Abstract
The nuclear METTL3-METTL14 enzyme complex transfers a methyl group from S-adenosyl-L-methionine (SAM) to the N6 amino group of an adenosine (A) base in RNA to convert it to m6A and in ssDNA to 6mA. m6A marks are prevalent in eukaryotic mRNAs and lncRNAs and modulate their stability and fate in a context-dependent manner. The cytoplasmic METTL3 can act as a m6A reader to regulate mRNA translation. However, the precise mechanism that actuates the switch from m6A writer to reader/sensor is unclear. Here, we present a ~2.5Å crystal structure of the methyltransferase core of human METTL3-METTL14 in complex with the reaction product, N6-methyladenosine monophosphate (m6A), representing a state post-catalysis but before the release of m6A. m6A occupies a novel evolutionarily conserved cryptic pocket in METTL3-METTL14 located ~16Å away from the SAM pocket that frequently mutates in cancer. We propose a two-step model of swiveling of target A upon conversion to m6A and sensing its methylation status by the cryptic pocket, enabling it to actuate enzymes’ switch from writer to an m6A-sensor. Cancer-associated mutations cannot distinguish methylated from unmethylated adenine and show impaired RNA binding, de-stacking, and defective m6A writing and sensing.
A heterodimer of Methyltransferase like-3 (METTL3) and its obligate partner METTL14 installs the majority of m6A (N6-methyladenosine) modification within the consensus DRACH (D=A/G/U, R=A/G, H=A/C/U) motif in eukaryotic mRNAs and lncRNAs1–5, chromosome-associated regulatory RNAs (carRNAs)6,7, and 7SK RNA8. METTL3 and METTL14 contain an MT-A70 family methyltransferase (MTase) core9 diverged from an ancestral β-class of bacterial MTases10–13. METTL3 hydrolyzes SAM to facilitate the transfer of its methyl group to the N6 amino group of the target adenine base in RNA (in vivo and in vitro)1,4,9 and ssDNA (in vitro)11,12. In contrast, catalytically deficient METTL14 stabilizes METTL3 and is thought to position RNA in METTL3’s active site14–17. m6A modifications modulate RNA stability and play essential roles in myriad biological processes, including but not limited to miRNA biogenesis, maintenance of neural stem cells, translation efficiency, transcription elongation, innate immune response, DNA break repair, circadian rhythm, and viral pathogenesis18,19. Consistently, severe growth defects observed in the cellular KO phenotype of METTL3 underscore METTL3’s essential role in the maintenance of cellular homeostasis during development20–22, cancer growth23–26, and viral infections, including SARS-CoV-227–29.
While m6A deposition in mRNAs occurs in the nucleus and elevated METTL3 levels are associated with survival of acute myeloid leukemia24,30, non-catalytic functions of METTL3 outside the nucleus benefit lung cancer cells23,25. Cytoplasmic METTL3 can act as an m6A reader to promote the translation of mRNA of known oncogenes, thereby facilitating the crosstalk between m6A-bound METTL3 at 3’-end to the translation initiation machinery that has engaged the 5’- mRNA cap23,25,31.
m6A marks are also present in genomes of RNA viruses such as hepatitis C, Zika, dengue, West Nile, yellow fever, and SARS-CoV-2, and modulate viral replication and host immune response27. Thus, METTL3 has emerged as an attractive drug target for anti-cancer and anti-viral therapy development. Consistently, pharmacologic inhibition of METTL3 limits the growth of acute myeloid leukemia26 and SARS-CoV-228,29. The first METTL3 inhibitor STC-15 that targets its SAM pocket has entered the Phase I clinical trial (NCT05584111).
Despite significant advancement in the m6A field and interest in targeting it for therapy, the structural details of RNA recognition and catalysis by METTL3-METTL14 are lacking. Here we present a ~2.5Å crystal structure of the methyltransferase core of METTL3-METTL14 bound to methyladenosine monophosphate (m6A), a product mimic of the methylation reaction (Fig. 1a). We show that m6A occupies a novel cryptic pocket constituted to a large extent by residues from METTL3 and an interface arginine (R298) of METTL14. This pocket is evolutionarily conserved in mammals, plants, and yeast (Fig. 1b). Importantly, residues that partake in interaction with m6A are mutated in gynecologic, stomach, kidney, and bladder cancers32 (Fig. 1b). When introduced into wild-type METTL3-METTL14, the mutant enzymes exhibit a significant loss in catalysis, perturbed RNA binding, and compromised ability of de-stacking of the target adenine for presentation to the active site. Our data suggest that the target base swivels ~180° after methylation for sensing by the cryptic pocket located ~16Å away from the point of methyl transfer. METTL3-METTL14 uses this unique mechanism to sense the methylation status before releasing the substrate RNA. This arrangement will require de-stacking of the target base during catalysis and sensing. We also show that the wild-type METTL3-METTL14, but not the mutant, binds more tightly to an m6A-modified RNA to distinguish it from the unmethylated RNA. Moreover, the enzyme harboring R298P mutation, the most frequent mutation in endometrial cancer32, exhibits sub-optimal RNA binding, catalysis, and base de-stacking ability. Our results uncover entirely unexpected operating principles underlying methyl transfer and m6A-sensing by METTL3-METTL14.
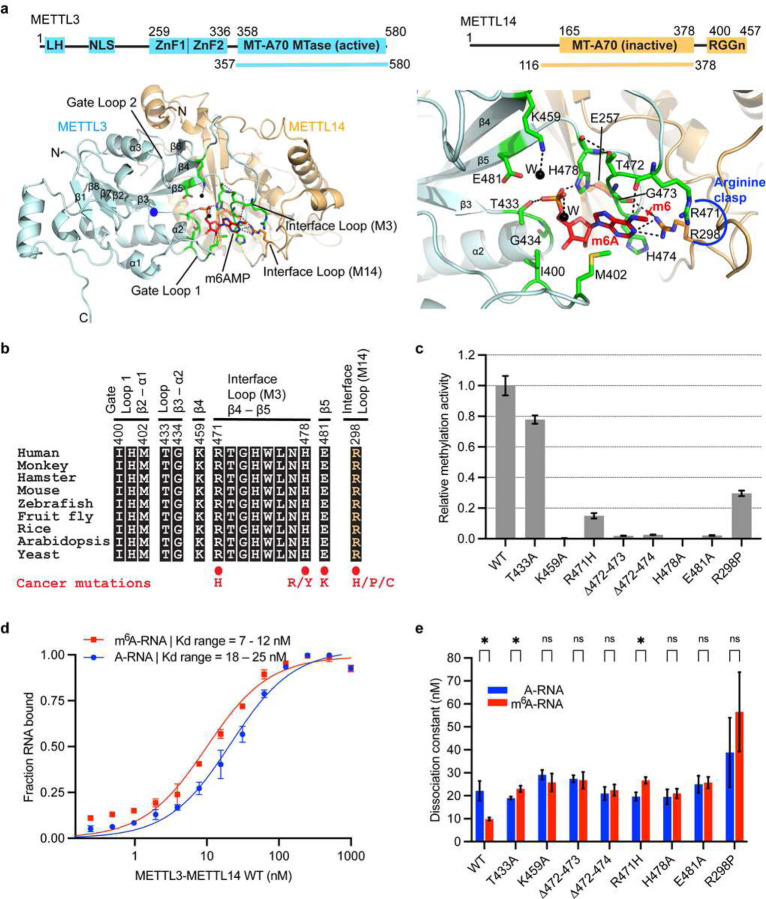
Figure 1 |. Structure of m6A bound human METTL3-METTL14 MTase core.
a, Domain architecture of METTL3 and METTL14; and boundaries of each used in crystallization are shown on top. Structure of the complex is shown in cartoon mode for METTL3 in cyan and METTL14 in orange; m6AMP (red) and interacting residues of METTL3 (green) and METTL14 (orange) are shown in stick mode. Blue dot, the position of N6 (in acceptor mode), i.e., ~3Å from the methyl group of the donor SAM. Methylated N6 of m6A is ~16Å away from its acceptor position in the catalytic pocket (blue dot). Black dots, water. Black dashes, h-bonds. The panel on right shows a close-up of the m6A interaction network, including the arginine clasp. b, An alignment of the regions participating in m6A confirms strict conservation of the interaction network throughout the evolution from yeast (Uniprot ID: P41833); arabidopsis (082486) and rice (Q6EU10); fruit fly (Q9VCE6), zebrafish (F1R777), mouse (Q8C3P7), hamster (A0A1U7R3Z3), and monkey (A0A8J8YGJ7); to human (Q86U44). c, Methyltransferase activity results of full-length human METTL3-METTL14 (wild-type, WT) and eight mutant enzymes as derived from three independent experiments, with error bars indicating the range of data points from these experiments (n = 3). d, Quantitative measurement of RNA (red, m6A-RNA; blue, A-RNA) binding (n = 3) by the WT enzyme shown as binding isotherms fitted with a one-site specific binding model. The equilibrium dissociation constant or Kd derived for each mutant enzyme is plotted along with Kd of the WT enzyme (e). ns, not significant (p >0.05), * denotes p ≤ 0.05. Source data for panels c-e are provided.
Overall structure
The MTase cores of human METTL3 (aa 358 – 580) and METTL14 (aa 116 – 378) form an obligate heterodimer. METTL3 acts as an active SAM-dependent MTase, whereas METTL14, an inactive MTase, stabilizes RNA14–17. We co-purified the MTase core of METTL3-METTL14 from E. coli. We succeeded in resolving its structure in the presence of N6-methyladenosine 5’-monophosphate (m6AMP, referred to as m6A), a product of methylation reaction, by soaking m6A into apo crystals (Fig. 1 a, Extended data Fig. 1a–c). The difference omit map showed clear and unbiased electron density for m6A, which was refined well with no discrepancies for the ligand, surrounding regions, or the rest of the protein (Extended data Fig. 1d–g and table 1). METTL3-METTL14-m6A model was refined to ~2.5Å resolution, with excellent stereochemistry and Rfree and Rwork of ~26.2 and 22.9%, respectively (Extended data Table 1). The final model contains one molecule each of METTL3 (aa 369 – 579), METTL14 (aa 116 – 402), one m6A, 90 water and two ethylene glycol.
The overall fold of METTL3-METTL14 is similar to those reported previously14–16, except for notable changes in the region around the m6A binding pocket. MTases adopt a β-class of MTase fold with a central β-sheet of seven parallel and one antiparallel β-strands flanked by three helices on each side. Three major loops (gate loops 1 and 2, and an interface loop) emanating from the central β-sheet of METTL3 participate in SAM, RNA, and METTL14 binding. While the two gate loops exhibit high flexibility upon SAM or SAH binding and release, the interface loop remains rigid due to extensive protein-protein contacts from METTL14 MTase (Fig. 1a).
Evolutionarily conserved m6A pocket plays an essential role in m6A sensing
Strikingly, m6A occupies a cryptic pocket ~16Å away from the methyl donor SAM pocket with its N6-methyl moiety in an energetically favored syn conformation, facing outward (Fig. 1a). Previously, this region was postulated to bind RNA due to its positive charge and polar nature14–16. m6A is stabilized by a vast network of specific interactions, mostly from METTL3 and R298 of METTL14. The purine ring of m6A is sandwiched between the side chain of M402 and the backbone atoms of the interface loop residues, R471, T472, G473, and H474. The two arginine residues (R471 of METTL3 and R298 of METTL14) act like a clasp to hold the N6-methyl moiety in place through a direct h-bond between R298 and N1, van der Waals and hydrophobic interactions between N6-methyl and its aliphatic portion, and the amino group of the R471 side chain, respectively. The carbonyl oxygen of G473 appears to neutralize the positive charge of the R298 residue. The carbonyl moiety of R471 embraces the N6 atom of m6A via a direct h-bond while the opposite side is stabilized by the side chain of H474 via a π-π interaction. Altogether, the arginine clasp, interface loop residues R471-H474 and M402, forms a partial closure around the methylated purine ring of m6A. The ribose in the C3’-endo conformation is stacked between the backbone atoms of G473 and H478 and the side chains of I400 and H478. The phosphate group of m6A is locked in place by multiple direct h-bonds with its phosphoryl oxygens and side chains of H478, E481, T433, and K459 (water-mediated) – all from METTL3, and another water-mediated interaction with E257 of METTL14. The side chain of H478 holds the m6A phosphate on one side and E257 of METTL14 on the other, thus acting as a hinge (Fig 1a, Extended data Fig. 1g). Strict conservation of the extensive interaction network of m6A in human, animal, plant, and yeast suggests that m6A sensing by this cryptic pocket is an evolutionarily conserved mechanism (Fig. 1b). Several key residues that partake in m6A binding, such as R471 and R298 of the arginine clasp, E481, and H478 that stabilize the N6-methyl and phosphate groups are recurrently mutated in endometrioid and adenocarcinoma32 (Fig. 1b). We introduced the R298P mutation in METTL14, a recurrent mutation event in endometrioid carcinoma32,33, and the R471H, E481A, T433A, K459A, and H478A mutations in METTL3. In addition, we generated two deletion mutants (∆472–473, ∆472–474) in which three residues of METTL3 (T472, G473, H474) that stack against the purine ring of m6A were deleted to shorten the interface loop.
We co-purified the full-length wild-type human METTL3-METTL14 and eight mutant enzymes from insect cells and probed their RNA methylation and binding activities. We used a 30-mer RNA oligo (NEAT2*) consisting of one canonical GGACU motif. Consistently, R298P and R471H mutants significantly reduced (up to 85%), whereas T433A resulted in ~20% loss in methyltransferase activity, agreeing with the reduced m6A levels observed in endometrial tumors harboring the R298P mutation33. The other five mutations in METTL3 (∆472–473, ∆472–474, K459A, E481A, and H478A) completely abolished the RNA methyltransferase activity of METTL3-METTL14 (Fig. 1c). Thus, the evolutionarily conserved m6A binding pocket is essential for efficient conversion of A to m6A.
Next, we quantitatively determined the binding affinities of wild-type (WT) and mutant enzymes to the substrate and a product RNA, wherein the target A base within GGACU is replaced by m6A. We covalently attached a fluoresceine moiety to the 5’-end of both the substrate NEAT2* (A-RNA) and product (m6A-RNA) RNAs and performed fluorescence polarization-based assays. The WT enzyme binds the m6A-RNA with a 2-fold stronger affinity than A-RNA (Kd = 9 vs. 20 nM) (Fig. 1 d,e), corroborating previous studies attributing the m6A-reader function to METTL3 in vivo23,25,31,34. In contrast, the mutants, although bound to both RNAs with weaker, yet nanonmolar affinity (Kd range = 18 – 30 nM), failed to distinguish m6A from A. One exception was the R298P mutant, and to some extent R471H (both mutate in cancers and belong to the arginine clasp motif that stablizes the m6A), that not only showed a significant loss in binding to both RNAs, but also switched the binding preference to unmodified (A-RNA) compared to the WT enzyme (Fig. 1e, Extended data Fig. 1h). Thus, its altered specificity (inability to sense and distinguish m6A) coupled with a significant loss in RNA methylation and binding affinity by the R298P mutation could promote tumorigenicity and growth of endometrial tumors as observed previously33. The nanomolar affinity of mutant enzymes suggests a significant contribution of flanking accessory motifs such as zinc fingers of METTL3 and RGG repeats of METTL14.
Base swiveling facilitates m6A sensing
The two loops in METTL3 (gate loops 1 and 2) surrounding the methyl donor SAM and acceptor base A pockets show varying degrees of flexibility upon SAM and SAH binding from their original positions in a ligand-free (apo) form14–16. Thus, we compared the m6A structure with three states (SAM, SAH, and apo). These loops also move in opposite directions upon m6A binding from their original positions in the SAM-bound METTL3 (Fig. 2a). Gate loop 1 (aa 398–409) moves ~ 5.7Å inward to the direction of m6A, whereas the gate loop 2 (aa 506–512) moves ~ 7.8Å outward, with several residues in this region, including H512, that flips ~180°. The invariant T433 and G434 from a small loop between β3 and α2 move ~ 2.1Å with the side chain of T433 rotating ~90° to stabilize the phosphate and ribose of m6A (Fig. 2a). This region remains unperturbed in SAH-bound METTL3, suggesting the m6A binding to this pocket occurs after hydrolysis of SAM (Fig. 2b). While the gate loop 2 in SAH remains in open confirmation, akin to SAM conformation, the position of gate loop 1 in m6A experiences significant repositioning of the M402 side chain (Fig 2c). Although m6A-bound METTL3 is most similar to the apo form with the smallest root mean square deviation for superposition of 1539 atoms of METTL3 achieved for apo (1.2), compared to SAH (1.5), and SAM (1.9), we observed notable changes in the m6A pocket (Fig. 2c).
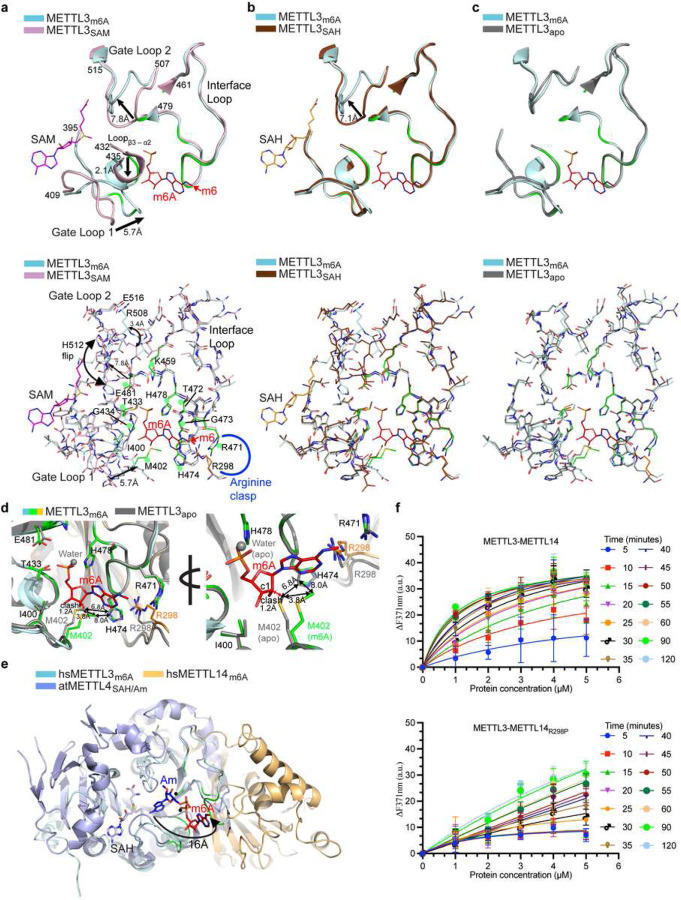
Figure 2 |. Base swiveling and loop orchestration.
a-c, Upper panels shows overlays of regions of METTL3 encompassing the catalytic motif, gate loops 1 and 2, and interface loop in METTL3 bound to m6A (red stick), SAM (pink stick), and SAH (orange stick). Arrows indicate the directional movement of loops. Lower panels: entire region of each overlay in stick mode. Green dots, the residues that form m6A interaction network. d, Close-up of an overlay of m6A and apo MTase of METTL3-METTL14 shown in two orientations for clarity. The exit channel between M402 and H474 in the m6A bound conformation becomes wider (up to 8Å) to stabilize m6A and avoid steric clashes with its purine and ribose moieties. e, An overlay of MTase cores of arabidopsis METTL4 (light blue cartoons)/SAH (light blue stick)/Am (blue stick) and METTL3 (cyan)-METTL14 (orange)/m6A (red stick) clarifies the ~180° pivot of the base around phosphate. Black dots, water molecules in the m6A structure help stabilize the m6A and compensate for the loss in binding energy in the site emptied by base pivoting. f, Change in emission fluorescence intensity upon titration of increasing concentration of WT (upper panel) and R298P mutant enzymes (lower panel) with 2-aminopurine (2-Ap) containing RNA (n=3). See the methods section and source data for details.
The side chain of M402 from gate loop 1 in the m6A structure stacks over the purine ring of m6A. In the SAM-bound form, this region is moved >5Å away, but in the SAH and apo forms, the M402 side chain will sterically clash with m6A ribose (distance between Cε of M402 and C4’ of ribose ~1.2 Å). To avoid this clash, the side chain of M402 in m6A-METTL3 rotates > 45°, resulting in a ~3.8Å gain in the distance for the Cε atom compared to its position in the apo structure. As a result of this repositioning, the inter-gate area between interface loop (H474) and gate loop 1 (M402) becomes wider, from 6.8Å in apo to 8.0 Å in the m6A structure (Fig. 2d). Thus, gate loop 1 from one side senses the SAM and targets the RNA base at the point of catalysis (395DPPW398 motif). It then swivels after SAM hydrolysis to facilitate the sensing of m6A status of the target base at the opposite or exit site.
Another change occurs in how the side chain of invariant R298 (METTL14) orients within the arginine clasp. The R298 side chain rotates ~180° around its Cβ, although the guanidino group shifts slightly to form a direct h-bond with N1 of m6A (Fig. 2d, Extended data Fig. 1g). The orientation of the gate loops suggests that m6A-METTL3 represents a state of enzyme post-catalysis before release of any product or enzyme reset.
How does m6A swivel ~16Å from the point of catalysis to occupy this novel pocket in METTL3? To answer this question, we superposed m6A-METTL3 over the structure of Arabidopsis METTL4, a member of the subclade of the MTA-70 family that possesses the substrate 2’-O methyladenosine (Am)35. The central β-sheet and the catalytic motif of the two enzymes (DPPW) overlay very closely. In this model, the acceptor N6 atom of Am resides at ~3Å or less from the methylsulfonium group of SAM for SN2 mechanism of direct methyl transfer. The phosphates of Am and m6A lie in close proximity (~1.2Å). However, their purine and ribose rings are rotated ~180° in opposite directions, suggesting the base (A) pivots after conversion into m6A (Fig. 2e). Such a rotation may necessitate the de-stacking of the target base for its presentation to catalytic pocket and or base swiveling.
A water molecule at the putative site of the substrate A base is present in the m6A structure to compensate for the loss in binding energy in the emptied site by rotation of m6A from this site post-catalysis. This water coordinates with K459, and its mutation to alanine abolishes the methylation activity (Fig. 1c). SAM-dependent DNA methyltransferases, including the ancestral members of MTA-70 family MTases such as EcoP15I, efficiently flip the target adenine base out of the DNA helix into the catalytic pocket36. Although METTL3-METTL14 does not methylate dsDNA and dsRNA11,12, it can still de-stack the target base from a single-stranded RNA into the catalytic pocket, similar to the m6A/m6Am eraser enzyme, FTO37,38, and the m6A reader, YTHDC139. To test this activity, we replaced the target A (6-aminopurine) in a GGACU in a 14-mer ssRNA with 2-aminopurine (2Ap), a fluorescent nucleobase used as a conformational probe due to its high sensitivity to changes in the local environment induced by DNA40 and RNA MTases41. As shown in Fig. 2f, the change in fluorescence intensity (at 371nm) with increasing enzyme concentrations was rapid for WT, but not the R298P mutant enzyme confirming diminished RNA binding and base de-stacking ability of the mutant enzyme. While cellular impact of R298P mutation has been studied in the context of cancer33, our data now uncover a precise mechanistic role of R298 in m6A sensing. Thus, an elegant orchestration of loops surrounding the SAM/SAH, substrate A, and product m6A binding pockets enables m6A base swiveling and sensing (see Movie 1).
METTL3-METTL14 acts as an atypical m6A sensor
The m6A-METTL3-METTL14 structure allowed us to gain valuable insights into how m6A writer (METTL3-METTL14), eraser (FTO), and reader (YTHDC1) proteins accommodate m6A. We examined their m6A pocket in detail (Fig. 3a–c). Despite the lack of obvious resemblance at the protein sequence, domain, and structure levels, we observed high similarity in the interaction networks of m6A in METTL3-METTL14 to the binding mode of 6mA in FTO (PDB: 5ZMD) and m6A in YTHDC1 (PDB: 4R3I) (Fig. 3). Of note, the purine ring of 6mA in FTO stacks between a hydrophobic amino acid, L109 (the equivalent of M402 in m6A), from the top and the backbone atoms of V228, S229, and H231 (the equivalent of T472, G473, and H474 in m6A) from the bottom. Interestingly, the arginine clasp we found in m6A-METTL3-METTL14 is also present in 6mA-FTO. Notably, the side chain of R96 in FTO forms a direct h-bond to N1 of 6mA, while the guanidino group of its R322 residue forms a van der Waals interaction with N6 methyl group, akin to identical interactions by R298 and R471 to stabilize m6A in m6A-METTL3-METTL14. Stacking interactions that lock the sugar moieties in place also display similarities. For example, the sugar of 6mA in FTO stacks between I85 and H231, whereas the sugar of m6A stacks between I400 and H478 of METTL3 (Fig. 3a, b).
Figure 3 |. Mode of m6A binding by writer/sensor, eraser, and reader.
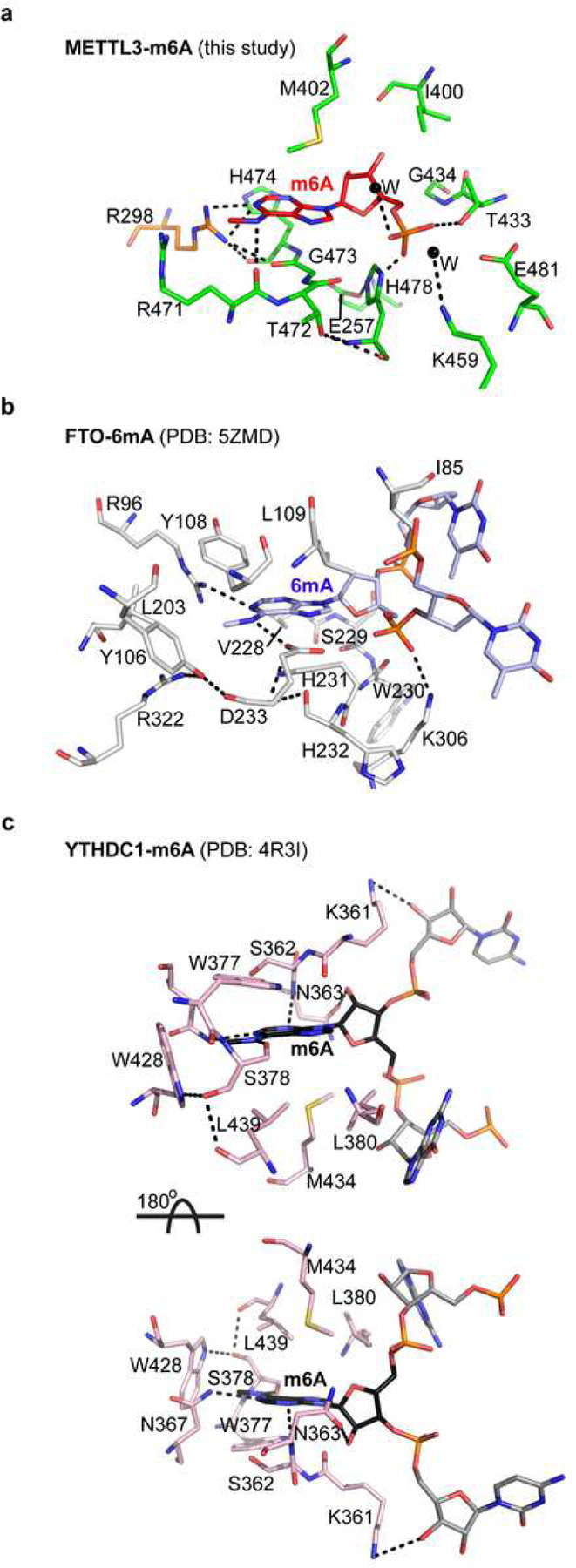
Interaction networks of m6A (red) binding to METTL3 (green), and METTL14 (a), 6mA (blue) binding to FTO (b), and m6A binding to YTH domain of YTHDC1 (c). The two nucleotides flanking the flipped methylated base in FTO and YTHDC1 are shown in light blue and grey, respectively. The hydrophobic stacking surface in YTHDC1 can only be aligned by rotating the molecule 180° around the x-axis, suggesting that reader proteins approach RNA from the opposite direction. The m6A pocket of METTL3-METTL14 harbors features that enable it to act as an atypical m6A sensor/reader during its switch from writer to reader. Dashed lines, h-bonds.
We found that m6A in METTL3-14 and YTHDC1 (PDB: 4R3I) had many similarities and striking differences, mainly in the orientation of the base (Fig. 3c). The N1 of m6A forms an h-bond with N367, whereas an h-bond with carbonyl of S378 akin to carbonyl of R471 of METTL3 stabilizes the N6. Additional hydrophobic interactions from W377 and W428 also support the N6 methyl group in YTHDC1. The nature of stacking interactions for the purine ring is also similar, i.e., hydrophobic residues M434, L380, and L439 on one side and backbone atoms of K361, S362, and N363 on the other. However, the orientation of the m6A base in YTHDC1 is reversed by 180° compared to 6mA in FTO and m6A in METTL3-14. As such, when the direction of sugars and phosphates of modified bases is aligned in three structures (facing downward in Fig. 3a, b, and upper panel of c), the hydrophobic residues (M434/L380/L439) in YTHDC1 stack from the bottom side and the backbone atoms of K361, S362, and N363 stack from the top side, in contrast to the base orientation in FTO and METTL3. A ~180° rotation of YTHDC1 will place the interacting residues in all three proteins in the same plane. However, the orientation of ribose and phosphate of m6A in YTHDC1 will also be reversed (facing upward, Fig. 3c lower panel). Thus, a m6A reader protein approaches the m6A entirely differently than a writer or eraser. This unique geometric difference may allow the reader to avoid clashes with a writer or eraser enzyme acting simultaneously on same transcript.
We show that METTL3 possesses features that enable it to act as an atypical m6A sensor/reader – a function ideally suited for its emerging non-catalytic functions, including crosstalk with eIF3H to promote mRNA circularization, thereby enhancing RNA translation as observed in lung cancer23,25 and bone marrow mesenchymal stem cells21. Consistently, METTL3 showed more robust binding to a methylated (m6A) RNA form, corroborating with previous results describing it as a ‘m6A reader’ for alternative mode of translation initiation during oncogenic translation23,25 and cellular stress (e.g., heat shock)34.
Methods
METTL3-METTL14 MTase core
The gene encoding the MTase domains of human METTL3 (aa 357–580aa) and METTL14 (aa 116–402) were cloned into a pETduet-1 vector and expressed in E. coli NiCo21(DE3) cells. The transformed cells were grown in Terrific Broth medium supplemented with 1 mM ampicillin at 37°C until OD600nm reached 0.6. Protein expression was then induced by adding 0.4 mM isopropyl β-D-thiogalactopyranoside, and the culture was grown at 18°C for 16 hrs. The cell pellets were harvested by centrifugation at 6000 r.p.m. at 4°C and resuspended in cold lysis buffer containing 25 mM Tris pH 8.0, 0.5 M NaCl, 10% glycerol, 5 mM imidazole, 0.1 mM TCEP, one tablet of protease inhibitor (Roche), lysozyme (0.1 mg/mL), and DNase I (5U/mL) and stirred gently at 4°C until achieving full homogeneity. Resuspended cells were lysed by two passages through a microfluidizer (Analytik, UK) and subjected to centrifugation at 41,000 r.p.m. for 50 min at 4°C. The clarified supernatant was filtered through a 0.22 µm filter and loaded onto a Nuvia IMAC column (Bio-Rad) pre-equilibrated with wash buffer (25 mM Tris pH 8.0, 0.5mM NaCl, 10% glycerol, 5 mM imidazole, and 0.1 mM TCEP). The His-tagged METTL3 was co-eluted with untagged METTL14 by increasing the imidazole concentration. The eluates were dialyzed in a buffer lacking imidazole overnight at 4°C in the presence of the ULP1 enzyme to remove the His-SUMO tag from METTL3 proteolytically. The dialyzed proteins were then re-loaded onto an IMAC column to remove un-cleaved proteins and the His-SUMO tag. Two successive passages through MonoQ and Hiload Superdex75 columns (Cytiva) further purified the tag-free complex. The fractions of a homogenous peak eluted in 20 mM Tris pH 8.0, 0.2 M NaCl, and 0.1mM TCEP were pooled, concentrated to 15 mg/ml, and either used immediately or flash-frozen in liquid nitrogen and then stored at −80°C.
Full-length METTL3-METTL14 and mutants
The full-length human METTL3 and METTL14 (wild-type and mutants) were expressed in insect cells (ExpiSF Expression System, Thermo Fisher) and purified using a protocol published earlier12. In brief, the METTL3 and METTL14 plasmids were transformed individually into Max Efficiency DH10Bac competent cells (Thermo Fisher) to generate the DNA bacmids. The successful insertion of genes was confirmed by PCR amplification using a pUC/M13 primer (Forward: 5’-CCCAGTCACGTTGTAAAACG −3’, Reverse: 5’ – AGCGGATAACAATTTCACACAGG −3’). The amounts of purified bacmids and ExpiFectamine SF transfection reagent (Thermo Fisher) were optimized as per the manufacturer’s recommendations (Thermo Fisher). The ExpiSf9 insect cells were cultured in ExpiCD medium (Thermo Fisher) at 125 r.p.m. and 27°C in a non-humidified, air-regulated environment. The cells were harvested 72 hrs post-infection by spinning at 300 × g for 5 min. The PBS-washed cells were resuspended in cold lysis buffer containing 0.5% Igepal, two tablets of protease inhibitor (Roche), and DNase I. Cells were lysed by passing through a microfluidizer (Analytick, UK) and clarified by centrifugation at 41,000 r.p.m. for 40 min.
The proteins were purified using a similar strategy as for the MTase core except for removing the His-tag from METTL3. This step was achieved by incubating proteins after the affinity column step with TEV protease for 3 hrs at room temperature. A second passage through a nickel IMAC column removed contaminants and any uncleaved fractions. The complex was then successfully purified by successive passages through HiTrap Heparin and Hiload Superdex 200 columns (Cytiva). Eluates from a homogenous peak of a Superdex column run in a buffer of 0.02 M Tris pH 8.0, 0.15 M NaCl, and 5% glycerol were pooled, concentrated to 1–3 mg/ml, and flash-frozen in liquid nitrogen and stored at −80°C. All full-length METTL3-METTL14 mutants (METTL3: T433A, K459A, R471H, Δ472–473; Δ472–474, H478A, E481A; METTL14: R298P) were generated by site-directed mutagenesis and purified by the same method as the wild-type protein.
Crystallization, data collection, and structure determination
The crystallization of the human METTL3-METTL14 MTase core (at 10 mg/mL concentration) was carried out by an OryxNano robotic system (Douglas Instruments) using the sitting-drop vapor diffusion method at 20°C. Initial crystals were grown in a solution containing 0.1 M MES pH 6.0, 1.0 M potassium sodium tartrate. After several rounds of optimization by varying pH and salt concentrations, large reproducible crystals were grown in seven days. The N6-methyladenosine monophosphate (m6AMP; Sigma, M2780) was soaked into native crystals (2.0 mM concentration) for 1 hr at 20°C. A complete diffraction dataset was measured to ~2.5Å at GMCAT 23ID-D beamline at Advanced Photon Source, Chicago, IL. The apo structure of METTL3-METTL14 MTase core (PDB: 5IL0) was used as a search model for molecular replacement in Phenix42. The structure was iteratively built and refined using Coot (Version 0.9.8.6)43, Phenix (Version 1.15.2-3472) and Buster (Version 2.10.4)44, respectively. The ligand geometry restraints were generated by Grade. All structure figures were generated using Pymol (Schrodinger Suite).
In vitro methyltransferase assays
5 µM [methyl-3H] SAM (PerkinElmer), 10 µM substrate RNA (NEAT2*: 5’ – GCCUAGUAGCAGAGAGGACUGCUCCUUGGU - 3’), and 2 µM purified WT or mutated METTL3-METTL14 were mixed and incubated at 37°C for 1 hr in a total volume of 5 µL in a reaction buffer (50 mM HEPES pH 7.5, 5 mM NaCl, and 1 mM DTT). The reactions were quenched by blotting 3 µL of each on the Hybond-N+ membrane (Amersham). The methylated substrates were then crosslinked by exposing them to ultraviolet light (254 nm) for 2 min. The membranes were washed three times with 1X PBS, followed by two 95% ethanol washes. Then the membranes were air-dried inside the hood for 15 minutes, and the RNA probe’s count per minute (c.p.m.) on each membrane were measured by a scintillation counter (Beckman LS6500). All results are reported as the means from three independent experiments (n=3) for each group, with one standard deviation (s.d.).
Fluorescence polarization
The reactions were carried out in a buffer containing 10 mM HEPES pH 7.5 and 50 mM KCl. The two 30-mer RNA probes (native RNA or A-RNA and its m6A-modified version or m6A-RNA) were synthesized with a fluoresceine moiety covalently attached to their 5’-end, de-protected, and purified using HPLC (HorizonDiscovery). The sequence of A-RNA was identical except the target A base within the characteristic motif (underlined and bold) in native A-RNA (Fl-NEAT2*: 5’ – [Fl]GCCUAGUAGCAGAGAGGACUGCUCCUUGGU - 3’), was replaced by N6-methyladenosine (m6A) in the modified RNA (Fl-NEAT2*-m6A: 5’ – [Fl]GCCUAGUAGCAGAGAGG[m6A]CUGCUCCUUGGU - 3’). A constant 5 nM of RNA probes were incubated with increasing concentrations of the purified WT or mutant METTL3-METTL14 enzymes in a 384-well plate. The fluorescence polrization values (excitation wavelength = 485 nm, emission wavelength = 530 nm) of each reaction were measured by PHERAstar FS (BMG Labtech). The affinity of RNA-protein binding was calculated by a simple one-site specific binding model (Y = Bmax*X/(Kd+X), X = protein concentration, Y = specific binding, Bmax = maximum specific binding, Kd = equilibrium dissociation constant). The results were analyzed and fitted by GraphPad Prism (GraphPad Software, San Diego, CA). Each experiment was repeated three times independently (n=3), and final Kd is reported as the mean of the three replicates with standard deviation (s.d.) for each RNA shown as error bars.
Steady-state fluorescence assays
For this experiment, we used a 14-mer single-stranded RNA probe in which the the target adenine base within the m6A motif was replaced with 2-aminopurine (2-Ap) (r6T*: 5’ – CUUCGG[2-Ap]CUCUGCU – 3’). In a 384-well plate format, 0.5 µM of RNA probe mixed with increasing concentrations (1 – 5 µM) of full-length human WT or mutant METTL3-METTL14 enzymes in a 20 µL reaction in the buffer containing 50 mM Tris pH 8.0, and 10 mM MgCl2 and incubated at room temperature. The reaction was excited at 320 nm with a 325 nm cut-off wavelength in a SpectraMax M5 microplate reader (MolecularDevices). The fluorescence emission was measured at 371 nm and 37°C every 5 minutes from 0 – 60 minutes and then every 30 minutes until the end time point (120 minutes). The data were analyzed and fitted by GraphPad Prism (GraphPad Software, San Diego, CA) using the Michaelis-Menten model (Y=Vmax*X/(Km+X), X = protein concentration, Y = enzyme velocity, Vmax = maximum enzyme velocity, Km = Michaelis-Menten constant). All results reported are mean values from three independent experiments with standard deviations (s.d.) shown as error bars.
Supplementary Material
Acknowledgments
This work was supported, in part, by funding from NIH/NIAID (R01AI161363) and the Welch Foundation (AQ-2101) to Y.K.G. We are thankful to the San Antonio Partnership for Precision Therapeutics, San Antonio Medical Foundation, and the Max and Minnie Tomerlin Voelcker Trust for their support. We thank Shailee Arya for TEV and ULP1 purification. GM/CA at APS has been funded by the National Cancer Institute (ACB-12002) and the National Institute of General Medical Sciences (AGM-12006, P30GM138396). This research used resources of the Advanced Photon Source, a U.S. Department of Energy (DOE) Office of Science User Facility operated for the DOE Office of Science by Argonne National Laboratory under Contract No. DE-AC02-06CH11357.
Footnotes
Additional Declarations: There is NO Competing Interest.
Competing interests
None
Data availability
The coding sequences of METTL3 (NCBI reference sequence GI: 33301371) and METTL14 (NCBI reference sequence GI: 172045930) used in this study are available at NCBI. We have provided source data as a separate Source Data file. Requests for additional material and information should be directly addressed to Y.K.G. (guptay@uthscsa.edu).
Movie 1 | Loop dynamics during conversion of A to m6A and sensing by METTL3-METTL14 An animation shows the motions in two gate loops and the interface loop of METTL3-METTL14. The model for catalysis and m6A sensing was generated by ChimeraX (UCSF). The position of A during methylation is modeled by overlaying METTL3-SAM (PDB: 5IL1) with METTL4-Am (PDB: 7CV6). The coordinates used for generating the morph movie are as follows: METTL3-METTL14: m6A (this study) apo (PDB: 5IL0), SAM (PDB: 5IL1), SAH (PDB: 5IL2).
References
- 1.Bokar J. A., Rath-Shambaugh M. E., Ludwiczak R., Narayan P. & Rottman F. Characterization and partial purification of mRNA N6-adenosine methyltransferase from HeLa cell nuclei. Internal mRNA methylation requires a multisubunit complex. J Biol Chem 269, 17697–17704 (1994). [PubMed] [Google Scholar]
- 2.Rottman F. M., Bokar J. A., Narayan P., Shambaugh M. E. & Ludwiczak R. N6-adenosine methylation in mRNA: substrate specificity and enzyme complexity. Biochimie 76, 1109–1114 (1994). [DOI] [PubMed] [Google Scholar]
- 3.Meyer K. D. et al. Comprehensive analysis of mRNA methylation reveals enrichment in 3’ UTRs and near stop codons. Cell 149, 1635–1646, doi: 10.1016/j.cell.2012.05.003 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Liu J. et al. A METTL3-METTL14 complex mediates mammalian nuclear RNA N6-adenosine methylation. Nat Chem Biol 10, 93–95, doi: 10.1038/nchembio.1432 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dominissini D. et al. Topology of the human and mouse m6A RNA methylomes revealed by m6A-seq. Nature 485, 201–206, doi: 10.1038/nature11112 (2012). [DOI] [PubMed] [Google Scholar]
- 6.Liu J. et al. N (6)-methyladenosine of chromosome-associated regulatory RNA regulates chromatin state and transcription. Science 367, 580–586, doi: 10.1126/science.aay6018 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ke S. et al. m(6)A mRNA modifications are deposited in nascent pre-mRNA and are not required for splicing but do specify cytoplasmic turnover. Genes Dev 31, 990–1006, doi: 10.1101/gad.301036.117 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Perez-Pepe M. et al. 7SK methylation by METTL3 promotes transcriptional activity. Sci Adv 9, eade7500, doi: 10.1126/sciadv.ade7500 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bokar J. A., Shambaugh M. E., Polayes D., Matera A. G. & Rottman F. M. Purification and cDNA cloning of the AdoMet-binding subunit of the human mRNA (N6-adenosine)-methyltransferase. RNA 3, 1233–1247 (1997). [PMC free article] [PubMed] [Google Scholar]
- 10.Bujnicki J. M., Feder M., Radlinska M. & Blumenthal R. M. Structure prediction and phylogenetic analysis of a functionally diverse family of proteins homologous to the MT-A70 subunit of the human mRNA:m(6)A methyltransferase. J Mol Evol 55, 431–444, doi: 10.1007/s00239-002-2339-8 (2002). [DOI] [PubMed] [Google Scholar]
- 11.Woodcock C. B. et al. Human MettL3-MettL14 complex is a sequence-specific DNA adenine methyltransferase active on single-strand and unpaired DNA in vitro. Cell Discov 5, 63, doi: 10.1038/s41421-019-0136-4 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Qi S. et al. RNA binding to human METTL3-METTL14 restricts N(6)-deoxyadenosine methylation of DNA in vitro. Elife 11, doi: 10.7554/eLife.67150 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Woodcock C. B., Horton J. R., Zhang X., Blumenthal R. M. & Cheng X. Beta class amino methyltransferases from bacteria to humans: evolution and structural consequences. Nucleic Acids Res 48, 10034–10044, doi: 10.1093/nar/gkaa446 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang X. et al. Structural basis of N(6)-adenosine methylation by the METTL3-METTL14 complex. Nature 534, 575–578, doi: 10.1038/nature18298 (2016). [DOI] [PubMed] [Google Scholar]
- 15.Wang P., Doxtader K. A. & Nam Y. Structural Basis for Cooperative Function of Mettl3 and Mettl14 Methyltransferases. Mol Cell 63, 306–317, doi: 10.1016/j.molcel.2016.05.041 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sledz P. & Jinek M. Structural insights into the molecular mechanism of the m(6)A writer complex. Elife 5, doi: 10.7554/eLife.18434 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang Y. et al. N6-methyladenosine modification destabilizes developmental regulators in embryonic stem cells. Nat Cell Biol 16, 191–198, doi: 10.1038/ncb2902 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shi H., Wei J. & He C. Where, When, and How: Context-Dependent Functions of RNA Methylation Writers, Readers, and Erasers. Mol Cell 74, 640–650, doi: 10.1016/j.molcel.2019.04.025 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Flamand M. N., Tegowski M. & Meyer K. D. The Proteins of mRNA Modification: Writers, Readers, and Erasers. Annu Rev Biochem 92, 145–173, doi: 10.1146/annurev-biochem-052521-035330 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Geula S. et al. Stem cells. m6A mRNA methylation facilitates resolution of naive pluripotency toward differentiation. Science 347, 1002–1006, doi: 10.1126/science.1261417 (2015). [DOI] [PubMed] [Google Scholar]
- 21.Wu Y. et al. Mettl3-mediated m(6)A RNA methylation regulates the fate of bone marrow mesenchymal stem cells and osteoporosis. Nat Commun 9, 4772, doi: 10.1038/s41467-018-06898-4 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang Y. et al. N(6)-methyladenosine RNA modification regulates embryonic neural stem cell self-renewal through histone modifications. Nat Neurosci 21, 195–206, doi: 10.1038/s41593-017-0057-1 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lin S., Choe J., Du P., Triboulet R. & Gregory R. I. The m(6)A Methyltransferase METTL3 Promotes Translation in Human Cancer Cells. Mol Cell 62, 335–345, doi: 10.1016/j.molcel.2016.03.021 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Barbieri I. et al. Promoter-bound METTL3 maintains myeloid leukaemia by m(6)A-dependent translation control. Nature 552, 126–131, doi: 10.1038/nature24678 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Choe J. et al. mRNA circularization by METTL3-eIF3h enhances translation and promotes oncogenesis. Nature 561, 556–560, doi: 10.1038/s41586-018-0538-8 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yankova E. et al. Small-molecule inhibition of METTL3 as a strategy against myeloid leukaemia. Nature 593, 597–601, doi: 10.1038/s41586-021-03536-w (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.McFadden M. J. & Horner S. M. N(6)-Methyladenosine Regulates Host Responses to Viral Infection. Trends Biochem Sci 46, 366–377, doi: 10.1016/j.tibs.2020.11.008 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Burgess H. M. et al. Targeting the m(6)A RNA modification pathway blocks SARS-CoV-2 and HCoV-OC43 replication. Genes Dev 35, 1005–1019, doi: 10.1101/gad.348320.121 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Li N. et al. METTL3 regulates viral m6A RNA modification and host cell innate immune responses during SARS-CoV-2 infection. Cell Rep 35, 109091, doi: 10.1016/j.celrep.2021.109091 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Vu L. P. et al. The N6-methyladenosine (m6A)-forming enzyme METTL3 controls myeloid differentiation of normal hematopoietic and leukemia cells. Nat Med, doi: 10.1038/nm.4416 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schumann U., Shafik A. & Preiss T. METTL3 Gains R/W Access to the Epitranscriptome. Mol Cell 62, 323–324, doi: 10.1016/j.molcel.2016.04.024 (2016). [DOI] [PubMed] [Google Scholar]
- 32.Forbes S. A. et al. COSMIC: exploring the world’s knowledge of somatic mutations in human cancer. Nucleic Acids Res 43, D805–811, doi: 10.1093/nar/gku1075 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Liu J. et al. m(6)A mRNA methylation regulates AKT activity to promote the proliferation and tumorigenicity of endometrial cancer. Nat Cell Biol 20, 1074–1083, doi: 10.1038/s41556-018-0174-4 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Coots R. A. et al. m(6)A Facilitates eIF4F-Independent mRNA Translation. Mol Cell 68, 504–514 e507, doi: 10.1016/j.molcel.2017.10.002 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Luo Q. et al. Structural insights into molecular mechanism for N(6)-adenosine methylation by MT-A70 family methyltransferase METTL4. Nat Commun 13, 5636, doi: 10.1038/s41467-022-33277-x (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gupta Y. K., Chan S. H., Xu S. Y. & Aggarwal A. K. Structural basis of asymmetric DNA methylation and ATP-triggered long-range diffusion by EcoP15I. Nat Commun 6, 7363, doi: 10.1038/ncomms8363 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhang X. et al. Structural insights into FTO’s catalytic mechanism for the demethylation of multiple RNA substrates. Proc Natl Acad Sci U S A 116, 2919–2924, doi: 10.1073/pnas.1820574116 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mauer J. et al. Reversible methylation of m(6)A(m) in the 5’ cap controls mRNA stability. Nature 541, 371–375, doi: 10.1038/nature21022 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Xu C. et al. Structural basis for selective binding of m6A RNA by the YTHDC1 YTH domain. Nat Chem Biol 10, 927–929, doi: 10.1038/nchembio.1654 (2014). [DOI] [PubMed] [Google Scholar]
- 40.Allan B. W. & Reich N. O. Targeted base stacking disruption by the EcoRI DNA methyltransferase. Biochemistry 35, 14757–14762, doi: 10.1021/bi9615708 (1996). [DOI] [PubMed] [Google Scholar]
- 41.Hamdane D., Guelorget A., Guerineau V. & Golinelli-Pimpaneau B. Dynamics of RNA modification by a multi-site-specific tRNA methyltransferase. Nucleic Acids Res 42, 11697–11706, doi: 10.1093/nar/gku820 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Adams P. D. et al. PHENIX: a comprehensive Python-based system for macromolecular structure solution. Acta Crystallogr D Biol Crystallogr 66, 213–221, doi: 10.1107/S0907444909052925 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Emsley P. & Cowtan K. Coot: model-building tools for molecular graphics. Acta Crystallogr D Biol Crystallogr 60, 2126–2132, doi: 10.1107/S0907444904019158 (2004). [DOI] [PubMed] [Google Scholar]
- 44.Bricogne G., B. E., Brandl M., Flensburg C., Keller P., Paciorek W., & Roversi P, S. A., Smart O.S., Vonrhein C., Womack T.O. BUSTER version 2.10.4. Cambridge, United Kingdom: Global Phasing Ltd. (2017). [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.