Abstract
Introduction
Extensive studies have studied the factors associated with subfoveal choroidal thickness (SFCT). However, studies of the association between lipid profile and SFCT in patients with proliferative diabetic retinopathy (PDR) in type 2 diabetes remain limited. Thus, we aimed to investigate the relationship between lipid profile and SFCT in patients with PDR.
Materials and Methods
This was a retrospective cross-sectional study. The included participants were inpatients who underwent vitrectomy for PDR with type 2 diabetes and contralateral eyes of PDR patients meeting the criteria. Multivariate linear regression analysis was used to determine the independent association between lipid profile and SFCT.
Results
A total of 131 participants with PDR were enrolled in the final analysis. The average age of the participants was 55.76 ± 9.88 years, and the average SFCT was 276.10 ± 92.92 μm. Multivariate linear regression model results showed that in the fully adjusted model, total cholesterol, high-density lipoprotein cholesterol (HDL-C), and low-density lipoprotein cholesterol (LDL-C) had a negative association with SFCT (β = −16.51, 95% CI: −29.57, −3.46; P = 0.0148; β = −42.65, 95% CI: −82.60, −2.70; P = 0.0390; β = −17.89, 95% CI: −33.24, −2.54; P = 0.0245, respectively), while triglyceride was not significantly associated with SFCT (β = 5.23, 95% CI: −18.57, 29.02; P = 0.6678). Furthermore, the results of stratified analysis showed that except for triglyceride, the trends of total cholesterol, HDL-C, LDL-C, and SFCT were consistent among different stratifications in participants.
Conclusion
The cholesterol profile had a significant negative association with SFCT in Chinese PDR patients, but triglyceride was not significantly associated with SFCT. This suggests that these systemic imbalances contribute to choroidal changes, and often coexist in diabetic patients.
Keywords: cholesterol, subfoveal choroidal thickness, proliferative diabetic retinopathy, low-density lipoprotein cholesterol, high-density lipoprotein cholesterol
Introduction
The choroid plays a crucial role in the visual process, accounting for more than 85% of the blood supply of the ophthalmic artery and the nutrient supply for both the retinal pigment epithelium (RPE) and photoreceptors.1 A considerable decrease in choroidal blood flow may lead to the destruction of photoreceptors and affect visual function.2 Recent advances in the development of spectral-domain optical coherence tomography (SD-OCT) and enhanced depth imaging (EDI)-OCT have facilitated the quantitative measurement of choroidal thickness.3
Diabetic retinopathy (DR) is the main cause of visual impairment and blindness in working age population.4–6 Modifiable (ie, hyperglycaemia, hypertension, hyperlipidaemia and obesity) and non-modifiable factors (ie, duration of diabetes and genetic susceptibility) are involved in the development of DR.7–9 In addition to the factors mentioned above, previous studies10–17 have proved that renal function impairment, especially low estimated glomerular filtration rate (eGFR), is involved in the development of DR. In addition to retinal changes, choroid abnormalities are also common in diabetes patients.
Extensive studies have shown that subfoveal choroidal thickness (SFCT) decreases substantially with age18–20 and ocular axial length.18,21,22 Moreover, SFCT is associated with various systemic diseases including hypertension,23 coronary artery disease,24 diabetes,25–27 carotid stenosis,28,29 heart failure,30 and hyperlipidemia.31 As such, SFCT is a useful biomarker for predicting cardiovascular risk.
More recently, studies have focussed on the relationship between cholesterol and SFCT.32–34 Wong et al found an association between hypercholesterolemia and increased SFCT in the healthy population.32 The authors speculate that lipid accumulation on the choroid and hypertrophy of vascular smooth muscle cells are responsible for this phenomenon. Aydin et al34 found that low-density lipoprotein cholesterol (LDL-C) levels were inversely correlated with SFCT in both eyes of patients with cardiovascular risk factors. However, they failed to find the correlation between cholesterol levels and SFCT. Abnormal cholesterol metabolism is closely associated with various ocular diseases, such as age-related macular degeneration (AMD),35 hypertensive retinopathy,36 DR,37–39 and diabetic macular edema (DME).40 Choroid is an important vascular tissue in the eye, which is also affected by diabetes and can cause visual impairment in diabetes patients.41 Yazgan et al found that macular choroidal thickness may be the earliest determiner to detect the onset of DR in patients with prediabetes.42 What’s more, a recent study has shown a positive correlation between normal cholesterol levels including LDL-C and high-density lipoprotein cholesterol (HDL-C), and SFCT in adolescent children with type 1 diabetes.33 This suggests lipid metabolism may play a role in SFCT in patients with DR.However, the relationship between lipid profile and SFCT in patients with proliferative diabetic retinopathy (PDR) secondary to type 2 diabetes remains underexplored. Here we conducted a cross-sectional study to explore the association between lipid profile and SFCT in PDR patients.
Materials and Methods
Study Population
This was a retrospective cross-sectional study. This study was performed in line with the principles of the Declaration of Helsinki and ethical approval was obtained from the West China Hospital of Sichuan University (2020–834). Data used were anonymized and the requirement for informed consent was therefore waived. The study participants were inpatients with type 2 diabetes who had definite indications for vitrectomy, no absolute contraindications, and underwent vitrectomy for PDR at the Ophthalmology Department of West China Hospital of Sichuan University from May 2020 to February 2022. And the contralateral eye in these PDR patients meeting the following criteria were included as subjects in the study. Firstly, the contralateral eyes of PDR patients were graded as PDR with clear refractive media, and no history of vitrectomy. Secondly, included patients had a fasting blood glucose lower than 8 mmol/L, blood glucose < 11 mmol/L 2 hours after 3 meals, and consistent blood glucose levels for at least 7 days. Finally, included participants had no missing serum lipid variables. Patients meeting any of the following criteria were excluded from the study. Firstly, refractive error exceeding ± 6.00 diopters, or an ocular axial length of more than 26.50 mm. Secondly, acquired immune deficiency syndrome, syphilis, or leukemia. Thirdly, type 1 diabetes. Iris neovascularization or neovascular glaucoma, uveitis, retinal vein occlusion, retinal artery occlusion, paracentral acute middle maculopathy, AMD, ocular trauma, endophthalmitis, and other eye conditions. Finally, poor quality EDI-OCT images with no discernable chorioscleral boundaries.
Laboratory Variables
All relevant laboratory tests were completed on admission. Fasting laboratory values included serum lipid profile, fasting blood glucose, preoperative glycosylated hemoglobin A1c (HbA1c), serum creatinine, and eGFR. Some studies have defined chronic kidney disease as an eGFR of < 60 mL/min/1.73m2 and/or urine albumin-creatinine ratio ≥ 30 mg/g.43 Therefore, eGFR was used as a stratification factor of renal function, which was divided into two groups (eGFR≥60 (mL/min×1.73 m2) group and eGFR<60 (mL/min×1.73 m2) group). In our study, the serum fasting lipid profile included triglyceride, total cholesterol, LDL-C, and HDL-C. According to the Chinese Adult Dyslipidemia Prevention Guide (2007 edition), hypercholesterolemia was defined as fasting serum total cholesterol concentrations greater than 6.22 mmol/L (240 mg/dL).44
EDI-OCT Imaging
All participants underwent comprehensive ocular examinations, including visual acuity, intraocular pressure (IOP), ocular axial length, slit-lamp examination, and EDI-OCT. The EDI-OCT examination was conducted after pupil dilation with compound tropicamide eye drops (Mydrin-P; Santen, Osaka, Japan). The standardized EDI-OCT scans were obtained using SD-OCT (Heidelberg Engineering; Heidelberg, Germany) and carried out by experienced technicians in the afternoon, avoiding diurnal variations. The EDI-OCT measurements of central macular thickness (CMT) and SFCT centered on the fovea were taken by horizontal/vertical scans. CMT was measured automatically, while SFCT was measured manually using digital calipers provided by the Heidelberg Eye Explorer software. CMT was defined as the perpendicular distance in the macula from the inner limiting membrane to the RPE. SFCT was defined as the distance in the macula from the outer border of the hyper-reflective line corresponding to the RPE perpendicular to the chorioscleral interface. EDI-OCT images of poor quality where it was difficult to distinguish chorioscleral boundaries were excluded. Two experienced physicians who were blinded to the patients clinical data performed measurements independently, and the average of all measurements was used for the final statistical analysis.
Other Variables
Relevant baseline characteristics important for the management of hospitalized patients with PDR were retrieved from the electronic medical record system. Demographic information included age, sex and education level. Education level was grouped into junior high school or below, senior high school, and college or above. Hypertension history was defined as a physician diagnosis or the use of antihypertensive medication. Diabetes mellitus was defined as physician diagnosis or the use of insulin/ oral glucose-lowering drugs. Relevant medical history included diabetes duration, hypertension history, chronic kidney disease history, stroke history, and heart disease history. The degree of panretinal photocoagulation (PRP) in the contralateral eyes of PDR patients and the history of anti-vascular endothelial growth factor (VEGF) therapy were collected. The degree of PRP was grouped into 3 categories, none, partial, and whole, according to the scope of the laser. The above data were all obtained from the self-reported medical conditions by participants. Systemic medication history including oral glucose-lowering drugs, insulin treatment, and oral antihypertensive drugs (eg calcium antagonist) was extracted. Physical characteristics at initial presentation including height, weight, and systolic and diastolic blood pressure (DBP) were extracted. The body mass index (BMI) was calculated as the weight (kilogram) divided by the height (meter) squared.
Statistical Analysis
Demographic characteristics and study outcomes were summarized using descriptive statistics. Continuous variables were summarized with means ± standard deviation, and the categorical variables with frequencies and percentages. The variation was described and evaluated using the χ²-test or Kruskal–Wallis test. Multivariate linear regression analysis was used to detect the independent association between lipid profile and SFCT, and smooth curve fitting was used to present the tendency. Candidate confounders were selected based on their associations with the outcomes of interest, or a change in effect estimate of more than 10%.45,46 Stratified analysis, using the multivariate linear regression model with smooth curve fitting, showed a nonlinear (after adjusting for all covariates) correlation between age and SFCT in patients with PDR. The inflection point was the age of 53 years (data not shown) using the two-piecewise multivariate linear regression model. Statistical analyses were performed using Empower Stats (http://www.empowerstats.com; X&Y Solutions Inc., Boston, MA) and R software, version 3.4.3 (http://www.R-project.org/, The R Foundation). A two-sided P < 0.05 was considered statistically significant.
Results
Baseline Characteristics of Participants
A total of 131 participants with PDR were enrolled in the final analysis. The average age of the participants was 55.76 ± 9.88 years old, and 63.36% of them were men. The average SFCT was 276.10 ± 92.92 μm and the average ocular axial length was 23.22 ± 1.02 mm. The participants were divided into 2 groups according to total cholesterol level.44 Except for triglyceride, total cholesterol, HDL-C, LDL-C, hypertension, and application of calcium antagonists, there was no statistically significant difference in other variables between the two groups. Baseline characteristics are listed in Table 1.
Table 1.
Baseline Characteristics of Participants with Proliferative Diabetic Retinopathy
| Mean±SD/n (%) | Total Cholesterol (mmol/L) | P-value | ||
|---|---|---|---|---|
| <6.22 | ≥6.22 | |||
| N | 131 | 119 | 12 | |
| Age (year) | 55.76 ± 9.88 | 55.55 ± 9.81 | 57.83 ± 10.79 | 0.449 |
| Male sex | 83 (63.36%) | 77 (64.71%) | 6 (50.00%) | 0.314 |
| SBP (mmHg) | 139.60 ± 20.31 | 138.88 ± 20.26 | 145.92 ± 20.53 | 0.257 |
| DBP (mmHg) | 85.08 ± 10.82 | 85.34 ± 11.05 | 82.75 ± 8.56 | 0.434 |
| BMI (kg/m2) | 24.24 ± 3.00 | 24.25 ± 3.03 | 24.19 ± 2.81 | 0.953 |
| Duration of diabetes (year) | 12.57 ± 6.99 | 12.47 ± 6.93 | 13.50 ± 7.75 | 0.629 |
| Visual acuity (logMAR) | 0.75 ± 0.54 | 0.72 ± 0.54 | 1.03 ± 0.45 | 0.056 |
| IOP (mmHg) | 16.27 ± 4.08 | 16.29 ± 4.14 | 16.04 ± 3.57 | 0.847 |
| Ocular axial length (mm) | 23.22 ± 1.02 | 23.25 ± 1.02 | 22.87 ± 0.97 | 0.347 |
| CMT (μm) | 289.83 ± 128.46 | 288.38 ± 128.51 | 304.19 ± 132.73 | 0.686 |
| SFCT (μm) | 276.10 ± 92.92 | 278.67 ± 86.68 | 250.57 ± 143.76 | 0.320 |
| Education level | 0.665 | |||
| Junior high school or below | 75 (57.25%) | 69 (57.98%) | 6 (50.00%) | |
| Senior high school | 26 (19.85%) | 24 (20.17%) | 2 (16.67%) | |
| College or above | 30 (22.90%) | 26 (21.85%) | 4 (33.33%) | |
| Systemic diseases | ||||
| Hypertension | 67 (51.15%) | 57 (47.90%) | 10 (83.33%) | 0.019 |
| Chronic kidney disease history | 35 (26.72%) | 31 (26.05%) | 4 (33.33%) | 0.587 |
| Stroke history | 8 (6.11%) | 8 (6.72%) | 0 (0.00%) | 0.354 |
| Heart disease history | 6 (4.58%) | 6 (5.04%) | 0 (0.00%) | 0.426 |
| Systemic medication history | ||||
| Insulin treatment | 80 (61.07%) | 71 (59.66%) | 9 (75.00%) | 0.299 |
| Oral glucose-lowering drugs | 98 (74.81%) | 90 (75.63%) | 8 (66.67%) | 0.495 |
| Calcium antagonists | 41 (31.30%) | 34 (28.57%) | 7 (58.33%) | 0.034 |
| Ophthalmological treatment history | ||||
| Anti-VEGF therapy | 40 (30.53%) | 37 (31.09%) | 3 (25.00%) | 0.662 |
| PRP | 0.333 | |||
| None | 57 (43.51%) | 54 (45.38%) | 3 (25.00%) | |
| Partial | 27 (20.61%) | 23 (19.33%) | 4 (33.33%) | |
| Whole | 47 (35.88%) | 42 (35.29%) | 5 (41.67%) | |
| Laboratory data | ||||
| Fasting blood glucose (mmol/L) | 7.22 ± 2.69 | 7.15 ± 2.76 | 7.93 ± 1.78 | 0.341 |
| HbA1c (%) | 7.66 ± 1.71 | 7.75 ± 1.77 | 6.86 ± 0.66 | 0.140 |
| Total cholesterol (mmol/L) | 4.60 ± 1.19 | 4.34 ± 0.89 | 7.14 ± 0.79 | <0.001 |
| HDL-C (mmol/L) | 1.31 ± 0.41 | 1.28 ± 0.34 | 1.60 ± 0.78 | 0.009 |
| LDL-C (mmol/L) | 2.68 ± 1.02 | 2.47 ± 0.76 | 4.77 ± 0.98 | <0.001 |
| Triglyceride (mmol/L) | 1.50 ± 0.75 | 1.44 ± 0.70 | 2.08 ± 1.02 | 0.004 |
| Serum creatinine (µmol/L) | 152.02 ± 189.46 | 147.48 ± 182.98 | 197.00 ± 250.25 | 0.390 |
| eGFR (mL/min×1.73m2) | 65.79 ± 30.04 | 67.38 ± 30.16 | 50.07 ± 24.71 | 0.057 |
Abbreviations: SBP, systolic blood pressure; DBP, diastolic blood pressure; BMI, body mass index; HbA1c, hemoglobin A1c; logMAR, logarithmic minimum resolution angle; IOP, intraocular pressure; HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol; eGFR, estimated glomerular filtration rate; CMT, central macular thickness; SFCT, subfoveal choroidal thickness; VEGF, vascular endothelial growth factor; PRP, panretinal photocoagulation.
Univariate Analysis
The results of univariate analysis revealed a significant negative association between SFCT and both total cholesterol and HDL-C in participants (β = −16.08, 95% CI: −29.25, −2.91; P = 0.0181; β = −42.66, 95% CI: −81.44, −3.89; P = 0.0329, respectively), while LDL and triglyceride were not significantly associated with SFCT (β = −15.27, 95% CI: −30.72, 0.17; P = 0.0548; β = −3.01, 95% CI: −24.36, 18.34; P = 0.7828, respectively). Compared to women, men were more likely to have higher SFCT (β = 38.91, 95% CI: 6.44, 71.38; P = 0.0203). There was a statistically significant difference in SFCT by age, DBP, chronic kidney disease history, oral glucose-lowering drugs, and calcium antagonists. We found that other factors were not statistically associated with SFCT. The results of univariate analysis are shown in Table 2.
Table 2.
Univariate Linear Regression Analysis of SFCT in Participants with Proliferative Diabetic Retinopathy
| β, 95% CI | P-value | |
|---|---|---|
| Age, per 1 year increase | −2.68 (−4.24, −1.13) | 0.0009 |
| Male vs female | 38.91 (6.44, 71.38) | 0.0203 |
| SBP, per 1 mmHg increase | 0.57 (−0.26, 1.41) | 0.1815 |
| DBP, per 1 mmHg increase | 1.85 (0.30, 3.40) | 0.0213 |
| BMI, per 1 kg/m2 increase | 2.72 (−2.90, 8.34) | 0.3451 |
| Duration of diabetes, per 1 year increase | −0.51 (−2.84, 1.82) | 0.6663 |
| Visual acuity, per 1 logMAR increase | 1.40 (−28.46, 31.27) | 0.9268 |
| IOP, per 1 mmHg increase | 1.04 (−2.91, 4.99) | 0.6073 |
| Ocular axial length, per 1 mm increase | −0.47 (−1.89, 0.94) | 0.5134 |
| CMT, per 1 μm increase | 0.02 (−0.10, 0.14) | 0.7524 |
| Education level | ||
| Junior high school or below | Reference | |
| Senior high school | −9.96 (−51.66, 31.74) | 0.6403 |
| College or above | 6.78 (−32.80, 46.36) | 0.7376 |
| Systemic diseases | ||
| Hypertension vs absent | −3.80 (−35.75, 28.15) | 0.8161 |
| Chronic kidney disease history vs absent | 38.58 (3.10, 74.07) | 0.0350 |
| Stroke history vs absent | −1.30 (−68.01, 65.41) | 0.9695 |
| Heart disease vs absent | 5.49 (−70.92, 81.89) | 0.8883 |
| Systemic medication history | ||
| Insulin vs absent | 22.16 (−10.38, 54.70) | 0.1842 |
| Oral glucose-lowering drugs vs absent | −43.36 (−79.39, −7.33) | 0.0198 |
| Calcium antagonists vs absent | 37.18 (3.33, 71.03) | 0.0332 |
| Ophthalmological treatment history | ||
| Anti-VEGF therapy vs absent | 32.04 (−2.20, 66.28) | 0.0690 |
| PRP | ||
| None | Reference | |
| Partial | 5.83 (−36.43, 48.09) | 0.7874 |
| Whole | 34.28 (−1.36, 69.92) | 0.0617 |
| Laboratory data | ||
| Fasting blood glucose, per 1 mmol/L increase | −2.10 (−8.04, 3.85) | 0.4902 |
| HbA1c, per 1% increase | 0.50 (−3.64, 4.64) | 0.8144 |
| Total cholesterol, per 1 mmol/L increase | −16.08 (−29.25, −2.91) | 0.0181 |
| HDL-C, per 1 mmol/L increase | −42.66 (−81.44, −3.89) | 0.0329 |
| LDL-C, per 1 mmol/L increase | −15.27 (−30.72, 0.17) | 0.0548 |
| Triglyceride, per 1 mmol/L increase | −3.01 (−24.36, 18.34) | 0.7828 |
| Serum creatinine, per 1 µmol/L increase | 0.08 (−0.00, 0.16) | 0.0648 |
| eGFR, per 1 mL/min×1.73m2 increase | −0.12 (−0.66, 0.41) | 0.6470 |
Abbreviations: CI, confidence interval; SBP, systolic blood pressure; DBP, diastolic blood pressure; BMI, body mass index; HbA1c, hemoglobin A1c; logMAR, logarithmic minimum resolution angle; IOP, intraocular pressure; HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol; eGFR, estimated glomerular filtration rate; CMT, central macular thickness; SFCT, subfoveal choroidal thickness; VEGF, vascular endothelial growth factor; PRP, panretinal photocoagulation.
The Relationship Between Lipid Profile and SFCT in Patients with PDR
We used a multivariate linear regression model to evaluate the associations between SFCT and total cholesterol, HDL-C, LDL-C and triglyceride (Table 3). Three adjusted models are shown in Table 3. In adjusted model I (adjusted age, sex), SFCT was not significantly associated with total cholesterol, HDL-C, LDL-C and triglyceride (β = −13.48, 95% CI: −26.87, −0.09; P = 0.0506; β = −33.94, 95% CI: −73.31, 5.43; P = 0.0936; β = −12.90, 95% CI: −28.60, 2.80; P = 0.1098; β = −1.71, 95% CI: −22.95, 19.52; P = 0.8747, respectively). In adjusted model II (adjusted age, sex, ocular axial length), the results revealed a significantly negative association between SFCT and both total cholesterol and HDL-C (β = −14.71, 95% CI: −27.42, −2.00; P = 0.0251; β = −42.38, 95% CI: −80.27, −5.40; P = 0.0267), while there was no relationship between SFCT and LDL-C or triglyceride (β = −14.98, 95% CI: −29.91, −0.06; P =0.0513; β = 3.60, 95% CI: −17.10, 24.29; P = 0.7340, respectively). Furthermore, in adjusted model III (adjusted age, gender, ocular axial length, PRP, duration of diabetes, serum creatinine, DBP, HbA1c, anti-VEGF therapy, oral glucose-lowering drugs, and oral calcium antagonists), SFCT had a significantly negative association with total cholesterol, HDL-C, and LDL-C (β = −16.51, 95% CI: −29.57, −3.46; P = 0.0148; β = −42.65, 95% CI: −82.60, −2.70; P = 0.0390; β = −17.89, 95% CI: −33.24, −2.54; P = 0.0245, respectively), while the result of triglyceride was consistent (β = 5.23, 95% CI: −18.57, 29.02; P = 0.6678). We divided participants by total cholesterol into 2 groups according to the Chinese Adult Dyslipidemia Prevention Guide (2007 edition).44 Compared to the group with total cholesterol < 6.22 mmol/L, the hypercholesterolemia group had lower SFCT in these different adjusted models. However, these trends did not show statistical differences.
Table 3.
Relationship Between Lipid Profile and SFCT in Participants with Proliferative Diabetic Retinopathy
| Exposure | Model I | Model II | Model III | |||
|---|---|---|---|---|---|---|
| β, 95% CI | P-value | β, 95% CI | P-value | β, 95% CI | P-value | |
| Total cholesterol (mmol/L) | −13.48 (−26.87, −0.09) | 0.0506 | −14.71 (−27.42, −2.00) | 0.0251 | −16.51 (−29.57, −3.46) | 0.0148 |
| HDL-C (mmol/L) | −33.94 (−73.31, 5.43) | 0.0936 | −42.38 (−80.27, −5.40) | 0.0267 | −42.65 (−82.60, −2.70) | 0.0390 |
| LDL-C (mmol/L) | −12.90 (−28.60, 2.80) | 0.1098 | −14.98 (−29.91, −0.06) | 0.0513 | −17.89 (−33.24, −2.54) | 0.0245 |
| Total cholesterol (mmol/L) group | ||||||
| <6.22 | Reference | Reference | Reference | |||
| ≥6.22 | −20.91 (−75.85, 34.03) | 0.4571 | −27.03 (−79.37, 25.31) | 0.3130 | −42.07 (−97.70, 13.55) | 0.1414 |
| Triglyceride (mmol/L) | −1.71 (−22.95, 19.52) | 0.8747 | 3.60 (−17.10, 24.29) | 0.7340 | 5.23 (−18.57, 29.02) | 0.6678 |
Notes: Model I adjusted for: age and sex. Model II adjusted for age, sex, and ocular axial length. Model III adjusted for age, gender, ocular axial length, PRP, duration of diabetes, serum creatinine, DBP, HbA1c, anti-VEGF therapy, oral glucose-lowering drugs, and oral calcium antagonists.
Abbreviations: CI, confidence interval; DBP, diastolic blood pressure; HbA1c, hemoglobin A1c; HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol; SFCT, subfoveal choroidal thickness; VEGF, vascular endothelial growth factor; PRP, panretinal photocoagulation.
Smooth Curve Fitting Between Lipid Profile and SFCT in PDR Patients
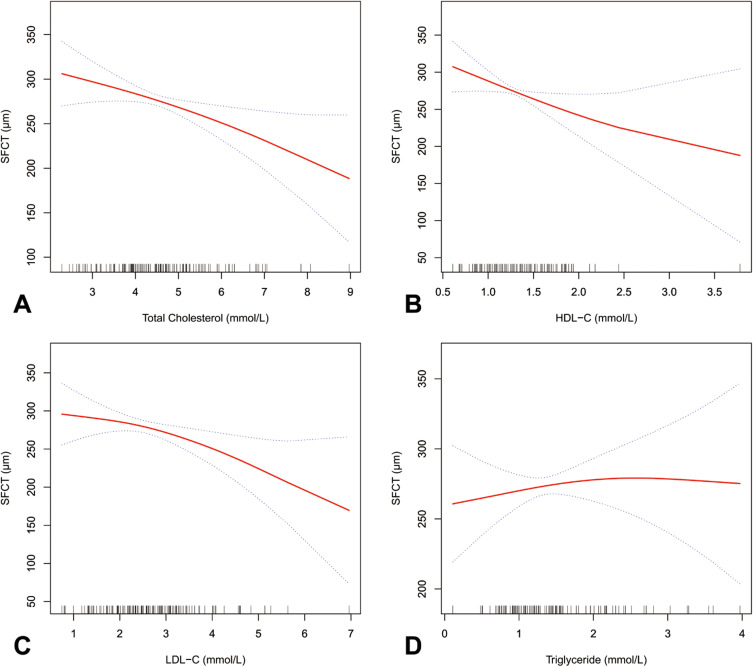
Smooth curve fitting showed that SFCT decreased with increasing concentration of total cholesterol, HDL-C, and LDL-C but not triglyceride, in patients with PDR (Figure 1).
Figure 1.
Smooth curve fitting between lipid profile and SFCT in patients with proliferative diabetic retinopathy. (A–D) present the tendency between total cholesterol, HDL-C, LDL-C, triglyceride, and SFCT, respectively.
The Results of Stratified Analyses Between Lipid Profile and SFCT in PDR Patients
Each stratification was adjusted for all factors (age, gender, ocular axial length, PRP, duration of diabetes, serum creatinine, DBP, HbA1c, anti-VEGF therapy, oral glucose-lowering drugs, and oral calcium antagonists), except the stratification factor itself. All the results of the stratified analysis were shown in Table 4. Except for triglyceride, the trends of total cholesterol, HDL-C, LDL-C, and SFCT were consistent among different stratifications in patients with PDR.
Table 4.
The Results of Stratified Analysis in Participants with Proliferative Diabetic Retinopathy
| Y= SFCT, μm | N | Total Cholesterol (mmol/L) | HDL-C (mmol/L) | LDL-C (mmol/L) | Triglyceride (mmol/L) | ||||
|---|---|---|---|---|---|---|---|---|---|
| β, 95% CI | P-value | β, 95% CI | P-value | β, 95% CI | P-value | β, 95% CI | P-value | ||
| Gender | |||||||||
| Female | 48 | −19.80 (−38.61, −0.99) | 0.0492 | −98.49 (−163.85, −33.14) | 0.0066 | −23.98 (−46.19, −1.77) | 0.0441 | 24.47 (−7.69, 56.63) | 0.1479 |
| Male | 83 | −12.18 (−30.17, 5.81) | 0.1896 | −31.20 (−82.95, 20.54) | 0.2420 | −11.63 (−32.92, 9.65) | 0.2884 | −1.72 (−35.40, 31.97) | 0.9208 |
| Age (year) | |||||||||
| <53 | 45 | −30.22 (−59.36, −1.08) | 0.0538 | −51.53 (−128.77, 25.71) | 0.2039 | −29.00 (−64.82, 6.82) | 0.1262 | −0.71 (−36.39, 34.97) | 0.9692 |
| ≥53 | 86 | −14.29 (−31.00, 2.41) | 0.0985 | −42.64 (−92.30, 7.03) | 0.0975 | −15.59 (−34.82, 3.63) | 0.1169 | 11.14 (−21.88, 44.16) | 0.5110 |
| PRP | |||||||||
| None | 57 | −13.47 (−32.77, 5.84) | 0.1793 | −95.84 (−161.33, −30.35) | 0.0066 | −12.65 (−35.52, 10.21) | 0.2848 | 34.69 (−9.48, 78.86) | 0.1318 |
| Partial | 27 | −9.92 (−44.14, 24.31) | 0.5879 | −12.18 (−102.96, 78.60) | 0.8002 | −14.04 (−58.44, 30.36) | 0.5550 | −14.62 (−72.10, 42.86) | 0.6334 |
| Whole | 47 | 1.88 (−28.89, 32.64) | 0.9058 | −45.95 (−133.77, 41.86) | 0.3141 | −3.94 (−34.69, 26.81) | 0.8036 | 36.86 (−6.00, 79.73) | 0.1034 |
| Oral glucose-lowering drugs | |||||||||
| No | 33 | −14.93 (−60.04, 30.18) | 0.5263 | −72.23 (−211.16, 66.69) | 0.3243 | −20.03 (−84.82, 44.76) | 0.5536 | 14.98 (−53.27, 83.23) | 0.6732 |
| Yes | 98 | −17.33 (−30.51, −4.14) | 0.0121 | −35.07 (−75.34, 5.20) | 0.0923 | −17.48 (−32.68, −2.28) | 0.0273 | −3.19 (−30.69, 24.31) | 0.8207 |
| Calcium antagonist | |||||||||
| No | 90 | −19.31 (−34.06, −4.56) | 0.0127 | −33.21 (−73.40, 6.97) | 0.1102 | −19.47 (−36.97, −1.97) | 0.0330 | −0.68 (−28.68, 27.32) | 0.9621 |
| Yes | 41 | −19.30 (−58.12, 19.52) | 0.3403 | −57.26 (−199.69, 85.16) | 0.4391 | −20.59 (−60.76, 19.58) | 0.3260 | 11.40 (−47.28, 70.08) | 0.7070 |
| Anti-VEGF therapy | |||||||||
| No | 91 | −16.38 (−31.59, −1.17) | 0.0386 | −53.00 (−96.93, −9.07) | 0.0210 | −16.84 (−34.77, 1.09) | 0.0703 | 10.08 (−16.99, 37.15) | 0.4681 |
| Yes | 40 | −24.29 (−50.84, 2.26) | 0.0881 | −33.63 (−137.75, 70.50) | 0.5339 | −25.21 (−57.15, 6.74) | 0.1377 | −30.42 (−78.58, 17.75) | 0.2301 |
| eGFR (mL/min×1.73m2) | |||||||||
| <60 | 55 | −19.87 (−42.49, 2.76) | 0.0952 | 14.42 (−95.03, 123.88) | 0.7979 | −23.18 (−48.02, 1.66) | 0.0771 | −25.61 (−68.01, 16.80) | 0.2456 |
| ≥60 | 76 | −18.26 (−35.43, −1.08) | 0.0421 | −51.84 (−95.40, −8.29) | 0.0236 | −17.90 (−38.59, 2.79) | 0.0960 | 24.39 (−9.94, 58.72) | 0.1697 |
| Hypertension history | |||||||||
| No | 64 | −15.95 (−34.62, 2.71) | 0.1016 | −54.18 (−114.53, 6.18) | 0.0860 | −13.82 (−33.67, 6.03) | 0.1799 | 18.59 (−17.86, 55.04) | 0.3234 |
| Yes | 67 | −20.32 (−45.64, 5.01) | 0.1230 | −33.20 (−96.86, 30.45) | 0.3122 | −22.99 (−52.89, 6.92) | 0.1391 | −4.63 (−43.84, 34.57) | 0.8179 |
| Chronic kidney disease history | |||||||||
| No | 96 | −14.57 (−28.57, −0.56) | 0.0453 | −39.69 (−76.36, −3.02) | 0.0374 | −12.86 (−29.24, 3.51) | 0.1280 | 7.64 (−17.68, 32.97) | 0.5560 |
| Yes | 35 | 8.25 (−43.39, 59.88) | 0.7597 | 18.59 (−191.86, 229.04) | 0.8654 | 7.30 (−58.29, 72.89) | 0.8309 | 13.03 (−50.67, 76.73) | 0.6955 |
Notes: Each stratification was adjusted for all factors (age, gender, ocular axial length, PRP, duration of diabetes, serum creatinine, DBP, HbA1c, anti-VEGF therapy, oral glucose-lowering drugs, and oral calcium antagonists), except the stratification factor itself.
Abbreviations: CI, confidence interval; SBP, systolic blood pressure; DBP, diastolic blood pressure; BMI, body mass index; HbA1c, hemoglobin A1c; HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol; eGFR, estimated glomerular filtration rate; SFCT, subfoveal choroidal thickness; VEGF, vascular endothelial growth factor; PRP, panretinal photocoagulation.
Discussion
Study results demonstrate a relationship between lipid profile and SFCT in patients with PDR. These results show a negative association between the concentration of cholesterol profile and SFCT in PDR patients, indicating that cholesterol may play an important role in choroidal thickness. However, triglyceride was not statistically associated with SFCT. To the best of our knowledge, this is the first study targeting at evaluating the association between lipid profile and SFCT in patients with PDR.
Recently, attention has been increasingly drawn to the relationship between abnormal lipid metabolism and ocular microvascular complications in diabetes. The association between abnormal cholesterol metabolism and DR39,47 was well-reviewed. The metabolically active retina obtains essential lipids by endogenous biosynthesis,48,49 and from systemic circulation.50,51 The cholesterol metabolism of retina and brain is different from that of other peripheral organs and is separated from the system circulation.52 In the healthy retina, most exogenous cholesterol is transmitted through the RPE layer and gradually spread throughout the entire retina. However, in the diabetic retina, the hyperglycemia-induced damage of the internal and external blood-retinal barriers makes the absorption of cholesterol in the retina abnormally increase. The choroid is one of the most complex vascular networks in the human body and choroidal circulation is essential for maintaining retinal homeostasis.53 Such findings illustrated that most exogenous cholesterol passes through the choroidal circulatory system, suggesting the choroidal circulatory system is susceptible to lipid metabolism.
Many studies have demonstrated the relationship between cholesterol profile and SFCT. In animal studies, choroidal atherosclerotic changes can occur in the presence of hypercholesterolemia.54–56 Salazar et al54 found that in rabbits, the choroid thickened with hypercholesterolemia. After normalization of serum cholesterol level, choroidal changes were not completely reversible. In addition to increased thickness, lipid accumulation on the choroid and hypertrophy of endothelial and vascular smooth muscle cells were seen. In addition to previous animal studies,54–56 Wong et al examined 6147 adults aged 45–84 years old from the MESA cohort and found the mean SFCT was thicker in healthy participants with hypercholesterolemia.32 Recently, Marta et al33 found the physiological cholesterol concentration was positively correlated with SFCT in children with type 1 diabetes during puberty. In contrast, in the present study, we found a negative correlation between cholesterol levels including total cholesterol, LDL-C and HDL-C, and SFCT in PDR patients. We speculate our results on the relationship between cholesterol concentration and SFCT differed from previous studies due to the different study populations. Our research population was middle-aged and elderly patients with PDR. Ageing is an established risk factor for a wide range of chorioretinal diseases. Studies53,57 have found an age-related accumulation of various cellular and blood-derived products in the RPE/choroid complex, such as lipids, and advanced glycation end-products. This indicates abnormalities in cholesterol homeostasis sharing phenotypic features with atherosclerosis. In addition to ageing, hyperglycemia can also cause choroidal vascular damage, such as arteriosclerotic changes.1 Bhutto et al confirmed microvascular changes, such as vascular tortuosity, vascular diameter changes, and arterial stenosis in the diabetic rat model.58 Several clinical trials59,60 have shown that choriocapillaris dropout in DR, especially in PDR eyes. Biswas et al61 found that translocator protein (TSPO), a cholesterol-binding protein involved in mitochondrial cholesterol transport, is expressed in choroidal endothelial cells (CECs). Furthermore, TSPO ligands promote cholesterol efflux and suppress oxidative stress and inflammation in CECs. In addition to increased exogenous cholesterol caused by age and hyperglycemia; abnormal cholesterol efflux caused by decreased TSPO arising from a decrease in CECs resulting from choriocapillaris dropout is another reason for choroidal atherosclerotic changes in PDR patients. Besides, as a common contributor to atherosclerosis, the existence of diabetes with or without hypertension and hypercholesterolemia can also cause choroidal vascular changes like those of atherosclerosis.62 The choroid may be prone to arteriosclerotic processes to the same degree as other organs, specifically, considering the choroid an extremely vascular terminal organ associated with the largest flow per mm3 in the body.63 Here, we speculate that these choroidal vascular changes induced by age and cholesterol profile in patients with PDR can cause tissue ischemia. In the early stage, ischemia can be compensated for by the continuous expansion of choroidal vessels, leading to choroidal thickening. In the late stage, ischemia can no longer be compensated for and leads to choroid blood vessel obstruction, resulting in choroidal thinning. Atherosclerosis-related diseases are associated with decreased SFCT.34,64–66 This supports our hypothesis, indicating prominent reductions of SFCT could provide a useful biomarker for predicting cardiovascular risk in patients of advanced age.
Interestingly, we found that the trend between SFCT and HDL-C was consistent with that between SFCT and both total cholesterol and LDL-C in PDR patients. In contrast to the atherogenic properties of total cholesterol and LDL-C, HDL-C is considered to have independent atheroprotective properties. HDL-C has both antioxidant and anti-inflammatory properties and plays a central role in reverse cholesterol transport and endothelial homeostasis.67 However, the anti-atherosclerotic effect of very high HDL-C in cardiovascular disease is currently controversial.68–70 In ophthalmology, several studies have shown that AMD is positively associated with HDL-C,71,72 although conflicting results have been found. A meta-analysis by Wang et al72 showed a significantly positive association between HDL-C and AMD, suggesting people with high HDL-C levels may be at an increased risk for AMD. In addition to AMD, HDL-C is also significantly associated with hypertensive retinopathy,36 suggesting extremely high HDL reflects a specific atherosclerotic condition. A plausible explanation for this discrepancy is that HDL-C can be modified via myeloperoxidase-associated mechanisms under certain conditions, thus losing its protective effect and becoming atherogenic.67,68,73 Myeloperoxidase-associated modification of HDL may be one of the mechanisms.73 Findings from NO BLIND study74 confirmed that high HDL cholesterol is a risk factor for DR. Therefore, we speculated that the negative correlation between HDL-C and SFCT may be due to modification of HDL-C causing HDL-C to become atherogenic in diabetes.
Conversely, the present study failed to find an association between triglyceride concentrations and SFCT in patients with PDR. Currently, to our knowledge, no studies targeted at the relationship between triglycerides and SFCT. Gupta et al found that lower triglyceride level was associated with peripapillary choroidal thickening.75 However, the study only proved a correlation between triglyceride level and peripapillary choroidal thickness, not a quantitative analysis. Hence, the mechanism remains unclear.
In the present study, we found that the use of specific drugs may also be one of the reasons for affecting SFCT. This was the case specifically for oral glucose-lowering drugs and calcium antagonists; thus, we took these factors into statistical analyses. According to the Chinese Adult Dyslipidemia Prevention Guide (2007 edition),44 the proportion of hypercholesterolemia in patients with PDR was not enough to determine a relationship between hypercholesterolemia and SFCT. Nevertheless, in this study, we observed a negative trend between them. Although the clinical significance of SFCT in PDR patients is not fully understood, cholesterol concentrations are a modifiable variable, which could be reduced by the cholesterol-lowering drugs, increasing SFCT in PDR patients. The findings of this study warrant further investigation through clinical, large-scale, and long-term prospective studies.
There are some limitations to our study. Firstly, our results were obtained in a homogeneous population of Chinese patients with PDR. Therefore, findings cannot be extrapolated to other populations. Secondly, we were unable to obtain the history related to cholesterol-lowering drugs. Cholesterol levels can be adjusted with cholesterol-lowering drugs. However, the use of cholesterol-lowering drugs is likely to be a confounding factor, due to being a contributing factor to the thickening of the SFCT in patients with PDR. It is however noteworthy that the lack of information on exposure as a result of such errors could bias results, thus resulting in an underestimation of the association between cholesterol profile and SFCT. Thirdly, in this study, the EDI-OCT measurements of SFCT centered on the fovea were taken by horizontal/vertical scans. However, the choroidal thickness varies at different locations in each person. Therefore, our results cannot be extrapolated to choroidal thickness in other locations. Furthermore, manually measured SFCT may have measurement errors. Considering this, future further study using automated quantification of SFCT would be beneficial.
Conclusions
In conclusion, our results showed a significant negative association between cholesterol profile and SFCT in Chinese patients with PDR. Cholesterol imbalances contribute to choroidal thickness changes and often coexist in diabetic patients. This would enable a full evaluation of the exact role of cholesterol profile in the SFCT in PDR patients. As such, further additional research in experimental models should also be undertaken.
Funding Statement
This work was supported by 1.3.5 project for disciplines of excellence, West China Hospital, Sichuan University (ZYJC21025), and Sichuan Provincial Science and Technology Support Project (no. 2021ZYD0110).
Abbreviations
SD-OCT, spectral-domain optical coherence tomography; EDI, enhanced depth imaging; SFCT, subfoveal choroidal thickness; LDL-C, low-density lipoprotein cholesterol; AMD, age-related macular degeneration; DR, diabetic retinopathy; DME, diabetic macular edema; HDL-C, high-density lipoprotein cholesterol; PDR, proliferative diabetic retinopathy; HbA1c, glycosylated hemoglobin A1c; eGFR, estimated glomerular filtration rate; BCVA, best-corrected visual acuity; IOP, intraocular pressure; CMT, central macular thickness; RPE, retinal pigment epithelium; PRP, panretinal photocoagulation; VEGF, vascular endothelial growth factor; DBP, diastolic blood pressure; TSPO, translocator protein; CECs, choroidal endothelial cells.
Data Sharing Statement
The primary data for this study is available from the corresponding author (Meixia Zhang) on reasonable request.
Ethical Approval and Consent to Participate
The trial has received ethical approval from the West China Hospital of Sichuan University. The data were anonymous and informed consent was waived by the approving Institutional Review Board because of the retrospective nature of the study.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
The authors declare that they have no competing interests.
References
- 1.Hidayat AA, Fine BS. Diabetic choroidopathy. Light and electron microscopic observations of seven cases. Ophthalmology. 1985;92(4):512–522. doi: 10.1016/S0161-6420(85)34013-7 [DOI] [PubMed] [Google Scholar]
- 2.Gaudric A, Coscas G, Bird AC. Choroidal ischemia. Am J Ophthalmol. 1982;94(4):489–498. doi: 10.1016/0002-9394(82)90242-2 [DOI] [PubMed] [Google Scholar]
- 3.Spaide RF, Koizumi H, Pozzoni MC. Enhanced depth imaging spectral-domain optical coherence tomography. Am J Ophthalmol. 2008;146(4):496–500. doi: 10.1016/j.ajo.2008.05.032 [DOI] [PubMed] [Google Scholar]
- 4.Cheung N, Mitchell P, Wong TY. Diabetic retinopathy. Lancet. 2010;376(9735):124–136. doi: 10.1016/S0140-6736(09)62124-3 [DOI] [PubMed] [Google Scholar]
- 5.Yau JW, Rogers SL, Kawasaki R, et al. Global prevalence and major risk factors of diabetic retinopathy. Diabetes Care. 2012;35(3):556–564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Frank RN. Diabetic retinopathy. N Engl J Med. 2004;350(1):48–58. doi: 10.1056/NEJMra021678 [DOI] [PubMed] [Google Scholar]
- 7.Milluzzo A, Maugeri A, Barchitta M, et al. Epigenetic mechanisms in type 2 diabetes retinopathy: a systematic review. Int J Mol Sci. 2021;22(19):10502. doi: 10.3390/ijms221910502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Milluzzo A, Barchitta M, Maugeri A, et al. Do nutrients and nutraceuticals play a role in diabetic retinopathy? A Systematic review. Nutrients. 2022;14(20):4430. doi: 10.3390/nu14204430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Milluzzo A, Barchitta M, Maugeri A, et al. Body mass index is related to short term retinal worsening in type 2 diabetes patients treated with anticancer drugs. Minerva Endocrinol. 2022. doi: 10.23736/S2724-6507.22.03653-3 [DOI] [PubMed] [Google Scholar]
- 10.Yamanouchi M, Mori M, Hoshino J, et al. Retinopathy progression and the risk of end-stage kidney disease: results from a longitudinal Japanese cohort of 232 patients with type 2 diabetes and biopsy-proven diabetic kidney disease. BMJ Open Diabetes Res Care. 2019;7(1):e000726. doi: 10.1136/bmjdrc-2019-000726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mule G, Vadalà M, La Blasca T, et al. Association between early-stage chronic kidney disease and reduced choroidal thickness in essential hypertensive patients. Hypertens Res. 2019;42(7):990–1000. doi: 10.1038/s41440-018-0195-1 [DOI] [PubMed] [Google Scholar]
- 12.Chung JO, Park S-Y, Cho DH, et al. Associations between serum apolipoproteins, urinary albumin excretion rate, estimated glomerular filtration rate, and diabetic retinopathy in individuals with type 2 diabetes. Medicine. 2019;98(20):e15703. doi: 10.1097/MD.0000000000015703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rodriguez-Poncelas A, Mundet-Tudurí X, Miravet-Jiménez S, et al. Chronic kidney disease and diabetic retinopathy in patients with type 2 diabetes. PLoS One. 2016;11(2):e0149448. doi: 10.1371/journal.pone.0149448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang J, Xin X, Luo W, et al. Anemia and diabetic kidney disease had joint effect on diabetic retinopathy among patients with type 2 diabetes. Invest Ophthalmol Vis Sci. 2020;61(14):25. doi: 10.1167/iovs.61.14.25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rajalakshmi R, Shanthi Rani CS, Venkatesan U, et al. Correlation between markers of renal function and sight-threatening diabetic retinopathy in type 2 diabetes: a longitudinal study in an Indian clinic population. BMJ Open Diabetes Res Care. 2020;8(1):e001325. doi: 10.1136/bmjdrc-2020-001325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cho A, Park HC, Lee Y-K, et al. Progression of diabetic retinopathy and declining renal function in patients with type 2 diabetes. J Diabetes Res. 2020;2020:8784139. doi: 10.1155/2020/8784139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhuang X, Cao D, Yang D, et al. Association of diabetic retinopathy and diabetic macular oedema with renal function in southern Chinese patients with type 2 diabetes mellitus: a single-centre observational study. BMJ Open. 2019;9(9):e031194. doi: 10.1136/bmjopen-2019-031194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Huo Y, GUO Y, Wang H, et al. Change regularity of adult subfoveal choroidal thickness with age and its influencing factors. Chin J Exp Ophthalmol. 2021;39(1):29–33. [Google Scholar]
- 19.Shao L, Wang Y, Xu J, et al. Subfoveal choroidal thickness of Chinese aged over 50 years and patients with diabetes mellitus and glaucoma. Chin J Ophthalmol. 2014;50(6):414–420. [PubMed] [Google Scholar]
- 20.Wei WB, Xu L, Jonas JB, et al. Subfoveal choroidal thickness: the Beijing Eye Study. Ophthalmology. 2013;120(1):175–180. doi: 10.1016/j.ophtha.2012.07.048 [DOI] [PubMed] [Google Scholar]
- 21.Moussa M, Sabry D, Soliman W. Macular choroidal thickness in normal Egyptians measured by swept source optical coherence tomography. BMC Ophthalmol. 2016;16:138. doi: 10.1186/s12886-016-0314-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Flores-Moreno I, Lugo F, Duker JS, et al. The relationship between axial length and choroidal thickness in eyes with high myopia. Am J Ophthalmol. 2013;155(2):314–319 e1. doi: 10.1016/j.ajo.2012.07.015 [DOI] [PubMed] [Google Scholar]
- 23.Asikgarip N, Temel E, Kıvrak A, et al. Choroidal structural changes and choroidal vascularity index in patients with systemic hypertension. Eur J Ophthalmol. 2022;32(4):2427–2432. doi: 10.1177/11206721211035615 [DOI] [PubMed] [Google Scholar]
- 24.Romero-Trevejo JL, Fernández-Romero L, Delgado J, et al. Choroidal thickness and granulocyte colony-stimulating factor in tears improve the prediction model for coronary artery disease. Cardiovasc Diabetol. 2022;21(1):103. doi: 10.1186/s12933-022-01538-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fernandez-Espinosa G, Orduna-Hospital E, Boned-Murillo A, et al. Choroidal and retinal thicknesses in type 2 diabetes mellitus with moderate diabetic retinopathy measured by swept source OCT. Biomedicines. 2022;10(9):2314. doi: 10.3390/biomedicines10092314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gun RD, Gümüş T, Kardaş ASY, et al. Acute effect of hyperbaric oxygen therapy on macular and choroidal thickness in patients with type 2 diabetes and diabetic foot ulcers: optical coherence tomography based study. Photodiagnosis Photodyn Ther. 2022;39:102926. doi: 10.1016/j.pdpdt.2022.102926 [DOI] [PubMed] [Google Scholar]
- 27.Wang W, Li L, Wang J, et al. Macular choroidal thickness and the risk of referable diabetic retinopathy in type 2 diabetes: a 2-year longitudinal study. Invest Ophthalmol Vis Sci. 2022;63(4):9. doi: 10.1167/iovs.63.4.9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Durusoy GK, Gumus G, Onay M, et al. Early choroidal structure and choroidal vascularity index change after carotid stenting. Photodiagnosis Photodyn Ther. 2022;38:102748. doi: 10.1016/j.pdpdt.2022.102748 [DOI] [PubMed] [Google Scholar]
- 29.Wan J, Kwapong WR, Tao. W, et al. Choroidal changes in carotid stenosis patients after stenting detected by swept-source optical coherence tomography angiography. Curr Neurovasc Res. 2022;19(1):100–107. doi: 10.2174/1567202619666220406092532 [DOI] [PubMed] [Google Scholar]
- 30.Altinkaynak H, Kara N, Sayın N, et al. Subfoveal choroidal thickness in patients with chronic heart failure analyzed by spectral-domain optical coherence tomography. Curr Eye Res. 2014;39(11):1123–1128. doi: 10.3109/02713683.2014.898310 [DOI] [PubMed] [Google Scholar]
- 31.Krajewski P, Turczyńska M, Gołębiewska J, et al. Optical coherence tomography angiography findings in patients with chronic thromboembolic pulmonary hypertension. Retina. 2022;42(12):2354–2360. doi: 10.1097/IAE.0000000000003607 [DOI] [PubMed] [Google Scholar]
- 32.Wong IY, Wong RL, Zhao P, et al. Choroidal thickness in relation to hypercholesterolemia on enhanced depth imaging optical coherence tomography. Retina. 2013;33(2):423–428. doi: 10.1097/IAE.0b013e3182753b5a [DOI] [PubMed] [Google Scholar]
- 33.Marta WM, Andrzej O, Marta B-W, et al. Choroidal thickness in children with type 1 diabetes depending on the pubertal status and metabolic parameters analyzed by optical coherence tomography. Sci Rep. 2021;11(1):19677. doi: 10.1038/s41598-021-97794-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Aydin E, Kazanci L, Balikoglu Yilmaz M, et al. Analysis of central macular thickness and choroidal thickness changes in patients with cardiovascular risk factors. Eye. 2020;34(11):2068–2075. doi: 10.1038/s41433-020-0775-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wang H, Ramshekar A, Kunz E, et al. 7-ketocholesterol induces endothelial-mesenchymal transition and promotes fibrosis: implications in neovascular age-related macular degeneration and treatment. Angiogenesis. 2021;24(3):583–595. doi: 10.1007/s10456-021-09770-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nakajima K, Higuchi R, Mizusawa K, et al. Association between extremely high high-density lipoprotein-cholesterol and hypertensive retinopathy: results of a cross-sectional study from Kanagawa investigation of total checkup data from the national database-6 (KITCHEN-6). BMJ Open. 2021;11(5):e043677. doi: 10.1136/bmjopen-2020-043677 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rema M, Srivastava BK, Anitha B, et al. Association of serum lipids with diabetic retinopathy in urban South Indians--the Chennai Urban Rural Epidemiology Study (CURES) Eye Study--2. Diabet Med. 2006;23(9):1029–1036. doi: 10.1111/j.1464-5491.2006.01890.x [DOI] [PubMed] [Google Scholar]
- 38.Ezhilvendhan K, Sathiyamoorthy A, Prakash B, et al. Association of dyslipidemia with diabetic retinopathy in type 2 diabetes mellitus patients: a hospital-based study. J Pharm Bioallied Sci. 2021;13(Suppl 2):S1062–S1067. doi: 10.4103/jpbs.jpbs_164_21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhang X, Wang K, Zhu L, et al. Reverse Cholesterol Transport Pathway and Cholesterol Efflux in Diabetic Retinopathy. J Diabetes Res. 2021;2021:8746114. doi: 10.1155/2021/8746114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Das R, Kerr R, Chakravarthy U, et al. Dyslipidemia and diabetic macular edema: a systematic review and meta-analysis. Ophthalmology. 2015;122(9):1820–1827. doi: 10.1016/j.ophtha.2015.05.011 [DOI] [PubMed] [Google Scholar]
- 41.Melancia D, Vicente A, Cunha JP, et al. Diabetic choroidopathy: a review of the current literature. Graefes Arch Clin Exp Ophthalmol. 2016;254(8):1453–1461. doi: 10.1007/s00417-016-3360-8 [DOI] [PubMed] [Google Scholar]
- 42.Yazgan S, Arpaci D, Celik HU, et al. Macular choroidal thickness may be the earliest determiner to detect the onset of diabetic retinopathy in patients with prediabetes: a prospective and comparative study. Curr Eye Res. 2017;42(7):1039–1047. doi: 10.1080/02713683.2016.1264606 [DOI] [PubMed] [Google Scholar]
- 43.KDOQI. KDOQI clinical practice guidelines and clinical practice recommendations for diabetes and chronic kidney disease. Am J Kidney Dis. 2007;49(2 Suppl 2):S12–154. doi: 10.1053/j.ajkd.2006.12.005 [DOI] [PubMed] [Google Scholar]
- 44.Joint Committee for Developing Chinese guidelines on Prevention and Treatment of Dyslipidemia in Adults. 中国成人血脂异常防治指南 [Chinese guidelines on prevention and treatment of dyslipidemia in adults]. Zhonghua Xin Xue Guan Bing Za Zhi. 2007;35(5):390–419. Chinese. [PubMed] [Google Scholar]
- 45.Jaddoe VW, de Jonge LL, Hofman A, et al. First trimester fetal growth restriction and cardiovascular risk factors in school age children: population based cohort study. BMJ. 2014;348:g14. doi: 10.1136/bmj.g14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kernan WN, Viscoli CM, Brass LM, et al. Phenylpropanolamine and the risk of hemorrhagic stroke. N Engl J Med. 2000;343(25):1826–1832. doi: 10.1056/NEJM200012213432501 [DOI] [PubMed] [Google Scholar]
- 47.Busik JV. Lipid metabolism dysregulation in diabetic retinopathy. J Lipid Res. 2021;62:100017. doi: 10.1194/jlr.TR120000981 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Fliesler SJ, Florman R, Rapp LM, et al. In vivo biosynthesis of cholesterol in the rat retina. FEBS Lett. 1993;335(2):234–238. doi: 10.1016/0014-5793(93)80736-E [DOI] [PubMed] [Google Scholar]
- 49.Fliesler SJ. Retinal degeneration in a rat model of Smith-Lemli-Opitz Syndrome: thinking beyond cholesterol deficiency. Adv Exp Med Biol. 2010;664:481–489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Elner VM. Retinal pigment epithelial acid lipase activity and lipoprotein receptors: effects of dietary omega-3 fatty acids. Trans Am Ophthalmol Soc. 2002;100:301–338. [PMC free article] [PubMed] [Google Scholar]
- 51.Tserentsoodol N, Sztein J, Campos M, et al. Uptake of cholesterol by the retina occurs primarily via a low density lipoprotein receptor-mediated process. Mol Vis. 2006;12:1306–1318. [PubMed] [Google Scholar]
- 52.Fliesler SJ, Bretillon L. The ins and outs of cholesterol in the vertebrate retina. J Lipid Res. 2010;51(12):3399–3413. doi: 10.1194/jlr.R010538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Brinks J, van Dijk EHC, Klaassen I, et al. Exploring the choroidal vascular labyrinth and its molecular and structural roles in health and disease. Prog Retin Eye Res. 2021;87:100994. doi: 10.1016/j.preteyeres.2021.100994 [DOI] [PubMed] [Google Scholar]
- 54.Salazar JJ, Ramírez AI, de Hoz R, et al. Alterations in the choroid in hypercholesterolemic rabbits: reversibility after normalization of cholesterol levels. Exp Eye Res. 2007;84(3):412–422. doi: 10.1016/j.exer.2006.10.012 [DOI] [PubMed] [Google Scholar]
- 55.Kouchi M, Ueda Y, Horie H, et al. Ocular lesions in Watanabe heritable hyperlipidemic rabbits. Veterinary Ophthalmology. 2006;9(3):145–148. doi: 10.1111/j.1463-5224.2006.00453.x [DOI] [PubMed] [Google Scholar]
- 56.Torres RJ, Maia M, Noronha L, et al. Avaliação das alterações precoces na coróide e esclera ocorridas em coelhos hipercolesterolêmicos. Estudo histológico e histomorfométrico [Evaluation of choroid and sclera early alterations in hypercholesterolemic rabbits: histologic and histomorphometric study]. Arq Bras Oftalmol. 2009;72(1):68–74. Portuguese. doi: 10.1590/S0004-27492009000100014 [DOI] [PubMed] [Google Scholar]
- 57.Ban N, Lee TJ, Sene A, et al. Impaired monocyte cholesterol clearance initiates age-related retinal degeneration and vision loss. JCI Insight. 2018;3(17). doi: 10.1172/jci.insight.120824 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Bhutto IA, Lu Z-Y, Takami Y, et al. Retinal and choroidal vasculature in rats with spontaneous diabetes type 2 treated with the angiotensin-converting enzyme inhibitor cilazapril: corrosion cast and electron-microscopic study. Ophthalmic Res. 2002;34(4):220–231. doi: 10.1159/000063877 [DOI] [PubMed] [Google Scholar]
- 59.Yang J, Wang E, Zhao X, et al. Optical coherence tomography angiography analysis of the choriocapillary layer in treatment-naïve diabetic eyes. Graefes Arch Clin Exp Ophthalmol. 2019;257(7):1393–1399. doi: 10.1007/s00417-019-04326-x [DOI] [PubMed] [Google Scholar]
- 60.Conti FF, Qin VL, Rodrigues EB, et al. Choriocapillaris and retinal vascular plexus density of diabetic eyes using split-spectrum amplitude decorrelation spectral-domain optical coherence tomography angiography. Br J Ophthalmol. 2019;103(4):452–456. doi: 10.1136/bjophthalmol-2018-311903 [DOI] [PubMed] [Google Scholar]
- 61.Biswas L, Farhan F, Reilly J, et al. TSPO ligands promote cholesterol efflux and suppress oxidative stress and inflammation in choroidal endothelial cells. Int J Mol Sci. 2018;19(12):3740. doi: 10.3390/ijms19123740 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Yeung SC, You Y, Howe KL, et al. Choroidal thickness in patients with cardiovascular disease: a review. Surv Ophthalmol. 2020;65(4):473–486. doi: 10.1016/j.survophthal.2019.12.007 [DOI] [PubMed] [Google Scholar]
- 63.Alm A, Bill A. Ocular and optic nerve blood flow at normal and increased intraocular pressures in monkeys (Macaca irus): a study with radioactively labelled microspheres including flow determinations in brain and some other tissues. Exp Eye Res. 1973;15(1):15–29. doi: 10.1016/0014-4835(73)90185-1 [DOI] [PubMed] [Google Scholar]
- 64.Kim JH, Kim S, Kim S, et al. Relationship between coronary artery calcification and central chorioretinal thickness in patients with subclinical atherosclerosis. Ophthalmologica. 2021;244(1):18–26. doi: 10.1159/000506056 [DOI] [PubMed] [Google Scholar]
- 65.Kocamaz M, Karadağ O, Onder SE. Comparison of choroidal thicknesses in patients with coronary artery disease and patients at risk of coronary artery disease. Int Ophthalmol. 2021;41(6):2117–2124. doi: 10.1007/s10792-021-01769-2 [DOI] [PubMed] [Google Scholar]
- 66.Ala-Kauhaluoma M, Koskinen SM, Silvennoinen H, et al. Subfoveal choroidal thickness in ipsi- and contralateral eyes of patients with carotid stenosis before and after carotid endarterectomy: a prospective study. Acta Ophthalmol. 2021;99(5):545–552. doi: 10.1111/aos.14648 [DOI] [PubMed] [Google Scholar]
- 67.Ossoli A, Pavanello C, Giorgio E, et al. Dysfunctional HDL as a therapeutic target for atherosclerosis prevention. Curr Med Chem. 2019;26(9):1610–1630. doi: 10.2174/0929867325666180316115726 [DOI] [PubMed] [Google Scholar]
- 68.Masuda D, Yamashita S. Very high levels of high-density lipoprotein cholesterol and cardiovascular events in Japanese population. J Atheroscler Thromb. 2016;23(7):771–772. doi: 10.5551/jat.ED049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Singh K, Rohatgi A. Examining the paradox of high high-density lipoprotein and elevated cardiovascular risk. J Thorac Dis. 2018;10(1):109–112. doi: 10.21037/jtd.2017.12.97 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Madsen CM, Nordestgaard BG. Is it time for new thinking about high-density lipoprotein? Arterioscler Thromb Vasc Biol. 2018;38(3):484–486. doi: 10.1161/ATVBAHA.118.310727 [DOI] [PubMed] [Google Scholar]
- 71.Burgess S, Davey Smith G. Mendelian randomization implicates high-density lipoprotein cholesterol-associated mechanisms in etiology of age-related macular degeneration. Ophthalmology. 2017;124(8):1165–1174. doi: 10.1016/j.ophtha.2017.03.042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Wang Y, Wang M, Zhang X, et al. The association between the lipids levels in blood and risk of age-related macular degeneration. Nutrients. 2016;8(10):8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Feng H, Li XA. Dysfunctional high-density lipoprotein. Curr Opin Endocrinol Diabetes Obes. 2009;16(2):156–162. doi: 10.1097/MED.0b013e32832922fc [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Sasso FC, Pafundi PC, Gelso A, et al. High HDL cholesterol: a risk factor for diabetic retinopathy? Findings from NO BLIND study. Diabetes Res Clin Pract. 2019;150:236–244. doi: 10.1016/j.diabres.2019.03.028 [DOI] [PubMed] [Google Scholar]
- 75.Gupta P, Jing T, Marziliano P, et al. Peripapillary choroidal thickness assessed using automated choroidal segmentation software in an Asian population. Br J Ophthalmol. 2015;99(7):920–926. doi: 10.1136/bjophthalmol-2014-306152 [DOI] [PubMed] [Google Scholar]