Abstract
Fracture healing is a complex cascade of cellular and molecular processes. These processes require the appropriate cellular and molecular environment to ensure the restoration of skeletal stability and resolution of inflammation. In order for fracture healing to occur, the necessary building blocks for bone metabolism and synthesis must be supplied through proper nutrition. Pharmacologic therapies aimed at modulating the inflammatory response to fractures have the potential to interfere with the synthesis of molecules needed for the production of bone. Infection can interfere with, and even prevent normal fracture healing from occurring. Cellular and genetic treatment strategies are actively being developed to target deficiencies, and bridge gaps that can influence how fractures heal. Evolving technologies, including nutritional supplementation, pharmacotherapies, antibiotics, surgical techniques, as well as genetic and cellular therapies, have the potential to enhance, optimize, and even revolutionize the process of fracture healing.
Keywords: cellular therapies, fracture healing, metabolics
1. Metabolic optimization—A missed opportunity in bone health? Calcium and vitamin D use
Vitamin D and calcium homeostasis play an integral role in fracture healing, muscle strength, and injury prevention.[1] Vitamin D promotes the uptake of calcium and phosphate from the kidneys and gastrointestinal tract, which are then deposited in bone. Vitamin D can also directly promote bone formation by activating the receptor activator of nuclear factor-κB ligand (RANKL) to induce osteoblast differentiation.
Vitamin D deficiency is prevalent in orthopaedic trauma patients, with studies estimating anywhere from 66% to 89%.[2,3] Unfortunately, there remains a lack of consensus on what constitutes vitamin D insufficiency and deficiency. The National Academy of Medicine defines vitamin D deficiency as <12 ng/mL and insufficiency as 13 to 19 ng/mL, whereas the Endocrine Society defines deficiency as <20 ng/mL and insufficiency as 20 to 29 ng/mL.[4,5] The relevance of these values has not been validated in orthopaedic trauma.
Brinker et al[2] showed that 68% of patients with nonunions without any identifiable cause were vitamin D deficient, bringing vitamin D to the forefront of the nonunion discussion. The prevalence of hypovitaminosis D and nonunion has been shown in several subsequent studies, but causation has been more difficult to discern, as a high percentage of trauma patients are vitamin D deficient, not just those with nonunions.
Bodendorfer et al[6] treated 201 trauma patients with vitamin D and found no difference in follow-up vitamin D levels between the union and nonunion groups. Gorter et al[7] found a higher union rate in a cohort with a corrected vitamin D level compared to a cohort with uncorrected levels, although there were only 3 nonunions in the uncorrected group and 2 in the corrected group. Haines et al[3] randomized 100 patients to either 1 high-dose vitamin D supplement or placebo. There was no statistical difference in union rates between the 2 cohorts, with 2 nonunions in each.
These studies highlight the difficulty in investigating the effect of Vitamin D on fracture healing. Controlling for other nonunion risk factors such as smoking, diabetes, infection, and non-steroidal anti-inflammatory drug (NSAID) use would require a large pool of patients. Additionally, there is significant heterogeneity in these studies regarding what constitutes vitamin D deficiency and the dose and duration of vitamin D supplementation investigated. A consensus on which patients need vitamin D supplementation and the optimal dose and duration of supplementation is lacking in the existing literature.
Author Mullis and colleagues developed a replacement protocol in collaboration with Pharmacy and Endocrinology at their institution (Table 1). However, a retrospective case-control study evaluating tibia shaft fractures treated by an intramedullary nail before and after protocol initiation showed there was no effect on nonunion rates.[8] Given the cost of screening, they now supplement all trauma patients with 2 days of 50,000 units of D3 orally, with continued over the counter daily supplementation with vitamin D and calcium until fracture consolidation. Vitamin D testing and a tailored treatment protocol are still used for established delayed unions or nonunions.
Table 1.
Vitamin D replacement protocol.
| Vitamin D 25-OH level | Ergocalciferol (vitamin D2) supplementation† | Primary care referral |
|---|---|---|
| >30 ng/mL | No treatment needed | No |
| 21–30 ng/mL | 50,000 units once weekly for 6 weeks | No∗ |
| 10-20 ng/mL | 50,000 units twice weekly for 6 weeks | No∗ |
| <10 ng/mL | 50,000 units 3 times weekly for 8 weeks | Yes |
If repeat serum 25 (OH)D levels are clinically indicated and performed after 8 weeks and are again abnormal, the patient should be referred to primary care.
Calcium supplementation has not been investigated in isolation for fracture healing, and recommendations on supplementation for trauma patients are typically extrapolated from osteoporosis data for fracture prevention. A meta-analysis of randomized trials showed that vitamin D in isolation did not prevent fractures, but it did when given in combination with calcium supplementation.[9] The typical recommendation is 1200 mg of calcium daily from diet and supplementation combined. When daily intake exceeds this value, patients are at increased risk of nephrolithiasis, cardiovascular disease, and stroke.[10]
Nonunion has been shown to significantly worsen functional outcomes, and amplify the cost of care.[11,12] However, measuring vitamin D levels can also be expensive, with a list charge of $365 at 1 academic medical center (reagent cost is $8 per test). Therefore, it may be more cost effective to treat all trauma patients with vitamin D supplementation given the safety and low cost of supplementation.[13]
We know that vitamin D and calcium play an important physiologic role in bone healing. There appears to be a correlation between vitamin D deficiency and nonunion; however, causation has yet to be convincingly proven. Should the threshold for supplementation be lower in trauma patients, who have a disproportionately high rate of hypovitaminosis D? What dose and duration of supplementation should be used? Should we also be providing calcium supplementation? Should we be screening at all or treating prophylactically? The answers to these questions will require high power studies to control for the many risk factors for nonunion but could potentially lower nonunion rates, which is a costly and debilitating complication.
2. NSAIDs and bone healing. What is the answer?
Fracture healing is a complex cascade of cellular and molecular processes. A fracture represents not only an injury to the bone, but also to the surrounding soft tissues. Fracture healing is influenced by the fracture/soft tissue injury pattern, the health of host, and the resultant biomechanical/biochemical environment. Two types of fracture healing typically occur: primary bone healing through direct remodeling of the lamellar bone, which requires anatomic reduction of the fracture and stable internal fixation, and secondary bone healing—the most common form of bone healing, which involves endochondral and intramembranous ossification.
Fracture healing begins with an acute inflammatory phase where interference by nonsteroidal anti-inflammatory drugs is thought to occur. This inflammatory phase peaks between 24 and 72 hours, and involves the release of cytokines including tumor necrosis factor-α (TNF-α) interleukin (IL)-1, -6, -11, and -18, as well as a variety of prostaglandins. These inflammatory factors recruit mesenchymal stem cells (MSCs) from the surrounding soft tissues, bone marrow, and systemic circulation. These cells promote vascular endothelial growth factor (VEGF), support angiogenesis, and stimulate the differentiation of osteoblasts and osteoclasts from progenitor cells.
The majority of inflammatory molecules originate from the cell membrane phospholipids. These phospholipids are metabolized to arachidonic acid, which is then metabolized by cyclooxygenase (COX) isoenzymes, COX-1 and COX-2, to prostaglandins (Figure 1). It is the COX-2 isoenzyme that is upregulated by an inflammatory state, and induces the release of prostaglandins specifically in response to injury. Historically, NSAIDs have been separated into 3 main classes: Classic NSAIDs, which are nonselective and block COX1 and COX-2 equally, Selective NSAIDs, which preferentially inhibit COX-2, and Coxibs, highly selective inhibitors of COX-2. The concern with administering NSAIDs in the setting of an acute fracture is that they will interfere with this necessary inflammatory pathway and disrupt fracture healing. Additionally, COX-2 has been shown to be necessary for MSCs to differentiate into osteoblasts. Inhibition of COX-2 has been shown to limit osteogenesis as well as angiogenesis, and block differentiation and proliferation of osteoblast precursors.
Figure 1.
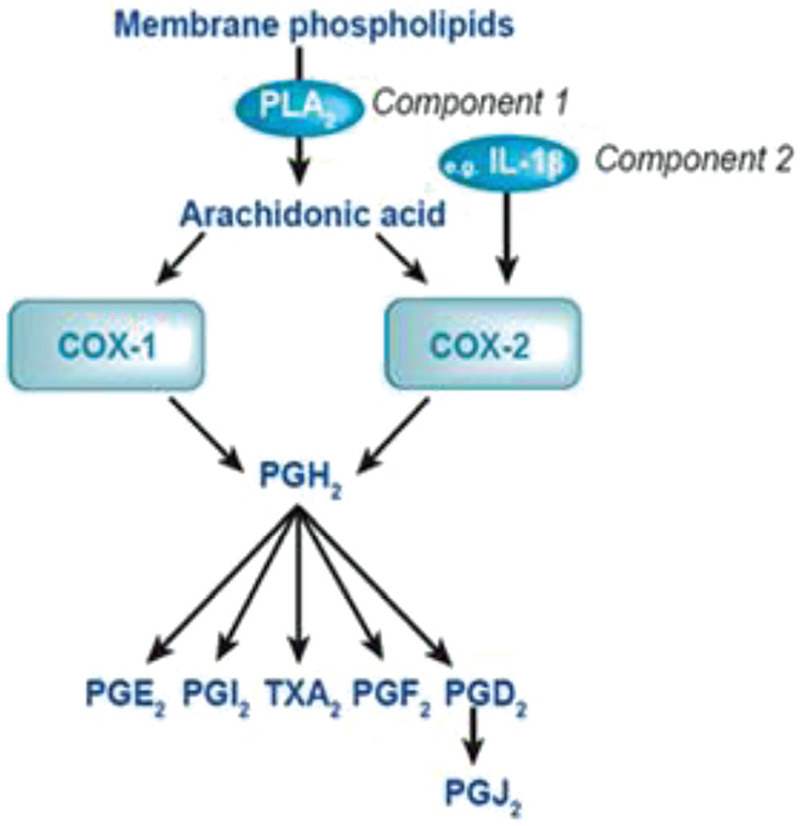
Prostaglandin production. COX-1 = cyclooxygenase-1; COX-2 = cyclooxygenase-2; PLA2 = phospholipase A2; IL-1β = interleukin-1β; PGH2 = prostaglandin H2; PGE2 = prostaglandin E2; PGI2 = prostaglandin I2; TXA2 = thromboxane A2; PGF2 = prostaglandin F2; PGD2 = prostaglandin D2; PGJ2 = prostaglandin J2.
Animal studies have historically shown a negative effect of NSAIDs on bone healing. These negative effects include: decreased prostaglandin production, decreased mineral content and matrix of the callus, inhibited Haversian remodeling, decreased bone stiffness and strength, and histologic evidence of increased fibrous tissue accumulation.[14]
Many investigators have studied the effects of NSAIDs on fracture healing in humans. Giannoudis et al[15] studied 32 patients with nonunion of a femoral diaphyseal fracture and compared them to 67 comparable patients whose fractures had united. Multiple factors that could have influenced healing, including exposure to NSAIDs, were assessed. These authors found a marked association between nonunion and the use of NSAIDs after injury. Borgeat et al systematically identified 3 prospective randomized controlled studies and 13 retrospective studies including 12,895 patients that did not identify any strong evidence that NSAIDs administered after fracture or spinal fusion lead to increased nonunion rates.[16] More recently, Whestley et al[17] studied the effect of NSAIDs on fracture healing rates in children and adults. There were a total of 2017 pediatric fractures, of which 1181 were exposed to NSAIDs. There were 12,030 adult fractures, of which 1349 were exposed to NSAIDs. In their analysis, a negative effect of NSAIDs on bone healing was identified in the adult population, but not the pediatric patients. The investigators felt that these effects in adults might be dose and time dependent, because low-dose/short-duration exposure did not affect union rates. Jeffcoach et al[18] reported on 1901 patients with femur, tibia, and/or humerus fractures, 12% of whom received NSAIDs. They found that long bone fracture patients who received NSAIDs in the postoperative period were twice as likely to suffer fracture healing complications, and therefore recommended avoiding NSAIDs in patients with long bone fractures.
It remains unclear whether a negative relationship exists between the use of NSAIDs and fracture healing. Although the animal and clinical studies indicate NSAIDs may have a detrimental effect on fracture healing, the effects appear to be dependent upon the type, dosage, timing, and the duration of NSAID use. General recommendations for the use of NSAIDs in the setting of fracture is to limit their use to ≤7 days, use the lowest effective dose, and the least selective NSAID.
3. Strategies to optimize fracture healing when competing with infection
What should we do for the patient who returns to clinic with an infection? There are principles and protocols that can be successful over 60% of the time.[19,20,21] The goals for treatment are to control infection, obtain fracture healing, avoid chronic osteomyelitis, and restore function.[22] The way these goals are reached vary based on patient and injury factors, timing of the infection, and resources available—both from a surgeon's technical expertise and support from Infectious Disease and soft tissue coverage colleagues.
The literature lacks a clear definition for musculoskeletal infections involving fractures. Similar to previous efforts for a Prosthetic Joint Infection (PJI), an international multidisciplinary expert panel was used to make consensus statements regarding infections involving fractures. Although there may be different treatment strategies for acute versus chronic infections, the anatomical location involved, superficial versus deep, and whether or not implants are involved, there should be only 1 definition. To improve uniformity in both communication and research, the term fracture-related infection (FRI) was unanimously agreed upon as the general term for infections involving fractures.[23]
Both confirmatory and suggestive criteria for FRI were determined. Confirmatory criteria included (1) fistula, sinus, or wound breakdown communicating with the bone or hardware, (2) purulent drainage or pus found during surgery, (3) phenotypically indistinguishable specimens identified by intraoperative culture from at least 2 separate deep tissue/implant (including sonication-fluid), and (4) intraoperative deep tissue cultures with microorganisms confirmed by histopathological examination using specific staining techniques for bacteria or fungi. It was recommended that at least 3 tissue cultures (joint aspiration with fluid sample if articular involvement) be taken during surgery. Suggestive criteria included clinical and radiographic signs consistent with infection, elevated inflammatory markers, a positive single tissue culture, and persistent wound drainage or joint effusion.
Patient and injury factors contribute to the risk of FRI.[22,23] Injury risk factors include open fractures with vascular and/or soft tissue compromise. Patient risk factors include medical co-morbidities that affect host physiology such as diabetes mellitus, peripheral vascular or cardiac disease, liver disease, renal disease, alcoholism, and malignancy. These risk factors—host and injury—not only determine the risk of infection, but also affect potential management strategies.
Standard radiographs of the fracture and laboratory evaluation should be performed. At minimum, a C-reactive protein (CRP) should be obtained. Usually, a complete blood count (CBC) and erythrocyte sedimentation rate (ESR) are obtained as well, although these are not necessarily specific or sensitive. When a nonunion is suspected, a metabolic evaluation is also performed to identify any modifiable risk factors for nonunion. With delayed infection (greater than 2 weeks) presentation, serial radiographs, computed tomography (CT), and magnetic resonance imaging (MRI) may be used to evaluate the depth of bone and soft tissue involvement. The use of other nuclear medicine scans and advance imaging in FRI may be helpful, but their reliability is inconclusive at this time.
Treatment strategies revolve around the goals of infection control, fracture healing, and functional restoration. Strategies vary based on time of symptom onset, fracture healing status, fracture location, host factors, pathogen, and type and status of indwelling implants. However, the common denominator is aggressive operative debridement and pathogen identification. Operative debridement includes debriding until viable, bleeding tissue is encountered, potentially creating bony and soft tissue defects to ensure a healthy healing environment. It is important to obtain at least 3 deep tissue cultures intraoperatively.[23] If 2 of the 3 cultures are positive for the same organism, then the diagnosis is reliable. Improving the ability to accurately detect bacteria has led to the use of polymerase chain reaction (PCR) testing. However, this only identifies the presence of bacterial DNA, not whether it is alive or dead, or the antibiotic sensitivities. Infection control consists of operative debridement, implant retention (if FRI is early and implants are stable) or acute/staged definitive implant exchange (if FRI is late, poor host or soft tissue envelope, implants unstable, indwelling intramedullary nail, previous failed retention attempt, or virulent organism/mature biofilm present), plus antibiotic therapy—systemic and/or local. Although this process may be long and arduous, these strategies can lead to successful fracture healing and a functional limb.
4. Orthopaedic biologics technology and cell-based therapies
Cell-based therapies are a frequently discussed and commonly utilized approach for augmenting challenging healing situations in orthopaedic trauma.[24,25,26] Access to autologous cell populations has improved recently.[27] However, questions still remain about the efficacy of cell-based approaches for enhancing bone regeneration in clinical applications. Nonetheless, clinicians remain interested in utilizing cellular augmentation, and a number of point-of-care techniques are available to obtain a variety of cell types. Bone marrow aspiration and intramedullary bone harvest represent the most highly accessible cell harvesting solutions for Orthopaedic surgeons, and advances in understanding of these technologies may improve their therapeutic benefit in the future.
While bone marrow aspiration requires specific techniques and experience to efficiently aspirate marrow versus blood,[28] commercially available systems are effective at concentrating aspirates at the point-of-care, representing low morbidity options for harvesting cell progenitors. Commercial bone marrow aspirate systems have been well studied and efficiently concentrate MSC populations, platelets, and growth factors, but not hematopoietic progenitor cells.[29] The importance of this efficiency in concentration cannot be understated because the number of MSCs in bone marrow is small, estimated to be around 0.02% of the resident cell population. Current needs for this technique include the development of BMAC quality control assays for the point-of-care to ensure that progenitor populations are being isolated before delivery.
Bone marrow aspirates have been well studied in orthopaedic trauma applications.[30] Nonetheless, important questions still remain regarding the benefit of bone marrow aspirate treatment as a way of harnessing cell-based treatment benefits. This is due to lack of uniform techniques for cell isolation, lack of a point of care assay for cell type, number, and viability from a broad range of donor types, and no clear understanding of the best way to deliver these cells.[28] Clinical trials will be necessary to correlate specific MSC subpopulations and growth factors with therapeutic efficacy.
Intramedullary bone graft harvest is a well-studied grafting option and, in theory, allows access to autogenous cells. Several publications have evaluated human reaming debris as a source of viable osteoblastic progenitor cells.[31,32] A large number of cells are found on the surface of the bony spicules liberated during the mechanical reaming process. In fact, reaming may represent a more efficient and reliable approach to cell harvesting versus bone marrow aspiration or cancellous crest harvest.[33]
More recently discussed is the concept that endosteal reaming may provide access to a potent bone progenitor cell population.[34] The endosteal region of bone has many progenitor cells with high proliferative capacity.[33] This finding suggests that mechanical reaming of the endosteal zone likely liberates a large number of mesenchymal progenitor cells. Studies are underway to optimize isolation, identification, and deployment of this specific cell population.[26]
Multiple studies demonstrate that the waste water effluent from reamer aspirator systems can provide a significant source of cells.[35] Interestingly, all of the Reamer-Irrigator-Aspirator (RIA – DePuy-Synthes/Johnson & Johnson Medical Devices Companies, West Chester, PA) samples had higher Colony Forming Units (CFU)-F counts than 55,000, the number referenced by Hernigou[36] as the threshold number needed for successful healing; in fact the average number of cells was over 300,000 representing a very high volume of liberated progenitors.[36] The development of point-of-care technology to capture and re-deploy these cells remains an unsolved clinical need.
The ability to harvest viable cells is currently technically feasible. However, a multitude of questions remains regarding the deployment and efficacy of these cells. Even more fundamental questions requiring further inquiry include the determination of the optimal cell progenitor type and the mechanism and function of harvested progenitor cells in bone regeneration. In theory, surgeons will soon have the ability to enrich structural and inductive grafts of choice with a large number of endogenous bone-forming progenitors, which may significantly improve healing rates in challenging clinical healing and bone loss scenarios.
5. Conclusion
Calcium, vitamin D, NSAIDs, infection, and mesenchymal progenitor cells all impact bone metabolism and fracture healing. Modification of each of these factors has been shown to have a significant impact, but not necessarily a definitive correlation, with timely and complete fracture healing. Calcium and vitamin D supplementation is indicated for patients with documented deficiencies. However, the role of supplementation in fracture patients without deficiencies, those with additional risk factors for delayed or nonunion, or in all musculoskeletal trauma patients, is less well defined. Data from animal studies and our understanding of biochemical molecular mechanisms suggest that NSAIDs have the potential to negatively impact fracture healing. Human studies have indicated that their effects are likely dosage-dependent and drug-specific, suggesting that they may be used safely in limited quantities, and for a limited duration. Fracture-related infection can be challenging to define, diagnose, and treat effectively. A clear understanding of the factors that can be optimized in the host, and treated effectively through antimicrobial and surgical strategies, will be most likely to lead to successful fracture healing. Emerging technologies that facilitate the targeted delivery of selected autologous progenitor cell populations into fractures have significant potential to address some of the greatest challenges in modern fracture care.
References
- 1. Abrams GD, Feldman D, Safran MR. Effects of vitamin D on skeletal muscle and athletic performance. J Am Acad Orthop Surg. 2018;26:278–285. [DOI] [PubMed] [Google Scholar]
- 2. Brinker MR, O’Connor DP, Monla YT, et al. Metabolic and endocrine abnormalities in patients with nonunions. J Orthop Trauma. 2007;21:557–570. [DOI] [PubMed] [Google Scholar]
- 3. Haines N, Kempton LB, Seymour RB, et al. The effect of a single early high-dose vitamin D supplement on fracture union in patients with hypovitaminosis D: a prospective randomised trial. Bone Joint J. 2017;99-B:1520–1525. [DOI] [PubMed] [Google Scholar]
- 4. Rosen CJ, Abrams SA, Aloia JF, et al. IOM committee members respond to endocrine society vitamin D guideline. J Clin Endocrinol Metab. 2012;97:1146–1152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Holick MF, Binkley NC, Bischoff-Ferrari HA, et al. Evaluation, treatment, and prevention of vitamin D deficiency: an endocrine society clinical practice guideline. J Clin Endocrinol Metab. 2011;96:1911–1930. [DOI] [PubMed] [Google Scholar]
- 6. Bodendorfer BM, Cook JL, Robertson DS, et al. Do 25-hydroxyvitamin D levels correlate with fracture complications? J Orthop Trauma. 2016;30:e312–317. [DOI] [PubMed] [Google Scholar]
- 7. Gorter EA, Krijnen P, Schipper IB. Vitamin D status and adult fracture healing. J Clin Orthop Trauma. 2017;8:34–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Jacobson J, Harris S, Zahn E, et al. Characterization of vitamin D deficiency and use of a standardized supplementation protocol in orthopaedic trauma patients. Poster Presented at the 2018 Orthopaedic Trauma Association Annual Meeting, Kissimmee, FL October 17-20. 2018. [Google Scholar]
- 9. Avenell A, Mak JCS, O’connell D. Vitamin D and vitamin D analogues for preventing fractures in post-menopausal women and older men. Cochrane Database Syst Rev. 2014;2014:CD000227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Cosman F, de Beur SJ, LeBoff MS, et al. Clinician's guide to prevention and treatment of osteoporosis. Osteoporos Int. 2014;25:2359–2381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Tay WH, de Steiger R, Richardson M, et al. Health outcomes of delayed union and nonunion of femoral and tibial shaft fractures. Injury. 2014;10:1653–1658. [DOI] [PubMed] [Google Scholar]
- 12. Kanakaris NK, Giannoudis PV. The health economics of the treatment of longbone non-unions. Injury. 2007;38:577–584. [DOI] [PubMed] [Google Scholar]
- 13. Patton CM, Powell AP, Patel AA. Vitamin D in orthopaedics. J Am Acad Orthop Surg. 2012;20:123–129. [DOI] [PubMed] [Google Scholar]
- 14. Pountos I, Georgouli T, Blokhuis TJ, et al. Pharmacological agents and impairment of fracture healing: what is the evidence? Injury. 2008;39:384–394. [DOI] [PubMed] [Google Scholar]
- 15. Giannoudis PV, MacDonald DA, Matthews SJ, et al. The influence of reaming and non-steroidal anti-inflammatory drugs. J Bone Joint Surg. 2000;82-B:655–658. [DOI] [PubMed] [Google Scholar]
- 16. Borgeat A, Ofner C, Saporito A, et al. The effect of nonsteriodal anti-inflammatory drugs on bone healing in humans: A qualitative, systemic review. J Clin Anesth. 2018;49:92–100. [DOI] [PubMed] [Google Scholar]
- 17. Wheatley BM, Nappo KE, Christensen DL, et al. Effect of NSAIDs on bone healing rates: a meta-analysis. J Am Acad Orthop Surg. 2019;27:e330–e336. [DOI] [PubMed] [Google Scholar]
- 18. Jeffcoach DR, Sams VG, Lawson CM, et al. Nonsteroidal anti-inflammatory drugs’ impact on nonunion and infection rates in long-bone fractures. J Trauma Acute Care Surg. 2014;76:779–783. [DOI] [PubMed] [Google Scholar]
- 19. Rightmire E, Zurakowski D, Vrahas M. Acute infections after fracture repair: management with hardware in place. Clin Orthop. 2008;466:466–472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Cho J-W, Kim J, Cho W-T, et al. Antibiotic coated hinged threaded rods in the treatment of infected nonunions and intramedullary long bone infections. Injury. 2018;49:1912–1921. [DOI] [PubMed] [Google Scholar]
- 21. Berkes M, Obremskey WT, Scannell B, et al. Maintenance of hardware after early postoperative infection following fracture internal fixation. J Bone Joint Surg Am. 2010;92:823–828. [DOI] [PubMed] [Google Scholar]
- 22. Metsemakers WJ, Kuehl R, Moriarty TF, et al. Infection after fracture fixation: current surgical and microbiological concepts. Injury. 2018;49:511–522. [DOI] [PubMed] [Google Scholar]
- 23. Metsemakers WJ, Morgenstern M, McNally MA, et al. Fracture-related infection: a consensus on definition from an international expert group. Injury. 2018;49:505–510. [DOI] [PubMed] [Google Scholar]
- 24. Palombella S, Lopa S, Gianola S, et al. Bone marrow-derived cell therapies to heal long-bone nonunions: a systematic review and meta-analysis—which is the best available treatment? Stem Cells Int. 2019;2019:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Marcucio RS, Nauth A, Giannoudis PV, et al. Stem cell therapies in orthopaedic trauma. J Orthop Trauma. 2015;29 (Suppl 12):S24–S27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Tare RS, Kanczler J, Aarvold A, et al. Skeletal stem cells and bone regeneration: translational strategies from bench to clinic. Proc Inst Mech Eng H. 2010;224:1455–1470. [DOI] [PubMed] [Google Scholar]
- 27. Ho-Shui-Ling A, Bolander J, Rustom LE, et al. Bone regeneration strategies: engineered scaffolds, bioactive molecules and stem cells current stage and future perspectives. Biomaterials. 2018;180:143–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Schottel PC, Warner SJ. Role of bone marrow aspirate in orthopedic trauma. Orthop Clin North Am. 2017;48:311–321. [DOI] [PubMed] [Google Scholar]
- 29. Schäfer R, DeBaun MR, Fleck E. Quantitation of progenitor cell populations and growth factors after bone marrow aspirate concentration. J Transl Med. 2019;17:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Imam MA, Holton J, Ernstbrunner L, et al. A systematic review of the clinical applications and complications of bone marrow aspirate concentrate in management of bone defects and nonunions. Int Orthop. 2019;41:2213–2220. [DOI] [PubMed] [Google Scholar]
- 31. Trinkaus K, Wenisch S, Siemers C, et al. [Reaming debris: a source of vital cells! First results of human specimens]. Unfallchirurg. 2005;108:650–656. [DOI] [PubMed] [Google Scholar]
- 32. Siclari VA, Zhu J, Akiyama K, et al. Mesenchymal progenitors residing close to the bone surface are functionally distinct from those in the central bone marrow. Bone. 2013;53:575–586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Baboolal TG, Boxall SA, El-Sherbiny YM, et al. Multipotential stromal cell abundance in cellular bone allograft: comparison with fresh age-matched iliac crest bone and bone marrow aspirate. Regen Med. 2014;9:593–607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Bakker AD, Kroeze RJ, Korstjens C, et al. Reaming debris as a novel source of autologous bone to enhance healing of bone defects. J Biomed Mater Res. 2011;97:457–465. [DOI] [PubMed] [Google Scholar]
- 35. Churchman SM, Kouroupis D, Boxall SA, et al. Yield optimisation and molecular characterisation of uncultured CD271+ mesenchymal stem cells in the Reamer Irrigator Aspirator waste bag. Eur Cell Mat. 2013;26:252–262. [DOI] [PubMed] [Google Scholar]
- 36. Hernigou P, Poignard A, Beaujean F, et al. Percutaneous autologous bone-marrow grafting for nonunions. Influence of the number and concentration of progenitor cells. J Bone Joint Surg Am. 2005;87:1430–1437. [DOI] [PubMed] [Google Scholar]


