Abstract
Behçet’s uveitis (BU), a vision-threatening manifestation of Behçet’s disease, poses substantial management challenges due to its chronic, relapsing nature and potential for vision loss. This review explores the role of biologic therapies in the treatment of BU, providing a comprehensive overview of their effectiveness, drawbacks, and future possibilities. Traditionally, management has relied heavily on corticosteroids and conventional immunosuppressants. However, their long-term use is frequently associated with systemic side effects and insufficient control of ocular inflammation. Biologic therapies, particularly TNF-alpha inhibitors like infliximab and adalimumab, have emerged as effective alternatives, offering better disease control and a more favorable safety profile. We critically evaluated these agents, noting their clinical efficacy in reducing inflammatory flares and preserving visual acuity. Despite their benefits, several issues remain. Accessibility, cost, and lack of long-term safety data limit their widespread use. Additionally, individual variability in treatment response necessitates personalized therapeutic strategies. Recent research has shown promise in addressing these challenges, with the emergence of novel biologic agents and personalized medicine approaches. In summary, biologic therapies represent a paradigm shift in BU management, contributing to better patient outcomes. Yet, there are significant challenges to be overcome. As we move forward, continued research, development of novel biologic agents, and a precision medicine approach will shape the future landscape of BU treatment.
Keywords: Behçet’s uveitis, Biologic, Anti-TNF-α, Infliximab, Adalimumab, IFN-α
Key Summary Points
| Biologic therapies are revolutionizing the treatment landscape for Behçet's uveitis (BU), demonstrating efficacy where conventional treatments have proven inadequate. |
| Despite their promising potential, biologic therapies also present unique challenges, including costs, accessibility, and adverse effects, requiring careful patient selection and ongoing monitoring. |
| TNF-α inhibitors, such as infliximab and adalimumab, have emerged as front-line therapies, with clinical trials showing strong efficacy in managing BU. |
| Continuous research and development efforts are essential to address the limitations of current biologic therapies as well as to explore novel potential therapeutic targets. |
| Global cooperation and meticulous data collection, via platforms like the AIDA International Registry, will significantly shape BU management, highlighting the potential of global health alliances. |
Introduction
Behçet’s uveitis (BU), a potent and recurrent ocular manifestation of the intricate systemic vasculitis known as Behçet’s disease, represents one of the most debilitating uveitic conditions, often culminating in considerable visual impairment and blindness [1]. This disease is intricately entwined with a multifaceted pathophysiology, hallmarked by chronic, recurrent bouts of inflammation [2, 3]. Historically, managing this incapacitating condition has proven complex because of its erratic disease progression, diverse clinical presentations, and variable responses to conventional immunosuppressive treatment regimens [4].
Heretofore, standard therapies including corticosteroids and non-biologic disease-modifying anti-rheumatic drugs (DMARDs) have served as the mainstay of treatment [5]. While these traditional therapies have borne some measure of success, they frequently yield inconsistent results, carry some side effect profiles, and risk disease resurgence upon dose reduction or discontinuation [5]. These predicaments underscore the dire necessity for more efficacious, safer, and better tolerated therapeutic strategies for BU management [6].
Recently, the advent and escalating acceptance of biologic therapies have drastically revolutionized the management landscape for BU. These avant-garde treatments, primarily interferon-α (IFN-α) and anti-tumor necrosis factor-α (anti-TNFα) antibodies, deliver targeted immune modulation and exhibit a favorable side-effect profile, thus offering a valuable alternative to traditional immunosuppressive therapies [7, 8]. Biologics reported for use in BU include anti-TNF agents (infliximab, adalimumab, golimumab, and etanercept), IFN-α 2a, anti-interleukin agents (anakinra, canakinumab, tocilizumab, secukinumab), anti-CD20—rituximab, and anti-CD52—alemtuzumab.
This review aims to deliver a thorough appraisal of the prevailing state of biologic therapies in the management of BU. Its principal objective lies in assessing the evidence supporting the efficacy and safety of these novel therapies. Specifically, this review will furnish a current synthesis of existing evidence, scrutinizing the benefits and potential pitfalls of these treatments compared with traditional therapies. In addition, it will elucidate the hurdles encountered during the implementation of these treatments and suggest potential trajectories for future research within this rapidly evolving field.
In providing a comprehensive, contemporary perspective on the present state and future potential of biologic therapies for BU, this review aspires to be a valuable tool for clinicians, researchers, and health policy-makers. Our overarching goal is to facilitate improved therapeutic decision-making and enhanced patient outcomes for this demanding and vision-threatening condition.
Methods
Literature Search Strategy
An exhaustive literature search was conducted independently by two authors (Biao Li and Qun Huang), spanning diverse databases such as Medline, Embase, the Cochrane Library, the Cochrane Controlled Trials Register, the Web of Science, and the Clinical Trials Registry, from their inception to March 1, 2023, devoid of linguistic constraints. Supplementary gray literature was harvested from corresponding databases. Correspondences were established with authors of published trials for potential supplementary data or unpublished evidence. Haoran Li meticulously devised, scrutinized, and implemented search strategies across databases.
To filter out irrelevant literature, the literature search was constrained to the abstract, keywords, and title fields. Search terms comprised “Behçet’s,” uveitis, iridocyclitis, retinitis, retinal vasculitis, panuveitis, uveit*, in addition to specific “biologics” such as adalimumab, etanercept, infliximab, anakinra, canakinumab, tocilizumab, secukinumab, rituximab, and alemtuzumab. Boolean operators AND, OR, and NOT were employed. Non-English articles were systematically translated with the support of an institutional librarian.
This article is based on previously conducted studies and does not contain any new studies with human participants or animals performed by any of the authors.
Inclusion and Exclusion Criteria
Given the limited number of randomized clinical trials, prospective studies, the disease's rareness, and therapeutic challenges, we incorporated both interventional and observational cohort studies into our review, irrespective of their prospective or retrospective, controlled or uncontrolled nature, appraising the efficacy of biologic agents for Behçet’s uveitis, regardless of their publication status. Single case studies and succinct case series were not excluded.
We eliminated letters to the editor, narrative and systematic literature reviews, meta-analyses, and studies addressing uveitis caused by other etiologies.
Study Selection Process
The relevance of studies for eligibility was independently appraised by two reviewers (Biao Li and Haoran Li), adhering to the defined inclusion criteria. Any arising disparities were resolved through discussion or, when required, mediation by a third reviewer.
Data Analysis
Owing to the restricted availability of randomized controlled trials (RCTs) and prospective studies, coupled with outcome measures' heterogeneity, results were not amalgamated for a meta-analysis.
Data Extraction
Data extraction was carried out autonomously by two authors (Biao Li and Haoran Li), who collected data from the selected articles using standardized forms. Extracted data were verified for accuracy. The harvested information encompassed the type of study design, patient count, age and gender distribution, follow-up period, and definitions of outcomes and results. For trials represented in multiple or sequential publications, data from the most recent or comprehensive publication were selected.
Results
Literature Search
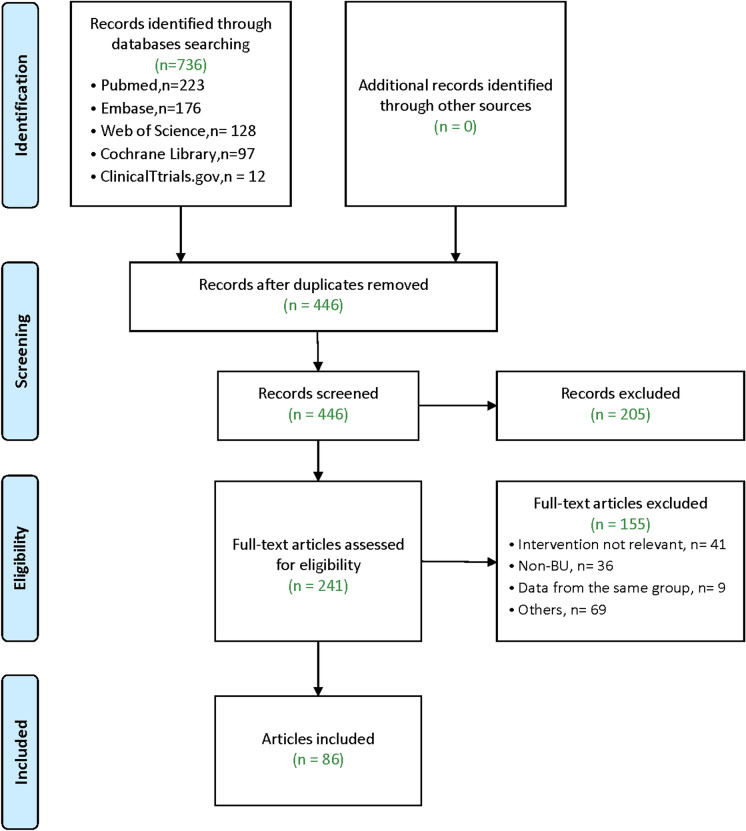
Electronic searches yielded a total of 736 records (Pubmed: 223; Embase: 176; Web of Science: 128; Cochrane Library: 97; ClinicalTrials.gov: 12). Neither alternate sources nor manual exploration unearthed additional references. Upon the removal of duplicates, a pool of 446 articles underwent screening based on their title and abstract, leading to the exclusion of 205 papers. Following a meticulous examination of the full text of the remaining 241 articles, we ultimately incorporated 86 articles into our review. A flow diagram elucidating the process of study selection, along with explicit exclusion criteria, is presented in Fig. 1. The comprehensive overview of the primary characteristics of biologic drugs used for Behçet's uveitis is concisely summarized in Table 1.
Fig. 1.
Flow diagram of the narrative review process
Table 1.
Main features of the biologic drugs for BU
| Drug names | Target | Route | Recommended dosage | Potential side effects |
|---|---|---|---|---|
| Infliximab | TNF-α | i.v | 5–10 mg/kg at baseline, 2nd and 6th week, then every 4–8 weeks | Infusion reactions, autoantibody formation, infection (particularly tuberculosis), demyelinating diseases |
| Adalimumab | TNF-α | s.c | Initial dose of 80 mg, followed by 40 mg every 2 weeks | Injection site reactions, infections, tuberculosis, demyelination, lupus-like syndrome |
| Golimumab | TNF-α | s.c | 50 mg per month | ANA positivity, development of resistance |
| Etanercept | TNF-α/β | s.c | 0.4 mg/kg twice a week | Exacerbate non-Behçet's syndrome ocular inflammation or even induce inflammation, infections, allergic responses |
| IFN-α | No specific target | s.c | 3–6 million units daily with slow taper | Flu-like symptoms, mild leukopenia/thrombocytopenia, elevated liver enzymes, depression, suicidal ideation, bone marrow suppression |
| Anakinra | IL-1 receptor | s.c | 100 mg/daily | Injection site reactions (for s.c.), infections, antibody development, allergic reactions |
| Canakinumab | IL-1β | s.c./i.v | 150 mg every 6 weeks | Injection site reactions (for s.c.), infections |
| Tocilizumab | IL-6R | i.v | 4 mg/kg every 4 weeks | Infections, viral hepatitis and TBC reactivation, elevated lipid parameters and transaminases, injection site reaction |
| Secukinumab | IL-17 | s.c./i.v | 150–300 mg every 4 weeks | Injection site reactions (for s.c.), infections, inflammatory bowel disease |
| Rituximab | B cells (CD20) | i.v | 375 mg/m2 body surface per week for 8 weeks, then every 4 weeks for 4 months | Infusion reactions, hepatotoxic, cardiovascular, fatigue, pruritis, nausea, urinary tract infections, antibody development |
| Alemtuzumab | Anti-CD52 | i.v | 30 mg, thrice a week for 12 months |
Infusion reactions, diminished thyroid function, bone marrow suppression, allergic reactions, reduced lymphocyte counts Abbreviations: BU Behçet’s uveitis; TNF-α/β tumor necrosis factor-alpha/beta; IL-1 interleukin-1; IFN-α interferon-α; IL-1β interleukin-1 beta; IL-6R interleukin-6 receptor; IL-17 interleukin-17; i.v. intravenous; s.c. subcutaneous; CD20 cluster of differentiation 20; CD52 cluster of differentiation 52; TBC tuberculosis |
Anti-TNF Agents
Tumor necrosis factor-alpha (TNF-α), a versatile cytokine implicated in numerous immune-mediated conditions, is chiefly secreted by monocytes-macrophages, Th1 cells, retinal parenchymal microglia, and pigment epithelial cells, playing a pivotal role in the pathogenesis and perpetuation of BU [9]. Inflammation in BU, predominantly orchestrated by Th1 lymphocytes, involves TNF-α, which contributes to retinal tissue damage by disrupting the blood-retinal barrier, exacerbating the inflammatory response, and interfering with effector T cell differentiation, as demonstrated by experimental autoimmune uveoretinitis (EAU) models [10]. Elevated TNF-α levels in serum and aqueous humor of BU patients [11], particularly during active inflammation [12], correlate with increased monocytes and T lymphocytes [13] and are markedly reduced following treatment with TNF-α inhibitors [14]. Numerous studies have shown that TNF-α inhibitors, such as IFX and adalimumab (ADA), yield significant improvements in BU outcomes, including inflammation remission, enhanced visual acuity, corticosteroid dose reduction, and diminished risk of severe visual loss [15, 16]. TNF-α inhibitors, initially employed for severe BU patients unresponsive to or intolerant of traditional immunosuppressive therapy, are now recommended as first-line treatment for visually impaired patients, reflecting augmented clinical experience and research [5]. Four commonly utilized anti-TNF therapies include IFX, ADA, golimumab, and etanercept (ETN).
Infliximab (IFX)
IFX, a chimeric anti-TNF-α monoclonal antibody, has exhibited rapid and effective treatment for BU and is approved for various autoimmune diseases, including rheumatoid arthritis, ankylosing spondylitis, and Crohn’s disease [17]. IFX functions by neutralizing membrane-bound and soluble TNF-α, suppressing TNF-α production, promoting Treg cell function, inhibiting activated T cells and monocytes, inducing T cell apoptosis, and ultimately impeding the downstream inflammatory cascade to alleviate inflammation [18].
In 2008, the EULAR recommended IFX for severe BU, leading to its approval in Japan for refractory cases and off-label use in other countries [19]. Besides, IFX effectively manages uveitis in patients requiring intraocular surgery, such as vitrectomy, while reducing the dosage of immunosuppressants and hormones, thereby providing favorable conditions for the surgery [20].
IFX, the most extensively studied and utilized TNF inhibitor for BU, is typically administered intravenously at a dosage of 3–5 mg/kg with an induction regimen at weeks 0, 2, and 6, with each medication completed within 3 h, followed by infusions every 8 weeks [21]. However, posterior segment involvement may necessitate shorter intervals to prevent recurrences [22]. Although its half-life is approximately 10 days, IFX’s downstream biological effects can persist for up to 8 weeks [23].
IFX has demonstrated efficacy in rapidly inducing remission and controlling ocular inflammation in BU in numerous studies [24], with advantages including minimal side effects, reduced immunosuppressive agent doses [25], and sustained remission in some cases after therapy cessation [26].
Initial studies confirmed IFX’s potent and rapid anti-inflammatory effect, demonstrating that repetitive infusions diminished the frequency and severity of ocular inflammatory episodes, improved or maintained visual acuity, and produced a significant corticosteroid-sparing effect. In 2001, Sfikakis et al. reported the first study using IFX for BU treatment, with all five refractory BU patients achieving complete resolution of uveitis within 1 week after receiving a single dose of IFX (5 mg/kg) infusion [27]. Since then, numerous investigations have attested to IFX's efficacy and safety in treating acute and recurrent BU. In 2004, Ohno et al. conducted the first properly structured multicenter clinical trial of anti-TNF treatment in BU [28]. Based on this trial, the Japanese Ministry of Health approved IFX in 2007 for treating refractory Behçet’s uveoretinitis. The accumulating evidence underscores the promising role of IFX in managing BU and enhancing patient outcomes.
Keino et al. reported that IFX effectively reduced not only the frequency and severity of ocular inflammatory episodes but also background retinal vascular and disc leakage in refractory BU [29]. A 2017 study by Japan and the US demonstrated that IFX treatment led to significantly decreased serum TNF-a levels and improved visual acuity in BU patients [14]. The therapeutic effect was more pronounced in patients with the first onset of the disease compared to those with recurrent cases, indicating the importance of early intervention. In 2021, Keino et al. supported these findings, showing that initiating IFX treatment early, when patients had better baseline visual acuity, resulted in improved long-term visual outcomes and sustained remission after discontinuation of the therapy [30]. In 2021, a large multicenter study involving 103 Spanish patients with recurrent BU found IFX treatment effectively controlled uveitis and maintained disease stability [25]. Remission was achieved in 76.5% of patients treated with IFX for an average of 31.5 ± 23.5 months, with progressive improvement observed in both patients who had therapy optimization after remission and those on the standard regimen during a mean follow-up of 46.6 ± 18.4 months. The study concluded that IFX optimization was effective, safe, and cost-effective, encouraging its use in countries where the cost of biologic treatment is a major concern. In 2022, Yamana et al. reported a continued decrease in ocular attacks and Behçet's Disease Ocular Attack Score 24 (BOS24) even after > 5 years of IFX therapy [31], while Kose et al. found that half of the patients who discontinued IFX in remission remained attack-free for 12–90 months [26]. These findings further substantiate the efficacy and long-term benefits of IFX in managing BU, with implications for improving the quality of life for affected patients.
IFX consistently outperforms conventional therapy in reducing inflammation, improving visual acuity, and decreasing ocular complications in BU patients. Markomichelakis et al. conducted a comparative study evaluating the efficacy of a single intravenous infusion of IFX versus intravitreal triamcinolone, discovering that IFX was not only more effective at diminishing total ocular and fundus inflammation but also exhibited a faster onset of action than corticosteroid therapy [32]. The prevalence of retinal vasculitis decreased from 79% at baseline to 15% by the 14-day follow-up in the IFX group, while the intravitreal triamcinolone acetonide group displayed a reduction from 100 to 87.5%. This prospective observational study demonstrated a significantly faster resolution in ocular inflammation scores for patients receiving IFX infusion compared to high-dose intravenous methylprednisolone or intravitreal triamcinolone acetonide administered during acute panuveitis attacks. In a 2008 nonrandomized, retrospective comparative study, BD patients treated with IFX exhibited substantially improved outcomes compared to those receiving conventional therapy (oral prednisone, cyclosporine, and azathioprine or methotrexate) [33]. The IFX group experienced fewer uveitis flares, diminished inflammation, enhanced visual acuity at 24 months, and a reduced number of relapses during the infusion period. In 2010, another study found that IFX was more effective than ciclosporin A (CsA) in mitigating acute uveitis episodes in patients with refractory uveoretinitis associated with BD, although no significant differences in visual acuity improvement were observed between the two therapies [34]. In 2019, a systematic review, which included nine studies encompassing 78 BU patients, revealed that IFX was more effective than cyclosporin in treating the condition [35]. Collectively, IFX has consistently demonstrated its ability to reduce inflammation, improve visual acuity, and decrease ocular complications in BD patients compared to conventional therapy.
Intravitreal administration of IFX, in addition to its intravenous application, has been explored as a potential method for treating BU. Numerous studies have demonstrated the safety and effectiveness of this approach in ameliorating ocular inflammation; however, it remains uncertain whether its efficacy surpasses that of systemic medication. Investigations of IFX aim to circumvent systemic adverse effects. Markomichelakis et al. reported that intravitreal IFX doses of 1–1.5 mg yielded improvements in intraocular inflammation, visual acuity, and central macular thickness in the short term, although the effect was not as rapid as that for intravenous IFX infusion [36]. Hamza et al. demonstrated the safety and efficacy of a single 1 mg/0.05 ml intravitreal IFX injection in 20 patients with refractory uveitis due to BD [37]. At 18 weeks of follow-up, they observed statistically significant improvements in mean visual acuity, reductions in mean central macular thickness, and decreased mean vitreous haze scores. In contrast, an open-label study of monthly intravitreal IFX injections reported a high ocular complication rate and failure to control BD uveitis, leading the authors to advice against this approach [38]. Studies have discovered that excessively high IFX concentrations in the vitreous cavity may provoke uveitis because of immune response; therefore, this therapy is recommended as an alternative treatment for patients who cannot tolerate systemic IFX medication [36].
IFX treatment for BU presents the challenge of recurrent inflammation, which often occurs 7–8 weeks post-medication and intensifies over time, necessitating multiple injections and abbreviated infusion intervals for some patients [39, 40]. Recurrence may be associated with diminished blood concentrations of IFX [41], rapid reduction of steroids and immunosuppressants [22], and the development of neutralizing antibodies against IFX with repeated infusions [42]. Concurrent treatment with conventional disease-modifying antirheumatic drugs (cDMARDs), such as azathioprine and/or cyclosporine A, has been advised to prevent secondary failure [5], although a study by Fabiani et al. found the infliximab retention rate remained high and unaffected by cDMARDs [43]. Sugita et al. proposed measuring blood concentrations of IFX to determine re-administration timing and prevent inflammation recurrence [41], noting that patients with a high population of Foxp3-expressing Treg cells experienced fewer acute uveitis episodes. Ueda et al. reported that patients with higher pre-treatment serum levels of IL-2, IL-6, and TNF-α responded better to IFX therapy [40], and initiating IFX therapy earlier led to better outcomes [44].
In uveitis treatment, IFX demonstrates favorable long-term tolerability and manageable adverse effects in numerous studies. Common side effects of IFX include infusion reactions, autoantibody formation, infection (particularly tuberculosis), and demyelinating diseases [42], necessitating pre-treatment TB screening and cautious use in individuals at risk for demyelinating disease [45]. Although serious side effects, such as malignancy development, multiple sclerosis, and lupus-like reactions, are exceedingly rare [46], patients susceptible to recurrent opportunistic infections should be closely monitored, and those with active infections should avoid IFX therapy [47]. The BRIGHT study reported a 32% incidence of adverse events and 6% of serious adverse events in IFX-treated BU patients in Japan [46], while another study found 28% and 13% incidence rates [48], respectively. Additional side effects encompass cancer, lupus-like syndrome, and allergic reactions [39].
In conclusion, IFX has proven to be a valuable therapeutic option for BU, demonstrating efficacy in controlling inflammation, preserving visual acuity, and enabling corticosteroid dose reduction. Early intervention with IFX is crucial for achieving better long-term outcomes and sustained remission after therapy cessation. Future research, including large-scale, randomized, controlled trials, is essential to establish optimal treatment strategies and determine the long-term safety profile of IFX for patients with BU.
Adalimumab (ADA)
ADA is a fully humanized monoclonal IgG1 antibody targeting TNF-α, binding to both soluble and transmembrane forms, and is currently the only approved biologic DMARD for non-infectious intermediate, posterior, and panuveitis [49]. Commonly used for autoimmune diseases like rheumatoid arthritis and non-infectious uveitis [50], ADA has proven highly effective as an alternative to IFX or when patients prefer subcutaneous infusions over intravenous injections [51]. ADA is approved for uveitis treatment with an initial 80 mg subcutaneous dose, followed by 40 mg every 2 weeks, and has been well tolerated [21]; intravitreal ADA at a 1.5-mg dose has been proposed as a potential adjunctive therapy for controlling breakthrough inflammation in BU patients on systemic ADA [52].
In 2007, Mushtaq et al. first reported the successful use of ADA in treating three BU patients unresponsive to other immunosuppressive agents and IFX [53]. All three patients maintained disease remission and visual acuity without relapse during a 24-month follow-up period after switching to ADA. Numerous studies have since confirmed ADA's efficacy and safety in treating BU, demonstrating comparable effectiveness and fewer side effects than IFX [54], while also enabling dosage reductions for concurrent immunosuppressive agents [55] and corticosteroids [56].
The FDA and EMA approved ADA for BU based on the VISUAL-1 [57] and VISUAL-2 [58] multicenter phase III randomized controlled trials (RCTs), which evaluated its efficacy and safety in active (VISUAL I) and inactive (VISUAL II) non-infectious uveitis patients. Both trials enrolled patients with various causes of uveitis, but only 7% had BU. VISUAL I involved 217 patients from 18 countries, while VISUAL II included 226 patients from 21 countries. The primary outcome in VISUAL I was time to treatment failure (TF), while in VISUAL II, the primary outcome was assessed at intervals until TF occurred. The efficacy of ADA in BU uveitis was not specifically evaluated in VISUAL I, and VISUAL II did not report primary outcomes by etiology, limiting their relevance for this review. In terms of retinal involvement, one RCT reported significant improvements with ADA, while another did not find differences between ADA and placebo in time to macular edema. For inflammatory activity, one RCT showed ADA significantly improved vitreous haze scores in patients with active uveitis, but no differences were observed in the inactive uveitis group. In terms of visual functioning, one RCT reported significant improvements in the ADA group compared to the placebo for the VFQ-25 score, near vision, and ocular pain subscores, but not for distance vision. However, no differences were found in patients with inactive uveitis between ADA and placebo groups.
In the phase 3, open-label, multicenter clinical trial extension (VISUAL III), researchers evaluated the safety and efficacy of ADA in patients with non-infectious intermediate, posterior, or panuveitis [59]. Participants included adults who either met treatment failure criteria or completed VISUAL I or II without treatment failure. Patients received ADA 40 mg every other week, and interim follow-up data were analyzed from weeks 0 to 78. Results indicated that 60% of patients with active uveitis achieved quiescence at week 78, while 66% of those were corticosteroid-free. Additionally, 74% of patients with inactive uveitis achieved quiescence at week 78, with 93% of those being corticosteroid-free. In patients with active uveitis, best-corrected visual acuity (BCVA) improved, and corticosteroid doses decreased. No new safety signals were identified. In the long-term extension study (VISUAL III), ADA treatment led to quiescence and reduced corticosteroid use in patients with active uveitis and maintained quiescence for those with inactive uveitis. Adverse events were consistent with the known safety profile of ADA.
In 2019, Atienza et al. conducted a large-scale, multicenter study to compare the efficacy of IFX and ADA in treating BU, including 177 patients with refractory BU [60]. Both treatment groups showed improvements in ocular parameters after 1 year, with the IFX group experiencing more rapid improvement in anterior chamber inflammation and vitritis. In contrast, the ADA group demonstrated significantly greater visual improvement and a higher drug retention rate.
In 2021, a retrospective analysis of treatment-naive BU patients who received ADA plus conventional therapy as an initial line of treatment or conventional therapy alone reported significantly better results in the ADA group, including lower relapse, better fluorescein angiography scores and visual acuity, and lower corticosteroid dose at the last visit [61].
ADA presents several advantages in treating BU, such as a reduced risk of anti-drug antibody development [62], more stable serum concentrations, and an ameliorated side effect profile [63]. Its subcutaneous administration facilitates self-administration at home, fostering improved patient compliance [55]. ADA is the only approved biologic for non-infectious uveitis and can achieve long-term control even without concomitant cDMARD treatment [64]. Moreover, ADA's marginally lower treatment cost compared to IFX renders it more favorable for patients' long-term adherence [65].
In conclusion, ADA shares a similar safety profile with IFX but exhibits a lower incidence of side effects, as it is a fully humanized monoclonal antibody, diminishing the risk of allergic reactions and anti-drug antibody formation. The most prevalent side effects encompass injection site reactions, infections, and heart failure, while other concerns include tuberculosis, demyelination, and lupus-like syndrome. Despite these potential side effects, the incidence of adverse events leading to drug discontinuation remains low.
Golimumab
Golimumab, a fully human monoclonal antibody characterized by low immunogenicity and an extended half-life, is administered subcutaneously at a standard dose of 50 mg every 4 weeks [66]. As the only TNF antagonist proven effective in patients refractory to other anti-TNF agents, golimumab neutralizes TNF activity by binding both soluble and transmembrane TNF, inhibiting its interaction with TNF receptors [67]. Golimumab is a novel, fully human anti-TNF-α monoclonal antibody approved for the treatment of rheumatoid arthritis, psoriatic arthritis, and ankylosing spondylitis, demonstrating highly promising results [66].
Golimumab has shown promise in managing refractory Behçet’s uveitis while exhibiting a side effect profile comparable to other anti-TNF-α therapies. In 2013, Mesquida et al. reported a case of BU refractory to IFX, successfully treated with golimumab injections [68]. The inflammation resolved, and the dosages of adjunctive cyclosporine-A and prednisone were reduced, resulting in quiescent uveitis and 6/6 visual acuity after 6 months of treatment. Golimumab’s side effect profile is similar to that of other anti-TNF-α therapies. In a 2016 study, Santos-Gómez et al. demonstrated golimumab's efficacy in four patients with BD-associated uveitis who had refractory uveitis unresponsive to ADA and/or IFX [69]. All four patients achieved complete remission of uveitis at 1 year of follow-up, and mean best-corrected visual acuity improved significantly from baseline to 3-month follow-up. The study suggests that golimumab may be effective in managing refractory BU uveitis and was well tolerated without serious side effects. Case reports by Vitale et al. [70] and Fabiani et al. [71] demonstrated golimumab’s effectiveness in alleviating intraocular inflammation in BU patients, reducing uveitis recurrence, and significantly decreasing the use of glucocorticosteroids, with adverse effects comparable to other TNF-α inhibitors. In 2017, Vitale et al. evaluated golimumab as a treatment option for BD in 17 patients in a retrospective study, with a mean treatment duration of 18.47 months [70]. The study found golimumab effectively controlled BD-related manifestations in 94.1% of cases, significantly improved the BD Current Activity Form (BDCAF) score, and exhibited a notable corticosteroid-sparing effect. In 2019, Fabiani et al. further confirmed golimumab's efficacy in managing BD-related uveitis, achieving complete control of intraocular inflammation in 87.5% of eyes at the 12-month follow-up [71]. The number of relapses reduced significantly after golimumab initiation, and active retinal vasculitis resolved in all affected eyes by the 3-month follow-up. The study confirms golimumab’s effectiveness in reducing intraocular inflammation in BD, by both decreasing the number of uveitis relapses and achieving prompt resolution of active retinal vasculitis.
Golimumab presents several advantages in the treatment of BU, including reduced cost, extended injection intervals, and a diminished risk of developing neutralizing antibodies compared to other anti-TNF agents such as IFX. As a fully human monoclonal antibody, golimumab exhibits a lower propensity for eliciting allergic infusion reactions and loss of efficacy, although resistance may still emerge. The therapy also benefits from a less frequent dosing schedule, permitting monthly subcutaneous self-administration, which circumvents the time and expenses associated with intravenous treatments. Golimumab's parenteral administration facilitates rapid absorption and an accelerated onset of action, evident after the first dose in various studies [66]. The drug’s rapid and sustained effect, combined with its lower immunogenicity compared to IFX, may contribute to enhanced long-term response maintenance without compromising efficacy.
Etanercept (ETN)
ETN, a humanized fusion protein composed of two p75 TNF receptors and an Fc molecule, competitively binds to TNF-α, disrupting its interaction with membrane receptors and inhibiting TNF-α-mediated immune responses. Commonly employed for juvenile arthritis and other autoimmune conditions, ETN has exhibited efficacy in treating mucocutaneous and articular manifestations in BD patients, yet demonstrates weaker effectiveness for BU compared to IFX [72]. Administered as a 20-mg subcutaneous injection biweekly, ETN has proven effective in managing BU as well as mucocutaneous and gastrointestinal disease manifestations [73].
Current evidence-based medicine for treating BU with ETN is limited, as only a few small case studies illustrate its efficacy in controlling ocular inflammation and reducing glucocorticosteroid dosage. Among these studies, the largest reported outcomes for ten patients with severe uveitis unresponsive to combination therapy with corticosteroids, azathioprine, and cyclosporine-A [74]. The addition of ETN to the treatment regimen led to decreased ocular inflammation, improved visual acuity, and corticosteroid dose reduction. However, uveitis recurred in all patients within 6 months of ETN discontinuation. Side effects experienced with ETN were akin to those observed with other anti-TNF-α agents.
ETN has been found to potentially exacerbate non-Behçet’s syndrome ocular inflammation, with the underlying pro-inflammatory mechanism remaining elusive [75]. Paradoxically, ETN-induced ocular inflammation has been reported in non-BD cohorts, and it may even aggravate the course of autoimmune uveitis [76]. Consequently, ETN is not routinely employed as a first-line agent in managing BU. A 2014 systematic review recommended ETN as a second-line treatment for refractory BU because of its lower success rates, reserving it for cases of intolerance to IFX or ADA [77].
Potential adverse reactions associated with etanercept in the treatment of Behçet's uveitis can be deduced from the data on other anti-TNF-α agents, as research specifically targeting etanercept for this condition remains scarce. Common side effects may include injection site reactions, infections, allergic responses, and heart failure, while less prevalent adverse events could encompass gastrointestinal complications, demyelinating disorders, and tumor development.
In conclusion, ETN demonstrates weaker effectiveness for BU compared to IFX and ADA and is recommended as a second-line treatment for refractory cases.
IFN-α
In addition to TNF-alpha inhibitors, EULAR endorses IFN-alpha, an early biological agent with antiviral and immunoregulatory properties [78], as a first-line therapy for BU [5], exhibiting comparable efficacy to TNF-alpha inhibitors in managing refractory cases. Commercially available recombinant IFN-alpha subtypes, IFN-alpha-2a and IFN-alpha-2b, have been utilized in subcutaneous injections for BD treatment, with IFN-alpha-2a demonstrating a superior response rate for ocular manifestations [78]. The precise mechanism of IFN-alpha in treating BU remains uncertain but may encompass immunomodulatory effects, inhibition of lymphocyte proliferation, and antagonism of TNF-alpha [79, 80]. Research has revealed that IFN-alpha reduces peripheral blood T cell numbers, impedes T cell adhesion, induces regulatory T cells, and can suppress IL-17 to alleviate uveitis, thereby enhancing long-term prognosis for severe cases [79, 81].
IFN-alpha 2a, approved for various conditions such as chronic viral hepatitis, myeloproliferative syndromes, and select solid tumors and lymphomas, is also recommended for refractory BU and specific instances of mucocutaneous and chronic joint involvement [5]. Currently, there is no consensus on the ideal IFN-alpha treatment regimen for BU, including dosage and therapy duration. An initial treatment generally involves a higher dose of 3–9 MIU/day via subcutaneous or intramuscular injection, followed by a gradual tapering to a maintenance dose of 3 MIU 2–3 times a week, with treatment typically lasting 12–18 months [79, 82]. Steroids and immunosuppressants may interfere with IFN-alpha therapy and should be discontinued or reduced to 10 mg/day prior to initiating treatment [83]. In cases of insufficient initial clinical response or relapses during dose reduction, increasing IFN-alpha doses has proven to be an effective management strategy.
IFN-α therapy has shown significant potential as a viable treatment for refractory BU, offering notable clinical outcomes and reduced corticosteroid usage with minimal complications. First employed in the mid-1980s for its antiviral properties, IFN-α therapy has demonstrated substantial efficacy in managing refractory BU, achieving enduring remissions, improving visual acuity, and reducing inflammation recurrence and corticosteroid usage, with success rates approximating 90% across numerous studies [84, 85]. Studies by Lightman et al. and Park et al. emphasized the substantial corticosteroid reduction effect of IFN-α on BU patients, with varying outcomes on visual acuity [82, 86]. In 2019, two Chinese investigations illustrated the efficacy of IFN-α-2a in treating refractory BU, reporting success rates of approximately 90%, diminished ocular inflammation recurrence, and marked reduction in steroid and immunosuppressive agent dosages, with minimal adverse reactions [84, 85]. The first study, a retrospective cohort involving 30 patients, achieved treatment success in 86.7% of cases [85]. In contrast, the second study, a prospective observational cohort encompassing 127 patients, observed an effectiveness rate of 91% in managing the condition [84]. These findings underscore the potential of IFN-α-2a as a viable treatment option for refractory BU, providing notable clinical outcomes with minimal complications.
IFN-α therapy has demonstrated long-term efficacy in BU patients, providing sustained inflammation control, improved visual acuity, and reduced recurrence rates even after treatment discontinuation. Deuter et al.’s studies demonstrated long-term remission of BU using IFN-2α therapy [87]. In their study, visual acuity improved or remained stable in 91 out of 96 eyes during a median follow-up of 6 years, and 50% of patients achieved complete remission of ocular inflammation 46 months after the first IFN-2α course. In 2010, Sobaci et al. assessed the safety and efficacy of IFN-αlpha-2a for treating refractory BU, finding an 84.9% response rate after 1 year, with reduced uveitis recurrence and improved visual acuity [88]. During the 2-year follow-up, 28.3% of patients discontinued treatment and remained disease-free for an average of 28.0 ± 13.1 months. Kavandi et al.’s 2016 study provided further evidence of long-term efficacy in BU patients treated with IFN-α, showing sustained inflammation control even after treatment discontinuation [89]. They reported that eight patients experienced improved or stable visual acuity, with the disease remaining in remission and no adverse effects 2 years after ceasing IFN-α-2a therapy.
IFN-α consistently outperforms conventional immunosuppressive treatments in reducing relapse rates and improving visual outcomes for BU patients, as evidenced by multiple studies. A 2017 retrospective study by Hasanreisoglu et al. discovered that IFN-α significantly reduced treatment failure and relapse rates, with side effects comparable to conventional therapy (AZA + CsA), except for fever and flu-like symptoms [90]. In a 12-month randomized, controlled, prospective trial, the efficacy and safety of IFN-α-2a and cyclosporine-A (CsA) were evaluated and compared in patients with refractory BU [91]. The study determined that the response and complete remission rates were higher in the IFN-α2a group (85.7% and 50.0%, respectively) compared to the CsA group (66.7% and 25.0%, respectively), with complete remission achieved more rapidly with IFN-α2a. Additionally, logMAR best-corrected visual acuity significantly improved in the IFN-α2a group but not in the CsA group, with the IFN-α2a group also demonstrating a lower BU attack score 24 (BOS24) compared to the CsA group. In conclusion, IFN-α2a plus corticosteroid appears to induce better treatment response, greater visual acuity improvement, and more stable remission of intraocular inflammation over a 12-month period compared to CsA plus corticosteroid. The major limitation of this trial was the small sample size, including 14 patients in the IFN-α group and 12 in the cyclosporine group.
IFN-alpha therapy for BU has demonstrated high response rates between 80 and 90% [84, 85], with low relapse rates upon treatment cessation, and long-term follow-up results reveal relapse-free rates of 50% and 76% [87, 92]. This therapy enables reduction of concurrent corticosteroid doses, improving patient quality of life, and can be safely used in patients with a history of viral infections such as viral hepatitis or latent tuberculosis [77, 86]. Compared to anti-TNF-alpha agents, IFN-alpha carries no risk of tuberculosis reactivation and can be used in patients with coexisting hepatitis B virus infections [85, 91]. PEGylated IFN-alpha, which has a longer serum half-life and requires fewer injections, has been used to maintain remission, but its efficacy and optimal dosage as induction therapy for BU remain unknown [86].
IFN-alpha therapy for BU has shown promising results, but side effects are common and can be severe, with flu-like symptoms occurring in approximately 90% of patients in the initial weeks of treatment [93]. Other side effects include mild leukopenia/thrombocytopenia, elevated liver enzymes, hair loss, depression, and suicidal ideation, with some serious side effects such as bone marrow suppression and injection-site reactions also reported [94]. Practical limitations of IFN-alpha therapy encompass the frequent occurrence of flu-like symptoms, the risk of depression, and reports of suicidal ideation. Despite these challenges, more clinical trials, ideally randomized, placebo-controlled ones, are needed to make an informed decision about the routine use of IFN-alpha in treating uveitis caused by BD.
In conclusion, IFN-α demonstrates promising efficacy in managing refractory BU, achieving high response rates and long-term remission while enabling corticosteroid dose reduction. However, its use can be limited by common and potentially severe side effects, such as flu-like symptoms and depression. Further randomized, placebo-controlled clinical trials are needed to fully establish IFN-alpha's role in treating BU.
Anti-Interleukin-1
Interleukin-1 (IL-1) is a pro-inflammatory cytokine primarily produced by macrophages, monocytes, and dendritic cells, playing a pivotal role in various autoimmune inflammatory diseases, including Behçet’s syndrome [95]. IL-1β operates through IL-1 receptor type I (IL-1RI) and accessory protein (IL-1RAcP) to augment the innate immune system's response, inducing inflammation and the expression of additional cytokines, chemokines, and pro-inflammatory molecules [95]. Elevated levels of IL-1β play a substantial role in numerous inflammatory diseases, including BD, where circulating monocytes produce copious amounts of this cytokine [95]. Studies have found that serum IL-1β and IL-1RA levels in BD patients are significantly higher than those in healthy controls, emphasizing the correlation between BD and IL-1β [96].
IL-1 blockade has a favorable therapeutic effect on BU, particularly in patients with longer disease duration and eye involvement, as evidenced by studies demonstrating sustained response to IL-1 inhibitors in these subsets of BD patients. IL-1 inhibition is an appealing therapeutic option for BU patients with multi-drug-resistant manifestations and may be a safer alternative to anti-TNF treatments due to a lower risk of opportunistic infections, including tuberculosis [97]. Studies suggest that IL-1 blockers, including anakinra and canakinumab, are better tolerated and safer, particularly in areas with high tuberculosis prevalence. However, the limited clinical experience and lack of large-sample randomized controlled trials warrant further investigation to determine the true efficacy and safety of IL-1 blockers in treating BU.
Anakinra (ANA)
Anakinra, a non-glycosylated recombinant IL-1 receptor antagonist, inhibits IL-1α and IL-1β to alleviate inflammation and modulate immune response. It is commonly employed to treat autoimmune diseases and juvenile arthritis-associated uveitis [95]. Although its use in BU has been reported primarily in small case studies, ANA has demonstrated success in treating various autoinflammatory conditions, including refractory cases in a preclinical experimental autoimmune uveitis mouse model [98]. ANA is typically administered as a 100–200 mg/day subcutaneous injection, with dose adjustments or switching to other biologic agents considered for patients exhibiting low response or partial results [39].
To date, there are limited reports on the use of ANAin BU. In 2013, Emmi et al. first demonstrated anakinra's efficacy in treating vitritis and restoring the retinal-blood barrier in both eyes of patients with resistant BU [99]. In 2015, Cantarini et al. treated nine BD patients, including five with uveitis, who were refractory to TNF inhibitors using daily subcutaneous ANA injections [100]. The treatment led to the resolution of ocular inflammation in all five uveitis patients and disease activity in eight out of nine patients within 4 weeks, without any reported adverse events. However, most patients experienced relapse within months, warranting further studies to determine whether increasing ANA dosage could prevent recurrence. In 2017, Orlando et al. used ANA to treat a patient with refractory BU, successfully relieving inflammation when the dose was increased to 150 mg/day, but subsequently switched to canakinumab because of drug-related generalized urticaria [97]. Conversely, Ugurlu et al. reported in a case study that ANA was ineffective against BU, while canakinumab effectively controlled the inflammatory response [101].
In conclusion, ANA has demonstrated potential for treating BU in limited case studies, with some patients experiencing resolution of ocular inflammation. However, the efficacy varies, and relapse is common, indicating a need for further research to determine optimal dosages or alternative biologic agents. Common side effects associated with ANA include injection site reactions and generalized urticaria.
Canakinumab
Canakinumab, a fully human monoclonal antibody, selectively targets IL-1β by competitively binding to the IL-1 receptor, inhibiting signal transduction and neutralizing the cytokine’s bioactivity in inflammatory disorders [102]. Canakinumab boasts encouraging clinical safety and pharmacokinetic properties, demonstrating potential in treating cryopyrin-associated periodic syndromes and possibly other complex inflammatory diseases, such as rheumatoid arthritis, systemic-onset juvenile idiopathic arthritis, and ocular diseases [103]. Presently, canakinumab is primarily employed for treating autoinflammatory diseases. The typical dosage for treating BU is a 150 mg subcutaneous injection every 6–8 weeks. Compared to anakinra, canakinumab has a longer treatment interval, potentially improving patient compliance [95].
Canakinumab has demonstrated potential in effectively managing refractory BU and reducing corticosteroid dependency in many studies. In 2012, Ugurlu et al. reported successful treatment of a 16-year-old woman with refractory BU using a single 150 mg subcutaneous injection of canakinumab [101]. The patient experienced complete resolution of ocular inflammation, significant improvement in visual acuity, and sustained relief for up to 8 weeks without severe adverse effects. This case showcased canakinumab’s potential efficacy in treating BD-associated panuveitis, though the report’s main limitations were the single case and brief follow-up duration. In 2017, Orlando et al. employed canakinumab (150 mg/6 weeks) to treat a patient with similarly refractory BU [97]. Over the 36-month follow-up period, inflammation was well controlled, no recurrence was observed, and the treatment exhibited a favorable safety profile. In a 2017 multicenter study, Fabiani et al. evaluated the efficacy of ANA and canakinumab in treating BU in 19 patients (31 eyes) over a 12-month period [104]. The study demonstrated a significant reduction in ocular inflammatory flares, from 200 episodes/100 patients/year to 48.87 episodes/100 patients/year (p < 0.0001), and a significant decrease in retinal vasculitis (p < 0.0001 and p = 0.001, respectively). Furthermore, systemic steroid dosage was significantly reduced at the 12-month visit compared to baseline (p = 0.02). The authors concluded that IL-1 inhibitor therapy, such as ANA and CAN, effectively manages refractory BD-related uveitis, providing long-term control of ocular inflammation and a corticosteroid-sparing effect.
In conclusion, Canakinumab has demonstrated potential in treating refractory BU, providing long-term control of ocular inflammation, a corticosteroid-sparing effect, and a favorable safety profile.
Anti-Interleukin-6—Tocilizumab
IL-6 is a cytokine with multifaceted activity, playing a pivotal role in modulating immune inflammatory responses by inducing B cell differentiation, promoting antibody production, and influencing T lymphocyte differentiation [105]. It directs CD4+ Th cell differentiation into Th17 cells and suppresses TGF-β-induced Treg differentiation [106], resulting in an imbalance between Th17 and Treg cells [107]. This Th17/Treg imbalance is implicated in the pathogenesis of various autoimmune diseases, including BU [108]. Tocilizumab, a humanized monoclonal antibody against the IL-6 receptor, effectively inhibits IL-6-mediated inflammatory reactions and regulates diverse lymphocyte-mediated immune responses [109]. By precluding IL-6 binding to both transmembrane and soluble IL-6 receptors, tocilizumab obstructs IL-6-mediated signal transduction and is approved for treating inflammatory diseases such as rheumatoid arthritis [110]. Research reveals elevated IL-6 concentrations in the vitreous humor of patients with non-infectious uveitis, encompassing idiopathic cases and those associated with systemic inflammatory diseases like sarcoidosis, Vogt-Koyanagi-Harada syndrome, and BU. These findings suggest potential therapeutic benefits of IL-6 inhibition in refractory uveitis cases [108].
Tocilizumab, an approved biological drug for treating rheumatoid arthritis, systemic and polyarticular juvenile idiopathic arthritis, and Castleman's disease [105], has exhibited efficacy in managing various refractory ocular inflammations, including BU [111]. Specifically, tocilizumab appears suitable for BU patients with moderate to severe background leakage and associated cystoid macular edema (CME). It is administered at a standard dose of 4–12 mg/kg every 2–4 weeks via intravenous infusions or 162 mg weekly subcutaneous injections, either as monotherapy or in combination with cDMARDs.
Tocilizumab has shown potential in treating refractory BU, improving visual acuity and reducing ocular attack frequency in some studies. In 2012, Hirano et al. reported a case of a BD patient with severe posterior uveitis unresponsive to conventional immunosuppressive treatment and resistant to IFX after 15 months [112]. Tocilizumab monotherapy (8 mg/kg, every 4 weeks) was initiated, resulting in remission of systemic manifestations such as genital ulcers and erythema nodosum, a significant decrease in ocular attack frequency and severity, and improved visual acuity, with the disease remaining quiescent for 12 months. The authors also observed serum IL-6 concentrations correlating with disease activity, suggesting tocilizumab's efficacy. This report marked the first successful treatment of refractory BU with tocilizumab, also alleviating other clinical manifestations. The main limitation of this study is its representation of a single case. Since then, numerous studies have demonstrated that tocilizumab is safe and effective in treating BU. A multicenter retrospective study of 25 patients with refractory cystoid macular edema (CME) secondary to non-infectious uveitis, including seven BU patients, found significant improvements in central macular thickness and visual acuity after 12 months of tocilizumab therapy [113]. The treatment facilitated a reduction in the daily dose of prednisone, with remission achieved in 14 patients and only minor side effects observed during the follow-up period. In the STOP-uveitis study, a randomized controlled trial, monthly intravenous infusions of 4 mg/kg and 8 mg/kg tocilizumab were found to be safe and equally effective in treating naïve and previously treated patients with non-infectious uveitis involving the posterior segment [114].
Several studies have reported favorable outcomes in recalcitrant BU when treated with tocilizumab [112, 113, 115, 116], particularly after failure of anti-TNFα therapy [117]. A systematic literature review investigating the effectiveness of tocilizumab in BD patients found significant improvement in almost all patients with BU (24/25) [117]. Tocilizumab also proved effective in reducing glucocorticoid doses, with glucocorticoid-free remission achieved in several cases. However, the treatment was not highly effective for other manifestations, such as mucocutaneous and articular involvement, and even worsened some oral or genital ulcers and skin lesions. A recent multicenter study of 16 refractory BD cases treated with tocilizumab primarily for ocular (14) and neurological (2) involvement demonstrated the variable effectiveness of tocilizumab on different BD manifestations [116]. Patients had previously received several conventional and/or biological immunosuppressants such as methotrexate, cyclosporine, ADA, or IFX before tocilizumab treatment. After a median follow-up of 20 months using tocilizumab, neurological and ocular domains improved, with most uveitis patients achieving complete remission. However, tocilizumab proved less effective for oral/genital ulcers, skin lesions, and intestinal manifestations, suggesting its primary effectiveness in BD cases with major clinical involvement.
Contrary to the aforementioned results, in a single-arm observational study, tocilizumab showed unsatisfactory results for acute BU attacks in three consecutive Chinese patients who had responded poorly to glucocorticosteroids or other biologics [118]. Despite previous case series indicating favorable tocilizumab efficacy in refractory BU patients, the study's clinical trial was terminated early due to uncontrolled uveitis attacks in all three patients. Further research is needed to identify the subgroup of refractory BU patients who may benefit from tocilizumab treatment.
In conclusion, tocilizumab has shown promise in treating refractory BU, especially in cases unresponsive to anti-TNFα therapy, with improvements in central macular thickness and visual acuity. However, tocilizumab's effectiveness for other BD manifestations remains variable, and further large-scale, randomized controlled trials are necessary to confirm optimal treatment duration and identify patient subgroups most likely to benefit from this therapy.
Anti-Interleukin-17—Secukinumab
BU patients exhibit an elevated Th17/Th1 ratio and increased IL-17 production, particularly IL-17A, one of the primary inflammatory cytokines secreted by Th17 cells [107]. IL-17A, a crucial inflammatory cytokine produced by Th17 cells, has been associated with immune-mediated diseases and heightened serum concentrations in BU patients compared to healthy controls [119]. Secukinumab, a selective high-affinity, fully human monoclonal antibody, targets IL-17A, neutralizing downstream signals that activate neutrophils and macrophages [120]. This therapeutic approach could potentially yield favorable results by specifically inhibiting IL-17A-mediated inflammation in BU patients.
Secukinumab's potential in treating BU has been explored, with mixed outcomes and some limitations in study design. A 2010 case report demonstrated the safety and effectiveness of secukinumab in treating patients with non-infectious uveitis, suggesting a role for IL-17A in the pathophysiology of these diverse inflammatory diseases [121]. The SHIELD study, a multicenter randomized controlled phase III trial, evaluated the efficacy of secukinumab for BU in 118 patients [119]. Although the primary endpoint of reducing uveitis recurrence or vitreous haze score alongside withdrawal of immunosuppressive therapy was not achieved, secondary endpoints, such as withdrawal or reduction of immunosuppressive medications, showed significant differences between the secukinumab and placebo groups. During the 24-week follow-up period, recurrence rates, visual acuity, and vitreous opacity in the secukinumab group did not significantly differ from the placebo group. However, the secukinumab group had a significantly lower dose of concomitant immunosuppressive drugs. Despite these results, the authors highlighted several limitations, including the small sample size, variability in disease severity, the complex interplay between cytokines in individual patients, and potentially confounding effects of concomitant immunosuppressive medications.
Intravenous secukinumab has shown higher efficacy and safety in treating non-infectious posterior segment uveitis compared to subcutaneous administration. In a phase II dose-ranging randomized controlled trial, intravenous (IV) secukinumab demonstrated higher efficacy and safety in treating non-infectious uveitis involving the posterior segment compared to subcutaneous (SC) administration [122]. Patients receiving IV secukinumab showed higher responder and remission rates, with the 30 mg/kg IV dose exhibiting statistical and clinical superiority over the 300 mg SC dose. The study suggested that SC administration might not provide sufficient drug concentration, and high-dose IV secukinumab may be necessary for therapeutic effectiveness.
In conclusion, secukinumab holds promise in treating BU by specifically inhibiting IL-17A-mediated inflammation. Although the SHIELD study did not meet its primary endpoint, secondary endpoints demonstrated some potential benefits, such as reducing concomitant immunosuppressive drug doses. Further research, including larger-scale randomized controlled trials and investigations into the optimal administration route and dosage, is needed to fully understand secukinumab’s efficacy and safety in treating BU.
Anti-CD20—Rituximab
Rituximab, a chimeric monoclonal antibody targeting CD20 on B cells, induces apoptosis and alleviates inflammation [78]. Although BU is predominantly a T cell-driven disease, findings support a potential pathogenic role for B cells, which may be targeted by rituximab [123]. Rituximab has been used off-label in BU patients and some T cell-mediated disorders, suggesting that interfering with the complex T and B cell interplay could yield therapeutic benefits [124]. However, the mechanisms of action for rituximab are not yet fully understood, warranting further investigation [125].
Rituximab, commonly employed to treat B cell hyperfunction and various autoimmune diseases, has demonstrated effectiveness in non-Hodgkin's lymphoma, rheumatoid arthritis, and ocular inflammatory diseases [126]. The dosage regimen for treating BU typically mirrors that of rheumatoid arthritis, with 1000 mg administered intravenously on days 1 and 15, followed by treatment every 6 months or as needed.
There is relatively limited published evidence supporting the use of rituximab in BU. Sadreddini et al. reported a case of a patient with refractory BU and visual loss due to retinal vasculitis who was successfully treated with rituximab, achieving 24 months of disease remission [127]. The study demonstrated rituximab's effectiveness in controlling ocular inflammation in BU for the first time, but could not determine if it was superior to conventional treatment. Davatchi et al. conducted a single-blind randomized controlled trial to assess the efficacy of rituximab combined with conventional immunosuppressive therapy in treating refractory BU [128]. Twenty patients with treatment-resistant BU were randomly assigned to either a rituximab group (rituximab + methotrexate + glucocorticosteroids) or a conventional treatment group (cyclophosphamide + azathioprine + prednisolone). After 6 months of follow-up, the rituximab group showed significant alleviation of uveitis and improvements in retinal, optic disc, and macular edema compared to the conventional treatment group. However, no significant differences were observed between the two groups in visual acuity, disease activity index for posterior uveitis and retinal vasculitis, and total inflammatory activity index. The authors concluded that rituximab is an effective treatment for intractable BU, at least as effective as conventional combination therapy. Although this small pilot study did not provide statistically significant differences between the treatment groups, it supports the need for a larger randomized controlled trial with an appropriate number of patients to determine efficacy. Kidd et al. described a case of severe, treatment-resistant neurological Behçet’s syndrome with concomitant occlusion of a branch retinal vein, which responded positively to rituximab [124]. The patient's vision improved to 6/12 after conventional therapies failed.
In conclusion, rituximab has demonstrated potential in treating BU, particularly in refractory cases. Although limited published evidence exists, case reports and a small pilot study suggest that rituximab may be an effective treatment option, at least as effective as conventional combination therapy. Further research, including larger-scale randomized controlled trials, is necessary to establish the optimal use and efficacy of rituximab in treating BU.
Anti-CD52—Alemtuzumab
Alemtuzumab, a humanized IgG1 monoclonal antibody targeting CD52, attenuates immune-inflammatory responses by inducing apoptosis in T and B cells [129]. This rapid and long-lasting T and B cell depletion results in a substantial reduction in these cells, with CD8+ T cells repopulating after 31 months and CD4+ T lymphocytes achieving full repopulation in approximately 60 months [130]. Alemtuzumab is primarily employed for the treatment of lymphocytic leukemia, multiple sclerosis, and related conditions.
Alemtuzumab treatment shows potential for long-term remission in severe, refractory BU, but monitoring and dosing adjustments may be necessary. Dick and colleagues treated ten patients with severe, refractory, non-infectious ocular inflammation using alemtuzumab for the first time [131]. The treatment resulted in long-term remission for eight patients, enabling a reduction in the dosage of other immunosuppressive agents. Subsequently, case reports by Lockwood et al. (2003) demonstrated that alemtuzumab has therapeutic effects on Behçet's syndrome-related ocular, cutaneous, and neurological symptoms [132]. Lockwood et al. investigated the therapeutic response to lymphocyte depletion using alemtuzumab in 18 BD patients, with 12 of them having uveitis and 4 exhibiting active ocular inflammation at baseline. At the 6-month follow-up, two out of four patients with active ocular inflammation experienced disease remission, while the other two showed partial remission. Although long-term remission was achieved in the majority of patients, relapses were observed in 54% of the cohort after an average of 25 months, indicating the necessity for close monitoring of lymphopenia. In a 20-year study involving 32 refractory BD patients, 21 of whom had uveitis, 60 courses of alemtuzumab were administered across three dosing regimens [133]. Remission was achieved in all patients with severe eye disease, but relapses were more common in the lowest-dose group. Alemtuzumab treatment led to remission in the majority of difficult-to-treat cases, with relapses potentially associated with lower dosing and adverse events, including infusion reactions and new autoimmunity. Infusion reactions were observed in 27% of cases, while thyroid dysfunction affected 25% of patients.
Alemtuzumab's primary adverse reactions encompass infusion reactions and diminished thyroid function, while other side effects include bone marrow suppression, allergic reactions, and reduced lymphocyte counts [133]. Studies have also demonstrated an association between alemtuzumab and thyroid dysfunctions, necessitating close monitoring for early diagnosis [134]. The drug’s efficacy may be attributed to its ability to induce long-term lymphopenia, although its non-negligible risk of infections calls for careful consideration, particularly in multi-drug failure cases. Opportunistic infections caused by alemtuzumab are less frequently reported, possibly because of the reduced use of glucocorticosteroids and immunosuppressants in its treatment.
In conclusion, alemtuzumab has shown promising results in treating severe, refractory BU by inducing apoptosis in T and B cells. While long-term remission has been achieved in many cases, potential relapses and adverse events, such as infusion reactions and thyroid dysfunction, necessitate close monitoring of patients. Further research and larger-scale studies are needed to determine optimal dosing and treatment strategies while minimizing adverse effects and infection risks in patients with BU.
Discussion
Summary
In recent years, biologic therapies, particularly IFN-α and anti-TNFα antibodies, have substantially improved visual prognosis and quality of life for BU patients, surpassing the outcomes achieved with traditional immunosuppressive treatments. These therapies offer targeted immune modulation and a favorable side effect profile, culminating in enhanced disease management and a marked improvement in patients' quality of life. This comprehensive review synthesizes the current evidence regarding the efficacy, safety, advantages, and disadvantages of biologic therapies for BU, providing an updated perspective on clinical management and future directions for this vision-threatening condition. Although no randomized controlled trials conclusively identify the most effective therapy specifically for BU, biologic medications represent promising and potent treatment options. Once considered alternative treatments after immunosuppressive and corticosteroid therapies, an accumulating body of evidence now supports their use as first-line agents.
Challenges
Despite the groundbreaking advances in biologic therapies for BU, persistent challenges include the scarcity of robust randomized controlled trials (RCTs), off-label utilization of potentially superior agents, and exorbitant costs.
Currently, there is a notable deficiency of RCTs explicitly designed for BU, and findings from trials involving assorted types of non-infectious uveitis may not be directly applicable to BU, a severe variant characterized by potentially unique immunopathology. The literature remains bereft of RCTs comparing the efficacy of disparate biologic therapies with each other or with conventional immunosuppressants, leaving a multitude of queries unanswered concerning initiation, agent and dosage selection, and treatment duration.
Moreover, the widespread adoption of biologic treatments for BU is constrained by their prohibitive costs and off-label employment in most countries. For instance, ADA is the sole approved biologic DMARD for non-infectious uveitis, encompassing BU, while IFX is exclusively approved in Japan for cases refractory to cDMARDs. As a result, due to the steep expenses and limited long-term experience, biologic therapies are typically reserved for uveitis cases unresponsive to traditional immunosuppressive treatments.
Future Directions
To enhance our understanding of BU and optimize biologic therapies, it is imperative to conduct robust, large-scale, multicenter randomized controlled trials (RCTs) that rigorously assess the efficacy, safety profiles, and long-term outcomes of diverse biologic treatments, ultimately facilitating the development of more precise and effective therapeutic strategies for patients.
Identifying clinical, laboratory, genetic, and proteomic biomarkers capable of predicting disease severity, treatment response, and prognosis in BU is essential for enabling personalized therapeutic approaches. Furthermore, incorporating uniform outcome measures and patient-reported outcomes, such as quality of life assessments, is crucial for comprehensive evaluations.
BU is a multifaceted disorder characterized by individual variability in severity and treatment responsiveness. Given that not all patients uniformly respond to available therapeutic regimens, there is an increasing demand for a diverse therapeutic arsenal, including alternative therapies with distinct modes of action. Certain biologic DMARDs, which have been unsuccessful in randomized controlled trials, such as secukinumab, may warrant further exploration at higher doses or alternative routes of administration, as promising results have been observed with intravenous infusions.
Long-term follow-up studies are vital for corroborating the efficacy and safety of novel agents. The ultimate objective is to develop a universally effective, rapidly acting, affordable biologic therapy with minimal side effects, thereby improving both clinical and quality of life outcomes for patients. To realize this goal, additional research is indispensable, and it is anticipated that advancements in personalized medicine will pave the way for more targeted treatments for BU in the future.
The relatively infrequent occurrence and geographical dispersion of Behçet's uveitis (BU) present formidable hurdles to both comprehensive clinical trials and practical clinical research. Overcoming such impediments calls for intensified global cooperation and meticulous data collection, encapsulated in platforms such as the AutoInflammatory Disease Alliance (AIDA) International Registry [135]. This instrument, defying the geographic limitations often linked to rare disorders like BU, furnishes in-depth understanding of the disease’s demographic profile, clinical characteristics, therapeutic methodologies, and socioeconomic repercussions. The trajectory of BU management will be significantly shaped by such international registries, underscoring the transformative potential of global healthcare alliances. With the forward march of precision medicine, these expansive data repositories will be pivotal in honing our understanding and management of BU.
Conclusion
In conclusion, biologic therapies have demonstrated significant potential in managing BU, transforming treatment outcomes, and improving patients’ quality of life. However, challenges such as the lack of robust randomized controlled trials, off-label use, and high costs persist. To overcome these challenges, large-scale, multicenter RCTs are essential for evaluating the efficacy, safety profiles, and long-term outcomes of various biologic treatments. Identifying clinical, laboratory, genetic, and proteomic biomarkers is crucial for personalized therapeutic approaches. The development of affordable, effective, and safe biologic therapies with minimal side effects will be paramount to advancing clinical and quality of life outcomes for patients. Continued investigation and innovation in this field hold promise for a brighter future in the management of BU.
Acknowledgements
Funding
No funding or sponsorship was received for this study or publication of this article. The Rapid Service Fee was funded by the authors.
Author Contributions
Conceptualization: Biao Li. Data curation: Biao Li and Haoran Li. Methodology: Biao Li and Qun Huang. Drafting of the article: Biao Li and Yanlin Zheng. Final approval: Yanlin Zheng.
Disclosures
Biao Li, Haoran Li, Qun Huang and Yanlin Zheng have nothing to disclose.
Compliance with Ethics Guidelines
This article is based on previously conducted studies and does not contain any new studies with human participants or animals performed by any of the authors.
Data Availability
Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study.
References
- 1.Tugal-Tutkun I, Onal S, Altan-Yaycioglu R, Altunbas HH, Urgancioglu M. Uveitis in Behçet disease: an analysis of 880 patients. Am J Ophthalmol. 2004;138(3):373–380. doi: 10.1016/j.ajo.2004.03.022. [DOI] [PubMed] [Google Scholar]
- 2.Maldini C, Lavalley M, Cheminant M, de Menthon M, Mahr A. Relationships of HLA-B51 or B5 genotype with Behcet’s disease clinical characteristics: systematic review and meta-analyses of observational studies. Rheumatology (Oxford) 2012;51(5):887–900. doi: 10.1093/rheumatology/ker428. [DOI] [PubMed] [Google Scholar]
- 3.Leccese P, Alpsoy E. Behçet’s disease: an overview of etiopathogenesis. Front Immunol. 2019;10:1067. doi: 10.3389/fimmu.2019.01067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tugal-Tutkun I, Onal S, Altan-Yaycioglu R, Huseyin Altunbas H, Urgancioglu M. Uveitis in Behçet disease: an analysis of 880 patients. Am J Ophthalmol. 2004;138(3):373–380. doi: 10.1016/j.ajo.2004.03.022. [DOI] [PubMed] [Google Scholar]
- 5.Hatemi G, Christensen R, Bang D, Bodaghi B, Celik A, Fortune F, et al. 2018 update of the EULAR recommendations for the management of Behçet’s syndrome. Ann Rheum Dis. 2018;77(6):808–818. doi: 10.1136/annrheumdis-2018-213225. [DOI] [PubMed] [Google Scholar]
- 6.Alibaz-Oner F, Direskeneli H. Advances in the treatment of Behcet’s disease. Curr Rheumatol Rep. 2021;23(6):47. doi: 10.1007/s11926-021-01011-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Calvo-Río V, Blanco R, Beltrán E, Sánchez-Bursón J, Mesquida M, Adán A, et al. Anti-TNF-α therapy in patients with refractory uveitis due to Behçet’s disease: a 1-year follow-up study of 124 patients. Rheumatology (Oxford) 2014;53(12):2223–2231. doi: 10.1093/rheumatology/keu266. [DOI] [PubMed] [Google Scholar]
- 8.Sakai T, Watanabe H, Kuroyanagi K, Akiyama G, Okano K, Kohno H, et al. Health- and vision-related quality of life in patients receiving infliximab therapy for Behcet uveitis. Br J Ophthalmol. 2013;97(3):338–342. doi: 10.1136/bjophthalmol-2012-302515. [DOI] [PubMed] [Google Scholar]
- 9.Rao N, Kimoto T, Zamir E, Giri R, Wang R, Ito S, et al. Pathogenic role of retinal microglia in experimental uveoretinitis. Invest Ophthalmol Vis Sci. 2003;44(1):22–31. doi: 10.1167/iovs.02-0199. [DOI] [PubMed] [Google Scholar]
- 10.Sugita S, Kawazoe Y, Imai A, Yamada Y, Horie S, Mochizuki M. Inhibition of Th17 differentiation by anti-TNF-alpha therapy in uveitis patients with Behcet’s disease. Arthritis Res Ther. 2012;14(3):R99. doi: 10.1186/ar3824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.El Menyawi M, Fawzy M, Al-Nahas Z, Edris A, Hussein H, Shaker O, et al. Serum tumor necrosis factor alpha (TNF-α) level in patients with Behçet’s disease: relation to clinical manifestations and disease activity. Egypt Rheumatol. 2014;36(3):139–143. doi: 10.1016/j.ejr.2014.01.004. [DOI] [Google Scholar]
- 12.El-Asrar A, Struyf S, Kangave D, Al-Obeidan S, Opdenakker G, Geboes K, et al. Cytokine profiles in aqueous humor of patients with different clinical entities of endogenous uveitis. Clin Immunol (Orlando, FL) 2011;139(2):177–184. doi: 10.1016/j.clim.2011.01.014. [DOI] [PubMed] [Google Scholar]
- 13.Evereklioglu C, Er H, Türköz Y, Cekmen M. Serum levels of TNF-alpha, sIL-2R, IL-6, and IL-8 are increased and associated with elevated lipid peroxidation in patients with Behçet’s disease. Mediat Inflamm. 2002;11(2):87–93. doi: 10.1080/09629350220131935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Takeuchi M, Karasawa Y, Harimoto K, Tanaka A, Shibata M, Sato T, et al. Analysis of Th cell-related cytokine production in behcet disease patients with uveitis before and after infliximab treatment. Ocul Immunol Inflamm. 2017;25(1):52–61. doi: 10.3109/09273948.2016.1158276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhou X, Shi X, Ren Y, Yan T, Ye Q. Anti-tumour necrosis factor-alpha agent therapy, compared with conventional therapy, reduces the relapse of uveitis in patients with Behçet’s disease: a systematic review of controlled trials. Front Pharmacol. 2022;13:912906. doi: 10.3389/fphar.2022.912906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Abolhasani S, Khabbazi A, Hosseini F, Gholizadeh-Ghaleh Aziz S, Alipour S. Effects of anti-TNF biologic drugs on uveitis severity in Behçet patients: systematic review and meta-analysis. Int J Ophthalmol. 2022;15(5):813–819. doi: 10.18240/ijo.2022.05.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sfikakis P, Kaklamanis P, Elezoglou A, Katsilambros N, Theodossiadis P, Papaefthimiou S, et al. Infliximab for recurrent, sight-threatening ocular inflammation in Adamantiades-Behçet disease. Ann Intern Med. 2004;140(5):404–406. doi: 10.7326/0003-4819-140-5-200403020-00025. [DOI] [PubMed] [Google Scholar]
- 18.Fabiani C, Vitale A, Rigante D, Emmi G, Lopalco G, Sota J, et al. Predictors of sustained clinical response in patients with Behcet’s disease-related uveitis treated with infliximab and adalimumab. Clin Rheumatol. 2018;37(6):1715–1720. doi: 10.1007/s10067-018-4092-4. [DOI] [PubMed] [Google Scholar]
- 19.Hatemi G, Silman A, Bang D, Bodaghi B, Chamberlain A, Gul A, et al. EULAR recommendations for the management of Behçet disease. Ann Rheum Dis. 2008;67(12):1656–1662. doi: 10.1136/ard.2007.080432. [DOI] [PubMed] [Google Scholar]
- 20.Sakai T, Kanetaka A, Noro T, Tsuneoka H. Intraocular surgery in patients receiving infliximab therapy for Behçet disease. Jpn J Ophthalmol. 2010;54(4):360. doi: 10.1007/s10384-010-0824-y. [DOI] [PubMed] [Google Scholar]
- 21.Cakar OP. Behcet’s uveitis: current diagnostic and therapeutic approach. Turk J Ophthalmol. 2020;50(3):169–182. doi: 10.4274/tjo.galenos.2019.60308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yamada Y, Sugita S, Tanaka H, Kamoi K, Takase H, Mochizuki M. Timing of recurrent uveitis in patients with Behcet’s disease receiving infliximab treatment. Br J Ophthalmol. 2011;95(2):205–208. doi: 10.1136/bjo.2009.168856. [DOI] [PubMed] [Google Scholar]
- 23.Sfikakis PP, Kaklamanis PH, Elezoglou A, Katsilambros N, Theodossiadis PG, Papaefthimiou S, et al. Infliximab for recurrent, sight-threatening ocular inflammation in Adamantiades-Behcet disease. Ann Intern Med. 2004;140(5):404–406. doi: 10.7326/0003-4819-140-5-200403020-00025. [DOI] [PubMed] [Google Scholar]
- 24.Ozguler Y, Leccese P, Christensen R, Esatoglu S, Bang D, Bodaghi B, et al. Management of major organ involvement of Behçet’s syndrome: a systematic review for update of the EULAR recommendations. Rheumatology (Oxford) 2018;57(12):2200–2212. doi: 10.1093/rheumatology/key242. [DOI] [PubMed] [Google Scholar]
- 25.Martín-Varillas J, Atienza-Mateo B, Calvo-Rio V, Beltrán E, Sánchez-Bursón J, Adán A, et al. Long-term follow-up and optimization of infliximab in refractory uveitis due to Behçet disease: national study of 103 white patients. J Rheumatol. 2021;48(5):741–750. doi: 10.3899/jrheum.200300. [DOI] [PubMed] [Google Scholar]
- 26.Köse H, Yalçındağ N. Clinical follow-up of patients with Behçet uveitis after discontinuation of infliximab therapy. Ocul Immunol Inflamm. 2022;30(1):203–207. doi: 10.1080/09273948.2020.1774907. [DOI] [PubMed] [Google Scholar]
- 27.Sfikakis PP, Theodossiadis PG, Katsiari CG, Kaklamanis P, Markomichelakis NN. Effect of infliximab on sight-threatening panuveitis in Behcet’s disease. Lancet. 2001;358(9278):295–296. doi: 10.1016/s0140-6736(01)05497-6. [DOI] [PubMed] [Google Scholar]
- 28.Ohno S, Nakamura S, Hori S, Shimakawa M, Kawashima H, Mochizuki M, et al. Efficacy, safety, and pharmacokinetics of multiple administration of infliximab in Behcet’s disease with refractory uveoretinitis. J Rheumatol. 2004;31(7):1362–1368. [PubMed] [Google Scholar]
- 29.Keino H, Okada AA, Watanabe T, Taki W. Long-term efficacy of infliximab on background vascular leakage in patients with Behcet’s disease. Eye (Lond) 2014;28(9):1100–1106. doi: 10.1038/eye.2014.138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Keino H, Watanabe T, Nakayama M, Komagata Y, Fukuoka K, Okada A. Long-term efficacy of early infliximab-induced remission for refractory uveoretinitis associated with Behçet’s disease. Br J Ophthalmol. 2021;105(11):1525–1533. doi: 10.1136/bjophthalmol-2020-316892. [DOI] [PubMed] [Google Scholar]
- 31.Yamana S, Hasegawa E, Takeda A, Yawata N, Sonoda K. Long-term outcomes of infliximab in patients with Behçet’s disease-associated uveitis. Int Ophthalmol. 2023;43(3):937–944. doi: 10.1007/s10792-022-02495-z. [DOI] [PubMed] [Google Scholar]
- 32.Markomichelakis N, Delicha E, Masselos S, Fragiadaki K, Kaklamanis P, Sfikakis P. A single infliximab infusion vs corticosteroids for acute panuveitis attacks in Behçet’s disease: a comparative 4-week study. Rheumatology (Oxford) 2011;50(3):593–597. doi: 10.1093/rheumatology/keq366. [DOI] [PubMed] [Google Scholar]
- 33.Tabbara KF, Al-Hemidan AI. Infliximab effects compared to conventional therapy in the management of retinal vasculitis in Behçet disease. Am J Ophthalmol. 2008;146(6):845–50.e1. doi: 10.1016/j.ajo.2008.09.010. [DOI] [PubMed] [Google Scholar]
- 34.Yamada Y, Sugita S, Tanaka H, Kamoi K, Kawaguchi T, Mochizuki M. Comparison of infliximab versus ciclosporin during the initial 6-month treatment period in Behcet disease. Br J Ophthalmol. 2010;94(3):284–288. doi: 10.1136/bjo.2009.158840. [DOI] [PubMed] [Google Scholar]
- 35.Urruticoechea-Arana A, Cobo-Ibanez T, Villaverde-Garcia V, Santos Gomez M, Loza E, Vargas-Osorio K, et al. Efficacy and safety of biological therapy compared to synthetic immunomodulatory drugs or placebo in the treatment of Behcet’s disease associated uveitis: a systematic review. Rheumatol Int. 2019;39(1):47–58. doi: 10.1007/s00296-018-4193-z. [DOI] [PubMed] [Google Scholar]
- 36.Markomichelakis N, Delicha E, Masselos S, Sfikakis PP. Intravitreal infliximab for sight-threatening relapsing uveitis in Behçet disease: a pilot study in 15 patients. Am J Ophthalmol. 2012;154(3):534–41.e1. doi: 10.1016/j.ajo.2012.03.035. [DOI] [PubMed] [Google Scholar]
- 37.Hamza MM, Macky TA, Sidky MK, Ragab G, Soliman MM. Intravitreal infliximab in refractory uveitis in Behcet’s disease: a safety and efficacy clinical study. Retina. 2016;36(12):2399–2408. doi: 10.1097/IAE.0000000000001109. [DOI] [PubMed] [Google Scholar]
- 38.Refaat M, Abdullatif A, Hamza M, Macky T, El-Agha M, Ragab G, et al. Monthly intravitreal infliximab in Behçet’s disease active posterior uveitis: a long-term safety study. Retina (Philadelphia, PA) 2021;41(8):1739–1747. doi: 10.1097/iae.0000000000003095. [DOI] [PubMed] [Google Scholar]
- 39.Neri P, Arapi I, Nicolai M, Pirani V, Saitta A, Luchetti MM, et al. Biologic therapy in inflammatory eye conditions (ophtalmology): safety profile. Curr Drug Saf. 2016;11(1):47–54. doi: 10.2174/1574886310666151014114925. [DOI] [PubMed] [Google Scholar]
- 40.Ueda S, Akahoshi M, Takeda A, Inoue Y, Omoto A, Ayano M, et al. Long-term efficacy of infliximab treatment and the predictors of treatment outcomes in patients with refractory uveitis associated with Behcet’s disease. Eur J Rheumatol. 2018;5(1):9–15. doi: 10.5152/eurjrheum.2017.17068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sugita S, Yamada Y, Mochizuki M. Relationship between serum infliximab levels and acute uveitis attacks in patients with Behcet disease. Br J Ophthalmol. 2011;95(4):549–552. doi: 10.1136/bjo.2009.174888. [DOI] [PubMed] [Google Scholar]
- 42.Al Rashidi S, Al Fawaz A, Kangave D, Abu El-Asrar AM. Long-term clinical outcomes in patients with refractory uveitis associated with Behcet disease treated with infliximab. Ocul Immunol Inflamm. 2013;21(6):468–474. doi: 10.3109/09273948.2013.779727. [DOI] [PubMed] [Google Scholar]
- 43.Fabiani C, Sota J, Vitale A, Emmi G, Vannozzi L, Bacherini D, et al. Ten-year retention rate of infliximab in patients with Behcet’s disease-related uveitis. Ocul Immunol Inflamm. 2019;27(1):34–39. doi: 10.1080/09273948.2017.1391297. [DOI] [PubMed] [Google Scholar]
- 44.Guzelant G, Ucar D, Esatoglu SN, Hatemi G, Ozyazgan Y, Yurdakul S, et al. Infliximab for uveitis of Behcet’s syndrome: a trend for earlier initiation. Clin Exp Rheumatol. 2017;35(Suppl 108):86–89. [PubMed] [Google Scholar]
- 45.Rubbert-Roth A. Assessing the safety of biologic agents in patients with rheumatoid arthritis. Rheumatology (Oxford) 2012;51(suppl 5):v38–47. doi: 10.1093/rheumatology/kes114. [DOI] [PubMed] [Google Scholar]
- 46.Ohno S, Umebayashi I, Matsukawa M, Goto T, Yano T. Safety and efficacy of infliximab in the treatment of refractory uveoretinitis in Behçet's disease: a large-scale, long-term postmarketing surveillance in Japan. Arthritis Res Ther. 2019;21(1):2. doi: 10.1186/s13075-018-1793-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cantarini L, Lopalco G, Caso F, Costa L, Iannone F, Lapadula G, et al. Effectiveness and tuberculosis-related safety profile of interleukin-1 blocking agents in the management of Behçet's disease. Autoimmun Rev. 2015;14(1):1–9. doi: 10.1016/j.autrev.2014.08.008. [DOI] [PubMed] [Google Scholar]
- 48.Vallet H, Seve P, Biard L, Baptiste Fraison J, Bielefeld P, Perard L, et al. Infliximab versus adalimumab in the treatment of refractory inflammatory uveitis: a multicenter study from the French uveitis network. Arthrit Rheumatol. 2016;68(6):1522–1530. doi: 10.1002/art.39667. [DOI] [PubMed] [Google Scholar]
- 49.Gueudry J, LeHoang P, Bodaghi B. New treatments in noninfectious uveitis. Karger Publishers; 2012. Anti-tumor necrosis factor-α agents in noninfectious uveitis; pp. 63–78. [DOI] [PubMed] [Google Scholar]
- 50.Hamam RN, Barikian AW, Antonios RS, Abdulaal MR, Alameddine RM, El Mollayess G, et al. Intravitreal adalimumab in active noninfectious uveitis: a pilot study. Ocul Immunol Inflamm. 2016;24(3):319–326. doi: 10.3109/09273948.2014.990041. [DOI] [PubMed] [Google Scholar]
- 51.Olivieri I, Leccese P, D’angelo S, Padula A, Nigro A, Palazzi C, et al. Efficacy of adalimumab in patients with Behçet’s disease unsuccessfully treated with infliximab. Clin Exp Rheumatol. 2011;29(4 Suppl 67):S54–S57. [PubMed] [Google Scholar]
- 52.Kheir WJ, Mehanna CJ, Abdul Fattah M, Al Ghadban S, El Sabban M, Mansour AM, et al. Intravitreal adalimumab for the control of breakthrough intraocular inflammation. Ocul Immunol Inflamm. 2018;26(8):1206–1211. doi: 10.1080/09273948.2017.1335756. [DOI] [PubMed] [Google Scholar]
- 53.Mushtaq B, Saeed T, Situnayake RD, Murray PI. Adalimumab for sight-threatening uveitis in Behcet’s disease. Eye (Lond) 2007;21(6):824–825. doi: 10.1038/sj.eye.6702352. [DOI] [PubMed] [Google Scholar]
- 54.Kunimi K, Usui Y, Asakage M, Maehara C, Tsubota K, Mitsuhashi R, et al. Anti-TNF-α therapy for refractory uveitis associated with Behçet’s syndrome and sarcoidosis: a single center study of 131 patients. Ocul Immunol Inflamm. 2020 doi: 10.1080/09273948.2020.1791346. [DOI] [PubMed] [Google Scholar]
- 55.Perra D, Alba MA, Callejas JL, Mesquida M, Ríos-Fernández R, Adan A, et al. Adalimumab for the treatment of Behçet’s disease: experience in 19 patients. Rheumatology. 2012;51(10):1825–1831. doi: 10.1093/rheumatology/kes130. [DOI] [PubMed] [Google Scholar]
- 56.Suhler EB, Lowder CY, Goldstein DA, Giles T, Lauer AK, Kurz PA, et al. Adalimumab therapy for refractory uveitis: results of a multicentre, open-label, prospective trial. Br J Ophthalmol. 2013;97(4):481–486. doi: 10.1136/bjophthalmol-2012-302292. [DOI] [PubMed] [Google Scholar]
- 57.Jaffe GJ, Dick AD, Brezin AP, Nguyen QD, Thorne JE, Kestelyn P, et al. Adalimumab in patients with active noninfectious uveitis. N Engl J Med. 2016;375(10):932–943. doi: 10.1056/NEJMoa1509852. [DOI] [PubMed] [Google Scholar]
- 58.Nguyen QD, Merrill PT, Jaffe GJ, Dick AD, Kurup SK, Sheppard J, et al. Adalimumab for prevention of uveitic flare in patients with inactive non-infectious uveitis controlled by corticosteroids (VISUAL II): a multicentre, double-masked, randomised, placebo-controlled phase 3 trial. Lancet (Lond, Engl) 2016;388(10050):1183–1192. doi: 10.1016/s0140-6736(16)31339-3. [DOI] [PubMed] [Google Scholar]
- 59.Suhler EB, Adán A, Brézin AP, Fortin E, Goto H, Jaffe GJ, et al. Safety and efficacy of adalimumab in patients with noninfectious uveitis in an ongoing open-label study: VISUAL III. Ophthalmology. 2018;125(7):1075–1087. doi: 10.1016/j.ophtha.2017.12.039. [DOI] [PubMed] [Google Scholar]
- 60.Atienza-Mateo B, Martín-Varillas JL, Calvo-Rió V, Demetrio-Pablo R, Beltrán E, Sánchez-Bursón J, et al. Comparative study of infliximab versus adalimumab in refractory uveitis due to Behçet’s disease. National multicenter study of 177 cases. Ann Rheum Dis. 2019;78:814–815. doi: 10.1136/annrheumdis-2019-eular.1606. [DOI] [PubMed] [Google Scholar]
- 61.Yang S, Huang Z, Hu Y, Zhang J, Liu X, Li H, et al. The efficacy of adalimumab as an initial treatment in patients with Behçet’s retinal vasculitis. Front Pharmacol. 2021;12:609148. doi: 10.3389/fphar.2021.609148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Fabiani C, Sota J, Vitale A, Rigante D, Emmi G, Vannozzi L, et al. Cumulative retention rate of adalimumab in patients with Behcet’s disease-related uveitis: a four-year follow-up study. Br J Ophthalmol. 2018;102(5):637–641. doi: 10.1136/bjophthalmol-2017-310733. [DOI] [PubMed] [Google Scholar]
- 63.Takase K, Ohno S, Ideguchi H, Uchio E, Takeno M, Ishigatsubo Y. Successful switching to adalimumab in an infliximab-allergic patient with severe Behçet disease-related uveitis. Rheumatol Int. 2011;31(2):243–245. doi: 10.1007/s00296-009-1178-y. [DOI] [PubMed] [Google Scholar]
- 64.Bitossi A, Bettiol A, Silvestri E, Di Scala G, Bacherini D, Lopalco G, et al. Adalimumab accounts for long-term control of noninfectious uveitis also in the absence of concomitant DMARD treatment: a multicenter retrospective study. Mediat Inflamm. 2019;2019:1623847. doi: 10.1155/2019/1623847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Scheinfeld N. Adalimumab: a review of side effects. Expert Opin Drug Saf. 2005;4(4):637–641. doi: 10.1517/14740338.4.4.637. [DOI] [PubMed] [Google Scholar]
- 66.Keystone E, Genovese M, Klareskog L, Hsia E, Hall S, Miranda P, et al. Golimumab in patients with active rheumatoid arthritis despite methotrexate therapy: 52-week results of the GO-FORWARD study. Ann Rheum Dis. 2010;69(6):1129–1135. doi: 10.1136/ard.2009.116319. [DOI] [PubMed] [Google Scholar]
- 67.Smolen J, Kay J, Landewé R, Matteson E, Gaylis N, Wollenhaupt J, et al. Golimumab in patients with active rheumatoid arthritis who have previous experience with tumour necrosis factor inhibitors: results of a long-term extension of the randomised, double-blind, placebo-controlled GO-AFTER study through week 160. Ann Rheum Dis. 2012;71(10):1671–1679. doi: 10.1136/annrheumdis-2011-200956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Mesquida M, Victoria Hernandez M, Llorenc V, Pelegrin L, Espinosa G, Dick AD, et al. Behcet disease-associated uveitis successfully treated with golimumab. Ocul Immunol Inflamm. 2013;21(2):160–162. doi: 10.3109/09273948.2012.741744. [DOI] [PubMed] [Google Scholar]
- 69.Santos-Gomez M, Calvo-Rio V, Blanco R, Beltran E, Mesquida M, Adan A, et al. The effect of biologic therapy different from infliximab or adalimumab in patients with refractory uveitis due to Behcet’s disease: results of a multicentre open-label study. Clin Exp Rheumatol. 2016;34(6 Supplement 102):S34–S40. [PubMed] [Google Scholar]
- 70.Vitale A, Emmi G, Lopalco G, Fabiani C, Gentileschi S, Silvestri E, et al. Long-term efficacy and safety of golimumab in the treatment of multirefractory Behçet’s disease. Clin Rheumatol. 2017;36(9):2063–2069. doi: 10.1007/s10067-017-3627-4. [DOI] [PubMed] [Google Scholar]
- 71.Fabiani C, Sota J, Rigante D, Vitale A, Emmi G, Vannozzi L, et al. Rapid and sustained efficacy of golimumab in the treatment of multirefractory uveitis associated with Behcet’s disease. Ocul Immunol Inflamm. 2019;27(1):58–63. doi: 10.1080/09273948.2017.1351573. [DOI] [PubMed] [Google Scholar]
- 72.Sanchez-Cano D, Callejas-Rubio JL, Ruiz-Villaverde R, Rios-Fernandez R, Ortego-Centeno N. Off-label uses of anti-TNF therapy in three frequent disorders: Behcet’s disease, sarcoidosis, and noninfectious uveitis. Mediators Inflamm. 2013;2013:286857. doi: 10.1155/2013/286857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Melikoglu M, Fresko I, Mat C, Ozyazgan Y, Gogus F, Yurdakul S, et al. Short-term trial of etanercept in Behcet’s disease: a double blind, placebo controlled study. J Rheumatol. 2005;32(1):98–105. [PubMed] [Google Scholar]
- 74.Sfikakis P. Behcet’s disease: a new target for anti-tumour necrosis factor treatment. Ann Rheum Dis. 2002;61(suppl 2):ii51–ii53. doi: 10.1136/ard.61.suppl_2.ii51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Gaujoux-Viala C, Giampietro C, Gaujoux T, Ea HK, Prati C, Orcel P, et al. Scleritis: a paradoxical effect of etanercept? Etanercept-associated inflammatory eye disease. J Rheumatol. 2012;39(2):233–239. doi: 10.3899/jrheum.110865. [DOI] [PubMed] [Google Scholar]
- 76.Galor A, Perez V, Hammel J, Lowder C. Differential effectiveness of etanercept and infliximab in the treatment of ocular inflammation. Ophthalmology. 2006;113(12):2317–2323. doi: 10.1016/j.ophtha.2006.04.038. [DOI] [PubMed] [Google Scholar]
- 77.Levy-Clarke G, Jabs DA, Read RW, Rosenbaum JT, Vitale A, Van Gelder RN. Expert panel recommendations for the use of anti-tumor necrosis factor biologic agents in patients with ocular inflammatory disorders. Ophthalmology. 2014;121(3):785–U215. doi: 10.1016/j.ophtha.2013.09.048. [DOI] [PubMed] [Google Scholar]
- 78.Reddy AK, Albini TA. A review of biologics for uveitis. Retin Today. 2015;1:54–56. [Google Scholar]
- 79.Gholijani N, Ataollahi MR, Samiei A, Aflaki E, Shenavandeh S, Kamali-Sarvestani E. An elevated pro-inflammatory cytokines profile in Behcet’s disease: a multiplex analysis. Immunol Lett. 2017;186:46–51. doi: 10.1016/j.imlet.2016.12.001. [DOI] [PubMed] [Google Scholar]
- 80.Plskova J, Greiner K, Muckersie E, Duncan L, Forrester J. Interferon-alpha: a key factor in autoimmune disease? Invest Ophthalmol Vis Sci. 2006;47(9):3946–3950. doi: 10.1167/iovs.06-0058. [DOI] [PubMed] [Google Scholar]
- 81.Albayrak O, Oray M, Can F, Uludag Kirimli G, Gul A, Tugal-Tutkun I, et al. Effect of interferon alfa-2a treatment on adaptive and innate immune systems in patients with Behcet disease uveitis. Invest Ophthalmol Vis Sci. 2019;60(1):52–63. doi: 10.1167/iovs.18-25548. [DOI] [PubMed] [Google Scholar]
- 82.Park JY, Chung YR, Lee K, Song JH, Lee ES. Clinical experience of interferon Alfa-2a treatment for refractory uveitis in Behcet's disease. Yonsei Med J. 2015;56(4):1158–1162. doi: 10.3349/ymj.2015.56.4.1158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Kötter I, Günaydin I, Zierhut M, Stübiger N. Seminars in arthritis and rheumatism. Elsevier; 2004. The use of interferon α in Behçet disease: review of the literature; pp. 320–335. [DOI] [PubMed] [Google Scholar]
- 84.Yang P, Huang G, Du L, Ye Z, Hu K, Wang C, et al. Long-term efficacy and safety of interferon Alpha-2a in the treatment of Chinese patients with Behcet’s uveitis not responding to conventional therapy. Ocul Immunol Inflamm. 2019;27(1):7–14. doi: 10.1080/09273948.2017.1384026. [DOI] [PubMed] [Google Scholar]
- 85.Shi J, Zhao C, Zhou J, Liu J, Wang L, Gao F, et al. Effectiveness and safety of interferon alpha2a as an add-on treatment for refractory Behcet’s uveitis. Ther Adv Chronic Dis. 2019;10:2040622319847881. doi: 10.1177/2040622319847881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Lightman S, Taylor SR, Bunce C, Longhurst H, Lynn W, Moots R, et al. Pegylated interferon-alpha-2b reduces corticosteroid requirement in patients with Behcet's disease with upregulation of circulating regulatory T cells and reduction of Th17. Ann Rheum Dis. 2015;74(6):1138–1144. doi: 10.1136/annrheumdis-2014-205571. [DOI] [PubMed] [Google Scholar]
- 87.Deuter CM, Zierhut M, Mohle A, Vonthein R, Stobiger N, Kotter I. Long-term remission after cessation of interferon-alpha treatment in patients with severe uveitis due to Behcet's disease. Arthrit Rheum. 2010;62(9):2796–2805. doi: 10.1002/art.27581. [DOI] [PubMed] [Google Scholar]
- 88.Sobaci G, Erdem U, Durukan A, Erdurman C, Bayer A, Köksal S, et al. Safety and effectiveness of interferon alpha-2a in treatment of patients with Behçet’s uveitis refractory to conventional treatments. Ophthalmology. 2010;117(7):1430–1435. doi: 10.1016/j.ophtha.2009.11.022. [DOI] [PubMed] [Google Scholar]
- 89.Kavandi H, Khabbazi A, Kolahi S, Hajialilo M, Shayan FK, Oliaei M. Long-term efficacy and safety of interferon α-2a therapy in severe refractory ophthalmic Behcet’s disease. Clin Rheumatol. 2016;35(11):2765–2769. doi: 10.1007/s10067-016-3318-6. [DOI] [PubMed] [Google Scholar]
- 90.Hasanreisoglu M, Cubuk MO, Ozdek S, Gurelik G, Aktas Z, Hasanreisoglu B. Interferon Alpha-2a therapy in patients with refractory Behcet uveitis. Ocul Immunol Inflamm. 2017;25(1):71–75. doi: 10.3109/09273948.2015.1133835. [DOI] [PubMed] [Google Scholar]
- 91.Qian Y, Qu Y, Gao F, Pei M, Liang A, Xiao J, et al. Comparison of the safety and efficacy of interferon Alpha-2a and cyclosporine-a when combined with glucocorticoid in the treatment of refractory Behçet’s uveitis: a randomized controlled prospective study. Front Pharmacol. 2021;12:699903. doi: 10.3389/fphar.2021.699903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Diwo E, Gueudry J, Saadoun D, Weschler B, LeHoang P, Bodaghi B. Long-term efficacy of interferon in severe uveitis associated with Behcet disease. Ocul Immunol Inflamm. 2017;25(1):76–84. doi: 10.1080/09273948.2016.1206204. [DOI] [PubMed] [Google Scholar]
- 93.Onal S, Kazokoglu H, Koc A, Akman M, Bavbek T, Direskeneli H, et al. Long-term efficacy and safety of low-dose and dose-escalating interferon alfa-2a therapy in refractory Behçet uveitis. Arch Ophthalmol. 2011;129(3):288–294. doi: 10.1001/archophthalmol.2011.3. [DOI] [PubMed] [Google Scholar]
- 94.Keskin Y, Seyahi E, Poyraz C, Ugurlu S, Ozyazgan Y, Yazici H. Interferon alfa-associated depression in patients with Behçet’s syndrome: a prospective controlled study. Clin Exp Rheumatol. 2014;32(4 Suppl 84):S175. [PubMed] [Google Scholar]
- 95.Dinarello CA, Simon A, van der Meer JW. Treating inflammation by blocking interleukin-1 in a broad spectrum of diseases. Nat Rev Drug Discov. 2012;11(8):633–652. doi: 10.1038/nrd3800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Duzgun N, Ayaslioglu E, Tutkak H, Aydintug OT. Cytokine inhibitors: soluble tumor necrosis factor receptor 1 and interleukin-1 receptor antagonist in Behcet’s disease. Rheumatol Int. 2005;25(1):1–5. doi: 10.1007/s00296-003-0400-6. [DOI] [PubMed] [Google Scholar]
- 97.Orlando I, Vitale A, Rigante D, Lopalco G, Fabiani C, Cantarini L. Long-term efficacy and safety of the interleukin-1 inhibitors anakinra and canakinumab in refractory Behcet disease uveitis and concomitant bladder papillary carcinoma. Intern Med J. 2017;47(9):1086–1088. doi: 10.1111/imj.13538. [DOI] [PubMed] [Google Scholar]
- 98.Teoh S, Sharma S, Hogan A, Lee R, Ramanan A, Dick A. Tailoring biological treatment: anakinra treatment of posterior uveitis associated with the CINCA syndrome. Br J Ophthalmol. 2007;91(2):263–264. doi: 10.1136/bjo.2006.0101477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Emmi G, Silvestri E, Cameli A, Bacherini D, Vannozzi L, Squatrito D, et al. Anakinra for resistant Behçet uveitis: why not? Clin Exp Rheumatol. 2013;31:152–153. [PubMed] [Google Scholar]
- 100.Cantarini L, Vitale A, Scalini P, Dinarello CA, Rigante D, Franceschini R, et al. Anakinra treatment in drug-resistant Behcet’s disease: a case series. Clin Rheumatol. 2015;34(7):1293–1301. doi: 10.1007/s10067-013-2443-8. [DOI] [PubMed] [Google Scholar]
- 101.Ugurlu S, Ucar D, Seyahi E, Hatemi G, Yurdakul S. Canakinumab in a patient with juvenile Behcet’s syndrome with refractory eye disease. Ann Rheum Dis. 2012;71(9):1589–1591. doi: 10.1136/annrheumdis-2012-201383. [DOI] [PubMed] [Google Scholar]
- 102.Blech M, Peter D, Fischer P, Bauer MM, Hafner M, Zeeb M, et al. One target—two different binding modes: structural insights into gevokizumab and canakinumab interactions to interleukin-1β. J Mol Biol. 2013;425(1):94–111. doi: 10.1016/j.jmb.2012.09.021. [DOI] [PubMed] [Google Scholar]
- 103.Cantarini L, Vitale A, Borri M, Galeazzi M, Franceschini R. Successful use of canakinumab in a patient with resistant Behçet’s disease. Clin Exp Rheumatol. 2012;30(Suppl 72):S115. [PubMed] [Google Scholar]
- 104.Fabiani C, Vitale A, Emmi G, Lopalco G, Vannozzi L, Guerriero S, et al. Interleukin (IL)-1 inhibition with anakinra and canakinumab in Behcet’s disease-related uveitis: a multicenter retrospective observational study. Clin Rheumatol. 2017;36(1):191–197. doi: 10.1007/s10067-016-3506-4. [DOI] [PubMed] [Google Scholar]
- 105.Kaly L, Rosner I. Tocilizumab—a novel therapy for non-organ-specific autoimmune diseases. Best Pract Res Clin Rheumatol. 2012;26(1):157–165. doi: 10.1016/j.berh.2012.01.001. [DOI] [PubMed] [Google Scholar]
- 106.Kimura A, Kishimoto T. IL-6: regulator of Treg/Th17 balance. Eur J Immunol. 2010;40(7):1830–1835. doi: 10.1002/eji.201040391. [DOI] [PubMed] [Google Scholar]
- 107.Geri G, Terrier B, Rosenzwajg M, Wechsler B, Touzot M, Seilhean D, et al. Critical role of IL-21 in modulating TH17 and regulatory T cells in Behçet disease. J Allergy Clin Immunol. 2011;128(3):655–664. doi: 10.1016/j.jaci.2011.05.029. [DOI] [PubMed] [Google Scholar]
- 108.Lin P. Targeting interleukin-6 for noninfectious uveitis. Clin Ophthalmol. 2015;9:1697–1702. doi: 10.2147/OPTH.S68595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Tanaka T, Narazaki M, Ogata A, Kishimoto T. Seminars in immunology. Elsevier; 2014. A new era for the treatment of inflammatory autoimmune diseases by interleukin-6 blockade strategy; pp. 88–96. [DOI] [PubMed] [Google Scholar]
- 110.Ogata A, Tanaka T. Tocilizumab for the treatment of rheumatoid arthritis and other systemic autoimmune diseases: current perspectives and future directions. Int J Rheumatol. 2012;2012:946048. doi: 10.1155/2012/946048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Calvo-Río V, Santos-Gómez M, Calvo I, González-Fernández MI, López-Montesinos B, Mesquida M, et al. Anti–interleukin-6 receptor tocilizumab for severe juvenile idiopathic arthritis-associated uveitis refractory to anti-tumor necrosis factor therapy: a multicenter study of twenty-five patients. Arthrit Rheumatol. 2017;69(3):668–675. doi: 10.1002/art.39940. [DOI] [PubMed] [Google Scholar]
- 112.Hirano T, Ohguro N, Hohki S, Hagihara K, Shima Y, Narazaki M, et al. A case of Behcet’s disease treated with a humanized anti-interleukin-6 receptor antibody, tocilizumab. Mod Rheumatol. 2012;22(2):298–302. doi: 10.1007/s10165-011-0497-5. [DOI] [PubMed] [Google Scholar]
- 113.Vegas-Revenga N, Calvo-Río V, Mesquida M, Adán A, Hernández M, Beltrán E, et al. Anti-IL6-receptor tocilizumab in refractory and noninfectious uveitic cystoid macular edema: multicenter study of 25 patients. Am J Ophthalmol. 2019;200:85–94. doi: 10.1016/j.ajo.2018.12.019. [DOI] [PubMed] [Google Scholar]
- 114.Hassan M, Sadiq M, Ormaechea M, Uludağ G, Halim M, Afridi R, et al. Utilisation of composite endpoint outcome to assess efficacy of tocilizumab for non-infectious uveitis in the STOP-uveitis study. Br J Ophthalmol. 2022 doi: 10.1136/bjophthalmol-2021-320604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Sepah YJ, Sadiq MA, Chu DS, Dacey M, Gallemore R, Dayani P, et al. Primary (month-6) outcomes of the STOP-uveitis study: evaluating the safety, tolerability, and efficacy of tocilizumab in patients with noninfectious uveitis. Am J Ophthalmol. 2017;183:71–80. doi: 10.1016/j.ajo.2017.08.019. [DOI] [PubMed] [Google Scholar]
- 116.Atienza-Mateo B, Beltrán E, Hernández-Garfella M, Valls Pascual E, Martínez-Costa L, Atanes A, et al. Tocilizumab in Behçet’s disease with refractory ocular and/or neurological involvement: response according to different clinical phenotypes. Clin Exp Rheumatol. 2021;5:37–42. doi: 10.55563/clinexprheumatol/9ipkcs. [DOI] [PubMed] [Google Scholar]
- 117.Akiyama M, Kaneko Y, Takeuchi T. Effectiveness of tocilizumab in Behcet's disease: a systematic literature review. Semin Arthrit Rheum. 2020;50(4):797–804. doi: 10.1016/j.semarthrit.2020.05.017. [DOI] [PubMed] [Google Scholar]
- 118.Zhao C, Liu J, Song H, Shi J, Zhang M, Zheng W. Anti-interleukin 6 receptor antibody tocilizumab was not satisfactory for acute attack of Behçet’s uveitis in three consecutive patients. Clin Exp Rheumatol. 2022;40(8):1600. doi: 10.55563/clinexprheumatol/xnb396. [DOI] [PubMed] [Google Scholar]
- 119.Dick AD, Tugal-Tutkun I, Foster S, Zierhut M, Melissa Liew SH, Bezlyak V, et al. Secukinumab in the treatment of noninfectious uveitis: results of three randomized, controlled clinical trials. Ophthalmology. 2013;120(4):777–787. doi: 10.1016/j.ophtha.2012.09.040. [DOI] [PubMed] [Google Scholar]
- 120.Sota J, Rigante D, Lopalco G, Frediani B, Franceschini R, Galeazzi M, et al. Biological therapies for the treatment of Behcet’s disease-related uveitis beyond TNF-alpha blockade: a narrative review. Rheumatol Int. 2018;38(1):25–35. doi: 10.1007/s00296-017-3775-5. [DOI] [PubMed] [Google Scholar]
- 121.Hueber W, Patel DD, Dryja T, Wright AM, Koroleva I, Bruin G, et al. Effects of AIN457, a fully human antibody to interleukin-17A, on psoriasis, rheumatoid arthritis, and uveitis. Sci Transl Med. 2010;2(52):52ra72. doi: 10.1126/scitranslmed.3001107. [DOI] [PubMed] [Google Scholar]
- 122.Letko E, Yeh S, Foster CS, Pleyer U, Brigell M, Grosskreutz CL, et al. Efficacy and safety of intravenous secukinumab in noninfectious uveitis requiring steroid-sparing immunosuppressive therapy. Ophthalmology. 2015;122(5):939–948. doi: 10.1016/j.ophtha.2014.12.033. [DOI] [PubMed] [Google Scholar]
- 123.Shaker O, Tawfic S, El-Tawdy A, El-Komy M, El Menyawi M, Heikal A. Expression of TNF-α, APRIL and BCMA in Behcet’s disease. J Immunol Res. 2014;2014:380405. doi: 10.1155/2014/380405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Kidd D. Rituximab is effective in severe treatment-resistant neurological Behçet’s syndrome. J Neurol. 2015;262(12):2676–2677. doi: 10.1007/s00415-015-7897-y. [DOI] [PubMed] [Google Scholar]
- 125.Caso F, Rigante D, Vitale A, Costa L, Bascherini V, Latronico E, et al. Long-lasting uveitis remission and hearing loss recovery after rituximab in Vogt-Koyanagi-Harada disease. Clin Rheumatol. 2015;34(10):1817–1820. doi: 10.1007/s10067-014-2781-1. [DOI] [PubMed] [Google Scholar]
- 126.Miserocchi E, Pontikaki I, Modorati G, Gattinara M, Meroni PL, Gerloni V. Anti-CD 20 monoclonal antibody (rituximab) treatment for inflammatory ocular diseases. Autoimmun Rev. 2011;11(1):35–39. doi: 10.1016/j.autrev.2011.07.001. [DOI] [PubMed] [Google Scholar]
- 127.Sadreddini S, Noshad H, Molaeefard M, Noshad R. Treatment of retinal vasculitis in Behcet’s disease with rituximab. Mod Rheumatol. 2008;18(3):306–308. doi: 10.1007/s10165-008-0057-9. [DOI] [PubMed] [Google Scholar]
- 128.Davatchi F, Shams H, Rezaipoor M, Sadeghi-Abdollahi B, Shahram F, Nadji A, et al. Rituximab in intractable ocular lesions of Behcet’s disease; randomized single-blind control study (pilot study) Int J Rheum Dis. 2010;13(3):246–252. doi: 10.1111/j.1756-185X.2010.01546.x. [DOI] [PubMed] [Google Scholar]
- 129.Salisbury JR, Rapson NT, Codd JD, Rogers MV, Nethersell AB. Immunohistochemical analysis of CDw52 antigen expression in non-Hodgkin’s lymphomas. J Clin Pathol. 1994;47(4):313–317. doi: 10.1136/jcp.47.4.313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Ruck T, Bittner S, Wiendl H, Meuth S. Alemtuzumab in multiple sclerosis: mechanism of action and beyond. Int J Mol Sci. 2015;16(7):16414–16439. doi: 10.3390/ijms160716414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Dick AD, Meyer P, James T, Forrester JV, Hale G, Waldmann H, et al. Campath-1H therapy in refractory ocular inflammatory disease. Br J Ophthalmol. 2000;84(1):107–109. doi: 10.1136/bjo.84.1.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Lockwood CM, Hale G, Waldman H, Jayne DR. Remission induction in Behcet’s disease following lymphocyte depletion by the anti-CD52 antibody CAMPATH 1-H. Rheumatology (Oxford) 2003;42(12):1539–1544. doi: 10.1093/rheumatology/keg424. [DOI] [PubMed] [Google Scholar]
- 133.Mohammad AJ, Smith RM, Chow YW, Chaudhry AN, Jayne DR. Alemtuzumab as remission induction therapy in Behcet disease: a 20-year experience. J Rheumatol. 2015;42(10):1906–1913. doi: 10.3899/jrheum.141344. [DOI] [PubMed] [Google Scholar]
- 134.Daniels GH, Vladic A, Brinar V, Zavalishin I, Valente W, Oyuela P, et al. Alemtuzumab-related thyroid dysfunction in a phase 2 trial of patients with relapsing-remitting multiple sclerosis. J Clin Endocrinol Metab. 2014;99(1):80–89. doi: 10.1210/jc.2013-2201. [DOI] [PubMed] [Google Scholar]
- 135.Vitale A, Autoinflammatory Diseases Alliance (AIDA) Network et al. Development and implementation of the AIDA International Registry for patients with Behçet’s disease. Intern Emerg Med. 2022;17(7):1977–1986. doi: 10.1007/s11739-022-03038-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study.