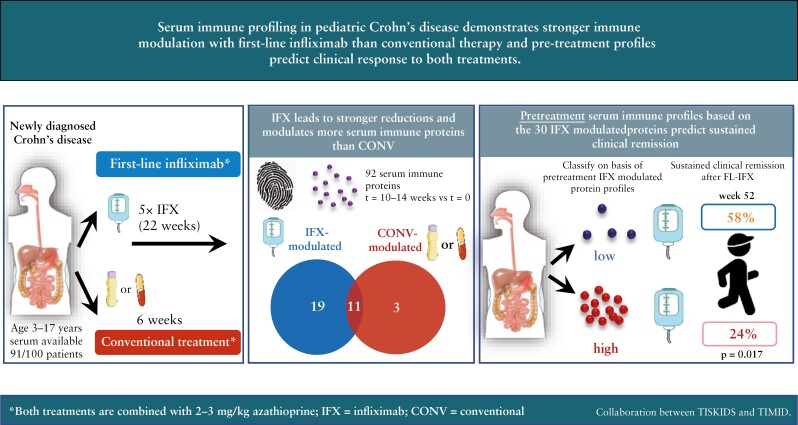
Abstract
Background
Despite its efficacy, rational guidance for starting/stopping first-line biologic treatment in individual paediatric Crohn’s disease [CD] patients is needed. We assessed how serum immune profiles before and after first-line infliximab [FL-IFX] or conventional [CONV] induction therapy associate with disease remission at week 52.
Methods
Pre- [n = 86], and 10–14-week post-treatment [n = 84] sera were collected from patients with moderate-to-severe paediatric CD in the TISKids trial, randomized to FL-IFX [n = 48; five 5-mg/kg infusions over 22 weeks] or CONV [n = 43; exclusive enteral nutrition or oral prednisolone]; both groups received azathioprine maintenance. The relative concentrations of 92 inflammatory proteins were determined with Olink Proteomics; fold changes [FC] with |log2FC| > 0.5 after false discovery rate adjustment were considered significant.
Results
FL-IFX modulated a larger number of inflammatory proteins and induced stronger suppression than CONV; 18/30 proteins modulated by FL-IFX were not regulated by CONV. Hierarchical clustering based on IFX-modulated proteins at baseline revealed two clusters of patients: CD-hi patients had significantly higher concentrations of 23/30 IFX-modulated proteins [including oncostatin-M, TNFSF14, HGF and TGF-α], and higher clinical disease activity, C-reactive protein and blood neutrophils at baseline than CD-lo patients. Only 24% of CD-hi FL-IFX-treated patients maintained remission without escalation at week 52 vs 58% of CD-lo FL-IFX-treated patients. Similarly, 6% of CD-hi CONV-treated patients achieved remission vs 20% of CONV-treated CD-lo patients. Clustering based on immune profiles post-induction therapy did not relate to remission at week 52.
Conclusion
FL-IFX leads to stronger reductions and modulates more immune proteins than CONV. Stratification on pre-treatment profiles of IFX-modulated proteins directly relates to maintenance of remission without treatment escalation.
Trial registration number
Keywords: Proteomics, inflammatory bowel disease, anti-TNF
Graphical Abstract
Graphical Abstract.
1. Introduction
The chronicity of Crohn’s disease [CD], gastrointestinal tract inflammation driven by aberrant inflammatory immune responses to commensal microbiota,1 is proposed to depend on the longevity of CD4+ memory T cells in the intestinal lamina propria,2 which are suppressed or eradicated during induction of disease remission.3 However, reactivation of inflammatory memory CD4+ T cells and inflammation inevitably occur during subsequent maintenance treatment, necessitating treatment escalation, at least transiently. This relapsing–remitting course causes irreversible tissue damage and increases the risk of fistulizing and stricturing disease behaviour.4 Frequent relapses increase the risk of surgical resection, especially in children who have more severe disease and less access to new biologics or small-molecule inhibitors.5
Early biologic therapy, especially anti-tumour necrosis factor [TNF]-α, is very effective and commonly used to prevent disease progression and complications.6–9 Anti-TNF mainly depletes TNF-expressing inflammatory CD4+ T cells by inducing apoptosis,10 reinforcing the postulate that deletion of activated memory CD4+ T cells may prevent chronic disease reactivation. Early biologic interventions remain relatively novel in both adult and paediatric CD11 due to risks of loss-of-response to treatment, overtreatment, high cost or, in paediatrics, the unavailability of other treatments when anti-TNF treatment fails. Recently, we established that first-line infliximab [FL-IFX] is superior to conventional treatment [CONV] for achieving and maintaining remission in moderate-to-severe newly diagnosed paediatric CD,12 and the European Crohn’s and Colitis Organization [ECCO] European Society for Paediatric Gastroenterology, Hepatology and Nutrition [ESPGHAN] consensus guideline was subsequently adapted.13
However, a rational and quantitative approach, tailored to decide which individual patients should receive first-line biologic therapy is lacking. This problem is further complicated by the clinical heterogeneity of CD.14 Selection of first-line anti-TNF is mostly based on presumably aggressive disease14 and, in children, pan-enteric inflammation, deep colonic ulcers, complicated disease and/or growth failure.13 Other indicators may include the baseline simple endoscopic score for CD [SES-CD],15 global histological disease activity score [GHAS]16 and faecal calprotectin17; however, these parameters of disease activity are not predictive for risk of a complicated course.
The increasing use of first-line anti-TNF in moderate-to-severe paediatric CD also raises the question of when to stop treatment and switch to more subtle immunomodulation or even dietary intervention. Currently, IFX is rarely stopped in paediatric or adult patients; moreover, guidance for stopping IFX remains scarce.18 In the TISKids trial, therapy-naïve paediatric CD patients started FL-IFX, with the aim to use a top-down strategy and stop after 22 weeks. In confirmation that stopping FL-IFX is preferable for some—but not all—patients, 41% of FL-IFX-treated patients did not restart IFX and were in sustained clinical remission at 1 year.12 From this observation, we set out to compare the immunological profiles of these patients with patients who needed treatment escalation.
Protein profiling is emerging as a powerful tool to discern immune heterogeneity.19 Multi-parameter profiles offer better discriminatory potential than single biomarkers and may provide mechanistic insights. Combined with reliable clinical parameters, protein profiling could possibly help to tailor treatment for patients at risk of clinically severe relapsing–remitting disease. Serum/plasma immune profiling studies have already yielded promising results. In a large newly diagnosed adult cohort, a five-protein model predicted a subgroup with a high risk for inflammatory bowel disease [IBD] needing treatment escalation [median follow-up, 518 days]20; this model had better accuracy for ulcerative colitis [UC] than for CD. In the paediatric RISK CD cohort, the baseline plasma concentrations of interleukin-7 [IL-7], matrix metallopeptidase-10, IL12p40 and C-C motif chemokine ligand-11 predicted stricturing [B2] disease, while TNF superfamily member 14, CCL4, IL-15 receptor subunit alpha, TNFβ and cluster of differentiation 40 [CD40] more accurately predicted internal penetrating [B3] disease than classical serology-only models.21 Although these inception cohort studies indicate the strength of plasma/serum profiling to classify IBD, the heterogenous cohorts and absence of strict protocols for treatment decisions may have confounded these analyses.
Thus, we compared the effects of FL-IFX and CONV induction treatment [with prednisolone or exclusive enteral nutrition], both combined with azathioprine maintenance therapy, on 92 inflammatory proteins and assessed whether pre-treatment baseline serum immune profiles of FL-IFX-modulated proteins relate to maintenance of remission at week 52. We focused on clinically moderate-to-severe paediatric CD, the subgroup of patients for whom clinical decision-making is most complex, using samples from the TISKids trial.12
2. Methods
2.1. TISKids cohort
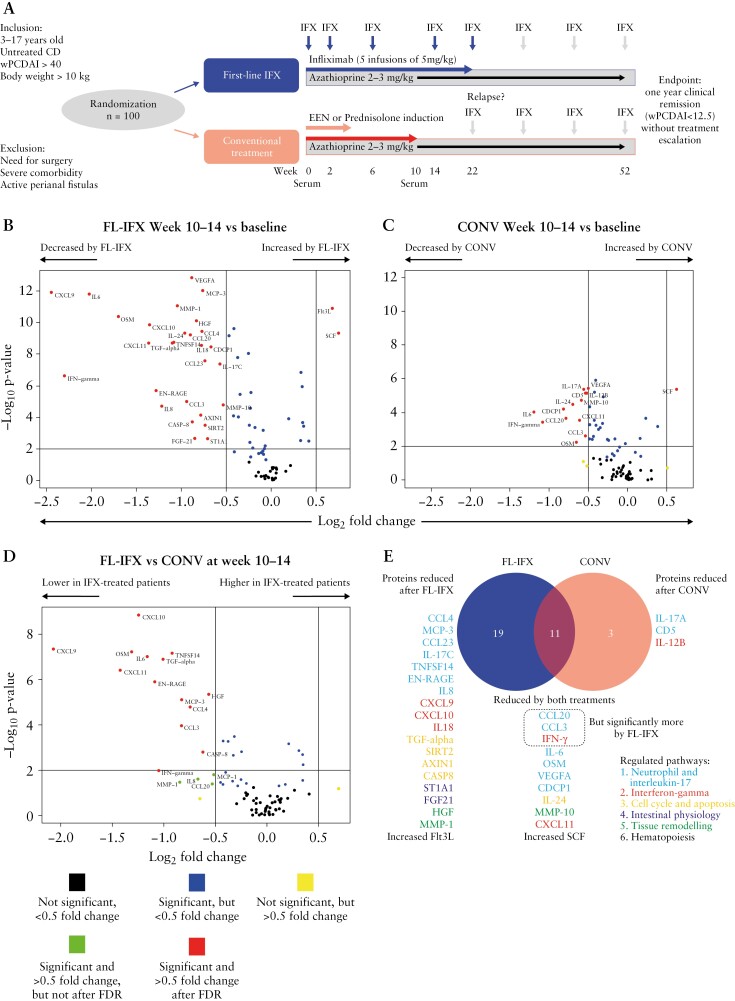
Details of the TISKids randomized control trial [ClinicalTrials.gov: NCT02517684; Figure 1A] of newly diagnosed, untreated, moderate-to-severe paediatric CD were previously published.12,22 This study included 91 patients for whom sera were available at diagnosis [i.e. baseline] and/or after 10–14 weeks of induction therapy. The FL-IFX group received five infusions of 5 mg/kg IFX [Inflectra, CT-P13]. CONV treatment was standard induction treatment with exclusive enteral nutrition [EEN; 6–8 weeks of polymeric feeding, then normal diet gradually reintroduced over 2–3 weeks] or oral prednisolone [1 mg/kg/day for 4 weeks; maximum 40 mg; tapered by 5 mg per week until stop].23 Both groups received oral azathioprine [AZA] as maintenance.
Figure 1.
First-line infliximab [FL-IFX] induces greater qualitative and quantitative differences in serum immune protein concentrations than conventional [CONV] induction treatment. [A] Summary of trial protocol. Untreated patients received either five infusions of 5 mg/kg infliximab at weeks 0, 2, 6, 14 and 22 [FL-IFX], or CONV treatment consisting of exclusive enteral nutrition [EEN] or oral prednisolone [1 mg/kg, maximum 40 mg]. Both groups received azathioprine maintenance therapy. Serum was collected at baseline [week 0] and weeks 10–14. The endpoint was clinical remission [wPCDAI < 12.5 at week 52, without treatment escalation]. [B–D] Volcano plots of the changes in the concentrations of 83 proteins after 10–14 weeks of induction treatment. Wilcoxon signed-rank test and Mann–Whitney U-test; corrected for false discovery rate [FDR] based on the Benjamini–Hochberg procedure; p < 0.05 was considered significant. [B] FL-IFX significantly reduced 28 proteins and enhanced two proteins [paired samples; n = 46; Wilcoxon signed-rank test]. [C] CONV significantly reduced 13 proteins and enhanced one protein [paired samples; n = 32; Wilcoxon signed-rank test]. [D] FL-IFX [n = 46] led to significantly greater reductions in the concentrations of 18 proteins compared to CONV [n = 32; Mann–Whitney U-test]. [E] Venn diagram of the 33/83 proteins significantly regulated by FL-IFX or CONV, and the six related pathways [for protein descriptions also see Supplementary Table S3]; 19 proteins were only modulated by FL-IFX; three proteins were only modulated by CONV; and 11 proteins were modulated by both treatments, but more strongly reduced by FL-IFX than CONV. A total of 30 proteins were significantly modulated by FL-IFX.
2.2. Samples and clinical data
Clinical data were collected at baseline [before induction], 10–14 weeks and 52 weeks, including weighted paediatric CD activity index [wPCDAI],24 routine blood laboratory analysis and sera. Endoscopy, histological disease activity [GHAS] and faecal calprotectin were conducted/determined at baseline and week 10 [see Supplementary Methods]. Clinical remission was defined as wPCDAI < 12.5 at week 52 without treatment escalation; any additional CD-related therapy/surgery was considered escalation.
2.3. Proximity extension assay [PEA]
Serum samples were analysed in one batch using PEA technology [Olink Proteomics]; the proSeek Multiplex Inflammation panel measures 92 inflammation-related proteins [see Supplementary Methods]. Relative normalized protein expression [NPX] values [on an arbitrary log2 scale] of individual proteins can be compared across samples; the NPX values of different proteins cannot be compared.
Nine and six of the 92 proteins were below the limit of detection [LOD] in <50% of serum samples, respectively, and were excluded [Supplementary Table S1].
Internal controls were added to samples to monitor assay performance and sample quality. Two serum samples [one baseline and one week 10–14 sample from a CONV-treated patient] failed quality control and were excluded. Allocation of protein function was performed on the basis of Uniprot, the human protein atlas and literature [detailed functional descriptions are provided in Supplementary Table S3].
2.4. Statistical analysis
Fold changes [FC] in mean NPX with |log2FC| > 0.5 were considered meaningful. Fold changes between timepoints, groups or clusters are presented as delta mean NPX. Continuous variables are presented as medians and interquartile ranges [IQR] and were compared using the Mann–Whitney U-test. Paired continuous variables were compared using the Wilcoxon signed-rank test. Categorical variables are presented as frequencies and percentages and were compared using χ2 or Fisher’s exact tests. Correction for multiple testing [83 proteins] was applied by false discovery rate [FDR] correction based on the Benjamini–Hochberg procedure; unless indicated, adjusted p values <0.05 were considered significant. Serum immune profile clusters were obtained as follows: serum protein concentrations were normalized to z-scores for each protein at each time point. Patients were stratified into two groups based on the protein concentrations of the 30 IFX-modulated proteins [high and low protein concentrations]. The clusters were defined using hierarchical clustering, Euclidean distance and Ward’s linkage. This resulted in, at baseline, the clusters CD-hi and CD-lo and the clusters CD#1 10–14 weeks and CD#2 10–14 weeks at the 10–14-week time point. Spearman’s correlation was used to assess relationships between clinical characteristics and FL-IFX-modulated proteins. Time-to-treatment escalation outcomes were analysed via the Kaplan–Meier method and log-rank tests. Multivariate binary logistic regression analyses were used to test the associations between variables: [1] treatment group [FL-IFX or CONV], or [2] the immune profiles [baseline CD-hi/CD-lo or CD#1 10–14 weeks/CD#2 10–14 weeks] or clinical disease activity at baseline [wPCDAI] with binary variables: {1] remission without treatment escalation at week 52 [yes/no], or [2] biological treatment at week 52 [yes/no], or [3] escalation within 52 weeks [yes/no]. All analyses were performed using R studio version 4.3.
3. Results
Sera were collected for 91/100 patients [91%]. Forty-eight [53%] patients received FL-IFX and 43 [47%] received CONV [Figure 1A; Supplementary Figure S1A]; the baseline characteristics of these groups were comparable [Table 1]. Paired baseline and 10–14-week samples were collected for 78/100 patients [Supplementary Table S2]. After FDR correction, baseline relative serum protein concentrations did not differ significantly between the FL-IFX [n = 47] and CONV [n = 38] groups; 12 proteins differed before FDR [Supplementary Figure Figure S1B and C, Table S1].
Table 1.
Baseline characteristics of all patients, stratified by treatment group.
| CONV [n = 43] | FL-IFX [n = 48] | p-value | ||
|---|---|---|---|---|
| Age at diagnosis, years | 14.1 [11.5–16.3] | 15.0 [11.8–16.5] | 0.266 | |
| Male gender | 24 [53%] | 23 [49%] | 0.596 | |
| Height, cm | 161 [143–172] | 166 [153–175] | 0.154 | |
| Weight, kg | 46.3 [30.5–55.0] | 47.3 [36.4–56.8] | 0.332 | |
| wPCDAI | 57.5 [47.5–72.5] | 57.5 [47.5–67.5] | 0.886 | |
| CRP, mg/L | 37.0 [22.0–65.5] | 29.5 [8.8–46.8] | 0.053 | |
| ESR, mm/h | 32.5 [22.0–63.5] | 34.0 [26.0–46.0] | 0.910 | |
| SES-CD | 15.0 [8–22] | 15.0 [9–21] | 0.843 | |
| Faecal calprotectin, µg/g | 1128 [IQR 592–1670] | 1114 [IQR 886–1800] | 0.553 | |
| Paris classification | ||||
| Age at diagnosis | <10 years | 7 [16%] | 4 [8%] | 0.481 |
| 10–17 years | 32 [74%] | 38 [79%] | ||
| 17–40 years | 4 [9%] | 6 [13%] | ||
| Disease location | L1 | 10 [23%] | 12 [25%] | 0.802 |
| L2 | 11 [26%] | 11 [23%] | ||
| L3 | 22 [51%] | 24 [50%] | ||
| Isolated L4 | — | 1 [2%] | ||
| Upper disease location | No upper GI | 22 [51%] | 28 [58%] | 0.789 |
| L4a | 19 [44%] | 18 [38%] | ||
| L4b | 2 [5%] | 2 [4%] | ||
| Disease behaviour | B1 | 38 [88%] | 45 [94%] | 0.366 |
| B2 | 5 [12%] | 3 [4%] | ||
| B3 | – | – | ||
| B2B3 | – | – | ||
| Perianal diseaseb | 9 [21%] | 5 [10%] | 0.165 | |
| Growth delay | 2 [0.5%] | 1 [0.2%]a | 0.509 | |
Data are n [%] or median [IQR]. Significance was assessed using the Mann–Whitney U-test for numerical variables or chi-square test for categorical variables; p < 0.05 was considered significant. FL-IFX, first-line infliximab; CONV, conventional induction therapy; wPCDAI, weighted paediatric Crohn’s disease activity index [range, 0‒125]; CRP, C-reactive protein; ESR, erythrocyte sedimentation rate; SES-CD, simple endoscopic score for Crohn’s disease [range, 0–60].
aOne missing data point.
bPerianal disease was defined as inactive fistula, skin tags or anal fissures.
3.1. FL-IFX induces greater qualitative and quantitative differences in serum immune profiles than conventional induction
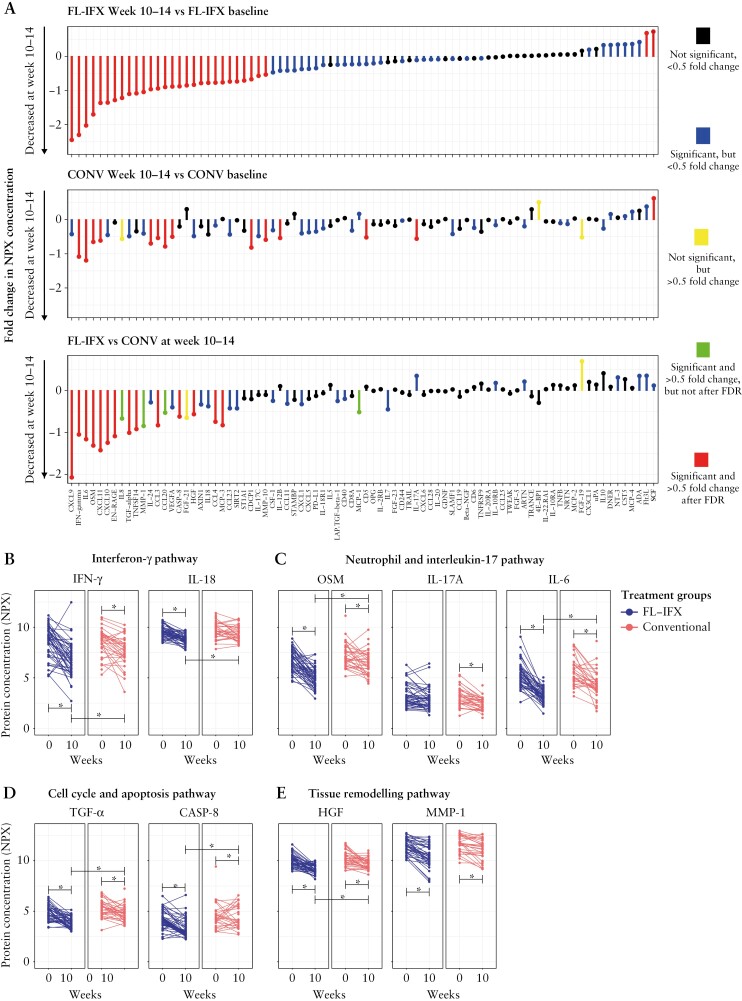
After 10–14 weeks, FL-IFX [n = 46 patients] significantly altered [|log2FC| > 0.5] the relative concentrations of 30 inflammatory proteins compared to baseline [Figure 1B]: 28 proteins decreased [log2FC < −0.5; FDR < 0.05], with multiple interferon [IFN]-γ pathway proteins exhibiting very large fold changes [Figures 1B and 2A]. Moreover, FL-IFX enhanced two proteins [Figures 1B and 2A]. Of note, TNF-α was excluded as formation of IFX–TNF complexes led to very high NPX values at weeks 10–14. Compared to FL-IFX, CONV [n = 32 patients] induced lower qualitative reductions [mean NPX values with a maximal change of log2FC, −1.3; Figures 1C and 2A] and modulated fewer proteins [Figures 1C, D and 2A], with significant reductions in 13 proteins, and only enhanced SCF [Figures 1C and 2A].
Figure 2.
First-line infliximab [FL-IFX] reduces proteins in the IFN-γ, neutrophil and IL-17, cell cycle and apoptosis, and tissue remodelling pathways. [A] Fold changes [FC] in the median serum concentrations of 83 immune proteins between baseline and after 10–14 weeks of FL-IFX or conventional [CONV] induction therapy, and differential FCs after FL-IFX and CONV at 10–14 weeks. FL-IFX induced greater qualitative [i.e. different proteins] and quantitative [i.e. the same proteins, but stronger reductions] changes than CONV. Wilcoxon signed-rank test and Mann-Whitney U-test with false discovery rate [FDR] correction based on the Benjamini–Hochberg procedure. [B–E] Comparison of the changes in protein concentrations between baseline and 10–14 weeks for the individual patients in both groups. Mann–Whitney U-test. [B] Proteins represented in the IFN-γ pathway were reduced more significantly by FL-IFX than CONV; both interferon-gamma [IFN-γ] and interleukin [IL]-18 were reduced more significantly by FL-IFX [p = 0.036 and 0.003, respectively]. [B] Proteins in the neutrophil and IL-17 pathway were significantly regulated by both FL-IFX and CONV; however, oncostatin-M [OSM] and IL-6 were reduced more significantly by FL-IFX [both p < 0.001], while IL-17A was only decreased by CONV [p = 0.080]. [D] Proteins in the cell cycle and apoptosis pathway were reduced more significantly by FL-IFX than by CONV; both TGF-α and caspase-8 [CASP-8] were reduced more significantly by FL-IFX [p < 0.001 and 0.007, respectively]. [E] Proteins in the tissue remodelling pathway were significantly regulated by both FL-IFX and CONV, although hepatocyte growth factor [HGF] was reduced more significantly by FL-IFX [p < 0.001]; the concentration of matrix metalloproteinase-1 [MMP-1] was comparable between groups [p = 0.089]. A value of p < 0.05 was considered significant for all tests with false discovery rate [FDR] correction based on the Benjamini–Hochberg procedure.
Eleven proteins were modulated by both treatments; 19 were selectively modulated by FL-IFX, and three were only reduced by CONV [Figure 1E].
These data demonstrate that FL-IFX more effectively reduces a larger number of circulating immune proteins than CONV. Indeed, the median concentrations of 18 proteins were significantly lower [log2FC < −0.5] after FL-IFX than CONV [Figures 1D and 2A; Supplementary Table S1].
3.2. FL-IFX-modulated immune pathways
Supervised pathway annotation of the 30 FL-IFX-modulated proteins at 10–14 weeks [Figure 1E; Supplementary Table S3] revealed strong enrichment of IFN-γ and neutrophil/IL-17 pathways, particularly T helper-1 cell [Th1]-derived IFN-γ and IFN-γ-induced T cell chemokines [CXCL9, CXCL10, CXCL11]. These IFN-γ-induced chemokines are secreted by structural cells, including epithelial cells, fibroblasts and high endothelial venules, and ligate CXCR3 to elicit T cell migration to lymph nodes or tissue. The strong reduction of IFN-γ and downstream signalling reflects the capacity of IFX to delete intestinal inflammatory T cells; these changes appeared weaker and more variable after CONV [Figure 2B]. FL-IFX more strongly suppressed IFN-γ than CONV, but did not suppress IL12p40, which was significantly suppressed by CONV. Notably, antigen-presenting cell-derived IL12p40 is shared by IL-12p70 and IL-23 and targeted by ustekinumab, an effective biologic used to treat CD. The strong inhibitory effect of FL-IFX on the auto-inflammatory cytokine IL-18 is notable [Figure 2B], as both enterocytes and myeloid cells secrete IL-18. Subsequently, IL-18 acts as a cofactor with IL-12p70 to elicit IFN-γ secretion by Th1 cells. IL-18 was suppressed more effectively by FL-IFX than by CONV [Figure 2B], indicating substantial regulation of epithelial and myeloid cell activation. Moreover, FL-IFX strongly suppressed multiple granulocyte/neutrophil-derived cytokines/chemokines, including: OSM, an IL-6 family member associated with complicated disease in CD; EN-RAGE, the antimicrobial calcium-binding protein A12 [S100A12]; and TNFSF14, which enhances neutrophil/monocyte bactericidal activity and drives IFN-γ-dominated Th1 responses [Figure 2].25 Notably, despite strong neutrophil regulation, IL-17A—an important driver of neutrophil activation—was not regulated by FL-IFX, though IL-17C was [Figure 2C]. CONV substantially decreased IL-17A [Figure 2C]; interestingly, IL-17A is mostly secreted by T cells, while IL-17C is inducibly expressed by intestinal epithelial cells. Neither the T helper 2 cell [Th2] cytokines IL-4 or IL-13, T-cell proliferation cytokine pathways, IL-2, interleukin 2 receptor subunit beta [IL-2RB], IL-7 or IL15RA were modulated by FL-IFX [Supplementary Table S3]. Remarkably, MCP-3 [CCL7], a monocyte and macrophage-derived chemokine that selectively recruits monocytes, was an FL-IFX-modulated protein, but was unaffected by CONV. Lastly, FL-IFX substantially regulated immune proteins involved in cell cycle, apoptosis [Figure 2D], vascular permeability [VEGF] and tissue remodelling pathways [Figure 2E], with marked reduction of pro-apoptotic IL-24. High IL-24 production in CD and UC lamina propria has been attributed to subepithelial myofibroblast IL-1β signalling.26
Overall, in moderate-to-severe CD, FL-IFX regulates a highly diverse network of immune processes involving granulocytes, monocytes, Th1 responses, epithelial cells and [myo]fibroblasts.
3.3. Comparison of the serum immune profiles of EEN- and prednisolone-treated patients
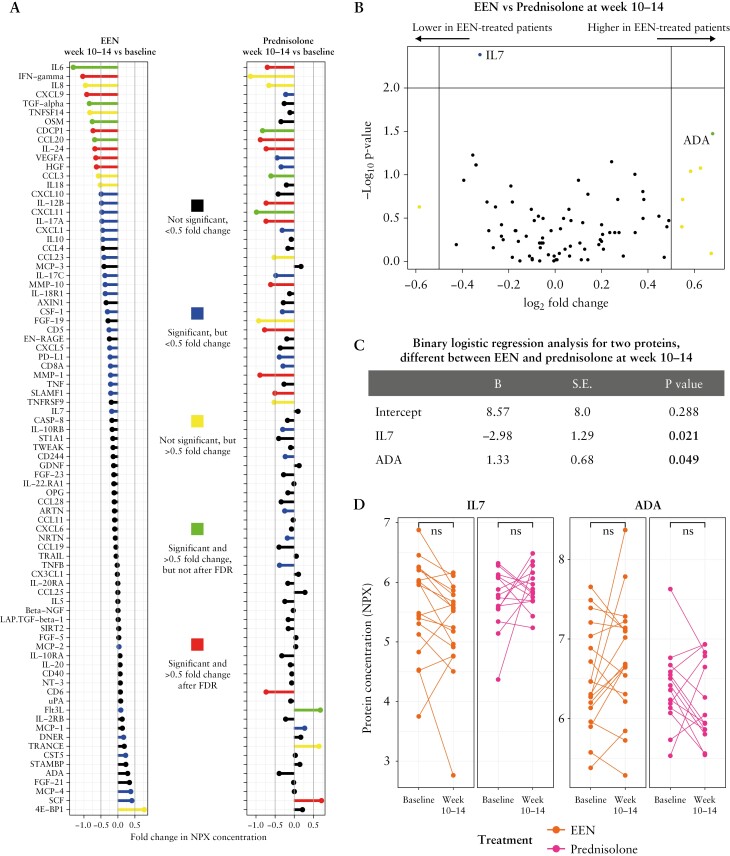
Within the CONV group, EEN- and prednisolone-treated patients had similar median protein concentrations at 10–14 weeks [Figure 3A, B]. Six of 83 proteins [VEGFA, CDCP1, CXCL9, HGF, IL-24 and IFN-γ] significantly reduced [log2FC| > 0.5] between baseline and weeks 10–14 in EEN-treated patients [n = 18]. When the four of 18 [22%] patients on EEN induction therapy who were intensified to prednisolone or IFX before the 10–14-week sample were excluded from these analysis, IL-17A, VEGFA, CDCP1, CXCL9, HFG and IFN-γ remained significantly reduced [|log2FC| > 0.5; data not shown]. The 10–14-week prednisolone treatment significantly decreased the concentrations of 11/83 proteins [|log2FC| > 0.5]: IL-6, IL-17A, CD6, SCF, SLAMF1, MMP-1, IL-12p40, IL-24, MMP-10, CD5 and CCL20 [Figure 3A]. There were no large differences in the serum immune profiles of EEN- and prednisolone-treated patients at weeks 10–14, except that IL-7 was significantly higher in prednisolone-treated patients, although the median fold change difference was below the threshold [NPX |log2FC| > 0.5; Figure 3B–D]. These data clearly establish that both EEN and prednisolone induction treatment have significant immunomodulatory effects.
Figure 3.
Patients within the conventional [CONV] treatment group treated with exclusive enteral nutrition [EEN] or oral prednisolone show similar immune signatures at 10–14 weeks. [A] Fold changes in the median protein concentrations of all 83 proteins between baseline and 10–14 weeks for patients who received EEN or prednisolone treatment; Wilcoxon signed-rank test. [B] Volcano plot of median protein concentrations at 10–14 weeks for EEN-treated patients [n = 18] compared to oral prednisolone-treated patients [n = 14]. None of the 83 proteins with |log2FC| < 0.5 were significantly different after FDR correction; Mann–Whitney U-test. [C] The concentration of interleukin [IL]-7 was higher in prednisolone patients while the concentration of adenosine deaminase [ADA] was lower in prednisolone- compared to EEN-treated patients at weeks 10–14; binary logistic regression analysis. [D] After FDR correction, the concentrations of IL-7 and ADA did not reduce significantly between baseline and after 10–14 weeks of induction treatment in either EEN- or prednisolone-treated patients; Wilcoxon signed-rank test; p < 0.05 was considered significant for all tests.
3.4. Baseline IFX-modulated immune profiles correlate with clinicopathological features
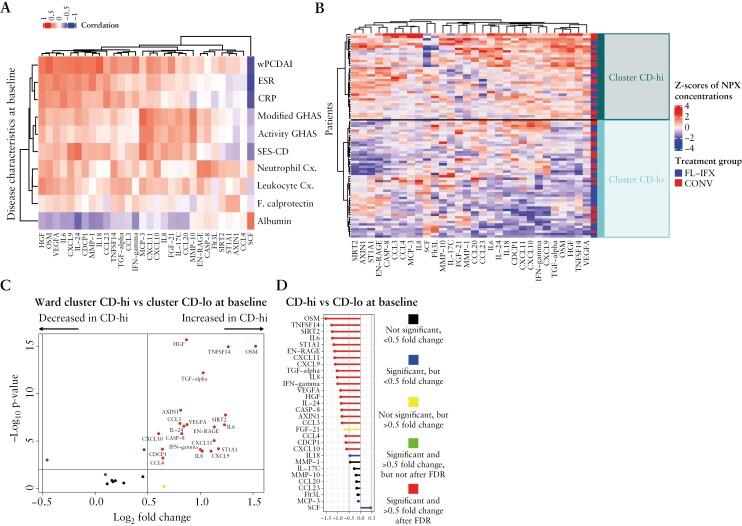
We next investigated the relationships between the 30 FL-IFX-modulated proteins and baseline clinicopathological features [Figure 4A]. Although the correlation coefficients were moderate [R ≤ 0.6], known relationships such as the correlation between C-reactive protein [CRP] and its inducing cytokine IL-6 were detected [R = 0.38, p < 0.001; Figure 4A; Supplementary Figures S2 and S3]. Investigating the correlations between various clinical characteristics of CD and the serum concentrations of the 30 FL-IFX-modulated proteins at baseline revealed that wPDCAI, erythrocyte sedimentation rate [ESR] and CRP were strongly positively associated with serum OSM, HGF, IL-6 and IL-24. SES-CD, modified GHAS and activity GHAS were more strongly associated with chemokine responses, including: monocyte-recruiting chemokine MCP-3; IFN-γ-induced chemokines CXCL10 and CXCL11; and CCL20, a ligand of chemokine receptor 6 [CCR6] on IL-17-producing T cells [Figure 4A; Supplementary Figures S2 and S3].
Figure 4.
Hierarchical clustering based on the baseline profiles of the 30 IFX-modulated proteins reveals two clusters of patients. [A] Heatmap of the Spearman’s correlation coefficient values between various clinical characteristics of CD and the serum concentrations of the 30 FL-IFX-modulated proteins at baseline [unpaired samples, n = 85]; wPCDAI, weighted paediatric Crohn’s disease activity score; ESR, erythrocyte sedimentation rate [mm/h]; CRP, C-reactive protein [mg/L]; modified GHAS, modified global histological activity score; activity GHAS, activity global histological activity score; SES-CD; simple endoscopic score for Crohn’s disease; neutrophil Cx., neutrophil concentrations [109/L]; leukocyte Cx., leukocyte concentrations [109/L]; F. calprotectin, faecal calprotectin [µg/mL]; albumin, serum albumin [g/L]. [B] Heatmap with hierarchical clustering [Euclidean distance, Ward method] of the 30 IFX-modulated proteins at baseline [paired samples, n = 78] revealed two patient clusters: CD-hi, with a high inflammation profile, and CD-lo, with a lower inflammation profile. The proportions of patients receiving first-line infliximab [FL-IFX] and conventional treatment [CONV] were not significantly different between clusters [p = 0.535]. [C] Volcano plot showing the serum concentrations of 23 of the 30 FL-IFX-modulated proteins were significantly higher in the CD-hi cluster than CD-lo cluster at baseline [unpaired samples, n = 85; Mann–Whitney U-test]. [D] Comparison of the fold changes in the median serum concentrations of the 30 FL-IFX-modulated proteins between the CD-hi and CD-lo clusters after induction treatment. A value of p < 0.05 was considered significant for all tests.
Thus, serum immune profiles relate to clinicopathological features.
3.5. Hierarchical clustering of baseline FL-IFX-modulated immune profiles reveals two patient clusters
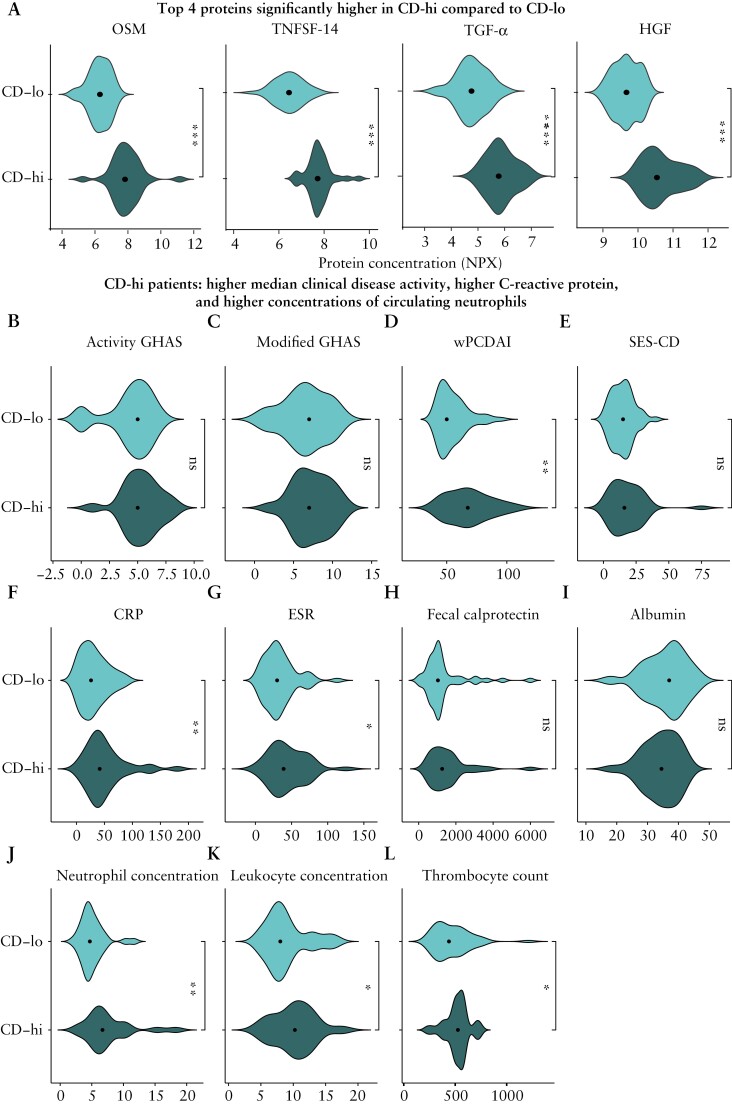
Since the 30 FL-IFX-modulated proteins provided additional information beyond clinicopathological parameters, we interrogated the patients’ baseline immune profiles. Unsupervised hierarchical clustering of baseline IFX-modulated proteins revealed two clear clusters. The CD-hi cluster [n = 36] had uniformly higher inflammatory profiles and elevated Th1 and neutrophil Th17 pathway protein concentrations, and the CD-lo cluster [n = 49] had lower overall inflammation and a more scattered pattern [Figure 4B]. By chance, the treatments were equally distributed [p = 0.54], with 50% [18/36] and 50% [18/36] of the CD-hi cluster and 59% [29/49] and 41% [20/49] of the CD-lo cluster receiving FL-IFX vs CONV. Compared to CD-lo, the CD-hi cluster had significantly higher concentrations of 23/30 proteins, in particular HGF, TNFSF14, OSM and TGF-α [Figures 4C, D and 5A]. The CD-hi cluster also had significantly higher median wPDCAI scores, CRP and neutrophil counts [after FDR correction], and higher ESR, thrombocyte counts and leukocyte counts [before correction; Supplementary Table S4], but no differences in disease location [p = 0.289], activity GHAS [p = 0.242], modified GHAS [p = 0.398] or faecal calprotectin [p = 0.157] [Figure 5B–L]. Thus, the baseline serum profiles of FL-IFX-modulated proteins identify two clusters, mostly discriminated by HGF, TNFSF14, OSM and TGF-α.
Figure 5.
CD-hi patients have significantly higher median clinical disease activity, C-reactive protein and neutrophil concentrations. [A] Violin plots of the top four most differently expressed proteins in the CD-hi and CD-lo clusters at baseline [unpaired samples, n = 85]. Oncostatin-M [OSM]; tumour necrosis factor ligand superfamily member 14 [TNFSF14]; hepatocyte growth factor [HGF]; transforming growth factor alpha [TGF-α], ***p < 0.001 after false discovery rate [FDR] correction. [B–L] Comparison of clinical disease parameters between the CD-hi and CD-lo clusters at baseline [unpaired samples, n = 85]. [B] Activity GHAS, activity global histological activity score; [C] modified GHAS; [D] wPCDAI, weighted paediatric Crohn’s disease activity score; [E] SES-CD, simple endoscopic score for Crohn’s disease; [F] CRP, C-reactive protein [mg/L]; [G] ESR, erythrocyte sedimentation rate [mm/h]; [H] faecal calprotectin [µg/mL]; [I] serum albumin [g/L]; [J] neutrophil concentration [109/L]; [K] leukocyte concentration [109/L]; [L] thrombocyte count [109/L]. Mann–Whitney U-test; **p < 0.05 after FDR correction; *p < 0.05 before FDR correction; ns, no significant difference.
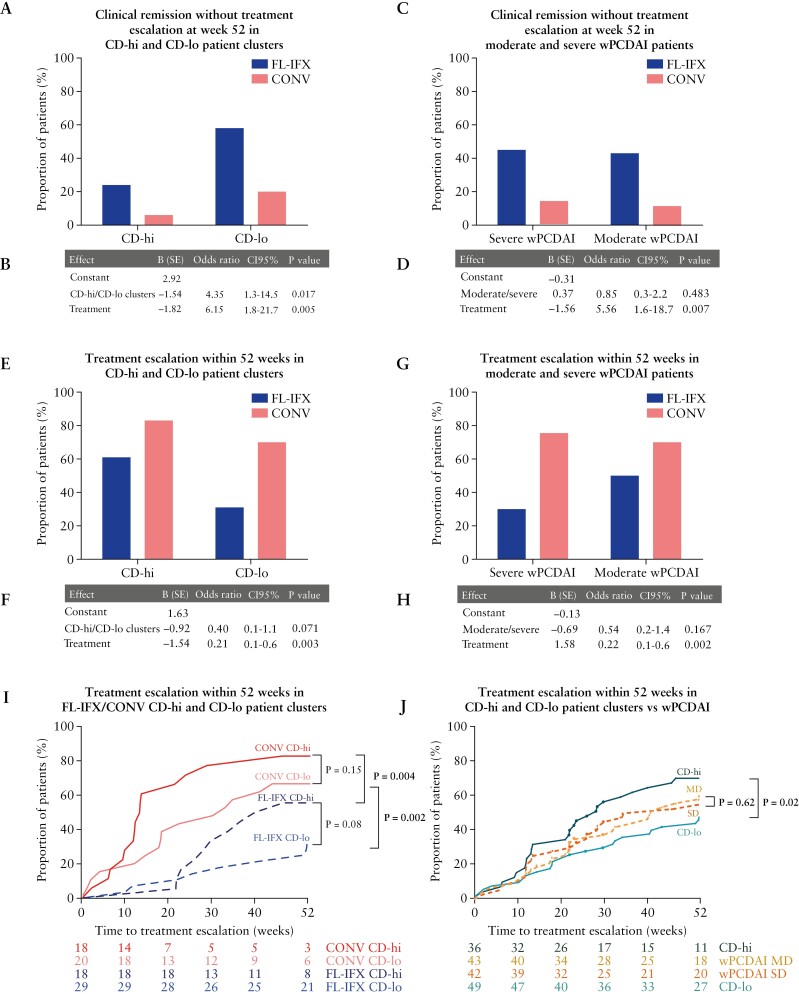
3.6. Baseline serum immune profiles are associated with clinical remission without treatment escalation at week 52
Next, we assessed whether the baseline CD-hi/CD-lo serum profiles relate to clinical remission without escalation at week 52. Crucially, irrespective of treatment, significantly fewer CD-hi patients maintained remission on AZA without escalation [Figure 6A, B, E, F]. Both baseline inflammatory protein profile (odds ratio [OR] 4.35; 95% confidence interval [CI] 1.3–14.5; p = 0.017) and type of induction therapy [OR 6.15; 95% CI 1.7–21.7; p = 0.005] significantly determined maintenance of remission without escalation: 58% of CD-lo patients and only 24% of CD-hi patients reached this primary endpoint after FL-IFX [Figure 6A, B]. Though CONV was clinically inferior in inducing remission, 20% of CD-lo patients and only 6% of CD-hi patients maintained remission after CONV [Figure 6A]. Overall—independent of induction treatment—clustering based on baseline FL-IFX-modulated proteins—but not baseline clinical disease activity [OR 0.85; 95% CI 0.3–2.2.6; p = 0.483]—was associated with remission [Figure 6C, D]. Conversely, CD-hi patients more rapidly required escalation than CD-lo patients, irrespective of induction treatment [Figure 6I, J]; baseline disease activity [wPDCAI] was not associated with this endpoint [Figure 6G, H, J; Supplementary Figure S4A]. Anti-TNF use within 52 weeks did not differ in the CD-hi and CD-lo patient groups or in groups based on baseline clinical disease activity [Supplementary Figure S4B–E].
Figure 6.
Baseline serum immune profiles are associated with clinical remission without treatment escalation at week 52. [A] Barplot with proportion of patients in the CD-hi cluster [high baseline inflammation profile] and CD-lo cluster [lower baseline inflammation profile] achieving clinical disease remission without treatment escalation at week 52 after FL-IFX or CONV treatment. Each bar is the proportion out of 100%. [B] Multivariate binary logistic regression analysis of clinical disease remission without treatment escalation at week 52. [C] Barplot with proportion of patients with moderate [>40–57.5] and severe [>57.5] weighted paediatric Crohn’s disease index [wPCDAI] scores at baseline achieving clinical disease remission without treatment escalation at week 52 after FL-IFX or CONV treatment. Each bar is the proportion out of 100%. [D] Multivariate binary logistic regression analysis of clinical disease remission without treatment escalation at week 52. [E] Barplot with proportion of patients in the CD-hi cluster and CD-lo cluster receiving additional treatment before week 52 after induction treatment with FL-IFX or CONV. Each bar is the proportion out of 100%. [F] Multivariate binary logistic regression analysis of patients who required treatment escalation within 52 weeks. A value of p < 0.05 was considered significant weeks. [G] Barplot of proportion of patients with a moderate wPCDAI [MD, 40–57.5] and severe wPCDAI [SD, >57.5] receiving additional treatment before week 52 after induction treatment with FL-IFX or CONV. Each bar is the proportion out of 100%. [H] Multivariate binary logistic regression analysis of patients who required treatment escalation within 52 weeks. [I, J] Kaplan–Meier analyses of treatment escalation in weeks [unpaired samples, n = 85; log-rank-test] stratified [E] by treatment group, CD-lo/CD-hi cluster and moderate/severe wPCDAI, and [F] by CD-lo/CD-hi cluster and moderate/severe wPCDAI.
3.7. CD-hi and CD-lo patients have similar serum immune profiles after induction therapy
CD-hi patients had higher serum immune profiles at baseline. This raised the question of whether failure to maintain remission until week 52 relates to less effective suppression of inflammatory immune proteins during the 10–14-week induction treatment. However, irrespective of the type of induction, the serum profiles of CD-hi and CD-lo patients were not significantly different at 10–14 weeks [Supplementary Figure S4F]. This indicates that the higher baseline serum immune protein concentrations in CD-hi patients were effectively reduced and reached concentrations comparable to those of CD-lo patients. Clinicopathological features were not significantly different between CD-hi and CD-lo patients after induction therapy [Supplementary Table S5], indicating the lower proportion of CD-hi patients maintaining remission at week 52 was not related to inadequate reduction of inflammatory cytokines after induction.
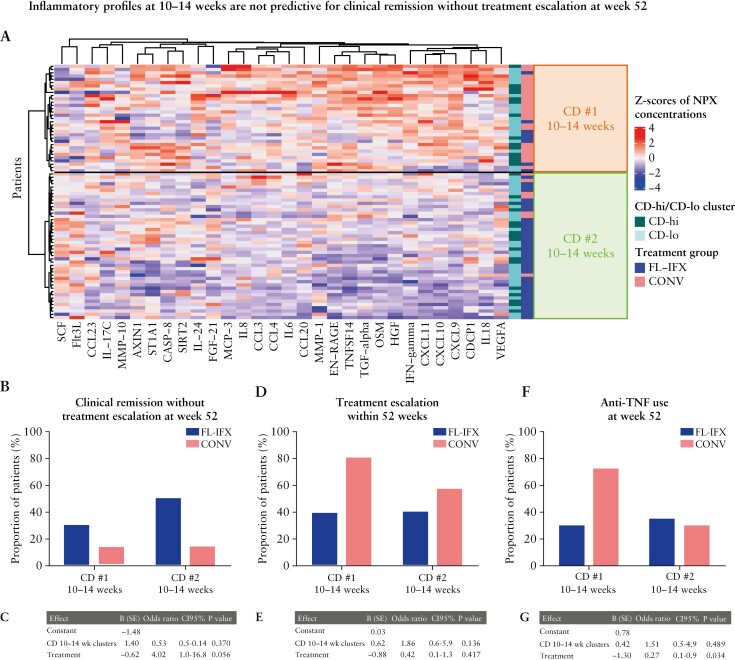
Hierarchical clusters based on IFX-modulated proteins at 10–14 weeks were not associated with clinical remission without treatment escalation at week 52.
The results above suggest induction therapy effectively suppressed inflammation, but AZA monotherapy was insufficient to maintain remission for most CD-hi patients. Thus, we investigated whether higher immune profiles after induction therapy are related to clinical outcomes, independent of baseline CD-hi/CD-lo clusters. Hierarchical clustering of immune profiles at 10–14 weeks independent of induction therapy revealed two clusters: cluster CD#1 10–14 weeks, with higher concentrations of 28/30 IFX-modulated proteins [Figure 7A; Supplementary Figure S4G], including OSM, HGF, CXCL9 and CXCL10 [log2FC, ~1.5]; and cluster CD#2 10–14 weeks, with a lower inflammatory profile [Figure 7A]. Cluster CD#1 10–14 weeks had significantly more severe clinicopathological features [Supplementary Table S6]. In line with CONV exerting weaker qualitative and quantitative suppression of serum proteins, 25/33 [76%] of the CD#1 10–14 weeks cluster received CONV vs only 7/45 [16%] of the CD#2 10–14 weeks cluster [Figure 7A]. Despite the clear clinicopathological differences, multivariate binary logistic regression analysis showed these clusters were not associated with remission without escalation [OR 0.53; 95% CI 0.1–2.1; p = 0.370, Figure 7B, C], escalation [Figure 7D, E] or use of anti-TNF [Figure 7F, G] within 52 weeks.
Figure 7.
Hierarchical clusters based on IFX-modulated proteins at 10–14 weeks are not associated with clinical remission without treatment escalation at week 52. [A] Heatmap with hierarchical clustering [Euclidean distance, Ward method] of the 30 IFX-modulated proteins at 10–14 weeks [paired samples, n = 78] revealed two clusters: CD#1 10–14 weeks [high inflammation profile] and CD#2 10–14 weeks [low inflammation profile]. The proportion of patients in the CD-lo [lower baseline inflammation profile] and CD-hi [high baseline inflammation profile] clusters [see Figure 4] was not significantly different between the CD#1 and CD#2 10–14 weeks clusters; however, the CD#2 10–14 weeks cluster contained a higher proportion of patients treated with FL-IFX than the CD#1 10–14 weeks cluster [p < 0.001]. [B] Barplot with the proportion of patients treated with FL-IFX or CONV in the CD#1 and CD#2 10–14 weeks cluster achieving clinical disease remission without treatment escalation at week 52. Each bar is the proportion out of 100%. [C] Multivariate binary logistic regression analysis of clinical disease remission without treatment escalation at week 52. [D] Barplot with the proportion of patients treated with FL-IFX or CONV in the CD#1 and CD#2 10–14 weeks cluster receiving additional treatment before week 52 after induction. Each bar is the proportion out of 100%. [E] Multivariate binary logistic regression analysis of treatment escalation at week 52. [F] Barplot with the proportion of patients treated with FL-IFX or CONV in the CD#1 and CD#2 10–14 weeks cluster [re]starting with anti-TNF within 52 weeks. Each bar is the proportion out of 100%. [G] Multivariate binary logistic regression analysis of patients [re]starting anti-TNF within 52 weeks; p < 0.05 was considered significant for all tests. FL-IFX, first-line infliximab; CONV, conventional induction therapy.
The fact that CONV and FL-IFX treatments are unequally distributed in the CD#1 and CD#2 10–14-week clusters may overshadow independent associations of the 30 IFX-modulated protein profiles with remission at week 52. Analysis of individual proteins revealed patients who did not achieve remission after FL-IFX had significantly higher serum IL-6 and VEGFA concentrations [corrected p-values] at weeks 10–14; CCL23, IFN-γ, MMP-1 and IL-18 were also significant before FDR correction [Supplementary Figure S5]. Moreover, CONV-treated patients who escalated to IFX within 1 year had significantly higher IL17A concentrations at 10–14 weeks [Supplementary Figure S5].
These results suggest that reduction of inflammation after induction therapy on the basis of the 30 IFX-modulated proteins is not predictive of maintenance of remission on AZA without treatment escalation, although significant associations with individual protein concentrations, including IL-6, were found.
4. Discussion
Early anti-TNF in moderate-to-severe CD is clinically very effective, and is now commonly used to prevent disease progression and complications. After only 10–14 weeks, three infusions of FL-IFX more effectively reduced inflammatory cytokines in peripheral blood than CONV in moderate-to-severe paediatric CD. FL-IFX reduced more proteins than CONV, and the proteins altered by both treatments were corrected more by FL-IFX. Key FL-IFX target pathways include the IFN-γ pathway [IFN-γ, CXCL9, CXCL10], neutrophil and IL-17 pathway [EN-RAGE, IL-8, CCL3, CCL23, CCL20, IL17C], cell cycle and apoptosis [TGF-α, SIRT2, caspase-8], tissue remodelling processes [HGF, MMP-1, MMP-10], intestinal physiology [ST1A1, FGF21] and haematopoiesis [SCF, Flt3L]. These data demonstrate that FL-IFX effectively restores peripheral blood immune homeostasis.
However, despite its effectiveness and relatively few side-effects, FL-IFX may introduce risks of over-treatment and leads to higher healthcare costs.27–33 Rational approaches should be devised to decide which individual patients should receive FL-IFX, and when to stop and provide alternative maintenance treatments.
The pre-treatment serum immune profiles based on 30 FL-IFX-modulated proteins, but not clinical disease score, related strongly to 1 year of maintenance of remission on AZA without escalation after induction of 22 weeks of remission with FL-IFX. Specifically, 58% of FL-IFX-treated patients with low pre-treatment serum concentrations of the 30 FL-IFX-modulated proteins [CD-lo cluster] maintained clinical remission vs 20% of CD-lo CONV patients, indicating that—even in patients with relatively low immune activation—the chance of sustained remission after CONV is low. Crucially, 22 weeks of FL-IFX improved the chance of maintaining remission among CD-lo patients. Thus, although almost all CD patients may benefit from FL-IFX, CD-lo patients may be more eligible to stop IFX after induction of remission. Studies in adult CD demonstrate discontinuing IFX after remission is effective and not associated with major complications.27,34 Most studies report 2-year relapse rates of 40–50% after stopping anti-TNF, though the maintenance immunomodulators varied.35–37 Crucially, a 10-year follow-up of adult patients demonstrated the time-to-flare after induction of remission was 4.5 years for early [first-line] IFX [6 weeks] vs 2.5 years for CONV.27 Similarly, our data emphasize CD-hi patients with high baseline immune profiles should not discontinue IFX, as only 24% maintained remission after 1 year.
Our data demonstrate FL-IFX is advantageous for all patients with moderate-to-severe CD, and low baseline serum concentrations of the 30 IFX-modulated proteins can support the decision to discontinue FL-IFX.
Ideally, in addition to baseline profiles, immune profiling after 14–22 weeks of induction FL-IFX could further corroborate the decision to stop FL-IFX. Surprisingly, despite clear differences in immune activation, higher concentrations of IFX-modulated proteins at weeks 10–14 [i.e. CD#1 10–14 weeks cluster] was not independently predictive [over the induction treatment] for remission at 1 year without escalation. This was an unexpected finding because previous studies did find a relationship between higher inflammatory clinicopathological features after induction treatment and risk of a disease relapse within 18 months and/or early surgery.5,38 Our failure to find this relationship may relate to the fact most FL-IFX-treated patients [38/45] had low serum immune protein concentrations at 10–14 weeks, whereas most CONV patients [25/33] still had elevated concentrations. Hence, the unequal distribution of FL-IFX and CONV in the CD#1- and CD#2- 10–14 weeks cluster may hamper assessments of whether serum immune profiles at 10–14 weeks predict 1-year outcomes independently of induction therapy. Therefore, we assessed whether specific proteins were associated with remission without escalation in each group. FL-IFX-treated patients who did not achieve remission at 1 year had significantly higher serum IL-6 and VEGFA concentrations at weeks 10–14. As IL-6 mediates CRP production in the liver, this finding agrees with our report that TISKids patients who required escalation had higher CRP concentrations after induction,12 confirming that CRP is strongly related to an active disease course. However, whether CRP and the high IL-6 concentrations at weeks 10–14 may be predictive for escalation is unclear as clinical decision-making to escalate is based on CRP, creating a confounder, and truly independent indicators of relapse are desired.20 Higher CCL23, IFN-γ, MMP-1 and IL-18 concentrations at weeks 10–14 were related to remission without escalation in FL-IFX-treated patients [before FDR], and thus future studies should further assess the value of immune profiling at 10–14 weeks to predict remission.
How do the distinctive 30 IFX-modulated protein profiles relate to CD pathogenesis? Many of the IFX-modulated proteins have been associated with CD pathogenesis. Fourteen of these proteins differed between IBD and controls in other cohorts, five have been associated with treatment escalation, and six have been associated with complications.20,21,39–41 Although accumulation of the IFN-γ-induced chemokines CXCL9 and CXCL10 is associated with CD, strikingly, the baseline concentrations of multiple proteins from this entire pathway, including CXCL11 and IFN-γ itself, predicted failure to maintain remission in our CD-hi cohort. IFN-γ-mediated Th1 responses and downstream phagocyte activation are major drivers of CD. This entire pathway is more active in CD-hi patients, who are more susceptible to disease relapse, than CD-lo patients, suggesting CD-hi patients have a different disease immunotype with a much stronger Th1 memory response that is resistant to maintenance immunomodulation, even after successful inhibition after 22 weeks of FL-IFX. In line with this, CD-hi patients with colonic disease involvement more often had high concentrations of CXCL9, CXCL10 and CXCL11 compared to CD-hi patients with isolated ileal disease. The strong Th1 signature in CD-hi patients is emphasized by the lack of differential abundance of IL-17A, IL17C or the related chemokine CCL20 between CD-hi and CD-lo patients. A second striking feature of CD-hi patients is the abundance of proteins produced by neutrophils or target neutrophils, in agreement with the neutrophilia observed in CD-hi patients. Despite effective suppression after 22 weeks of FL-IFX, high baseline TNFSF-14, OSM and IL-6 concentrations were highly related to failure to maintain remission. The pleiotropic cytokine IL-6 is produced by structural cells such as tissue fibroblasts and regulates haematopoiesis, favouring neutrophil mobilization from bone marrow. OSM, an IL-6 family cytokine, shares the gp130 signalling cascade with IL-6 but is produced by immune cells; neutrophils carry a preformed stock of this protein.42 High intestinal OSM has been associated with resistance to IFX and poorer prognosis.43,44 TNFSF-14 [LIGHT], a pro/anti-inflammatory protein produced by neutrophils and T cells, has not previously been associated with failure to maintain remission. Intriguingly, high circulating TNFSF14 concentrations were associated with a decreased risk of B3 complications in the RISK-paediatric CD cohort21 and TNFSF-14-deficient mice are more susceptible to experimental dextran saline sodium [DSS] colitis and T cell-mediated transfer colitis, arguing that TNFSF-14 may be protective.45 However, TNFSF14 co-stimulates T cells to enhance T cell proliferation and IFN-γ production,46 and transgenic overexpression of TNFSF-14 in mice leads to colitis with enhanced Th1 cytokine activity.47,48 Further support for this strong neutrophil signature in CD-hi patients includes the higher concentrations of the neutrophil chemoattractant IL-8 [CXCL8] and neutrophil products VEGF and CUB domain-containing protein 1 [CDCP1]. Notably, IL-8 and CDCP1 concentrations were significantly higher in ileocolonic than isolated ileal disease. Thus, the serum immune profiles that differentiate moderate-to-severe CD with a poor chance of maintaining remission after FL-IFX delineate a strong Th1–neutrophil immunotype with a low abundance of Th17 cytokines.
Overall, serum immune profiles combined with clinical parameters demonstrate the efficacy of FL-IFX in therapy-naive paediatric CD. FL-IFX achieves stronger reductions and modulates more proteins than CONV. FL-IFX is superior to CONV for patients with high and lower concentrations of IFX-modulated proteins at baseline. However, patients with high concentrations of IFX-modulated proteins at baseline [i.e. CD-hi] have a lower chance of maintaining remission without escalation at 1 year compared to CD-lo; therefore, maintenance treatment with AZA only, after stopping FL-IFX, is probably insufficient in these patients. Serum profiling is fast and relatively affordable, and provides a rational approach to start/stop IFX after induction. However, consensus on which proteins provide most information across cohorts and disease stages must be obtained before immune profiling can be integrated into clinical practice.
Supplementary Material
Acknowledgments
We thank all children and adolescents with CD who participated in this study, the research teams at all participating centres, as well as M. A. Aardoom and S. A. Vuijk, Department of Anesthesiology, and J. C. de Graaff of the Department of Paediatric Gastroenterology at Erasmus University Medical Center-Sophia Children’s Hospital, Rotterdam, the Netherlands. We also thank B. Li [Sketchy Pipette, the Netherlands] for graphical assistance with the final manuscript and A. Devlin from Science Editing Experts for editorial assistance.
Contributor Information
Maria M E Jongsma, Department of Pediatric Gastroenterology, Erasmus University Medical Center/Sophia Children’s Hospital, Rotterdam, the Netherlands.
Lea M M Costes, Laboratory of Pediatrics, Division of Gastroenterology and Nutrition, Erasmus University Medical Center/Sophia Children’s Hospital, Rotterdam, the Netherlands.
Irma Tindemans, Laboratory of Pediatrics, Division of Gastroenterology and Nutrition, Erasmus University Medical Center/Sophia Children’s Hospital, Rotterdam, the Netherlands.
Martinus A Cozijnsen, Department of Pediatric Gastroenterology, Erasmus University Medical Center/Sophia Children’s Hospital, Rotterdam, the Netherlands.
Rolien (H) C Raatgreep, Laboratory of Pediatrics, Division of Gastroenterology and Nutrition, Erasmus University Medical Center/Sophia Children’s Hospital, Rotterdam, the Netherlands.
Merel van Pieterson, Department of Pediatric Gastroenterology, Erasmus University Medical Center/Sophia Children’s Hospital, Rotterdam, the Netherlands.
Yunlei Li, Department of Pathology & Clinical Bioinformatics, Erasmus University Medical Center/Erasmus MC Cancer Institute, Rotterdam, the Netherlands.
Johanna C Escher, Department of Pediatric Gastroenterology, Erasmus University Medical Center/Sophia Children’s Hospital, Rotterdam, the Netherlands.
Lissy de Ridder, Department of Pediatric Gastroenterology, Erasmus University Medical Center/Sophia Children’s Hospital, Rotterdam, the Netherlands.
Janneke N Samsom, Laboratory of Pediatrics, Division of Gastroenterology and Nutrition, Erasmus University Medical Center/Sophia Children’s Hospital, Rotterdam, the Netherlands.
Conference Presentation
Part of this study was presented at the 16th congress of ECCO, 2021, Virtual, UEGweek 2021, Virtual, and 17th congress of ECCO, 2022, Virtual.
Funding
This investigator-initiated trial was financially supported by ZonMw [the Netherlands Organization for Health Research and Development] under project number 113202001, Crocokids [a Dutch fundraising organization that supports research on IBD in children], and an Investigator-Sponsored Research Award from Pfizer [Study ID WI213008]. The work presented in this paper was supported by stichting Dalijn and the collaborative TIMID project [LSHM18057-SGF] financed by the PPP allowance made available by Top Sector Life Sciences & Health to Samenwerkende Gezondheidsfondsen [SGF] to stimulate public–private partnerships and co-financing by health foundations that are part of the SGF. The funders of the study had no role in the study design, data collection, statistical analysis, interpretation or writing of the report.
Conflict of Interest
JCE received consultancy fees from Abbvie and Janssen, as well as research support from MSD and Nutricia. LdR: collaboration [e.g. involved in industry-sponsored studies, investigator-initiated study, consultancy] with Abbvie, Lilly, Takeda, Janssen and Pfizer; grant from ZonMw, ECCO, Pfizer. JS, HCR, IT, LMMC, MAC, MMEJ, MvP and YL declare no competing interests.
Author Contributions
JS, JCE, LdR and MMEJ contributed to the study concept and design. JS and MMEJ had full access to the data in the trial and take responsibility for the integrity of the data and the accuracy of the data analysis. All authors contributed to acquisition, analysis or interpretation of the data. MMEJ and JS contributed to drafting of the manuscript. All authors contributed to critical revision of the manuscript and provided important intellectual content. YL and MMEJ contributed to the statistical analysis. JS and LdR supervised the study. All authors approved the final version of the manuscript.
Patient and Public Involvement
Patients and/or the public were not involved in the design, conduct, reporting or dissemination plans of this research.
Ethics Approval
Medical ethical approval was obtained for each site. Eudra CT number: NL52030.078.15 2014-00570237, Medical Ethical Trial Committee Erasmus MC: MEC-2015-080.
Data Accessibility Statement
The dataset that supports the findings of this study is openly available in the Supplemental Data file: supplemental_clinical_serum_data_complete.xlsx.
References
- 1. Torres J, Mehandru S, Colombel JF, Peyrin-Biroulet L.. Crohn’s disease. Lancet 2017;389:1741–55. [DOI] [PubMed] [Google Scholar]
- 2. Duchmann R, Kaiser I, Hermann E, Mayet W, Ewe K, Meyer zum Büschenfelde KH.. Tolerance exists towards resident intestinal flora but is broken in active inflammatory bowel disease (IBD). Clin Exp Immunol 1995;102:448–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Tindemans I, Joosse ME, Samsom JN.. Dissecting the heterogeneity in t-cell mediated inflammation in IBD. Cells 2020;9:110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Rieder F, Zimmermann EM, Remzi FH, Sandborn WJ.. Crohn’s disease complicated by strictures: A systematic review. Gut 2013;62:1072–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Levine A, Chanchlani N, Hussey S, et al. Complicated disease and response to initial therapy predicts early surgery in paediatric Crohn’s disease: Results from the Porto Group Growth Study. J Crohns Colitis 2020;14:71–8. [DOI] [PubMed] [Google Scholar]
- 6. Danese S, Fiorino G, Peyrin-Biroulet L.. Early intervention in Crohn’s disease: Towards disease modification trials. Gut 2017;66:2179–87. [DOI] [PubMed] [Google Scholar]
- 7. Kang B, Choe YH.. Early biologic treatment in pediatric Crohn’s disease: Catching the therapeutic window of opportunity in early disease by treat-to-target. Pediatr Gastroenterol Hepatol Nutr 2018;21:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Singh H, Nguyen T, Pho C, Giles E.. Early infliximab in Crohn’s is associated with decreased intestinal surgery and similar health care costs. Scand J Gastroenterol 2021;56:397–402. [DOI] [PubMed] [Google Scholar]
- 9. Ma C, Beilman CL, Huang VW, et al. Anti-TNF therapy within 2 years of Crohn’s disease diagnosis improves patient outcomes: A retrospective cohort study. Inflamm Bowel Dis 2016;22:870–9. [DOI] [PubMed] [Google Scholar]
- 10. Veltkamp C, Anstaett M, Wahl K, et al. Apoptosis of regulatory T lymphocytes is increased in chronic inflammatory bowel disease and reversed by anti-TNFα treatment. Gut 2011;60:1345–53. [DOI] [PubMed] [Google Scholar]
- 11. Berg DR, Colombel JF, Ungaro R.. The role of early biologic therapy in inflammatory bowel disease. Inflamm Bowel Dis 2019;25:1896–905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Jongsma MME, Aardoom MA, Cozijnsen MA, et al. First -line treatment with infliximab versus conventional treatment in children with newly diagnosed moderate-to-severe Crohn’s disease: An open-label multicentre randomised controlled trial. Gut 2022;71(1):34–42. doi: 10.1136/gutjnl-2020-322339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. van Rheenen PF, Aloi M, Assa A, et al. The medical management of paediatric Crohn’s disease: An ECCO-ESPGHAN guideline update. J Crohns Colitis 2020. [DOI] [PubMed] [Google Scholar]
- 14. Noor NM, Verstockt B, Parkes M, Lee JC.. Personalised medicine in Crohn’s disease. Lancet Gastroenterol Hepatol 2020;5:80–92. [DOI] [PubMed] [Google Scholar]
- 15. Daperno M, D’Haens G, Van Assche G, et al. Development and validation of a new, simplified endoscopic activity score for Crohn’s disease: The SES-CD. Gastrointest Endosc 2004;60:505–12. [DOI] [PubMed] [Google Scholar]
- 16. D’Haens GR, Geboes K, Peeters M, Baert F, Penninckx F, Rutgeerts P.. Early lesions of recurrent Crohn’s disease caused by infusion of intestinal contents in excluded ileum. Gastroenterology 1998;114:262–7. [DOI] [PubMed] [Google Scholar]
- 17. Turner D, Griffiths AM, Wilson D, et al. Designing clinical trials in paediatric inflammatory bowel diseases: A PIBDNET commentary. Gut 2019. [DOI] [PubMed] [Google Scholar]
- 18. Doherty G, Katsanos KH, Burisch J, et al. European Crohn’s and Colitis Organisation topical review on treatment withdrawal [‘exit strategies’] in inflammatory bowel disease. J Crohns Colitis 2018;12:17–31. [DOI] [PubMed] [Google Scholar]
- 19. Atreya R, Neurath MF.. Can serum proteomic profiling annunciate individual disease progression in newly diagnosed inflammatory bowel disease patients? J Crohns Colitis 2021;15:697–8. [DOI] [PubMed] [Google Scholar]
- 20. Kalla R, Adams AT, Bergemalm D, et al. Serum proteomic profiling at diagnosis predicts clinical course, and need for intensification of treatment in inflammatory bowel disease. J Crohns Colitis 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Ungaro RC, Hu L, Ji J, et al. Machine learning identifies novel blood protein predictors of penetrating and stricturing complications in newly diagnosed paediatric Crohn’s disease. Aliment Pharmacol Ther 2021;53:281–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Cozijnsen MA, van Pieterson M, Samsom JN, Escher JC, de Ridder L.. Top-down infliximab study in kids with Crohn’s disease (TISKids): An international multicentre randomised controlled trial. BMJ Open Gastroenterol 2016;3:e000123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Ruemmele FM, Veres G, Kolho KL, et al. ; European Crohn's and Colitis Organisation. Consensus guidelines of ECCO/ESPGHAN on the medical management of pediatric Crohn’s disease. J Crohns Colitis 2014;8:1179–207. [DOI] [PubMed] [Google Scholar]
- 24. Turner D, Griffiths AM, Walters TD, et al. Mathematical weighting of the pediatric Crohn’s disease activity index [PCDAI] and comparison with its other short versions. Inflamm Bowel Dis 2012;18:55–62. [DOI] [PubMed] [Google Scholar]
- 25. Wang J, Anders RA, Wang Y, et al. The critical role of light in promoting intestinal inflammation and Crohn’s disease. J Immunol 2005;174:8173–82. [DOI] [PubMed] [Google Scholar]
- 26. Andoh A, Shioya M, Nishida A, et al. Expression of IL-24, an activator of the JAK1/STAT3/SOCS3 cascade, is enhanced in inflammatory bowel disease. J Immunol 2009;183:687–95. [DOI] [PubMed] [Google Scholar]
- 27. Hoekman DR, Stibbe JA, Baert FJ, et al. ; BIRD (Belgian Inflammatory Bowel Disease Research and Development) Group; North-Holland Gut Club. Long-term outcome of early combined immunosuppression versus conventional management in newly diagnosed Crohn’s disease. J Crohns Colitis 2018;12:517–24. [DOI] [PubMed] [Google Scholar]
- 28. Ashton JJ, Ennis S, Beattie RM.. Infliximab at diagnosis: Moving towards personalisation in paediatric inflammatory bowel disease. Gut 2021. [DOI] [PubMed] [Google Scholar]
- 29. Hyams J, Crandall W, Kugathasan S, et al. ; REACH Study Group. Induction and maintenance infliximab therapy for the treatment of moderate-to-severe Crohn’s disease in children. Gastroenterology 2007;132:863–73; quiz 1165. [DOI] [PubMed] [Google Scholar]
- 30. Hyams JS, Dubinsky MC, Baldassano RN, et al. Infliximab is not associated with increased risk of malignancy or hemophagocytic lymphohistiocytosis in pediatric patients with inflammatory bowel disease. Gastroenterology 2017;152:1901–1914.e3. [DOI] [PubMed] [Google Scholar]
- 31. Aardoom MA, Veereman G, de Ridder L.. A review on the use of anti-tnf in children and adolescents with inflammatory bowel disease. Int J Mol Sci 2019;20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. van der Valk ME, Mangen MJ, Leenders M, et al. ; COIN study group and the Dutch Initiative on Crohn and Colitis. Healthcare costs of inflammatory bowel disease have shifted from hospitalisation and surgery towards anti-TNFα therapy: Results from the coin study. Gut 2014;63:72–9. [DOI] [PubMed] [Google Scholar]
- 33. Pierre N, Baiwir D, Huynh-Thu VA, et al. Discovery of biomarker candidates associated with the risk of short-term and mid/long-term relapse after infliximab withdrawal in Crohn’s patients: a proteomics-based study. Gut 2020. [DOI] [PubMed] [Google Scholar]
- 34. Reenaers C, Mary JY, Nachury M, et al. ; Groupe d’Etude Therapeutique des Affections Inflammatoires du tube Digestif. Outcomes 7 years after infliximab withdrawal for patients with Crohn’s disease in sustained remission. Clin Gastroenterol Hepatol 2018;16:234–243.e2. [DOI] [PubMed] [Google Scholar]
- 35. Chapman TP, Gomes CF, Louis E, Colombel JF, Satsangi J.. De-escalation of immunomodulator and biological therapy in inflammatory bowel disease. Lancet Gastroenterol Hepatol 2020;5:63–79. [DOI] [PubMed] [Google Scholar]
- 36. Torres J, Boyapati RK, Kennedy NA, Louis E, Colombel J-F, Satsangi J.. Systematic review of effects of withdrawal of immunomodulators or biologic agents from patients with inflammatory bowel disease. Gastroenterology 2015;149:1716–30. [DOI] [PubMed] [Google Scholar]
- 37. Buhl S, Steenholdt C, Brynskov J, et al. Discontinuation of infliximab therapy in patients with Crohn’s disease. NEJM Evidence 2022;1:EVIDoa2200061. [DOI] [PubMed] [Google Scholar]
- 38. Ziv-Baran T, Hussey S, Sladek M, et al. Response to treatment is more important than disease severity at diagnosis for prediction of early relapse in new-onset paediatric Crohn’s disease. Aliment Pharmacol Ther 2018;48:1242–50. [DOI] [PubMed] [Google Scholar]
- 39. Bourgonje AR, Hu S, Spekhorst LM, et al. The effect of phenotype and genotype on the plasma proteome in patients with inflammatory bowel disease. J Crohns Colitis 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Walshe M, Nayeri S, Ji J, et al. A role for CXCR3 ligands as biomarkers of post-operative Crohn’s disease recurrence. J Crohns Colitis 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Bergemalm D, Andersson E, Hultdin J, et al. ; IBD Character Consortium. Systemic inflammation in preclinical ulcerative colitis. Gastroenterology 2021;161:1526–1539.e9. [DOI] [PubMed] [Google Scholar]
- 42. Grenier A, Dehoux M, Boutten A, et al. Oncostatin M production and regulation by human polymorphonuclear neutrophils. Blood 1999;93:1413–21. [PubMed] [Google Scholar]
- 43. West NR, Hegazy AN, Owens BMJ, et al. ; Oxford IBD Cohort Investigators. Oncostatin M drives intestinal inflammation and predicts response to tumor necrosis factor-neutralizing therapy in patients with inflammatory bowel disease. Nat Med 2017;23:579–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Verstockt S, Verstockt B, Machiels K, et al. Oncostatin M is a biomarker of diagnosis, worse disease prognosis, and therapeutic nonresponse in inflammatory bowel disease. Inflamm Bowel Dis 2021;27:1564–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Krause P, Zahner SP, Kim G, Shaikh RB, Steinberg MW, Kronenberg M.. The tumor necrosis factor family member TNFSF14 [LIGHT] is required for resolution of intestinal inflammation in mice. Gastroenterology 2014;146:1752–62.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Tamada K, Shimozaki K, Chapoval AI, et al. LIGHT, a TNF-like molecule, costimulates T cell proliferation and is required for dendritic cell-mediated allogeneic T cell response. J Immunol 2000;164:4105–10. [DOI] [PubMed] [Google Scholar]
- 47. Shaikh RB, Santee S, Granger SW, et al. Constitutive expression of LIGHT on T cells leads to lymphocyte activation, inflammation, and tissue destruction. J Immunol 2001;167:6330–7. [DOI] [PubMed] [Google Scholar]
- 48. Wang J, Lo JC, Foster A, et al. The regulation of T cell homeostasis and autoimmunity by T cell-derived LIGHT. J Clin Invest 2001;108:1771–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The dataset that supports the findings of this study is openly available in the Supplemental Data file: supplemental_clinical_serum_data_complete.xlsx.