Abstract
Nodal explants of Holarrhena pubescens, an important medicinal tree, were cultured on Murashige and Skoog’s medium (MS) containing 15 µM BA (control) alone and on medium supplemented with different concentrations (0, 1, 5, 25, 50, 100 and 200 mg/L) of heavy metals such as NiCl2, CoCl2, As2O3 and CrO3 to study their toxic effect. After 28 days of treatments, the nodal segments were harvested to assess the average number of shoots per explants, average shoot length, malondialdehyde content, proline content, conessine accumulation and antioxidant enzymatic activity. Among all the metals tried, best morphogenic response was achieved at 5 mg/L CrO3 where 80% culture differentiated an average of 3.21 ± 0.08 shoots per explant having 0.95 ± 0.018 cm average shoot length. Highest concentration (200 mg/L) of all the heavy metals proved lethal for morphogenesis. Maximum inhibition in average shoot number and average shoot length was observed in nodal explants treated with 25 mg/L As2O3 where an average of 0.49 ± 0.047 shoots having an average shoot length of 0.3 ± 0.02 cm. Contrarily, addition of heavy metals in culture medium proved strong elicitors, exhibiting significant enhancement in the biosynthesis of conessine, an important bioactive compound. HPLC analysis of the crude extract of in vitro grown untreated nodal cultures revealed an average of 117.06 ± 2.59 µg/g d. w. of conessine, whereas those treated with 100 mg/L of CoCl2 accounted for 297.1 ± 7.76 µg/g d. w. (an increase of 156% over control). Among the heavy metals tried, CoCl2 proved to be the best for conessine enhancement which was in the order of CoCl2 > Cr2O3 > NiCl2 > As2O3 in the nodal explants. Concomitantly, MDA content, the antioxidant enzymes activities of catalase (CAT), superoxide dismutase (SOD), glutathione peroxidase (GR) and ascorbate peroxidase (APX) were also observed to be differentially expressed with the increase in the heavy metals concentration from 1 to 200 mg/L. Free proline, too, increased up to 3.5-fold over control. The results obtained during the present investigation revealed that the overall response of the nodal explants in terms of morphogenesis, conessine content and antioxidant enzyme activities was metal specific as well as dose dependent.
Keywords: Heavy metal, Conessine, Antioxidant enzymes, Morphogenesis, High-performance liquid chromatography
Introduction
Industrial wastes such as metals, chemical solvents, paints, pigments, radioactive wastes, ash, paper products and sludge produced by chemical industries, mining industries, paint industries and metal processing industries are the important sources of soil contamination (Chowdhary et al. 2020). In recent times, problem related to agriculture land pollution is becoming very damaging due to the use of domestic and industrial wastes for agricultural purpose in India (Xia et al. 2020). Among them, heavy metals display persistence behaviour in the soils due to their non-biodegradable nature and toxic effect to animal, plant and human. The increased concentration of heavy metal poses a toxic effect into the plant cells, such as substitution of essential functional groups, cellular damage, generation of reactive oxygen species (ROS) and disturbance in the various metabolic reactions by altering the enzymatic activity (Hu et al. 2021; Pishchik et al. 2021). However, ROS production is primary event in the elicitation of compounds through tissue culture using heavy metal stress (Chen et al. 2019), but it causes severe damaging effects, including damage of lipid membranes, protein and DNA. To encounter and minimize the detrimental effects of ROS, plants have developed many detoxification mechanisms, including various enzymes (CAT, SOD, GPX and APX) and non-enzymatic antioxidant (such as tannins, flavonoids and phenols) (Rajput and Agrawal 2020). Plants produce a vast variety of metabolites, which do not have direct role in growth, development or reproduction of plants, are commonly known as secondary metabolites (SM). These compounds are differentially distributed among plants or plant kingdoms and differ from the primary metabolites (amino acid, carbohydrates, protein, lipids) and has restricted distribution in the plant kingdom. Biologically, these compounds are very active, and hence, they are also known as bioactive compounds (Porokhovinova et al. 2022). Primary metabolites are required for the basic functioning of all living organisms including plants. Secondary metabolites are produced in trace amounts, but the production gets enhanced employing different physical and chemical tools (elicitors). The production of secondary metabolites is influenced by certain factors including nutrient supply, oxygenation, temperature elicitors, signal compounds and pH. At the industrial level, these factors are controlled for optimum production of secondary metabolites. The interest in SMs has increased in the recent years because various investigations with respect to human nutrition pointed out that long-term intake of certain SMs will have a major impact on preventing incidences of cancers, antimalarial drugs and many chronic diseases (Elshafie et al. 2023). Although various elicitors are available for enhancement of bioactive compounds, the heavy metals-based elicitors are predominantly considered for enhancement, because at the low level they serve as a beneficial role in plant development, growth and productivity. Holarrhena pubescens Wall. ex G. Don is a medicinally important tree species distributed throughout the tropical and subtropical regions in the world. It is commonly known as Kurchi or Indrajav and belongs to the family Apocynaceae. It has a wide range of application in pharmaceutical industries for curing serious human diseases such as amoebic dysentery haemorrhoids, diarrhoea, dyspepsia, toothache and diuresis (Kumar et al. 2018a, b). The major active ingredients are conessine isolated from dry bark of H. pubescens which has a wide range of medicinal properties including anthelmintic, astringent and to cure dysentery, biliousness, leprosy, fatigue, skin diseases, bleeding piles, bronchopneumonia, gastric disorders, dyspepsia and diarrhoea (Zahara et al. 2020). It has also been reported that conessine possess anticancer, antiparasitic, antibacterial and antidiabetic activities. The effect of various heavy metals on antioxidant potential and secondary metabolite accumulation remains largely untested. However, very few reports were published concerning the effect of heavy metals on in vitro propagation of nodal explants of H. pubescens (Kumar et al. 2005; Agrawal and Sharma 2006). Therefore, in the present study, we investigated the role of heavy metals (NiCl2, CoCl2, CrO3 and As2O3) on production of conessine and antioxidant potential of the nodal cultures of H. pubescens.
Materials and methods
Plant materials
Healthy and fresh twigs of Holarrhena pubescens Wall. ex G. Don (Syn: Holarrhena antidysenterica (L.) Wall.) were collected from the tree growing near the Department of Physics, University of Delhi (Latitude 28.68° N; Longitude 77.21° E), India. The excised twigs were dipped in the 1% citric acid solution to minimize the browning effect.
Establishment of nodal culture and stress treatment
Twigs of Holarrhena pubescens were cut into 0.5–1.0 cm from the nodal segments and used as nodal explants. These explants were washed thoroughly under running tap water for 15–20 min, followed by rinsing with 5% v/v teepol (Reckitt and Colman, Mumbai, India) for 10–15 min and subsequently treated with 2% citric acid followed by 2% bavistin (BASF, India Ltd., Mumbai, India) for 10 min each. The explants were surface sterilized with 0.1% HgCl2 for 2 min in laminar air flow. Sterilized explants were cultured on full strength Murashige and Skoog (MS) medium containing 3% sucrose and 0.8% agar supplemented with 15 µM N6-Benzyladenine (BA) as previously standardized in our laboratory (Kumar et al. 2005; Murashige and Skoog 1962). Further, nodal explants were exposed to different concentrations (0, 1, 5, 25, 50, 100 and 200 mg/L) of various metals including chromium, arsenic, nickel and cobalt for 28 days.
Harvest and measurement of parameters
Growth parameters
The plants were harvested after 28 days of treatment and analysed for various parameters. Plant growth was evaluated in terms of the average number of shoots per explants and the average shot length.
Malondialdehyde (MDA) content
Membrane permeability was measured by thiobarbituric acid-reactive-substances assay described by Hodges et al. (1997). 100 mg of dried plant material was extracted in 3 mL of 80% methanol and added 0.5% thiobarbituric acid (TBA) prepared with 20% trichloroacetic acid (TCA) without TCA at 95 °C for 20 min. The mixture was centrifuged at 12,000×g at 4 °C for 10 min after stopping the reaction. After centrifugation, supernatant was collected and absorbance was taken at 532 nm. MDA concentration was determined using equation developed by Hodges et al. (1999) after subtracting the non-specific absorbance at 440 nm and 600 nm:
Equation
Proline
Proline (Pro) content was determined using freeze dried material using liquid nitrogen, according to the previously published ninhydrin-acetic acid method (Bates et al. 1973). Freeze dried sample (0.05 g) was homogenized in 2 mL 3% sulpho-salicylic acid and centrifuged at 13,000×g for 15 min at 25 °C and filtered through Whatman Filter Paper No.1. The reaction mixture (1 mL of supernatant, 1 mL of acid ninhydrin reagent and 1 mL of glacial acetic acid) was boiled at 100 °C for 1 h and the reaction was stopped by incubating the reaction mixture on ice bath for 10 min. After that 2 mL of toluene was added in each reaction mixture and vortexed vigorously for 2 min. A brick red coloured chromophore of proline-ninhydrin complex was formed and extracted from the aqueous phase at room temperature Absorbance of the sample was taken at 520 nm using toluene as a blank. Proline concentration was expressed as μmol g−1 DW.
Protein extraction and quantification
Proteins were extracted as per the protocol of Gill et al. (2013). Frozen tissue (100 mg) of each sample was powdered in chilled pestle and mortar and homogenized with 1 mL of extraction buffer [20 mM Hepes, pH 7.5, 50 mM KCl, 1 mM EDTA, 0.1%(v/v) Triton X-100, 0.2% (w/v) polyvinylpyrrolidone, 0.2% (w/v) polyvinylpolypyrrolidone and 5% (v/v) glycerol] followed by the addition of 200 µm 225 mM Hepes, pH 7.5, 1.5 M KCl and 22.5 mM MgCl2). The homogenate was centrifuged at 20,000 rpm for 20 min at 4 °C. The supernatant was used to determine protein concentration at 595 nm using bovine serum albumin as the standard. 50 µL of the supernatant was reacted with 2.5 mL of Bradford’s reagent for 15 min. The intensity of blue colour developed in the reaction was measured at 595 nm using Beckman Coulter DU 730 UV/Vis Spectrophotometer. Protein concentration in the extracts was determined by the method of Bradford (1976), using the Bio-Rad commercial reagent and bovine serum albumin (BSA) as a standard.
Antioxidant enzymes assays
Catalase (CAT) activity was estimated according to Aebi (1984), by monitoring the rate of disappearance of hydrogen peroxide by the decline in absorbance at 240 nm for 3 min. CAT activity was expressed as mmol H2O2 decomposed min−1 mg−1 protein. CAT activity was assayed in 3 mL of reaction mixture containing 30 mM H2O2, 100 µL of crude extract and 100 mM sodium phosphate buffer (pH 7.0). Superoxide dismutase (SOD) activity was assayed spectrophotometrically at 560 nm using a modified nitroblue tetrazolium (NBT) method of Beyer and Fridovich (1987). The activity was expressed as Units/mg protein. One unit of enzyme activity is defined as SOD enzyme required for inhibition of NBT by 50% of its initial volume. 3 mL of SOD reaction contained 100 µL crude extract, 0.1 mM EDTA, 12 mM l-methionine, 10 µM NBT, 50 mM sodium phosphate buffer with pH 7.6 and 10 µM riboflavin. Glutathione reductase (GR) activities were assayed in one mL assay mixture containing 100 mM Hepes (pH 7.5) 1 mM EDTA, 3 mM MgCl2, 0.5 mM GSSG, 0.2 mM NADPH and 25 µL of plant extract following the protocol of Connell and Mullet (1986). NADPH was added last to initiate the reaction and the decrease in absorbance was recorded for 25 min. The oxidation of NADPH in the reaction was monitored spectrophotometrically at 340 nm and the enzyme activity was expressed as mmol NADPH/min/mg protein. Ascorbate peroxidase activity (APX) activity was determined from the decrease in absorbance at 290 nm due to oxidation of ascorbate in the reaction following the protocol of Nakano and Asada (1981). One mL solution having 25 µL of the enzyme extract, 0.5 mM ascorbate 0.1 mM H2O2 and pH 7.0 of 50 mM potassium phosphate. H2O2 was added last to initiate the reaction and the decreased in absorbance was recorded for 3 min. One unit of APX activity is defined as mmol ascorbate oxidized (min−1 mg−1 protein).
Quantification of conessine using high-performance liquid chromatography
Standard stock solution of conessine was prepared at concentration 1 mg/mL and it was diluted to different concentrations such as 500 µg/mL and 125 µg/mL in methanol. The metal-treated nodal cultures of Holarrhena pubescens were ground into a fine powder using mortar and pestle. Subsequently, 2 g powder of each plant part was extracted with 50 mL methanol and was subjected to shaking in an incubator shaker at room temperature for 24 h. The extract was filtered through Whatman filter paper No.1 and then concentrated and subjected to HPLC analysis. The HPLC unit (Waters, USA) equipped with PDA (photo diode array) detector was operated under the following parameters: C18 Column, Zorbax ODS (Octadecyl silane); solvent: acetonitrile:water 95:5 v/v (HPLC grade); injection volume: 20 µL; flow rate: 0.6 mL/min; UV detection: 254 nm.
Statistical analysis
One-way analysis of variance (ANOVA) was used to determine differences among the treatments for all the recorded data followed by Duncan’s multiple range test (DMRT) using software SPSS. The values are presented as mean ± standard deviation (SD). p ≤ 0.05 was used to determine the significance of differences.
Result
Effect of metal stress on morphogenesis
Addition of different concentrations (0, 1, 5, 25, 50, 100 and 200 mg/L) of nickel, cobalt, chromium and arsenic in the growth media exhibited varying toxicity in the nodal culture of H. pubescens. Higher concentrations of NiCl2 exhibited a decline in morphogenic response, but its lower concentrations proved beneficial. Optimum response was achieved at 5 mg/L NiCl2, where 95.66% cultures differentiated an average of 2.88 ± 0.22 shoots (ASN) having average shoot length (ASL) of 1.63 ± 0.05 cm (Table 1; Fig. 1B). Beyond 5 mg/L, a gradual decline in shoot number and shoot length was observed, which dropped significantly at 200 mg/L NiCl2 where the ASN was 0.63 ± 0.06 and ASL 0.12 ± 0.027 cm (Table 1; Fig. 1C). However, under control, i.e. without any metal stress, 91% cultures developed an average of 2.43 ± 0.2 shoots per explant with an average of 0.78 ± 0.03 cm shoot length (Table 1; Fig. 1A). Cobalt chloride is an essential micronutrient for plant growth. In vitro nodal cultures of H. pubescens when reared on media containing different concentrations of (0, 1, 5, 25, 50, 100 and 200 mg/L) of CoCl2 did not show any improvement in morphogenesis cultures and average shoot number over control, contrarily, exhibited a gradual decline along all the observed parameters. Extremely low response was observed at 100 mg/L of CoCl2 where ASN and ASL were 0.56 ± 0.023 and 0.16 ± 0.034 cm,(Table 1; Fig. 1E) respectively, compared to control having ASN (2.43 ± 0.2 and ASL (0.78 ± 0.03 cm (Table 1; Fig. 1A). No response was observed at 200 mg/L (Table 1). When the nodal explants of H. pubescens were exposed to various concentrations (1, 5, 25, 50, 100 and 200 mg/L) of CrO3, explants showed dose-dependent response in terms of morphogenic response (ASN and ASL). Optimum morphogenic response (91.66) was observed in untreated nodal culture of H. pubescens having ASN (2.43 ± 0.2) and ASL (0.78 ± 0.03 cm). At lower concentration (1 mg/L) of CrO3, the ASN and ASL were 2.75 ± 0.11 and 0.8 ± 0.02 cm, respectively (Table 1; Fig. 1F). Optimum response was observed at 5 mg/L of CrO3 where ASN and ASL were 3.21 ± 0.08 and 0.95 ± 0.018 cm, respectively (Table 1). In subsequent concentrations, declining trend was observed at its higher concentrations and maximum decline was at 100 mg/L where the ASN and ASL were 0.43 ± 0.09 and 0.14 ± 0.02 cm, respectively (Table 1; Fig. 1G). The nodal explants of H. pubescens survived only up to 25 mg/L among all concentration of As2O3 tried, (Table 1; Fig. 1H, I) and beyond this concentration no morphogenic activity was observed. ASN and ASL were 0.49 ± 0.047 and 0.3 ± 0.02 cm, at 25 mg/L of As2O3, respectively (Fig. 1).
Table 1.
Morphogenic response of nodal explant from in vitro H. antidysenterica shoots cultured on MS + 15 µM N6-Benzyladenine supplemented with various concentrations of different metals (Ni, Co, Cr and As) for 28d (*-means of 24 replicates ± SE, **-means of 3 replicates ± SE)
| Metal | Concentrations (mg/L) | *Percentage explants developing shoots | *Average no of shoots per explants | *Average shoot length (cm) | **Conessine content µg/g d. w.) |
|---|---|---|---|---|---|
| 0 | 91.66 ± 1.290d | 2.43 ± 0.200 | 0.78 ± 0.030 | 117.06 ± 2.590 | |
| 1 | 93.01 ± 1.640d | 2.59 ± 0.120 | 1.01 ± 0.059 | 119.60 ± 3.320 | |
| 5 | 95.66 ± 1.210d | 2.88 ± 0.220 | 1.63 ± 0.050 | 154.00 ± 4.490 | |
| NiCl2 | 25 | 80.00 ± 1.290c | 1.27 ± 0.030 | 0.58 ± 0.020 | 185.60 ± 7.350 |
| 50 | 76.30 ± 1.370c | 1.35 ± 0.120 | 0.51 ± 0.030 | 233.90 ± 4.510 | |
| 100 | 46.00 ± 1.450b | 1.02 ± 0.080 | 0.26 ± 0.025 | 253.70 ± 5.670 | |
| 200 | 33.34 ± 1.730a | 0.63 ± 0.060 | 0.12 ± 0.027 | 243.10 ± 4.870 | |
| 1 | 91.60 ± 1.710e | 2.36 ± 0.300 | 0.48 ± 0.030 | 104.00 ± 4.310 | |
| 5 | 83.00 ± 1.510d | 2.21 ± 0.050 | 0.34 ± 0.150 | 217.80 ± 6.750 | |
| CoCl2 | 25 | 75.00 ± 1.100c | 1.88 ± 0.100 | 0.30 ± 0.020 | 227.40 ± 7.120 |
| 50 | 68.00 ± 1.970b | 1.50 ± 0.110 | 0.29 ± 0.060 | 253.30 ± 5.510 | |
| 100 | 37.85 ± 1.630a | 0.56 ± 0.023 | 0.16 ± 0.034 | 297.20 ± 7.760 | |
| 200 | – | – | – | – | |
| 1 | 86.00 ± 1.570e | 2.75 ± 0.110 | 0.80 ± 0.020 | 134.40 ± 3.220 | |
| 5 | 80.00 ± 1.570d | 3.21 ± 0.080 | 0.95 ± 0.018 | 146.40 ± 5.320 | |
| CrO3 | 25 | 76.00 ± 1.520c | 1.47 ± 0.100 | 0.45 ± 0.035 | 264.90 ± 4.400 |
| 50 | 69.00 ± 1.280b | 0.90 ± 0.080 | 0.31 ± 0.027 | 296.90 ± 6.490 | |
| 100 | 42.40 ± 1.620a | 0.43 ± 0.090 | 0.14 ± 0.020 | 234.40 ± 3.650 | |
| 200 | – | – | – | – | |
| 1 | 90.00 ± 1.400c | 1.94 ± 0.050 | 0.68 ± 0.030 | 117.60 ± 3.120 | |
| 5 | 73.00 ± 1.590b | 1.63 ± 0.066 | 0.55 ± 0.050 | 143.20 ± 5.080 | |
| As2O3 | 25 | 64.00 ± 1.380a | 0.49 ± 0.047 | 0.30 ± 0.020 | 177.50 ± 5.320 |
| 50 | – | – | – | – | |
| 100 | – | – | – | – | |
| 200 | – | – | – | – |
Value in the column followed by the same superscript are not significantly different as determined by one-way ANOVA at p ≤ 0.05
Fig. 1.

Effect of increasing Ni, Co, Cr and As concentrations (1, 5, 25, 50, 100 and 200 mg/L) on in vitro grown nodal culture of Holarrhena pubescens raised on Murashige and Skoog medium (MS) supplemented with 15 µM N6-Benzyladenine (BA) after 28 days of exposure. A Explant differentiated into multiple shoots MS + 15 µM BA medium (control). B Explants showing number of multiple shoots at 5 mg/L of NiCl2. C Explants showing gradual decline in the shoot number and shoot length at 200 mg/L of NiCl2. D Explants showing better growth at 1 mg/L of CoCl2. E Explants showing significant decline in shoot number and shoot length at 100 mg/L of CoCl2. F Explants showing optimum number of multiple shoots and shoot length at 1 mg/L of CrO3. G Significant decline in shoot number and shoot length in at 100 mg/L of CrO3. H Explants showing moderate growth in terms of number of multiple shoots at 1 mg/L of As2O3. I Explants with stunted shoot growth, lesser number of multiple shoots and decreased shoot length at 25 mg/L of As2O3
Effect of metals on conessine accumulation
A gradual increase in conessine content was observed with the increase in concentrations of CrO3 from 1 to 50 mg/L and maximum amount (296.9 ± 6.49 µg/g d. w.) was reported at 50 mg/L. (Table 1, Fig. 2A). Different concentrations of NiCl2 added to the growth medium, too, elicited the conessine content in the nodal explants of H. pubescens. Conessine content increased with the increasing concentrations of NiCl2, being maximum 253.7 ± 5.61 µg/g d. wt. at 100 mg/L (Table 1, Fig. 2A). Similarly, CoCl2 also proved effective for the enhancement of conessine content and its optimum concentration was 297.2 ± 7.76 µg/g d. wt. at 100 mg/L (Table 1, Fig. 2A). As2O3 displayed extreme toxicity for nodal cultures and its lower amount (25 mg/L of As2O3) elicited optimum amount of conessine (177.5 ± 5.32 µg/g d. w.) (Table 1, Fig. 2A); beyond this concentration culture did not survive, turned brown, and dead.
Fig. 2.
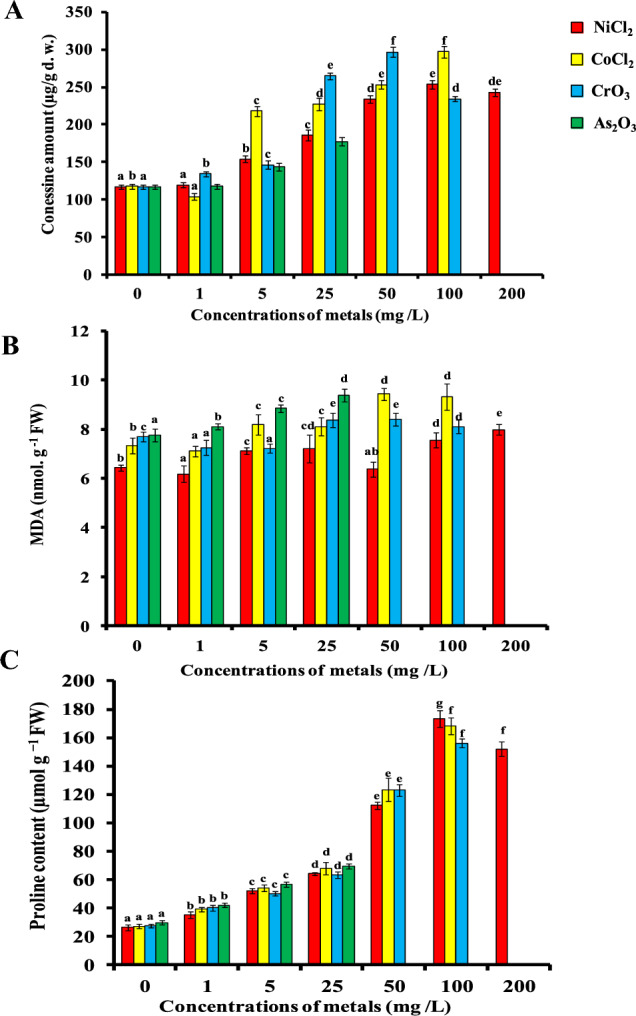
Effect of increasing Ni, Co, Cr and As concentrations (1, 5, 25, 50, 100 and 200 mg/L) on A conessine B MDA and C proline content in 28-day-old nodal culture of Holarrhena pubescens raised on Murashige and Skoog medium supplemented with 15 µM N6-Benzyladenine (BA). Vertical bars indicate ± SE, and values having different letters are significantly different (p < 0.05)
Effect of metal stress on lipid peroxidation
A gradual and significant increase in MDA content was observed under the heavy metals (NiCl2, CoCl2 and CrO3)-treated nodal cultures of H. pubescens as compared to control. The respective percentages of enhancement in the MDA content in the nodal explants of H. pubescens were 24.1%, 46.65% and 30.79% at 200 mg/L NiCl2, 50 mg/L CoCl2 and 50 mg/L CrO3 compared to control. The level of MDA content was metal and concentration specific (Fig. 2B). Contrary to above, MDA content increased gradually and significantly with increasing concentration of As2O3 in the medium (Fig. 2B). At 25 mg/L As2O3, nearly, 45.72% (9.37 nmol g−1 FW) MDA content was increased over the control.
Effect of metal stress on proline content
Proline content in the nodal culture of H. pubescens increased steadily up to 173.56 µmol g−1 FW at 100 mg/L NiCl2 in the culture medium (Fig. 2C). Beyond 100 mg/L, no increment in proline content was observed and decline in level was evident at 200 mg/L (Fig. 2C). A concentration-dependent proline accumulation was observed––when the explants were exposed to different concentrations of CoCl2, 1, 5, 25, 50 and 100 mg/L, the proline content was 39.1, 54.19, 68.11, 123.43 and 168.32 µmol g−1 FW, respectively (Fig. 2C). Similarly, when nodal explants were treated with 1, 5, 25, 50 and 100 mg/L CrO3, the proline accumulation increased by 40.25, 50.21, 63.37, 123.11 and 156.11 µmol g−1 FW (Fig. 2C), respectively, as compared to control (27.7 µmol g−1 FW). As2O3proved to be the most toxic element among the heavy metals and its lower concentration (25 mg/L) could significantly enhanced proline accumulation (69.37 ± 1.76 µmol g−1 FW) over the control explants (Fig. 2C).
Effect of metal stress on the antioxidant enzymatic activity
Generally, heavy metal stress induced the antioxidant enzyme activities and it was directly proportional to the metal concentrations. The antioxidant enzymatic components which are associated with the stress responses included catalase, superoxide dismutase, glutathione peroxidase and ascorbate peroxidase. CAT activity in nodal explants subjected to different concentrations of heavy metals (NiCl2, CoCl2, CrO3 and As2O3) stress increased over the control explants. CAT activity increased by 85%, 76%, 83.3% and 25% at 100 mg/L NiCl2, 50 mg/L CoCl2, 100 mg/L CrO3 and 5 mg/L As2O3, respectively, as compared to control (Fig. 3A). Beyond above concentration decreasing trend in CAT activity was observed. However, CAT activity was metal specific and dose dependent. The activity of SOD was increased by providing an excessive supply of each heavy metal in the growth medium. Arsenic was more efficient for enhancing the SOD activity in the nodal explants of H. pubescens compared to NiCl2, CoCl2 and CrO3. Under heavy metal stress, SOD activity increased up to 84%, 145.4%, 85.5% and 233% at 50 mg/L NiCl2, 50 mg/L CoCl2, 50 mg/L CrO3 and 25 mg/L As2O3, respectively, as compared to control (Fig. 3B). Similar trends were also followed in case of APX activities, whereas a maximum APX activity (58.48%) was reported at 25 mg/L As2O3, over the other heavy metals (NiCl2, CoCl2, and CrO3) (Fig. 3C). Increasing concentrations of heavy metals caused an initial increase in GR activity which is subsequently decreased slightly at higher concentrations. GR activity increased by 61.3%, 79%, 11.11% and 6% at 100 mg/L NiCl2, 25 mg/L CoCl2, 50 mg/L CrO3 and 25 mg/L As2O3, respectively, as compared to control (Fig. 3D). As exhibit most toxic effect as its lower concentrations (25 mg/L) induced optimum GR activity beyond that concentration explant was not survived.
Fig. 3.
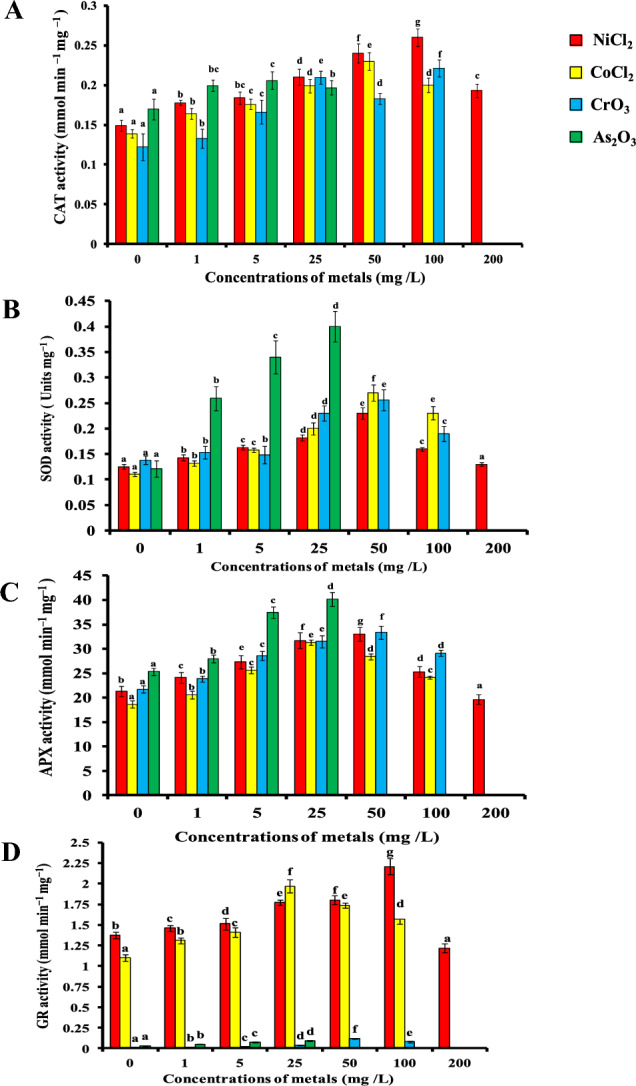
Effect of increasing Ni, Co, Cr and As concentrations (1, 5, 25, 50, 100 and 200 mg/L) on antioxidant enzyme activity of A catalase, B superoxide dismutase, C ascorbate peroxidase and D glutathione reductase in 28-day-old nodal culture of Holarrhena pubescens raised on MS + 15 µM BA medium. Vertical bars indicate ± SE, and values having different letters are significantly different (p < 0.05)
Discussion
Heavy metals enter the ecological system by natural sources, e.g. urban runoff, soil erosion, mining, insecticides applied to disease control of crops, industrial effluents, weathering of the earth’s crust, livestock manure, and wastewater and through anthropogenic activities (Alengebawy et al. 2021; Zhang and Wang 2020). These metals accumulate in the soil beyond their normal level and affect the microbial composition and their activities (Akoto et al. 2022), which leads to loss in the soil fertility and crop productivity. This study for the first time highlights the toxic effect of heavy metals, e.g. NiCl2, CoCl2, CrO3 and As2O3 on growth and productivity of H. pubescens through nodal cultures. Our observation corroborates with those of Hu et al. (2021) which report that Co at lower concentration promotes growth of plants but at higher concentration causes toxicity.
Plant growth
Reduction of shoot length is one of the most important parameters while studying the toxic effect of heavy metals on plant due to lack of defence mechanisms (Tanveer et al. 2022). In the present study, it has been observed that both the shoot length and shoot number of H. pubescens decreased at high concentrations of metals (NiCl2, CoCl2, CrO3 and As2O3) while their lower concentrations showed growth promoting effect. This could be attributed to heavy metal-induced metabolic disorders like interaction with cell wall polysaccharides, thereby decreasing cell wall plasticity. Nodal explants treated with As showed maximum toxicity followed by CrO3, CoCl2 and NiCl2. As2O3 was most toxic elements among the all four metals tried, as no further growth was observed beyond 25 mg/L. The toxicity of arsenic was due to its chemical similarity to phosphorus, which participates in many cellular reactions. High concentrations of arsenic or arsenites interfere physiological and biochemical processes of the cell by replacing the phosphorus groups of DNAs which are essential for plant growth (Suriyagoda et al. 2018). Irem et al. (2019), while working on Oryza sativa, reported that higher concentration of arsenic reduced the shoot length. Similar observations of showing negative effect of As concentration on shoot length of Brassica juncea have been reported by Ansari et al. (2016). Plants growing under high concentrations of CrO3 exhibited visible symptoms such as necrosis, inhibition of shoot and root length, chlorosis and anatomical disorders (Sinha et al. 2018). This leads to retardation of plant growth and adverse effects on morphogenesis. Park (2020) reported that the addition of Cr (III) in the hydroponic culture reduced the leaf and root growth in Lettuce (Lactuca sativa) plant. Likewise, increasing the concentration of chromium from 0 to 50 mg/kg leads to a decrease in shoot plant height, root dry weight, shoot dry weight, leaf area and number of leaves per plant in Sunflower (Helianthus annuus L.) (Ma et al. 2022). Cobalt and nickel being essential trace elements are required for normal growth and development. Nickel exhibits optimum nodal explant growth at lower concentrations up to 5 mg/L and above that concentration a decline in growth was observed over the control explants. Elevated concentrations of nickel cause nutrient imbalance, stunted growth and chlorosis and alterations of defence mechanism such as synthesis of bioactive compounds, accumulation of proline and MDA and change in SOD, POD and CAT enzyme activities (Gupta et al. 2017). Previous finding also showed that NiCl2 infestation in the culture medium reduced shoot significantly (Zurayk et al. 2002; Maheshwari and Dubey 2009). Morphogenic response of nodal explants significantly changed with manipulation of CoCl2 in the medium (Hand and Reed 2014). In Solanum pennellii, cobalt exhibited no effect on shoot regeneration potential which might be due to mode of regeneration and narrow range of metal concentration used by authors. It is also proved that requirement of micronutrient varies with species to species (Trujillo-Moya and Gisbert 2012). In our observations, the decrease in shoot length and shoot number with increasing concentrations might be because the range of concentrations selected was not according to the plant system. Rajaei and Mohamad (2013), while working on Brassica napus L., reported that 3 mM concentration of CoCl2 reduced the shoot length (3.4 ± 0.2) compared with control (4.8 ± 0.2). CoCl2 blocked the ethylene synthesis by inhibiting the 1-Aminocyclopropane-1-carboxylic acid (ACC) that caused increasing shoot length and decreasing shoot length (Rajaei and Mohamad 2013). Few reports showed that increasing concentrations (10, 50, 100 and 150 mM) of heavy metals (Ni and Co) decreased the rate of shoot and root length, protein and phenolics content and seed germination in Vicia faba (Mahey et al. 2020). Low concentrations of NiCl2 promote the plant growth, but high concentrations inhibit the growth significantly (Hu et al. 2021). Similar finding was also reported in Oryza sativa L. (Girija and Abirami 2022) and Landoltia punctata (Guo et al. 2017).
MDA content
Plasma membrane may be considered as the first alive component that is a target for heavy metal toxicity. The cell membrane may play an important role in ion homeostasis either through efflux mechanism or by selective entry of metal ions into the cell (Verma and Sharma 2017). Heavy metals increase the levels of ‘reactive oxygen species’ (ROS), therefore causing oxidative stress in plants by disrupting normal metabolism of plant (Raza et al. 2022; Naveen et al. 2023). Disintegration of plasma membrane is a consequence of oxidative stress and is manifested by increase in concentration of ion leakage and malondialdehyde (Shankar et al. 2016). In the present study, we observed that there is a dose-dependent increase in lipid peroxidation and ion leakage in the H. pubescens nodal culture when exposed to different concentrations of metals. Damage to the plasma membrane and increase in the level of MDA due to metal stress have been reported for As in wheat (Triticum aestivum L.) (Maghsoudi et al. 2020), Cr in Brassica oleracea L. (Ahmad et al. 2020), NiCl2 in Solanum lycopersicum L. (Altaf et al. 2021) and CoCl2 in wheat grains Triticum aestivum L. (Mohamed and Hassan 2019). The toxic effect on MDA was metal specific and dose dependent. In the present case, the minimum MDA content was reported in As followed by CrO3, NiCl2 and CoCl2. Such damage could result from various mechanisms including the oxidation and cross-linking of protein thiols, inhibition of key membrane proteins such as the H+-ATPase, or changes in the composition and fluidity of membrane lipids (Shankar et al. 2016). Exposure of plant to excess amount of As2O3 generates oxidative stress by increasing the production of ROS in plant (Cortes-Gomez et al. 2018). High chromium concentration also induces ROS and MDA content in maize (Anjum et al. 2017). Ali et al. (2019) reported that oxidative stress damages the micro-molecules (protein, DNA carbohydrates) and disrupts the metabolic pathways.
Proline content
Proline is a major osmolyte in many plant species, and its oxidative stress-induced accumulation represents an important mechanism of response to abiotic stresses (Wang et al. 2018). Accumulation of proline under metal stress has been directly correlated with heavy metal tolerance as reported by Kandziora-Ciupa et al. (2016) in Pinus sylvestris. In the present study, we observed that proline content in the H. pubescens nodal culture increased in a dose-dependent manner or increased with the increasing of concentrations of metals in the growth medium. Proline accumulation in plants exposed to metal stress leads to decrease in water potential. Therefore, proline plays an important role in water balance. Increase in proline concentration during present study may also result either due to degradation or de novo synthesis or both (Singh et al. 2018). Proline accumulation increases the tolerance of plants towards heavy metals through mechanisms, such as protection of enzymes against degradation, denaturation, osmoregulation and stabilization of protein synthesis (Bhagyawant et al. 2019). Accumulation of proline in response to arsenic stress has been reported in Oryza sativa L. by Majumder et al. (2019). Addition of arsenic in the growth media limits the other nutrients uptake which is essential for plant growth and physiological functioning of the plant, which leads to stunted growth. When nodal culture of Holarrhena pubescens was exposed to arsenic stress, visible symptoms of toxicity have been started in the form of decrease in shoot length and reduction in shoot number. Shoot length and shoot number decreased with increasing concentrations of arsenic. Proline and MDA content steadily increased with increasing concentrations of arsenic up to 25 mg/L and above the 25 mg/L concentration of arsenic, browning in tissue occurred and no green tissue was left for analysis of different parameters, such as shot number and shoot length, and proline content. So 25 mg/L is considered as threshold value for lethal effect. The arsenic negatively interferes with the plant metabolism that affects uptake and translocation of various micro- and macronutrients in plant (Samanta and Roychoudhury 2022), which leads to stunted growth. Stunted growth of root and shoot was observed when Cicer arietinum and Oryza sativa seedlings were exposed to increasing concentration of arsenic (Abbas et al. 2018). Similar result was also observed in arsenic-treated Vigna mungo which exhibits marked enhancement in MDA, proline and antioxidant enzyme activity and reduction of shoot length (Srivastava and Sharma 2013). Similarly, increase in the level of proline and reduction in shoot length with increasing concentrations of arsenic were also reported in different plant species (Pandey and Gupta 2015; Panthri and Gupta 2019). Similarly, proline accumulation has also been increased in Glycine max (Ganesh et al. 2009), Landoltia punctata (Guo et al. 2017) and Brassica juncea (Thakur and Sharma 2016) with the increasing concentration of CrO3, CoCl2 and NiCl2, respectively.
Antioxidant enzyme assays
Excessive amount of the heavy metals causes the overproduction of reactive oxygen species (ROS Al Mahmud et al. 2019), which increases the lipid peroxidation and the damage to DNA, protein and carbohydrates (Abbas et al. 2018). Plants have well-developed defence system consisting of enzymatic and non-enzymatic antioxidant to minimize the negative effect of metals. These antioxidants play a major role in the cellular defence strategy against oxidative stress (Nanda and Agrawal 2016, 2018), inducing resistance to metals by protecting labile macromolecules (Kandziora-Ciupa et al. 2016). The specific activities of SOD, CAT, APX and GR increased significantly with the increasing concentrations of metals (As2O3, CrO3, CoCl2 and NiCl2) in the nodal culture of H. pubescens. It can be concluded that activation of theses enzymes may be contributed to minimize the toxic effect of ROS induced by metal stress (Kumar et al. 2017). Superoxide dismutase (SOD) is a most effective intracellular enzyme present in all sub-cellular organs like mitochondria, chloroplast and peroxisomes. It is considered first line of defence against oxidative stress or elevated level of ROS (Juybari et al. 2018). By dismutation reaction, SOD reduced O2· to H2O2 and other oxidized to O2 by Haber–Weiss reaction (Abbas et al. 2018). Increase in SOD activity is positively related to superoxide radical concentration to maintain study state level (Shankar et al. 2016). SOD activity increased in response to different metals (Cd, Cr, Cu, Fe, Mn, Ni, Pb, and Zn) in Alternanthera sessilis and Ipomoea aquatica as reported by Mazumdar and Das (2021). Similar to above study, increase in SOD activity under Fe, Cu, Ni, Co, Zn and Cr metal stress has also been reported in Marigold (Calendula officinalis L.) by Varshney et al. (2023). SOD activity decreases at higher level of metals, which may be due to either by binding of metals to active site of enzymes or by inactivation of enzymes by H2O2. Catalase is an important enzyme in peroxisomes and chloroplast which decomposes H2O2 to water and oxygen. Increase in CAT activity is related to high level of substrate or to maintain the level of H2O2 as adaptive mechanism of plants (Hasanuzzaman et al. 2018). In our experiments, CAT activity increased with the increasing concentration of metals up to certain level after that decline trend was observed. The decline in CAT activity might be due to degradation of enzymes or inactivation of enzyme by ROS or decrease in synthesis of enzyme (Kurek et al. 2019). APX has higher affinity towards the H2O2 as compared to CAT and plays an essential role in ROS scavenging during metal stress. APX reduces the H2O2 to water and oxygen using ascorbate. GR is a flavoprotein oxidoreductase responsible for maintaining high-level antioxidant molecule, glutathione (Abbas et al. 2018). Glutathione reductase (GR) plays an important role in plant defence system against ROS by maintaining the reduced level of GSH (Hasanuzzaman et al. 2018). GSSG produced during the reaction converted back to GSH by GR enzyme.
Conessine content
Generally, heavy metal stress stimulates the production of reactive oxygen species (ROS) in medicinal plants. ROS trigger to the production of highly active signalling molecules that induce the formation of secondary metabolites, proline, alkaloids, phenolics, sugar and polyamines (Jothimani et al. 2017). Some researchers reported that the heavy metals play an important role in the induction of secondary metabolites genes (Kumar et al. 2017). However, the exact mechanism by which metals stimulate the secondary metabolites production still remains unclear. During our study, of the different heavy metals tried such as NiCl2, CoCl2, CrO3 and As2O3, optimum enhancement in conessine content 297.1 ± 7.76 µg/g d. w. was achieved at 100 mg/L CoCl2 compared to control (117.06 ± 2.59 µg/g d. w.). Similar to our finding, Saad-Allah and Elhaak (2017) reported the positive correlation between alkaloid content and heavy metal (Cr, Pb, Fe, Al, and Zn) concentrations in Solanum nigrum plant. Ahmad and Misra (2014), while working on Catharanthus roseus, reported that alkaloids content increased with the increasing concentration of Chromium. High levels of nickel enhanced the production of alkaloids in Catharanthus roseus (ArefiFard 2017). The present study also suggests that different heavy metals increased the concentration of conessine in H. pubescens, which might be due to upregulation of genes involved in conessine synthesis or oxidative burst.
Conclusion
The present study was undertaken with a view to understand and assess the effect of heavy metals such as NiCl2, CoCl2, As2O3 and CrO3 on in vitro regeneration of Holarrhena pubescens. The lower concentrations of NiCl2 and CrO3 enhanced the morphogenetic response and their higher concentrations inhibited the response in terms of plant growth and number of shoot per explants. Higher concentrations of CoCl2, and As2O3 inhibited the plant growth by disrupting the physiological process including photosynthesis, water and nutrient uptake, respiration and biochemical process such as ROS production, antioxidants, enzymes, proteins and secondary metabolites accumulations. Increasing the antioxidant enzyme activity, MDA and proline content were directly correlated to maintaining osmotic as well as nutrient balance by decreasing membrane damage and ROS production. Hence, enhanced levels of antioxidant enzymes (SOD, CAT, APX and GR), MDA and proline will enhance the tolerance capacity of plants towards metal stress.
Acknowledgements
The authors are grateful to the University Grants Commission (New Delhi, India) and University of Delhi, India for their financial assistance. DK is indebted to Indian Council of Medical Research and India for awarding Research Associate fellowship, Dyal Singh College, Delhi University and Director of ICMR-National Institute of Malaria Research, Dwarka Sector 8, for providing necessary infrastructure and support. BS is indebted to CSIR for awarding JRF research fellowship.
Author contributions
All the authors have equally contributed in designing, writing, obtaining and analysing the data in this manuscript. All the authors read and approved the manuscript.
Funding
Not applicable.
Data availability
All data are available upon request.
Declarations
Conflict of interest
The authors declare no competing interests.
Ethical approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
References
- Abbas G, Murtaza B, Bibi I, Shahid M, Niazi NK, Khan MI, Amjad M, Hussain M. Arsenic uptake, toxicity, detoxification, and speciation in plants: physiological, biochemical, and molecular aspects. Int J Environ Res Public Health. 2018;15:59. doi: 10.3390/ijerph15010059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aebi H. Catalase in vitro. Methods Enzymol. 1984;105:121–126. doi: 10.1016/S0076-6879(84)05016-3. [DOI] [PubMed] [Google Scholar]
- Agrawal V, Sharma K. Phytotoxic effects of Cu, Zn, Cd and Pb on in vitro regeneration and concomitant protein changes in Holarrhena antidysenterica. Biol Plant. 2006;50:307–310. doi: 10.1007/s10535-006-0027-z. [DOI] [Google Scholar]
- Ahmad R, Misra N (2014) Evaluation of phytoremediation potential of Catharanthus roseus with respect to chromium contamination. Am J Plant Sci 5:2378–2388
- Ahmad R, Ali S, Abid M, Rizwan M, Ali B, Tanveer A, Ahmad I, Azam M, Ghani MA. Glycine betaine alleviates the chromium toxicity in Brassica oleracea L. by suppressing oxidative stress and modulating the plant morphology and photosynthetic attributes. Environ Sci Pollut Res. 2020;27:1101–1111. doi: 10.1007/s11356-019-06761-z. [DOI] [PubMed] [Google Scholar]
- Akoto R, Anning AK, Belford EJD. Effects of ethylenediaminetetraacetic acid-assisted phytoremediation on soil physicochemical and biological properties. Int J Environ Sci Technol. 2022;19:8995–9010. doi: 10.1007/s13762-021-03770-9. [DOI] [Google Scholar]
- Al Mahmud J, Bhuyan MHMB, Anee TI, Nahar K, Fujita M, Hasanuzzaman M (2019) Reactive oxygen species metabolism and antioxidant defense in plants under metal/metalloid stress. In: Hasanuzzaman M, Hakeem K, Nahar K, Alharby H (eds) Plant abiotic stress tolerance. Springer, US, Cham 221–257. 10.1007/978-3-030-06118-0_10
- Alengebawy A, Abdelkhalek ST, Qureshi SR, Wang MQ. Heavy metals and pesticides toxicity in agricultural soil and plants: ecological risks and human health implications. Toxics. 2021;9:42. doi: 10.3390/toxics9030042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ali MA, Fahad S, Haider I, Ahmed N, Ahmad S, Hussain S, Arshad M (2019) Oxidative stress and antioxidant defense in plants exposed to metal/metalloid toxicity. In: Hasanuzzaman M, Fotopoulos V, Nahar K, Fujita M (eds) Reactive oxygen, nitrogen and sulfur species in plants: production, metabolism, signaling and defense mechanisms. John Wiley & Sons Ltd US, pp 353–370
- Altaf MA, Shahid R, Ren MX, Altaf MM, Jahan MS, Khan LU. Melatonin mitigates nickel toxicity by improving nutrient uptake fluxes, root architecture system, photosynthesis, and antioxidant potential in tomato seedling. J Soil Sci Plant Nutr. 2021;21:1842–1855. doi: 10.1007/s42729-021-00484-2. [DOI] [Google Scholar]
- Anjum SA, Ashraf U, Imran KH, Tanveer M, Shahid M, Shakoor A, Longchang WA. Phyto-toxicity of chromium in maize: oxidative damage, osmolyte accumulation, anti-oxidative defense and chromium uptake. Pedosphere. 2017;27:262–273. doi: 10.1016/S1002-0160(17)60315-1. [DOI] [Google Scholar]
- Ansari MKA, Zia MH, Ahmad A, Aref IM, Fatma T, Iqbal M, Owens G. Status of antioxidant defense system for detoxification of arsenic in Brassica juncea (L.) Ecoprint: Int J Ecol. 2016;22:7–19. doi: 10.3126/eco.v22i0.15466. [DOI] [Google Scholar]
- ArefiFard M (2017) The study of the effect of nickel heavy metal on some growth parameters and production of alkaloids in catharanthus roseus. In: Naeem M, Aftab T, Khan M (eds) Catharanthus roseus. Springer, Cham, Switzerland, pp 399–412. 10.1007/978-3-319-51620-2_18
- Bates LS, Waldren RP, Teare ID. Rapid determination of free proline for water stress studies. Plant Soil. 1973;39:205–207. doi: 10.1007/BF00018060. [DOI] [Google Scholar]
- Beyer Jr WF, Fridovich I (1987) Assaying for Superoxide dismutase activity: Some large consequences of minor changes in conditions. Anal Biochem 161:559–566 [DOI] [PubMed]
- Bhagyawant SS, Narvekar DT, Gupta N, Bhadkaria A, Koul KK, Srivastava N. Variations in the antioxidant and free radical scavenging under induced heavy metal stress expressed as proline content in chickpea. Physiol Mol Biol Plants. 2019;25:683–696. doi: 10.1007/s12298-019-00667-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford MM. A rapid and sensitive method for the quantization of microgram quantities of protein utilizing the principle of the protein dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Chen D, Shao Q, Yin L, Younis A, Zheng B. Polyamine function in plants: metabolism, regulation on development, and roles in abiotic stress responses. Front Plant Sci. 2019;9:1945. doi: 10.3389/fpls.2018.01945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chowdhary P, Bharagava RN, Mishra S, Khan N (2020) Role of industries in water scarcity and its adverse effects on environment and human health. In: Shukla V, Kumar N (eds) Environmental concerns and sustainable development. Springer, Singapore, vol 1, pp 235–256. 10.1007/978-981-13-5889-0_12
- Connell JP, Mullet JE (1986) Pea chloroplast glutathione reductase: purification and characterization. Plant Physiol 82:351–356 [DOI] [PMC free article] [PubMed]
- Cortes-Gomez AA, Morcillo P, Guardiola FA, Espinosa C, Esteban MA, Cuesta A, Girondot M, Romero D. Molecular oxidative stress markers in olive ridley turtles (Lepidochelys olivacea) and their relation to metal concentrations in wild populations. Environ Pollut. 2018;233:156–167. doi: 10.1016/j.envpol.2017.10.046. [DOI] [PubMed] [Google Scholar]
- Elshafie HS, Camele I, Mohamed AA. A Comprehensive review on the biological, agricultural and pharmaceutical properties of secondary metabolites based-plant origin. Int J Mol Sci. 2023;24:3266. doi: 10.3390/ijms24043266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganesh SK, Baskaran AL, Chidambaram A, Sundaramoorthy P. Influence of chromium stress on proline accumulation in soybean (Glycine max L. Merr.) genotypes. Glob J Environ Res. 2009;3:106–108. [Google Scholar]
- Gill R, Boscaiu M, Lull C, Bautista I, Lidon A, Vicente O. Are soluble carbohydrates ecologically relevant for salt tolerance in halophytes? Funct Plant Biol. 2013;40:805–818. doi: 10.1071/FP12359. [DOI] [PubMed] [Google Scholar]
- Girija D, Abirami K. Evaluation of seed germination and early seedling growth under heavy metals stress conditions in coastal red rice (Oryza sativa L.) crop. J Stress Physiol Biochem. 2022;18:17–31. [Google Scholar]
- Guo L, Ding Y, Xu Y, Li Z, Jin Y, He K, Fang Y, Zhao H. Responses of Landoltia punctata to cobalt and nickel: removal, growth, photosynthesis, antioxidant system and starch metabolism. Aquat Toxicol. 2017;190:87–93. doi: 10.1016/j.aquatox.2017.06.024. [DOI] [PubMed] [Google Scholar]
- Gupta V, Jatav PK, Verma R, Kothari SL, Kachhwaha S. Nickel accumulation and its effect on growth, physiological and biochemical parameters in millets and oats. Environ Sci Pollut Res. 2017;24:23915–23925. doi: 10.1007/s11356-017-0057-4. [DOI] [PubMed] [Google Scholar]
- Hand C, Reed BM. Minor nutrients are critical for the improved growth of Corylus avellana shoot cultures. Plant Cell Tissue Organ Cult. 2014;119:427–439. doi: 10.1007/s11240-014-0545-x. [DOI] [Google Scholar]
- Hasanuzzaman M, Nahar K, Anee TI, Khan MIR, Fujita M. Silicon-mediated regulation of antioxidant defense and glyoxalase systems confers drought stress tolerance in Brassica napus L. S Afr J Bot. 2018;115:50–57. doi: 10.1016/j.sajb.2017.12.006. [DOI] [Google Scholar]
- Hodges DM, Andrews CJ, Johnson DA, Hamilton RI (1997) Antioxidant enzyme responses to chilling stress in differentially sensitive inbred maize lines. J Exp Bot 48:1105–1113
- Hodges DM, DeLong JM, Forney CF, Prange RK. Improving the thiobarbituric acid-reactive-substances assay for estimating lipid peroxidation in plant tissues containing anthocyanin and other interfering compounds. Planta. 1999;207:604–611. doi: 10.1007/s004250050524. [DOI] [PubMed] [Google Scholar]
- Hu X, Wei X, Ling J, Chen J. Cobalt: an essential micronutrient for plant growth? Front Plant Sci. 2021 doi: 10.3389/fpls.2021.768523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irem S, Islam E, Maathuis FJ, Niazi NK, Li T. Assessment of potential dietary toxicity and arsenic accumulation in two contrasting rice genotypes: effect of soil amendments. Chemosphere. 2019;225:104–114. doi: 10.1016/j.chemosphere.2019.02.202. [DOI] [PubMed] [Google Scholar]
- Jothimani K, Arulbalachandran D, Yasmin K (2017) Amelioration of environmental stress for sustainable crop productivity. In: Dhanarajan A (ed) Sustainable agriculture towards food security. Springer, Singapore, pp 327–348. 10.1007/978-981-10-6647-4_17
- Juybari KB, Ebrahimi G, Moghaddam MAM, Asadikaram G, Torkzadeh-Mahani M, Akbari M, Mirzamohammadi S, Karimi A, Nematollahi MH. Evaluation of serum arsenic and its effects on antioxidant alterations in relapsing-remitting multiple sclerosis patients. Mult Scler Relat Disord. 2018;19:79–84. doi: 10.1016/j.msard.2017.11.010. [DOI] [PubMed] [Google Scholar]
- Kandziora-Ciupa M, Ciepał R, Nadgorska-Socha A, Barczyk G. Accumulation of heavy metals and antioxidant responses in Pinus sylvestris L. needles in polluted and non-polluted sites. Ecotoxicology. 2016;25:970–981. doi: 10.1007/s10646-016-1654-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar R, Sharma K, Agrawal V. In vitro clonal propagation of Holarrhena antidysenterica (L.) Wall. through nodal explants from mature trees. In Vitro Cell Dev Biol Plant. 2005;41:137–144. doi: 10.1079/IVP2004624. [DOI] [Google Scholar]
- Kumar D, Al Hassan M, Naranjo MA, Agrawal V, Boscaiu M, Vicente O (2017) Effects of salinity and drought on growth, ionic relations, compatible solutes and activation of antioxidant systems in oleander (Nerium oleander L.). PLoS ONE 12:9. p.e0185017 [DOI] [PMC free article] [PubMed]
- Kumar D, Kumar G, Agrawal V. Green synthesis of silver nanoparticles using Holarrhena antidysenterica (L.) Wall. bark extract and their larvicidal activity against dengue and filariasis vectors. Parasitol Res. 2018;117:377–389. doi: 10.1007/s00436-017-5711-8. [DOI] [PubMed] [Google Scholar]
- Kumar D, Kumar G, Das R, Kumar R, Agrawal V. In vitro elicitation, isolation, and characterization of conessine biomolecule from Holarrhena antidysenterica (L.) Wall. callus and its larvicidal activity against malaria vector, Anopheles stephensi Liston. Environ Sci Pollut Res. 2018;25:6783–6796. doi: 10.1007/s11356-017-1038-3. [DOI] [PubMed] [Google Scholar]
- Kurek K, Plitta-Michalak B, Ratajczak E. Reactive oxygen species as potential drivers of the seed aging process. Plants. 2019;8:174. doi: 10.3390/plants8060174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma J, Alshaya H, Okla MK, Alwasel YA, Chen F, Adrees M, Hussain A, Hameed S, Shahid MJ (2022) Application of cerium dioxide nanoparticles and chromium-resistant bacteria reduced chromium toxicity in sunflower plants. Front Plant Sci 13:876119 [DOI] [PMC free article] [PubMed]
- Maghsoudi K, Arvin MJ, Ashraf M. Mitigation of arsenic toxicity in wheat by the exogenously applied salicylic acid, 24-epi-brassinolide and silicon. J Soil Sci Plant Nutr. 2020;20:577–588. doi: 10.1007/s42729-019-00147-3. [DOI] [Google Scholar]
- Maheshwari R, Dubey R. Nickel-induced oxidative stress and the role of antioxidant defence in rice seedlings. Plant Growth Regul. 2009;59:37–49. doi: 10.1007/s10725-009-9386-8. [DOI] [Google Scholar]
- Mahey S, Kumar R, Sharma M, Kumar V, Bhardwaj R. A critical review on toxicity of cobalt and its bioremediation strategies. SN Appl Sci. 2020;2:1–12. doi: 10.1007/s42452-020-3020-9. [DOI] [Google Scholar]
- Majumder B, Das S, Mukhopadhyay S, Biswas AK. Identification of arsenic-tolerant and arsenic-sensitive rice (Oryza sativa L.) cultivars on the basis of arsenic accumulation assisted stress perception, morpho-biochemical responses, and alteration in genomic template stability. Protoplasma. 2019;256:193–211. doi: 10.1007/s00709-018-1290-5. [DOI] [PubMed] [Google Scholar]
- Mazumdar K, Das S. Phytoremediation of soil treated with metalliferous leachate from an abandoned industrial site by Alternanthera sessilis and Ipomoea aquatica: metal extraction and biochemical responses. Ecol Eng. 2021;170:106349. doi: 10.1016/j.ecoleng.2021.106349. [DOI] [Google Scholar]
- Mohamed HE, Hassan AM (2019) Role of salicylic acid in alleviating cobalt toxicity in wheat (Triticum aestivum L.) seedlings. J Agric Sci 11(10):112
- Murashige T, Skoog F. A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol Plant. 1962;15:473–497. doi: 10.1111/j.1399-3054.1962.tb08052.x. [DOI] [Google Scholar]
- Nakano Y, Asada K. Hydrogen peroxide is scavenged by ascorbate-specific peroxidase in spinach chloroplasts. Plant Cell Physiol. 1981;22:867–880. [Google Scholar]
- Nanda R, Agrawal V. Piriformospora indica, an excellent system for heavy metal sequestration and amelioration of oxidative stress and DNA damage in Cassia angustifolia Vahl under copper stress. Ecotoxicol Environ Saf. 2018;156:409–419. doi: 10.1016/j.ecoenv.2018.03.016. [DOI] [PubMed] [Google Scholar]
- Naveen J, Hithamani G, Pushpalatha HG (2023) Stress and its influence on the generation of reactive oxygen species and oxidative damage in plants. In: Desai NH, Patil, M, Pawar UR (eds) Mechanisms, responses, and adaptation strategies. Apple Academic Press, New York. pp 219–248. 10.1201/9781003304869
- Pandey C, Gupta M (2015) Selenium and auxin mitigates arsenic stress in rice (Oryza sativa L.) by combining the role of stress indicators, modulators and genotoxicity assay. J Hazard Mater 287:384–391 [DOI] [PubMed]
- Panthri M, Gupta M. Facets of iron in arsenic exposed Oryza sativa varieties: a manifestation of plant’s adjustment at morpho-biochemical and enzymatic levels. Environ Pollut. 2019;255:113289. doi: 10.1016/j.envpol.2019.113289. [DOI] [PubMed] [Google Scholar]
- Park JH. Contrasting effects of Cr(III) and Cr(VI) on lettuce grown in hydroponics and soil: chromium and manganese speciation. Environ Pollut. 2020;266:115073. doi: 10.1016/j.envpol.2020.115073. [DOI] [PubMed] [Google Scholar]
- Pishchik V, Mirskaya G, Chizhevskaya E, Chebotar V, Chakrabarty D. Nickel stress-tolerance in plant-bacterial associations. Peer J. 2021 doi: 10.7717/peerj.12230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porokhovinova EA, Shelenga TV, Kerv YA, Khoreva VI, Konarev AV, Yakusheva TV, Pavlov AV, Slobodkina AA, Brutch NB. Features of profiles of biologically active compounds of primary and secondary metabolism of lines from VIR flax genetic collection, contrasting in size and color of seeds. Plants. 2022;11:750. doi: 10.3390/plants11060750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajaei P, Mohamad N. Effect of beta aminobutyric acid (BABA), ABA and ethylene synthesis inhibitor (CoCl2) on seed germination and seedling growth of Brassica napus L. Eur J Exp Biol. 2013;3:437–440. [Google Scholar]
- Nanda R, Agrawal V. Elucidation of zinc and copper induced oxidative stress, DNA damage and activation of defence system during seed germination in Cassia angustifolia Vahl. Environ Exp Bot. 2016;125:31–41. doi: 10.1016/j.envexpbot.2016.02.001. [DOI] [Google Scholar]
- Rajput S, Agrawal V. Micropropagation of Atropa acuminata Royle ex Lindl. (a critically endangered medicinal herb) through root callus and evaluation of genetic fidelity, enzymatic and non-enzymatic antioxidant activity of regenerants. Acta Physiol Plant. 2020;42:160. doi: 10.1007/s11738-020-03145-6. [DOI] [Google Scholar]
- Raza A, Salehi H, Rahman MA, Zahid Z, Madadkar Haghjou M, Najafi-Kakavand S, Charagh S, Osman HS, Albaqami M, Zhuang Y, Siddique KH (2022) Plant hormones and neurotransmitter interactions mediate antioxidant defenses under induced oxidative stress in plants. Front Plant Sci 13:961872. 10.3389/fpls.2022.961872 [DOI] [PMC free article] [PubMed]
- Saad-Allah KM, Elhaak MA. Hyperaccumulation activity and metabolic responses of Solanum nigrum in two differentially polluted growth habitats. J Saudi Soc Agric Sci. 2017;16:227–235. [Google Scholar]
- Samanta S, Roychoudhury A (2022) Arsenic stress and mineral nutrition in plants. In: Kumar V, Srivastava AK, Suprasanna P (eds) Plant nutrition and food security in the era of climate change. Elsevier, Academic Press, Cambridge. 10.1016/B978-0-12-822916-3.00002-0, pp 361–375
- Shankar V, Kumar D, Agrawal V. Assessment of antioxidant enzyme activity and mineral nutrients in response to NaCl stress and its amelioration through glutathione in Chickpea. Appl Biochem Biotechnol. 2016;178:267–284. doi: 10.1007/s12010-015-1870-1. [DOI] [PubMed] [Google Scholar]
- Singh A, Sengar K, Sharma MK, Sengar RS, Garg SK (2018) Proline metabolism as sensor of abiotic stress in sugarcane. In: Sengar K (eds) Biotechnology to enhance sugarcane productivity and stress tolerance. CRS press, US, pp 281–300. 10.1201/9781315152776-12
- Sinha V, Pakshirajan K, Chaturvedi R. Chromium tolerance, bioaccumulation and localization in plants: an overview. J Environ Manag. 2018;206:715–730. doi: 10.1016/j.jenvman.2017.10.033. [DOI] [PubMed] [Google Scholar]
- Srivastava S, Sharma YK (2013) Impact of arsenic toxicity on black gram and its amelioration using phosphate. ISRN Toxicol. V. 2013, 34092. 10.1155/2013/340925 [DOI] [PMC free article] [PubMed]
- Suriyagoda LD, Dittert K, Lambers H. Mechanism of arsenic uptake, translocation and plant resistance to accumulate arsenic in rice grains. Agric Ecosyst Environ. 2018;253:23–37. doi: 10.1016/j.agee.2017.10.017. [DOI] [Google Scholar]
- Tanveer Y, Yasmin H, Nosheen A, Ali S, Ahmad A. Ameliorative effects of plant growth promoting bacteria, zinc oxide nanoparticles and oxalic acid on Luffa acutangula grown on arsenic enriched soil. Environ Pollut. 2022;300:118889. doi: 10.1016/j.envpol.2022.118889. [DOI] [PubMed] [Google Scholar]
- Thakur S, Sharma SS. Characterization of seed germination, seedling growth, and associated metabolic responses of Brassica juncea L. cultivars to elevated nickel concentrations. Protoplasma. 2016;253:571–580. doi: 10.1007/s00709-015-0835-0. [DOI] [PubMed] [Google Scholar]
- Trujillo-Moya C, Gisbert C. The influence of ethylene and ethylene modulators on shoot organogenesis in tomato. Plant Cell Tissue Organ Cult. 2012;111:41–48. doi: 10.1007/s11240-012-0168-z. [DOI] [Google Scholar]
- Varshney A, Dahiya P, Mohan S. Antioxidant activity of pot marigold (Calendula officinalis L.) in response to metal (loid) induced oxidative stress from fly ash amended soil. J Plant Growth Regul. 2023;30:1–7. [Google Scholar]
- Verma N, Sharma R. Bioremediation of toxic heavy metals: a patent review. Recent Pat Biotechnol. 2017;11:171–187. doi: 10.2174/1872208311666170111111631. [DOI] [PubMed] [Google Scholar]
- Wang R, Mei Y, Xu L, Zhu X, Wang Y, Guo J, Liu L. Genome-wide characterization of differentially expressed genes provides insights into regulatory network of heat stress response in radish (Raphanus sativus L.) Funct Integr Genom. 2018;18:225–239. doi: 10.1007/s10142-017-0587-3. [DOI] [PubMed] [Google Scholar]
- Xia Y, Zhang M, Tsang DC, Geng N, Lu D, Zhu L, Igalavithana AD, Dissanayake PD, Rinklebe J, Yang X, Ok YS. Recent advances in control technologies for non-point source pollution with nitrogen and phosphorous from agricultural runoff: current practices and future prospects. Appl Biol Chem. 2020;63:1–13. doi: 10.1186/s13765-020-0493-6. [DOI] [Google Scholar]
- Zahara K, Panda SK, Swain SS, Luyten W. Metabolic diversity and therapeutic potential of Holarrhena pubescens: an important ethnomedicinal plant. Biomolecules. 2020;10:1341. doi: 10.3390/biom10091341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Q, Wang C. Natural and human factors affect the distribution of soil heavy metal pollution: a review. Water Air Soil Pollut. 2020;231:1–3. doi: 10.1007/s11270-020-04728-2. [DOI] [Google Scholar]
- Zurayk R, Sukkariyah B, Baalbaki R, Ghanem DA. Ni phytoaccumulation in Mentha aquatica L. and Mentha sylvestris L. Water Air Soil Pollut. 2002;139:355–364. doi: 10.1023/A:1015840601761. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data are available upon request.


