Abstract
Retinopathy of prematurity (ROP), a leading cause of childhood blindness, has historically been associated with blindness from overgrowth of blood vessels from the retina into the vitreous that lead to complex retinal detachments. Our understanding of ROP has evolved with the survival of extremely low-birthweight infants and includes not only overgrowth of blood vessels, but also insufficient developmental retinal vascular growth in early phases of the disease. Our current treatments of ROP have focused on methods to improve perinatal and prenatal care, reduce premature birth, and prevent early phases of ROP. Nonetheless, addressing vasoproliferation in treatment-warranted eyes remains the mainstay of management. Two main treatment strategies co-exist today: laser treatment, which has been the standard of care since the 1990s, and anti-VEGF injections, which have been used since early reports in 2007 (Travassos et al. in Ophthalmic Surg Lasers Imaging, 38:233–237, 10.3928/15428877-20070501-09, 2007, Shah et al. in Indian J Ophthalmol 55:75–76, 10.4103/0301-4738.29505, 2007, Quiroz-Mercado et al. in Semin Ophthalmol 22:109–125, 10.1080/08820530701420082, 2007).
Keywords: Vitreoretinal surgery, Laser, Anti-VEGF, Retinopathy of prematurity, ROP
Key Summary Points
| ROP has changed in appearance since the first description in 1942 and varies world wide. |
| The hypothesis of the pathophysiology of ROP has been refined and with it the understanding that regulation of vascular endothelial growth factor can cause regression of treatment-warranted ROP while allowing vascularization of the peripheral avascular retina. |
| This is a review of major recent clinical trials and meta-analyses to develop a reasonable approach to manage treatment-warranted ROP considering laser or anti-VEGF based on outcomes of efficacy, reactivation, safety, persistent avascular retina, and refractive status. |
| Our assessment supports strong consideration of anti-VEGF agents in zone I treatment-warranted ROP and careful informed consent for zone II treatment-warranted ROP. |
Introduction
Retinopathy of prematurity (ROP) is a leading cause of vision loss throughout the world and may be increasing with rising estimates of premature birth [4]. However, differences in the infant age at presentation and appearance of ROP throughout the world have been attributed to several factors, including prenatal and perinatal nutrition and care, oxygen regulation, resources to provide care in nurseries, genetics and epigenetics, and inflammation/oxidation. ROP was originally identified as retrolental fibroplasia (RLF)—for the worst stage of current ROP—in larger infants of older gestational ages and attributed to unregulated oxygen [5–7]. Ashton described the pathophysiology of RLF in a two-phase hypothesis as oxygen-induced damage of already developed retinal capillaries in phase I followed by vasoproliferation into the vitreous at the junctions of vascular and the hypoxic, avascular retina in phase II [8]. As technological advances allowed infants of extremely low-birthweight and young gestational age to survive [9], and for oxygen to be monitored and regulated, ROP again changed in appearance and the original hypothesis was refined to include compromised vascularity and delayed physiologic retinal vascular development in phase I and vasoproliferation in phase I I[10, 11]. Imaging with wide-angle photography and angiography have allowed more accurate characterization of the retinal vasculature, aided by comparison with representative animal models of the pathophysiology [10, 12].
This article is based on previously conducted studies and does not contain any new studies with human participants or animals performed by any of the authors.
Clinical Classification
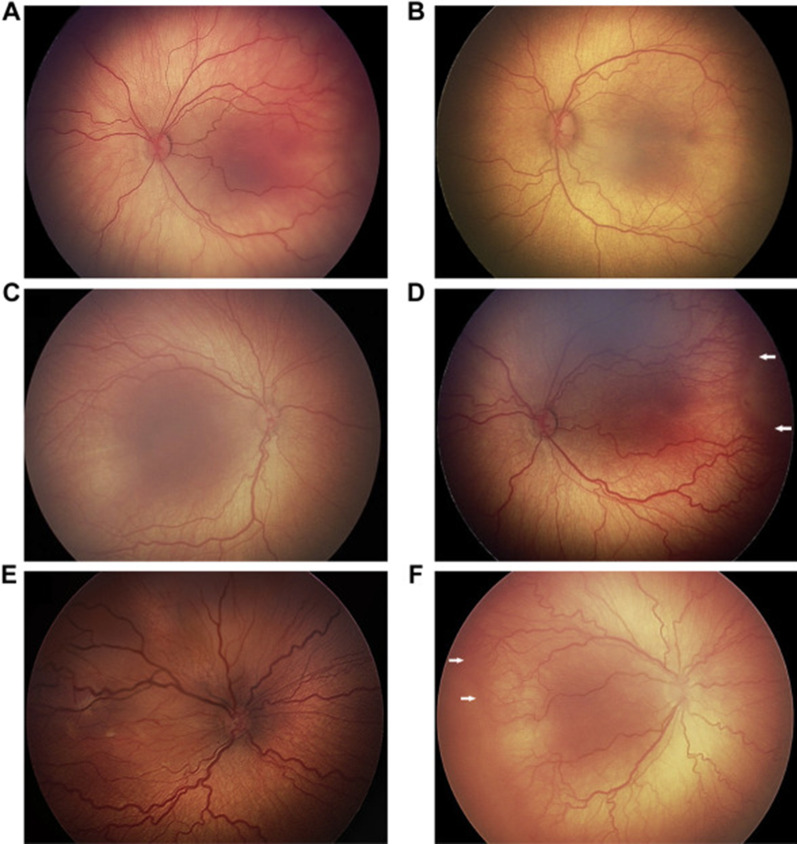
The International Classification of ROP (ICROP) provided a classification of early stages of ROP that preceded vasoproliferation and the stages involving partial or total retinal detachment. ICROP enabled research studies and severity scales to assess effects of treatment on outcomes. The classification includes: stages of severity (stages 1–5); zones of involvement (region on retina where stages occur), clock hour extent of stage, and plus disease (the presence or absence of vascular tortuosity and dilation (Fig. 1). Treatment-warranted ROP generally involves zone I or II, stage 2 or 3 ROP, and often with plus disease. Stages 4 and 5 ROP generally require surgical intervention using vitreoretinal techniques but are fortunately rare compared with treatment-warranted ROP amenable to laser or anti-VEGF. Now, in its third edition, ICROP3 includes aggressive forms of ROP such as those in larger babies that do not progress through the typical chronological order of stages and can be associated with vascular dropout peripherally and/or posteriorly with vascular loops and “flat” neovascularization (Fig. 2) [13].
Fig. 1.
Spectrum of preplus and plus disease in different left eyes: mild preplus through preplus but insufficient for plus (A–C), and plus disease from mild to severe (D–F). Reprinted from Ophthalmology, Fig. 2A–F), volume 128, Chiang MF et al. [13], with permission from Elsevier
Fig. 2.
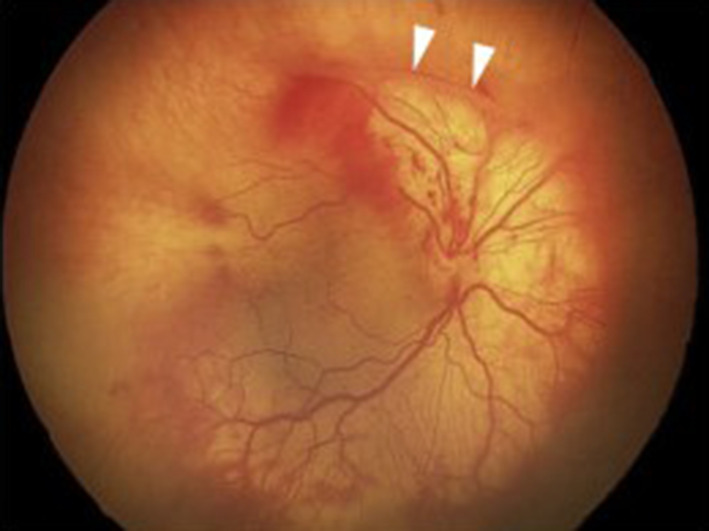
Aggressive ROP (A-ROP) in right eye showing image of severe plus disease with flat neovascularization and vitreous hemorrhage noted near vitreoretinal traction (arrowheads). Reprinted from Ophthalmology, Fig. 7C), volume 128, Chiang MF et al. [13], with permission from Elsevier
Importance of Early Clinical Trials in Management
Vasoproliferation in ROP has been treated by methods to ablate the peripheral avascular retina in order to reduce the hypoxic stimulus for vasoproliferation. The first multicenter study tested cryotherapy to ablate the peripheral avascular retina and reported efficacy for a more severe level of treatment-warranted ROP than typically treated today [14, 15]. However, forms of A-ROP and zone 1 treatment-warranted ROP had much poorer outcomes than zone II treatment-warranted ROP [16]. The Early Treatment of ROP study later tested and reported that treatment to ablate the peripheral retina at a less severe level of treatment-warranted ROP (type 1 ROP) improved outcomes compared with conventional treatment [17].
Paradigm Shift in Treatment
Based on basic research using oxygen-induced retinopathy (OIR) models, it was found that activation of a receptor in the vascular endothelial growth factor (VEGF) signaling pathway in retinal endothelial cells caused vasoproliferation and also thwarted normal retinal vascular development [18, 19]. In contrast to the experimental studies that used subretinal injections to regulate VEGF receptor (VEGFR2) signaling specifically in retinal endothelial cells, the method adopted clinically is through the use of intravitreal neutralizing antibodies or fusion proteins that bind ligands, such as VEGF [and placental growth factor (PlGF), in the case of aflibercept]. These methods are specific to neither VEGF receptor nor cell type in the retina. Therefore, adverse effects may occur when the supportive effects of VEGF are removed from other cell types. Experimentally, in animal models, a neutralizing antibody to VEGF was associated with thinning of the neural retina, reduced weight gain, and retinal capillary dropout [20, 21]. These findings increased existing concerns of potential adverse events [22]. There have been some reports of adverse events in clinical studies, for example, in association with pulmonary hypertension [23]. Large retrospective cohort studies in Canada [24] and the USA [25] have reported reduced cognitive development or death, respectively. These studies recognized potential bias with infants having more severe courses frequently treated with anti-VEGF than laser. However, the report in the USA attempted to statistically control for potential confounders and still found greater risk of death in infants treated with the anti-VEGF agent bevacizumab [25]. Nonetheless, other studies having smaller sample sizes did not find reduced cognitive function after anti-VEGF treatment [26–29]. It is difficult to assess the effect of anti-VEGF on cognitive function since the infants at greatest risk of ROP are also often those at great risk of poor neurocognitive development and poor survival. In addition, there are limitations of neurocognitive testing in young children. Anti-VEGF agents differ by half-life and effect on systemic VEGF levels [28, 30–32], with ranibizumab having a significantly shorter half-life than bevacizumab or aflibercept. These differences may have different influences on later cognitive development. For example, randomized clinical trials found no effect of ranibizumab on systemic VEGF levels compared to laser treatment or between doses of ranibizumab [28, 30]. Nonetheless, concerns persist, and long-term data on efficacy and long-term safety from clinical trials are awaited.
Changing “Natural History” of ROP
Clinical data support the findings in experimental research that regulation of the angiogenic factor, VEGF, not only reduced vasoproliferation, but also supported ongoing more normal intraretinal vascular growth. However, reactivation of ROP occurs, and adverse outcomes have been reported over a year after a single intravitreal administration [33]. Current guidelines recommend continued dilated retinal examinations for reactivation and additional treatment through at least 65 weeks PGA [34] or in Germany through 69 weeks PGA or 35 weeks after the last injection [35].
Clinical Trials
Initially, clinical trials for anti-VEGF agents showed efficacy in adults for the treatment of diabetic macular edema, macular edema associated with retinal vein occlusion, and neovascular age-related macular degeneration (AMD). A number of clinical studies demonstrated the remarkable effects of an intravitreal injection of an anti-VEGF agent in treatment-warranted ROP [1–3]. These case reports and series have been followed by several clinical trials that reported outcomes using different anti-VEGF agents [30, 31, 36]. The agents tested in adult studies were bevacizumab, a humanized monoclonal antibody against the full-length VEGFA, ranibizumab, the active F(ab) fragment of the antibody against VEGF, and aflibercept, which is a fusion protein of domains from receptors, VEGFR1 and VEGFR2, which traps VEGFA and placental growth factor (PlGF). These agents have been tested in clinical trials for treatment-warranted ROP and work by binding the VEGF ligand (and PlGF in the case of aflibercept) to interfere with the ligand–receptor interaction and activation.
Below are summaries of efficacy, reactivation, safety, refractive error, and persistent avascular retina (PAR) from anti-VEGF clinical trials.
Efficacy of Trials
The first clinical trial for treatment-warranted ROP was Bevacizumab Eliminates the Angiogenic Threat in ROP (BEAT-ROP) study [36]. Treatment-warranted ROP was considered stage 3+ ROP in zone I or II. The investigators used reactivation at 54 weeks as an outcome and found significantly reduced need for retreatment of eyes (4%) treated with 0.625 mg intravitreal bevacizumab compared with laser (22%) when stage 3+ ROP occurred in zone I or posterior zone II but not in mid or anterior zone II ROP. The RAnibizumab compared with laser therapy for the treatment of INfants BOrn prematurely With retinopathy of prematurity (RAINBOW) trial was unable to statistically prove superiority of ranibizumab 0.2 mg or 0.1 mg compared with laser, although the success rates with ranibizumab were numerically higher with ranibizumab compared with laser (p = 0.051) [30]. The outcomes after laser were lower (66%) than those reported in other studies, such as 82% in FIREFLEYE and over 90% in ETROP. In FIREFLEYE, aflibercept 0.4 mg did not meet noninferiority to laser based on success at week 24, but similar to RAINBOW, success rates with aflibercept were numerically higher compared with laser [31]. The RAINBOW and FIREFLEYE trial results led to the approval of ranibizumab for the treatment of ROP by the European Medicine Agency (EMA) in 2019 and the approval of aflibercept by the EMA and the FDA in 2022. The Pediatric Eye Disease Investigative Group (PEDIG) of the National Eye Institute (NEI) is enrolling for a bevacizumab versus laser study testing 1/10 the dose used in BEAT-ROP or comparing two doses of bevacizumab for zone I or posterior zone II eyes with type I ROP[37]. In a study of Bayesian network meta-analyses (Bayesian NM) of randomized clinical trials testing anti-VEGF agents for ROP, single treatment success, defined as the likelihood of needing no further treatment, reported 89.3% for laser, 87% for bevacizumab, 80.7% for aflibercept, and 74% for ranibizumab. For zone I ROP, bevacizumab had the highest success (91.2%) and laser the lowest (65.9%) [38].
Reactivation
Reactivation after treatment is harder to discern from clinical trials, in part because the outcomes for the trials were determined prior to the definition of reactivation in ICROP3. Evidence suggests that timing of reactivation may be based on the half-life or dose of the agents. The half-life of ranibizumab is shorter than for bevacizumab [39–41]. The BEAT-ROP study found only 4% high-dose bevacizumab required retreatment of posterior zone II by 54 weeks compared with 22% of laser-treated eyes. In a later study that included BEAT-ROP patients and patients enrolled in a separate cohort, 8.3% bevacizumab-treated eyes reactivated by 65 weeks PGA [42]. PEDIG reported 27% additional treatments usually by 2–3 months after treatment in their 12-month outcomes of the de-escalating dose study testing from 0.25 to 0.002 mg bevacizumab [43]. In RAINBOW, retreatments were common by 28 days (27–28%) and were needed in 15–17% by about 9 weeks compared with 2% of laser-treated infants [44]. The Bayesian NM analyses reported reactivations on average at 9.29 weeks for ranibizumab, 11.36 weeks for bevacizumab, and 12.96 weeks for aflibercept. Post-hoc testing demonstrated a longer time to retreatment for aflibercept and bevacizumab compared with ranibizumab [38].
Safety
VEGF is not only protective of endothelial cells, but also neurons and glia [45]. Reports from the Canadian and US Neonatal Networks of reduced neurocognitive scores or increased death in premature infants treated with anti-VEGF (bevacizumab) compared with laser raised concerns [24, 25]. However, the studies were retrospective, and it is known that very sick infants may not be able to undergo the duration of time needed for adequate laser treatment, which is much longer than for intravitreal injections [46], and this observation introduces a selection bias favoring anti-VEGF treatment for severely sick infants. As a means to assess safety, studies have reported on the amount of anti-VEGF agent that leaks into the blood and/or the concentrations of systemic VEGF. Early studies reported significant declines in circulating VEGF following an intravitreal bevacizumab injection [32], and the reduction persisted for over 2 months. There were also concerns that an experimental method using gene therapy to specifically knock down overexpressed full-length VEGFA mRNA and reduce vitreous VEGF to the minimum level effective at inhibiting vasoproliferation still led to thinning of the outer nuclear layers [21]. However, using the same gene therapy strategy to knock down VEGF164, thereby allowing some of the soluble forms of VEGF to access the retina through the vitreous, had no effect on the outer nuclear layer, compared with control gene therapy injection [21]. This finding supported the idea that an appropriate dose of anti-VEGF agent may be efficacious and safe and is being tested by the PEDIG.
Plasma VEGF increases in premature infants as they develop treatment-warranted ROP [47, 48]. Although bevacizumab reduces VEGF in the bloodstream by 50%, laser alone can reduce VEGF by 30%. This observation suggests that some of the increase in plasma VEGF originates from the eye. Following an injection, plasma bevacizumab was found to peak at 2 weeks and require about 2 months to clear [41]. PEDIG reported that plasma bevacizumab was associated with the intravitreal dose given at 2 and 4 weeks; however, VEGF decreased by > 50% at both 2 and 4 weeks after injection, and the decrease was unassociated with the bevacizumab dose [47]. Ranibizumab peaks in the blood stream at 1.3 days but has a very short half-life [40]; there were no differences in VEGF in the blood stream between laser and either ranibizumab dose [49] in the RAINBOW study and no suppression of systemic VEGF with ranibizumab in the CARE-ROP study [28]. In adults, aflibercept reduced VEGF in the plasma that remained reduced for 30 days [50]. Systemic VEGF levels were not measured in the FIREFLEYE study, but systemic free aflibercept was measured in the circulation up to 4 weeks after intravitreal injection [31].
As indicated earlier, reports on neurocognitive function and pulmonary hypertension have been raised. The issue of low VEGF levels in the bloodstream of premature infants should be on the list of potential adverse effects of some anti-VEGF drugs and deserves attention in future clinical studies.
Refractive Error
Many studies reported that eyes treated with anti-VEGF agents are associated with lower levels of myopia than those treated with laser. Studies differ on the dosages of agents and ages of infants when they are treated. A meta-analysis of 1850 eyes from randomized clinical trials and observational studies reported that anti-VEGF therapies resulted in 1.8D less myopia than eyes treated with laser[51]. Follow-up from a cohort of infants from BEAT-ROP reported mean spherical equivalent of −1.51 in zone I eyes treated with bevacizumab compared with −8.44 in eyes treated with laser and −0.58 in zone II eyes treated with bevacizumab compared with −5.83 in eyes treated with laser [52]. In the 12-month follow-up from the bevacizumab de-escalating dose study from PEDIG, the median refraction at 12 months was −0.31 and strabismus was present in 29% of infants [43]. In the RAINBOW study, high myopia (−5D or more) was reported in 5% of ranibizumab-treated eyes compared with 20% of laser-treated eyes [53].
Persistent Avascular Retina (PAR)
Concerns about persistent avascular retina (PAR) include the potential for reactivation from avascular, presumed hypoxic, retina in the infant, and later sequelae from vitreoretinal traction tearing the thinned atrophic avascular retina and leading to retinal detachments in childhood and teenage years. Retinal detachments can be missed and present late. The detachments are complex with firm abnormal vitreoretinal adhesions and may be associated with proliferative vitreoretinopathy and combined tractional/rhegmatogenous components. These eyes have poorer anatomic and visual outcomes following surgical correction. Therefore, some specialists recommend an examination under anesthesia (EUA) with fluorescein angiography and treatment of the peripheral avascular retina with laser once an infant becomes too large and active to examine adequately in the clinic, particularly with evidence of a vascularized ridge. It is helpful to remember that reactivation does not necessarily appear as recurrence of type 1 ROP. Nonetheless, it is unknown what the risk of later retinal detachments actually is, because series include cases referred to retina specialists and may not have included all cases of resolved ROP with clinically unrecognized PAR. Nor is it known if the laser will reduce the risk of later retinal detachment. Peripheral avascular retina has been recognized for many years prior to the use of anti-VEGF agents. The ability to diagnose PAR is improved with wide-angle fluorescein angiography showing the cessation of vascularization in the peripheral retina, but not all studies performed this routinely. Estimates from PEDIG 12-month outcome study were that 27% of eyes were treated with laser for PAR [43].
Where We Are Now with Treatment
We have better tools to manage ROP than 30 years ago. From the studies, anti-VEGF appears superior to laser for efficacy in treatment-warranted ROP especially in zone I, posterior zone II, and aggressive ROP, and has improved overall refractive outcomes. Given the ability to extend peripheral retinal vascularization to a degree, anti-VEGF treated eyes may have expanded visual fields, but long-term data are needed. For treatment-warranted ROP in mid or anterior zone II, laser or anti-VEGF can be considered and should be weighed against each other with careful informed consent.
Different anti-VEGF agents have different reported outcomes based on the meta-analyses. Bevacizumab has better single treatment success, and lower dosages are being assessed for safety and efficacy. However, bevacizumab reduces VEGF levels in the circulation for extended duration. Ranibizumab tends to require retreatments more frequently and earlier but does not substantially affect systemic VEGF levels. Aflibercept has become the first FDA-approved anti-VEGF drug for premature infants with ROP but shows sustained systemic drug exposure. More studies and long-term follow-up are ongoing for these agents.
We have long-term outcomes and more experience with laser, which has been used for a longer period of time than anti-VEGF agents. Laser treatment also requires fewer retreatments and does not introduce a needle into the eye thereby reducing iatrogenic damage or endophthalmitis. Follow-up is generally shorter than for anti-VEGF agents [34]. Laser treatment avoids systemic drug exposure and possible, albeit unproven, risks associated with that. In addition, full treatment with laser to the peripheral avascular zone removes PAR, although there are reports of later-onset vitreous hemorrhage and retinal detachment in laser-treated eyes [54].
There are downsides to laser. It is destructive and does not permit vascularization of peripheral avascular retina (Fig. 3). The treatment is longer and can be more difficult for premature infants. Adequate treatment requires experienced and careful treaters. Studies show eyes treated with laser more often have high myopia than eyes treated with anti-VEGF agents.
Fig. 3.
Right eye of regressed ROP (A) with black arrows showing a line in the place of the previous ridge and stage 3 ROP, and red arrows pointing out vascularization into the peripheral retina 6 weeks after 0.25 mg bevacizumab injection for type 1 ROP (39 weeks PMA). Left eye with type 2 ROP at 37 weeks PMA (B) with spontaneous regression of the ridge and stage 3 ROP followed by vascularization of the peripheral retina at 42 weeks PMA (C). Courtesy of Melissa Chandler, BS, CRA, OCT-c, COT
Conversely, treatment with anti-VEGF agents does not destroy retina, allows vascularization of the peripheral avascular retina with the potential of expanded visual field and appears to have a lower risk of myopia. However, reports of late adverse outcomes are concerning and have in some cases led to unfavorable outcomes and stage 5 ROP. However, if reactivations are identified and treated, whether with anti-VEGF reinjection or laser, the outcomes are not worse than in eyes without reactivations [28, 55]. There is no consensus as to what constitutes reactivation warranting retreatment, but most believe that plus disease and/or a vascularized thickened ridge are important criteria. Examinations can be difficult to perform adequately in the older, larger active infant in clinic. Long-term effects of anti-VEGF on the neural retina and developing infant remain currently unknown as longer-duration follow-up studies are underway, and there is a risk of lens injury and endophthalmitis.
We have learned a great deal about ROP over the past 50 years, and our treatment options have expanded significantly, but we continue to have questions, particularly as ever smaller and younger premature infants are able to survive.
Acknowledgements
Funding
Grant support: NIH/NEI R01EY015130, R01EY017011 and R21EY033579 to Mary Elizabeth Hartnett. No funding or sponsorship was received for publication of this article.
Author Contributions
Mary Elizabeth Hartnett wrote primary draft Mary Elizabeth Hartnett and Andreas Stahl provided critical input and revisions.
Disclosures
Mary Elizabeth Hartnett is PI for NEI/NIH support for R01EY107011 and R01EY105130, R21EY033579 and receives partial salary support. She receives departmental support from Research to Prevent Blindness. Mary Elizabeth Hartnett serves on the Scientific Advisory Boards for nonprofit Knights Templar Eye Foundation, and as chair of the Macula Society and the Jack McGovern Coats’ Disease Foundation. Mary Elizabeth Hartnett’s institution received financial support for the Regeneron BUTTERFLEYE study for IRB development. Otherwise there is no support from commercial sponsors. Andreas Stahl is a Speaker for Allergan, Bayer, and Novartis and is on the following Scientific advisory boards: Bayer, Novartis, and Roche. Andreas Stahl receives Research grants from Bayer and Novartis and performs Clinical trials for Bayer and Novartis. Andreas Stahl is on the Board of Directors: SemaThera Inc.
Compliance with Ethics Guidelines
This article is based on previously conducted studies and does not contain any new studies with human participants or animals performed by any of the authors.
Data Availability
Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study.
Contributor Information
M. Elizabeth Hartnett, Email: me.hartnett@stanford.edu.
Andreas Stahl, Email: andreas.stahl@med.uni-greifswald.de.
References
- 1.Travassos A, Teixeira S, Ferreira P, Regadas I, Travassos AS, Esperancinha FE, Prieto I, Pires G, van Velze R, Valido A, et al. Intravitreal bevacizumab in aggressive posterior retinopathy of prematurity. Ophthalmic Surg Lasers Imaging. 2007;38:233–237. doi: 10.3928/15428877-20070501-09. [DOI] [PubMed] [Google Scholar]
- 2.Shah PK, Narendran V, Tawansy KA, Raghuram A, Narendran K. Intravitreal bevacizumab (Avastin) for post laser anterior segment ischemia in aggressive posterior retinopathy of prematurity. Indian J Ophthalmol. 2007;55:75–76. doi: 10.4103/0301-4738.29505. [DOI] [PubMed] [Google Scholar]
- 3.Quiroz-Mercado H, Ustariz-González O, Martinez-Castellanos MA, Covarrubias P, Dominguez F, Sanchez-Huerta V. Our experience after 1765 intravitreal injections of bevacizumab: the importance of being part of a developing story. Semin Ophthalmol. 2007;22:109–125. doi: 10.1080/08820530701420082. [DOI] [PubMed] [Google Scholar]
- 4.Chawanpaiboon S, Vogel JP, Moller AB, Lumbiganon P, Petzold M, Hogan D, Landoulsi S, Jampathong N, Kongwattanakul K, Laopaiboon M, et al. Global, regional, and national estimates of levels of preterm birth in 2014: a systematic review and modelling analysis. Lancet Glob Health. 2019;7:e37–e46. doi: 10.1016/s2214-109x(18)30451-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ashton N, Cook C. Direct observation of the effect of oxygen on developing vessels: preliminary report. Br J Ophthalmol. 1954;38:433–440. doi: 10.1136/bjo.38.7.433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Patz A. Oxygen studies in retrolental fibroplasia. Am J Ophthalmol. 1954;38:291–308. doi: 10.1016/0002-9394(54)90845-4. [DOI] [PubMed] [Google Scholar]
- 7.Campbell K. Intensive oxygen therapy as a possible cause of retrolental fibroplasia. A clinical approach. Med J Australia. 1951;2:48. doi: 10.5694/j.1326-5377.1951.tb109040.x. [DOI] [PubMed] [Google Scholar]
- 8.Ashton N, Ward B, Serpell G. Effect of oxygen on developing retinal vessels with particular reference to the problem of retrolental fibroplasia. Br J Ophthalmol. 1954;38:397–432. doi: 10.1136/bjo.38.7.397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Allen MB, Donohue PK, Dusman AE. The limit of viability—neonatal outcome of infants born at 22 to 25 weeks' gestation. N Engl J Med. 1993;329:1597–1601. doi: 10.1056/NEJM199311253292201. [DOI] [PubMed] [Google Scholar]
- 10.Hartnett ME. Pathophysiology and mechanisms of severe retinopathy of prematurity. Ophthalmology. 2015;122:200–210. doi: 10.1016/j.ophtha.2014.07.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hartnett ME, Penn JS. Mechanisms and management of retinopathy of prematurity. N Engl J Med. 2012;367:2515–2526. doi: 10.1056/NEJMra1208129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hartnett ME, Toth CA. Experimental evidence behind clinical trial outcomes in retinopathy of prematurity. Ophthalmic Surg Lasers Imaging Retina. 2019;50:228–234. doi: 10.3928/23258160-20190401-05. [DOI] [PubMed] [Google Scholar]
- 13.Chiang MF, Quinn GE, Fielder AR, Ostmo SR, Paul Chan RV, Berrocal A, Binenbaum G, Blair M, Peter Campbell J, Capone A, Jr, et al. International classification of retinopathy of prematurity, third edition. Ophthalmology. 2021;128:e51–e68. doi: 10.1016/j.ophtha.2021.05.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Multicenter trial of cryotherapy for retinopathy of prematurity. Preliminary results. Cryotherapy for Retinopathy of Prematurity Cooperative Group. Arch Ophthalmol. 1988; 106: 471–479. [DOI] [PubMed]
- 15.Palmer EA, Hardy RJ, Dobson V, Phelps DL, Quinn GE, Summers CG, Krom CP, Tung B. 15-Year outcomes following threshold retinopathy of prematurity: final results from the multicenter trial of cryotherapy for retinopathy of prematurity. Arch Ophthalmol. 2005;123:311–318. doi: 10.1001/archopht.123.3.311. [DOI] [PubMed] [Google Scholar]
- 16.Vander JF, Handa J, McNamara JA, Trese M, Spencer R, Repka MX, Rubsamen P, Li H, Morse LS, Tasman WS. Early treatment of posterior retinopathy of prematurity: a controlled trial. Ophthalmology. 1997;104:1731–1735. doi: 10.1016/s0161-6420(97)30034-7. [DOI] [PubMed] [Google Scholar]
- 17.Good WV, Hardy RJ, Dobson V, Palmer EA, Phelps DL, Tung B, Redford M. Final visual acuity results in the early treatment for retinopathy of prematurity study. Arch Ophthalmol. 2010;128:663–671. doi: 10.1001/archophthalmol.2010.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Simmons AB, Bretz CA, Wang H, Kunz E, Hajj K, Kennedy C, Yang Z, Suwanmanee T, Kafri T, Hartnett ME. Gene therapy knockdown of VEGFR2 in retinal endothelial cells to treat retinopathy. Angiogenesis. 2018;21:751–764. doi: 10.1007/s10456-018-9618-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hartnett ME. Discovering mechanisms in the changing and diverse pathology of retinopathy of prematurity: the Weisenfeld award lecture. Invest Ophthalmol Vis Sci. 2019;60:1286–1297. doi: 10.1167/iovs.18-25525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.McCloskey M, Wang H, Jiang Y, Smith GW, Strange J, Hartnett ME. Anti-VEGF antibody leads to later atypical intravitreous neovascularization and activation of angiogenic pathways in a rat model of retinopathy of prematurity. Invest Ophthalmol Vis Sci. 2013;54:2020–2026. doi: 10.1167/iovs.13-11625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Becker S, Wang H, Simmons AB, Suwanmanee T, Stoddard GJ, Kafri T, Hartnett ME. Targeted knockdown of overexpressed VEGFA or VEGF164 in Muller cells maintains retinal function by triggering different signaling mechanisms. Sci Rep. 2003;2018:8. doi: 10.1038/s41598-018-20278-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Good WV. Bevacizumab for retinopathy of prematurity: treatment when pathology is embedded in a normally developing vascular system. Ophthalmology. 2016;123:1843–1844. doi: 10.1016/j.ophtha.2016.06.054. [DOI] [PubMed] [Google Scholar]
- 23.Nitkin CR, Bamat NA, Lagatta J, DeMauro SB, Lee HC, Patel RM, King B, Slaughter JL, Campbell JP, Richardson T, et al. Pulmonary hypertension in preterm infants treated with laser vs anti-vascular endothelial growth factor therapy for retinopathy of prematurity. JAMA Ophthalmol. 2022;140:1085–1094. doi: 10.1001/jamaophthalmol.2022.3788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Morin J, Luu TM, Superstein R, Ospina LH, Lefebvre F, Simard MN, Shah V, Shah PS, Kelly EN. Neurodevelopmental outcomes following bevacizumab injections for retinopathy of prematurity. Pediatrics. 2016 doi: 10.1542/peds.2015-3218. [DOI] [PubMed] [Google Scholar]
- 25.Natarajan G, Shankaran S, Nolen TL, Sridhar A, Kennedy KA, Hintz SR, Phelps DL, DeMauro SB, Carlo WA, Gantz MG, et al. Neurodevelopmental outcomes of preterm infants with retinopathy of prematurity by treatment. Pediatrics. 2019 doi: 10.1542/peds.2018-3537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kennedy KA, Mintz-Hittner HA. Medical and developmental outcomes of bevacizumab versus laser for retinopathy of prematurity. J AAPOS. 2018;22:61–65.e61. doi: 10.1016/j.jaapos.2017.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wallace DK, Hercinovic A, Freedman SF, Crouch ER, Bhatt AR, Hartnett ME, Yang MB, Rogers DL, Hutchinson AK, Good WV, et al. Ocular and developmental outcomes of a dosing study of bevacizumab for retinopathy of prematurity. J AAPOS. 2023;27(10):e11–10.e18. doi: 10.1016/j.jaapos.2022.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Stahl A, Bründer MC, Lagrèze WA, Molnár FE, Barth T, Eter N, Guthoff R, Krohne TU, Pfeil JM. Ranibizumab in retinopathy of prematurity—one-year follow-up of ophthalmic outcomes and two-year follow-up of neurodevelopmental outcomes from the CARE-ROP study. Acta Ophthalmol. 2022;100:e91–e99. doi: 10.1111/aos.14852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chiang MC, Chen YT, Kang EY, Chen KJ, Wang NK, Liu L, Chen YP, Hwang YS, Lai CC, Wu WC. Neurodevelopmental outcomes for retinopathy of prematurity: a Taiwan premature infant follow-up network database study. Am J Ophthalmol. 2023;247:170–180. doi: 10.1016/j.ajo.2022.10.020. [DOI] [PubMed] [Google Scholar]
- 30.Stahl A, Krohne TU, Eter N, Oberacher-Velten I, Guthoff R, Meltendorf S, Ehrt O, Aisenbrey S, Roider J, Gerding H, et al. Comparing alternative ranibizumab dosages for safety and efficacy in retinopathy of prematurity: a randomized clinical trial. JAMA Pediatr. 2018 doi: 10.1001/jamapediatrics.2017.4838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Stahl A, Sukgen EA, Wu WC, Lepore D, Nakanishi H, Mazela J, Moshfeghi DM, Vitti R, Athanikar A, Chu K, et al. Effect of intravitreal aflibercept vs laser photocoagulation on treatment success of retinopathy of prematurity: the FIREFLEYE randomized clinical trial. JAMA. 2022;328:348–359. doi: 10.1001/jama.2022.10564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sato T, Wada K, Arahori H, Kuno N, Imoto K, Iwahashi-Shima C, Kusaka S. Serum concentrations of bevacizumab (avastin) and vascular endothelial growth factor in infants with retinopathy of prematurity. Am J Ophthalmol. 2012;153:327–333e321. doi: 10.1016/j.ajo.2011.07.005. [DOI] [PubMed] [Google Scholar]
- 33.Hu J, Blair MP, Shapiro MJ, Lichtenstein SJ, Galasso JM, Kapur R. Reactivation of retinopathy of prematurity after bevacizumab injection. Arch Ophthalmol. 2012;130:1000–1006. doi: 10.1001/archophthalmol.2012.592. [DOI] [PubMed] [Google Scholar]
- 34.Fierson WM. Screening examination of premature infants for retinopathy of prematurity. Pediatrics. 2018 doi: 10.1542/peds.2018-3061. [DOI] [PubMed] [Google Scholar]
- 35.German Society of, O.; German Retina Society e.,V.; Professional Association of German, O Statement of the German Society of Ophthalmology, the German Retina Society, and the Professional Association of German Ophthalmologists on anti-VEGF therapy of retinopathy of prematurity. Der Ophthalmol. 2021;118:68–77. doi: 10.1007/s00347-020-01250-y. [DOI] [PubMed] [Google Scholar]
- 36.Mintz-Hittner HA, Kennedy KA, Chuang AZ. Efficacy of intravitreal bevacizumab for stage 3+ retinopathy of prematurity. N Engl J Med. 2011;364:603–615. doi: 10.1056/NEJMoa1007374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kraker RT, Wallace DK, Beck RW, Saunders CT, Lorenzi E, Melia BM, Li Z. Choice of dose level for a randomized clinical trial of low-dose bevacizumab vs laser for type 1 retinopathy of prematurity. JAMA Ophthalmol. 2021;139:1143–1144. doi: 10.1001/jamaophthalmol.2021.3192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chang E, Josan AS, Purohit R, Patel CK, Xue K. A network meta-analysis of retreatment rates following bevacizumab, ranibizumab, aflibercept, and laser for retinopathy of prematurity. Ophthalmology. 2022;129:1389–1401. doi: 10.1016/j.ophtha.2022.06.042. [DOI] [PubMed] [Google Scholar]
- 39.Lu JF, Bruno R, Eppler S, Novotny W, Lum B, Gaudreault J. Clinical pharmacokinetics of bevacizumab in patients with solid tumors. Cancer Chemother Pharmacol. 2008;62:779–786. doi: 10.1007/s00280-007-0664-8. [DOI] [PubMed] [Google Scholar]
- 40.Avery RL, Castellarin AA, Steinle NC, Dhoot DS, Pieramici DJ, See R, Couvillion S, Nasir MA, Rabena MD, Maia M, et al. Systemic pharmokinetics and pharmacodynamics of intravitreal aflibercept, bevacizumab, and ranibizumab. Retina (Philadelphia, Pa.) 2017;37:1847–1858. doi: 10.1097/iae.0000000000001493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kong L, Bhatt AR, Demny AB, Coats DK, Li A, Rahman EZ, Smith OE, Steinkuller PG. Pharmacokinetics of bevacizumab and its effects on serum VEGF and IGF-1 in infants with retinopathy of prematurity. Invest Ophthalmol Vis Sci. 2015;56:956–961. doi: 10.1167/iovs.14-15842. [DOI] [PubMed] [Google Scholar]
- 42.Mintz-Hittner HA, Geloneck MM, Chuang AZ. Clinical management of recurrent retinopathy of prematurity after intravitreal bevacizumab monotherapy. Ophthalmology. 2016;123:1845–1855. doi: 10.1016/j.ophtha.2016.04.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Freedman SF, Hercinovic A, Wallace DK, Kraker RT, Li Z, Bhatt AR, Boente CS, Crouch ER, Hubbard GB, Rogers DL, et al. Low- and very low-dose bevacizumab for retinopathy of prematurity: reactivations, additional treatments, and 12-month outcomes. Ophthalmology. 2022;129:1120–1128. doi: 10.1016/j.ophtha.2022.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Fleck BW, Reynolds JD, Zhu Q, Lepore D, Marlow N, Stahl A, Li J, Weisberger A, Fielder AR. Time course of retinopathy of prematurity regression and reactivation after treatment with ranibizumab or laser in the RAINBOW trial. Ophthalmol Retina. 2022;6:628–637. doi: 10.1016/j.oret.2022.02.006. [DOI] [PubMed] [Google Scholar]
- 45.Cool J, DeFalco TJ, Capel B. Vascular-mesenchymal cross-talk through Vegf and Pdgf drives organ patterning. Proc Natl Acad Sci USA. 2011;108:167–172. doi: 10.1073/pnas.1010299108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Barnett JM, Hubbard GB. Complications of retinopathy of prematurity treatment. Curr Opin Ophthalmol. 2021;32:475–481. doi: 10.1097/icu.0000000000000783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hartnett ME, Wallace DK, Dean TW, Li Z, Boente CS, Dosunmu EO, Freedman SF, Golden RP, Kong L, Prakalapakorn SG, et al. Plasma levels of bevacizumab and vascular endothelial growth factor after low-dose bevacizumab treatment for retinopathy of prematurity in infants. JAMA Ophthalmol. 2022;140:337–344. doi: 10.1001/jamaophthalmol.2022.0030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hellgren G, Löfqvist C, Hård AL, Hansen-Pupp I, Gram M, Ley D, Smith LE, Hellström A. Serum concentrations of vascular endothelial growth factor in relation to retinopathy of prematurity. Pediatr Res. 2016;79:70–75. doi: 10.1038/pr.2015.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Fidler M, Fleck BW, Stahl A, Marlow N, Chastain JE, Li J, Lepore D, Reynolds JD, Chiang MF, Fielder AR. Ranibizumab population pharmacokinetics and free VEGF pharmacodynamics in preterm infants with retinopathy of prematurity in the RAINBOW trial. Transl Vis Sci Technol. 2020;9:43. doi: 10.1167/tvst.9.8.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wang X, Sawada T, Sawada O, Saishin Y, Liu P, Ohji M. Serum and plasma vascular endothelial growth factor concentrations before and after intravitreal injection of aflibercept or ranibizumab for age-related macular degeneration. Am J Ophthalmol. 2014;158:738–744.e731. doi: 10.1016/j.ajo.2014.06.009. [DOI] [PubMed] [Google Scholar]
- 51.Kong Q, Ming WK, Mi XS. Refractive outcomes after intravitreal injection of antivascular endothelial growth factor versus laser photocoagulation for retinopathy of prematurity: a meta-analysis. BMJ Open. 2021;11:e042384. doi: 10.1136/bmjopen-2020-042384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Geloneck MM, Chuang AZ, Clark WL, Hunt MG, Norman AA, Packwood EA, Tawansy KA, Mintz-Hittner HA. Refractive outcomes following bevacizumab monotherapy compared with conventional laser treatment: a randomized clinical trial. JAMA Ophthalmol. 2014;132:1327–1333. doi: 10.1001/jamaophthalmol.2014.2772. [DOI] [PubMed] [Google Scholar]
- 53.Marlow N, Stahl A, Lepore D, Fielder A, Reynolds JD, Zhu Q, Weisberger A, Stiehl DP, Fleck B. 2-year outcomes of ranibizumab versus laser therapy for the treatment of very low birthweight infants with retinopathy of prematurity (RAINBOW extension study): prospective follow-up of an open label, randomised controlled trial. Lancet Child Adolesc Health. 2021;5:698–707. doi: 10.1016/s2352-4642(21)00195-4. [DOI] [PubMed] [Google Scholar]
- 54.Hwang CK, Hubbard GB, Hutchinson AK, Lambert SR. Outcomes after intravitreal bevacizumab versus laser photocoagulation for retinopathy of prematurity: a 5-year retrospective analysis. Ophthalmology. 2015;122:1008–1015. doi: 10.1016/j.ophtha.2014.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Stahl A, Lepore D, Fielder A, Fleck B, Reynolds JD, Chiang MF, Li J, Liew M, Maier R, Zhu Q, et al. Ranibizumab versus laser therapy for the treatment of very low birthweight infants with retinopathy of prematurity (RAINBOW): an open-label randomised controlled trial. Lancet (London, England) 2019;394:1551–1559. doi: 10.1016/s0140-6736(19)31344-3. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study.