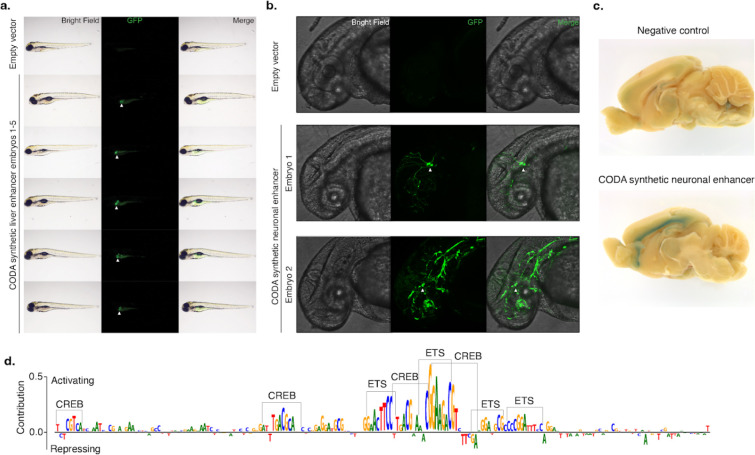
Figure 4. In vivo validation of synthetic elements using zebrafish and mouse.
(a) A synthetic liver-specific CRE drives transgene expression in the larval zebrafish liver. Brightfield, GFP, and merged whole animal imaging 96 hours post-fertilization indicates that the synthetic CRE reproducibly drives transgene expression in zebrafish liver (white arrows). Lateral view, anterior to the left, dorsal up. (b) CODA-designed SK-N-SH-specific CRE drives GFP expression in embryonic zebrafish neurons (white arrows). Brightfield, GFP, and merged imaging of the brain and anterior spinal region of animals 48 hours post-fertilization show transgene expression in the developing brain and spinal cord. Embryo 2 shows additional incidental off-target expression in vascular tissue. Lateral view, anterior to the left, dorsal up. (c) Synthetic SK-N-SH-specific CRE drives transgene expression in 5-week-old postnatal mice. X-Gal staining for LacZ of the medial section of the brain reveals specific transgene expression at layer 6 of the neocortex. (d) Malinois contribution scores reveal the role of ETS and CREB-like binding domains in mediating synthetic CRE activity in neurons. Subsequences of high predicted contribution to SK-N-SH activity overlap with ETS- and CREB-like binding motifs based on visual inspection.