Abstract
Uracil DNA glycosylase (UNG) removes mutagenic uracil base from DNA to initiate base excision repair (BER). The result is an abasic site (AP site) that is further processed by the high-fidelity BER pathway to complete repair and maintain genome integrity. The gammaherpesviruses (GHVs), human Kaposi sarcoma herpesvirus (KSHV), Epstein-Barr virus (EBV), and murine gammaherpesvirus 68 (MHV68) encode functional UNGs that have a role in viral genome replication. Mammalian and GHVs UNG share overall structure and sequence similarity except for a divergent amino-terminal domain and a leucine loop motif in the DNA binding domain that varies in sequence and length. To determine if divergent domains contribute to functional differences between GHV and mammalian UNGs, we analyzed their roles in DNA interaction and catalysis. By utilizing chimeric UNGs with swapped domains we found that the leucine loop in GHV, but not mammalian UNGs facilitates interaction with AP sites and that the amino-terminal domain modulates this interaction. We also found that the leucine loop structure contributes to differential UDGase activity on uracil in single- versus double-stranded DNA. Taken together we demonstrate that the GHV UNGs evolved divergent domains from their mammalian counterparts that contribute to differential biochemical properties from their mammalian counterparts.
Keywords: Uracil DNA glycosylase, gammaherpesvirus, abasic site
1. INTRODUCTION
Genomic uracil arises from spontaneous or enzymatic cytosine deamination and misincorporation of dUTP during DNA replication. The U:G mismatches that result from deamination are mutagenic and result in C to T transition mutations if left unrepaired [1, 2]. The primary role of mammalian UNG is the removal of genomic uracil. It cleaves the N-glycosidic bond, creating an abasic site (AP site) and initiates the base excision repair (BER) pathway that facilitates high-fidelity repair [2, 3]. In addition, mammalian UNG plays a critical role in antibody diversification [4], but does so by error-prone repair of uracils created by activation-induced cytidine deaminase (AID) during B cell development [5–8]. An AP site is a requisite intermediate of base removal in BER pathway that arises from many sources and is a common DNA lesion [9]. AP sites must be rapidly repaired as they inhibit transcription and stall the high-fidelity DNA polymerases during DNA replication [9]. Alternatively, translesion DNA polymerases can bypass AP site during replication but often introduce mutations [10].
DNA glycosylases are initiators of BER and channel the AP site intermediate to AP endonuclease 1 (APE1) to create a nick, which is sequentially repaired by DNA polymerases and DNA ligases [11]. High-fidelity repair is facilitated by coordination of UNG and BER machinery. This can be accomplished by a model where intermediate products are handed off to the next component in a “pass the baton” fashion to promote error-free repair [12, 13] or as a preassembled complex of glycosylases with high-fidelity BER machinery that accomplishes this process [2, 14]. The amino-terminus of mammalian UNG contains regulatory phosphorylation sites and binding motifs for proliferating cell nuclear antigen (PCNA) and replication protein A (RPA), that may contribute to repair function [2]. In mammalian cells, there are four glycosylases capable of removing uracil in DNA: UNG, single-strand selective monofunctional uracil DNA glycosylase 1 (SMUG1), thymine DNA glycosylase (TDG) and Methyl-CpG Binding Domain 4 (MBD4). Each has affinity for AP sites except for UNG [15–17]. The high affinity of glycosylases to AP sites is thought to protect them from further detrimental modification or repair by the translesion repair which introduces mutations. The basis for differential AP site affinity and the functional consequence of UNG in this process is not understood.
In addition to prokaryotes and eukaryotes, many viruses encode their own viral homologs of UNG. All herpesviruses examined thus far encode an enzymatically active homolog of UNG [18–22]. Viral UNG (vUNG) promotes herpesvirus replication in cell culture and pathogenesis in vivo [23–26]. The murine gammaherpesvirus 68 (MHV68) pathogen requires vUNG (ORF46) to expand in the lung tissue [22], and vUNG (UL2) of herpes simplex virus type 1 (HSV-1) promotes replication in neuronal tissues of mice [23]. The vUNG is believed to support viral genomic integrity by enzymatic removal of uracils, and it may contribute to the progression of viral DNA replication via interactions with viral replication factors [19, 20, 27]. The vUNG and murine UNG (mUNG) have overlapping but non-redundant roles. While GHV UNG can process host genomic uracils when introduced into B lymphocytes [22], the mUNG cannot fully compensate for the absence of vUNG [28].
The GHVs, murine gammaherpesvirus 68 (MHV68, MuHV-4, GHV68), Epstein-Barr virus (EBV, HHV-4) and Kaposi sarcoma herpesvirus (KSHV, HHV-8) are genetically related viruses that are the etiologic agents of lymphomas and lymphoproliferative disorders [29, 30] as well as other malignancies [31]. These large dsDNA viruses encode their own viral polymerase and replicative accessory factors including UNG [32, 33]. At the protein sequence and structural level, GHV UNG and mammalian UNGs are highly conserved except for the amino terminal domain and a leucine loop motif within the DNA binding domain [21, 34–36]. The leucine loop begins with the conserved motif HPSPL, but then diverges in sequence and the length as a leucine loop extension. In mammalian UNGs there are five additional amino acids, in GHVs there are twelve additional amino acids. The leucine in the HPSPL motif functions to flip the uracil base out of dsDNA [37]. A structure of the KSHVUNG in complex with double-stranded DNA indicates that the leucine loop extension is responsible for flipping the widowed base, opposite the uracil on the complementary strand [36]. This widowed base flipping mechanism is absent in mammalian UNG [37]. The biological consequence of the leucine loop extension and widowed base flipping by the GHV UNGs are not known. In this study we investigated the functional differences of the divergent mammalian and GHV UNG domains on substrate catalysis and DNA binding. Our investigation uncovered substantial differences in AP site binding and substrate recognition between GHVUNG and mUNG. We report the unexpected finding that the leucine loop extension enables GHVUNG binding to AP sites and this interaction is modulated by the N-terminus. We also found that the leucine loop extension contributes to a distinct substrate DNA specificity.
2. MATERIAL AND METHODS
2.1. Bacterial expression constructs and primers
pet-DUET-1 was used for all recombinant UNGs and APE1 expression. Full-length, truncated and chimeric mutants of GHV UNGs and murine UNG were cloned into EcoRI and SalI restriction sites of the expression vector and confirmed with sanger sequencing.
Primers used in this study (Supplementary table 1)
The list of mutant and chimeric UNG proteins used in this study. The name of the mutant and the description of amino acids for each mutant or incorporated from the denoted UNG is listed.
mUNG (1–306)
MHV68UNG (1–249)
EBVUNG (1–255)
KSHVUNG (1–255)
MHV68UNGcat (D85N, H207L)
m/MHV68UNG (mUNG1–86/MHV68UNG24–249)
MHV68/mUNG (MHV68UNG1–23/mUNG87–306)
MHV68UNG-Ls (MHV68UNG 1–211/mUNG 275–279/MHV68UNG 224–249)
mUNG-Ls (mUNG1–274/MHV68UNG212–223/mUNG280–306)
MHV68UNGΔN (MHV68UNG 24–249)
mUNGΔN (mUNG 87–306)
EBVUNGΔN (EBVUNG 30–255)
KSHVUNGΔN (KSHVUNG 27–255)
MHV68UNGΔL-Loop (MHV68UNG 1–211/MHV68UNG219–249)
EBVUNGΔN-N220L (EBVUNG 30–255-N220L)
EBVUNGΔN-QNST219–222SLGG (EBVUNG30–218/KSHVUNG216–219/EBV223–255).
2.2. Recombinant UNGs and APE1 expression and purification
E. coli BL21 (DE3) cells (Invitrogen™ One Shot™ BL21 Star™ (DE3) Chemically Competent E. coli) were transformed with pet-DUET-1 containing UNG variants or APE1. Single colonies were inoculated into LB with 100 μg/ml ampicillin overnight. 10 ml overnight culture was used to inoculate 1 L LB media with 100 μg/ml ampicillin. 0.2 mM IPTG was added to BL21 expression culture containing pet-DUET-1-UNGs or pet-DUET-1-APE1 when OD at 600 nM reached 0.6. BL21 cells were harvested after overnight induction with IPTG and lysed by sonication in lysis buffer (20 mM Tris-HCl, 150 mM NaCl pH 7.5) and centrifuged at 26,916 g (Sorvall Lynx 6000 centrifuge, rotor F21–8X50Y, 15,000 rpm/26,916 g) for 1 hour. 500 μl HisPur Cobalt resin (Thermo Cat. # 89964) was incubated with 250 ml cleared lysate, equally divided into 6 X 50 ml conical tubes, rotated overnight in cold room with Fisherbrand™ Mini-Tube Rotators at speed 12 rpm. Cobalt resin was pelleted and resuspended with lysis buffer and loaded onto Poly-Prep Chromatography column from Bio-RAD (Cat. # 731–1550), which was equilibrated (conditioned) with 5 ml lysis buffer before addition of 500 μl HisPur Cobalt resin, and washed with 2ml 1% triton X-100 followed by 10ml lysis buffer, then 2 ml 5 mM imidazole followed by 10 ml lysis buffer. His-tagged proteins were eluted with 500 μl 150 mM imidazole in lysis buffer and dialyzed against 20 mM Tris-HCl (pH 7.5), 50 mM NaCl, 1 mM dithiothreitol, 1 mM EDTA, and 10% glycerol. Proteins were stored at 50 μM to 200 μM in dialysis buffer at −80 °C. Proteins were quantified after SDS-PAGE and Coomassie blue staining by band intensity quantification through comparison with BSA standard using Image J software (version 1.49) (Supplementary Figure 1).
2.3. Electrophoretic mobility shift assay (EMSA)
The AP site containing DNA was derived from an uracil containing oligo using previously published methods [38, 39]. 20 μM biotin-dT-labeled ssDNA containing a single U was incubated with 20 nM mUNGΔN at 37°C for 2 hours in buffer (20 mM Tris-HCl (pH 8.0), 1 mM DTT, 1 mM EDTA). Reaction was incubated in boiling water and slowly cooled to room temperature to deactivate UNG activity and anneal with complementary strand containing a G opposite the U (i.e., AP site after UNG treatment) : 5′-AGAAAAGGGGAAAGUAAAGAGGAAAGG(biotin-dT)GAGGAGGT-3′. Quality control check was performed on the dsDNA biotin-dT oligo to ensure complete conversion to AP site and to check for integrity of the oligo (Supplemental Figure 2A). Complete deactivation of UNG and confirmation that no UDGase activity remained in the oligo preparation was confirmed (Supplemental Figure 2B). For the EMSA, Biotin labeled AP: G dsDNA (5′-AGAAAAGGGGAAAG(AP)AAAGAGGAAAGG (biotin-dT) GAGGAGGT-3′) annealed with complementary strand) was incubated with varying amounts of UNGs (0–500 nM) for 20 min at room temperature. Samples (11 μl) were resolved on a 6% native polyacrylamide gel (37.5:1, BioRAD cat# 1610148 ) that was cast with 0.5× TBE plus 5% glycerol and run with 0.5× TBE for 160 min, 100 V, at 4°C. DNA and DNA-protein complexes were transferred to nylon membrane with 0.5x TBE and crosslinked with stratagene crosslinker using auto crosslinking program. Chemiluminescent based nucleic acids detection strategy was used for signal detection. (Thermo Fisher Scientific, cat# 89880). Uncropped gel images included in supplemental data.
2.4. In vitro UDGase assay to measure UNG-specific Activity on U in ssDNA or G:U mismatch in dsDNA
Activities of WT and mutants UNG proteins were measured using Fluorescein-labeled 36-nt ssDNA 5′-AGAAAAGGGGAAAGUAAAGAGGAAAGG(FdT)GAGGAGGT-3′ or the same Uracil-containing oligo annealed to complementary strand with a G opposite U for dsDNA. UDGase reactions (30-μl volume) were carried out in a buffer containing 50 mM potassium acetate, 20 mM Tris-acetate (pH 7.9), 10 mM magnesium acetate, 1 mM DTT, 333 nm of the substrate DNA. Following incubation with UNG at 37 °C, the reactions were quenched by a double extraction with phenol/chloroform/isoayl alcohol (25:24:1). For reactions comparing MHV68UNG and MHV68UNGΔL-Loop, a buffer containing 20 mM Tris-HCl, 1 mM EDTA (pH 8.0), 1 mM DTT was used. 50 nM NaOH was added to extracted DNA and heated for 5min at 95 °C to accomplish cleavage of AP site. UDGase reactions after NaOH treatment were mixed with equal volume of stop solution (9.5 ml formamide, 0.4 ml 0.5 M EDTA pH 8.0 and 0.1 ml H2O) and resolved with 8 M Urea containing denaturing 16% PAGE in 1XTBE buffer. A Typhoon scanner FLA900 (GE Amersham) biomolecular imager was used to image gels and determine DNA band intensity.
Quantification was via Image J (version 1.49) and specific activity was calculated as picomoles of uracil removed from DNA substrate/min/μg of enzyme. This was determined in the linear range of protein concentration and incubation times. Analysis with catalytic mutant MHV68UNG (D85N, H207L) confirmed the absence of glycosylase contamination from E. coli (Supplementary Figure 3A). Western blot with anti- MHV68UNG sera confirmed equal loading of UNG in the activity assay (Supplementary Figure 3B). For the measurement of the effects of APE1 on MHV68UNG turnover, we used a buffer containing 20 mM Tris-HCl, 1 mM EDTA (pH 8.0), 1 mM DTT. APE1 at 0, 150, 300, 600 nM was preincubated with DNA substrate containing U:G mismatch before the addition of MHV68UNG. Summary of activity was determined from three independent experiments and error bars were calculated based on standard deviation. Uncropped gel images included in supplemental data.
2.5. Fluorescence anisotropy assays
Binding of recombinant 6XHis-tagged UNG variants to dsDNA containing a single AP:G mismatch was monitored by changes in steady-state fluorescence depolarization (rotational anisotropy). 20 μM fluorescein-labeled (FdT) ssDNA substrate containing a single U was incubated with 20 nM mUNGΔN at 37°C for 2 hours in buffer (20 mM Tris-HCl (pH 8.0), 1 mM DTT, 1 mM EDTA) before annealing with a complementary strand containing a G opposite the U (or AP site after UNG treatment) : 5′-AGAAAAGGGGAAAGUAAAGAGGAAAGG(FdT)GAGGAGGT-3′. Reaction mixture (50 μl), containing fluorescein-labeled DNA (10 nM) in buffer (50 mM potassium acetate, 20 mM Tris-acetate (pH 7.9), 10 mM magnesium acetate, 1 mM DTT), was incubated with increasing concentrations (0–10000 nM) of UNG at room temperature. Rotational anisotropy was measured using a BioTek™ Synergy™ H4 Hybrid Microplate Reader. Samples were excited with vertically polarized light at 485 nm, and both vertical and horizontal emission were monitored at 528 nm (20 nm band pass). The microscopic dissociation constant (Kd) is defined as the concentration of UNG at which half of the total AP:G dsDNA is bound. Apparent dissociation constants (Kd) were obtained by fitting the data to a rectangular hyperbolic curve for noncooperative binding using Graphpad Prism 9.0 software. The data were calculated from three independent experiments.
3. RESULTS
3.1. MHV68UNG interacts with AP site containing DNA
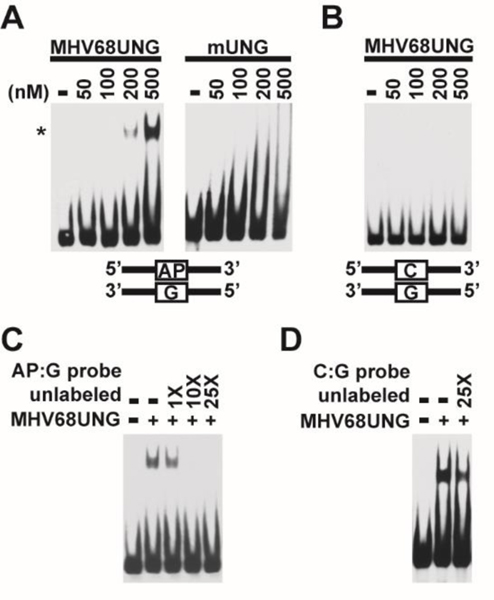
One common biochemical feature of DNA glycosylases is their high affinity to AP sites, the requisite product of base lesion removal. In the UDG family, TDG, MBD4, SMUG1 are reported to bind to the AP site by either EMSA [38, 40] or rotational anisotropy assays [41]. Human UNG (hUNG) is the exception [18]. EMSA and anisotropy binding analysis of full length hUNG (nuclear form, UNG2) [38] or the hUNG catalytic domain demonstrate low affinity for the AP site [18, 39]. We investigated whether, the GHVUNGs, with divergent domains, interact with labeled AP site containing DNA that would mimic the product of uracil removal. An EMSA assay was performed after incubation of recombinant MHV68UNG with an oligonucleotide containing a single AP:G mismatch in a DNA duplex. A shifted band was observed when MHV68UNG concentration reached 200 nM, and a stronger shift was achieved when protein concentration was increased to 500 nM (Figure 1A). However, even at 500 nM no shifted band was observed with mUNG, consistent with low affinity of hUNG to the AP site observed in previous studies[18, 38]. No band shift by MHV68UNG was detected on perfectly matched substrates containing C:G base pair in replacement of AP:G mismatch (Figure 1B). Titration of 10-fold excess of unlabeled AP:G dsDNA blocked the interaction between MHV68UNG and labeled AP:G dsDNA (Figure 1C), whereas unlabeled dsDNA homoduplex did not affect the MHV68UNG-AP site interaction (Figure 1D). This indicates that the interaction between MHV68UNG and dsDNA is AP site specific.
Figure 1.
GHV UNG AP site binding. (A) EMSA assay with dsDNA probe containing an AP site (AP:G) and indicated amounts of purified recombinant MHV68UNG or mUNG. Shifted (*) probe is indicated. (B) EMSA assay with equivalent non-AP containing probe (C:G). (C) EMSA assay with AP:G probe, 500nM MHV68 UNG and indicated fold addition of unlabeled AP:G probe DNA or (D) unlabeled C:G probe DNA. Uncropped gel images included in supplemental data.
3.2. Catalytic domain is conserved between GHV and mammalian UNG except the leucine loop extension and the unstructured N-terminus
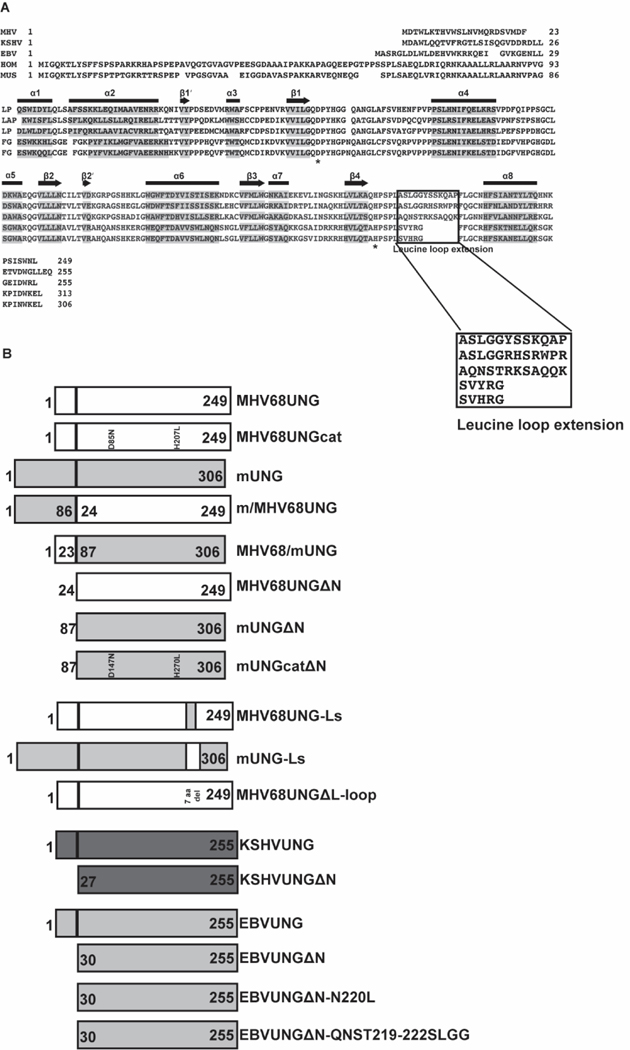
Sequence alignment of UNGs from GHV (MHV, EBV and KSHV), human (HOM) and mouse (MUS) demonstrates a highly conserved catalytic core and two diverse domains, which consist of the N-terminus and leucine loop motif in the DNA binding domain (Figure 2A). The catalytic core is conserved in primary sequence and three-dimensional crystal structure based on data from human [37], EBV [21] and KSHV [36] UNG. The N-terminus is highly diverse in sequence and length between the UNGs with the GHVs having a much shorter domain. Within the DNA binding domain, a leucine loop begins with the conserved motif HPSPL, but then diverges in sequence. This leucine loop extension in humans and mice consists of five amino acids, in GHVs it contains twelve additional amino acids. Based on identity, the five amino acids of the mammalian leucine loop extension align to each other but do not align to the twelve amino acids of GHVs. MHV68UNG has higher sequence identity with KSHVUNG than EBVUNG within the leucine loop extension. Although the sequence identity is low between EBVUNG and KSHVUNG/MHV68UNG, the leucine loop in GHV UNGs appears to be structurally conserved [36]. With the idea that the diverse domains contribute to the differences in DNA interaction, we generated mutants that either deleted the N-terminus and leucine loop extension or chimeric UNGs that swapped either the N-terminus or the leucine loop extensions (Figure 2B, Supplementary Figure 1).
Figure 2.
Alignment of GHV UNGs and mammalian UNGs and diagram for domain swap mutants of UNGs. (A) Sequence alignment of uracil-DNA glycosylases encoded by MHV68, KSHV, EBV, human, and mouse. Denotation of secondary structural elements, alpha helix (horizontal bar) and beta sheet (arrow) are based on PDB structures of KSHV, EBV and human UNG. Sequence of these elements are shaded. Catalytic site aspartic acid and histidine residues are labeled with asterisk. Leucine loop extension is boxed. (B) Diagram for UNG variants used in this study. MHV68UNG sequence is depicted as white bar and mUNG with gray bar. Numbers on the bar denote the start and stop amino acids for chimeric UNGs as indicated.
3.3. N-terminal domain and AP site binding
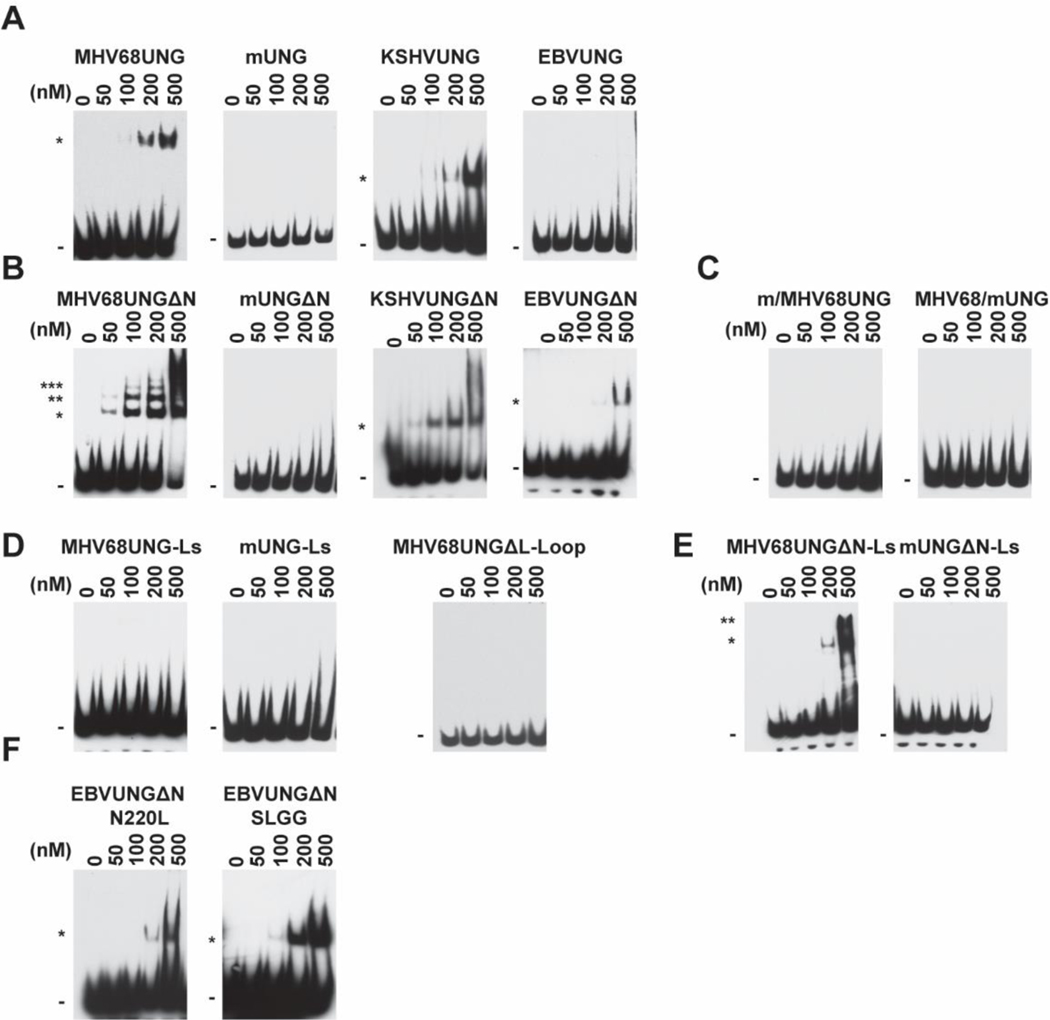
Within GHV there is less identity in the N-terminus and the leucine loop extension sequence than the catalytic core. To examine this, we next investigated whether the human GHV UNGs interact with AP site DNA. A gel shift assay was performed on AP site dsDNA for KSHVUNG and EBVUNG. MHV68UNG and KSHVUNG shifted AP site-containing oligo, but EBVUNG did not even at 500 nM (Figure 3A). To further explore the contribution of the N-terminus to AP site binding, we then assayed mutants with the N-terminal truncations (Figure 2B). While mUNGΔN did not associate with the AP site, MHV68UNGΔN AP site binding was noted at lower protein concentrations. We also noticed the appearance of multiple shifted bands, which may indicate MHV68UNGΔN oligomerizes on DNA (Figure 3B). Similar to MHV68UNGΔN, KSHVUNGΔN AP site binding was noted with lower protein concentrations and EBVUNGΔN interaction with AP site was detected (Figure 3B). The N-terminus swap mutants did not bind to the AP site, suggesting the N-terminus does not positively contribute to AP site binding (Figure 3C). We therefore conclude, that all GHV UNG can bind to AP sites and the N-terminus of GHVUNG has a suppressive effect on AP site binding.
Figure 3.
Contribution of leucine loop extension and N-terminus of GHV UNG to AP sites binding. EMSA assays with dsDNA probe containing an AP site (AP:G) and indicated amounts of recombinant UNGs corresponding to (A) MHV68UNG, mUNG, KSHVUNG and EBVUNG. (B) Amino-terminal deletion mutants ΔN, (C) Chimeras with the N-terminus swapped (m/MHV68UNG, MHV68/mUNG) (D) Chimeras with the leucine loop extension swapped (MHV68UNG-Ls, mUNG-Ls) or 7 amino acids of the leucine loop extension motif deleted (MHV68UNGΔL-Loop) (E) ΔN mutants with leucine loop extension swapped (MHV68UNG ΔN-Ls, mUNG ΔN-Ls) or (F) EBVUNG ΔN with indicated leucine loop extension residues to mutated to the equivalent residues in KSHVUNG, N220L and 219QNST222 to SLGG. All panels representative of n = 3 independent experiments. Shifted (*) probe is indicated. Uncropped gel images included in supplemental data.
3.4. Leucine loop is necessary but not sufficient for AP site binding
To determine whether the leucine loop extension contributes to AP site binding, we examined mutants that swap the leucine loop extension between MHV68UNG and mUNG in the EMSA assay. Both MHV68UNG-Ls and mUNG-Ls lost AP site binding ability indicating that the leucine loop extension is necessary but not sufficient for AP site binding (Figure 3D). When the first 7 amino acids at the leucine loop extension were deleted (MHV68UNGΔL-loop) AP site binding was undetectable in this assay, suggesting that the first 7 amino acids have a role in AP site binding (Figure 3D). Although MHV68UNG-Ls AP site interaction was undetectable in the EMSA, upon N-terminus deletion AP site interaction was restored with MHV68UNGΔN-Ls (Figure 3E). This further supports our observation that the leucine loop extension contributes to, but is not sufficient for, AP site binding. To determine if a specific sequence in the leucine loop extension motif contributes to AP site binding differences between MHV68UNG, KSHVUNG and EBVUNG, we examined mutants of EBVUNGΔN within the leucine loop extension. Substitution of N220 to L or QNST219–222 to SLGG in EBVUNGΔN converts these residues to those of MHV68UNG and KSHVUNG (Figure 2A). Both mutants enable the detection of AP site binding by EBVUNGΔN at lower UNG concentrations (Figure 3F). To ensure that ECL exposure time does not affect the comparison between the high affinity binder MHV68UNG and low affinity binder, swap mutants, we have run WT and chimeras or mutants’ side by side on the same gel (blot) (Supplementary Figure 4). Combined with the gel shift data from the mutant MHV68UNGΔL-loop (Figure 3D), we conclude that the conserved amino acids motif SLGG within the leucine loop extension of MHV68UNG and KSHVUNG contribute to AP site binding.
3.5. Measuring UNG-AP site interaction in equilibrium
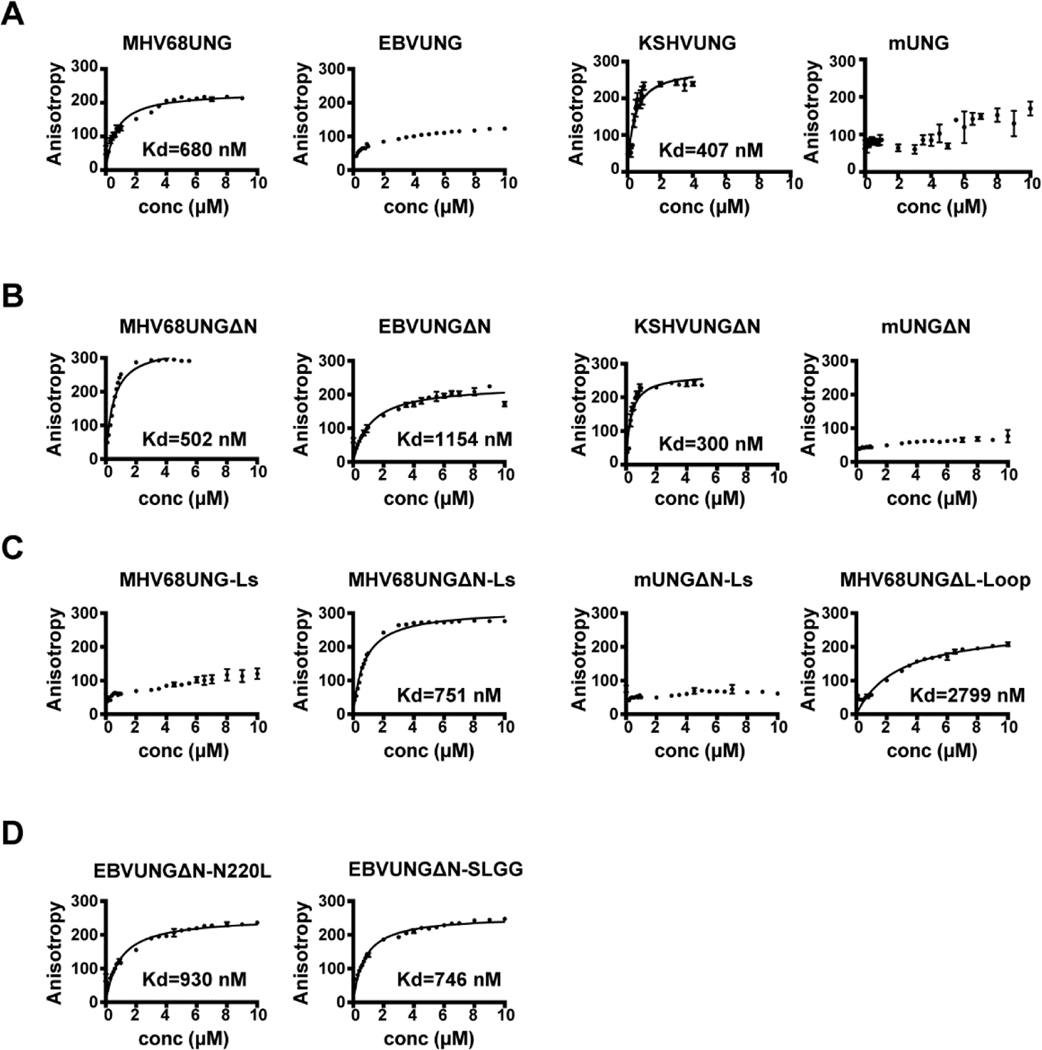
We measured and compared the dissociation constant between the full length, mutant and chimeric UNGs with dsDNA oligo containing AP:G by fluorescence anisotropy, which measures molecular interactions under equilibrium conditions without the need to separate free oligo and protein-DNA complexes. For wild-type MHV68UNG, anisotropy increased linearly with added protein in the initial part of the curve (Figure 4A) until the anisotropy changes became smaller and reached a plateau. The data fit a hyperbolic curve with a relatively high dissociation constant (Kd=680 nM). This AP site binding profile was also noticed for KSHVUNG. In contrast to wild-type MHV68UNG and KSHVUNG, EBVUNG and mUNG had no plateau in its binding isotherm within the same protein concentration range. The data were not fitted because the upper plateau was not well defined (Figure 4A). The binding profiles for wild-type MHV68UNG, EBVUNG, KSHVUNG, and mUNG agree with the EMSA gel shift data (Figure 3A). Upon deletion of the N-terminus, higher affinity to AP site was achieved for all GHVUNGs as indicated by the decreased Kd value (Figure 4B). In contrast, the gain of anisotropy was slow with titration of concentrated mUNGΔN, and never reached saturation. In agreement with the gel shift data, MHV68UNGΔN-Ls shows an intermediate binding isotherm (Kd=751 nM) between MHV68UNG and MHV68UNG-Ls (Figure 4C). We observe a significantly increased Kd value for MHV68UNGΔL-loop (Kd=2799 nM). This parallels the results of the EMSA analysis where no MHV68UNGΔL-loop DNA binding was detected (Figure 3E), and reflects sensitivity differences of the two assays. Altogether these data support the critical role of the leucine loop extension in AP site interaction (Figure 4C). For EBVUNGΔN with amino acid substitutions to those of the MHV68/KSHVUNG leucine loop extension, the Kd values decreased (Figure 4D). This anisotropy data is consistent with the EMSA analysis (Figure 3F) and further supports that the SLGG within the leucine loop extension of MHV68UNG and KSHVUNG contributes to AP site binding.
Figure 4.
UNGs binding to AP:G in dsDNA monitored by fluorescence anisotropy. Anisotropy data for equilibrium binding of recombinant UNGs to 36-nt fluorescein-dT labeled dsDNA containing AP:G. Kd determined by nonlinear hyperbolic binding mode. Anisotropy profiles for (A) full length MHV68UNG, EBVUNG, KSHVUNG AND mUNG. (B) Amino-terminal deletion mutants ΔN. (C) Chimeras with the leucine loop extension swapped (MHV68UNG-Ls), ΔN mutants with leucine loop extension swapped (MHV68UNG ΔN-Ls, mUNG ΔN-Ls) or 7 amino acids of the leucine loop extension motif deleted (MHV68UNGΔL-Loop). (D) EBVUNG ΔN with indicated leucine loop extension residues to mutated to the equivalent residues in KSHVUNG, N220L and 219QNST222 to SLGG. Data represents average of n=3 independent experiments.
3.6. GHVUNG and mUNG UDGase activity
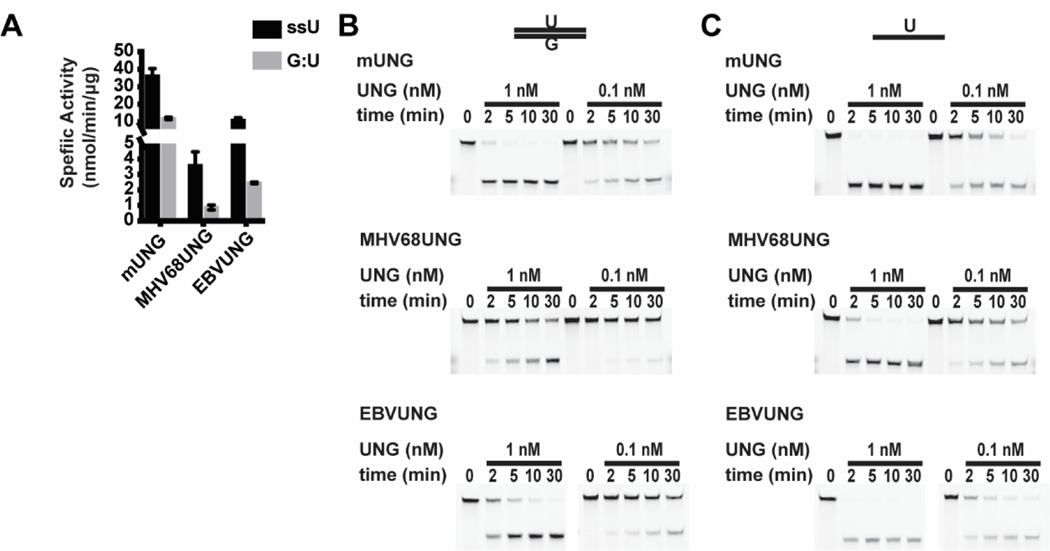
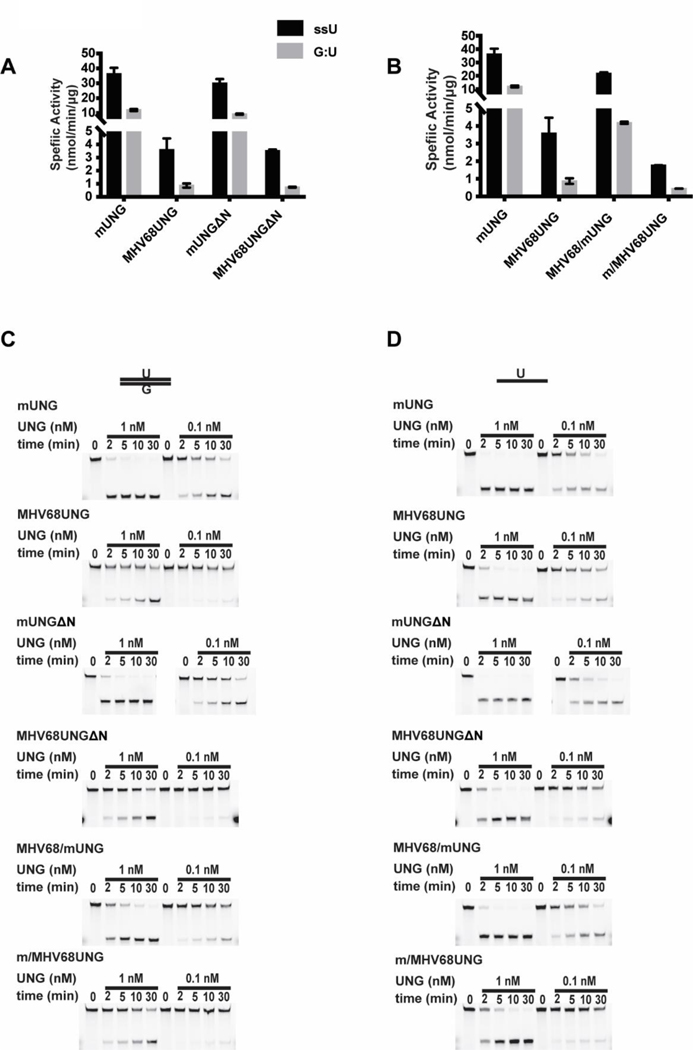
We next determined if the divergent domains of GHVUNG and mUNG contribute to differences in substrate recognition. We performed a UDGase assay by incubating recombinant UNGs with an uracil containing fluorescein labeled DNA substrate in either ssDNA or dsDNA (U:G mismatch) format. After various incubation times, uracil removal was detected via a hot alkaline based cleavage assay and resolved by denaturing PAGE. UDGase activity was determined by conversion of full-length substrate to the shorter oligo. The mUNG had higher specific activity than either MHV68UNG or EBVUNG on both ssDNA (ssU) or U:G mismatch substrates (Figure 5A–C, Table 1). The difference reflected overall activity and was proportional on both ssDNA and dsDNA substrates (ratio of ssU/G:U mUNG=3 and MHV68UNG=4.1) (Table 1). To confirm that our recombinant UNG preparation is free from E. coli UDG contamination, we tested the UDGase activity of the catalytic mutant MHV68UNG(D85N,H207L), and found no activity for either uracil in ssDNA or U:G mismatch dsDNA when the mutant was used at much higher concentration (supplementary Figure 3A). We next determined if the divergent N-terminus of the UNGs contributed to the activity differences on the ssDNA or dsDNA U:G mismatch substrates. The UDGase assay was performed using recombinant UNGs without the N-terminal domains (MHV68UNGΔN and mUNGΔN) or recombinant chimeras that swapped the N-terminal domains (MHV68/mUNG and m/MHV68UNG) (Figure 6A–D, Table 1). We observed little difference in activity between the full length UNGs and N-terminal deletion mutants on either ssDNA (ssU) or dsDNA substrates (Figure 6A, 6C, 6D, Table 1). Specific activity of both MHV68/mUNG and m/MHV68UNG chimeras were slightly lower than the full length UNGs (~50%), but proportionally decreased on both ssDNA and dsDNA substrates (Figure 6B, 6C, 6D, Table 1). This indicates that the N-terminus is not a determinant for substrate specificity activity in vitro.
Figure 5.
UDGase activity of mUNG vs. GHVUNG. (A) Summary of specific activity of mUNG, MHV68UNG, and EBVUNG on uracil in ssDNA (black bar) or G:U mismatch in dsDNA (grey bar) in UDGase assay from n=3 experiments. (B) Representative denaturing PAGE gels from the UDGase assay resolving indicated dosage of mUNG, MHV68UNG, or EBVUNG incubated with 333 nM G:U mismatch in dsDNA or (C) the ssDNA substrate with uracil incubated for the indicated time. Upper band (intact substrate) and lower band (cleaved product of UDGase activity) are resolved on the gel images. Uncropped gel images included in supplemental data.
Table 1.
Comparison of specific activities against U in ssDNA or G:U in dsDNA by mUNG, MHV68UNG and domain swapped chimeric UNGs.
| Specific Activity | (nmol/min/μg) | |||
|---|---|---|---|---|
|
| ||||
| Enzyme | U in ssDNA | G:U in dsDNA | ratio (ssU/G:U) | Reaction buffer |
|
| ||||
| mUNG | 36.2 ±4.2 | 12.0 ±0.7 | 3.0 | NEB buffer 4 |
| MHV68UNG | 3.6 ±0.9 | 0.9 ±0.2 | 4.1 | NEB buffer 4 |
| EBVUNG | 11.0 ±1.4 | 2.5 ±0.1 | 4.5 | NEB buffer 4 |
| mUNG-Ls | 15.8 ±0.3 | 0.5 ±0.1 | 29.0 | NEB buffer 4 |
| MHV68UNG-Ls | 14.4 ±0.5 | 3.1 ±0.1 | 4.6 | NEB buffer 4 |
| mUNGΔN | 30.0 ±2.9 | 9.3 ±0.4 | 3.2 | NEB buffer 4 |
| MHV68UNGΔN | 3.5 ±0.1 | 0.7 ±0.1 | 4.8 | NEB buffer 4 |
| MHV68/mUNG | 21.8 ±0.8 | 4.2 ±0.1 | 5.2 | NEB buffer 4 |
| m/MHV68UNG | 1.8 ±0.1 | 0.4 ±0.0 | 4.0 | NEB buffer 4 |
| MHV68UNG | 2.00±0.26 | 0.13±0.05 | 16.2 | NEB UDG buffer |
| MHV68UNG-ΔL-loop | 0.59±0.01 | 0.28±0.08 | 2.1 | NEB UDG buffer |
Figure 6.
Effects of N-terminus motif on UDGase activity of mUNG and MHV68UNG. Summary of specific activity on uracil in ssDNA (black bar) or G:U mismatch in dsDNA (grey bar) in UDGase assay of mUNG and MHV68UNG compared to (A) mutants with deleted amino-terminal domains (mUNGΔ, MHV68UNGΔ) or (B) chimeras with swapped amino-terminal domains (m/MHV68UNG, MHV68UNG/mUNG). Average from n=3 independent experiments. (C) Representative denaturing PAGE gels from the UDGase assay resolving indicated dosage of mUNG, MHV68UNG or mutant incubated with 333 nM G:U mismatch in dsDNA or (D) ssDNA substrate containing an uracil for the indicated time. Upper band (intact substrate) and lower band (cleaved product of UDGase activity) are resolved on the gel images. Uncropped gel images included in supplemental data.
3.7. Leucine loop extension determines DNA substrate specificity
The leucine loop is thought to be involved in substrate specificity since it is required for widening the minor groove of double stranded DNA containing uracils and a mutation within hUNG leucine loop extension (R276E) decreases its activity on dsDNA [42]. To determine if there is a difference in substrate specificity between the UNGs we generated leucine loop swap chimeras by exchanging the leucine loop extension motif between mUNG and MHV68UNG. The leucine loop extensions, consisting of the 12 amino acids of MHV68UNG or 5 amino acids of mUNG that follow the conserved motif HPSPL, were swapped (Figure 2B). The leucine loop extension had a significant effect on substrate specificity in the UDGase assay (Figure 7A–C). The specific activity of mUNG was 3-fold higher on ssDNA versus dsDNA (Table 1). The mUNG with the MHV68 leucine loop extension (mUNG-Ls) had a modest decrease in activity on ssDNA substrate (~50%) but a substantial drop in activity on dsDNA G:U substrate, resulting in a 29-fold activity difference on ssDNA versus dsDNA (Table 1). Activity of mUNG-Ls on dsDNA substrate more closely resembled that of MHV68UNG than mUNG (Figure 7A). On the other hand, MHV68UNG with its leucine loop extension swapped with that of mUNG, gained approximately 4-fold activity on both ssU and G:U substrates. (Figure 7A, Table 1). The difference in leucine loop extension length and sequence could contribute to the differences. To further dissect how the structure of the leucine loop extension affects activity on specific substrates, we deleted the 7 amino acids following the conserved HPSPL motif in the leucine loop extension of MHV68UNG (MHV68UNGΔL-loop). We then compared specific activity of MHV68UNG and MHV68UNGΔL-loop in the UDGase assay. Strikingly, activity on the dsDNA substrate increased 2.5-fold, whereas activity on ssU substrate decreased 4-fold (Figure 7D–F). Taken altogether, this data demonstrates that the leucine loop extension contributes to both catalytic activity and substrate specificity.
Figure 7.
Effects of leucine loop extension motif on UDGase activity of mUNG and MHV68UNG. Summary of specific activity on uracil in ssDNA (black bar) or G:U mismatch in dsDNA (grey bar) in UDGase assay of mUNG and MHV68UNG compared to chimeras with leucine loop extension motif swapped from the other UNG (mUNG-Ls, MHV68UNG-Ls) from n=3 independent experiments. (B) Representative denaturing PAGE gels from the UDGase assay resolving indicated dosage of mUNG, or mUNG-Ls with 333 nM G:U mismatch in dsDNA (upper panels) or the ssDNA substrate with uracil (lower panels) incubated for the indicated time. Upper band (intact substrate) and lower band (cleaved product of UDGase activity) are resolved on the gel images. (C) Plot of percentage uracil removal as a function of time. The percentage of processed substrates at each time point for the indicated concentrations are displayed. (D) Specific activity of MHV68UNG compared to MHV68ΔL-Loop (deletion of the first 7 amino acids of the 12 amino acid leucine loop extension) on uracil in ssDNA (ssU) or G:U mismatch in dsDNA from n=2 independent experiments. (E) Representative denaturing PAGE gels from the UDGase assay and (F) Plot of percentage uracil removal as a function of time. Uncropped gel images included in supplemental data.
3.8. MHV68UNG mediated uracil turnover by APE1
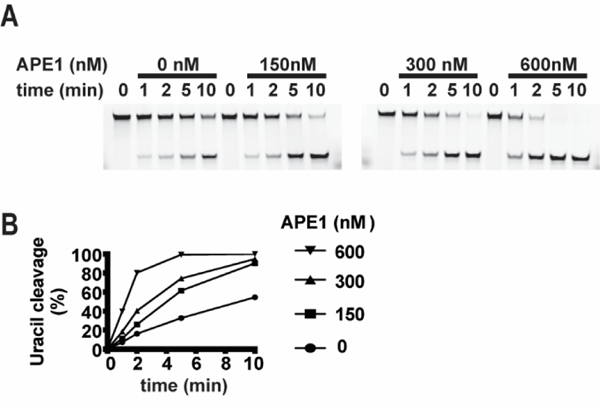
High affinity to AP site is a kinetic barrier for the release of most DNA glycosylases following lesion excision [39]. APE1 has affinity to AP sites [43] and it incises the DNA phosphodiester bond at the AP site. APE1 can displace glycosylases binding to the AP site through a mechanism that does not require APE1 catalytic activity [44]. It promotes turnover of the DNA glycosylases and prevents retrograde binding of glycosylase to the AP site [39]. We therefore measured MHV68UNG dsDNA substrate processing in the presence of increasing concentrations of recombinant APE1 (Figure 8A,8B). We find that APE1 increases MHV68UNG U:G substrate conversion in a dose-dependent manner. This assay was performed in the absence of magnesium, which is essential for APE1 catalytic activity. This suggests the increase in glycosylase activity is the result of greater MHV68UNG turnover and is consistent with an ability of APE1 to displace MHV68UNG from AP sites.
Figure 8.
Stimulation of MHV68UNG turnover by recombinant murine APE1. (A) denaturing PAGE gels from the UDGase assay resolving 333 nM G:U mismatch incubated with 5 nM MHV68UNG for the indicated time in the presence of 0, 150nM, 300 nM or 600 nM murine APE1. (B) Plot of percentage uracil removal as a function of time. Uncropped gel images included in supplemental data.
4. DISCUSSION
A wealth of studies on biochemical, structural, and biological functions of mammalian and herpesvirus UNGs have advanced the understanding of UNG function in base excision repair [18–21, 36, 45], yet a detailed role for substrate targeting or biological function of the leucine loop extension has not been described. In this study, we make the novel observation that GHVUNGs bind AP sites, and our structure-function analysis demonstrates it is through the leucine loop extension motif. In contrast, mammalian UNGs do not have significant AP site binding activity based on EMSA and fluorescence anisotropy assays. This is consistent with a previous study of hUNG interaction with AP site using EMSA [38]. Studies using equilibrium binding assays demonstrated a low affinity between the catalytic domain of hUNG (without the N-terminus) and dsDNA AP site with reported Kd values of 9 uM [18] and 50 uM [39]. Here, our anisotropy measurement for the catalytic domain of mUNG (mUNGΔN) did not achieve saturation binding even at 10 uM.
In this study we find that the N-terminus of GHVUNG modulates the AP site interaction. Little else is known regarding the specific function for this GHVUNG domain. The N-terminus of hUNG contains numerous protein interaction motifs, sites for posttranslational modifications and facilitates diffusion of hUNG under macromolecular crowding conditions [46, 47]. Interaction with RPA through the N-terminus facilitates targeting uracils at the junction of dsDNA and ssDNA [48]. Two recent studies have demonstrated that a portion of the N-terminus of mouse UNG interacts with FAM72A (Ugene) to maintain the steady state level of UNG in B lymphocyte which facilitates SHM and CSR [49, 50]. The N-terminus is predicted to be intrinsically disordered [51], and the only structural information is a complex between the short RPA binding motif of hUNG and the winged-helix domain of the RPA32 subunit of the RPA complex [52]. The GHVUNG N-terminus is highly divergent in sequence and length with none of the interaction motifs and functions ascribed to its mammalian counterpart. In our study we find a suppressive effect of the N-terminus on GHVUNG-AP site binding. Since this was in the absence of other cellular and viral factors it suggests MHV68UNG regulates an aspect of its activity via intra- or intermolecular interaction. We note that other DNA glycosylases are known to exhibit this self-regulation. Neil1, a DNA glycosylase involved in the repair of oxidatively damaged bases in mammalian cells, is regulated through the intramolecular interaction between folded glycosylase domain with its disordered C-terminus [53]. Both NMR and crystal structural studies have revealed that the N-terminus proximal to catalytic core of TDG interacts with the catalytic core and regulates its activity [54, 55].
We find that the leucine loop extension is a determinant of AP site binding. Following the conserved motif HPSPL, the leucine loop extension is 5 amino acids in mammalian and 12 in GHVUNGs (Figure 2A). There is no clear sequence conservation between the leucine loop extension of mammalian and GHVUNG. The crystal structure of the KSHVUNG catalytic core bound to a dsDNA AP site revealed that the leucine loop extension displaced the widowed based on the opposite strand [36]. A biological role has not been connected to this widowed base displacement. In contrast, the crystal structure for hUNG did not reveal leucine loop extension displacement of the widowed base on the opposite strand [37]. Deletion of 7 amino acids consisting of residues 5–11 in the KSHVUNG leucine loop extension ablated DNA binding and enzymatic activity [36]. In this study we deleted residues 1–7 of this motif in MHV68UNG and found that enzymatic activity was retained (Figure 7D), but AP-site affinity was significantly reduced (Figure 3D). This suggested that sequence context in addition to length was an important determinant of leucine loop extension function. Within the gammaherpesvirus subfamily, KSHV and MHV are both rhadinoviruses, while EBV is a lymphocryptovirus. The leucine loop extension of KSHVUNG and MHV68UNG has closer sequence conservation, than to EBVUNG (Figure 2A). In particular, the first 5 amino acids of KSHVUNG and MHV68UNG (motif ASLGG) are identical, while EBVUNG (motif AQNST) only shares one residue (Figure 2A). Since KSHVUNG and MHV68UNG bind AP sites with higher affinity than EBVUNG (Figure 4B), we hypothesized that the first 5 residues of the leucine loop extension were key contributors. We tested this idea by changing residues of EBVUNG to that of the MHV68UNG/KSHVUNG leucine loop extension. We did this in the context of N-terminal deletions, since EBVUNGΔN has detectable AP site affinity, while full length EBVUNG did not (Figure 4A,4B). Converting a single EBVUNG residue, N220L, increased affinity slightly (Kd=1154 nM, to Kd=930 nM, Figure 4B,4D). Converting four continuous EBVUNG residues 219-QNST-222 to SLGG, the equivalent motif in both KSHVUNG and MHV68UNG, increased affinity even further (Fig 4D Kd=746 nM). This definitively demonstrates that the first five amino acids of the leucine loop extension are key determinants of AP site binding affinity.
UNG processes uracils in the context of both ssDNA and dsDNA. Other glycosylases such as TDG and MBD4 do not act on ssDNA [56, 57]. UNG activity on ssDNA is several-fold higher than on dsDNA. The extent to which domains of UNG contribute to different activity on substrates has not been investigated. We used the difference in specific activity between mUNG and MHV68UNG to investigate the structural elements involved. We find the N-terminus has little influence on substrate specificity. However, through swapping the leucine loop extension motifs we determined that this region contributes to substrate recognition. In general, loss or change of a fragment of an enzyme could potentially affect the natural folding. This could be caused by loss of thermal stability which would be reflected in an activity decrease. Comparative analysis of activity between full length MHV68UNG and mUNG with their respective N-terminus deletion mutants revealed no significant difference in activity on either ssDNA or dsDNA substrates (Table 1). A two-fold decrease in specific activity of mUNG-Ls on ssDNA uracil containing substrate was observed. While a small decrease, such a change in activity could potentially be caused by reduced thermal stability of mUNG-Ls. Since we did not analyze thermal stability, it is a limitation of our studies. However, in contrast to the 2-fold decrease in ssDNA activity, the mUNG-Ls displayed an approximately 20-fold decrease in dsDNA substrate activity, suggesting a specific role for the leucine loop extension in DNA substrate preference. Further supporting this, was the gain of specific activity on both ssDNA and dsDNA substrates with the MHV68UNG-Ls versus MHV68UNG (Table 1). Altogether, these data argue the differential is not an artifact of thermal stability issues. The mUNG-Ls mutant had a proportionally larger activity decrease on dsDNA than the ssDNA substrate, which support a role for the leucine loop extension in DNA substrate preference. In contrast, the MHV68UNGΔL-loop mutant had the opposite effect, a decrease in ssDNA activity and gain in dsDNA activity. This demonstrated that amino acids in the leucine loop extension differentially supported ssDNA and dsDNA substrate recognition. Consistent with this, hUNG with R276 mutated to E, located in the leucine loop extension, lost dsDNA activity and but retains single strand activity [42]. Mechanistically, this mutant is deficient in uracil base flipping [58].
UNG removes uracils in DNA by glycosidic bond breakage resulting in AP site, an intermediate dangerous to genome integrity. If encountered during replication, AP sites may be processed by translesion polymerases that risk incorporation of mutation [59, 60]. AP sites in close proximity on opposite strands of dsDNA can result in a double strand break [61] or the intrinsic reactive properties of AP sites can cause interstrand cross-links [62, 63], both potentially catastrophic lesions that can lead to genome disruption. Therefore, it is critical that AP sites be quickly and properly processed to maintain genome integrity. Among the herpesvirus family, the longer leucine loop extension is unique to GHV. Alpha- and beta- herpesviruses express vUNGs that have leucine loop extensions with similar length to mammalian UNGs. However, the sequences that comprise the leucine loop extensions share little conservation with either the GHVs or mammalian UNGs [36], the biochemical function of which remain to be comprehensively characterized. AP sites are prevalent in the mammalian genome. Although it is unknown whether the gHV genome accumulates AP sites and further converts them into double strand breaks, interstrand cross-links when it encounters other cellular repair factors. We can interpret that since the virus resides in the same intracellular environment where the host genome consistently undergoes DNA damage including the AP site, the gHV genome may suffer from the same sets of DNA damage.
Therefore, a unique AP site binding activity may be beneficial to viral genome stability and viral homeostasis. In line with this, a recent biochemical study indicates that a mammalian DNA glycosylase AAG can shield the AP site from interstrand cross-link [64]. This novel finding of AP site-vUNG interaction awaits the development of an adequate cellular virus infection model to further define other cellular factors and repair pathways. While mouse models may not recapitulate all aspects of human gHVs they can give insight into how vUNG biochemical function may impact viral pathogenesis. A proper mouse infection model may also provide insights to the mechanism. It is known KSHV LANA recruits the host UNG to support viral persistence [65] and that KSHV and EBV UNGs with mutations at catalytic sites are efficient in viral replication [26, 45]. Our finding could support a non-catalytic function of gHVUNG in the viral life cycle through protection of the viral genome from DNA damage.
During its lifecycle, GHVs are exposed to mutagenic environments that can create uracils or AP sites in the viral genome. Limiting nucleotide pools upon EBV infection of B cells results in replication stress and impaired transformation [66]. GHV have evolved viral dUTPases that can potentially limit uracil incorporation [28, 67]. MHV68 vDUT has activity in dUTPase assays and MHV68 displays increased genome instability with combined loss of both MHV68UNG AND dUTPase [28]. GHV infection induces expression of APOBECs and AID but has evolved defenses against these cytidine deaminases such as BORF2 in EBV that inhibits APOBECs [68], and miRs expressed by KSHV that target AID [66]. AID directly impacts viral fitness by inhibiting lytic reactivation [66]. We speculate that the unique structural features of GHVUNGs and activity that they facilitate, evolved to maintain viral genome integrity in the presence of the mutagenic environment the virus encounters during its lifecycle.
Supplementary Material
Highlights.
Gammaherpesvirus UNG binds abasic sites while mammalian UNG does not.
Divergent structures in the leucine loop of gammaherpesvirus UNG mediate abasic site binding.
The N-terminus of UNG modulates abasic site binding.
UNG leucine loop structures mediate differential UDGase activity on uracil in ss-versus dsDNA.
ACKNOWLEDGEMENT
We would express our gratitude to Dr. Xiaodong Cheng (UTMDACC) for discussion of the fluorescence anisotropy assay. We also would like to thank Dr. Eun Jeong Cho from The Targeted Therapeutic Drug Discovery & Development Program (TTP) core facility at University of Texas, Austin for instruction on the usage of microplate reader for anisotropy assay. We thank Dr. Richard Wood (UTMDACC) for advice and project feedback. We are grateful to Dr. Mark Bedford and the Protein Analysis and Array Core (PAAC) at UTMDACC and the Recombinant Antibody Production Core (RAPC) at UTMDACC. We thank Ben Daniel, Allie Hebertson, David Aghado, Dr. Shalaka Lotlikar, Dr Melissa Simper, Dr. Xiaoling Liu and Dr. Somnath Paul for their thoughtful input on these studies.
FUNDING
Yunxiang Mu was supported by a fellowship from the UTMDACC Center for Cancer Epigenetics. National Institutes of Health [R01AI12539 to K.M.M.; R21 Al111129 to L.T.K and K.M.M.]. Protein Analysis and Array Core [CPRIT RP180804] and Recombinant Antibody Production Core [CPRIT RP190507]. Virginia Harris Cockrell endowment fund. Texas Tobacco Settlement – Molecular Mechanisms of Tobacco Carcinogenesis. Funding for open access charge UTMDACC.
DATA AVAILABILITY
Data that support the findings of this study are available from the corresponding author upon request.
Footnotes
CONFLICT OF INTEREST. None declared
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- [1].Shalhout S, Haddad D, Sosin A, Holland TC, Al-Katib A, Martin A, Bhagwat AS, Genomic uracil homeostasis during normal B cell maturation and loss of this balance during B cell cancer development, Mol Cell Biol, 34 (2014) 4019–4032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Krokan HE, Saetrom P, Aas PA, Pettersen HS, Kavli B, Slupphaug G, Error-free versus mutagenic processing of genomic uracil--relevance to cancer, DNA Repair (Amst), 19 (2014) 38–47. [DOI] [PubMed] [Google Scholar]
- [3].Safavi S, Larouche A, Zahn A, Patenaude AM, Domanska D, Dionne K, Rognes T, Dingler F, Kang SK, Liu Y, Johnson N, Hebert J, Verdun RE, Rada CA, Vega F, Nilsen H, Di Noia JM, The uracil-DNA glycosylase UNG protects the fitness of normal and cancer B cells expressing AID, NAR Cancer, 2 (2020) zcaa019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Sarno A, Lundbaek M, Liabakk NB, Aas PA, Mjelle R, Hagen L, Sousa MML, Krokan HE, Kavli B, Uracil-DNA glycosylase UNG1 isoform variant supports class switch recombination and repairs nuclear genomic uracil, Nucleic Acids Res, 47 (2019) 4569–4585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Di Noia JM, Neuberger MS, Molecular mechanisms of antibody somatic hypermutation, Annu Rev Biochem, 76 (2007) 1–22. [DOI] [PubMed] [Google Scholar]
- [6].Stavnezer J, Guikema JE, Schrader CE, Mechanism and regulation of class switch recombination, Annu Rev Immunol, 26 (2008) 261–292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Peled JU, Kuang FL, Iglesias-Ussel MD, Roa S, Kalis SL, Goodman MF, Scharff MD, The biochemistry of somatic hypermutation, Annu Rev Immunol, 26 (2008) 481–511. [DOI] [PubMed] [Google Scholar]
- [8].Kavli B, Iveland TS, Buchinger E, Hagen L, Liabakk NB, Aas PA, Obermann TS, Aachmann FL, Slupphaug G, RPA2 winged-helix domain facilitates UNG-mediated removal of uracil from ssDNA; implications for repair of mutagenic uracil at the replication fork, Nucleic Acids Res, 49 (2021) 3948–3966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Thompson PS, Cortez D, New insights into abasic site repair and tolerance, DNA Repair (Amst), 90 (2020) 102866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Prakash S, Johnson RE, Prakash L, Eukaryotic translesion synthesis DNA polymerases: specificity of structure and function, Annu Rev Biochem, 74 (2005) 317–353. [DOI] [PubMed] [Google Scholar]
- [11].Krokan HE, Bjoras M, Base excision repair, Cold Spring Harb Perspect Biol, 5 (2013) a012583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Wilson SH, Kunkel TA, Passing the baton in base excision repair, Nat Struct Biol, 7 (2000) 176–178. [DOI] [PubMed] [Google Scholar]
- [13].Prasad R, Shock DD, Beard WA, Wilson SH, Substrate channeling in mammalian base excision repair pathways: passing the baton, J Biol Chem, 285 (2010) 40479–40488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Akbari M, Otterlei M, Pena-Diaz J, Aas PA, Kavli B, Liabakk NB, Hagen L, Imai K, Durandy A, Slupphaug G, Krokan HE, Repair of U/G and U/A in DNA by UNG2-associated repair complexes takes place predominantly by short-patch repair both in proliferating and growth-arrested cells, Nucleic Acids Res, 32 (2004) 5486–5498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Kavli B, Sundheim O, Akbari M, Otterlei M, Nilsen H, Skorpen F, Aas PA, Hagen L, Krokan HE, Slupphaug G, hUNG2 is the major repair enzyme for removal of uracil from U:A matches, U:G mismatches, and U in single-stranded DNA, with hSMUG1 as a broad specificity backup, J Biol Chem, 277 (2002) 39926–39936. [DOI] [PubMed] [Google Scholar]
- [16].Manvilla BA, Maiti A, Begley MC, Toth EA, Drohat AC, Crystal structure of human methyl-binding domain IV glycosylase bound to abasic DNA, J Mol Biol, 420 (2012) 164–175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Waters TR, Gallinari P, Jiricny J, Swann PF, Human thymine DNA glycosylase binds to apurinic sites in DNA but is displaced by human apurinic endonuclease 1, J Biol Chem, 274 (1999) 67–74. [DOI] [PubMed] [Google Scholar]
- [18].Krusong K, Carpenter EP, Bellamy SR, Savva R, Baldwin GS, A comparative study of uracil-DNA glycosylases from human and herpes simplex virus type 1, J Biol Chem, 281 (2006) 4983–4992. [DOI] [PubMed] [Google Scholar]
- [19].Ranneberg-Nilsen T, Dale HA, Luna L, Slettebakk R, Sundheim O, Rollag H, Bjoras M, Characterization of human cytomegalovirus uracil DNA glycosylase (UL114) and its interaction with polymerase processivity factor (UL44), J Mol Biol, 381 (2008) 276–288. [DOI] [PubMed] [Google Scholar]
- [20].Lu CC, Huang HT, Wang JT, Slupphaug G, Li TK, Wu MC, Chen YC, Lee CP, Chen MR, Characterization of the uracil-DNA glycosylase activity of Epstein-Barr virus BKRF3 and its role in lytic viral DNA replication, J Virol, 81 (2007) 1195–1208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Geoui T, Buisson M, Tarbouriech N, Burmeister WP, New insights on the role of the gamma-herpesvirus uracil-DNA glycosylase leucine loop revealed by the structure of the Epstein-Barr virus enzyme in complex with an inhibitor protein, J Mol Biol, 366 (2007) 117–131. [DOI] [PubMed] [Google Scholar]
- [22].Minkah N, Macaluso M, Oldenburg DG, Paden CR, White DW, McBride KM, Krug LT, Absence of the uracil DNA glycosylase of murine gammaherpesvirus 68 impairs replication and delays the establishment of latency in vivo, J Virol, 89 (2015) 3366–3379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Pyles RB, Thompson RL, Evidence that the herpes simplex virus type 1 uracil DNA glycosylase is required for efficient viral replication and latency in the murine nervous system, J Virol, 68 (1994) 4963–4972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Pyles RB, Thompson RL, Mutations in accessory DNA replicating functions alter the relative mutation frequency of herpes simplex virus type 1 strains in cultured murine cells, J Virol, 68 (1994) 4514–4524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Courcelle CT, Courcelle J, Prichard MN, Mocarski ES, Requirement for uracil-DNA glycosylase during the transition to late-phase cytomegalovirus DNA replication, J Virol, 75 (2001) 7592–7601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Morgens DW, Nandakumar D, Didychuk AL, Yang KJ, Glaunsinger BA, A Two-tiered functional screen identifies herpesviral transcriptional modifiers and their essential domains, PLoS Pathog, 18 (2022) e1010236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Bogani F, Corredeira I, Fernandez V, Sattler U, Rutvisuttinunt W, Defais M, Boehmer PE, Association between the herpes simplex virus-1 DNA polymerase and uracil DNA glycosylase, J Biol Chem, 285 (2010) 27664–27672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Dong Q, Smith KR, Oldenburg DG, Shapiro M, Schutt WR, Malik L, Plummer JB, Mu Y, MacCarthy T, White DW, McBride KM, Krug LT, Combinatorial Loss of the Enzymatic Activities of Viral Uracil-DNA Glycosylase and Viral dUTPase Impairs Murine Gammaherpesvirus Pathogenesis and Leads to Increased Recombination-Based Deletion in the Viral Genome, mBio, 9 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Sunil-Chandra NP, Arno J, Fazakerley J, Nash AA, Lymphoproliferative disease in mice infected with murine gammaherpesvirus 68, Am J Pathol, 145 (1994) 818–826. [PMC free article] [PubMed] [Google Scholar]
- [30].Tarakanova VL, Suarez F, Tibbetts SA, Jacoby MA, Weck KE, Hess JL, Speck SH, H.W.t. Virgin, Murine gammaherpesvirus 68 infection is associated with lymphoproliferative disease and lymphoma in BALB beta2 microglobulin-deficient mice, J Virol, 79 (2005) 14668–14679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Bjornevik K, Cortese M, Healy BC, Kuhle J, Mina MJ, Leng Y, Elledge SJ, Niebuhr DW, Scher AI, Munger KL, Ascherio A, Longitudinal analysis reveals high prevalence of Epstein-Barr virus associated with multiple sclerosis, Science, 375 (2022) 296–301. [DOI] [PubMed] [Google Scholar]
- [32].Zelazowska MA, McBride K, Krug LT, Dangerous Liaisons: Gammaherpesvirus Subversion of the Immunoglobulin Repertoire, Viruses, 12 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Wang Y, Tibbetts SA, Krug LT, Conquering the Host: Determinants of Pathogenesis Learned from Murine Gammaherpesvirus 68, Annu Rev Virol, 8 (2021) 349–371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Savva R, Targeting uracil-DNA glycosylases for therapeutic outcomes using insights from virus evolution, Future Med Chem, 11 (2019) 1323–1344. [DOI] [PubMed] [Google Scholar]
- [35].Savva R, The Essential Co-Option of Uracil-DNA Glycosylases by Herpesviruses Invites Novel Antiviral Design, Microorganisms, 8 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Earl C, Bagneris C, Zeman K, Cole A, Barrett T, Savva R, A structurally conserved motif in gamma-herpesvirus uracil-DNA glycosylases elicits duplex nucleotide-flipping, Nucleic Acids Res, 46 (2018) 4286–4300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Parikh SS, Mol CD, Slupphaug G, Bharati S, Krokan HE, Tainer JA, Base excision repair initiation revealed by crystal structures and binding kinetics of human uracil-DNA glycosylase with DNA, EMBO J, 17 (1998) 5214–5226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Pettersen HS, Sundheim O, Gilljam KM, Slupphaug G, Krokan HE, Kavli B, Uracil-DNA glycosylases SMUG1 and UNG2 coordinate the initial steps of base excision repair by distinct mechanisms, Nucleic Acids Res, 35 (2007) 3879–3892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Esadze A, Rodriguez G, Cravens SL, Stivers JT, AP-Endonuclease 1 Accelerates Turnover of Human 8-Oxoguanine DNA Glycosylase by Preventing Retrograde Binding to the Abasic-Site Product, Biochemistry, 56 (2017) 1974–1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Hendrich B, Hardeland U, Ng HH, Jiricny J, Bird A, The thymine glycosylase MBD4 can bind to the product of deamination at methylated CpG sites, Nature, 401 (1999) 301–304. [DOI] [PubMed] [Google Scholar]
- [41].Morgan MT, Maiti A, Fitzgerald ME, Drohat AC, Stoichiometry and affinity for thymine DNA glycosylase binding to specific and nonspecific DNA, Nucleic Acids Res, 39 (2011) 2319–2329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Chen CY, Mosbaugh DW, Bennett SE, Mutational analysis of arginine 276 in the leucine-loop of human uracil-DNA glycosylase, J Biol Chem, 279 (2004) 48177–48188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Masuda Y, Bennett RA, Demple B, Dynamics of the interaction of human apurinic endonuclease (Ape1) with its substrate and product, J Biol Chem, 273 (1998) 30352–30359. [DOI] [PubMed] [Google Scholar]
- [44].Vidal AE, Hickson ID, Boiteux S, Radicella JP, Mechanism of stimulation of the DNA glycosylase activity of hOGG1 by the major human AP endonuclease: bypass of the AP lyase activity step, Nucleic Acids Res, 29 (2001) 1285–1292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Su MT, Liu IH, Wu CW, Chang SM, Tsai CH, Yang PW, Chuang YC, Lee CP, Chen MR, Uracil DNA glycosylase BKRF3 contributes to Epstein-Barr virus DNA replication through physical interactions with proteins in viral DNA replication complex, J Virol, 88 (2014) 8883–8899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Perkins JL, Zhao L, The N-terminal domain of uracil-DNA glycosylase: Roles for disordered regions, DNA Repair (Amst), 101 (2021) 103077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Rodriguez G, Esadze A, Weiser BP, Schonhoft JD, Cole PA, Stivers JT, Disordered N-Terminal Domain of Human Uracil DNA Glycosylase (hUNG2) Enhances DNA Translocation, ACS Chem Biol, 12 (2017) 2260–2263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Weiser BP, Rodriguez G, Cole PA, Stivers JT, N-terminal domain of human uracil DNA glycosylase (hUNG2) promotes targeting to uracil sites adjacent to ssDNA-dsDNA junctions, Nucleic Acids Res, 46 (2018) 7169–7178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Feng Y, Li C, Stewart JA, Barbulescu P, Seija Desivo N, Alvarez-Quilon A, Pezo RC, Perera MLW, Chan K, Tong AHY, Mohamad-Ramshan R, Berru M, Nakib D, Li G, Kardar GA, Carlyle JR, Moffat J, Durocher D, Di Noia JM, Bhagwat AS, Martin A, FAM72A antagonizes UNG2 to promote mutagenic repair during antibody maturation, Nature, 600 (2021) 324–328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Rogier M, Moritz J, Robert I, Lescale C, Heyer V, Abello A, Martin O, Capitani K, Thomas M, Thomas-Claudepierre AS, Laffleur B, Jouan F, Pinaud E, Tarte K, Cogne M, Conticello SG, Soutoglou E, Deriano L, Reina-San-Martin B, Fam72a enforces error-prone DNA repair during antibody diversification, Nature, 600 (2021) 329–333. [DOI] [PubMed] [Google Scholar]
- [51].Hegde ML, Hazra TK, Mitra S, Functions of disordered regions in mammalian early base excision repair proteins, Cell Mol Life Sci, 67 (2010) 3573–3587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Mer G, Bochkarev A, Gupta R, Bochkareva E, Frappier L, Ingles CJ, Edwards AM, Chazin WJ, Structural basis for the recognition of DNA repair proteins UNG2, XPA, and RAD52 by replication factor RPA, Cell, 103 (2000) 449–456. [DOI] [PubMed] [Google Scholar]
- [53].Hegde ML, Tsutakawa SE, Hegde PM, Holthauzen LM, Li J, Oezguen N, Hilser VJ, Tainer JA, Mitra S, The disordered C-terminal domain of human DNA glycosylase NEIL1 contributes to its stability via intramolecular interactions, J Mol Biol, 425 (2013) 2359–2371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Smet-Nocca C, Wieruszeski JM, Chaar V, Leroy A, Benecke A, The thymine-DNA glycosylase regulatory domain: residual structure and DNA binding, Biochemistry, 47 (2008) 6519–6530. [DOI] [PubMed] [Google Scholar]
- [55].Coey CT, Malik SS, Pidugu LS, Varney KM, Pozharski E, Drohat AC, Structural basis of damage recognition by thymine DNA glycosylase: Key roles for N-terminal residues, Nucleic Acids Res, 44 (2016) 10248–10258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Petronzelli F, Riccio A, Markham GD, Seeholzer SH, Stoerker J, Genuardi M, Yeung AT, Matsumoto Y, Bellacosa A, Biphasic kinetics of the human DNA repair protein MED1 (MBD4), a mismatch-specific DNA N-glycosylase, J Biol Chem, 275 (2000) 32422–32429. [DOI] [PubMed] [Google Scholar]
- [57].Visnes T, Doseth B, Pettersen HS, Hagen L, Sousa MM, Akbari M, Otterlei M, Kavli B, Slupphaug G, Krokan HE, Uracil in DNA and its processing by different DNA glycosylases, Philos Trans R Soc Lond B Biol Sci, 364 (2009) 563–568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Chen CY, Mosbaugh DW, Bennett SE, Mutations at Arginine 276 transform human uracil-DNA glycosylase into a single-stranded DNA-specific uracil-DNA glycosylase, DNA Repair (Amst), 4 (2005) 793–805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Roy U, Scharer OD, Involvement of translesion synthesis DNA polymerases in DNA interstrand crosslink repair, DNA Repair (Amst), 44 (2016) 33–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Yang W, Gao Y, Translesion and Repair DNA Polymerases: Diverse Structure and Mechanism, Annu Rev Biochem, 87 (2018) 239–261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Dianov GL, Sleeth KM, Dianova II, Allinson SL, Repair of abasic sites in DNA, Mutat Res, 531 (2003) 157–163. [DOI] [PubMed] [Google Scholar]
- [62].Housh K, Jha JS, Haldar T, Amin SBM, Islam T, Wallace A, Gomina A, Guo X, Nel C, Wyatt JW, Gates KS, Formation and repair of unavoidable, endogenous interstrand cross-links in cellular DNA, DNA Repair (Amst), 98 (2021) 103029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Semlow DR, Walter JC, Mechanisms of Vertebrate DNA Interstrand Cross-Link Repair, Annu Rev Biochem, 90 (2021) 107–135. [DOI] [PubMed] [Google Scholar]
- [64].Admiraal SJ, O’Brien PJ, Base excision repair enzymes protect abasic sites in duplex DNA from interstrand cross-links, Biochemistry, 54 (2015) 1849–1857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Verma SC, Bajaj BG, Cai Q, Si H, Seelhammer T, Robertson ES, Latency-associated nuclear antigen of Kaposi’s sarcoma-associated herpesvirus recruits uracil DNA glycosylase 2 at the terminal repeats and is important for latent persistence of the virus, J Virol, 80 (2006) 11178–11190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Hafez AY, Messinger JE, McFadden K, Fenyofalvi G, Shepard CN, Lenzi GM, Kim B, Luftig MA, Limited nucleotide pools restrict Epstein-Barr virus-mediated B-cell immortalization, Oncogenesis, 6 (2017) e349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Tarbouriech N, Buisson M, Seigneurin JM, Cusack S, Burmeister WP, The monomeric dUTPase from Epstein-Barr virus mimics trimeric dUTPases, Structure, 13 (2005) 1299–1310. [DOI] [PubMed] [Google Scholar]
- [68].Cheng AZ, Yockteng-Melgar J, Jarvis MC, Malik-Soni N, Borozan I, Carpenter MA, McCann JL, Ebrahimi D, Shaban NM, Marcon E, Greenblatt J, Brown WL, Frappier L, Harris RS, Epstein-Barr virus BORF2 inhibits cellular APOBEC3B to preserve viral genome integrity, Nat Microbiol, 4 (2019) 78–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Bekerman E, Jeon D, Ardolino M, Coscoy L, A Role for Host Activation-Induced Cytidine Deaminase in Innate Immune Defense against KSHV. PLoS Pathog 9 (2013) e1003748. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data that support the findings of this study are available from the corresponding author upon request.