Abstract
The two β-arrestins, β-arrestin-1 and -2 (systematic names: arrestin-2 and -3, respectively), are multifunctional intracellular proteins that regulate the activity of a very large number of cellular signaling pathways and physiologic functions. The two proteins were discovered for their ability to disrupt signaling via G protein-coupled receptors (GPCRs) via binding to the activated receptors. However, it is now well recognized that both β-arrestins can also act as direct modulators of numerous cellular processes via either GPCR-dependent or -independent mechanisms. Recent structural, biophysical, and biochemical studies have provided novel insights into how β-arrestins bind to activated GPCRs and downstream effector proteins. Studies with β-arrestin mutant mice have identified numerous physiologic and pathophysiological processes regulated by β-arrestin-1 and/or -2. Following a short summary of recent structural studies, this review primarily focuses on β-arrestin-regulated physiologic functions, with particular focus on the central nervous system and the roles of β-arrestins in carcinogenesis and key metabolic processes including the maintenance of glucose and energy homeostasis. This review also highlights potential therapeutic implications of these studies and discusses strategies that could prove useful for targeting specific β-arrestin-regulated signaling pathways for therapeutic purposes.
Significance Statement
The two β-arrestins, structurally closely related intracellular proteins that are evolutionarily highly conserved, have emerged as multifunctional proteins able to regulate a vast array of cellular and physiological functions. The outcome of studies with β-arrestin mutant mice and cultured cells, complemented by novel insights into β-arrestin structure and function, should pave the way for the development of novel classes of therapeutically useful drugs capable of regulating specific β-arrestin functions.
I. Introduction
Arrestins represent a family of relatively small cytoplasmic proteins (average size: approximately 45 kDa) that consist of four distinct subtypes. The name “arrestin” stems from the observation that these proteins function to terminate (“arrest”) signaling through G protein-coupled receptors (GPCRs) (DeWire et al., 2007; Gurevich and Gurevich, 2020). Two arrestin subtypes, also knowns as arrestin-1 and -4, or rod and cone arrestins, respectively, are referred to as visual arrestins since their expression is largely confined to photoreceptors of the eye. Arrestin-2 and -3 are ubiquitously expressed and can bind to and regulate the activity of hundreds of different GPCRs (DeWire et al., 2007; Peterson and Luttrell, 2017). These two arrestins are also referred to as β-arrestin-1 (βarr1) and β-arrestin-2 (βarr2), respectively, since their function was first studied by analyzing their role in regulating the activity of β-adrenergic receptors (β-ARs) (DeWire et al., 2007; Peterson and Luttrell, 2017).
The two β-arrestins play key roles in the homologous desensitization of GPCRs, which involves two steps: phosphorylation of the activated receptor by one or more specialized GPCR kinases (GRKs), followed by the binding of arrestin(s) to the active phosphorylated receptor, thus interfering with productive receptor/G protein coupling (DeWire et al., 2007; Gurevich and Gurevich, 2020). After a brief summary of recent insights into structural and functional aspects of β-arrestins, this review focuses on the physiologic and pathophysiological roles of βarr1 and βarr2, with particular emphasis on their ability to regulate various functions of the central nervous system (CNS), tumor formation, and several important metabolic functions. Due to space limitations, we refer the reader to several excellent reviews that cover other important physiologic β-arrestin functions. Wherever possible, we emphasize the potential translational relevance of β-arrestin-mediated cellular functions.
II. Arrestin Structure and Function
A. Conformational Flexibility of Arrestins
Different conformational states of most proteins have distinct biologic functions. Therefore, it is important to elucidate conformational states of arrestins and determine their functional capabilities. Available evidence suggests that arrestins exist in four different states: free monomers, free oligomers, GPCR-bound, and microtubule-bound. Among the four arrestin subtypes, only arrestin-4 does not oligomerize (Gurevich and Gurevich, 2022).
Arrestins are elongated molecules consisting of two domains, usually termed N- and C-domains (Fig. 1). In their basal conformation, all four vertebrate arrestins have a very similar structure (Scheerer and Sommer, 2017; Chen et al., 2018). Receptor binding induces similar, but not identical, conformational rearrangements in arrestin-1 and β−arrestins, as revealed by biophysical methods (Hanson et al., 2006b; Zhuang et al., 2013; Zhuo et al., 2014) and high-resolution structures of arrestin/GPCR complexes. It should be noted in this context that all high-resolution structures of arrestin-GPCR complexes solved so far contained either arrestin-1 (Kang et al., 2015; Zhou et al., 2017) or βarr1 (Yin et al., 2019; Lee et al., 2020; Staus et al., 2020; Bous et al., 2022; Cao et al., 2022). No high high-resolution structures of βarr2 bound to GPCRs have been published so far.
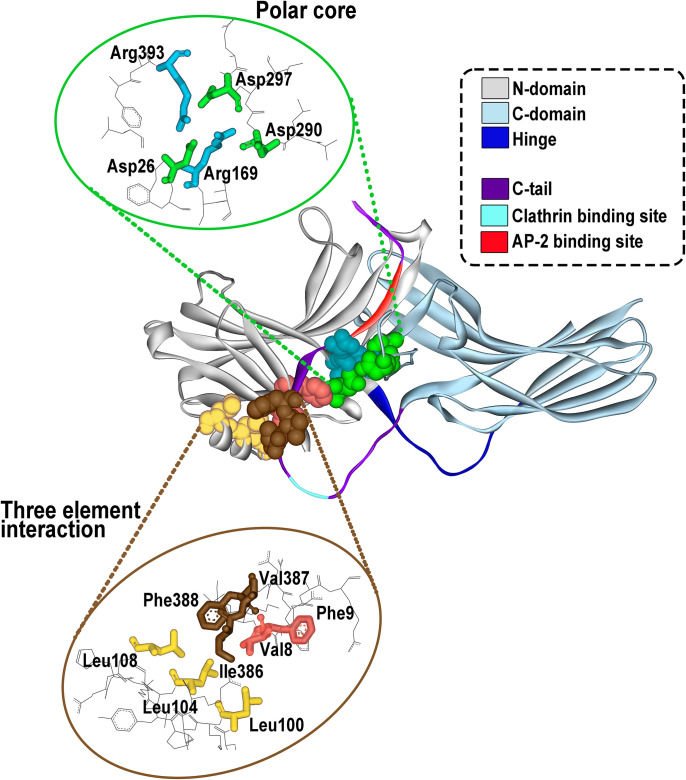
Fig. 1.
Arrestin structure and important functional elements. All arrestins consist of the N-domain (gray), the C-domain (blue-gray), and the C-terminus (C-tail, magenta) that emerges from the C-domain and is anchored to the N-domain via the so-called three-element interaction and the polar core (side chains participating in these two interactions are shown as CPK models on the structure; their spatial arrangement is shown in the insets). The N- and C-domains are connected by a 12-residue hinge region (dark blue). The C-terminus contains binding sites for clathrin (light blue) and clathrin adaptor AP2 (red). The basal arrestin conformation is stabilized by two intramolecular interactions. One is the polar core, an arrangement of five charged side chains, two of which are supplied by the N-domain, two by the C-domain, and one by the C-terminus (upper inset). The other one is the three-element interaction mediated by hydrophobic side chains of β-strand I and α-helix I of the N-domain and β-strand XX of the C-terminus (lower inset). Both of these interactions are destabilized by GPCR binding, which results in the release of the C-terminus and twisting of the two domains relative to each other by approximately 20°.
Structural studies have identified two major GPCR/arrestin interaction sites. The arrestin N-domain binds to phosphorylated regions of the receptor (Shukla et al., 2013; Zhuo et al., 2014; Thomsen et al., 2016; Cahill et al., 2017), and the arrestin finger loop region inserts into the cytoplasmic cavity formed by the GPCR transmembrane core (Kang et al., 2015; Yin et al., 2019; Böttke et al., 2020; Staus et al., 2020; Bous et al., 2022; Cao et al., 2022). Moreover, recent studies have demonstrated that loops within the C-edge of arrestin can function as a membrane anchor, thus enhancing the stability of GPCR/arrestin complexes (Lally et al., 2017; Staus et al., 2020).
Structural studies suggest that β-arrestins can bind to activated GPCRs in two different modes. One mode of binding involves the interaction of arrestins with the phosphorylated C-terminus of GPCRs only (“hanging” complex) (Shukla et al., 2014; Cahill et al., 2017; Nguyen et al., 2019). The other one represents a high affinity β-arrestin/GPCR complex involving interactions with the finger loop region and the C-edge loops (Kang et al., 2015; Zhou et al., 2017; Yin et al., 2019; Lee et al., 2020; Staus et al., 2020; Bous et al., 2022; Cao et al., 2022). While this latter complex interferes with the binding of heterotrimeric G proteins, the GPCR–β-arrestin complex in the “hanging” configuration still allows for productive receptor-G protein interactions (Thomsen et al., 2016). In this structure, arrestin interacted exclusively with the phosphorylated C-terminal segment of the receptor, whereas the G protein engaged the cytoplasmic cavity formed by the transmembrane receptor core. It is of considerable interest to explore whether agonist-bound native GPCRs form similar trimeric complexes. The outcome of such studies is expected to have important implications for the concept of biased GPCR signaling, which implies that GPCRs can preferentially engage either G proteins or arrestins, depending on the structural properties of the activating ligand (Lefkowitz and Shenoy, 2005; Rajagopal et al., 2010b; Smith et al., 2018; Gurevich and Gurevich, 2020; Seyedabadi et al., 2022).
The conformation of microtubule-bound arrestins appears to be different from those of both basal and GPCR-bound conformations (Hanson et al., 2006a, 2007). Biochemical and biophysical studies have shown that arrestins are endowed with considerable conformational flexibility, suggesting that this feature contributes to the ability of β-arrestins to interact with a vast array of cellular proteins (see Gurevich and Gurevich, 2014a; Peterson and Luttrell, 2017, and references therein). In addition to several subtle structural rearrangements, GPCR binding to arrestins induces the release of the arrestin C-terminus and a twist of the two arrestin domains relative to each other (Hanson et al., 2006b; Shukla et al., 2013; Zhuo et al., 2014; Scheerer and Sommer, 2017; Maharana et al., 2022). The release of the C-terminus makes the arrestin binding sites for clathrin and adaptor protein 2 (AP2) more accessible, ensuring that free arrestins do not compete with the receptor-arrestin complexes that should be internalized. In contrast to arrestin-1, where the C-terminus released upon receptor binding appears to “flop around” without any preferred position (Hanson et al., 2006b), the C-terminus in receptor-bound β-arrestins appears to occupy a particular position (Zhuo et al., 2014), possibly more than one (Asher et al., 2022), a finding predicted to be of functional significance.
GPCR-bound arrestins are often called active, implying that free arrestins are not. Existing evidence suggests that this notion is unfounded. Arrestins in the so-called basal conformation can affect cellular activity. For example, some cellular proteins including E3 ubiquitin ligases Mdm2 (Song et al., 2006) and parkin (Ahmed et al., 2011) preferentially interact with the basal arrestin conformation. Moreover, certain cellular proteins, exemplified by Jun N-terminal kinase (JNK) 3 do not show a clear preference for either free or receptor-bound arrestins (Song et al., 2006). An arrestin mutant “frozen” in a basal-like state that cannot bind GPCRs facilitates JNK3 activation in cells, whereas another mutant with significantly enhanced ability to bind GPCRs is inactive in this regard (Breitman et al., 2012). In the same cells where the activation status of endogenous β2-AR greatly affects the level of extracellular signal-regulated kinases (ERK) 1/2 activation, it does not affect the activation of JNK3, which is solely determined by a particular form of βarr2 expressed (Breitman et al., 2012).
GPCRs show significant sequence differences in arrestin-binding elements. Also, GPCRs vary considerably in the position of serine and threonine residues present within these regions that are potential targets for GRK-mediated phosphorylation required for efficient arrestin recruitment (Ranjan et al., 2017). These differences might explain recent observations that arrestins can display distinct conformations when bound to different GPCRs or after stimulation of the same GPCR by structurally different activating ligands (Ranjan et al., 2017). It is conceivable that distinct conformations of GPCR-bound arrestins lead to different cellular responses by interacting with different sets of intracellular signaling proteins. More detailed structural and biochemical studies are needed to elucidate the molecular mechanisms by which arrestins can efficiently interact with different GPCRs.
Most GPCRs have many more potential phosphorylation sites on their cytoplasmic regions than required for tight arrestin binding. This observation has led to the proposal that different phosphorylation patterns of the same receptor can determine the functional outcome of β-arrestin recruitment (barcode hypothesis; Kim et al., 2005; Nobles et al., 2011). The same GPCR might exhibit cell type-specific phosphorylation patterns, depending on which GRKs are preferentially expressed in a certain cellular context (Butcher et al., 2012). The results of a recent study are consistent with the barcode hypothesis. Using sophisticated biophysical techniques, the authors showed that βarr1 and βarr2 can adopt distinct phosphorylation-dependent conformations after recruitment by the same GPCR (parathyroid hormone 1 receptor) (Haider et al., 2022). Additional work is needed to explore whether the findings obtained with this receptor subtype apply to other GPCRs.
B. Biologic Functions of β-Arrestins
The two β-arrestins have no enzymatic activity. Their only function is to bind other proteins, often several at the same time, as exemplified by arrestin scaffolding of three-tiered mitogen-activated protein kinase activation cascades (see Gurevich and Gurevich, 2006; DeWire et al., 2007; Peterson and Luttrell, 2017, and references therein). Thus, if one of the arrestin-binding partners has a particular localization, β-arrestins localize the other partners that simultaneously interact with them to the same subcellular compartment. Since GPCRs are integral membrane proteins, proteins that bind GPCR-associated β-arrestins are predicted to act in proximity to the plasma membrane. Similarly, via binding to polymerized tubulin, β-arrestins localize signaling proteins to microtubules, directing their activity to microtubule-associated targets (Hanson et al., 2007).
β-Arrestins regulate a remarkable array of cellular functions (see Lefkowitz and Shenoy, 2005; Peterson and Luttrell, 2017; Gurevich and Gurevich, 2019b, and references therein). Numerous studies revealed that receptor-bound β-arrestins can initiate another wave of GPCR-driven signaling and that free β-arrestins in the cytoplasm also regulate many signaling pathways (reviewed in Lefkowitz and Shenoy, 2005; Gurevich and Gurevich, 2006; Peterson and Luttrell, 2017). It has been demonstrated that both βarr1 and βarr2 can interact with hundreds of different GPCRs, certain growth factor receptors (Lin et al., 1998; Dalle et al., 2001; Girnita et al., 2014), as well as >100 nonreceptor signaling and trafficking proteins (Xiao et al., 2007). The list of arrestin-interacting partners includes the trafficking proteins clathrin and clathrin adaptor AP2, as well as numerous signaling proteins, including kinases (e.g., members of the Src family, ERK1/2, p38, or JNK1/2/3), phosphatases, and E3 ubiquitin ligases (see Gurevich and Gurevich, 2014b; Peterson et al., 2015, and references therein). Moreover, the two β−arrestins have been implicated in regulating the post-endocytic fate of GPCRs via ubiquitination and deubiquitination (Shenoy et al., 2001; Jean-Charles et al., 2016), cell spreading and motility (Cleghorn et al., 2015, 2018), apoptotic cell death (Kook et al., 2014, 2019), and many other fundamental cellular processes.
C. β-Arrestins and Extracellular Signal-Regulated Kinases 1/2 Activation
One of the most common responses observed after GPCR-mediated arrestin recruitment is the activation of ERK1/2 (DeWire et al., 2007; Peterson and Luttrell, 2017). As discussed in the previous paragraph, βarr1 and βarr2 can act as scaffolding proteins to facilitate ERK1/2 activation (DeWire et al., 2007; Peterson and Luttrell, 2017). The molecular mechanisms underlying β-arrestin scaffolding of the Raf1-MEK1-ERK1/2 cascade (Qu et al., 2021), and likely differences between βarr1 and βarr2 in mediating this process (Perry-Hauser et al., 2022a) are currently the subject of intense investigation. Interestingly, recent data suggest that different GPCR/βarr1 complexes, besides acting as scaffolding proteins, can directly activate, in an allosteric fashion, the protein kinases Src and C-Raf (Pakharukova et al., 2020; Zang et al., 2021).
Somewhat surprisingly, recent studies showed that deletion or inactivation of multiple functional G protein α-subunits abolished ERK1/2 activation by essentially all GPCR–ligand pairs examined (Grundmann et al., 2018), suggesting that β-arrestin-mediated ERK1/2 activation requires the presence of functional G proteins. Notably, ligand-activated GPCRs were still able to recruit β-arrestins in the absence of functional G proteins (Grundmann et al., 2018). These observations led to the hypothesis that β-arrestins primarily act as modulators (“rheostats”) of GPCR-mediated ERK1/2 activation by regulating the intensity and temporal and spatial pattern of ERK1/2 signaling (Gutkind and Kostenis, 2018). In a subsequent study, Luttrell et al. (Luttrell et al., 2018) reported that independently generated βarr1/2 knockout (KO) clonal HEK293 cell lines showed variable ERK1/2 responses after stimulation of the β2-AR and other GPCRs including the β1-AR and the V2 vasopressin and follicle-stimulating hormone receptors. This finding, complemented by βarr1/2 small interfering siRNA knockdown and overexpression studies, raised the possibility that clonal variation and potential “rewiring” of intracellular signaling pathways caused by the lack of specific Gα or arrestin subtypes may affect the outcome of ERK1/2 signaling assays (Luttrell et al., 2018). Further studies are needed to shed more light on these seemingly discrepant findings. Importantly, such studies should focus on cell types other than HEK293 and include the analysis of signaling pathways in vivo.
III. Tools for Studying Arrestin Function and Physiology
In the following, we briefly review strategies that are most commonly employed to explore the cellular and biologic functions of βarr1 and βarr2 in vitro and in vivo.
A. Knockdown or Knockout of Arrestin Expression
The knockdown of βarr1 and βarr2 expression via siRNA technology in cultured cells has provided important insights into the cellular function of these two signaling proteins. However, the use of siRNA or related tools usually does not lead to a complete suppression of protein expression, making negative data difficult to interpret. More recently, CRISPR/Cas9 technology has been employed to generate cell lines that completely lack βarr1 and/or βarr2 (O’Hayre et al., 2017; Grundmann et al., 2018; Luttrell et al., 2018). A potential caveat associated with the use of this approach is that it may lead to the inadvertent selection of atypical cells that do not require the targeted protein for survival (Luttrell et al., 2018; Gurevich and Gurevich, 2020).
In vivo studies with whole body βarr1 and βarr2 KO mice have demonstrated that the two β-arrestins play critical roles in regulating numerous physiologic functions, including key activities of the CNS and many important functions of peripheral tissues and organs (Schmid and Bohn, 2009; Zhao and Pei, 2013; Porter-Stransky and Weinshenker, 2017). One disadvantage associated with the use of whole-body KO mice is that this approach does not provide clear information about the specific cell types and potential cellular mechanisms underlying the observed phenotypes. Moreover, the whole-body β-arrestin KO mice that have been analyzed in the past lack βarr1 or βarr2 throughout development, raising the possibility that at least some of the observed phenotypes may be affected by compensational developmental changes (Kovacs et al., 2009; Philipp et al., 2013). It should also be noted that the first whole-body βarr1 (Conner et al., 1997) and βarr2 (Bohn et al., 1999) KO mice that were generated had a mixed genetic background, which can affect the outcome of mouse phenotyping studies (Gerlai, 1996).
The recent development of floxed βarr1 or βarr2 mutant mice has greatly advanced our knowledge about the in vivo functions of the two β-arrestins in specific cell types or tissues (Urs et al., 2016; Kim et al., 2018). By crossing floxed βarr1 or βarr2 mice with specific Cre driver lines, it has been possible to generate mice that lack βarr1 or βarr2 only in distinct cell types (Pydi et al., 2022). In many cases, these mutations were induced in adult animals by using Cre driver lines in which Cre activity can be stimulated in a tamoxifen-dependent fashion (Pydi et al., 2022).
B. Arrestin-Biased G Protein-Coupled Receptor Ligands
During the past two decades, GPCR ligands have been identified that are unable or impaired in their ability to promote receptor-mediated G protein activation but can recruit β-arrestins and initiate arrestin-dependent signaling with high efficacy (Luttrell et al., 2015; Peterson and Luttrell, 2017; Smith et al., 2018; Seyedabadi et al., 2019; Gurevich and Gurevich, 2020). These so-called arrestin-based ligands are widely used to explore mechanisms of arrestin-mediated signaling in vitro and in vivo. The in vivo use of these agents has led to important novel findings regarding the physiologic and pathophysiological roles of various arrestin-regulated signaling pathways (Peterson and Luttrell, 2017; Smith et al., 2018). In some cases, arrestin-biased ligands have been shown to mimic the (potential) therapeutic effects of GPCR agonists, while ligand-induced G protein activation was associated with unwanted side effects (Luttrell et al., 2015; Peterson and Luttrell, 2017; Smith et al., 2018). GPCR ligands that preferentially activate G proteins but are impaired in their ability to recruit β-arrestins are referred to as G protein-biased agonists (Luttrell et al., 2015; Peterson and Luttrell, 2017; Smith et al., 2018). Several studies suggest that the use of this class of GPCR ligands may offer therapeutic advantages under conditions where GPCR-mediated arrestin recruitment and/or signaling may cause unwanted side effects. Recent studies have shown that GPCRs are highly dynamic proteins and that structurally different GPCR ligands have the potential to preferentially stabilize distinct GPCR conformations (Smith et al., 2018; Wingler and Lefkowitz, 2020). The conformational heterogeneity of GPCRs thus provides a structural basis for the identification of arrestin- and G protein-biased GPCR ligands. Examples for the potential therapeutic usefulness of arrestin- and G protein-biased GPCR agonists are given throughout this review.
C. Caveats Associated with the Use of Arrestin-Biased G Protein-Coupled Receptor Ligands
Several caveats must be considered when interpreting the outcome of studies involving the use of biased GPCR agonists. For example, the expression levels and localization of GPCRs and downstream effector and regulatory proteins may vary in different cell types and may be altered under specific physiologic or pathophysiological conditions (Seyedabadi et al., 2019). Moreover, it is important to test the potential clinical efficacy of a particular biased GPCR ligand in an experimental setting that closely mimics the clinical condition for which this drug is being developed (Seyedabadi et al., 2019). Also, many published studies reported ligand bias for β-arrestins simply based on GPCR-arrestin recruitment assays without exploring arrestin- dependent changes in intracellular signaling (Gurevich and Gurevich, 2020). Such studies do not provide convincing information regarding ligand-dependent biased signaling. As discussed in detail recently (Gurevich and Gurevich, 2020), ligand-dependent changes in the kinetics of GPCR internalization and trafficking can complicate the identification of ligands that show intrinsic bias for facilitating GPCR-dependent arrestin signaling. However, to the best of our knowledge, no unambiguous readout for arrestin-dependent signaling currently exists to assess arrestin activity in signaling assays for screening purposes (Gurevich and Gurevich, 2020). The development of such assays is complicated by the multitude of signaling pathways modulated by both G proteins and β-arrestins, as well as by overlapping activities of these two signaling arms.
D. Arrestin-Biased G Protein-Coupled Receptors
Interestingly, mutant versions of the angiotensin II AT1 receptor (AT1R) (Wei et al., 2003) and the β2-adrenergic (Shenoy et al., 2006) receptor have been described that, following agonist binding, fail to activate heterotrimeric G proteins but can recruit β-arrestins and stimulate ERK in a β-arrestin-dependent fashion. More recently, Nakajima et al. (Nakajima and Wess, 2012) developed an M3 muscarinic receptor-based designer GPCR (DREADD: Designer Receptor Exclusively Activated by a Designer Drug) that contained a point mutation within the “DRY” motif of the receptor. This point mutation disrupted receptor/G protein coupling but still allowed recruitment of β-arrestins by the receptor in the presence of clozapine-N-oxide (CNO), a DREADD agonist (Urban and Roth, 2015). CNO stimulation of mouse β-cells expressing the arrestin-biased designer receptor resulted in a significant increase in insulin release, implicating arrestin-dependent pathways in the regulation of insulin secretion (Nakajima and Wess, 2012). However, CNO treatment of mice expressing this new designer receptor in hepatocytes and other metabolically important cell types in vivo has not resulted in significant phenotypic changes so far (J. Wess, unpublished data).
Similarly, more recent studies also generated mutant versions of other GPCRs, including the D2 dopamine receptor (Peterson et al., 2015; Donthamsetti et al., 2020), that show a strong bias for the recruitment of β-arrestins. In vivo studies with this new class of receptors should provide important novel insights into the physiologic relevance of β-arrestin-dependent signaling cascades. Specific examples for this approach are given under “Psychoactive Drugs.”
E. Dominant Negative β-Arrestin Mutants
Dominant-negative mutants of βarr1 and βarr2 represent useful tools to selectively interfere with distinct β-arrestin functions. An early study (Luttrell et al., 1999) reported the generation of βarr1 mutants, impaired either in c-Src binding or their ability to target GPCRs to clathrin-coated pits, that act as dominant negative inhibitors of β2-AR-mediated stimulation of ERK1/2. More recently, dominant negative mutant versions of βarr1 and βarr2 or minigene fragments of βarr1 have been used to explore mechanisms underlying β-arrestin-dependent GPCR endocytosis (Kang et al., 2014; Ghosh et al., 2017). Interestingly, a βarr2 mutant containing a series of alanine substitutions of key receptor-binding residues (“KNC mutant”) displays dominant negative activity, competitively decreasing JNK activation by wild-type (WT) βarr2 (Breitman et al., 2012). In addition, introduction of a single point mutation (R307A) into βarr1 is able to prevent βarr1-dependent ERK1/2 activation by selectively reducing βarr1 binding to c-Raf1 (Coffa et al., 2011). A related study demonstrated that a minigene fragment of βarr1 (residues 25–161) interferes with βarr1 binding to signal transducing adaptor molecule 1 (Malik and Marchese, 2010). A subsequent study using this minigene fragment as a tool showed that formation of the βarr1– signal transducing adaptor molecule 1 complex is essential for C-X-C chemokine receptor (CXCR) 4-dependent activation of focal adhesion kinase and chemotaxis (Alekhina and Marchese, 2016; Zhuo et al., 2020). These findings indicate that it is feasible to design dominant negative β-arrestin mutants that selectively suppress specific β-arrestin functions.
F. β-Arrestins with Altered G Protein-Coupled Receptor Binding Affinity and/or Selectivity
Following activation by agonist ligands, phosphorylated GPCRs are able to bind β-arrestins with high affinity via destabilization of two key interactions that keep β-arrestins in their basal state: the polar core and the three-element interaction between β-strand I, β-strand XX of the C-terminus, and α-helix I (Gurevich and Gurevich, 2019a; Karnam et al., 2021) (Fig. 1). These intramolecular interactions in βarr1 and βarr2 can be destabilized by mutations, yielding mutant β-arrestins that bind active phosphorylated and even unphosphorylated GPCRs more readily than WT β-arrestins (Gurevich and Gurevich, 2019a; Karnam et al., 2021). Gain-of-function mutations that can occur in numerous GPCRs are known to cause various human disorders (Schöneberg et al., 2004; Stoy and Gurevich, 2015; Arang and Gutkind, 2020). Arrestin mutants that can bind to overactive receptors with greater affinity than to WT receptors have the potential to suppress excessive G protein-mediated signaling by disease-causing mutant GPCRs (Song et al., 2009; Samaranayake et al., 2018). In particular, arrestin mutants that do not require GPCR phosphorylation for tight binding to the receptor can compensate for defects in receptor phosphorylation. For example, structure-function studies identified several “enhanced” mutant versions of arrestin-1 that bind all active forms of rhodopsin more readily and interact with high affinity with active unphosphorylated rhodopsin. One of these arrestin-1 mutants was able to partially compensate for defects of rhodopsin phosphorylation in vivo, improving photoreceptor function and survival in mice (Song et al., 2009; Samaranayake et al., 2018).
Enhanced phosphorylation-independent mutants of βarr1 and βarr2 have also been constructed and characterized in vitro (Gurevich et al., 1997; Kovoor et al., 1999; Celver et al., 2002; Pan et al., 2003), but their therapeutic potential has yet to be tested. As discussed earlier, βarr1 and βarr2 interact with a very large number of activated GPCRs. Mutant versions of βarr2 that show a certain degree of GPCR binding selectivity have also been described (Gimenez et al., 2012, 2014). This observation may stimulate the development of novel, GPCR subtype-biased β-arrestins that may prove useful both as novel research tools and also for therapeutic purposes. It is likely that the recently published high-resolution structures of GPCR-arrestin-1/βarr1 complexes (Kang et al., 2015; Zhou et al., 2017; Yin et al., 2019; Huang et al., 2020; Lee et al., 2020; Staus et al., 2020; Bous et al., 2022; Cao et al., 2022) will promote the rational design of such novel classes of mutant β-arrestins.
G. β-Arrestin Mutants with Altered Signaling Properties
The two β-arrestins interact, directly or indirectly, with a very large number of effector proteins (e.g., (Xiao et al., 2007)), thus regulating numerous intracellular signaling pathways. Several mutant versions of βarr1 and βarr2 have been identified that selectivity interfere with distinct arrestin/effector protein interactions. For example, βarr1 or βarr2 mutants have been described that fail to interact with distinct effector proteins, including MEK1 (Meng et al., 2009) and c-Raf1 (Coffa et al., 2011) or do not facilitate JNK3 activation (Breitman et al., 2012). ERK1/2 activation usually promotes cell proliferation, whereas activated JNKs cause antiproliferative and sometimes proapoptotic effects. For this reason, these β-arrestins mutants may become therapeutically useful in disorders associated with excessive proliferation (e.g., cancer) or excessive cell death including neurodegenerative diseases, such as Alzheimer’s and Parkinson’s disease. Since methods of targeted gene delivery are being rapidly developed and improved (Alnasser, 2021), the cell-type specific expression of certain β-arrestin mutants for the treatment of life-threatening diseases may become feasible at some point in the future.
H. β-Arrestin-Derived Peptides
Arrestin-derived peptides also represent useful tools for modulating arrestin function in a more targeted fashion. For example, a cell-permeable 25 amino acid βarr1 peptide encompassing the MEK1-binding site can block βarr1-MEK1 interactions and inhibit ERK1/2 phosphorylation (Meng et al., 2009). Recently, short βarr2 peptides have been developed that promote activation of the ASK1-MKK4/7-JNK3 signaling cascade (Zhan et al., 2016; Perry-Hauser et al., 2022b). Since short peptides are rapidly degraded by cytoplasmic exopeptidases, these peptides were fused to the Venus protein to enhance their stability (Zhan et al., 2016; Perry-Hauser et al., 2022b). The βarr2 peptides can be considered “mono-functional” since they lack most receptor-binding residues and are unlikely to interfere with any other βarr2 functions. As discussed in the previous paragraph, activation of JNKs has the potential to suppress excessive proliferation or induce apoptosis of cancer cells (Bubici and Papa, 2014). Thus, these βarr2-based peptides capable of activating JNK signaling should be of considerable translational interest.
In sum, recent studies have demonstrated the feasibility of creating arrestin-based tools for targeted manipulation of cell signaling and, potentially, for therapeutic purposes. However, given the remarkable multifunctionality of βarr1 and βarr2, the development and further evaluation of these agents for therapeutic utility represents a daunting task. Most importantly, future work in this area requires the identification of arrestin-binding sites for numerous other signaling proteins known as β-arrestin interaction partners. It is likely that such studies will lead to the development of novel tools that are able to alter arrestin-dependent functions in a more targeted fashion, a prerequisite for the potential evaluation of arrestin-based therapeutic strategies.
IV. Role of β-Arrestins in Modulating Key Functions of the Central Nervous System
Neurotransmission via the chemical synapses is essential for the functioning of the CNS. Synaptic signals are carried by neurotransmitter molecules that interact with plasma membrane receptors, initiating a cascade of signaling events eventually resulting in a specific neural response. Most neurotransmitter receptors belong to the GPCR superfamily, including, for example, all dopamine and opioid receptor subtypes. Since β-arrestins are critical regulators of GPCR function, it is not surprising that β-arrestins make important contributions to many neural processes.
A. Opioid Receptor-Mediated Analgesia
Acute and chronic pain has emerged as a major societal challenge (Davis et al., 2020). The most commonly used drugs endowed with superior analgesic activity target GPCRs of the opioid receptor family, which consists of three major subtypes, μ, κ, and δ (Fig. 2) (Stein, 2016). These receptors, which preferentially interact with G proteins of the Gi/o family, are widely expressed in the CNS and are also present in various peripheral tissues (Stein, 2016).
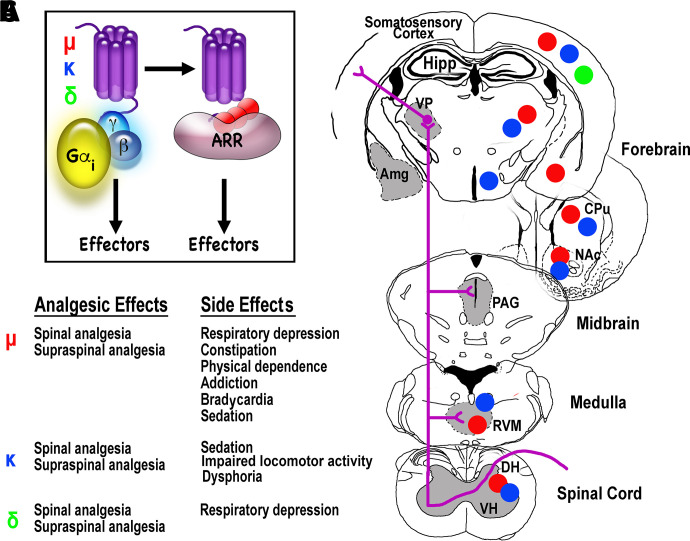
Fig. 2.
Opioid receptor subtypes: signal transduction, agonist effects, and localization. (A) The three opioid receptor subtypes (μ, κ, and δ) are G protein-coupled receptors that primarily couple to the Gi/o subfamily of G proteins. The ligand-activated receptors are phosphorylated by G protein-coupled receptor kinases, resulting in the recruitment of β-arrestins (ARR), followed by receptor desensitization and/or internalization, and, most likely, β-arrestin-dependent modulation of intracellular signaling. (B) The analgesic effects of μ, κ, and δ opioid receptor agonists are usually accompanied by a series of side effects. The potential involvement of β-arrestin-dependent signaling in mediating these side effects is currently a hotly debated issue (Gurevich and Gurevich, 2020). (C) A simplified diagram of the ascending pain pathway (for details see De Ridder et al., 2021; Wang et al., 2022a) showing the brain regions with the highest expression levels of the three opioid receptor subtypes (red circles, μ; blue, κ; green, δ). Although the striatum (CPu and NAc) is technically not part of the pain pathway, this brain region expresses high levels of μ and κ receptors. Amg, amygdala; CPu, caudate-putamen; DH, dorsal horn of the spinal cord; Hipp, hippocampus; NAc, nucleus accumbens; PAG, periaqueductal gray; RVM, rostroventral medulla; VH, ventral horn of the spinal cord; VP, ventral posterior nucleus of the thalamus.
1. μ-Opioid Receptors
The most frequently used agents to treat moderate to severe pain are μ-opioid receptor (MOR) agonists, including morphine and oxycodone. However, the use of morphine and its derivatives causes major side effects, including respiratory depression, constipation, tolerance, and addiction (Fig. 2B) (Darcq and Kieffer, 2018). The severity of the side effects associated with the use of MOR agonists is underscored by the staggering loss of life resulting from the current opioid epidemic in the United Sates (about 75,000 deaths per year in 2020/2021; https://www.cdc.gov/nchs/pressroom/nchs_press_releases/2021/20211117.htm). For this reason, novel classes of MOR agonists endowed with an improved therapeutic window are urgently needed.
Following the generation of whole-body βarr2 KO mice, Caron and coworkers demonstrated that βarr2 deficiency led to a pronounced potentiation and prolongation of the analgesic effects of morphine (Bohn et al., 1999) and that tolerance did not occur after chronic morphine treatment of βarr2 KO mice (Bohn et al., 2000). More recently, Manglik et al. (Manglik et al., 2016) reported the development of a novel, G protein-biased MOR agonist, PZM21, that shows no structural similarity to known opioid drugs. This agent displays strong analgesic activity but is devoid of many of the side effects associated with the use of morphine-like analgesics (Manglik et al., 2016). Based on these findings, many academic and industry laboratories focused on developing clinically useful MOR agonists that promote MOR-mediated activation of G proteins but do not trigger β-arrestin recruitment to the receptor. These efforts led to the development of novel G protein-biased MOR agonists, which, as was hoped, retained strong analgesic activity but displayed an improved side-effect profile, as compared with morphine (Schmid et al., 2017). For example, chronic treatment of mice with one of these compounds (SR-17018) did not cause antinociceptive tolerance or MOR desensitization, effects typically observed after chronic administration of morphine (Grim et al., 2020). Similarly, in vivo studies in rodents showed that a G protein-biased MOR agonist, TRV130 (alternative name: oliceridine) had strong analgesic activity but was less likely than morphine to cause respiratory depression, constipation, or tolerance upon chronic administration (DeWire et al., 2013; Altarifi et al., 2017). Following the successful completion of a series of clinical studies, oliceridine was approved by the Food and Drug Administration in 2020 for intravenous use in moderate to severe pain in adults (Azzam and Lambert, 2022). As reviewed recently (Azzam and Lambert, 2022), oliceridine possesses potent analgesic activity but appears to have less abuse potential and an improved side-effect profile as compared with traditional opioid drugs.
In contrast to the findings that led to the successful development of oliceridine as a novel analgesic agent, two recent studies using independently generated βarr2 KO mice failed to reproduce the original result that βarr2 mediates respiratory depression and constipation caused by morphine or other opioids (Kliewer et al., 2020; Bachmutsky et al., 2021). Moreover, recent evidence suggests that effective morphine-induced recruitment of βarr2 by a mutant MOR can improve morphine analgesia without exacerbating respiratory depression (He et al., 2021). One possible reason for these discrepant findings is that the analyzed βarr2 KO strains differed in their genetic background. The βarr2 KO mice analyzed by Caron and coworkers had a mixed genetic background (129/SvJ x C57BL/6) (Bohn et al., 1999, 2000). In contrast, Kliewer et al. (Kliewer et al., 2020) analyzed βarr2 KO mice that had been extensively backcrossed to the C57BL/6J background. In fact, previous work has demonstrated considerable variation among different mouse strains in opioid-induced antinociception, respiratory depression, and tolerance Kelly et al. (2023). Recent data link the beneficial pharmacological profile of oliceridine or other G protein-biased MOR agonists to the partial agonist properties of these compounds (Azevedo Neto et al., 2020; Gillis et al., 2020a,b; Singleton et al., 2021; Kelly et al., 2023). However, as discussed recently, partial agonist activity alone cannot account for the improved side-effect profiles of the new class of MOR agonists described previously (Stahl and Bohn, 2022).
Clearly, the recently developed novel MOR agonists display an improved therapeutic window and expand the spectrum of clinically useful drugs that can suppress severe pain. However, additional studies are needed to explore to which extent changes in β-arrestin recruitment or signaling (or the lack thereof) affect the pharmacological profile of this new class of analgesic agents.
2. κ-Opioid Receptors
Similar to MOR, κ-opioid receptors (KOR) are widely expressed in the CNS and mediate analgesia following agonist activation (Mores et al., 2019; French and van Rijn, 2022). KOR agonists are also useful for the treatment of intractable itch (Bohn and Aubé, 2017). Importantly, KOR agonists do not induce respiratory depression and lack abuse potential, making them an attractive alternative to MOR agonists to treat moderate to severe pain (Mores et al., 2019; French and van Rijn, 2022). However, KOR agonists can induce central side effects such as sedation, lack of motor coordination, and dysphoria, which limit the utility of these agents as analgesic drugs (Fig. 2B) (Mores et al., 2019; French and van Rijn, 2022).
Several reports, including studies with βarr2 KO mice, suggest that βarr2 signaling may play a role in KOR-mediated dysphoria (Chavkin et al., 2014). In contrast, the antipruritic and analgesic effects of KOR agonists are not affected in βarr2 KO mice (Morgenweck et al., 2015; White et al., 2015). Based on these findings, several laboratories set out to develop G protein-biased KOR agonists (Bohn and Aubé, 2017; Mores et al., 2019; Faouzi et al., 2020) with reduced side effects such as dysphoria, sedation, and other KOR-mediated central side effects. As reviewed recently (Bohn and Aubé, 2017), the outcomes of many animal studies are consistent with the concept that KOR agonists, which can efficiently activate G proteins but are impaired in the ability to recruit β-arrestins, are endowed with an increased therapeutic window. It remains to be seen whether these preclinical studies will eventually lead to the development of G protein-biased KOR agonists that will prove clinically useful as novel analgesic and antipruritic drugs.
3. δ-Opioid Receptors
δ-Opioid receptor (DOR) agonists are a poor substitute for MOR agonists for the treatment of acute pain but are highly efficacious in chronic pain models (Quirion et al., 2020). Importantly, the use of DOR agonists is not associated with significant abuse potential or severe side effects (Quirion et al., 2020). However, the use of DOR agonists in various animal models is generally associated with the development of significant analgesic tolerance upon repeated drug administration (Pradhan et al., 2011). DOR activity is regulated by interactions of the activated receptor with both β-arrestins (see, for example, Pradhan et al., 2016). Studies with β-arrestin KO mice indicate that agonist-activated DORs interact with β-arrestins in a ligand-specific manner (Pradhan et al., 2016; Vicente-Sanchez et al., 2018). Interestingly, the tolerance induced by DOR agonists that induce DOR internalization with high efficacy has been linked to DOR recruitment of βarr1 (Vicente-Sanchez et al., 2018). Based on these findings, the development of DOR agonists that induce DOR conformations that are less likely to interact with βarr1 represents an attractive goal. Such agents may offer the advantage that they can suppress chronic pain in humans but are less prone to cause tolerance upon repeated administration.
B. Dopaminergic Signaling
1. Schizophrenia
Most antipsychotic drugs are thought to exert their therapeutic actions primarily by blocking D2-class dopamine (DA) receptors, in agreement with the concept that enhanced dopaminergic neurotransmission plays a central role in the pathophysiology of schizophrenia (Seeman, 2021). Pharmacological studies have shown that activation of D2-type DA receptors impairs central Akt activity, resulting in enhanced glycogen synthase kinase-3 signaling in the striatum (Beaulieu et al., 2009). Studies with βarr2 KO mice demonstrated that βarr2 plays an important role in regulating the activity of this signaling cascade (Beaulieu et al., 2009). Specifically, activation of D2-class DA receptors stimulates the formation of a protein complex containing Akt, βarr2, and protein phosphatase 2A (Beaulieu et al., 2005). This multiprotein complex is predicted to facilitate DA-induced dephosphorylation (inactivation) of Akt (Beaulieu et al., 2005). Various lines of evidence indicate that this signaling complex is involved in regulating several important DA-dependent behaviors, including locomotor hyperactivity, responsiveness to psychostimulants (e.g., amphetamines), and amphetamine (AMPH)-induced disruption of sensorimotor gating, an animal model of psychosis commonly used to test drugs for potential antipsychotic activity (Beaulieu et al., 2009).
The Akt/glycogen synthase kinase-3 signaling cascade is also involved in mediating the therapeutic effects of lithium (Beaulieu et al., 2009), a drug commonly used for the treatment of bipolar disorder (also known as manic depression) (Volkmann et al., 2020). Interestingly, βarr2 KO mice are unresponsive to both acute and chronic treatment with lithium in various behavioral and biochemical tests. For example, Caron and coworkers demonstrated that lithium is unable to stimulate Akt activity in βarr2 KO mice, in contrast to findings obtained with WT mice (Beaulieu et al., 2008). Mechanistic studies showed that lithium disrupts the formation of the Akt/βarr2/protein phosphatase 2A complex both in vitro and in vivo (Beaulieu et al., 2008), suggesting that at least some of the behavioral effects of lithium are mediated via this mechanism.
Besides enhanced striatal DA release, schizophrenia is also associated with reduced cortical DA tone (Slifstein et al., 2015). Current antipsychotic drugs primarily act by reducing elevated DA signaling in the striatum. A recent study (Urs et al., 2016) tested the hypothesis that βarr2-biased D2 receptor partial agonists, including UNC9994, may be able to improve cortical hypodopaminergia. Studies with mutant mice lacking βarr2 in specific neuronal subpopulations demonstrated that the antipsychotic-like effects of UNC9994A are due to a combination of striatal antagonism and cortical agonism of D2 receptor-βarr2 signaling (Urs et al., 2016). These findings suggest that βarr2-biased D2 receptor ligands could prove beneficial as a novel class of antipsychotic drugs.
2. Psychostimulants
Psychostimulant drugs such as cocaine and AMPH enhance striatal dopaminergic neurotransmission, causing stimulation of striatal DA receptors and, as a result, increased locomotor activity (Kohno et al., 2022). Repeated administration of these agents leads to a further stimulation of locomotor activity, a phenomenon referred to as behavioral sensitization (Steketee and Kalivas, 2011). AMPH-induced behavioral sensitization (augmentation of locomotor activity) is considered a useful animal model to explore the neural basis of drug addiction (Steketee and Kalivas, 2011).
Recent work demonstrated that βarr2 KO mice are deficient in AMPH-induced locomotor sensitization (Zurkovsky et al., 2017), suggesting that βarr2 plays a key role in facilitating this behavior. The data presented in this study suggest that βarr2 regulates AMPH-induced locomotor sensitization via βarr2-dependent cellular signaling rather than βarr2-mediated DA receptor desensitization. The precise neuronal and molecular mechanisms underlying this type of regulation remain to be explored in future studies.
Most drugs of abuse, including psychostimulants and opioids, stimulate DA neurotransmission in the nucleus accumbens by activating D1‐ and D2‐like DA receptors expressed by medium spiny neurons (MSNs) (Pierce and Kumaresan, 2006). A recent study demonstrated that mice selectively lacking βarr1 in D1 receptor-expressing cells showed similar behavioral responses as control mice following treatment with cocaine or morphine (Porter-Stransky et al., 2020). In contrast, mice lacking βarr2 in D2 receptor-expressing neurons showed significantly reduced hyperlocomotor activity in response to both drugs, attenuated locomotor sensitization in response to cocaine, and blunted cocaine-seeking behavior (Porter-Stransky et al., 2020). Electrophysiological data indicated that the lack of βarr2 impaired the ability of DA to inhibit D2-receptor-expressing MSNs of the nucleus accumbens (Porter-Stransky et al., 2020). These findings indicate that βarr2 plays a key role in the excitability of this neuronal population, suggesting that this deficit may contribute to the behavioral changes observed after psychostimulant treatment of mice lacking βarr2 in this subclass of neurons. However, it should be noted that the βarr2 mutant mice used in this study (Porter-Stransky et al., 2020) lacked βarr2 in all D2 (or D1) receptor-expressing neurons, raising the possibility that other neuronal subpopulations (e.g., certain striatal neurons) may also be involved in the observed phenotypes.
Interestingly, the Caron laboratory developed mutant D2 DA receptors that were biased for either G protein or β-arrestin interactions (Peterson et al., 2015). Expression of the arrestin–biased designer receptor in mouse GABAergic MSNs of the striatum resulted in a pronounced augmentation in locomotor activity in response to AMPH, whereas overexpression of the G protein-biased D2 mutant receptor in the same set of neurons had little effect on this AMPH response (Peterson et al., 2015). One major caveat associated with this approach is that the functions of the D2 designer receptors are explored in the presence of endogenous D2 receptors, which may affect the nature of the phenotypes observed with the mutant mice. Nevertheless, these newly developed designer GPCRs represent excellent novel tools to explore the relative contributions of G protein and arrestin signaling pathways to the physiologic and pathophysiological functions of the D2 DA receptor.
In a related study, an arrestin-biased D2 designer receptor was expressed in “indirect pathway” medium spiny neurons (iMSNs) of the nucleus accumbens of whole-body D2 receptor KO mice (Donthamsetti et al., 2020). Strikingly, expression of this mutant receptor in this neuronal population resulted in normal (WT-like) locomotor activity and cocaine-induced locomotor activity (Donthamsetti et al., 2020). In contrast, the reward effect of cocaine could only be restored by expression of the WT D2 receptor. These data support the concept that D2-receptor mediated β-arrestin recruitment can modulate locomotor activity without simultaneous activation of G proteins.
Using a similar strategy as the Javitch laboratory (Donthamsetti et al., 2020), Caron and colleagues expressed β-arrestin- or G protein-biased mutant D2 receptors in D2 receptor-deficient iMSNs of the dorsal (caudate–putamen) and ventral striatum (nucleus accumbens) (Rose et al., 2018). Behavioral studies indicated that coordinated G protein and arrestin actions were required to restore proper basal locomotion and WT-like AMPH- or cocaine-induced hyperlocomotion in these mutant mice (Rose et al., 2018). These data suggest that D2 receptor-dependent control of locomotor activity relies on the proper balance between G protein and β-arrestin activities in iMSNs. Possible reasons for the seemingly discrepant findings by the Javitch and Caron laboratories (Rose et al., 2018; Donthamsetti et al., 2020) may be differences in receptor expression levels, coupling efficacy of the various designer D2 receptors, the type of targeted neuronal subpopulations, or other changes in experimental conditions that remain to be explored.
C. Learning, Memory, and Mood
Relatively little is known about the potential roles of β-arrestins in regulating cognitive and memory functions.
1. Memory Reconsolidation
Behavioral studies demonstrated that βarr2 KO mice show deficits in memory reconsolidation (Liu et al., 2015), a process that enhances, updates, or reduces a previously acquired memory after recall (Lee et al., 2017). Studies with various β-AR antagonists including carvedilol, an agent that retains the ability to stimulate β-arrestin-dependent signaling (Wisler et al., 2007), suggested that the β1-AR/βarr2/ERK signaling module plays a key role in mediating memory reconsolidation (Liu et al., 2015). Strikingly, selective expression of βarr2 in the entorhinal cortex of βarr2 KO mice greatly improved impaired memory reconsolidation in an object-recognition paradigm (Liu et al., 2015). The outcome of this study is of considerable clinical relevance since disruption of memory reconsolidation can potentially erase pathophysiological memories including symptoms associated with posttraumatic stress disorder. For this reason, β-AR antagonists that can selectively interfere with β-AR-mediated arrestin recruitment/signaling may prove more efficacious and cause fewer side effects than propranolol and other conventional β-AR blockers in the treatment of posttraumatic stress disorder and related pathophysiological conditions (Liu et al., 2015).
2. Working Memory
DA modulates working memory largely via activation of the cortical D1 receptors, and D1 receptor agonists have been shown to improve cognitive performance in a dose-dependent manner (Arnsten et al., 2015, 2017; Wang et al., 2019). Recently, Yang et al. (Yang et al., 2021) identified two D1 receptor-selective compounds (2MDHX and CY208,243) that stimulated G protein signaling with similar intrinsic activity but showed marked differences in their ability to recruit β-arrestins (2MDHX > CY208,243). Behavioral studies showed that treatment with 2MDHX resulted in a slight improvement in working memory (decrease in decision-making time), as compared with CY208,243. 2MDHX administration also led to greater improvements at the electrophysiological level (Yang et al., 2021). To strengthen the concept that β-arrestins play a role in mediating the beneficial cognitive effects of 2MDHX, it will be important to study the activity of 2MDHX in β-arrestin mutant mice.
3. Metabotropic Glutamate Receptors and Cognition
Group I metabotropic glutamate receptors, mGluR1 and mGluR5, are widely expressed at central excitatory synapses (Gregory and Goudet, 2021) where they mediate changes in synaptic plasticity, which are closely linked to memory formation (Ménard and Quirion, 2012; Crupi et al., 2019). For example, mGluRs mediate intermediate-term potentiation of excitatory synapses in CA3 hippocampal neurons (Frausto et al., 2011). A recent study demonstrated that this form of synaptic plasticity is absent in βarr2 KO mice but preserved in βarr1 KO mice (Eng et al., 2016). Electrophysiological studies demonstrated that mGluR-dependent depression of synaptic transmission in CA1 pyramidal neurons was also dependent on the presence of βarr2 (Eng et al., 2016). In contrast, classic long-term potentiation of the mossy fibers-CA3 synapses remained unaffected by the lack of βarr2. Immunoprecipitation studies indicated that βarr2 can associate with both mGluR1 and mGluR5 in the mouse hippocampus. These data suggest that βarr2 plays a key role in meditating various forms of hippocampal synaptic plasticity in response to mGluR1/5 activation, most likely by facilitating c-Src and ERK1/2 signaling (Eng et al., 2016).
The function of mGluR5 is critically involved in the pathology of fragile X syndrome (FXS), the most common form of heritable mental retardation and the leading identified cause of autism (Stoppel et al., 2021). FXS is caused by the transcriptional silencing of the gene encoding fragile X mental retardation protein. However, many pathologic features seem to result from the hyperactivity of mGluR5 receptors (Stoppel et al., 2021). At the neural level, FXS mouse models show enhanced mGluR5-dependent long-term depression (Dölen and Bear, 2008), which requires the presence of βarr2 (Eng et al., 2016) and enhanced protein synthesis (Dölen and Bear, 2008; Osterweil et al., 2010). The increase in mGluR5-dependent protein synthesis was absent in mice with heterozygous deletion of βarr2, suggesting that βarr2 acts as a mediator of mGluR5 signaling to protein translation (Stoppel et al., 2017). Importantly, reducing βarr2 expression levels reversed the behavioral and synaptic deficits in a mouse model of FXS without affecting G protein signaling or causing psychosis-like effects (Stoppel et al., 2017). These findings are of considerable translational relevance for the development of novel drugs useful for the treatment of FXS.
4. Muscarinic Receptor-Mediated Cognitive Improvements
Interestingly, a knockin mouse strain expressing a phosphorylation-deficient version of the M3 muscarinic receptor showed deficits in fear conditioning, which requires hippocampus-dependent learning and memory (Poulin et al., 2010). The mutant M3 receptor was able to activate Gq/11 proteins normally but failed to recruit β-arrestins, suggesting that β-arrestin-dependent pathways play a role in the cognition-enhancing effects of M3 receptor signaling, at least under certain experimental conditions (Poulin et al., 2010).
In a related study, Scarpa et al. (Scarpa et al., 2021) analyzed knockin mice expressing a phosphorylation-deficient version of the M1 muscarinic receptor. This mutant receptor couples normally to Gq/11 but is deficient in β-arrestin recruitment (Bradley et al., 2020). Interestingly, the authors found that mouse prion disease progresses more rapidly in the M1 receptor mutant mice as compared with WT littermates (Scarpa et al., 2021). Mouse prion disease, a progressive terminal neurodegenerative disease, displays many of the hallmarks of human Alzheimer’s disease (AD) (Mallucci et al., 2003). Based on these observations, muscarinic agonists that promote M1 receptor phosphorylation/β-arrestin-dependent signaling are predicted to be endowed with neuroprotective properties and may be able to slow down the progression of neurodegenerative diseases such as AD (Scarpa et al., 2021).
5. Potential Role of β-Arrestins in Tauopathies
The β2-AR and mGluR2 have been shown to mediate the hyperphosphorylation of tau, a key feature of AD (see Woo et al., 2021, and references therein). A recent study reported that β-arrestins are required for the ability of these two GPCRs to promote the abundance of pathogenic tau (Woo et al., 2021). Interestingly, enhanced levels of βarr1 promoted the accumulation of pathogenic tau, whereas genetic reduction of βarr1 expression alleviated tauopathy and certain cognitive deficits in mice (Woo et al., 2021). Mechanistic data indicated that βarr1 causes tauopathy by various molecular mechanisms including the dissociation of tau from microtubules (Woo et al., 2021). These new data suggest that strategies aimed at decreasing βarr1 expression levels or βarr1 activity may become clinically relevant for the treatment of tauopathies such as AD.
6. Drug Addiction and Associative Learning
Drug addiction involves associative learning, a process that attributes excessive motivational value to distinct stimuli or environments that are paired with drug use (Meyer et al., 2016). The infralimbic prefrontal cortex (IL-PFC) is thought to play a key role in extinction learning and the attenuation of the original associative memory, reducing the craving for drugs of abuse such as cocaine (Huang et al., 2018a). A recent study reported that infusion of propranolol, a nonbiased β-AR blocker, into the IL-PFC interfered with extinction learning of cocaine-induced conditioned place preference in mice (Huang et al., 2018a). This effect was not observed after infusion of carvedilol, a β-AR blocker that is able to stimulate arrestin-dependent signaling (Wisler et al., 2007). Moreover, the lack of βarr2 in IL-PFC excitatory neurons disrupted extinction learning of cocaine-conditioned place preference, while overexpression of βarr2 in IL-PFC facilitated this behavior (Huang et al., 2018a). βarr2 knockdown in mouse IL-PFC excitatory neurons also interfered with extinction learning of cocaine self-administration memory. These finding suggest that a β-AR/βarr2 signaling pathway that is operative in IL-PFC excitatory neurons is required for extinction learning of cocaine-associated memories.
Recently, the Caron laboratory reported that SBI-553, a small molecule binding to the neurotensin receptor 1 (NTSR1), functions as a β-arrestin-biased allosteric NTSR1 agonist and promotes β-arrestin recruitment to the neurotensin-occupied NTSR1 while inhibiting G protein coupling (Slosky et al., 2020). Importantly, SBI-553 demonstrated efficacy in various animal models of psychostimulant abuse but did not cause the side effects typically observed after administration of nonbiased NTSR1 agonists (Slosky et al., 2020). Clearly, these observations are of considerable clinical relevance for the development of novel approaches toward the treatment of drug addiction.
7. Anxiety Disorders
Aberrant stimulation of neural circuits that regulate anxiety and fear behaviors can cause psychiatric disorders, including posttraumatic stress disorder and various phobias (Tovote et al., 2015). Anxiety and fear are complex behaviors that are under the control of several neurotransmitter systems (Tovote et al., 2015; Hare and Duman, 2020; Chen et al., 2022). In agreement with this concept, drugs targeting multiple GPCRs can modulate the expression of fear and anxiety (Takahashi, 2001; Kindt et al., 2009; de la Mora et al., 2010; Mores et al., 2019; French and van Rijn, 2022). For example, DOR agonists can reduce anxiety and fear expression (Saitoh et al., 2004; Sugiyama et al., 2019; Yamada et al., 2019). Interestingly, treatment of mice with a β-arrestin–biased DOR agonist (SNC80) (Chiang et al., 2016) resulted in reduced anxiety-like and fear-related behaviors (Ko et al., 2021). This effect was absent in βarr2 KO mice, indicative of a central role of βarr2 in mediating these behaviors (Ko et al., 2021). Moreover, the SNC80-induced activation of ERK1/2 in limbic brain structures was not observed in βarr2 KO mice. Studies with a MEK1/2 inhibitor indicated that ERK1/2 signaling is required for SNC80-mediated anxiolysis (Ko et al., 2021). In contrast, additional studies with G protein- or arrestin-biased DOR agonists and βarr1 and βarr2 KO mice demonstrated that both β-arrestins are involved in regulating fear-related behaviors (Ko et al., 2021). These findings may guide the development of more efficacious drugs useful for the treatment of fear and anxiety disorders. As mentioned earlier, G protein-biased DOR agonists are currently being developed as novel analgesic drugs (Quirion et al., 2020). The study by the van Rijn laboratory (Ko et al., 2021) therefore raises the caveat that the use such agents for the treatment of pain could affect neuronal circuits involved in anxiety- and fear-related behaviors.
8. Alcohol Intake and Depression
DOR agonists endowed with analgesic activity are also being developed for the treatment of depression and alcohol dependence (Pradhan et al., 2011; Chu Sin Chung and Kieffer, 2013). Studies with DOR agonists differing in their efficacy to recruit β-arrestins showed that the efficacy of these drugs to increase the intake of alcohol positively correlated with their efficacy to recruit β-arrestins (Chiang et al., 2016). Interestingly, the ability of SNC80, a DOR agonist that can recruit β-arrestins with high efficacy, to enhance alcohol intake was abolished in βarr2 KO mice. In contrast, TAN67, a G protein-biased DOR agonist, suppressed alcohol intake in a β-arrestin-independent fashion. Both SNC80 and TAN67 displayed antidepressive properties in the forced swimming paradigm, in agreement with previous results (Saitoh et al., 2004; Saitoh and Yamada, 2012). The antidepressive effect of SNC80 but not that of TAN67 was significantly attenuated in βarr2 KO mice (Chiang et al., 2016). Interestingly, the antidepressant effects of fluoxetine, a selective serotonin reuptake inhibitor, were also reduced in βarr2 KO mice (David et al., 2009). The finding that DOR agonists that are able to recruit βarr2 with high efficacy can promote alcohol intake suggest that drug development efforts should focus on DOR agonists that are unable to recruit βarr2 efficiently.
9. Caveats
In most studies reviewed here, the precise molecular and cellular mechanisms through which β-arrestins modulate the actions of neurotropic drugs remain incompletely understood. One complicating factor is that receptor agonists or mutant GPCRs biased toward G protein- or β-arrestin-mediated signaling affect both signaling branches via arrestin recruitment. This could be one of the reasons for the seemingly inconsistent results observed in related studies. Thus, a receptor biased toward G proteins is likely to induce exaggerated G protein signaling, which may involve altered receptor trafficking and recycling patterns. Similarly, an arrestin-biased receptor may cause specific signaling and/or behavioral effects not only via arrestin-dependent signaling but also via altered G protein signaling due to impaired G protein recruitment. The role of β-arrestins in GPCR recycling and resensitization is also often overlooked. Similar caveats apply to the use of biased GPCR ligands.
Interestingly, a recent study with βarr1/βarr2 double mutant mice revealed a complex interplay between the two arrestin isoforms regarding the behavioral effects of AMPH (Zurkovsky et al., 2017). It remains to be explored whether this finding is of more general relevance for β-arrestin-modulated actions of neurotropic drugs. The potential roles of βarr1, which is highly expressed in most neuronal subpopulations (Gurevich et al., 2002), in the behavioral and/or therapeutic effects of psychotropic drugs remain largely unexplored.
V. Modulation of Cardiovascular Functions by β-Arrestins
The potential roles of β-arrestins in regulating various cardiovascular functions have been reviewed in detail recently (Lymperopoulos, 2018; Jiang et al., 2022; Lino and Barreto-Chaves, 2022). In the following, we only briefly discuss the therapeutic potential of arrestin-biased AT1R agonists. Angiotensin II regulates cardiovascular functions primarily via activation of AT1Rs (Balakumar and Jagadeesh, 2014; Lino and Barreto-Chaves, 2022). Abnormal signaling by AT1R is involved in multiple cardiovascular pathologies including hypertension and heart failure, as well as various other pathophysiological conditions (Balakumar and Jagadeesh, 2014; Karnik et al., 2015). Various lines of evidence suggest that arrestin signaling mediates the beneficial effects of AT1R activation on cardiac contractility while limiting the deleterious effects of excessive adrenergic and AT1R stimulation leading to heart failure (Monasky et al., 2013; Ryba et al., 2017; Capote et al., 2021; Lino and Barreto-Chaves, 2022). For this reason, arrestin-biased AT1R agonists are predicted to have considerable potential for the treatment of various cardiovascular disorders.
In a recent study, an arrestin-biased ATR1 agonist TRV027 was administered in a phase IIb clinical trial to patients with acute heart failure (Pang et al., 2017). Disappointingly, TRV027 did not lead to any clinically significant improvements at any of the three doses tested (Pang et al., 2017). Possible reasons for the negative outcome of this study have been discussed recently (Lino and Barreto-Chaves, 2022). Given the vast amount of encouraging preclinical and clinical data that have been obtained with arrestin-biased AT1R agonists (Monasky et al., 2013; Ryba et al., 2017; Capote et al., 2021; Lino and Barreto-Chaves, 2022), other members of this drug family endowed with different pharmacokinetic and pharmacodynamic properties may prove clinically beneficial in different patient cohorts suffering from heart failure and other cardiovascular conditions.
VI. Cancer and β-Arrestins
A. General Comments
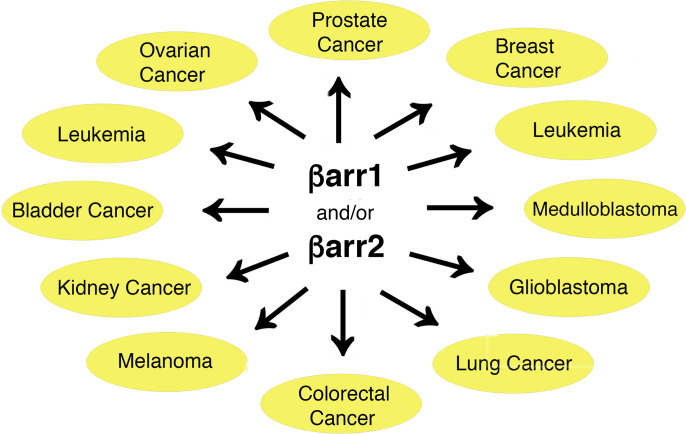
Many studies have shown that altered GPCR signaling can contribute to cancer progression at multiple levels (Arang and Gutkind, 2020). Since βarr1 and βarr2 are important regulators of GPCR activity, it is not surprising that these two proteins have been implicated in the pathogenesis of various types of cancer (Bagnato and Rosanò, 2019; Shukla and Dwivedi-Agnihotri, 2020; Aamna et al., 2022; Tian et al., 2022) (Fig. 3).
Fig. 3.
β-arrestins play important roles in regulating carcinogenic processes. The cancer types shown in this figure are briefly discussed in the text. In most but not all cases, β-arrestins contribute to tumor formation by stimulating processes that promote cell proliferation. See text for details.
Moreover, β-arrestins can modulate various signaling pathways involved in cell proliferation, carcinogenesis, and metastasis (Peterson and Luttrell, 2017). As reviewed recently (Peterson and Luttrell, 2017), Ras-mediated activation of mitogenic ERK1/2 signaling, transactivation of EGF receptors, and cytoskeletal rearrangements represent three major cellular pathways or mechanisms through which β-arrestins can promote cell cycle progression. In line with these findings, many studies have shown that β-arrestins play a role in cancer initiation and progression (Bagnato and Rosanò, 2019; Shukla and Dwivedi-Agnihotri, 2020; Aamna et al., 2022; Tian et al., 2022).
In the following, we briefly review several recent studies in the β-arrestin/cancer field. Cancer is a highly heterogeneous disease that is endowed with distinct molecular signatures depending on tumor stage and initial site of formation (Dagogo-Jack and Shaw, 2018). This heterogeneity, combined with the various different in vivo and in vitro tumor models used by different laboratories, may explain that the two β-arrestins can both promote or inhibit cancer formation and progression under specific experimental conditions.
B. Roles of β-Arrestin-1 in Carcinogenesis
1. Ovarian Cancer
Activation of the two endothelin receptor (ETR) subtypes (ETA and ETB) can promote tumorigenesis and metastatic progression (Tocci et al., 2019). Accumulating evidence suggests that ETR/βarr1 signaling plays an important role in mediating these processes. In ovarian cancer, for example, the ETR/βarr1 signaling module stimulates cellular processes, including changes in gene transcription, that promote tumorigenesis, suggesting that strategies aimed at interfering with ETR/βarr1 signaling may prove useful for the treatment of this type of cancer (Tocci et al., 2019). A related study reported that hMENA, a member of the actin-regulatory protein ENA/VASP family, can bind to βarr1 and that this interaction is required for ETRA-mediated invadopodial function during the progression of serous ovarian cancer (SOC) (Di Modugno et al., 2018). Interestingly, ligand activation of ETRA in cultured SOC cells promoted the binding of βarr1 to hMENA/hMENAΔv6, resulting in the activation of RhoC, cortactin, and other cellular pathways, triggering the maturation of invadopodia and the spread of SOC cells (Di Modugno et al., 2018). Moreover, treatment with an ETRA antagonist interfered with the formation of the βarr1/hMENA complex, resulting in impaired invadopodial maturation (Di Modugno et al., 2018). This finding suggests that ETRA antagonists may prove clinically useful to prevent the progression of ovarian cancer.
2. Prostate Cancer
Several reports suggest that βarr1 signaling may also play a role in the pathogenesis of prostate cancer (see, for example, Zecchini et al., 2014). A recent study demonstrated that βarr1 can promote cell growth by inhibiting the expression of FOXO3a in prostate cancer cells in vitro and in vivo (Kong et al., 2018). Mechanistic data suggested that βarr1 inhibits the transcriptional activity of FOXO3a via Akt- and ERK1/2-dependent pathways and stimulates the degradation of FOXO3a via MDM2-mediated ubiquitination (Kong et al., 2018). These findings support the concept that strategies aimed at suppressing the expression and/or activity of βarr1 may lead to novel anticancer drugs.
Androgen receptor (AR)-mediated signaling drives all stages of prostate cancer, including castration-resistant prostate cancer (CRPC), the lethal and drug-resistant form of the disease (Feng and He, 2019). Interestingly, a recent study showed that the expression of βarr1 is upregulated in CRPC and that nuclear βarr1 promotes prostate cancer cell migration and invasion in vitro and stimulates prostate tumor growth in vivo (Purayil et al., 2021). Mechanistic data suggest that these effects are mediated by a βarr1/β-catenin complex that controls the expression of AR-regulated genes that drive CRPC (Purayil et al., 2021). Approaches capable of inhibiting the formation of this nuclear complex may lead to new drugs useful for the treatment of CRPC.
Upregulation of βarr1 expression in human prostate cancer, including CRPC, is also positively correlated with increased expression and function of the glucocorticoid receptor (GR) (Purayil and Daaka, 2022). Biochemical studies have shown that βarr1 forms a complex with GR in the nucleus of CRPC cells and that downregulation of βarr1 expression inhibits GR function and CRPC growth and invasion both in vitro and in vivo (Purayil and Daaka, 2022). These data suggest that the βarr1/GR complex represents a potential target for the treatment CRPC.
3. Breast Cancer
An early study (Lundgren et al., 2011) demonstrated the potential importance of βarr1 as a prognostic and treatment-predictive marker in breast cancer. A related study reported that βarr1 levels are reduced while βarr2 levels are elevated during breast cancer progression and that these changes in β-arrestin expression levels correlate with a poor clinical outcome (Michal et al., 2011). Moreover, Shenoy et al. (Shenoy et al., 2012) showed that βarr1 can interact with hypoxia-induced factor-1α (HIF-1α) in breast carcinoma cells and that this interaction is critical for HIF-1α-dependent expression of vascular endothelial growth factor (VEGF) A. Interestingly, βarr1 and VEGF-A expression levels were positively correlated in metastatic human breast cancer tissues, suggesting that βarr1 modulates gene transcription under hypoxic conditions to promote breast cancer cell growth in a VEGF-dependent fashion (Shenoy et al., 2012).
A recent study demonstrated that suppression of the expression of miR-374a-5p, a miRNA upregulated in triple negative breast cancer (TNBC), reduced tumor progression, and growth in TNBC cell lines (Son et al., 2019). Moreover, inhibition of miR-374a-5p expression resulted in increased βarr1 expression in TNBC cell lines and in xenograft mouse models. Additional studies demonstrated that overexpression of βarr1 inhibited the growth and migration of TNBC cell lines in an AMPK-dependent fashion (Son et al., 2019), suggesting that βarr1 acts as a tumor suppressor in TNBC. In contrast to this finding, several other studies reported that βarr1 expression is upregulated in different types of cancer (Zecchini et al., 2014; Yang et al., 2015; Purayil and Daaka, 2018). These data suggest that the ability of βarr1 to promote or suppress cancer cell growth and metastasis may depend on the type of cancer under investigation.
Consistent with the findings by Son et al. (Son et al., 2019), a recent study demonstrated that knockdown of βarr1 or βarr2 expression in TNBC cell lines stimulated cell proliferation and invasion, whereas overexpression of βarr1 or βarr2 inhibited these processes (Bostanabad et al., 2021). Overexpression of the two β-arrestins resulted in cell cycle S-phase arrest and the altered expression of many cell cycle genes (Bostanabad et al., 2021). The authors also reported a positive correlation between low βarr1 expression levels and poorer prognosis in breast cancer patients (Bostanabad et al., 2021). These data support the concept that βarr1 functions as a tumor suppression in TNBC.
4. Leukemia
Human telomerase reverse transcriptase (hTERT) is highly expressed in many malignant tumors including certain types of leukemia (Liu et al., 2017a; Leão et al., 2018). Interestingly, knockdown of βarr1 expression promoted cell senescence in cells mimicking a certain subtype of leukemia-initiating cells in vivo and in vitro (Liu et al., 2017a). Mechanistic data indicated that βarr1 stimulates hTERT transcription by facilitating the binding of P300-Sp1 to the hTERT promoter (Liu et al., 2017a). Moreover, elevated βarr1 levels in senile leukemia-initiating cells from acute lymphoblastic leukemia patients were predictive of poor prognosis (Liu et al., 2017a). These findings suggest that βarr1 acts as tumor-promoting factor in certain forms of leukemia.
Activating mutations of NOTCH1, a transmembrane receptor linked to many signaling pathways involved in tumorigenesis (Gharaibeh et al., 2020), are observed in most cases of T-cell acute lymphoblastic leukemia (T-ALL) (Liu et al., 2017b), the most common type of childhood cancer. A recent study reported that overexpression of βarr1 inhibited the progression of T-ALL in vivo and in vitro (Shu et al., 2020). RNA sequencing data suggested that βarr1 probably exerts its tumor-suppressive activity, at least in part, by reducing the expression of genes associated with the NOTCH1 signaling pathway (Shu et al., 2020). Additional data obtained with cultured cells indicated that βarr1 is also able to promote the degradation of NOTCH1 by facilitating NOTCH1 ubiquitination (Shu et al., 2020). These findings establish βarr1 as a tumor suppressor in T-ALL. In contrast, as reviewed in this section, many recent studies have shown that βarr1 signaling contributes to the formation and progression of various other types of cancer.
5. Medulloblastoma
Medulloblastoma (MB) is the most common pediatric malignant brain tumor (Massimino et al., 2011). In about one quarter of patients, MB is driven by aberrant sonic hedgehog/Gli signaling (SHH-MB) (Miele et al., 2017). A recent study (Miele et al., 2017) using a mouse model of SHH-MB reported the downregulation of βarr1 in cancer stem cells derived from SHH-MB. Interestingly, the expression of miR-326, which is localized to the first intron of the mouse βarr1 gene, was also significantly reduced in these cells (Miele et al., 2017). Additional studies showed that miR-326 suppresses sonic hedgehog (HH)/Gli signaling by targeting various components of this signaling cascade and that βarr1 interferes with Gli1 transcriptional activity by facilitating p300-dependent Gli1 acetylation, resulting in a further impairment of the sonic HH/Gli signaling module (Miele et al., 2017). These new findings suggest that βarr1 represents a potential target for the treatment of SHH-MB.
6. Glioblastoma
Glioblastoma is the most common type of malignant brain tumor among adults (Tan et al., 2020). Several studies have demonstrated that neurokinin-1 receptor (NK1R) agonists promote glioblastoma cell proliferation and that NK1R antagonists can slow down glioma cell growth (for references, see Zhang et al., 2017). Knockdown of βarr1 expression in human glioblastoma cells inhibited NK1R-mediated cell proliferation and caused G2/M phase cell cycle arrest, associated with the downregulation of several genes involved in cell cycle progression. In glioblastoma cells, NK1R activation resulted in a prolonged phosphorylation of ERK1/2 and Akt in an βarr1-dependent fashion (Zhang et al., 2017). This response was absent in glioblastoma cells with reduced βarr1 expression, suggesting that inhibition of these signaling pathways contribute to impaired cell cycle progression and cell proliferation. Interestingly, following knockdown of βarr1 expression, glioblastoma cells showed increased sensitivity to treatment with NK1R antagonists (Zhang et al., 2017), suggesting that inhibition of NK1R-dependent βarr1-signaling may prove beneficial for the therapy of glioblastoma.
7. Lung Cancer
The G protein–coupled bile acid receptor (GPBAR) regulates many important physiologic and pathophysiological functions including carcinogenesis (Jia et al., 2018). The GPBAR is highly expressed in non–small cell lung cancer (NSCLC), and a positive correlation exists between GPBAR expression levels and the clinical progression of NSCLC (Liu et al., 2018). Ma et al. (Ma et al., 2022) recently identified a novel GPBAR agonist, R399, that preferentially promotes βarr1 signaling. Studies with NSCLC cells showed that R399 stimulated YAP signaling and cell proliferation in a βarr1-dependent fashion (Ma et al., 2022). In contrast, treatment of NSCLC cells with a G protein-biased GPBAR agonist, INT-777, interfered with YAP signaling, inhibited cell proliferation, and induced apoptosis (Ma et al., 2022). These new data are highly relevant for the design of novel anticancer agents targeting the GPBAR.
A recent study reported that PLEKHH2, a member of the pleckstrin homology domain-containing family H, is also highly expressed in NSCLC (Wang et al., 2022b). Studies with NSCLC cells showed that high expression of PLEKHH2 facilitated cell proliferation, migration, and invasion (Wang et al., 2022b). Biochemical studies showed that PLEKHH2 binds to βarr1 through its FERM domain, resulting in the activation of the FAK/PI3K/AKT signaling cascade and the stimulation of NSCLC cell proliferation, migration, and invasion (Wang et al., 2022b). These findings indicate that βarr1 can modulate the progression of NSCLC via multiple cellular mechanisms that could be targeted for therapeutic purposes.
C. Roles of β-Arrestin-2 in Carcinogenesis
1. Breast Cancer
Previous work has demonstrated that βarr2 can be covalently modified via SUMOylation (Wyatt et al., 2011; Xiao et al., 2015). Recent data (Dong et al., 2020) showed that a SUMOylation-deficient mutant version of βarr2 slowed the migration of breast cancer cells but had little effect on cell proliferation. This effect was accompanied by changes in the expression levels of a series of metabolically important genes, suggesting that βarr2 SUMOylation represents an important factor regulating breast cancer progression (Dong et al., 2020).
A recent study reported that βarr2 plays an important role in kisspeptin receptor-dependent formation of invadopodia in breast cancer cells (Goertzen et al., 2016). Invadopodia are actin-rich protrusions of the plasma membrane involved in cancer invasiveness and metastasis (Linder et al., 2022). Specifically, agonist stimulation of kisspeptin receptors stimulated the formation of invadopodia via an βarr2- and ERK1/2-dependent mechanism (Goertzen et al., 2016). These data suggest that inhibition of βarr2 binding to kisspeptin receptors may lead to the development of novel anticancer drugs.
The Iverson laboratory (Perry et al., 2019) recently demonstrated that βarr2 can directly interact with the kinase domain of maternal embryonic leucine zipper kinase (MELK). The expression of MELK, a serine/threonine kinase known to play an important role in cell cycle regulation and proliferation, is increased in various cancer cells including breast cancer cells (Ganguly et al., 2015). Strikingly, coexpression of βarr2 and MELK led to a significant decrease in the number of cells in the S-phase, indicating that βarr2 can interfere with cell proliferation via this mechanism (Perry et al., 2019). More detailed mechanistic studies are needed to further explore how βarr2 affects MELK function and cellular localization where it exerts its antiproliferative activity.
2. Leukemia
Chronic myelogenous leukemia (CML) is a myeloproliferative form of cancer that is defined by the unrestrained proliferation of pluripotent bone marrow stem cells (Thompson et al., 2015). Studies with β-arrestin KO mice demonstrated that βarr2 is essential for the progression of CML, most likely due to its ability to stabilize β-catenin, thus promoting Wnt/β-catenin signaling (Fereshteh et al., 2012). This observation raises the possibility that βarr2 also plays a role in the pathogenesis of other solid cancers characterized by aberrant Wnt signaling (Fereshteh et al., 2012).
Primary myelofibrosis (PMF), which is closely related to CML, represents another form of chromic leukemia characterized by myeloproliferation and bone marrow fibrosis (Abdel-Wahab and Levine, 2009). Studies with a mouse model of PMF showed that conditional deletion of βarr2 from established PMF interferes with the progression of this disease, possibly due to the antiapoptotic properties of βarr2 (Rein et al., 2017).
3. Ovarian Cancer
Ovarian cancer is the most fatal gynecologic cancer (Stewart et al., 2019). It is often referred to as the “silent killer,” since it is frequently not diagnosed until it has progressed to advanced stages (Stewart et al., 2019). A recent study reported a positive correlation between cytoplasmic βarr2 expression levels in ovarian cancer samples and reduced overall survival (Czogalla et al., 2020). In agreement with this observation, βarr2 overexpression enhanced the viability of an ovarian cancer cell line (A2780) in vitro (Czogalla et al., 2020). These findings suggest that βarr2 expression levels in ovarian cancer are of potential prognostic value and that strategies that can interfere with βarr2 expression or activity may have potential in this type of cancer.
4. Colorectal Cancer
Colorectal cancer (CRC) is among the most frequent types of cancer and most common causes of cancer death worldwide (Favoriti et al., 2016). 5-Fluorouracil (5-FU) represents the first-line treatment of CRC (Vodenkova et al., 2020). A recent study found that βarr2 levels were increased in CRC tissues compared with normal colon tissues (Ren et al., 2018). In vitro studies with CRC cell lines showed that knockdown of βarr2 expression resulted in a decrease in 5-FU-induced apoptosis, while overexpression of βarr2 promoted cancer cell apoptosis (Ren et al., 2018). Moreover, following 5-FU treatment, downregulation of βarr2 expression led to reduced levels of pro-apoptotic proteins (cleaved caspase-3 and Bax) and increased expression of Bcl-2, an anti-apoptotic protein (Ren et al., 2018). These data indicate that βarr2 plays a key role in mediating 5-FU-stimulated apoptotic responses in CRC, a finding that is of considerable translational relevance.
5. Medulloblastoma
Suppressor of fused (SuFu) is a highly conserved protein that functions as an inhibitor of the HH signaling pathway, a major determinant of cell differentiation and proliferation (Huang et al., 2018b). Mutations in the human SuFu gene predispose to sonic HH medulloblastoma (Guerrini-Rousseau et al., 2018). A recent study reported that a complex between Itch, a E3 ubiquitin ligase, and βarr2 promotes the ubiquitination of SuFu, resulting in impaired HH signaling (Infante et al., 2018). This observation indicates that βarr2 is an important regulator of the tumor suppressor functions of SuFu, suggesting that βarr2 may represent a potential target for the treatment of medulloblastoma.
6. Kidney Cancer
Kidney cancer is among the most common cancers worldwide (Scelo and Larose, 2018). Most kidney cancers represent renal cell carcinomas (RCCs). Masannat et al. (Masannat et al., 2018) recently reported that ARRB2 (gene encoding human βarr2) transcript levels are increased in RCC and that a positive correlation exists between ARRB2 expression levels and patient survival rate. Deletion of ARRB2 inhibited the rate of RCC cell proliferation and migration both in vitro and in vivo (Masannat et al., 2018). Additional studies showed that βarr2 regulates RRC cell cycle progression through c-Src activation and cyclin A expression (Masannat et al., 2018). In sum, βarr2 regulates the progression of RCC and represents a potential target for new drugs targeting this type of cancer.
7. Glioblastoma
High expression levels of βarr2 correlate with reduced tumorigenesis in glioblastoma and increased survival probability in glioblastoma patients (Bae et al., 2021). Recent data suggest that βarr2 promotes the degradation of HIF-1α, the master regulator of the body's response to low oxygen concentrations (Bae et al., 2021). Functional studies showed that βarr2 interacts with HIF-1α and stimulates its proteasomal degradation by recruiting PHD2 and pVHL, two key factors intimately involved in ubiquitin-dependent HIF-1α degradation (Bae et al., 2021). Overexpression of βarr2 in human glioblastoma cells resulted in impaired HIF-1α signaling, tumor growth, and angiogenesis (Bae et al., 2021), supporting the concept that βarr2 can regulate the stability of HIF-1α in certain human cancers such as glioblastoma, a finding that is of potential translational relevance.
D. Roles of β-Arrestin-1/β-Arrestin-2 in Carcinogenesis
1. Atypical Chemokine Receptors
Chemokines are involved in the pathophysiology of various cancers, due to their ability to modulate cell migration and proliferation (Caronni et al., 2016; Liu et al., 2020). Recent reports have implicated atypical chemokine receptors (ACKRs) in tumor initiation and metastasis (Mollica Poeta et al., 2019; Lokeshwar et al., 2020; Sjöberg et al., 2020; Torphy et al., 2022). ACKRs, following the binding of distinct chemokine ligands, are unable to initiate classic G protein-mediated signaling (Nibbs and Graham, 2013; Torphy et al., 2022). In agreement with this notion, ligand activation of the ACKR3 stimulates arrestin-mediated pathways, without activating heterotrimeric G proteins (Rajagopal et al., 2010a; Gustavsson et al., 2017). The expression of ACKR3 protein or mRNA is increased in numerous cancers (Sjöberg et al., 2020). Because of this finding, this chemokine receptor subtype is considered a potential target for novel anticancer drugs (Sjöberg et al., 2020). ACKR3 activation can lead to the stimulation of ERK1/2, Akt, and other pathways able to promote tumor formation in a β-arrestin-dependent fashion (Sjöberg et al., 2020), suggesting that inhibition of the ACKR3/β-arrestin module might prove beneficial clinically in certain types of cancer.
2. Bladder Cancer
A recent study reported that specimens from human bladder cancer show increased βarr1 but reduced βarr2 expression, as compared with specimens from normal bladder tissues (Kallifatidis et al., 2019). Both changes in β-arrestin gene expression positively correlated with bladder cancer metastasis. Knockdown of βarr2 expression in bladder cancer cell lines resulted in an increase in cancer stem cell markers, while overexpression of βarr2 had the opposite effect (Kallifatidis et al., 2019). In contrast, deletion of the βarr1 gene in bladder cancer cells resulted in reduced expression of several cancer stem cell markers. The authors also presented data suggesting that βarr1/2 expression levels can be predictive of the response to chemotherapy in bladder cancer (Kallifatidis et al., 2019). These findings suggest that βarr1 and βarr2 can affect bladder cancer progression in an opposing fashion, most likely by interacting with different sets of cellular signaling proteins.
3. Leukemia
Chronic lymphoproliferative disorder of natural killer cells is a form of leukemia defined by the clonal expansion of natural killer cells. Baer et al. (Baer et al., 2022) recently reported that somatic mutations in a chemokine gene (CCL22) are found in many cases of chronic lymphoproliferative disorder of natural killer cells. These mutations interfered with the ability of CCL22 to promote the internalization of the chemokine receptor 4 subtype, due to impaired β-arrestin recruitment (Baer et al., 2022). This deficit resulted in increased cell chemotaxis and enhanced natural killer cell proliferation in vitro and in vivo, indicating that impaired chemokine receptor 4-mediated β-arrestin recruitment can promote tumor formation.
4. Melanoma
The incidence of malignant melanoma has increased dramatically over the past few decades (Lopes et al., 2022). Interestingly, a recent study reported that the expression of the oxytocin receptor (OTR) was increased in malignant melanoma (Ji et al., 2019). In vitro and in vivo studies showed that ligand-dependent OTR activation facilitated melanoma cell migration, invasion, and angiogenesis via a βarr2-dependent ERK-VEGF-matrix metalloproteinase-2 signaling network (Ji et al., 2019). These findings raise the possibility that OTR-mediated βarr2 recruitment may play a role in the pathophysiology of at least certain forms of melanoma.
A related study reported that CXCR7 was the most highly expressed chemokine receptor in mouse melanoma cell lines and that CXCR7 expression levels were positively correlated with melanoma progression in human melanoma samples (Xu et al., 2019). Overexpression of CXCR7 stimulated melanoma proliferation in vitro and in vivo, whereas inactivation of the CXCR7 gene resulted in opposite effects (Xu et al., 2019). Additional studies showed that CXCR7-mediated stimulation of melanoma cell proliferation required βarr2-dependent activation of Src (Xu et al., 2019). CXCR7 activation also promoted the secretion of VEGF and melanoma angiogenesis via upregulation of the expression of HIF-1α. These findings suggest that the CXCR7 receptor subtype represents a potential target for novel anticancer drugs.
Several studies have shown that the apelin receptor is overexpressed in different types of cancer including melanoma (Wysocka et al., 2018). Loss-of-function mutations in the apelin receptor are linked to impaired CD8+ T cell cytotoxicity, reduced interferon-γ (IFN-γ) signaling in tumor cells, and decreased efficacy of cancer immunotherapies (Patel et al., 2017). Liu et al. (Liu et al., 2022) recently reported that activation of the apelin receptor in melanoma cells stimulates IFN-γ signaling via recruitment of βarr1 and that βarr1 binding to STAT1 results in impaired STAT1 phosphorylation and reduced IFN-γ signaling (Liu et al., 2022). These data suggest that the effectiveness of melanoma immunotherapies may depend on the activity of the apelin receptor/βarr1 signaling module.
E. Conclusions
The studies summarized here indicate that βarr1 and βarr2 play important roles in regulating the formation and progression of many different types of cancer. Depending on the specific tumor models employed, these effects can be stimulatory or inhibitory in nature. Clearly, the outcome of this work strongly suggests that it should be possible to target β-arrestins and the signaling pathways through which they regulate cell proliferation and survival for the development of novel anticancer drugs.
VII. Regulation of Metabolic Functions by β-Arrestins
During the past decades, the prevalence of type 2 diabetes (T2D) has reached epidemic proportions worldwide, primarily resulting from changes in lifestyle that include the consumption of energy-dense refined food and reduced physical activity (Roden and Shulman, 2019; Eizirik et al., 2020). Although various drugs are in clinical use to reduce pathologically elevated blood glucose levels, the hallmark of T2D, the disease is still linked to a high degree of morbidity and mortality (Roden and Shulman, 2019; Eizirik et al., 2020). It is likely that a better understanding of the cellular and molecular mechanisms that regulate the function of the various cell types involved in maintaining euglycemia will lead to more efficacious antidiabetic drugs with an improved side effect profile.
A. Cell Type-Specific β-Arrestin Mutant Mice as Novel Tools
Whole-body βarr1 and βarr2 KO mice exhibit major metabolic phenotypes, suggesting that the two β-arrestins regulate several important metabolic functions including glucose tolerance and insulin sensitivity (Zhao and Pei, 2013). Since the two β-arrestins are widely expressed throughout the body, the identification of specific cell types and signaling pathways responsible for the observed metabolic phenotypes remains a challenging task. The interpretation of the metabolic phenotypes displayed by whole-body β-arrestin KO mice is further complicated by the fact that β-arrestins regulate important developmental functions (Kovacs et al., 2009; Philipp et al., 2013), raising the possibility that compensatory pathways affect glucose and energy homeostasis in adult βarr1 and βarr2 KO mice.
To overcome these difficulties, recent studies focused on generating and analyzing β-arrestin mutant mice that lack either βarr1 or βarr2 in specific cell types that are known to play key roles in regulating energy and glucose homeostasis (Pydi et al., 2022). These studies took advantage of the recent development of floxed βarr1 and βarr2 mutant mice (Urs et al., 2016; Kim et al., 2018). In mouse hepatocytes, myocytes, and pancreatic β-cells, the genes coding for βarr1 and βarr2 were inactivated in adult mice in a tamoxifen-dependent fashion by using Cre driver lines in which Cre activity is inducible by repeated tamoxifen injections (Pydi et al., 2022). In the following, we briefly discuss the major metabolic phenotypes of these cell-type specific β-arrestin KO mice, the underlying cellular and molecular mechanisms, and potential therapeutic targets suggested by the outcome of these studies.
B. Hepatocytes
1. β-Arrestin-2
Hepatocytes express many GPCRs including the glucagon receptor (GCGR), which is expressed at particularly high levels (Regard et al., 2008). Glucagon-mediated activation of hepatic GCGRs leads to the activation of the Gs/PKA signaling cascade, which triggers the stimulation of gluconeogenesis and glycogen breakdown and ultimately results in enhanced hepatic glucose production and output. While this signaling pathway plays a key role in maintaining euglycemia under fasting conditions, elevated hepatic GCGR signaling is thought to contribute to the pathophysiology of T2D and related metabolic disorders (D’Alessio, 2011; Capozzi et al., 2022).
Metabolic studies with mutant mice lacking βarr2 selectively in hepatocytes (hep-βarr2 KO mice) demonstrated that hep-βarr2 KO mice showed impaired glucose tolerance associated with enhanced hepatic GCGR signaling, both in vivo and in vitro (Zhu et al., 2017b). In agreement with this finding, glucagon treatment led to more pronounced elevations in blood glucose levels in hep-βarr2 KO mice than in control littermates. Similarly, glucagon-stimulated cAMP levels and increases in hepatic glucose output were significantly augmented in primary hepatocytes lacking βarr2 (Zhu et al., 2017b), indicating that βarr2 functions as a negative regulator of GCGR signaling in hepatocytes (Fig. 4).
Fig. 4.
Metabolic roles of βarr1 and βarr2 in metabolically important cell types. The overview given in this figure is based on data obtained with mutant mice selective lacking or overexpressing either of the two β-arrestins in hepatocytes, adipocytes, pancreatic β-cells, or AgRP neurons of the arcuate nucleus of the hypothalamus (Pydi et al., 2022).
In vitro studies showed that glucagon treatment of primary hepatocytes prepared from control mice resulted in a marked decrease in the number of cell surface GCGRs (Zhu et al., 2017b). In striking contrast, this response was absent in primary hepatocytes lacking βarr2 (Zhu et al., 2017b), clearly indicating that βarr2 is required for the internalization of hepatic GCGRs. These observations suggest that hepatic βarr2 functions to dampen GCGR signaling under physiologic conditions by promoting GCGR internalization, a so-called canonical or conventional function of βarr2.
In contrast to hep-βarr2 KO mice, mice overexpressing βarr2 selectively in hepatocytes (hep-βarr2-OE mice) displayed significant improvements in glucose homeostasis, in particular when hep-βarr2-OE mice were maintained on a calorie-rich high-fat diet (HFD) (Zhu et al., 2017b). Under these conditions, hep-βarr2-OE mice showed reduced blood glucose levels and improved glucose tolerance, as compared with their control littermates. Additional studies demonstrated that these beneficial metabolic effects were due to reduced GCGR signaling caused by elevated hepatic βarr2 expression (Zhu et al., 2017b). These findings suggest that agents capable of enhancing the activity or expression of hepatic βarr2 may prove therapeutically beneficial under conditions of impaired glucose homeostasis.
2. β-Arrestin-1
Mutant mice lacking βarr1 selectively in hepatocytes (hep-βarr1 KO mice) did not display any significant metabolic phenotypes (Zhu et al., 2017b). This observation suggests that the physiologic functions of βarr1 and βarr2 are not redundant in this cell type.
C. Adipocytes
1. β-Arrestin-2
The ongoing obesity epidemic is the major driver of the high prevalence of T2D in most parts of the world (Klein et al., 2022). Obesity is associated with the enlargement of adipocytes (adipocyte hypertrophy), inflammatory processes in fat tissue, and enhanced secretion of biologically active agents that have a negative impact on glucose homeostasis (Klein et al., 2022). These factors, which include free fatty acids (FFAs) and proinflammatory cytokines, interfere with the ability of peripheral tissues to properly respond to insulin. This phenomenon, referred to as peripheral insulin resistance, represents a hallmark of T2D.
In mammals, adipose tissues contain two major types of adipocytes, white and brown adipocytes (Wang and Seale, 2016). White adipocytes primarily function to store excess calories in the form of triglycerides. In contrast, brown adipocytes consume nutrients such as FFAs and glucose to generate heat, in particular after activation of the sympathetic nervous system (Wang and Seale, 2016). Interestingly, a third class of adipocytes, referred to as “beige adipocytes,” can arise from precursor cells in white adipose tissue (WAT) or through conversion of white adipocytes (Wang and Seale, 2016). Like brown adipocytes, beige adipocytes express uncoupling protein 1 and several other thermogenic genes and function to dissipate chemical energy stored in nutrients into heat.
To explore the role of βarr2 in regulating adipocyte function in vivo, Pydi et al. (Pydi et al., 2019) generated and analyzed mice that selectivity lacked βarr2 in white adipocytes (adipo-βarr2-KO mice). When adipo-βarr2-KO mice were maintained on a HFD, the mutant mice gained significantly less body weight than their control littermates, due to reduced fat accumulation (Pydi et al., 2019). Moreover, the mutant mice were largely resistant to the metabolic deficits that are normally caused by the consumption of a HFD. Additional studies showed that these phenotypes resulted from an increase in energy expenditure caused by WAT βarr2 deficiency (Pydi et al., 2019). Consistent with this finding, the expression of uncoupling protein 1 and other thermogenic genes was significantly increased in inguinal (subcutaneous) WAT lacking βarr2, indicating that βarr2 deficiency stimulates the beiging of WAT.
Adipocytes express numerous GPCRs that are linked to different functional classes of heterotrimeric G proteins (Ceddia and Collins, 2020). Since the activation of adipocyte β-ARs promotes lipolysis and the beiging of WAT (Collins, 2022), this class of receptors has been studied in considerable detail. The β3-AR is the predominant β-AR subtype expressed by mouse adipocytes, and activation of this receptor subtype in vivo (e.g., by administration of CL316243, a selective β3-AR agonist) promotes the beiging of WAT (Collins, 2022). Interestingly, CL316243 treatment of white adipocytes prepared from adipo-βarr2 KO mice led to a more robust increase in cAMP levels, as compared with CL316243-treated control adipocytes (Pydi et al., 2019), suggesting that βarr2 acts as an inhibitor of β3-AR signaling in WT adipocytes (Fig. 4). Thus, this βarr2 function is consistent with its conventional role as a terminator of GPCR G protein signaling.
CL316243 treatment of a cultured mouse adipocytes (3T3-F442A cells) resulted in the rapid disappearance of β3-ARs from the cell surface (receptor internalization) (Pydi et al., 2019). Strikingly, siRNA-mediated knockdown of βarr2 expression in these cells completely blocked this CL316243 response (Pydi et al., 2019), strongly suggesting that the lack of β3-AR internalization is responsible for the increase in β3-AR signaling observed with adipo-βarr2 KO mice.
The metabolic improvements observed with HFD adipo-βarr2-KO mice were absent after treatment with propanol, a β-AR blocker, or when the mutant mice were kept at thermoneutrality (30°C). At thermoneutrality, the activity of the sympathetic nervous system is strongly attenuated. In addition, adipocyte-specific deletion of PRDM16, a master transcriptional coregulator required for the beiging of WAT (Wang and Seale, 2016), prevented all metabolic changes resulting from the lack of βarr2 in adipocytes. Taken together, these findings indicate that the metabolic improvements displayed by the adipo-βarr2-KO mice require the beiging of WAT, which is mediated by enhanced G protein signaling via adipocyte β3-ARs.
Human WAT cells also express β3-ARs, although β1- and β2-AR are expressed at considerable higher levels (Collins, 2022). In any case, the data reported by Pydi et al. (Pydi et al., 2019) suggest that G protein-biased, selective β3-AR agonists may prove clinically useful for the treatment of obesity and impaired glucose homeostasis.
2. β-Arrestin-1
To explore the potential metabolic roles of βarr1 expressed by adipocytes, Pydi et al. (Pydi et al., 2020b) studied mutant mice lacking βarr1 selectively in adipocytes (adipo-βarr1-KO mice). Unlike their HFD adipo-βarr2-KO counterparts, HFD adipo-βarr1-KO mice showed striking metabolic impairments including hyperglycemia, impaired glucose tolerance, and reduced insulin sensitivity. Also, in contrast to the HFD βarr2 mutant mice, the HFD adipo-βarr1-KO mice did not display significant changes in body fat mass and total energy expenditure, as compared with their control littermates (Pydi et al., 2020b). Additional studies showed that mutant mice that overexpressed βarr1 in adipocytes were protected from HFD-induced metabolic deficits (Pydi et al., 2020b).
RNA sequencing and quantitative reverse transcription polymerase chain reaction studies showed that the expression levels of Mstn (gene encoding myostatin or short Mstn) and various other myogenic genes were significantly increased in brown adipose tissue (BAT), but not in WAT, of HFD adipo-βarr1-KO mice. In contrast, the BAT expression levels of Ucp1 and other thermogenic genes remained largely unaffected by the lack of βarr1. In agreement with the gene expression data, plasma Mstn levels were significantly elevated in adipo-βarr1-KO mice (Pydi et al., 2020b).
Mstn, a member of the transforming growth factor-β superfamily, is named after its ability to suppress skeletal muscle growth (Lee, 2023). Lineage tracing studies have shown that BAT cells are derived from Myf5-positive precursor cells that also give rise to skeletal muscle cells (Sanchez-Gurmaches and Guertin, 2014), explaining why genetic manipulation of BAT cell function can activate the transcription of genes that are normally expressed by skeletal muscle cells.
Several lines of evidence suggest that Mstn can also modulate skeletal muscle-independent physiologic functions. For example, Mstn treatment of mice can induce peripheral insulin resistance (Pydi et al., 2020b). Strikingly, chronic treatment of HFD adipo-βarr1-KO mice with an anti-Mstn antibody led to significant improvements in glucose homeostasis including improved glucose tolerance and reduced blood glucose levels (Pydi et al., 2020b).
Biochemical studies with cultured mouse BAT cells demonstrated that nuclear βarr1 can form a complex with PPARγ (Pydi et al., 2020b). Additional mechanistic data indicated that βarr1 interacts with the PPARγ/RXRα complex in the nucleus of brown adipocytes, thus interfering with the ability of PPARγ to activate the Mstn promoter.
In sum, the data presented by Pydi et al. (Pydi et al., 2020b) indicate that βarr1 represents an important negative regulator of Mstn expression in BAT. The ability of nuclear βarr1 to alter gene expression profiles in BAT provides a striking example for the ability of nuclear βarr1 to regulate whole-body glucose homeostasis via a nonconventional mechanism of action (Fig. 4). As discussed, the activity of nuclear βarr1 has also been implicated in carcinogenesis and other important physiologic functions. Additional studies are needed to address the question of whether the ability of βarr1 to inhibit the expression of myogenic genes in BAT is regulated by the activity of specific GPCRs. In any case, the development of novel strategies to modulate the expression or activity of nuclear βarr1 may prove beneficial in the treatment of various human diseases including metabolic disorders such as T2D.
D. Pancreatic β-Cells
The islets of Langerhans of the endocrine pancreas contain multiple cell types including the insulin-producing β-cells, which release insulin after food intake to lower blood glucose levels. Besides peripheral insulin resistance, impaired β-cell function plays a key role in the pathogenesis of T2D (Vetere et al., 2014; Eizirik et al., 2020). Due to the deleterious effects of chronically elevated plasma glucose and lipid levels, β-cell function and number continue to decline, and insulin production eventually becomes inadequate to maintain euglycemia (Vetere et al., 2014; Eizirik et al., 2020). For this reason, pancreatic β-cells are considered a prime target for the development of novel antidiabetic drugs.
An early study (Kong et al., 2010) supported the concept that β-arrestins play a role in regulating the release of insulin from pancreatic β-cells. Specifically, a knockin mouse strain expressing a phosphorylation-deficient mutant version of the M3 muscarinic receptor showed impaired insulin secretion (Kong et al., 2010). Mechanistic data indicated that this deficit was most likely due to the absence of phosphorylation/β-arrestin-dependent coupling of β-cell M3 receptors to protein kinase D1 (Kong et al., 2010).
1. β-Arrestin-2
Mutant mice lacking βarr2 selectively in pancreatic β-cells (β-βarr2-KO mice) displayed several major metabolic impairments in vitro and in vivo (Zhu et al., 2017a). For example, in vitro studies demonstrated that glucose-stimulated insulin section (GSIS) was greatly reduced in islets from β-βarr2-KO islets, as compared with control islets (Zhu et al., 2017a). Similar results were obtained with EndoC-βH1 cells, an immortalized human pancreatic β-cell line (Scharfmann et al., 2014), following siRNA-mediated knockdown of βarr2 expression. Electrophysiological studies indicated that βarr2 deficiency resulted in impaired glucose-stimulated Ca2+ entry into β-cells due to reduced activity of voltage-dependent Ca2+ channels, associated with a resulting decrease action potential firing frequency (Zhu et al., 2017a). In agreement with the outcome of the in vitro studies, β-βarr2-KO mice showed striking metabolic deficits in vivo, in particular when the mutant mice were maintained on a HFD (Zhu et al., 2017a). In HFD β-βarr2-KO, GSIS was dramatically reduced, resulting in hyperglycemia and impaired glucose tolerance.
Additional studies strongly suggested that the various deficits associated with the lack of β-cell βarr2 are caused by impaired activity of β-cell CAMKII, a multifunctional serine/threonine protein kinase that promotes insulin secretion via phosphorylation of various signaling proteins (Dadi et al., 2014). Zhu et al. (Zhu et al., 2017a) observed that the biochemical, electrophysiological, and metabolic deficits caused by β-cell βarr2 deficiency are very similar to those displayed by a mouse strain expressing a dominant negative version of CAMKII selectively in β-cells (Dadi et al., 2014). Moreover, biochemical studies indicated that βarr2 is able to form a complex with CAMKII, thus facilitating CAMKII signaling in β-cells (Zhu et al., 2017a). Taken together, these observations strongly support the concept that βarr2 regulation of β-cell CAMKII activity is critical for proper β-cell function.
In agreement with the metabolic deficits observed with mice lacking βarr2 in β-cells, mutant mice that overexpressed βarr2 selectively in β-cells (β-βarr2-OE mice) showed major metabolic improvements. For example, HFD β-βarr2-OE mice displayed a striking increase in GSIS, leading to significantly improved glucose tolerance (Zhu et al., 2017a). Moreover, HFD β-βarr2-OE mice were resistant against most metabolic deficits associated with the consumption of a HFD (Fig. 4).
It remains to be explored whether the formation of β-cell βarr2/CAMKII complexes is regulated by signaling pathways activated by specific β-cell GPCRs. In any case, the striking phenotypes displayed by β-cell βarr2 mutant mice suggest the possibility that strategies capable of promoting βarr2/CAMKII interactions in β-cells may prove useful to restore euglycemia in T2D.
2. β-Arrestin-1—Role in Sulphonylurea-Induced Insulin Secretion
With one exception (see later discussion), mutant mice lacking βarr1 selectively in pancreatic β-cells (β-βarr1-KO mice) did not display any significant metabolic deficits when consuming regular chow (Barella et al., 2019). However, surprisingly, the ability of certain sulphonylurea drugs (SUs), including glibenclamide and tolbutamide, to promote insulin secretion was significantly reduced in β-βarr1-KO mice (Barella et al., 2019). Previous studies have shown that glibenclamide, tolbutamide, and various other SUs can bind to and activate Epac2 in β-cells, thus contributing to SU-mediated insulin secretion (Zhang et al., 2009). Epac2 is a cAMP binding protein that promotes the activation of Rap1 by acting as a guanine nucleotide exchange factor, a process known to facilitate trafficking of insulin granules to the plasma membrane (Shibasaki et al., 2007). Insulin release studies carried out in the presence of a specific Epac2 inhibitor suggested that the presence of βarr1 is required for the proper function of Epac2 in β-cells (Barella et al., 2019). Moreover, the lack of β-cell βarr1 interfered with the ability of glibenclamide to stimulate Rap1 activation.
Coimmunoprecipitation assays revealed the existence of a βarr1/Epac2 complex in cultured mouse β-cells and demonstrated that glibenclamide is able to promote the formation of this complex (Barella et al., 2019). Pull-down assays with purified proteins showed that βarr1 is capable of binding to Epac2 in a direct fashion. In sum, these observations strongly suggest that certain SUs, including glibenclamide and tolbutamide, promote the formation of a βarr1/Epac2 complex in β-cells which in turn promotes Rap1 activation and enhanced insulin exocytosis (Fig. 4). The development of strategies that can promote or stabilize βarr1/Epac2 interactions in β-cells may prove useful for stimulating insulin release for therapeutic purposes.
3. β-Arrestin-1—Role in β-Cell Mass Expansion During Obesity
It is well known that obesity promotes β-cell hypertrophy and proliferation, resulting in an increase in β-cell mass (Sachdeva and Stoffers, 2009; Golson et al., 2010; Aguayo-Mazzucato and Bonner-Weir, 2018). Before the development of overt T2D, this increase in β-cell mass can maintain euglycemia despite peripheral insulin resistance (Sachdeva and Stoffers, 2009). Strikingly, Barella et al. (Barella et al., 2021) recently demonstrated that β-cell βarr1 plays a critical role in obesity-induced β-cell mass expansion. Specifically, HFD β-βarr1-KO mice (β-βarr1-KO mice) showed a pronounced reduction in β-cell mass due to greatly reduced β-cell proliferation, as compared with HFD control mice. As a result, insulin content was reduced by approximately 50% in islets from HFD β-βarr1-KO mice. In agreement with these findings, HFD β-βarr1-KO mice showed pronounced metabolic deficits in vivo, including hyperglycemia, glucose intolerance, and greatly reduced GSIS.
Western blotting studies showed that the expression of Pdx1 was significantly decreased in the absence of β-cell βarr1 (HFD β-βarr1-KO mice) (Barella et al., 2021). In the mature endocrine pancreas, Pdx1 acts as a key transcription factor required for the maintenance of proper β-cell function and for the increase in β-cell mass triggered by peripheral insulin resistance (Kulkarni et al., 2004; Brissova et al., 2005). In agreement with this finding, overexpression of Pdx1 in islets from HFD β-βarr1-KO mice restored control-like GSIS (Barella et al., 2021). Interestingly, siRNA-mediated knockdown of BARR1 expression in cultured human β-cells (EndoC-βH1 cells) also resulted in a significant downregulation of PDX1 expression. This effect was accompanied by an almost complete loss in GSIS, suggesting that βarr1 regulates similar cellular function in mouse and human β-cells (Barella et al., 2021).
Expression of a Pdx1 promoter construct in cultured mouse β-cells showed that the presence of βarr1 is required for efficient transcription from the Pdx1 promoter (Barella et al., 2021). Moreover, chromatin immunoprecipitation experiments with cultured mouse β-cells demonstrated that nuclear βarr1 promotes Pdx1 gene expression, most likely by facilitating the formation of a complex with p300, a histone acetyltransferase, and other nuclear factors. In agreement with this finding, Kang et al. (Kang et al., 2005) reported previously that nuclear βarr1 can stimulate the transcription of various other genes via binding to p300.
Barella et al. (Barella et al., 2021) also analyzed mice that selectively overexpressed βarr1 in β-cells (β-βarr1-OE mice). When maintained on a HFD, β-βarr1-OE mice displayed phenotypic changes that were opposite to those observed with HFD β-βarr1-KO mice. Islets prepared from HFD β-βarr1-OE mice showed a significant increase in insulin content and β-cell mass, as compared with islets from WT littermates. Consistent with these findings, HFD β-βarr1-OE mice displayed a pronounced increase in GSIS, reduced blood glucose levels, and improved glucose tolerance (Barella et al., 2021).
In sum, metabolic studies with β-cell-specific βarr1 mutant mice strongly suggest that nuclear βarr1 plays an important role in regulating transcriptional processes required for β-cell mass expansion and replication under conditions of metabolic stress (Fig. 4). This observation raises the possibility that agents that are able to promote the translocation of βarr1 into the nucleus may prove useful to stimulate β-cell replication in T2D and related disorders.
E. Skeletal Muscle
Skeletal muscle (SKM) is the major tissue responsible for insulin-induced glucose disposal and utilization (Merz and Thurmond, 2020). Moreover, the inability of SKM to properly respond to insulin is considered the primary defect in the progression to T2D (DeFronzo and Tripathy, 2009). To explore the role of β-arrestins in regulating SKM function in the context of glucose homeostasis, Meister et al. (Meister et al., 2019) generated and analyzed mutant mice that lacked βarr1 and/or βarr2 selectively in SKM tissues. Somewhat surprisingly, SKM βarr1 and/or βarr2 deficiency had little or no effect on modulating whole-body glucose homeostasis, SKM insulin sensitivity, and exercise performance (Meister et al., 2019). These findings do not rule out the possibility that β-arrestins may modulate other SKM functions under different experimental conditions (see the following paragraph).
Clenbuterol is a β2-AR agonist that is used by some bodybuilders to enhance SKM mass due to its anabolic effects (Spiller et al., 2013). Interestingly, βarr1 deficiency reduced the increase in SKM mass and strength observed after chronic clenbuterol treatment of WT mice, suggesting that βarr1 plays a key role in mediating β2AR-dependent SKM growth and strength (Kim et al., 2018). In agreement with this finding, chronic treatment of mice with carvedilol, a β-arrestin-biased β2-AR agonist, was able to enhance the contractile force of SKM in a βarr1-dependent fashion (Kim et al., 2020). Clearly, these observations are translationally relevant for the development of novel classes of drugs useful for the treatment of various diseases characterized by SKM wasting.
F. Agouti-Related Peptide Neurons
Neurons contained within the arcuate nucleus of the hypothalamus play key roles in regulating appetite as well as glucose and energy homeostats (Deem et al., 2022). Agouti-related peptide (AgRP) neurons of the arcuate nucleus are best known for their role in stimulating food intake due to their ability to synthesize and release several appetite-inducing agents, including AgRP, NPY, and GABA (Deem et al., 2022). Modulation of the activity of AgRP neurons can also lead to altered peripheral glucose metabolism and carbohydrate utilization independent of the orexigenic activity of these neurons (Steculorum et al., 2016; Cavalcanti-de-Albuquerque et al., 2019). Like other cell types, AgRP neurons express numerous GPCRs, which, following their activation by agonist ligands, are likely to recruit β-arrestins ( Cowley et al., 2003; Ren et al., 2012; Nakajima et al., 2016).
To investigate the potential physiologic relevance of the two β-arrestins expressed by AgRP neurons, Pydi et al. generated mutant mice that lacked βarr1 or βarr2 selectively in AgRP neurons (Pydi et al., 2020a). Elimination of βarr2 in AgRP neurons had no detectable effect on whole-body glucose homeostasis, insulin sensitivity, and several other metabolic parameters, independent of the diet that the mice consumed (regular chow or HFD) (Pydi et al., 2020a). In contrast, mutant mice lacking βarr1 selectively in AgRP neurons (AgRP-βarr1-KO mice) displayed significant deficits in glucose tolerance, enhanced hepatic glucose production (HGP), and impaired insulin sensitivity when maintained on a HFD. Interestingly, these metabolic phenotypes were not observed after surgical dissection of the hepatic branch of the vagus nerve, suggesting that the lack of βarr1 in AgRP neurons enhances vagal outflow to the liver, resulting in increased HGP (Pydi et al., 2020a). Metabolic studies also suggested that βarr1 expressed by AgRP neurons suppresses the activity of a neuronal pathway that causes the sympathetic activation of adipose tissue, explaining why plasma FFA levels were significantly increased in HFD AgRP-βarr1-KO mice.
It is well known that insulin hyperpolarizes AgRP neurons, resulting in various metabolic changes including the suppression of vagus-mediated HGP (Könner et al., 2007; Steculorum et al., 2016; Huang et al., 2018c). Strikingly, insulin was unable to hyperpolarize AgRP neurons lacking βarr1 (Pydi et al., 2020a). Additional electrophysiological studies showed that βarr1 is required for proper insulin signaling in AgRP neurons upstream of PI3 kinase, most likely at the level of IRS-1 (Pydi et al., 2020a) (Fig. 4). However, the precise molecular mechanisms underlying this βarr1 activity remain to be explored.
In contrast to HFD AgRP-βarr1-KO mice, HFD mice that overexpressed βarr1 in AgRP neurons (AgRP-βarr1-OE mice) showed beneficial metabolic outcomes, including improved glucose tolerance and insulin sensitivity, as compared with HFD control mice (Pydi et al., 2020a). This observation suggests that strategies aimed at enhancing βarr1 expression levels in AgRP neurons could prove clinically useful to treat impairments in glucose homeostasis.
In sum, these findings demonstrate that the lack of a single β-arrestin isoform in a single neuronal subpopulation can have striking effects on whole-body glucose homeostasis. It should be of considerable interest to explore the potential roles of βarr1 and βarr2 in modulating the activity of other neuronal subpopulations known to regulate key metabolic functions.
VIII. Targeting β-Arrestins for Therapeutic Purposes
As shown in Fig. 1, β-arrestins consist of two cup-like domains. GPCRs engage residues on the concave side of both domains, while most nonreceptor signaling partners are predicted to bind to the opposite side of the molecule. The C-terminal portion of β-arrestins contains binding sites for trafficking proteins such as clathrin and its adaptor AP2 (Fig. 1). This distinct pattern of functionally relevant arrestin regions should make it possible to independently manipulate the receptor-binding surface, the effector-binding side, or the elements that engage trafficking proteins.
A. Biased G Protein-Coupled Receptor Ligands
The therapeutic potential of G protein- or arrestin-biased GPCR ligands has been discussed in several excellent review articles (Rajagopal et al., 2010b; Shonberg et al., 2014; Peterson and Luttrell, 2017; Eiger et al., 2022). Likewise, the mechanistic underpinnings of GPCR signaling bias and the potential caveats associated with the development of biased GPCR ligands as novel therapeutic agents have been reviewed in detail recently (Gurevich and Gurevich, 2020; Seyedabadi et al., 2022). Examples for the potential therapeutic use of G protein- or arrestin-biased GPCR ligands are given throughout this review.
B. Other Small Molecules
β-Arrestins are multifunctional cytoplasmic proteins that are expressed by virtually all cell types. As a result, modulating β-arrestin function for therapeutic purposes appears to be a very challenging task. However, accumulating evidence suggests that different β-arrestin interfaces are involved in facilitating interactions with different intracellular signaling proteins (Chaturvedi et al., 2018; Shukla and Dwivedi-Agnihotri, 2020). This observation suggests that it might be possible to develop small molecules that target distinct β-arrestin subdomains to modulate specific signaling cascades for therapeutic purposes. The potential feasibility of this approach is exemplified by the recent discovery of barbadin, a small molecule that inhibits the interaction of β-arrestins with the β2-adaptin subunit of the clathrin adaptor protein AP2 via binding to β2-adaptin (Beautrait et al., 2017). Since β2-adaptin is a key component of clathrin-coated pits, barbadin interferes with the internalization of various GPCRs without affecting receptor-mediated β-arrestin recruitment (Beautrait et al., 2017). Studies with selected GPCRs demonstrated that barbadin treatment of cultured cells can affect the magnitude of activation and kinetics of distinct intracellular signaling cascades (Beautrait et al., 2017). Moreover, additional signaling studies involving the use of barbadin confirmed the concept that GPCR-mediated cAMP signaling persists after activation of Gs-coupled receptors (Beautrait et al., 2017), suggesting that GPCR endocytosis can promote GPCR signaling in certain cases.
C. Aptamers
Recent studies suggest that the use of RNA aptamers (short sequences of artificial DNA or RNA that bind a specific target molecule) may also prove useful to target β-arrestins in a direct fashion (Chaturvedi et al., 2018). For example, a sophisticated screening strategy led to the identification of RNA aptamers that can bind β-arrestins with high affinity (Kotula et al., 2014). Several of these aptamers displayed pronounced selectivity for βarr2, as compared with βarr1, and were able to inhibit distinct intracellular signaling pathways in cultured cells (Kotula et al., 2014). Although this hypothesis has not been tested experimentally, it may be feasible to develop aptamers that target specific β-arrestin surfaces, thus modifying β-arrestin-mediated functions in a more targeted fashion. Clearly, studies in this area are of considerable therapeutic interest.
D. Synthetic Intrabodies
A recent study identified several antigen-binding fragments (Fabs) that were able to distinguish between βarr1 and βarr2 (Ghosh et al., 2017). Several of these Fabs selectively modulated the interaction of β-arrestins with clathrin and the ERK signaling cascade. One of the newly identified Fabs selectively interfered with βarr2-clathrin binding (Ghosh et al., 2017). An intrabody (an antibody that works within the cell) derived from this Fab strongly inhibited the agonist-dependent endocytosis of several GPCRs but did not interfere with GPCR-induced ERK signaling (Ghosh et al., 2017). These findings suggest the possibility that intrabodies that can selectivity interfere with distinct β-arrestin functions may prove useful for the treatment of various diseases characterized by abnormal β-arrestin expression and/or function.
IX. Conclusion
As discussed in this review article, βarr1 and βarr2 are involved in a very large number of physiologic functions, and altered βarr1/2 expression levels or activity are predicted to play key roles in the pathogenesis of many important pathophysiological conditions. On the basis of these findings, it should be possible to design novel therapeutic strategies that target β-arrestins or their associated signaling molecules and networks for therapeutic purposes. The identification of βarr1/2-regulated signaling cascades that show increased or decreased activity in specific cell types or tissues should greatly aid this endeavor.
Acknowledgments
The authors would like to thank all present and past members of the Wess and Gurevich laboratories who contributed to some of the work covered in this review. Moreover, the authors apologize to all whose work they were unable to cite due to space limitations.
Abbreviations
- 5-FU
5-fluorouracil
- ACKR
atypical chemokine receptor
- AD
Alzheimer’s disease
- AgRP
agouti-related peptide
- AMPH
amphetamine
- AP2
adaptor protein 2
- AR
androgen receptor
- AT1R
angiotensin II AT1 receptor
- β-AR
β-adrenergic receptor
- βarr1
β-arrestin-1
- βarr2
β-arrestin-2
- BAT
brown adipose tissue
- CML
chronic myelogenous leukemia
- CNO
clozapine N-oxide
- CNS
central nervous system
- CRC
colorectal cancer
- CRPC
castration-resistant prostate cancer
- CXCR
C-X-C chemokine receptor
- DA
dopamine
- DOR
δ-opioid receptor
- ERK
extracellular signal-regulated kinases
- ETR
endothelin receptor
- FFA
free fatty acid
- FXS
fragile X syndrome
- GCGR
glucagon receptor
- GPBAR
G protein-coupled bile acid receptor
- GPCR
G protein-coupled receptor
- GR
glucocorticoid receptor
- GRKs
GPCR kinases
- GSIS
glucose-stimulated insulin section
- HFD
high-fat diet
- HGP
hepatic glucose production
- HH
hedgehog
- HIF-1α
hypoxia-inducible factor 1α
- hTERT
human telomerase reverse transcriptase
- IL-PFC
infralimbic prefrontal cortex
- IFN-γ
interferon-γ
- JNK
Jun N-terminal kinase
- KO
knockout
- KOR
κ-opioid receptor
- MB
medulloblastoma
- MELK
maternal embryonic leucine zipper kinase
- mGluR
metabotropic glutamate receptor
- MOR
μ-opioid receptor
- MSN
medium spiny neuron
- iMSN
“indirect pathway” medium spiny neuron
- Mstn
myostatin
- NK1R
neurokinin-1 receptor
- NSCLC
non–small cell lung cancer
- NTSR1
neurotensin receptor 1
- OTR
oxytocin receptor
- PMF
primary myelofibrosis
- RCC
renal cell carcinoma
- SHH-MB
MB driven by aberrant sonic hedgehog/Gli signaling
- siRNA
small interfering RNA
- SKM
skeletal muscle
- SOC
serous ovarian cancer
- SU
sulphonylurea drug
- SuFu
Suppressor of fused
- T-ALL
T-cell acute lymphoblastic leukemia
- T2D
type 2 diabetes
- TNBC
triple negative breast cancer
- VEGF
vascular endothelial growth factor
- WAT
white adipose tissue
- WT
wild-type
Authorship Contributions
Wrote or contributed to the writing of the manuscript: Wess, Oteng, Rivera-Gonzalez, EV Gurevich, VV Gurevich.
Footnotes
This work was supported by funding through the National Institute of Diabetes and Digestive and Kidney Diseases Intramural Program (J.W., A.-B.O., and O.R.-G.), as well as National Institutes of Health National Institute of General Medical Sciences [Grant GM122491] and National Eye Institute [Grant EY011500], and Cornelius Vanderbilt Endowed Chair (E.V.G. and V.V.G.).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- Aamna B, Kumar Dan A, Sahu R, Behera SK, Parida S (2022) Deciphering the signaling mechanisms of β-arrestin1 and β-arrestin2 in regulation of cancer cell cycle and metastasis. J Cell Physiol 237:3717–3733. [DOI] [PubMed] [Google Scholar]
- Abdel-Wahab OI, Levine RL (2009) Primary myelofibrosis: Update on definition, pathogenesis, and treatment. Annu Rev Med 60:233–245. [DOI] [PubMed] [Google Scholar]
- Aguayo-Mazzucato C, Bonner-Weir S (2018) Pancreatic β cell regeneration as a possible therapy for diabetes. Cell Metab 27:57–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmed MR, Zhan X, Song X, Kook S, Gurevich VV, Gurevich EV (2011) Ubiquitin ligase parkin promotes Mdm2-arrestin interaction but inhibits arrestin ubiquitination. Biochemistry 50:3749–3763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alekhina O, Marchese A (2016) β-Arrestin1 and signal-transducing adaptor molecule 1 (STAM1) cooperate to promote focal adhesion kinase autophosphorylation and chemotaxis via the chemokine receptor CXCR4. J Biol Chem 291:26083–26097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alnasser SM (2021) Review on mechanistic strategy of gene therapy in the treatment of disease. Gene 769:145246. [DOI] [PubMed] [Google Scholar]
- Altarifi AA, David B, Muchhala KH, Blough BE, Akbarali H, Negus SS (2017) Effects of acute and repeated treatment with the biased mu opioid receptor agonist TRV130 (oliceridine) on measures of antinociception, gastrointestinal function, and abuse liability in rodents. J Psychopharmacol 31:730–739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arang N, Gutkind JS (2020) G protein-coupled receptors and heterotrimeric G proteins as cancer drivers. FEBS Lett 594:4201–4232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnsten AF, Girgis RR, Gray DL, Mailman RB (2017) Novel dopamine therapeutics for cognitive deficits in schizophrenia. Biol Psychiatry 81:67–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnsten AF, Wang M, Paspalas CD (2015) Dopamine’s actions in primate prefrontal cortex: challenges for treating cognitive disorders. Pharmacol Rev 67:681–696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asher WB, Terry DS, Gregorio GGA, Kahsai AW, Borgia A, Xie B, Modak A, Zhu Y, Jang W, Govindaraju A, et al. (2022) GPCR-mediated β-arrestin activation deconvoluted with single-molecule precision. Cell 185:1661–1675.e16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azevedo Neto J, Costanzini A, De Giorgio R, Lambert DG, Ruzza C, Calò G (2020) Biased versus partial agonism in the search for safer opioid analgesics. Molecules 25:3870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azzam AAH, Lambert DG (2022) Preclinical discovery and development of oliceridine (Olinvyk®) for the treatment of post-operative pain. Expert Opin Drug Discov 17:215–223. [DOI] [PubMed] [Google Scholar]
- Bachmutsky I, Wei XP, Durand A, Yackle K (2021) ß-arrestin 2 germline knockout does not attenuate opioid respiratory depression. eLife 10:e62552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bae WY, Choi JS, Nam S, Jeong JW (2021) β-arrestin 2 stimulates degradation of HIF-1α and modulates tumor progression of glioblastoma. Cell Death Differ 28:3092–3104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baer C, Kimura S, Rana MS, Kleist AB, Flerlage T, Feith DJ, Chockley P, Walter W, Meggendorfer M, Olson TL, et al. (2022) CCL22 mutations drive natural killer cell lymphoproliferative disease by deregulating microenvironmental crosstalk. Nat Genet 54:637–648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bagnato A, Rosanò L (2019) New routes in GPCR/β-arrestin-driven signaling in cancer progression and metastasis. Front Pharmacol 10:114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balakumar P, Jagadeesh G (2014) A century-old renin-angiotensin system still grows with endless possibilities: AT1 receptor signaling cascades in cardiovascular physiopathology. Cell Signal 26:2147–2160. [DOI] [PubMed] [Google Scholar]
- Barella LF, Rossi M, Pydi SP, Meister J, Jain S, Cui Y, Gavrilova O, Fulgenzi G, Tessarollo L, Wess J (2021) β-Arrestin-1 is required for adaptive β-cell mass expansion during obesity. Nat Commun 12:3385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barella LF, Rossi M, Zhu L, Cui Y, Mei FC, Cheng X, Chen W, Gurevich VV, Wess J (2019) β-Cell-intrinsic β-arrestin 1 signaling enhances sulfonylurea-induced insulin secretion. J Clin Invest 129:3732–3737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beaulieu JM, Gainetdinov RR, Caron MG (2009) Akt/GSK3 signaling in the action of psychotropic drugs. Annu Rev Pharmacol Toxicol 49:327–347. [DOI] [PubMed] [Google Scholar]
- Beaulieu JM, Marion S, Rodriguiz RM, Medvedev IO, Sotnikova TD, Ghisi V, Wetsel WC, Lefkowitz RJ, Gainetdinov RR, Caron MG (2008) A beta-arrestin 2 signaling complex mediates lithium action on behavior. Cell 132:125–136. [DOI] [PubMed] [Google Scholar]
- Beaulieu JM, Sotnikova TD, Marion S, Lefkowitz RJ, Gainetdinov RR, Caron MG (2005) An Akt/beta-arrestin 2/PP2A signaling complex mediates dopaminergic neurotransmission and behavior. Cell 122:261–273. [DOI] [PubMed] [Google Scholar]
- Beautrait A, Paradis JS, Zimmerman B, Giubilaro J, Nikolajev L, Armando S, Kobayashi H, Yamani L, Namkung Y, Heydenreich FM, et al. (2017) A new inhibitor of the β-arrestin/AP2 endocytic complex reveals interplay between GPCR internalization and signalling. Nat Commun 8:15054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohn LM, Aubé J (2017) Seeking (and finding) biased ligands of the kappa opioid receptor. ACS Med Chem Lett 8:694–700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohn LM, Gainetdinov RR, Lin FT, Lefkowitz RJ, Caron MG (2000) Mu-opioid receptor desensitization by beta-arrestin-2 determines morphine tolerance but not dependence. Nature 408:720–723. [DOI] [PubMed] [Google Scholar]
- Bohn LM, Lefkowitz RJ, Gainetdinov RR, Peppel K, Caron MG, Lin FT (1999) Enhanced morphine analgesia in mice lacking beta-arrestin 2. Science 286:2495–2498. [DOI] [PubMed] [Google Scholar]
- Bostanabad SY, Noyan S, Dedeoglu BG, Gurdal H (2021) Overexpression of β-arrestins inhibits proliferation and motility in triple negative breast cancer cells. Sci Rep 11:1539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Böttke T, Ernicke S, Serfling R, Ihling C, Burda E, Gurevich VV, Sinz A, Coin I (2020) Exploring GPCR-arrestin interfaces with genetically encoded crosslinkers. EMBO Rep 21:e50437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bous J, Fouillen A, Orcel H, Trapani S, Cong X, Fontanel S, Saint-Paul J, Lai-Kee-Him J, Urbach S, Sibille N, et al. (2022) Structure of the vasopressin hormone-V2 receptor-β-arrestin1 ternary complex. Sci Adv 8:eabo7761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradley SJ, Molloy C, Valuskova P, Dwomoh L, Scarpa M, Rossi M, Finlayson L, Svensson KA, Chernet E, Barth VN, et al. (2020) Biased M1-muscarinic-receptor-mutant mice inform the design of next-generation drugs. Nat Chem Biol 16:240–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breitman M, Kook S, Gimenez LE, Lizama BN, Palazzo MC, Gurevich EV, Gurevich VV (2012) Silent scaffolds: inhibition of c-Jun N-terminal kinase 3 activity in cell by dominant-negative arrestin-3 mutant. J Biol Chem 287:19653–19664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brissova M, Blaha M, Spear C, Nicholson W, Radhika A, Shiota M, Charron MJ, Wright CV, Powers AC (2005) Reduced PDX-1 expression impairs islet response to insulin resistance and worsens glucose homeostasis. Am J Physiol Endocrinol Metab 288:E707–E714. [DOI] [PubMed] [Google Scholar]
- Bubici C, Papa S (2014) JNK signalling in cancer: in need of new, smarter therapeutic targets. Br J Pharmacol 171:24–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butcher AJ, Kong KC, Prihandoko R, Tobin AB (2012) Physiological role of G-protein coupled receptor phosphorylation. Handb Exp Pharmacol 208:79–94. [DOI] [PubMed] [Google Scholar]
- Cahill TJ 3rd, Thomsen AR, Tarrasch JT, Plouffe B, Nguyen AH, Yang F, Huang LY, Kahsai AW, Bassoni DL, Gavino BJ, et al. (2017) Distinct conformations of GPCR-β-arrestin complexes mediate desensitization, signaling, and endocytosis. Proc Natl Acad Sci USA 114:2562–2567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao C, Barros-Álvarez X, Zhang S, Kim K, Dämgen MA, Panova O, Suomivuori CM, Fay JF, Zhong X, Krumm BE, et al. (2022) Signaling snapshots of a serotonin receptor activated by the prototypical psychedelic LSD. Neuron 110:3154–3167.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capote AE, Batra A, Warren CM, Chowdhury SAK, Wolska BM, Solaro RJ, Rosas PC (2021) B-arrestin-2 signaling is important to preserve cardiac function during aging. Front Physiol 12:696852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capozzi ME, D’Alessio DA, Campbell JE (2022) The past, present, and future physiology and pharmacology of glucagon. Cell Metab 34:1654–1674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caronni N, Savino B, Recordati C, Villa A, Locati M, Bonecchi R (2016) Cancer and chemokines. Methods Mol Biol 1393:87–96. [DOI] [PubMed] [Google Scholar]
- Cavalcanti-de-Albuquerque JP, Bober J, Zimmer MR, Dietrich MO (2019) Regulation of substrate utilization and adiposity by Agrp neurons. Nat Commun 10:311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ceddia RP, Collins S (2020) A compendium of G-protein-coupled receptors and cyclic nucleotide regulation of adipose tissue metabolism and energy expenditure. Clin Sci (Lond) 134:473–512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Celver J, Vishnivetskiy SA, Chavkin C, Gurevich VV (2002) Conservation of the phosphate-sensitive elements in the arrestin family of proteins. J Biol Chem 277:9043–9048. [DOI] [PubMed] [Google Scholar]
- Chaturvedi M, Schilling J, Beautrait A, Bouvier M, Benovic JL, Shukla AK (2018) Emerging paradigm of intracellular targeting of G protein-coupled receptors. Trends Biochem Sci 43:533–546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chavkin C, Schattauer SS, Levin JR (2014) Arrestin-mediated activation of p38 MAPK: molecular mechanisms and behavioral consequences. Handb Exp Pharmacol 219:281–292. [DOI] [PubMed] [Google Scholar]
- Chen Q, Iverson TM, Gurevich VV (2018) Structural basis of arrestin-dependent signal transduction. Trends Biochem Sci 43:412–423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Hu N, Yang J, Gao T (2022) Prefrontal cortical circuits in anxiety and fear: an overview. Front Med 16:518–539. [DOI] [PubMed] [Google Scholar]
- Chiang T, Sansuk K, van Rijn RM (2016) β-Arrestin 2 dependence of δ opioid receptor agonists is correlated with alcohol intake. Br J Pharmacol 173:332–343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu Sin Chung P, Kieffer BL (2013) Delta opioid receptors in brain function and diseases. Pharmacol Ther 140:112–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleghorn WM, Branch KM, Kook S, Arnette C, Bulus N, Zent R, Kaverina I, Gurevich EV, Weaver AM, Gurevich VV (2015) Arrestins regulate cell spreading and motility via focal adhesion dynamics. Mol Biol Cell 26:622–635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleghorn WM, Bulus N, Kook S, Gurevich VV, Zent R, Gurevich EV (2018) Non-visual arrestins regulate the focal adhesion formation via small GTPases RhoA and Rac1 independently of GPCRs. Cell Signal 42:259–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coffa S, Breitman M, Spiller BW, Gurevich VV (2011) A single mutation in arrestin-2 prevents ERK1/2 activation by reducing c-Raf1 binding. Biochemistry 50:6951–6958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins S (2022) β-Adrenergic receptors and adipose tissue metabolism: Evolution of an old story. Annu Rev Physiol 84:1–16. [DOI] [PubMed] [Google Scholar]
- Conner DA, Mathier MA, Mortensen RM, Christe M, Vatner SF, Seidman CE, Seidman JG (1997) Beta-arrestin1 knockout mice appear normal but demonstrate altered cardiac responses to beta-adrenergic stimulation. Circ Res 81:1021–1026. [DOI] [PubMed] [Google Scholar]
- Cowley MA, Smith RG, Diano S, Tschöp M, Pronchuk N, Grove KL, Strasburger CJ, Bidlingmaier M, Esterman M, Heiman ML, et al. (2003) The distribution and mechanism of action of ghrelin in the CNS demonstrates a novel hypothalamic circuit regulating energy homeostasis. Neuron 37:649–661. [DOI] [PubMed] [Google Scholar]
- Crupi R, Impellizzeri D, Cuzzocrea S (2019) Role of metabotropic glutamate receptors in neurological disorders. Front Mol Neurosci 12:20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czogalla B, Partenheimer A, Jeschke U, von Schönfeldt V, Mayr D, Mahner S, Burges A, Simoni M, Melli B, Benevelli R, et al. (2020) β-arrestin 2 is a prognostic factor for survival of ovarian cancer patients upregulating cell proliferation. Front Endocrinol (Lausanne) 11:554733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Alessio D (2011) The role of dysregulated glucagon secretion in type 2 diabetes. Diabetes Obes Metab 13(Suppl 1):126–132. [DOI] [PubMed] [Google Scholar]
- Dadi PK, Vierra NC, Ustione A, Piston DW, Colbran RJ, Jacobson DA (2014) Inhibition of pancreatic β-cell Ca2+/calmodulin-dependent protein kinase II reduces glucose-stimulated calcium influx and insulin secretion, impairing glucose tolerance. J Biol Chem 289:12435–12445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dagogo-Jack I, Shaw AT (2018) Tumour heterogeneity and resistance to cancer therapies. Nat Rev Clin Oncol 15:81–94. [DOI] [PubMed] [Google Scholar]
- Dalle S, Ricketts W, Imamura T, Vollenweider P, Olefsky JM (2001) Insulin and insulin-like growth factor I receptors utilize different G protein signaling components. J Biol Chem 276:15688–15695. [DOI] [PubMed] [Google Scholar]
- Darcq E, Kieffer BL (2018) Opioid receptors: drivers to addiction? Nat Rev Neurosci 19:499–514. [DOI] [PubMed] [Google Scholar]
- David DJ, Samuels BA, Rainer Q, Wang JW, Marsteller D, Mendez I, Drew M, Craig DA, Guiard BP, Guilloux JP, et al. (2009) Neurogenesis-dependent and -independent effects of fluoxetine in an animal model of anxiety/depression. Neuron 62:479–493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis KD, Aghaeepour N, Ahn AH, Angst MS, Borsook D, Brenton A, Burczynski ME, Crean C, Edwards R, Gaudilliere B, et al. (2020) Discovery and validation of biomarkers to aid the development of safe and effective pain therapeutics: challenges and opportunities. Nat Rev Neurol 16:381–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de la Mora MP, Gallegos-Cari A, Arizmendi-García Y, Marcellino D, Fuxe K (2010) Role of dopamine receptor mechanisms in the amygdaloid modulation of fear and anxiety: Structural and functional analysis. Prog Neurobiol 90:198–216. [DOI] [PubMed] [Google Scholar]
- De Ridder D, Adhia D, Vanneste S (2021) The anatomy of pain and suffering in the brain and its clinical implications. Neurosci Biobehav Rev 130:125–146. [DOI] [PubMed] [Google Scholar]
- Deem JD, Faber CL, Morton GJ (2022) AgRP neurons: regulators of feeding, energy expenditure, and behavior. FEBS J 289:2362–2381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeFronzo RA, Tripathy D (2009) Skeletal muscle insulin resistance is the primary defect in type 2 diabetes. Diabetes Care 32(Suppl 2):S157–S163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeWire SM, Ahn S, Lefkowitz RJ, Shenoy SK (2007) Beta-arrestins and cell signaling. Annu Rev Physiol 69:483–510. [DOI] [PubMed] [Google Scholar]
- DeWire SM, Yamashita DS, Rominger DH, Liu G, Cowan CL, Graczyk TM, Chen XT, Pitis PM, Gotchev D, Yuan C, et al. (2013) A G protein-biased ligand at the μ-opioid receptor is potently analgesic with reduced gastrointestinal and respiratory dysfunction compared with morphine. J Pharmacol Exp Ther 344:708–717. [DOI] [PubMed] [Google Scholar]
- Di Modugno F, Caprara V, Chellini L, Tocci P, Spadaro F, Ferrandina G, Sacconi A, Blandino G, Nisticò P, Bagnato A, et al. (2018) hMENA is a key regulator in endothelin-1/β-arrestin1-induced invadopodial function and metastatic process. Proc Natl Acad Sci USA 115:3132–3137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dölen G, Bear MF (2008) Role for metabotropic glutamate receptor 5 (mGluR5) in the pathogenesis of fragile X syndrome. J Physiol 586:1503–1508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong C, Li Y, Niu Q, Fang H, Bai J, Yan Y, Gu C, Xiao N (2020) SUMOylation involves in β-arrestin-2-dependent metabolic regulation in breast cancer cell. Biochem Biophys Res Commun 529:950–956. [DOI] [PubMed] [Google Scholar]
- Donthamsetti P, Gallo EF, Buck DC, Stahl EL, Zhu Y, Lane JR, Bohn LM, Neve KA, Kellendonk C, Javitch JA (2020) Arrestin recruitment to dopamine D2 receptor mediates locomotion but not incentive motivation. Mol Psychiatry 25:2086–2100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eiger DS, Pham U, Gardner J, Hicks C, Rajagopal S (2022) GPCR systems pharmacology: a different perspective on the development of biased therapeutics. Am J Physiol Cell Physiol 322:C887–C895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eizirik DL, Pasquali L, Cnop M (2020) Pancreatic β-cells in type 1 and type 2 diabetes mellitus: different pathways to failure. Nat Rev Endocrinol 16:349–362. [DOI] [PubMed] [Google Scholar]
- Eng AG, Kelver DA, Hedrick TP, Swanson GT (2016) Transduction of group I mGluR-mediated synaptic plasticity by β-arrestin2 signalling. Nat Commun 7:13571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faouzi A, Varga BR, Majumdar S (2020) Biased opioid ligands. Molecules 25:4257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Favoriti P, Carbone G, Greco M, Pirozzi F, Pirozzi RE, Corcione F (2016) Worldwide burden of colorectal cancer: a review. Updates Surg 68:7–11. [DOI] [PubMed] [Google Scholar]
- Feng Q, He B (2019) Androgen receptor signaling in the development of castration-resistant prostate cancer. Front Oncol 9:858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fereshteh M, Ito T, Kovacs JJ, Zhao C, Kwon HY, Tornini V, Konuma T, Chen M, Lefkowitz RJ, Reya T (2012) β-Arrestin2 mediates the initiation and progression of myeloid leukemia. Proc Natl Acad Sci USA 109:12532–12537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frausto SF, Ito K, Marszalec W, Swanson GT (2011) A novel form of low-frequency hippocampal mossy fiber plasticity induced by bimodal mGlu1 receptor signaling. J Neurosci 31:16897–16906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- French AR, van Rijn RM (2022) An updated assessment of the translational promise of G-protein-biased kappa opioid receptor agonists to treat pain and other indications without debilitating adverse effects. Pharmacol Res 177:106091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganguly R, Mohyeldin A, Thiel J, Kornblum HI, Beullens M, Nakano I (2015) MELK-a conserved kinase: functions, signaling, cancer, and controversy. Clin Transl Med 4:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerlai R (1996) Gene-targeting studies of mammalian behavior: is it the mutation or the background genotype? Trends Neurosci 19:177–181. [DOI] [PubMed] [Google Scholar]
- Gharaibeh L, Elmadany N, Alwosaibai K, Alshaer W (2020) Notch1 in cancer therapy: possible clinical implications and challenges. Mol Pharmacol 98:559–576. [DOI] [PubMed] [Google Scholar]
- Ghosh E, Srivastava A, Baidya M, Kumari P, Dwivedi H, Nidhi K, Ranjan R, Dogra S, Koide A, Yadav PN, et al. (2017) A synthetic intrabody-based selective and generic inhibitor of GPCR endocytosis. Nat Nanotechnol 12:1190–1198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillis A, Gondin AB, Kliewer A, Sanchez J, Lim HD, Alamein C, Manandhar P, Santiago M, Fritzwanker S, Schmiedel F, et al. (2020a) Low intrinsic efficacy for G protein activation can explain the improved side effect profiles of new opioid agonists. Sci Signal 13:eaaz3140.. [DOI] [PubMed] [Google Scholar]
- Gillis A, Sreenivasan V, Christie MJ (2020b) Intrinsic efficacy of opioid ligands and its importance for apparent bias, operational analysis, and therapeutic window. Mol Pharmacol 98:410–424. [DOI] [PubMed] [Google Scholar]
- Gimenez LE, Babilon S, Wanka L, Beck-Sickinger AG, Gurevich VV (2014) Mutations in arrestin-3 differentially affect binding to neuropeptide Y receptor subtypes. Cell Signal 26:1523–1531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gimenez LE, Vishnivetskiy SA, Baameur F, Gurevich VV (2012) Manipulation of very few receptor discriminator residues greatly enhances receptor specificity of non-visual arrestins. J Biol Chem 287:29495–29505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Girnita L, Worrall C, Takahashi S, Seregard S, Girnita A (2014) Something old, something new and something borrowed: emerging paradigm of insulin-like growth factor type 1 receptor (IGF-1R) signaling regulation. Cell Mol Life Sci 71:2403–2427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goertzen CG, Dragan M, Turley E, Babwah AV, Bhattacharya M (2016) KISS1R signaling promotes invadopodia formation in human breast cancer cell via β-arrestin2/ERK. Cell Signal 28:165–176. [DOI] [PubMed] [Google Scholar]
- Golson ML, Misfeldt AA, Kopsombut UG, Petersen CP, Gannon M (2010) High fat diet regulation of β-cell proliferation and β-cell mass. Open Endocrinol J 4:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregory KJ, Goudet C (2021) International union of basic and clinical pharmacology. CXI. Pharmacology, signaling, and physiology of metabotropic glutamate receptors. Pharmacol Rev 73:521–569. [DOI] [PubMed] [Google Scholar]
- Grim TW, Schmid CL, Stahl EL, Pantouli F, Ho JH, Acevedo-Canabal A, Kennedy NM, Cameron MD, Bannister TD, Bohn LM (2020) A G protein signaling-biased agonist at the μ-opioid receptor reverses morphine tolerance while preventing morphine withdrawal. Neuropsychopharmacology 45:416–425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grundmann M, Merten N, Malfacini D, Inoue A, Preis P, Simon K, Rüttiger N, Ziegler N, Benkel T, Schmitt NK, et al. (2018) Lack of beta-arrestin signaling in the absence of active G proteins. Nat Commun 9:341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerrini-Rousseau L, Dufour C, Varlet P, Masliah-Planchon J, Bourdeaut F, Guillaud-Bataille M, Abbas R, Bertozzi AI, Fouyssac F, Huybrechts S, et al. (2018) Germline SUFU mutation carriers and medulloblastoma: clinical characteristics, cancer risk, and prognosis. Neuro-oncol 20:1122–1132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurevich EV, Benovic JL, Gurevich VV (2002) Arrestin2 and arrestin3 are differentially expressed in the rat brain during postnatal development. Neuroscience 109:421–436. [DOI] [PubMed] [Google Scholar]
- Gurevich VV, Gurevich EV (2006) The structural basis of arrestin-mediated regulation of G-protein-coupled receptors. Pharmacol Ther 110:465–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurevich VV, Gurevich EV (2014a) Extensive shape shifting underlies functional versatility of arrestins. Curr Opin Cell Biol 27:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurevich VV, Gurevich EV (2014b) Overview of different mechanisms of arrestin-mediated signaling. Curr Protoc Pharmacol 67:2.10.11–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurevich VV, Gurevich EV (2019a) Arrestin mutations: some cause diseases, others promise cure. Prog Mol Biol Transl Sci 161:29–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurevich VV, Gurevich EV (2019b) Plethora of functions packed into 45 kDa arrestins: biological implications and possible therapeutic strategies. Cell Mol Life Sci 76:4413–4421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurevich VV, Gurevich EV (2020) Biased GPCR signaling: possible mechanisms and inherent limitations. Pharmacol Ther 211:107540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurevich VV, Gurevich EV (2022) Solo vs. chorus: monomers and oligomers of arrestin proteins. Int J Mol Sci 23:7253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurevich VV, Pals-Rylaarsdam R, Benovic JL, Hosey MM, Onorato JJ (1997) Agonist-receptor-arrestin, an alternative ternary complex with high agonist affinity. J Biol Chem 272:28849–28852. [DOI] [PubMed] [Google Scholar]
- Gustavsson M, Wang L, van Gils N, Stephens BS, Zhang P, Schall TJ, Yang S, Abagyan R, Chance MR, Kufareva I, et al. (2017) Structural basis of ligand interaction with atypical chemokine receptor 3. Nat Commun 8:14135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutkind JS, Kostenis E (2018) Arrestins as rheostats of GPCR signalling. Nat Rev Mol Cell Biol 19:615–616. [DOI] [PubMed] [Google Scholar]
- Haider RS, Matthees ESF, Drube J, Reichel M, Zabel U, Inoue A, Chevigné A, Krasel C, Deupi X, Hoffmann C (2022) β-arrestin1 and 2 exhibit distinct phosphorylation-dependent conformations when coupling to the same GPCR in living cells. Nat Commun 13:5638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanson SM, Cleghorn WM, Francis DJ, Vishnivetskiy SA, Raman D, Song X, Nair KS, Slepak VZ, Klug CS, Gurevich VV (2007) Arrestin mobilizes signaling proteins to the cytoskeleton and redirects their activity. J Mol Biol 368:375–387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanson SM, Francis DJ, Vishnivetskiy SA, Klug CS, Gurevich VV (2006a) Visual arrestin binding to microtubules involves a distinct conformational change. J Biol Chem 281:9765–9772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanson SM, Francis DJ, Vishnivetskiy SA, Kolobova EA, Hubbell WL, Klug CS, Gurevich VV (2006b) Differential interaction of spin-labeled arrestin with inactive and active phosphorhodopsin. Proc Natl Acad Sci USA 103:4900–4905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hare BD, Duman RS (2020) Prefrontal cortex circuits in depression and anxiety: contribution of discrete neuronal populations and target regions. Mol Psychiatry 25:2742–2758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He L, Gooding SW, Lewis E, Felth LC, Gaur A, Whistler JL (2021) Pharmacological and genetic manipulations at the µ-opioid receptor reveal arrestin-3 engagement limits analgesic tolerance and does not exacerbate respiratory depression in mice. Neuropsychopharmacology 46:2241–2249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang B, Li Y, Cheng D, He G, Liu X, Ma L (2018a) β-Arrestin-biased β-adrenergic signaling promotes extinction learning of cocaine reward memory. Sci Signal 11:eaam5402. [DOI] [PubMed] [Google Scholar]
- Huang D, Wang Y, Tang J, Luo S (2018b) Molecular mechanisms of suppressor of fused in regulating the hedgehog signalling pathway. Oncol Lett 15:6077–6086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang W, Masureel M, Qu Q, Janetzko J, Inoue A, Kato HE, Robertson MJ, Nguyen KC, Glenn JS, Skiniotis G, et al. (2020) Structure of the neurotensin receptor 1 in complex with β-arrestin 1. Nature 579:303–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Y, He Z, Gao Y, Lieu L, Yao T, Sun J, Liu T, Javadi C, Box M, Afrin S, Guo H, Williams KW (2018c) PI3K is integral for the acute activity of leptin and insulin in arcuate NPY/AgRP neurons in males. J Endocr Soc 2:518–532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Infante P, Faedda R, Bernardi F, Bufalieri F, Lospinoso Severini L, Alfonsi R, Mazzà D, Siler M, Coni S, Po A, et al. (2018) Itch/β-arrestin2-dependent non-proteolytic ubiquitylation of SuFu controls Hedgehog signalling and medulloblastoma tumorigenesis. Nat Commun 9:976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jean-Charles PY, Snyder JC, Shenoy SK (2016) Chapter one—ubiquitination and deubiquitination of G protein-coupled receptors. Prog Mol Biol Transl Sci 141:1–55. [DOI] [PubMed] [Google Scholar]
- Ji H, Liu N, Li J, Chen D, Luo D, Sun Q, Yin Y, Liu Y, Bu B, Chen X, et al. (2019) Oxytocin involves in chronic stress-evoked melanoma metastasis via β-arrestin 2-mediated ERK signaling pathway. Carcinogenesis 40:1395–1404. [DOI] [PubMed] [Google Scholar]
- Jia W, Xie G, Jia W (2018) Bile acid-microbiota crosstalk in gastrointestinal inflammation and carcinogenesis. Nat Rev Gastroenterol Hepatol 15:111–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang H, Galtes D, Wang J, Rockman HA (2022) G protein-coupled receptor signaling: transducers and effectors. Am J Physiol Cell Physiol 323:C731–C748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kallifatidis G, Smith DK, Morera DS, Gao J, Hennig MJ, Hoy JJ, Pearce RF, Dabke IR, Li J, Merseburger AS, et al. (2019) β-Arrestins regulate stem cell-like phenotype and response to chemotherapy in bladder cancer. Mol Cancer Ther 18:801–811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang DS, Tian X, Benovic JL (2014) Role of β-arrestins and arrestin domain-containing proteins in G protein-coupled receptor trafficking. Curr Opin Cell Biol 27:63–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang J, Shi Y, Xiang B, Qu B, Su W, Zhu M, Zhang M, Bao G, Wang F, Zhang X, et al. (2005) A nuclear function of beta-arrestin1 in GPCR signaling: regulation of histone acetylation and gene transcription. Cell 123:833–847. [DOI] [PubMed] [Google Scholar]
- Kang Y, Zhou XE, Gao X, He Y, Liu W, Ishchenko A, Barty A, White TA, Yefanov O, Han GW, et al. (2015) Crystal structure of rhodopsin bound to arrestin by femtosecond X-ray laser. Nature 523:561–567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karnam PC, Vishnivetskiy SA, Gurevich VV (2021) Structural basis of arrestin selectivity for active phosphorylated G protein-coupled receptors. Int J Mol Sci 22:12481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karnik SS, Unal H, Kemp JR, Tirupula KC, Eguchi S, Vanderheyden PM, Thomas WG (2015) International Union of Basic and Clinical Pharmacology. XCIX. Angiotensin receptors: interpreters of pathophysiological angiotensinergic stimuli [corrected]. Pharmacol Rev 67:754–819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly E, Conibear A, Henderson G (2023) Biased agonism: lessons from studies of opioid receptor agonists. Annu Rev Pharmacol Toxicol 63:491–515. [DOI] [PubMed] [Google Scholar]
- Kim J, Ahn S, Ren XR, Whalen EJ, Reiter E, Wei H, Lefkowitz RJ (2005) Functional antagonism of different G protein-coupled receptor kinases for beta-arrestin-mediated angiotensin II receptor signaling. Proc Natl Acad Sci USA 102:1442–1447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J, Grotegut CA, Wisler JW, Li T, Mao L, Chen M, Chen W, Rosenberg PB, Rockman HA, Lefkowitz RJ (2018) β-arrestin 1 regulates β2-adrenergic receptor-mediated skeletal muscle hypertrophy and contractility. Skelet Muscle 8:39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J, Grotegut CA, Wisler JW, Mao L, Rosenberg PB, Rockman HA, Lefkowitz RJ (2020) The β-arrestin-biased β-adrenergic receptor blocker carvedilol enhances skeletal muscle contractility. Proc Natl Acad Sci USA 117:12435–12443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kindt M, Soeter M, Vervliet B (2009) Beyond extinction: erasing human fear responses and preventing the return of fear. Nat Neurosci 12:256–258. [DOI] [PubMed] [Google Scholar]
- Klein S, Gastaldelli A, Yki-Järvinen H, Scherer PE (2022) Why does obesity cause diabetes? Cell Metab 34:11–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kliewer A, Gillis A, Hill R, Schmiedel F, Bailey C, Kelly E, Henderson G, Christie MJ, Schulz S (2020) Morphine-induced respiratory depression is independent of β-arrestin2 signalling. Br J Pharmacol 177:2923–2931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ko MJ, Chiang T, Mukadam AA, Mulia GE, Gutridge AM, Lin A, Chester JA, van Rijn RM (2021) β-Arrestin-dependent ERK signaling reduces anxiety-like and conditioned fear-related behaviors in mice. Sci Signal 14:eaba0245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohno M, Dennis LE, McCready H, Hoffman WF (2022) Dopamine dysfunction in stimulant use disorders: mechanistic comparisons and implications for treatment. Mol Psychiatry 27:220–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong KC, Butcher AJ, McWilliams P, Jones D, Wess J, Hamdan FF, Werry T, Rosethorne EM, Charlton SJ, Munson SE, et al. (2010) M3-muscarinic receptor promotes insulin release via receptor phosphorylation/arrestin-dependent activation of protein kinase D1. Proc Natl Acad Sci USA 107:21181–21186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong Z, Deng T, Zhang M, Zhao Z, Liu Y, Luo L, Cai C, Wu W, Duan X (2018) β-Arrestin1-medieated inhibition of FOXO3a contributes to prostate cancer cell growth in vitro and in vivo. Cancer Sci 109:1834–1842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Könner AC, Janoschek R, Plum L, Jordan SD, Rother E, Ma X, Xu C, Enriori P, Hampel B, Barsh GS, et al. (2007) Insulin action in AgRP-expressing neurons is required for suppression of hepatic glucose production. Cell Metab 5:438–449. [DOI] [PubMed] [Google Scholar]
- Kook S, Vishnivetskiy SA, Gurevich VV, Gurevich EV (2019) Cleavage of arrestin-3 by caspases attenuates cell death by precluding arrestin-dependent JNK activation. Cell Signal 54:161–169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kook S, Zhan X, Cleghorn WM, Benovic JL, Gurevich VV, Gurevich EV (2014) Caspase-cleaved arrestin-2 and BID cooperatively facilitate cytochrome C release and cell death. Cell Death Differ 21:172–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotula JW, Sun J, Li M, Pratico ED, Fereshteh MP, Ahrens DP, Sullenger BA, Kovacs JJ (2014) Targeted disruption of β-arrestin 2-mediated signaling pathways by aptamer chimeras leads to inhibition of leukemic cell growth. PLoS One 9:e93441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovacs JJ, Hara MR, Davenport CL, Kim J, Lefkowitz RJ (2009) Arrestin development: emerging roles for beta-arrestins in developmental signaling pathways. Dev Cell 17:443–458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovoor A, Celver J, Abdryashitov RI, Chavkin C, Gurevich VV (1999) Targeted construction of phosphorylation-independent beta-arrestin mutants with constitutive activity in cells. J Biol Chem 274:6831–6834. [DOI] [PubMed] [Google Scholar]
- Kulkarni RN, Jhala US, Winnay JN, Krajewski S, Montminy M, Kahn CR (2004) PDX-1 haploinsufficiency limits the compensatory islet hyperplasia that occurs in response to insulin resistance. J Clin Invest 114:828–836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lally CC, Bauer B, Selent J, Sommer ME (2017) C-edge loops of arrestin function as a membrane anchor. Nat Commun 8:14258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leão R, Apolónio JD, Lee D, Figueiredo A, Tabori U, Castelo-Branco P (2018) Mechanisms of human telomerase reverse transcriptase (hTERT) regulation: clinical impacts in cancer. J Biomed Sci 25:22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JLC, Nader K, Schiller D (2017) An update on memory reconsolidation updating. Trends Cogn Sci 21:531–545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SJ (2023) Myostatin: a skeletal muscle chalone. Annu Rev Physiol 85:269–291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee Y, Warne T, Nehmé R, Pandey S, Dwivedi-Agnihotri H, Chaturvedi M, Edwards PC, García-Nafría J, Leslie AGW, Shukla AK, et al. (2020) Molecular basis of β-arrestin coupling to formoterol-bound β1-adrenoceptor. Nature 583:862–866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lefkowitz RJ, Shenoy SK (2005) Transduction of receptor signals by beta-arrestins. Science 308:512–517. [DOI] [PubMed] [Google Scholar]
- Lin FT, Daaka Y, Lefkowitz RJ (1998) Beta-arrestins regulate mitogenic signaling and clathrin-mediated endocytosis of the insulin-like growth factor I receptor. J Biol Chem 273:31640–31643. [DOI] [PubMed] [Google Scholar]
- Linder S, Cervero P, Eddy R, Condeelis J (2022) Mechanisms and roles of podosomes and invadopodia. Nat Rev Mol Cell Biol 24:86–106. [DOI] [PubMed] [Google Scholar]
- Lino CA, Barreto-Chaves ML (2022) Beta-arrestins in the context of cardiovascular diseases: Focusing on angiotensin II type 1 receptor (AT1R). Cell Signal 92:110253. [DOI] [PubMed] [Google Scholar]
- Liu H, Yang Z, Lu W, Chen Z, Chen L, Han S, Wu X, Cai T, Cai Y (2020) Chemokines and chemokine receptors: a new strategy for breast cancer therapy. Cancer Med 9:3786–3799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu S, Liu H, Qin R, Shu Y, Liu Z, Zhang P, Duan C, Hong D, Yu J, Zou L (2017a) The cellular senescence of leukemia-initiating cells from acute lymphoblastic leukemia is postponed by β-arrestin1 binding with P300-Sp1 to regulate hTERT transcription. Cell Death Dis 8:e2756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Chen B, You W, Xue S, Qin H, Jiang H (2018) The membrane bile acid receptor TGR5 drives cell growth and migration via activation of the JAK2/STAT3 signaling pathway in non-small cell lung cancer. Cancer Lett 412:194–207. [DOI] [PubMed] [Google Scholar]
- Liu X, Ma L, Li HH, Huang B, Li YX, Tao YZ, Ma L (2015) β-Arrestin-biased signaling mediates memory reconsolidation. Proc Natl Acad Sci USA 112:4483–4488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Easton J, Shao Y, Maciaszek J, Wang Z, Wilkinson MR, McCastlain K, Edmonson M, Pounds SB, Shi L, et al. (2017b) The genomic landscape of pediatric and young adult T-lineage acute lymphoblastic leukemia. Nat Genet 49:1211–1218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Ma X, Yang H, Li X, Ma Y, Ason B, Liu S, Hu LA (2022) APLNR regulates IFN-γ signaling via β-arrestin 1 mediated JAK-STAT1 pathway in melanoma cells. Biochem J 479:385–399. [DOI] [PubMed] [Google Scholar]
- Lokeshwar BL, Kallifatidis G, Hoy JJ (2020) Atypical chemokine receptors in tumor cell growth and metastasis. Adv Cancer Res 145:1–27. [DOI] [PubMed] [Google Scholar]
- Lopes J, Rodrigues CMP, Gaspar MM, Reis CP (2022) Melanoma management: from epidemiology to treatment and latest advances. Cancers (Basel) 14:4652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundgren K, Tobin NP, Lehn S, Stål O, Rydén L, Jirström K, Landberg G (2011) Stromal expression of β-arrestin-1 predicts clinical outcome and tamoxifen response in breast cancer. J Mol Diagn 13:340–351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luttrell LM, Ferguson SS, Daaka Y, Miller WE, Maudsley S, Della Rocca GJ, Lin F, Kawakatsu H, Owada K, Luttrell DK, et al. (1999) Beta-arrestin-dependent formation of beta2 adrenergic receptor-Src protein kinase complexes. Science 283:655–661. [DOI] [PubMed] [Google Scholar]
- Luttrell LM, Maudsley S, Bohn LM (2015) Fulfilling the promise of “biased” G protein-coupled receptor agonism. Mol Pharmacol 88:579–588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luttrell LM, Wang J, Plouffe B, Smith JS, Yamani L, Kaur S, Jean-Charles PY, Gauthier C, Lee MH, Pani B, et al. (2018) Manifold roles of β-arrestins in GPCR signaling elucidated with siRNA and CRISPR/Cas9. Sci Signal 11:eaat7650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lymperopoulos A (2018) Arrestins in the cardiovascular system: an update. Prog Mol Biol Transl Sci 159:27–57. [DOI] [PubMed] [Google Scholar]
- Ma L, Yang F, Wu X, Mao C, Guo L, Miao T, Zang SK, Jiang X, Shen DD, Wei T, et al. (2022) Structural basis and molecular mechanism of biased GPBAR signaling in regulating NSCLC cell growth via YAP activity. Proc Natl Acad Sci USA 119:e2117054119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maharana J, Banerjee R, Yadav MK, Sarma P, Shukla AK (2022) Emerging structural insights into GPCR-β-arrestin interaction and functional outcomes. Curr Opin Struct Biol 75:102406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malik R, Marchese A (2010) Arrestin-2 interacts with the endosomal sorting complex required for transport machinery to modulate endosomal sorting of CXCR4. Mol Biol Cell 21:2529–2541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mallucci G, Dickinson A, Linehan J, Klöhn PC, Brandner S, Collinge J (2003) Depleting neuronal PrP in prion infection prevents disease and reverses spongiosis. Science 302:871–874. [DOI] [PubMed] [Google Scholar]
- Manglik A, Lin H, Aryal DK, McCorvy JD, Dengler D, Corder G, Levit A, Kling RC, Bernat V, Hübner H, et al. (2016) Structure-based discovery of opioid analgesics with reduced side effects. Nature 537:185–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masannat J, Purayil HT, Zhang Y, Russin M, Mahmud I, Kim W, Liao D, Daaka Y (2018) βArrestin2 mediates renal cell carcinoma tumor growth. Sci Rep 8:4879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massimino M, Giangaspero F, Garrè ML, Gandola L, Poggi G, Biassoni V, Gatta G, Rutkowski S (2011) Childhood medulloblastoma. Crit Rev Oncol Hematol 79:65–83. [DOI] [PubMed] [Google Scholar]
- Meister J, Bone DBJ, Godlewski G, Liu Z, Lee RJ, Vishnivetskiy SA, Gurevich VV, Springer D, Kunos G, Wess J (2019) Metabolic effects of skeletal muscle-specific deletion of beta-arrestin-1 and -2 in mice. PLoS Genet 15:e1008424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ménard C, Quirion R (2012) Group 1 metabotropic glutamate receptor function and its regulation of learning and memory in the aging brain. Front Pharmacol 3:182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng D, Lynch MJ, Huston E, Beyermann M, Eichhorst J, Adams DR, Klussmann E, Houslay MD, Baillie GS (2009) MEK1 binds directly to betaarrestin1, influencing both its phosphorylation by ERK and the timing of its isoprenaline-stimulated internalization. J Biol Chem 284:11425–11435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merz KE, Thurmond DC (2020) Role of skeletal muscle in insulin resistance and glucose uptake. Compr Physiol 10:785–809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer PJ, King CP, Ferrario CR (2016) Motivational processes underlying substance abuse disorder. Curr Top Behav Neurosci 27:473–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michal AM, Peck AR, Tran TH, Liu C, Rimm DL, Rui H, Benovic JL (2011) Differential expression of arrestins is a predictor of breast cancer progression and survival. Breast Cancer Res Treat 130:791–807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miele E, Po A, Begalli F, Antonucci L, Mastronuzzi A, Marras CE, Carai A, Cucchi D, Abballe L, Besharat ZM, et al. (2017) β-Arrestin1-mediated acetylation of Gli1 regulates Hedgehog/Gli signaling and modulates self-renewal of SHH medulloblastoma cancer stem cells. BMC Cancer 17:488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mollica Poeta V, Massara M, Capucetti A, Bonecchi R (2019) Chemokines and chemokine receptors: new targets for cancer immunotherapy. Front Immunol 10:379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monasky MM, Taglieri DM, Henze M, Warren CM, Utter MS, Soergel DG, Violin JD, Solaro RJ (2013) The β-arrestin-biased ligand TRV120023 inhibits angiotensin II-induced cardiac hypertrophy while preserving enhanced myofilament response to calcium. Am J Physiol Heart Circ Physiol 305:H856–H866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mores KL, Cummins BR, Cassell RJ, van Rijn RM (2019) A review of the therapeutic potential of recently developed G protein-biased kappa agonists. Front Pharmacol 10:407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgenweck J, Frankowski KJ, Prisinzano TE, Aubé J, Bohn LM (2015) Investigation of the role of βarrestin2 in kappa opioid receptor modulation in a mouse model of pruritus. Neuropharmacology 99:600–609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakajima K, Cui Z, Li C, Meister J, Cui Y, Fu O, Smith AS, Jain S, Lowell BB, Krashes MJ, et al. (2016) Gs-coupled GPCR signalling in AgRP neurons triggers sustained increase in food intake. Nat Commun 7:10268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakajima K, Wess J (2012) Design and functional characterization of a novel, arrestin-biased designer G protein-coupled receptor. Mol Pharmacol 82:575–582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen AH, Thomsen ARB, Cahill TJ 3rd, Huang R, Huang LY, Marcink T, Clarke OB, Heissel S, Masoudi A, Ben-Hail D, et al. (2019) Structure of an endosomal signaling GPCR-G protein-β-arrestin megacomplex. Nat Struct Mol Biol 26:1123–1131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nibbs RJ, Graham GJ (2013) Immune regulation by atypical chemokine receptors. Nat Rev Immunol 13:815–829. [DOI] [PubMed] [Google Scholar]
- Nobles KN, Xiao K, Ahn S, Shukla AK, Lam CM, Rajagopal S, Strachan RT, Huang TY, Bressler EA, Hara MR, et al. (2011) Distinct phosphorylation sites on the β(2)-adrenergic receptor establish a barcode that encodes differential functions of β-arrestin. Sci Signal 4:ra51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Hayre M, Eichel K, Avino S, Zhao X, Steffen DJ, Feng X, Kawakami K, Aoki J, Messer K, Sunahara R, et al. (2017) Genetic evidence that β-arrestins are dispensable for the initiation of β2-adrenergic receptor signaling to ERK. Sci Signal 10:eaal3395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osterweil EK, Krueger DD, Reinhold K, Bear MF (2010) Hypersensitivity to mGluR5 and ERK1/2 leads to excessive protein synthesis in the hippocampus of a mouse model of fragile X syndrome. J Neurosci 30:15616–15627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pakharukova N, Masoudi A, Pani B, Staus DP, Lefkowitz RJ (2020) Allosteric activation of proto-oncogene kinase Src by GPCR-beta-arrestin complexes. J Biol Chem 295:16773–16784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan L, Gurevich EV, Gurevich VV (2003) The nature of the arrestin x receptor complex determines the ultimate fate of the internalized receptor. J Biol Chem 278:11623–11632. [DOI] [PubMed] [Google Scholar]
- Pang PS, Butler J, Collins SP, Cotter G, Davison BA, Ezekowitz JA, Filippatos G, Levy PD, Metra M, Ponikowski P, et al. (2017) Biased ligand of the angiotensin II type 1 receptor in patients with acute heart failure: a randomized, double-blind, placebo-controlled, phase IIB, dose ranging trial (BLAST-AHF). Eur Heart J 38:2364–2373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel SJ, Sanjana NE, Kishton RJ, Eidizadeh A, Vodnala SK, Cam M, Gartner JJ, Jia L, Steinberg SM, Yamamoto TN, et al. (2017) Identification of essential genes for cancer immunotherapy. Nature 548:537–542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry NA, Fialkowski KP, Kaoud TS, Kaya AI, Chen AL, Taliaferro JM, Gurevich VV, Dalby KN, Iverson TM (2019) Arrestin-3 interaction with maternal embryonic leucine-zipper kinase. Cell Signal 63:109366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry-Hauser NA, Hopkins JB, Zhuo Y, Zheng C, Perez I, Schultz KM, Vishnivetskiy SA, Kaya AI, Sharma P, Dalby KN, et al. (2022a) The two non-visual arrestins engage ERK2 differently. J Mol Biol 434:167465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry-Hauser NA, Kaoud TS, Stoy H, Zhan X, Chen Q, Dalby KN, Iverson TM, Gurevich VV, Gurevich EV (2022b) Short arrestin-3-derived peptides activate JNK3 in cells. Int J Mol Sci 23:8679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson SM, Pack TF, Wilkins AD, Urs NM, Urban DJ, Bass CE, Lichtarge O, Caron MG (2015) Elucidation of G-protein and β-arrestin functional selectivity at the dopamine D2 receptor. Proc Natl Acad Sci USA 112:7097–7102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson YK, Luttrell LM (2017) The diverse roles of arrestin scaffolds in G protein-coupled receptor signaling. Pharmacol Rev 69:256–297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Philipp M, Evron T, Caron MG (2013) The role of arrestins in development. Prog Mol Biol Transl Sci 118:225–242. [DOI] [PubMed] [Google Scholar]
- Pierce RC, Kumaresan V (2006) The mesolimbic dopamine system: the final common pathway for the reinforcing effect of drugs of abuse? Neurosci Biobehav Rev 30:215–238. [DOI] [PubMed] [Google Scholar]
- Porter-Stransky KA, Petko AK, Karne SL, Liles LC, Urs NM, Caron MG, Paladini CA, Weinshenker D (2020) Loss of β-arrestin2 in D2 cells alters neuronal excitability in the nucleus accumbens and behavioral responses to psychostimulants and opioids. Addict Biol 25:e12823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porter-Stransky KA, Weinshenker D (2017) Arresting the development of addiction: the role of β-arrestin 2 in drug abuse. J Pharmacol Exp Ther 361:341–348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poulin B, Butcher A, McWilliams P, Bourgognon JM, Pawlak R, Kong KC, Bottrill A, Mistry S, Wess J, Rosethorne EM, et al. (2010) The M3-muscarinic receptor regulates learning and memory in a receptor phosphorylation/arrestin-dependent manner. Proc Natl Acad Sci USA 107:9440–9445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pradhan AA, Befort K, Nozaki C, Gavériaux-Ruff C, Kieffer BL (2011) The delta opioid receptor: an evolving target for the treatment of brain disorders. Trends Pharmacol Sci 32:581–590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pradhan AA, Perroy J, Walwyn WM, Smith ML, Vicente-Sanchez A, Segura L, Bana A, Kieffer BL, Evans CJ (2016) Agonist-specific recruitment of arrestin isoforms differentially modify delta opioid receptor function. J Neurosci 36:3541–3551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purayil HT, Daaka Y (2018) β-Arrestin1 mediates hMENA expression and ovarian cancer metastasis. Proc Natl Acad Sci USA 115:2856–2858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purayil HT, Daaka Y (2022) βArrestin1 regulates glucocorticoid receptor mitogenic signaling in castration-resistant prostate cancer. Prostate 82:816–825. [DOI] [PubMed] [Google Scholar]
- Purayil HT, Zhang Y, Black JB, Gharaibeh R, Daaka Y (2021) Nuclear βarrestin1 regulates androgen receptor function in castration resistant prostate cancer. Oncogene 40:2610–2620. [DOI] [PubMed] [Google Scholar]
- Pydi SP, Barella LF, Zhu L, Meister J, Rossi M, Wess J (2022) β-Arrestins as important regulators of glucose and energy homeostasis. Annu Rev Physiol 84:17–40. [DOI] [PubMed] [Google Scholar]
- Pydi SP, Cui Z, He Z, Barella LF, Pham J, Cui Y, Oberlin DJ, Egritag HE, Urs N, Gavrilova O, et al. (2020a) Beneficial metabolic role of β-arrestin-1 expressed by AgRP neurons. Sci Adv 6:eaaz1341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pydi SP, Jain S, Barella LF, Zhu L, Sakamoto W, Meister J, Wang L, Lu H, Cui Y, Gavrilova O, Wess J (2020b) β-arrestin-1 suppresses myogenic reprogramming of brown fat to maintain euglycemia. Sci Adv 6:eaba1733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pydi SP, Jain S, Tung W, Cui Y, Zhu L, Sakamoto W, Jain S, Abel BS, Skarulis MC, Liu J, et al. (2019) Adipocyte β-arrestin-2 is essential for maintaining whole body glucose and energy homeostasis. Nat Commun 10:2936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qu C, Park JY, Yun MW, He QT, Yang F, Kim K, Ham D, Li RR, Iverson TM, Gurevich VV, et al. (2021) Scaffolding mechanism of arrestin-2 in the cRaf/MEK1/ERK signaling cascade. Proc Natl Acad Sci USA 118:e2026491118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quirion B, Bergeron F, Blais V, Gendron L (2020) The delta-opioid receptor; a target for the treatment of pain. Front Mol Neurosci 13:52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajagopal S, Kim J, Ahn S, Craig S, Lam CM, Gerard NP, Gerard C, Lefkowitz RJ (2010a) Beta-arrestin—but not G protein-mediated signaling by the “decoy” receptor CXCR7. Proc Natl Acad Sci USA 107:628–632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajagopal S, Rajagopal K, Lefkowitz RJ (2010b) Teaching old receptors new tricks: biasing seven-transmembrane receptors. Nat Rev Drug Discov 9:373–386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranjan R, Dwivedi H, Baidya M, Kumar M, Shukla AK (2017) Novel structural insights into GPCR-β-arrestin interaction and signaling. Trends Cell Biol 27:851–862. [DOI] [PubMed] [Google Scholar]
- Regard JB, Sato IT, Coughlin SR (2008) Anatomical profiling of G protein-coupled receptor expression. Cell 135:561–571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rein LA, Wisler JW, Kim J, Theriot B, Huang L, Price T, Yang H, Chen M, Chen W, Sipkins D, et al. (2017) β-Arrestin2 mediates progression of murine primary myelofibrosis. JCI Insight 2:e98094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren H, Orozco IJ, Su Y, Suyama S, Gutiérrez-Juárez R, Horvath TL, Wardlaw SL, Plum L, Arancio O, Accili D (2012) FoxO1 target Gpr17 activates AgRP neurons to regulate food intake. Cell 149:1314–1326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren W, Wang T, He X, Zhang Q, Zhou J, Liu F, Gao F, Zhang Y, Liu Y (2018) β-arrestin2 promotes 5-FU-induced apoptosis via the NF-κB pathway in colorectal cancer. Oncol Rep 39:2711–2720. [DOI] [PubMed] [Google Scholar]
- Roden M, Shulman GI (2019) The integrative biology of type 2 diabetes. Nature 576:51–60. [DOI] [PubMed] [Google Scholar]
- Rose SJ, Pack TF, Peterson SM, Payne K, Borrelli E, Caron MG (2018) Engineered D2R variants reveal the balanced and biased contributions of G-protein and β-arrestin to dopamine-dependent functions. Neuropsychopharmacology 43:1164–1173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryba DM, Li J, Cowan CL, Russell B, Wolska BM, Solaro RJ (2017) Long-term biased β-arrestin signaling improves cardiac structure and function in dilated cardiomyopathy. Circulation 135:1056–1070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sachdeva MM, Stoffers DA (2009) Minireview: meeting the demand for insulin: molecular mechanisms of adaptive postnatal beta-cell mass expansion. Mol Endocrinol 23:747–758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saitoh A, Kimura Y, Suzuki T, Kawai K, Nagase H, Kamei J (2004) Potential anxiolytic and antidepressant-like activities of SNC80, a selective delta-opioid agonist, in behavioral models in rodents. J Pharmacol Sci 95:374–380. [DOI] [PubMed] [Google Scholar]
- Saitoh A, Yamada M (2012) Antidepressant-like effects of δ opioid receptor agonists in animal models. Curr Neuropharmacol 10:231–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samaranayake S, Song X, Vishnivetskiy SA, Chen J, Gurevich EV, Gurevich VV (2018) Enhanced mutant compensates for defects in rhodopsin phosphorylation in the presence of endogenous arrestin-1. Front Mol Neurosci 11:203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez-Gurmaches J, Guertin DA (2014) Adipocyte lineages: tracing back the origins of fat. Biochim Biophys Acta 1842:340–351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scarpa M, Molloy C, Jenkins L, Strellis B, Budgett RF, Hesse S, Dwomoh L, Marsango S, Tejeda GS, Rossi M, et al. (2021) Biased M1 muscarinic receptor mutant mice show accelerated progression of prion neurodegenerative disease. Proc Natl Acad Sci USA 118:e2107389118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scelo G, Larose TL (2018) Epidemiology and risk factors for kidney cancer. J Clin Oncol 36:JCO2018791905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scharfmann R, Pechberty S, Hazhouz Y, von Bülow M, Bricout-Neveu E, Grenier-Godard M, Guez F, Rachdi L, Lohmann M, Czernichow P, et al. (2014) Development of a conditionally immortalized human pancreatic β cell line. J Clin Invest 124:2087–2098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheerer P, Sommer ME (2017) Structural mechanism of arrestin activation. Curr Opin Struct Biol 45:160–169. [DOI] [PubMed] [Google Scholar]
- Schmid CL, Bohn LM (2009) Physiological and pharmacological implications of beta-arrestin regulation. Pharmacol Ther 121:285–293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmid CL, Kennedy NM, Ross NC, Lovell KM, Yue Z, Morgenweck J, Cameron MD, Bannister TD, Bohn LM (2017) Bias factor and therapeutic window correlate to predict safer opioid analgesics. Cell 171:1165–1175.e13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schöneberg T, Schulz A, Biebermann H, Hermsdorf T, Römpler H, Sangkuhl K (2004) Mutant G-protein-coupled receptors as a cause of human diseases. Pharmacol Ther 104:173–206. [DOI] [PubMed] [Google Scholar]
- Seeman MV (2021) History of the dopamine hypothesis of antipsychotic action. World J Psychiatry 11:355–364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seyedabadi M, Ghahremani MH, Albert PR (2019) Biased signaling of G protein coupled receptors (GPCRs): molecular determinants of GPCR/transducer selectivity and therapeutic potential. Pharmacol Ther 200:148–178. [DOI] [PubMed] [Google Scholar]
- Seyedabadi M, Gharghabi M, Gurevich EV, Gurevich VV (2022) Structural basis of GPCR coupling to distinct signal transducers: implications for biased signaling. Trends Biochem Sci 47:570–581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shenoy SK, Drake MT, Nelson CD, Houtz DA, Xiao K, Madabushi S, Reiter E, Premont RT, Lichtarge O, Lefkowitz RJ (2006) Beta-arrestin-dependent, G protein-independent ERK1/2 activation by the beta2 adrenergic receptor. J Biol Chem 281:1261–1273. [DOI] [PubMed] [Google Scholar]
- Shenoy SK, Han S, Zhao YL, Hara MR, Oliver T, Cao Y, Dewhirst MW (2012) β-Arrestin1 mediates metastatic growth of breast cancer cells by facilitating HIF-1-dependent VEGF expression. Oncogene 31:282–292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shenoy SK, McDonald PH, Kohout TA, Lefkowitz RJ (2001) Regulation of receptor fate by ubiquitination of activated beta 2-adrenergic receptor and beta-arrestin. Science 294:1307–1313. [DOI] [PubMed] [Google Scholar]
- Shibasaki T, Takahashi H, Miki T, Sunaga Y, Matsumura K, Yamanaka M, Zhang C, Tamamoto A, Satoh T, Miyazaki J, et al. (2007) Essential role of Epac2/Rap1 signaling in regulation of insulin granule dynamics by cAMP. Proc Natl Acad Sci USA 104:19333–19338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shonberg J, Lopez L, Scammells PJ, Christopoulos A, Capuano B, Lane JR (2014) Biased agonism at G protein-coupled receptors: the promise and the challenges—a medicinal chemistry perspective. Med Res Rev 34:1286–1330. [DOI] [PubMed] [Google Scholar]
- Shu Y, Wang Y, Lv WQ, Peng DY, Li J, Zhang H, Jiang GJ, Yang BJ, Liu S, Zhang J, et al. (2020) ARRB1-promoted NOTCH1 degradation is suppressed by OncomiR miR-223 in T-cell acute lymphoblastic leukemia. Cancer Res 80:988–998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shukla AK, Dwivedi-Agnihotri H (2020) Structure and function of β-arrestins, their emerging role in breast cancer, and potential opportunities for therapeutic manipulation. Adv Cancer Res 145:139–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shukla AK, Manglik A, Kruse AC, Xiao K, Reis RI, Tseng WC, Staus DP, Hilger D, Uysal S, Huang LY, et al. (2013) Structure of active β-arrestin-1 bound to a G-protein-coupled receptor phosphopeptide. Nature 497:137–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shukla AK, Westfield GH, Xiao K, Reis RI, Huang L-Y, Tripathi-Shukla P, Qian J, Li S, Blanc A, Oleskie AN, et al. (2014) Visualization of arrestin recruitment by a G-protein-coupled receptor. Nature 512:218–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singleton S, Baptista-Hon DT, Edelsten E, McCaughey KS, Camplisson E, Hales TG (2021) TRV130 partial agonism and capacity to induce anti-nociceptive tolerance revealed through reducing available μ-opioid receptor number. Br J Pharmacol 178:1855–1868. [DOI] [PubMed] [Google Scholar]
- Sjöberg E, Meyrath M, Chevigné A, Östman A, Augsten M, Szpakowska M (2020) The diverse and complex roles of atypical chemokine receptors in cancer: from molecular biology to clinical relevance and therapy. Adv Cancer Res 145:99–138. [DOI] [PubMed] [Google Scholar]
- Slifstein M, van de Giessen E, Van Snellenberg J, Thompson JL, Narendran R, Gil R, Hackett E, Girgis R, Ojeil N, Moore H, et al. (2015) Deficits in prefrontal cortical and extrastriatal dopamine release in schizophrenia: a positron emission tomographic functional magnetic resonance imaging study. JAMA Psychiatry 72:316–324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slosky LM, Bai Y, Toth K, Ray C, Rochelle LK, Badea A, Chandrasekhar R, Pogorelov VM, Abraham DM, Atluri N, et al. (2020) β-Arrestin-biased allosteric modulator of NTSR1 selectively attenuates addictive behaviors. Cell 181:1364–1379.e14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith JS, Lefkowitz RJ, Rajagopal S (2018) Biased signalling: from simple switches to allosteric microprocessors. Nat Rev Drug Discov 17:243–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Son D, Kim Y, Lim S, Kang HG, Kim DH, Park JW, Cheong W, Kong HK, Han W, Park WY, et al. (2019) miR-374a-5p promotes tumor progression by targeting ARRB1 in triple negative breast cancer. Cancer Lett 454:224–233. [DOI] [PubMed] [Google Scholar]
- Song X, Raman D, Gurevich EV, Vishnivetskiy SA, Gurevich VV (2006) Visual and both non-visual arrestins in their “inactive” conformation bind JNK3 and Mdm2 and relocalize them from the nucleus to the cytoplasm. J Biol Chem 281:21491–21499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song X, Vishnivetskiy SA, Gross OP, Emelianoff K, Mendez A, Chen J, Gurevich EV, Burns ME, Gurevich VV (2009) Enhanced arrestin facilitates recovery and protects rod photoreceptors deficient in rhodopsin phosphorylation. Curr Biol 19:700–705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spiller HA, James KJ, Scholzen S, Borys DJ (2013) A descriptive study of adverse events from clenbuterol misuse and abuse for weight loss and bodybuilding. Subst Abus 34:306–312. [DOI] [PubMed] [Google Scholar]
- Stahl EL, Bohn LM (2022) Low intrinsic efficacy alone cannot explain the improved side effect profiles of new opioid agonists. Biochemistry 61:1923–1935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staus DP, Hu H, Robertson MJ, Kleinhenz ALW, Wingler LM, Capel WD, Latorraca NR, Lefkowitz RJ, Skiniotis G (2020) Structure of the M2 muscarinic receptor-β-arrestin complex in a lipid nanodisc. Nature 579:297–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steculorum SM, Ruud J, Karakasilioti I, Backes H, Engström Ruud L, Timper K, Hess ME, Tsaousidou E, Mauer J, Vogt MC, et al. (2016) AgRP neurons control systemic insulin sensitivity via myostatin expression in brown adipose tissue. Cell 165:125–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein C (2016) Opioid receptors. Annu Rev Med 67:433–451. [DOI] [PubMed] [Google Scholar]
- Steketee JD, Kalivas PW (2011) Drug wanting: behavioral sensitization and relapse to drug-seeking behavior. Pharmacol Rev 63:348–365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart C, Ralyea C, Lockwood S (2019) Ovarian cancer: an integrated review. Semin Oncol Nurs 35:151–156. [DOI] [PubMed] [Google Scholar]
- Stoppel DC, McCamphill PK, Senter RK, Heynen AJ, Bear MF (2021) mGluR5 negative modulators for fragile X: treatment resistance and persistence. Front Psychiatry 12:718953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoppel LJ, Auerbach BD, Senter RK, Preza AR, Lefkowitz RJ, Bear MF (2017) β-Arrestin2 couples metabotropic glutamate receptor 5 to neuronal protein synthesis and is a potential target to treat fragile X. Cell Rep 18:2807–2814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoy H, Gurevich VV (2015) How genetic errors in GPCRs affect their function: possible therapeutic strategies. Genes Dis 2:108–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugiyama A, Yamada M, Furuie H, Gotoh L, Saitoh A, Nagase H, Oka JI, Yamada M (2019) Systemic administration of a delta opioid receptor agonist, KNT-127, facilitates extinction learning of fear memory in rats. J Pharmacol Sci 139:174–179. [DOI] [PubMed] [Google Scholar]
- Takahashi LK (2001) Role of CRF(1) and CRF(2) receptors in fear and anxiety. Neurosci Biobehav Rev 25:627–636. [DOI] [PubMed] [Google Scholar]
- Tan AC, Ashley DM, López GY, Malinzak M, Friedman HS, Khasraw M (2020) Management of glioblastoma: state of the art and future directions. CA Cancer J Clin 70:299–312. [DOI] [PubMed] [Google Scholar]
- Thompson PA, Kantarjian HM, Cortes JE (2015) Diagnosis and treatment of chronic myeloid leukemia in 2015. Mayo Clin Proc 90:1440–1454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomsen ARB, Plouffe B, Cahill TJ 3rd, Shukla AK, Tarrasch JT, Dosey AM, Kahsai AW, Strachan RT, Pani B, Mahoney JP, et al. (2016) GPCR-G protein-β-arrestin super-complex mediates sustained G protein signaling. Cell 166:907–919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian X, Wang J, Jiang L, Jiang Y, Xu J, Feng X (2022) Chemokine/GPCR signaling-mediated EMT in cancer metastasis. J Oncol 2022:2208176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tocci P, Rosanò L, Bagnato A (2019) Targeting endothelin-1 receptor/β-arrestin-1 axis in ovarian cancer: from basic research to a therapeutic approach. Front Endocrinol (Lausanne) 10:609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torphy RJ, Yee EJ, Schulick RD, Zhu Y (2022) Atypical chemokine receptors: emerging therapeutic targets in cancer. Trends Pharmacol Sci 43:1085–1097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tovote P, Fadok JP, Lüthi A (2015) Neuronal circuits for fear and anxiety. Nat Rev Neurosci 16:317–331. [DOI] [PubMed] [Google Scholar]
- Urban DJ, Roth BL (2015) DREADDs (designer receptors exclusively activated by designer drugs): chemogenetic tools with therapeutic utility. Annu Rev Pharmacol Toxicol 55:399–417. [DOI] [PubMed] [Google Scholar]
- Urs NM, Gee SM, Pack TF, McCorvy JD, Evron T, Snyder JC, Yang X, Rodriguiz RM, Borrelli E, Wetsel WC, et al. (2016) Distinct cortical and striatal actions of a β-arrestin-biased dopamine D2 receptor ligand reveal unique antipsychotic-like properties. Proc Natl Acad Sci USA 113:E8178–E8186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vetere A, Choudhary A, Burns SM, Wagner BK (2014) Targeting the pancreatic β-cell to treat diabetes. Nat Rev Drug Discov 13:278–289. [DOI] [PubMed] [Google Scholar]
- Vicente-Sanchez A, Dripps IJ, Tipton AF, Akbari H, Akbari A, Jutkiewicz EM, Pradhan AA (2018) Tolerance to high-internalizing δ opioid receptor agonist is critically mediated by arrestin 2. Br J Pharmacol 175:3050–3059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vodenkova S, Buchler T, Cervena K, Veskrnova V, Vodicka P, Vymetalkova V (2020) 5-Fluorouracil and other fluoropyrimidines in colorectal cancer: past, present and future. Pharmacol Ther 206:107447. [DOI] [PubMed] [Google Scholar]
- Volkmann C, Bschor T, Köhler S (2020) Lithium treatment over the lifespan in bipolar disorders. Front Psychiatry 11:377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L-H, Ding W-Q, Sun Y-G (2022a) Spinal ascending pathways for somatosensory information processing. Trends Neurosci 45:594–607. [DOI] [PubMed] [Google Scholar]
- Wang M, Datta D, Enwright J, Galvin V, Yang ST, Paspalas C, Kozak R, Gray DL, Lewis DA, Arnsten AFT (2019) A novel dopamine D1 receptor agonist excites delay-dependent working memory-related neuronal firing in primate dorsolateral prefrontal cortex. Neuropharmacology 150:46–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang R, Wang S, Li Z, Luo Y, Zhao Y, Han Q, Rong XZ, Guo YX, Liu Y (2022b) PLEKHH2 binds β-arrestin1 through its FERM domain, activates FAK/PI3K/AKT phosphorylation, and promotes the malignant phenotype of non-small cell lung cancer. Cell Death Dis 13:858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang W, Seale P (2016) Control of brown and beige fat development. Nat Rev Mol Cell Biol 17:691–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei H, Ahn S, Shenoy SK, Karnik SS, Hunyady L, Luttrell LM, Lefkowitz RJ (2003) Independent beta-arrestin 2 and G protein-mediated pathways for angiotensin II activation of extracellular signal-regulated kinases 1 and 2. Proc Natl Acad Sci USA 100:10782–10787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White KL, Robinson JE, Zhu H, DiBerto JF, Polepally PR, Zjawiony JK, Nichols DE, Malanga CJ, Roth BL (2015) The G protein-biased κ-opioid receptor agonist RB-64 is analgesic with a unique spectrum of activities in vivo. J Pharmacol Exp Ther 352:98–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wingler LM, Lefkowitz RJ (2020) Conformational basis of G protein-coupled receptor signaling versatility. Trends Cell Biol 30:736–747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wisler JW, DeWire SM, Whalen EJ, Violin JD, Drake MT, Ahn S, Shenoy SK, Lefkowitz RJ (2007) A unique mechanism of beta-blocker action: carvedilol stimulates beta-arrestin signaling. Proc Natl Acad Sci USA 104:16657–16662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woo JA, Yan Y, Kee TR, Cazzaro S, McGill Percy KC, Wang X, Liu T, Liggett SB, Kang DE (2021) β-arrestin1 promotes tauopathy by transducing GPCR signaling, disrupting microtubules and autophagy. Life Sci Alliance 5::e202101183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyatt D, Malik R, Vesecky AC, Marchese A (2011) Small ubiquitin-like modifier modification of arrestin-3 regulates receptor trafficking. J Biol Chem 286:3884–3893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wysocka MB, Pietraszek-Gremplewicz K, Nowak D (2018) The role of apelin in cardiovascular diseases, obesity and cancer. Front Physiol 9:557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao K, McClatchy DB, Shukla AK, Zhao Y, Chen M, Shenoy SK, Yates JR 3rd, Lefkowitz RJ (2007) Functional specialization of beta-arrestin interactions revealed by proteomic analysis. Proc Natl Acad Sci USA 104:12011–12016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao N, Li H, Mei W, Cheng J (2015) SUMOylation attenuates human β-arrestin 2 inhibition of IL-1R/TRAF6 signaling. J Biol Chem 290:1927–1935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu S, Tang J, Wang C, Liu J, Fu Y, Luo Y (2019) CXCR7 promotes melanoma tumorigenesis via Src kinase signaling. Cell Death Dis 10:191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada D, Yanagisawa S, Yoshizawa K, Yanagita S, Oka JI, Nagase H, Saitoh A (2019) Selective agonists of the δ-opioid receptor, KNT-127 and SNC80, act differentially on extinction learning of contextual fear memory in mice. Neuropharmacology 160:107792. [DOI] [PubMed] [Google Scholar]
- Yang Y, Guo Y, Tan S, Ke B, Tao J, Liu H, Jiang J, Chen J, Chen G, Wu B (2015) β-Arrestin1 enhances hepatocellular carcinogenesis through inflammation-mediated Akt signalling. Nat Commun 6:7369. [DOI] [PubMed] [Google Scholar]
- Yang Y, Lee SM, Imamura F, Gowda K, Amin S, Mailman RB (2021) D1 dopamine receptors intrinsic activity and functional selectivity affect working memory in prefrontal cortex. Mol Psychiatry 26:645–655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin W, Li Z, Jin M, Yin YL, de Waal PW, Pal K, Yin Y, Gao X, He Y, Gao J, et al. (2019) A complex structure of arrestin-2 bound to a G protein-coupled receptor. Cell Res 29:971–983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zang Y, Kahsai AW, Pakharukova N, Huang LY, Lefkowitz RJ (2021) The GPCR-β-arrestin complex allosterically activates C-Raf by binding its amino terminus. J Biol Chem 297:101369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zecchini V, Madhu B, Russell R, Pértega-Gomes N, Warren A, Gaude E, Borlido J, Stark R, Ireland-Zecchini H, Rao R, et al. (2014) Nuclear ARRB1 induces pseudohypoxia and cellular metabolism reprogramming in prostate cancer. EMBO J 33:1365–1382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhan X, Stoy H, Kaoud TS, Perry NA, Chen Q, Perez A, Els-Heindl S, Slagis JV, Iverson TM, Beck-Sickinger AG, et al. (2016) Peptide mini-scaffold facilitates JNK3 activation in cells. Sci Rep 6:21025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang CL, Katoh M, Shibasaki T, Minami K, Sunaga Y, Takahashi H, Yokoi N, Iwasaki M, Miki T, Seino S (2009) The cAMP sensor Epac2 is a direct target of antidiabetic sulfonylurea drugs. Science 325:607–610. [DOI] [PubMed] [Google Scholar]
- Zhang YX, Li XF, Yuan GQ, Hu H, Song XY, Li JY, Miao XK, Zhou TX, Yang WL, Zhang XW, et al. (2017) β-Arrestin 1 has an essential role in neurokinin-1 receptor-mediated glioblastoma cell proliferation and G2/M phase transition. J Biol Chem 292:8933–8947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao J, Pei G (2013) Arrestins in metabolic regulation. Prog Mol Biol Transl Sci 118:413–427. [DOI] [PubMed] [Google Scholar]
- Zhou XE, He Y, de Waal PW, Gao X, Kang Y, Van Eps N, Yin Y, Pal K, Goswami D, White TA, et al. (2017) Identification of phosphorylation codes for arrestin recruitment by G protein-coupled receptors. Cell 170:457–469.e13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu L, Almaça J, Dadi PK, Hong H, Sakamoto W, Rossi M, Lee RJ, Vierra NC, Lu H, Cui Y, et al. (2017a) β-Arrestin-2 is an essential regulator of pancreatic β-cell function under physiological and pathophysiological conditions. Nat Commun 8:14295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu L, Rossi M, Cui Y, Lee RJ, Sakamoto W, Perry NA, Urs NM, Caron MG, Gurevich VV, Godlewski G, et al. (2017b) Hepatic β-arrestin 2 is essential for maintaining euglycemia. J Clin Invest 127:2941–2945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhuang T, Chen Q, Cho M-K, Vishnivetskiy SA, Iverson TM, Gurevich VV, Sanders CR (2013) Involvement of distinct arrestin-1 elements in binding to different functional forms of rhodopsin. Proc Natl Acad Sci USA 110:942–947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhuo Y, Gurevich VV, Vishnivetskiy SA, Klug CS, Marchese A (2020) A non-GPCR-binding partner interacts with a novel surface on β-arrestin1 to mediate GPCR signaling. J Biol Chem 295:14111–14124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhuo Y, Vishnivetskiy SA, Zhan X, Gurevich VV, Klug CS (2014) Identification of receptor binding-induced conformational changes in non-visual arrestins. J Biol Chem 289:20991–21002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zurkovsky L, Sedaghat K, Ahmed MR, Gurevich VV, Gurevich EV (2017) Arrestin-2 and arrestin-3 differentially modulate locomotor responses and sensitization to amphetamine. Neuropharmacology 121:20–29. [DOI] [PMC free article] [PubMed] [Google Scholar]