Abstract
Malignancy in heart transplant recipients is a grave complication. Post-transplant lymphoproliferative disorder (PTLD) is the second most common tumour in adults and commonest in children. The incidence varies with the transplanted organ from 1 to 2% following kidney transplantation to as high as 10% following thoracic organ transplantation due to different immunosuppression intensity. PTLD include a wide spectrum of diseases ranging from benign proliferation of lymphoid tissue to frank malignancy with aggressive behaviour (lymphoma). Epstein-Barr virus (EBV) infection and prolonged immunosuppressant therapy are implicated in the pathogenesis of PTLD. The incidence of PTLD varies from 2.6% at 1 year to 28% at 10 years post-transplant. Seronegativity for EBV in recipients with seropositive donors increases the risk of PTLD in recipients. The majority of early-onset PTLDs (85%) are of B-cell origin and associated with EBV. Timely and accurate diagnosis with histological examination of lymphoid tissue is essential for early intervention. Reduction of immunosuppressive therapy (IST) and rituximab usually are effective in remission of PTLD. In resistant cases, chemotherapy is given with or without rituximab. Adoptive T-cell transfer represents a promising therapeutic approach. Early PTLD respond well to lowering immunosuppression and has a favourable prognosis compared to late PTLD. Five-year survival is 30% for high-grade lymphomas. The prognosis of EBV-negative lymphomas is worse. One out of 40 heart transplant recipients followed up in our centre developed PTLD. He was treated to remission and we describe this case here.
Keywords: Post-transplant lymphoproliferative disorder (PTLD), Heart transplant, Immunosuppressive therapy, Epstein-Barr Virus
Introduction
Post-transplant lymphoproliferative disorder (PTLD) is a life-threatening complication in heart transplant recipients (HTRs), a consequence of long-term immunosuppressant therapy (IST). Immunosuppressant drugs impair immune response against malignant cells and oncogenic viruses, thereby predisposing the transplant recipients to cancer [1, 2]. The chance of developing cancer in post-transplant patients is 3 to 4 times more than the age-matched controls in the general population [3]. The incidence of PTLD increases with time, affecting 28% of HTRs 10 years post-transplant, but high-grade lymphomas are seen in 1.8% [4]. PTLD is a serious complication leading to high mortality in HTRs [5, 6]. Two main aetiologies of PTLD are Epstein-Barr virus (EBV) infection and prolonged immunosuppression [1, 2, 7, 8].
The symptoms are nonspecific like fever, weight loss, anorexia, and night sweats which may be confused with allograft rejection or infections. A high index of suspicion leads to early diagnosis and treatment of PTLD. Confirmation of diagnosis is done from lymphoid tissue biopsy, and quantitative estimation of EBV deoxyribonucleic acid (DNA) from blood reversed transcriptase polymerised chain reaction (RT-PCR) [1, 8–10]. Reduction and modification of IST is the cornerstone of treatment [1, 2, 7–9]. Cluster of differentiate (CD)20 monoclonal antibody (rituximab) therapy combined with reduction of IST achieves remission of the disease [1, 7, 8]. In resistant high-grade lymphomas, chemotherapy is required in addition to the above treatment modalities [1, 8]. The most important challenge is to balance between the reduced immunosuppressive drug levels and avoiding allograft rejection which can cause sudden death. One of our HTRs, now 5.5 years post heart transplant, developed PTLD and we could treat him successfully to remission and this case is described here.
Case report
A 22-year-old male diagnosed with dilated cardiomyopathy was under treatment for advanced heart failure in our coronary care unit. He suffered refractory cardiac arrest, and was resuscitated with veno-arterial extracorporeal membrane oxygenation (ECMO). He was bridged with ECMO to heart transplantation (HTx) in February 2017 and recovered. He was on maintenance IST with tacrolimus, mycophenolate mofetil (MMF), and prednisolone. Our immunosuppressive regime is triple therapy (tacrolimus, MMF, and prednisolone) as maintenance for all our HTRs in accordance with the International Society for Heart and Lung Transplantation (ISHLT) guidelines and we do not use induction agents for our patients. He has been on regular follow-up since the last 5.5 years. He has not encountered allograft rejection on surveillance endomyocardial biopsy (EMB) and he has good biventricular function on echocardiogram to date. Our EMB protocol is 1st biopsy at 2 weeks after heart transplant (before hospital discharge) and subsequent biopsies are done at 3 months, 6 months, 1 year, and then yearly. Any further EMB is done if the patient presents with clinical features of heart failure and/or ventricular dysfunction on echocardiography.
The patient was infected with severe acute respiratory syndrome coronavirus (SARS CoV-2) and developed moderate COVID-19 illness in November 2020. He was treated with oxygen supplementation, repurposed medications (azithromycin, ivermectin, zinc, and vitamin C) for corona virus disease (COVID) and dose reduction of IST. He recovered uneventfully and his IST was resumed to previous levels.
Five months post COVID recovery, patient presented with recurrent episodes of diarrhoea, treated with anti-protozoal medication. As the diarrhoea did not resolve completely, anti-cytomegalovirus (CMV) medication (valganciclovir) was started empirically and the diarrhoea subsided. In the next 6 months, he gradually developed loss of appetite, and weight loss of 10 kg. On examination, he had anaemia and axillary lymphadenopathy and no other abnormal clinical findings.
The routine haematological and biochemical tests were normal except for low hemoglobin at 9 g/dl. Fluoro deoxyglucose (FDG) positive emission tomographic (PET) scan showed metabolically active lymphadenopathy in axillary, paratracheal, mediastinal, retroperitoneal, and inguino-femoral regions (Fig. 1). Bone marrow biopsy did not show any abnormality. Tuberculosis was also ruled out. Plasma RT-PCR was positive for anti-EBV Viral Capsid Antigen (VCA) IgM. RT-PCR for EBV DNA detected with viral load-181 copies/ml.
Fig. 1.

PET images: metabolically active lymph node (red arrows) in both sides of diaphragm (Before therapy)
Axillary lymph node biopsy showed partial effacement of architecture with expansion of para-cortical zone which showed mature plasma cells admixed with immunoblasts and a few large cells with prominent nucleoli. The large cells were positive for CD20, CD79a, and CD30, and few of them showed immunopositivity for EBV-latent membrane protein 1 (LMP1). Plasma cells were immune-positive for CD 138 and showed admixture of kappa and lambda cells by immunohistochemistry. These features confirmed the diagnosis of destructive type, a polymorphous variant of PTLD (World Health Organisation 2017 classification).
Treatment was started with modification of IST, which included omission of MMF and reduction of tacrolimus dose targeting a plasma level of 3–4 ng/dl. He was started with everolimus 0.5 mg daily (aiming a plasma levels of 3–4 ng/dl) and prednisolone 5 mg daily was continued. Everolimus was started to ward off the allograft rejection in lieu of lowering tacrolimus and omitting MMF. In addition to immunosuppressive action, everolimus is reported to have anti-cancer effects [3].
Treatment for PTLD was started in consultation with the medical oncologist. Rituximab (anti-CD20 monoclonal antibody) infusion at 375 mg/m2 was given once a week for 4 weeks (4 doses, 1st cycle). The patient became febrile and developed herpes labialis after the first dose of rituximab and was treated with intravenous acyclovir. PET scan was repeated 1 month after the 1st cycle of rituximab therapy. Compared to the previous scan, there was decrease in size and metabolic activity of axillary, mediastinal, abdominal, and pelvic lymph nodes which suggested a partial response, indicating incomplete remission.
The patient was maintained on low IST with tacrolimus, everolimus, and prednisolone. He was closely monitored clinically and echocardiographically for allograft rejection. The second cycle of rituximab at the same weekly dose was given to the patient over 4 weeks. Routine haematological and biochemistry including the blood levels of tacrolimus and everolimus were carried out at regular intervals. PET scan was repeated 4 weeks following the 2nd cycle. Some more residual active lymph nodes in retroperitoneal region were detected again.
The remaining residual lesion was treated with the 3rd cycle of rituximab. Repeat PET scan after 4 weeks showed complete resolution of lymphadenopathy in all locations and no metabolic activity seen (Fig. 2). The patient showed significant symptomatic improvement, regained his appetite and weight, and resumed his normal activities at 6 months follow-up after remission.
Fig. 2.
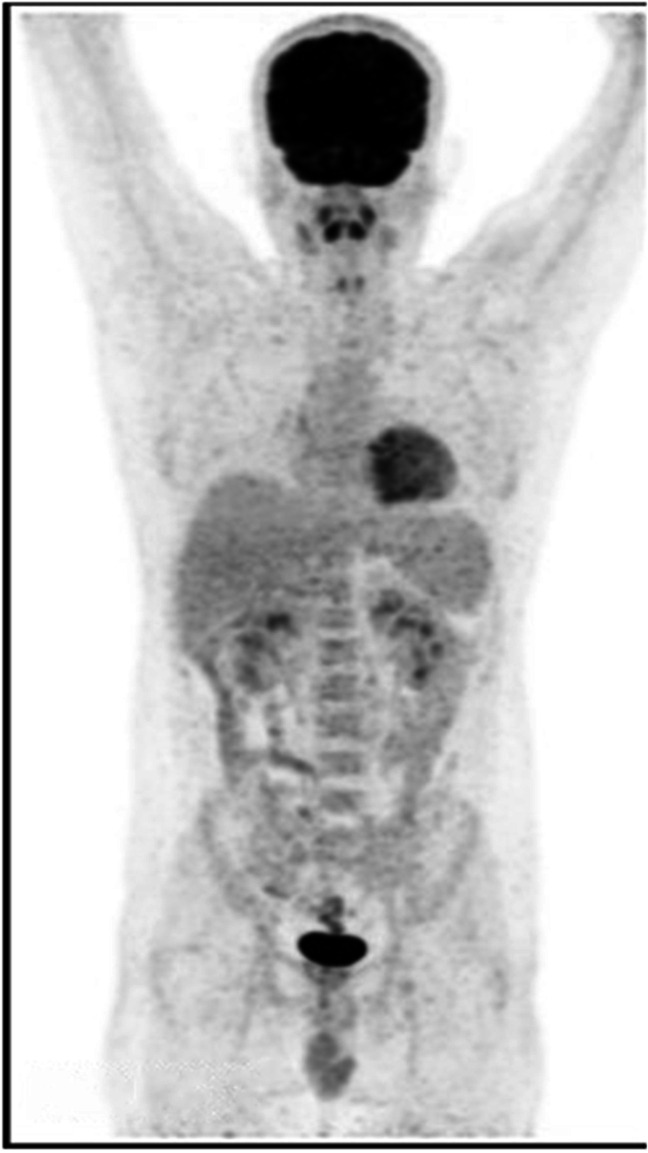
Resolution indicated by no uptake on follow-up PET scan (After completion of therapy)
Discussion
Excellent short- and long-term survival have been achieved after heart transplantation for end-stage heart failure patients. With passing time, HTRs face many life-threatening complications including malignancy. PTLD is a devastating complication with high mortality among all malignancies [4–6]. Five years post-transplant, about 24% of deaths are directly caused by malignancy [4] and in those with lymphomas, survival is only 30% [7].
Cancer risk in HTRs depends on recipient age, duration, and intensity of IST [4, 5]. Risk factors for PTLD include EBV infection, high-intensity IST, and induction therapy using OKT3 (murine monoclonal anti-T cell antibody) [5, 7]. Lund et al. described some strong risk factors of non-skin malignancies following heart transplant, which included antibody induction agents (hazard ratio (HR) 2.38, 95% confidence interval (CI) 1.40–4.07), donor history of cancer (HR 1.95, 95% CI 1.09–3.48), female sex (HR 1.87, 95% CI 1.26–2.78), etc. [4]. Antilymphocyte agents like anti-thymocyte globulin (ATG), anti-CD3 monoclonal antibodies, and azathioprine cause profound T-cell depletion and increase the risk of PTLD by 25% as compared to normal individuals [1, 7, 9]. None of these factors was associated with our patient; he was a young male and we did not use any induction agent.
PTLDs developing early after transplantation are mostly caused by EBV infection, and typically affect B-cells. Late-onset PTLD are likely to be EBV negative and of non-B cell origin [1, 8, 10]. In contrast, our patient developed PTLD 4.5 years after HTx and he was found to be EBV positive from the lymphoid tissue biopsy and blood. Though he suffered from COVID infection 6 months before, we do not know the associations between SARS-CoV-2 and malignancy in HTRs. Also our patient had multiple episodes of diarrhoea, which responded to anti-protozoal and anti-cytomegalovirus (CMV) medications. CMV infection has been implicated in the development of PTLD [9, 10].
Blood PCR for detecting Epstein-Barr nuclear antigen (EBNA) IgG and IgM antibodies are specific for diagnosing EBV infection [7, 9, 10]. Similarly finding EBNA in lymph node tissue biopsy histology is highly sensitive and specific [1, 8–10]. Viral load determination by quantitative PCR in serum, plasma, or whole blood is recommended [9, 10]. PET scan is recommended for diagnostic stratification, and evaluation of response to treatment [9]. In our patient, PTLD was diagnosed from lymphadenopathy in whole-body PET scan, lymph node biopsy, and positive EBV DNA from blood serology analysis.
Treatment of PTLDs is to reduce the lymphoid tissue proliferation to halt the progression of bulk lymphoma disease while preserving allograft function. Reducing the immunosuppressant dose and anti-CD20 monoclonal antibody is the mainstay treatment for PTLDs [1, 7, 8]. This modified IST (dose reduction of tacrolimus, omission of MMF) has proven to be efficacious in most B-cell type PTLDs either alone or in conjunction with rituximab therapy [1, 8–10]. Chemotherapy with CHOP (cyclophosphamide, doxorubicin, vincristine, prednisone) regime is the last option if the PTLD does not respond to modified IST + rituximab [1, 7–10]. CHOP regimen is used to treat high-grade non-Hodgkin’s lymphoma along with rituximab and reduction of IST [1, 7, 9, 10]. We reduced the tacrolimus dose, replaced MMF with everolimus, and continued prednisolone at 5 mg per day for our patient. We targeted the trough blood levels of both tacrolimus and everolimus to 3–4 ng/ml. Achieving state of disease remission without causing graft rejection can be quite challenging in PTLD. But with early treatment, remission of PTLD occurred in our patient and also his allograft was working normally, not affected by reduction of IST.
Conclusion
PTLD is a fatal complication arising in transplant recipients as a side effect of immunosuppression. Remission occurred with modification and reduction of IST and treatment with rituximab in our patient. His cardiac function is normal by echocardiography and has not shown any rejection from EMB to date. PET scan showed complete resolution of the lymphadenopathy and there was no radio-isotope uptake by the lymphoid tissue. At 6 months follow-up after remission, our patient has regained his appetite and weight and doing good clinically.
Funding
None.
Declarations
Ethics approval
Not applicable being a case report.
Informed consent statement
Informed consent was obtained from the patient regarding publication of this case.
Conflict of interest
There are no conflicts of interest.
Statement of human and animal rights
All procedures performed in this study involving human participant were in accordance with the ethical standards of the institutional research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. This article does not contain any studies with animals performed by any of the authors.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Liu L, Liu Q, Feng S. Management of Epstein- Barr virus- related post-transplant lympho-proliferative disorder after allogeneic hematopoietic stem cell transplantation. Ther Adv Hematol. 2020 doi: 10.1177/2040620720910964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dierickx D, Habermann TM. Post-transplantation lymphoproliferative disorders in adults. N Engl J Med. 2018;378:549–562. doi: 10.1056/NEJMra1702693. [DOI] [PubMed] [Google Scholar]
- 3.Ross K. For organ transplant recipients, cancer threatens long-term survival. J Natl Cancer Inst. 2007;99:421–422. doi: 10.1093/jnci/djk141. [DOI] [PubMed] [Google Scholar]
- 4.Lund LH, Edwards LB, Kucheryavaya AY, Dipchand AI, Benden C, Christie JD, et al. The registry of the international society for heart and lung transplantation: thirtieth official adult heart transplant report 2013, focus theme: age. J Heart Lung Transplant. 2013;32:951–964. doi: 10.1016/j.healun.2013.08.006. [DOI] [PubMed] [Google Scholar]
- 5.Crespo-Leiro MG, Alonso-Pulpón L, Vázquez de Prada JA, Almenar L, Arizón JM, Brossa V, et al. Malignancy after heart transplantation: incidence, prognosis and risk factors. Am J Transplant. 2008; 8:1031–9. [DOI] [PubMed]
- 6.Hayers D, Jr, Tumin D, Foraker RE, Tobias JD. Posttransplant lymphoproliferative disease and survival in adult heart transplant recipients. J Cardiol. 2017;69:144–148. doi: 10.1016/j.jjcc.2016.02.010. [DOI] [PubMed] [Google Scholar]
- 7.Végso G, Hajdu M, Sebestyén A. Lymphoproliferative disorders after solid organ transplantation-classification, incidence, risk factors, early detection and treatment options. Pathol Oncol Res. 2011; 17: 443–54. [DOI] [PubMed]
- 8.Abbas F, El Kossi M, Shaheen IS, Sharma A, Halawa A. Post-transplantation lymphoproliferative disorders: current concepts and future therapeutic approaches. World J Transplant. 2020;10:29–46. doi: 10.5500/wjt.v10.i2.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gupta D, Mendonca S, Chakraborty S, Chatterjee T. Post transplant lymphoproliferative disorder. Indian J Hematol Blood Transfus. 2020;36:229–237. doi: 10.1007/s12288-019-01182-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Allen U, Preiksaitis J. Epstein-Barr virus and posttransplant lymphoproliferative disorder in solid organ transplant recipients. Am J Transplant. 2009;9:S87–S96. doi: 10.1111/j.1600-6143.2009.02898.x. [DOI] [PubMed] [Google Scholar]


