Abstract
Purpose
Several studies report decreased hospital admissions for acute exacerbations of COPD (AECOPD) during the COVID-19 pandemic. However, there are no studies that compare AECOPD admissions with admissions for respiratory infections, including COVID-19. This study aimed to examine hospital admission rates for AECOPD, pneumonia, influenza, and COVID-19 among COPD patients, before and during the COVID-19 pandemic.
Patients and Methods
We obtained anonymized data on hospital admissions of patients with COPD and a primary diagnosis code for AECOPD, pneumonia, influenza, or COVID-19, from the hospital patient admission register at a large Swedish hospital. The study compared the pandemic period (February 2020–March 2022) to a period before the pandemic (June 2017–January 2020). Sequential phases of the pandemic were evaluated separately. Monthly admission rates were compared using Poisson regression, controlling for admission month.
Results
Comparing monthly admission rates during the pandemic with the prepandemic period, incidence rate ratios were 0.72 for AECOPD (95% CI 0.67–0.77; p<0.001), 0.56 for pneumonia (95% CI 0.49–0.62; p<0.001), 0.18 for influenza during the winter period (95% CI 0.10–0.30; p<0.001) and 0.79 for total COPD admissions, including COVID-19 (95% CI 0.75–0.84; p<0.001). The study showed significantly lower rate ratios for AECOPD, pneumonia, and total COPD admissions during the first, second, third, and fifth (Omicron) waves. No significant effect on admissions was seen after the withdrawal of restriction measures.
Conclusion
There was a significant reduction in the overall rate of hospital admissions among COPD patients for AECOPD, pneumonia, and respiratory viral infections during the pandemic despite the rise in COVID-19 admissions. However, prepandemic admission levels returned in the post-restriction period.
Keywords: COPD, acute exacerbation of COPD, respiratory viruses, influenza virus, COVID-19, epidemiology
Introduction
Chronic obstructive pulmonary disease (COPD) is a significant cause of morbidity and death globally.1 In Sweden, a study reported a high average direct cost for COPD patients in 2013 (€13,719).2 A large part of these costs was associated with hospitalizations. Acute exacerbations of chronic obstructive pulmonary disease (AECOPDs), are closely linked to hospitalizations in COPD and hospital admission may be accompanied by acute respiratory failure. Exacerbations are related to disease progression, impaired pulmonary function, and mortality in COPD patients.3,4 Frequent hospitalizations for AECOPD are associated with poor long-term prognosis and increased mortality.4
Respiratory viral infections are the primary triggers for AECOPDs, although bacterial infections and environmental factors can also provoke them.5 Viral detection of agents such as influenza virus is associated with poor clinical outcomes.6 In addition, pneumonia with or without AECOPD is a common cause of hospitalization in patients with COPD.7,8
Since the pandemic spread of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), COPD has been identified as an independent risk factor of severe coronavirus disease 2019 (COVID-19), requiring hospitalization.9 More severe disease and increased mortality among COVID-19 patients with COPD have also been described in several studies.10–13
Decreased frequency of hospital admissions for AECOPD during the COVID-19 pandemic has been reported in previous studies.14 The exact cause for the decreased admission rate due to AECOPD is unknown. However, it has been hypothesized that non-pharmaceutical interventions such as masking and social distancing, implemented to mitigate the spread of SARS-CoV-2, may have reduced the incidence of AECOPD caused by respiratory viral infections other than COVID-19. In line with this, non-pharmaceutical interventions have been associated with reduced transmission of various common circulating viral respiratory agents during the pandemic, across different geographic regions.15 In Sweden, the influenza season of 2019–2020 was unusually mild, with a reported national total of 7941 cases, significantly lower than the previous three-year average of approximately 15,800 cases.16
During the COVID-19 pandemic in Sweden, public health strategies to mitigate the spread of SARS-CoV-2 included widespread testing, contact tracing, and social distancing.17 Additionally, restrictions on large gatherings and events were implemented. However, general restriction measures were based on voluntary recommendations rather than regulations and laws. Furthermore, lockdowns were not imposed, elementary schools were kept open and the general use of face masks was not recommended. Thus, the effect of non-pharmaceutical interventions on the transmission of circulating respiratory viral infections may have differed from other countries, since public health measures were less strict in Sweden.
The elderly in Sweden over the age of 70 was initially during the pandemic advised to minimize personal contact.17 In a recent register study on a large Swedish cohort of COPD patients, the mean age was 71.5.18 The percentage of patients reporting at least one exacerbation the last year was 28.1% and a high rate of cardiovascular comorbidity was observed at 18%. Thus, although being vulnerable to COVID-19, many COPD patients in Sweden were subjected to social shielding during the pandemic, Further, one study reported increased use of telemedicine for respiratory tract infections in 2020.19 Consequently, the exact impact of the pandemic on respiratory viral infections among COPD patients remains uncertain.
Thus, reduced AECOPD admission rates during the pandemic have been shown, although exacerbations have not been studied in relation to hospital admissions for COVID-19 and other lower respiratory tract infections such as influenza and pneumonia among COPD patients. Also, the observation period during the ongoing pandemic was limited in previous studies. This study aimed to examine if the proportion of respiratory infections and AECOPD with hospitalization in COPD patients were different between prepandemic and pandemic periods, and how admission rates varied over time throughout the pandemic.
Materials and Methods
Study Design
The objective of this study was to investigate the impact of the COVID-19 pandemic and related interventions on the total burden of hospital admissions for AECOPD, pneumonia, influenza, and COVID-19 in COPD patients. We wanted to measure the rate of admissions during a prolonged pandemic period and compare it with a prepandemic period. Therefore, we conducted a retrospective epidemiological study evaluating the rate of hospitalizations by diagnosis, covering five years, from June 2017 through August 2022. The sample size for each diagnosis was considered sufficient to address the research questions.
Study Subjects
We collected anonymized epidemiologic data on hospital admissions from the hospital inpatient register at Sahlgrenska University Hospital, a 2000-bed tertiary care teaching hospital in Western Sweden, with a catchment area of approximately 700 000 inhabitants. We used ICD-10 diagnostic codes to identify hospital inpatient admissions with a COPD diagnosis and a primary diagnosis code for either “AECOPD”, “pneumonia”, “influenza” or “COVID-19”, at discharge (Supplementary Table 1). The validity of the Swedish National Inpatient Register is in general high for several diagnoses.20 6166 inpatient hospital admissions in COPD patients met these criteria and were considered eligible for inclusion in the study. We screened hospital admissions with a primary AECOPD diagnosis code for a secondary COVID-19 diagnosis code. Also, anonymized data on all inpatients at Sahlgrenska University Hospital with a primary diagnosis code for “COVID-19” at discharge, regardless of the secondary diagnosis, were obtained. The data was included in the study as a reference. The Swedish Ethical Review Authority exempted the study from review since no personal data was collected.
Exposure
The Supplementary Methods section discusses the definitions of periods. We defined two major periods: the prepandemic period spanning from June 2017 through January 2020, and the pandemic period, from February 2020 through March 2022. Additionally, a third period was defined and referred to as the “post-restriction period”. This period spanned from April 2022 through August 2022, signifying the period after the withdrawal of general public health measures. The pandemic period was compared to the prepandemic period. Moreover, the post-restriction period and various phases during the pandemic period were analyzed separately and compared with the prepandemic period.
The phases of the pandemic were defined using our data regarding the different waves of hospital admissions (Supplemental Figure 1), as well as reports of novel COVID-19 variants being detected in Sweden.
Thus, based on these data, the following phases were defined: the “first wave” from February – June 2020, the low-incidence “intermediate period” from July – September 2020, the “second wave” from October 2020 – February 2021, the “third wave” from March – May 2021, the “fourth wave” from August – October 2021 followed by the “fifth wave” from December 2021 – March 2022.
Outcome
Outcomes were defined as the mean monthly rate of admissions for AECOPD, pneumonia, and influenza, respectively. In addition, a combined outcome of “total COPD admissions” was defined, indicating the mean number of admissions per month related to either AECOPD, pneumonia, influenza, or COVID-19.
Statistical Analysis
Admission rates per month by diagnosis were plotted over time to visually evaluate trends. Incidence rate ratios per month were calculated to assess the pandemic effect on admission rates over time, adjusting for seasonal variations. The analysis included AECOPD, pneumonia, influenza, and total COPD admissions (AECOPD, pneumonia, influenza, or COVID-19). The admission rates of the individual months starting in January 2020 were divided with the mean admission rates of the same months during the period before 2020. The incidence rate ratios per month were then plotted over time.
For statistical analyses, we used the SPSS Statistics software package for Macintosh, Version 28.0 (IBM Corp, Armonk, NY). Admission rates were compared between the pandemic and prepandemic periods for AECOPD, influenza during the winter period (November - April), pneumonia, COVID-19, and all COPD patients in the study group. To control for seasonal variations in admission rate, Poisson regression was used to calculate p-values, 95% confidence intervals, and incidence rate ratios, controlling for admission month. The size of the population of COPD patients within the catchment area of the hospital was presumed to be stable across the study period and the incidence rate ratios were therefore considered to be reliable.
Admission rates during the various defined phases of the pandemic were compared with the corresponding months during the prepandemic period. To control for an uneven distribution of corresponding prepandemic months, Poisson regression was used to calculate incidence rate ratios, 95% confidence intervals, and p-values.
Results
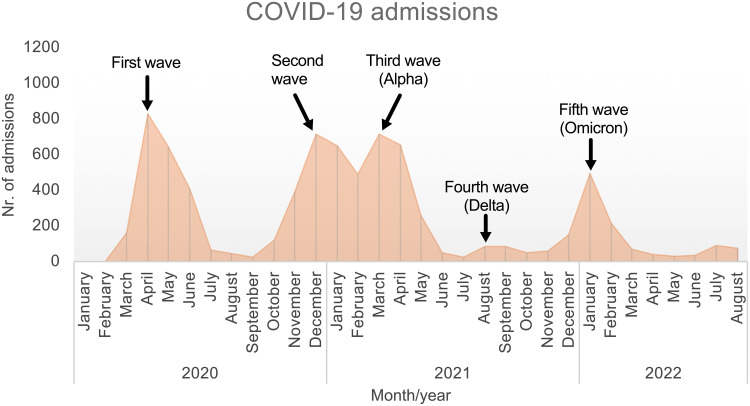
Admissions rates for all COVID-19 patients during the study period are shown in Figure 1. A total number of 7789 patients were admitted for COVID-19 at the hospital during the entire pandemic study period. Five distinct waves of hospital admissions were identified during the pandemic period until the withdrawal of general public health measures on April 1st, 2022.
Figure 1.
Total monthly number of hospital admissions due to COVID-19 at Sahlgrenska University Hospital throughout the pandemic (regardless of COPD diagnosis). Five separate waves of COVID-19 admission were identified.
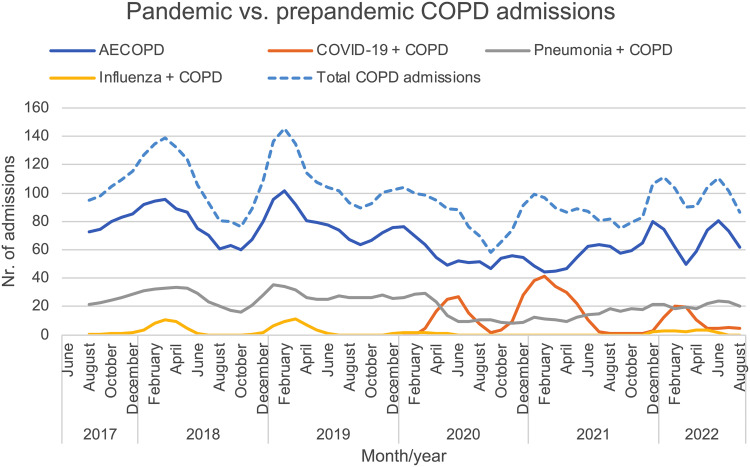
The distribution of monthly admission rates among COPD patients for AECOPD, pneumonia, influenza, and COVID-19, before and during the pandemic are presented in Figure 2 (original unprocessed data in Supplemental Figure 2). A total of 4302 AECOPD admissions, 1348 pneumonia admissions, 107 influenza admissions, and 409 COVID-19 admissions in COPD patients during the period from June 2017 through August 2022 were identified. Thirty-three patients with a primary AECOPD diagnosis also had a secondary COVID-19 diagnosis, whereof twenty-one had a diagnosis indicating current infection. Reduced admission rates for AECOPD, pneumonia, and influenza were seen at the beginning of the pandemic, coinciding with the rise in COVID-19 admissions. AECOPD and pneumonia admissions were stabilized at a low level until after the third wave of COVID-19 when there was a tendency for increased admission rates. After March 2020, there were no registered influenza admissions until December 2021, and there were in total 18 influenza admissions registered during the entire winter season of 2021–2022. A marked decrease in AECOPD admissions was seen during the fifth wave when the omicron variant of SARS-CoV-2 was predominant.
Figure 2.
Linear graphs showing the three-month moving average number of hospital admissions according to primary diagnosis (acute exacerbations, pneumonia, influenza, or COVID-19) in patients with chronic obstructive pulmonary disease, during the prepandemic, pandemic, and post-restriction periods. A combined outcome including all of the primary diagnoses above is also shown (total COPD admissions, dashed line).
Abbreviation: AECOPD, acute exacerbation of chronic obstructive pulmonary disease.
The comparison of admission rates before and during the pandemic is displayed in Table 1. Comparing monthly admission rates during the pandemic with the prepandemic period, incidence rate ratios were 0.72 for AECOPD (95% CI 0.67–0.77; p<0.001), 0.56 for pneumonia (95% CI 0.49–0.62; p<0.001), 0.18 for influenza during the winter period (95% CI 0.10–0.30; p<0.001) and 0.79 for total COPD admissions (95% CI 0.75–0.84; p<0.001). The mean monthly admission rate for COVID-19 in COPD patients during the pandemic period was 15 patients.
Table 1.
Average Monthly Admission Rates in COPD Patients According to Primary Diagnosis During the Pandemic, Compared with a Prepandemic Period
| Primary Diagnosis | Monthly Number of Prepandemic Admissions | Monthly Number of Pandemic Admissions | Admission IRR | Pa |
|---|---|---|---|---|
| Mean (95% CI) | Mean (95% CI) | (95% CI) | ||
| AECOPD | 78 (75–81) | 56 (53–59) | 0.72 (0.67–0.77) | <0.001 |
| Pneumonia | 27 (25–28) | 15 (13–16) | 0.56 (0.49–0.62) | <0.001 |
| Influenzab | 3.8 (2.5–5.5) | 0.7 (0.4–1.2) | 0.18 (0.10–0.30) | <0.001 |
| COVID-19 | 0 | 15 | ||
| Total COPD admissionsc | 107 (103–111) | 85 (81–89) | 0.79 (0.75–0.84) | <0.001 |
Notes: ap-values and incidence rate ratios with 95% confidence intervals were calculated using Poisson regression. bMean admission rates for influenza were calculated for the winter period, as defined in the method section. cCombined outcome of all COPD admissions for AECOPD, pneumonia, influenza, or COVID-19.
Abbreviations: AECOPD, acute exacerbation of chronic obstructive pulmonary disease; IRR, incidence rate ratio.
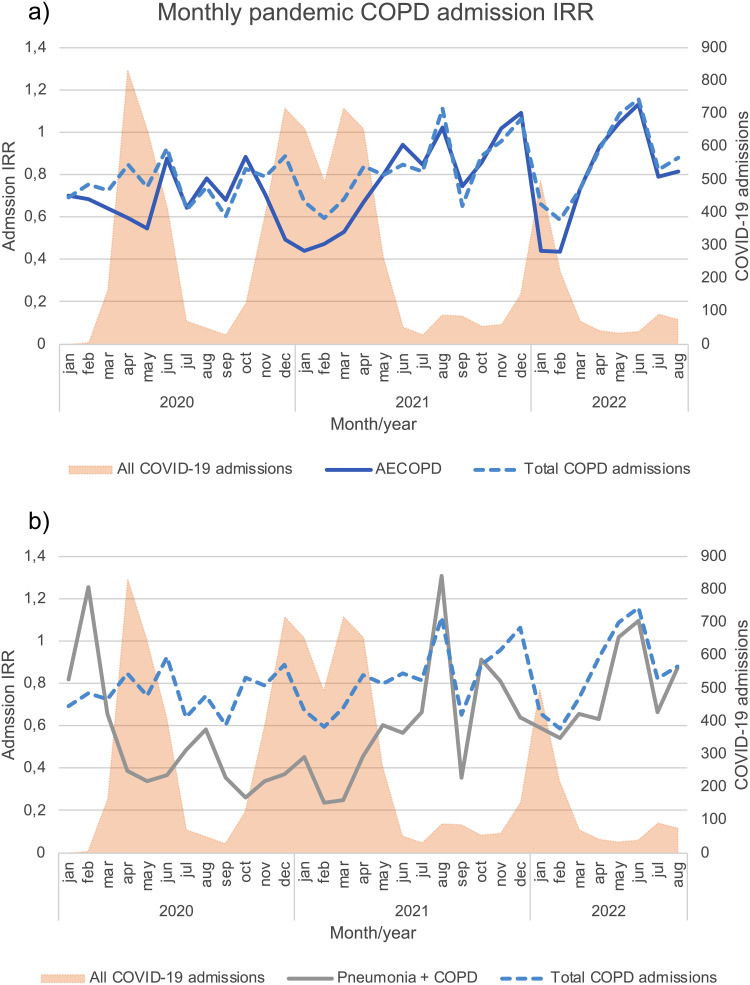
Monthly incidence rate ratios during the pandemic are depicted in Figure 3 (original data in Supplemental Figure 3). Incidence rate ratios for AECOPD, pneumonia, and total COPD admissions in January 2020 were 0.70, 0.82, and 0.69 respectively, and remained <1 until after the third wave. In the same month, the incidence rate ratio for influenza was 0.19 (Supplemental Figure 3c). The incidence ratios for total COPD admissions mirrored those of AECOPD, except during the first, second, third, and Omicron waves. During these waves, incidence rate ratios for total COPD admissions were higher than those of AECOPD, although still well below 1.
Figure 3.
Hospital admission incidence rate ratios for (a) acute exacerbations of chronic obstructive pulmonary disease and (b) pneumonia, from January 2020 through August 2022. An incidence rate ratio below 1.0 indicates a decrease in the rate of hospital admissions during the pandemic, compared with the prepandemic period. The incidence rate ratios for the combined outcome of total COPD admissions (acute exacerbations, pneumonia, influenza, or COVID-19) are also shown (dashed blue line). For reference, the total number of all registered COVID-19 admissions at the hospital is shown on the secondary y-axis (shaded Orange graph).
Abbreviations: AECOPD, acute exacerbation of chronic obstructive pulmonary disease; IRR, incidence rate ratio.
Table 2 shows a comparison of admission incidence rates during different waves of the pandemic. Significantly reduced admission rates were seen for AECOPD, pneumonia, and total COPD admissions during the first, second, and third waves as well as for the fifth wave. Lower IRR for total COPD admissions was also seen during the smaller fourth wave, but this did not reach significance (p=0.06). Reduction in admission rates for AECOPD and pneumonia were most pronounced during the second wave of COVID-19. Significant reductions in admissions were also seen during the intermediate period between the first and the second wave. This period saw the most significant decrease in total COPD admissions. There were no significant reductions in admission rates during the post-restriction period.
Table 2.
Incidence Rate Ratios for Admissions of COPD Patients by Primary Diagnosis During the Different Waves of the Pandemic Compared with the Prepandemic Period
| Phasea | AECOPD Admissions | Pneumonia Admissions | Total COPD Admissionsb | |||
|---|---|---|---|---|---|---|
| IRR (95% CI) | Pc | IRR (95% CI) | Pc | IRR (95% CI) | Pc | |
| First wave | 0.67 (0.58–0.77) | <0.001 | 0.61 (0.48–0.78) | <0.001 | 0.80 (0.71–0.89) | <0.001 |
| Intermediate period | 0.70 (0.58–0.84) | <0.001 | 0.47 (0.32–0.69) | <0.001 | 0.65 (0.56–0.77) | <0.001 |
| Second wave | 0.59 (0.52–0.68) | <0.001 | 0.35 (0.26–0.46) | <0.001 | 0.77 (0.69–0.85) | <0.001 |
| Third wave (Alpha) | 0.66 (0.55–0.79) | <0.001 | 0.43 (0.30–0.61) | <0.001 | 0.77 (0.67–0.89) | <0.001 |
| Fourth wave (Delta) | 0.90 (0.79–1.04) | 0.16 | 0.83 (0.65–1.06) | 0.15 | 0.89 (0.79–1.01) | 0.06 |
| Fifth wave (Omicron) | 0.69 (0.60–0.80) | <0.001 | 0.62 (0.48–0.80) | <0.001 | 0.78 (0.70–0.88) | <0.001 |
| Post-restriction period | 0.95 (0.84–1.08) | 0.42 | 0.86 (0.69–1.07) | 0.17 | 0.98 (0.88–1.09) | 0.70 |
Notes: Chronologically presented phases of the COVID-19 pandemic, compared to the corresponding prepandemic months. aDefinitions of the various phases of the pandemic are described in the methods section and Figure 1. bCombined outcome of all COPD admissions for AECOPD, pneumonia, influenza, or COVID-19. cp-values and incidence rate ratios with 95% confidence intervals were calculated using Poisson regression.
Abbreviations: AECOPD, acute exacerbation of chronic obstructive pulmonary disease; IRR, incidence rate ratio.
Discussion
This study shows a significant decrease in hospital admissions for AECOPD, influenza, and pneumonia in COPD patients during a prolonged pandemic study period, compared to the prepandemic period. Moreover, the current results show a significant reduction in total COPD admissions within the study group, even when accounting for COVID-19 admissions during the pandemic. The effect was seen across the different waves of the pandemic but was less pronounced and not significant during the smaller fourth wave. However, the admission rate returned to prepandemic rates in the post-restriction period.
The reasons for decreased admissions for AECOPD during the pandemic period have been discussed in previous studies. This decline in admissions has been attributed to reduced exposure to respiratory viral infections because of interventions such as social distancing and the use of face masks.21–28 Other possible causes could be a changed healthcare-seeking behavior among COPD patients and a relative lack of available hospital beds due to an increase in COVID-19 admissions. This could result in a higher rate of outpatient exacerbation treatment during this period. However, the latter hypothesis is contradicted in this study by the observed reduced incidence rate of AECOPD and pneumonia admissions during the period between the first and second wave. In this period there was a low incidence of COVID-19 admissions. Interestingly, as described in our results, there was a reduction in incidence rate ratios for AECOPD and the total studied COPD admissions already before the start of the pandemic in January 2020. Hospital admissions for influenza among COPD patients during the same month were most markedly reduced, maybe reflecting the low incidence season for influenza in 2019–2020. Supporting this observation, the national incidence of influenza in January 2020 was lower than in the three preceding seasons.16
Comparing effects on admission rates, pneumonia admissions decreased more than admissions for AECOPD (IRR 0.56 vs 0.72). The actual admission rate for AECOPD might be slightly lower considering that there were registered cases with a primary AECOPD diagnosis and a secondary diagnosis code for COVID-19. Yet, it is also plausible that these cases could represent COVID-19-triggered AECOPD, although we could not examine it with this data set. The two conditions are difficult to distinguish since the symptoms are similar. According to Global Initiative for Obstructive Lung Disease (GOLD) guidelines, a SARS-CoV-2 positive COPD patient requiring altered maintenance therapy due to respiratory symptoms would meet the criteria.5
Influenza admissions during the winter period were highly affected, illustrated by the fact that after March 2020 there were no influenza admissions registered at all among COPD patients, until December 2021. A decrease in the transmission of respiratory viral infections other than SARS-CoV-2 could explain a greater effect on the incidence of pneumonia than that of AECOPD. In line with this, it has been reported that the spread of respiratory viral infections commonly associated with viral pneumonia such as influenza and respiratory syncytial virus was lower during the pandemic.15 However, the incidence of rhinovirus infection (the most common respiratory viral agent causing AECOPD) was only transiently decreased during the beginning of the pandemic. Furthermore, there have been reports of reduced incidence of non-SARS-CoV-2 related community-acquired pneumonia during the pandemic, including changes in both bacterial and viral aetiologies.29,30 These studies have observed decreased prevalence of pneumococci and most respiratory viruses except rhinovirus. It is also important to note that AECOPD can be caused by bacterial infections as well as other non-infectious reasons.
As illustrated in our results, the impact of the pandemic on admission rates varied across different waves. The initial effect on admission rates of COPD patients was waning during the fourth wave. The reasons for this are not known. During the pandemic, different interventions were used as public health measures to reduce the transmission of SARS-CoV-2 in Sweden. Still, throughout the pandemic, a total lockdown of the community did not occur, elementary schools were kept open, face masks were not mandatory in most settings and most public health measures were based on recommendations rather than obligations. It could be speculated that after the vaccination campaign in the spring of 2021 when most of the vulnerable population and a substantial part of the population without risk factors were vaccinated, social distancing practices decreased. This might have led to increased transmission of other respiratory agents. Supporting this, starting in late summer and culminating in the autumn of 2021, respiratory syncytial virus infections in Sweden were reaching record levels, out of the normal season.31,32 Hospital admissions for COVID-19 during this period were low because of the vaccination campaign, despite the contagious spread of the Delta variant of SARS-CoV-2.
The fifth wave represented a shift in the pandemic. The spread of the Omicron variant of SARS-CoV-2 resulted in an exponential increase of the confirmed COVID-19 cases in Sweden, probably due to vaccine escape mutations in the viral genome. As a result, COVID-19 admissions increased, also in COPD patients. However, we show that admissions for AECOPD, pneumonia, and all study groups were again significantly decreased during this period compared to the prepandemic period. Once more, it is plausible that it could be related to reduced transmission of other respiratory agents due to reinforced public health interventions. After the fifth wave and withdrawal of public health measures in April 2022, hospitalization incidence rates for COPD returned to prepandemic rates.
There are several limitations to this study. First, the study was based on discharge ICD-10 diagnosis codes and did not include virological data in patients admitted for AECOPD and pneumonia. It is however routine clinical praxis to verify COVID-19 and influenza based on airway swab PCR sampling.
Second, due to a lack of microbial data and the retrospective design, it is not possible to establish a causal association between reduced transmissions of respiratory agents and a reduction in AECOPD admissions. Also, since outpatient data were not available, it was not possible to measure if the overall burden of AECOPD decreased.
Third, the study only included COPD admissions due to respiratory tract diseases and did not include other discharge diagnoses in COPD patients as a control. Due to all the above limitations, many confounding variables could explain reduced admission rates for AECOPD. Yet, the concurrent reduction of admissions for pneumonia and influenza during periods of restriction measures in this study provides further support for the suspicion of a causal correlation. Moreover, the significantly low total admissions rate during the period of low COVID-19 incidence between the first and second waves suggests that reduced admission rates were unlikely to be explained by hospital overcrowding.
Fourth, the study did not include demographic data on patient characteristics such as age, gender, or the GOLD stage. Hence, the characteristics of admitted patients cannot be compared across different periods. However, we presumed that the prevalence and disease severity of COPD patients in the catchment area was rather consistent across the study period and this should not affect the rate of admissions.
Fifth, we did not have access to data on clinical variables and outcomes. Thus, it was not feasible to compare variables such as oxygen requirement, non-invasive ventilation, disease severity, length of stay, readmissions, and mortality between different periods.
The strength of this study is its relatively large study cohort including admissions for exacerbation in COPD patients and admissions due to pneumonia, influenza, and COVID-19. Also, the extended follow-up period before and during the pandemic made it possible to compare admission rates during various phases of the pandemic.
Conclusions
We show that the overall burden of admissions for AECOPD or respiratory infections (pneumonia, influenza, or COVID-19) was significantly reduced during the COVID-19 pandemic, despite the inclusion of admissions with a primary diagnosis of COVID-19. The hospitalization rates returned to prepandemic rates in the post-restriction period. Our results support the need for surveillance of all respiratory pathogens and that public health measures could be of benefit to COPD patients during periods of peak incidence of any viral respiratory infection.
Acknowledgments
We would like to thank Michel Paulli, logistician at the Analysis and Project Unit at Sahlgrenska University Hospital, for helping with data extraction. The abstract of this paper was presented at the ERS International Conference 2022 as a poster presentation with interim findings. The poster’s abstract was published in “ERS International Congress 2022 abstracts” in European Respiratory Journal 04 September 2022; volume 60, issue suppl 66: https://erj.ersjournals.com/content/60/suppl_66/1929.
Funding Statement
The study was financed by grants from the Swedish state under the agreement between the Swedish government and the county councils, the ALF-agreement (ALFGBG-965569) to J.W. and (ALFGBG-824371) to L.E.G.W.V. and by grants from the Family Kamprad Foundation (20190024) and the Swedish Heart and Lung Foundation (20200150) to L.E.G.W.V.
Disclosure
Dr Lowie EGW Vanfleteren reports personal fees from AstraZeneca, GSK, Boehringer, Novartis, Chiesi, Pulmonx, and Resmed, outside the submitted work. The authors report no other conflicts of interest in this work.
References
- 1.Roth GA, Abate D, Abate KH, et al. Global, regional, and national age-sex-specific mortality for 282 causes of death in 195 countries and territories, 1980–2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet. 2018;392(10159):1736–1788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lisspers K, Larsson K, Johansson G, et al. Economic burden of COPD in a Swedish cohort: the Arctic study. Int J Chron Obstruct Pulmon Dis. 2018;13:275–285. doi: 10.2147/COPD.S149633 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Halpin DM, Decramer M, Celli B, Kesten S, Liu D, Tashkin DP. Exacerbation frequency and course of COPD. Int J Chron Obstruct Pulmon Dis. 2012;7:653–661. doi: 10.2147/COPD.S34186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Soler-Cataluna JJ, Martinez-Garcia MA, Roman Sanchez P, Salcedo E, Navarro M, Ochando R. Severe acute exacerbations and mortality in patients with chronic obstructive pulmonary disease. Thorax. 2005;60(11):925–931. doi: 10.1136/thx.2005.040527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.GOLD (Global Initiative for Chronic Obstructive Lung Disease). Global strategy for prevention, diagnosis, management, and prevention of chronic obstructive pulmonary disease (2023 REPORT); 2023. Available from: https://goldcopd.org/wp-content/uploads/2023/03/GOLD-2023-ver-1.3-17Feb2023_WMV.pdf. Accessed May 16, 2023.
- 6.Linden D, Guo-Parke H, Coyle PV, et al. Respiratory viral infection: a potential “missing link” in the pathogenesis of COPD. Eur Respir Rev. 2019;28(151):180063. doi: 10.1183/16000617.0063-2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ramirez JA, Wiemken TL, Peyrani P, et al. Adults hospitalized with pneumonia in the United States: incidence, epidemiology, and mortality. Clin Infect Dis. 2017;65(11):1806–1812. doi: 10.1093/cid/cix647 [DOI] [PubMed] [Google Scholar]
- 8.Ryan M, Suaya JA, Chapman JD, Stason WB, Shepard DS, Thomas CP. Incidence and cost of pneumonia in older adults with COPD in the United States. PLoS One. 2013;8(10):e75887. doi: 10.1371/journal.pone.0075887 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hippisley-Cox J, Young D, Coupland C, et al. Risk of severe COVID-19 disease with ACE inhibitors and angiotensin receptor blockers: cohort study including 8.3 million people. Heart. 2020;106(19):1503–1511. doi: 10.1136/heartjnl-2020-317393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Aveyard P, Gao M, Lindson N, et al. Association between pre-existing respiratory disease and its treatment, and severe COVID-19: a population cohort study. Lancet Respir Med. 2021;9(8):909–923. doi: 10.1016/S2213-2600(21)00095-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Karlsson Sundbaum J, Vanfleteren L, Konradsen JR, Nyberg F, Ekberg-Jansson A, Stridsman C. Severe COVID-19 among patients with asthma and COPD: a report from the Swedish National Airway Register. Ther Adv Respir Dis. 2021;15:17534666211049738. doi: 10.1177/17534666211049738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Halpin DMG, Rabe AP, Loke WJ, et al. Epidemiology, healthcare resource utilization, and mortality of asthma and COPD in COVID-19: a systematic literature review and meta-analyses. J Asthma Allergy. 2022;15:811–825. doi: 10.2147/JAA.S360985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Andreen N, Andersson LM, Sundell N, Gustavsson L, Westin J. Mortality of COVID-19 is associated with comorbidity in patients with chronic obstructive pulmonary disease. Infect Dis. 2022;54(7):508–513. doi: 10.1080/23744235.2022.2050422 [DOI] [PubMed] [Google Scholar]
- 14.Alqahtani JS, Oyelade T, Aldhahir AM, et al. Reduction in hospitalised COPD exacerbations during COVID-19: a systematic review and meta-analysis. PLoS One. 2021;16(8):e0255659. doi: 10.1371/journal.pone.0255659 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chow EJ, Uyeki TM, Chu HY. The effects of the COVID-19 pandemic on community respiratory virus activity. Nat Rev Microbiol. 2022;2022:1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Folkhälsomyndigheten (Public Health Organisation of Sweden). Influensasäsongen 2019–2020; 2020. Available from: https://www.folkhalsomyndigheten.se/contentassets/607a64370a29485a8ad1426684ccd4e3/influensasasongen-2019-2020-sasongssammanfattning-final-v3.pdf. Accessed May 22, 2023.
- 17.Ludvigsson JF. How Sweden approached the COVID-19 pandemic: summary and commentary on the national commission inquiry. Acta Paediatr. 2023;112(1):19–33. doi: 10.1111/apa.16535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vanfleteren L, Lindberg A, Zhou C, Nyberg F, Stridsman C. Exacerbation risk and mortality in COPD GOLD group A and B patients with and without exacerbation history. Am J Respir Crit Care Med. 2023;208(2):163–175. doi: 10.1164/rccm.202209-1774OC [DOI] [PubMed] [Google Scholar]
- 19.Milos Nymberg V, Ellegård LM, Kjellsson G, et al. Trends in remote health care consumption in Sweden: comparison before and during the first wave of the COVID-19 pandemic. JMIR Hum Factors. 2022;9(1):e33034. doi: 10.2196/33034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ludvigsson JF, Andersson E, Ekbom A, et al. External review and validation of the Swedish national inpatient register. BMC Public Health. 2011;11(1):450. doi: 10.1186/1471-2458-11-450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Huh K, Kim YE, Ji W, et al. Decrease in hospital admissions for respiratory diseases during the COVID-19 pandemic: a nationwide claims study. Thorax. 2021;76(9):939–941. doi: 10.1136/thoraxjnl-2020-216526 [DOI] [PubMed] [Google Scholar]
- 22.Chan KPF, Ma TF, Kwok WC, et al. Significant reduction in hospital admissions for acute exacerbation of chronic obstructive pulmonary disease in Hong Kong during coronavirus disease 2019 pandemic. Respir Med. 2020;171:106085. doi: 10.1016/j.rmed.2020.106085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tan JY, Conceicao EP, Wee LE, Sim XYJ, Venkatachalam I. COVID-19 public health measures: a reduction in hospital admissions for COPD exacerbations. Thorax. 2021;76(5):512–513. doi: 10.1136/thoraxjnl-2020-216083 [DOI] [PubMed] [Google Scholar]
- 24.Saeed MI, Sivapalan P, Eklof J, et al. Social distancing in relation to severe exacerbations of chronic obstructive pulmonary disease: a nationwide semi-experimental study during the COVID-19 pandemic. Am J Epidemiol. 2022;191(5):874–885. doi: 10.1093/aje/kwab292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Alsallakh MA, Sivakumaran S, Kennedy S, et al. Impact of COVID-19 lockdown on the incidence and mortality of acute exacerbations of chronic obstructive pulmonary disease: national interrupted time series analyses for Scotland and Wales. BMC Med. 2021;19(1):124. doi: 10.1186/s12916-021-02000-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Poucineau J, Delory T, Lapidus N, et al. Hospital admissions and mortality for acute exacerbations of COPD during the COVID-19 pandemic: a nationwide study in France. Front Med. 2022;9:995016. doi: 10.3389/fmed.2022.995016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sarc I, Lotric Dolinar A, Morgan T, et al. Mortality, seasonal variation, and susceptibility to acute exacerbation of COPD in the pandemic year: a nationwide population study. Ther Adv Respir Dis. 2022;16:17534666221081047. doi: 10.1177/17534666221081047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.So JY, O’Hara NN, Kenaa B, et al. Population decline in COPD admissions during the COVID-19 pandemic associated with lower burden of community respiratory viral infections. Am J Med. 2021;134(10):1252–1259.e1253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dahne T, Bauer W, Essig A, et al. The impact of the SARS-CoV-2 pandemic on the prevalence of respiratory tract pathogens in patients with community-acquired pneumonia in Germany. Emerg Microbes Infect. 2021;10(1):1515–1518. doi: 10.1080/22221751.2021.1957402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Huang C. The COVID-19 pandemic and the incidence of the non-COVID-19 Pneumonia in adults. Front Med. 2021;8:737999. doi: 10.3389/fmed.2021.737999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Folkhälsomyndigheten (Public Health Organisation of Sweden). RSV-säsongen 2021–2022; 2022. Available from: https://www.folkhalsomyndigheten.se/globalassets/statistik-uppfoljning/smittsamma-sjukdomar/veckorapporter-rsv/rsv-sasongen-2021-2022_final_uppdaterat-12-aug.pdf. Accessed November 18, 2022.
- 32.van Summeren J, Meijer A, Aspelund G, et al. Low levels of respiratory syncytial virus activity in Europe during the 2020/21 season: what can we expect in the coming summer and autumn/winter? Euro Surveill. 2021;26(29):2100639. doi: 10.2807/1560-7917.ES.2021.26.29.2100639 [DOI] [PMC free article] [PubMed] [Google Scholar]