Abstract
Objectives:
To determine the representation of women, minorities, and the elderly groups in clinical trials and whether participation has changed over time.
Methods:
Retrospective study in the National Cancer Institute (NCI) Clinical Data Update System and Center for Disease Control and Prevention United States Cancer Statistics 2000 to 2019. We compared cancer incidence proportion to proportion of patients enrolled in an NCI trial when stratified by race/ethnicity, sex, and age. We performed multivariable analysis to determine the odds of participating in a clinical trial in 2015 to 2019 when compared to 2000 to 2004.
Results:
This study included 14,094 patients, 12,169 (86.3%) non-Hispanic White patients, 662 (4.7%) Black patients, and 660 (4.7%) Hispanic patients. There were 3,701 (26.3%) female patients and 10,393 (73.7%) male patients. For bladder cancer clinical trials, Black patients and Hispanic patients were underrepresented in clinical trials compared to Non-Hispanic White patients (odds ratio [OR] 0.71, 95% confidence interval [CI] 0.57–0.88, P = 0.002) and (OR 0.69, 95%CI 0.54–0.88, P = 0.003), respectively. For kidney cancer trials, Black and Hispanic patients were underrepresented in clinical trials compared to Non-Hispanic White patients (OR 0.42, OR 0.33–0.54, P < 0.001) and (OR 0.68, 95% CI 0.55–0.83, P < 0.001), respectively. Women were underrepresented in kidney cancer trials compared to men (OR 0.80, 95% CI 0.72–0.89) and similarly for bladder cancer trials (OR 0.72, 95% CI 0.64–0.81, P < 0.001). For bladder cancer trials, the participation of Black patients over time (OR 1.04, P = 0.814) and female patients over time (OR 1.03, P = 0.741) were unchanged. For kidney cancer trials, the participation of Black patients over time (OR 1.17, P = 0.293) and female patients over time (OR 1.03, P = 0.663) participation was also unchanged.
Conclusion:
In this study of clinical trials in bladder and kidney cancer, we identified that Blacks, Hispanics, and females were underrepresented. Additionally, Black and female participation was unchanged over the span of 20 years.
Keywords: Health services research, Bladder cancer, Kidney cancer, Clinical trials
1. Introduction
The Revitalization Act was passed by the National Institute of Health (NIH) in 1993. The purpose of this act was to increase the participation of women and minority patients in publicly funded clinical trials [1]. In JAMA 2004, Murthy et al’s influential work demonstrated that significant disparities still exist in breast, colorectal, lung, and prostate clinical trials and do not adequately represent cancer incidence [2]. Since then National Cancer Institute (NCI) has placed considerable effort to mediate these disparities and created initiatives.
In our recent work, we identified that some disparities have improved over time, particularly for Black and Hispanic prostate cancer patients who were more likely to participate in a clinical trial in recent years [3]. However, outside of the 4 major cancers in the United States., there has been scant literature thus far into enrollment trends in other genitourinary malignancies, such as kidney and bladder cancer. The purpose of this study was to evaluate trends in age, sex, and race/ethnic groups in clinical trial enrollment between 2000 and 2019 for other genitourinary cancer trials sponsored by an NCI Clinical Trial Cooperative Group.
2. Methods
2.1. Data collection
In this study we followed the STROBE guidelines for reporting observational cohort studies [4]. The investigators filed a data request with the Freedom of Information Office of the NCI [5]. Through this request, data for all therapeutic NCI-sponsored clinical trials with bladder or kidney as the lead disease were acquired from 2000 to 2019. This data is populated from the NCI Clinical Data Update System, which is a database that contains enrollment information about clinical trial participants [6]. Due to limitations of the database, therapeutic modality (surgery, chemotherapy, etc.) could not be identified. Additionally factors such as socioeconomic status, insurance, geographic area, cancer stage including localized vs. metastatic disease variables, randomization, number of arms were not available.
The investigators requested cancer incidence data (2000–2017) from by the Centers for Disease Control and Prevention (CDC) who manages the United States Cancer Statistics [7]. The United States Cancer Statistics combine the NCI’s Surveillance, Epidemiology, and End Results (SEER) Program [8] with the CDC’s National Program of Cancer Registries (NPCR) [9]. This database is projected to cover 100% of the United States population for incidence cancer cases [9,10]. The study was determined to be exempt by the institutional review board in coordination with the ethics committee at UC San Diego. Informed consent was waived as and the database was deidentified.
2.2. Study participants
We included all patients over the age of 18 who participated in a bladder or kidney cancer trial. Of note our group requested data on testicular cancer, however due to low number of patients (<100) this data was not analyzed. Designation of race and ethnicity was coded in the database, we created 5 mutually exclusive groups, Non-Hispanic White, Non-Hispanic Black (Black), Non-Hispanic Asian/Pacific Islander (Asian/Pacific Islander), Non-Hispanic American Indian/Alaska Native (American Indian/Alaska Native), Non-Hispanic Other/Unknown (Other), and any race Hispanic (Hispanic) [2]. For age, we stratified patients as elderly, older than 65 and non-elderly, younger than 65 as described in Duma et al. [11]. Lastly, for sex, patients were listed as male or female. Patients with missing data were excluded from the study, in total we excluded 143 patients from the initial cohort, which is rate of 1.0%.
2.3. Statistical analysis
We determined the representation of each subgroup by calculating an enrollment fraction, previously described by Murthy et al. [2]. Enrollment Fraction is defined as the number of patients enrolled in a trial over the total cancer incidence in the corresponding years. For example, 10 patients enrolled in a clinical trial / 10,000 patients with bladder cancer would yield an Enrollment Fraction of 0.10%. Thus, we analyzed the representation of racial/ethnic, age, and sex groups in the year 2015 to 2019 and performed Pearson’s χ2 of independence. Lastly, we calculated crude odds ratios for each subgroup. Non-Hispanic White was used as the referent for race/ethnicity, patients younger than 65 were used as the referent for age, and patients who were male were used as the referent for the analysis involving sex. For instance, if the odds ratio of participation in a clinical trial is 0.5 for Hispanic patients with bladder cancer, this would indicate that the odds of a Hispanic patient participating in a clinical trial would be 50% less likely compared to a Non-Hispanic White patient.
In our secondary analysis, we performed multivariable logistic regression analysis to determine odds of participating in a clinical trial for 2015 to 2019 when compared to 2000 to 2004. Our model was adjusted for race/ethnicity, age, and sex.
The statistical analysis was performed using IBMSPSS Version 27 and R version 3.6.1.
3. Results
The final cohort of patients included 14,094 patients, which included 12,169 (86.3%) non-Hispanic White patients, 662 (4.7%) Black patients, 660 (4.7%), Hispanic patients, 312 (2.2%) Asian/Pacific Islander patients, 61 (0.4%) American Indian/Alaska Native patients, and 230 (1.6%) other (Table 1). Patients were overall younger than 65 years, (56.1%), predominantly male (73.7%), and non-Hispanic white (86.3%) (Table 1). In Fig. 1A and B, the proportion of patients who were male and female are presented in 5-year intervals. In Fig. 1C and D, the proportion of patients who were Hispanic, Black and Asian are presented in 5 year intervals.
Table 1.
Participants in bladder and kidney national cancer institute cooperative group trials and proportion of incidence cancer patients in the United States according to race/ethnicity, age, and sex, 2000 to 2019.
| Characteristic | Bladder and kidney cancer n = 14,094 | Percent incident cancer in U.S. | Bladder cancern = 5,468 | Percent incident cancer in U.S. | Kidney cancer n = 8,626 | Percent incident cancer in U.S. |
|---|---|---|---|---|---|---|
| Race/ethnicity | No (%) | % | No (%) | % | No (%) | % |
| Non-Hispanic White | 12,169 (86.3%) | 83.25% | 4,780 (87.4%) | 88.02% | 7,389 (85.7%) | 76.84% |
| Black | 662 (4.7%) | 7.69% | 232 (4.2%) | 5.15% | 420 (5.0%) | 11.0% |
| Hispanic | 660 (4.7%) | 6.06% | 197 (3.6%) | 4.03% | 463 (5.4%) | 8.79% |
| Asian/Pacific Islander | 312 (2.2%) | 1.75% | 143 (2.6%) | 1.56% | 169 (2.0%) | 1.99% |
| American Indian/Alaska Native | 61 (0.4%) | 0.51% | 15 (0.3%) | 0.29% | 46 (0.5%) | 0.81% |
| Other | 230 (1.6%) | 0.74% | 101 (1.8%) | 0.94% | 129 (1.5%) | 0.48% |
| Age, years | ||||||
| <65 | 7,902 (56.1%) | 36.52% | 2,196 (40.2%) | 27.06% | 5,706 (66.1%) | 49.24% |
| >65 | 6,192 (43.9%) | 63.47% | 3,272 (59.8%) | 72.93% | 2,920 (33.9%) | 50.75% |
| Sex | ||||||
| Female | 3,701 (26.3%) | 30.08% | 1,134 (20.7%) | 24.56% | 2,567 (29.8%) | 37.49% |
| Male | 10,393 (73.7%) | 69.91% | 4,334 (79.3%) | 75.43% | 6,059 (70.2%) | 62.5% |
Fig. 1.
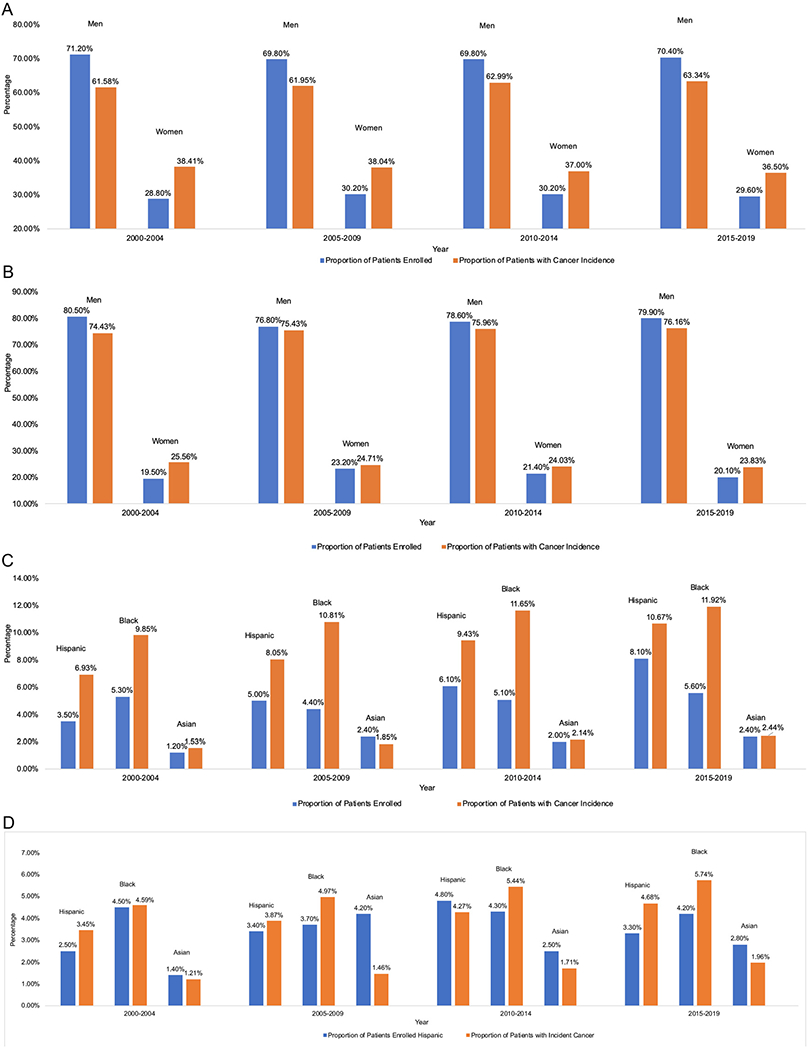
(A) Comparison of proportion of clinical trial enrollment vs. proportion of cancer incidence by sex for kidney cancer trials. (B) Comparison of proportion of clinical trial enrollment vs. proportion of cancer incidence by sex for bladder cancer trials. (C) Comparison of proportion of clinical trial enrollment vs. proportion of cancer Incidence by race/ethnicity for kidney cancer trials. (D) Comparison of proportion of clinical trial enrollment vs. proportion of cancer incidence by race/ethnicity for bladder cancer trials.
We evaluated clinical trial enrollment from 2015 to 2019 and compared it to the cancer incidence from 2015 to 2017 (Table 2). In our unadjusted model, for bladder cancer, both Black patients and Hispanic patients were underrepresented compared to non-Hispanic White patients, (OR 0.71, 95%CI 0.57–0.88, P = 0.002) and (OR 0.69, 95%CI 0.54–0.88, P = 0.003) respectively. Similarly, for kidney cancer, Black (OR 0.42, OR 0.33–0.54, P < 0.001) and Hispanic patients (OR 0.68, 95% CI 0.55–0.83, P < 0.001) were underrepresented compared to Non-Hispanic White patients.
Table 2.
Trial enrollment for minorities vs. non-hispanic white for bladder and kidney cancer trials, 2015 to 2019.
| Race/ethnicity | No. of Trial participants | Enrollment fractiona | Odds ratio (95% CI) | P value |
|---|---|---|---|---|
| Bladder cancer | ||||
| Non-Hispanic White | 1,813 | 0.93% | Referent | |
| Black | 87 | 0.67% | 0.71 (0.57–0.88) | 0.002 |
| Hispanic | 69 | 0.65% | 0.69 (0.54–0.88) | 0.003 |
| Asian/Pacific Islander | 59 | 1.33% | 1.43 (1.10–1.86) | 0.006 |
| American Indian/Alaska Native | 4 | 0.45% | 0.48 (0.18–1.30) | 0.154 |
| Kidney cancer | ||||
| Non-Hispanic White | 1,076 | 0.76% | Referent | |
| Black | 75 | 0.32% | 0.42 (0.33–0.54) | <0.001 |
| Hispanic | 107 | 0.52% | 0.68 (0.55–0.83) | <0.001 |
| Asian/Pacific Islander | 32 | 0.68% | 0.89 (0.62–1.27) | 0.533 |
| American Indian/Alaska Native | 7 | 0.40% | 0.52 (0.24–1.10) | 0.090 |
Enrollment fraction – Defined as patients enrolled in trials/total cancer incidence for corresponding years.
When evaluating age (Table 3) we found that elderly patients were underrepresented compared to younger patients in both kidney and bladder cancer trials (OR 0.65, 95% CI 0.59–0.71, P < 0.001) and (OR 0.62, 95% CI 0.56–0.69, P < 0.001) respectively. For sex (Table 4), female patients were underrepresented compared to males in both kidney (OR 0.80, 95% CI 0.72–0.89) and bladder cancer (OR 0.72, 95% CI 0.64–0.81, P < 0.001).
Table 3.
Trial enrollment fraction for elderly vs. nonelderly cancer for bladder and kidney cancer trials, 2015 to 2019.
| Age | No. of trial participants | Enrollment fractiona | Odds ratio (95% CI) | P value |
|---|---|---|---|---|
| Bladder cancer | ||||
| <65 | 708 | 1.24% | Referent | |
| >65 | 1370 | 0.81% | 0.65 (0.59–0.71) | <0.001 |
| Kidney cancer | ||||
| <65 | 805 | 0.85% | Referent | |
| >65 | 524 | 0.53% | 0.62(0.56–0.69) | <0.001 |
Enrollment fraction – Defined as patients enrolled in trials/total cancer incidence for corresponding years.
Table 4.
Trial enrollment fraction according for sex for bladder and kidney cancer trials, 2015 to 2019.
| Sex | No. of trial participants | Enrollment fractiona | Odds ratio (95% CI) | P value |
|---|---|---|---|---|
| Bladder cancer | ||||
| Male | 1660 | 0.96% | Referent | |
| Female | 418 | 0.73% | 0.80 (0.72–0.89) | <0.001 |
| Kidney cancer | ||||
| Male | 935 | 0.76% | Referent | |
| Female | 394 | 0.56% | 0.72 (0.64–0.81) | <0.001 |
Enrollment fraction – Defined as patients enrolled in trials/total cancer incidence for corresponding years.
We performed multivariable logistic regression analysis comparing the years 2000 to 2004 to 2015 to 2019 and adjusting for sex, age, and race/ethnicity (Table 5). For bladder cancer trials, Hispanic and Black patient and enrollment were unchanged (OR 1.04, P = 0.814) and (OR 1.45, P = 0 = 1.04). The participation of elderly patients increased (OR 1.80, 95%CI 1.55–2.09), and the participation of women was unchanged (OR 1.03, P = 0.741). For kidney cancer trials, the participation of Black patients was unchanged (OR 1.17, P = 0.293) and increased for Hispanic patients (OR 2.54, 95% CI 1.88–3.43, P < 0.001) and Asian/Pacific Islander patients (OR 2.27, 95%CI 1.34–3.83, P = 0.002). The participation of elderly patients increased (OR 1.20, 95%CI 1.04–1.38, P = 0.011), and for women was unchanged (OR 1.03, 95% CI 0.89–1.20, P = 0.663).
Table 5.
Multivariable logistic regressiona for trial enrollment 2000 to 2004 vs. 2015 to 2019.
| Characteristic | Bladder | P value | Kidney | P value |
|---|---|---|---|---|
| Race/ethnicity | OR (95% CI)b | OR (95% CI) | ||
| Non-Hispanic White | Referent | Referent | ||
| Black | 1.04 (0.73–1.49) | 0.814 | 1.17(0.87–1.58) | 0.293 |
| Hispanic | 1.45(0.92–2.27) | 0.104 | 2.54(1.88–3.43) | <0.001 |
| Asian/Pacific Islander | 2.02 (1.15–3.55) | 0.013 | 2.27 (1.34–3.83) | 0.002 |
| American Indian/Alaska Native | 2.42 (0.27–21.86) | 0.429 | 1.46 (0.54–3.93) | 0.454 |
| Other | 1.58 (0.89–2.78) | 0.112 | 1.77 (1.08–2.89) | 0.023 |
| Age | ||||
| <65 | Referent | Referent | ||
| >65 | 1.80 (1.55–2.09) | <0.001 | 1.20 (1.04–1.38) | 0.011 |
| Sex | ||||
| Male | Referent | Referent | ||
| Female | 1.03 (0.85–1.24) | 0.741 | 1.03 (0.89–1.20) | 0.663 |
Multivariable model adjusts for age, sex, and race/ethnicity.
Reference time period is 2000 to 2004.
4. Discussion
In this study, we present clinical trial enrollment for bladder and kidney cancer patients that spans 2 decades, 149 trials, and 14,094 patients. We found that clinical trial enrollment for Black patients and women is unchanged in 20 years of clinical trials for both kidney and bladder cancer trials. We found that Hispanic participation increased for kidney cancer trials but was unchanged for bladder cancer trials. Collectively, Blacks, Hispanics, and women were still underrepresented in bladder and kidney cancer trials when compared to their incidence. Lastly, we found that elderly participation increased for both kidney and bladder cancer trials but were still underrepresented. These findings indicate that clinical trials disproportionately represent some groups of patients and may not reflect cancer incidence.
A focus of disparity research to date has largely focused on the 4 major organ systems of breast, colorectal, lung and prostate cancer. We previously reported our findings on prostate cancer earlier this year [3]. Our major finding was that Black and Hispanic patients are underrepresented in prostate cancer clinical trials but that participation has increased since 2000. In 2017, Duma et al. evaluated 2,020 kidney cancer patients from 2003 to 2016. The authors identified that Black patients, Hispanic patients, and women were underrepresented, but there was no subanalysis by age. In our study, we evaluated 8,626 patients from 2000 to 2019 and our results mirror those of Duma et al., and additionally found that elderly patients were underrepresented. Our findings are novel indicating an increase in Hispanic participation trials over the last 5 years as well as elderly patients, but there no changes in the participation of Black patients and women. In the end, all of these groups remain underrepresented compared to their cancer incidence.
For other genitourinary malignancies, there is scarce literature on the participation of these groups in clinical trials. We identified that Hispanic patients, Black patients, women, and elderly patients are underrepresented in bladder cancer clinical trials over the 20 year span. We did identify an increase in the participation of elderly patients in bladder cancer trials recently, but no changes for Hispanic patients, Black patients, and women. Dymanus et al. recently published similar findings about the underrepresentation of women with respect to bladder cancer when evaluating clinical trial from 2014 to 2019 [10]. Our findings mirror those of Dymanus et al., and additionally indicate a lack of change in this representation since the early 2000s. Additionally, Fletcher et al. recently reported that race/ethnic minorities were underrepresented in non-BCG responsive bladder cancer trials by a cumulatively evaluation of years1998 to 2021 [12]. Our work expands upon both prior publications by identifying when evaluating year-to-year trends, there has been no increase in the participation in participation of Black patients, Hispanic patients, or women in bladder cancer clinical trials.
Our findings are concerning, as we found a significantly disproportionate representation of men compared to women in both kidney and bladder cancer trials. Gender disparities in clinical trial enrollment are well known [13]. There have been few reasons hypothesized for this including caregiver and family responsibilities that may differentially affect women, although this has not been validated [10]. Additionally, there has been little effort with recruitment strategies surrounding women for kidney and bladder cancer trials.
An additional notable finding of our study was a rise in the proportion of Asian/Pacific Islander minorities in clinical trials when compared to Non-Hispanic White patients. In our prior study, we noted the same finding amongst colorectal and lung cancer clinical trials [3]. Our study is amongst the first to identify this finding, and while the significance is unclear it illustrates that some minority participation has increased in clinical trials over time.
These findings call for further efforts are needed to increase the participation of these groups as inclusion may counter safety concerns about treatment and abate differences in survival outcomes
4.1. Study limitations
Our study has limitations. From 2000 to 2019, 36% of trials were industry sponsored [14]. The NCI does not collect industry only sponsored trials in their database. Second, we could account for clerical errors in the data set. Third, due to the small number of American Indian/Alaska Native patients we could speak further to the validity of these findings. Fourth, the NCI Clinical Data Update System has limited information, we did not have access to therapeutic modality (surgery, chemotherapy, etc.), factors such as socioeconomic status, insurance status, cancer stage, or trial information such as randomization, or number of arms. Lastly, we compared cancer incidence to clinical trial enrollment population, while not all patients were eligible for these trials, this should nonetheless continue to serve as a target for primary investigators.
5. Conclusion
In summary, our study adds to the existing literature by expanding upon clinical trial enrollment differences for kidney and bladder cancer trials over the last 20 years. We found that Black patients, Hispanic patients, women and elderly patients are underrepresented in bladder and kidney trials and there has been little change since the early 2000s.
Acknowledgment
Access to Data statement: Dr. Rose and Dr. Javier-DesLoges had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Role of Funder
The Department of Defense was not involved in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Funding:
Funding Department of Defense, grant number W81XWH-17-PCRP-PRA.
Footnotes
Conflict of interest
None.
Supplementary materials
Supplementary material associated with this article can be found in the online version at https://doi.org/10.1016/j.urolonc.2022.03.013.
References
- [1].“NOT-OD-18-014: Revision: NIH policy and guidelines on the inclusion of women and minorities as subjects in clinical research.” https://grants.nih.gov/grants/guide/notice-files/NOT-OD-18-014.html (Accessed February 05, 2021).
- [2].Murthy VH, Krumholz HM, Gross CP. Participation in cancer clinical trials: race-, sex-, and age-based disparities. JAMA 2004;291 (22):2720–6. 10.1001/jama.291.22.2720. [DOI] [PubMed] [Google Scholar]
- [3].Javier-DesLoges J, Nelson TJ, Murphy JD, McKay RR, Pan E, Parsons JK, et al. Disparities and trends in the participation of minorities, women, and the elderly in breast, colorectal, lung, and prostate cancer clinical trials. Cancer 2021. 10.1002/cncr.33991. [DOI] [PubMed] [Google Scholar]
- [4].“The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) Statement: guidelines for reporting observational studies | The EQUATOR Network.” https://www.equator-network.org/reporting-guidelines/strobe/ (Accessed May 13, 2021). [Google Scholar]
- [5].F. of I. Act (FOIA), “FOIA.gov (Freedom of Information Act) Home page.” https://www.foia.gov/ (Accessed March 30, 2021).
- [6].“NCI’s National Clinical Trials Network (NCTN) - National Cancer Institute,” 2014. https://www.cancer.gov/research/infrastructure/clinical-trials/nctn(Accessed March 30, 2021). [Google Scholar]
- [7].“United States Cancer Statistics Cancer | CDC,” 2021. https://www.cdc.gov/cancer/uscs/index.htm (Accessed April 08, 2021). [Google Scholar]
- [8].“SEER incidence data, 1975 - 2017.” https://seer.cancer.gov/data/ (Accessed March 30, 2021).
- [9].“National Program of Cancer Registries (NPCR) | CDC,” Mar. 31, 2021. https://www.cdc.gov/cancer/npcr/index.htm (Accessed April 08, 2021). [Google Scholar]
- [10].Dymanus KA, Butaney M, Magee DE, Hird AE, Luckenbaugh AN, Ma MW, et al. Assessment of gender representation in clinical trials leading to FDA approval for oncology therapeutics between 2014 and 2019: A systematic review-based cohort study,”. Cancer 2021. 10.1002/cncr.33533. [DOI] [PubMed] [Google Scholar]
- [11].Duma N, Vera Aguilera J, Paludo J, Haddox CL, Gonzalez Velez M, Wang Y, et al. Representation of minorities and women in oncology clinical trials: review of the past 14 years. J Oncol Pract 2017;14 (1):e1–e10. 10.1200/JOP.2017.025288. [DOI] [PubMed] [Google Scholar]
- [12].Fletcher SA, Bivalacqua TJ, Brawley OW, Kates M. Race, ethnicity, and gender reporting in North American clinical trials for BCG-unresponsive non-muscle invasive bladder cancer. Urol Oncol 2021:(21):S1078–439. 10.1016/j.urolonc.2021.11.015:00514-7. [DOI] [PubMed] [Google Scholar]
- [13].Liu KA, Mager NAD. Women’s involvement in clinical trials: historical perspective and future implications. Pharm Pract 2016;14 (1):708. 10.18549/PharmPract.2016.01.708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Gresham G, Meinert JL, Gresham AG, Meinert CL. Assessment of trends in the design, accrual, and completion of trials registered in ClinicalTrials.gov by sponsor type, 2000-2019. JAMA Netw Open 2020;3 (8):e2014682. 10.1001/jamanetworkopen.2020.14682. [DOI] [PMC free article] [PubMed] [Google Scholar]


