Abstract
Marital quality shares ties to inflammation-related conditions like cardiovascular disease and diabetes. Lab-based studies implicate hostility during marital conflict as a mechanism via inflammatory reactivity, but little attention has been paid to the inflammatory aftermath of other marital exchanges. A spouse’s emotional distress is an important but overlooked context for middle-aged and older couples, as conflict declines and networks shrink. To examine the links of spousal distress to changes in proinflammatory gene expression, 38 adults ages 40–81 witnessed their spouse relive an upsetting personal memory aloud, rated their mood before and after, and provided blood samples at baseline and twice post-task; they also shared their own upsetting memory and discussed a marital problem in the interim. Those whose spouse disclosed their upsetting memory with greater emotional intensity showed larger elevations in proinflammatory gene expression 30–40 min and 80–90 min after the task. The association replicated for listeners whose negative mood increased more in response to spousal disclosure. Findings were robust to behavior in the other emotional tasks, race, gender, age, alcohol, smoking, comorbidities, and sagittal abdominal diameter. These novel results identify spousal distress as a key marital context that may escalate inflammation-related health risks.
Keywords: Proinflammatory gene expression, Couples, Marriage, Partner effects, Spousal distress, Aging
1. Introduction
Marital quality shares clear ties to inflammation and its associated chronic conditions. Indeed, according to data from large national studies, people who rate their marriage as less supportive and satisfying have higher circulating interleukin (IL)-6 and C-reactive protein (CRP) levels compared to their happily married counterparts (Donoho et al., 2013; Whisman and Sbarra, 2012). In tandem, the unhappily married carry greater risks for cardiovascular events, diabetes, and earlier mortality (Liu and Waite, 2014; Robles et al., 2014; Whisman et al., 2014). Lab-based studies of marital interaction have converged on one prominent mechanism to explain this pattern: hostility during marital conflict. In two studies, couples who discussed problems in their relationship with more criticism, disgust, and blaming had higher IL-6 and tumor necrosis factor (TNF)-α relative to couples who conversed with less hostility (Kiecolt-Glaser et al., 2015; Kiecolt-Glaser et al., 2005). According to the dominant conceptual framework (McEwen, 1998), more hostile exchanges trigger longer and more frequent inflammatory responses, leading to greater inflammatory burden and higher disease risk (Kiecolt-Glaser et al., 2020).
However, hostile marital conflict may decline as couples age. According to social-emotional aging theories, as adults age, they increasingly prioritize emotional fulfillment and close relationships over future-oriented instrumental goals (Carstensen, 1995). For this reason, older adults tend to avoid overt conflict and positively reframe stressors more than their younger counterparts (Charles, 2010). Although the evidence for this pattern in married couples is mixed, the longest prospective study of middle-aged and older couples’ observed interaction showed that negative emotional behavior during marital conflict discussions decreased over 13 years, whereas positive behavior increased (Verstaen et al., 2018). In other work, older couples rated their spouse’s behavior during conflict as more positive than outside coders judged them to be (Story et al., 2007). An increasingly prevalent phenomenon wherein couples divorce in middle or older age after decades-long marriage, “gray divorce” magnifies the pattern of diminishing negativity as dissatisfied couples opt out of marriage in later years, leaving the intact subset of older couples happier than younger counterparts (Brown and Lin, 2012).
If marital conflict does decline in frequency and intensity with older age, other stressful marital exchanges may pose greater challenges to older couples’ health and well-being than overt hostility. A spouse’s emotional distress represents one such experience that is likely to persist over the adult lifespan, as the stressors of midlife—e.g., work, family, and competing demands—give way to those of older age—e.g., the loss of loved ones and emergence of health problems. Such unavoidable stressors represent a vulnerability in older adults’ emotion regulation and, thus, their physiological reactivity (Charles, 2010). Indeed, listening to a spouse’s disclosure, watching a spouse in pain, or even talking about the suffering of one’s spouse can increase blood pressure (Monin et al., 2010) and disrupt sleep (Kane et al., 2014). Specifically, in older, long-married couples, spousal caregivers’ blood pressure and heart rate increased more after witnessing their own partner complete painful tasks compared to watching a stranger in pain (Monin et al., 2010). In addition, satisfied and dissatisfied spouses showed equal amounts of reactivity to their partner’s pain. In other evidence, husbands whose wives disclosed more of their thoughts and feelings had poorer sleep efficiency (Kane et al., 2014), a pattern the authors attributed to stress contagion between the partners. Nevertheless, whether a spouse’s emotional distress is also sufficient to evoke changes in inflammatory biology remains unknown.
Understanding how the social environment impacts middle-aged and older adults’ health is critical for a few reasons. With longer life expectancies and declining birth rates, much of the world’s population is rapidly aging (Wang et al., 2020). Moreover, so-called age-related diseases such as cardiovascular disease and diabetes manifest with increasing prevalence in middle and older years, and toxic psychosocial stress may further hasten this health decline (Epel, 2020). In this way, middle and older age are critical periods of unprecedented health risk and disease burden, which relationships may further exacerbate or buffer, depending on relationship characteristics and quality.
1.1. Current study
To examine the links between a spouse’s emotional distress and subsequent changes in cellular aspects of inflammatory biology, we assayed pro-inflammatory gene expression in circulating blood cells from middle-aged and older adults before and after they relived an upsetting personal memory in front of their partner. As part of a larger parent study, participants also discussed a marital problem with their spouse. We hypothesized that proinflammatory gene expression would increase more among those whose spouse disclosed their upsetting memory with greater emotional intensity, controlling for their emotional behavior during their own disclosure and the marital conflict. In tandem, we predicted that those who experienced greater increases in negative affect while listening to their spouse would also show larger rises in proinflammatory gene expression, above and beyond emotional responses to the other tasks. We chose to examine changes in proinflammatory gene expression in part due to their rapid response times (as gene expression changes can take place within minutes and precede protein production) and in part to capture changes in circulating immune cell components of inflammation (whereas plasma cytokine levels often derive from other cellular sources such as adipose tissue or lymphoid organs). To this end, we focused on a well-validated multigene profile of proinflammatory RNA transcripts in order to smooth over idiosyncratic variations in activity of specific individual gene transcripts and capture overall inflammatory “tone” in the pool of circulating immune cells.
2. Method
2.1. Participants
Middle-aged and older heterosexual couples were recruited for a parent study on molecular aging. Interested couples in a marriage or marriage-like relationship completed online and in-person screens to determine eligibility. Couples were excluded if they were together and cohabiting fewer than three years, were younger than 40 years old, and had sensory or cognitive impairments that would interfere with study completion. To reduce bias in physiological data, couples were also excluded if either partner had a range of chronic health problems (e.g., cancer, autoimmune conditions, diabetes [Hba1c > 6.5], chronic obstructive pulmonary disease, liver failure), smoked, abused substances, or took prescription medication that affected the immune system, e.g., steroids, methotrexate. A total of 412 interested individuals were excluded because they or their partner did not meet the stringent health criteria. From the parent sample, 38 participants in 19 couples provided gene expression data and were, thus, included in the present analyses. Although budget constraints limited the size of the subsample, the hypotheses were adequately powered. Indeed, a comparable study wherein individuals witnessed their spouse’s pain reported effects on blood pressure and heart rate that were large in magnitude (η2 =.12–.19) (Monin et al., 2010). With N = 38 participants, a minimal expected couple-level intraclass coefficient (ICC =.01) (Kiecolt-Glaser et al., 2015), and α = .05, the current study had 80% to detect an association of moderate magnitude (r = .40). This value provides a very conservative power estimate given the study’s repeated-measures design, with three blood samples and expression of multiple proinflammatory genes, for a total of up to n = 1824 observations.
The subsample consisted of mostly non-Hispanic white participants (92.1%) with a bachelor’s degree (42.1%) or graduate education (44.7%) who had never smoked (73.7%) and had no chronic conditions (73.7%). They ranged 40–81 years old (M = 58.6, SD = 11.2) and had been married 1–55 years (M = 30.6, SD = 14.3). On average, they were highly satisfied in their relationship (MCouple Satisfaction Index = 129.1, SD = 28.6), though marital satisfaction scores spanned a large range (43− 158), with 18.4% of the sample meeting criteria for clinically significant relationship distress (<104.5). There were no differences between the analytic subsample and the parent study’s participants on any demographic, relationship, or health dimension (i.e., race, age, education, comorbidities, smoking history, alcohol intake, sagittal abdominal diameter, marital satisfaction), except that couples in the present study had lived together longer than those in the parent sample (t(212) = − 2.31, p = .022; Manalytic sample = 33.3, SD = 14.2; Mparent sample = 27.6, SD = 13.9).
2.2. Data collection procedure
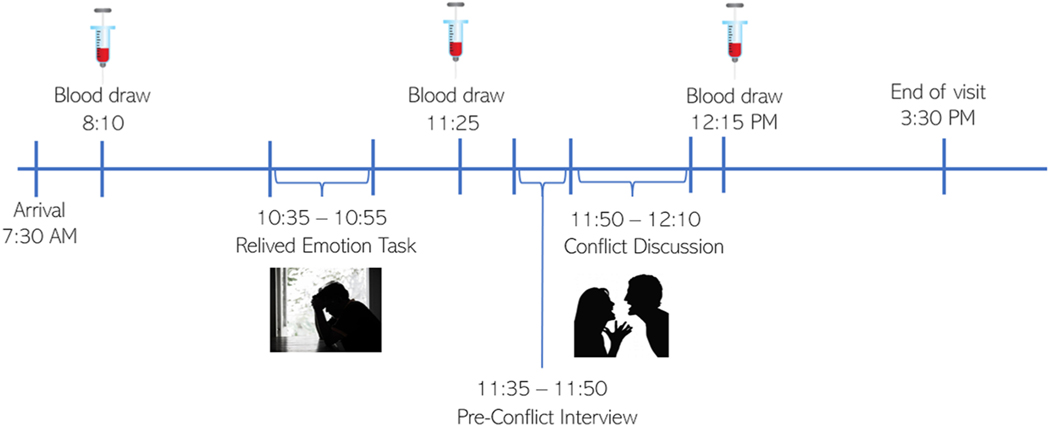
Study procedures were approved by the Ohio State University Institutional Review Board; participants provided written informed consent before completing study activities. When participants arrived at the hospital research unit, both partners were fit with a venous catheter. Following a brief relaxation period, participants provided baseline blood samples and rated their moods. Then, in a variation of the relived emotion task (Levenson et al., 1991; Richter et al., 2011) adapted for couples, each person took a turn reliving a distressing personal memory aloud for 8 min in their partner’s presence—in randomized order, balanced by sex. The event had to be one that continued to elicit strong emotion, and that they experienced as an individual (e.g., death of a parent or sibling), not jointly with the partner (e.g., illness of a child). Before the task, participants were directed away from events that triggered clinically significant posttraumatic symptoms according to a PTSD screener (Prins et al., 2016). The discloser was asked to focus on reliving their emotions rather than reporting the facts—how they felt at the time, what made it difficult, what it felt like in their body, how they felt in the present moment—reviewing each vivid scene in emotional detail. The listener was asked to silently take in the discloser’s experience without interrupting or conversing (Langer et al., 2007; Lewis and Manusov, 2009). Immediately afterward, both participants rated their moods, then they switched roles and, again, rated their moods. Because it takes ~20–25 min for proinflammatory gene expression to meaningfully change following a stimulus (Cole, 2010), after 30 min had passed, both participants provided a blood sample. In accordance with the parent study’s aims, thereafter, the couple discussed one or two of their most important marital disagreements (e.g., money, communication, or in-laws) for 20 min, then rated their moods. The research team remained out of sight while videotaping each task. Final blood samples were collected 5 min after the conflict discussion, 80–90 min after the disclosures. See the timeline of study activities visualized in Fig. 1.
Fig. 1. Study Visit Timeline.
Note. Approximately 2.5 h following the baseline blood draw, each partner completed the 8-minute relived emotion task, in randomized order balanced by sex, and rated their mood after each turn. Approximately 40 min after the end of the first disclosure and 30 min after the second disclosure, both partners provided another blood sample. Following an unstructured interview to identify the most important areas of disagreement in their relationship, the couple discussed one or two marital problems for 20 min then rated their mood. The final blood draw was taken 5 min after the end of the marital conflict discussion, 80–90 min after the disclosures.
2.3. Self-report measures
Participants rated their negative mood at baseline and after each task on a scale of 0 (not at all) to 100 (extremely). Items included “sad or blue”, “gloomy”, “angry or irritable”, and “upset,” given their reliability and sensitivity to an emotional video (Richter et al., 2011). Cronbach’s α ranged .81–.89 for these items in the present study. To examine negative mood reactivity as a predictor of post-task gene expression, difference scores subtracted baseline negative mood from each post-task rating so that higher scores reflected larger increases.
2.4. Behavioral coding
Each participant’s disclosure received a global rating for its emotional intensity. The score took into account any observable cue that indicated emotional upset—crying (e.g., tears welling up, wiping of the eyes), facial expressions (e.g., eyebrows furrowing in sadness or anger), vocal cues (e.g., cracking, wavering), physical gestures (e.g., covering face with hands, using hands or arms for emphasis), and emotional language (e.g., using negative emotion words such as sad, devastated, etc.). Scores ranged 0, i.e., not at all emotionally upset while strictly reporting the facts or events, to 3, i.e., intensely upset. Independent coders achieved reliable ratings (Finn’s r = 0.85).
Marital disagreement discussions were coded using the Rapid Marital Interaction Coding System (RMICS), which discriminates well between distressed and nondistressed couples (Heyman, 2004). Consistent with prior work (Kiecolt-Glaser et al., 2005), the composite hostility score summed the four most negative RMICS codes of both partners in the conflict discussion: psychological abuse (e.g., disgust, contempt), distress-maintaining attributions (e.g., “You were being mean on purpose”), hostility (e.g., criticism), and withdrawal (e.g., behaviors that suggest not listening). Interrater agreement was sufficient, with a Holley and Guilford’s G of 0.86.
2.5. Gene expression assays
Immediately after each blood draw, technicians pipetted 200 μL of whole blood onto Whatman #903 filter paper, allowed it to dry for 24 h, then stored at − 80°C. This method mirrored that of a follow-up study wherein serial blood draws from a venous catheter would not be feasible. Following RNA extraction, samples were tested for suitable mass and integrity, converted to cDNA using a high-efficiency mRNA-targeted reverse transcription system (Lexogen QuantSeq 3’ FWD), and sequenced on an Illumina NextSeq system, all following the manufacturers’ standard protocols for low-mass RNA samples (Cole et al., 2011; McDade et al., 2016; Moieni et al., 2015). Assays targeted 4 million reads per sample (achieved median = 4.2), each of which was mapped to the GRCh38 reference human transcriptome (median mapping rate = 91.6%) and quantified as gene transcripts per million total mapped reads using the STAR aligner. Read counts were log2-transformed to stabilize variance and analyzed as described below, focusing on a well-validated set of 19 proinflammatory genes, in accordance with prior work (Cole et al., 2015). Of the 19 genes in our targeted set, 16 produced detectable expression (mean transcript counts per million total reads > 1) and sufficient variability for analysis (CXCL8, FOS, FOSB, FOSL2, IL1B, JUN, JUNB, JUND, NFKB1, NFKB2, PTGS1, PTGS2, REL, RELA, RELB, TNF).
2.6. Analytic approach
Hypotheses were evaluated in SAS version 9.4 (Cary, NC) with linear mixed models, which allowed explicit modeling of within-person correlations across blood samples and gene transcripts using random person-level intercepts. We attempted to estimate couple-level intercepts to account for possible within-couple correlation, but the variance at this level was too small for the model to converge. To facilitate model estimation, log2-transformed gene expression abundance data were z-scored (Moieni et al., 2015; Schwaiger et al., 2016).
In the first set of models, we examined whether individuals whose partners disclosed with greater emotional intensity had larger subsequent increases in average proinflammatory gene expression from baseline, using two-way interactions between time and partners’ emotional intensity. Couples provided the first post-task blood sample 30 min following the second partner’s disclosure and 40 min after the first partner’s disclosure. Because it takes ~20–25 min for proinflammatory gene expression to meaningfully change following a stimulus (Cole, 2010), this time point captured reactivity to the disclosure task. The final blood sample was collected 80 min after the second disclosure and 90 min after the first disclosure. Because responses in proinflammatory gene expression can resolve in approximately 60 min (Cole, 2010), this sample enabled us to examine whether more emotionally intense partner disclosures were associated with higher sustained reactivity; a non-significant effect would signal recovery. To account for the differing time intervals in the partners’ disclosures relative to the blood sampling, models controlled for disclosure order. To tease apart the reactivity associated with partners’ emotional disclosure from that of individuals’ own disclosure and marital conflict, we controlled for the emotional intensity of individuals’ own disclosure (i.e., the actor effect in this indistinguishable-dyad actor-partner model) and couples’ hostile behavior during the conflict discussion. Other demographic and health-related covariates included the fixed effects of gene, gender (female or male), race (person of color or white), age, smoking history (previously smoked or never smoked), number of alcoholic drinks per week, comorbid conditions (a score of 1 or 2 on the Charlson comorbidity index versus none), and sagittal abdominal diameter. In a second step, we examined whether associations with partner emotional intensity were explained by leukocyte redistribution by controlling for expression of mRNA markers of major leukocyte subsets: CD3D, CD19, CD4, CD8A, FCGR3A, NCAM1, and CD14 (Cole et al., 2015).
A second set of models substituted the listener’s self-reported negative emotional reactivity as the focal predictor in place of the discloser’s coded emotional intensity. By examining two separate indicators of the hypothesized associations, we sought to leverage the unique predictive power of observed behavior alongside individuals’ emotional changes, thereby gauging the robustness of the findings. These models replicated the structure and sequence of the first, and treated negative emotional reactivity to individuals’ own disclosure and the conflict discussion as covariates to parallel the predictor. Finally, supplemental analyses in both sets of models tested the interactions between time (i.e., blood sample) and the other emotional tasks, seeking to further tease apart the unique variance associated with partner’s emotional intensity and changes in proinflammatory gene expression.
3. Results
More than half of spouses (52.6%) in the analytic sample showed overwhelming emotion as they recalled their upsetting memory (a score of 3 on the 0–3 behavioral coding scale), but emotional intensity spanned the entire range. Likewise, on average, listeners’ negative emotion rose 21 points on the 0–100 self-report scale, but ranged widely between individuals (−2.5 to 77.5). All chosen memories were meaningful to the participants: on a scale of 1–7, the topics averaged a score of 6.3 (SD = 0.90). Most couples had already discussed their chosen memories at least a handful of times (81.6%), and some reported discussing the topic frequently (36.8%). As shown in Table 1, spouses’ observed emotional intensity and listeners’ self-reported emotional responses correlated with moderate strength (r = 0.47, p = .003). In addition, negative emotional responses to disclosing an upsetting memory and listening to the spouse’s disclosure tracked together (r = 0.57, p = .0002). Those who showed more hostile behavior during the conflict also endorsed larger increases in self-reported negative emotion post-conflict (r = 0.69, p < .0001). However, behavior and emotional responses to the disclosure tasks were unrelated to those of marital conflict. Observed behavior was not correlated across the three tasks.
Table 1.
Description of Primary Continuous Variables
| Variable | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | M (SD) |
|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||
| 1. Spouse’s Observed Emotional Intensity | - | 2.21 (0.96) | ||||||||
| 2. Own Observed Emotional Intensity | 0.07 | - | 2.21 (0.96) | |||||||
| 3. Hostility Behavior in Conflict | −0.01 | −0.01 | - | 16.17 (24.50) | ||||||
| 4. Negative Emotional Response to Spouse’s Disclosure | 0.47* | 0.30 | −0.22 | - | 21.25 (22.00) | |||||
| 5. Negative Emotional Response to Own Disclosure | 0.24 | 0.36* | 0.04 | 0.57* | - | 31.58 (28.82) | ||||
| 6. Negative Emotional Response to Conflict | −0.03 | −0.08 | 0.69* | −0.02 | 0.28 | - | 11.18 (24.92 | |||
| 7. Baseline Negative Emotion | 0.31 | 0.13 | −0.19 | −0.13 | −0.25 | −0.30 | - | 3.95 (8.55) | ||
| 8. Age | −0.21 | −0.26 | 0.23 | −0.19 | −0.08 | 0.19 | −0.14 | - | 58.82 (11.18) | |
| 9. Sagittal Abdominal Diameter | 0.04 | 0.15 | −0.19 | 0.10 | 0.19 | 0.01 | 0.10 | −0.09 | - | 22.34 (3.49) |
| 10. Alcoholic Drinks per Week | −0.32* | −0.29 | −0.06 | −0.10 | 0.02 | 0.07 | −0.19 | 0.32* | −0.07 | 4.00 (3.81) |
Note.
p < .05.
3.1. Changes in proinflammatory gene expression as a function of the spouse’s observed emotional intensity
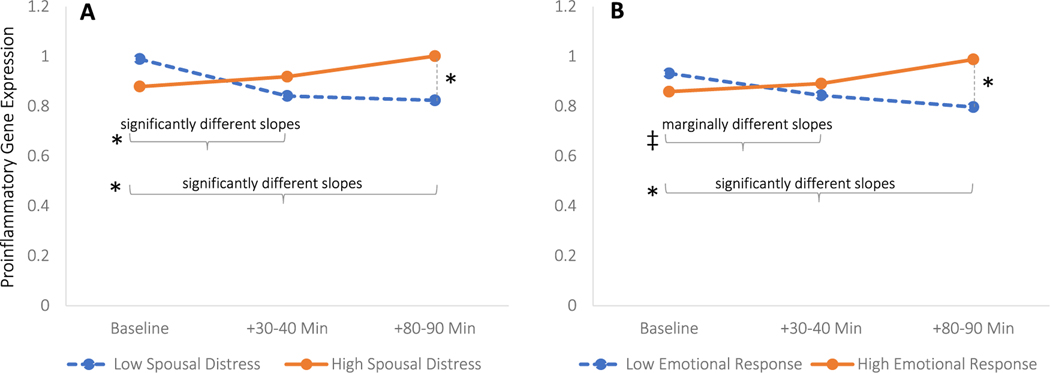
Accounting for the contributions of health covariates, demographics, and the other emotional exchanges, those whose spouse disclosed their upsetting memory with greater emotional intensity had significantly larger increases in proinflammatory gene expression from baseline 30–40 min following the disclosure (Table 2, B = 0.170, SE = 0.060, p = .005, Cohen’s f2 = 0.01), and this association remained significantly elevated from baseline 80–90 min after the disclosure (B = 0.202, SE = 0.060, p = .001, Cohen’s f2 = 0.01). These associations remained significant after controlling for the expression of major leukocyte subset markers at both 30–40 min (B = 0.149, SE = 0.061, p = .014, Cohen’s f2 = 0.01) and 80–90 min after the spouse’s disclosure (B = 0.226, SE = 0.062, p = .0003, Cohen’s f2 = 0.01), which suggests that the changes cannot be attributed to the effects of leukocyte redistribution alone. Associations were also robust to other emotional tasks’ interactions with time, which were not statistically significant (Table S1).
Table 2.
Associations between Partners’ Observed Emotional Intensity and Proinflammatory Gene Expression
| Predictors | Primary Model | Leukocyte Redistribution | ||||
|---|---|---|---|---|---|---|
|
|
|
|||||
| B | SE | p | B | SE | p | |
|
| ||||||
| Intercept | −0.285 | 0.173 | 0.1133 | −0.430 | 0.192 | 0.0351 |
| CXCL8 | −0.007 | 0.132 | 0.9608 | −0.007 | 0.132 | 0.9606 |
| FOS | 0.016 | 0.132 | 0.9046 | 0.016 | 0.132 | 0.9041 |
| FOSB | −0.004 | 0.132 | 0.9780 | −0.004 | 0.132 | 0.9779 |
| FOSL2 | 0.048 | 0.132 | 0.7159 | 0.048 | 0.132 | 0.7145 |
| IL1B | 0.016 | 0.132 | 0.9017 | 0.016 | 0.132 | 0.9011 |
| JUN | 0.001 | 0.132 | 0.9944 | 0.001 | 0.132 | 0.9944 |
| JUNB | −0.014 | 0.132 | 0.9137 | −0.014 | 0.132 | 0.9133 |
| JUND | 0.022 | 0.132 | 0.8693 | 0.022 | 0.132 | 0.8686 |
| NFKB1 | −0.007 | 0.132 | 0.9601 | −0.007 | 0.132 | 0.9599 |
| NFKB2 | 0.004 | 0.132 | 0.9761 | 0.004 | 0.132 | 0.9760 |
| PTGS1 | 0.030 | 0.132 | 0.8208 | 0.030 | 0.132 | 0.8199 |
| PTGS2 | 0.013 | 0.132 | 0.9221 | 0.013 | 0.132 | 0.9217 |
| REL | 0.004 | 0.132 | 0.9760 | 0.004 | 0.132 | 0.9759 |
| RELA | 0.013 | 0.132 | 0.9224 | 0.013 | 0.132 | 0.9220 |
| RELB TNF (Reference) | 0.018 | 0.132 | 0.8927 | 0.018 | 0.132 | 0.8922 |
| Age | 0.006 | 0.004 | 0.1152 | 0.006 | 0.004 | 0.1529 |
| Female | 0.127 | 0.090 | 0.1578 | 0.119 | 0.092 | 0.1960 |
| Race/ethnicity | −0.240 | 0.155 | 0.1215 | −0.251 | 0.157 | 0.1114 |
| SAD | −0.034 | 0.013 | 0.0065 | −0.028 | 0.013 | 0.0335 |
| Comorbidities | 0.007 | 0.093 | 0.9421 | 0.056 | 0.096 | 0.5585 |
| Smoking | −0.053 | 0.099 | 0.5898 | −0.081 | 0.102 | 0.4260 |
| Alcohol | 0.013 | 0.011 | 0.2413 | 0.012 | 0.011 | 0.2946 |
| Disclosure Order | −0.025 | 0.106 | 0.8154 | 0.012 | 0.112 | 0.9150 |
| Time (Baseline) | 0.444 | 0.144 | 0.0021 | 0.490 | 0.149 | 0.0010 |
| Time (Post +30–40 Min) | −0.019 | 0.141 | 0.8908 | 0.107 | 0.146 | 0.4666 |
| Time (Post +80–90 Min, Reference) | ||||||
| Leukocyte Redistribution | ||||||
| CD3D | 0.013 | 0.014 | 0.3565 | |||
| CD19 | 0.056 | 0.058 | 0.3395 | |||
| CD4 | 0.066 | 0.027 | 0.0142 | |||
| CD8A | 0.060 | 0.023 | 0.0088 | |||
| FCGR3A | 0.005 | 0.018 | 0.7815 | |||
| NCAM1 | 0.063 | 0.052 | 0.2261 | |||
| CD14 | −0.008 | 0.017 | 0.6143 | |||
| Behavioral Measures | ||||||
| Partner Emotional Intensity | 0.142 | 0.054 | 0.0089 | 0.141 | 0.055 | 0.0107 |
| Own Emotional Intensity | 0.080 | 0.045 | 0.0730 | 0.073 | 0.046 | 0.1132 |
| Couple Hostile Behavior | −0.002 | 0.002 | 0.2351 | −0.002 | 0.002 | 0.2599 |
| Partner Emotional Intensity * Time (Baseline) | −0.202 | 0.060 | 0.0008 | −0.226 | 0.062 | 0.0003 |
| Partner Emotional Intensity * Time (Post +30–40 Min) | −0.032 | 0.059 | 0.5882 | −0.077 | 0.061 | 0.2104 |
| Contrasts | ||||||
| Partner Emotional Intensity * Baseline vs. Post-task (Both Points) | 0.186 | 0.052 | 0.0004 | 0.188 | 0.053 | 0.0004 |
| Partner Emotional Intensity * Baseline vs. Post +30–40 Min | 0.170 | 0.060 | 0.0049 | 0.149 | 0.061 | 0.0144 |
| Partner Emotional Intensity * Baseline vs. Post +80–90 Min | 0.202 | 0.060 | 0.0008 | 0.226 | 0.062 | 0.0003 |
| Partner Emotional Intensity * Post +30–40 Min vs. Post +80–90 Min | 0.032 | 0.059 | 0.5882 | 0.077 | 0.061 | 0.2104 |
3.2. Changes in proinflammatory gene expression as a function of the listener’s self-reported emotional response to the spouse’s disclosure
In parallel, listeners who experienced more severe negative emotional responses following their spouse’s disclosure also showed larger increases in proinflammatory gene expression from baseline 30–40 min later (Table 3, B = 0.006, SE = 0.003, p = .024, Cohen’s f2 = 0.01), as well as 80–90 min later (B = 0.010, SE = 0.003, p < .0001, Cohen’s f2 = 0.01). In contrast to findings with spouses’ observed emotional intensity, controlling for leukocyte subset markers reduced the association at 30–40 min post-disclosure to a non-significant trend (B = 0.005, SE = 0.003, p = .077). However, controlling for leukocyte subsets did not attenuate the association at 80–90 min post-disclosure (B = 0.010, SE = 0.003, p = .0001, Cohen’s f2 = 0.01). These associations did not change with the inclusion of other emotional tasks’ interactions with time (Table S2), one of which was statistically significant: those who had larger emotional responses to conflict also had greater changes in proinflammatory gene expression thereafter (B = 0.006, SE = 0.003, p = .039, Cohen’s f2 = 0.004).
Table 3.
Associations between Emotional Responses to Partners’ Upsetting Disclosure and Proinflammatory Gene Expression
| Predictors | Primary Model | Leukocyte Redistribution | ||||
|---|---|---|---|---|---|---|
|
|
|
|||||
| B | SE | p | B | SE | p | |
|
| ||||||
| Intercept | −0.150 | 0.132 | 0.2673 | −0.328 | 0.146 | 0.0332 |
| CXCL8 | −7.61E-15 | 0.127 | 1.0000 | −6.33E-15 | 0.127 | 1.0000 |
| FOS | −7.37E-15 | 0.127 | 1.0000 | −6.09E-15 | 0.127 | 1.0000 |
| FOSB | −7.54E-15 | 0.127 | 1.0000 | −6.25E-15 | 0.127 | 1.0000 |
| FOSL2 | −7.39E-15 | 0.127 | 1.0000 | −6.10E-15 | 0.127 | 1.0000 |
| IL1B | −7.37E-15 | 0.127 | 1.0000 | −6.09E-15 | 0.127 | 1.0000 |
| JUN | −7.68E-15 | 0.127 | 1.0000 | −6.41E-15 | 0.127 | 1.0000 |
| JUNB | −7.65E-15 | 0.127 | 1.0000 | −6.37E-15 | 0.127 | 1.0000 |
| JUND | −7.61E-15 | 0.127 | 1.0000 | −6.33E-15 | 0.127 | 1.0000 |
| NFKB1 | −7.33E-15 | 0.127 | 1.0000 | −6.04E-15 | 0.127 | 1.0000 |
| NFKB2 | −7.56E-15 | 0.127 | 1.0000 | −6.27E-15 | 0.127 | 1.0000 |
| PTGS1 | −8.47E-15 | 0.127 | 1.0000 | −7.19E-15 | 0.127 | 1.0000 |
| PTGS2 | −7.42E-15 | 0.127 | 1.0000 | −6.14E-15 | 0.127 | 1.0000 |
| REL | −7.58E-15 | 0.127 | 1.0000 | −6.30E-15 | 0.127 | 1.0000 |
| RELA | −7.37E-15 | 0.127 | 1.0000 | −6.10E-15 | 0.127 | 1.0000 |
| RELB | −7.37E-15 | 0.127 | 1.0000 | −6.10E-15 | 0.127 | 1.0000 |
| TNF (Reference) | ||||||
| Age | 0.011 | 0.004 | 0.0063 | 0.009 | 0.004 | 0.0262 |
| Female | 0.181 | 0.092 | 0.0485 | 0.161 | 0.089 | 0.0718 |
| Race/ethnicity | −0.139 | 0.157 | 0.3748 | −0.182 | 0.153 | 0.2337 |
| SAD | −0.024 | 0.014 | 0.0853 | −0.020 | 0.014 | 0.1580 |
| Comorbidities | 0.004 | 0.104 | 0.9677 | 0.061 | 0.101 | 0.5483 |
| Smoking | −0.041 | 0.101 | 0.6825 | −0.088 | 0.099 | 0.3784 |
| Alcohol | 0.006 | 0.012 | 0.6222 | 0.008 | 0.011 | 0.4915 |
| Disclosure Order | −0.089 | 0.103 | 0.3888 | −0.023 | 0.102 | 0.8182 |
| Time (Baseline) | 0.229 | 0.079 | 0.0038 | 0.226 | 0.081 | 0.0053 |
| Time (Post +30–40 Min) | 0.027 | 0.078 | 0.7312 | 0.082 | 0.080 | 0.3044 |
| Time (Post +80–90 Min, Reference) | ||||||
| Leukocyte Redistribution | ||||||
| CD3D | 0.020 | 0.014 | 0.1618 | |||
| CD19 | −0.013 | 0.037 | 0.7164 | |||
| CD4 | 0.068 | 0.026 | 0.0089 | |||
| CD8A | 0.053 | 0.022 | 0.0189 | |||
| FCGR3A | 0.004 | 0.017 | 0.8093 | |||
| NCAM1 | 0.045 | 0.051 | 0.3757 | |||
| CD14 | 0.003 | 0.015 | 0.8659 | |||
| Behavioral Measures | ||||||
| Emotional Response to Partner Disclosure | 0.007 | 0.003 | 0.0052 | 0.007 | 0.003 | 0.0051 |
| Emotional Response to Own Disclosure | 0.001 | 0.002 | 0.7500 | 0.001 | 0.002 | 0.7705 |
| Emotional Response to Conflict | −0.004 | 0.002 | 0.0325 | −0.003 | 0.002 | 0.1434 |
| Emotional Response to Partner Disclosure * Time (Baseline) | −0.010 | 0.003 | <.0001 | −0.010 | 0.003 | 0.0001 |
| Emotional Response to Partner Disclosure * Time (Post +30–40 Min) | −0.005 | 0.003 | 0.0726 | −0.005 | 0.003 | 0.0369 |
| Contrasts | ||||||
| Emotional Response to Partner Disclosure * Baseline vs. Post-task (Both Points) | 0.008 | 0.002 | 0.0003 | 0.007 | 0.002 | 0.0013 |
| Emotional Response to Partner Disclosure * Baseline vs. Post +30–40 Min | 0.006 | 0.003 | 0.0237 | 0.005 | 0.003 | 0.0772 |
| Emotional Response to Partner Disclosure * Baseline vs. Post +80–90 Min | 0.010 | 0.003 | <.0001 | 0.010 | 0.003 | 0.0001 |
| Emotional Response to Partner Disclosure * Post +30–40 Min vs. Post +80–90 Min | 0.005 | 0.003 | 0.0726 | 0.005 | 0.003 | 0.0369 |
4. Discussion
In a sample of healthy middle-aged and older couples, individuals whose spouse showed greater emotional distress while recounting an upsetting personal memory had larger increases in proinflammatory gene expression 30–40 min later, and these differences persisted 80–90 min after the disclosure. These results were robust with respect to health-related confounds, demographic differences, the emotional dynamics of other tasks in the study visit, and markers of leukocyte redistribution. Associations with listeners’ negative emotional reactivity largely mirrored findings with spouses’ observed distress. This study provides the first evidence that witnessing a spouse’s emotional distress can trigger proinflammatory responses. Thus, spousal distress may play a key role in the way close relationships shape health in the second half of life.
Prior work on spousal distress associations has largely focused on suffering related to painful or degenerative conditions, such as osteoarthritis and Alzheimer’s disease, and its links to a partner’s well-being, sleep, and blood pressure (Martire et al., 2013; Monin and Schulz, 2010; Monin et al., 2010). The current findings extend these patterns to emotional distress and inflammatory processes in healthy couples, providing a novel route by which spousal distress may contribute to inflamm-aging, if repeated over time (Franceschi et al., 2000; Kiecolt-Glaser et al., 2020). In terms of the temporal dynamics, gene expression changes can appear as early as 20 min after a stressful stimulus and resolve in approximately 60 min, if the psychosocial dynamics promote recovery (e.g., Cole, 2010; Moieni et al., 2015). Our results showed no evidence of downregulation: proinflammatory gene expression continued to rise in association with greater spousal distress 80–90 min later. This is especially striking in light of the fact that most couples had discussed their upsetting memories many times before. To the extent that these persisting effects prolong the production of new inflammatory proteins, such effects may hold greater potential for health relevance than if the response had resolved more rapidly. Couples discussed a marital problem immediately before the final blood draw, but models accounted for hostile behavior during that discussion. Findings were largely consistent between observed spousal distress and listeners’ self-reported emotional reactivity, although results with observed emotional intensity were larger and more robust to covariates than those of individuals’ emotional responses 30–40 min post-task. This reflects a pattern commonly seen in lab-based paradigms: Directly observed behavior (spouse distress in this case) more strongly predicts health-related outcomes than does self-report (e.g., Wilson et al., 2020), likely due to the self-presentation biases and measurement limitations of self-ratings (Fazio and Olson, 2003).
The current study’s results are relevant for middle-aged and older couples alike, but may be especially important in older age. As social networks shrink across adulthood, the marital relationship may grow more influential for the health of both partners (Kiecolt-Glaser and Wilson, 2017). In addition, normative challenges faced in older age, such as the loss of loved ones and the emergence of health problems, present unavoidable sources of distress. On the other hand, longitudinal evidence suggests that hostile marital conflict diminishes over time (Verstaen et al., 2018), and some of the most conflict-ridden marriages may dissolve by older age (Brown and Lin, 2012). Thus, in comparison to conflict—the most widely studied and understood behavioral mechanism linking marriage to health—a spouse’s distress may stand to exact greater health costs as couples navigate the difficulties of aging. Indeed, supplemental analyses revealed that spousal distress shared larger and more consistent associations with inflammatory responses than did marital conflict or even individuals’ own upsetting memories.
Moreover, the findings remained significant after accounting for couples’ hostility during conflict, the gold-standard behavioral marker of marital distress. Thus, the pattern applies to satisfied and dissatisfied couples alike. Indeed, the sample was highly satisfied on average, but a notable subset of couples met criteria for clinically significant relationship distress (18.4%). Likewise, almost one third of couples (27.8%) exchanged more than 30 hostile remarks (i.e., more than one per minute), and up to 83 acts of hostility during the 20-minute discussion. In this way, the fact that associations with spousal distress held after controlling for hostile marital behavior is meaningful. It also provides a novel pathway by which happy couples may face health risks in the relationship, particularly in periods of suffering and distress. In addition, emotional reactivity to the partner’s distress was unrelated to hostile behavior during conflict, suggesting that it offers unique information about the marital relationship. Therefore, examining a person’s emotional and physiological responses to their partner’s distress sheds new light on how marriage affects health.
The couples in this sample were recruited for their good health, to avoid disease- and medication-related confounds—both a strength and a limitation. It will be important for future work to replicate the study in a larger sample with more health conditions and greater diversity to gauge generalizability. In another limitation, due to time constraints in the laboratory, we had to statistically separate the contributions of listening to the partner’s disclosure from those of their own disclosure and conflict. Future work should seek to replicate the findings with more time and separate blood samples between tasks. Also, the associations of interest were smaller in magnitude than expected. Nevertheless, the study had greater power to detect smaller associations than anticipated given the minimal correlations within-person and within-couple, paired with the repeated measures design. In addition, a few genes’ expression levels (e.g., IL6, IL1A) could not be detected, in part because circulating leukocytes do not actively transcribe IL6 except during acute infection. This limitation is generic to all methods that collect circulating leukocytes and assay them by the highly specific methodology of RNA sequencing or RT-PCR. Finally, future research in larger samples must document the potential health impacts of the RNA biomarker changes observed here, as well as conduct well-powered genome-wide discovery analyses to uncover additional genomic response beyond the inflammatory dynamics targeted here.
In conclusion, proinflammatory gene expression rose with spousal distress and persisted up to 90 min later, with no evidence of recovery during the study visit. Repeated over time, this dynamic may contribute a unique role to inflamm-aging for adult couples of all ages, and may grow increasingly central to couples’ health with older age. The findings highlight a novel risk that may even lurk in satisfying marriages, raising the possibility of a happy marriage’s diminishing returns in later life.
Supplementary Material
Fig. 2. Spouses’ observed emotional distress and listeners’ emotional reactivity predicting changes in proinflammatory gene expression.
Note. Proinflammatory gene expression values represent z-scores and were back-transformed from the log 2 transformation to aid visual interpretation. Plotted estimates adjust for all covariates: disclosure order, emotional valence of individuals’ own disclosure and marital problem discussion, gene, gender, race, age, smoking history, alcohol use, comorbidities, and sagittal abdominal diameter, and gene expression values associated with leukocyte redistribution. Panel A shows changes in association with emotional intensity of the spouse’s disclosure. Trajectories of gene expression relative to baseline were significantly different for those whose spouse disclosed with high emotional intensity (+1 SD, score of 3) versus low intensity (− 1 SD, score of 1) at both time points. Panel B shows changes in association with listeners’ emotional reactivity to the spouse’s disclosure. Trajectories of gene expression relative to baseline were marginally different 30–40 min post-disclosure and significantly different 80–90 min post-disclosure for listeners who emotionally responded (+1 SD, increase of 43) compared to those who did not (− 1 SD, increase of 0). Levels of gene expression were significantly different 80–90 min after spousal disclosure as a function of the discloser’s emotional intensity and listener’s emotional reactivity. *, p < .05, ‡, p < .077.
Acknowledgments
This work was supported by the National Institutes of Health (KL2TR002530 (PI S. Robb) and UL1TR002529 (Co-PIs S. Wiehe, S. Moe) support M.R.S., UL1RR025755, R01 AG057032 to J.K.K., K05 CA172296 to J.K.K., R00 AG056667 to S.J.W., and L30 AG060525 to S.J.W.) and an Ohio State University Presidential Postdoctoral Scholars Fellowship to M.R.S.
Footnotes
Appendix A. Supporting information
Supplementary data associated with this article can be found in the online version at doi:10.1016/j.psyneuen.2023.106116.
Conflicts of Interest
The authors have no conflicts of interest to declare.
References
- Brown SL, Lin IF, 2012. The gray divorce revolution: rising divorce among middle-aged and older adults, 1990–2010. J. Gerontol. Ser. B, Psychol. Sci. Soc. Sci 67 (6), 731–741. 10.1093/geronb/gbs089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carstensen LL, 1995. Evidence for a life-span theory of socioemotional selectivity. Curr. Dir. Psychol. Sci 4 (5), 151–156. 10.2307/20182356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charles ST, 2010. Strength and vulnerability integration: a model of emotional well-being across adulthood. Psychol. Bull 136 (6), 1068–1091. 10.1037/a0021232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole SW, 2010. Elevating the perspective on human stress genomics. Psychoneuroendocrinology 35 (7), 955–962. 10.1016/j.psyneuen.2010.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole SW, Hawkley LC, Arevalo JMG, Cacioppo JT, 2011. Transcript origin analysis identifies antigen-presenting cells as primary targets of socially regulated gene expression in leukocytes. Proc. Natl. Acad. Sci 108 (7), 3080–3085. 10.1073/pnas.1014218108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole SW, Levine ME, Arevalo JM, Ma J, Weir DR, Crimmins EM, 2015. Loneliness, eudaimonia, and the human conserved transcriptional response to adversity. Psychoneuroendocrinology 62, 11–17. 10.1016/j.psyneuen.2015.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donoho CJ, Crimmins EM, Seeman TE, 2013. Marital quality, gender, and markers of inflammation in the MIDUS Cohort. J. Marriage Fam 75 (1), 127–141. 10.1111/j.1741-3737.2012.01023.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epel ES, 2020. The geroscience agenda: toxic stress, hormetic stress, and the rate of aging. Ageing Res. Rev 63, 101167 10.1016/j.arr.2020.101167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fazio RH, Olson MA, 2003. Implicit measures in social cognition research: their meaning and use. Annu. Rev. Psychol 54, 297–327. 10.1146/annurev.psych.54.101601.145225. [DOI] [PubMed] [Google Scholar]
- Franceschi C, Bonafe M, Valensin S, Olivieri F, De Luca M, Ottaviani E, De Benedictis G, 2000. Inflamm-aging. An evolutionary perspective on immunosenescence. Ann. N. Y. Acad. Sci 908, 244–254. 10.1111/j.1749-6632.2000.tb06651.x. [DOI] [PubMed] [Google Scholar]
- Heyman RE, 2004. Rapid Marital Interaction Coding System (RMICS). In: Kerig PK, Baucom DH (Eds.), Couple Observational Coding Systems. Lawrence Erlbaum Associates., pp. 67–94 [Google Scholar]
- Kane HS, Slatcher RB, Reynolds BM, Repetti RL, Robles TF, 2014. Daily self-disclosure and sleep in couples. Health Psychol. 33 (8), 813–822. 10.1037/hea0000077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiecolt-Glaser JK, Wilson SJ, 2017. Lovesick: how couples’ relationships influence health. Annu. Rev. Clin. Psychol 17, 421–443. 10.1146/annurevclinpsy-032816-045111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiecolt-Glaser JK, Loving TJ, Stowell JR, Malarkey WB, Lemeshow S, Dickinson SL, Glaser R, 2005. Hostile marital interactions, proinflammatory cytokine production, and wound healing. Arch. Gen. Psychiatry 62 (12), 1377–1384. 10.1001/archpsyc.62.12.1377. [DOI] [PubMed] [Google Scholar]
- Kiecolt-Glaser JK, Jaremka L, Andridge R, Peng J, Habash D, Fagundes CP, Glaser R, Malarkey WB, Belury MA, 2015. Marital discord, past depression, and metabolic responses to high-fat meals: interpersonal pathways to obesity. Psychoneuroendocrinology 52, 239–250. 10.1016/j.psyneuen.2014.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiecolt-Glaser JK, Renna ME, Shrout MR, Madison AA, 2020. Stress reactivity: what pushes us higher, faster, and longer - and why it matters. Curr. Dir. Psychol. Sci 29 (5), 492–498. 10.1177/0963721420949521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langer SL, Rudd ME, Syrjala KL, 2007, 2007. Protective buffering and emotional desynchrony among spousal caregivers of cancer patients. Health Psychol. 26, 635–643. [DOI] [PubMed] [Google Scholar]
- Levenson RW, Carstensen LL, Friesen WV, Ekman P, 1991. Emotion, physiology, and expression in old age. Psychol. Aging 6, 28–35. 〈http://psycnet.apa.org/journals/pag/6/1/28.pdf〉. [DOI] [PubMed] [Google Scholar]
- Lewis T, Manusov V, 2009. Listening to another’s distress in everyday relationships. Commun. Q 57 (3), 282–301. 10.1080/01463370903107279. [DOI] [Google Scholar]
- Liu H, Waite L, 2014. Bad marriage, broken heart? Age and gender differences in the link between marital quality and cardiovascular risks among older adults. J. Health Soc. Behav 55 (4), 403–423. 10.1177/0022146514556893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martire LM, Keefe FJ, Schulz R, Stephens MAP, Mogle JA, 2013. The impact of daily arthritis pain on spouse sleep. Pain 154 (9), 1725–1731. 10.1016/j.pain.2013.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDade TW, K MR, R LF, Arevalo JM, Ma J, Miller GE, Cole SW, 2016. Genome-wide profiling of RNA from dried blood spots: convergence with bioinformatic results derived from whole venous blood and peripheral blood mononuclear cells. Biodemogr. Soc. Biol 62 (2), 182–197. 10.1080/19485565.2016.1185600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwen BS, 1998. Stress, adaptation, and disease: allostasis and allostatic load. Ann. N. Y. Acad. Sci 840, 33–44. 〈http://www.ncbi.nlm.nih.gov/pubmed/9629234〉. [DOI] [PubMed] [Google Scholar]
- Moieni M, Irwin MR, Jevtic I, Breen EC, Cho HJ, Arevalo JMG, Ma J, Cole SW, Eisenberger NI, 2015. Trait sensitivity to social disconnection enhances proinflammatory responses to a randomized controlled trial of endotoxin. Psychoneuroendocrinology 62, 336–342. 10.1016/j.psyneuen.2015.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monin JK, Schulz R, 2010. The effects of suffering in chronically Ill older adults on the health and well-being of family members involved in their care: the role of emotion- related processes. GeroPsych 23 (4), 207–213. 10.1024/1662-9647/a000024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monin JK, Schulz R, Martire LM, Jennings JR, Lingler JH, Greenberg MS, 2010. Spouses’ cardiovascular reactivity to their partners’ suffering. J. Gerontol. Ser. B Psychol. Sci. Soc. Sci 65B (2), 195–201. 10.1093/geronb/gbp133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prins A, Bovin MJ, Smolenski DJ, Marx BP, Kimerling R, Jenkins-Guarnieri MA, Kaloupek DG, Schnurr PP, Kaiser AP, Leyva YE, Tiet QQ, 2016. The primary care PTSD screen for DSM-5 (PC-PTSD-5): development and evaluation within a veteran primary care sample. J. Gen. Intern. Med 31 (10), 1206–1211. 10.1007/s11606-016-3703-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richter D, Dietzel C, Kunzmann U, 2011. Age differences in emotion recognition: the task matters. J. Gerontol. B Psychol. Sci. Soc. Sci 66 (1), 48–55. 10.1093/geronb/gbq068. [DOI] [PubMed] [Google Scholar]
- Robles TF, Slatcher RB, Trombello JM, McGinn MM, 2014. Marital quality and health: a meta-analytic review. Psychol. Bull 140 (1), 140–187. 10.1037/a0031859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwaiger M, Grinberg M, Moser D, Zang JCS, Heinrichs M, Hengstler JG, Rahnenführer J, Cole S, Kumsta R, 2016. Altered Stress-Induced Regulation of Genes in Monocytes in Adults with a History of Childhood Adversity. Neuropsychopharmacol.: Off. Publ. Am. Coll. Neuropsychopharmacol 41 (10), 2530–2540. 10.1038/npp.2016.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Story TN, Berg CA, Smith TW, Beveridge R, Henry NJ, Pearce G, 2007. Age, marital satisfaction, and optimism as predictors of positive sentiment override in middle-aged and older married couples. Psychol. Aging 22 (4), 719–727. 10.1037/0882-7974.22.4.719. [DOI] [PubMed] [Google Scholar]
- Verstaen A, Haase CM, Lwi SJ, Levenson RW, 2018. Age-related changes in emotional behavior: Evidence from a 13-year longitudinal study of long-term married couples. Emot., Adv. Online Publ. 10.1037/emo0000551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Abbas KM, Abbasifard M, Abbasi-Kangevari M, Abbastabar H, Abd-Allah F, Abdelalim A, Abolhassani H, Abreu LG, Abrigo MRM, Abushouk AI, Adabi M, Adair T, Adebayo OM, Adedeji IA, Adekanmbi V, Adeoye AM, Adetokunboh OO, Advani SM, Afshin A, Aghaali M, Agrawal A, Ahmadi K, Ahmadieh H, Ahmed MB, Al-Aly Z, Alam K, Alam T, Alanezi FM, Alanzi TM, Alcalde-Rabanal JE, Ali M, Alicandro G, Alijanzadeh M, Alinia C, Alipour V, Alizade H, Aljunid SM, Allebeck P, Almadi MAH, Almasi-Hashiani A, Al-Mekhlafi HM, Altirkawi KA, Alumran AK, Alvis-Guzman N, Amini-Rarani M, Aminorroaya A, Amit AML, Ancuceanu R, Andrei CL, Androudi S, Angus C, Anjomshoa M, Ansari F, Ansari I, Ansari-Moghaddam A, Antonio CAT, Antony CM, Anvari D, Appiah SCY, Arabloo J, Arab-Zozani M, Aravkin AY, Aremu O, Ärnlöv J, Aryal KK, Asadi-Pooya AA, Asgari S, Asghari Jafarabadi M, Atteraya MS, Ausloos M, Avila-Burgos L, Avokpaho EFGA, Ayala Quintanilla BP, Ayano G, Ayanore MA, Azarian G, Babaee E, Badiye AD, Bagli E, Bahrami MA, Bakhtiari A, Balassyano S, Banach M, Banik PC, Barker-Collo SL, Bärnighausen TW, Barzegar A, Basu S, Baune BT, Bayati M, Bazmandegan G, Bedi N, Bell ML, Bennett DA, Bensenor IM, Berhe K, Berman AE, Bertolacci GJ, Bhageerathy R, Bhala N, Bhattacharyya K, Bhutta ZA, Bijani A, Biondi A, Bisanzio D, Bisignano C, Biswas RK, Bjørge T, Bohlouli S, Bohluli M, Bolla SRR, Borzì AM, Borzouei S, Brady OJ, Braithwaite D, Brauer M, Briko AN, Briko NI, Bumgarner BR, Burugina Nagaraja S, Butt ZA, Caetano dos Santos FL, Cai T, Callender CSKH, Camera LLAA, Campos-Nonato IR, Cárdenas R, Carreras G, Carrero JJ, Carvalho F, Castaldelli-Maia JM, Castelpietra G, Castro F, Catalá-Ĺopez F, Cederroth CR, Cerin E, Chattu VK, Chin KL, Chu D-T, Ciobanu LG, Cirillo M, Comfort H, Costa VM, Cowden RG, Cromwell EA, Croneberger AJ, Cunningham M, Dahlawi SMA, Damiani G, D’Amico E, Dandona L, Dandona R, Dargan PI, Darwesh AM, Daryani A, Das Gupta R, das Neves J, Davletov K, De Leo D, Denova-Gutiérrez E, Deribe K, Dervenis N, Desai R, Dhungana GP, Dias da Silva D, Diaz D, Dippenaar IN, Djalalinia S, Do HT, Dokova K, Doku DT, Dorostkar F, Doshi CP, Doshmangir L, Doyle KE, Dubljanin E, Duraes AR, Edvardsson D, Effiong A, El Sayed I, El Tantawi M, Elbarazi I, El-Jaafary SI, Emamian MH, Eskandarieh S, Esmaeilzadeh F, Estep K, Farahmand M, Faraj A, Fareed M, Faridnia R, Faro A, Farzadfar F, Fattahi N, Fazaeli AA, Fazlzadeh M, Feigin VL, Fereshtehnejad S-M, Fernandes E, Ferreira ML, Filip I, Fischer F, Flohr C, Foigt NA, Folayan MO, Fomenkov AA, Freitas M, Fukumoto T, Fuller JE, Furtado JM, Gad MM, Gakidou E, Gallus S, Gebrehiwot AM, Gebremedhin KB, Gething PW, Ghamari F, Ghashghaee A, Gholamian A, Gilani SA, Gitimoghaddam M, Glushkova EV, Gnedovskaya EV, Gopalani SV, Goulart AC, Gugnani HC, Guo Y, Gupta R, Gupta SS, Haagsma JA, Haj-Mirzaian A, Haj-Mirzaian A, Halvaei I, Hamadeh RR, Hamagharib Abdullah K, Han C, Handiso DW, Hankey GJ, Haririan H, Haro JM, Hasaballah AI, Hassanipour S, Hassankhani H, Hay SI, Heibati B, Heidari-Soureshjani R, Henny K, Henry NJ, Herteliu C, Heydarpour F, Hole MK, Hoogar P, Hosgood HD, Hossain N, Hosseinzadeh M, Hostiuc M, Hostiuc S, Househ M, Hoy DG, Hu G, Huda TM, Ibitoye SE, Ikuta KS, Ilesanmi OS, Ilic IM, Ilic MD, Imani-Nasab MH, Islam M, Iso H, Iwu CJ, Jaafari J, Jacobsen KH, Jahagirdar D, Jahanmehr N, Jalali A, Jalilian F, James SL, Janjani H, Jenabi E, Jha RP, Jha V, Ji JS, Jonas JB, Joukar F, Jozwiak JJ, Jürisson M, Kabir Z, Kalani H, Kalankesh LR, Kamiab Z, Kanchan T, Kapoor N, Karch A, Karimi SE, Karimi SA, Kassebaum NJ, Katikireddi SV, Kawakami N, Kayode GA, Keiyoro PN, Keller C, Khader YS, Khalid N, Khan EA, Khan M, Khang Y-H, Khater AM, Khater MM, Khazaei S, Khazaie H, Khodayari MT, Khubchandani J, Kianipour N, Kim C.-i, Kim Y-E, Kim YJ, Kinfu Y, Kisa A, Kisa S, Kissimova-Skarbek K, Kivimäki M, Komaki H, Kopec JA, Kosen S, Koul PA, Koyanagi A, Kravchenko MA, Krishan K, Krohn KJ, Kuate Defo B, Kumar GA, Kumar M, Kumar P, Kumar V, Kusuma D, Kyu HH, La Vecchia C, Lacey B, Lal DK, Lalloo R, Lami FH, Lansky S, Larson SL, Larsson AO, Lasrado S, Lassi ZS, Lazarus JV, Lee PH, Lee SWH, Leever AT, LeGrand KE, Leonardi M, Li S, Lim L-L, Lim SS, Linn S, Lodha R, Logroscino G, Lopez AD, Lopukhov PD, Lotufo PA, Lozano R, Lu A, Lunevicius R, Madadin M, Maddison ER, Magdy Abd El Razek H, Magdy Abd El Razek M, Mahasha PW, Mahdavi MM, Malekzadeh R, Mamun AA, Manafi N, Mansour-Ghanaei F, Mansouri B, Mansournia MA, Mapoma CC, Martini S, Martins-Melo FR, Masaka A, Mastrogiacomo CI, Mathur MR, May EA, McAlinden C, McGrath JJ, McKee M, Mehndiratta MM, Mehri F, Mehta KM, Meitei WB, Memiah PTN, Mendoza W, Menezes RG, Mengesha EW, Mensah GA, Meretoja A, Meretoja TJ, Mestrovic T, Michalek IM, Mihretie KM, Miller TR, Mills EJ, Milne GJ, Mirrakhimov EM, Mirzaei H, Mirzaei M, Mirzaei-Alavijeh M, Misganaw AT, Moazen B, Moghadaszadeh M, Mohamadi E, Mohammad DK, Mohammad Y, Mohammad Gholi Mezerji N, Mohammadbeigi A, Mohammadian-Hafshejani A, Mohammadpourhodki R, Mohammed H, Mohammed S, Mohebi F, Mohseni Bandpei MA, Mokari A, Mokdad AH, Momen NC, Monasta L, Mooney MD, Moradi G, Moradi M, Moradi-Joo M, Moradi-Lakeh M, Moradzadeh R, Moraga P, Moreno Velasquez I, Morgado-da-Costa J, Morrison SD, Mosser JF, ´ Mouodi S, Mousavi SM, Mousavi Khaneghah A, Mueller UO, Musa KI, Muthupandian S, Nabavizadeh B, Naderi M, Nagarajan AJ, Naghavi M, Naghshtabrizi B, Naik G, Najafi F, Nangia V, Nansseu JR, Ndwandwe DE, Negoi I, Negoi RI, Ngunjiri JW, Nguyen HLT, Nguyen TH, Nigatu YT, Nikbakhsh R, Nikpoor AR, Nixon MR, Nnaji CA, Nomura S, Noubiap JJ, Nouraei Motlagh S, Nowak C, Oţoiu A, Odell CM, Oh I-H, Oladnabi M, Olagunju AT, Olusanya BO, Olusanya JO, Omar Bali A, Ong KL, Onwujekwe OE, Ortiz A, Otstavnov N, Otstavnov SS, Øverland S, Owolabi MO, P A M, Padubidri JR, Pakshir K, Palladino R, Pana A, Panda-Jonas S, Park J, Pasupula DK, Patel JR, Patel SK, Patton GC, Paulson KR, Pazoki Toroudi H, Pease SA, Peden AE, Pepito VCF, Peprah EK, Pereira A, Pereira DM, Perico N, Pigott DM, Pilgrim T, Pilz TM, Piradov MA, Pirsaheb M, Pokhrel KN, Postma MJ, Pourjafar H, Pourmalek F, Pourshams A, Poznaǹska A, Prada SI, Prakash S, Preotescu L, Quazi Syed Z, Rabiee M, Rabiee N, Radfar A, Rafiei A, Raggi A, Rahman MA, Rajabpour- Sanati A, Ram P, Ranabhat CL, Rao SJ, Rasella D, Rashedi V, Rastogi P, Rathi P, Rawal L, Remuzzi G, Renjith V, Renzaho AMN, Resnikoff S, Rezaei N, Rezai M. s, Rezapour A, Rickard J, Roever L, Ronfani L, Roshandel G, Rostamian M, Rubagotti E, Rwegerera GM, Sabour S, Saddik B, Sadeghi E, Sadeghi M, Saeedi Moghaddam S, Safari Y, Safi S, Safiri S, Sagar R, Sahebkar A, Sahraian MA, Sajadi SM, Salahshoor MR, Salama JS, Salamati P, Salem MRR, Salimi Y, Salomon JA, Salz I, Samad Z, Samy AM, Sanabria J, Santric-Milicevic MM, Saraswathy SYI, Sartorius B, Sarveazad A, Sathian B, Sathish T, Sattin D, Saylan M, Schaeffer LE, Schiavolin S, Schwebel DC, Schwendicke F, Sekerija M, Senbeta AM, Senthilkumaran S, Sepanlou SG, Serván-Mori E, Shabani M, Shahabi S, Shahbaz M, Shaheen AA, Shaikh MA, Shalash AS, Shams-Beyranvand M, Shamsi M, Shamsizadeh M, Shannawaz M, Sharafi K, Sharafi Z, Sharara F, Sharma R, Shaw DH, Sheikh A, Shin JI, Shiri R, Shrime MG, Shuval K, Siabani S, Sigfusdottir ID, Sigurvinsdottir R, Silva DAS, Simonetti B, Simpson KE, Singh JA, Skiadaresi E, Skryabin VY, Soheili A, Sokhan A, Sorensen RJD, Soriano JB, Sorrie MB, Soyiri IN, Spurlock EE, Sreeramareddy CT, Stockfelt L, Stokes MA, Stubbs JL, Sudaryanto A, Sufiyan M. a B., Suliankatchi Abdulkader R, Sykes BL, Tabarés-Seisdedos R, Tabb KM, Tadakamadla SK, Taherkhani A, Tang M, Taveira N, Taylor HJ, Teagle WL, Tehrani-Banihashemi A, Teklehaimanot BF, Tessema ZT, Thankappan KR, Thomas N, Thrift AG, Titova MV, Tohidinik HR, Tonelli M, Topor-Madry R, Topouzis F, Tovani-Palone MRR, Traini E, Tran BX, Travillian R, Trias-Llimós S, Truelsen TC, Tudor Car L, Unnikrishnan B, Upadhyay E, Vacante M, Vakilian A, Valdez PR, Valli A, Vardavas C, Vasankari TJ, Vasconcelos AMN, Vasseghian Y, Veisani Y, Venketasubramanian N, Vidale S, Violante FS, Vlassov V, Vollset SE, Vos T, Vujcic IS, Vukovic A, Vukovic R, Waheed Y, Wallin MT, Walters MK, Wang H, Wang Y-P, Watson S, Wei J, Weiss J, Weldesamuel GT, Werdecker A, Westerman R, Whiteford HA, Wiangkham T, Wiens KE, Wijeratne T, Wiysonge CS, Wojtyniak B, Wolfe CDA, Wondmieneh AB, Wool EE, Wu A-M, Wu J, Xu G, Yamada T, Yamagishi K, Yano Y, Yaya S, Yazdi-Feyzabadi V, Yearwood JA, Yeheyis TY, Yilgwan CS, Yip P, Yonemoto N, Yoon S-J, Yoosefi Lebni J, York HW, Younis MZ, Younker TP, Yousefi Z, Yousefinezhadi T, Yousuf AY, Yusefzadeh H, Zahirian Moghadam T, Zakzuk J, Zaman SB, Zamani M, Zamanian M, Zandian H, Zhang Z-J, Zheng P, Zhou M, Ziapour A, Murray CJL, 2020. Global age-sex-specific fertility, mortality, healthy life expectancy (HALE), and population estimates in 204 countries and territories, 1950–2019: a comprehensive demographic analysis for the Global Burden of Disease Study 2019. In: The Lancet, 396, pp. 1160–1203. 10.1016/S0140-6736(20)30977-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whisman MA, Sbarra DA, 2012. Marital adjustment and interleukin-6 (IL-6). J. Fam. Psychol 26 (2), 290–295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whisman MA, Li A, Sbarra DA, Raison CL, 2014. Marital quality and diabetes: results from the health and retirement study. Health Psychol. 33 (8), 832–840. 10.1037/hea0000064. [DOI] [PubMed] [Google Scholar]
- Wilson SJ, Peng J, Andridge R, Jaremka LM, Fagundes CP, Malarkey WB, Belury MA, Kiecolt-Glaser JK, 2020. For better and worse? The roles of closeness, marital behavior, and age in spouses’ cardiometabolic similarity. Psychoneuroendocrinology 120, 104777. 10.1016/j.psyneuen.2020.104777. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.