Abstract
The Janus kinase/signal transducer and activator of transcription (JAK/STAT) axis is implicated in cancer, inflammation, and immunity. Numerous cytokines/growth factors affect JAK/STAT signaling. JAKs (JAK1, JAK2, JAK3, and TYK2) noncovalently associate with cytokine receptors, mediate receptor tyrosine phosphorylation, and recruit ≥1 STAT proteins (STAT1, STAT2, STAT3, STAT4, STAT5a, STAT5b, and STAT6). Tyrosine-phosphorylated STATs dimerize and are then transported into the nucleus to function as transcription factors. Signaling is attenuated by specific suppressor of cytokine signaling (SOCS) proteins, creating a negative feedback loop. Both germline mutations and polymorphisms of JAK family members correlate with specific diseases: systemic lupus erythematosus (TYK2 polymorphisms); severe combined immunodeficiency (JAK3 mutations); pediatric acute lymphoblastic leukemia (TYK2 mutations); and hereditary thrombocytosis (JAK2 mutations). Somatic gain-of-function JAK mutations mainly occur in hematologic malignancies, with the activating JAK2 V617F being a myeloproliferative disorder hallmark; it is also seen in clonal hematopoiesis of indeterminate potential. Several T-cell malignancies, as well as B-cell acute lymphoblastic leukemia, and acute megakaryoblastic leukemia also harbor JAK family somatic alterations. On the other hand, JAK2 copy number loss is associated with immune checkpoint inhibitor resistance. JAK inhibitors (jakinibs) have been deployed in many conditions with JAK activation; they are approved in myeloproliferative disorders, rheumatoid and psoriatic arthritis, atopic dermatitis, ulcerative colitis, graft-versus-host disease, alopecia areata, ankylosing spondylitis, and in patients hospitalized for COVID-19. Clinical trials are investigating jakinibs in multiple other autoimmune/inflammatory conditions. Furthermore, dermatologic and neurologic improvement has been observed in children with Aicardi-Goutieres syndrome (a genetic interferonopathy) treated with JAK inhibitors.
Keywords: JAK, cytokines, myelofibrosis, autoimmune
Introduction
Janus kinase or “just another kinase” (JAK) is a family of intracellular, non-receptor tyrosine kinases that transduce cytokine-mediated signals via the JAK-signal transducers and activators of transcription (JAK-STAT) pathway (1,2). The name Janus originates from the two-faced Roman god of duality, because JAKs possess two similar phosphate-transferring domains, one of which displays the kinase enzymatic activity, while the other motif negatively regulates the kinase activity of the first in a feedback loop (1).
JAK-STAT signaling is composed of three major proteins: cell-surface receptors, JAKs, and STATs. JAKs function as tyrosine kinase enzymes that are bound to the cytoplasmic regions of type I and II cytokine receptors. Once a ligand binds to the receptor, JAKs add phosphates to the receptor. Two STAT proteins then bind to the phosphates and form a dimer. The dimer enters the nucleus, binds to DNA, and transcription of target genes ensues. Overall, the JAK-STAT pathway is vital for the proliferation and survival of cancer cells and may contribute to drug resistance (2).
The JAK-STAT pathway is activated by over 50 different pro-cytokine receptors that control hematopoiesis, the immune response, embryogenesis, and inflammation through the signaling pathway (3,4,5,6). This pathway has an important role in the pathogenesis of a variety of immune-mediated diseases and malignant processes.
JAK inhibitors are a class of medications that function to block signaling through the JAK-STAT pathway (3). JAK inhibitors (also called jakinibs) function by preventing the JAK protein from phosphorylating, therefore attenuating intracellular signaling. The most extensive clinical studies for JAK inhibitors have been in rheumatoid arthritis (RA) and myelofibrosis (MF), but JAK inhibitors have also been applied in dermatology for treatment of conditions such as psoriasis/psoriatic arthritis, atopic dermatitis, alopecia areata, vitiligo, and dermatomyositis (5–22).
JAK: Structure and function
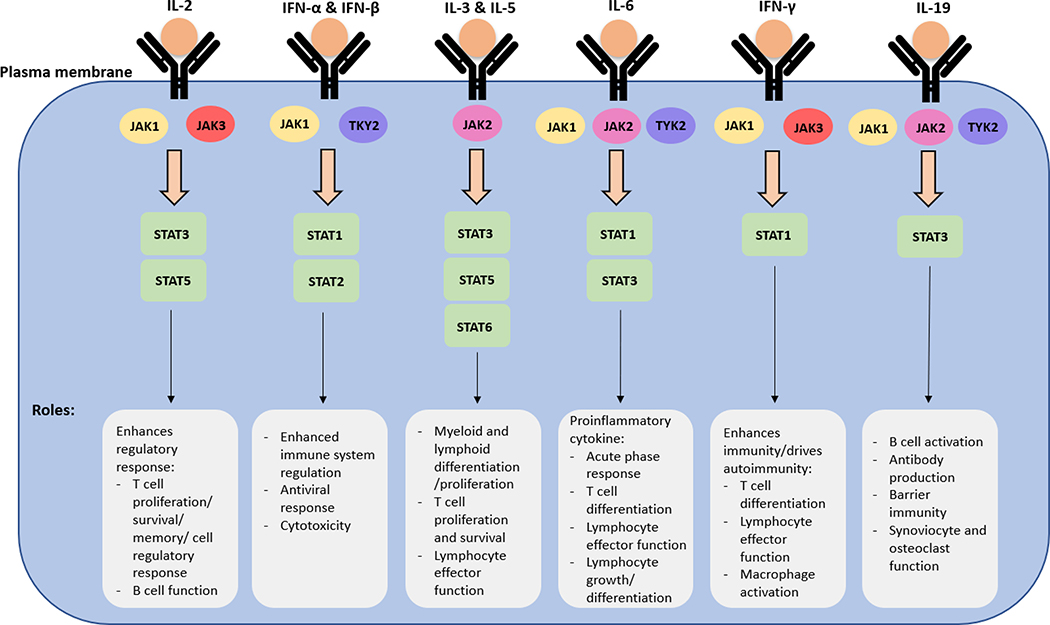
Over 50 cytokines signal via the JAK/STAT pathway to orchestrate hematopoiesis, regulate immunity, and induce inflammation. The main function of the JAK-STAT pathway is to transfer signals from the receptors on the cell membrane, which can be categorized as including interleukin (IL) receptors, interferon (IFN) receptors, or colony stimulating factor receptors (CSFRs), to the nucleus. The pathway is necessary for cytokines and growth factor function, leading to essential cellular processes such as myeloid and lymphoid differentiation/proliferation/hematopoiesis, lactation, development of the immune system, and proinflammatory response (Figure 1) (23–28).
Figure 1: Functional Roles of JAK1, JAK2, JAK3, TYK2/STAT Pathways:
There are several members of the JAK family and the TYK protein that are involved in the JAK/STAT pathway. Some examples are shown. Important roles of these pathways include enhanced regulatory response and immune system regulation, myeloid and lymphoid differentiation/proliferation, and proinflammatory response (25–27)
Abbreviations: IFN- interferon; IL- interleukin; JAK- Janus kinase; STAT- signal transducer and activator of transcription; TYK- tyrosine kinase
Cytokines are secreted glycoproteins that operate as intercellular messengers, stimulating differentiation, proliferation, and programmed cell death of their target cells. They act by binding to specific receptors on the target cell surface and switching on a phosphotyrosine-based intracellular signaling cascade initiated by kinase enzymes and then propagated by SH2 domain-containing transcription factors. As cytokine signaling is proliferative and often inflammatory, it’s amplitude and duration is tightly controlled.
Most cytokines are small helical-bundle proteins, generally 150–200 amino acids in length (4). They are divided into two classes based on elements discerned in their receptors. Class I cytokines consist of four α-helices in an up-up-down-down configuration. Some of these, such as IL-5, exist as dimers, but the topology is conserved. The up-up-down-down conformation requires two long loops to connect the up-up and down-down pairs. In class II cytokines, one or both of these loops is replaced by an extra α-helix resulting in five to six helices arranged in an anti-parallel manner.
Class I cytokines signaling through JAK-STAT include, but are not limited to IL-2, IL-3 family, IL-4, IL-6 family, IL-7, IL-9, IL-12, IL-13, IL-15, IL-21, IL-23, G-CSF, GM-CSF, EPO, TPO (4). Class II cytokines include, but are not limited to interferon-alpha, -beta, -epsilon, -kappa, -omega, -gamma, IL-10, IL-19, IL-20, IL-22, IL-24, IL-26 (4).
The cytokine signaling cascade needs only three elements (receptor, kinase, and transcription factor) to elicit a response. Each cytokine binds to a specific receptor on its target cell surface. These receptors contain intracellular motifs that are constitutively associated with members of the JAK family of tyrosine kinase enzymes. The four JAK family members are: JAK1, JAK2, JAK3 and tyrosine kinase 2 (TYK2). JAKs are inactive prior to cytokine exposure. However, once the cytokine binds to its receptor, it induces their auto-activation by transphosphorylation. Once activated, JAKs phosphorylate the intracellular tails of the receptors on specific tyrosines, which in turn act as docking sites for member of the STAT family of transcription factors. The STAT family is composed of seven members STAT1, STAT2, STAT3, STAT4, STAT5a, STAT5b, STAT6, which mainly act, as mentioned, as transcription factors. Receptor-localized STATs are then phosphorylated by JAK, which leads to their disassociation from the receptor and translocation to the nucleus, where they drive the expression of cytokine-responsive genes. To guarantee that signaling is properly switched off, a number of proteins act to dampen cytokine signaling at numerous levels of the pathway. Importantly, the suppressors of cytokine signaling (SOCS) family are negative feedback inhibitors of the signaling cascade. A general rule of cytokine signaling is that each cytokine binds to a specific receptor, which promotes activation of specific JAK(s) and STAT(s), and signaling is switched off by a specific SOCS protein.
Germline JAK family mutations
JAK family members--JAK1, JAK2, JAK3, and TYK2---are implicated in cell growth, survival, and development, and are critically important for differentiation of a variety of cells including immune cells and hematopoietic elements. Therefore, it is not surprising that germline mutations or specific polymorphisms in these genes are associated with disease. A striking phenotype associated with inactivating JAK3 germline mutations is severe combined immunodeficiency syndrome (Table 1) (29–36). JAK3 missense mutations have also been found in patients with clear cell renal cell cancer. Pediatric acute lymphoblastic leukemia has been associated anecdotally with germline TYK2 mutations, and hereditary thrombocytosis with JAK2 germline mutations. Finally, the autoimmune disease systemic lupus erythematosus has been linked to TYK2 polymorphisms.
Table 1:
Examples of Diseases Associated with Germline JAK Mutations or Polymorphisms
| Mutation | Syndrome | Features | Frequency and inheritance | References |
|---|---|---|---|---|
| JAK3 | Severe combined immunodeficiency disorder | Lack of T and NK cells, impaired B-cell function, severe and recurrent opportunistic infections Loss of functional JAK3 protein results in the absence of T cells and natural killer cells and a normal number of poorly functioning B cells |
7–14% due to germline JAK3 mutations Autosomal recessive |
(29) |
| JAK3 | Clear cell renal cell cancer | Patients with JAK3 mutations more frequently presented with metastases (3 out of 4 [75%] vs. 4 out of 46 [9%]; P = 0.004) and had poorer survival (P = 0.049) | Four different JAK3 germline missense mutations (p.Gln13Lys; p.Arg925Ser; p.Ala677Thr, p.Val722Ile) were found in a total of 7 patients (14%), but in none of the controls (P = 0.0006). Note: The cohort was small and the data has not been replicated |
(30) |
| TYK2 | Pediatric acute lymphoblastic leukemia (ALL). | Children presenting with multiple de novo leukemias are more likely to suffer from genetic predisposition | Anecdotal For instance, in two patients, germline mutations in TYK2, were located in two adjacent codons of the pseudokinase domain (p.Pro760Leu and p.Gly761Val). |
(31) |
| JAK2 | Hereditary thrombocytosis | Familial myeloproliferative disorder with clinical features resembling sporadic essential thrombocythemia. |
JAK2 germline mutation In some families, germline mutations have been identified in the genes for thrombopoietin and its receptor, MPL. |
(32, 33) |
| JAK2 | Myeloproliferative neoplasms | Familial myeloproliferative | Germline JAK2 mutations causing familial myeloproliferative neoplasms have been reported and also specific constitutional JAK2 haplotype (46/1) was associated with the predisposition to myeloproliferative neoplasms with somatic JAK2 V617F mutations | (34,35) |
| TYK2 | Systemic lupus erythematosus (SLE) | Autoimmune disease |
TYK2 and IFN regulatory factor 5 (IRF5) genes showed polymorphisms linked with SLE TYK2 binds to the type I IFN receptor complex and IRF5 is a regulator of type I IFN gene expression |
(36) |
Diseases associated with somatic JAK family mutations
Both activating and loss-of-function somatic alterations in JAK genes can occur. Activating JAK alterations are associated with development of mostly hematologic malignancies. Loss-of function JAK2 alterations are associated with immune checkpoint blockade resistance (37–57).
JAK activating alterations:
Somatic alterations in various JAK genes are predominantly associated with a variety of hematologic malignancies (Table 2) (37–57). As an example, the acquired somatic mutation JAK2 V617F, which constitutively activates JAK2 kinase, is the most frequent molecular event in myeloproliferative disorders. It is observed in about 95% of cases of polycythemia vera and in 55–60% cases of essential thrombocytosis and primary myelofibrosis. JAK2 V617F is also found in the normal population. Among 49,488 individuals from the Copenhagen General Population Study, 63 (0.1%) tested positive for the JAK2V617F mutation. Of these, 48 were eventually diagnosed with a myeloproliferative neoplasm (49). JAK2 V617F mutations in healthy people are referred to as clonal hematopoiesis of indeterminate potential (CHIP), which defines the presence of a clonally expanded hematopoietic stem cell caused by a leukemogenic mutation in individuals without evidence of dysplasia, cytopenia or hematologic malignancy. CHIP correlates with a 0.5–1.0% risk per year of leukemia. Importantly, CHIP also confers a two-fold increase in cardiovascular risk independent of conventional risk factors and is also associated with venous thromboembolism and other inflammatory states. Other CHIP mutations (in addition to JAK2 V617F) occur in DNA damage repair genes PPM1D and TP53, epigenetic regulators ASXL1, DNMT3A, TET2, and mRNA spliceosome components SF3B1, and SRSF2, as well as other genes (50). Interestingly, patients with Erdheim Chester Disease, a non-Langerhans histiocytosis, appear to have elevated rates of myeloproliferative disorders, sometimes heralded by a JAK2 V617F mutation (51). JAK family mutations are also observed in a subset of patients with a variety of T-cell malignancies, including T-ALL, T-PLL and Sezary syndrome, as well as childhood B-cell precursory ALL, and acute megakaryoblastic leukemia (with and without Down syndrome (37–48). An activating JAK1 mutation has been reported in Castleman’s disease as well and may explain an exceptional response to the anti-IL6 antibody siltuximab in the reported patient in the absence of elevated IL6 levels, since the JAK1 mutation may have sensitized the receptor to the IL6 ligand (42). Finally, a TEL-JAK2 fusion protein that includes the catalytic domain of JAK2 and the TEL-specific oligomerization domain, resulting in constitutive activation of its tyrosine kinase activity, has been seen in ALL and in atypical chronic myelogenous leukemia (44).
Table 2:
Examples of Diseases Associated with Somatic JAK family Mutations (37–57)
| Disease | Mutations and Frequency | References |
|---|---|---|
| T-ALL | Constitutive activation of JAK-STAT signaling seen in one-third of T-ALL; caused by activating mutations IL7R, JAK1 or JAK3, or in STAT5B A t(9;12)(p24;p13) chromosomal translocation in a T-cell childhood ALL patient fused the 3’ portion of JAK2 to the 5’ region of TEL, a gene encoding a member of the ETS transcription factor family. The TEL-JAK2 protein included the JAK2 catalytic domain and the TEL-specific oligomerization domain, resulting in constitutive tyrosine kinase activity Also seen in atypical chronic myelogenous leukemia |
(37) (44) |
| Childhood B-cell precursor ALL | JAK2 mutations in 3.5% of cases | (38) |
| B-ALL | JAK2 mutations are frequent in the specific subtype of B-ALL (“Ph-like”) and JAK2 fusion genes with various partner genes, such as ETV6-JAK2, were also found in this type of B-ALL. | (39) |
| BCR-ABL1-negative, high-risk pediatric ALL | Activating JAK1, JAK2 and JAK3 mutations in 10.7% BCR-ABL1-negative, high-risk pediatric ALL cases. | (40) |
| Myeloproliferative disorders |
JAK2 V617F mutation is considered the most important criterion in the diagnosis of Bcr-Abl-negative neoplasms and is thus used as a clonal marker. Seen in 95% in polycythemia vera; 55–60% in essential thrombocytosis and primary myelofibrosis. |
(41) |
| Castleman disease | Anecdotal case of JAK1 mutation Patient had exceptional response to siltuximab (anti-IL-6) without evidence of increased blood levels of IL-6, perhaps because JAK1 mutation sensitized JAK-STAT signaling to IL-6 |
(42) |
| Sezary syndrome (T-cell lymphoma type) | Gain-of-function mutations in JAK1, JAK3, STAT3 and STAT5B (JAK/STAT total ∼11% of patients). | (43) |
| Acute megakaryoblastic leukemia | Activating mutations of the JAK3 gene in both Down syndrome and non-Down syndrome-associated acute megakaryoblastic leukemia. These mutations include A572V and V722I substitutions in the JH2 pseudokinase domain, and a P132T substitution in the JH6 part of the receptor-binding domain. | (45–47) |
| T-cell prolymphocytic leukemia (T-PLL) | Overall, 62.1% of cases harbored mutated JAK or STAT genes. Most frequently, JAK1 (6.3%), JAK3 (36.4%), and STAT5B (18.8%) carried somatic single-nucleotide variants, with missense mutations in the SH2 or pseudokinase domains being most common. Importantly, these lesions were predominantly subclonal. | (48) |
| Loss- or gain-of-function JAK alterations | ||
| Resistance to immune checkpoint blockade | JAK2 deletions (often accompanied by deletions in PDL1) (on the same amplicon) | (52–55) |
| Sensitivity to immune checkpoint blockade | JAK2 amplifications may accompany PDL1 amplification (on the same amplicon | (56,57) |
Abbreviations: ALL = acute lymphoblastic leukemia; T-ALL = T cell acute lymphoblastic leukemia
JAK loss-of-function alterations:
Somatic genomic copy number alterations, involving loss of the key interferon-γ gene function inducer JAK2, are a pivotal driver of immune resistance and escape. Acquired resistance to immune checkpoint therapy, including PD-1 blockade, in patients with advanced melanoma and other cancers has been associated with JAK2 deletion and loss-of-function mutations, respectively (52–55). In depth study showed that the deletion of JAK2 and PD-LI, two neighboring genes found on chromosome 9p24, was associated with primary resistance to anti-PD-1 immunotherapy in recurrent human papilloma virus-negative head and neck squamous cancers (54). The latter findings regarding resistance to immune checkpoint blockade were confirmed (55), and CAP/CLIA validated for use in the clinic to select optimal therapy in this setting. Therefore, lack of JAK2-mediated interferon-γ responsiveness allows cancer cells to escape from antitumor T cells and, in the context of anti-PD-1/L1 immunotherapy, abrogates the antitumor efficacy of this approach. Consistent with these PD-L1-JAK2 co-deletion resistance findings, JAK2, PD-L1 (and PDL2) (9p24.1) amplification, or 9p copy number gain, have been associated with opposite effects, namely anti–PD-1 immunotherapy benefit. Increased PD-L1 expression, which can be directly caused by 9p24.1 amplification (or 9p copy number gain) and indirectly caused by increased JAK2 signaling, has been associated with immunotherapy response against tumors with 9p amplification (56,57). These results may apply to other tumors/sites and therapies, especially given the pivotal, broad role of JAK2 in cancer cell sensitivity to IFN-γ, impaired antigen presentation, T cell sensitivity, and evasion.
JAK inhibitors in the clinic
A wealth of functional data provided strong motivation for the generation of inhibitors that block the enzymatic activity of JAK as a new type of immunomodulatory medication. Preclinical disease models, including in arthritis, transplantation, graft-versus-host disease and other autoimmune conditions led to clinical trials. Moreover, the discovery of gain-of-function mutations of JAK2 in myeloproliferative neoplasms, including myelofibrosis, polycythemia vera and essential thrombocytosis, provided additional impetus for developing JAK inhibitors (jakinibs) for the clinic (Table 3) (58–67).
Table 3:
| Drug | JAKs inhibited with IC50 <150 nM* | Approval indication | References |
|---|---|---|---|
| Abrocitinib | JAK1 = 29 nM | Atopic dermatitis (Significant improvements vs placebo (≥75% improvement in lesion extent and severity) were shown for patients with moderate to severe topic dermatitis (generally over 40 to 50% at 200 mg doses) | (66) |
| Baricitinib |
JAK1 = 5.9 nM JAK2 = 5.7 nM |
Rheumatoid arthritis, alopecia areata Rheumatoid arthritis More patients who received baricitinib than those who received placebo achieved ACR20, ACR50, and ACR70 responses at week 12 and week 24 (ACR response is scored as a percentage improvement, comparing disease activity at two discrete time points (usually baseline and post-baseline comparison)). Alopecia areata: 17% to 35% of patients showed improvement The FDA issued an Emergency Use Authorization for baricitinib in combination with remdesivir, and subsequently alone, for the treatment of suspected or laboratory confirmed COVID-19 in hospitalized patients |
(62,63) |
| Fedratinib | JAK2 = 3 nM | Myelofibrosis (~40% responders) | (64) |
| Ruxolitinib |
JAK1 = 3.3 nM JAK2 = 2.8 nM |
Myelofibrosis (~50% of patients are responders) Polycythemia vera (~20% are responders) Graft-versus-host disease (~70% are responders) Topical cream for atopic dermatitis (~50% are responders) |
(60,61, 67) |
| Tofacitinib |
JAK1 = 112nM, JAK2 = 20 nM JAK3 = 1 nM |
Rheumatoid arthritis (~60% are responders) Psoriatic arthritis (~30 to 50% are responders) Ulcerative colitis (~20% remission by week 8) Polyarticular juvenile idiopathic arthritis (At week 44, the tofacitinib-treated patients who achieved a response at the end of the study’s first phase had a statistically significant lower occurrence of disease flare (31%) than placebo-treated patients (55%; P=0.0007)). |
(58,59) |
| Upadacitinib |
JAK1 = 45 nM JAK2 = 109 nM |
Rheumatoid arthritis (~50% are responders) Psoriatic arthritis (~35% are responders) Atopic dermatitis (>50% are responders) Ankylosing spondylitis (~50%are responders) |
(65) |
Information from: JAK Inhibition | JAK Inhibitor Review (selleckchem.com)
Abbreviations: IC50= half-maximal inhibitory concentration
The first JAK inhibitor to receive approval was the JAK1/JAK2 inhibitor ruxolitinib (50,61,67). Based on the identification of gain-of-function JAK2 mutations in myelofibrosis, ruxolitinib was approved in 2011 for this indication. Subsequently, ruxolitinib has also been approved for steroid-refractory acute graft-versus-host disease, and a cream form for atopic dermatitis. More recently, fedratinib, a selective JAK2 inhibitor, was also approved for myelofibrosis (64).
Tofacitinib, a JAK1/JAK2/JAK3 inhibitor, is approved for rheumatoid arthritis, psoriatic arthritis, polyarticular course juvenile idiopathic arthritis, and ulcerative colitis (57,58). Baricitinib is a JAK1/JAK2 inhibitor approved for rheumatoid arthritis and more recently for alopecia areata. It is also approved for the treatment of atopic dermatitis (AD) in Europe and has recently received emergency FDA approval, with and without the antiviral remdesivir, for the treatment of COVID-19 in hospitalized patients (62,63). Upadacitinib has a degree of selectivity for JAK1 over JAK2 and is approved for the treatment of rheumatoid arthritis, psoriatic arthritis and atopic dermatitis (63). Abrocitinb is also a selective JAK1 inhibitor approved for atopic dermatitis (64). Several other JAK inhibitors have been authorized for similar indications in Europe or Japan: filgotinib, peficitinib, delgocitinib, and oclacitinib (66).
Ongoing clinical trials are investigating the efficacy of jakinibs in a wide variety of additional conditions such as alopecia areata, ankylosing spondylitis, inflammatory bowel disease, dermatomyositis, interstitial lung disease, lupus, vasculitides, vitiligo and myasthenia gravis. Finally, recent results suggest improvement in dermatologic abnormalities and in neurologic function in children with Aicardi-Goutieres syndrome (a genetic interferonopathy associated with severe disability and death) (68).
JAK inhibitors carry an increased risk of infection as a side effect. The incidence of common infections such as upper respiratory tract, lower respiratory tract (including tuberculosis), and urinary tract infections are higher compared with the general population (69). In addition, for those JAK inhibitors used to treat chronic inflammatory conditions, the FDA requires warning about increased risk of cardiac events, cancer, and blood clots (70,71).
Interaction of JAK/STAT with cross talking signaling pathways: Implications for future therapeutic interventions
Different components of the JAK/STAT pathway, such as receptors, JAK, STAT, and gene transcription factors cross talk with other signaling pathways. These signaling cross talks play very important roles in pluripotency, differentiation, immune regulation, and tumorigenesis, which may be important for future therapeutic developments (72,73). Specifically, components of the transforming growth factor-β (TGFβ) signaling pathway, which is involved in embryonic development and cell homeostasis, interact with components in the JAK/STAT pathway, and can either upregulate or downregulate the pathway (72,73). SMAD proteins, the modulators of the TGFβ pathway, and STAT proteins often share the same transcription complex. One example is the STAT3 and SMAD1 complex linked by p300, which induces astrocyte differentiation.
The TGFβ signaling pathway is modulated by the Notch pathway, which is involved in cell proliferation, differentiation, and cell death (72, 73). Components of the Notch pathway also cross talk with the JAK/STAT pathway and have been studied in organ development in Drosophila. The Notch pathway suppresses JAK/STAT signals through interference of STAT translocation to the DNA domain (72). For example, in the development of the Drosophila central nervous system, Hes proteins (downstream effectors of Notch) facilitate JAK2 phosphorylation of STAT3 (72, 73).
The cross talk between these pathways suggests that TGFβ and Notch pathway modulators may deserve consideration in the future when developing JAK pathway inhibitors.
Conclusions
Janus kinase or “just another kinase” (JAK) is a family of intracellular, non-receptor tyrosine kinase enzymes that convert cytokine-mediated signals via the JAK- STAT pathway into nuclear effects. Over 50 cytokines signal via the JAK/STAT pathway to coordinate hematopoiesis, immune function, and inflammation. The four JAK family members are: JAK1, JAK2, JAK3 and TYK2. The STAT family, which mainly act as transcription factors, is composed of seven members STAT1, STAT2, STAT3, STAT4, STAT5a, STAT5b, STAT6. The cytokine signaling cascade requires only three elements (receptor, kinase, and transcription factor) to elicit a biologic impact. Each cytokine binds to a specific receptor, which promotes activation of specific JAK(s) and STAT(s); receptor-localized STATs are phosphorylated by JAK, which leads to their disassociation from the receptor and translocation to the nucleus, where they induce the expression of cytokine-responsive genes; signaling is switched off by a specific SOCS protein via a negative feedback loop.
JAK family members are associated with both germline mutations and polymorphisms that correlate with specific disease manifestations (Table 1) (29–36). The autoimmune disease systemic lupus erythematosus has been linked to TYK2 polymorphisms. JAK3 germline mutations have been seen in severe combined immunodeficiency syndrome and in clear cell renal cell cancer. Pediatric acute lymphoblastic leukemia has been associated with germline TYK2 mutations, and hereditary thrombocytosis with JAK2 germline mutations.
Somatic gain-of-function mutations in JAK genes are predominantly associated with hematologic malignancies (Table 2) (37–48). The somatic mutation JAK2 V617F, which constitutively activates JAK2 kinase, is a hallmark of myeloproliferative disorders including polycythemia vera, essential thrombocytosis and primary myelofibrosis. JAK2 V617F is also found in the normal population and increases with aging as part of a phenomenon known as clonal hematopoiesis of indeterminate potential, which enhances risk for development of leukemia as well as for cardiac events and thromboembolism. JAK family somatic mutations are also observed in a subset of patients with a variety of T-cell malignancies--T-ALL, T-PLL and Sezary syndrome--as well as in childhood B-cell precursory ALL, and acute megakaryoblastic leukemia (with and without Down syndrome,) and in Castleman’s disease. Finally, an activating TEL-JAK2 fusion protein has been reported in ALL and in atypical chronic myelogenous leukemia (42).
JAK2 copy number loss, resulting in abrogation of JAK2 function, is also important. In particular, JAK2 gene loss is a pivotal driver of immune resistance and escape in cancer, and is associated with resistance to immunotherapy.
Because JAK family gain-of-function aberrant proteins are implicated in a spectrum of illnesses, JAK inhibitors have been deployed to treat these conditions. JAK inhibitors or jakinibs are now approved for a variety of medical problems including, but not limited to myeloproliferative disorders (ruxolitinib and fedratinib), graft-versus-host disease (ruxolitinib), rheumatoid arthritis (tofacitinib, baricitinib, and upadacitinib), psoriatic arthritis (tofactitinib and upadacitinib) atopic dermatitis (abrocitinib, upadacitinib and topical ruxolitinib), ulcerative colitis (tofacitinib), ankylosing spondylitis (tofacitinib, upadacitinib), and COVID-19 in hospitalized patients (baricitinib) (Table 3) (58–67), as well as most recently, baricitinib for alopecia (74). Future research will elucidate the potential of jakinibs in numerous other autoimmune illnesses including, but not limited to alopecia areata, ankylosing spondylitis, inflammatory bowel disease, dermatomyositis, lupus, vasculitides, vitiligo and myasthenia gravis. Interestingly, recent observations suggest improvement in neurologic function and in skin abnormalities in children with Aicardi-Goutieres syndrome (a genetic interferonopathy resulting in severe disability and death). Interactions of JAK molecules with other signaling pathways such as TGF-β and notch may also need to be considered. Taken together, JAK inhibitors are transforming outcomes for many disorders, from cancer to autoimmunity and beyond.
Acknowledgement:
This work was supported in part by NIH P30 CA023100 (S Lippman).
Footnotes
Disclosures: Dr. Lippman is on the scientific advisory board for Biological Dynamics and co-founded io9. Dr. Kurzrock has received research funding from Biological Dynamics, Boehringer Ingelheim, Debiopharm, Foundation Medicine, Genentech, Grifols, Guardant, Incyte, Konica Minolta, Medimmune, Merck Serono, Omniseq, Pfizer, Sequenom, Takeda, and TopAlliance; as well as consultant and/or speaker fees and/or advisory board for Actuate Therapeutics, AstraZeneca, Bicara Therapeutics, Biological Dynamics, Daiichi Sankyo, Inc., EISAI, EOM Pharmaceuticals, Iylon, Merck, NeoGenomics, Neomed, Pfizer, Prosperdtx, Roche, TD2/Volastra, Turning Point Therapeutics, X-Biotech; has an equity interest in CureMatch Inc., CureMetrix, and IDbyDNA; serves on the Board of CureMatch and CureMetrix,and is a co-founder of CureMatch.
References
- [1].Muller R JAK inhibitors in 2019, synthetic review in 10 points. Eur J Intern Med 2019;66:9–17. doi: 10.1016/j.ejim.2019.05.022. [DOI] [PubMed] [Google Scholar]
- [2].Bousoik E, Montazeri Aliabadi H . Do We Know Jack About JAK? A Closer Look at JAK/STAT Signaling Pathway. Front Oncol 2018;8:287. doi: 10.3389/fonc.2018.00287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Loh CY, Arya A, Naema AF, Wong WF, Sethi G, Looi CY. Signal Transducer and Activator of Transcription (STATs) Proteins in Cancer and Inflammation: Functions and Therapeutic Implication . Front Oncol 2019;9:48. doi: 10.3389/fonc.2019.00048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Morris R, Kershaw NJ, Babon JJ. The molecular details of cytokine signaling via the JAK/STAT pathway. Protein Sci 2018;27:1984–2009. doi: 10.1002/pro.3519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Fragoulis GE, McInnes IB, Siebert S. JAK-inhibitors. New players in the field of immune-mediated diseases, beyond rheumatoid arthritis. Rheumatology (Oxford) 2019;58(Suppl 1):i43–i54. doi: 10.1093/rheumatology/key276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Brooks AJ, Putoczki T. JAK-STAT Signalling Pathway in Cancer. Cancers (Basel) 2020;12:1971. doi: 10.3390/cancers12071971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Tanaka Y Recent progress and perspective in JAK inhibitors for rheumatoid arthritis: from bench to bedside. J Biochem 2015;158:173–9. doi: 10.1093/jb/mvv069. [DOI] [PubMed] [Google Scholar]
- [8].Mogul A, Corsi K, McAuliffe L. Baricitinib. The Second FDA-Approved JAK Inhibitor for the Treatment of Rheumatoid Arthritis. Ann Pharmacother 2019;53:947–953. doi: 10.1177/1060028019839650. [DOI] [PubMed] [Google Scholar]
- [9].Bhagwat Neha, Matthew D Keller Raajit K. Rampal, Shank Kaitlyn, Elisa de Stanchina Kristine Rose, Amakye Dereck, Ross L. Levine; Improved Efficacy Of Combination Of JAK2 and Hedgehog Inhibitors In Myelofibrosis. Blood 2013;122:666. doi: 10.1182/blood.V122.21.666.66623794067 [DOI] [Google Scholar]
- [10].Dhillon S Tofacitinib: A Review in Rheumatoid Arthritis. Drugs 2017;77:1987–2001. doi: 10.1007/s40265-017-0835-9. [DOI] [PubMed] [Google Scholar]
- [11].Mascarenhas J, Hoffman R. Ruxolitinib: the first FDA approved therapy for the treatment of myelofibrosis. Clin Cancer Res 2012;18:3008–14. doi: 10.1158/1078-0432.CCR-11-3145. [DOI] [PubMed] [Google Scholar]
- [12].Raedler LA. Jakafi (Ruxolitinib): First FDA-Approved Medication for the Treatment of Patients with Polycythemia Vera. Am Health Drug Benefits 2015;8(Spec Feature):75–9. [PMC free article] [PubMed] [Google Scholar]
- [13].Cinats A, Heck E, Robertson L. Janus Kinase Inhibitors: A Review of Their Emerging Applications in Dermatology. Skin Therapy Lett 2018;23:5–9. [PubMed] [Google Scholar]
- [14].He Y, Wong AY, Chan EW, Lau WC, Man KK, Chui CS, et al. Efficacy and safety of tofacitinib in the treatment of rheumatoid arthritis: a systematic review and meta-analysis. BMC Musculoskelet Disord 2013;14:298. doi: 10.1186/1471-2474-14-298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Serhal L, Edwards CJ. Upadacitinib for the treatment of rheumatoid arthritis. Expert Rev Clin Immunol 2019;15:13–25. doi: 10.1080/1744666X.2019.1544892. [DOI] [PubMed] [Google Scholar]
- [16].Gladman D, Rigby W, Azevedo VF, Behrens F, Blanco R, Kaszuba A, et al. Tofacitinib for Psoriatic Arthritis in Patients with an Inadequate Response to TNF Inhibitors. N Engl J Med 2017;377:1525–1536. doi: 10.1056/NEJMoa1615977. [DOI] [PubMed] [Google Scholar]
- [17].Rodrigues MA, Torres T. JAK/STAT inhibitors for the treatment of atopic dermatitis. J Dermatolog Treat 2020;31:33–40. doi: 10.1080/09546634.2019.1577549. [DOI] [PubMed] [Google Scholar]
- [18].Wang EHC, Sallee BN, Tejeda CI, Christiano AM. JAK Inhibitors for Treatment of Alopecia Areata. J Invest Dermatol 2018;138:1911–1916. doi: 10.1016/j.jid.2018.05.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Niu C, Xie HX, Aisa HA. Janus Kinase Inhibitors: A Review of Their Application in the Vitiligo. Mini Rev Med Chem 2021;21:3203–3218. doi: 10.2174/1389557521666210325120233. [DOI] [PubMed] [Google Scholar]
- [20].Paudyal A, Zheng M, Lyu L, Thapa C, Gong S, Yang Y, et al. JAK-inhibitors for dermatomyositis: A concise literature review. Dermatol Ther 2021;34:e14939. doi: 10.1111/dth.14939. [DOI] [PubMed] [Google Scholar]
- [21].Paik JJ, Casciola-Rosen L, Shin JY, Albayda J, Tiniakou E, Leung DG, et al. Study of Tofacitinib in Refractory Dermatomyositis: An Open-Label Pilot Study of Ten Patients. Arthritis Rheumatol 2021;73:858–865. doi: 10.1002/art.41602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Hornung T, Janzen V, Heidgen FJ, Wolf D, Bieber T, Wenzel J. Remission of recalcitrant dermatomyositis treated with ruxolitinib. N Engl J Med 2014;371:2537–8. doi: 10.1056/NEJMc1412997. [DOI] [PubMed] [Google Scholar]
- [23].Seif F, Khoshmirsafa M, Aazami H, Mohsenzadegan M, Sedighi G, Bahar M. The role of JAK-STAT signaling pathway and its regulators in the fate of T helper cells. Cell Commun Signal 2017;15:23. doi: 10.1186/s12964-017-0177-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Caffarel MM, Zaragoza R, Pensa S, Li J, Green AR, Watson CJ. Constitutive activation of JAK2 in mammary epithelium elevates Stat5 signalling, promotes alveologenesis and resistance to cell death, and contributes to tumourigenesis. Cell Death Differ 2012;19:511–22. doi: 10.1038/cdd.2011.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Schwartz DM, Kanno Y, Villarino A, Ward M, Gadina M, O’Shea JJ. JAK inhibition as a therapeutic strategy for immune and inflammatory diseases. Nat Rev Drug Discov 2017;17:78. doi: 10.1038/nrd.2017.267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Luo W, Li YX, Jiang LJ, Chen Q, Wang T, Ye DW. Targeting JAK-STAT Signaling to Control Cytokine Release Syndrome in COVID-19. Trends Pharmacol Sci 2020;41:531–543. doi: 10.1016/j.tips.2020.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Fleming SB. Viral Inhibition of the IFN-Induced JAK/STAT Signalling Pathway: Development of Live Attenuated Vaccines by Mutation of Viral-Encoded IFN-Antagonists. Vaccines (Basel) 2016;4:23. doi: 10.3390/vaccines4030023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Fasouli ES, Katsantoni E. JAK-STAT in Early Hematopoiesis and Leukemia. Front Cell Dev Biol 2021;9:669363. doi: 10.3389/fcell.2021.669363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].MedlinePlus. JAK3-deficient severe combined immunodeficiency, https://medlineplus.gov/genetics/condition/jak3-deficient-severe-combined-immunodeficiency/#inheritance; 2020. [accessed 20 January 2022]. [Google Scholar]
- [30].de Martino M, Gigante M, Cormio L, Prattichizzo C, Cavalcanti E, Gigante M, et al. JAK3 in clear cell renal cell carcinoma: mutational screening and clinical implications. Urol Oncol 2013;31:930–7. doi: 10.1016/j.urolonc.2011.07.001. [DOI] [PubMed] [Google Scholar]
- [31].Waanders E, Scheijen B, Jongmans MC, Venselaar H, van Reijmersdal SV, van Dijk AH, et al. Germline activating TYK2 mutations in pediatric patients with two primary acute lymphoblastic leukemia occurrences. Leukemia 2017;31:821–828. doi: 10.1038/leu.2016.277. [DOI] [PubMed] [Google Scholar]
- [32].Mead AJ, Rugless MJ, Jacobsen SE, Schuh A. Germline JAK2 mutation in a family with hereditary thrombocytosis. N Engl J Med 2012;366:967–9. doi: 10.1056/NEJMc1200349. [DOI] [PubMed] [Google Scholar]
- [33].Han EY, Catherwood M, McMullin MF. Hereditary thrombocytosis: the genetic landscape. Br J Haematol 2021; 194:1098–1105. doi: 10.1111/bjh.17741. [DOI] [PubMed] [Google Scholar]
- [34].Etheridge SL, Cosgrove ME, Sangkhae V, Corbo LM, Roh ME, Seeliger MA, Chan EL, Hitchcock IS. A novel activating, germline JAK2 mutation, JAK2R564Q, causes familial essential thrombocytosis. Blood. 2014. Feb 13;123(7):1059–68. doi: 10.1182/blood-2012-12-473777. [DOI] [PubMed] [Google Scholar]
- [35].Jones AV, Chase A, Silver RT, Oscier D, Zoi K, Wang YL, Cario H, Pahl HL, Collins A, Reiter A, Grand F, Cross NC. JAK2 haplotype is a major risk factor for the development of myeloproliferative neoplasms. Nat Genet. 2009. Apr;41(4):446–9. doi: 10.1038/ng.334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Sigurdsson S, Nordmark G, Göring HH, Lindroos K, Wiman AC, Sturfelt G, et al. Polymorphisms in the tyrosine kinase 2 and interferon regulatory factor 5 genes are associated with systemic lupus erythematosus. Am J Hum Genet 2005;76:528–37. doi: 10.1086/428480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Govaerts I, Jacobs K, Vandepoel R, Cools J. JAK/STAT Pathway Mutations in T-ALL, Including the STAT5B N642H Mutation, are Sensitive to JAK1/JAK3 Inhibitors. Hemasphere 2019;3:e313. doi: 10.1097/HS9.0000000000000313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Steeghs EMP, Jerchel IS, de Goffau-Nobel W, Hoogkamer AQ, Boer JM, Boeree A, et al. JAK2 aberrations in childhood B-cell precursor acute lymphoblastic leukemia. Oncotarget 2017;8:89923–89938. doi: 10.18632/oncotarget.21027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Roberts KG, Li Y, Payne-Turner D. et al. Targetable kinase-activating lesions in Ph-like acute lymphoblastic leukemia. N Engl J Med. 2014. Sep 11;371(11):1005–15. doi: 10.1056/NEJMoa1403088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Mullighan CG, Zhang J, Harvey RC, Collins-Underwood JR, Schulman BA, Phillips LA, et al. JAK mutations in high-risk childhood acute lymphoblastic leukemia. Proc Natl Acad Sci U S A 2009;106:9414–8. doi: 10.1073/pnas.0811761106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].de Freitas RM, da Costa Maranduba CM. Myeloproliferative neoplasms and the JAK/STAT signaling pathway: an overview. Rev Bras Hematol Hemoter 2015;37:348–53. doi: 10.1016/j.bjhh.2014.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Patel M, Ikeda S, Pilat SR, Kurzrock R. JAK1 Genomic Alteration Associated With Exceptional Response to Siltuximab in Cutaneous Castleman Disease. JAMA Dermatol 2017;153:449–452. doi: 10.1001/jamadermatol.2016.5554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Kiel MJ, Sahasrabuddhe AA, Rolland DCM, Velusamy T, Chung F, Schaller M, et al. Genomic analyses reveal recurrent mutations in epigenetic modifiers and the JAK-STAT pathway in Sézary syndrome. Nat Commun 2015;6:8470. doi: 10.1038/ncomms9470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Lacronique V, Boureux A, Valle VD, Poirel H, Quang CT, Mauchauffé M, et al. A TEL-JAK2 fusion protein with constitutive kinase activity in human leukemia. Science 1997;278:1309–12. doi: 10.1126/science.278.5341.1309. [DOI] [PubMed] [Google Scholar]
- [45].Kiyoi H, Yamaji S, Kojima S, Naoe T. JAK3 mutations occur in acute megakaryoblastic leukemia both in Down syndrome children and non-Down syndrome adults. Leukemia 2007;21:574–6. doi: 10.1038/sj.leu.2404527. [DOI] [PubMed] [Google Scholar]
- [46].Walters DK, Mercher T, Gu TL, O’Hare T, Tyner JW, Loriaux M, et al. Activating alleles of JAK3 in acute megakaryoblastic leukemia. Cancer Cell 2006;10:65–75. doi: 10.1016/j.ccr.2006.06.002. [DOI] [PubMed] [Google Scholar]
- [47].Klusmann JH, Reinhardt D, Hasle H, Kaspers GJ, Creutzig U, Hahlen K, et al. Janus kinase mutations in the development of acute megakaryoblastic leukemia in children with and without Down’s syndrome. Leukemia 2007;21:1584–7. doi: 10.1038/sj.leu.2404694. [DOI] [PubMed] [Google Scholar]
- [48].Wahnschaffe L, Braun T, Timonen S, Giri AK, Schrader A, Wagle P, et al M . JAK/STAT-Activating Genomic Alterations Are a Hallmark of T-PLL. Cancers (Basel 2019;11:1833. doi: 10.3390/cancers11121833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Nielsen C, Bojesen SE, Nordestgaard BG, Kofoed KF, Birgens HS. JAK2V617F somatic mutation in the general population: myeloproliferative neoplasm development and progression rate. Haematologica 2014;99:1448–55. doi: 10.3324/haematol.2014.107631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Marnell CS, Bick A, Natarajan P. Clonal hematopoiesis of indeterminate potential (CHIP): Linking somatic mutations, hematopoiesis, chronic inflammation and cardiovascular disease. J Mol Cell Cardiol 2021;161:98–105. doi: 10.1016/j.yjmcc.2021.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Choi MY, Kato S, Wang HY, Lin JH, Lanman RB, Kurzrock R. JAK2 V617F mutation in plasma cell-free DNA preceding clinically overt myelofibrosis: Implications for early diagnosis. Cancer Biol Ther 2018;19:664–668. doi: 10.1080/15384047.2018.1450120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Zaretsky JM, Garcia-Diaz A, Shin DS, Escuin-Ordinas H, Hugo W, Hu-Lieskovan S, et al. Mutations Associated with Acquired Resistance to PD-1 Blockade in Melanoma. N Engl J Med 2016;375:819–29. doi: 10.1056/NEJMoa1604958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Shin DS, Zaretsky JM, Escuin-Ordinas H, Garcia-Diaz A, Hu-Lieskovan S, Kalbasi A, et al. Primary Resistance to PD-1 Blockade Mediated by JAK1/2 Mutations. Cancer Discov 2017;7:188–201. doi: 10.1158/2159-8290.CD-16-1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].William WN Jr, Zhao X, Bianchi JJ, Lin HY, Cheng P, Lee JJ, et al. Immune evasion in HPV- head and neck precancer-cancer transition is driven by an aneuploid switch involving chromosome 9p loss. Proc Natl Acad Sci U S A 2021;118:e2022655118. doi: 10.1073/pnas.2022655118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Han G, Yang G, Hao D, Lu Y, Thein K, Simpson BS, et al. 9p21 loss confers a cold tumor immune microenvironment and primary resistance to immune checkpoint therapy. Nat Commun 2021;12:5606. doi: 10.1038/s41467-021-25894-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Goodman AM, Piccioni D, Kato S, Boichard A, Wang HY, Frampton G, et al. Prevalence of PDL1 Amplification and Preliminary Response to Immune Checkpoint Blockade in Solid Tumors. JAMA Oncol 2018;4:1237–1244. doi: 10.1001/jamaoncol.2018.1701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Gupta S, Cheville JC, Jungbluth AA, Zhang Y, Zhang L, Chen YB, et al. JAK2/PD-L1/PD-L2 (9p24.1) amplifications in renal cell carcinomas with sarcomatoid transformation: implications for clinical management. Mod Pathol 2019;32:1344–1358. doi: 10.1038/s41379-019-0269-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Drugs.com. Xeljanz FDA Approval History. https://www.drugs.com/history/xeljanz.html; 2021. [accessed 25 January 2022] [Google Scholar]
- [59].Harrington R, Al Nokhatha SA, Conway R. JAK Inhibitors in Rheumatoid Arthritis: An Evidence-Based Review on the Emerging Clinical Data. J Inflamm Res 2020;13:519–531. doi: 10.2147/JIR.S219586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Plosker GL. Ruxolitinib: a review of its use in patients with myelofibrosis. Drugs 2015;75:297–308. doi: 10.1007/s40265-015-0351-8. [DOI] [PubMed] [Google Scholar]
- [61].Papp K, Szepietowski JC, Kircik L, Toth D, Eichenfield LF, Leung DYM, et al. Efficacy and safety of ruxolitinib cream for the treatment of atopic dermatitis: Results from 2 phase 3, randomized, double-blind studies. J Am Acad Dermatol 2021;85:863–872. doi: 10.1016/j.jaad.2021.04.085. [DOI] [PubMed] [Google Scholar]
- [62].Al-Salama ZT, Scott LJ. Baricitinib: A Review in Rheumatoid Arthritis. Drugs 2018;78:761–772. doi: 10.1007/s40265-018-0908-4. [DOI] [PubMed] [Google Scholar]
- [63].Marconi VC, Ramanan AV, de Bono S, Kartman CE, Krishnan V, Liao R, et al. Efficacy and safety of baricitinib for the treatment of hospitalised adults with COVID-19 (COV-BARRIER): a randomised, double-blind, parallel-group, placebo-controlled phase 3 trial. Lancet Respir Med 2021;9:1407–1418. doi: 10.1016/S2213-2600(21)00331-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Talpaz M, Kiladjian JJ. Fedratinib, a newly approved treatment for patients with myeloproliferative neoplasm-associated myelofibrosis. Leukemia 2021;35:1–17. doi: 10.1038/s41375-020-0954-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Cohen SB, van Vollenhoven RF, Winthrop KL, Zerbini CAF, Tanaka Y, Bessette L, et al. Safety profile of upadacitinib in rheumatoid arthritis: integrated analysis from the SELECT phase III clinical programme. Ann Rheum Dis 2020;80:304–11. doi: 10.1136/annrheumdis-2020-218510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Meher BR, Mohanty RR, Padhy BM. Efficacy and safety of abrocitinib for the treatment of moderate-to-severe atopic dermatitis: a meta-analysis of randomized clinical trials. J Dermatolog Treat 2021;13:1–9. doi: 10.1080/09546634.2021.1961997. [DOI] [PubMed] [Google Scholar]
- [67].Drugs.com. Jakafi FDA Approval. https://www.drugs.com/history/jakafi.html; 2021. [accessed 25 January 2022] [Google Scholar]
- [68].Spinelli FR, Meylan F, O’Shea JJ, Gadina M. JAK inhibitors: Ten years after. Eur J Immunol 2021;51:1615–1627. doi: 10.1002/eji.202048922. [DOI] [PubMed] [Google Scholar]
- [69].Vanderver A, Adang L, Shults J. Janus kinase inhibition in the Aicardi-Goutieres syndrome. New Engl J Med 2020:382: 986–988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Winthrop KL. The emerging safety profile of JAK inhibitors in rheumatic disease. Nat Rev Rheumatol 2017;13:234–243. doi: 10.1038/nrrheum.2017.23. [DOI] [PubMed] [Google Scholar]
- [71].Kotyla PJ, Islam MA, Engelmann M. Clinical Aspects of Janus Kinase (JAK) Inhibitors in the Cardiovascular System in Patients with Rheumatoid Arthritis. Int J Mol Sci 2020;21:7390. doi: 10.3390/ijms21197390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Hu X, li J, Fu M et al. The JAK/STAT signaling pathway: from bench to clinic. Sig Transduct Target Ther 2021: 6, 402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Akhurst R, Hata A Targeting the TGFβ signalling pathway in disease. Nat Rev Drug Discov 2012: 11, 790–811 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].King B, Ohyama M, Kwon O,, Zlotogorski A., Ko J., et al. Two Phase 3 Trials of Baricitinib for Alopecia Areata N Engl J Med 2022; 386:1687–1699 [DOI] [PubMed] [Google Scholar]