Abstract
Purpose of Review
Dysphagia affects the majority of individuals with Parkinson disease (PD) and is not typically diagnosed until later in disease progression. This review will cover the current understanding of PD pathophysiology, and provides an overview of dysphagia in PD including diagnostic practices, gaps in knowledge, and future directions.
Recent Findings
Many non-motor and other motor signs of PD appear in the prodrome prior to the manifestation of hall- mark signs and diagnosis. While dysphagia often presents already in the prodrome, it is not routinely addressed in standard neurology examinations.
Summary
Dysphagia in PD can result in compromised efficiency and safety of swallowing, which significantly contributes to malnutrition and dehydration, decrease quality of life, and increase mortality. The heterogeneous clinical presentation of PD complicates diagnostic procedures which often leads to delayed treatment. Research has advanced our knowledge of mechanisms underlying PD, but dysphagia is still largely understudied, especially in the prodromal stage.
Keywords: Parkinson disease, Swallowing, Dysphagia, Pathophysiology, Diagnosis, Saliva, Non-motor, Motor
Introduction
Parkinson disease (PD) is a progressive, degenerative, multi-system disorder. Signs of disease include the traditional hallmark motor signs – bradykinesia, resting tremor, rigidity, and postural instability – as well as multiple non-motor and ‘other’ motor signs such as orthostatic hypotension, cardiac dysautonomia, gastrointestinal dysmotility, and deficits in higher order functions such as communication, cognition, and affect [1]. Impaired swallowing, or dysphagia, is a significant concern in PD, as functional swallowing is controlled by multiple somatic and autonomic sensorimotor functions and requires appropriate cognitive function. Many non-motor or other motor signs including dysphagia manifest early in the disease process, and predate the emergence of hallmark motor disturbances by decades. Dysphagia affects over 90% of people with PD, significantly impacting health outcomes by contributing to malnutrition, dehydration, and increased mortality due to aspiration, which can lead to aspiration pneumonia [2–4]. Even though aspiration pneumonia is the leading cause of death in PD [4], dysphagia is not routinely identified until it is more severe, typically in the mid-to-late stages. Despite current research advancements, understanding of the prodromal and early stages of the disease process and the mechanisms underlying dysphagia in PD remains limited. Here, we briefly review pathophysiology, current diagnostic practices, gaps in knowledge, as well as future directions for PD-associated dysphagia research and clinical practice.
Classic Pathology
PD was originally described as a movement disorder characterized by progressive loss of dopaminergic neurons in the nigrostriatal pathway and aggregation of Lewy bodies in the remaining neurons [5]. The loss of dopaminergic neurons within the substantia nigra was thought to be the primary cause for cardinal motor signs such as rigidity, tremor, and bradykinesia, although other neurotransmitter systems appear to be affected as well [6]. Research has shifted the focus from motor signs to non-motor and ‘other’ motor features of PD, revealing that PD is a multi-system disease affecting the central as well as the peripheral nervous systems [1]. Current diagnostic practices, however, depend heavily on motor symptoms only, even though many non-motor signs manifest earlier in disease progression, which causes for tremendous delays in diagnosis and treatment. In fact, motor features are estimated to appear after 50-60% of dopaminergic neurons have already been lost [7]. However, hyposmia, sleep disruptions, constipation, and depression can precede dopamine deficiency related symptoms by years and mark the prodromal stage of disease [1]. Further, fatigue, diplopia, anxiety, and dysphagia might occur during mid-stage disease, often still prior to diagnosis [1]. PD-specific dementia and cognitive dysfunction occur much later and mark the late stages in the progression of PD [1]. Most motor and non-motor signs worsen over the time course of the disease. Cognitive deficits, although often present at diagnosis, worsen in the later years of the disease and with age [8]. Diagnosis is often delayed due to the broad spectrum of non-specific signs in the prodromal and early stages and occurs during mid-to late stage of disease progression, when hallmark motor signs appear. The duration of each stage of the disease is variable as well as the order of the appearance of non-motor symptoms [1]. However, non-motor signs can appear throughout the course of the disease and often worsen over time. Figure 1 shows a timeline by which non-motor PD features are expected to manifest [1].
Figure 1.
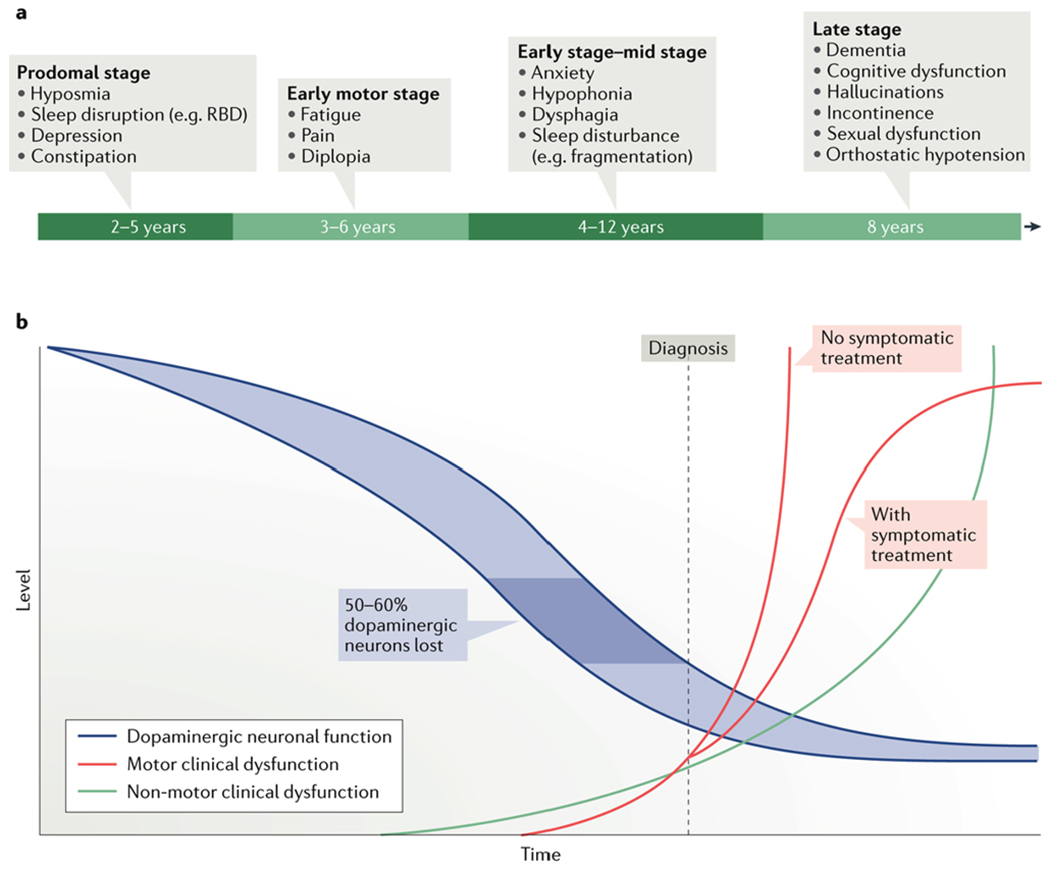
Schematic timeline of non-motor features of PD [1]
Pathophysiology
The etiology of PD is not yet fully understood, but there have been substantial advances in identifying risk factors, genetic contributions, and early pathophysiology. PD is heterogeneous in its presentation, and there may be several different subtypes of PD based on age of onset, clinical presentation, sex, genetics, and pathology [9]. The average age of diagnosis is 60–65 years [10] but diagnoses are sometimes made earlier (early onset-45 years) or later in elderly populations (late onset-75 years) [9,11]. PD can also be subtyped into cases that are tremor-dominant versus akinetic-rigid (sometimes referred to as the postural instability and gait dysfunction, PIGD, type) [12]. Further, the prevalence and wide array of non-motor signs often precede hallmark motor signs, suggesting a non-motor subtyping – those with non-motor-dominant phenotypes or those with a mixed motor and non-motor phenotype [13].
There are also sex-based differences in how PD manifests. Overall, males are twice as likely to develop PD, with earlier age of onset [14] and more severe clinical presentation compared to females [14,15]. Males and females also differ based on predominant signs of disease. Females are more likely to present with depression, constipation, and tremor-predominant phenotype [16,17], whereas males are more affected by deficits in verbal fluency, rigidity, and daytime sleepiness [16,17]. In the context of swallowing, those with PD have larger pharyngeal areas compared to healthy individuals, an indicator of pharyngeal muscle atrophy [18]. A recent study reported that this may be more pronounced in males, thereby contributing to worse swallowing function [19]. Another study reported that male sex is one predictive clinical risk factor for increased penetration and aspiration in PD [20]. However, further research is needed to confirm and analyze sex differences in PD-associated dysphagia.
Research on mechanisms underlying non-motor signs of PD have led to subtyping based on neurotransmitter systems, including cholinergic, noradrenergic, serotonergic, and mixed dopaminergic [1,9,13]. Additionally, while the vast majority of cases are still considered idiopathic, more than 20 genes have been identified in PD, including GBA, LRRK2, SNCA, PINK1, PRKN, and DJ-1 [21]. About 10% of individuals with PD carry a GBA mutation, and these carriers are more likely to have earlier symptom onset and a predominantly PIGD phenotype with non-motor signs including depression, anxiety, and cognitive impairment [22]. LRRK2, especially the G2019S mutation, is one of the most common genes linked to PD. Approximately 1% are associated with sporadic and 4% with familial PD [23]. Cases associated with LRRK2 have a slower decline in motor signs and a lack of cognitive impairment [24].
Post-mortem analyses, biopsy, and animal research suggest subdividing PD into a brain-first or body-first disorder, whereby pathology originates either first in the brain and spreads to other neural regions and periphery (brain-first), or originates in the periphery and spreads to the brain (body-first) [25]. The brain-first hypothesis posits that pathology originates in the brain prior to spreading elsewhere. In line with this directionality of spread hypothesis, some propose an “amygdala-centered” pattern, where pathology is most abundant in the center of the brain (amygdala, entorhinal cortex, and substantia nigra), compared to lower brainstem regions, spinal intermediolateral column, and neocortical regions. Borghammer and colleagues indicate that an amygdala-centered profile appears to be one of two common patterns of Lewy pathology, as evidenced by postmortem studies [25].
Another proposed pattern is a caudo-rostral pattern, which is consistent with the body-first hypothesis. The Braak staging system is in line with the body-first hypothesis [25,26] as pathology may originate outside of the brain (e.g. the gut or olfactory bulb). It also suggests that pathology propagates in a caudal-to-rostral direction, affecting lower brainstem regions before higher/cortical regions. More specifically, caudal brainstem regions such as the dorsal motor nucleus of the vagus (Braak stage 1) show Lewy body pathology earlier than the substantia nigra or cortex (Braak stage 3–6). Figure 2 illustrates the Braak’s proposed disease staging system. Interestingly, brainstem regions associated with swallow function are located in the caudal brainstem. For example, nucleus ambiguus nerve fibers provide motor innervation for swallowing, including muscles of the pharynx, upper esophagus, and intrinsic laryngeal muscles [27]. The nucleus tractus solitarius integrates sensory information from vagally-mediated peripheral regions such as the larynx, pharynx, and esophagus, and synchronizes peristaltic activity during swallowing [27]. These swallowing brainstem regions are heavily connected to caudal brainstem regions affected early in disease progression according to Braak staging. Clinical studies and animal models reveal that changes to swallowing often occur in the prodrome, prior to hallmark motor disturbances and nigrostriatal dopamine depletion [1,28–30].
Figure 2.
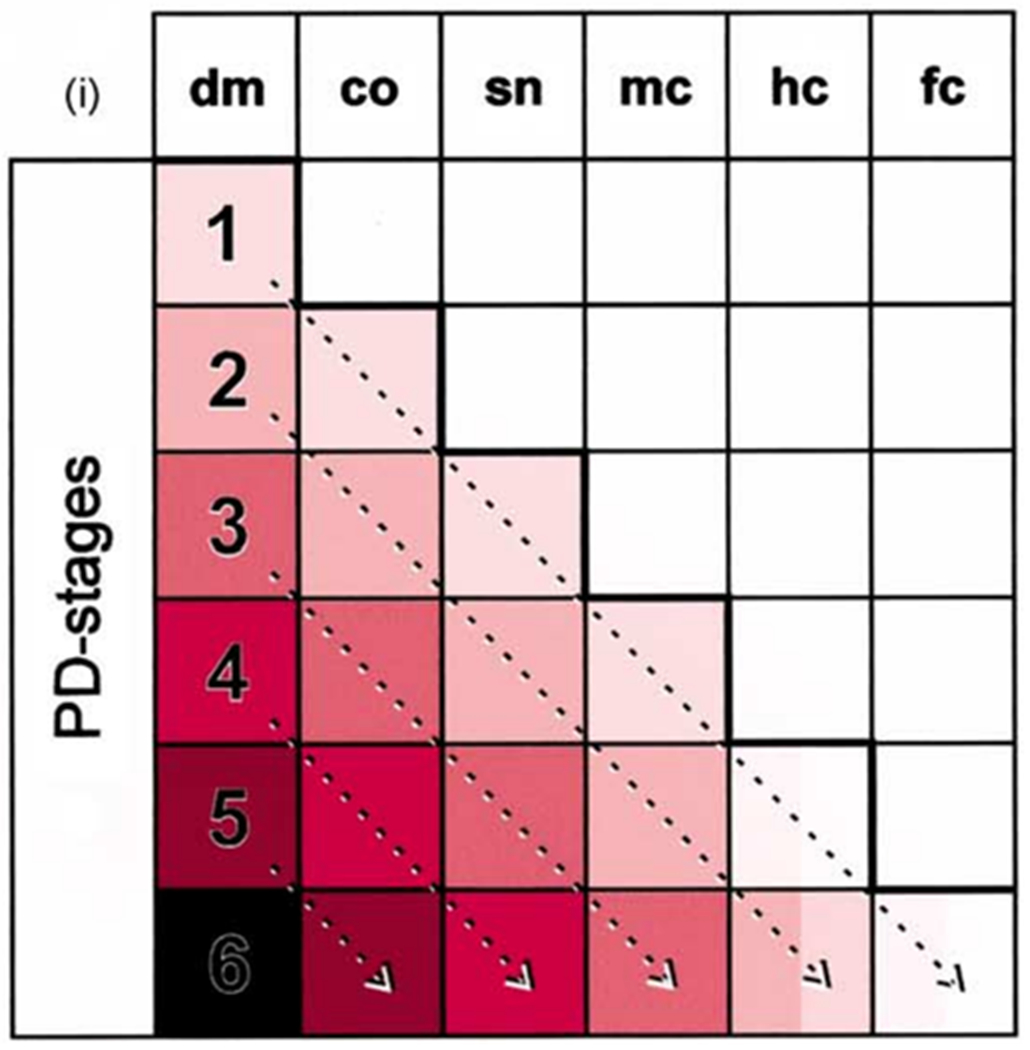
Braak’s proposed disease staging system [35]; List of abbreviations: co, coeruleus–subcoeruleus complex; dm, dorsal motor nucleus of the glossopharyngeal and vagal nerves; fc, first order sensory association areas, premotor areas, and primary sensory/motor field; hc, high order sensory association areas and prefrontal fields; mc, anteromedial temporal mesocortex; sn, substantia nigra.
Hallmark pathologic markers of PD are inclusion bodies known as Lewy Bodies or Lewy Neurites. A major component of these bodies is an aggregated form of the protein alpha-synuclein (α-syn). Abnormal α-syn aggregation is consistently seen throughout the nervous system in many different subtypes of PD. Pathologic aggregation of α-syn leads to soluble oligomeric and insoluble fibrillary forms. Although Lewy Bodies have traditionally been identified in the central nervous system, there is also peripheral involvement, including findings in the upper aerodigestive [31,32] and gastrointestinal tracts [33,34]. This supports the hypothesis that PD onset may manifest earliest, in many cases, within the periphery [34].
Although not all cases present uniformly, it is very likely that PD onset begins decades prior to diagnosis, and that dysfunction at time of diagnosis is not reflective of the full pathophysiology occurring earlier in the disease process. This is because diagnosis is heavily reliant on motor signs, response to dopamine replacement, and/or fluorodopa imaging studies [36–38]. Yet, earlier non-motor and other motor signs of disease, including dysphagia, do not respond to favorably to dopamine replacement [39,40]. Some of the earliest and most common signs of PD include hyposmia, depression, sleep disturbances, gastrointestinal dysfunction, diplopia, anxiety, hypophonia, and dysphagia [1]. Dysphagia ultimately affects over 90% of patients and is associated with reduced quality of life, increased medical costs, increased social isolation, poor patient outcomes, and complications such as malnutrition, dehydration, and mortality [3,4,41,42]. Deficits include impaired sensation and movement for the safe and efficient transport of food, liquid, and saliva from mouth to stomach. Compromised swallow function often results in residue and airway compromise, which increases risk for aspiration pneumonia – the leading cause of death in PD [4,43,44]. However, the pathophysiology underlying swallowing dysfunction in PD is poorly understood. Current understanding points to dopaminergic and non-dopaminergic mechanisms being impaired in PD-related swallow dysfunction, with noradrenergic and serotonergic systems being implicated sooner [1].
Dysphagia in Parkinson disease
Dysphagia is prevalent in PD, estimated in 40-80% of individuals worldwide – depending on the type of diagnostic procedure [44], and can occur across all stages of disease [45]. Even though dysphagia typically worsens over time and becomes more apparent in later stages, early signs can occur in the prodromal and early stages [1]. However, dysphagia typically remains undiagnosed until later in disease progression. The hallmark features of dysphagia in PD include festinated/repetitive tongue movement (also termed tongue pumping) [46,47], reduced mastication speed and coordination [48], significantly prolonged oropharyngeal transit time [46,49], pharyngeal ‘spillage’ [49], and delayed swallow initiation [50]. Additionally, studies report an increased number of swallows to clear the pharynx of residue [46], slowed hyolaryngeal movements [51], and esophageal dysmotility [52,53]. Due to deficits in proprioception across sensorimotor functions [54,55], many individuals with PD are unaware of their swallowing issues [4,56]. Further, reduced laryngeal sensation can contribute to silent aspiration [46,53] and aspiration pneumonia, which is a major cause of hospitalization in patients with PD [57,58]. Deficits in airway protection are also very commonly evidenced by decreased expiratory cough airflow rates [59–61], decreased cough reflex [56,60,61], airway sensory deficits [54,55], and upper airway obstruction [62]. Additionally, esophageal abnormalities, as well as achalasia [63] and reflux [64] are commonly seen in this population.
The consequences of impaired safety and efficiency of swallowing in PD significantly and adversely affect quality of life and can lead to economic burden. Weight loss, malnutrition, and dehydration, as well as an increased risk for aspiration contribute to increased mortality rates [65]. However, we currently do not fully understand how the incidence and prevalence of aspiration pneumonia contributes to morbidity and mortality. Müller and colleagues reported an estimated mean survival time of 24 months after onset of dysphagia symptoms [66]. Additionally, individuals with PD often experience salivary changes during disease progression, described below, which can impact swallowing function, overall health, and quality of life.
Saliva
Salivation plays a key role in oral health and swallowing physiology, including: (1) moistening and formation of the bolus [67], (2) coating and lubrication of dental and mucosal surfaces [68–71], and (3) clearance of food debris from the oral cavity [72,73]. The major structures associated with salivary secretion are the paired parotid, sublingual, and submandibular glands [74]. These glands produce more than 90% of the salivary volume [75], and produce varying volumes and compositions of saliva depending on age [76], medications [77], and gland size [78].
About 50% of individuals with PD experience hyposalivation [79,80] and xerostomia [79], and 50% experience sialorrhea [44,81]. These two phenomena may appear contradictory because drooling may suggest excessive production of saliva. However, PD largely reduces salivary flow [82]. This reduction in flow may result from the high density of α-syn aggregation within salivary glands [83]. Excessive drooling in PD does not correspond with hypersalivation, but rather unsuccessful clearance of saliva from the oral cavity, such as reduced spontaneous swallow frequency [84,85]. In fact, individuals with PD may spontaneously swallow 39% less frequently than younger healthy controls [86]. However, a reduction in swallow frequency alone does not capture the complexity of salivary dysfunction, given that individuals with PD who complain of drooling swallow significantly more often than those with no complaints of drooling [87]. Instead, the primary predictor of drooling is facial and oropharyngeal hypokinesia. Therefore, an increase in swallowing frequency may be a compensatory response to reduce drooling and improve swallow efficiency [87]. Additionally, drooling may have a cognitive component, as individuals with PD swallow less frequently and drool more often when cognitively distracted [88].
In addition to flow, salivary composition is also altered. Individuals with PD have salivary microbiota profiles distinct from healthy controls, suggesting that oral dysbiosis may precede a decline in oral health in PD [89]. These early changes to the oral microbiota may predispose individuals to dental caries and inflammation, thereby contributing to the oral health issues experienced by individuals with PD [79]. Further, oral dysbiosis in PD may be linked to the dysbiosis in the gut [90], and could also increase the risk for respiratory infection in those patients who aspirate due to oropharyngeal dysphagia [91].
Diagnosis and Disease Monitoring in PD
Diagnosis of PD
The heterogeneous clinical presentation of PD complicates the diagnostic procedure. Diagnosis is primarily based on the presence of hallmark motor signs (i.e. resting tremor, bradykinesia, muscle rigidity, postural instability), response to dopamine replacement [92], and brain imaging [38]. However, a definitive diagnosis can typically only be verified postmortem. As aforementioned, many non-motor and other motor features of PD, including swallowing dysfunction, manifest in the early stages and often remain undiagnosed until later in the disease process [1,28,64].
Disease Severity Rating Scales
Clinical differentiation among disease stages is difficult and various scales are used to evaluate disease progression and severity during the diagnostic process. The Unified Parkinson Disease Rating Scale (UPDRS) is the most common tool used to measure disease severity in PD. It consists of three subsections with multiple individually scored signs using a scale from 0 to 4 with the lowest number representing no dysfunction and the highest number representing severe dysfunction. The scale measures 1) mentation, behavior, and mood, 2) activities of daily living, and 3) motor control [93,94]. Unfortunately, the UPDRS has only one question focused on swallowing and one focused on salivation, which does not represent the full complexity or severity of dysphagia in PD. The Hoehn and Yahr Scale (H&Y) is another measure of disease severity based on the UPDRS to assess motor deficits in PD [95]. Five stages of injury and disability are defined by this scale as five stages of disease progression: from no signs of disease to wheelchair bound or bedridden, unless aided [96]. Because swallowing dysfunction appears early in the disease process prior to time of diagnosis, this scale also does not fully reflect the severity and complexity of swallowing deficits relative to staging. Early Braak Stages (1-2) encompass pathology prior to nigrostriatal dopamine depletion; however, this framework does not account for earlier peripheral involvement such as α-syn aggregation in muscles and organs [1,97–100]. Further, Braak staging is not used clinically and is more often reported in research. Although dysphagia generally worsens with disease progression, this is not linear nor is it often associated with a particular disease stage due to the inherent heterogeneous presentation of PD and the lack of clinical tools used in neurology that include robust measures of swallowing function across stages.
Diagnosis of Dysphagia in PD
Dysphagia is not routinely addressed in standard neurology consultations despite the fact that dysphagia may present as one of the first signs of PD [101,102]. However, only 20–40% of patients are aware of their swallowing issues, in part because laryngeal sensation is impaired [4,56], contributing to silent aspiration in this population. Deficits in proprioception across most sensorimotor functions are a major feature of PD [54,55]. Therefore, a comprehensive approach to diagnosis is paramount to identify PD-associated swallowing changes in the early stages of disease [64].
Clinical Screening and Evaluation
Standardized self-report questionnaires are helpful in the diagnosis of swallowing dysfunction in PD and can serve as an initial screening for swallowing impairment. Of all available questionnaires, the dysphagia-specific Swallowing Quality of Life Questionnaire (SWAL-QOL) is the most commonly used to assess the impact of dysphagia on quality of life within PD [103]. The SWAL-QOL has also been a useful tool in research and clinical trials [3,104–106]. Additionally, the Sydney Swallow Questionnaire, a 17-item visual-analog scale self-report questionnaire used to evaluate several areas of an individual’s swallowing performance, is often used clinically and in research [107] .
Clinical examinations of swallowing using trials with solid and liquid boluses are also paramount for diagnosis of dysphagia in PD [4]. Protocols such as the ‘standard water swallow test’ can screen for signs of penetration or aspiration [4]; however, instrumented assessments are required to specify the nature and degree of impairment in the general diagnosis of dysphagia in this population [56, 108,109].
Instrumented Evaluations
In contrast to clinical screens and evaluations, instrumented assessments allow for visualization of structures in the oropharynx and yield functional measurement of swallowing physiology impairments. With instrumented tools, swallowing dysfunction can be detected in more than 50% of asymptomatic PD patients [50]. The two most commonly used tools to evaluate oropharyngeal dysphagia are the Videofluoroscopic Swallow Study (VFSS) [4] and Fiberoptic Endoscopic Evaluation of Swallowing (FEES).
VFSS allows for examination of the swallow using video x-ray technology to observe the oral, pharyngeal, and esophageal stages of the swallow, including bolus passage through the upper esophageal sphincter [110–112]. Additionally, the entire esophagus can be visualized during VFSS to assess esophageal dysfunction such as achalasia [63]. This procedure is usually performed in radiology (although mobile units can be used at bedside), and involves small amounts of radiation [112]. Lateral and anterior/posterior views allow for identification of anatomic and physiologic impairments related to swallowing. Individuals with PD often develop oropharyngeal swallow deficits (see Table 1), that contribute to issues with bolus clearance [50], and airway compromise/aspiration [46,53].
Table 1.
Typical findings from a VFSS in PD.
| Finding | Selection of Articles |
|---|---|
| Repetitive tongue elevations (pumping) | [46,47] |
| Reduced mastication speed and coordination | [48] |
| Prolonged oropharyngeal transit time | [46,49] |
| Pharyngeal spillage | [49,50] |
| Delayed swallow initiation | [50] |
| Increased number of swallows to clear pharynx | [46] |
| Slowed hyolaryngeal movement | [51] |
| Esophageal dysmotility | [52,53] |
FEES uses a flexible endoscope that is inserted into the nasal passage and passes through the hypopharynx, allowing visualization of pharyngeal and laryngeal structures [113]. Swallow safety (penetration/aspiration/clearance after cough) and efficiency (pharyngeal residue), as well as the anatomy and physiology of the velopharynx, posterior pharyngeal wall, epiglottis, vocal folds, pyriform sinuses, and the tongue base, can be assessed. Further, secretions and respiration can be evaluated, and sensory testing can be performed. FEES is arguably more sensitive especially for penetration and aspiration than VFSS [114,115]. Table 2 shows major findings of FEES in individuals with PD.
Table 2.
Typical findings of FEES in PD.
High-Resolution Manometry (HRM) is useful for assessing pressure and timing events in the pharynx and esophagus [124]. A catheter with circumferential pressure sensors is passed through the nasal cavity into the esophagus. Spatio-temporal pressure measurements along the aerodigestive pathway provide quantitative descriptions of pressure and timing differentials during a swallow. HRM is a useful tool in identifying subclinical swallowing impairment, especially in the early stages of PD [4]. A multimodal approach using HRM in combination with the Sydney Swallow Questionnaire, has shown to be a robust method for the identification of even subtle sub-clinical changes in swallowing function and could identify individuals with early-stage PD with the highest accuracy compared to VFSS findings [28]. Further, intraluminal impedance can detect speed and direction of bolus movements [125] and is, in combination with pressure measurements, an effective assessment tool for motility and flow luminal content [126]. A combination of manometry and impedance has been used to evaluate esophageal motility disorders [127] and for the assessment of pharyngeal swallow [127]. Overall, impedance is a highly reliable tool when combined with HRM pressure/timing measurements and can assess pharyngeal residue [128].
Deficits in airway protection often result in worsening of health outcomes impacting quality of life; therefore, cough function assessment, including thorough pulmonary and airway examination, is recommended for PD-associated dysphagia [129]. Table 3 shows major findings of airway examinations in individuals with PD.
Table 3.
Typical findings of airway examinations in PD.
For a detailed analysis of disturbances in the oral, pharyngeal, and esophageal phases of swallowing in PD, a combination of tools are often used [4], including thorough airway and cough examination. Frequent oral phase findings in PD-associated dysphagia are repetitive tongue pumping, oral residue, premature spillage, and piecemeal deglutition [131]. Findings in the pharyngeal stage are residue in vallecula and pyriform sinuses, penetration, aspiration, somatosensory deficits, and reduced swallow rate in spontaneous swallowing [132]. In the esophageal phase, PD patients often present with esophageal hypomobility, spasms, and contractions [133].
Existing Gaps in Knowledge and Future Directions
A challenge in the diagnosis of PD is the considerable overlap in disease presentation with other similarly presenting conditions, such as essential tremor, dementia with Lewy bodies, and Huntington’s, to name a few [134]. Additionally, disambiguating the normal aging process from PD-related changes in swallowing function is difficult [135]. Efforts have been made to improve differential diagnosis of PD with the use of biomarkers such as detection of α-syn in cerebral spinal fluid, blood, and saliva [136,137]. While currently there is no established laboratory test or single biomarker that provides adequate sensitivity and specificity to identify PD, particularly in the early stages, these methods show promise for future clinical utility.
Lastly, gaps in knowledge remain concerning potential differences in clinical presentation between sexes in terms of phenotype, age of onset, and disease progression, especially with regard to dysphagia. This minimizes the generalizability of research on standard diagnostic procedures that may be biased toward male subjects. Future basic and translational research in the prodromal stage, with particular attention to both sexes, is necessary to collectively advance diagnosis and treatment options of dysphagia in PD.
Conclusion
Parkinson disease is a complex progressive condition involving the central and peripheral nervous systems. Often, individuals with PD present with motor and non-motor signs besides the hallmark motor deficits which are the primary target of clinical diagnosis. Further, the heterogeneous clinical presentation of PD often complicates diagnosis. Changes in swallow function can occur in the prodromal stage and often present as one of the first signs; however, dysphagia is typically not addressed during initial clinical diagnostic practices, which can delay prevention and treatment of swallowing impairments. Early comprehensive swallow assessments that combine functional assessments, patient reported outcomes/questionnaires, and instrumented evaluations are highly recommended to asses PD-related swallowing changes – especially in the early stages of disease. Early monitoring, assessment, and treatment may help combat the devastating sequela of untreated dysphagia and broaden treatment options. Since biological sex may play a role in PD phenotype, disease progression, and age of onset, diagnostic practices might require re-evaluation and refinement in order to address these potential differences. Unfortunately, only few studies have investigated sex differences and more research is needed to address this gap in knowledge. The second article in this set – “Dysphagia in Parkinson disease: Part II – Treatment Practices and Contributions from Animal Research,” addresses current treatments for PD and PD-associated dysphagia, and discusses insights from animal research.
Funding
This work was supported by the National Institute on Deaf- ness and other Communication Disorders of the National Institutes of Health, United States: R01DC018584 (Ciucci); R01DC014358 (Ciucci); 1K76AG068590 (Rogus-Pulia); T32DC009401 (Krasko), the University of Wisconsin-Madison, and the William S. Middleton Veteran Affairs Hospital in Madison, WI.
Footnotes
Conflict of Interest Dr. Michelle R. Ciucci is on the board of directors of the National Foundation of Swallowing Disorders (NFOSD) and receives no compensation as member of the board of directors.
Statements on the Welfare of Animals This article does not contain any studies with human participants or animals performed by any of the authors.
Disclaimer The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
References
- 1.Schapira AHV, Chaudhuri KR, Jenner P. Non-motor features of Parkinson disease. Nat Rev Neurosci. 2017;18:435–50. 10.1038/NRN.2017.62. [DOI] [PubMed] [Google Scholar]
- 2.Fernandez HH, Lapane KL. Predictors of mortality among nurs- ing home residents with a diagnosis of Parkinson’s disease. Med Sci Monit. 2002;8:241–6. [PubMed] [Google Scholar]
- 3.Plowman-Prine EK, Sapienza CM, Okun MS, Pollock SL, Jacob- son C, Wu SS, Rosenbek JC. The relationship between qual- ity of life and swallowing in Parkinson’s disease. Mov Disord. 2009;24:1352–8. 10.1002/mds.22617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Suttrup I, Warnecke T. Dysphagia in Parkinson’s disease. Dys- phagia. 2016;31:24–32. 10.1007/S00455-015-9671-9/FIGURES/1. [DOI] [PubMed] [Google Scholar]
- 5.Samii A, Nutt JG, Ransom BR. Parkinson’s disease. Lancet. 2004;363:1783–93. 10.1016/S0140-6736(04)16305-8. [DOI] [PubMed] [Google Scholar]
- 6.Antony PMA, Diederich NJ, Krüger R, Balling R. The hallmarks of Parkinson’s disease. FEBS J. 2013;280:5981–93. 10.1111/FEBS.12335. [DOI] [PubMed] [Google Scholar]
- 7.Gibb WRG, Lees AJ. Anatomy, pigmentation, ventral and dor- sal subpopulations of the substantia Nigra, and differential cell death in Parkinson’s disease. J Neurol Neurosurg Psychiatry. 1991;54:388–96. 10.1136/JNNP.54.5.388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kempster PA, O’Sullivan SS, Holton JL, Revesz T, Lees AJ. Relationships between age and late progression of Parkinson’s disease: a clinico-pathological study. Brain. 2010;133:1755–62. 10.1093/BRAIN/AWQ059. [DOI] [PubMed] [Google Scholar]
- 9.Marras C, Chaudhuri KR, Titova N, Mestre TA. Therapy of Par- kinson’s disease subtypes. Neurotherapeutics. 2020;17(4):1366–77. 10.1007/S13311-020-00894-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ball N, Teo WP, Chandra S, Chapman J. Parkinson’s disease and the environment. Front Neurol. 2019;10:218. 10.3389/fneur.2019.00218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pagano G, Ferrara N, Brooks DJ, Pavese N. Age at onset and Par- kinson disease phenotype. Neurology. 2016;86:1400–7. 10.1212/WNL.0000000000002461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jankovic J, McDermott M, Carter J, Gauthier S, Goetz C, Golbe L, Huber S, Koller W, Olanow C, Shoulson I, et al. Variable expression of Parkinson’s disease: a base-line analysis of the datatop cohort. Neurology. 1990;40:1529–34. 10.1212/wnl.40.10.1529. [DOI] [PubMed] [Google Scholar]
- 13.Marras C, Chaudhuri KR. Nonmotor features of Parkinson’s dis- ease subtypes. Mov Disord. 2016;31:1095–102. 10.1002/mds.26510. [DOI] [PubMed] [Google Scholar]
- 14.Gillies GE, Pienaar IS, Vohra S, Qamhawi Z. Sex differences in Parkinson’s disease. Front Neuroendocrinol. 2014;35:370–84. 10.1016/J.YFRNE.2014.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Haaxma CA, Bloem BR, Borm GF, Oyen WJG, Leenders KL, Eshuis S, Booij J, Dluzen DE, Horstink MWIM. Gender dif- ferences in Parkinson’s disease. J Neurol Neurosurg Psychiatry. 2007;78:819–24. 10.1136/JNNP.2006.103788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Martinez-Martin P, Pecurariu CF, Odin P, van Hilten JJ, Antonini A, Rojo-Abuin JM, Borges V, Trenkwalder C, Aars- land D, Brooks DJ, et al. Gender-related differences in the bur- den of non-motor symptoms in Parkinson’s disease. J Neurol. 2012;259:1639–47. 10.1007/S00415-011-6392-3. [DOI] [PubMed] [Google Scholar]
- 17.Miller IN, Cronin-Golomb A. Gender differences in Parkinson’s disease: clinical characteristics and cognition. Mov Disord. 2010;25:2695. 10.1002/MDS.23388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Iwaki H, Blauwendraat C, Leonard HL, Makarious MB, Kim JJ, Liu G, Maple-Grødem J, Corvol JC, Pihlstrøm L, van Nimwegen M, et al. Differences in the presentation and progression of Par- kinson’s disease by sex. MovDisord. 2021;36:106–17. 10.1002/MDS.28312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang P, Wang B, Chen X, Xiong B, Xie F, Wu S, Tang Y, Chen S, Ding X, Liu P, et al. Six-year follow-up of dysphagia in patients with Parkinson’s disease. Dysphagia. 2022;37:1271–8. 10.1007/S00455-021-10387-0/TABLES/5. [DOI] [PubMed] [Google Scholar]
- 20.Nienstedt JC, Bihler M, Niessen A, Plaetke R, Pötter-Nerger M, Gerloff C, Buhmann C, Pflug C. Predictive clinical factors for pen- etration and aspiration in Parkinson’s disease. Neurogastroenterol Motil. 2019;31:e13524. 10.1111/NMO.13524. [DOI] [PubMed] [Google Scholar]
- 21.Blauwendraat C, Nalls MA, Singleton AB. The genetic archi- tecture of Parkinson’s disease. Lancet Neurol. 2020;19:170–8. 10.1016/S1474-4422(19)30287-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Swan M, Doan N, Ortega RA, Barrett M, Nichols W, Ozelius L, Soto-Valencia J, Boschung S, Deik A, Sarva H, et al. Neuropsy- chiatric characteristics of GBA-associated Parkinson disease. J Neurol Sci. 2016;370:63–9. 10.1016/j.jns.2016.08.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Healy DG, Falchi M, O’Sullivan SS, Bonifati V, Durr A, Bress- man S, Brice A, Aasly J, Zabetian CP, Goldwurm S, et al. Phe- notype, genotype, and worldwide genetic penetrance of LRRK2- associated Parkinson’s disease: a case-control study. Lancet Neurol. 2008;7:583–90. 10.1016/S1474-4422(08)70117-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Alcalay RN, Mirelman A, Saunders-Pullman R, Tang MX, Mejia Santana H, Raymond D, Roos E, Orbe-Reilly M, Gurevich T, Bar Shira A, et al. Parkinson disease phenotype in Ashkenazi Jews with and without LRRK2 G2019S mutations. Mov Disord. 2013;28:1966–71. 10.1002/mds.25647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Borghammer P, Horsager J, Andersen K, Van Den Berge N, Raunio A, Murayama S, Parkkinen L, Myllykangas L. Neuro- pathological evidence of body-first vs brain-first Lewy body dis- ease. Neurobiol Dis. 2021;161:105557. 10.1016/j.nbd.2021.105557. [DOI] [PubMed] [Google Scholar]
- 26.Braak H, Ghebremedhin E, Rüb U, Bratzke H, del Tredici K. Stages in the development of Parkinson’s disease-related pathol- ogy. Cell Tissue Res. 2004;318:121–34. 10.1007/s00441-004-0956-9. [DOI] [PubMed] [Google Scholar]
- 27.Bieger D, Neuhuber W. Neural circuits and mediators regulating swallowing in the brainstem. GI Motility Online. 2006; 1–19. 10.1038/GIMO74. [DOI] [Google Scholar]
- 28.Jones CA, Ciucci MR. Multimodal swallowing evaluation with high-resolution manometry reveals subtle swallowing changes in early and mid-stage Parkinson disease. J Parkinsons Dis. 2016;6:197–208. 10.3233/JPD-150687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sung HY, Kim J-S, Lee K-S, Kim Y-I, Song I-U, Chung S-W, Yang D-W, Cho YK, Park JM, Lee IS, et al. The prevalence and patterns of pharyngoesophageal dysmotility in patients with early stage Parkinson’s disease. Mov Disord. 2010;25:2361–8. 10.1002/mds.23290. [DOI] [PubMed] [Google Scholar]
- 30.Cullen KP, Grant LM, Kelm-Nelson CA, Brauer AFL, Bickel- haupt LB, Russell JA, Ciucci MR. Pink1 −/− rats show early- onset swallowing deficits and correlative brainstem pathol- ogy. Dysphagia. 2018;33:749–58. 10.1007/s00455-018-9896-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Braak H, del Tredici K, Rüb U, de Vos RAI, Jansen Steur ENH, Braak E. Staging of brain pathology related to sporadic Parkin- son’s disease. Neurobiol Aging. 2003;24:197–211. 10.1016/S0197-4580(02)00065-9. [DOI] [PubMed] [Google Scholar]
- 32.Mu L, Chen J, Sobotka S, Nyirenda T, Benson B, Gupta F, Sand- ers I, Adler CH, Caviness JN, Shill HA, et al. Alpha-synuclein pathology in sensory nerve terminals of the upper aerodigestive tract of Parkinson’s disease patients. Dysphagia. 2015;30:404–17. 10.1007/s00455-015-9612-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mu L, Sobotka S, Chen J, Su H, Sanders I, Adler CH, Shill HA, Caviness JN, Samanta JE, Beach TG. Alpha-synuclein pathology and axonal degeneration of the peripheral motor nerves innervat- ing pharyngeal muscles in Parkinson disease. J Neuropathol Exp Neurol. 2013;72:119–29. 10.1097/NEN.0b013e3182801cde. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shannon KM, Keshavarzian A, Mutlu E, Dodiya HB, Daian D, Jaglin JA, Kordower JH. Alpha-synuclein in colonic sub- mucosa in early untreated Parkinson’s disease. Mov Disord. 2012;27:709–15. 10.1002/mds.23838. [DOI] [PubMed] [Google Scholar]
- 35.Braak H, De Vos RAI, Bohl J, Del Tredici K. Gastric α-Synuclein immunoreactive inclusions in Meissner’s and Auerbach’s Plex- uses in cases staged for Parkinson’s disease-related brain pathol- ogy. Neurosci Lett. 2006;396:67–72. 10.1016/j.neulet.2005.11.012. [DOI] [PubMed] [Google Scholar]
- 36.Armstrong MJ, Okun MS. Diagnosis and treatment of Parkinson disease: a review. JAMA. 2020;323:548–60. 10.1001/jama.2019.22360. [DOI] [PubMed] [Google Scholar]
- 37.Postuma RB, Berg D, Stern M, Poewe W, Olanow CW, Oertel W, Obeso J, Marek K, Litvan I, Lang AE, et al. MDS clini- cal diagnostic criteria for Parkinson’s disease. Mov Disord. 2015;30:1591–601. 10.1002/mds.26424. [DOI] [PubMed] [Google Scholar]
- 38.Sioka C, Fotopoulos A, Kyritsis AP. Recent advances in PET imaging for evaluation of Parkinson’s disease. Eur J Nucl Med Mol Imaging. 2010;37:1594–603. 10.1007/s00259-009-1357-9. [DOI] [PubMed] [Google Scholar]
- 39.Del Tredici K, Braak H. Dysfunction of the locus coeruleus-nor- epinephrine system and related circuitry in Parkinson’s disease- related dementia. J Neurol Neurosurg Psychiatry. 2013;84:774–83. 10.1136/jnnp-2011-301817. [DOI] [PubMed] [Google Scholar]
- 40.Beattie DT, Smith JAM. Serotonin pharmacology in the gastroin- testinal tract: a review. Naunyn Schmiedebergs Arch Pharmacol. 2008;377:181–203. 10.1007/s00210-008-0276-9. [DOI] [PubMed] [Google Scholar]
- 41.Findley LJ. The economic impact of Parkinson’s disease. Parkin- sonism Relat Disord. 2007;13:S8–12. 10.1016/j.parkreldis.2007.06.003. [DOI] [PubMed] [Google Scholar]
- 42.Farri A, Accornero A, Burdese C. Social importance of dyspha- gia: its impact on diagnosis and therapy. Acta Otorhinolaryngol Ital. 2007;27:83–6. [PMC free article] [PubMed] [Google Scholar]
- 43.Singer RB. Mortality in patients with Parkinson’s disease treated with Dopa. J Insur Med. 1992;24:126–7. [PubMed] [Google Scholar]
- 44.Kalf JG, de Swart BJM, Bloem BR, Munneke M. Prevalence of oropharyngeal dysphagia in Parkinson’s disease: a meta-analysis. Parkinsonism Relat Disord. 2012;18:311–5. 10.1016/J.PARKRELDIS.2011.11.006. [DOI] [PubMed] [Google Scholar]
- 45.Pflug C, Bihler M, Emich K, Niessen A, Nienstedt JC, Flügel T, Koseki JC, Plaetke R, Hidding U, Gerloff C, et al. Critical dys- phagia is common in Parkinson disease and occurs even in early stages: a prospective cohort study. Dysphagia. 2018;33:41–50. 10.1007/S00455-017-9831-1. [DOI] [PubMed] [Google Scholar]
- 46.Bird MR, Woodward MC, Gibson EM, Phyland DJ, Fonda D. Asymptomatic swallowing disorders in elderly patients with Parkinson’s disease: a description of findings on clinical exami- nation and videofluoroscopy in sixteen patients. Age Ageing. 1994;23:251–4. 10.1093/AGEING/23.3.251. [DOI] [PubMed] [Google Scholar]
- 47.Argolo N, Sampaio M, Pinho P, Melo A, Nóbrega AC. Swallow- ing disorders in Parkinson’s disease: impact of lingual pumping. Int J Lang Commun Disord. 2015;50:659–64. 10.1111/1460-6984.12158. [DOI] [PubMed] [Google Scholar]
- 48.Leopold NA, Kagel MC. Prepharyngeal dysphagia in Parkinson’s disease. Dysphagia. 1996;11:14–22. 10.1007/BF00385794. [DOI] [PubMed] [Google Scholar]
- 49.Umemoto G, Tsuboi Y, Kitashima A, Furuya H, Kikuta T. Impaired food transportation in Parkinson’s disease related to lingual bradykinesia. Dysphagia. 2011;26:250–5. 10.1007/S00455-010-9296-Y. [DOI] [PubMed] [Google Scholar]
- 50.Fuh JL, Lee RC, Wang SJ, Lin CH, Wang PN, Chiang JH, Liu HC. Swallowing difficulty in Parkinson’s disease. Clin Neurol Neurosurg. 1997;99:106–12. 10.1016/S0303-8467(97)00606-9. [DOI] [PubMed] [Google Scholar]
- 51.Kim YH, Oh BM, Jung IY, Lee JC, Lee GJ, Han TR. Spati- otemporal characteristics of swallowing in Parkinson’s disease. Laryngoscope. 2015;125:389–95. 10.1002/LARY.24869. [DOI] [PubMed] [Google Scholar]
- 52.Ali GN, Wallace KL, Schwartz R, DeCarle DJ, Zagami AS, Cook IJ. Mechanisms of oral-pharyngeal dysphagia in patients with Parkinson’s disease. Gastroenterology. 1996;110:383–92. 10.1053/gast.1996.v110.pm8566584. [DOI] [PubMed] [Google Scholar]
- 53.Stroudley J, Walsh M. Radiological assessment of dysphagia in Parkinson’s disease. Br J Radiol. 1991;64:890–3. 10.1259/0007-1285-64-766-890. [DOI] [PubMed] [Google Scholar]
- 54.Hammer MJ, Murphy CA, Abrams TM. Airway somatosensory deficits and dysphagia in Parkinson’s disease. J Parkinsons Dis. 2013;3:39–44. 10.3233/JPD-120161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Clark JP, Adams SG, Dykstra AD, Moodie S, Jog M. Loudness perception and speech intensity control in Parkinson’s disease. J Commun Disord. 2014;51:1–12. 10.1016/j.jcomdis.2014.08.001. [DOI] [PubMed] [Google Scholar]
- 56.Troche MS, Brandimore AE, Okun MS, Davenport PW, Hegland KW. Decreased cough sensitivity and aspiration in Parkinson dis- ease. Chest. 2014;146:1294–9. 10.1378/CHEST.14-0066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Fujioka S, Fukae J, Ogura H, Mishima T, Yanamoto S, Higuchi MA, Umemoto G, Tsuboi Y. Hospital-based study on emergency admission of patients with Parkinson’s disease. eNeurologicalSci. 2016;4:19–21. 10.1016/J.ENSCI.2016.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Martinez-Ramirez D, Almeida L, Giugni JC, Ahmed B, Higuchi M aki, Little CS, Chapman JP, Mignacca C, Shukla AW, Hess CW, et al. Rate of aspiration pneumonia in hospitalized Parkin- son’s disease patients: a cross-sectional study. BMC Neurol. 2015; 15. 10.1186/S12883-015-0362-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Silverman EP, Carnaby G, Singletary F, Hoffman-Ruddy B, Yeager J, Sapienza C. Measurement of voluntary cough produc- tion and airway protection in Parkinson disease. Arch Phys Med Rehabil. 2016;97:413–20. 10.1016/J.APMR.2015.10.098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wheeler Hegland K, Troche MS, Brandimore AE, Davenport PW, Okun MS. Comparison of voluntary and reflex cough effec- tiveness in Parkinson’s disease. Parkinsonism Relat Disord. 2014;20:1226–30. 10.1016/J.PARKRELDIS.2014.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Silverman EP, Carnaby-Mann G, Pitts T, Davenport P, Okun MS, Sapienza C. Concordance and discriminatory power of cough measurement devices for individuals with Parkinson dis- ease. Chest. 2014;145:1089–96. 10.1378/CHEST.13-0596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Sabaté M, González I, Ruperez F, Rodríguez M. Obstructive and restrictive pulmonary dysfunctions in Parkinson’s disease. J Neurol Sci. 1996;138:114–9. 10.1016/0022-510X(96)00003-2. [DOI] [PubMed] [Google Scholar]
- 63.Schima W, Ryan JM, Harisinghani M, Schober E, Pokieser P, Denk DM, Stacher G. Radiographic detection of acha- lasia: diagnostic accuracy of videofluoroscopy. Clin Radiol. 1998;53:372–5. 10.1016/S0009-9260(98)80012-3. [DOI] [PubMed] [Google Scholar]
- 64.Broadfoot CK, Abur D, Hoffmeister JD, Stepp CE, Ciucci MR. Research-based updates in swallowing and communication dys- function in Parkinson disease: implications for evaluation and management. Perspect ASHA Spec Interest Groups. 2019;4:825. 10.1044/2019_PERS-SIG3-2019-0001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Umemoto G, Furuya H. Management of dysphagia in patients with Parkinson’s disease and related disorders. Intern Med. 2020;59:7. 10.2169/INTERNALMEDICINE.2373-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Müller J, Wenning GK, Verny M, McKee A, Chaudhuri KR, Jellinger K, Poewe W, Litvan I. Progression of dysarthria and dysphagiain postmortem-confirmed Parkinsonian disorders. Arch Neurol. 2001;58:259–64. 10.1001/ARCHNEUR.58.2.259. [DOI] [PubMed] [Google Scholar]
- 67.Mosca AC, Chen J. Food-saliva interactions: mechanisms and implications. Trends Food Sci Technol. 2017;66:125–34. 10.1016/j.tifs.2017.06.005. [DOI] [Google Scholar]
- 68.Pramanik R, Osailan SM, Challacombe SJ, Urquhart D, Proctor GB. Protein and mucin retention on oral mucosal surfaces in dry mouth patients. Eur J Oral Sci. 2010;118:245–53. 10.1111/j.1600-0722.2010.00728.x. [DOI] [PubMed] [Google Scholar]
- 69.Bongaerts JHH, Rossetti D, Stokes JR. The lubricating properties of human whole saliva. Tribol Lett. 2007;27:277–87. 10.1007/s11249-007-9232-y. [DOI] [Google Scholar]
- 70.Hahn Berg IC, Lindh L, Arnebrant T. Intraoral lubrication of PRP-1, statherin and mucin as studied by AFM. Biofouling. 2004;20:65–70. 10.1080/08927010310001639082. [DOI] [PubMed] [Google Scholar]
- 71.Xu X, He J, Xue J, Wang Y, Li K, Zhang K, Guo Q, Liu X, Zhou Y, Cheng L, et al. Oral cavity contains distinct niches with dynamic microbial communities. Environ Microbiol. 2015;17:699–710. 10.1111/1462-2920.12502. [DOI] [PubMed] [Google Scholar]
- 72.Tabak LA. In defense of the oral cavity: the protective role of the salivary secretions. Pediatr Dent. 2006;28:110–7. [PubMed] [Google Scholar]
- 73.Dawes C, Wong DTW. Role of saliva and salivary diagnostics in the advancement of oral health. J Dent Res. 2019;98:133–41. 10.1177/0022034518816961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Holsinger FC, Bui DT. Anatomy, Function, and Evaluation of the Salivary Glands. In Myers EN, Ferris RL, editors. Salivary Gland Disorders. Berlin: Springer Berlin Heidelberg; 2007. pp. 1–16 ISBN 9783540470700. [Google Scholar]
- 75.Dawes C, Wood CM. The contribution of oral minor mucous gland secretions to the volume of whole saliva in man. Arch Oral Biol. 1973;18:337–42. 10.1016/0003-9969(73)90156-8. [DOI] [PubMed] [Google Scholar]
- 76.Affoo RH, Foley N, Garrick R, Siqueira WL, Martin RE. Meta- analysis of salivary flow rates in young and older adults. J Am Geriatr Soc. 2015;63:2142–51. 10.1111/jgs.13652. [DOI] [PubMed] [Google Scholar]
- 77.Laugisch O, Holtfreter B, Pink C, Samietz S, Völzke H, Kocher T. Polypharmacy and saliva volumes in the Northeast of Ger- many – the study of health in pomerania. Community Dent Oral Epidemiol. 2022;50:139–46. 10.1111/cdoe.12644. [DOI] [PubMed] [Google Scholar]
- 78.Inoue H, Ono K, Masuda W, Morimoto Y, Tanaka T, Yokota M, Inenaga K. Gender difference in unstimulated whole saliva flow rate and salivary gland sizes. Arch Oral Biol. 2006;51:1055–60. 10.1016/j.archoralbio.2006.06.010. [DOI] [PubMed] [Google Scholar]
- 79.Barbe AG, Heinzler A, Derman S, Hellmich M, Timmermann L, Noack MJ. Hyposalivation and xerostomia among Parkin- son’s disease patients and its impact on quality of life. Oral Dis. 2017;23:464–70. 10.1111/odi.12622. [DOI] [PubMed] [Google Scholar]
- 80.Bagheri H, Damase-Michel C, Lapeyre-Mestre M, Cismondo S, O’Connell D, Senard JM, Rascol O, Montastruc JL. A study of salivary secretion in Parkinson’s disease. Clin Neurophar- macol. 1999;22:213–5. [PubMed] [Google Scholar]
- 81.Kalf JG, De Swart BJM, Borm GF, Bloem BR, Munneke M. Prevalence and definition of drooling in Parkinson’s disease: a systematic review. J Neurol. 2009;256:1391–6. 10.1007/s00415-009-5098-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Verhoeff MC, Koutris M, de Vries R, Berendse HW, Dijk KD, van Lobbezoo F. Salivation in Parkinson’s disease: a scoping review. Gerodontology. 2022. 10.1111/ger.12628. [DOI] [PubMed] [Google Scholar]
- 83.Beach TG, Adler CH, Sue LI, Vedders L, Lue LF, White CL, Akiyama H, Caviness JN, Shill HA, Sabbagh MN, et al. Multi- organ distribution of phosphorylated α-synuclein histopathol- ogy in subjects with lewy body Disorders. Acta Neuropathol. 2010;119:689–702. 10.1007/s00401-010-0664-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Isaacson J, Patel S, Torres-Yaghi Y, Pagán F. Sialorrhea in Par- kinson’s disease. Toxins (Basel). 2020; 12. 10.3390/toxins12110691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.South AR, Somers SM, Jog MS. Gum chewing improves swal- low frequency and latency in Parkinson patients: a preliminary study. Neurology. 2010;74:1198–202. 10.1212/WNL.0b013e3181d9002b. [DOI] [PubMed] [Google Scholar]
- 86.Bulmer JM, Ewers C, Drinnan MJ, Ewan VC. Evaluation of spontaneous swallow frequency in healthy people and those with, or at risk of developing, dysphagia: a review. Gerontol Geriatr Med. 2021;7:23337214211041800. 10.1177/23337214211041801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Kalf JG, Munneke M, van den Engel-Hoek L, de Swart BJ, Borm GF, Bloem BR, Zwarts MJ. Pathophysiology of diurnal drooling in Parkinson’s disease. Mov Disord. 2011;26:1670–6. 10.1002/mds.23720. [DOI] [PubMed] [Google Scholar]
- 88.Reynolds H, Miller N, Walker R. Drooling in Parkinson’s disease: evidence of a role for divided attention. Dysphagia. 2018;33:809–17. 10.1007/s00455-018-9906-7. [DOI] [PubMed] [Google Scholar]
- 89.Fleury V, Zekeridou A, Lazarevic V, Gaïa N, Giannopoulou C, Genton L, Cancela J, Girard M, Goldstein R, Bally JF, et al. Oral dysbiosis and inflammation in Parkinson’s disease. J Parkinsons Dis. 2021;11:619–31. 10.3233/JPD-202459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Jo S, Kang W, Hwang YS, Lee SH, Park KW, Kim MS, Lee H, Yoon HJ, Park YK, Chalita M, et al. Oral and gut dysbiosis leads to functional alterations in Parkinson’s disease. NPJ Parkinsons Dis. 2022;8:87. 10.1038/s41531-022-00351-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Ortega O, Sakwinska O, Combremont S, Berger B, Sauser J, Parra C, Zarcero S, Nart J, Carrión S, Clavé P. High prevalence of colonization of oral cavity by respiratory pathogens in frail older patients with oropharyngeal dysphagia. Neurogastroenterol Motil. 2015;27:1804–16. 10.1111/NMO.12690. [DOI] [PubMed] [Google Scholar]
- 92.Pfeiffer RF. Non-motor symptoms in Parkinson’s disease. Par- kinsonism Relat Disord. 2016;22:S119–22. 10.1016/J.PARKRELDIS.2015.09.004. [DOI] [PubMed] [Google Scholar]
- 93.Steffen T, Seney M. Test-retest reliability and minimal detectable change on balance and ambulation tests, the 36-item short-form health survey, and the unified Parkinson disease rating scale in people with Parkinsonism. Phys Ther. 2008;88:733–46. 10.2522/PTJ.20070214. [DOI] [PubMed] [Google Scholar]
- 94.Goetz CC. The Unified Parkinson’s Disease Rating Scale (UPDRS): status and recommendations. Mov Disord. 2003;18:738–50. 10.1002/MDS.10473. [DOI] [PubMed] [Google Scholar]
- 95.Opara JA, Małecki A, Małecka E, Socha T. Motor assessment in Parkinson`s disease. Ann Agric Environ Med. 2017;24:411–5. 10.5604/12321966.1232774. [DOI] [PubMed] [Google Scholar]
- 96.Hoehn MM, Yahr MD. Parkinsonism: onset, progression and mortality. Neurology. 1967;17:427–42. 10.1212/WNL.17.5.427. [DOI] [PubMed] [Google Scholar]
- 97.Htike TT, Mishra S, Kumar S, Padmanabhan P, Gulyás B. Periph- eral biomarkers for early detection of Alzheimer’s and Parkin- son’s diseases. Mol Neurobiol. 2019;56:2256–77. 10.1007/S12035-018-1151-4. [DOI] [PubMed] [Google Scholar]
- 98.Braak H, Müller CM, Rüb U, Ackermann H, Bratzke H, de Vos RAI, del Tredici K. Pathology associated with sporadic Parkin- son’s disease--where does it end? J Neural Transm Suppl. 2006; 89–97. 10.1007/978-3-211-45295-0_15. [DOI] [PubMed] [Google Scholar]
- 99.Braak H, Bohl JR, Müller CM, Rüb U, de Vos RAI, del Tredici K. Stanley Fahn lecture 2005: the staging procedure for the inclu- sion body pathology associated with sporadic Parkinson’s disease reconsidered. Mov Disord. 2006;21:2042–51. 10.1002/MDS.21065. [DOI] [PubMed] [Google Scholar]
- 100.Bloem BR, Okun MS, Klein C. Parkinson’s disease. Lancet. 2021;397:2284–303. 10.1016/S0140-6736(21)00218-X. [DOI] [PubMed] [Google Scholar]
- 101.Noyce AJ, Silveira-Moriyama L, Gilpin P, Ling H, Howard R, Lees AJ. Severe dysphagia as a presentation of Parkinson’s disease. Mov Disord. 2012;27:457–8. 10.1002/MDS.24006. [DOI] [PubMed] [Google Scholar]
- 102.Thomas M, Haigh RA. Dysphagia, a reversible cause not to be forgotten. Postgrad Med J. 1995;71:94. 10.1136/PGMJ.71.832.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.McHorney CA, Bricker DE, Kramer AE, Rosenbek JC, Robbins JA, Chignell KA, Logemann JA, Clarke C. The SWAL-QOL out- comes tool for oropharyngeal dysphagia in adults: I. Conceptual foundation and item development. Dysphagia. 2000;15(3):115–21. 10.1007/S004550010012. (2014). [DOI] [PubMed] [Google Scholar]
- 104.Carneiro D, das Graças Wanderley de Sales Coriolano M, Belo LR, de Marcosrabelo AR, Asano AG, Lins OG. Quality of life related to swallowing in Parkinson’s disease. Dysphagia. 2014;29:578–82. 10.1007/S00455-014-9548-3. [DOI] [PubMed] [Google Scholar]
- 105.Patel DA, Sharda R, Hovis KL, Nichols EE, Sathe N, Penson DF, Feurer ID, McPheeters ML, Vaezi MF, Francis DO. Patient- reported outcome measures in dysphagia: a systematic review of instrument development and validation. Dis Esophagus. 2017;30:1–23. 10.1093/DOTE/DOW028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Leow LP, Huckabee ML, Anderson T, Beckert L. The impact of dysphagia on quality of life in ageing and Parkinson’s disease as measured by the Swallowing Quality of Life (SWAL-QOL) questionnaire. Dysphagia. 2010;25:216–20. 10.1007/S00455-009-9245-9. [DOI] [PubMed] [Google Scholar]
- 107.Wallace KL, Middleton S, Cook IJ. Development and valida- tion of a self-report symptom inventory to assess the severity of oral-pharyngeal dysphagia. Gastroenterology. 2000;118:678–87. 10.1016/S0016-5085(00)70137-5. [DOI] [PubMed] [Google Scholar]
- 108.Lam K, Lam FKY, Kwok KL, Yiu KC, Kan EYL, Woo J, Fat KW, Ko A. Simple clinical tests may predict severe oro- pharyngeal dysphagia in Parkinson’s disease. Mov Disord. 2007;22:640–4. 10.1002/MDS.21362. [DOI] [PubMed] [Google Scholar]
- 109.Ebihara S, Saito H, Kanda A, Nakajoh M, Takahashi H, Arai H, Sasaki H. Impaired efficacy of cough in patients with Parkin- son disease. Chest. 2003;124:1009–15. 10.1378/CHEST.124.3.1009. [DOI] [PubMed] [Google Scholar]
- 110.Logemann JA, Blonsky ER, Boshes B. Dysphagia in Parkinson- ism. JAMA. 1975;231:69–70. 10.1001/JAMA.1975.03240130051031. [DOI] [PubMed] [Google Scholar]
- 111.Bushmann M, Dobmeyer SM, Leeker L, Perlmutter JS. Swallow- ing abnormalities and their response to treatment in Parkinson’s disease. Neurology. 1989;39:1309–14. 10.1212/WNL.39.10.1309. [DOI] [PubMed] [Google Scholar]
- 112.Martin-Harris B, Bonilha HS, Brodsky MB, Francis DO, Fynes MM, Martino R, O’Rourke AK, Rogus-Pulia NM, Spinazzi NA, Zarzour J. The modified barium swallow study for oropharyngeal dysphagia: recommendations from an interdisciplinary expert panel. Perspect ASHA Spec Interest Groups. 2021;6:610–9. 10.1044/2021_PERSP-20-00303. [DOI] [Google Scholar]
- 113.Fattori B, Nacci A, Farneti D, Ceravolo R, Santoro A, Bastiani L, Simoni F, Pagani R, de Bortoli N. Dysphagia in Parkinson’s disease: pharyngeal manometry and fiberoptic endoscopic evalu- ation. Auris Nasus Larynx. 2022;49:986–94. 10.1016/J.ANL.2022.03.016. [DOI] [PubMed] [Google Scholar]
- 114.Langmore SE. History of fiberoptic endoscopic evaluation of swallowing for evaluation and management of pharyngeal dys- phagia: changes over the years. Dysphagia. 2017;32(1):27–38. 10.1007/S00455-016-9775-X. [DOI] [PubMed] [Google Scholar]
- 115.Kelly AM, Drinnan MJ, Leslie P. assessing penetration and aspiration: how do videofluoroscopy and fiberoptic endo- scopic evaluation of swallowing compare? Laryngoscope. 2007;117:1723–7. 10.1097/MLG.0B013E318123EE6A. [DOI] [PubMed] [Google Scholar]
- 116.Pisegna JM, Langmore SE. Parameters of instrumental swallow- ing evaluations: describing a diagnostic dilemma. Dysphagia. 2016;31:462–72. 10.1007/S00455-016-9700-3. [DOI] [PubMed] [Google Scholar]
- 117.Fattori B, Nacci A, Farneti D, Ceravolo R, Santoro A, Bastiani L, Simoni F, Pagani R, De Bortoli N (2022) Dysphagia in Parkin- son’s disease: Pharyngeal manometry and fiberoptic endoscopic evaluation. Auris, nasus, larynx, 49(6):986–994. 10.1016/j.anl.2022.03.016 [DOI] [PubMed] [Google Scholar]
- 118.Warnecke T, Ringelstein EB, Dziewas R. Neurologische endosko- pische dysphagiediagnostik untersuchungstechnik einsatzmögli- chkeiten und typische befunde. Klinische Neurophysiologie. 2009;40:194–203. 10.1055/S-0029-1220750/ID/82. [DOI] [Google Scholar]
- 119.Leder SB, Sasaki CT, Burrell MI. Fiberoptic endoscopic evalu- ation of dysphagia to identify silent aspiration. Dysphagia. 1998;13:19–21. 10.1007/PL00009544. [DOI] [PubMed] [Google Scholar]
- 120.Leder SB. Serial fiberoptic endoscopic swallowing evalua- tions in the management of patients with dysphagia. Arch Phys Med Rehabil. 1998;79:1264–9. 10.1016/S0003-9993(98)90273-8. [DOI] [PubMed] [Google Scholar]
- 121.da Silva AP, Lubianca Neto JF, Santoro PP. Comparison between videofluoroscopy and endoscopic evaluation of swallowing for the diagnosis of dysphagia in children. Otolaryngol Head Neck Surg. 2010;143:204–9. 10.1016/J.OTOHNS.2010.03.027. [DOI] [PubMed] [Google Scholar]
- 122.Wu CH, Hsiao TY, Chen JC, Chang YC, Lee SY. Evaluation of swallowing safety with fiberoptic endoscope: comparison with videofluoroscopic technique. Laryngoscope. 1997;107:396–401. 10.1097/00005537-199703000-00023. [DOI] [PubMed] [Google Scholar]
- 123.Kelly AM, Leslie P, Beale T, Payten C, Drinnan MJ. Fibreoptic endoscopic evaluation of swallowing and videofluoroscopy: does examination type influence perception of pharyngeal residue severity? Clin Otolaryngol. 2006;31:425–32. 10.1111/J.1749-4486.2006.01292.X. [DOI] [PubMed] [Google Scholar]
- 124.Suttrup I, Suttrup J, Suntrup-Krueger S, Siemer ML, Bauer J, Hamacher C, Oelenberg S, Domagk D, Dziewas R, Warnecke T. Esophageal dysfunction in different stages of Parkinson’s disease. Neurogastroenterol Motil. 2017; 29. 10.1111/NMO.12915. [DOI] [PubMed] [Google Scholar]
- 125.Fass J, Silny J, Braun J, Heindrichs U, Dreuw B, Schumpelick V, Rau G. Measuring esophageal motility with a new intraluminal impedance. Device first clinical results in reflux patients. Scand J Gastroenterol. 1994;29:693–702. 10.3109/00365529409092496. [DOI] [PubMed] [Google Scholar]
- 126.Omari TI, Rommel N, Szczesniak MM, Fuentealba S, Dinning PG, Davidson GP, Cook IJ. Assessment of intraluminal imped- ance for the detection of pharyngeal bolus flow during swallow- ing in healthy adults. Am J Physiol Gastrointest Liver Physiol. 2006; 290. 10.1152/AJPGI.00011.2005. [DOI] [PubMed] [Google Scholar]
- 127.Tutuian R, Castell DO. Combined multichannel intraluminal impedance and manometry clarifies esophageal function abnor- malities: study in 350 patients. Am J Gastroenterol. 2004;99:1011–9. 10.1111/J.1572-0241.2004.30035.X. [DOI] [PubMed] [Google Scholar]
- 128.Hoffman MR, Mielens JD, Omari TI, Rommel N, Jiang JJ, McCulloch TM. Artificial neural network classification of phar- yngeal high-resolution manometry with impedance data. Laryn- goscope. 2013;123:713. 10.1002/LARY.23655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Troche MS, Schumann B, Brandimore AE, Okun MS, Hegland KW. Reflex cough and disease duration as predictors of swallow- ing dysfunction in Parkinson’s disease. Dysphagia. 2016;31:757–64. 10.1007/S00455-016-9734-6/TABLES/3. [DOI] [PubMed] [Google Scholar]
- 130.Hegland KW, Okun MS, Troche MS. Sequential voluntary cough and aspiration or aspiration risk in Parkinson’s disease. Lung. 2014;192:601–8. 10.1007/S00408-014-9584-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Kwon M, Lee J-H. Oro-pharyngeal dysphagia in Parkin- son’s disease and related movement disorders. J Mov Disord. 2019;12:152. 10.14802/JMD.19048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Nóbrega AC, Rodrigues B, Melo A. Silent aspiration in Par- kinson’s disease patients with diurnal sialorrhea. Clin Neurol Neurosurg. 2008;110:117–9. 10.1016/J.CLINEURO.2007.09.011. [DOI] [PubMed] [Google Scholar]
- 133.Castell JA, Johnston BT, Colcher A, Li Q, Gideon RM, Castell DO. Manometric abnormalities of the oesophagus in patients with Parkinson’s disease. Neurogastroenterol Motil. 2001;13:361–4. 10.1046/J.1365-2982.2001.00275.X. [DOI] [PubMed] [Google Scholar]
- 134.O’Keeffe GC, Michell AW, Barker RA. Biomarkers in Hunting- ton’s and Parkinson’s disease. Ann N Y Acad Sci. 2009;1180:97–110. 10.1111/J.1749-6632.2009.04943.X. [DOI] [PubMed] [Google Scholar]
- 135.Collier TJ, Kanaan NM, Kordower JH. Aging and Parkin- son’s disease: different sides of the same coin? Mov Disord. 2017;32:983–90. 10.1002/MDS.27037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Parnetti L, Castrioto A, Chiasserini D, Persichetti E, Tambasco N, El-Agnaf O, Calabresi P. Cerebrospinal fluid biomarkers in Parkinson disease. Nat Rev Neurol. 2013;9(3):131–40. 10.1038/nrneurol.2013.10. [DOI] [PubMed] [Google Scholar]
- 137.Pawlik P, Błochowiak K. The role of salivary biomarkers in the early diagnosis of Alzheimer’s disease and Parkinson’s disease. Diagnostics. 2021;11:371. 10.3390/DIAGNOSTICS11020371. [DOI] [PMC free article] [PubMed] [Google Scholar]


