Abstract
Atrial fibrillation (AF) poses a serious healthcare burden on society due to its high morbidity and the resulting serious complications such as thrombosis and heart failure. The principle of catheter ablation is to achieve electrical isolation by linear destruction of cardiac tissue, which makes AF a curable disease. Currently, catheter ablation does not have a high long-term success rate. The current academic consensus is that inflammation and fibrosis are central mechanisms in the progression of AF. However, artificially caused inflammatory cell death by catheter ablation may have a significant impact on structural and electrical remodeling, which may affect the long-term prognosis. This review first focused on the inflammatory response induced by apoptosis, necrosis, necroptosis, pyroptosis, ferroptosis and their interaction with arrhythmia. Then, we compared the differences in cell death induced by radiofrequency ablation, cryoballoon ablation and pulsed-field ablation. Finally, we discussed the structural and electrical remodeling caused by inflammation and the association between inflammation and the recurrence of AF after catheter ablation. Collectively, pulsed-field ablation will be a revolutionary innovation with faster, safer, better tissue selectivity and less inflammatory response induced by apoptosis-dominated cell death.
Keywords: atrial fibrillation, inflammatory cell death, radiofrequency ablation, cryoballoon ablation, electroporation
Introduction
Atrial fibrillation (AF) is a common arrhythmia with high morbidity and mortality.1 Without proper intervention, this arrhythmia may develop into a permanent condition. Current therapeutic regimens include catheter ablation and drug therapy. Although antiarrhythmic drugs can help maintain sinus rhythm, most of them are ineffective and associated with intolerable side effects. AF and heart failure are closely related and mutually reinforcing. Evidence supported that catheter ablation reduces all-cause mortality in patients with AF combined with heart failure compared with drug therapy.2
Since Haissaguerre identified the pulmonary veins as the main trigger of AF,3 isolation of the electrical activity of the pulmonary veins by utilizing various form of energy (radiofrequency, cryoballoon, laser, ultrasound, pulsed-field) has become the cornerstone of catheter ablation. The primary goal of catheter ablation is to eliminate the symptoms associated with AF and improve quality of life. However, numerous clinical evidences have shown that the success rate of catheter ablation is not very high. After weighing complications such as atrioesophageal fistula, cardiac tamponade, phrenic nerve injury, esophageal injury, and pulmonary vein contracture, catheter ablation is not a routine choice as a first-line option.4 This awkward positioning of catheter ablation suggests that there is much room for improvement and optimization.
Catheter ablation leads to the membrane-breaking cell death of localized cardiomyocytes, releasing diverse bioactive substances. Undoubtedly, the local pro-inflammatory microenvironment may create a substrate for structural and electrical remodeling. This review will focus on the possible inflammatory response induced by cell death as well as arrhythmias, and attempt to clarify the differences in catheter ablation induced cell death at a microscopic level, with a view to translate the available evidence into clinical guides.
Cell Death and AF
Excessive cardiomyocytes death with limited regenerative capacity leads to a variety of cardiac diseases, including myocardial infarction, malignant arrhythmia, heart failure, and sudden cardiac death. Currently, known modes of cell death include, but are not limited to, apoptosis, necrosis, necroptosis, pyroptosis, and ferroptosis, which occur independently and interact with each other. Dead cells can exhibit features of autophagy, apoptosis, pyroptosis, and necrosis simultaneously.5 Except for apoptosis, all other membrane-breaking cell deaths (necrosis, necroptosis, pyroptosis, and ferroptosis) all occur in conjunction with an inflammatory response. More importantly, the loss of cardiomyocytes may not only imply diminished systolic function, but also activate the cascade of inflammatory responses, which may further contribute to the development of arrhythmia (Figure 1). Additionally, arrhythmia can exist as a single or cardiomyopathy-related disorder, and myocardial injury associated with arrhythmia is usually accompanied by a temporary or permanent inflammatory response.6
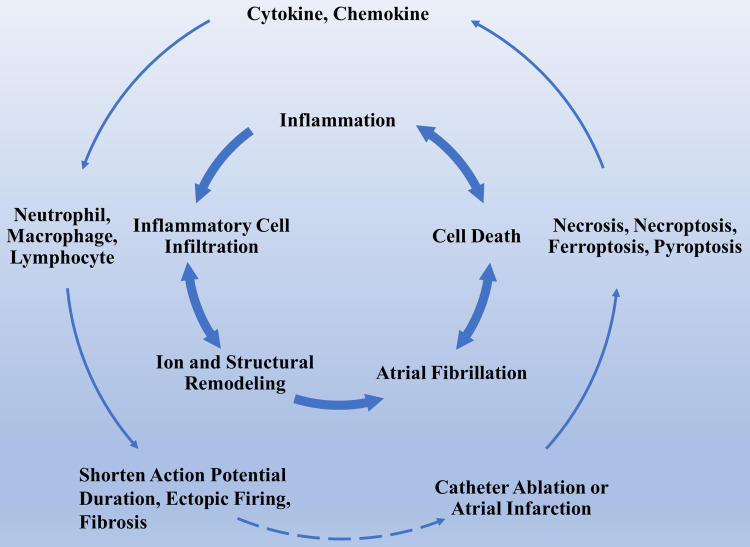
Figure 1.
The association between inflammatory cell death and AF. This figure briefly recapitulates the association between inflammatory cell death and AF induce inflammatory cell death. Inflammatory cell death caused by artificial therapeutic regimens releases inflammatory factors that lead to structural and electrical remodeling, thereby contributing to the permanent progression of the arrhythmia. The cross-link between AF and inflammatory cell death creates a vicious loop.
Abbreviation: AF, atrial fibrillation.
Apoptosis and AF
Apoptosis is required for the clearance of damaged or senescent cells. Since 1972, the ultra-structural features of apoptosis have been characterized as the integrity of the cell membrane structure and the absence of a significant inflammatory response.7 Apoptosis has been shown to be associated with ischemia/reperfusion (I/R) injury, heart failure, and atherosclerosis. In 1994, evidence of apoptosis in the I/R myocardial tissue of rabbits was obtained by transmission electron microscopy and DNA gel electrophoresis.8 Apoptosis was present in the sinoatrial node, atrioventricular node, and conduction tissues during embryonic development and the postnatal weeks of development. Excessive, insufficient, or delayed apoptosis can cause arrhythmia.9 James et al observed typical apoptosis in surgically resected sinus nodes in patients who were recurrent ventricular arrhythmia combined with syncopal long Q-T syndrome, suggesting that arrhythmia is implicated in apoptosis, with limited, non-inflammatory degeneration in the sinus node.10 Thus, an imbalance of apoptosis may underlie the pathogenesis of some arrhythmia.
Sustained tachycardia and rapid pacing promote cardiomyocytes apoptosis, which have been corroborated by extensive clinical data.11–13 Knocking down caspase-3 in a porcine AF model could prevent intra-atrial conduction delay and inhibit the development of sustained AF.14 Additionally, ATG5 and M30, which are associated with autophagy and apoptosis, were elevated in the serum of patients with paroxysmal AF.15 Besides, epicardial isolation of pulmonary veins also reduced the apoptosis indicators in AF.16
Apoptosis may be the ultimate culmination of various unfavorable stimuli. However, how apoptosis without inflammatory response contributes to the progression of AF has not been investigated clearly. A possible explanation is that widespread apoptosis of cardiomyocytes or conduction system implies a weakening of atria contractile function which causes the atria to enlarge, ultimately leading to structural remodeling and electrical remodeling.
Necrosis and AF
Necrosis is usually considered a non-programmed, unregulated cell death process resulting from exposure to external violent stimuli or severe imbalance in the homeostasis of the internal environment. Necrosis is characterized by cell swelling, rupture, and spillage of cell contents, which triggers an inflammatory response.
Necrotic myocardium can trigger innate and adaptive immunity.17 Damage-associated molecular patterns (DAMP) bind to pattern recognition receptors to induce activation of downstream signaling pathways on cardiomyocytes or resident immune cells (Figure 2). Many DAMPs have been identified, including high-mobility group box 1 (HMGB1), uric acid, double-stranded DNA, amyloid-β-peptide, heat shock proteins (HSP), interleukin-1α (IL-1α), and IL-33. Ultimately, the cascade leads to the upregulation of chemokines and cytokines.18 The inflammatory response is also exacerbated because of elevated fibrinogen, fibronectin, bilirubin, metallopeptidase 2 (MMP-2), tissue inhibitors of metalloproteinase 2 (TIMP-2), and thrombospondin 2 in neutrophils or polarized macrophages.19–21
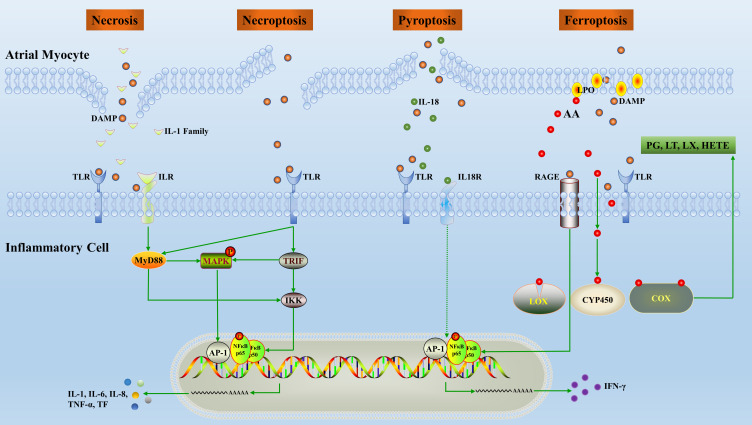
Figure 2.
Overview of the inflammatory pathways induced by inflammatory cell death. Release of inflammatory factors, mainly DAMPs, following cardiomyocytes death promotes polarization and chemotaxis of inflammatory cells. In turn, inflammatory cells secrete inflammatory factors which alter the structure and electrical conduction activity of the local microenvironment.
Abbreviations: AA, arachidonic acid; TF, tissue factor; AP, activating protein; NF, nuclear factor; IFN, interferon; IL, interleukin; PG, prostaglandin; LT, leukotriene; LX, lipoxin; CYP450, cytochrome P450; MyD, myeloid differentiation factor; LOX, lipoxygenase; COX, cyclooxygenase; TNF, tumor necrosis factor; IKK, inhibitor of kappa B kinase; TLR, toll-like receptors; LPO, lipid hydroperoxide; DAMP, damage-associated molecular patterns; RAGE, the receptor of advanced glycation end products; HETE, hydroxyeicosatetraenoic acid; MAPK, mitogen-activated protein kinase; TRIF, TIR-domain-containing adaptor inducing interferon β.
There are few direct studies of necrosis on arrhythmia, but we can draw an analogy to myocardial infarction as a more severe form of myocardial necrosis. Studies have found that larger infarct sizes in the heart are more prone to arrhythmia.22–24 The cumulative morbidity of AF over 5 years after acute myocardial infarction is 6%-21%, compared to 3% in the general population.25 Recent studies have shown that the morbidity of supraventricular tachycardia in atrial infarction patients is 27%.26 A prospective cohort study has shown that artificial atrial coronary occlusion causes atrial arrhythmias and delayed intra-atrial conduction.27 Moreover, the more atrial branches are blocked, the higher morbidity of AF.28,29 It has been verified that atrial infarction is associated with slower atrial conduction velocity, increased conduction heterogeneity, increased susceptibility to AF, and a longer duration of AF.30 Coincidentally, in the canine model of chronic atrial infarction, researchers found an approximately 50-fold increase in spontaneous atrial ectopic activity and conduction abnormalities, and significant fibrosis at the border of the atrial infarct region. Patch-clamp implied that the initiating activity of atrial myocytes in infarct boundary areas was enhanced, with faster decay of caffeine-induced Ca2+ transients and enhanced Na+-Ca2+ exchange currents.31 A refined study by Uma et al reported that ligation of the atrial branch of the left anterior descending coronary artery resulted in spontaneous AF. Optical mapping observed focal spontaneous discharges and conduction delays at the border of the ischemic/normal zone, which provided substrates for reentry. The possible mechanism involved is that the physiological binding of calmodulin to ryanodine receptor type 2 (RyR) is altered by hypoxic and inflammatory responses.32 Taken together, these results suggest that local myocardial tissue necrosis has profound effects on electrophysiology. However, little is known about whether catheter ablation of the pulmonary veins and extensive linear ablation in the atria could cause atrial infarction by damaging the corresponding arteries and whether it has any effect on the recurrence of AF after the procedure.
Of course, researchers have also conducted a series of mechanistic explorations to find interventions. Previous research prompted that C1q/TNF-related protein 9 may reduce atrial inflammation and fibrosis effectively and reduce the incidence of concomitant AF after myocardial infarction through its inhibitory effects on TLR4/NF-κB and Smad2/3 signaling pathways.33 Relaxin exerts beneficial anti-fibrotic and anti-inflammatory effects and reduces the susceptibility of AF after myocardial infarction.34
Necroptosis and AF
Necroptosis is a newly discovered mechanism of cell death that combines the features of necrosis and apoptosis.35 A cascade of physicochemical factors stimulates the activation of autophosphorylation of receptor-interacting serine/threonine protein kinase. It leads to the release of DAMPs, which promote the inflammatory response (Figure 2).
Several studies have shown that necroptosis plays an accelerating role in myocardial infarction, I/R injury, and heart failure.36–38 Inhibition of necroptosis was found to reduce the susceptibility to CaCl2-acetylcholine or high lipid-induced AF and reverse structural remodeling of the atria.39 TGF-β activated kinase 1 acts as a key survival factor by antagonizing necroptosis directly, which is essential for maintaining myocardial homeostasis and preventing unfavorable myocardial remodeling.40 A recent study demonstrated that tumor necrosis factor receptor-associated factor 2-mediated NF-κB independent pro-survival pathway inhibited necrotic signaling, and it could be used as a therapeutic target for ventricular remodeling and heart failure.37 More studies are needed to gain a better understanding of the relationship between necroptosis and arrhythmia.
Ferroptosis and AF
Ferroptosis is a regulated cell death characterized by iron-dependent lipid peroxidation (LPO) accumulation.41 Ferroptosis has been shown to release mitochondrial and intranuclear material,42 but the study of inflammation in ferroptosis is still in the early stage.43
Ferroptotic cells were found to be extremely pro-inflammatory by recruiting macrophages through chemokine ligand 2.44 Ferroptotic cells release HMGB145 and anti-HMGB1-neutralizing antibodies mitigated the inflammatory response of macrophages.46 Inhibition of ferroptosis has been reported to inhibit leukocyte extravasation.47 The activation of the toll-like receptors (TLR) inflammatory response led to neutrophil accumulation and myocardial injury in cardiac transplantation, and Ferrostatin (selective ferroptosis inhibitor) reversed this alteration.48 4-hydroxynonenal, which is the LPO product, is a ligand for the TLR4 receptor and triggers an inflammatory response.49 In addition, LPO drives an increase in modified low-density lipoprotein, which promotes inflammation through macrophage polarization.50 Reducing glutathione peroxidase 4 (GPX4) expression up-regulates the expression of 12-lipoxygenase (ALOX12) and cyclooxygenase 1 (COX-1).51,52 (Figure 2) In contrast, GPX4 activation inhibits ferroptosis and inflammatory responses by attenuating arachidonic acid oxidation and NF-κB pathway activation.53 Arachidonic acid is an unsaturated fatty acid that is metabolized by COX, LOX, cytochrome P450, and monooxygenases to synthesize biologically active inflammatory mediators such as prostaglandins, leukotrienes, epoxyeicosatrienoic acid, and hydroxyeicosatetraenoic acid.54 Excessive reactive oxygen species (ROS) and iron overload are strongly associated with arrhythmia.55,56 A recent study reported that SARS-CoV-2 induced ferroptosis, leading to the dysfunction of human embryonic stem cell-derived sinoatrial node-like pacemaker cells.57 AF is a common cause of death in β-thalassemia patients, and it can be hypothesized that the presence of ferroptosis in β-thalassemia patients promotes the development of AF.58 Basic research hinted that rapid pacing cardiac fibroblast-derived exosomes exacerbated cardiomyocytes ferroptosis, and AF could be prevented by intervening in exosomal miRNAs.59 In addition, LPS-induced endotoxemia,60 excessive alcohol consumption,61 and obesity-related gut flora dysbiosis62 could increase AF susceptibility through ferroptosis.
Pyroptosis and AF
Pyroptosis is an inflammatory form of programmed cell death involving the activation of caspase-1 by the inflammasome. Pyroptosis exhibits a plasma membrane blistering morphology and therefore is usually considered a monocyte-specific form of apoptosis. However, the recent discovery of Gasdermin-D of pore-forming activity, which is a key executor of pyroptosis, has redefined pyroptosis as a necrotic form of cell death.
Pyroptosis is a type of pro-inflammatory cell death that is closely associated with the activation of NOD-like receptor thermal protein domain associated protein 3 (NLRP3) inflammasome and is intensively involved in AF.63 A previous report claimed the NLRP3 inhibitor glibenclamide reversed atrial remodeling partially and prevented AF in diabetic rabbits.64 Yao et al found that NLRP3 inflammasome activity was enhanced significantly in atrial myocytes from AF patients and dogs with atrial tachycardia pacing.63 Over activation of the NLRP3 signaling pathway promotes the expression of apoptotic mechanisms mediated by RyR2 and caspase-1, resulting in increased Ca2+ release from the sarcoplasmic reticulum and increased secretion of inflammatory cytokines.63,65 At the same time, enhanced transcription of Kcna5 leads to an increase of Kv1.5-current, shortening the effective refractory period and forming a reentry substrate.63 Cold exposure increased trimethylamine N-oxide by enhancing gut-derived trimethylamine production, which promoted M1 macrophage infiltration, induced the pyroptosis of cardiomyocytes in rats, exacerbates atrial structural remodeling, and ultimately led to AF.66 LncRNA x–inactive specific transcript (XIST) may promote Arl2 expression through the uptake of miR-214-3p to blunt the pyroptosis of cardiomyocytes, providing encouraging insights for XIST-based AF-targeted therapy.67
Pulsed-Field Ablation (PFA) versus Conventional Catheter Ablation
Catheter ablation has formed a scenario in which radiofrequency ablation (RFA) is the mainstay, supplemented by cryoballoon, laser, and ultrasound ablation during the past decades. The success rates of RFA, cryoballoon ablation (CBA), and PFA for paroxysmal AF within 1 year are 61.1%–92%, 51.7%–88% and 66.2%–90% respectively (Table 1). Although a shoulder-to-shoulder comparison of the effectiveness of RFA and PFA is not yet available, the published data from small samples of PFA is worth envisioning. Unlike RFA and CBA, which use extreme temperatures to destroy myocardial tissue, PFA utilizes strong electric fields to disrupt the phospholipid bilayer integrity of cell membranes or organelles to induce cell death.68 Cardiomyocytes have the lowest electroporation threshold of all tissues, which makes this technique particularly appropriate for cardiac ablation.69 This difference in sensitivity between cardiomyocytes and other non-target tissues may reduce the risk of collateral damage to the esophagus and phrenic nerve.
Table 1.
Summary of Success Rate After Different Ablation Procedures of Atrial Fibrillation
| Reference | Total Number | Age (Year) | LVEF% | Type of AF | Treatment Method | 1-Year Success Rate | Follow-Up Time (Success Rate) |
|---|---|---|---|---|---|---|---|
| [70] | 150 | 63.4±9.9 | 60.3±4.8 | Par | PFA | 66.2% | – |
| [71] | 121 | 57.4±10.3 | 62.5±5.7 | Par | PFA | 78.5% | – |
| [72] | 186 | 59.4±10.2 | 60.8±5.8 | Par | PFA | 78.9% | – |
| [73] | 1568 | 64.5±11.5 | 60 | Par/Per | PFA | 78.1% | – |
| [74] | 52 | 65±10 | 56±11 | Par/Per | PFA | – | 0.5 years (85%) |
| [75] | 200 | 71 (62–77) | – | Par/Per | PFA | 80.3%(Par)/66.8%(Per) | – |
| [76] | 191 | 69±12 | 60±10 | Par/Per | PFA | – | 0.5 years (94.2%) |
| [77] | 138 | 67±12 | 52±10 | Par/Per | PFA | 90%(Par)/60%(Per) | – |
| [70] | 150 | 66.0±9.0 | 57.6±6.4 | Per | PFA | 55.1% | – |
| [78] | 45 | 67.1±10 | 56.5±11.2 | Per | PFA | – | 0.3 years (80%) |
| [79] | 253 | 61±9 | 65.8±4.4 | Par | RFA | 82.7% | 2.1 years (79.6%) |
| [80] | 25 | 59±9 | 58±6 | Par | RFA | 92% | – |
| [81] | 100 | 60.0±10.1 | 58.9±8.0 | Par | RFA | 61.1% | – |
| [82] | 260 | 58.3±8.7 | – | Par | RFA | – | 1.9 years (57.3%) |
| [83] | 66 | 54.3±1.3 | 60.8±7 | Par | RFA | – | 2 years (45.5%) |
| [84] | 146 | 56±9 | – | Par | RFA | 82.2% | 2 years (84.9%) |
| [85] | 159 | 60 (54–67) | – | Par | RFA | 70.7% | – |
| [86] | 121 | 59.8±9.7 | – | Par | RFA | – | 4.8 years (46.6%) |
| [87] | 210 | 56.6±10 | 61.8±6.1 | Par/Per | RFA | 80.6% | – |
| [88] | 61 | 63.4±10.5 | 67.1±6.6 | Par/Per | RFA | 96.7% | 3 years (88.5%) |
| [89] | 136 | 58.2±10.8 | – | Par/Per | RFA | 57.4% | – |
| [90] | 63 | 56.0±7.2 | 55.5±8.2 | Par/Per | RFA | 38.1% | 7 years (13%) |
| [91] | 150 | 58.2±10 | 57.±10.8 | Par/Per | RFA | 84% | – |
| [92] | 356 | 59 (51–65) | 60 (57–62) | Par/Per | RFA | ~67.5% | 5 years (45%) |
| [93] | 100 | 55.7±9.6 | 70±11 | Par/Per | RFA | 40% | 5 years (29%) |
| [94] | 229 | – | – | Par/Per | RFA | 77.3% | – |
| [95] | 50 | 62.4±9.5 | 56.3±4.1 | Per | RFA | 54% | – |
| [96] | 83 | 65±8 | 27.8±9.5 | Per | RFA | 73.5% | – |
| [97] | 25 | 60±7 | 55 (50–60) | Per | RFA | 68% | – |
| [98] | 49 | 64.0±8.7 | 56.8±8.1 | Per | RFA | 61.2% | – |
| [99] | 67 | 58±10 | 55±11 | Per | RFA | – | 1.5 years (59%) |
| [100] | 61 | 62.1±9.9 | - | Per | RFA | 54% | – |
| [101] | 202 | 61±9 | 60±7 | Per | RFA | 35.1% | 4.6 years (20.3%) |
| [102] | 191 | 58.2±12.9 | 57±11 | Per | RFA | – | 1.1 years (32%) |
| [103] | 104 | 60.4±11.2 | 60.9±0.6 | Par | CBA | 74.6% | – |
| [104] | 154 | 57.7±12.3 | 59.6±7.0 | Par | CBA | 57.1% | – |
| [105] | 116 | 58.6±9.2 | 59.1±6.6 | Par | CBA | 51.7% | – |
| [106] | 163 | 57±9 | 60±6 | Par | CBA | 69.9% | – |
| [107] | 107 | 50.5±13.1 | 62.8±5.4 | Par | CBA | 82.2% | – |
| [79] | 247 | 60.8±10.0 | 65.9±5.4 | Par | CBA | 79.8% | 2.2 years (70.9%) |
| [108] | 163 | 58 (50, 64) | 62 (57, 65) | Par | CBA | 69% | 5 years (53%) |
| [80] | 25 | 58±9 | 60±5 | Par | CBA | 88% | – |
| [109] | 55 | 62.9±8.7 | 61.7±5.4 | Par/Per | CBA | 61.8% | – |
| [81] | 101 | 62.9±8.9 | 58.0±9.0 | Par | CBA | 60.3% | – |
| [82] | 136 | 57±13.3 | – | Par | CBA | – | 1.9 years (63.2%) |
| [110] | 120 | 61.9±9.3 | 55.9±8.5 | Par/Per | CBA | 74.2% | – |
| [111] | 1742 | 58.0±10.4 | – | Par/Per | CBA | 78.5% | – |
| [112] | 100 | 66.5±9.4 | 61.5±5.6 | Par/Per | CBA | 80% | – |
| [113] | 80 | 66±10 | 57±10 | Par/Per | CBA | 68% | – |
| [114] | 256 | 58±10.9 | 65±6.5 | Par/Per | CBA | 85.5% | 5 years (59.4%) |
| [87] | 200 | 57.5±9.8 | 61.1±7.1 | Par/Per | CBA | 72.7% | – |
| [115] | 299 | 60±11 | 59±8 | Par/Per | CBA | 83.9%(Par)/61.6%(Per) | – |
| [88] | 70 | 64.1±10.1 | 68.0±9.1 | Par/Per | CBA | 82.8% | 3 years (70.0%) |
| [116] | 236 | 54.6±10.45 | 64.5±5.8 | Par/Per | CBA | – | 1.5 years (74.5%) |
| [89] | 133 | 59.7±9.9 | – | Par/Per | CBA | 75.2% | – |
| [117] | 133 | 66±10 | 51±8 | Per | CBA | 67% | – |
| [118] | 49 | 63±10 | – | Per | CBA | 69% | 1.1 years (69%) |
| [119] | 100 | 63±10 | 57.6±6.4 | Per | CBA | – | 0.9 years (67%) |
| [95] | 50 | 62.4±9.8 | 57.5±3.7 | Per | CBA | 56% | – |
| [120] | 69 | 59.4±8.1 | – | Per | CBA | 59% | 1.7 years (50%) |
| [121] | 917 | – | – | Per | CBA | – | 1.4 years (68.9%) |
Abbreviations: AF, atrial fibrillation; RFA, radiofrequency ablation; PFA, pulsed field ablation; CBA, cryoballoon ablation; Par, Paroxysmal; Per, Persistent.
Cell Death in RFA and CBA
RFA uses radiofrequency currents to generate high temperature to cause damage to the target tissue, while CBA uses argon to absorb surrounding heat as it expands rapidly to cause a rapid drop in the temperature of the surrounding tissue. Both RFA and CBA are energy-based ablations that utilize extreme temperatures, resulting in irreversible cellular damage.
RFA causes a high temperature and then transmits it to the surrounding tissues, forming the main characteristic lesion centered on coagulative necrosis. When the temperature exceeds 60 °C, the time to reach irreversible damage decreases exponentially. The inactivation of important enzymes is the initial feature of the damage, and proteins are denatured quickly and followed by cytotoxicity causing coagulative necrosis (Figure 3). At temperatures around 40–45 °C, irreversible cellular damage only occurs with prolonged exposure (30–60 minutes). Temperature gradients caused by catheter can induce apoptosis and lead to the progression of injury. Besides, stimulation of apoptosis can be triggered by various cytokines and can induce direct alterations in the tissue microenvironment.122 In particular, endothelial pyroptosis after RFA of liver tissue has been reported to be closely associated with systemic inflammatory response syndrome, confirming that pyroptosis occurs with thermal injury.123,124 There is little ferroptosis reported from RFA or thermal injury. However, ferroptosis mediated alkali burn-induced corneal injury has been reported in ophthalmology.125 In sum, cell death in the injury zone is a complex process and various types of cell death may need to be further explored.
Figure 3.
Comparison of cell death patterns due to RFA and PFA. RFA causes damage by localized cell necrosis, whereas PFA causes damage by apoptotic cell death. By comparing the schematic diagrams, RFA causes widespread necrosis, and the area of necrosis may contain patterns of death including necrosis, necroptosis, pyroptosis, and ferroptosis. A large accumulation of inflammatory cells such as neutrophils, monocytes and lymphocytes are chemotactic to the migrated area, which in turn induces a strong inflammatory response. Whereas the necrosis by PFA is limited to the area close to the catheter, the wider damage is predominantly apoptotic. There is also relatively little infiltration of inflammatory cells in the migratory zone.
Abbreviations: RFA, radiofrequency ablation; PFA, pulsed field ablation.
CBA creates an injury similar to RFA, a pattern of injury centered on necrosis.126 Ice crystals can form inside and outside the cells during CBA and are accelerated by nucleation. Spontaneous nucleation of cells can occur at −5°C to −15°C.127 The percentage of intracellular ice increases during rapid temperature reduction. Cell damage occurs due to shearing, and the damage caused during rapid temperature reduction is lethal.128 Animal studies verified that the sublethal temperature in the peripheral zone causes cells to activate apoptosis.129,130 Regrettably, the existence of other types of cell death in the migratory region has not yet been reported clearly.
Cell Death in PFA
Unlike RFA and CBA, which are energy-based ablations, PFA uses multiple strong pulses to generate an electric field that causes irreversible cell membrane damage.131 Electric fields are most commonly generated by a high-voltage direct current delivered between two or more electrodes. When a sufficiently strong electric field is applied to a cell and increases the permeability of its membrane, electroporation occurs, leading to increased ion transport and overall membrane instability.132 Its nonthermal mechanism of ablation allows PFA to ablate the atrial myocardium because it has a lower threshold for injury compared to the phrenic nerve and esophagus which are close to the heart. At a certain distance from the electrode, there will be an area of tissue at the PFA threshold that forms the edge of the lesion. With the use of higher voltages or increasing other exposure metrics (including pulse duration and the number of pulses), the lesion volume will increase. Cell death due to field exposure above the PFA threshold and permanent hyperpermeability is attributed to adenosine triphosphate depletion, ion channel failure, calcium overload, and a general loss of intracellular homeostasis.133–135
This non-thermal damage maintains the integrity of the extracellular matrix and thus reduces structural remodeling. Apoptosis has been reported widely as the most common form of cell death by PFA.136 (Figure 3) Animal models showed that the injury zone is delineated well and bounded by the surrounding tissue clearly.137 This ensures that tissues outside the lesion are less affected during ablation and reduces the occurrence of inflammation. The PFA delivery energy is determined by the voltage, the pulse duration, and the number of pulses. In the region close to the electrodes, where the electric field reaches high amplitude, cells may undergo thermal damage.138,139 Sometimes, PFA-ablated tissues experience necrosis, pyroptosis, or necroptosis rather than apoptosis.140–142 Therefore, appropriate electroporation parameters could mitigate the degree of necrosis and the inflammatory response.
Inflammation and AF
The inflammatory response in local myocardial tissue leads to structural remodeling, which slows down atrial myocardial conduction velocity and prolongs the effective induction period, ultimately leading to the AF toward permanent arrhythmia. In patients with an acute systemic inflammation such as sepsis, the morbidity of AF can be up to 23%.143 In patients with more severe septic shock, the morbidity of new-onset AF can be up to 46%.144 Other chronic systemic inflammatory diseases such as rheumatoid arthritis,145 psoriasis,146 and inflammatory bowel disease147 have all reported high incidences of AF. In addition, AF can lead to the release of inflammatory factors. Elevated IL-6 and tumor necrosis factor (TNF) were detected in both atrial tissue and peripheral blood in canines with atrial rapid pacing.148 Recent studies have shown that T-cell-mediated inflammation can be regulated by modulating gut flora, which reduces atrial structural remodeling in turn and the development of age-related AF.149
In addition to elevated serum inflammatory factors in AF, the local inflammatory status of myocardial tissue was also validly assessed. Epicardial adipose tissue from persistent AF patients expressed IL-1β in large abundances at the transcriptional level.150 High expression of tissue factors was produced by inflammatory stimuli in the left atrial appendage obtained from AF patients.151 Narducci et al showed high expression of CRP in atrial septum specimens from patients with paroxysmal AF.152 Local inflammation produces abundantly pentraxin 3. Markedly elevated pentraxin 3 expression by serology and immunohistochemistry confirmed an increased local inflammatory burden in AF.153
Inflammation and Electrical Remodeling
Inflammatory cytokines such as platelet derived growth factor (PDGF), IL-2, and TNF-α regulate cellular ion channels and calcium homeostasis. PDGF from myofibroblasts can reduce the action potential duration and Ca2+ transients.154 TNF-α can increase the potential for pulmonary veins to cause arrhythmogenesis and induce abnormalities in calcium homeostasis, leading to inflammation-associated AF.155 It has been shown that mice overexpressing TNF-α have prolonged action potentials and Ca2+ transients, higher diastolic Ca2+ currents, and lower systolic Ca2+ currents.156,157
Inflammation, in addition to regulating cellular ion channels and calcium homeostasis, has been associated with the heterogeneity of atrial conduction. Heterogeneous conduction may be the result of altered expression or distribution of connexin (Cx) 40, Cx43, or atrial fibrosis. In a canine model of aseptic pericarditis, reduced expression and transmural gradients of Cx40 and Cx43 (both absent in the epicardium, reduced in the pericardium, and normal in the endocardium) were associated with marked atrial conduction abnormalities and the induction and maintenance of AF.158 One study found that the serum IL-6 level is negatively correlated with the expression level of Cx43. The expression of Cx40 and Cx43 decreased rapidly after direct exposure of IL-6 to HL-1 cells, and their expression recovered rapidly after the cessation of IL-6 exposure.159 Ishii et al found that the degree of atrial inflammation was associated with the atrial conduction heterogeneity and the duration of AF.160 Topical application of arachidonic acid to the atria of canines produced heterogeneous conduction, whereas methyl-prednisolone reversed it.161 Additionally, endurance athletes have increased risk for AF, and a longitudinal study of athletes participating in marathons found that prolonged signal-averaged P-wave duration was accompanied by transient increases of high-sensitivity C-reactive proteins (hs-CRP), pro-inflammatory cytokines, leukocytes, neutrophils, pro-cardiac natriuretic peptide, and high-sensitivity troponin, suggesting that acute changes in inflammatory cytokines are associated with atrial electrical conduction and susceptibility to AF.162
Inflammation and Structural Remodeling
Inflammation is also closely related to structural remodeling of the heart, and clinical and experimental studies of AF have revealed that atrial fibrosis is the most important histopathological change in AF.163 PDGF-A promotes cell proliferation and collagen expression in cardiac fibroblasts and increases susceptibility to atrial fibrosis and AF, which can be reversed by mast cell stabilizers and PDGF-A blockers.164 PDGF and its receptors are expressed in atrial fibroblasts more strongly compared to ventricular fibroblasts. Whereas induction of HSPs, especially HSPA1A, attenuates the remodeling of atrial substrates.165 TNF-α/TGF-β signaling pathway could increase secretion of MMPs which is involved in the pathogenesis of mouse atrial fibrosis.166 In contrast, anti-TNF-α treatment reduced the activation of MMP-2 and MMP-9 and prevented collagen synthesis and deposition.167 Recruitment of macrophages is critical for tissue fibrosis. Macrophages can stimulate IL-6 in cardiac fibroblasts, which leads to TGF-β1 in cardiac fibroblasts and the phosphorylation of Smad3, stimulating cardiac fibrosis.168 In clinical studies, high levels of CRP, IL-6, and IL-18 and low levels of HSP27 were associated with increased atrial size and susceptibility to AF, which further supports the role of inflammation in atrial remodeling.169–171
Inflammatory Response After Ablation Procedures
There is no doubt that invasive catheter ablation procedures impart inflammatory response to the tissue, but this inflammatory response may be intimately correlated with the form of energy.
The ablated tissue or cells and extracellular matrix may release pro-inflammatory cytokines.172 Serum levels of IL-1β, IL-6, IL-8, and TNF-α are shown to be elevated after RFA.173–175 Infiltration of inflammatory cells such as neutrophils, macrophages, dendritic cells, and natural killer cells has been reported in migratory zone.176–178 There is controversy regarding the cellular damage and inflammatory response produced after CBA. Some studies have shown a weak systemic inflammatory response after CBA,179 while others have shown comparable cellular damage and inflammatory response between CBA and RFA.180 Antolič et al found that myocardial damage was more pronounced with CBA than RFA. However, there was no significant difference between the two ablation techniques in terms of inflammatory response and coagulation activation.181 Nonetheless, Shinsuke et al found that RFA and CBA groups had comparable peak blood hs-CRP and produced comparable inflammatory responses, and CBA caused greater myocardial damage.182
The inflammatory response after PFA has been less studied. Massimo et al reported a detailed histological analysis of atrial lesions after PFA and performed the first comparative study of the histological findings at 7 and 30 days, finding that the degree of tissue inflammation was evident at 7 days and reduced with almost no inflammatory cells observed at 30 days, indicating a shorter duration of the inflammatory response.183 Another study found that significant ablation-related necrosis, inflammation and fibrosis were seen in all tissue sections of RFA compared with PFA, and they speculated that RFA causes a greater inflammatory burden compared with PFA.184 Comparing the inflammatory response generated by the organism after PFA and RFA in patients with hepatocellular carcinoma, it was found that the serum of macrophage migration inhibitory factor was elevated immediately after PFA, while this phenomenon was not observed in the RFA group, which may promote the early repair process after PFA and lead to a significant reduction of the ablation zone and alleviate the inflammatory burden in the organism.185 Further studies are still needed regarding the strength of the inflammatory response after different ablation procedures for AF.
There is little direct histological evidence of inflammation in local myocardial tissue post catheter ablation, mainly due to concerns about the safety of invasive myocardial biopsy procedures. However, advances in imaging technology have made it possible to assess atrial inflammation in a non-invasive manner. Xie et al proposed that 18F-fluorodeoxyglucose positron emission tomography/computed tomography (PET-CT) would reflect favorably the degree of inflammatory burden after catheter ablation.186 Late-gadolinium enhancement magnetic resonance imaging (LGE-MRI) is superior for assessing fibrosis as the final terminus of local inflammation. Clinical studies have reported that the higher the grade of fibrosis appraised by LGE-MRI after catheter ablation, the increased the rate of recurrence. In addition, enhanced CT assessment epicardial adipose tissue associated with local inflammation has been extensively utilized in clinical practice.187–189 Artificial intelligence algorithms can effectively predict the recurrence of AF after catheter ablation. By evaluating patient data, artificial intelligence algorithms can select the most effective AF treatment based on the patient’s unique characteristics.190 These tools considerably enrich the means of objectively appraise the local inflammatory response after catheter ablation. Early identification of excessive inflammatory responses and interventions facilitate a better prognosis.
Relationship Between Inflammation and Recurrence After Ablation Procedures
The primary goal of catheter ablation is to eliminate triggers that cause fibrillation, such as reentry, or to eliminate areas that lead to multiple depolarization, but it does not address the underlying process of myocardial remodeling.
The report published by Andrade et al suggested that early recurrence is thought to be due to inadequate early structural remodeling after pulmonary vein isolation (PVI), leading to leakage of the electrical impulses. Late recurrence may be the ectopic foci shifting from the pulmonary veins to other sites.191 In addition to other comorbidities, there is a fact that the levels of markers associated with myocardial injury like creatine kinase, creatine kinase-MB and troponin are high after catheter ablation.192 Troponin has been shown to be the most sensitive marker for detecting myocardial injury.193 A systemic review found that the level of troponin was closely associated with AF recurrence after RFA.194 During PVI, both radiofrequency ablation and cryoballoon ablation indiscriminately damage myocardial tissue, including blood vessels, nerves and myocardium. Complete PVI inevitably damages the left coronary artery branch, and stenosis with thrombosis causes myocardial tissue damage to extend from the ablation line to other areas. As mentioned above, ectopic foci activity is enhanced by subsequent atrial infarction and inflammation. The increase of local inflammation after catheter ablation may contribute to recurrence.
Several studies identified lymphocytes/neutrophils as a reliable predictor of recurrence after catheter ablation.195,196 Galectin-3 is a biomarker of AF that mediates atrial inflammation and may reflect progressive atrial fibrosis in AF. More studies have shown that galectin-3 is an independent predictor of recurrence after catheter ablation of AF,197–199 and Takemoto et al demonstrated that inhibition of galectin-3 could improve outcomes in persistent AF treated with catheter ablation.200 Besides, it has been reported that uric acid/albumin,201 myeloperoxidase,202,203 monocyte/HDL,204 TLR4,205 MMP-2 and TNF-α,206 and CRP207 were associated with higher recurrence after catheter ablation. The inhibitory effects of anti-inflammatory drugs such as steroids, colchicine, angiotensin-converting enzyme inhibitor/angiotensin receptor blocker, and statins on AF are summarized in a review by Kensuke et al in detail.208 In addition, the AntaEP study indicated that antazoline may be suitable for pharmacological cardioversion of AF occurring during PVI, hinting the potential participation of histamine in the progression of AF.209
Conclusion
Inflammatory cell death is strongly associated with the development and recurrence of AF. Therefore, avoiding inflammatory and unnecessary cell death in myocardial tissue is essential to prevent the recurrence of AF. In the current review, PFA, a new energy source of non-thermal irreversible electroporation, has great advantages over traditional catheter ablation. From the cellular perspective, catheter ablation uses extreme temperatures to cause necrosis of diseased tissues and cells, and the inflammatory response generated places a certain burden on the organism. In contrast, PFA benefits patients by protecting adjacent tissues and inducing apoptosis with a less inflammatory response on the organism while ensuring significant efficacy of durable PVI. However, different parameters cause different types of cell death in the tissues. There is still a lack of clinical trials on PFA, and further research is needed to investigate its safety and effects.
Funding Statement
This work was supported by National Natural Science Foundation of China (81970277, 82170312).
Disclosure
Dishiwen Liu and Yajia Li are co-first authors for this study. The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- 1.Santhanakrishnan R, Wang N, Larson MG, et al. Atrial fibrillation begets heart failure and vice versa: temporal associations and differences in preserved versus reduced ejection fraction. Circulation. 2016;133(5):484–492. doi: 10.1161/CIRCULATIONAHA.115.018614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Şaylık F, Çınar T, Akbulut T, Hayıroğlu M. Comparison of catheter ablation and medical therapy for atrial fibrillation in heart failure patients: a meta-analysis of randomized controlled trials. Heart Lung. 2023;57:69–74. [DOI] [PubMed] [Google Scholar]
- 3.Haïssaguerre M, Jaïs P, Shah DC, et al. Spontaneous initiation of atrial fibrillation by ectopic beats originating in the pulmonary veins. N Engl J Med. 1998;339(10):659–666. doi: 10.1056/NEJM199809033391003 [DOI] [PubMed] [Google Scholar]
- 4.Nogami A, Kurita T, Abe H, et al. JCS/JHRS 2019 guideline on non-pharmacotherapy of cardiac arrhythmias. Circ J. 2021;85(7):1104–1244. doi: 10.1253/circj.CJ-20-0637 [DOI] [PubMed] [Google Scholar]
- 5.Li P, Dong XR, Zhang B, et al. Molecular mechanism and therapeutic targeting of necrosis, apoptosis, pyroptosis, and autophagy in cardiovascular disease. Chin Med J. 2021;134(22):2647–2655. doi: 10.1097/cm9.0000000000001772 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ji N, Qi Z, Wang Y, et al. Pyroptosis: a new regulating mechanism in cardiovascular disease. J Inflamm Res. 2021;14:2647–2666. doi: 10.2147/jir.S308177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kerr JF, Wyllie AH, Currie AR. Apoptosis: a basic biological phenomenon with wide-ranging implications in tissue kinetics. Br J Cancer. 1972;26(4):239–257. doi: 10.1038/bjc.1972.33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gottlieb RA, Burleson KO, Kloner RA, Babior BM, Engler RL. Reperfusion injury induces apoptosis in rabbit cardiomyocytes. J Clin Invest. 1994;94(4):1621–1628. doi: 10.1172/jci117504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.James TN. Normal and abnormal consequences of apoptosis in the human heart. From postnatal morphogenesis to paroxysmal arrhythmias. Circulation. 1994;90(1):556–573. [PubMed] [Google Scholar]
- 10.James TN, Terasaki F, Pavlovich ER, Vikhert AM. Apoptosis and pleomorphic micromitochondriosis in the sinus nodes surgically excised from five patients with the long QT syndrome. J Lab Clin Med. 1993;122(3):309–323. [PubMed] [Google Scholar]
- 11.Kuramochi Y, Guo X, Sawyer DB, Lim CC. Rapid electrical stimulation induces early activation of kinase signal transduction pathways and apoptosis in adult rat ventricular myocytes. Exp Physiol. 2006;91(4):773–780. doi: 10.1113/expphysiol.2006.033894 [DOI] [PubMed] [Google Scholar]
- 12.Aimé-Sempé C, Folliguet T, Rücker-Martin C, et al. Myocardial cell death in fibrillating and dilated human right atria. J Am Coll Cardiol. 1999;34(5):1577–1586. doi: 10.1016/s0735-1097(99)00382-4 [DOI] [PubMed] [Google Scholar]
- 13.Zhao J, Li J, Li W, et al. Effects of spironolactone on atrial structural remodelling in a canine model of atrial fibrillation produced by prolonged atrial pacing. Br J Pharmacol. 2010;159(8):1584–1594. doi: 10.1111/j.1476-5381.2009.00551.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Trappe K, Thomas D, Bikou O, et al. Suppression of persistent atrial fibrillation by genetic knockdown of caspase 3: a pre-clinical pilot study. Eur Heart J. 2013;34(2):147–157. doi: 10.1093/eurheartj/ehr269 [DOI] [PubMed] [Google Scholar]
- 15.Fedai H, Altiparmak IH, Tascanov MB, et al. The relationship between oxidative stress and autophagy and apoptosis in patients with paroxysmal atrial fibrillation. Scand J Clin Lab Invest. 2022;82(5):391–397. doi: 10.1080/00365513.2022.2100274 [DOI] [PubMed] [Google Scholar]
- 16.Osmancik P, Peroutka Z, Budera P, et al. Decreased apoptosis following successful ablation of atrial fibrillation. Cardiology. 2010;116(4):302–307. doi: 10.1159/000319619 [DOI] [PubMed] [Google Scholar]
- 17.Adamo L, Rocha-Resende C, Prabhu SD, Mann DL. Reappraising the role of inflammation in heart failure. Nat Rev Cardiol. 2020;17(5):269–285. doi: 10.1038/s41569-019-0315-x [DOI] [PubMed] [Google Scholar]
- 18.Mann DL, Topkara VK, Evans S, Barger PM. Innate immunity in the adult mammalian heart: for whom the cell tolls. Trans Am Clin Climatol Assoc. 2010;121:34–50. [PMC free article] [PubMed] [Google Scholar]
- 19.Mouton AJ, DeLeon-Pennell KY, Rivera Gonzalez OJ, et al. Mapping macrophage polarization over the myocardial infarction time continuum. Basic Res Cardiol. 2018;113(4):26. doi: 10.1007/s00395-018-0686-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Daseke MJ, Valerio FM, Kalusche WJ, Ma Y, DeLeon-Pennell KY, Lindsey ML. Neutrophil proteome shifts over the myocardial infarction time continuum. Basic Res Cardiol. 2019;114(5):37. doi: 10.1007/s00395-019-0746-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rurik JG, Aghajanian H, Epstein JA. Immune Cells and immunotherapy for cardiac injury and repair. Circ Res. 2021;128(11):1766–1779. doi: 10.1161/circresaha.121.318005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Majidi M, Kosinski AS, Al-Khatib SM, et al. Reperfusion ventricular arrhythmia ‘bursts’ in TIMI 3 flow restoration with primary angioplasty for anterior ST-elevation myocardial infarction: a more precise definition of reperfusion arrhythmias. Europace. 2008;10(8):988–997. doi: 10.1093/europace/eun123 [DOI] [PubMed] [Google Scholar]
- 23.Majidi M, Kosinski AS, Al-Khatib SM, et al. Reperfusion ventricular arrhythmia ‘bursts’ predict larger infarct size despite TIMI 3 flow restoration with primary angioplasty for anterior ST-elevation myocardial infarction. Eur Heart J. 2009;30(7):757–764. doi: 10.1093/eurheartj/ehp005 [DOI] [PubMed] [Google Scholar]
- 24.van der Weg K, Majidi M, Haeck JD, et al. Ventricular arrhythmia burst is an independent indicator of larger infarct size even in optimal reperfusion in STEMI. J Electrocardiol. 2016;49(3):345–352. doi: 10.1016/j.jelectrocard.2016.03.013 [DOI] [PubMed] [Google Scholar]
- 25.Belkouche A, Yao H, Putot A, et al. The multifaceted interplay between atrial fibrillation and myocardial infarction: a review. J Clin Med. 2021;10(2). doi: 10.3390/jcm10020198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bocharov AV, Popov LV, Lagkuev MD. The frequency of atrial infarction in patients with supraventricular arrhythmias. Kardiologiia. 2022;62(3):28–31. doi: 10.18087/cardio.2022.3.n1648 [DOI] [PubMed] [Google Scholar]
- 27.Álvarez-García J, Vives-Borrás M, Gomis P, et al. Electrophysiological effects of selective atrial coronary artery occlusion in humans. Circulation. 2016;133(23):2235–2242. doi: 10.1161/circulationaha.116.021700 [DOI] [PubMed] [Google Scholar]
- 28.Biccirè FG, Pastori D, Torromeo C, et al. Acute atrial ischemia associates with early but not late new-onset atrial fibrillation in STEMI patients treated with primary PCI: relationship with in-hospital outcomes. J Cardiol. 2021;78(5):368–374. doi: 10.1016/j.jjcc.2021.05.013 [DOI] [PubMed] [Google Scholar]
- 29.Shiba T, Kondo Y, Senoo K, et al. Proximal occlusion in the right coronary artery involving the atrial branch as a strong predictor of new-onset atrial fibrillation in acute myocardial infarction. Int Heart J. 2019;60(6):1308–1314. doi: 10.1536/ihj.18-713 [DOI] [PubMed] [Google Scholar]
- 30.Alasady M, Shipp NJ, Brooks AG, et al. Myocardial infarction and atrial fibrillation: importance of atrial ischemia. Circ Arrhythm Electrophysiol. 2013;6(4):738–745. doi: 10.1161/circep.113.000163 [DOI] [PubMed] [Google Scholar]
- 31.Nishida K, Qi XY, Wakili R, et al. Mechanisms of atrial tachyarrhythmias associated with coronary artery occlusion in a chronic canine model. Circulation. 2011;123(2):137–146. doi: 10.1161/circulationaha.110.972778 [DOI] [PubMed] [Google Scholar]
- 32.Avula UMR, Hernandez JJ, Yamazaki M, et al. Atrial infarction-induced spontaneous focal discharges and atrial fibrillation in sheep: role of dantrolene-sensitive aberrant ryanodine receptor calcium release. Circ Arrhythm Electrophysiol. 2018;11(3):e005659. doi: 10.1161/circep.117.005659 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Liu M, Li W, Wang H, et al. CTRP9 ameliorates atrial inflammation, fibrosis, and vulnerability to atrial fibrillation in post-myocardial infarction rats. J Am Heart Assoc. 2019;8(21):e013133. doi: 10.1161/jaha.119.013133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Beiert T, Tiyerili V, Knappe V, et al. Relaxin reduces susceptibility to post-infarct atrial fibrillation in mice due to anti-fibrotic and anti-inflammatory properties. Biochem Biophys Res Commun. 2017;490(3):643–649. doi: 10.1016/j.bbrc.2017.06.091 [DOI] [PubMed] [Google Scholar]
- 35.Zhou W, Yuan J. Necroptosis in health and diseases. Semin Cell Dev Biol. 2014;35:14–23. doi: 10.1016/j.semcdb.2014.07.013 [DOI] [PubMed] [Google Scholar]
- 36.Koudstaal S, Oerlemans MI, Van der Spoel TI, et al. Necrostatin-1 alleviates reperfusion injury following acute myocardial infarction in pigs. Eur J Clin Invest. 2015;45(2):150–159. doi: 10.1111/eci.12391 [DOI] [PubMed] [Google Scholar]
- 37.Guo X, Yin H, Li L, et al. Cardioprotective role of tumor necrosis factor receptor-associated factor 2 by suppressing apoptosis and necroptosis. Circulation. 2017;136(8):729–742. doi: 10.1161/circulationaha.116.026240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Szobi A, Gonçalvesová E, Varga ZV, et al. Analysis of necroptotic proteins in failing human hearts. J Transl Med. 2017;15(1):86. doi: 10.1186/s12967-017-1189-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fu Y, Jiang T, Sun H, et al. Necroptosis is required for atrial fibrillation and involved in aerobic exercise-conferred cardioprotection. J Cell Mol Med. 2021;25(17):8363–8375. doi: 10.1111/jcmm.16796 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Li L, Chen Y, Doan J, Murray J, Molkentin JD, Liu Q. Transforming growth factor β-activated kinase 1 signaling pathway critically regulates myocardial survival and remodeling. Circulation. 2014;130(24):2162–2172. doi: 10.1161/circulationaha.114.011195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dixon SJ, Lemberg KM, Lamprecht MR, et al. Ferroptosis: an iron-dependent form of nonapoptotic cell death. Cell. 2012;149(5):1060–1072. doi: 10.1016/j.cell.2012.03.042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Friedmann Angeli JP, Schneider M, Proneth B, et al. Inactivation of the ferroptosis regulator Gpx4 triggers acute renal failure in mice. Nat Cell Biol. 2014;16(12):1180–1191. doi: 10.1038/ncb3064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Proneth B, Conrad M. Ferroptosis and necroinflammation, a yet poorly explored link. Cell Death Differ. 2019;26(1):14–24. doi: 10.1038/s41418-018-0173-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wang Y, Quan F, Cao Q, et al. Quercetin alleviates acute kidney injury by inhibiting ferroptosis. J Adv Res. 2021;28:231–243. doi: 10.1016/j.jare.2020.07.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wen Q, Liu J, Kang R, Zhou B, Tang D. The release and activity of HMGB1 in ferroptosis. Biochem Biophys Res Commun. 2019;510(2):278–283. doi: 10.1016/j.bbrc.2019.01.090 [DOI] [PubMed] [Google Scholar]
- 46.Luo X, Gong HB, Gao HY, et al. Oxygenated phosphatidylethanolamine navigates phagocytosis of ferroptotic cells by interacting with TLR2. Cell Death Differ. 2021;28(6):1971–1989. doi: 10.1038/s41418-020-00719-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Linkermann A, Skouta R, Himmerkus N, et al. Synchronized renal tubular cell death involves ferroptosis. Proc Natl Acad Sci U S A. 2014;111(47):16836–16841. doi: 10.1073/pnas.1415518111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Li W, Feng G, Gauthier JM, et al. Ferroptotic cell death and TLR4/Trif signaling initiate neutrophil recruitment after heart transplantation. J Clin Invest. 2019;129(6):2293–2304. doi: 10.1172/jci126428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wang Y, Wang W, Yang H, Shao D, Zhao X, Zhang G. Intraperitoneal injection of 4-hydroxynonenal (4-HNE), a lipid peroxidation product, exacerbates colonic inflammation through activation of Toll-like receptor 4 signaling. Free Radic Biol Med. 2019;131:237–242. doi: 10.1016/j.freeradbiomed.2018.11.037 [DOI] [PubMed] [Google Scholar]
- 50.Yu Y, Yan Y, Niu F, et al. Ferroptosis: a cell death connecting oxidative stress, inflammation and cardiovascular diseases. Cell Death Discov. 2021;7(1):193. doi: 10.1038/s41420-021-00579-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Chen CJ, Huang HS, Chang WC. Depletion of phospholipid hydroperoxide glutathione peroxidase up-regulates arachidonate metabolism by 12S-lipoxygenase and cyclooxygenase 1 in human epidermoid carcinoma A431 cells. FASEB J. 2003;17(12):1694–1696. doi: 10.1096/fj.02-0847fje [DOI] [PubMed] [Google Scholar]
- 52.Sakamoto H, Imai H, Nakagawa Y. Involvement of phospholipid hydroperoxide glutathione peroxidase in the modulation of prostaglandin D2 synthesis. J Biol Chem. 2000;275(51):40028–40035. doi: 10.1074/jbc.M003191200 [DOI] [PubMed] [Google Scholar]
- 53.Li C, Deng X, Xie X, Liu Y, Friedmann Angeli JP, Lai L. Activation of glutathione peroxidase 4 as a novel anti-inflammatory strategy. Front Pharmacol. 2018;9:1120. doi: 10.3389/fphar.2018.01120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Calder PC. Polyunsaturated fatty acids, inflammation, and immunity. Lipids. 2001;36(9):1007–1024. doi: 10.1007/s11745-001-0812-7 [DOI] [PubMed] [Google Scholar]
- 55.Xie LH, Gwathmey JK, Zhao Z. Cardiac adaptation and cardioprotection against arrhythmias and ischemia-reperfusion injury in mammalian hibernators. Pflugers Arch. 2021;473(3):407–416. doi: 10.1007/s00424-020-02511-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Gordan R, Wongjaikam S, Gwathmey JK, Chattipakorn N, Chattipakorn SC, Xie LH. Involvement of cytosolic and mitochondrial iron in iron overload cardiomyopathy: an update. Heart Fail Rev. 2018;23(5):801–816. doi: 10.1007/s10741-018-9700-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Jacobs W, Lammens M, Kerckhofs A, et al. Fatal lymphocytic cardiac damage in coronavirus disease 2019 (COVID-19): autopsy reveals a ferroptosis signature. ESC Heart Fail. 2020;7(6):3772–3781. doi: 10.1002/ehf2.12958 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Nomani H, Bayat G, Sahebkar A, et al. Atrial fibrillation in β-thalassemia patients with a focus on the role of iron-overload and oxidative stress: a review. J Cell Physiol. 2019;234(8):12249–12266. doi: 10.1002/jcp.27968 [DOI] [PubMed] [Google Scholar]
- 59.Liu D, Yang M, Yao Y, et al. Cardiac fibroblasts promote ferroptosis in atrial fibrillation by secreting exo-miR-23a-3p targeting SLC7A11. Oxid Med Cell Longev. 2022;2022:3961495. doi: 10.1155/2022/3961495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Fang J, Kong B, Shuai W, et al. Ferroportin-mediated ferroptosis involved in new-onset atrial fibrillation with LPS-induced endotoxemia. Eur J Pharmacol. 2021;913:174622. doi: 10.1016/j.ejphar.2021.174622 [DOI] [PubMed] [Google Scholar]
- 61.Dai C, Kong B, Qin T, et al. Inhibition of ferroptosis reduces susceptibility to frequent excessive alcohol consumption-induced atrial fibrillation. Toxicology. 2022;465:153055. doi: 10.1016/j.tox.2021.153055 [DOI] [PubMed] [Google Scholar]
- 62.Kong B, Fu H, Xiao Z, Zhou Y, Shuai W, Huang H. Gut microbiota dysbiosis induced by a high-fat diet increases susceptibility to atrial fibrillation. Can J Cardiol. 2022. doi: 10.1016/j.cjca.2022.08.231 [DOI] [PubMed] [Google Scholar]
- 63.Yao C, Veleva T, Scott L, et al. Enhanced cardiomyocyte NLRP3 inflammasome signaling promotes atrial fibrillation. Circulation. 2018;138(20):2227–2242. doi: 10.1161/circulationaha.118.035202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wu X, Liu Y, Tu D, et al. Role of NLRP3-inflammasome/caspase-1/galectin-3 pathway on atrial remodeling in diabetic rabbits. J Cardiovasc Transl Res. 2020;13(5):731–740. doi: 10.1007/s12265-020-09965-8 [DOI] [PubMed] [Google Scholar]
- 65.Chen G, Chelu MG, Dobrev D, Li N. Cardiomyocyte inflammasome signaling in cardiomyopathies and atrial fibrillation: mechanisms and potential therapeutic implications. Front Physiol. 2018;9:1115. doi: 10.3389/fphys.2018.01115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Luo Y, Zhang Y, Han X, et al. Akkermansia muciniphila prevents cold-related atrial fibrillation in rats by modulation of TMAO induced cardiac pyroptosis. EBioMedicine. 2022;82:104087. doi: 10.1016/j.ebiom.2022.104087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Yan B, Liu T, Yao C, Liu X, Du Q, Pan L. LncRNA XIST shuttled by adipose tissue-derived mesenchymal stem cell-derived extracellular vesicles suppresses myocardial pyroptosis in atrial fibrillation by disrupting miR-214-3p-mediated Arl2 inhibition. Lab Invest. 2021;101(11):1427–1438. doi: 10.1038/s41374-021-00635-0 [DOI] [PubMed] [Google Scholar]
- 68.Lavee J, Onik G, Mikus P, Rubinsky B. A novel nonthermal energy source for surgical epicardial atrial ablation: irreversible electroporation. Heart Surg Forum. 2007;10(2):E162–E167. doi: 10.1532/hsf98.20061202 [DOI] [PubMed] [Google Scholar]
- 69.Ramirez FD, Reddy VY, Viswanathan R, Hocini M, Jaïs P. Emerging Technologies for Pulmonary Vein Isolation. Circ Res. 2020;127(1):170–183. doi: 10.1161/circresaha.120.316402 [DOI] [PubMed] [Google Scholar]
- 70.Verma A, Haines DE, Boersma LV, et al. Pulsed field ablation for the treatment of atrial fibrillation: PULSED AF pivotal trial. Circulation. 2023. doi: 10.1161/CIRCULATIONAHA.123.063988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Reddy VY, Dukkipati SR, Neuzil P, et al. Pulsed field ablation of paroxysmal atrial fibrillation: 1-year outcomes of IMPULSE, PEFCAT, and PEFCAT II. JACC Clin Electrophysiol. 2021;7(5):614–627. doi: 10.1016/j.jacep.2021.02.014 [DOI] [PubMed] [Google Scholar]
- 72.Duytschaever MA-O, De Potter TA-OX, Grimaldi MA-O, et al. Paroxysmal atrial fibrillation ablation using a novel variable-loop biphasic pulsed field ablation catheter integrated with a 3-dimensional mapping system: 1-year outcomes of the multicenter inspIRE study. Circulation. 2023;16(3):e011780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Turagam MA-O, Neuzil PA-O, Schmidt BA-O, et al. Safety and effectiveness of pulsed field ablation to treat atrial fibrillation: one-year outcomes from the MANIFEST-PF Registry. Circulation. 2023;2023:1. [DOI] [PubMed] [Google Scholar]
- 74.Badertscher P, Weidlich S, Serban T, et al. Pulsed-field ablation versus single-catheter high-power short-duration radiofrequency ablation for atrial fibrillation: procedural characteristics, myocardial injury, and mid-term outcomes. Heart Rhythm. 2023. doi: 10.1016/j.hrthm.2023.05.007 [DOI] [PubMed] [Google Scholar]
- 75.Urbanek LA-OX, Bordignon SA-O, Schaack DA-O, et al. Pulsed field versus cryoballoon pulmonary vein isolation for atrial fibrillation: efficacy, safety, and long-term follow-up in a 400-patient cohort. Circulation. 2023;16(7):389–398. [DOI] [PubMed] [Google Scholar]
- 76.Schmidt B, Bordignon S, Tohoku S, et al. 5S study: safe and simple single shot pulmonary vein isolation with pulsed field ablation using sedation. Circ Arrhythm Electrophysiol. 2022;15(6):e010817. doi: 10.1161/circep.121.010817 [DOI] [PubMed] [Google Scholar]
- 77.Lemoine MD, Fink T, Mencke C, et al. Pulsed-field ablation-based pulmonary vein isolation: acute safety, efficacy and short-term follow-up in a multi-center real world scenario. Clin Res Cardiol. 2022. doi: 10.1007/s00392-022-02091-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Davong B, Adeliño R, Delasnerie H, et al. Pulsed-field ablation on mitral isthmus in persistent atrial fibrillation: preliminary data on efficacy and safety. JACC. 2023. doi: 10.1016/j.jacep.2023.03.021 [DOI] [PubMed] [Google Scholar]
- 79.Wei Y, Chen L, Cao J, et al. Long-term outcomes of a time to isolation - based strategy for cryoballoon ablation compared to radiofrequency ablation in patients with symptomatic paroxysmal atrial fibrillation. Pacing Clin Electrophysiol. 2022;45(9):1015–1023. doi: 10.1111/pace.14556 [DOI] [PubMed] [Google Scholar]
- 80.Kühne M, Suter Y, Altmann D, et al. Cryoballoon versus radiofrequency catheter ablation of paroxysmal atrial fibrillation: biomarkers of myocardial injury, recurrence rates, and pulmonary vein reconnection patterns. Heart Rhythm. 2010;7(12):1770–1776. doi: 10.1016/j.hrthm.2010.08.028 [DOI] [PubMed] [Google Scholar]
- 81.Wasserlauf J, Pelchovitz DJ, Rhyner J, et al. Cryoballoon versus radiofrequency catheter ablation for paroxysmal atrial fibrillation. Pacing Clin Electrophysiol. 2015;38(4):483–489. doi: 10.1111/pace.12582 [DOI] [PubMed] [Google Scholar]
- 82.Mugnai G, Chierchia GB, de Asmundis C, et al. Comparison of pulmonary vein isolation using cryoballoon versus conventional radiofrequency for paroxysmal atrial fibrillation. Am J Cardiol. 2014;113(9):1509–1513. doi: 10.1016/j.amjcard.2014.01.425 [DOI] [PubMed] [Google Scholar]
- 83.Morillo CA, Verma A, Connolly SJ, et al. Radiofrequency ablation vs antiarrhythmic drugs as first-line treatment of paroxysmal atrial fibrillation (RAAFT-2): a randomized trial. JAMA. 2014;311(7):692–700. doi: 10.1001/jama.2014.467 [DOI] [PubMed] [Google Scholar]
- 84.Cosedis Nielsen J, Johannessen A, Raatikainen P, et al. Radiofrequency ablation as initial therapy in paroxysmal atrial fibrillation. N Engl J Med. 2012;367(17):1587–1595. doi: 10.1056/NEJMoa1113566 [DOI] [PubMed] [Google Scholar]
- 85.Luik A, Radzewitz A, Kieser M, et al. Cryoballoon versus open irrigated radiofrequency ablation in patients with paroxysmal atrial fibrillation: the prospective, randomized, controlled, noninferiority FreezeAF study. Circulation. 2015;132(14):1311–1319. doi: 10.1161/circulationaha.115.016871 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Ouyang F, Tilz R, Chun J, et al. Long-term results of catheter ablation in paroxysmal atrial fibrillation: lessons from a 5-year follow-up. Circulation. 2010;122(23):2368–2377. doi: 10.1161/circulationaha.110.946806 [DOI] [PubMed] [Google Scholar]
- 87.Choi JH, Park SJ, Park KM, Kim JS, On YK. Efficacy and safety of cryoballoon pulmonary vein isolation for paroxysmal and persistent atrial fibrillation: a comparison with radiofrequency ablation. PLoS One. 2022;17(7):e0265482. doi: 10.1371/journal.pone.0265482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Tanaka N, Tanaka K, Ninomiya Y, et al. Comparison of the safety and efficacy of automated annotation-guided radiofrequency ablation and 2nd-generation cryoballoon ablation in paroxysmal atrial fibrillation. Circ J. 2019;83(3):548–555. doi: 10.1253/circj.CJ-18-1035 [DOI] [PubMed] [Google Scholar]
- 89.Buist TJ, Adiyaman A, Smit JJJ, Ramdat Misier AR, Elvan A. Arrhythmia-free survival and pulmonary vein reconnection patterns after second-generation cryoballoon and contact-force radiofrequency pulmonary vein isolation. Clin Res Cardiol. 2018;107(6):498–506. doi: 10.1007/s00392-018-1211-9 [DOI] [PubMed] [Google Scholar]
- 90.Castellá M, Kotecha D, van Laar C, et al. Thoracoscopic vs catheter ablation for atrial fibrillation: long-term follow-up of the FAST randomized trial. Europace. 2019;21(5):746–753. doi: 10.1093/europace/euy325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Shin DG, Ahn J, Han SJ, Lim HE. Efficacy of high-power and short-duration ablation in patients with atrial fibrillation: a prospective randomized controlled trial. Europace. 2020;22(10):1495–1501. doi: 10.1093/europace/euaa144 [DOI] [PubMed] [Google Scholar]
- 92.Wójcik M, Erkapic D, Berkowitsch A, et al. Ipsilateral circumferential radiofrequency ablation of atrial fibrillation with irrigated tip catheter: long-term outcome and pre-procedural predictors. Circ J. 2013;77(9):2280–2287. doi: 10.1253/circj.cj-13-0275 [DOI] [PubMed] [Google Scholar]
- 93.Weerasooriya R, Khairy P, Litalien J, et al. Catheter ablation for atrial fibrillation: are results maintained at 5 years of follow-up? J Am Coll Cardiol. 2011;57(2):160–166. doi: 10.1016/j.jacc.2010.05.061 [DOI] [PubMed] [Google Scholar]
- 94.Bertaglia E, Tondo C, De Simone A, et al. Does catheter ablation cure atrial fibrillation? Single-procedure outcome of drug-refractory atrial fibrillation ablation: a 6-year multicentre experience. Europace. 2010;12(2):181–187. doi: 10.1093/europace/eup349 [DOI] [PubMed] [Google Scholar]
- 95.Ciconte G, Baltogiannis G, de Asmundis C, et al. Circumferential pulmonary vein isolation as index procedure for persistent atrial fibrillation: a comparison between radiofrequency catheter ablation and second-generation cryoballoon ablation. Europace. 2015;17(4):559–565. doi: 10.1093/europace/euu350 [DOI] [PubMed] [Google Scholar]
- 96.Kuck KH, Merkely B, Zahn R, et al. Catheter ablation versus best medical therapy in patients with persistent atrial fibrillation and congestive heart failure: the randomized AMICA trial. Circ Arrhythm Electrophysiol. 2019;12(12):e007731. doi: 10.1161/circep.119.007731 [DOI] [PubMed] [Google Scholar]
- 97.Pavlović N, Sticherling C, Knecht S, et al. One-year follow-up after irrigated multi-electrode radiofrequency ablation of persistent atrial fibrillation. Europace. 2016;18(1):85–91. doi: 10.1093/europace/euv020 [DOI] [PubMed] [Google Scholar]
- 98.Shi LB, Rossvoll O, Tande P, Schuster P, Solheim E, Chen J. Cryoballoon vs radiofrequency catheter ablation: insights from Norwegian randomized study of PERSistent Atrial Fibrillation (NO-PERSAF study). Europace. 2022;24(2):226–233. doi: 10.1093/europace/euab281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Verma A, Jiang C-Y, Betts TR, et al. Approaches to catheter ablation for persistent atrial fibrillation. N Engl J Med. 2015;372(19):1812–1822. doi: 10.1056/NEJMoa1408288 [DOI] [PubMed] [Google Scholar]
- 100.Fink T, Schlüter M, Heeger CH, et al. Stand-alone pulmonary vein isolation versus pulmonary vein isolation with additional substrate modification as index ablation procedures in patients with persistent and long-standing persistent atrial fibrillation: the randomized Alster-Lost-AF Trial (Ablation at St. Georg Hospital for Long-Standing Persistent Atrial Fibrillation). Circ Arrhythm Electrophysiol. 2017;10(7). doi: 10.1161/circep.117.005114 [DOI] [PubMed] [Google Scholar]
- 101.Tilz RR, Rillig A, Thum A-M, et al. Catheter ablation of long-standing persistent atrial fibrillation: 5-year outcomes of the Hamburg Sequential Ablation Strategy. J Am Coll Cardiol. 2012;60(19):1921–1929. doi: 10.1016/j.jacc.2012.04.060 [DOI] [PubMed] [Google Scholar]
- 102.McCready JW, Smedley T, Lambiase PD, et al. Predictors of recurrence following radiofrequency ablation for persistent atrial fibrillation. Europace. 2011;13(3):355–361. doi: 10.1093/europace/euq434 [DOI] [PubMed] [Google Scholar]
- 103.Wazni OM, Dandamudi G, Sood N, et al. Cryoballoon ablation as initial therapy for atrial fibrillation. N Engl J Med. 2021;384(4):316–324. doi: 10.1056/NEJMoa2029554 [DOI] [PubMed] [Google Scholar]
- 104.Andrade JG, Wells GA, Deyell MW, et al. Cryoablation or drug therapy for initial treatment of atrial fibrillation. N Engl J Med. 2021;384(4):305–315. doi: 10.1056/NEJMoa2029980 [DOI] [PubMed] [Google Scholar]
- 105.Andrade JG, Champagne J, Dubuc M, et al. Cryoballoon or radiofrequency ablation for atrial fibrillation assessed by continuous monitoring: a randomized clinical trial. Circulation. 2019;140(22):1779–1788. doi: 10.1161/circulationaha.119.042622 [DOI] [PubMed] [Google Scholar]
- 106.Packer DL, Kowal RC, Wheelan KR, et al. Cryoballoon ablation of pulmonary veins for paroxysmal atrial fibrillation: first results of the North American Arctic Front (STOP AF) pivotal trial. J Am Coll Cardiol. 2013;61(16):1713–1723. doi: 10.1016/j.jacc.2012.11.064 [DOI] [PubMed] [Google Scholar]
- 107.Kuniss M, Pavlovic N, Velagic V, et al. Cryoballoon ablation vs antiarrhythmic drugs: first-line therapy for patients with paroxysmal atrial fibrillation. Europace. 2021;23(7):1033–1041. doi: 10.1093/europace/euab029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Neumann T, Wójcik M, Berkowitsch A, et al. Cryoballoon ablation of paroxysmal atrial fibrillation: 5-year outcome after single procedure and predictors of success. Europace. 2013;15(8):1143–1149. doi: 10.1093/europace/eut021 [DOI] [PubMed] [Google Scholar]
- 109.Schiavone M, Gasperetti A, Montemerlo E, et al. Long-term comparisons of atrial fibrillation ablation outcomes with a cryoballoon or laser-balloon: a propensity-matched analysis based on continuous rhythm monitoring. Hell J Cardiol. 2022;65:1–7. doi: 10.1016/j.hjc.2022.03.006 [DOI] [PubMed] [Google Scholar]
- 110.Wu S-J, Li C-H, Weng C-J, et al. Efficacy of cryoballoon ablation for atrial fibrillation and recurrence predictors in an Asian cohort. J Pers Med. 2022;12(5):732. doi: 10.3390/jpm12050732 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Ferrero-De-Loma-Osorio Á, Cózar R, García-Alberola A, et al. Primary results of the Spanish Cryoballoon Ablation Registry: acute and long-term outcomes of the RECABA study. Sci Rep. 2021;11(1):17268. doi: 10.1038/s41598-021-96655-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Chun JKR, Bordignon S, Last J, et al. Cryoballoon versus laserballoon: insights from the first prospective randomized balloon trial in catheter ablation of atrial fibrillation. Circ Arrhythm Electrophysiol. 2021;14(2):e009294. doi: 10.1161/circep.120.009294 [DOI] [PubMed] [Google Scholar]
- 113.Knecht S, Sticherling C, Roten L, et al. Efficacy and safety of a novel cryoballoon ablation system: multicentre comparison of 1-year outcome. Europace. 2022. doi: 10.1093/europace/euac094 [DOI] [PubMed] [Google Scholar]
- 114.Chen X, Xia Y, Lin Y, et al. Cryoballoon ablation for treatment of atrial fibrillation in a Chinese population: five-year outcomes and predictors of recurrence after a single procedure. Front Cardiovasc Med. 2022;9:836392. doi: 10.3389/fcvm.2022.836392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Lim HE, Oh IY, Kueffer FJ, van Bragt KA, On YK. Cryoballoon catheter ablation in Korean patients with paroxysmal and persistent atrial fibrillation: one year outcome from the Cryo Global Registry. Korean Circ J. 2022;52(10):755–767. doi: 10.4070/kcj.2022.0127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Aytemir K, Oto A, Canpolat U, et al. Immediate and medium-term outcomes of cryoballoon-based pulmonary vein isolation in patients with paroxysmal and persistent atrial fibrillation: single-centre experience. J Interv Card Electrophysiol. 2013;38(3):187–195. doi: 10.1007/s10840-013-9834-2 [DOI] [PubMed] [Google Scholar]
- 117.Yalin K, Abdin A, Lyan E, et al. Safety and efficacy of persistent atrial fibrillation ablation using the second-generation cryoballoon. Clin Res Cardiol. 2018;107(7):570–577. doi: 10.1007/s00392-018-1219-1 [DOI] [PubMed] [Google Scholar]
- 118.Lemes C, Wissner E, Lin T, et al. One-year clinical outcome after pulmonary vein isolation in persistent atrial fibrillation using the second-generation 28 mm cryoballoon: a retrospective analysis. Europace. 2016;18(2):201–205. doi: 10.1093/europace/euv092 [DOI] [PubMed] [Google Scholar]
- 119.Koektuerk B, Yorgun H, Hengeoez O, et al. Cryoballoon ablation for pulmonary vein isolation in patients with persistent atrial fibrillation: one-year outcome using second generation cryoballoon. Circ Arrhythm Electrophysiol. 2015;8(5):1073–1079. doi: 10.1161/circep.115.002776 [DOI] [PubMed] [Google Scholar]
- 120.Guhl EN, Siddoway D, Adelstein E, Voigt A, Saba S, Jain SK. Efficacy of cryoballoon pulmonary vein isolation in patients with persistent atrial fibrillation. J Cardiovasc Electrophysiol. 2016;27(4):423–427. doi: 10.1111/jce.12924 [DOI] [PubMed] [Google Scholar]
- 121.Omran H, Gutleben KJ, Molatta S, et al. Second generation cryoballoon ablation for persistent atrial fibrillation: an updated meta-analysis. Clin Res Cardiol. 2018;107(2):182–192. doi: 10.1007/s00392-017-1171-5 [DOI] [PubMed] [Google Scholar]
- 122.Nikfarjam M, Muralidharan V, Christophi C. Mechanisms of focal heat destruction of liver tumors. J Surg Res. 2005;127(2):208–223. doi: 10.1016/j.jss.2005.02.009 [DOI] [PubMed] [Google Scholar]
- 123.Yang M, Yang X, Wang S, et al. HMGB1-induced endothelial cell pyroptosis is involved in systemic inflammatory response syndrome following radiofrequency ablation of hepatic hemangiomas. Am J Transl Res. 2019;11(12):7555–7567. [PMC free article] [PubMed] [Google Scholar]
- 124.Wang S, Yang M, Yang X, et al. Endothelial pyroptosis underlies systemic inflammatory response following radiofrequency ablation of hepatic hemangiomas. Scand J Clin Lab Invest. 2019;79(8):619–628. doi: 10.1080/00365513.2019.1689428 [DOI] [PubMed] [Google Scholar]
- 125.Wang K, Jiang L, Zhong Y, et al. Ferrostatin-1-loaded liposome for treatment of corneal alkali burn via targeting ferroptosis. Bioeng Transl Med. 2022;7(2):e10276. doi: 10.1002/btm2.10276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Chu KF, Dupuy DE. Thermal ablation of tumours: biological mechanisms and advances in therapy. Nat Rev Cancer. 2014;14(3):199–208. doi: 10.1038/nrc3672 [DOI] [PubMed] [Google Scholar]
- 127.Gao D, Critser JK. Mechanisms of cryoinjury in living cells. ILAR J. 2000;41(4):187–196. doi: 10.1093/ilar.41.4.187 [DOI] [PubMed] [Google Scholar]
- 128.Hoffmann NE, Bischof JC. The cryobiology of cryosurgical injury. Urology. 2002;60(2 Suppl 1):40–49. doi: 10.1016/s0090-4295(02)01683-7 [DOI] [PubMed] [Google Scholar]
- 129.Hanai A, Yang WL, Ravikumar TS. Induction of apoptosis in human colon carcinoma cells HT29 by sublethal cryo-injury: mediation by cytochrome c release. Int J Cancer. 2001;93(4):526–533. doi: 10.1002/ijc.1359 [DOI] [PubMed] [Google Scholar]
- 130.Yang WL, Addona T, Nair DG, Qi L, Ravikumar TS. Apoptosis induced by cryo-injury in human colorectal cancer cells is associated with mitochondrial dysfunction. Int J Cancer. 2003;103(3):360–369. doi: 10.1002/ijc.10822 [DOI] [PubMed] [Google Scholar]
- 131.Davalos RV, Mir IL, Rubinsky B. Tissue ablation with irreversible electroporation. Ann Biomed Eng. 2005;33(2):223–231. doi: 10.1007/s10439-005-8981-8 [DOI] [PubMed] [Google Scholar]
- 132.Yarmush ML, Golberg A, Serša G, Kotnik T, Miklavčič D. Electroporation-based technologies for medicine: principles, applications, and challenges. Annu Rev Biomed Eng. 2014;16:295–320. doi: 10.1146/annurev-bioeng-071813-104622 [DOI] [PubMed] [Google Scholar]
- 133.Freeman SA, Wang MA, Weaver JC. Theory of electroporation of planar bilayer membranes: predictions of the aqueous area, change in capacitance, and pore-pore separation. Biophys J. 1994;67(1):42–56. doi: 10.1016/s0006-3495(94)80453-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Chen W, Zhongsheng Z, Lee RC. Supramembrane potential-induced electroconformational changes in sodium channel proteins: a potential mechanism involved in electric injury. Burns. 2006;32(1):52–59. doi: 10.1016/j.burns.2005.08.008 [DOI] [PubMed] [Google Scholar]
- 135.Frandsen SK, Gissel H, Hojman P, Tramm T, Eriksen J, Gehl J. Direct therapeutic applications of calcium electroporation to effectively induce tumor necrosis. Cancer Res. 2012;72(6):1336–1341. doi: 10.1158/0008-5472.Can-11-3782 [DOI] [PubMed] [Google Scholar]
- 136.Batista Napotnik T, Polajžer T, Miklavčič D. Cell death due to electroporation - A review. Bioelectrochemistry. 2021;141:107871. doi: 10.1016/j.bioelechem.2021.107871 [DOI] [PubMed] [Google Scholar]
- 137.Lee EW, Loh CT, Kee ST. Imaging guided percutaneous irreversible electroporation: ultrasound and immunohistological correlation. Technol Cancer Res Treat. 2007;6(4):287–294. doi: 10.1177/153303460700600404 [DOI] [PubMed] [Google Scholar]
- 138.Kim HB, Sung CK, Baik KY, et al. Changes of apoptosis in tumor tissues with time after irreversible electroporation. Biochem Biophys Res Commun. 2013;435(4):651–656. doi: 10.1016/j.bbrc.2013.05.039 [DOI] [PubMed] [Google Scholar]
- 139.Long G, Bakos G, Shires PK, et al. Histological and finite element analysis of cell death due to irreversible electroporation. Technol Cancer Res Treat. 2014;13(6):561–569. doi: 10.7785/tcrtexpress.2013.600253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.van den Bos W, Jurhill RR, de Bruin DM, et al. Histopathological outcomes after irreversible electroporation for prostate cancer: results of an ablate and resect study. J Urol. 2016;196(2):552–559. doi: 10.1016/j.juro.2016.02.2977 [DOI] [PubMed] [Google Scholar]
- 141.Jose A, Sobrevals L, Ivorra A, Fillat C. Irreversible electroporation shows efficacy against pancreatic carcinoma without systemic toxicity in mouse models. Cancer Lett. 2012;317(1):16–23. doi: 10.1016/j.canlet.2011.11.004 [DOI] [PubMed] [Google Scholar]
- 142.Zhang Y, Lyu C, Liu Y, Lv Y, Chang TT, Rubinsky B. Molecular and histological study on the effects of non-thermal irreversible electroporation on the liver. Biochem Biophys Res Commun. 2018;500(3):665–670. doi: 10.1016/j.bbrc.2018.04.132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Klein Klouwenberg PM, Frencken JF, Kuipers S, et al. Incidence, predictors, and outcomes of new-onset atrial fibrillation in critically ill patients with sepsis. A cohort study. Am J Respir Crit Care Med. 2017;195(2):205–211. doi: 10.1164/rccm.201603-0618OC [DOI] [PubMed] [Google Scholar]
- 144.Meierhenrich R, Steinhilber E, Eggermann C, et al. Incidence and prognostic impact of new-onset atrial fibrillation in patients with septic shock: a prospective observational study. Crit Care. 2010;14(3):R108. doi: 10.1186/cc9057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Bacani AK, Crowson CS, Roger VL, Gabriel SE, Matteson EL. Increased incidence of atrial fibrillation in patients with rheumatoid arthritis. Biomed Res Int. 2015;2015:809514. doi: 10.1155/2015/809514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Ahlehoff O, Gislason GH, Jørgensen CH, et al. Psoriasis and risk of atrial fibrillation and ischaemic stroke: a Danish Nationwide Cohort Study. Eur Heart J. 2012;33(16):2054–2064. doi: 10.1093/eurheartj/ehr285 [DOI] [PubMed] [Google Scholar]
- 147.Choi YJ, Choi EK, Han KD, et al. Increased risk of atrial fibrillation in patients with inflammatory bowel disease: a nationwide population-based study. World J Gastroenterol. 2019;25(22):2788–2798. doi: 10.3748/wjg.v25.i22.2788 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Zhao Q, Zhang S, Zhao H, et al. Median nerve stimulation prevents atrial electrical remodelling and inflammation in a canine model with rapid atrial pacing. Europace. 2018;20(4):712–718. doi: 10.1093/europace/eux003 [DOI] [PubMed] [Google Scholar]
- 149.Zhang Y, Sun D, Zhao X, et al. Bacteroides fragilis prevents aging-related atrial fibrillation in rats via regulatory T cells-mediated regulation of inflammation. Pharmacol Res. 2022;177:106141. doi: 10.1016/j.phrs.2022.106141 [DOI] [PubMed] [Google Scholar]
- 150.Liu Q, Zhang F, Yang M, Zhong J. Increasing level of Interleukin-1β in epicardial adipose tissue is associated with persistent atrial fibrillation. J Interferon Cytokine Res. 2020;40(1):64–69. doi: 10.1089/jir.2019.0098 [DOI] [PubMed] [Google Scholar]
- 151.Nakamura Y, Nakamura K, Fukushima-Kusano K, et al. Tissue factor expression in atrial endothelia associated with nonvalvular atrial fibrillation: possible involvement in intracardiac thrombogenesis. Thromb Res. 2003;111(3):137–142. doi: 10.1016/s0049-3848(03)00405-5 [DOI] [PubMed] [Google Scholar]
- 152.Narducci ML, Pelargonio G, Dello Russo A, et al. Role of tissue C-reactive protein in atrial cardiomyocytes of patients undergoing catheter ablation of atrial fibrillation: pathogenetic implications. Europace. 2011;13(8):1133–1140. doi: 10.1093/europace/eur068 [DOI] [PubMed] [Google Scholar]
- 153.Soeki T, Bando S, Uematsu E, et al. Pentraxin 3 is a local inflammatory marker in atrial fibrillation. Heart Vessels. 2014;29(5):653–658. doi: 10.1007/s00380-013-0400-8 [DOI] [PubMed] [Google Scholar]
- 154.Musa H, Kaur K, O’Connell R, et al. Inhibition of platelet-derived growth factor-AB signaling prevents electromechanical remodeling of adult atrial myocytes that contact myofibroblasts. Heart Rhythm. 2013;10(7):1044–1051. doi: 10.1016/j.hrthm.2013.03.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Lee SH, Chen YC, Chen YJ, et al. Tumor necrosis factor-alpha alters calcium handling and increases arrhythmogenesis of pulmonary vein cardiomyocytes. Life Sci. 2007;80(19):1806–1815. doi: 10.1016/j.lfs.2007.02.029 [DOI] [PubMed] [Google Scholar]
- 156.Saba S, Janczewski AM, Baker LC, et al. Atrial contractile dysfunction, fibrosis, and arrhythmias in a mouse model of cardiomyopathy secondary to cardiac-specific overexpression of tumor necrosis factor-{alpha}. Am J Physiol Heart Circ Physiol. 2005;289(4):H1456–H1467. doi: 10.1152/ajpheart.00733.2004 [DOI] [PubMed] [Google Scholar]
- 157.Sawaya SE, Rajawat YS, Rami TG, et al. Downregulation of connexin40 and increased prevalence of atrial arrhythmias in transgenic mice with cardiac-restricted overexpression of tumor necrosis factor. Am J Physiol Heart Circ Physiol. 2007;292(3):H1561–H1567. doi: 10.1152/ajpheart.00285.2006 [DOI] [PubMed] [Google Scholar]
- 158.Ryu K, Li L, Khrestian CM, et al. Effects of sterile pericarditis on connexins 40 and 43 in the atria: correlation with abnormal conduction and atrial arrhythmias. Am J Physiol Heart Circ Physiol. 2007;293(2):H1231–H1241. doi: 10.1152/ajpheart.00607.2006 [DOI] [PubMed] [Google Scholar]
- 159.Lazzerini PE, Laghi-Pasini F, Acampa M, et al. Systemic inflammation rapidly induces reversible atrial electrical remodeling: the role of interleukin-6-mediated changes in connexin expression. J Am Heart Assoc. 2019;8(16):e011006. doi: 10.1161/jaha.118.011006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Ishii Y, Schuessler RB, Gaynor SL, et al. Inflammation of atrium after cardiac surgery is associated with inhomogeneity of atrial conduction and atrial fibrillation. Circulation. 2005;111(22):2881–2888. doi: 10.1161/circulationaha.104.475194 [DOI] [PubMed] [Google Scholar]
- 161.Tselentakis EV, Woodford E, Chandy J, Gaudette GR, Saltman AE. Inflammation effects on the electrical properties of atrial tissue and inducibility of postoperative atrial fibrillation. J Surg Res. 2006;135(1):68–75. doi: 10.1016/j.jss.2006.03.024 [DOI] [PubMed] [Google Scholar]
- 162.Wilhelm M, Zueger T, De Marchi S, et al. Inflammation and atrial remodeling after a mountain marathon. Scand J Med Sci Sports. 2014;24(3):519–525. doi: 10.1111/sms.12030 [DOI] [PubMed] [Google Scholar]
- 163.Burstein B, Nattel S. Atrial fibrosis: mechanisms and clinical relevance in atrial fibrillation. J Am Coll Cardiol. 2008;51(8):802–809. doi: 10.1016/j.jacc.2007.09.064 [DOI] [PubMed] [Google Scholar]
- 164.Liao CH, Akazawa H, Tamagawa M, et al. Cardiac mast cells cause atrial fibrillation through PDGF-A-mediated fibrosis in pressure-overloaded mouse hearts. J Clin Invest. 2010;120(1):242–253. doi: 10.1172/jci39942 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Hoogstra-Berends F, Meijering RA, Zhang D, et al. Heat shock protein-inducing compounds as therapeutics to restore proteostasis in atrial fibrillation. Trends Cardiovasc Med. 2012;22(3):62–68. doi: 10.1016/j.tcm.2012.06.013 [DOI] [PubMed] [Google Scholar]
- 166.Liew R, Khairunnisa K, Gu Y, et al. Role of tumor necrosis factor-α in the pathogenesis of atrial fibrosis and development of an arrhythmogenic substrate. Circ J. 2013;77(5):1171–1179. doi: 10.1253/circj.cj-12-1155 [DOI] [PubMed] [Google Scholar]
- 167.Li YY, Feng YQ, Kadokami T, et al. Myocardial extracellular matrix remodeling in transgenic mice overexpressing tumor necrosis factor alpha can be modulated by anti-tumor necrosis factor alpha therapy. Proc Natl Acad Sci U S A. 2000;97(23):12746–12751. doi: 10.1073/pnas.97.23.12746 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Ma F, Li Y, Jia L, et al. Macrophage-stimulated cardiac fibroblast production of IL-6 is essential for TGF β/Smad activation and cardiac fibrosis induced by angiotensin II. PLoS One. 2012;7(5):e35144. doi: 10.1371/journal.pone.0035144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Hu YF, Yeh HI, Tsao HM, et al. Electrophysiological correlation and prognostic impact of heat shock protein 27 in atrial fibrillation. Circ Arrhythm Electrophysiol. 2012;5(2):334–340. doi: 10.1161/circep.111.965996 [DOI] [PubMed] [Google Scholar]
- 170.Psychari SN, Apostolou TS, Sinos L, Hamodraka E, Liakos G, Kremastinos DT. Relation of elevated C-reactive protein and interleukin-6 levels to left atrial size and duration of episodes in patients with atrial fibrillation. Am J Cardiol. 2005;95(6):764–767. doi: 10.1016/j.amjcard.2004.11.032 [DOI] [PubMed] [Google Scholar]
- 171.Luan Y, Guo Y, Li S, et al. Interleukin-18 among atrial fibrillation patients in the absence of structural heart disease. Europace. 2010;12(12):1713–1718. doi: 10.1093/europace/euq321 [DOI] [PubMed] [Google Scholar]
- 172.Sabel MS. Cryo-immunology: a review of the literature and proposed mechanisms for stimulatory versus suppressive immune responses. Cryobiology. 2009;58(1):1–11. doi: 10.1016/j.cryobiol.2008.10.126 [DOI] [PubMed] [Google Scholar]
- 173.Ahmad F, Gravante G, Bhardwaj N, et al. Changes in interleukin-1β and 6 after hepatic microwave tissue ablation compared with radiofrequency, cryotherapy and surgical resections. Am J Surg. 2010;200(4):500–506. doi: 10.1016/j.amjsurg.2009.12.025 [DOI] [PubMed] [Google Scholar]
- 174.Erinjeri JP, Thomas CT, Samoilia A, et al. Image-guided thermal ablation of tumors increases the plasma level of interleukin-6 and interleukin-10. J Vasc Interv Radiol. 2013;24(8):1105–1112. doi: 10.1016/j.jvir.2013.02.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Fietta AM, Morosini M, Passadore I, et al. Systemic inflammatory response and downmodulation of peripheral CD25+Foxp3+ T-regulatory cells in patients undergoing radiofrequency thermal ablation for lung cancer. Hum Immunol. 2009;70(7):477–486. doi: 10.1016/j.humimm.2009.03.012 [DOI] [PubMed] [Google Scholar]
- 176.Zerbini A, Pilli M, Laccabue D, et al. Radiofrequency thermal ablation for hepatocellular carcinoma stimulates autologous NK-cell response. Gastroenterology. 2010;138(5):1931–1942. doi: 10.1053/j.gastro.2009.12.051 [DOI] [PubMed] [Google Scholar]
- 177.Wissniowski TT, Hänsler J, Neureiter D, et al. Activation of tumor-specific T lymphocytes by radio-frequency ablation of the VX2 hepatoma in rabbits. Cancer Res. 2003;63(19):6496–6500. [PubMed] [Google Scholar]
- 178.Dromi SA, Walsh MP, Herby S, et al. Radiofrequency ablation induces antigen-presenting cell infiltration and amplification of weak tumor-induced immunity. Radiology. 2009;251(1):58–66. doi: 10.1148/radiol.2511072175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Oswald H, Gardiwal A, Lissel C, Yu H, Klein G. Difference in humoral biomarkers for myocardial injury and inflammation in radiofrequency ablation versus cryoablation. Pacing Clin Electrophysiol. 2007;30(7):885–890. doi: 10.1111/j.1540-8159.2007.00776.x [DOI] [PubMed] [Google Scholar]
- 180.Herrera Siklódy C, Arentz T, Minners J, et al. Cellular damage, platelet activation, and inflammatory response after pulmonary vein isolation: a randomized study comparing radiofrequency ablation with cryoablation. Heart Rhythm. 2012;9(2):189–196. doi: 10.1016/j.hrthm.2011.09.017 [DOI] [PubMed] [Google Scholar]
- 181.Antolič B, Pernat A, Cvijić M, Žižek D, Jan M, Šinkovec M. Radiofrequency catheter ablation versus balloon cryoablation of atrial fibrillation: markers of myocardial damage, inflammation, and thrombogenesis. Wien Klin Wochenschr. 2016;128(13–14):480–487. doi: 10.1007/s00508-016-1002-0 [DOI] [PubMed] [Google Scholar]
- 182.Miyazaki S, Kuroi A, Hachiya H, et al. Early recurrence after pulmonary vein isolation of paroxysmal atrial fibrillation with different ablation technologies - prospective comparison of radiofrequency vs second-generation cryoballoon ablation. Circ J. 2016;80(2):346–353. doi: 10.1253/circj.CJ-15-1051 [DOI] [PubMed] [Google Scholar]
- 183.Grimaldi M, Di monaco A, Gomez T, et al. Time course of irreversible electroporation lesion development through short- and long-term follow-up in pulsed-field ablation-treated hearts. Circ Arrhythm Electrophysiol. 2022;15(7):e010661. doi: 10.1161/circep.121.010661 [DOI] [PubMed] [Google Scholar]
- 184.Koruth JS, Kuroki K, Kawamura I, et al. Focal pulsed field ablation for pulmonary vein isolation and linear atrial lesions: a preclinical assessment of safety and durability. Circ Arrhythm Electrophysiol. 2020;13(6):e008716. doi: 10.1161/circep.120.008716 [DOI] [PubMed] [Google Scholar]
- 185.Sugimoto K, Kakimi K, Takeuchi H, et al. Irreversible electroporation versus radiofrequency ablation: comparison of systemic immune responses in patients with hepatocellular carcinoma. JVIR. 2019;30(6):845–853.e846. doi: 10.1016/j.jvir.2019.03.002 [DOI] [PubMed] [Google Scholar]
- 186.Xie B, Chen BX, Nanna M, et al. 18F-fluorodeoxyglucose positron emission tomography/computed tomography imaging in atrial fibrillation: a pilot prospective study. Eur Heart J Cardiovasc Imaging. 2021;23(1):102–112. doi: 10.1093/ehjci/jeab088 [DOI] [PubMed] [Google Scholar]
- 187.Chang TY, Hsiao YW, Guo SM, et al. Resistin as a biomarker for the prediction of left atrial substrate and recurrence in patients with drug-refractory atrial fibrillation undergoing catheter ablation. Int Heart J. 2020;61(3):517–523. doi: 10.1536/ihj.19-680 [DOI] [PubMed] [Google Scholar]
- 188.Kusayama T, Furusho H, Kashiwagi H, et al. Inflammation of left atrial epicardial adipose tissue is associated with paroxysmal atrial fibrillation. J Cardiol. 2016;68(5):406–411. doi: 10.1016/j.jjcc.2015.11.005 [DOI] [PubMed] [Google Scholar]
- 189.Yorgun H, Canpolat U, Aytemir K, et al. Association of epicardial and peri-atrial adiposity with the presence and severity of non-valvular atrial fibrillation. Int J Cardiovasc Imaging. 2015;31(3):649–657. doi: 10.1007/s10554-014-0579-5 [DOI] [PubMed] [Google Scholar]
- 190.Hayıroğlu Mİ, Altay SAO. The role of artificial intelligence in coronary artery disease and atrial fibrillation. Balkan Med J. 2023;40(3):151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Andrade JG, Khairy P, Macle L, et al. Incidence and significance of early recurrences of atrial fibrillation after cryoballoon ablation. Circulation. 2014;7(1):69–75. doi: 10.1161/circep.113.000586 [DOI] [PubMed] [Google Scholar]
- 192.Hernández-Romero D, Marín F, Roldán V, et al. Comparative determination and monitoring of biomarkers of necrosis and myocardial remodeling between radiofrequency ablation and cryoablation. Pacing Clin Electrophysiol. 2013;36(1):31–36. doi: 10.1111/pace.12017 [DOI] [PubMed] [Google Scholar]
- 193.Del Rey JM, Madrid AH, Novo L, et al. Evaluación de marcadores de lesión miocárdica tras la ablación con radiofrecuencia. Utilidad de la troponina I [Evaluation of biochemical markers of myocardial lesion after radiofrequency ablation. Value of troponin I]. Rev Esp Cardiol. 1997;50(8):552–560. Spanish. doi: 10.1016/s0300-8932(97)73263-9 [DOI] [PubMed] [Google Scholar]
- 194.Bai Y, Guo SD, Liu Y, Ma CS, Lip GYH. Relationship of troponin to incident atrial fibrillation occurrence, recurrence after radiofrequency ablation and prognosis: a systematic review, meta-analysis and meta-regression. Biomarkers. 2018;23(6):512–517. doi: 10.1080/1354750x.2018.1463562 [DOI] [PubMed] [Google Scholar]
- 195.Canpolat U, Aytemir K, Yorgun H, et al. Role of preablation neutrophil/lymphocyte ratio on outcomes of cryoballoon-based atrial fibrillation ablation. Am J Cardiol. 2013;112(4):513–519. doi: 10.1016/j.amjcard.2013.04.015 [DOI] [PubMed] [Google Scholar]
- 196.Guo X, Zhang S, Yan X, et al. Postablation neutrophil/lymphocyte ratio correlates with arrhythmia recurrence after catheter ablation of lone atrial fibrillation. Chin Med J. 2014;127(6):1033–1038. [PubMed] [Google Scholar]
- 197.Ruan ZB, Gao RF, Wang F, et al. Circulating galectin-3 and aldosterone for predicting atrial fibrillation recurrence after radiofrequency catheter ablation. Cardiovasc Ther. 2022;2022:6993904. doi: 10.1155/2022/6993904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Clementy N, Benhenda N, Piver E, et al. Serum galectin-3 levels predict recurrences after ablation of atrial fibrillation. Sci Rep. 2016;6:34357. doi: 10.1038/srep34357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Berger WR, Jagu B, van den Berg NWE, et al. The change in circulating galectin-3 predicts absence of atrial fibrillation after thoracoscopic surgical ablation. Europace. 2018;20(5):764–771. doi: 10.1093/europace/eux090 [DOI] [PubMed] [Google Scholar]
- 200.Takemoto Y, Ramirez RJ, Yokokawa M, et al. Galectin-3 regulates atrial fibrillation remodeling and predicts catheter ablation outcomes. Basic Transl Sci. 2016;1(3):143–154. doi: 10.1016/j.jacbts.2016.03.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Karataş MAO, Durmuş G, Zengin A, et al. Association of uric acid albumin ratio with recurrence of atrial fibrillation after cryoballoon catheter ablation. Medicina. 2022;58(12):1872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Li SB, Yang F, Jing L, et al. Myeloperoxidase and risk of recurrence of atrial fibrillation after catheter ablation. J Investig Med. 2013;61(4):722–727. doi: 10.2310/JIM.0b013e3182857fa0 [DOI] [PubMed] [Google Scholar]
- 203.Richter B, Gwechenberger M, Socas A, et al. Markers of oxidative stress after ablation of atrial fibrillation are associated with inflammation, delivered radiofrequency energy and early recurrence of atrial fibrillation. Clin Res Cardiol. 2012;101(3):217–225. doi: 10.1007/s00392-011-0383-3 [DOI] [PubMed] [Google Scholar]
- 204.Canpolat U, Aytemir K, Yorgun H, et al. The role of preprocedural monocyte-to-high-density lipoprotein ratio in prediction of atrial fibrillation recurrence after cryoballoon-based catheter ablation. Europace. 2015;17(12):1807–1815. doi: 10.1093/europace/euu291 [DOI] [PubMed] [Google Scholar]
- 205.Gurses KM, Kocyigit D, Yalcin MU, et al. Monocyte toll-like receptor expression in patients with atrial fibrillation. Am J Cardiol. 2016;117(9):1463–1467. doi: 10.1016/j.amjcard.2016.02.014 [DOI] [PubMed] [Google Scholar]
- 206.Kimura T, Takatsuki S, Inagawa K, et al. Serum inflammation markers predicting successful initial catheter ablation for atrial fibrillation. Heart Lung Circ. 2014;23(7):636–643. doi: 10.1016/j.hlc.2014.02.003 [DOI] [PubMed] [Google Scholar]
- 207.Meyre PB, Sticherling C, Spies F, et al. C-reactive protein for prediction of atrial fibrillation recurrence after catheter ablation. BMC Cardiovasc Disord. 2020;20(1):427. doi: 10.1186/s12872-020-01711-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Ihara K, Sasano T. Role of inflammation in the pathogenesis of atrial fibrillation. Front Physiol. 2022;13:862164. doi: 10.3389/fphys.2022.862164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Farkowski MM, Maciag A, Kowalik I, Konka M, Szwed H, Pytkowski M. Intravenous antazoline, a first-generation antihistaminic drug with antiarrhythmic properties, is a suitable agent for pharmacological cardioversion of atrial fibrillation induced during pulmonary vein isolation due to the lack of influence on atrio-venous conduction and high clinical effectiveness (AntaEP Study). Br J Clin Pharmacol. 2019;85(7):1552–1558. doi: 10.1111/bcp.13940 [DOI] [PMC free article] [PubMed] [Google Scholar]