Abstract
The aim of this study was to compare outcomes between dexmedetomidine and propofol for sedation after cardiac surgery in patients requiring mechanical ventilation. This meta-analysis was performed according to Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines. Online databases, including EMBASE, PubMed, and the Cochrane Library, were comprehensively searched to identify relevant randomized controlled trials (RCTs) comparing the safety and efficacy of dexmedetomidine and propofol in patients undergoing cardiac surgery and requiring mechanical ventilation. The examined outcomes included the mean length of intensive care unit (ICU) stay in hours, duration of mechanical ventilation in hours, length of hospital stay in days, and number of patients diagnosed with delirium. A total of 14 studies were included in the present meta-analysis while 1360 patients undergoing cardiac surgery were involved in these studies. Pooled results showed that the duration of mechanical ventilation was lower in the dexmedetomidine group compared to the propofol group (mean difference (MD): 0.75, 95% confidence interval (CI): 0.06-1.44, p-value: 0.03). We also found a significantly low length of stay in ICU in the dexmedetomidine group compared to the propofol (MD: 0.89, 95% CI: 0.04-1.74, p-value: 0.04). The length of hospital stay was also significantly lower in patients receiving dexmedetomidine as compared to the propofol group (MD: 0.51, 95% CI: 0.32-0.70, p-value<0.001). Risk of delirium was significantly higher in patients receiving propofol compared to patients receiving dexmedetomidine (RR: 2.02, 95% CI: 1.48-2.74, p-value<0.001). In conclusion, our meta-analysis provides evidence of the beneficial impacts of dexmedetomidine on clinical outcomes in patients undergoing cardiac surgery. Dexmedetomidine was associated with a significant reduction in the duration of mechanical ventilation, length of stay in the intensive care unit (ICU) and hospital, and the risk of delirium.
Keywords: meta-analysis, cardiac surgery, post-operative outcomes, propofol, dexmedetomidine
Introduction and background
Every year, more than two-million cardiac surgeries are performed worldwide [1]. Although cardiac surgery is commonly utilized to treat complications arising from ischemic heart disease, correct congenital heart disease, or address valvular heart disease caused by factors such as endocarditis, rheumatic heart disease, and atherosclerosis, these procedures come with several drawbacks [2]. The performance of cardiac surgery is known to carry significant risks of cardiovascular complications and other unfavorable outcomes, often resulting in extended hospital stays and even mortality [3-5]. Despite notable advancements in equipment, techniques, and medical care that have led to reduced rates of major complications and mortality [6-7], there remains a need for effective and safe perioperative medication to further minimize these adverse events [8].
While propofol is widely employed as a sedative agent in operating rooms and intensive care units (ICUs), it can potentially lead to hypotension, bradycardia, respiratory depression, and even apnea, depending on the dosage used for infusion [9-10]. On the other hand, dexmedetomidine is a selective agonist of α2 receptors. Its utilization in fast-track procedures is on the rise due to its advantageous properties, including sedation, analgesia, and anxiolysis, without causing respiratory depression. Dexmedetomidine induces a sedative effect that closely resembles natural sleep patterns when examined using electroencephalography, thereby preserving cognitive functions. Patients can be easily awakened, allowing for improved cooperation. However, it should be noted that depending on the infusion dosage, dexmedetomidine may result in hypotension and bradycardia [11-12].
An earlier meta-analysis attempted to elucidate the role of these sedatives in post-cardiac surgical sedation [13]. However, the study's findings were limited due to methodology issues and the inclusion criteria, as pointed out in a letter addressed to the editor [14]. Despite this, propofol was preferred to benzodiazepines for sedation among patients in the cardiac surgical ICU, according to the 2018 Clinical Practise Guidelines for the Prevention and Management of Pain, Agitation/Sedation, Delirium, Immobility, and Sleep Disruption in Adult Patients in the ICU (PADIS) guidelines [15]. However, there was a lack of sufficient data on the efficacy of dexmedetomidine versus propofol in this population. A meta-analysis conducted by Heybati et al. reported that the use of dexmedetomidine did not have a significant effect on the length of stay in the ICU when compared to propofol. However, it did lead to a significant decrease in the duration of mechanical ventilation and a reduced risk of delirium among patients undergoing cardiac surgery [16]. Nonetheless, this meta-analysis included both cardiac surgery patients and non-cardiac surgery patients. Furthermore, since the publication of this meta-analysis, certain new randomized control trials have been published that reported postoperative outcomes between patients who received dexmedetomidine and propofol. Therefore, we conducted this meta-analysis to compare outcomes between dexmedetomidine and propofol for sedation after cardiac surgery in patients requiring mechanical ventilation.
Review
Methodology
This meta-analysis was performed according to Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines. Online databases, including EMBASE, PubMed, and the Cochrane Library, were comprehensively searched to identify relevant randomized controlled trials (RCTs) comparing the safety and efficacy of dexmedetomidine and propofol in patients undergoing cardiac surgery and requiring mechanical ventilation. The following Medical Subject Heading (MeSH) terms and free texts were utilized in different combinations to select eligible articles: "dexmedetomidine," "propofol," "cardiac surgery," "heart surgery," and "coronary artery bypass grafting," without placing restrictions on the year of publication. Our search was restricted to studies published in the English language only. To further expand the search, the reference lists of all included articles were manually searched. Two authors independently screened all records obtained from the online database search. The first-level screening was done based on titles and abstracts, followed by full-text screening. For this purpose, full texts of eligible records were obtained, and a detailed assessment was done based on predefined inclusion and exclusion criteria. Any disagreement in the study selection process was resolved through discussion.
Inclusion and Exclusion Criteria
According to the Participants, Interventions, Comparisons, Outcomes, and Study (PICOS) design protocol, the following criteria were used.
Participants: We included patients undergoing cardiac surgery, including coronary artery bypass grafting (CABG), aortic surgery, percutaneous coronary intervention, valve surgery, and others.
Intervention and comparison: Studies comparing dexmedetomidine and propofol.
Outcomes: Postoperative outcomes, including the mean length of ICU stay in hours, duration of mechanical ventilation in hours, length of hospital stay in days, and number of patients diagnosed with delirium.
Study design: Only randomized controlled trials (RCTs) were included in this meta-analysis to ensure the quality of pooled results. We excluded observational studies, reviews, editorials, and animal studies. Studies without a control group were also excluded.
Data Extraction
Two reviewers independently extracted the characteristics and relevant endpoint data from the included studies. The baseline information included details such as the first author, publication year, patient numbers in each group, average age, gender distribution, and type of cardiac surgery. The examined outcomes included the mean length of ICU stay in hours, duration of mechanical ventilation in hours, length of hospital stay in days, and number of patients diagnosed with delirium.
Quality Assessment
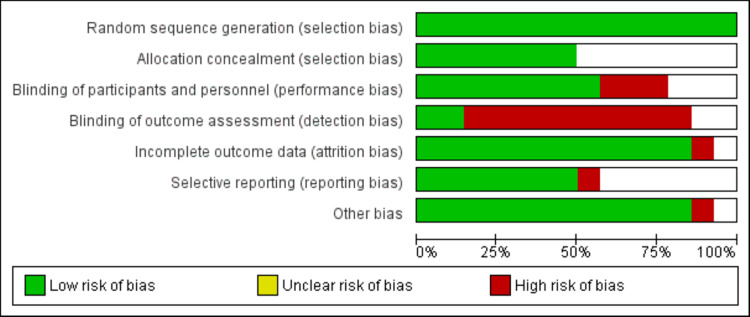
Two researchers independently evaluated the overall quality following the guidelines for quality assessment found in the Cochrane Handbook for Systematic Reviews of Interventions. In each included study, a number of quality issues were investigated, including selection bias, blinding bias, inadequate outcome data bias, selective reporting prejudice, and other possible biases. In the event of a dispute, a third investigator was brought in to settle the matter. The final quality was assigned one of three levels of bias risk based on the findings of the overall quality assessment: low, high, or unclear risk of bias.
Statistical Analysis
Mean and standard deviation were used to present the continuous data. A mean difference (MD) with a 95% confidence interval (CI) was used to compare continuous outcomes between the two groups. For dichotomous outcomes, a risk ratio (RR) with 95% CI was reported. Heterogeneity was assessed using the I-square statistics and Q test, with a p-value of less than 0.1 considered significant for heterogeneity. Based on the findings of the heterogeneity analysis, the random-effects model was employed when significant heterogeneity was reported. Conversely, if no significant heterogeneity was found, the fixed-effects model was utilized. The meta-analysis was performed using RevMan version 5.4.1 (The Cochrane Collaboration, London, United Kingdom).
Results
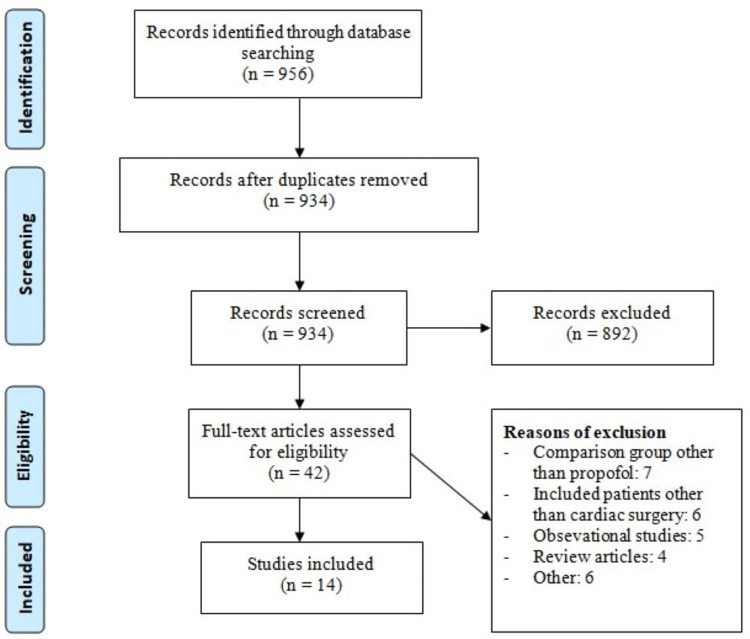
Figure 1 shows the PRISMA flowchart of study selection. A total of 956 records were retrieved through online database searching and the studies were screened according to the predefined inclusion and exclusion criteria. Eight hundred ninety-two of these studies were excluded after the initial screening process using abstract and title. The full text of 42 studies was obtained and a detailed evaluation was done. Out of these studies, 14 studies fulfilled the eligibility criteria and were included in the present meta-analysis. A total of 1360 patients undergoing cardiac surgery were involved in these studies. The baseline characteristics of the included studies are shown in Table 1. Figure 2 shows the risk of bias assessment of all included studies.
Table 1. Characteristics of included studies.
CABG: coronary artery bypass surgery; CPB: cardiopulmonary bypass; NR: not reported
| Author Name | Publication Year | Region | Surgery Type | Groups | Number of Participants | Mean Age (Years) | Males (%) |
| Abdallah et al [17] | 2021 | Egypt | CABG or valve replacement | Propofol | 49 | NR | NR |
| Dexmedetomidine | 49 | ||||||
| Corbett et al [18] | 2005 | United States | CABG | Propofol | 46 | 62.4/ 63.6 | 82.6/ 81.4 |
| Dexmedetomidine | 43 | ||||||
| Djaiani et al [19] | 2016 | United States | CABG or valve replacement | Propofol | 92 | 72.4/ 72.7 | 76/ 74.7 |
| Dexmedetomidine | 91 | ||||||
| Elgebaly et al [20] | 2018 | Egypt | Open heart surgery | Propofol | 25 | 52.5/ 53.7 | 30/ 50 |
| Dexmedetomidine | 25 | ||||||
| Eremenko et al [21] | 2014 | Russia | CABG or valve replacement | Propofol | 27 | NR | NR |
| Dexmedetomidine | 28 | ||||||
| Karaman et al [22] | 2015 | Turkey | CABG | Propofol | 31 | 63.9/ 62.5 | 87.9/ 83.8 |
| Dexmedetomidine | 33 | ||||||
| Liu et al [23] | 2016 | China | Elective cardiac surgery with CPB | Propofol | 44 | 56.5/ 53 | 31.8/ 47.7 |
| Dexmedetomidine | 44 | ||||||
| Maldonado et al [24] | 2009 | United States | Elective cardiac surgery with CPB | Propofol | 30 | 58/ 55 | 58/ 65 |
| Dexmedetomidine | 30 | ||||||
| Mogahd et al [25] | 2017 | United States | CABG | Propofol | 35 | 54.8/ 53.4 | 57/51.4 |
| Dexmedetomidine | 35 | ||||||
| Patil et al [26] | 2021 | India | CABG | Propofol | 30 | NR | NR |
| Dexmedetomidine | 30 | ||||||
| Sharaf et al [27] | 2022 | Egypt | Elective cardiac surgery | Propofol | 75 | 68.9/ 67.9 | 50.7/ 52 |
| Dexmedetomidine | 75 | ||||||
| Sheikh et al [28] | 2018 | India | Elective open heart surgery | Propofol | 16 | 35.6/ 33.6 | NR |
| Dexmedetomidine | 16 | ||||||
| Subramaniam et al [29] | 2019 | United States | CABG | Propofol | 61 | 69/ 71 | 83.3/ 86.7 |
| Dexmedetomidine | 59 | ||||||
| Susheela et al [30] | 2017 | United States | Elective cardiac surgery | Propofol | 6 | NR | NR |
| Dexmedetomidine | 6 |
Figure 1. PRISMA flowchart of study selection.
PRISMA: Preferred Reporting Items for Systematic Reviews and Meta-Analyses
Figure 2. Risk of bias graph.
Meta-analysis of outcomes
Duration of Mechanical Ventilation (In Hours)
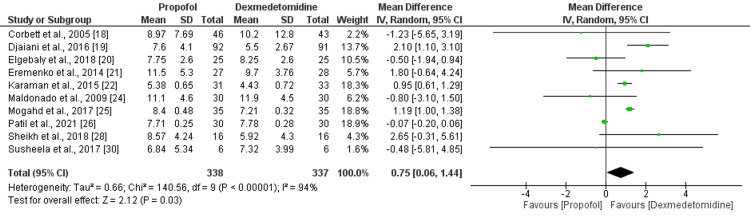
A meta-analysis of 10 studies on dexmedetomidine versus propofol found that the duration of mechanical ventilation was significantly lower in the dexmedetomidine group compared to the propofol group (MD: 0.75, 95% CI: 0.06-1.44, p-value: 0.03) as shown in Figure 3. As significant heterogeneity was there across these studies (I-square: 94%, p-value: 0.03), the random effect model was used. A sensitivity analysis was performed to identify the source of heterogeneity, and the result showed that the study of Patil et al. [26] might be responsible for it, as excluding this study reduced heterogeneity from 94% to 48% as shown in Appendix A.
Figure 3. Duration of mechanical ventilation (hours).
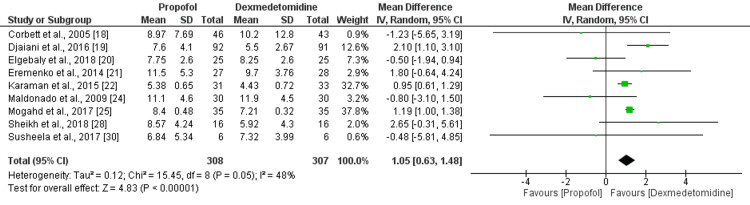
Length of Stay in ICU (In Hours)
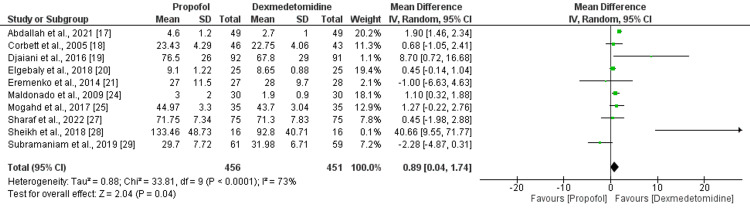
Ten studies provided data on the length of stay in the ICU. Statistical heterogeneity was there among the studies (I-square: 73%, p-value<0.001). Combined results from the 10 RCTs of dexmedetomidine versus propofol found a significantly low length of stay in ICU in the dexmedetomidine group compared to the propofol (MD: 0.89, 95% CI: 0.04-1.74, p-value: 0.04) as shown in Figure 4. The sensitivity analysis was performed to identify the source of heterogeneity, and the result demonstrated that the study conducted by Abdallah et al., 2021, could be responsible for it [17].
Figure 4. Length of ICU stay (hours).
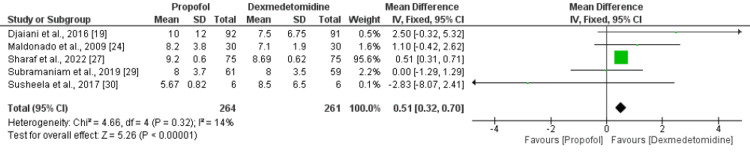
Length of Hospital Stay (In Days)
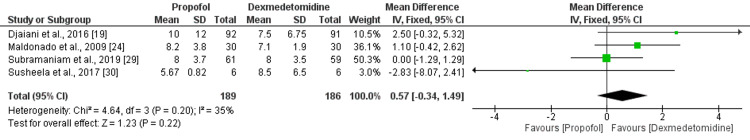
Five RCTs compared the data on the length of hospital stay in patients undergoing cardiac surgery. No significant heterogeneity was reported among the study results (I-square: 14%, p-value: 0.32) and the fixed effect model was used. The combined results of five RCTs suggested that the length of hospital stay was significantly lower in patients receiving dexmedetomidine compared to the propofol group (MD: 0.51, 95% CI: 0.32-0.70, p-value<0.001) as shown in Figure 5. As most of the weight in this outcome was carried by Sharaf et al., 2022, we performed a sensitivity analysis by removing this study. As shown in Appendix B, the total hospital stay was lower in the dexmedetomidine group but the difference was insignificant.
Figure 5. Length of hospital stay (in days).
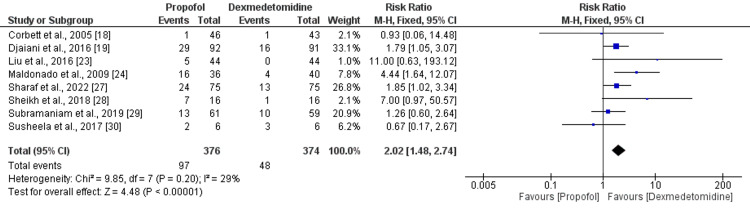
Risk of Delirium
Eight studies were included in the pooled analysis of the risk of delirium. As shown in Figure 6, the risk of delirium was significantly higher in patients receiving propofol compared to patients receiving dexmedetomidine (RR: 2.02, 95% CI: 1.48-2.74, p-value<0.001). No significant heterogeneity was reported among the study results (I-square: 29%, p-value: 0.20).
Figure 6. Risk of delirium.
Safety Events
We compared the risk of bradycardia and atrial fibrillation between two groups and the results are shown in Table 2. The risk of bradycardia was higher in the dexmedetomidine group compared to the placebo group but the difference was statistically insignificant. Similarly, the risk of atrial fibrillation was not significantly different between the two groups.
Table 2. Safety outcomes.
RR: risk ratio; CI: confidence interval
| Outcome | RR (95% CI) | I-square |
| Bradycardia | 0.32 (0.07-1.57) | 0% |
| Atrial fibrillation | 1.54 (0.59-4.02) | 70% |
| Hypotension | 0.59 (0.41-0.86) | 0% |
Discussion
Despite significant advancements in cardiac surgery that have reduced complications and mortality rates, there is still a need for effective drugs to benefit patients undergoing these procedures. Our meta-analysis indicates that dexmedetomidine may have beneficial effects on clinical outcomes in cardiac surgery patients, including reducing the duration of mechanical ventilation, length of stay in the intensive care unit (ICU) and hospital, and risk of delirium.
Our study found a significantly shorter duration of mechanical ventilation in the dexmedetomidine group compared to the propofol group. Similar findings were reported in a review by Heybati et al. [16]. Another study by Hu et al. demonstrated that patients who received dexmedetomidine were extubated three hours earlier compared to those given propofol [31]. These results align with the known sedative properties of dexmedetomidine, which promote consciousness, patient compliance, improved communication, and enhanced pain management. Dexmedetomidine's shorter duration of action and minimal impact on the urge to breathe contribute to these benefits, distinguishing it from propofol [32].
Our meta-analysis also revealed a shorter length of stay in the ICU and hospital for the dexmedetomidine group compared to the propofol group. Prolonged stays in the ICU and shorter hospital stays carry implications such as increased susceptibility to infections, unfavorable outcomes, and financial concerns [13]. Therefore, management plans should address these aspects as well.
In a recent meta-analysis of cardiac surgery patients, no significant reduction in the occurrence of delirium was observed [33]. However, when trials administering dexmedetomidine as adjuncts were excluded, a notable reduction in ICU delirium was seen among patients who received dexmedetomidine compared to propofol. Previous reviews have also concluded that dexmedetomidine sedation may lead to a lower incidence of ICU delirium compared to propofol [34-35]. Our high-certainty evidence from the meta-analysis demonstrated a significant reduction in the risk of ICU delirium in cardiac surgical patients who received dexmedetomidine. The majority of studies included in this meta-analysis used the confusion assessment method (CAM) to assess delirium. The precise mechanisms by which dexmedetomidine reduces the likelihood of delirium and the underlying pathophysiology of delirium are not fully understood. However, studies attribute this advantage to dexmedetomidine's sparing activity on gamma-aminobutyric acid receptors, minimal impact on respiration, ability to mimic normal sleep patterns, lack of anticholinergic activity, and potential to reduce the need for opioid medications [36-37].
The potential cardiovascular complications associated with dexmedetomidine sedation should be considered. The increased risk of bradycardia observed in patients undergoing cardiac surgery aligns with the findings reported by Abowali et al. [13]. This significant finding is consistent across patients undergoing medical procedures or other surgeries, and those with sepsis [16]. Our meta-analysis indicated a higher risk of bradycardia in patients receiving dexmedetomidine compared to the propofol group, although the difference was not statistically significant, potentially due to the limited number of studies assessing this outcome. While bradycardia has been reported with the use of dexmedetomidine, it can be effectively resolved with fluid boluses. Wu et al. also suggested a potential association between cardiovascular effects and high-dose dexmedetomidine infusion or the use of a loading dose in a previous review [38]. Therefore, close monitoring of patients in this specific subgroup, regardless of the sedation agent used, is advised, employing advanced techniques such as continuous heart rhythm and non-invasive blood pressure monitoring. The administration of boluses should also be approached with caution.
Our review included a larger number of trials with a more substantial patient sample, resulting in a comprehensive and updated evaluation of treatment effects compared to previous meta-analyses. Additionally, significant variability was observed in the dosage and duration of analgesic therapy administered during the post-surgical period, with inadequate documentation in some cases. Similar variability was noted in the use of other sedatives, such as benzodiazepines, during surgeries, indicating a lack of standardized protocols for general anesthesia. This variability may have influenced time-dependent outcomes, particularly delirium, which can be influenced by various contributing factors. Future trials should address these aspects and aim to follow standardized protocols. Furthermore, important outcomes like bradycardia and atrial fibrillation were not consistently assessed in the majority of studies, highlighting the need for future research to focus on the safety aspects of these drugs.
Conclusions
In conclusion, our meta-analysis provides evidence of the beneficial impacts of dexmedetomidine on clinical outcomes in patients undergoing cardiac surgery. Dexmedetomidine was associated with a significant reduction in the duration of mechanical ventilation, length of stay in the ICU and hospital, and the risk of delirium. While dexmedetomidine is associated with potential cardiovascular complications, such as bradycardia, close monitoring of patients undergoing cardiac surgery is essential regardless of the sedation agent used. Advanced monitoring techniques, such as continuous heart rhythm and non-invasive blood pressure monitoring, should be employed to ensure patient safety.
Acknowledgments
Lubna Sattar, Ibrahim Reyaz, and Anurag Rawat contributed to the conception and design of the study. Raam Mannam and Ibrahim Reyaz developed the search strategy and performed online database searching. Abhimanyu Karumanchi and Anurag Rawat performed study selection and data extraction. Venu Gopal Reddy Depa and Saima Batool performed the quality assessment. Lubna Sattar and Muhammad Usama performed the statistical analysis. Anurag Rawat, Ibrahim Reyaz, Abhimanyu Karumanchi, Venu Gopal Reddy Depa, and Saima Batool wrote the initial draft. Lubna Sattar reviewed the article and performed the final editing. All authors agreed to the final version of the manuscript.
Appendices
Appendix A
Figure 7. Forest plot comparison of mechanical ventilation after removing the study conducted by Patil et al.
Appendix B
Figure 8. Forest plot comparison of the length of hospital stay after removing the study conducted by Sharaf et al.
The authors have declared that no competing interests exist.
References
- 1.Cardiac surgery-associated acute kidney injury. Mao H, Katz N, Ariyanon W, et al. Cardiorenal Med. 2013;3:178–199. doi: 10.1159/000353134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.The effects of dexmedetomidine on inflammatory mediators after cardiopulmonary bypass. Ueki M, Kawasaki T, Habe K, Hamada K, Kawasaki C, Sata T. Anaesthesia. 2014;69:693–700. doi: 10.1111/anae.12636. [DOI] [PubMed] [Google Scholar]
- 3.A review of the peri-operative risk stratification assessment tools used for the prediction of cardiovascular complications in non-cardiac surgery. Magapu P, Haskard D, Fisher M. Perfusion. 2016;31:358–365. doi: 10.1177/0267659115615207. [DOI] [PubMed] [Google Scholar]
- 4.Cardiovascular complications of obesity surgery in patients with increased preoperative cardiac risk. Afolabi BA, Novaro GM, Szomstein S, Rosenthal RJ, Asher CR. Surg Obes Relat Dis. 2009;5:653–656. doi: 10.1016/j.soard.2009.06.009. [DOI] [PubMed] [Google Scholar]
- 5.Gastrointestinal complications after cardiac surgery: a nationwide population-based analysis of morbidity and mortality predictors. Chaudhry R, Zaki J, Wegner R, Pednekar G, Tse A, Sheinbaum R, Williams GW. J Cardiothorac Vasc Anesth. 2017;31:1268–1274. doi: 10.1053/j.jvca.2017.04.013. [DOI] [PubMed] [Google Scholar]
- 6.Detailed insight into the impact of postoperative neuropsychiatric complications on mortality in a cohort of cardiac surgery subjects: a 23,000-patient-year analysis. Krzych LJ, Wybraniec MT, Krupka-Matuszczyk I, Skrzypek M, Bolkowska A, Wilczyński M, Bochenek AA. J Cardiothorac Vasc Anesth. 2014;28:448–457. doi: 10.1053/j.jvca.2013.05.005. [DOI] [PubMed] [Google Scholar]
- 7.Impact of severe postoperative complications after cardiac surgery on mortality in patients aged over 80 years. Kamiya H, Tanzeem N, Akhyari P, Pedraza A, Kallenbach K, Lichtenberg A, Karck M. Ann Thorac Cardiovasc Surg. 2014;20:383–389. doi: 10.5761/atcs.oa.13-02268. [DOI] [PubMed] [Google Scholar]
- 8.Dexmedetomidine pharmacology in neonates and infants after open heart surgery. Su F, Gastonguay MR, Nicolson SC, DiLiberto M, Ocampo-Pelland A, Zuppa AF. Anesth Analg. 2016;122:1556–1566. doi: 10.1213/ANE.0000000000000869. [DOI] [PubMed] [Google Scholar]
- 9.Propofol in anesthesia. Mechanism of action, structure-activity relationships, and drug delivery. Trapani G, Altomare C, Liso G, Sanna E, Biggio G. Curr Med Chem. 2000;7:249–271. doi: 10.2174/0929867003375335. [DOI] [PubMed] [Google Scholar]
- 10.Propofol: a review of its use in intensive care sedation of adults. McKeage K, Perry CM. CNS Drugs. 2003;17:235–272. doi: 10.2165/00023210-200317040-00003. [DOI] [PubMed] [Google Scholar]
- 11.Assessing the depth of dexmedetomidine-induced sedation with electroencephalogram (EEG)-based spectral entropy. Maksimow A, Snapir A, Särkelä M, et al. Acta Anaesthesiol Scand. 2007;51:22–30. doi: 10.1111/j.1399-6576.2006.01174.x. [DOI] [PubMed] [Google Scholar]
- 12.Pharmacokinetics of dexmedetomidine infusions for sedation of postoperative patients requiring intensive care. Venn RM, Karol MD, Grounds RM. Br J Anaesth. 2002;88:669–675. doi: 10.1093/bja/88.5.669. [DOI] [PubMed] [Google Scholar]
- 13.Critical review and meta-analysis of postoperative sedation after adult cardiac surgery: dexmedetomidine versus propofol. Abowali HA, Paganini M, Enten G, Elbadawi A, Camporesi EM. J Cardiothorac Vasc Anesth. 2021;35:1134–1142. doi: 10.1053/j.jvca.2020.10.022. [DOI] [PubMed] [Google Scholar]
- 14.Current evidence demonstrates a significant reduction in the incidence of delirium with postoperative dexmedetomidine versus propofol sedation. Heybati K, Deng J, Zhou F, Ali S. J Cardiothorac Vasc Anesth. 2022;36:347–348. doi: 10.1053/j.jvca.2021.06.026. [DOI] [PubMed] [Google Scholar]
- 15.Clinical practice guidelines for the prevention and management of pain, agitation/sedation, delirium, immobility, and sleep disruption in adult patients in the ICU. Devlin JW, Skrobik Y, Gélinas C, et al. Crit Care Med. 2018;46:0–73. doi: 10.1097/CCM.0000000000003299. [DOI] [PubMed] [Google Scholar]
- 16.Outcomes of dexmedetomidine versus propofol sedation in critically ill adults requiring mechanical ventilation: a systematic review and meta-analysis of randomised controlled trials. Heybati K, Zhou F, Ali S, Deng J, Mohananey D, Villablanca P, Ramakrishna H. Br J Anaesth. 2022;129:515–526. doi: 10.1016/j.bja.2022.06.020. [DOI] [PubMed] [Google Scholar]
- 17.Dexmedetomidine versus propofol in reducing atrial fibrillation after cardiac surgery. Abdallah O, Salem MI, Gomaa M. Egypt J Anaesth. 2022;31:72–77. [Google Scholar]
- 18.Dexmedetomidine does not improve patient satisfaction when compared with propofol during mechanical ventilation. Corbett SM, Rebuck JA, Greene CM, Callas PW, Neale BW, Healey MA, Leavitt BJ. Crit Care Med. 2005;33:940–945. doi: 10.1097/01.ccm.0000162565.18193.e5. [DOI] [PubMed] [Google Scholar]
- 19.Dexmedetomidine versus propofol sedation reduces delirium after cardiac surgery: a randomized controlled trial. Djaiani G, Silverton N, Fedorko L, Carroll J, Styra R, Rao V, Katznelson R. Anesthesiology. 2016;124:362–368. doi: 10.1097/ALN.0000000000000951. [DOI] [PubMed] [Google Scholar]
- 20.Sedation effects by dexmedetomidine versus propofol in decreasing duration of mechanical ventilation after open heart surgery. Elgebaly AS, Sabry M. Ann Card Anaesth. 2018;21:235–242. doi: 10.4103/aca.ACA_168_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Comparison of dexmedetomidine and propofol for short-term sedation in early postoperative period after cardiac surgery (Article in Russian) Eremenko AA, Chemova EV. https://pubmed.ncbi.nlm.nih.gov/25055491/ Anesteziol Reanimatol. 2014:37–41. [PubMed] [Google Scholar]
- 22.Effects of dexmedetomidine and propofol on sedation in patients after coronary artery bypass graft surgery in a fast-track recovery room setting. Karaman Y, Abud B, Tekgul ZT, Cakmak M, Yildiz M, Gonullu M. J Anesth. 2015;29:522–528. doi: 10.1007/s00540-015-1975-2. [DOI] [PubMed] [Google Scholar]
- 23.Dexmedetomidine sedation reduces atrial fibrillation after cardiac surgery compared to propofol: a randomized controlled trial. Liu X, Zhang K, Wang W, Xie G, Fang X. Crit Care. 2016;20:298. doi: 10.1186/s13054-016-1480-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dexmedetomidine and the reduction of postoperative delirium after cardiac surgery. Maldonado JR, Wysong A, van der Starre PJ, Block T, Miller C, Reitz BA. Psychosomatics. 2009;50:206–217. doi: 10.1176/appi.psy.50.3.206. [DOI] [PubMed] [Google Scholar]
- 25.Safety and efficacy of ketamine-dexmedetomidine versus ketamine-propofol combinations for sedation in patients after coronary artery bypass graft surgery. Mogahd MM, Mahran MS, Elbaradi GF. Ann Card Anaesth. 2017;20:182–187. doi: 10.4103/aca.ACA_254_16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Comparing dexmedetomidine and propofol for sedation and hemodynamic stability in cardio-thoracic intensive care unit for patients following off-pump coronary artery bypass graft surgery. Patil VP, Abraham J, George GM. Int J Basic Clin Pharmacol. 2021;10:153–159. [Google Scholar]
- 27.Comparative study between propofol and dexmedetomidine sedation in reducing delirium after cardiac surgery in elderly patients. Sharaf SI, Hefny SS, Anis SG, Elfar MM, Rashed AA. Ain Shams Med J. 2020;1:465–474. [Google Scholar]
- 28.A comparative study evaluating effects of intravenous sedation by dexmedetomidine and propofol on patient hemodynamics and postoperative outcomes in cardiac surgery. Sheikh TA, Dar BA, Akhter N, Ahmad N. Anesth Essays Res. 2018;12:555–560. doi: 10.4103/aer.AER_46_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Effect of intravenous acetaminophen vs placebo combined with propofol or dexmedetomidine on postoperative delirium among older patients following cardiac surgery. The DEXACET randomized clinical trial. Subramaniam B, Shankar P, Shaefi S, et al. JAMA. 2019;321:686–696. doi: 10.1001/jama.2019.0234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.The use of dexmedetomidine and intravenous acetaminophen for the prevention of postoperative delirium in cardiac surgery patients over 60 years of age: a pilot study. Susheela AT, Packiasabapathy S, Gasangwa DV, Patxot M, O'Neal J, Marcantonio E, Subramaniam B. F1000Res. 2017;6:1842. doi: 10.12688/f1000research.12552.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Comparison between dexmedetomidine and propofol on outcomes after coronary artery bypass graft surgery: a retrospective study. Hu J, Lv B, West R, Chen X, Yan Y, Pac Soo C, Ma D. BMC Anesthesiol. 2022;22:51. doi: 10.1186/s12871-022-01589-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Alpha-2 adrenergic receptor agonists: a review of current clinical applications. Giovannitti JA Jr, Thoms SM, Crawford JJ. Anesth Prog. 2015;62:31–39. doi: 10.2344/0003-3006-62.1.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Clinical benefits of dexmedetomidine versus propofol in adult intensive care unit patients: a meta-analysis of randomized clinical trials. Xia ZQ, Chen SQ, Yao X, Xie CB, Wen SH, Liu KX. J Surg Res. 2013;185:833–843. doi: 10.1016/j.jss.2013.06.062. [DOI] [PubMed] [Google Scholar]
- 34.Dexmedetomidine versus propofol sedation in reducing delirium among older adults in the ICU: a systematic review and meta-analysis. Pereira JV, Sanjanwala RM, Mohammed MK, Le ML, Arora RC. Eur J Anaesthesiol. 2020;37:121–131. doi: 10.1097/EJA.0000000000001131. [DOI] [PubMed] [Google Scholar]
- 35.Prevalence of delirium with dexmedetomidine compared with morphine based therapy after cardiac surgery: a randomized controlled trial (DEXmedetomidine COmpared to Morphine-DEXCOM Study) Shehabi Y, Grant P, Wolfenden H, Hammond N, Bass F, Campbell M, Chen J. Anesthesiology. 2009;111:1075–1084. doi: 10.1097/ALN.0b013e3181b6a783. [DOI] [PubMed] [Google Scholar]
- 36.Sedative, amnestic, and analgesic properties of small-dose dexmedetomidine infusions. Hall JE, Uhrich TD, Barney JA, Arain SR, Ebert TJ. Anesth Analg. 2000;90:699–705. doi: 10.1097/00000539-200003000-00035. [DOI] [PubMed] [Google Scholar]
- 37.The Society of Thoracic Surgeons Adult Cardiac Surgery Database: 2019 update on outcomes and quality. D'Agostino RS, Jacobs JP, Badhwar V, Fernandez FG, Paone G, Wormuth DW, Shahian DM. Ann Thorac Surg. 2019;107:24–32. doi: 10.1016/j.athoracsur.2018.10.004. [DOI] [PubMed] [Google Scholar]
- 38.Perioperative dexmedetomidine reduces delirium after cardiac surgery: A meta-analysis of randomized controlled trials. Wu M, Liang Y, Dai Z, Wang S. J Clin Anesth. 2018;50:33–42. doi: 10.1016/j.jclinane.2018.06.045. [DOI] [PubMed] [Google Scholar]