Abstract
Construction of in vitro vascular models is of great significance to various biomedical research, such as pharmacokinetics and hemodynamics, and thus is an important direction in tissue engineering field. In this work, a standing surface acoustic wave field was constructed to spatially arrange suspended endothelial cells into a designated acoustofluidic patterning. The cell patterning was maintained after the acoustic field was withdrawn within solidified hydrogel. Then, interstitial flow was provided to activate vessel tube formation. In this way, a functional vessel network with specific vessel geometry was engineered on-chip. Vascular function, including perfusability and vascular barrier function, was characterized by microbeads loading and dextran diffusion, respectively. A computational atomistic simulation model was proposed to illustrate how solutes cross vascular lipid bilayer. The reported acoustofluidic methodology is capable of facile and reproducible fabrication of the functional vessel network with specific geometry and high resolution. It is promising to facilitate the development of both fundamental research and regenerative therapy.
Keywords: Acoustofluidics, surface acoustic wave, vessel-on-a-chip, biofabrication, vascular barrier function
INTRODUCTION
Vascular system is one of the most important circulatory systems in the human body1. Blood vessels are not only a necessary pathway for physiological metabolism, material exchange, and nutrient delivery, but also an important route for most pharmacokinetic drug delivery2,3. In order to match specific different structures and functions of each organ, the blood vessels have significant organ specificity4,5. For example, the vascular structure in the hepatic lobule has a hexagonal distribution6, while the vessels in muscle tissue run in parallel lines7. Therefore, the in vitro reconstruction of functional vascular models has always been one of the key research topics in the field of tissue engineering8.
At present, self-assembly nature of endothelial cells (ECs) is mostly used for in vitro capillary vessel network construction9–13, with inevitable randomness and low reproducibility14. In an attempt to control the vessel shape, researchers have been building blood vessels by coating the inner surface of hollow gel channels with ECs15–17. However, limited by the template resolution and difficulty of removing mold template without destroying channel, it is difficult to achieve micron-scale blood vessels with slightly complex shapes18,19. Multiphoton ablation technology reported by Prof. Zheng et al. can generate hollow gel channels with intricate patterns of a few microns, thus accurately control the shape and size of blood vessels at the same time20–22. However, the complexity and cost of the laser machine system may limit its widespread adoption. Due to various strengths, such as remote control, biocompatibility, non-contact and easy operation, acoustofluidics has gradually developed into a popular biomedical technology23,24, and widely used in the fields of disease diagnosis25–30, drug delivery31–34, tissue engineering35–40 and biophysical characterization41,42. Here, we report a standing surface acoustic wave (SSAW)-based acoustofluidic methodology as an alternate of engineering vessel-on-a-chip. First, the suspended endothelial cells were acoustically patterned into parallel lines topography in the hydrogel matrix. Then, the patterned endothelial cells were activated by the interstitial flow and developed into functional vessel tubes along the previous acoustic patterning geometry. The permeability difference between the mono-vessel and fibroblast-supported vessel was evaluated and a computational model was proposed for interpretation. Last, by characterizing the compound response of neurons in the pure matrix and in the vessel-associated matrix, the influence of blood vessels was demonstrated. The reported acoustofluidic vessel engineering demonstrates that without any physical channel as a guide, the HUVECs can still assembled into the vessel network with the designated specific geometry. Besides, the cost of the acoustofluidic system is more lab-friendly than photon laser facility. Thus, it is promising to be an alternative method for facile and high-resolution construction of in vitro vessel model.
MATERIALS AND METHODS
Acoustofluidic device fabrication
The SSAW device was fabricated through standard lithography and lift-off process43. A 15-μm-thick photoresist layer (AZ512, Kayaku Advanced Materials, Inc.) was spin-coated on a 500-μm-thick, double-side polished, piezoelectric LiNbO3 wafer (Precision Micro-Optics, Inc.). Then, the designed interdigital transducers (IDT) of double metal layers (Cr/100 Å, Au/600 Å) with a 125 μm finger width were transferred from the customized plastic mask to the substrate by metal deposition44. The finger pair number of the IDT is 30. The resonant frequency of the obtained SSAW device was measured as around 8.21 MHz. The microfluidic chamber mold was fabricated with soft lithography45. A 150-μm-thick SU8–2150 (Kayaku Advanced Materials, Inc.) photoresist mold was created on a 3-inch silicon wafer. After being peeled off from the mold, the polydimethylsiloxane (PDMS) chambers were plasma-bonded on a 1 mm thick glass coverslip46. All the fabrication processes were finished in the Center for Photonics and Nanoelectronics (CPN) at Lehigh University.
Cell culture, experimental setup and immune-fluorescence staining
Human Umbilical Vein Endothelial Cells (HUVEC) and Normal Human Lung Fibroblasts (NHLF) were purchased from LONZA47. HUVECs were cultured in an endothelial cell growth medium supplemented with EGM-2 SingleQuot kit supply and growth factors (EGM-2, LONZA). NHLF were cultured in fibroblast growth basal medium supplemented with FGM™-2 BulletKitTM (FGM-2, LONZA)48. The generation of excitatory human forebrain neurons was accomplished using over expression of Neurogenin-2 (NGN2) transcription factor, following the protocol49,50. The neurons were cultured with complete mTeSR1 medium, differentiation to neuron happened with introducing dox to the culture (N2 supplement + doxycycline and growth factors in DMEM/F12 media) and maintained in culture with 50x B27 supplement and 2 mM L-Glutamine in Neurobasal medium (Thermo Fisher Scientific). All cells were cultured at 37 °C in a humidified 5% CO2 environment.
For the patterning experiment, bovine fibrinogen (CAS-9001-32-5, Sigma) was dissolved in Dulbecco’s phosphate buffered saline (DPBS) to 2 mg/ml solution. The cell-laden fibrinogen pre gel solution was mixed with 2U/ml bovine thrombin (CAS-9002-044, Sigma) and injected into the chamber through chamber loading ports51. For the pure HUVEC condition, the cell concentration was 5 million/ml. For the coculture situation, the HUVEC concentration retained with 5 million/ml, while the concentration of another cell was 0.2 million/ml. During the cell patterning experiment, a radio frequency signal from a function generator (AFG3102, Tektronix, USA) was amplified by an amplifier (ACS-230-25W, Com-Power, USA), and transferred to the IDT pair. The input voltages on the devices were from 50 Vpp. A coupling water layer was placed between the SSAW device and the PDMS-glass-based microfluidic chamber for acoustic wave conduction52,53. The HUVECs were aligned into parallel lines in PDMS channels during 3 minutes of acoustic patterning process. Then the PDMS chambers were relocated into the incubator for the gel solidification and further in vitro cell culture54.
For immunofluorescence staining of the vessel model, detailed method can be found in our previous publication13. For the neural cells-vessel coculture system, 1.0 mM monosodium glutamate (MSG) (Sigma-Aldrich) was dissolved in EGM medium and loaded into the vessel tube. c-Fos (sc-166940-AF488, Santa Cruz)55 immunostaining was executed 10 min after loading the monosodium glutamate solution. All the fluorescent images and confocal scanning were acquired with a Nikon C2+ laser scanning confocal microscope in the Health Research Hub Center, Lehigh University. The image processing was conducted with ImageJ software from National Institutes of Health (USA).
On-chip vessel network construction and vascular permeability characterization
After the gel curing, a pipette tip containing 50 μL culture medium were inserted into one of the chamber loading ports to provide interstitial flow and hydrostatic, while an empty pipette tip was inserted into the other chamber loading port for medium collection13,47. The culture medium in pipette tips were refreshed every day. The permeability of the vessels was measured with solute diffusion across the vessel wall. 70kDa FITC conjugated dextran (CAS-60842-46-8, Sigma) was dissolved in the EGM-2 medium to prepare the solution56,57.
Computational simulation
COMSOL® Multiphysics 5.6 was exploited to simulate the defined central area resulted from the interference of two opposing SAW. A “Pressure Acoustic, Frequency Domain” is used to analyze the pressure distribution of one-dimensional standing acoustic field58–62:
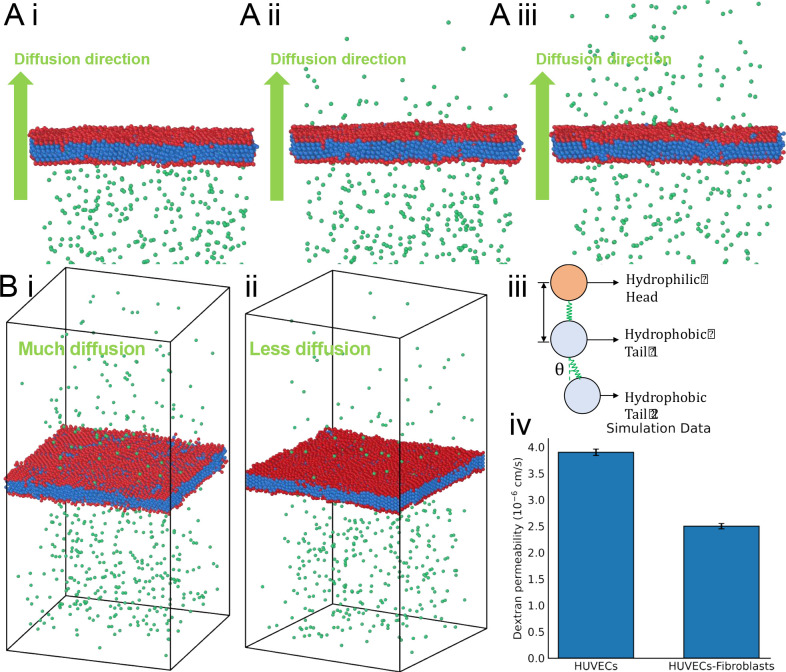
where is the angular frequency, is the sound speed, is the density, and is the pressure. In the permeability computational model, the 70 kDa FITC-dextran solute is represented as a series of discrete beads uniformly distributed on one side of the simulation domain (Fig. 4A green beads). Cooke-Deserno three-bead membrane representation model is implemented to simulate vascular membrane, which offers an elegant balance between computational simplicity and biological realism63. The interaction between these entities is defined by employing the Lennard-Jones potential, allowing for the manifestation of Brownian motion among the beads. The use of the Lennard-Jones potential not only provides a mechanism for interaction between the beads but also models the diffusion of these bead-represented solutes through the membrane64,65. Within this framework, each lipid is depicted as three discrete beads, representing the hydrophilic head group(red in fig. 4) and the two hydrophobic tail groups(blue in fig. 4), effectively capturing the amphiphilic nature of lipids. These head and tail coarse-grained beads are interconnected via a finite extensible nonlinear elastic bond, thus capturing the essential amphiphilic character of lipids. Additionally, a harmonic angular potential is employed to maintain the straightened configuration of the lipid molecule. The lipid bilayer membrane representative of HUVECs (Human Umbilical Vein Endothelial Cells) and fibroblasts is modeled using two distinct membrane layers, with each layer consisting of three beads. To closely emulate the experimentally observed diffusion rates of membrane permeability, the area per lipid in the membrane was optimized by adjusting the Lennard-Jones parameters. Several foundational assumptions were made to enhance computational efficiency and ensure clarity of the simulation. we conceptualized the lipid bilayer as a seamless, uniform surface, intentionally omitting intricate molecular details reduce computational complexity. Furthermore, the complexities of lipid head-groups and tails are abstracted into coarse-grained beads, encapsulating essential interaction properties. Lastly, the behavior and dynamics of the membrane are primarily driven by a balance between curvature elasticity and thermal fluctuations. The membrane representation and the associated parameters was described in our previous publication66.
Figure 4.
Computational vascular barrier function modeling with/without supporting fibroblasts. A) Modeling of the dextran diffusion process before (i), during (ii) and after (iii) the vessel membrane crossing. B) Modelling the final diffusion result of pure vessel condition within the time unit (i). Modelling the final diffusion result of fibroblast-supported condition within the time unit (ii). (iii) The detailed composition of simulated vascular membrane. (iv) Simulation result of the dextran permeability speed.
RESULTS AND DISCUSSION
Acoustofluidic patterning of the suspended HUVECs into acoustic pressure nodes
Figure. 1 illustrates the methodology workflow of the acoustofluidic vessel-on-a-chip engineering. Once signal of the resonant frequency from the signal generator was amplified and introduced to the acoustofluidic device, two identical but opposite-propagating traveling surface acoustic waves (SAW) were released from the IDT pair, and the SSAW field was constructed on the piezoelectric LiNbO3 wafer surface67,68. Thus, a periodic distribution of pressure nodes and antinodes with minimum and maximum pressure amplitudes was presenting respectively (Fig. 2B)69–71. With the conduction of the coupling water layer, the acoustic field was introduced into the chamber72. As a result, the originally randomly distributed cells was relocated to the nearest pressure nodes, thus forming parallel linear arrays73. Here, the half wavelength of the SSAW was designed as 250 μm, and the final patterned cell line spacing was approximately 150 μm (Fig. 2C). Fibrinogen is currently the most suitable hydrogel for culturing vascular tissue in vitro, and it was selected as the biological matrix in this experiment74,75. The thrombin concentration was reduced to prolong the time required for the gel solidification, until the cells could be fully patterned. In the experiment, it was observed that the fibrinogen gel began to coagulate after being mixed with thrombin for 3 minutes at room temperature, at which time the cells rarely moved. Thus, after 3 minutes, the sample was moved into the incubator for fully solidification. After 3-minute-patterning, the microfluidic chambers were moved into the incubator to fully solidify the gel. In this way, the original cell patterning topography was maintained by the solidified gel could after the acoustic field was withdrawn76.
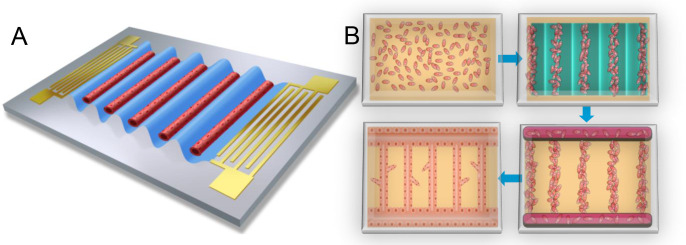
Figure 1.
Schematics of acoustofluidic engineering functional vessel-on-a-chip. A) As shown schematically, standing surface acoustic wave is generated on-chip to pattern the cells and promote the desired vessel formation. B) Flow chart of the acoustofluidic engineering of vessel formation. The suspended cells are patterned into the acoustic pressure node and aligned into parrel line array. After the acoustic field disappears, the solidified gel can still maintain the original patterning shape of the cells. Due to the nature of adherent growth, some excess vascular cells will grow along the wall of the PDMS chamber, forming transverse vessels perpendicular to the patterned longitudinal vessel tubes, thereby connecting these parallel arrays of vascular tubes. In the end, functional vessel-on-chip can be formed with the interstitial flow stimulation.
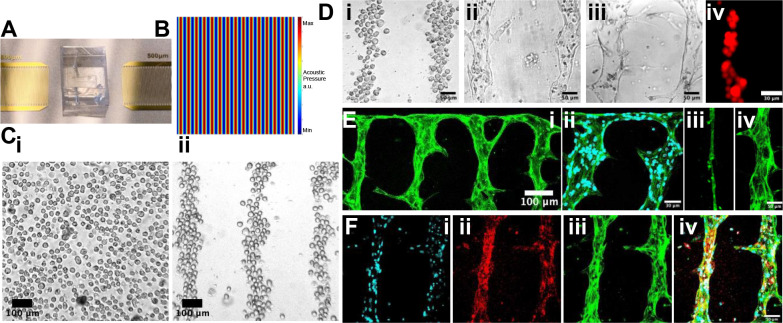
Figure 2.
Acoustofluidic engineering of suspended HUVECs into functional vessel-on-a chip. A) The photo of the SSAW device and microfluidic chamber. B) The simulation of the SSAW distribution. C) Before patterning (i), the cells are randomly distributed. After patterning (ii), the cells are aligned into parallel straight-line distribution. D) The separated cells (i) self-assemble at hour 24 (ii) and formed parallel perfusable vessel tubes at hour 48 (iii). 10-um-fluorescent beads can be loaded into the vessel tube (iv). E) The geometry of vessel-on-a-chip(i). The HUVECs are labeled with 488 fluorescence-conjugated-Factin. (ii) The anastomosis structure between the parallel vessel tubes and the side tube perpendicular to them. Cell nuclei is labeled with DAPI. (iii) The vessel tube under static culture condition. (iv) The vessel tube under interstitial flow-stimulated culture condition. F) The vessel tubes are characterized with the immunostaining. Cell nuclei is labeled with DAPI (i). Vessel biomarker is labeled with red CD31 (ii). Cytoskeleton is labeled with green Factin (iii). The overlap image is presented as (iv).
Patterned vessel-on-a-chip formation under interstitial flow-assisted in vitro cell culture
Prior to this project, several papers have been published on the construction of vascular models by acoustic cell patterning77–79. For example, Kang et al. acoustically patterned endothelial cells in hyaluronic acid to construct vascular tissue for ischemia therapy80. The authors transplanted the in vitro vascular tissue into a mouse model. After the vascular tissue was anastomosed with the rat’s own blood vessels, the vessel persusability was verified in vivo. Besides, Zhang et al. reported that for in vitro vascular models, the key to making vessel tubes perfusable is to provide a stimulus of interstitial flow81. The interstitial flow can promote the expression of matrix metalloproteinase-2 protein to enhance the vasculogenic capacity of endothelial cells. Based on these inspiring works, we hypothesized that the perfusable patterned vascular models could be remodeled in vitro by delivering the patterned ECs with interstitial flow stimulation.
As control, a group of patterned HUVECs and random-seeded HUVECs were cultured in the static culture condition, respectively (Fig. S2). Although HUVECs could also self-assemble into vessel tubes in both groups, these blood vessels are essentially thin and non-perfused. For the experiment group, the patterned HUVECs were applied with hydraulic pressure by loading 50 μl of medium into a 200 μl pipette tip to induce interstitial flow. The patterned ECs began to interconnect in 24 hours (Fig. 2D). The ECs in the lumen not involved in vascularization were washed away in 48 hours (Fig. 2Diii). Parts of ECs migrated to the chamber wall, along which the ECs grew into the horizontal vessel tube to connect all the vertical parallel patterned vessel tubes (Fig. 2Eii). After 48 hours in vitro culture, a vascular network was formed, maintaining the shape of the previous acoustic patterning (Fig. 2Ei). The vessel perfusability was demonstrated by loaded the 10 μm red fluorescent beads into the vessel tube (Fig. 2Div). From the cytoskeleton characterization (Fig. 2E), it can be clearly seen that the blood vessels formed under hydraulic pressure stimuli were much wider than the static cultured blood vessels82 (Fig. 2Eiii&iv, Fig. S4). To verify the HUVEC identity, the patterned vessel tubes were stained with red fluorescence conjugated CD31 antibody (Fig. 2F)83.
Vascular barrier function test of the vessel tube with/without the supporting fibroblasts
In addition to substance transportation, another important function of blood vessels is lateral permeability, which enables the exchange of substances with surrounding tissue, as known as vascular barrier function84,85. To evaluate the vascular barrier function in different scenarios, the monocultured vessel was set as control and the fibroblast-supported vessel was set as the experiment group86. First, the suspended fibroblasts were mixed into the HUVEC suspension, and went through the acoustofluidic engineering process (Fig. 3A). After 2 days culture, HUVECs could still interconnect and assemble into the straight vessel tubes while the fibroblasts were located on the outer wall of the vessel tube (Fig. S3A). Figure. 3B shows the confocal scanning of the fibroblast-supported vessel structure. The fibroblasts were labeled with red fluorescence conjugated α-smooth muscle actin antibody87.
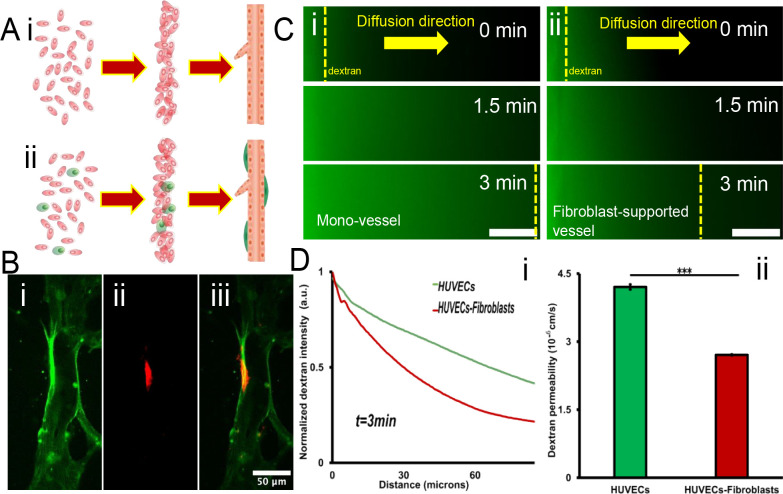
Figure 3.
Vascular barrier function characterization with/without supporting fibroblasts. A) Schematics drawing of the vessel formation from the suspended cells. (i) Pure HUVECs situation, and (ii) mixture of UVECs and fibroblasts. B) Coculture of the vessel (i) and the fibroblast (ii). The overlap image (iii) showed the fibroblast is on the outer wall of the vessel. C) Vessel permeability test in mono vessel tube (i) and fibroblast supported vessel tube (ii). Scale bar: 20um. D) (i)70 kDa dextran diffusion profiles at t = 3 min for vessels in various culture conditions. (ii)70 Kda dextran permeability values calculated for vessels in various culture conditions. Error bars show the standard deviation. t-test, *p< 0.05, **p< 0.01, ***p< 0.001, n= 3.
Then, the vascular barrier function was quantified by visualizing the diffusion of 70 KDa FITC-dextran solute88. The dextran solution was perfused into the parallel patterned vessel tubes through the anastomosis zone (Fig. 2Eii) and permeated into the surrounding matrix. The monocultured vessel was leakier compared with the fibroblast-supported vessel (Fig. 3C). The lateral diffusion rate of dextran in the monocultured vessel was almost twice that of the fibroblast-supported vessel (Fig. 3D). For potential interpretation of the interaction between the solute and vascular cell membrane, an in silico atomistic model was proposed to visualize how the solute crossing the endothelial cell membrane (Fig. 4).
Vascular structure influence compound transport in the vessel-associated matrix
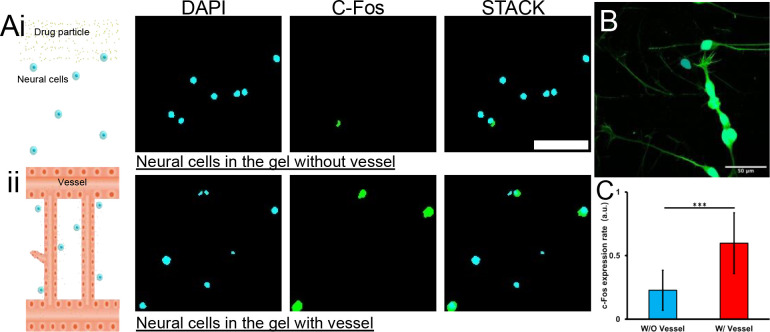
In addition to demonstrating that surrounding stromal cells can affect blood vessel, it was further shown that the vessel structure can also affect cells in the surrounding environment. Here, a functional assay was designed to show how vascular structure affects compound transport and influences neural activity. Induced pluripotent stem cells (iPSC)-derived neural cells were utilized89, which express glutamate receptors50 (Fig. 5). As a control, the iPSC-derived neural cells were resuspended in the pure fibrinogen gel in the microfluidic chamber (Fig. 5Ai). For the experiment group, the neural cells were cocultured with the patterned vessel network (Fig. 5Aii). Glutamate is one of the major excitatory neurotransmitters in the mammalian central nervous system90. For the pure hydrogel matrix, solution containing glutamate was loaded into the microfluidic chamber through one inject port. The compound travels through the matrix to reach the suspended cells by passive diffusion91. For the vessel-associated matrix, the compound was delivered through the patterned vessel network channel. After 10 minutes of treatment, the samples were washed to remove compound solution. After 20 minutes, the immune-staining for c-Fos was performed. The c-fos gene expression can be activated by a wide range of stimuli and is a reliable marker of neural activity92. Its transcription was in rapid manner93. In the group in which the compound was delivered by blood vessels, the level of C-fos expression in nerve cells was almost double that of the passive diffusion group (Fig. 5C). In a pure matrix without blood vessels, the substances are transported by passive diffusion, implying slow transport rates, limited transport distances and spatial substance gradients. In a matrix with blood vessel networks, substances are transported directly in vessel tubes, which serve as highway, and reaches the bulk of the matrix more quickly94.
Figure 5.
C-Fos expression show reduced how glutamate influence the neural cell without/without vessel transport. A) The neural cells seeded in the hydrogel respond to glutamate stimuli without (i) and with (ii) the vessel transport. Scale bar: 50 microns. B) The neural cells grow in adherence state. C) The normalized c-Fos expression rate with/without vessel transport the compound.
Conclusion
The reported acoustofluidic engineering of vessel-on-a-chip not only inherited the advantages of previous acoustophoretic publications80,95, but also incorporated technical points from the literatures about hydraulic pressure activating vessel formation96,97. To demonstrate the function of the acoustofluidic engineered vascular structure, the perfusability and permeability of the vessel-on-a-chip was verified. Further, a molecular dynamic computational model was proposed to illustrate how the compound crossing the vascular lipid layer. Lastly, a vessel-neural cells coculture system was established to demonstrate that the vascular structure played the important role in compound delivery process. In general, we reported an acoustofluidic methodology to engineering in vitro vessel-on-a-chip model. It is believed that the proposed method can contribute to tissue engineering and regenerative medicine application. In the future, this method has the opportunity to further combine different patterning modes of the sound field (Fig. S5A) to create functional vascular tissues of the specific shapes98,99.
Acknowledgements
This work was supported by National Institute of Health grant R01HL131750, R21EB033102, National Science Foundation grant CBET 2039310, OAC 2215789, Pennsylvania Department of Health Commonwealth Universal Research Enhancement Program (CURE), and Pennsylvania Infrastructure Technology Alliance (PITA).
Footnotes
The authors declare no competing financial interest.
Supplementary Materials
Supplementary Figure: Various HUVEC self-assembly behavior under different in vitro culture conditions.
References
- (1).Pugsley M. K.; Tabrizchi R. The Vascular System. An Overview of Structure and Function. J. Pharmacol. Toxicol. Methods 2000, 44 (2), 333–340. [DOI] [PubMed] [Google Scholar]
- (2).Banks W. A. From Blood-Brain Barrier to Blood-Brain Interface: New Opportunities for CNS Drug Delivery. Nat. Rev. Drug Discov. 2016, 15 (4), 275–292. [DOI] [PubMed] [Google Scholar]
- (3).Carmeliet P.; Conway E. M. Growing Better Blood Vessels. Nat. Biotechnol. 2001, 19 (11), 1019–1020. [DOI] [PubMed] [Google Scholar]
- (4).Hellmut G A.; Gou Young K. Organotypic Vasculature: From Descriptive Heterogeneity to Functional Pathophysiology. Science 2017, 357 (6353), eaal2379. [DOI] [PubMed] [Google Scholar]
- (5).Potente M.; Mäkinen T. Vascular Heterogeneity and Specialization in Development and Disease. Nat. Rev. Mol. Cell Biol. 2017, 18 (8), 477–494. [DOI] [PubMed] [Google Scholar]
- (6).Lorente S.; Hautefeuille M.; Sanchez-Cedillo A. The Liver, a Functionalized Vascular Structure. Sci. Rep. 2020, 10 (1), 16194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (7).Jain R. K.; Au P.; Tam J.; Duda D. G.; Fukumura D. Engineering Vascularized Tissue. Nat. Biotechnol. 2005, 23 (7), 821–823. [DOI] [PubMed] [Google Scholar]
- (8).Kwak T. J.; Lee E. In Vitro Modeling of Solid Tumor Interactions with Perfused Blood Vessels. Sci. Rep. 2020, 10 (1), 20142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (9).Haase K.; Piatti F.; Marcano M.; Shin Y.; Visone R.; Redaelli A.; Rasponi M.; Kamm R. D. Physiologic Flow-Conditioning Limits Vascular Dysfunction in Engineered Human Capillaries. Biomaterials 2022, 280 (121248), 121248. [DOI] [PubMed] [Google Scholar]
- (10).Yu J.; Lee S.; Song J.; Lee S.-R.; Kim S.; Choi H.; Kang H.; Hwang Y.; Hong Y.-K.; Jeon N. L. Perfusable Micro-Vascularized 3D Tissue Array for High-Throughput Vascular Phenotypic Screening. Nano Converg. 2022, 9 (1), 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (11).Paek J.; Park S. E.; Lu Q.; Park K.-T.; Cho M.; Oh J. M.; Kwon K. W.; Yi Y.-S.; Song J. W.; Edelstein H. I.; Ishibashi J.; Yang W.; Myerson J. W.; Kiseleva R. Y.; Aprelev P.; Hood E. D.; Stambolian D.; Seale P.; Muzykantov V. R.; Huh D. Microphysiological Engineering of Self-Assembled and Perfusable Microvascular Beds for the Production of Vascularized Three-Dimensional Human Microtissues. ACS Nano 2019, 13 (7), 7627–7643. [DOI] [PubMed] [Google Scholar]
- (12).Moya M. L.; Hsu Y.-H.; Lee A. P.; Hughes C. C. W.; George S. C. In Vitro Perfused Human Capillary Networks. Tissue Eng. Part C Methods 2013, 19 (9), 730–737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (13).Zhou Y.; Wu Y.; Paul R.; Qin X.; Liu Y. Hierarchical Vessel Network-Supported Tumor Model-on-a-Chip Constructed by Induced Spontaneous Anastomosis. ACS Appl. Mater. Interfaces 2023. 10.1021/acsami.2c19453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (14).Campisi M.; Shin Y.; Osaki T.; Hajal C.; Chiono V.; Kamm R. D. 3D Self-Organized Microvascular Model of the Human Blood-Brain Barrier with Endothelial Cells, Pericytes and Astrocytes. Biomaterials 2018, 180, 117–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (15).De LumeNEXT: A Practical Method to Pattern Luminal Structures in ECM Gels. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (16).de Graaf M. N. S.; Vivas A.; Kasi D. G.; van den Hil F. E.; van den Berg A.; van der Meer A. D.; Mummery C. L.; Orlova V. V. Multiplexed Fluidic Circuit Board for Controlled Perfusion of 3D Blood Vessels-on-a-Chip. Lab Chip 2022, 23 (1), 168–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (17).Wu Y.; Zhou Y.; Paul R.; Qin X.; Islam K.; Liu Y. Adaptable Microfluidic Vessel-on-a-Chip Platform for Investigating Tumor Metastatic Transport in Bloodstream. Anal. Chem. 2022. 10.1021/acs.analchem.2c02556. [DOI] [PubMed] [Google Scholar]
- (18).Zhao N.; Guo Z.; Kulkarni S.; Norman D.; Zhang S.; Chung T. D.; Nerenberg R. F.; Linville R.; Searson P. Engineering the Human Blood-Brain Barrier at the Capillary Scale Using a Double-Templating Technique. Adv. Funct. Mater. 2022, 32 (30), 2110289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (19).Paul R.; Zhao Y.; Coster D.; Qin X.; Islam K.; Wu Y.; Liu Y. Rapid Prototyping of High-Resolution Large Format Microfluidic Device through Maskless Image Guided in-Situ Photopolymerization. Nat. Commun. 2023, 14 (1), 4520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (20).Rayner S. G.; Howard C. C.; Mandrycky C. J.; Stamenkovic S.; Himmelfarb J.; Shih A. Y.; Zheng Y. Multiphoton-Guided Creation of Complex Organ-Specific Microvasculature. Adv. Healthc. Mater. 2021, 10 (10), e2100031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (21).Arakawa C.; Gunnarsson C.; Howard C.; Bernabeu M.; Phong K.; Yang E.; DeForest C. A.; Smith J. D.; Zheng Y. Biophysical and Biomolecular Interactions of Malaria-Infected Erythrocytes in Engineered Human Capillaries. Sci. Adv. 2020, 6 (3), eaay7243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (22).Arakawa C. K.; Badeau B. A.; Zheng Y.; DeForest C. A. Multicellular Vascularized Engineered Tissues through User-Programmable Biomaterial Photodegradation. Adv. Mater. 2017, 29 (37). 10.1002/adma.201703156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (23).Ozcelik A.; Rufo J.; Guo F.; Gu Y.; Li P.; Lata J.; Huang T. J. Acoustic Tweezers for the Life Sciences. Nat. Methods 2018, 15 (12), 1021–1028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (24).Yang S.; Rufo J.; Zhong R.; Rich J.; Wang Z.; Lee L. P.; Huang T. J. Acoustic Tweezers for High-Throughput Single-Cell Analysis. Nat. Protoc. 2023, 18 (8), 2441–2458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (25).Li P.; Mao Z.; Peng Z.; Zhou L.; Chen Y.; Huang P.-H.; Truica C. I.; Drabick J. J.; El-Deiry W. S.; Dao M.; Suresh S.; Huang T. J. Acoustic Separation of Circulating Tumor Cells. Proc. Natl. Acad. Sci. U. S. A. 2015, 112 (16), 4970–4975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (26).Wu M.; Ouyang Y.; Wang Z.; Zhang R.; Huang P.-H.; Chen C.; Li H.; Li P.; Quinn D.; Dao M.; Suresh S.; Sadovsky Y.; Huang T. J. Isolation of Exosomes from Whole Blood by Integrating Acoustics and Microfluidics. Proc. Natl. Acad. Sci. U. S. A. 2017, 114 (40), 10584–10589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (27).Gu Y.; Chen C.; Mao Z.; Bachman H.; Becker R.; Rufo J.; Wang Z.; Zhang P.; Mai J.; Yang S.; Zhang J.; Zhao S.; Ouyang Y.; Wong D. T. W.; Sadovsky Y.; Huang T. J. Acoustofluidic Centrifuge for Nanoparticle Enrichment and Separation. Sci Adv 2021, 7 (1). 10.1126/sciadv.abc0467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (28).Hao N.; Pei Z.; Liu P.; Bachman H.; Naquin T. D.; Zhang P.; Zhang J.; Shen L.; Yang S.; Yang K.; Zhao S.; Huang T. J. Acoustofluidics-Assisted Fluorescence-SERS Bimodal Biosensors. Small 2020, 16 (48), e2005179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (29).Wang Z.; Li F.; Rufo J.; Chen C.; Yang S.; Li L.; Zhang J.; Cheng J.; Kim Y.; Wu M.; Abemayor E.; Tu M.; Chia D.; Spruce R.; Batis N.; Mehanna H.; Wong D. T. W.; Huang T. J. Acoustofluidic Salivary Exosome Isolation: A Liquid Biopsy Compatible Approach for Human Papillomavirus-Associated Oropharyngeal Cancer Detection. J. Mol. Diagn. 2020, 22 (1), 50–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (30).Ma Z.; Zhou Y.; Collins D. J.; Ai Y. Fluorescence Activated Cell Sorting via a Focused Traveling Surface Acoustic Beam. Lab Chip 2017, 17 (18), 3176–3185. [DOI] [PubMed] [Google Scholar]
- (31).Xu J.; Cai H.; Wu Z.; Li X.; Tian C.; Ao Z.; Niu V. C.; Xiao X.; Jiang L.; Khodoun M.; Rothenberg M.; Mackie K.; Chen J.; Lee L. P.; Guo F. Acoustic Metamaterials-Driven Transdermal Drug Delivery for Rapid and on-Demand Management of Acute Disease. Nat. Commun. 2023, 14 (1), 869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (32).Ramesan S.; Rezk A. R.; Dekiwadia C.; Cortez-Jugo C.; Yeo L. Y. Acoustically-Mediated Intracellular Delivery. Nanoscale 2018, 10 (27), 13165–13178. [DOI] [PubMed] [Google Scholar]
- (33).Banerjee T.; Gosai A.; Yousefi N.; Garibay O. O.; Seal S.; Balasubramanian G. Examining Sialic Acid Derivatives as Potential Inhibitors of SARS-CoV-2 Spike Protein Receptor Binding Domain. J. Biomol. Struct. Dyn. 2023, 1–17. [DOI] [PubMed] [Google Scholar]
- (34).Yousefi N.; Yazdani-Jahromi M.; Tayebi A.; Kolanthai E.; Neal C. J.; Banerjee T.; Gosai A.; Balasubramanian G.; Seal S.; Ozmen Garibay O. BindingSite-AugmentedDTA: Enabling a next-Generation Pipeline for Interpretable Prediction Models in Drug Repurposing. Brief. Bioinform. 2023, 24 (3). 10.1093/bib/bbad136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (35).Wu Z.; Ao Z.; Cai H.; Li X.; Chen B.; Tu H.; Wang Y.; Lu R. O.; Gu M.; Cheng L.; Lu X.; Guo F. Acoustofluidic Assembly of Primary Tumor-Derived Organotypic Cell Clusters for Rapid Evaluation of Cancer Immunotherapy. J. Nanobiotechnology 2023, 21 (1), 40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (36).Ao Z.; Wu Z.; Cai H.; Hu L.; Li X.; Kaurich C.; Chang J.; Gu M.; Cheng L.; Lu X.; Guo F. Rapid Profiling of Tumor-Immune Interaction Using Acoustically Assembled Patient-Derived Cell Clusters. Adv. Sci. (Weinh.) 2022, 9 (22), e2201478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (37).Armstrong J. P. K.; Pchelintseva E.; Treumuth S.; Campanella C.; Meinert C.; Klein T. J.; Hutmacher D. W.; Drinkwater B. W.; Stevens M. M. Tissue Engineering Cartilage with Deep Zone Cytoarchitecture by High-Resolution Acoustic Cell Patterning. Adv. Healthc. Mater. 2022, e2200481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (38).Armstrong J. P. K.; Puetzer J. L.; Serio A.; Guex A. G.; Kapnisi M.; Breant A.; Zong Y.; Assal V.; Skaalure S. C.; King O.; Murty T.; Meinert C.; Franklin A. C.; Bassindale P. G.; Nichols M. K.; Terracciano C. M.; Hutmacher D. W.; Drinkwater B. W.; Klein T. J.; Perriman A. W.; Stevens M. M. Engineering Anisotropic Muscle Tissue Using Acoustic Cell Patterning. Adv. Mater. 2018, 30 (43), e1802649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (39).Bouyer C.; Chen P.; Güven S.; Demirtaş T. T.; Nieland T. J. F.; Padilla F.; Demirci U. A Bio-Acoustic Levitational (BAL) Assembly Method for Engineering of Multilayered, 3D Brain-like Constructs, Using Human Embryonic Stem Cell Derived Neuro-Progenitors. Adv. Mater. 2016, 28 (1), 161–167. [DOI] [PubMed] [Google Scholar]
- (40).Chen B.; Wu Z.; Wu Y.; Chen Y.; Zheng L. Controllable Fusion of Multicellular Spheroids Using Acoustofluidics. Microfluid. Nanofluidics 2023, 27 (7). 10.1007/s10404-023-02660-5. [DOI] [Google Scholar]
- (41).Link A.; Franke T. Acoustic Erythrocytometer for Mechanically Probing Cell Viscoelasticity. Lab Chip 2020, 20 (11), 1991–1998. [DOI] [PubMed] [Google Scholar]
- (42).Cai H.; Ao Z.; Wu Z.; Nunez A.; Jiang L.; Carpenter R. L.; Nephew K. P.; Guo F. Profiling Cell-Matrix Adhesion Using Digitalized Acoustic Streaming. Anal. Chem. 2020, 92 (2), 2283–2290. [DOI] [PubMed] [Google Scholar]
- (43).Gai J.; Nosrati R.; Neild A. High DNA Integrity Sperm Selection Using Surface Acoustic Waves. Lab Chip 2020, 20 (22), 4262–4272. [DOI] [PubMed] [Google Scholar]
- (44).Gai J.; Devendran C.; Neild A.; Nosrati R. Surface Acoustic Wave-Driven Pumpless Flow for Sperm Rheotaxis Analysis. Lab Chip 2022, 22 (22), 4409–4417. [DOI] [PubMed] [Google Scholar]
- (45).Liao Q.-Q.; Zhao S.-K.; Cai B.; He R.-X.; Rao L.; Wu Y.; Guo S.-S.; Liu Q.-Y.; Liu W.; Zhao X.-Z. Biocompatible Fabrication of Cell-Laden Calcium Alginate Microbeads Using Microfluidic Double Flow-Focusing Device. Sens. Actuators A Phys. 2018, 279, 313–320. [Google Scholar]
- (46).Guo F.; Xie Y.; Li S.; Lata J.; Ren L.; Mao Z.; Ren B.; Wu M.; Ozcelik A.; Huang T. J. Reusable Acoustic Tweezers for Disposable Devices. Lab Chip 2015, 15 (24), 4517–4523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (47).Wu Y.; Zhao Y.; Zhou Y.; Islam K.; Liu Y. Microfluidic Droplet-Assisted Fabrication of Vessel-Supported Tumors for Preclinical Drug Discovery. ACS Appl. Mater. Interfaces 2023, 15 (12), 15152–15161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (48).Hajal C.; Offeddu G. S.; Shin Y.; Zhang S.; Morozova O.; Hickman D.; Knutson C. G.; Kamm R. D. Engineered Human Blood-Brain Barrier Microfluidic Model for Vascular Permeability Analyses. Nat. Protoc. 2022, 17 (1), 95–128. [DOI] [PubMed] [Google Scholar]
- (49).Pak C.; Pak C.; Grieder S.; Yang N.; Zhang Y.; Wernig M.; Sudhof T. Rapid Generation of Functional and Homogeneous Excitatory Human Forebrain Neurons Using Neurogenin-2 (Ngn2). Protoc. Exch. 2018. 10.1038/protex.2018.082. [DOI] [Google Scholar]
- (50).Zhang Y.; Pak C.; Han Y.; Ahlenius H.; Zhang Z.; Chanda S.; Marro S.; Patzke C.; Acuna C.; Covy J.; Xu W.; Yang N.; Danko T.; Chen L.; Wernig M.; Südhof T. C. Rapid Single-Step Induction of Functional Neurons from Human Pluripotent Stem Cells. Neuron 2013, 78 (5), 785–798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (51).Yeon J. H.; Ryu H. R.; Chung M.; Hu Q. P.; Jeon N. L. In Vitro Formation and Characterization of a Perfusable Three-Dimensional Tubular Capillary Network in Microfluidic Devices. Lab Chip 2012, 12 (16), 2815–2822. [DOI] [PubMed] [Google Scholar]
- (52).Chen B.; Wu Y.; Ao Z.; Cai H.; Nunez A.; Liu Y.; Foley J.; Nephew K.; Lu X.; Guo F. High-Throughput Acoustofluidic Fabrication of Tumor Spheroids. Lab Chip 2019, 19 (10), 1755–1763. [DOI] [PubMed] [Google Scholar]
- (53).Lata J. P.; Guo F.; Guo J.; Huang P.-H.; Yang J.; Huang T. J. Surface Acoustic Waves Grant Superior Spatial Control of Cells Embedded in Hydrogel Fibers. Adv. Mater. 2016, 28 (39), 8632–8638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (54).Wu Z.; Chen B.; Wu Y.; Xia Y.; Chen H.; Gong Z.; Hu H.; Ding Z.; Guo S. Scaffold-Free Generation of Heterotypic Cell Spheroids Using Acoustofluidics. Lab Chip 2021. 10.1039/d1lc00496d. [DOI] [PubMed] [Google Scholar]
- (55).Katche C.; Bekinschtein P.; Slipczuk L.; Goldin A.; Izquierdo I. A.; Cammarota M.; Medina J. H. Delayed Wave of C-Fos Expression in the Dorsal Hippocampus Involved Specifically in Persistence of Long-Term Memory Storage. Proc. Natl. Acad. Sci. U. S. A. 2010, 107 (1), 349–354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (56).Virumbrales-Muñoz M.; Chen J.; Ayuso J.; Lee M.; Abel E. J.; Beebe D. J. Organotypic Primary Blood Vessel Models of Clear Cell Renal Cell Carcinoma for Single-Patient Clinical Trials. Lab Chip 2020, 20 (23), 4420–4432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (57).Ingram P. N.; Hind L. E.; Jiminez-Torres J. A.; Huttenlocher A.; Beebe D. J. An Accessible Organotypic Microvessel Model Using IPSC-Derived Endothelium. Adv. Healthc. Mater. 2018, 7 (2), 1700497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (58).Ni Z.; Yin C.; Xu G.; Xie L.; Huang J.; Liu S.; Tu J.; Guo X.; Zhang D. Modelling of SAW-PDMS Acoustofluidics: Physical Fields and Particle Motions Influenced by Different Descriptions of the PDMS Domain. Lab Chip 2019, 19 (16), 2728–2740. [DOI] [PubMed] [Google Scholar]
- (59).Cai H.; Ao Z.; Hu L.; Moon Y.; Wu Z.; Lu H.-C.; Kim J.; Guo F. Acoustofluidic Assembly of 3D Neurospheroids to Model Alzheimer’s Disease. Analyst 2020, 145 (19), 6243–6253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (60).Pan H.; Mei D.; Xu C.; Han S.; Wang Y. Bisymmetric Coherent Acoustic Tweezers Based on Modulation of Surface Acoustic Waves for Dynamic and Reconfigurable Cluster Manipulation of Particles and Cells. Lab Chip 2023, 23 (2), 215–228. [DOI] [PubMed] [Google Scholar]
- (61).Nama N.; Barnkob R.; Mao Z.; Kähler C. J.; Costanzo F.; Huang T. J. Numerical Study of Acoustophoretic Motion of Particles in a PDMS Microchannel Driven by Surface Acoustic Waves. Lab Chip 2015, 15 (12), 2700–2709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (62).Hsu J.-C.; Chao C.-L. Acoustophoretic Patterning of Microparticles in a Microfluidic Chamber Driven by Standing Lamb Waves. Appl. Phys. Lett. 2021, 119 (10), 103504. [Google Scholar]
- (63).Cooke I. R.; Kremer K.; Deserno M. Tunable Generic Model for Fluid Bilayer Membranes. Phys. Rev. E Stat. Nonlin. Soft Matter Phys. 2005, 72 (1 Pt 1), 011506. [DOI] [PubMed] [Google Scholar]
- (64).Razizadeh M.; Nikfar M.; Paul R.; Liu Y. Coarse-Grained Modeling of Pore Dynamics on the Red Blood Cell Membrane under Large Deformations. Biophys. J. 2020, 119 (3), 471–482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (65).Jorgensen C.; Ulmschneider M. B.; Searson P. C. Atomistic Model of Solute Transport across the Blood-Brain Barrier. ACS Omega 2022, 7 (1), 1100–1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (66).Islam K.; Razizadeh M.; Liu Y. Coarse-Grained Molecular Simulation of Extracellular Vesicle Squeezing for Drug Loading. Phys. Chem. Chem. Phys. 2023, 25 (17), 12308–12321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (67).Guo F.; Li P.; French J. B.; Mao Z.; Zhao H.; Li S.; Nama N.; Fick J. R.; Benkovic S. J.; Huang T. J. Controlling Cell-Cell Interactions Using Surface Acoustic Waves. Proc. Natl. Acad. Sci. U. S. A. 2015, 112 (1), 43–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (68).Guo F.; Mao Z.; Chen Y.; Xie Z.; Lata J. P.; Li P.; Ren L.; Liu J.; Yang J.; Dao M.; Suresh S.; Huang T. J. Three-Dimensional Manipulation of Single Cells Using Surface Acoustic Waves. Proc. Natl. Acad. Sci. U. S. A. 2016, 113 (6), 1522–1527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (69).Ding X.; Shi J.; Lin S.-C. S.; Yazdi S.; Kiraly B.; Huang T. J. Tunable Patterning of Microparticles and Cells Using Standing Surface Acoustic Waves. Lab Chip 2012, 12 (14), 2491–2497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (70).Mao Z.; Xie Y.; Guo F.; Ren L.; Huang P.-H.; Chen Y.; Rufo J.; Costanzo F.; Huang T. J. Experimental and Numerical Studies on Standing Surface Acoustic Wave Microfluidics. Lab Chip 2016, 16 (3), 515–524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (71).Guo F.; Zhou W.; Li P.; Mao Z.; Yennawar N. H.; French J. B.; Huang T. J. Precise Manipulation and Patterning of Protein Crystals for Macromolecular Crystallography Using Surface Acoustic Waves. Small 2015, 11 (23), 2733–2737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (72).Wu Y.; Ao Z.; Chen B.; Muhsen M.; Bondesson M.; Lu X.; Guo F. Acoustic Assembly of Cell Spheroids in Disposable Capillaries. Nanotechnology 2018, 29 (50), 504006. [DOI] [PubMed] [Google Scholar]
- (73).Li S.; Guo F.; Chen Y.; Ding X.; Li P.; Wang L.; Cameron C. E.; Huang T. J. Standing Surface Acoustic Wave Based Cell Coculture. Anal. Chem. 2014, 86 (19), 9853–9859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (74).Chen M. B.; Whisler J. A.; Fröse J.; Yu C.; Shin Y.; Kamm R. D. On-Chip Human Microvasculature Assay for Visualization and Quantification of Tumor Cell Extravasation Dynamics. Nat. Protoc. 2017, 12 (5), 865–880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (75).Wan Z.; Zhong A. X.; Zhang S.; Pavlou G.; Coughlin M. F.; Shelton S. E.; Nguyen H. T.; Lorch J. H.; Barbie D. A.; Kamm R. D. A Robust Method for Perfusable Microvascular Network Formation in Vitro. Small Methods 2022, 6 (6), e2200143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (76).Tian Z.; Wang Z.; Zhang P.; Naquin T. D.; Mai J.; Wu Y.; Yang S.; Gu Y.; Bachman H.; Liang Y.; Yu Z.; Huang T. J. Generating Multifunctional Acoustic Tweezers in Petri Dishes for Contactless, Precise Manipulation of Bioparticles. Sci. Adv. 2020, 6 (37). 10.1126/sciadv.abb0494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (77).Petta D.; Basoli V.; Pellicciotta D.; Tognato R.; Barcik J.; Arrigoni C.; Bella E. D.; Armiento A. R.; Candrian C.; Richards R. G.; Alini M.; Moretti M.; Eglin D.; Serra T. Sound-Induced Morphogenesis of Multicellular Systems for Rapid Orchestration of Vascular Networks. Biofabrication 2020, 13 (1), 015004. [DOI] [PubMed] [Google Scholar]
- (78).Di Marzio N.; Ananthanarayanan P.; Guex A. G.; Alini M.; Riganti C.; Serra T. Sound-Based Assembly of a Microcapillary Network in a Saturn-like Tumor Model for Drug Testing. Mater. Today Bio 2022, 16 (100357), 100357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (79).Garvin K. A.; Dalecki D.; Yousefhussien M.; Helguera M.; Hocking D. C. Spatial Patterning of Endothelial Cells and Vascular Network Formation Using Ultrasound Standing Wave Fields. J. Acoust. Soc. Am. 2013, 134 (2), 1483–1490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (80).Kang B.; Shin J.; Park H.-J.; Rhyou C.; Kang D.; Lee S.-J.; Yoon Y.-S.; Cho S.-W.; Lee H. High-Resolution Acoustophoretic 3D Cell Patterning to Construct Functional Collateral Cylindroids for Ischemia Therapy. Nat. Commun. 2018, 9 (1). 10.1038/s41467-018-07823-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (81).Zhang S.; Wan Z.; Pavlou G.; Zhong A. X.; Xu L.; Kamm R. D. Interstitial Flow Promotes the Formation of Functional Microvascular Networks in Vitro through Upregulation of Matrix Metalloproteinase-2. Adv. Funct. Mater. 2022, 2206767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (82).Winkelman M. A.; Kim D. Y.; Kakarla S.; Grath A.; Silvia N.; Dai G. Interstitial Flow Enhances the Formation, Connectivity, and Function of 3D Brain Microvascular Networks Generated within a Microfluidic Device. Lab Chip 2021, 22 (1), 170–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (83).Vila Cuenca M.; Cochrane A.; van den Hil F. E.; de Vries A. A. F.; Lesnik Oberstein S. A. J.; Mummery C. L.; Orlova V. V. Engineered 3D Vessel-on-Chip Using HiPSC-Derived Endothelial- and Vascular Smooth Muscle Cells. Stem Cell Reports 2021, 16 (9), 2159–2168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (84).Offeddu G. S.; Haase K.; Gillrie M. R.; Li R.; Morozova O.; Hickman D.; Knutson C. G.; Kamm R. D. An On-Chip Model of Protein Paracellular and Transcellular Permeability in the Microcirculation. Biomaterials 2019, 212, 115–125. [DOI] [PubMed] [Google Scholar]
- (85).Ho Y. T.; Adriani G.; Beyer S.; Nhan P.-T.; Kamm R. D.; Kah J. C. Y. A Facile Method to Probe the Vascular Permeability of Nanoparticles in Nanomedicine Applications. Sci. Rep. 2017, 7 (1). 10.1038/s41598-017-00750-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (86).Margolis E. A.; Cleveland D. S.; Kong Y. P.; Beamish J. A.; Wang W. Y.; Baker B. M.; Putnam A. J. Stromal Cell Identity Modulates Vascular Morphogenesis in a Microvasculature-on-a-Chip Platform. Lab Chip 2021, 21 (6), 1150–1163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (87).Pal D.; Ghatak S.; Singh K.; Abouhashem A. S.; Kumar M.; El Masry M. S.; Mohanty S. K.; Palakurti R.; Rustagi Y.; Tabasum S.; Khona D. K.; Khanna S.; Kacar S.; Srivastava R.; Bhasme P.; Verma S. S.; Hernandez E.; Sharma A.; Reese D.; Verma P.; Ghosh N.; Gorain M.; Wan J.; Liu S.; Liu Y.; Castro N. H.; Gnyawali S. C.; Lawrence W.; Moore J.; Perez D. G.; Roy S.; Yoder M. C.; Sen C. K. Identification of a Physiologic Vasculogenic Fibroblast State to Achieve Tissue Repair. Nat. Commun. 2023, 14 (1), 1129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (88).Zheng Y.; Chen J.; Craven M.; Choi N. W.; Totorica S.; Diaz-Santana A.; Kermani P.; Hempstead B.; Fischbach-Teschl C.; López J. A.; Stroock A. D. In Vitro Microvessels for the Study of Angiogenesis and Thrombosis. Proc. Natl. Acad. Sci. U. S. A. 2012, 109 (24), 9342–9347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (89).Hasan M. F.; Berdichevsky Y. Neuron and Astrocyte Aggregation and Sorting in Three-Dimensional Neuronal Constructs. Commun. Biol. 2021, 4 (1), 587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (90).Zhou Y.; Danbolt N. C. Glutamate as a Neurotransmitter in the Healthy Brain. J. Neural Transm. (Vienna) 2014, 121 (8), 799–817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (91).Adriani G.; Ma D.; Pavesi A.; Kamm R. D.; Goh E. L. K. A 3D Neurovascular Microfluidic Model Consisting of Neurons, Astrocytes and Cerebral Endothelial Cells as a Blood–Brain Barrier. Lab Chip 2017, 17 (3), 448–459. [DOI] [PubMed] [Google Scholar]
- (92).Joo J.-Y.; Schaukowitch K.; Farbiak L.; Kilaru G.; Kim T.-K. Stimulus-Specific Combinatorial Functionality of Neuronal c-Fos Enhancers. Nat. Neurosci. 2016, 19 (1), 75–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (93).Bullitt E. Expression of C-Fos-like Protein as a Marker for Neuronal Activity Following Noxious Stimulation in the Rat. J. Comp. Neurol. 1990, 296 (4), 517–530. [DOI] [PubMed] [Google Scholar]
- (94).Rademakers T.; Horvath J. M.; van Blitterswijk C. A.; LaPointe V. L. S. Oxygen and Nutrient Delivery in Tissue Engineering: Approaches to Graft Vascularization. J. Tissue Eng. Regen. Med. 2019, 13 (10), 1815–1829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (95).Hu X.; Zheng J.; Hu Q.; Liang L.; Yang D.; Cheng Y.; Li S.-S.; Chen L.-J.; Yang Y. Smart Acoustic 3D Cell Construct Assembly with High-Resolution. Biofabrication 2022, 14 (4), 045003. [DOI] [PubMed] [Google Scholar]
- (96).Wan H.-Y.; Chen J. C. H.; Xiao Q.; Wong C. W.; Yang B.; Cao B.; Tuan R. S.; Nilsson S. K.; Ho Y.-P.; Raghunath M.; Kamm R. D.; Blocki A. Stabilization and Improved Functionality of Three-Dimensional Perfusable Microvascular Networks in Microfluidic Devices under Macromolecular Crowding. Biomater. Res. 2023, 27 (1), 32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (97).Wan Z.; Floryan M. A.; Coughlin M. F.; Zhang S.; Zhong A. X.; Shelton S. E.; Wang X.; Xu C.; Barbie D. A.; Kamm R. D. New Strategy for Promoting Vascularization in Tumor Spheroids in a Microfluidic Assay. Adv. Healthc. Mater. 2022, e2201784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (98).Pan H.; Mei D.; Xu C.; Li X.; Wang Y. Acoustic Tweezers Using Bisymmetric Coherent Surface Acoustic Waves for Dynamic and Reconfigurable Manipulation of Particle Multimers. J. Colloid Interface Sci. 2023, 643, 115–123. [DOI] [PubMed] [Google Scholar]
- (99).Hu X.; Zhu J.; Zuo Y.; Yang D.; Zhang J.; Cheng Y.; Yang Y. Versatile Biomimetic Array Assembly by Phase Modulation of Coherent Acoustic Waves. Lab Chip 2020, 20 (19), 3515–3523. [DOI] [PubMed] [Google Scholar]