olympus, Tokyo, Japan). sfGFP was used to record fluorescence intensities from a GFP filter (Ex, 460–480 nm; Em 495–540 nm) keeping a constant camera exposure time of 100 ms. The outputs from the 100X objective were recorded on XM10 CCD sensor (Olympus, Tokyo, Japan) using cellSens software (v1.6).
FACS
WT and ΔfepA P cells harboring various reporter plasmid constructs, were grown till 0.5 OD600 in LB at 37°C, then collected by centrifugation, and resuspended in PBS. Cells were sorted on a BD Aria Fusion flow cytometer with BD FACSDiva (v.9.0.1) software (BD Biosciences) using a 488 nm excitation laser (530/30 band pass) and a gating strategy as illustrated in Extended Data Fig. 5a (see also Fig. 4c). The FACS sorter cannot deposit mother cells directly on a plate, so each sorted cell was deposited in one well of a 96-well plate prefilled with 20 μl LB, and then used for follow-up experiments as illustrated in Fig. 4d.
Swarm
For G0 mother data, each sorted cell was immediately collected from its well and spotted on a swarm plate. For G4-G7 data, 180 μl of LB was added to each sorted cell in its well. The 96-well plate was sealed and incubated as before to get G4 and G7 daughters.
Biofilm
A standard microplate biofilm assay61 was performed with minor modifications. Briefly, 80 μl of LB was added to each sorted cell in its well. The 96-well plates were then sealed with parafilm and incubated in 37°C for 24h. The plates were washed three times by dipping them into a bucket filled with deionized water pouring out the residual water. Each well was then filled with 125μl of 0.1% crystal violet and incubated for 15 min in RT. The plates were again washed with water and then incubated in 42°C without a lid for 1.5h. 125μl of 30% acetic acid was added to each well and plates incubated at RT for 15min. The solubilized crystal violet in each well was transferred to a new well of a fresh 96-well plate. The A550 reading was used as a measure of biofilm formation with 30% acetic acid serving as a blank control.
Antibiotic survival
To measure the survival of sorted cell in sub-MIC levels or antibiotic, each sorted cell was incubated with 2 μg/ml Kan or 1.5 μg/ml Cam in a final volume of 100 μl LB. The plates were sealed as before and incubated at 37°C with 200 RPM shaking for 4h and the growth was measured as OD600.
Swim assays
Fischer agar plates (0.3%) were used for swim motility assays by stabbing 4 μL of the cell suspension at the center, followed by incubation at 37°C.
Statistics and visualization
Statistical analyses were performed using Prizm v9 (Graphpad), Python (ggplot and numpy packages) using Jupyter, and Microsoft Excel. The data was visualized in Prizm and Python.
PCA
To identify environmental or genetic conditions that alter the swarming heterogeneity of G0 mothers, a PCA (Principal Component Analysis) was performed on the swarm diameter data shown in Fig. 3a. The calculated mean , SD , and the coefficient of variation of each sample was used as three variables for PCA48. The resulting PC1 (60.06%) could separate fepA and fur samples from others. The PCA biplot indicated that and influenced PC1 the most, while and influenced PC2 the most (Supplementary Fig. 3b).
Growth and motility clustering
The differences in growth and motility profiles of swarm colonies were measured and compared as illustrated in Extended Data Fig 3a. The images of swarm plates were processed on ImageJ with equal thresholding to measure the total area . The mean pixel intensity was measured using the IntDen function of ImageJ. The growth variable , i.e., the total pixel intensity of a swarm colony, was calculated as . The motility variable , i.e., the maximum diameter of a swarm colony was measured using a ruler. The and variables were min-max normalized within each cohort and clustered using KMeans package of sklearn module in Python.
Noise calculation and F-test
The coefficient of variation was calculated to measure noise for each sample group as where and denote standard deviation and mean respectively. The significance of differences among the values across samples were measured by calculating p values from F-test109 involving the following equation
where is sample size, ; 1 and 2 represent groups 1 and 2 respectively.
Dendrogram
To illustrate how a cell type can generate other cell types across generations, a dendrogram was created using the RAWGraphs package110. The fractions of different types of daughters after each generation, obtained from our mathematical modeling data, were used to calculate the approximate number of each type.
Mathematical Modeling
Classifying cells as poor (< 35 mm, XS), moderate (35–65 mm, M), and efficient swarmers (> 65 mm, L), we analyzed data from single mothers to quantify the population phenotypic heterogeneity with approximately 52%XS, 35% M, and 13% L swarmers. Tracking the state of individual cells in an expanding lineage reveals that the state of the initial single cell is maintained for approximately 4 generations (Fig. 2). However, this memory is transient and by 7 generations the lineage recreates the original population heterogeneity. We capture this transient memory using the following differential equation model:
where and represent the fraction of cells within a single-cell derived colony at time that are XS, M, and L swarmers, respectively. Here, time is normalized to the cell-cycle time so corresponds to one generation. The four parameters , are the kinetic rates of transitions between the different swarming states with being the transition rate from XS to M swarmer, and so on. To fit the model to data we further consider a delay, where the initial cell retains its state for a certain time before starting to switch. This can be incorporated into the model by considering if (when the initial cell is XS), if (when the initial cell is M) and if (when the initial cell is L). Outside these delays, these rates take constant values that are chosen such that and to ensure that after some time the steady-state fractions converge to 52% XL, 35% M, and 13% L swarmers. To estimate the parameters , we perform a least-squares fit between the model prediction at and data at G4 (Fig. 2), and model prediction at and the observed population heterogeneity of 52% XS, 35% M, and 13% L. The least-square fitting was done in Microsoft Excel using the Solver toolbox using the GRC Nonlinear solving method. The results of fitting are shown in Fig. 6b–d where the model captures the data at G4 and also recapitulate the population heterogeneity by the seventh generation. Based on the fitting we estimate the delays to be generations, generations per unit time.
Extended Data
Extended Data Fig. 1 |. Validation of dilution-to-extinction based single cell isolation.
a, Flow chart summarizing the single cell isolation protocol. The CFU counts of the 10−6 dilution () provided a mean estimate of cells per ml. This number was used to calculate the expected number of cells if diluted further. The desired dilution of 0.5 cell per 4 μl (=125 cells per ml) is obtained in the final dilution. A CFU count of this last dilution is designated ‘observed’ number of cells. b, A comparison of the observed and expected CFUs. The confidence interval values (CI 95%) are indicated. c, Flow chart summarizing the steps involved in swarm assays from SCIs (Single Cell Inoculums). Since this is the first report of such methods that involve swarming, the dilution was validated in every step. d, The dilution of 0.5 cells/ 4 μl was further validated by seeding each well of a 96-well plate with 0.5 cells. The expected positive number of events (wells that received cells) was calculated from a Poisson distribution (μ=0.5, n=96) and compared with the observed values, i.e., number of wells with visible growth after 18h. e, The expected positive number of events (plates that received cells), was also calculated from a Poisson distribution (μ=0.5, n=100) and compared with the observed values, i.e., number of plates with swarm colonies after 18h.
Extended Data Fig. 2 |. Swarm assays from single mother cells and their daughters from various generations of growth.
a-c, Infographic showing the standardization of time points for collecting daughters from different generations. a, The dilution of 0.5 cells per 4 μl was used to seed single mothers in wells (colored wells). From Poisson, it is expected that only ~40% of the wells would have received cells. CFU counts (y-axis) were performed on 10 wells at each time point (x axis) and the data from only positive events are shown in the dot plot. The expected number of cells after each generation are indicated. Only P mother data are shown here but both the P and S cells were in G4 and G7 at 100 min and 175 min respectively. b, The number of wells (from a) with no CFU (from P samples). Each dot represents a well. c, The confidence intervals of the observed number of cells in a. The data from S mothers are also shown here. d-f, Infographic showing the steps involved in swarm assays of SCIs collected from G4 or G7. d, The collected P or S cells were designated G0 immediately after the final dilution of 0.5 cells per 4 μl was made. Some G0 mothers were inoculated on swarm plates (e, μ=0.5, n=125 for both P and S), while other G0 mothers were seeded in wells and grown till G4 or G7. The total number of cells are very low at these time points, so no growth was visible. To get data from at least 25 mothers, 65 wells were randomly picked in each generation and processed as shown. A comparison of expected and observed positive outcomes is shown in f.
Extended Data Fig. 3 |. Growth and motility-based clustering of swarm data.
a, Infographic showing the steps involved in the clustering process. The images of swarm plates were processed to measure the variables, growth (VG) and motility (VM) as the total pixel intensity and the maximum diameter of a swarm respectively (see Methods). A K-means clustering was performed within each group. b, The results of clustering analysis of the different datasets as labeled: 1P, planktonic mothers at G0 (); 1P-G4 & 1P-G7, daughters of two random L mothers each from G4 and G7 (ñ=32 in each); 100P & 10000P, planktonic 100- and 10000-cell inoculums ( in each); 1S, swarm mothers at G0 (). The elbow method was used to identify the correct K value. Inset, the corresponding elbow plot showing the within-cluster sum of squares for different K values. Different clusters in each group are color-coded. b, Images of representative swarm plates from each cluster.
Extended Data Fig. 4 |. Growth & motility of iron-deficient strains.
a-c, Time-course measurements of various phenotypes of the indicated strains (): a, Growth curves in LB media at 37°C; b, Swimming motility measured (diameter) in 0.3% soft agar plates at 37°C; c, Swarm diameter measured on 0.5% agar at 30°C. d, Representative images of swarm plates of indicated strains at 20h. The numbers indicate replicates. e, Microscopic images of a WT strain with GFP driven from the fepA promoter, treated with FeCl3 or DFO.
Extended Data Fig. 5 |. Flow cytometry of iron-biosensor strains.
a, Flow cytometry scatter plots of singlet events, which were gated based on SSC-A (side scatter channel - area) and GFP intensity. Both x and y axes scales are biexponential. The number of events for each strain and associated singlet percentages are indicated. The heatmap is based on the kernel density estimate of the events. As a gating strategy, any singlet falling within the maximum value obtained in the GFP-A channel for the strain without any GFP (top row) was considered a negative event. The singlets above this threshold were considered positive events and sorted in individual wells of 96-well plates (see Supplementary Fig. 4 for sorting data). b, Frequency distributions of GFP intensity (x axis, log scale) from the corresponding strains in a. Note that the y-axis scales are not identical. A Gaussian fit and associated R2 value are shown.
Supplementary Material
Acknowledgements
We thank the following colleagues at UT Austin for their help – Alexandra Mey for suggestions with the iron biosensor design, Richard Salinas for assisting with FACS, and Yunesahng Hwang for assisting with biofilm assays. This work was supported by Public Health Service Grant GM118085 to R.M.H.
Footnotes
Declaration of Interests
The authors declare no competing interests.
References
- 1.Levins R. Evolution in changing environments. (Princeton University Press, Princeton. , 1968). [Google Scholar]
- 2.Agrawal A. A. Phenotypic plasticity in the interactions and evolution of species. Science 294, 321–326, doi: 10.1126/science.1060701 (2001). [DOI] [PubMed] [Google Scholar]
- 3.Balazsi G., van Oudenaarden A. & Collins J. J. Cellular decision making and biological noise: from microbes to mammals. Cell 144, 910–925, doi: 10.1016/j.cell.2011.01.030 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kandel E. R. The molecular biology of memory storage: a dialogue between genes and synapses. Science 294, 1030–1038, doi: 10.1126/science.1067020 (2001). [DOI] [PubMed] [Google Scholar]
- 5.Casadesus J. & D’Ari R. Memory in bacteria and phage. BioEssays : news and reviews in molecular, cellular and developmental biology 24, 512–518, doi: 10.1002/bies.10102 (2002). [DOI] [PubMed] [Google Scholar]
- 6.Chaudhuri R. & Fiete I. Computational principles of memory. Nature neuroscience 19, 394–403, doi: 10.1038/nn.4237 (2016). [DOI] [PubMed] [Google Scholar]
- 7.Thompson R. F. & Kim J. J. Memory systems in the brain and localization of a memory. Proceedings of the National Academy of Sciences of the United States of America 93, 13438–13444, doi: 10.1073/pnas.93.24.13438 (1996). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dunsmoor J. E. & Kroes M. C. W. Episodic memory and Pavlovian conditioning: ships passing in the night. Current opinion in behavioral sciences 26, 32–39, doi: 10.1016/j.cobeha.2018.09.019 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Squire L. R. & Zola S. M. Structure and function of declarative and nondeclarative memory systems. Proceedings of the National Academy of Sciences of the United States of America 93, 13515–13522, doi: 10.1073/pnas.93.24.13515 (1996). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dickinson A. & Mackintosh N. J. Classical conditioning in animals. Annual review of psychology 29, 587–612, doi: 10.1146/annurev.ps.29.020178.003103 (1978). [DOI] [PubMed] [Google Scholar]
- 11.Nguyen J., Lara-Gutierrez J. & Stocker R. Environmental fluctuations and their effects on microbial communities, populations and individuals. FEMS microbiology reviews 45, doi: 10.1093/femsre/fuaa068 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Norman T. M., Lord N. D., Paulsson J. & Losick R. Stochastic Switching of Cell Fate in Microbes. Annual review of microbiology 69, 381–403, doi: 10.1146/annurev-micro-091213-112852 (2015). [DOI] [PubMed] [Google Scholar]
- 13.Aertsen A. & Michiels C. W. Stress and how bacteria cope with death and survival. Critical reviews in microbiology 30, 263–273, doi: 10.1080/10408410490884757 (2004). [DOI] [PubMed] [Google Scholar]
- 14.Ferrell J. E. Jr. Self-perpetuating states in signal transduction: positive feedback, double-negative feedback and bistability. Current opinion in cell biology 14, 140–148, doi: 10.1016/s0955-0674(02)00314-9 (2002). [DOI] [PubMed] [Google Scholar]
- 15.Balaban N. Q., Merrin J., Chait R., Kowalik L. & Leibler S. Bacterial persistence as a phenotypic switch. Science 305, 1622–1625, doi: 10.1126/science.1099390 (2004). [DOI] [PubMed] [Google Scholar]
- 16.Ratcliff W. C. & Denison R. F. Bacterial persistence and bet hedging in Sinorhizobium meliloti. Communicative & integrative biology 4, 98–100, doi: 10.4161/cib.4.1.14161 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wolf D. M. et al. Memory in microbes: quantifying history-dependent behavior in a bacterium. PloS one 3, e1700, doi: 10.1371/journal.pone.0001700 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gokhale C. S., Giaimo S. & Remigi P. Memory shapes microbial populations. PLoS computational biology 17, e1009431, doi: 10.1371/journal.pcbi.1009431 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Letourneau J. et al. Ecological memory of prior nutrient exposure in the human gut microbiome. The ISME journal 16, 2479–2490, doi: 10.1038/s41396-022-01292-x (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Miyaue S. et al. Bacterial Memory of Persisters: Bacterial Persister Cells Can Retain Their Phenotype for Days or Weeks After Withdrawal From Colony-Biofilm Culture. Frontiers in microbiology 9, 1396, doi: 10.3389/fmicb.2018.01396 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lee C. K. et al. Multigenerational memory and adaptive adhesion in early bacterial biofilm communities. Proceedings of the National Academy of Sciences of the United States of America 115, 4471–4476, doi: 10.1073/pnas.1720071115 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yang C. Y. et al. Encoding Membrane-Potential-Based Memory within a Microbial Community. Cell systems 10, 417–423 e413, doi: 10.1016/j.cels.2020.04.002 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Skanata A. & Kussell E. Ecological memory preserves phage resistance mechanisms in bacteria. Nature communications 12, 6817, doi: 10.1038/s41467-021-26609-w (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.van der Woude M., Braaten B. & Low D. Epigenetic phase variation of the pap operon in Escherichia coli. Trends in microbiology 4, 5–9, doi: 10.1016/0966-842x(96)81498-3 (1996). [DOI] [PubMed] [Google Scholar]
- 25.Braaten B. A., Nou X., Kaltenbach L. S. & Low D. A. Methylation patterns in pap regulatory DNA control pyelonephritis-associated pili phase variation in E. coli. Cell 76, 577–588, doi: 10.1016/0092-8674(94)90120-1 (1994). [DOI] [PubMed] [Google Scholar]
- 26.Chai Y., Norman T., Kolter R. & Losick R. An epigenetic switch governing daughter cell separation in Bacillus subtilis. Genes & development 24, 754–765, doi: 10.1101/gad.1915010 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gosztolai A. & Barahona M. Cellular memory enhances bacterial chemotactic navigation in rugged environments. Communications Physics 3, 47, doi: 10.1038/s42005-020-0312-8 (2020). [DOI] [Google Scholar]
- 28.Vladimirov N. & Sourjik V. Chemotaxis: how bacteria use memory. Biological chemistry 390, 1097–1104, doi: 10.1515/BC.2009.130 (2009). [DOI] [PubMed] [Google Scholar]
- 29.Parkinson J. S. Signal transduction schemes of bacteria. Cell 73, 857–871, doi: 10.1016/0092-8674(93)90267-t (1993). [DOI] [PubMed] [Google Scholar]
- 30.Harshey R. M. Bacterial motility on a surface: many ways to a common goal. Annual review of microbiology 57, 249–273, doi: 10.1146/annurev.micro.57.030502.091014 (2003). [DOI] [PubMed] [Google Scholar]
- 31.Harshey R. M. Bees aren’t the only ones: swarming in gram-negative bacteria. Molecular microbiology 13, 389–394, doi: 10.1111/j.1365-2958.1994.tb00433.x (1994). [DOI] [PubMed] [Google Scholar]
- 32.Kearns D. B. A field guide to bacterial swarming motility. Nature reviews. Microbiology 8, 634–644, doi: 10.1038/nrmicro2405 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Partridge J. D. & Harshey R. M. Swarming: flexible roaming plans. Journal of bacteriology 195, 909–918, doi: 10.1128/JB.02063-12 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Verstraeten N. et al. Living on a surface: swarming and biofilm formation. Trends in microbiology 16, 496–506, doi: 10.1016/j.tim.2008.07.004 (2008). [DOI] [PubMed] [Google Scholar]
- 35.Andrews S. C., Robinson A. K. & Rodriguez-Quinones F. Bacterial iron homeostasis. FEMS microbiology reviews 27, 215–237, doi: 10.1016/S0168-6445(03)00055-X (2003). [DOI] [PubMed] [Google Scholar]
- 36.McCarter L. & Silverman M. Iron regulation of swarmer cell differentiation of Vibrio parahaemolyticus. Journal of bacteriology 171, 731–736, doi: 10.1128/jb.171.2.731-736.1989 (1989). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lin C. S. et al. An iron detection system determines bacterial swarming initiation and biofilm formation. Scientific reports 6, 36747, doi: 10.1038/srep36747 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wang Q., Frye J. G., McClelland M. & Harshey R. M. Gene expression patterns during swarming in Salmonella typhimurium: genes specific to surface growth and putative new motility and pathogenicity genes. Molecular microbiology 52, 169–187, doi: 10.1111/j.1365-2958.2003.03977.x (2004). [DOI] [PubMed] [Google Scholar]
- 39.Tague J. G., Regmi A., Gregory G. J. & Boyd E. F. Fis Connects Two Sensory Pathways, Quorum Sensing and Surface Sensing, to Control Motility in Vibrio parahaemolyticus. Frontiers in microbiology 12, 669447, doi: 10.3389/fmicb.2021.669447 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Matilla M. A. et al. Temperature and pyoverdine-mediated iron acquisition control surface motility of Pseudomonas putida. Environmental microbiology 9, 1842–1850, doi: 10.1111/j.1462-2920.2007.01286.x (2007). [DOI] [PubMed] [Google Scholar]
- 41.Gode-Potratz C. J., Kustusch R. J., Breheny P. J., Weiss D. S. & McCarter L. L. Surface sensing in Vibrio parahaemolyticus triggers a programme of gene expression that promotes colonization and virulence. Molecular microbiology 79, 240–263, doi: 10.1111/j.1365-2958.2010.07445.x (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pearson M. M., Rasko D. A., Smith S. N. & Mobley H. L. Transcriptome of swarming Proteus mirabilis. Infection and immunity 78, 2834–2845, doi: 10.1128/IAI.01222-09 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tremblay J. & Deziel E. Gene expression in Pseudomonas aeruginosa swarming motility. BMC genomics 11, 587, doi: 10.1186/1471-2164-11-587 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bhattacharyya S., Walker D. M. & Harshey R. M. Dead cells release a ‘necrosignal’ that activates antibiotic survival pathways in bacterial swarms. Nature communications 11, 4157, doi: 10.1038/s41467-020-17709-0 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kearns D. B. & Losick R. Swarming motility in undomesticated Bacillus subtilis. Molecular microbiology 49, 581–590, doi: 10.1046/j.1365-2958.2003.03584.x (2003). [DOI] [PubMed] [Google Scholar]
- 46.Ishii S., Tago K. & Senoo K. Single-cell analysis and isolation for microbiology and biotechnology: methods and applications. Applied microbiology and biotechnology 86, 1281–1292, doi: 10.1007/s00253-010-2524-4 (2010). [DOI] [PubMed] [Google Scholar]
- 47.Butler M. T., Wang Q. & Harshey R. M. Cell density and mobility protect swarming bacteria against antibiotics. Proceedings of the National Academy of Sciences of the United States of America 107, 3776–3781, doi: 10.1073/pnas.0910934107 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Johnstone I. M. & Lu A. Y. On Consistency and Sparsity for Principal Components Analysis in High Dimensions. Journal of the American Statistical Association 104, 682–693, doi: 10.1198/jasa.2009.0121 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Toguchi A., Siano M., Burkart M. & Harshey R. M. Genetics of swarming motility in Salmonella enterica serovar typhimurium: critical role for lipopolysaccharide. Journal of bacteriology 182, 6308–6321, doi: 10.1128/jb.182.22.6308-6321.2000 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bhattacharyya S. et al. Efflux-linked accelerated evolution of antibiotic resistance at a population edge. Molecular cell 82, 4368–4385 e4366, doi: 10.1016/j.molcel.2022.10.024 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mey A. R., Gomez-Garzon C. & Payne S. M. Iron Transport and Metabolism in Escherichia, Shigella, and Salmonella. EcoSal Plus 9, eESP00342020, doi: 10.1128/ecosalplus.ESP-0034-2020 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hirayama T. & Nagasawa H. Chemical tools for detecting Fe ions. Journal of clinical biochemistry and nutrition 60, 39–48, doi: 10.3164/jcbn.16-70 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zheng T. & Nolan E. M. Siderophore-based detection of Fe(III) and microbial pathogens. Metallomics : integrated biometal science 4, 866–880, doi: 10.1039/c2mt20082a (2012). [DOI] [PubMed] [Google Scholar]
- 54.McHugh J. P. et al. Global iron-dependent gene regulation in Escherichia coli. A new mechanism for iron homeostasis. The Journal of biological chemistry 278, 29478–29486, doi: 10.1074/jbc.M303381200 (2003). [DOI] [PubMed] [Google Scholar]
- 55.Inoue T. et al. Genome-wide screening of genes required for swarming motility in Escherichia coli K-12. Journal of bacteriology 189, 950–957, doi: 10.1128/JB.01294-06 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sarvan S., Butcher J., Stintzi A. & Couture J. F. Variation on a theme: investigating the structural repertoires used by ferric uptake regulators to control gene expression. Biometals : an international journal on the role of metal ions in biology, biochemistry, and medicine 31, 681–704, doi: 10.1007/s10534-018-0120-8 (2018). [DOI] [PubMed] [Google Scholar]
- 57.Pedelacq J. D., Cabantous S., Tran T., Terwilliger T. C. & Waldo G. S. Engineering and characterization of a superfolder green fluorescent protein. Nature biotechnology 24, 79–88, doi: 10.1038/nbt1172 (2006). [DOI] [PubMed] [Google Scholar]
- 58.Kosman D. J. Energy metabolism, oxygen flux, and iron in bacteria: The Mossbauer report. The Journal of biological chemistry 294, 63–64, doi: 10.1074/jbc.H118.006703 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wu Y. & Outten F. W. IscR controls iron-dependent biofilm formation in Escherichia coli by regulating type I fimbria expression. Journal of bacteriology 191, 1248–1257, doi: 10.1128/JB.01086-08 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Oliveira F., Rohde H., Vilanova M. & Cerca N. The Emerging Role of Iron Acquisition in Biofilm-Associated Infections. Trends in microbiology 29, 772–775, doi: 10.1016/j.tim.2021.02.009 (2021). [DOI] [PubMed] [Google Scholar]
- 61.O’Toole G. A. Microtiter dish biofilm formation assay. Journal of visualized experiments : JoVE, doi: 10.3791/2437 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Mehi O. et al. Perturbation of iron homeostasis promotes the evolution of antibiotic resistance. Molecular biology and evolution 31, 2793–2804, doi: 10.1093/molbev/msu223 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kohanski M. A., Dwyer D. J. & Collins J. J. How antibiotics kill bacteria: from targets to networks. Nature reviews. Microbiology 8, 423–435, doi: 10.1038/nrmicro2333 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kohanski M. A., Dwyer D. J., Hayete B., Lawrence C. A. & Collins J. J. A common mechanism of cellular death induced by bactericidal antibiotics. Cell 130, 797–810, doi: 10.1016/j.cell.2007.06.049 (2007). [DOI] [PubMed] [Google Scholar]
- 65.Kramer J., Ozkaya O. & Kummerli R. Bacterial siderophores in community and host interactions. Nature reviews. Microbiology 18, 152–163, doi: 10.1038/s41579-019-0284-4 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Bergmiller T. et al. Biased partitioning of the multidrug efflux pump AcrAB-TolC underlies long-lived phenotypic heterogeneity. Science 356, 311–315, doi: 10.1126/science.aaf4762 (2017). [DOI] [PubMed] [Google Scholar]
- 67.Peixoto L. L. et al. Memory acquisition and retrieval impact different epigenetic processes that regulate gene expression. BMC genomics 16 Suppl 5, S5, doi: 10.1186/1471-2164-16-S5-S5 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Riber L. & Hansen L. H. Epigenetic Memories: The Hidden Drivers of Bacterial Persistence? Trends in microbiology 29, 190–194, doi: 10.1016/j.tim.2020.12.005 (2021). [DOI] [PubMed] [Google Scholar]
- 69.Wang W. & Zou X. Modeling the role of altruism of antibiotic-resistant bacteria. Journal of mathematical biology 68, 1317–1339, doi: 10.1007/s00285-013-0668-4 (2014). [DOI] [PubMed] [Google Scholar]
- 70.Jaruszewicz-Blonska J. & Lipniacki T. Genetic toggle switch controlled by bacterial growth rate. BMC systems biology 11, 117, doi: 10.1186/s12918-017-0483-4 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Brunet Y. R., Bernard C. S., Gavioli M., Lloubes R. & Cascales E. An epigenetic switch involving overlapping fur and DNA methylation optimizes expression of a type VI secretion gene cluster. PLoS genetics 7, e1002205, doi: 10.1371/journal.pgen.1002205 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Singh A. & Saint-Antoine M. Probing transient memory of cellular states using single-cell lineages. Frontiers in microbiology 13, 1050516, doi: 10.3389/fmicb.2022.1050516 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kaern M., Elston T. C., Blake W. J. & Collins J. J. Stochasticity in gene expression: from theories to phenotypes. Nature reviews. Genetics 6, 451–464, doi: 10.1038/nrg1615 (2005). [DOI] [PubMed] [Google Scholar]
- 74.Swain P. S., Elowitz M. B. & Siggia E. D. Intrinsic and extrinsic contributions to stochasticity in gene expression. Proceedings of the National Academy of Sciences of the United States of America 99, 12795–12800, doi: 10.1073/pnas.162041399 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Elowitz M. B., Levine A. J., Siggia E. D. & Swain P. S. Stochastic gene expression in a single cell. Science 297, 1183–1186, doi:DOI 10.1126/science.1070919 (2002). [DOI] [PubMed] [Google Scholar]
- 76.Eldar A. & Elowitz M. B. Functional roles for noise in genetic circuits. Nature 467, 167–173, doi: 10.1038/nature09326 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Sorek M., Balaban N. Q. & Loewenstein Y. Stochasticity, Bistability and the Wisdom of Crowds: A Model for Associative Learning in Genetic Regulatory Networks. PLoS computational biology 9, doi:ARTN e1003179 10.1371/journal.pcbi.1003179 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Carrasco-Pujante J. et al. Associative Conditioning Is a Robust Systemic Behavior in Unicellular Organisms: An Interspecies Comparison. Frontiers in microbiology 12, doi:Artn 707086 10.3389/Fmicb.2021.707086 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Zhang H. Q. et al. Programming a Pavlovian-like conditioning circuit in Escherichia coli. Nature communications 5, doi:Artn 3102 10.1038/Ncomms4102 (2014). [DOI] [PubMed] [Google Scholar]
- 80.Huh D. & Paulsson J. Random partitioning of molecules at cell division. Proceedings of the National Academy of Sciences of the United States of America 108, 15004–15009, doi: 10.1073/pnas.1013171108 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Baik A. H. et al. Oxygen toxicity causes cyclic damage by destabilizing specific Fe-S cluster-containing protein complexes. Molecular cell 83, 942-+, doi: 10.1016/j.molcel.2023.02.013 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Imlay J. A. Iron-sulphur clusters and the problem with oxygen. Molecular microbiology 59, 1073–1082, doi: 10.1111/j.1365-2958.2006.05028.x (2006). [DOI] [PubMed] [Google Scholar]
- 83.Sen S., Ghosh P. & Ray D. S. Reaction-diffusion systems with stochastic time delay in kinetics. Physical Review E 81, doi:Artn 056207 10.1103/Physreve.81.056207 (2010). [DOI] [PubMed] [Google Scholar]
- 84.Yosef N. & Regev A. Impulse Control: Temporal Dynamics in Gene Transcription. Cell 144, 886–896, doi: 10.1016/j.cell.2011.02.015 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Pyragas K. Continuous Control of Chaos by Self-Controlling Feedback. Phys Lett A 170, 421–428, doi:Doi 10.1016/0375-9601(92)90745-8 (1992). [DOI] [Google Scholar]
- 86.Lam F. H., Steger D. J. & O’Shea E. K. Chromatin decouples promoter threshold from dynamic range. Nature 453, 246–U216, doi: 10.1038/nature06867 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Semsey S. et al. Genetic regulation of fluxes: iron homeostasis of Escherichia coli. Nucleic acids research 34, 4960–4967, doi: 10.1093/nar/gkl627 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.De Lorenzo V., Herrero M., Giovannini F. & Neilands J. B. Fur (ferric uptake regulation) protein and CAP (catabolite-activator protein) modulate transcription of fur gene in Escherichia coli. European journal of biochemistry 173, 537–546, doi: 10.1111/j.1432-1033.1988.tb14032.x (1988). [DOI] [PubMed] [Google Scholar]
- 89.Zheng M., Doan B., Schneider T. D. & Storz G. OxyR and SoxRS regulation of fur. Journal of bacteriology 181, 4639–4643, doi: 10.1128/JB.181.15.4639-4643.1999 (1999). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Seo S. W., Kim D., O’Brien E. J., Szubin R. & Palsson B. O. Decoding genome-wide GadEWX-transcriptional regulatory networks reveals multifaceted cellular responses to acid stress in Escherichia coli. Nature communications 6, 7970, doi: 10.1038/ncomms8970 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Garcia P. S. et al. An early origin of iron-sulfur cluster biosynthesis machineries before Earth oxygenation. Nature ecology & evolution 6, 1564-+, doi: 10.1038/s41559-022-01857-1 (2022). [DOI] [PubMed] [Google Scholar]
- 92.Nixon S. L., Bonsall E. & Cockell C. S. Limitations of microbial iron reduction under extreme conditions. FEMS microbiology reviews 46, doi: 10.1093/femsre/fuac033 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Beauchene N. A. et al. Impact of Anaerobiosis on Expression of the Iron-Responsive Fur and RyhB Regulons. mBio 6, doi:ARTN e01947–15 10.1128/mBio.01947-15 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Wofford J. D., Bolaji N., Dziuba N., Outten F. W. & Lindahl P. A. Evidence that a respiratory shield in Escherichia coli protects a low-molecular-mass Fe-II pool from O-2-dependent oxidation. Journal of Biological Chemistry 294, 50–62, doi: 10.1074/jbc.RA118.005233 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Thomas M. D. et al. Too much of a good thing Adaption to iron (II) intoxication in Escherichia coli. Evol Med Public Hlth 9, 53–67, doi: 10.1093/emph/eoaa051 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Chan D. C. K., Guo I. & Burrows L. L. Forging New Antibiotic Combinations under Iron-Limiting Conditions. Antimicrobial agents and chemotherapy 64, doi:ARTN e01909–19 10.1128/AAC.01909-19 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Cohen D. Optimizing Reproduction in a Randomly Varying Environment. Journal of theoretical biology 12, 119-&, doi:Doi 10.1016/0022-5193(66)90188-3 (1966). [DOI] [PubMed] [Google Scholar]
- 98.Birzu G., Matin S., Hallatschek O. & Korolev K. S. Genetic drift in range expansions is very sensitive to density dependence in dispersal and growth. Ecology letters 22, 1817–1827, doi: 10.1111/ele.13364 (2019). [DOI] [PubMed] [Google Scholar]
- 99.Mehi O. et al. Perturbation of Iron Homeostasis Promotes the Evolution of Antibiotic Resistance. Molecular biology and evolution 31, 2793–2804, doi: 10.1093/molbev/msu223 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Barber M. F. & Elde N. C. Escape from bacterial iron piracy through rapid evolution of transferrin. Science 346, 1362–1366, doi: 10.1126/science.1259329 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Chu B. C. et al. Siderophore uptake in bacteria and the battle for iron with the host; a bird’s eye view. Biometals : an international journal on the role of metal ions in biology, biochemistry, and medicine 23, 601–611, doi: 10.1007/s10534-010-9361-x (2010). [DOI] [PubMed] [Google Scholar]
- 102.Raines D. J. et al. Bacteria in an intense competition for iron: Key component of the Campylobacter jejuni iron uptake system scavenges enterobactin hydrolysis product. Proceedings of the National Academy of Sciences of the United States of America 113, 5850–5855, doi: 10.1073/pnas.1520829113 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Cornelis P., Wei Q., Andrews S. C. & Vinckx T. Iron homeostasis and management of oxidative stress response in bacteria. Metallomics : integrated biometal science 3, 540–549, doi: 10.1039/c1mt00022e (2011). [DOI] [PubMed] [Google Scholar]
- 104.Baba T. et al. Construction of Escherichia coli K-12 in-frame, single-gene knockout mutants: the Keio collection. Molecular systems biology 2, 2006 0008, doi: 10.1038/msb4100050 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Sternberg N. L. & Maurer R. Bacteriophage-mediated generalized transduction in Escherichia coli and Salmonella typhimurium. Methods in enzymology 204, 18–43, doi: 10.1016/0076-6879(91)04004-8 (1991). [DOI] [PubMed] [Google Scholar]
- 106.Schneider C. A., Rasband W. S. & Eliceiri K. W. NIH Image to ImageJ: 25 years of image analysis. Nature methods 9, 671–675, doi: 10.1038/nmeth.2089 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Kitagawa M. et al. Complete set of ORF clones of Escherichia coli ASKA library (a complete set of E. coli K-12 ORF archive): unique resources for biological research. DNA research : an international journal for rapid publication of reports on genes and genomes 12, 291–299, doi: 10.1093/dnares/dsi012 (2005). [DOI] [PubMed] [Google Scholar]
- 108.Gibson D. G. et al. Enzymatic assembly of DNA molecules up to several hundred kilobases. Nature methods 6, 343–U341, doi: 10.1038/Nmeth.1318 (2009). [DOI] [PubMed] [Google Scholar]
- 109.Forkman J. Estimator and Tests for Common Coefficients of Variation in Normal Distributions. Commun Stat-Theor M 38, 233–251, doi:Pii 905074839 10.1080/03610920802187448 (2009). [DOI] [Google Scholar]
- 110.Mauri M., Elli T., Caviglia G., Uboldi G. & Azzi M. in Proceedings of the 12th Biannual Conference on Italian SIGCHI Chapter Article 28 (Association for Computing Machinery, Cagliari, Italy, 2017). [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.