Abstract
Nontyphoidal Salmonella can cause gallbladder empyema and disseminated disease in patients with suppressed immune systems. We are reporting a unique case of concomitant gallbladder empyema and epidural abscess due to Salmonella enterica subsp enterica serovar Enteritidis in a patient who was appropriately treated for the primary Salmonella infection complicated by bacteremia. A high degree of suspicion is needed in high-risk patients as timely intervention can avoid life-threatening complications.
Keywords: nontyphoidal Salmonella epidural abscess, nontyphoidal Salmonella gallbladder empyema, nontyphoidal salmonellosis, Salmonella Enteritidis
Nontyphoidal Salmonella (NTS) infections are 1 of the leading causes of foodborne illness worldwide and are estimated to cause approximately 150 million cases of gastroenteritis and 60 000 deaths globally each year [1]. According to the Centers for Disease Control and Prevention (CDC), Salmonella causes around 1.35 million infections, with 26 500 hospitalizations and 420 deaths in the United States annually [2]. Salmonellosis is a zoonotic disease associated with consumption of food or water contaminated with animal feces. In immunocompetent individuals, illness is commonly manifested as acute gastroenteritis lasting 4–7 days, and most people recover without treatment. Up to 8% of patients with NTS gastroenteritis develop bacteremia but invasive infection is uncommon, occurring only in 5%–10% of patients who have bacteremia [3]. Risk factors for invasive disease include extreme ages; underlying immunocompromising conditions, particularly human immunodeficiency virus (HIV); malignancy; and hemoglobinopathies. With an underlying suppressed immune system, infection can disseminate to other body sites including bones, joints, skin, heart valves, and, rarely, body spaces and meninges. Gallbladder colonization by NTS is a rare phenomenon as compared to Salmonella enterica subsp enterica serovar Typhi (S Typhi) and has been reported to cause disseminated foci of infection even after resolution of primary infection [4]. Epidural abscess due to NTS is rare, and all the cases that are reported so far had disseminated disease during the episode of primary infection [5]. We present a unique case of gallbladder empyema and epidural abscess due to Salmonella enterica subsp enterica serovar Enteritidis (S Enteritidis) in a patient who was treated for the primary Salmonella infection and yet developed disseminated disease.
CASE REPORT
A 52-year-old Black woman presented after sustaining a fall, which she attributed to bilateral lower extremity (LE) weakness. The patient was unable to stand up and remained on the floor for 3 hours before an ambulance arrived. Approximately 2 days prior to admission, she developed watery diarrhea and associated abdominal pain after eating watermelon and ham that was possibly spoiled. Her past medical history included uncontrolled diabetes mellitus (DM) type 2, hypertension, morbid obesity, and chronic back pain due to remote motor vehicle accident.
Initial vital signs demonstrated tachypnea of 30 breaths/minute, tachycardia of 130 beats/minute, and fever of 38.4°C. Physical examination revealed a power of 4 of 5 in both LEs with no sensory deficits. She had tenderness in the right upper quadrant of the abdomen. She was noted to have urinary retention and thus required placement of a urinary catheter. Her laboratory tests showed normal leukocyte count, mild anemia with hemoglobin of 11 g/dL, acute kidney injury (AKI) with elevation of creatinine to 2.18 mg/dL from baseline of 1 mg/dL, elevated random blood sugar of 349 mg/dL, alanine aminotransferase of 54 IU/L, aspartate aminotransferase of 131 IU/L, and hemoglobin A1c of 12.7%. Alkaline phosphatase and total bilirubin were normal. Ultrasound of the abdomen showed cholelithiasis with changes suggestive of cholecystitis. Both of 2 sets of blood culture grew gram-negative bacilli that were identified as S Enteritidis. Ceftriaxone was started. Two days later, her stool culture also grew S Enteritidis. Susceptibility results showed that the organism was susceptible to ampicillin, ceftriaxone, and trimethoprim-sulfamethoxazole (TMP-SMX). A transthoracic echocardiogram showed no signs of endocarditis. Magnetic resonance imaging (MRI) of the lumbar spine without contrast showed degenerative disc disease at the L3–L5 level with mild spinal stenosis at the L5–S1 level. Patient was evaluated by neurosurgery; her back pain and LE weakness were attributed to degenerative disc disease with no surgical intervention recommended. Her abdominal pain resolved over the next few days and the patient was discharged to a rehabilitation facility on TMP-SMX for 14 days with a plan to undergo elective cholecystectomy as an outpatient.
The patient returned 1 month later due to fall and progressive bilateral LE weakness and numbness of the toes. She had abdominal pain but did not have fever. She had minimal improvement in her functional status after discharge from the rehabilitation facility. Neurological examination showed muscle strength of 3 of 5 in both lower extremities with diminished patellar reflex bilaterally. Sensation was intact bilaterally and there was no saddle anesthesia. Tenderness was noted in the right upper quadrant. Laboratory results showed normal leukocyte count, anemia with hemoglobin of 9.9 g/dL, and mild AKI with creatinine of 1.54 mg/dL. Serum bilirubin and liver enzymes were within normal range. An MRI of the thoracic spine with contrast showed discitis of T10–T11 disc space level with epidural fluid collection causing spinal cord compression (Figure 1). Blood cultures showed no growth. The patient underwent thoracic laminectomy with evacuation of the epidural abscess. Based on the recent history of Salmonella bacteremia, the patient was started on ceftriaxone. The epidural fluid grew S Enteritidis. Patient continued to have right upper quadrant pain and, because of high risk of surgery, underwent cholecystostomy with drainage of purulent bile, which also grew S Enteritidis. Susceptibility results from bile and epidural fluid showed that the Salmonella was susceptible to ampicillin, TMP-SMX, fluoroquinolones, and ceftriaxone. Later, she underwent cholecystectomy and was found to have gallbladder empyema with extensive necrosis. She recovered from surgeries well and did not have residual neurological deficit. The patient improved clinically and was discharged on ceftriaxone 2 g daily for 6 weeks. She was doing well at 1-week follow-up after discharge. Antibiotics were extended for a total of 12 weeks based on her elevated inflammatory markers. At the end of the course, she did not have any neurological deficit.
Figure 1.
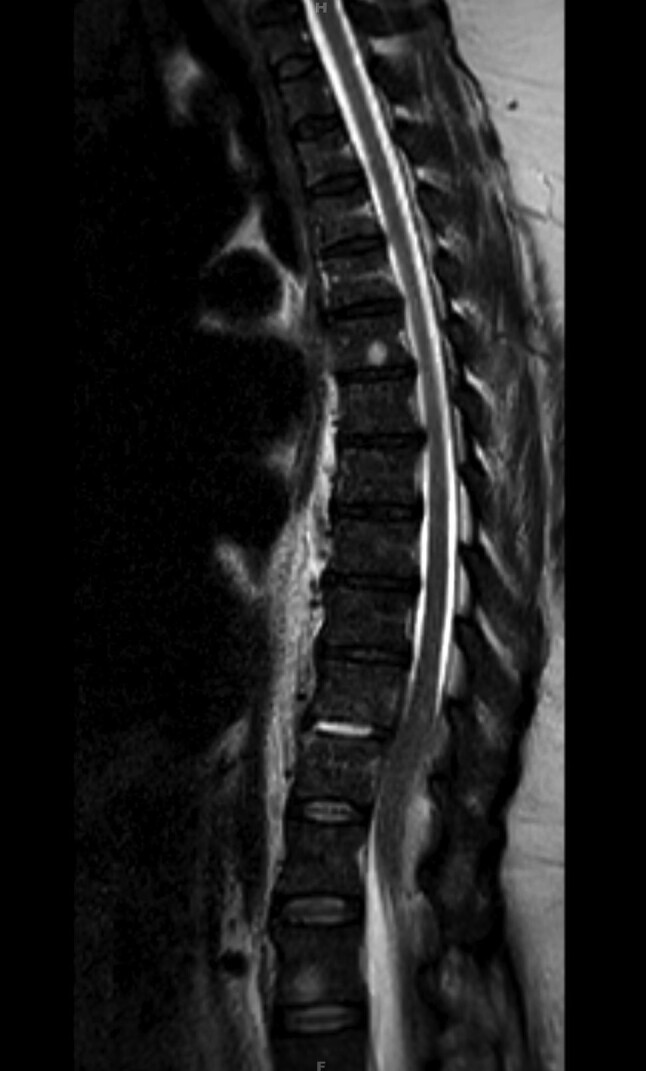
Sagittal view of contrast-enhanced magnetic resonance imaging of thoracic and lumbar spine shows discitis at the T10–T11 disc space level. Abnormal meningeal enhancement is noted at T10–T12, causing some spinal cord compression.
DISCUSSION AND REVIEW OF THE LITERATURE
Salmonellae are gram-negative, non-spore-forming, facultative anaerobic bacilli belonging to the Enterobacteriaceae family and subdivided into 2 species: Salmonella bongori and Salmonella enterica. Among these 2 species, >2500 serovars have been reported based on antigenic diversity of somatic O antigens (component of lipopolysaccharide) and flagellar (H) antigens. Almost all of the serotypes pathogenic for humans belong to S enterica species. NTS infection is caused by Salmonella species other than S Typhi and S enterica subsp enterica serovar Paratyphi. The incidence of NTS infections is highest in summers in temperate climates and rainy seasons in tropical areas.
NTS can be acquired from multiple animal reservoirs, and transmission to humans can occur by ingestion of contaminated food of animal origin, especially eggs, poultry, undercooked meat, dairy products, and fresh produce contaminated with manure. Human or animal waste can contaminate the surface of fruits and vegetables and, if not removed by washing, can be introduced into the food. In 2019, the CDC reported a multistate foodborne outbreak caused by Salmonella associated with precut melons that infected 137 people from 10 states [6]. Once the bacteria are ingested with contaminated food or water, it has the ability to display an adaptive acid tolerance, which facilitates its survival in the stomach and passage to the small intestines. Salmonella invades the intestinal epithelial cells by bacteria-mediated endocytosis with the help of an encoded protein called type III secreting system within Salmonella pathogenicity island 1 (SPI-1 T3SS) [7]. NTS also induces a secretory response in the intestinal epithelium initiating recruitment and transmigration of neutrophils into the intestinal lumen causing intestinal inflammation, which contributes to fluid secretion and diarrhea through disrupted epithelial barrier. Studies have demonstrated that Salmonella also uses SPI-1 T3SS to downregulate the host inflammatory response to intestinal invasion, contributing to asymptomatic colonization of the intestines typically seen with NTS infections. Bacteria can enter the biliary tract via portal vein bloodstream, directly from small intestines via the duodenal papilla or direct transfer into the gallbladder from the liver. Salmonella Typhi is known to colonize gallbladder due to its ability to form biofilms on the gallstones and cholesterol-coated surfaces as demonstrated by Crawford et al [8]. It is proposed that nontyphoidal strains can also form gallstone biofilm in similar fashion, leading to chronic carriage in the gallbladder, but this is infrequent and reported in only 0.2%–0.6% of adults infected by NTS as compared to S Typhi, which causes chronic carriage in 1%–4% of patients [9]. Immunosuppressive conditions and cholelithiasis are considered predisposing risk factors for gallbladder colonization [10].
The biliary tract including liver and gallbladder are the most common extraintestinal sites of Salmonella infection. Although acute cholecystitis is a well-reported complication of infection caused by S Typhi, it is rarely seen with NTS and only 10 cases of acute cholecystitis are reported in literature. Empyema of gallbladder is a much rarer complication. In the literature, only 4 cases of empyema of gallbladder due to NTS are reported and only 1 of them had invasive infection after treatment of primary infection (Table 1). That patient had underlying chronic granulomatous disease and was initially treated with 10 days of intravenous ceftriaxone for gastroenteritis and bacteremia due to S Enteritidis. After initial improvement, she relapsed and presented after 2 weeks with gallbladder empyema and did well after laparoscopic cholecystectomy [4].
Table 1.
Summary of Published Cases of Empyema of the Gallbladder Caused by Salmonella Enteritidis
| Study, Year [Reference] | Age, y |
Sex | Presenting Symptoms | Risk Factors | Source of Positive Culture | Salmonella Species | Surgical Intervention | Antibiotics | Outcome |
|---|---|---|---|---|---|---|---|---|---|
| Yamashita et al, 2018 [4] | 40 | F | Fever, abdominal pain, diarrhea | CGD | Blood, stool, bile | S Enteritidis | PTC, cholecystectomy | Levofloxacin, ceftriaxone | R |
| Lianos et al, 2019 [11] | 32 | M | Fever, abdominal pain, vomiting, diarrhea | None | Stool, bile | Not reported | Cholecystectomy | CRO, ciprofloxacin |
R |
| Juma et al, 2019 [12] | 41 | M | Fever, abdominal pain, vomiting, diarrhea | Travel to Guinea | Bile | S enterica subsp salamae | PTC, cholecystectomy | Piperacillin-tazobactam, CRO, ciprofloxacin | R |
| Zhao et al, 2020 [13] | 90 | M | Fever, abdominal pain, vomiting, diarrhea | Eating oysters | Blood, stool, bile | Salmonella group D | Cholecystectomy | CRO, meropenem | R |
| Present study | 52 | F | Leg weakness, abdominal pain, fever, diarrhea | Uncontrolled DM, morbid obesity | Blood, stool, bile, epidural abscess | S Enteritidis | Cholecystectomy, drainage of epidural abscess | CRO, TMP-SMX | R |
Abbreviations: CGD, chronic granulomatous disease; CRO, ceftriaxone; DM, diabetes mellitus; F, female; M, male; PTC, percutaneous transhepatic cholecystostomy; R, recovered; TMP-SMX, trimethoprim-sulfamethoxazole.
Epidural abscess is another rare clinical manifestation of NTS infection, with only 16 cases reported in the literature (Table 2). Sickle cell disease, connective tissue disease, HIV infection, poorly controlled DM, and myelofibrosis were reported as underlying risk factors for disseminated disease. The most common location identified was the lumbar spine followed by thoracic and cervical spine. Salmonella Enteritidis was the most common species identified. Commonly used antibiotics were third-generation cephalosporins, ciprofloxacin, penicillin, and TMP-SMX. Seven patients required surgical intervention in addition to the antibiotic.
Table 2.
Summary of Published Cases of Epidural Abscess Caused by Nontyphoidal Salmonella
| Study, Year [Reference] | Age, y |
Sex | Presenting Symptoms | Risk Factors | Source of Positive Culture | Location | Salmonella Species | Surgical Intervention | Antibiotics | Other Foci of Infection |
|---|---|---|---|---|---|---|---|---|---|---|
| Gardner, 1985 [14] | 2 | M | Fever, irritability | SCD | Blood, epidural fluid, CSF | L3 | S Enteritidis | Laminectomy and evacuation of epidural abscess | Ampicillin (4 wk) and chloramphenicol (3 wk) | Meningitis, lumbar, and rib osteomyelitis |
| Akagi et al, 1998 [15] | 53 | M | Diarrhea, paresthesia, weakness of bilateral upper and lower extremities | Alcohol abuse | Stool, epidural tissue | C5–C7 | S Enteritidis | Decompression and anterior cervical fusion with bone graft | Cefmetazole and amikacin | None |
| Diebold et al, 2003 [16] | 5 | M | Fever, diarrhea, headache | SCD | Blood, stool, frontal subcutaneous fluid | Frontal (Intracranial) | S Enteritidis | Frontal bone resection and drainage of epidural abscess followed by reconstruction | Amoxicillin-clavulanic acid (2 wk) followed by CRO (2 wk) and then amoxicillin-clavulanic acid (2 wk) | Frontal osteitis, Frontal skin abscess |
| Ozturk et al, 2006 [17] | 53 | F | Leg pain | SLE | Epidural tissue and fluid, trochanteric abscess | T8–T10 | S Typhimurium | Hemilaminectomy, drainage of abscess | Ciprofloxacin | Trochanteric abscess |
| Blázquez et al, 2009 [18] | 2 | F | Fever, diarrhea, vomiting | Brain surgery, dexamethasone use | Epidural fluid, CSF, stool | Parietal (intracranial) | S Enteritidis | Not done | Ceftazidime (1 wk), chloramphenicol (4 wk), cefotaxime (2 wk) | Brain abscess |
| Hachfi et al, 2009 [19] | 37 | M | Fever, headache | HIV | Frontal epidural fluid | Intracranial | S Typhimurium | Craniotomy and evacuation of abscess | Ciprofloxacin (12 wk) | Frontal osteomyelitis |
| Choi et al, 2010 [20] | 62 | M | Back pain | None | Epidural fluid | L3 | Salmonella group D | None | CRO, ciprofloxacin | None |
| de Araujo et al, 2012 [21] | 69 | F | Back pain, fever | SLE | Blood, epidural fluid | T6–T7 | S Enteritidis | Evacuation of abscess | Cefepime | None |
| Oki et al, 2016 [22] | 54 | M | Fever, diarrhea, back pain | Alcohol abuse | Blood, stool, CSF | L4–L5 | S Enteritidis | None | CRO, ciprofloxacin | Meningitis |
| Fareed et al, 2017 [23] | 50 | M | Fever, diarrhea, back pain | Myelofibrosis | Blood | L4–L5 | Salmonella group D | Laminectomy, drainage of epidural abscess | CRO (8 wk) | None |
| Dahlberg et al, 2018 [24] | 54 | M | Back pain, LE numbness and weakness | None | Epidural tissue and fluid | L5–S1 | S Agbeni | Decompression laminectomy, evacuation of epidural abscess | CRO | None |
| Majid et al, 2018 [25] | 54 | F | Back pain, LE numbness and weakness | None | Blood, epidural fluid, urine | T12–L3 | S Enteritidis | Hemilaminectomy and drainage of epidural abscess | CRO, ciprofloxacin | Endocarditis, UTI |
| Hirai et al, 2020 [5] | 52 | M | Fever, back pain | Well-controlled DM | Blood, stool, epidural fluid | L1–L5 | S Altona | Drainage of epidural abscess | CRO, ciprofloxacin | Psoas abscess, spinal osteomyelitis |
| Elnour et al, 2022 [26] | 27 | M | Fever, back pain | SCD, splenectomy | Blood, sputum, epidural fluid | T4–T7 | Salmonella (non- Typhi) | Decompression, posterior T4–T7 laminectomy, and drainage of epidural abscess | CRO (4 wk) followed by TMP-SMX (2 wk) | Pneumonia, discitis |
| Hsu et al, 2022 [27] | 85 | F | Fever, back pain | DM | Blood, urine | T12–S1 | Salmonella group C | Surgical debridement, decompression, and drainage of epidural abscess | Doripenem (8 wk) followed by colistin (5 wk) | Discitis |
| Daggett et al, 2022 [28] | 1 | M | Fever, diarrhea, vomiting, difficulty ambulating | Travel to Florida | Surgical tissue | S1–S2 | S Enteritidis | Hemilaminectomy and evacuation of epidural abscess | CRO (6 wk) followed by amoxicillin (3 mo) | Spinal osteomyelitis |
| Present study | 52 | F | Leg weakness, abdominal pain, fever, diarrhea | Uncontrolled DM | Blood, stool, bile, epidural fluid | T10–T12 | S Enteritidis | Cholecystectomy, drainage of epidural abscess | CRO, TMP-SMX | Gallbladder empyema |
Abbreviations: CSF, cerebrospinal fluid; CRO, ceftriaxone; DM, diabetes mellitus; F, female; HIV, human immunodeficiency virus; LE, lower extremity; M, male; SCD, sickle cell disease; SLE, systemic lupus erythematosus; TMP-SMX, trimethoprim-sulfamethoxazole; UTI, urinary tract infection.
In our literature review, we have found only 1 patient who developed epidural abscess and gallbladder empyema due to S Enteritidis after treatment of primary infection and, to our knowledge, this is the first case reported. We can postulate here that cholelithiasis and poorly controlled DM might have contributed to colonization of gallbladder in our patient. The reason for developing invasive disease despite treatment of primary infections could be explained by the fact that antibiotics might not be effective in eliminating carriage state and instead prolong the duration of carriage state. Also, poorly controlled DM and morbid obesity could have contributed toward invasive NTS disease as these factors are known to cause immunosuppression. The definitive study to diagnose epidural abscess is contrast-enhanced MRI, which was delayed in this case due to AKI. In our literature review, DM was reported in 2 cases as the underlying risk factor for invasive NTS disease, whereas morbid obesity has not been reported as a risk factor for invasive NTS disease.
Uncomplicated NTS gastroenteritis is a self-limiting disease, and antimicrobials should not be routinely used as they may not eliminate the bacteria and may select for resistant strains. Antimicrobial therapy should be considered in certain populations who are at increased risk of developing invasive infections and include neonates; patients aged >50 years; and patients with underlying immunosuppressed condition, cardiac valvular or endovascular abnormalities, or prosthetic joints. Due to increasing reported resistance, patients with bacteremia should be empirically treated with third-generation cephalosporins or fluoroquinolones unless the susceptibilities are known. Recommended antimicrobial therapy for epidural abscess is intravenous ceftriaxone or ampicillin for 6 weeks in addition to source control if indicated.
Treatment of patients with asymptomatic carriage of NTS is controversial; a randomized, placebo-controlled trial conducted on food workers in Thailand using two 5-day regimens (norfloxacin 500 mg twice daily and azithromycin 500 mg daily) showed no benefits among the 2 groups but instead increased the risk of reinfection and antimicrobial resistance [29].
We hypothesize, based on our case and review of the literature, about the potential for colonization of NTS among patients with diseased gallbladder and an immunocompromised state that can put them at risk for invasive disease even after adequate treatment [10]. Therefore we suggest careful monitoring and follow-up for these at-risk patients with NTS bacteremia.
CONCLUSIONS
NTS can cause colonization of the gallbladder in patients with immunocompromised conditions and cholelithiasis. These patients can develop invasive NTS disease even after the adequate treatment of primary infection as antibiotics are not effective in eliminating the colonization state. Careful monitoring and follow-up should be considered for these at-risk patients with NTS bacteremia. Prompt diagnosis, treatment, and early interventions for invasive NTS disease are important to prevent life-threatening complications.
Contributor Information
Ambreen Malik, Division of Infectious Disease, Ascension St John Hospital, Grosse Pointe, Michigan, USA.
Mamta Sharma, Division of Infectious Disease, Ascension St John Hospital, Grosse Pointe, Michigan, USA; Division of Infectious Disease, Wayne State University School of Medicine, Detroit, Michigan, USA.
Leonard B Johnson, Division of Infectious Disease, Ascension St John Hospital, Grosse Pointe, Michigan, USA; Division of Infectious Disease, Wayne State University School of Medicine, Detroit, Michigan, USA.
Ashish Bhargava, Division of Infectious Disease, Ascension St John Hospital, Grosse Pointe, Michigan, USA; Division of Infectious Disease, Wayne State University School of Medicine, Detroit, Michigan, USA; Division of Infectious Disease, Thomas Mackey Center for Infectious Disease Research, Detroit, Michigan, USA.
Notes
Patient consent. Written informed consent was obtained from the patient. The design of the study conforms to the standards currently applied in the country.
References
- 1. Centers for Disease Control and Prevention . Salmonellosis, nontyphoidal. Travel-associated infections and diseases. CDC Yellow Book 2024: Health Information for International Travelers. New York: Oxford University Press;2023.
- 2. Centers for Disease Control and Prevention . Salmonella. 2023. Available at: https://www.cdc.gov/salmonella/index.html. Accessed 9 June 2023.
- 3. Hsu RB, Tsay YG, Chen RJ, Chu SH. Risk factors for primary bacteremia and endovascular infection in patients without acquired immunodeficiency syndrome who have nontyphoid salmonellosis. Clin Infect Dis 2003; 36:829–34. [DOI] [PubMed] [Google Scholar]
- 4. Yamashita Y, Kimura T, Tanaka N, et al. Salmonella Enteritidis cholecystitis with chronic granulomatous disease. IDCases 2018; 12:49–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Hirai N, Kasahara K, Yoshihara S, et al. Spinal epidural abscess caused by non-typhoidal Salmonella: a case report and literature review. J Infect Chemother 2020; 26:1073–7. [DOI] [PubMed] [Google Scholar]
- 6. Centers for Disease Control and Prevention . Outbreak of Salmonella infections linked to pre-cut melons. 2019. Available at: https://www.cdc.gov/salmonella/carrau-04-19/index.html. Accessed 9 June 2023.
- 7. Francis CL, Starnbach MN, Falkow S. Morphological and cytoskeletal changes in epithelial cells occur immediately upon interaction with Salmonella Typhimurium grown under low-oxygen conditions. Mol Microbiol 1992; 6:3077–87. [DOI] [PubMed] [Google Scholar]
- 8. Crawford RW, Rosales-Reyes R, Ramírez-Aguilar M, Chapa-Azuela O, Alpuche-Aranda C, Gunn JS. Gallstones play a significant role in Salmonella spp. gallbladder colonization and carriage. Proc Natl Acad Sci U S A 2010; 107:4353–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Pegues DA, Miller SI. Salmonella species. In: Bennett JE, Dolin R, Blaser MJ, eds. Principles and practice of infectious diseases. 9th ed. Philadelphia, PA: Elsevier/Saunders; 2020:2725–35. [Google Scholar]
- 10. Han T, Sokal JE, Neter E. Salmonellosis in disseminated malignant diseases. A seven–year review (1959–1965). N Engl J Med 1967; 276:1045–52. [DOI] [PubMed] [Google Scholar]
- 11. Lianos GD, Drosou P, Souvatzoglou R, et al. Acute acalculous cholecystitis with empyema due to salmonellosis. Case Rep Gastrointest Med 2019; 2019:5185314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Juma F, Cave JJ, Gonzales H, Moore LSP. Non-typhoidal salmonellosis presenting as acute calculus cholecystitis. BMJ Case Rep 2019; 12:e230186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Zhao Y, Zhang L, Xing F, Zhang R, Huang J. Synchronous acute acalculous cholecystitis and appendicitis due to Salmonella group D: a rare case report from China and review of literature. Front Med 2020; 7:406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Gardner RV. Salmonella vertebral osteomyelitis and epidural abscess in a child with sickle cell anemia. Pediatr Emerg Care 1985; 1:87–9. [PubMed] [Google Scholar]
- 15. Akagi S, Hirofumi S, Ishashi K, Takanori S, Royokei O. Cervical spondylitis and epidural abscess caused by Salmonella Enteritidis with tetraplegia. Orthopedics 1998; 21:1289. [DOI] [PubMed] [Google Scholar]
- 16. Diebold P, Humbert J, Djientcheu V, Gudinchet F, Rilliet B. Salmonella epidural abscess in sickle cell disease: failure of the nonsurgical treatment. J Natl Med Assoc 2003; 95:1095–8. [PMC free article] [PubMed] [Google Scholar]
- 17. Ozturk C, Tezer M, Mirzanli C, Bilen FE, Aydogan M, Hamzaoglu A. An uncommon cause of paraplegia: Salmonella spondylodiscitis. J Spinal Cord Med 2006; 29:234–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Blázquez D, Munoz M, Gil C, Ruibal J. Brain abscess and epidural empyema caused by Salmonella Enteritidis in a child: successful treatment with ciprofloxacin: a case report. Cases J 2009; 2:7131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Hachfi W, Bellazreg F, Ladib M, et al. Salmonella Typhimurium epidural empyema in an HIV infected patient. Infect Dis Rep 2009; 1:e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Choi YS, Cho WJ, Yun SH, et al. A case of back pain caused by Salmonella spondylitis—a case report. Korean J Anesthesiol 2010; 59(Suppl):S233–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. de Araujo DB, Daolio L, Szajubok J, Araujo N, Chahade W. Epidural abscess due to Salmonella Enteritidis in a patient with systemic lupus erythematosus. Lupus 2012; 21:1356–8. [DOI] [PubMed] [Google Scholar]
- 22. Oki M, Ueda A, Tsuda A, Yanagi H, Ozawa H, Takagi A. Salmonella enterica serotype Enteritidis vertebral osteomyelitis and epidural abscess complicated with meningitis. Tokai J Exp Clin Med 2016; 41:169–71. [PubMed] [Google Scholar]
- 23. Fareed S, Nashwan A, Abu JS, et al. Spinal abscess caused by Salmonella bacteremia in a patient with primary myelofibrosis. Am J Case Rep 2017; 18:859–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Dahlberg RK, Lyvers ME, Dahlberg TK. Diagnostic quandary: Salmonella Agbeni vertebral osteomyelitis and epidural abscess. Case Rep Orthop 2018; 2018:1091932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Majid R, Demla V, Mohammed A, et al. Salmonella Enteritidis concurrent spinal epidural abscess, urinary tract infection and endocarditis in an immunocompetent host: case report and a review of the literature. J Trop Dis 2018; 6:3. [Google Scholar]
- 26. Elnour S, Hashim M, Ibrahim H. Disseminated non typhoidal Salmonella infection with Salmonella pneumonia and vertebral osteomyelitis in sickle cell disease: a case report. IDCases 2022; 27:e01390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Hsu TL, Yang CJ, Pao JL. Salmonella spondylodiscitis and epidural abscess successfully treated with unilateral biportal endoscopic discectomy and debridement: a rare case report. J Int Med Res 2022; 50:3000605221085405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Daggett A, Heston AL, Anderson MB, Qubain LM, Veesenmeyer AF. A rare case of Salmonella osteomyelitis in immunocompetent toddler without risk factors. Cureus 2022; 14:e30642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Sirinavin S, Thavornnunth J, Sakchainanont B, Bangtrakulnonth A, Chongthawonsatid S, Junumporn S. Norfloxacin and azithromycin for treatment of nontyphoidal Salmonella carriers. Clin Infect Dis 2003; 37:685–91. [DOI] [PubMed] [Google Scholar]


