Abstract
Introduction:
Sepsis is a life-threatening medical emergency and a leading cause of morbidity and mortality worldwide. Reductions in time to antibiotics in patients presenting with sepsis or septic shock are associated with reduced mortality, and Surviving Sepsis Campaign guidelines recommend antibiotics within one hour of recognition. Pharmacists are well-equipped to help navigate the therapeutic and operational challenges associated with achieving this goal.
Objectives:
To assess the association of pharmacist involvement in sepsis response with time to antibiotics in hospitalized patients with sepsis and septic shock.
Methods:
A systematic review of the following databases was conducted: PubMed/MEDLINE, Embase, Cumulative Index to Nursing and Allied Health Literature (CINAHL), and Web of Science. Studies must have included a designated role of an individual pharmacist in the management of sepsis or septic shock and not be considered an operational change. The primary outcome of interest was time to antibiotic administration, with secondary outcomes including intensive care unit (ICU) and hospital length of stay as well as in-hospital mortality.
Results:
We identified 10 studies including 1772 patients with sepsis or septic shock that evaluated a sepsis response in which a pharmacist was included. Studies included patients in the ICU, emergency department, and hospital ward setting. Seven studies demonstrated a significant reduction in time to antibiotics, with two other studies supporting this conclusion in extrapolation or sensitivity analysis. There was not a consistent reduction in ICU or hospital length of stay nor in-hospital mortality between those interventions involving a pharmacist compared with their defined control groups.
Conclusion:
Pharmacist involvement in sepsis response, often as part of a multi-professional team-based approach to sepsis care, is associated with a reduced time to antibiotic administration for hospitalized patients with sepsis or septic shock.
Keywords: pharmacist, sepsis, septic shock, antibiotic, systematic review
Sepsis is a leading cause of death in hospitalized patients with mortality ranging from 27–42% depending on severity, and is a leading cause of death worldwide responsible for up to 20% of all global deaths.1, 2 Although there is no targeted therapy for sepsis, early recognition and treatment are paramount to improving clinical outcomes. Delays in antibiotic therapy are associated with increasing mortality,3 including an estimated decrease in survival of 7.6% for every hour delay of antibiotics in patients with septic shock.4 This recognition has prompted the Surviving Sepsis Campaign to offer a strong recommendation to administer antimicrobials immediately (ideally within one hour of recognition) in adults with possible septic shock or a high likelihood for sepsis.5
Many institutions have implemented sepsis treatment bundles and other quality improvement initiatives aimed at improving the recognition of sepsis and time to appropriate triage and antibiotics. However, ensuring the ordering of antibiotics leads to timely administration of antibiotics continues to be a challenge for health-systems,6 leading some to suggest antibiotic order-to-infusion time as a potential quality metric in septic shock.7 Potential challenges to this include staff shortages, drug shortages, patient transport, and other potential operational barriers, as well as attempts to balance the need for early recognition and antibiotics with appropriate antimicrobial stewardship. Given the focus on optimizing antimicrobial selection for the appropriate suspected pathogens as well as navigating the institutional logistics of medication preparation and delivery, pharmacists are uniquely suited to participate in these medical emergencies.5 Thus, we conducted a systematic review of interventions that included pharmacist involvement in sepsis response and time to antibiotics.
METHODS
Our objective was to evaluate the published literature to determine if pharmacist involvement, including participation in a sepsis response team, was associated with a reduced time to antibiotics in patients presenting with sepsis or septic shock. Adult patients with a diagnosis of sepsis or septic shock represented the population of interest. For the purpose of this review, we defined pharmacist involvement as inclusion of a pharmacist with a defined role in the sepsis response. Furthermore, we focused on specific activities requiring participation of an individual pharmacist in sepsis response rather than operational changes (e.g., stocking antibiotics in automated dispensing cabinets, change of an order set making all antibiotics STAT). Comparator groups were study specific and were not strictly defined for inclusion into this review. The primary outcome of interest was time to antibiotics. The review protocol was registered on the International Platform of Registered Systematic Review and Meta-analysis Protocols8 and reported according to the guidelines set forth by the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) criteria.9
Search Strategy
With the assistance of an experienced medical librarian, we performed a systematic literature search using combinations of controlled vocabulary, title, and abstract keywords using Boolean operators. We searched the following databases from inception to June 28, 2022: PubMed MEDLINE (PubMed.gov), Embase (Elsevier), Cumulative Index to Nursing and Allied Health Literature – CINAHL (EBSCO), and Web of Science Core Collection (Clarivate Analytics). The complete search strategy employed is presented in Supporting Information, Table 1. Bond University’s Polyglot Search Translator tool was used to assist in translating the PubMed MEDLINE search into the syntax of the other databases.10 Reference lists of relevant articles were also reviewed for pertinent literature. Studies were sorted and duplicates removed using EndNote X9 (Clarivate Analytics, London, United Kingdom).
Table 1.
Overview of Included Studies
| Reference | Study Design | Time Period | Sample Size (total) | Patient Population | Comparator Group | Bundle Elements (Intervention Group) | Specific Pharmacist Intervention & Associated Activities |
|---|---|---|---|---|---|---|---|
| Sarani et al. 200819 | Retrospective | 2006–2007 | 62 | Hospital wards with rapid response activation in tertiary care, academic medical center | Patients with ‘STAT’ antibiotics without a MET activation | MET activation including: critical care nursing coordinator, respiratory therapist, clinical pharmacist (includes 24 hours per day coverage), and lead physician | 1) MET pharmacist verifies verbal antibiotic orders at bedside, notifies the pharmacy, retrieves antibiotics, and hand delivers to bedside nurse 2) Assists with drug selection, dosing, and drug interactions |
| LaRosa et al. 201216 | Prospective cohort | 2009 | 58 | Tertiary care, urban, teaching hospital (performed in single ICU) | Patients in whom an overhead sepsis alert was not triggered | Patients meeting criteria on screening tool triggered an overhead alert that brought responders to the bedside – including the ICU physician, ICU nurse, respiratory therapist, and pharmacist – to implement sepsis resuscitation and management bundle elements using a standardized order set | Paged to bedside to work with response team on sepsis resuscitation and bundle elements |
| Flynn et al. 201414 | Retrospective | 2008–2011 | 108 | Tertiary care academic medical center (wards, ED, and ICU) | Historical comparison based on DRG codes | Included electronic order sets, deployment of pharmacy and nursing personnel to bedside, and placement of sepsis carts around hospital stocked to facilitate resuscitation | 1) Pharmacy resident on-call responds to bedside, evaluates case with team to optimize antibiotic selection and dosing, and assists with preparation, acquisition, and administration of antibiotics in collaboration with nursing 2) Reviews fluid resuscitation, vasopressor selection, and corticosteroid candidacy |
| Beardsley et al. 201612 | Retrospective | 2012 | 283 | Tertiary care academic medical center (non-critical care units) | Historical comparison (prior to Code Sepsis initiative) | Code Sepsis activated by rapid response nurse, including: inpatient pharmacy, respiratory, blood gas laboratory, and ICU triage nurse response | 1) Pharmacist to call nursing unit 15 minutes after Code Sepsis activation if no antibiotics ordered 2) Protocol for pharmacist to select antibiotics if provider engaged in other aspects of care 3) Implementation of “Code Sepsis” verbal hand-offs at critical junctures of antibiotic ordering and delivery |
| Moussavi et al. 201618 | Retrospective | 2014 | 186 | ED of university teaching hospital | No pharmacist present in ED | Presence of a clinical pharmacist in the ED | 1) ED clinical pharmacist and technician physically present daily from 0900–1930 2) Numerous responsibilities of ED pharmacist, not limited to evaluating medication orders and facilitating medication preparation and delivery |
| Laine et al. 201815 | Retrospective | 2012–2014 | 76 | Tertiary care academic medical center | No comparator group | Included electronic order sets, and deployment of pharmacy and nursing personnel to bedside (evaluation limited to septic shock patients) | 1) Pharmacy resident on-call responds to bedside, evaluates case with team to optimize antibiotic selection and dosing, and assists with preparation, acquisition, and administration of antibiotics in collaboration with nursing 2) Reviews fluid resuscitation, vasopressor selection, and corticosteroid candidacy |
| Chanas et al. 201913 | Prospective cohort | 2016–2017 | 161 | Surgical/trauma/burn ICU of tertiary care academic medical center | Historical standard of care without BPA to provider | Electronic BPA for septic shock offered provider option to page medical residents, clinical pharmacist, and charge nurse to bedside to review case and facilitate care | Pharmacist reports to bedside with team to assist with antibiotic selection and facilitate administration in collaboration with nursing |
| MacMillan et al. 201917 | Retrospective | 2015–2016 | 160 | Medical ICU of a community teaching hospital | Historical standard of care prior to implementation of quality improvement initiative | If sepsis suspected based on electronic and/or manual assessment, charge nurse activated an overhead alert which sent verbal pages to supervising resident physician, critical care pharmacist, and phlebotomist, all of whom were expected to report to bedside within 15 minutes for group consultation. | Pharmacist expected at bedside within 15 minutes of alert to work with physician to order appropriate antibiotics and facilitate preparation/delivery to bedside nurse |
| Yarbrough et al. 201921 | Prospective, two-arm, parallel design | 2017 | 80 | ED of a quaternary community hospital | Sepsis alerts without a pharmacist present | Sepsis alert generated once patient meets criteria and multidisciplinary sepsis alert team paged to patient’s room, including the pharmacist | Pharmacist reports to patient’s room, reviews patient’s medical history, and uses a standardized checklist and empiric antibiotic guide to make recommendations and ensure bundle completion |
| Tarabichi et al. 202220 | Randomized, controlled, quality improvement initiative | 2019 | 598 | ED of academic, safety-net health care system | Standard sepsis care | Sepsis care augmented with EWS, which included notification on ED patient tracking tool and message sent to ED pharmacist | Pharmacist (available daily from 1000–2100) reviews chart, huddles with ED provider, and facilitates timely and appropriate blood work and fluid/antibiotic administration |
BPA = best-practice advisory; DRG = diagnosis-related group; ED = emergency department; EWS = Early warning system; ICU = intensive care unit; MET = medical emergency team.
Study Selection and Outcome of Interest
We included cohort studies (retrospective or prospective) and randomized controlled trials or other randomized initiatives. To be considered further for inclusion, studies focused on sepsis and time to antibiotics must have clearly defined an active, intentional role for the pharmacist in the sepsis response. This required the attention of a unique, individual pharmacist rather than an operational change to how antibiotics were ordered or stocked in the hospital. We excluded conference abstracts, reviews, and editorials. Two authors (PEA and AHF) independently screened articles at the title and abstract level with full text review, if necessary. Any discrepancies were resolved through detailed discussion with consultation from a third author (MLTB), if necessary.
Data Extraction, Outcome Definitions, and Quality Assessment
Two authors (PEA and AHF) independently extracted identified data elements from included studies. The following data points were extracted from each included article: study author, publication year, study design, time period study was conducted, patient population, bundle elements of the intervention group, specific role of the pharmacist as described, and definitions of the comparator group. If pertinent data were not reported, corresponding authors of published manuscripts were contacted via e-mail with requests for additional information, if available.
The primary outcome was time to antibiotic administration as defined by each study. Due to variations in the quantitative reporting of this outcome (mean vs. median vs. proportion), a meta-analysis was not performed. Secondary outcomes included intensive care unit (ICU) length of stay, hospital length of stay, and in-hospital mortality. Any additional findings deemed notable by the data extractors regarding the pharmacist response were recorded as well during data extraction.
Two authors (MLTB and MEL) independently performed a quality assessment for each study. Discrepancies were resolved via consultation with a third reviewer (AHF). Given the types of study designs anticipated for this research question, we used the Newcastle-Ottawa Scale for observational cohort studies.11 This scale assesses eight different components of study design among three main categories: selection of patients, comparability of cohorts, and outcome assessment. Points were totaled for each study and studies scoring 7–9 were assessed as low risk of bias, those scoring 4–6 assessed as high risk of bias, and those scoring 0–3 as very high risk of bias.
RESULTS
Studies Included
After removal of duplicates, the search strategy yielded 2107 citations from the databases searched. Following the application of inclusion and exclusion criteria, 9 studies were retained for inclusion. An additional study was identified from the review of bibliographies of these previously retained studies for a total of 10 studies included in this systematic review (Figure 1).12–21 These 10 studies include 1772 patients with sepsis or septic shock, were conducted during 2006–2019, and published during the time period of 2008–2022.
Figure 1.
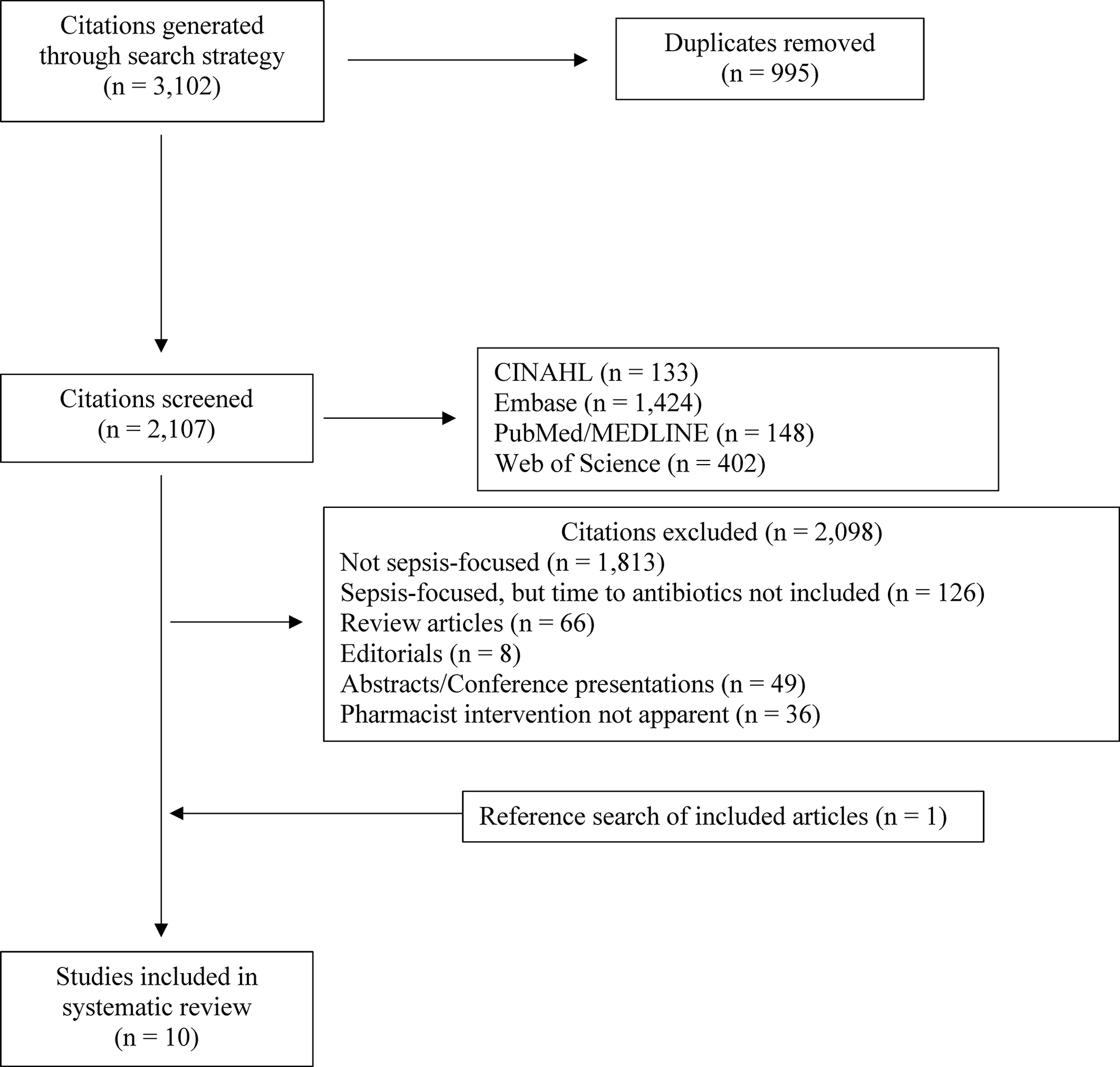
Study inclusion and exclusion. CINAHL = Cumulative Index to Nursing and Allied Health Literature.
Study Characteristics
An overview of included studies is presented in Table 1. Six studies were retrospective, observational cohorts,12, 14, 15, 17–19 three studies were prospective, observational cohorts,13, 16, 21 and a single study was a randomized, controlled, quality improvement initiative.20 All of the studies occurred in hospitalized, adult patients. Eight of the identified studies were conducted in academic medical centers12–16, 18–20 while two were performed at community hospitals.17, 21 Three studies evaluated septic patients in the emergency department,18, 20, 21 three studies were performed in a single intensive care unit,13, 16, 17 two studies were performed in non-critically ill patients,12, 19 and two studies were not restricted by location in the hospital.14, 15 The quality assessment for each study is shown in Supporting Information, Table 2. Two studies were assessed as low risk of bias (score of 7–9),16, 20 while the remaining eight studies were assessed as high risk of bias (score of 4–6).12–15, 17–19, 21 No studies were assessed as very high risk of bias (score of 0–3).
Table 2.
Study Outcomes
| Reference | Definition of Time to Antibiotics | Time to Antibiotics | ICU Length of Stay | Hospital Length of Stay | In-hospital Mortality | Other Notable Findings |
|---|---|---|---|---|---|---|
| Sarani et al. 200819 | Time from antibiotic order to first dose of antibiotic administration | Median of 54 minutes (IQR, 40–91 minutes) in MET group vs. 157 minutes (IQR, 113–258 minutes) in non-MET group; p<0.01 | NR | NR | NR | Non-MET group without pharmacist involvement: 15 minutes median time between CPOE and order verification, 82 minutes between order verification and delivery to nursing, and 60 minutes between drug delivery to administration |
| LaRosa et al. 201216 | Time to administration determined by binary assessment of compliance: antibiotics given within 3 hours of ED triage time and within 1 hour for non-ED admission | Antibiotic administration meeting time-based compliance: 91% in sepsis alert group vs. 54% in control; p<0.001 | NR | NR | 3/34 (9%) in sepsis alert group vs. 7/24 (29%) in control; p<0.05 | None |
| Flynn et al. 201414 | Time from sepsis recognition to antibiotic administration (pathogen susceptible) | Median of 0.65 hours in intervention vs. 2.4 hours in control | Median of 8 days (IQR 3–15 days) in intervention vs. 8 days (IQR 2–17 days) in control; p=0.83 | Median of 15 days (IQR 8–44 days) in intervention vs. 12 days (IQR 7–24 days) in control; p=0.14 | 48.9% intervention vs. 54.2% in control; p=0.586 | 77.5% of patients received appropriate antibiotics within 1 hour of sepsis recognition in the intervention group vs. 23.7% in the control group. |
| Beardsley et al. 201612 | Time from rapid response nurse arrival to the bedside and first dose of antibiotic administered | Mean of 53 minutes in Code Sepsis group vs. 396 minutes in historical group | NR | NR | NR | 1) Initiative associated with reduction in institution’s adjusted mortality index for sepsis by 16% 2) Pharmacists ordered antibiotics in 28% of Code Sepsis episodes in which antibiotics were ordered 3) Upon further roll out, mean time from Code Sepsis page to antibiotic delivery (including ordering and pharmacist verification) was 14.1 ± 13.7 minutes |
| Moussavi et al. 201618 | Time from antibiotic order to first documented dose of antibiotic administration | Median 0.61 hours (IQR, 0.37–0.95 hours) in pharmacist group vs. 0.88 hours (IQR, 0.525–1.23) in non-pharmacist group; p=0.001 | Median of 4 days (IQR, 2–11 days) in pharmacist group vs. 3.5 days (IQR, 2–8 days) in non-pharmacist group; p=0.37 | Median of 10.5 days (IQR, 5–16.8 days) in pharmacist group vs. 8.5 days (IQR, 5–15 days) in non-pharmacist group; p=0.437 | 19/92 (21%) in pharmacist group vs. 23/94 (24%) in non-pharmacist group; p=0.534 | 1) ED pharmacist presence improved proportion of patients receiving antibiotics within 3 hours: 100% vs. 95%; p=0.025 2) ED pharmacist presence improved proportion of patients receiving appropriate initial antibiotics: 97% vs. 81%; p=0.0008 |
| Laine et al. 201815 | Time from sepsis bundle order activation to first dose of documented antimicrobial | Median time to first dose of antibiotics was 43 minutes (no comparator group but control group from Flynn et al14 could be extrapolated) | Median of 11 days in pharmacist responder group (no comparator group) | Median of 18 days in pharmacist responder group (no comparator group) | 40/76 (53%) in pharmacist responder group (no comparator group) | 1) 66% of patients had antibiotics ordered at the time of sepsis bundle activation that covered the eventual pathogen identified; following pharmacist intervention this was increased to 80% (p=0.04) 2) Common documented interventions by pharmacist included changing original antimicrobial, adding double coverage for gram-negative pathogens, and adding empiric antifungals |
| Chanas et al. 201913 | Time from best practice alert trigger to administration of first new antibiotic | Median 4.2 hours in BPA group vs. 7.4 hours pre-BPA implementation; p=0.057) | Median of 5 days (IQR, 2–12 days) (no comparator group) | Median of 15 days (IQR, 8–32 days) (no comparator group) | 24/97 (25%) (no comparator group) | 1) Time to antibiotics significantly shorter when pharmacist responded to the bedside: 1.2 vs. 4.2 hours; p=0.046 2) When a pharmacist responded to the bedside, more patients had antibiotics started within 1 hour (36% vs. 14%; p=0.021) and 3 hours (60% vs. 34%; p=0.031) 3) Pharmacists responded to 67% of alerts, which could represent staffing limitations |
| MacMillan et al. 201917 | Time to administration defined per the 2012 Surviving Sepsis Campaign Guidelines | Antibiotic administration within 3 hours: 59.1% post-quality improvement initiative vs. 51.4% before; p=0.342 | Mean 5.6 ± 5.3 days post-quality improvement initiative vs. 5.9 ± 7.6 days before; p=0.808 | Mean 9.9 ± 8.0 days post-quality improvement initiative vs. 10.0 ± 10.3 days before; p=0.121 | 24/88 (27.3%) post-quality improvement initiative vs. 23/72 (31.9%) before; p=0.518 | None |
| Yarbrough et al. 201921 | Time to administration defined per SEP-1 criteria | Median of 46 minutes (IQR, 31–61.5 minutes) in pharmacist responder group vs. 68 minutes (IQR, 49–96 minutes) in the comparator group; p=0.009 | NR | NR | NR | Pharmacist response was associated with greater proportion of patients receiving antibiotics within 1 hour: 73% vs. 33%; p=0.002 |
| Tarabichi et al. 202220 | Time to first dose of antibiotics from arrival in ED | Median of 2.3 hours (IQR, 1.4–4.7 hours) in intervention vs. 3.0 hours (IQR, 1.6–5.5 hours) in control; p=0.039 | Median of 3.6 days (IQR, 2.0–5.4 days) in intervention vs. 3.4 days (IQR, 2.0–6.0 days) in control; p=0.937 | Median of 3.2 days (IQR, 1.1–6.2 days) in intervention vs. 4.0 days (1.4–7.0 days) in control; p=0.124 | 13/285 (4.6%) in intervention vs. 25/313 (8.0%) in control; p=0.086 | 1) Patients with intervention had greater days alive and out of hospital vs. control: median 24.1 vs. 22.5; p=0.011 2) Patients with intervention demonstrated shorter median time from alert to antibiotic ordering of 0.6 hours (IQR, 0.0–2.6 hours) vs. 1.4 hours (IQR, 0.2–3.9 hours; p=0.043) and shorter median time from antibiotic order placement to administration of 0.4 hours (IQR, 0.2–0.9 hours) vs 0.7 hours (IQR, 0.3–1.4 hours; p=0.001) 3) Randomized nature ultimately ceased to allow pharmacist notification as new standard of care |
BPA = best-practice advisory; CPOE = computerized physician order entry; ED = emergency department; IQR = interquartile range; MET = medical emergency team; NR = not reported; SEP-1 = Centers for Medicare and Medicaid Services Severe Sepsis/Septic Shock.
Pharmacists were specifically identified as key participants in all included studies per the inclusion criteria of the systematic review. Eight studies specifically noted a pharmacist responding to the bedside and interacting with other members of the health care team to consult and facilitate antibiotic order placement and verification,13–19, 21 one study described pharmacists “huddling” with providers when notified of a sepsis notification,20 and one study described pharmacists calling the nursing unit if no antibiotics were placed after 15 minutes of a sepsis alert and facilitating antibiotic ordering if needed, including a protocol which allowed for the pharmacist to select antibiotics if the provider was engaged in other aspects of care.12 Six studies compared a sepsis bundle response or sepsis quality improvement initiative which included a pharmacist in some capacity, with the comparator group representing prior or concurrent care of sepsis patients without the bundle or quality improvement initiative (e.g., historical control).12–14, 16, 17, 19 Two studies more directly compared the presence or involvement of a pharmacist as the sole intervention (the control group was theoretically similar care only without a pharmacist present),18, 21 while pharmacist involvement was one of two defined interventions (in addition to an icon change on a status board in the emergency department) in one study.20 A single study included no comparator group.15 Complete details of pharmacist intervention and comparator groups are provided in Table 1.
The definition of “time to antibiotics” was defined by each study and represented the time interval between a specific index event and documented antibiotic administration. The index events which initiated the antibiotic timing measurement were a combination of: time of patient arrival with sepsis (4 studies),16, 17, 20, 21 time of sepsis response triggered (4 studies),12–15 or time of antibiotic order placement (2 studies).18, 19 Eight studies reported the time to antibiotics as a continuous measurement (mean or median)12–15, 18–21 and two studies as a proportion meeting a defined timing threshold.16, 17 Complete information for this measurement and study outcomes are provided in Table 2.
Time to Antibiotics
Seven of the ten studies included in our review reported a significant reduction in time to antibiotics with sepsis response teams or protocols which included a pharmacist with defined involvement in sepsis resuscitation.12, 14, 16, 18–21 One study did not find a difference in any outcomes assessed, and considered variable education and use of the alert for all participating groups as a potential explanatory factor.17 In a study by Chanas and colleagues, a reduction in time to antibiotics was not observed; however, pharmacists only responded to 67% of sepsis notifications.13 In a secondary analysis, the authors reported when a pharmacist responded and significantly more patients received antibiotics within 1 and 3 hours of the sepsis notification compared with sepsis alerts without a pharmacist present (see Table 2 for further details), providing support for the pharmacist’s role in particular rather than the sepsis notification system as a whole.13 A study by Laine and colleagues did not include a comparator group to assess a baseline time to antibiotics.15 This was a follow-up study (years evaluated: 2012–2014) of a different cohort of patients than described by Flynn and colleagues in the same institution (years evaluated: 2008–2011).14 While no control group was included in Laine and colleagues, the median time to first dose of antibiotics was 43 minutes, which is a numeric reduction when compared with the control group from Flynn and colleagues (2.4 hours) at the same institution.14, 15
In addition to improving the time to administration, two studies reported the pharmacist intervention was associated with improved selection of antimicrobial therapy.15, 18 In Moussavi and colleagues, pharmacist interventions were associated with an improved proportion of patients receiving appropriate initial antibiotics in sepsis per guidelines (97% vs. 81%; p=0.0008).18 Laine and colleagues showed that at the time of sepsis bundle activation, the antibiotics ordered would have covered the eventual pathogen isolated 66% of the time. However, after documented pharmacist intervention, this was increased to 80% (p=0.04).15
Secondary Outcomes
Of the 10 included studies, 6 reported on ICU and hospital length of stay.13–15, 17, 18, 20 Of those 6 studies, 4 included a comparator group for ICU and hospital length of stay. There were no significant differences reported in ICU or hospital length of stay in any of these four studies.14, 17, 18, 20 Of the 10 included studies, 7 reported on in-hospital mortality.13–18, 20 Of those 7 studies, 5 included a comparator group for in-hospital mortality. Of these 5 studies, one study found an association with reduced in-hospital mortality16 while 4 other studies did not.14, 17, 18, 20 Of note, with a slightly different measurement, Beardsley and colleagues noted a reduction in their institution’s adjusted mortality index for sepsis.12
DISCUSSION
Our systematic review found a majority of studies which included a pharmacist as part of sepsis response improved the time to antibiotics in hospitalized patients with sepsis or septic shock. This improvement in time to antibiotics did not necessarily carry over to other outcomes consistently, such as length of stay or in-hospital mortality, although these outcomes were not reported by all studies and the individual studies may have been underpowered to assess these clinical outcomes.
The metric of time to antibiotics in sepsis and septic shock has been widely debated for years, and readers are referred elsewhere to detailed critiques of this metric in sepsis.22, 23 Nevertheless, guidelines from the Surviving Sepsis Campaign recommend immediate antimicrobial administration for patients with possible septic shock or a high likelihood for sepsis, ideally within one hour of recognition (strong recommendation, low quality of evidence for septic shock; strong recommendation, very low quality of evidence for sepsis without shock).5 Upon specific review of data presented in Table 2, many studies were not only able to improve the time to antibiotics with inclusion of a pharmacist in the sepsis response, but also were able to meet this lofty, often difficult to obtain, one-hour metric as assessed by the mean/median time to antibiotics. Although the Surviving Sepsis Campaign guidelines includes a provision that “…application of pharmacokinetic/pharmacodynamic principles can be aided by clinical pharmacists”,5 results from this systematic review suggest pharmacists can play an even more fundamental role in sepsis response in terms of operationalizing correct and rapid antibiotic administration. Patients included in this systematic review spanned the geography of the hospital environment, and pharmacists maintain practice settings in a diversity of areas such as hospital wards, ICUs, and EDs, and are available to navigate nuances of medication use in specific environments of the hospital.
Due to their extensive medication knowledge, including specific therapeutic as well as logistic knowledge of medication ordering and dispensing in the hospital environment, pharmacists are well-equipped to help teams expedite antibiotics for these high-risk patients. This is in many ways analogous to pharmacists’ documented role in improving time to other critical therapies, such as thrombolytics in ischemic stroke and reversal agents in bleeding.24, 25 Ascertaining which specific steps in the medication use process were impacted by pharmacists is difficult to assess from the studies included, however, a few studies provide insight. For example, in a study by Beardsley and colleagues which involved pharmacists making contact when antibiotics were not ordered within 15 minutes, pharmacists ordered antibiotics in 28% of cases. This supports the notion that while other health care providers acknowledge the importance of early antibiotics, this patient population is high acuity and often has competing priorities for care (i.e., patient transport, procedures, diagnostics) that may detract from antibiotics being ordered in a timely fashion.12 This reduction in time to ordering antibiotics was similarly demonstrated by Tarabichi and colleagues,20 who also showed what other included studies reported:18, 19 pharmacists improve the time interval from antibiotic order placement to administration in sepsis. This is likely to be a combination of awareness of the urgency for antibiotics in a sepsis patient, increased attention to rapid order verification, and facilitating antibiotic product into the hands of nursing for rapid administration. Further, operational improvements in pharmacy dispensing and workflow continue to be important in improving time to antibiotics.12, 26–29 Of equal if not greater importance, pharmacists also have a documented impact on improving the appropriate selection of antimicrobials, thus optimizing the chance patients receive not only rapid, but appropriate antimicrobial coverage in this medical emergency, particularly septic shock.15, 18 Inappropriate initial antimicrobial therapy is associated with increases in mortality, particularly for bacterial infections, but supporting data for fungal infections exist as well.30–32 Pharmacists are well-equipped to assess important factors involved in this selection such as recent infectious episodes, prior culture results, risk factors for resistant organisms, and patient allergies, as well as assist with other aspects of sepsis management such as assisting the team with the assessment and initiation of fluids and vasopressors. In addition, delays in the second dose of antibiotics in patients admitted from the emergency department are an increasingly recognized issue and associated with worse clinical outcomes, including mortality.33 Although not formally evaluated in this review, pharmacists are naturally positioned to reduce or eliminate these delays in further doses during a patient’s transition of care.
This is the first systematic review of pharmacist involvement in sepsis response and association with an important metric in time to antibiotics. The review is strengthened by a comprehensive search strategy and assessment of pharmacist involvement on other related outcomes, such as appropriate antibiotic selection. There are also noted limitations important to recognize. First and foremost, only two studies evaluated the pharmacist as the sole intervention in comparison to a defined control group. In the other studies, pharmacists were included to varying degrees as part of larger interventions and sepsis response teams. Control groups were often different between studies and included historical or concurrent controls, depending on the study. Given the retrospective nature of the majority of the studies, we are unable to dissect the contribution of pharmacist participation while holding the effect of other team members as part of the sepsis response team constant. Given that early recognition is the rate-limiting step to early antibiotic administration, pharmacist interventions in sepsis care as described in this review are likely optimized when there is a coordinated sepsis response within the institution. Second, only one study included randomization while the remainder were cohort designs, often of smaller sample size, indicating residual confounding or selection bias may still be present. Most of the studies were classified as high risk of bias when formally assessed, primarily for lacking definitive assessments of comparability and follow-up. Third, time to antibiotics was defined uniquely by each study, which may influence how the findings are interpreted. In addition to this and variations in the metric reporting time to antibiotics (mean vs. median vs. proportion), we were unable to conduct a formal meta-analysis. Fourth, publication bias may be present given the relative lack of “neutral” studies observed. Lastly, the majority of studies were conducted at academic medical centers. Translating the benefit of pharmacist involvement in sepsis response to other types of hospitals and health care systems deserves further research.
CONCLUSION
Pharmacist involvement in sepsis response, often as part of a multi-professional team-based approach to sepsis care, is associated with a reduction in time to antibiotics. This reduction appears to be driven by a combination of reduction in time to ordering antibiotics, time to verifying ordered antibiotics, and facilitating antibiotic delivery to the bedside. In addition, pharmacist participation may improve the appropriate selection of antibiotics in this high-risk patient population.
Supplementary Material
Funding:
This project was supported in part by the National Institutes of Health under award K23DK128562 (PI: AHF). The funding source had no role in study design; data collection, analysis, or interpretation; writing the report; or the decision to submit the report for publication. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the NIH.
Footnotes
Conflict of Interest Statement: The authors declare no conflicts of interest.
REFERENCES
- 1.Rudd KE, Johnson SC, Agesa KM, et al. Global, regional, and national sepsis incidence and mortality, 1990–2017: analysis for the Global Burden of Disease Study. Lancet 2020;10219:200–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fleischmann-Struzek C, Mellhammar L, Rose N, et al. Incidence and mortality of hospital- and ICU-treated sepsis: results from an updated and expanded systematic review and meta-analysis. Intensive Care Med 2020;8:1552–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Liu VX, Fielding-Singh V, Greene JD, et al. The Timing of Early Antibiotics and Hospital Mortality in Sepsis. Am J Respir Crit Care Med 2017;7:856–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kumar A, Roberts D, Wood KE, et al. Duration of hypotension before initiation of effective antimicrobial therapy is the critical determinant of survival in human septic shock. Crit Care Med 2006;6:1589–96. [DOI] [PubMed] [Google Scholar]
- 5.Evans L, Rhodes A, Alhazzani W, et al. Surviving Sepsis Campaign: International Guidelines for Management of Sepsis and Septic Shock 2021. Crit Care Med 2021;11:e1063–e143. [DOI] [PubMed] [Google Scholar]
- 6.Kashiouris MG, Zemore Z, Kimball Z, et al. Supply Chain Delays in Antimicrobial Administration After the Initial Clinician Order and Mortality in Patients With Sepsis. Crit Care Med 2019;10:1388–95. [DOI] [PubMed] [Google Scholar]
- 7.Klompas M, Rhee C. Antibiotic Order-to-Infusion Time for Patients With Septic Shock: A Potential New Quality Metric. Crit Care Med 2019;10:1467–70. [DOI] [PubMed] [Google Scholar]
- 8.Atkins PE, Thompson Bastin ML, Laine ME, Flannery AH. Pharmacist Involvement in Sepsis Response and Time to Antibiotics: A Systematic Review. Inplasy protocol 202270039. doi: 10.37766/inplasy2022.7.0039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ 2021. Mar 29;372:n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Clark JM, Sanders S, Carter M, et al. Improving the translation of search strategies using the Polyglot Search Translator: a randomized controlled trial. J Med Libr Assoc 2020;2:195–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ottawa Hospital Research Institute: The Newcastle-Ottawa Scale (NOS) for Assessing the Quality of Nonrandomised Studies in Metaanalyses. Available at: https://www.ohri.ca//programs/clinical_epidemiology/oxford.asp. Accessed June 2, 2022.
- 12.Beardsley JR, Jones CM, Williamson J, Chou J, Currie-Coyoy M, Jackson T. Pharmacist involvement in a multidisciplinary initiative to reduce sepsis-related mortality. Am J Health Syst Pharm 2016;3:143–9. [DOI] [PubMed] [Google Scholar]
- 13.Chanas T, Volles D, Sawyer R, Mallow-Corbett S. Analysis of a new best-practice advisory on time to initiation of antibiotics in surgical intensive care unit patients with septic shock. J Intensive Care Soc 2019;1:34–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Flynn JD, McConeghy KW, Flannery AH, Nestor M, Branson P, Hatton KW. Utilization of Pharmacist Responders as a Component of a Multidisciplinary Sepsis Bundle. Ann Pharmacother 2014;9:1145–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Laine ME, Flynn JD, Flannery AH. Impact of Pharmacist Intervention on Selection and Timing of Appropriate Antimicrobial Therapy in Septic Shock. J Pharm Pract 2018;1:46–51. [DOI] [PubMed] [Google Scholar]
- 16.Larosa JA, Ahmad N, Feinberg M, Shah M, Dibrienza R, Studer S. The use of an early alert system to improve compliance with sepsis bundles and to assess impact on mortality. Crit Care Res Pract 2012;980369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.MacMillan A, Rudinsky D, Han G, Elliott JO, Jordan K. Multidisciplinary Approach to Improve Sepsis Outcomes. J Healthc Qual 2019;4:220–27. [DOI] [PubMed] [Google Scholar]
- 18.Moussavi K, Nikitenko V. Pharmacist impact on time to antibiotic administration in patients with sepsis in an ED. Am J Emerg Med 2016;11:2117–21. [DOI] [PubMed] [Google Scholar]
- 19.Sarani B, Brenner SR, Gabel B, et al. Improving sepsis care through systems change: the impact of a medical emergency team. Jt Comm J Qual Patient Saf 2008;3:179–82, 25. [DOI] [PubMed] [Google Scholar]
- 20.Tarabichi Y, Cheng A, Bar-Shain D, et al. Improving Timeliness of Antibiotic Administration Using a Provider and Pharmacist Facing Sepsis Early Warning System in the Emergency Department Setting: A Randomized Controlled Quality Improvement Initiative. Crit Care Med 2022;3:418–27. [DOI] [PubMed] [Google Scholar]
- 21.Yarbrough N, Bloxam M, Priano J, Louzon Lynch P, Hunt LN, Elfman J. Pharmacist impact on sepsis bundle compliance through participation on an emergency department sepsis alert team. Am J Emerg Med 2019;4:762–63. [DOI] [PubMed] [Google Scholar]
- 22.Singer M Antibiotics for Sepsis: Does Each Hour Really Count, or Is It Incestuous Amplification? Am J Respir Crit Care Med 2017;7:800–02. [DOI] [PubMed] [Google Scholar]
- 23.Weinberger J, Rhee C, Klompas M. A Critical Analysis of the Literature on Time-to-Antibiotics in Suspected Sepsis. J Infect Dis 2020;Suppl 2:S110–s18. [DOI] [PubMed] [Google Scholar]
- 24.Masic D, Hidalgo DC, Kuhrau S, Chaney W, Rech MA. Pharmacist Presence Decreases Time to Prothrombin Complex Concentrate in Emergency Department Patients with Life-Threatening Bleeding and Urgent Procedures. J Emerg Med 2019;5:620–28. [DOI] [PubMed] [Google Scholar]
- 25.Rech MA, Bennett S, Donahey E. Pharmacist Participation in Acute Ischemic Stroke Decreases Door-to-Needle Time to Recombinant Tissue Plasminogen Activator. Ann Pharmacother 2017;12:1084–89. [DOI] [PubMed] [Google Scholar]
- 26.Horng M, Brunsman AC, Smoot T, Starosta K, Smith ZR. Using lean methodology to optimize time to antibiotic administration in patients with sepsis. Am J Health Syst Pharm 2018;5 Suppl 1:S13–s23. [DOI] [PubMed] [Google Scholar]
- 27.Hunt A, Nakajima S, Hall Zimmerman L, Patel M. Impact of prospective verification of intravenous antibiotics in an ED. Am J Emerg Med 2016;12:2392–96. [DOI] [PubMed] [Google Scholar]
- 28.Lo A, Zhu JN, Richman M, Joo J, Chan P. Effect of adding piperacillin-tazobactam to automated dispensing cabinets on promptness of first-dose antibiotics in hospitalized patients. Am J Health Syst Pharm 2014;19:1663–7. [DOI] [PubMed] [Google Scholar]
- 29.Panosh N, Rew R, Sharpe M. Effect of closed-loop order processing on the time to initial antimicrobial therapy. Am J Health Syst Pharm 2012;16:1423–6. [DOI] [PubMed] [Google Scholar]
- 30.Bassetti M, Rello J, Blasi F, et al. Systematic review of the impact of appropriate versus inappropriate initial antibiotic therapy on outcomes of patients with severe bacterial infections. Int J Antimicrob Agents 2020;6:106184. [DOI] [PubMed] [Google Scholar]
- 31.Garnacho-Montero J, Díaz-Martín A, Cantón-Bulnes L, et al. Initial Antifungal Strategy Reduces Mortality in Critically Ill Patients With Candidemia: A Propensity Score-Adjusted Analysis of a Multicenter Study. Crit Care Med 2018;3:384–93. [DOI] [PubMed] [Google Scholar]
- 32.Bassetti M, Righi E, Ansaldi F, et al. A multicenter study of septic shock due to candidemia: outcomes and predictors of mortality. Intensive Care Med 2014;6:839–45. [DOI] [PubMed] [Google Scholar]
- 33.Leisman D, Huang V, Zhou Q, et al. Delayed Second Dose Antibiotics for Patients Admitted From the Emergency Department With Sepsis: Prevalence, Risk Factors, and Outcomes. Crit Care Med 2017;6:956–65. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


