Abstract
Angiosarcomas (ASs) are very rare and constitute 1–2% of soft tissue malignancies. Primary pleural AS (PPAS) is a very rare neoplasm, with only 50 cases reported in the literatüre, and is a tumor with a high tendency for local recurrence and metastasis, with an aggressive course and a generally poor prognosis unless diagnosed early. It originates from the endothelial cells of small blood vessels and therefore can affect many organs. The etiology and definitive method in the treatment is still unclear. Patients usually present with nonspecific symptoms such as cough, dyspnea, chest pain, and hemoptysis. Recurrent exudative or hemorrhagic pleural effusion may develop due to its pleural location. The diagnosis can be made by histopathological and immunohistochemical examinations of excisional biopsy specimens. The effectiveness of chemotherapy and radiotherapy is weak and can be applied for palliative purposes. Surgical approach can be used for diagnostic and palliative purposes. Due to the high degree of malignancy and insidious course of PPAS, patients usually die within months after diagnosis. In these patients, surgical exploration is important for the diagnosis and palliative/definitive treatment of the disease. We present a 61-year-old male patient who presented with dyspnea, chest pain, and massive pleural effusion findings in the left hemithorax and was diagnosed with PPAS as a result of pleural biopsy.
Keywords: Primary pleural angiosarcoma, Spontaneous hemothorax, Pleural nodules
Introduction
Soft tissue tumors are malignancies seen in muscle, nerve, connective, skin, and subcutaneous tissues. While they constitute approximately 6.6% of all malignancies in childhood, this rate is 1% in the general population [1]. Angiosarcomas (ASs) are very rare and constitute 1–2% of soft tissue malignancies. They originate from the endothelial cells of small blood vessels and therefore can affect many organs. Primary pleural AS (PPAS) is a tumor with a high tendency for local recurrence and metastasis, with an aggressive course and a generally poor prognosis unless diagnosed early [2]. We present a case who presented with dyspnea, chest pain, and massive pleural effusion findings in the left hemithorax and was diagnosed with PPAS as a result of pleural biopsy.
Case report
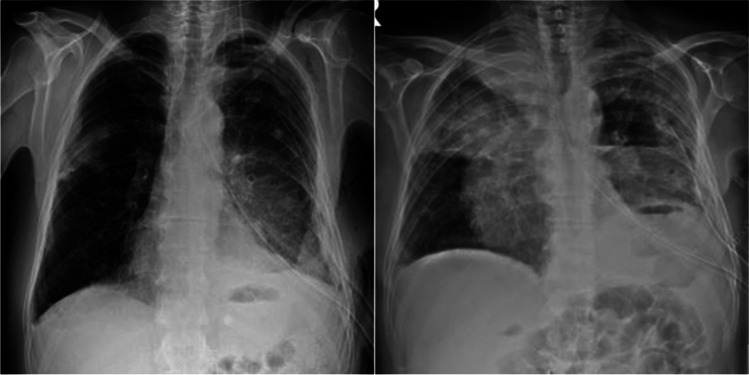
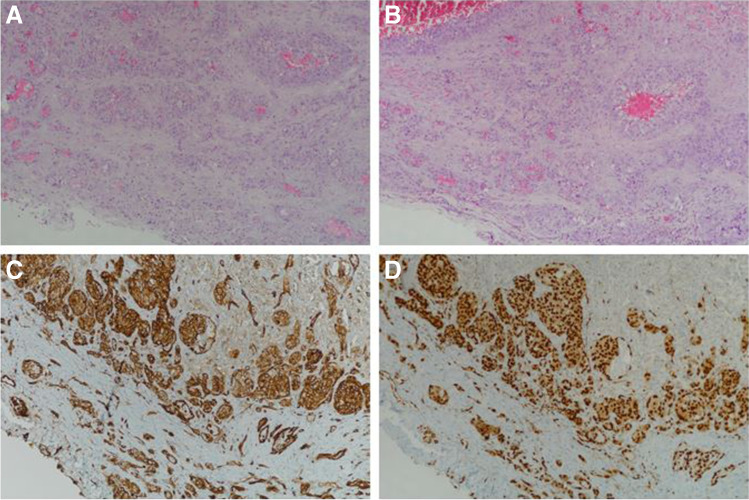
A 61-year-old male patient presented with dyspnea and chest pain. On physical examination, respiratory sounds were decreased in the lower zone of the left hemithorax. Except for a 30 pack/year smoking history, he had no history of environmental or occupational exposure or drug use. In his biochemical tests, hemoglobin (hg) was 7 g/dL and hematocrit (hct) was 20.6. Thoracic computered tomography reported massive pleural effusion on the left side and bilateral pleural nodules, more prominent on the right side. Biochemical examination of the pleural fluid was unremarkable, and 1000 ml of serohemorrhagic fluid drainage was observed daily from the pleurocan catheter applied for the left hemithorax. For discerning the etiology of the pleural effusion, pleural biopsy was performed via video-assisted thoracic surgery. In the perioperative exploration, widespread brown-black-colored foci in the left parietal pleura and parenchyma areas of the lung and 2000 ml of free hemorrhagic fluid originating from these foci were observed. After sampling from the lesions that were observed in the pleura and parenchyma, all bleeding foci were cauterized. Multiple blood and blood product replacement were required as hemorrhagic drainage, and hg-hct decrease continued in the postoperative intensive care follow-up. On the 4th postoperative day, due to an increase in hemorrhagic fluid drainage, the patient was operated again. Exploration with thoracotomy revealed bleeding in the form of leakage from parietal pleural lesions as well as intrathoracic free blood. Total pleurectomy was applied to the case because it was not possible to control bleeding from all surfaces. The patient was operated for the third time due to the continuation of hemorrhagic fluid drainage in the postoperative follow-up. In the exploration, bleeding foci in the form of leakage were detected from all intrathoracic surfaces, and especially from residual tumoral areas on the diaphragmatic surface. Bleeding foci were tried to be controlled with cauterization, talc, and fibrin-containing anti-bleeding solutions. Radiopacity area that started in the right hemithorax middle zone and progressed in the follow-up was noted in the posteroanterior chest X-ray of the patient with decreased hemorrhagic drainage after the operation (Fig. 1). It was thought that this appearance might be due to hemorrhagic foci originating from nodular areas in the right parietal pleura. Chemotherapy could not be planned because the pathological diagnosis could not be made and radiotherapy could not be planned because the disease was widespread on all pleural surfaces. Despite the replacement of 84 units of blood and blood products in total, the patient, whose general condition deteriorated and hypercapnia developed in arterial blood gas, was taken to the intensive care unit and followed up with mechanical ventilation. The patient remained hypotensive despite inotropic support and hypercapnic state did not regress in arterial blood gas, and died after 2 days of intensive care follow-up. Histopathological examination revealed a tumor characterized by solid islands formed by atypical cells with epithelioid appearance on the hemorrhagic background. Immunohistochemical examination revealed membranous positivity with CD31 and nuclear positivity with ETS (specific for erythroblast transformation)-related gene, and the diagnosis of angiosarcoma was reached in the light of these findings (Fig. 2A–D).
Fig. 1.
Radiopacity area that starts in the middle zone of the right hemithorax on the chest X-ray and progresses rapidly in the follow-up (the time between two chest X-rays is 11 days)
Fig. 2.
A, B Tumor characterized by solid islands formed by atypical cells with epithelioid appearance on a hemorrhagic background. C Membrane positivity with CD31 in immunohistochemical examination. D Nuclear positivity with ERG
Discussion
PPAS is a very rare neoplasm and only 50 cases have been reported in the literature [3]. Average age of reported cases is 55 years, with the male/female ratio of 15/4. Although the etiology is still unclear, factors such as tuberculosis, pyothorax, lymphedema, viral infections, radiation therapy, and asbestos are thought to predispose to the disease. Patients usually present with nonspecific symptoms such as cough, dyspnea, chest pain, and hemoptysis. Recurrent exudative or hemorrhagic pleural effusion may develop due to its pleural location. Non-specific findings such as pleural thickening, mass, or pleural effusion may be observed on chest radiographs. Computered tomography can show pleural effusion, thickening, mass, nodularity, and pulmonary or hilar/mediastinal lymph node metastases [4]. Radiographically, unilateral pleural effusion and bilateral pleural nodules were observed in our patient who presented with dyspnea and chest pain and anemia was found in biochemical tests. Positron emission tomography could not be done because the general condition of the patient was not suitable.
Since pleural fluid cytological examinations are generally insufficient for diagnosis, the diagnosis can be made by histopathological and immunohistochemical examinations of excisional biopsy specimens. Histopathological examination of the biopsy specimens of the case who underwent pleural biopsy and total pleurectomy resulted in the diagnosis of AS. The patient was diagnosed with PPAS after radiological absence of any other lesion and exclusion of other malignancies.
AS can be morphologically confused with other pleural tumors such as metastatic adenocarcinoma or mesothelioma, but vascular markers are negative in these two tumors [5]. PPASs are histologically seen in two different types, classical and epithelioid type [6]. Cytokeratin positivity in immunohistochemical examinations is indicative of epithelioid type AS, as in our case. Although epithelioid AS is mostly associated with radiotherapy, our case did not have a history of radiotherapy. The histopathological examination of the nodule detected in the lung parenchyma and removed by wedge resection as AS supports the lung metastasis of PPAS.
A definitive method for the treatment of PPAS has not yet been determined. In AS cases presenting as massive hemothorax, pleural or subpleural parenchymal tumor invasion may cause continuous bleeding. Therefore, it is recommended to treat cases as massive hemothorax in the first place. Apart from this, early diagnosis and treatment are very important because PPAS is a high-grade malignancy. Surgery is indicated for bleeding control and reduction of tumor volume by pleurectomy, including pneumonectomy/rib excision, in patients with localized lesions and/or presenting with hemothorax. Radiotherapy can be applied as adjuvant therapy in cases without extensive disease and/or incomplete resection with surgery [7]. Although it has been reported recently that response can be obtained with ifosfamide, the effectiveness of chemotherapy is weak and it can be applied for palliative purposes. Due to the massive hemothorax presenting in our case, surgery was performed for bleeding control and diagnostic purposes, and the subsequent operations were performed due to the persistence of massive hemorrhage, when total pleurectomy and almost complete resection were performed. However, radiotherapy was not applied because of the widespread disease, similar radiological symptoms in the contralateral hemithorax, and the possibility of metastatic disease. Chemotherapy was not considered because the general condition of the patient was not in a position to handle the chemotherapy, even if it was palliative.
Conclusion
PPAS is an aggressive malignant neoplasm with a very rapid and fatal clinical course despite various therapeutic modalities (surgery, radiotherapy, and chemotherapy). The prognosis is extremely poor and patients usually die within 24 months (average 7 months) after diagnosis [3]. Surgical exploration is important for the diagnosis and palliative/definite treatment of the disease in these patients.
Author contribution
Hasan Yavuz: study design, literature review, collecting data, writing, and editing
Ahmet Kayahan Tekneci: study design, collecting data, writing, and editing
Tevfik İlker Akçam: study design, writing, editing, and supervision
Kutsal Turhan: study design, supervision, and editing
Taner Akalın: study design, writing, editing, and supervision
Funding
None.
Data Availability
Our data is freely and openly accessable.
Declarations
Informed consent
Written informed consent was obtained from the patient for the study.
Human and animal rights statement
All stages of the study were completed ethically in accordance with the Declaration of Helsinki. No animals were involved.
Ethics committee approval
Not applicable.
Conflict of interest
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Yang Z, Zheng R, Zhang S, Zeng H, Li H, Chen W. Incidence, distribution of histological subtypes and primary sites of soft tissue sarcoma in China. Cancer Biol Med. 2019;16:565–574. doi: 10.20892/j.issn.2095-3941.2019.0041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Karki SD, Westhoff M, Maschek H, Augustyniak J, Gupta V, Welter S. A rare diagnostic challenge in a female patient with a rapid recurrent pleural effusion: autopsy revealed cardiac angiosarcoma with bilateral pleural and pulmonary metastases. A case report. Int J Surg Case Rep. 2021;78:278–283. [DOI] [PMC free article] [PubMed]
- 3.Abu-Zaid A, Mohammed S. Primary pleural angiosarcoma in a 63-year-old gentleman. Case Rep Pulmonol. 2013;2013:974567. doi: 10.1155/2013/974567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Azzakhmam M, Elktaibi A, El Ochi MR, Allaoui M, Albouzidi A, Oukabli M. Primary epitheloid angiosarcoma of the pleura: an exceptional tumor location. Pan Afr Med J. 2019;33:327. doi: 10.11604/pamj.2019.33.327.18145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen CY, Wu YC, Chou TY, Yang KY. Pleural angiosarcoma mimicking pleural haematoma. Interact Cardiovasc Thorac Surg. 2013;17:886–888. doi: 10.1093/icvts/ivt269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Drake BK, Timothy SM. Epithelioid angiosarcoma, a case review. Pathology Case Reviews. 2008;13:264–268. doi: 10.1097/PCR.0b013e31818bb9b9. [DOI] [Google Scholar]
- 7.Lorentziadis M, Sourlas A. Primary de novo angiosarcoma of the pleura. Ann Thorac Surg. 2012;93:996–998. doi: 10.1016/j.athoracsur.2011.07.023. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Our data is freely and openly accessable.