Abstract
Introduction
Clinical trials in neovascular age-related macular degeneration (nAMD) using anti-vascular endothelial growth factor (ant-VEGF) injections use disease activity (DA) criteria to shorten, maintain or increase the interval between injections. Differences in these DA criteria may contribute to differences in the proportions of patients with macular fluid at key time points or achieving extended dosing intervals in these trials. We identified, collated and evaluated DA criteria from pivotal anti-VEGF nAMD trials to understand how differences impact on these studies and real-world visual acuity and extending dosing outcomes.
Methods
This was a systematic review of literature on Pubmed for randomised clinical trials in nAMD using a proactive treatment regimen. We excluded case reports, review articles and studies on fewer than 50 participants.
Results
Twelve clinical trials (LUCAS, VIEW, TREX-AMD, FLUID, TREND, RIVAL, ALTAIR, CANTREAT, ARIES, TREX-Conbercept, HAWK & HARRIER, TENAYA & LUCERNE) investigating anti-VEGF treatment of nAMD were identified according to our search strategy. Different studies utilised a different combination of DA criteria. Specifically, six trials included visual acuity change; four included macular thickness change; one included visual acuity change if associated with macular thickness change; one with qualitative optical coherence tomography (OCT) features; four with qualitative OCT features if also associated with visual acuity change; 10 with macular haemorrhage and five with other fluorescein angiographic features.
Conclusion
Different clinical trials use different DA criteria when altering the interval between anti-VEGF injections. This makes it difficult to draw meaningful conclusions about secondary outcomes such as proportion of patients treated at extended dosing intervals or proportions of eyes with persistent subretinal or intraretinal fluid. Standardising DA criteria in clinical trials and preferentially using those easily applied in a real-world setting would lead to results more achievable in real-world settings and for a meaningful comparison of treatment durability.
Keywords: Age-related macular degeneration, Treatment outcomes, Clinical trial
Key Summary Points
| We identified 12 randomised, controlled clinical trials investigating treatment of neovascular age-related macular degeneration with anti-vascular endothelial growth factor treatments using a proactive treatment regimen. |
| Disease activity criteria were visual acuity change in 6 trials; 4 included macular thickness change; 1 trial included visual acuity change if associated with macular thickness change; 1 with qualitative optical coherence tomography (OCT) features; 4 with qualitative OCT features if also associated with visual acuity change; 10 with macular haemorrhage and 5 with other fluorescein angiographic features. Meaningful comparison of secondary outcomes relating to treatment durability across clinical trials, such as the proportion of eyes with macular fluid or extended treatment intervals at time points, can therefore not be made. |
| New approaches to clinical trial disease activity criteria are needed to help generalisability of findings to real-world settings and allow better comparison of treatment durability outcomes across clinical trials and real-world settings. |
Introduction
We have witnessed huge strides in the fight against vision loss caused by neovascular age-related macular degeneration (nAMD) through the introduction of agents which target and block all isoforms of vascular endothelial growth factor (VEGF). These anti-VEGF agents delivered by intravitreal injection have been licensed for use after positive safety and efficacy data in phase 3 clinical trials. Through reports of treatment outcomes in clinical practice, over the past 10–15 years we have learned that real-world visual acuity outcomes do not reach the heights seen in clinical trials and that a range of factors can underlie this “efficacy gap”. Although some of these factors such as age, a wider range of lesion and baseline characteristics and less compliance with regular follow-up are outside the control of ophthalmologists, one of the biggest factors we can control is the treatment paradigms used in real-world clinical settings. Proactive treat and extend paradigms have become popular in an effort to reduce or eliminate periods of nAMD disease activity between injections which could compromise visual acuity outcomes. Indeed, recent analysis of treat and extend regimens in nAMD has suggested similar visual acuity improvement to fixed dosing regimens and better visual acuity outcomes than pro re nata (PRN) regimens [1]. The specific disease activity criteria used to shorten, maintain or extend the interval between anti-VEGF injections in treat and extend dosing regimens merit scrutiny. Optimising these criteria and standardising them within large hospital or clinic providers of nAMD treatment (e.g. in the UK’s National Health Service) so that different clinicians involved in the care of these patients use same criteria may help us to get closer to the visual acuity outcomes we see in clinical trials. This in turn has benefits for patients and payors by helping to maximize the efficacy and cost-effectiveness of anti-VEGF agents in real-world settings.
In this current study we systematically review the literature describing disease activity criteria used to alter treatment intervals with anti-VEGF agents in clinical trials which used a treat and extend paradigm or modified treat and extend approach to treatment. The methodology and findings of eligible studies are summarised and appraised with a focus on identifying agreement between studies on commonly used disease activity criteria and commenting on potential barriers to clinical implementation of these criteria in modern, forward-looking treat and extend retreatment strategies to optimise treatment outcomes for patients with nAMD.
Methods
Study Design
This systematic review was completed in accordance with the Preferred Reporting Items for Systematic review and Meta-Analysis (PRISMA) guidelines. This article is based on previously conducted studies and does not contain any new studies with human participants or animals performed by any of the authors.
Systematic Search
Randomised controlled trials reporting treatment of nAMD using anti-VEGF treatment (including other isoforms and other mechanisms of nAMD neoangiogenesis) were identified from PubMed literature search using the terms “wet age-related macular degeneration”, “neovascular age-related macular degeneration”, “anti-VEGF”, “anti-vascular endothelial growth factor” and/or “anti-angiogenic”. Literature searches also included “macular degeneration” and/or “intravitreal injection”. We excluded review articles, case studies, diseases other than nAMD, preclinical/imaging studies and non-GA studies.
Study Selection
The titles and abstracts were fully screened for relevant articles. We included randomised controlled trials (phase III or phase IIIb) where at least one arm of the clinical trial included treatment delivered using a personalised treatment interval, or capped PRN regimens (with minimum and maximum treatment intervals) or treat and extend retreatment strategy.
We excluded studies which only used fixed dosing throughout the study duration in all arms of the study (and which by definition would not have retreatment criteria or disease activity criteria) or studies where PRN treatment regimens were used without a cap for a minimum of maximum treatment interval as these groups of studies are less relevant regarding proactive treatment regimens in use in clinical practice today.
Study Assessment
The studies were assessed for study number, intravitreal agent assessed, history of previous treatment/treatment naïve, treatment criteria used to inform interval decision (visual acuity, OCT, fluorescein angiography and fundus examination), interval extension/reduction choice and outcome of study.
Results
Studies Selected
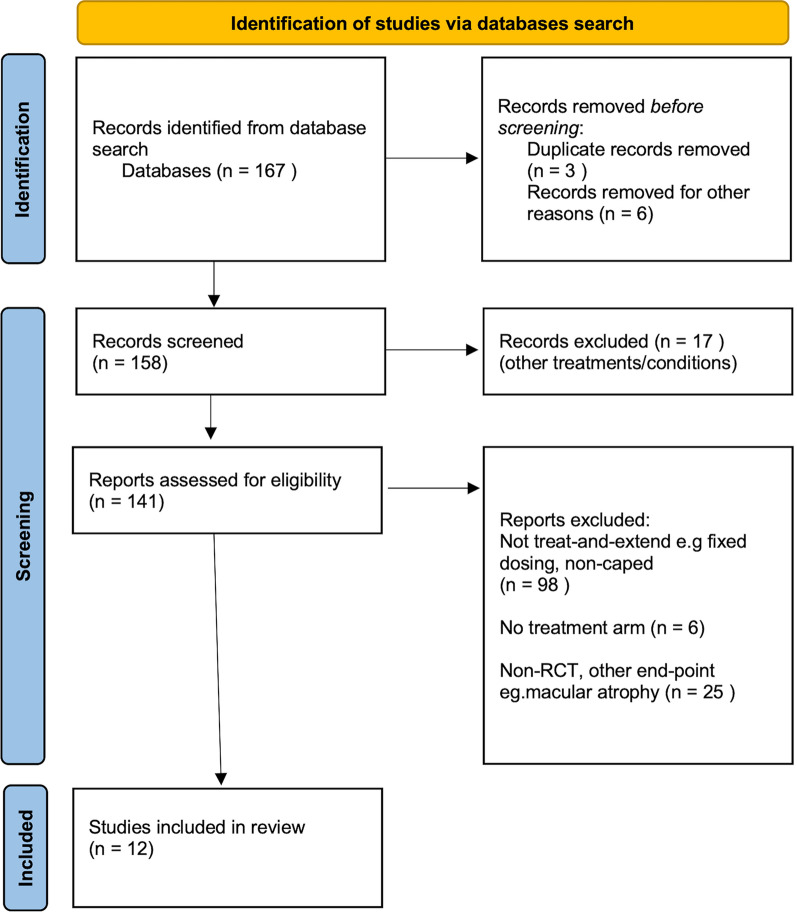
The search strategy resulted in 167 studies after removal of duplicate studies (see PRISMA Fig. 1). We then identified 12 clinical trials (LUCAS, VIEW, TREX-AMD, FLUID, TREND, RIVAL, ALTAIR, CANTREAT, ARIES, TREX-Conbercept, HAWK & HARRIER, TENAYA & LUCERNE) investigating anti-VEGF treatment of nAMD according to our search strategy [2–13]. These were multicentre phase 3/4 clinical trials in multiple locations worldwide. Treatment discontinuation was possible in the trials e.g. after adverse event.
Fig. 1.
PRISMA flowchart for study identification and selection, including exclusion numbers and reasons for exclusion
Treatment Regimens Utilised
We identified three studies using a proactive capped PRN or treat and extend regimen (Table 1). This included VIEW that utilised fixed dosing in the first year followed by a capped PRN regimen in the second year of treatment as well as two studies (HAWK & HARRIER, TENAYA & LUCERNE) that utilised a fixed loading regimen of monthly injections for 4 months followed by differing fixed-dose regimens (e.g. at 8-, 12- or 16-week intervals) depending on disease activity assessment at specific time points.
Table 1.
Phase 3 randomised controlled trials investigating treatment of neovascular age-related macular degeneration with intravitreal anti-vascular endothelial growth factors using a proactive capped pro re nata or treat and extend regimen
| Study | Study type | Intravitreal medication and treatment | DA criteria | Treatment regimen alteration | Primary end-point and outcome | Percentage of eyes with no macular fluid at 12 months |
|---|---|---|---|---|---|---|
| VIEW (2012) |
Multicentre phase 3 RCT N = 2419 |
Aflibercept 0.5 or 2 mg monthly or 2-monthly vs ranibizumab 0.5 mg monthly From week 52 to 96, IVT every 12 weeks with monthly evaluation for interim injections (capped PRN) |
1. Increase in central retinal thickness of ≥ 100 μm 2. Loss of ≥ 5 ETDRS letters from best previous with recurrent fluid 3. New onset classic neovascularisation 4. New or persistent leak on fluorescein angiography 5. New macular haemorrhage Time lapse of 12 weeks since previous injections |
Monthly evaluation |
Non-inferiority of visual acuity At 96 weeks, gain in visual acuity (letters) 7.9 (ranibizumab) 7.6 (aflibercept) |
62.0 (ranibizumab) 72.4 (2 mg aflibercept monthly) 60.3 (0.5 mg aflibercept monthly) 67.7 (2 mg aflibercept bi-monthly) |
| HAWK & HARRIER (2019) |
Multicentre phase 3 RCT N = 1817 |
Brolucizumab (3 mg or 6 mg) and aflibercept 2 mg Randomised to brolucizumab at every 12 weeks after loading phase decreased to fixed 8-weekly treatment if signs of disease activity and aflibercept 2 mg (fixed bi-monthly dosing) |
Week 16 1. Decrease in BCVA ≥ 5 letters compared to baseline 2. Decrease in BCVA ≥ 3 letters and CST increase of ≥ 75 compared with week 12 3. Decrease in BCVA ≥ 5 letters due to nAMD disease activity compared to week 12 4. New or worse intraretinal cysts or intraretinal fluid Weeks 20, 28, 32, 40, 44 BCVA decline compared to week 12 |
Brolucizumab 3-monthly injections With default 12-weekly treatment Patients assessed at week 16 and 20 visit Additional assessment week 28 and week 40 (HARRIER) If signs of disease activity, permanent switch to 8-weekly if stable |
Non-inferiority of BCVA Percentage of patients attaining 12-weekly treatment Gain in visual acuity (letters) HAWK 6.1 (brolucizumab 3 mg) 6.6 (brolucizumab 6 mg) 6.8 (aflibercept) HARRIER 6.9 (brolucizumab 6 mg) 7.6 (aflibercept) |
HAWK 68.9 (brolucizumab) 55.3 (aflibercept) HARRIER 74.1 (brolucizumab) 56.4 (aflibercept) |
| TENAYA & LUCERNE (2022) |
Multicentre phase 3 RCTs N = 273 |
Faricimab and aflibercept 2 mg Faricimab (fixed regime 8-, 12- or 16-weekly depending on week 20 and 24 assessment) and aflibercept 2 mg (fixed dosing) |
1. At discretion of masked observer 2. Increase of > 50 μm in CST compared with average CST over previous 2 visits 3. Increase of > 75 μm in CST compared with lowest CST of 2 previous visit 4. Decrease of ≥ 5 letters compared with average BCVA over previous 2 visits, due to nAMD activity 5. Decrease of ≥ 10 letters compared with highest BCVA of previous 2 visits, due to nAMD activity 6. Presence of new macular haemorrhage |
Four-monthly treatments of faricimab Then disease assessments for any sign of DA Week 20: active disease, treated with 8-weekly fixed dosing until week 60 Week 24: active disease, treated with 12-weekly fixed dosing until week 60 Week 20 and 24: no disease activity, 16-weekly fixed dosing until week 60 At week 24, presence of DA that does not meet disease activity criteria but requires immediate treatment in opinion of investigator received 12-weekly treatment |
Mean change in BCVA Gain in visual acuity (letters) TENAYA 5.8 (faricimab) 5.1 (aflibercept) LUCERNE 6.6 (faricimab) 6.6 (aflibercept) |
Not recorded |
BCVA best corrected visual acuity, CST central subfield thickness, DA disease activity, ETDRS Early Treatment of Diabetic Retinopathy Study, OCT optical coherence tomography, PRN pro re nata, RCT randomised controlled trial, RF intraretinal fluid, SRF subretinal fluid, VA visual acuity
We identified nine studies (LUCAS, TREX-AMD, FLUID, TREND, RIVAL, ALTAIR, CANTREAT, ARIES, TREX-Conbercept) that utilised a proactive treat and extend to treat patients with nAMD in at least one study arm (Table 2). These studies all utilised a fixed loading regimen of monthly injections (3 or 4 months) followed by a treat and extend regimen of varying treatment extension periods and corresponding criteria to maintain, extend or reduce the required treatment interval depending on disease assessment activity.
Table 2.
Phase 3b and 4 randomised controlled trials investigating treatment of neovascular age-related macular degeneration with intravitreal anti-vascular endothelial growth factors using a proactive treat and extend regimen
| Study | Study type | Intravitreal medication and treatment regimen | DA criteria | Treatment regimen alteration | Primary end-point | Percentage of eyes with no macular fluid at 12 months |
|---|---|---|---|---|---|---|
| LUCAS (2015) |
Multicentre phase 4 RCT, 24 months N = 441 |
Ranibizumab 0.5 mg vs bevacizumab 1.25 mg Randomised 1:1 Using same treat and extend regimen |
1. Any fluid on OCT 2. New or persistent haemorrhage 3. Dye leakage or increased lesion size on FFA NB BCVA not used as sign of DA |
Treat and extend after 3-monthly loading phase: Maintain: Continue at 4-weekly intervals after loading phase if any DA Extend: no DA, extend by 2 weeks at each visit until maximum of 12 weeks Reduce: after extension beyond 4 weeks, reduce by 2 weeks each visit until no DA After second attempt at extension, maintained at 2 weeks less than the maximum interval of disease recurrence If no response 4 weeks after third injection, removed from study (non-responders) |
Non-inferiority of visual acuity at 1 year Gain in visual acuity (letters) 7.9 (bevacizumab) 8.2 (ranibizumab) |
47.0 (bevacizumab) 65.2 (ranibizumab) |
| TREX-AMD (2015) |
Phase 3b multicentre RCT, 12 months N = 60 |
Ranibizumab 0.5 mg Randomised 1:1 fixed monthly vs treat and extend |
1. Presence of IRF or SRF 2. Macular haemorrhage (subretinal or intraretinal) |
Treat and extend after 3-monthly Maintain: Continue at 4-week intervals after loading phase if any DA Extend: no DA, extend by 2 weeks at each visit until maximum of 12 weeks Reduce: 1 DA: reduce by 2 weeks until stable - Extend after at 1 week if no DA - Reduce by 1 week again if recur, then maintain for a further visit before any attempt at extension If recurrent DA at same interval three times, continue at next shorter interval 3 times before resuming protocol |
Mean BCVA from baseline 12 months Proportion with gain in visual acuity of 10 letters 45% (fixed monthly) 33% (treat and extend) |
Not recorded |
| FLUID (2016) |
Multicentre phase 4 RCT, 24 months N = 349 |
Ranibizumab 0.5 mg Randomised 1:1 to two regimens (i) Intense: complete resolution of all retinal fluid (ii) Relaxed: resolution of IRF and/or subfoveal > 200 μm only at subfoveal location |
1. Loss of VA ≤ 5 letters 2. New retinal haemorrhage 3. Presence of SRF or IRF (intense) Presence of IRF and/or SRF > 200 μm at subfoveal location (relaxed) |
Treat and extend after 3-monthly loading phase Maintain: Continue at 4-weekly intervals after loading phase if any DA Extend: no DA, extend by 2 weeks at each visit until maximum of 12-weekly Reduce: after extension beyond 4 weeks, if 1 DA: reduce by 2 weeks ≥ 2 DA: return to 4-week interval After second attempt at extension, maintain at 2 weeks less than the maximum interval of disease recurrence If returned to 4 weeks twice, then maintain at 4 weeks |
Mean change in visual acuity from baseline to month 24 Gain in visual acuity (letters) 3 (intensive) 2.6 (relaxed) |
70.5 (relaxed) 78.5 (intense) |
| TREND (2017) |
Multicentre phase 3b RCT, 12 months N = 650 |
Ranibizumab 0.5 mg Randomised: monthly vs treat and extend regimen |
1. Presence of SRF or IRF |
Treat and extend after 2- monthly Maintain: Continue at 4-weekly intervals after loading phase if any DA Extend: no DA, extend by 2 weeks at each visit until maximum of 12 weeks Reduce: 1 DA: reduce by 2 weeks DA plus reduced VA: reduce to 4-weekly treatment interval After second attempt at extension, maintained at 2 weeks less than the maximum interval of disease recurrence |
Non-inferiority of BCVA of monthly and treat and extend regimen Gain in visual acuity (letters) 8.1 (monthly) 6.2 (treat and extend) |
62.3% (treat and extend) 60.1% (monthly) |
| CANTREAT (2019) |
Phase 4 RCT, 1 year N = 526 |
Ranibizumab 0.5 mg Randomised 1:1 fixed monthly versus treat and extend regimen |
1. Loss of VA ≥ 5 ETDRS letters 2. Any fluid 3. Any new haemorrhage 4. Progression of neovascularisation |
Treat and extend after 3-monthly Maintain: Continue at 4-weekly intervals after loading phase if any DA Extend: no DA by 2 weeks until maximum of 12 weeks Reduce: any sign of DA, reduce by 2 weeks if 6 or 8 weeks or 4 weeks if 10 or 12 weeks - Maintain once stable for 1 visit before extending - If fail again at same disease interval, then keep last stable interval throughout study |
Mean BCVA from baseline Gain in visual acuity (letters) 8.4 (treat and extend) 6 (monthly) |
Not recorded |
| RIVAL (2019) |
Phase 4 RCT, 12 months N = 281 |
Ranibizumab 0.5 mg vs aflibercept 2 mg Randomised 1:1 Using same treat and extend regimen |
1. Loss of VA ≤ 5 letters 2. New retinal haemorrhage 3. Presence of SRF or IRF |
Treat and extend after 3-monthly Maintain: Continue at 4-weekly intervals after loading phase if any DA Extend: no DA, extend by 2 weeks at each visit until maximum of 12 weeks Reduce: after extension beyond 4 weeks, if 1 DA: reduce by 2 weeks 2 DA: reduce to 4-weekly treatment interval After second attempt at extension, maintained at 2 weeks less than the maximum interval of disease recurrence |
Mean change in BCVA and number of injections at 12 months Gain in visual acuity (letters) 6.9 (ranibizumab) 5.2 (aflibercept) |
56.0 (ranibizumab) 64.0 (aflibercept) |
| ALTAIR (2020) |
Phase 4 RCT, 96 weeks N = 123 |
Aflibercept 2 mg Randomised 1:1 treat and extend 2-weekly and 4-weekly extension |
1. New or persistent fluid with unchanged or increased fluid volume compared to previous treatment visit 2. Loss of > 5 ETDRS letters in conjunction with recurrent fluid 3. Increase in CRT 100 µm in central 1 mm compared with lowest previous 4. New neovascularisation (fundus examination and multimodal imaging) 5. New macular haemorrhage |
Treat and extend after 4-monthly By 2 or 4 weeks Maintain: no DA and residual fluid decreased from previous visit Extend: no DA and no fluid to maximum of 16-weekly Reduce: any DA - If any reduction occurred in 4-weekly group, subsequent extension was by 2 weeks only |
Mean BCVA from baseline Gain in visual acuity (letters) 9 (2-week extension) 8.4 (4-week extension) |
68.3 (2-week extension) 69.1 (4-week extension) |
| ARIES (2020) |
Phase 3 RCT, 104 weeks N = 271 |
Aflibercept 2 mg Randomised 1:1 Treat and extend early (after 3-monthly) or late (after 1-year fixed bi-monthly dosing) |
1. Presence of IRF 2. New neovascularisation or haemorrhage 3. Subretinal fluid > 50 μm |
Treat and extend regimen after 4-monthly Early: from week-16, adjust by 2 weeks to max of 16-weekly. At week 16 can be extended by 4 weeks (next visit week 28) then subsequently 2-week adjustments only Late: adjust by 2 weeks to a max of 16 weeks Excluded from study if required more frequent than 2q8 interval before or at 16 weeks Extend: No DA Reduce: Any DA – reduce to last effective treatment interval |
Mean BCVA from baseline Change in visual acuity (letters) − 2.1 (early treat and extend) − 0.4 (late treat and extend) |
Not recorded |
| TREX-Conbercept (2022) |
Phase 3 RCT, 24 weeks N = 141 |
Conbercept 3-month loading then randomised treat and extend or PRN regimen |
1. Loss of VA > 5 letters 2. New macular haemorrhage 3. Any intraretinal fluid 4. Subretinal fluid more than 200 μm at subfoveal centre |
Treat and extend after 3-monthly Maintain: Continue at 4-weekly intervals after loading phase if any DA Extend: no DA, extend by 2 weeks at each visit until maximum of 12 weeks Reduce: 1 DA: reduce by 2 weeks until stable Regimen for multiple attempts at extension not stated |
Non-inferiority of visual acuity at 24 months Gain in visual acuity (letters) 4.0 (treat and extend) 5.1 (PRN) |
Not stated |
BCVA best corrected visual acuity, CST central subfield thickness, DA disease activity, ETDRS Early treatment of Diabetic Retinopathy Study, FFA fundus fluorescein angiogram, IRF intraretinal fluid, OCT optical coherence tomography, PRN pro re nata, RCT randomised controlled trial, SRF subretinal fluid, VA visual acuity
Treat and Extend Regimen Intervals
ALTAIR, ARIES, TENAYA & LUCERNE and HAWK & HARRIER had a maximum treatment interval of 16 weeks. FLUID, LUCAS, RIVAL, TREND, TREX-AMD, TREX-Conbercept and CANTREAT had a maximal treatment interval of 12 weeks.
LUCAS, RIVAL, FLUID, TREND, TREX-AMD and TREX-Conbercept adjusted treatment intervals (reduce or extend) by 2 weeks depending on disease activity. FLUID, RIVAL and TREND also reduced treatment intervals by 4 weeks if there were several signs of disease activity. CANTREAT and ARIES reduced treatment intervals by 4 weeks if current treatment was at a more prolonged treatment interval but 2 weeks at a less prolonged interval. ALTAIR was randomised to compare 2-week and 4-week alteration in treatment intervals. TENAYA & LUCERNE utilised a fixed 8-, 12- or 16-week dosing regimen up to week 60 after disease assessment at weeks 20 and 24. HAWK & HARRIER had a fixed 12-weekly dosing regimen that could be reduced to 8 weeks if signs of disease activity were noted.
The studies also had different approaches to treatment intervals in the event of multiple attempts at treatment interval expansion. In FLUID, LUCAS, RIVAL and TREND, the treatment interval was maintained at 2 weeks less than the maximum treatment interval of disease recurrence for the rest of the study at the second attempt of treatment interval extension. In TREX-AMD, if there was a recurrent disease activity at the same interval three times, treatment was given at the next shorter disease interval before recommencement of the treatment protocol. In CANTREAT, treatment was continued at the last stable interval after attempting to increase the treatment interval a single previous time only. In TENAYA & LUCERNE, patients had fixed dosing treatment for the whole study after 4-monthly treatments whilst default 12-weekly dosing was used in HAWK & HARRIER that could be reduced to fixed 8-weekly treatment if disease activity was found.
Disease Activity Criteria
Table 3 summarises the different disease activity criteria used in these studies. We included all the disease activity criteria used in the studies, including visual acuity, OCT-derived macular thickness, OCT qualitative features of disease activity, macular haemorrhage and angiographic features of disease activity.
-
(i)
Visual Acuity: Seven studies (FLUID, RIVAL, CANTREAT, ALTAIR, TENAYA & LUCERNE, HAWK & HARRIER, TREX-Conbercept) utilised visual acuity in their treatment criteria, all using a change (loss) of visual acuity of at least five ETDRS letters from the last visit except for TENAYA & LUCERNE which specified at least five letters lost from the average of the last two visits or at least 10 letters lost from the highest of the preceding two attendances. Furthermore, HAWK & HARRIER specified a loss of five letters compared with baseline as well as a loss of three letters combined with an increase in central subfield thickness (CST) of 75 µm or loss of five letters to week 12 attendance due to nAMD disease activity. Four studies (LUCAS, TREND, TREX-AMD, ARIES) did not utilise visual acuity in their treatment criteria.
-
(ii)
OCT macular thickness: Five studies (FLUID, ALTAIR, VIEW, ARIES, TENAYA & LUCERNE) utilised an increase in macular thickness as a treatment criterion. TENAYA & LUCERNE specified an increase of 50 µm compared with the two preceding visits. ALTAIR and VIEW specified an increase of 100 µm in the central retinal thickness (central 1 mm) compared with the lowest previous measurement. The definition of retinal thickness measurement (internal limiting membrane to Bruch membrane or retinal pigment epithelium) was not fully specified. FLUID employed an increase in a fluid compartment (subretinal and/or intraretinal fluid) of more than 200 µm at the subfoveal location. ARIES utilised an increase in the subretinal fluid compartment of > 50 µm (location not specified).
-
(iii)
Visual acuity and OCT macular thickness: HAWK & HARRIER alone utilised a combination of OCT macular thickness as a single disease criterion, specifically a loss of three letters combined with an increase in CST of 75 µm.
-
(iv)
Qualitative biomarkers of nAMD disease on structural OCT imaging: All the studies except for VIEW and TENAYA & LUCERNE utilised a change in nAMD disease biomarkers on OCT imaging such as the presence of subretinal or intraretinal fluid as a criterion for disease activity.
-
(v)
Visual acuity and qualitative OCT nAMD biomarkers: Two studies (ALTAIR and VIEW) utilised a combination of visual acuity and qualitative OCT nAMD biomarkers as a criterion of disease activity. Both studies described a loss of more than five letters in association with presence of retinal fluid on OCT imaging. Two further studies (TENAYA & LUCERNE, HAWK & HARRIER) employed a reduction in visual acuity due to disease activity.
-
(vi)
Macular haemorrhage: Ten studies (FLUID, LUCAS, VIEW, RIVAL, TREX-AMD, TREX-Conbercept, CANTREAT, ALTAIR, ARIES, TENAYA & LUCERNE) utilised presence of new macular haemorrhage secondary to nAMD as a disease activity criterion.
-
(vii)
Increase in lesion size on fundus fluorescein angiogram: Six studies (LUCAS, FLUID, CANTREAT, ALTAIR, ARIES, VIEW) specified a change in lesion size on fundus fluorescein angiogram (FFA) as a recommendation for treatment.
Table 3.
Summary of disease assessment criteria identified in proactive treatment regimen in randomised controlled trials of neovascular age-related macular degeneration
| Study | Type of disease assessment criteria used to modify treatment interval | ||||||
|---|---|---|---|---|---|---|---|
| OCT change in morphology: qualitative (e.g. increase in IRF) | New haemorrhage due to nAMD | Visual acuity reduction only | Increase in lesion size on FFA or other retinal imaging modality | OCT change in morphology with visual acuity reduction | OCT thickness increase only | Both visual acuity reduction and CST thickness increase | |
| LUCAS (2015) | Yes | Yes | No | Yes | No | No | No |
| VIEW (2012) | No | Yes | Yes | Yes | Yes | Yes | No |
| TREX-AMD (2015) | Yes | Yes | No | No | No | No | No |
| FLUID (2016) | Yes | Yes | Yes | Yes | No | Yes | No |
| TREND (2017) | Yes | No | No | No | No | No | No |
| RIVAL (2019) | Yes | Yes | Yes | No | No | No | No |
| CANTREAT (2019) | Yes | Yes | Yes | Yes | No | No | No |
| ALTAIR (2020) | Yes | Yes | No | Yes | Yes | Yes | No |
| ARIES (2020) | Yes | Yes | No | Yes | No | Yes | No |
| HAWK & HARRIER (2019) | Yes | No | Yes | No | Yes | No | Yes |
| TENAYA & LUCERNE (2022) | No | Yes | No | No | Yes | Yes | No |
| TREX-Conbercept (2022) | Yes | Yes | Yes | No | No | No | No |
| Number of studies using this type of disease assessment criterion | 10 | 10 | 6 | 6 | 4 | 5 | 1 |
CST central subfield thickness, FFA fundus fluorescein angiogram, IRF intraretinal fluid, nAMD neovascular age-related macular degeneration, OCT optical coherence tomography
Discussion
One of the biggest challenges in the treatment of nAMD is to achieve treatment outcomes close to those seen in well-conducted, large-scale RCTs despite the many and varied factors in which real-world clinical care differs from clinical trials. Some of these factors are those we cannot easily influence relating to patient factors such as intercurrent illness leading to missed nAMD treatment and a wider range of baseline lesion characteristics in real-world clinics [14]. Other factors are also responsible for variation in treatment outcomes in these clinical trials. These factors could include different intravitreal treatment agents, differences in baseline visual acuity, lesion size and baseline disease activity. In this systematic review we identified randomised controlled, clinical trials of anti-VEGF treatment for nAMD using proactive or treat and extend treatment paradigm, and listed the disease activity criteria used in clinical trials to change the interval between anti-VEGF injections. Our aim was to consider the similarities and differences in these criteria which in turn affect key secondary trial outcomes relating to treatment durability (proportion of eyes treated at q12 or q16 week dosing intervals) [15]. Also, we consider which of these disease activity criteria map across well to use in real-world clinics and could therefore be used in the next generation of clinical trials to help make future clinical trial results more generalisable and achievable in real-world settings so enabling patients to achieve visual acuity results closer to those seen in clinical trials with benefits for patients, payors and clinicians.
Visual Acuity Change
Six of the 12 clinical trials we considered used a reduction in VA as a disease activity criterion. However, VA measurements are notoriously subject to measurement variability in clinical settings, particularly in patients with AMD [16]. Moreover, VA is a lagging indicator of worsening nAMD compared to morphological changes seen on structural OCT imaging [17]. It is well established that there is a ceiling effect in visual acuity change, in which eyes with worse baseline visual acuity frequently demonstrate better visual acuity gain [18].
OCT Macular Thickness Change
Six of the 12 clinical trials we considered used an increase in macular thickness as a disease activity criterion either with or without VA change. In the presence of nAMD lesion components which affect detection of the retinal pigment epithelium by automated segmentation algorithms, accurate and repeatable measurement of macular thickness is difficult and would need manual correction or verification of boundary detection which is time consuming and not routinely done in clinic [19, 20]. However, if or when treatment has led to a stable well-treated lesion there is improved repeatability of retinal thickness measurement making macular thickness change a reasonable criterion to use to help detect reactivation of nAMD lesions during any maintenance phase of anti-VEGF treatment [21, 22].
Qualitative Biomarkers of nAMD Disease on Structural OCT Imaging
Nine of the 12 clinical trials used a change in macular fluid as a disease activity criterion. Qualitative interpretation of structural OCT images for nAMD disease biomarkers is well established in clinical practice as a way of determining improvement, stability or worsening of nAMD disease activity [23]. However, a recent review identified 11 features or biomarkers, some with more robust evidence base to support their use over other less well-established biomarkers where consensus is still lacking [23]. Even when considering intraretinal fluid as an imaging biomarker of nAMD disease activity, clinicians need to attempt to distinguish IRF associated with atrophy and tubulation from IRF associated with worsening exudative, nAMD.
Macular Haemorrhage
Ten of the 12 clinical trials included presence of new macular haemorrhage secondary to nAMD as a disease activity criterion suggesting that detection of haemorrhage is an important marker of nAMD disease activity. Macular haemorrhage can be detected in clinical trials and clinical practice in real-world settings using clinical examination, colour fundus photography and also OCT imaging (larger haemorrhages). This means undertreatment due to missed macular haemorrhage in real-world settings is unlikely particularly if multimodal imaging is used in clinical evaluation. Post hoc analysis of the HARBOR trial assessing macular haemorrhage suggested that macular haemorrhage without OCT detectable intraretinal fluid did not require treatment, but further randomised trials would be necessary [24].
Increase in Lesion Size
Only 6 out of 12 clinical trials used an increase in lesion size as a disease activity criterion suggesting there is a spectrum of opinion in the value of growth of lesion size as a disease activity criterion independent of other criteria. Moreover, FFA is not routinely used in clinical practice to assess response to treatment so the judgement on lesion size would fall on an assessment made through analysis of OCT imaging (e.g. increase in pigment epithelial detachment (PED) height or lateral growth of PED). The use of OCT angiography may help to both detect and quantify lesion size and activity in the future [25].
Other Disease Activity Considerations
Evidence from post hoc analyses of nAMD clinical trials and from analysis of real-world datasets suggests minimising fluctuations in retinal thickness is associated with better visual acuity outcomes. Also, results from clinical trials do not show additional benefits of a loading phase of treatment or extended fixed dosing when initiating treatment [10, 26]. These findings suggest further individualisation of treatment intervals could be considered on the basis of an extension in treatment intervals once maximum improvements in nAMD disease activity have been reached and to then minimise fluctuations in retinal thickness (and by inference, minimise fluctuation in all imaging biomarkers of nAMD which contribute to retinal thickness). Application of different disease activity criteria depends on multiple factors, including the experience of treating clinicians, imaging technology availability and variation and cost. This arguably suggests that the most feasible would include visual acuity (despite its inherent measurement difficulties) and qualitative OCT biomarkers of disease activity. The use of emerging disease biomarkers of nAMD has the potential to improve visual acuity outcomes and aid treatment decisions by improved characterisation of disease activity. Establishing their role in refining treatment paradigms in the real world is required, particularly in large volume retinal clinics with multiple clinicians. Artificial intelligence may have a role in the detection of disease activity including in retinal fluid analysis [27].
Conclusions
Clinical trials use different sets of disease activity criteria when altering the interval between anti-VEGF injections. This makes it difficult to draw meaningful conclusions about secondary outcomes relating to structural outcomes (e.g. proportion of eyes without macular fluid at a time point or proportion of eyes tolerating a q12 or q16 week dosing regimen). Moreover, some of these disease activity criteria are less robust when applied in a clinical setting because of the variability of clinical measurements, e.g. visual acuity measurements or retinal thickness change (due to OCT scan-related segmentation error affecting macular thickness measurements).
One way forward to help get closer to achieving clinical trial outcomes in real-world settings would be to adopt a treat-to-stability approach when initiating treatment with monthly injections until maximum improvement in anatomical signs of disease activity followed by a gradual extension in treatment intervals while still maintaining anatomical stability and minimising fluctuations in macular thickness, OCT and clinical biomarkers of nAMD disease activity (e.g. increasing intraretinal fluid, subretinal fluid, enlargement of a PED, new or increasing subretinal hyper-reflective material (SHRM), new retinal or subretinal haemorrhage). When an increase in macular thickness or disease activity biomarker is seen, the treatment interval can be shortened to re-achieve maximal improvement (e.g. a fluid-free macula or minimal, stable, subretinal fluid) after which further extensions in treatment interval may be cautiously considered (Table 4). The added benefit of this approach would be to reduce fluctuations in retinal thickness and nAMD disease biomarker thickness or volume through the maintenance phase of treatment as greater fluctuation in retinal thickness with follow-up has been associated with worse visual acuity outcomes in post hoc analyses of clinical trials and in real-world patient cohorts [21, 22]. We do note, however, that these suggested criteria need full evaluation in clinical trials before they can be used widely. Use of next-generation, proactive treat and extend paradigms can help bridge the “efficacy gap” between clinical trials of anti-VEGF treatment in nAMD and clinical practice. Disease activity criteria used in clinical trials of new and existing nAMD treatments need to be easy to implement allowing widespread uptake in real-world clinical settings.
Table 4.
Proposed disease assessment criteria to maintain, extend or reduce treatment intervals in future trials in neovascular age-related macular degeneration
| Treat with monthly injections to stability then assess for signs of disease activity as below | |
|---|---|
| Extend interval between injections (e.g. by 2 weeks at a time or by 4 weeks if no disease activity features present) |
If no worsening in qualitative features of disease activity: • No increase in subretinal fluid or intraretinal fluid • No new subretinal hyperreflective material or retinal/subretinal haemorrhage • No change in pigment epithelial detachment height or lateral growth and no worsening in quantitative features of disease activity: • No significant increase in OCT central subfield macular thickness compared to last visit (less than 50-μm change compared to last visit and within 75 μm of best, minimal thickness recorded) |
| Reduce interval between injections (e.g. by 2 weeks at a time or by 4 weeks if 2 or more features of worsening disease detected) |
If worsening in qualitative features of disease activity detected: • Increase in subretinal fluid or intraretinal fluid • New subretinal hyperreflective material or retinal/subretinal haemorrhage • Change in pigment epithelial detachment height or lateral growth or worsening in quantitative features of disease activity: • Significant increase in OCT central subfield macular thickness compared to last visit (less than 50-μm change compared to last visit and within 75 μm of best, minimal thickness recorded) |
| Consider maintaining treatment interval for a series of injections (e.g. 3) if extension of treatment interval is followed immediately by need to shorten interval again in 2 successive treatment extension phases (i.e. if extend, shorten, extend, shorten then follow this by maintaining treatment interval at shortened interval for 3 injections before thinking about extending again) | |
Acknowledgements
Funding
No funding or sponsorship was received for this study or publication of this article.
Author Contributions
Daren Hanumunthadu and Praveen J. Patel were responsible for initial article conception and design, literature review, drafting manuscript and reviewing the final manuscript. Pablo Villavicencio also completed literature review. All authors have final approval of version to be published.
Disclosures
The views expressed in the publication are those of the authors and not necessarily those of the Department of Health. Dr Praveen Patel has received speaker fees from Novartis, Bayer, Topcon and Heidelberg, consulting fees from Novartis, Bayer, Oxford Bioelectronics and Roche and research support from Bayer. Dr Daren Hanumunthadu has received consultancy fees from Roche. Dr Pablo Villavincencio has nothing to disclose.
Compliance with Ethics Guidelines
This was a systematic review based on reporting PRISMA Guidelines. This article is based on previously conducted studies and does not contain any new studies with human participants or animals performed by any of the authors.
Data Availability
Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study.
References
- 1.Rosenberg D, Deonarain DM, Gould J, et al. Efficacy, safety, and treatment burden of treat-and-extend versus alternative anti-VEGF regimens for nAMD: a systematic review and meta-analysis. Eye (Lond) 2023;37(1):6–16. doi: 10.1038/s41433-022-02020-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Heier JS, Brown DM, Chong V, et al. Intravitreal aflibercept (VEGF trap-eye) in wet age-related macular degeneration. Ophthalmology. 2012;119(12):2537–2548. doi: 10.1016/j.ophtha.2012.09.006. [DOI] [PubMed] [Google Scholar]
- 3.Berg K, Pedersen TR, Sandvik L, Bragadóttir R. Comparison of ranibizumab and bevacizumab for neovascular age-related macular degeneration according to LUCAS treat-and-extend protocol. Ophthalmology. 2015;122(1):146–152. doi: 10.1016/j.ophtha.2014.07.041. [DOI] [PubMed] [Google Scholar]
- 4.Wykoff CC, Ou WC, Brown DM, et al. Randomized trial of treat-and-extend versus monthly dosing for neovascular age-related macular degeneration: 2-year results of the TREX-AMD study. Ophthalmol Retina. 2017;1(4):314–321. doi: 10.1016/j.oret.2016.12.004. [DOI] [PubMed] [Google Scholar]
- 5.Arnold JJ, Markey CM, Kurstjens NP, Guymer RH. The role of sub-retinal fluid in determining treatment outcomes in patients with neovascular age-related macular degeneration–a phase IV randomised clinical trial with ranibizumab: the FLUID study. BMC Ophthalmol. 2016;16:31. doi: 10.1186/s12886-016-0207-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Silva R, Berta A, Larsen M, Macfadden W, Feller C, Monés J. Treat-and-extend versus monthly regimen in neovascular age-related macular degeneration: results with ranibizumab from the TREND study. Ophthalmology. 2018;125(1):57–65. doi: 10.1016/j.ophtha.2017.07.014. [DOI] [PubMed] [Google Scholar]
- 7.Guymer RH, Markey CM, McAllister IL, Gillies MC, Hunyor AP, Arnold JJ. Tolerating subretinal fluid in neovascular age-related macular degeneration treated with ranibizumab using a treat-and-extend regimen: FLUID study 24-month results. Ophthalmology. 2019;126(5):723–734. doi: 10.1016/j.ophtha.2018.11.025. [DOI] [PubMed] [Google Scholar]
- 8.Ohji M, Takahashi K, Okada AA, Kobayashi M, Matsuda Y, Terano Y. Efficacy and safety of intravitreal aflibercept treat-and-extend regimens in exudative age-related macular degeneration: 52- and 96-week findings from ALTAIR: a randomized controlled trial. Adv Ther. 2020;37(3):1173–1187. doi: 10.1007/s12325-020-01236-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kertes PJ, Galic IJ, Greve M, et al. Efficacy of a treat-and-extend regimen with ranibizumab in patients with neovascular age-related macular disease: a randomized clinical trial. JAMA Ophthalmol. 2020;138(3):244–250. doi: 10.1001/jamaophthalmol.2019.5540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mitchell P, Holz FG, Hykin P, et al. Efficacy and safety of intravitreal aflibercept using a treat-and-extend regimen for neovascular age-related macular degeneration: the ARIES study: a randomized clinical trial. Retina. 2021;41(9):1911–1920. doi: 10.1097/IAE.0000000000003128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jia H, Lu B, Yuan Y, et al. A randomized, controlled trial of treat-and-extend vs. pro re nata regimen for neovascular age-related macular degeneration. Front Med (Lausanne) 2022;9:852519. doi: 10.3389/fmed.2022.852519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dugel PU, Koh A, Ogura Y, et al. HAWK and HARRIER: phase 3, multicenter, randomized, double-masked trials of brolucizumab for neovascular age-related macular degeneration. Ophthalmology. 2020;127(1):72–84. doi: 10.1016/j.ophtha.2019.04.017. [DOI] [PubMed] [Google Scholar]
- 13.Heier JS, Khanani AM, Quezada Ruiz C, et al. Efficacy, durability, and safety of intravitreal faricimab up to every 16 weeks for neovascular age-related macular degeneration (TENAYA and LUCERNE): two randomised, double-masked, phase 3, non-inferiority trials. Lancet. 2022;399(10326):729–740. doi: 10.1016/S0140-6736(22)00010-1. [DOI] [PubMed] [Google Scholar]
- 14.Karampelas M, Pefkianaki M, Rees A, et al. Missed hospital appointments of patients receiving ranibizumab therapy for neovascular age-related macular degeneration. Ophthalmol Ther. 2015;4(1):43–49. doi: 10.1007/s40123-015-0031-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Amoaku WM, Chakravarthy U, Gale R, et al. Defining response to anti-VEGF therapies in neovascular AMD. Eye (Lond) 2015;29(6):721–731. doi: 10.1038/eye.2015.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Patel PJ, Chen FK, Rubin GS, Tufail A. Intersession repeatability of visual acuity scores in age-related macular degeneration. Invest Ophthalmol Vis Sci. 2008;49(10):4347–4352. doi: 10.1167/iovs.08-1935. [DOI] [PubMed] [Google Scholar]
- 17.Staurenghi G, Garweg JG, Gerendas BS, et al. Functional versus functional and anatomical criteria-guided ranibizumab treatment in patients with neovascular age-related macular degeneration—results from the randomized, phase IIIb OCTAVE study. BMC Ophthalmol. 2020;20(1):18. doi: 10.1186/s12886-019-1251-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Preston E, Hamilton RD, Mahroo OA. Exploring regression to the mean in visual acuities by investigating measurements at two consecutive time points in untreated fellow eyes. Eye (Lond) 2022 doi: 10.1038/s41433-022-02349-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Patel PJ, Chen FK, Ikeji F, et al. Repeatability of stratus optical coherence tomography measures in neovascular age-related macular degeneration. Invest Ophthalmol Vis Sci. 2008;49(3):1084–1088. doi: 10.1167/iovs.07-1203. [DOI] [PubMed] [Google Scholar]
- 20.Hanumunthadu D, Ilginis T, Restori M, et al. Repeatability of swept-source optical coherence tomography retinal and choroidal thickness measurements in neovascular age-related macular degeneration. Br J Ophthalmol. 2017;101(5):603–608. doi: 10.1136/bjophthalmol-2016-308999. [DOI] [PubMed] [Google Scholar]
- 21.Evans RN, Reeves BC, Maguire MG, et al. Associations of variation in retinal thickness with visual acuity and anatomic outcomes in eyes with neovascular age-related macular degeneration lesions treated with anti-vascular endothelial growth factor agents. JAMA Ophthalmol. 2020;138(10):1043–1051. doi: 10.1001/jamaophthalmol.2020.3001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chakravarthy U, Havilio M, Syntosi A, et al. Impact of macular fluid volume fluctuations on visual acuity during anti-VEGF therapy in eyes with nAMD. Eye (Lond) 2021;35(11):2983–2990. doi: 10.1038/s41433-020-01354-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hanson RLW, Airody A, Sivaprasad S, Gale RP. Optical coherence tomography imaging biomarkers associated with neovascular age-related macular degeneration: a systematic review. Eye (Lond) 2022 doi: 10.1038/s41433-022-02360-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Patel Y, Miller DM, Fung AE, Hill LF, Rosenfeld PJ. Are dilated fundus examinations needed for OCT-guided retreatment of exudative age-related macular degeneration? Ophthalmol Retina. 2020;4(2):141–147. doi: 10.1016/j.oret.2019.09.006. [DOI] [PubMed] [Google Scholar]
- 25.Lindner M, Fang PP, Steinberg JS, et al. OCT angiography-based detection and quantification of the neovascular network in exudative AMD. Invest Ophthalmol Vis Sci. 2016;57(14):6342–6348. doi: 10.1167/iovs.16-19741. [DOI] [PubMed] [Google Scholar]
- 26.Martin DF, Maguire MG, Fine SL, et al. Ranibizumab and bevacizumab for treatment of neovascular age-related macular degeneration: two-year results. Ophthalmology. 2012;119(7):1388–1398. doi: 10.1016/j.ophtha.2012.03.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pucchio A, Krance SH, Pur DR, Miranda RN, Felfeli T. Artificial intelligence analysis of biofluid markers in age-related macular degeneration: a systematic review. Clin Ophthalmol. 2022;16:2463–2476. doi: 10.2147/OPTH.S377262. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study.