Abstract
Coxsackievirus infection causes severe pancreatitis and myocarditis in humans, often leading to death in young or immunocompromised individuals. In susceptible strains of mice, coxsackievirus strain CB4 causes lethal hypoglycemia. To investigate the potential of gamma interferon (IFN-γ) in protection and clearance of the viral infection, IFN-γ knockout mice and transgenic (Tg) mice specifically expressing IFN-γ in their pancreatic β cells were infected with CB4. Lack of IFN-γ in mice normally resistant to CB4-mediated disease resulted in hypoglycemia and rapid death. However, expression of IFN-γ in the β cells of Tg mice otherwise susceptible to lethal infection allowed for survival and protected them from developing the accompanying hypoglycemia. While all the mice had high levels of viral replication in their pancreata and comparable tissue pathology following viral infection, the Tg mice had significantly lower levels of virus at the peak of infection, significantly higher numbers of activated macrophages before and after infection, and less damage to their acinar tissue. Additionally, despite having increased levels of inducible nitric oxide synthetase (iNOS) expression, treatment of Tg mice with the iNOS inhibitor aminoguanidine did not alter the level of protection afforded by IFN-γ expression. In conclusion, IFN-γ protects from lethal coxsackievirus infection by activating macrophages in an iNOS-independent manner.
Induced in response to immune stimulation, tissue damage, and viral replication, gamma interferon (IFN-γ) is an important mediator of cellular inflammation. As a pluripotent inducer of cellular and immune processes, IFN-γ has been shown not only to protect tissue from virus-mediated damage (21) but also to induce tissue damage on its own (16, 24). The ability of this cytokine to both directly protect and damage tissue is most likely associated with the local host factors within the target tissue compartment.
Numerous infectious agents have been associated with acute pancreatitis, and there is well-documented clinical evidence linking coxsackievirus B4 (CB4) to the development of both pancreatitis and diabetes in humans (4, 32). CB4 was isolated from patients suffering from both pancreatitis and diabetes and, after passage through murine cells, was subsequently demonstrated to infect mice (14, 31, 32). Following CB4 infection, the virus replicates in a number of tissues, specifically targeting the acinar tissue of the pancreas for pathology, causing severe pancreatitis similar to that observed in humans. However, the role of cytokines and inflammatory mediators in the development of pancreatitis has not been investigated thoroughly. Since IFN-γ is an important mediator of immune responses in vivo, it is a likely participant in the CB4-mediated immunopathogenesis.
To determine whether IFN-γ has a role in this virus-mediated disease, mice lacking systemic expression of IFN-γ (IFN-γ knockout mice [GKO]) (7) and transgenic mice overexpressing IFN-γ in the pancreas (NOD–IFN-γ) (12) were infected with CB4. The ability of IFN-γ to control the infection and to protect mice from the resulting CB4-mediated pancreatitis was then tested.
MATERIALS AND METHODS
Mice.
NOD/SHI and NOD/SCID mice were obtained from the rodent breeding colony at The Scripps Research Institute (La Jolla, Calif.). GKO mice of the H-2b haplotype were provided by D. Dalton (Trudeau Institute, Saranac, N.Y.) (7). Heterozygous GKO (+/−) mice were crossed with (129/SvEv × C57BL/6)F1 mice in our animal facility to generate homozygous (−/−) GKO (129/SvEv × C57BL/6)F2 mice. (129/SvJ × C57BL/6)F2 mice were obtained from The Jackson Laboratory (Bar Harbor, Maine), bred, and maintained in our colony. In addition, C57BL/6 mice were used as controls and showed results similar to those of the (129/SvJ × C57BL/6)F2 mice presented herein. Both 129/SvEv and 129/SvJ mice are derived from the same parental strain. The difference between the two substrains is that the SvEv was crossed once with C3H and the F1 was backcrossed 14 times to the Sv parental strain, but the SvEv substrain is 99.99% similar to SvJ (27).
The NOD–IFN-γ Tg mice were previously developed in our laboratory (12) and were bred and maintained there as well. Blood glucose was measured in tail vein or eye bleeds from nonfasting mice at various times postinfection with a standard glucometer with a range of 20 to 400 mg/dl.
NOD mice were used in this study because the transgene was derived on the NOD major histocompatibility complex (MHC) background. Female NOD mice spontaneously develop diabetes at a rate of 85% (30% for males) by 16 to 20 weeks of age in our colony. All mice were infected between 6 and 8 weeks of age and were tested prior to infection to ensure that they were not diabetic.
Virus.
Virus stocks of coxsackievirus group B type 4 Edwards strain 2 (CB4 strain E2) were obtained from Charles Gauntt (University of Texas—San Antonio) and were derived from stocks originating from Roger Loria (Medical College of Virginia, Virginia Commonwealth University) (31).
Virus stocks of CB4 strain E2 were prepared in monolayers of HeLa cells using a multiplicity of infection (MOI) of 0.1 PFU/cell in Dulbecco’s modified Eagle medium. Virus was harvested by freeze-thawing and stored at −80°C. Viral titers were determined on HeLa cell monolayers by using a standard plaque assay technique. Mice were infected at 6 to 8 weeks of age intraperitoneally (i.p.) with 104 PFU of CB4. The observed 50% lethal dose for this virus stock in NOD mice was calculated to be 100 PFU. Virus was plaque assayed in tissue from individual organs, aseptically weighed, and homogenized in diluent.
Immunohistochemical staining.
Mice were anesthetized before their organs were removed, placed in 10% formaldehyde, and processed for paraffin sectioning. Additionally, organs were snap frozen in O.C.T. on dry ice. Immunohistochemistry was performed on 4-μm-thick paraffin sections or 7-μm-thick frozen sections prepared and blocked with avidin and biotin (Vector Laboratories, Burlingame, Calif.). Staining was done with primary antibody to insulin (Dako, Carpinteria, Calif.), inducible nitric oxide synthetase (iNOS) (Transduction Laboratories, Lexington, Ky.), CB4 (American Type Culture Collection, Rockville, Md.), or F4/80 (Serotec, Washington, D.C.). The second antibody was a biotinylated anti-guinea pig immunoglobulin G (IgG), anti-rabbit IgG, anti-rat IgG, or anti-horse IgG used in conjunction with the Vectastain ABC (peroxidase) kits (Vector Laboratories). Staining was detected by using diaminobenzidine as a chromagen. Sections were counterstained in Mayer’s hematoxylin (Sigma, St. Louis, Mo.) and mounted in Permount (Fisher, Pittsburgh, Pa.).
AG treatment of mice.
Mice were divided into multiple groups: CB4-infected NOD and NOD–IFN-γ mice receiving aminoguanidine (AG) (Sigma) or phosphate-buffered saline (PBS) and sham-infected animals receiving AG or PBS. AG (8 mg dissolved in 0.5 ml of PBS) or 0.5 ml of PBS was injected i.p. followed by i.p. CB4 inoculation as described above. Thereafter, the mice were injected twice daily with 4 mg of AG or PBS for a total of 8 mg/day for 14 days. Mice were sacrificed on days 4, 7, and 14, and tissues were divided in half for plaque assay and histological analysis. In order to determine if AG was able to block in vivo iNOS activity, mice were bled prior to treatment and 24 h postinfection and posttreatment. Plasma samples were obtained through Ultrafree-MC columns (Millipore Corp., Bedford, Mass.) at 14,000 × g for 30 min at 4°C. Levels of NO2− and NO3− (NO2−/NO3−), the stable degradation products of nitric oxide (NO), were determined in plasma by using the Cayman nitrite/nitrate assay kit (Alexis Biochemicals, San Diego, Calif.). Treatment with AG in both NOD and NOD–IFN-γ Tg mice did not lead to increased rates of mortality in infected or uninfected mice.
Statistical analysis.
The unpaired Student t test was performed for all viral plaque assay analyses, and significant differences and P values are indicated in the figures and figure legends.
RESULTS
Susceptibility to CB4 infection in mice lacking IFN-γ.
CB4 infects mice with a variety of clinical outcomes. While high doses of virus (104 to 106 PFU per mouse) introduced i.p. to SJL, C57BL/6, and B10 mice lead to viral clearance by 2 weeks and survival, a similar inoculum in BALB/c and NOD mice led to rapid death (within 1 week). The primary tissue target for destruction of CB4 is the pancreas, as mice are hypoglycemic following infection and surviving mice suffer from severe pancreatitis with the loss of acinar tissue. While the pancreatic islets appear infected, islet tissue loss is not observed. Additionally, other tissues are infected, and mice suffer from mild myocarditis as well as inflammation in the lungs, kidneys, and liver. As a result of the severe pancreatitis, all infected mice exhibit an initial period of hypoglycemia following infection, from which susceptible mice (BALB/c and NOD) never recover.
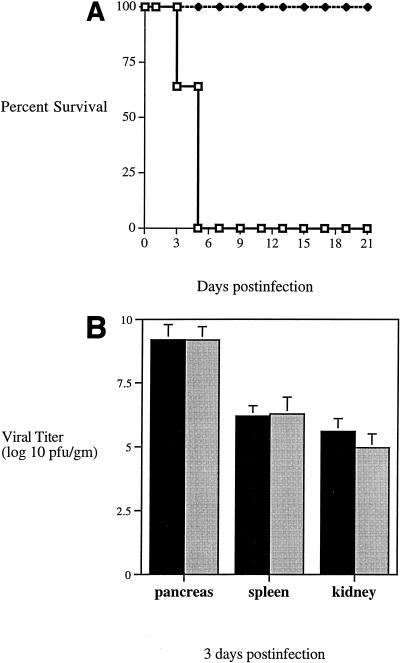
To evaluate the role of IFN-γ in susceptibility of mice to tissue damage following CB4 infection, GKO (7) and genetically matched control mice [(129/SvJ × C57BL/6)F2] were infected with CB4. Although initially hypoglycemic at 1 week after infection, the normally resistant control mice were healthy and survived for more than 3 months. On the other hand, the MHC-matched GKO mice exhibited hypoglycemic blood glucose levels (<50 mg/dl; n = 8), and none survived past day 5 postinfection (Fig. 1A).
FIG. 1.
(A) Survival of GKO mice following CB4 infection. Mice were given 104 PFU of coxsackievirus B4 and were monitored over time for survival. Both male (n = 4) and female (n = 7) GKO mice (□) were infected, while equal numbers of male (n = 4) and female (n = 4) C57BL/6 × 129 mice (⧫) were infected (n = 8). (B) Viral recovery from infected GKO mice. Mice were infected with CB4 and sacrificed at 3 days postinfection. Organs were harvested and assayed for virus recovery by standard plaque assay. Four GKO (■) and eight C57BL/6 × 129 (░⃞) mice were assayed. Standard deviations are indicated by error bars. No significant differences were observed.
We next measured the replication of CB4 to determine whether the poor survival of mice lacking IFN-γ correlated with an increased virus load. Quantitation of viral replication by plaque assay demonstrated that, despite the lack of IFN-γ and differences in clinical outcome, no significant differences in viral titer were observed at either 3 (Fig. 1B) or 4 (data not shown) days postinfection for GKO and control mice in the pancreata, spleens, or kidneys. This indicated that IFN-γ does not promote survival by limiting virus replication.
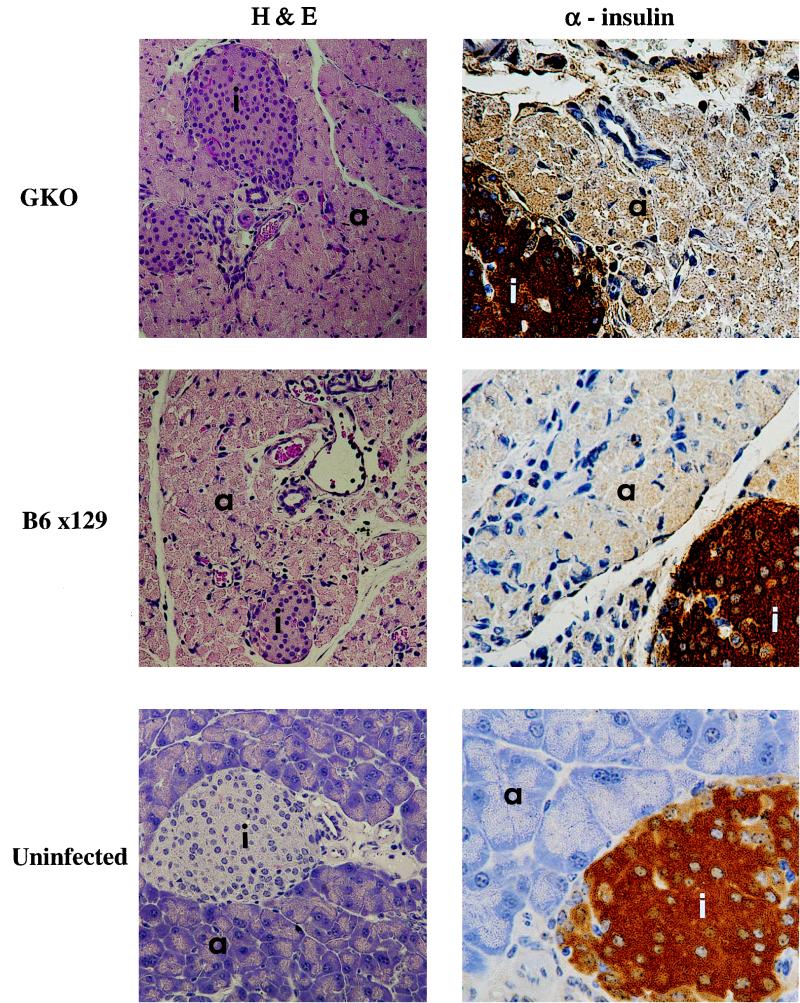
We further studied the histopathology of the pancreata from infected GKO and control mice to assess the damage resulting from CB4 infection. Four days after infection, GKO and control mice had similar tissue pathology within the pancreas (Fig. 2). Although the dropout of acinar tissue was not typically observed by 5 days postinfection, a dramatic loss of exocrine tissue structure and atrophy of the exocrine pancreas was observed at this time. Additionally, significant numbers of infiltrating lymphocytes and macrophages were easily identified. Islets remained functionally intact at 5 days postinfection in both GKO and control mice as demonstrated by immunostaining for insulin (Fig. 2), glucagon, and somatostatin (data not shown). By 2 weeks postinfection, the control mice had significant loss and dropout of their acinar tissue, although islets remained intact and produced functional insulin. Fat replacement of some of the acinar tissue could be observed by 3 months postinfection. In general, no obvious pathologic differences between CB4-infected GKO mice and other strains of mice were observed.
FIG. 2.
Histological analysis of pancreata of GKO mice following viral infection. Representative hematoxylin-and-eosin (H&E)-stained sections of the pancreas from a GKO and C57BL/6 × 129 mouse reveal the large inflammatory insult, necrosis, and damage to the pancreas following infection (×172). The mice were sacrificed at 4 days postinfection. Islet integrity and function were confirmed by immunohistochemistry using antibody to insulin (×344). Islets are designated with an “i.” Acinar tissue is designated with an “a.” Brown insulin staining is present over islets, demonstrating functionality following infection.
IFN-γ induces a number of responses within the tissue to help clear viral infections including increasing the expression of MHC molecules and iNOS. However, immunohistochemical analysis of infected pancreatic tissue from GKO mice revealed no obvious difference in the cell surface expression of MHC class I or II or in the production of iNOS in the coxsackievirus-infected, inflamed tissue (data not shown).
Local expression of IFN-γ protects from lethal CB4 infection.
The results described above demonstrate that mice required a systemic source of IFN-γ to survive lethal viral infection. Is local expression of the cytokine required for protection of the pancreas, or is it protecting by modulating systemic immune responses? To address this question, we infected transgenic mice expressing IFN-γ in the β cells of the pancreas (12) with a lethal dose (104 PFU) of CB4. These NOD–IFN-γ Tg mice were previously derived and offer a local concentration of IFN-γ near the primary site of CB4 infection. Following infection with a lethal dose of CB4, less than 50% of NOD mice survived to 6 days postinfection, and the remaining mice did not survive to day 10 (20 of 20) (Fig. 3A). However, their NOD–IFN-γ Tg counterparts were protected from lethal infection (18 of 18) and lived for more than 6 months. Additionally, at 3 days postinfection non-Tg mice developed hypoglycemia (75 ± 10; n = 6), while NOD–IFN-γ Tg mice remain normoglycemic (120 ± 12; n = 6). The ability to maintain normal glycemic levels and survive infection was directly linked to β-cell expression of IFN-γ.
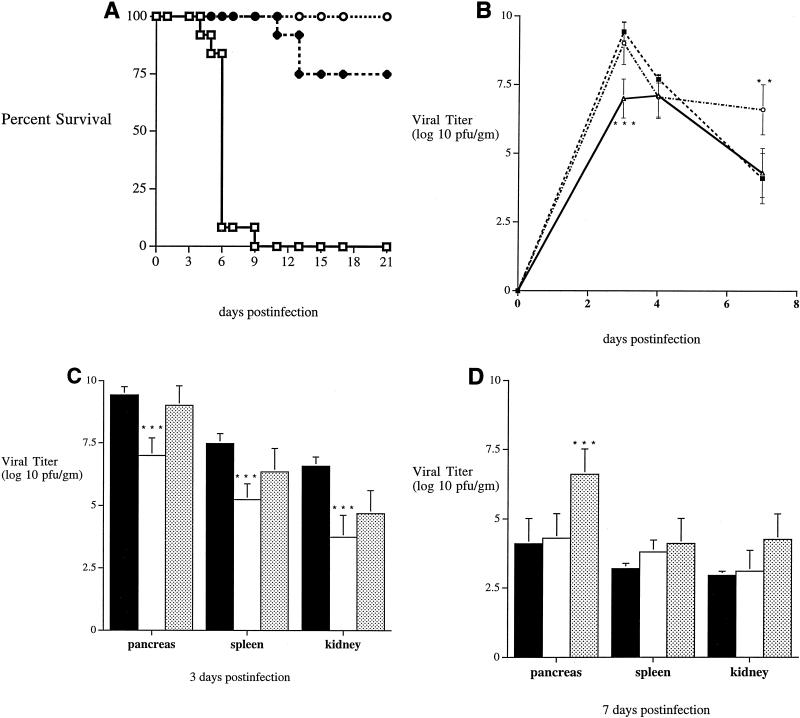
FIG. 3.
(A) Survival of NOD (□) (n = 20), NOD/SCID (⧫) (n = 20), and NOD–IFN-γ Tg (○) (n = 18) mice following CB4 infection. Mice were given 104 PFU of coxsackievirus B4 and were monitored over time for survival. Equal numbers of male (n = 9 or 10) and female (n = 9 or 10) mice from each group were infected. (B) Changes in pancreatic viral replication over time. NOD (■), NOD/SCID (○), and NOD–IFN-γ Tg (▵) mice were infected with CB4 and sacrificed 3, 4, or 7 days postinfection. Pancreata were harvested and assayed for virus recovery by standard plaque assay. At least four mice from each strain were assayed at each time point. Standard deviations are indicated by error bars. At day 3 postinfection, NOD–IFN-γ Tg mice were significantly lower in viral titer than both NOD and NOD/SCID mice at a P value of <0.0001. Additionally, NOD/SCID mice had a significantly higher titer at day 7 at a P value of <0.003. Asterisks denote statistically significant differences. (C) Viral recovery from infected NOD (■) (n = 8), NOD/SCID (░⃞) (n = 6), and NOD–IFN-γ Tg (□) (n = 5) mice. Mice were infected with CB4 and sacrificed 3 days postinfection. Organs (pancreas, spleen, and kidney) were harvested and assayed for virus recovery by standard plaque assay. Standard deviations are indicated by error bars. Significant differences in NOD–IFN-γ mice versus NOD mice were observed at P values of <0.0001 for all three organs. Asterisks denote statistically significant differences. (D) Viral recovery from infected NOD (■) (n = 8), NOD/SCID (░⃞) (n = 8), and NOD–IFN-γ Tg (□) (n = 4) mice at 7 days postinfection. Mice were infected with CB4 and sacrificed at 7 days following infection. Organs were harvested and assayed for virus recovery by standard plaque assay. Standard deviations are indicated by error bars. Significant differences in NOD/SCID mice versus the other mice were observed at P values of <0.003 (pancreas) and <0.05 (spleen and kidney). Asterisks denote statistically significant differences.
To determine whether this local expression of IFN-γ protected mice from lethal infection by reducing the viral load in the pancreas, we quantitated virus by plaque assays on tissues from both Tg and non-Tg mice 3, 4, and 7 days postinfection with CB4 (Fig. 3B). While high levels of replicating virus were observed in the pancreas of both Tg and non-Tg mice following infection, Tg mice had significantly lower levels of virus at the peak of infection (day 3). Despite this difference at day 3 postinfection, by day 4 equivalent levels of virus were found in the pancreata of both types of mice and ultimately virus was cleared at the same rate in both Tg and non-Tg mice. Analogously, lower levels of CB4 were found in the spleen and kidneys of Tg mice at the peak of infection (day 3), viral titers were relatively equivalent by day 4 postinfection, and clearance of virus in Tg and non-Tg mice was similar. The differences in IFN-γ expression and clinical outcome were associated with a difference in the level of viral replication at the peak of infection. Since the pancreas is the primary site of replication, reductions in the level of virus in the pancreas most likely reflect the ability of the virus to circulate and replicate in other organs.
To better define the precise role of lymphocytes following CB4 infection, we compared NOD/SCID mice, which lack both T and B cells, to NOD mice with an intact immune response. Infection with a lethal dose of CB4 led to only 25% mortality in NOD/SCID mice by day 21, although greater than 95% succumbed by day 28 (Fig. 3A). In comparison, all the NOD mice died within 10 days of infection. Therefore, the lethal effect of CB4 was to a great extent lymphocyte mediated, although if left unchecked, this cytopathic virus eventually resulted in death. The ability of CB4 to replicate in NOD/SCID mice was also quantitated by plaque assay. Although similar levels of CB4 were observed in the pancreata of NOD/SCID and NOD mice at both 3 and 4 days postinfection, considerably less virus was recovered from both the spleens and kidneys of NOD/SCID mice (Fig. 3C and D). Additionally, virus levels decreased over time in the surviving NOD mice, eventually leading to clearance by 7 to 10 days postinfection. However, viral clearance was observed in the NOD/SCID mice with a quite substantial and significant level of virus remaining in pancreata at 7 days postinfection (Fig. 3D). Although virus levels were eventually reduced in the pancreas, clearance was never observed. Thus, the absence of a competent immune response with T and B cells resulted in at least a temporary resistance to lethal infection.
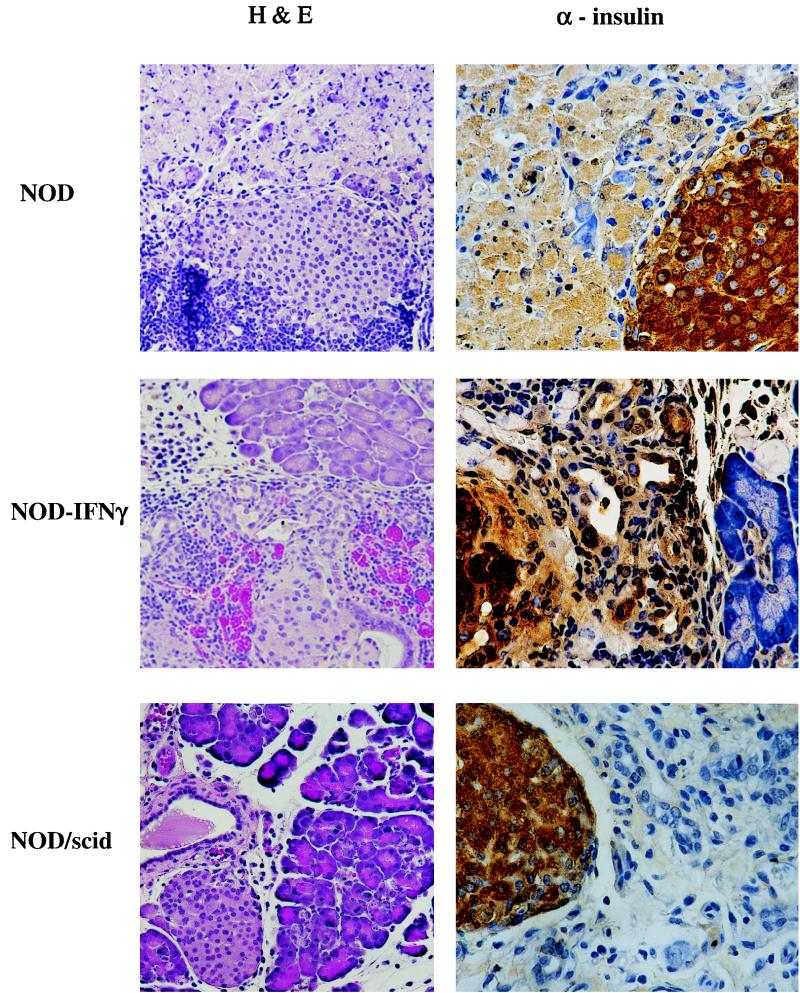
To distinguish between the pathology of infection in NOD, NOD–IFN-γ, and NOD/SCID mice, mice were sacrificed 4 days after infection for histological examination of their pancreata. All three groups of mice presented with pronounced immune infiltration of the pancreas, most notably in the acinar tissue. Additionally, loss of cells and damage were observed in all three mouse strains, but to the greatest extent in the NOD mice (Fig. 4). Despite the pronounced pathology, NOD–IFN-γ mice did have areas with significantly less acinar tissue damage and many surviving acini. Immunohistochemical staining with antibody to insulin (Fig. 4) and to somatostatin and glucagon (data not shown) revealed that the infection did not compromise the production of islet hormones. Clearly, most of the damage was to the acinar tissue.
FIG. 4.
Histological analysis of pancreata of NOD, NOD/SCID, and NOD–IFN-γ Tg mice following CB4 infection. Representative hematoxylin-and-eosin (H&E)-stained sections of the pancreas from NOD, NOD/SCID, and NOD–IFN-γ Tg mice reveal the large inflammatory insult, necrosis, and damage to the pancreas following infection (×172). The mice were sacrificed at 4 days postinfection. Islet integrity and function were confirmed by immunohistochemistry using antibody to insulin (×344). Brown insulin staining is present over islets, demonstrating functionality following infection.
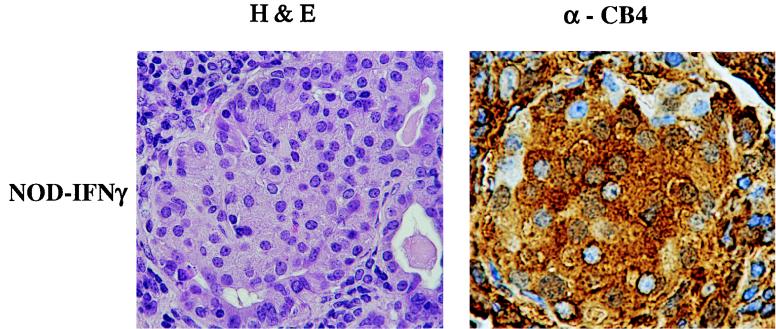
NOD–IFN-γ mice have a unique pancreatic morphology as a result of transgene expression alone; they exhibit a pronounced inflammatory insult, which precludes the effects of viral infection (12). This IFN-γ-mediated insult is directed at the islets, and despite the obvious inflammatory damage to the pancreas, blood glucose balance is maintained, for the most part due to islet cell regeneration originating from proliferating ducts where the new islets are sequestered. To determine whether these regenerated islets were protected from CB4 infection as they had been from infiltrating lymphocytes, we performed immunostaining of pancreatic sections with antibody to CB4. Infected islet cells were noted in the pancreata of both infected NOD and NOD–IFN-γ Tg mice at 4 days postinfection (Fig. 5). So while the duct wall restricts lymphocytes from entering, there is no noticeable block in the penetration of CB4 into intraductal islets.
FIG. 5.
Immunohistological analysis of the islets from CB4-infected NOD–IFN-γ Tg mice for viral antigen. A representative immunostained section of the islets from the pancreas of a NOD–IFN-γ Tg mouse reveals islet cells staining positively for viral antigen (×344). The mice were sacrificed at 4 days postinfection. A serial hematoxylin-and-eosin (H&E)-stained section is provided for comparison (×344). Brown anti-CB4 staining is present over islets, demonstrating viral infection.
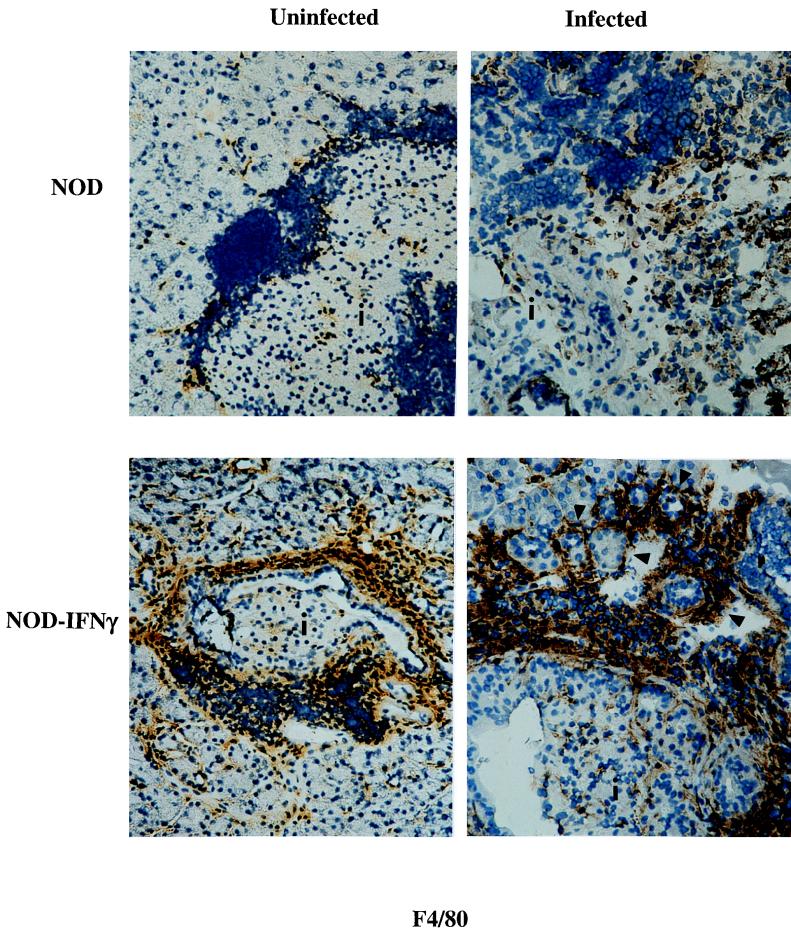
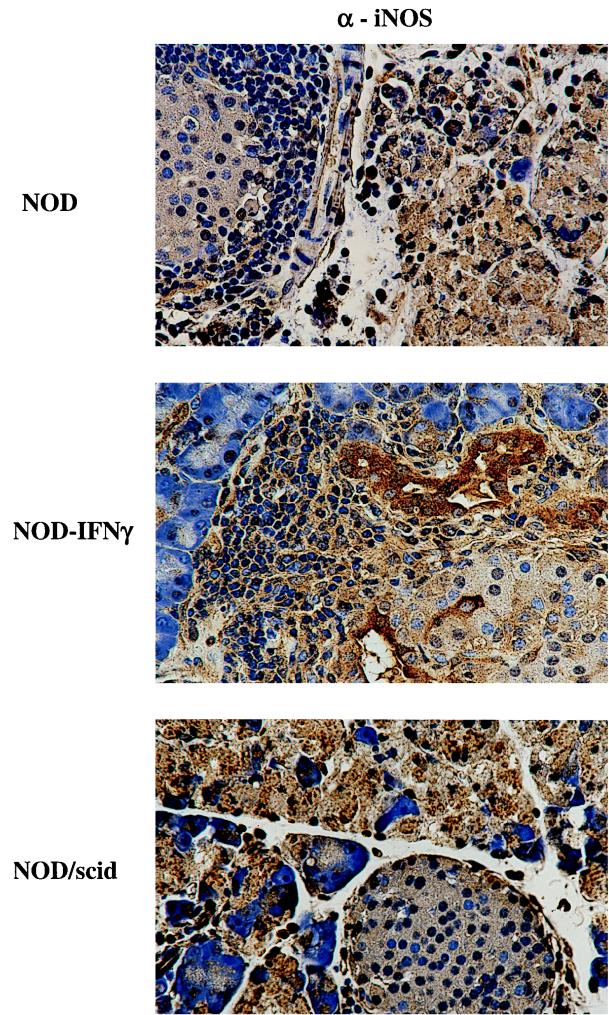
The mechanism of IFN-γ-mediated protection was then investigated by analyzing the constituents of the immune infiltration as well as by looking for changes in expression of the immune response molecules, MHC molecules, and iNOS. Prior to infection, pancreatic expression of IFN-γ by NOD–IFN-γ Tg mice induces the expression of high levels of endogenous MHC and iNOS as well as an array of infiltrating immune cells. Comparatively, NOD mice exhibit a more modest level of endogenous immune cell infiltration and immune response molecule expression, while NOD/SCID mice lack both endogenous immune cell infiltration and immune response molecule expression. However, following CB4 infection, a number of immune response molecules are upregulated and the number of infiltrating inflammatory cells has increased. While similar numbers of both CD4+ and CD8+ T cells were observed (data not shown) within the infected pancreata of NOD and NOD–IFN-γ mice, the levels of activated macrophages were quite different (Fig. 6). Not only were significantly more activated macrophages observed prior to infection in the Tg mice, but by day 3 postinfection, the level of activated macrophages as measured by F4/80 staining appeared to further increase in both the pancreatic acinar and islet tissue. After infection, macrophages appeared to mobilize out into the acinar tissue and to encircle and possibly protect individual acini. This activation of macrophages was not observed in infected NOD mice. The observation of high numbers of activated macrophages within the acinar tissue at day 3 postinfection is directly associated with the reduced viral load in the pancreas at day 3, survival, and transgene expression. To determine whether protection could also be associated with increases in local and/or macrophage expression of iNOS, pancreatic tissue sections were, further, immunostained for iNOS from Tg and non-Tg mice following infection. High levels of iNOS expression were observed in both Tg and non-Tg mice (Fig. 7). While no clear difference was observed, it is quite possible that expression of iNOS in the activated macrophages is mediating protection from the viral infection.
FIG. 6.
Immunohistological analysis of pancreata from CB4-infected NOD and NOD–IFN-γ Tg mice for macrophages. Representative immunostained sections of the pancreas from uninfected and 3-day-postinfection NOD and NOD–IFN-γ Tg mice reveal differences in the number and activation state of resident macrophages. Antibody to the macrophage activation marker, F4/80, was used to identify activated macrophages which, following infection, appear to have mobilized into the acinar tissue and encircled groups of acini (×324). Islets are designated with an “i.” Brown antimacrophage staining is present over macrophages. Activated macrophages can be observed encircling acini, and some are marked with arrowheads.
FIG. 7.
Immunohistological analysis of pancreata from CB4-infected NOD and NOD–IFN-γ Tg mice for iNOS. Representative immunostained sections of the islets from the pancreata of NOD and NOD–IFN-γ Tg mice show cells of both the acinar and islet location that stained positively for iNOS. Additionally, immune infiltrating cells also stained positively for iNOS. The mice were sacrificed at 3 days postinfection (×348). Brown anti-iNOS staining is present over many cell types in the three mouse strains, demonstrating upregulation of iNOS following infection.
NO not involved in protection from lethal infection.
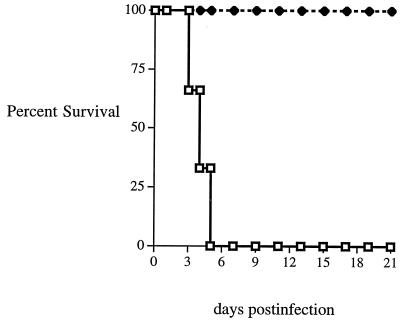
To address whether IFN-γ was able to protect the pancreas from the effects of lethal coxsackievirus infection through upregulation of iNOS and subsequent increases in the level of NO, mice were treated with AG before and after infection. AG is a selective inhibitor of iNOS activity, and treatment of rodents with AG has been shown to effectively neutralize iNOS (6). Daily administration of AG had no effect on the protection from lethal infection of NOD–IFN-γ mice, since all seven of the mice tested survived past the 14th day of CB4 infection (Fig. 8). Simultaneously, all the non-Tg NOD littermate control mice treated with AG (n = 6) and those mice mock-treated with PBS (n = 6) died from lethal infection by day 6. Furthermore, AG treatment did not affect the clearance of virus from either set of mice (data not shown). The AG treatment was sufficient to block in vivo iNOS activity, as AG-treated animals were unable to produce increased levels of NO2−/NO3− in plasma following CB4 infection (data not shown). Additionally, histological examination and immunohistochemical staining revealed no differences in pathology between AG-treated and untreated mice (data not shown). Therefore, neither iNOS nor NO plays a primary role in the protection from lethal infection or clearance of CB4 in these mice.
FIG. 8.
Survival of NOD (□) and NOD–IFN-γ Tg (⧫) mice infected with CB4 during AG treatment. Six groups of mice were examined. The figure presents the comparison of survival between two groups: NOD (n = 6) and NOD–IFN-γ Tg (n = 7) mice given 104 PFU of coxsackievirus B4 and 8 mg of AG per day. In addition, uninfected NOD (n = 4) and NOD–IFN-γ Tg (n = 4) mice given 8 mg of AG and infected NOD (n = 4) and NOD–IFN-γ Tg (n = 4) mice given PBS in place of AG were monitored for survival over time. Of these, only the infected PBS-treated NOD mice succumbed to lethal infection (4 of 4 died by day 7). The rest of the mice lived longer than 21 days.
DISCUSSION
In this report, we demonstrate that IFN-γ is required to protect against lethal coxsackievirus infection. The presence of systemic IFN-γ was required for survival following infection as was demonstrated by the lack of survival of GKO mice. This lack of IFN-γ successfully switched an otherwise resistant mouse to one with susceptibility to lethal infection, implying that IFN-γ and/or IFN-γ-induced host factors are responsible for the ability to resist lethal infection. More significantly, we have demonstrated here that local overexpression of IFN-γ in the pancreata of susceptible mice offset the lethal effects of the virus. The mechanism of this IFN-γ-mediated protection is most likely the result of resident activated macrophages that are easily mobilized to defend the pancreas from the invading virus. Additionally these studies demonstrate that this defense is not dependent on NO, as the administration of the iNOS inhibitor AG did not override protection. The importance of inflammatory cytokines like IFN-γ in the control of cytopathic infections is further underscored by these results, which support the possible therapeutic benefits of local administration to combat viral pathogenesis.
Coxsackievirus frequently infects humans, which results in debilitating pancreatitis and myocarditis and has also been implicated in the development of insulin-dependent diabetes mellitus (1, 30). This report is the first study to describe organ-specific IFN-γ-mediated protection from lethal viral infection, yet the mechanism by which coxsackievirus rapidly kills susceptible animals is not known. The protective nature of the local transgene expression is evidence for the cause of death as a result of pancreatic failure and/or destruction. Preceding death, systemic blood glucose levels dropped to less than 50 mg/dl, and this hypoglycemia alone may quite possibly have led to loss of life. Even though the pancreatic islets continued to function and produce high levels of insulin, glucagon, and somatostatin, the much-affected acinar tissue was lost, decreasing the production of digestive enzymes, including amylase, that directly contribute to the blood glucose level provided by the gut. Alternatively, the hypoglycemia may simply be indicative of the rapid destruction of exocrine tissue that provides the necessary digestive enzymes for the mice to gain nourishment and retain both their metabolic homeo- and thermostasis. Further, this rapid cytolysis of acinar tissue may have allowed large concentrations of digestive enzymes to empty not into the gut but into the peritoneal cavity, causing havoc to many of the organs that sustain life. This may well be the case, as numerous organs from the peritoneal cavity (i.e., kidney, spleen, and liver) were observed to exhibit degradation along their outer edges (data not shown). Alternatively, protected mice maintained intact portions of their acinar tissue and were subjected to a smaller viral load.
On the basis of studies of several viruses, it has been proposed that noncytopathic viruses are controlled by the host mainly by specific lytic defense whereas cytopathic viruses like coxsackievirus are controlled by cytokines and neutralizing antibodies (17). The results presented herein are consistent with the latter notion. In considering mechanisms that account for the systemic protection, it seems significant that NOD/SCID mice were temporarily protected from lethal coxsackievirus but that genetically matched immunocompetent mice succumbed quickly, implying a role for the innate response in mediating protection. In general, SCID mice have a stronger innate response that allows them to compensate for their lack of T and B cells with increased levels of macrophages and natural killer (NK) cells. This in turn may account for their increased survival and further suggests that resistant strains of mice are likely to have stronger innate responses than susceptible mice. Since IFN-γ was clearly required for survival, we can postulate that the innate response including activated macrophages and NK cells requires IFN-γ for its antiviral defense. Thus IFN-γ, elicited by high levels of macrophages and NK cells, may mediate early survival of CB4 infection, whereas lymphocytes are eventually required for complete viral clearance. Current studies are focused on examining the role of various immune cells through depletion analysis.
The mechanism of protection afforded by endogenous systemic IFN-γ and Tg-directed pancreatic IFN-γ may be quite different. IFN-γ is pluripotent in its function, inducing cellular processes that activate the macrophage inflammatory response pathway (8, 16, 24, 25, 28) as well as direct the protection (9, 21) and even growth of tissues (11, 12). The rapid nature of CB4-mediated destruction and the ability of IFN-γ to protect from lethal infection most likely reflect a strong dampening of the viral or immune-directed cytolysis. Viral replication rates in the pancreas were affected by the presence of IFN-γ by day 3, and although a considerably large and equivalent viral load was still present at day 4, this may suggest that protection is due to a simple reduction in early viral spread and damage. However, this did not appear to be the case with the GKO mice and their resistant MHC counterparts, as no difference in viral titer was observed at these early time points. Nevertheless, reduction in early spread of virus may be one way to allow enough of the acinar tissue to escape infection and survive and/or conversely to reduce the amount of degradative enzymes released from the damaged tissue. This rapid protection is most likely explained by the predisposing environment of the Tg pancreas, which contains a large number of activated macrophages and NK, T, and B cells ready and willing to defend the tissue. In fact, the massive and rapid mobilization of activated macrophages in the pancreas of the Tg mice implicates a macrophage-dependent mechanism in protection. Further, the inability of AG to alter such protection further implies that the mechanism of CB4 protection is NO independent. Nonetheless, macrophages have multiple mechanisms by which to defend against infections. However, prior studies with coxsackievirus B3 (CB3) have demonstrated a clear role for NO in the antiviral mechanism, as inhibition of iNOS resulted in increased viral replication and mortality (18). Although CB3 and CB4 are quite similar in structure, receptor usage, and the peptide sequence of many gene products, they differ markedly in their primary targets of pathology (1, 30), as well as the nature of their induced immune responses (2, 15, 22). In fact, the patterns of resistance and susceptibility of BALB/c and C57BL/6 mice are reversed with respect to CB3 and CB4 (1, 30). Moreover, differences of single amino acids distinguish CB4 variants resulting in different degrees of virulence and pathology (22). Thus, although the NO pathway was not responsible for the IFN-γ-mediated protection in this model, it certainly may participate in the defense against other infections.
The IFN-γ-mediated tissue protection in Tg mice may simply be explained by this preactivated local innate response but may also involve additional and distinct IFN-γ-induced protective mechanisms. For instance, the higher levels of MHC expression in Tg mice (data not shown) may increase immune surveillance and recognition of infected cells by immune cells within the pancreas tissue. Additionally, the presence of increased numbers of T cells in the pancreata of IFN-γ-expressing Tg mice preceding infection is well established (12). Along with the observed increases in inflammatory T cells, increases in the numbers of regulatory T cells and gamma/delta T cells are observed (data not shown). Such cells may reduce the activity of the virus-specific T cells in the pancreas enough to decrease their cytolytic activity while retaining their ability to clear virus. Moreover, a number of IFN-induced cellular proteins, including PML, 2′,5′-oligoadenylate synthetase, p68, and the Mx proteins, have been shown to display antiviral properties (23, 26, 29). Overexpression of some of these proteins can provide resistance to certain viruses while having no effect whatsoever on others (3, 5, 19, 20). Similarly, IFN-γ has reduced the replication of hepatitis B virus via distinct intracellular mechanisms (13). IFN-γ may also induce host factors to stabilize the acinar tissue during infection or downregulate viral virulence factors and, in either case, limit the pathology of infection. Finally, IFN-γ can alter expression of the apoptosis-related proteins Bcl-2, Bcl-x, and Bax (10), which enable cells to survive cytokine-mediated cell death. While our results point strongly to a macrophage-mediated mechanism, they do not rule out the possibility that multiple protective mechanisms are also in play.
ACKNOWLEDGMENTS
We thank Charles Gauntt (University of Texas—San Antonio) for his generous gift of coxsackievirus B4 Edwards strain 2, Howard Fox for the use of his virus facilities, and Gail Patstone for technical help. We also thank Howard Fox, Michael Buchmeier, Cecile King, and Michelle Krakowski for helpful discussions.
M.S.H. was a recipient of a Juvenile Diabetes Foundation International Postdoctoral Fellowship Award and an American Diabetes Association Career Development Award. N.S. was supported by a Diabetes Interdisciplinary Research Program grant from the Juvenile Diabetes Foundation International.
Footnotes
This is manuscript number 11674-IMM from The Scripps Research Institute.
REFERENCES
- 1.Chapman N M, Ramsingh A I, Tracy S. Genetics of Coxsackievirus virulence. Curr Top Microbiol Immunol. 1997;223:227–258. doi: 10.1007/978-3-642-60687-8_11. [DOI] [PubMed] [Google Scholar]
- 2.Chatterjee N, Hou J, Dockstader P, Charbonneau T. Coxsackievirus B4 infection alters thymic, splenic, and peripheral lymphocyte repertoire preceding onset of hyperglycemia in mice. J Med Virol. 1992;38:124–131. doi: 10.1002/jmv.1890380210. [DOI] [PubMed] [Google Scholar]
- 3.Chelbi-Alix M K, Quignon F, Pelicano L, Koken M H M, de Thé H. Resistance to virus infection conferred by the interferon-induced promyelocytic leukemia protein. J Virol. 1998;72:1043–1051. doi: 10.1128/jvi.72.2.1043-1051.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Clements G, Galbraith D, Taylor K. Coxsackie B virus infection and onset of childhood diabetes. Lancet. 1995;346:221–223. doi: 10.1016/s0140-6736(95)91270-3. [DOI] [PubMed] [Google Scholar]
- 5.Coccia E M, Romeo G, Nissim A, Marziali G. A full-length 2-5A synthetase cDNA transfected in NIH-3T3 cells impairs EMCV but not VSV replication. Virology. 1990;179:228–233. doi: 10.1016/0042-6822(90)90292-y. [DOI] [PubMed] [Google Scholar]
- 6.Corbett J A, Tilton R G, Chang K, Hasan K S, Ido Y, Wang J L, Sweetland M A, Lancaster J R, Jr, Williamson J R, McDaniel M L. Aminoguanidine inhibits nitric oxide formation and prevents diabetic vascular dysfunction. Diabetes. 1992;41:552–556. doi: 10.2337/diab.41.4.552. [DOI] [PubMed] [Google Scholar]
- 7.Dalton D K, Pitts-Meek S, Keshav S, Figari I S, Bradley A, Stewart T A. Multiple defects of immune cell function in mice with disrupted interferon-gamma genes. Science. 1993;259:1739–1742. doi: 10.1126/science.8456300. [DOI] [PubMed] [Google Scholar]
- 8.Geiger K, Howes E, Gallina M, Huang X J, Travis G H, Sarvetnick N. Transgenic mice expressing IFN-γ in the retina develop inflammation and photoreceptor loss. Investig Ophthalmol Vis Sci. 1994;35:2667–2681. [PubMed] [Google Scholar]
- 9.Geiger K, Howes E L, Sarvetnick N. Ectopic expression of gamma interferon in the eye protects transgenic mice from intraocular herpes simplex virus type 1 infections. J Virol. 1994;68:5556–5567. doi: 10.1128/jvi.68.9.5556-5567.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Geiger K D, Nash T, Sawyer S, Krahl T, Patstone G, Reed J C, Krajewski S, Dalton D, Buchmeier M J, Sarvetnick N. Interferon-γ protects against herpes simplex virus type 1-mediated neuronal death. Virology. 1997;238:189–197. doi: 10.1006/viro.1997.8841. [DOI] [PubMed] [Google Scholar]
- 11.Gu D-L, Lee M-S, Krahl T, Sarvetnick N. Transitional cells in the regenerating pancreas. Development. 1994;120:1873–1881. doi: 10.1242/dev.120.7.1873. [DOI] [PubMed] [Google Scholar]
- 12.Gu D-L, Sarvetnick N. Epithelial cell proliferation and islet neogenesis in IFN-γ transgenic mice. Development. 1993;118:33–46. doi: 10.1242/dev.118.1.33. [DOI] [PubMed] [Google Scholar]
- 13.Guidotti L G, Ishikawa T, Hobbs M V, Matzke B, Shreiber R, Chisari F V. Intracellular inactivation of the hepatitis B virus by cytotoxic T lymphocytes. Immunity. 1996;4:25–36. doi: 10.1016/s1074-7613(00)80295-2. [DOI] [PubMed] [Google Scholar]
- 14.Hartig P, Madge G, Webb S. Diversity within a human isolate of coxsackie B4: relationship to viral-induced diabetes. J Med Virol. 1983;11:23–30. doi: 10.1002/jmv.1890110104. [DOI] [PubMed] [Google Scholar]
- 15.Henke A, Huber S, Stelzner A, Whitton J L. The role of CD8+T lymphocytes in coxsackievirus B3-induced myocarditis. J Virol. 1995;69:6720–6728. doi: 10.1128/jvi.69.11.6720-6728.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Horwitz M S, Evans C F, McGaver D B, Rodriguez M, Oldstone M B A. Primary demyelination in transgenic mice expressing interferon-gamma. Nat Med. 1997;3:1037–1041. doi: 10.1038/nm0997-1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kagi D, Hengartner H. Different roles for cytotoxic T cells in the control of infections with cytopathic versus noncytopathic viruses. Curr Opin Immunol. 1996;8:472–477. doi: 10.1016/s0952-7915(96)80033-1. [DOI] [PubMed] [Google Scholar]
- 18.Lowenstein C J, Hill S L, Lafond-Walker A, Wu J, Allen G, Landavere M, Rose N R, Herskowitz A. Nitric oxide inhibits viral replication in murine myocarditis. J Clin Investig. 1996;97:1837–1843. doi: 10.1172/JCI118613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Meurs E, Watanabe Y, Kadereit S, Barber G, Katze M, Chong K, Williams B, Hovanessian A. Constitutive expression of human double-stranded RNA-activated p68 kinase in murine cells mediates phosphorylation of eukaryotic initiation factor 2 and partial resistance to encephalomyocarditis virus growth. J Virol. 1992;66:5805–5814. doi: 10.1128/jvi.66.10.5805-5814.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pavlovic J, Zürcher T, Haller O, Staeheli P. Resistance to influenza virus and vesicular stomatitis virus conferred by expression of human MxA protein. J Virol. 1990;64:3370–3375. doi: 10.1128/jvi.64.7.3370-3375.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ramsay A J, Ruby J, Ramshaw I A. A case for cytokines as effector molecules in the resolution of virus infection. Immunol Today. 1993;14:155–157. doi: 10.1016/0167-5699(93)90277-R. [DOI] [PubMed] [Google Scholar]
- 22.Ramsingh A I, Lee W T, Collins D N, Armstrong L E. Differential recruitment of B and T cells in coxsackievirus B4-induced pancreatitis is influenced by a capsid protein. J Virol. 1997;71:8690–8697. doi: 10.1128/jvi.71.11.8690-8697.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Samuel C E. Antiviral-regulated cellular proteins and their surprisingly selective antiviral activities. Virology. 1991;183:1–11. doi: 10.1016/0042-6822(91)90112-o. [DOI] [PubMed] [Google Scholar]
- 24.Sarvetnick N, Liggitt D, Pitts S, Hansen S, Stewart T. Insulin-dependent diabetes mellitus induced in transgenic mice by ectopic expression of class II MHC and interferon-gamma. Cell. 1988;52:773–782. doi: 10.1016/0092-8674(88)90414-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sarvetnick N, Shizuru J, Liggitt D, Martin L, McIntyre B, Gregory A, Parslow T, Stewart T. Loss of pancreatic islet tolerance induced by beta-cell expression of interferon-gamma. Nature. 1990;346:844–847. doi: 10.1038/346844a0. [DOI] [PubMed] [Google Scholar]
- 26.Sen G C, Ransohoff R M. Interferon-induced antiviral actions and their regulation. Adv Virus Res. 1993;42:57–102. doi: 10.1016/s0065-3527(08)60083-4. [DOI] [PubMed] [Google Scholar]
- 27.Simpson E M, Linder C C, Sargent E E, Davisson M T, Mobraaten L E, Sharp J J. Genetic variation among 129 substrains and its importance for targeted mutagenesis in mice. Nat Genet. 1997;16:19–27. doi: 10.1038/ng0597-19. [DOI] [PubMed] [Google Scholar]
- 28.Spitalny G L, Havell E A. Monoclonal antibody to murine gamma interferon inhibits lymphokine-induced antiviral and macrophage tumoricidal activities. J Exp Med. 1984;158:1560–1565. doi: 10.1084/jem.159.5.1560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Staeheli P, Pitossi F, Pavlovic J. Mx proteins: GTPases with antiviral activity. Trends Cell Biol. 1993;3:268–272. doi: 10.1016/0962-8924(93)90055-6. [DOI] [PubMed] [Google Scholar]
- 30.Tracy S, Chapman N M, Romero J, Ramsingh A I. Genetics of coxsackievirus B cardiovirulence and inflammatory heart muscle disease. Trends Microbiol. 1996;4:175–179. doi: 10.1016/0966-842x(96)10026-3. [DOI] [PubMed] [Google Scholar]
- 31.Webb S, Loria R, Madge G, Kibrick S. Susceptibility of mice to group B coxsackie virus is influenced by the diabetic gene. J Exp Med. 1976;143:1239–1248. doi: 10.1084/jem.143.5.1239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yoon J, Austin M, Onodera T, Notkins A. Isolation of a virus from the pancreas of a child with diabetic ketoacidosis. N Engl J Med. 1979;300:1173–1179. doi: 10.1056/NEJM197905243002102. [DOI] [PubMed] [Google Scholar]