Abstract
Background:
Residual tumor at the resection margins after surgery for gastric and gastroesophageal junction (GEJ) adenocarcinoma is a known prognostic factor. In this single-center, retrospective cohort study in a tertiary referral center, the authors aimed to evaluate the relevance of intraoperative pathology consultation (IOC) and consecutive extension of surgery on patient survival.
Study design:
Of 737 consecutive patients undergoing (sub)total gastrectomy for gastric or GEJ adenocarcinoma, 679 cases with curative intent surgery between 05/1996 and 03/2019 were included. Patients were categorized into: R0 without further resection (direct R0), R0 after positive IOC and extension of resection (converted R0), and R1.
Results:
IOC was performed in 242 (35.6%) patients, in 216 (89.3%) at the proximal resection margin. Direct R0-status was achieved in 598 (88.1%), converted R0 in 26 (3.8%) of 38 (5.6%) patients with positive IOC and R1 in 55 (8.1%) patients. The median follow-up was 29 months for surviving patients. 3-year survival rate (3-YSR) was significantly higher for direct R0 compared to converted R0 with 62.3% compared to 21.8% (hazard ratio=0.298; 95% CI=0.186–0.477, P<0.001). 3-YSR was similar between converted R0 and R1 (21.8 vs. 13.3%; hazard ratio =0.928; 95% CI=0.526–1.636, P=0.792). In multivariate analysis, advanced T (P<0.001), N (P<0.001), R (P=0.003), and M1 status (P<0.001) were associated with worse overall survival.
Conclusion:
IOC and consecutive extended resection for positive resection margins in gastrectomy for the proximal gastric and GEJ adenocarcinoma does not achieve long-term survival benefits in advanced tumor stages.
Keywords: esophagogastric adenocarcinoma, excision margin, extended resection, gastric adenocarcinoma, gastric cancer, gastroesophageal junction adenocarcinoma, histopathology, intraoperative consultation, intraoperative consultation (IOC), prognosis, R1, resection margin, residual tumor, stomach neoplasm
Introduction
Highlights
A single-center, retrospective cohort study concerning residual tumors at the resection margins after oncological surgery of the gastric and gastroesophageal junction.
Seven hundred thirty-seven consecutive patients undergoing (sub)total gastrectomy for gastric or gastroesophageal junctional adenocarcinoma with 679 cases with curative intent surgery between 05/1996 and 03/2019 were included.
Intraoperative pathology consultation and consecutive extended resection for positive resection margins in proximal gastric and gastroesophageal junction adenocarcinoma does not achieve long-term survival benefits in advanced tumor stages.
Adenocarcinoma of the stomach and the gastroesophageal junction (GEJ) are highly prevalent causes of cancer related death1–4. Despite advances in the management of both malignancies, curative intent therapy still relies on the resection of the primary tumor and on oncological lymphadenectomy5. One of the prognostic factors in gastric/GEJ adenocarcinoma is the resection margin status. Achievement of an R0 resection is well-known to be associated with an improved overall survival (OS)6.
A common practice for enhancing the R0 resection rate is the utilization of intraoperative pathology consultation (IOC). In the case of a positive IOC result, resection can be extended in order to achieve a tumor-free resection margin7. Usage of IOC on the oral margin is recommended where the distance of transection from the grossly visible tumor edge is under 5 cm in intestinal type and under 8 cm in diffuse type carcinoma8. Some authors even recommend routine execution of IOC in every patient7. Although the concept suggests an improvement of operative management, little is known about the impact of IOC and consecutive extended resection.
A significant issue for GEJ II carcinoma is, if IOC and consecutive extension of resection for ensuring a tumor-free oral margin is reliable, or whether the transthoracic approach should be prioritized in cases with a higher risk of positive margins. IOC may also yield false negative results9. Rather than the resection margin status, OS should be considered as the more important outcome parameter. Recently, it has been shown that false negative IOC results do not lead to a decrease in disease-specific survival compared to cases with true positive IOC results9. This brings up the important question, whether intraoperative detection of microscopic tumor infiltration at the resection margin and the subsequent change of operative management generate a survival benefit.
The aim of this study is to reveal the prognostic value of IOC and extended resection in gastric and GEJ adenocarcinoma.
Methods
Study population
This retrospective cohort study was performed at our tertiary referral center for tumors of the upper gastrointestinal tract. Since 1st May 1996 medical charts of patients who undergo surgery for gastric or GEJ adenocarcinomas have been collected in a department internal database for research analyses.
In this study, patients undergoing curative surgery between 1st May 1996 and 31st March 2019 with (sub)total gastrectomy for gastric or GEJ adenocarcinoma were included. Palliative resections and resections with transthoracic esophagectomy and gastric pull-up were excluded. Informed consent to be included in the study was obtained from all patients. The study was approved by the local ethics committee (application number: 19-1480).
Study variables
Demographics, perioperative treatment, survival, and clinicopathologic data were obtained. Medical records were reviewed and complications were categorized according to the Clavien–Dindo classification.
Depending on IOCs, extended resection and the final pathology report a treatment flow-chart was created (Fig. 1): Patients were grouped on IOC received (yes/no), IOC positive (yes/no), extended resection (yes/no), second IOC (IOC2; yes/no), IOC2 positive (yes/no), and final pathology results (R0/R1). The decision to perform IOC and to carry out extended resection in the case of a positive IOC was at the discretion of the treating surgeon. Patients were then categorized into three subgroups according to final R-status: R0 after extended resection (i.e. converted R0), R0 without extended resection (i.e. direct R0), and R1.
Figure 1.
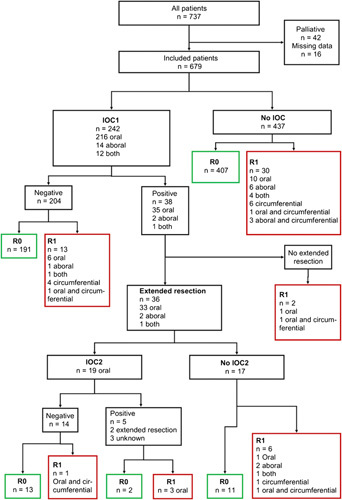
Study cohort flow: Cases were classified according to intraoperative pathology consultation, extension of resection, and final resection margin status.
Histopathology
IOC was performed on hematoxylin and eosin (HE) stained optimal cutting temperature compound embedded frozen sections, and IOC margin status was categorized as positive or negative. Final pathological reports were based on HE stained paraffin-embedded resection specimens.
Statistical analysis
Statistical analyses were performed with SPSS 25.0 software (IBM Inc.). For categorical variables χ 2 statistics and for continuous variables Student’s t-test, the analysis of variance (ANOVA)-Test and the Dunn-Bonferroni-Test were used. Kaplan–Meier survival plots were calculated for OS. Univariate and multivariate Cox log-rank regression analyses were performed to assess independent predictors of prognosis. OS was defined as the time from tumor resection to death. The threshold for statistical significance and the cut off value for inclusion in the multivariate model was predefined as two-sided P<0.05.
Results
Demographics and clinicopathological features
Six hundred and seventy-nine (92.1%; median age 65, 458 men, 221 women) of 737 consecutive patients undergoing (subtotal) gastrectomy due to adenocarcinoma of the stomach or GEJ were included (Table 1). Fourty-two (5.7%) patients receiving palliative resection due to tumor related complications (e.g. bleeding, perforation, ileus) and 16 (2.2%) patients with insufficient data were excluded.
Table 1.
Demographic, operative, postoperative, and pathological characteristics of the study cohort.
| Variable | All Patients (n=679) | Direct R0 (n=598) | R1 (n=55) | Converted R0 (n=26) | P |
|---|---|---|---|---|---|
| Sex | |||||
| Male | 458 (67.5%) | 404 (67.6%) | 35 (63.6%) | 19 (73.1%) | 0.690 |
| Female | 221 (32.5%) | 194 (32.4%) | 20 (36.4%) | 7 (26.9%) | |
| Age, years | 63.8 | 64.1±0.5 | 61.0±1.7 | 63.2±2.4 | 0.198 |
| Tumor site | |||||
| GEJ | 258 (38.1%) | 218 (36.6%) | 21 (38.2%) | 19 (73.1%) | <0.001 |
| Proximal stomach | 53 (7.8%) | 43 (7.2%) | 7( 12.7%) | 3 (11.5%) | |
| Body | 180 (26.6%) | 166 (27.9%) | 12 (21.8%) | 2 (7.7%) | |
| Distal | 145 (21.4%) | 136 (22.8%) | 8 (14.5%) | 1 (3.8%) | |
| Whole stomach | 15 (2.2%) | 9 (1.5%) | 5 (9.1%) | 1 (3.8%) | |
| Gastric stump | 26 (3.8%) | 24 (4.0%) | 2 (3.6%) | 0 (0.0%) | |
| Operation type | |||||
| Transhiatal extended | 282 (41.5%) | 235 (39.3%) | 27 (49.1%) | 20 (76.9%) | <0.001 |
| Total | 296 (43.6%) | 272 (45.5%) | 21 (38.2%) | 3 (11.5%) | |
| Subtotal | 61 (9.0%) | 59 (9.9%) | 2 (3.6%) | 0 (0.0%) | |
| Resection of remnant stomach | 30 (4.4%) | 27 (4.5%) | 3 (5.5%) | 0 (0.0%) | |
| Total gastrectomy and subtotal esophagectomy | 9 (1.3%) | 4 (0.7) | 2 (3.6%) | 3 (11.5%) | |
| Fundus resection | 1 (0.1%) | 1 (0.2%) | 0 (0.0%) | 0 (0.0%) | |
| Reconstruction | |||||
| Roux-en-Y with Pouch | 97 (14.5%) | 86 (14.7%) | 11 (20.4%) | 0 (0.0%) | 0.004 |
| Roux-en-Y without Pouch | 550 (82.5%) | 487 (83.0%) | 40 (74.1%) | 23 (88.5%) | |
| Billroth II | 7 (1.0%) | 6 (1.0%) | 1 (1.9%) | 0 (0.0%) | |
| Colon interposition | 10 (1.5%) | 5 (0.9%) | 2 (3.7%) | 3 (11.5%) | |
| Other | 3 (0.4%) | 3 (0.5%) | 0 (0.0%) | 0 (0.0%) | |
| LAD | |||||
| <D2 | 7 (1.1%) | 5 (0.9%) | 2 (3.8%) | 0 (0.0%) | 0.197 |
| ≥ D2 | 651 (98.9%) | 575 (99.1%) | 51 (96.2%) | 25 (100%) | |
| (y)pT | |||||
| 0 | 16 (2.4%) | 16 (2.7%) | 0 (0.0%) | 0 (0.0%) | <0.001 |
| 1 | 142 (21.4%) | 139 (23.8%) | 1 (1.9%) | 2 (7.7%) | |
| 2 | 82 (12.3%) | 80 (13.7%) | 2 (3.8%) | 0 (0.0%) | |
| 3 | 277 (41.7%) | 239 (40.9%) | 22 (41.5%) | 16 (61.5%) | |
| 4 | 147 (22.1%) | 111 (19.0%) | 28 (52.8%) | 8 (30.8%) | |
| (y)pN | |||||
| 0 | 306 (45.3%) | 289 (48.6%) | 11 (20.4%) | 6 (23.1%) | <0.001 |
| 1 | 110 (16.3%) | 101 (17.0%) | 3 (5.6%) | 6 (23.1%) | |
| 2 | 94 (13.9%) | 80 (13.4%) | 10 (18.5%) | 4 (15.4%) | |
| 3 | 165 (24.4%) | 125 (21.0%) | 30 (55.6%) | 10 (38.5%) | |
| M | |||||
| M0 | 521 (86.7%) | 466 (88.6%) | 36 (73.5%) | 19 (73.1%) | 0.006 |
| M1 | 71 (11.8%) | 52 (9.9%) | 13 (26.5%) | 6 (23.1%) | |
| Mx | 9 (1.5%) | 8 (1.5%) | 0 (0.0%) | 1 (3.8%) | |
| UICC Stage | |||||
| I | 188 (28.7%) | 185 (32.1%) | 1 (1.9%) | 2 (7.7%) | <0.001 |
| II | 176 (26.9%) | 161 (28.0%) | 9 (17.3%) | 6 (23.1%) | |
| III | 205 (31.3%) | 164 (28.5%) | 29 (55.8%) | 12 (46.2%) | |
| IV | 71 (10.9%) | 52 (9.0%) | 13 (25.0%) | 6 (23.1%) | |
| (y)pT0 | 14 (2.1%) | 14 (2.4%) | 0 (0.0%) | 0 (0.0%) | |
| Lauren Subtype | |||||
| Diffuse | 211 (52.0%) | 174 (48.7%) | 33 (84.6%) | 4 (40.0) | <0.001 |
| Intestinal | 160 (39.4%) | 153 (42.9%) | 3 (7.7%) | 4 (40.0) | |
| Mixed | 35 (8.6%) | 30 (8.4%) | 3 (7.7%) | 2 (20%) | |
| Neoadjuvant therapy | |||||
| CTx | 0.350 | ||||
| PLF | 30 (10.4%) | 27 (10.8%) | 3 (11.5%) | 0 (0.0%) | |
| MAGIC | 59 (20.4%) | 49 (19.6%) | 7 (26.9%) | 3 (23.1%) | |
| FLOT | 113 (39.1%) | 102 (40.8%) | 6 (23.1%) | 5 (38.5%) | |
| Unknown | 47 (16.3%) | 41 (16.4%) | 5 (19.2%) | 1 (7.7%) | |
| Other | 4 (1.4%) | 3 (1.2%) | 1 (3.8%) | 0 (0.0%) | |
| RCTx | 36 (12.3%) | 28 (11.2%) | 4 (15.4%) | 4 (30.8%) | |
| Clavien–Dindo | |||||
| No complications | 323 (50.3%) | 295 (52.2%) | 20 (38.5%) | 8 (32.0%) | 0.194 |
| 1 | 76 (11.8%) | 65 (11.5%) | 9 (17.3%) | 2 (8.0%) | |
| 2 | 89 (13.9%) | 80 (14.2%) | 5 (9.6%) | 4 (16.0%) | |
| 3a | 63 (9.8%) | 52 (9.2%) | 7 (13.5%) | 4 (16.0%) | |
| 3b | 62 (9.7%) | 49 (8.7%) | 8 (15.4%) | 5 (20.0%) | |
| 4a | 9 (1.4%) | 8 (1.4%) | 1 (1.9%) | 0 (0.0%) | |
| 4b | 4 (0.6%) | 3 (0.5%) | 1 (1.9%) | 0 (0.0%) | |
| 5 | 16 (2.5%) | 13 (2.3%) | 1 (1.9%) | 2 (8.0%) | |
Continuous data shown as mean±SD, frequency data reported as absolute numbers and percentages.
CTx, Chemotherapy; GEJ, Gastroesophageal junction; LAD, Lymphadenectomy; RCTx, Chemoradiation; UICC, Union internationale contre le cancer.
Three hundred and eleven (45.9%) patients had a tumor at the GEJ or proximal stomach. The majority of the patients received a total gastrectomy with (n=282, 41.5%) or without (n=296, 43.6%) transhiatal extension. D2-level lymphadenectomy (LAD) was performed in 432 (65.7%) patients. 219 (33.3%) underwent greater than D2 LAD.
Most patients had locally advanced tumor disease [(y)pT3/4, n=424, 63.9%] and/or nodal invasion (n=369, 54.7%). Neoadjuvant treatment was administered to 289 (42.6%) patients. Most frequently, the FLOT regimen10 was used (n=113, 39.1%), followed by chemotherapy according to the MAGIC trial11 (n=59, 20.4%). In 47 patients (16.3%) the exact chemotherapy regimen could not be determined.
Patients without IOC
Four hundred and thirty-seven patients did not receive IOC. The rate of final R1 was 6.9% (30 of 437) for these patients. In 20 cases (66.7%) final R1 was within oral/aboral resection margins, six cases (20%) within circumferential margins and in four cases (13.3%) both were affected (Fig. 1).
Patients with IOC
Two hundred and forty-two patients (35.6%) received IOC: 216 (89.3%) for oral, 14 (5.8%) for aboral, and 12 (5.0%) for both margins. The rate of final R1 was 10.3% (25 of 242) among cases receiving IOC (Fig. 1). IOC was more frequently performed for GEJ and proximal stomach tumors (83.9%, P<0.001), during resection with transhiatal extension (78.5%, P=0.004) and after neoadjuvant treatment (51.2%, P=0.001).
Negative IOC
In 204 (84.3%) patients IOC was negative. 13 (6.4%) patients with negative IOC had a final R1.
Positive IOC
In 38 (15.7%) cases, IOC was positive and resection was extended in 36 (94.7%) cases with a final R0 achieved in 26 (72.2%) patients. 28.6% of the IOCs in midbody tumors were positive, the rate was 16.7% for GEJ and proximal stomach tumors. Extended resection was performed in 33 patients (91.7%) at the oral, in 2 (5.6%) patients at the aboral, and in one (2.8%) case at both resection margins. After extension, a second IOC (IOC2) was performed in 19 (52.8%) patients and waived in 17 (47.2%). For cases without a second IOC, the rate of final R1 was 35.3% (6 of 17) with tumor infiltration exclusively at the oral/aboral resection margins in four (66.7%) patients.
All 19 IOC2s were performed at the oral resection margin and were negative for 14 (73.7%) and positive for 5 (26.3%) patients. Two (40%) patients could be converted to final R0 with a second extension of resection, however, one (7.1%) patient had a final R1 including the oral margin despite a negative IOC2.
False negative results
Thirteen (6.4%) of 204 patients with negative IOC had a final R1. Considering that 4 (30.8%) of these 13 patients did not show tumor infiltration at the oral and aboral resection margins but were found to be only positive at the circumferential margin in the final pathology, the false negative rate for IOC was 4.4% (9 of 204).
Direct R0, converted R0 and R1
Five hundred and ninty-eight (88.1%) patients had a direct R0 resection, 26 (3.8%) were converted to R0, and 55 (8.1%) ended up with an R1 resection. Thirty patients with a final R1 status did not have an IOC (since the surgeon had no awareness of residual tumor at the margins). Thirteen R1 patients had a false negative IOC and therefore did not undergo extended surgical resection. Two patients did not undergo extended surgical resection. In 10 patients in the R1 cohort, a positive IOC resulted in extended resection without achieving final R0-status. Nevertheless, achieving R0-status was goal in all patients during surgery. However, factors such as the intraoperative status of the patients or the possibility of reconstruction after evaluation of the morbidity and benefit associated with the procedure had been taken into account limiting the extension of the surgical approach. Nineteen (34.5%) of 55 patients with R1 showed tumor infiltration at - but in eight (42.1%) cases not limited- to the circumferential margin.
Advanced pT-status ([y]pT3/4) was detected in 59.9% direct R0 patients, in 92.3% converted R0 patients and in 94.3% R1 patients (P<0.001, Table 1). Similarly, nodal metastases were seen in 51.4% direct R0 patients, in 77.0% converted R0 patients and in 79.7% R1 patients (P<0.001). An advanced UICC stage (stage III/IV) was diagnosed in 37.5% direct R0 patients, in 69.3% converted R0 patients and in 80.8% R1 patients (P<0.001, Table 1).
Transhiatal extended gastrectomy was performed in 39.3% direct R0 patients, in 76.9% converted R0 patients and in 49.1% R1 patients (P<0.001). 11.5% of converted R0 patients underwent colonic interposition as part of a more extensive resection versus 0.9% in the direct R0 cohort and 3.7% in the R1 group (P<0.001).
A higher proportion of patients in the converted group received neoadjuvant therapy compared to the direct R0 group (Table 1, Fig. 2), although the difference was statistically not significant. The significant differences and similarities between the three groups (direct R0, converted R0, and R1) are summarized in Figure 2.
Figure 2.
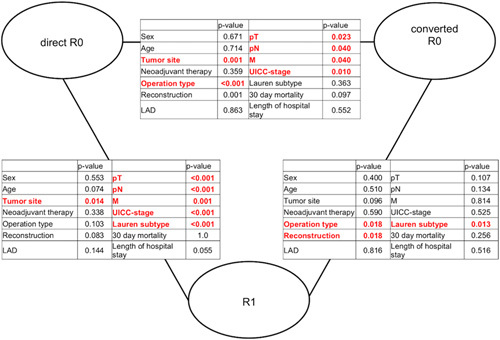
Head-to-head comparison: Demographic, operative, and postoperative parameters and pathology depending on resection margin status.
Survival analysis
The Median follow-up time for surviving patients was 29 months. Five-year survival rate (5-YSR) was 54.1% for the direct R0 group and 14.5% for the converted R0 (P<0.001; Fig. 3). Three-years survival rate (3-YSR) was also significantly higher for direct R0 compared to converted R0 (62.3 vs. 21.8%, hazard ratio (HR)=0.298; 95% CI=0.186–0.477, P<0.001).
Figure 3.
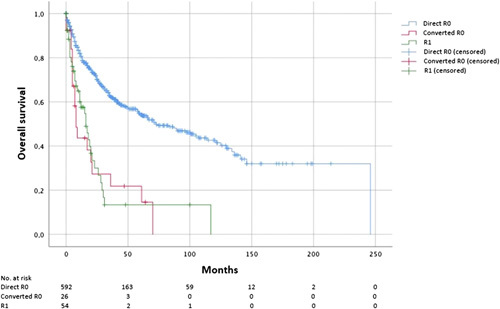
Overall survival depending on resection margin status: A significantly better survival for direct R0 margin was observed, whereas no significant difference in survival is shown between converted R0 and R1 margin.
There was no significant difference in OS between the converted R0 and the R1 group (3-YSR 21.8 vs. 13.3%, respectively, HR=0.928; 95% CI=0.526–1.636, P=0.792).
Even when tested among different histological subtypes (among non-diffuse type: P=0.310, HR=0.489, 95% CI= 0.113–2.116; among diffuse type: P=0.0814, HR=1.115, 95% CI=0.342–3.904), among patients with advanced tumor infiltration depth (pT3–pT4, P=0.929, HR=1.027, 95% CI=0.564–1.869) and among patients with nodal invasion (N+, P=0.864, HR=1.059, 95% CI=0.544–2.060), difference in OS remained nonsignificant between converted R0 and R1 patients. OS also remained statistically nonsignificant between converted R0 and R1 patients (P>0.99 HR=0.997, 95% CI=0.544–1.826) when cases with colon interposition were excluded (data not shown).
Tumor location at GEJ or proximal stomach was associated with a significantly poorer survival compared to more distal tumor locations (P=0.014).
Multivariate analysis
pT-, pN-, M-, and R-status, tumor location, surgical approach, type of reconstruction, and Lauren subtype were included in the multivariate analysis (Table 2). pT (P<0.001), pN (P<0.001), R (P=0.003), and M-status (P<0.001) proved to be significant independent variables associated with prognosis.
Table 2.
Multivariate analysis of factors associated with prognosis.
| Univariate | Multivariate | |||||||
|---|---|---|---|---|---|---|---|---|
| 95% CI for HR | 95% CI for HR | |||||||
| Variable | HR | lower limit | upper limit | P | HR | lower limit | upper limit | P |
| T stage | ||||||||
| Overall | <0.001 | <0.001 | ||||||
| (y)pT4 | Ref. | Ref. | ||||||
| (y)pT0 | 0.094 | 0.013 | 0.674 | 0.019 | 0.000 | 0.000 | 5.56E+125 | 0.951 |
| (y)pT1 | 0.142 | 0.093 | 0.217 | <0.001 | 0.271 | 0.151 | 0.488 | <0.001 |
| (y)pT2 | 0.269 | 0.174 | 0.414 | <0.001 | 0.320 | 0.162 | 0.631 | 0.001 |
| (y)pT3 | 0.543 | 0.415 | 0.709 | <0.001 | 0.507 | 0.357 | 0.721 | <0.001 |
| N Stage | ||||||||
| Overall | <0.001 | <0.001 | ||||||
| (y)pN3 | Ref. | Ref. | ||||||
| (y)pN0 | 0.186 | 0.139 | 0.248 | <0.001 | 0.406 | 0.264 | 0.626 | <0.001 |
| (y)pN1 | 0.281 | 0.192 | 0.412 | <0.001 | 0.408 | 0.235 | 0.709 | 0.001 |
| (y)pN2 | 0.480 | 0.342 | 0.673 | <0.001 | 0.550 | 0.352 | 0.860 | 0.009 |
| M Stage | ||||||||
| M1 | Ref. | Ref. | ||||||
| M0 | 0.227 | 0.162 | 0.318 | <0.001 | 0.405 | 0.254 | 0.646 | <0.001 |
| R-Status | ||||||||
| Overall | <0.001 | 0.003 | ||||||
| PS R1 | Ref. | Ref. | ||||||
| PS R0 | 0.316 | 0.220 | 0.454 | <0.001 | 0.660 | 0.413 | 1.055 | 0.083 |
| Converted R0 | 1.070 | 0.611 | 1.872 | 0.814 | 2.254 | 0.939 | 5.408 | 0.069 |
| Histological subtype | ||||||||
| Overall | 0.001 | – | – | – | 0.631 | |||
| Intestinal type | Ref. | – | – | – | Ref. | |||
| Diffuse type | 1.528 | 1.138 | 2.052 | 0.005 | – | – | – | – |
| Mixed | 2.394 | 1.479 | 3.876 | <0.001 | – | – | – | – |
| Surgical approach | ||||||||
| Overall | <0.001 | – | – | – | 0.807 | |||
| Transhiatal extended | Ref. | – | – | – | Ref. | |||
| Total gastrectomy | 0.741 | 0.576 | 0.954 | 0.020 | – | – | – | – |
| Subtotal gastrectomy | 0.560 | 0.354 | 0.887 | 0.013 | – | – | – | – |
| Fundus resection | 1.292 | 0.181 | 9.248 | 0.799 | – | – | – | – |
| Resection of the remnant stomach | 0.722 | 0.391 | 1.335 | 0.299 | – | – | – | – |
| Total gastrectomy and subtotal esophagectomy | 3.895 | 1.808 | 8.389 | 0.001 | – | – | – | – |
| Localization | ||||||||
| Overall | 0.045 | – | – | – | 0.365 | |||
| GEJ | Ref. | – | – | – | Ref. | |||
| Proximal | 1.403 | 0.944 | 2.083 | 0.094 | – | – | – | – |
| Corpus | 0.712 | 0.522 | 0.970 | 0.031 | – | – | – | – |
| Distal | 0.910 | 0.668 | 1.240 | 0.552 | – | – | – | – |
| Whole stomach | 1.295 | 0.604 | 2.779 | 0.507 | – | – | – | – |
| Stump | 0.755 | 0.383 | 1.488 | 0.417 | – | – | – | – |
| Reconstruction | ||||||||
| Overall | 0.003 | – | – | – | 0.544 | |||
| Roux-en-Y with pouch | Ref. | – | – | – | Ref. | |||
| Roux-en-Y without pouch | 1.118 | 0.798 | 1.565 | 0.516 | – | – | – | – |
| Billroth II | 2.049 | 0.868 | 4.838 | 0.102 | – | – | – | – |
| Colon interposition | 4.494 | 1.889 | 10.691 | 0.001 | – | – | – | – |
GEJ, Gastroesophageal junction; HR, Hazard ratio.
Discussion
Defining the resection margins in upper gastrointestinal cancer is a critical step for tumor removal. IOC is used to objectify the intraoperative decision-making. Our findings of a prognostic advantage for R0 resection margin are in accordance with previous studies6,12. However, not only the final pathology result, but also the way how this result is achieved, is of prognostic importance. If R0-status is accomplished only after extended resection, no significant long-term survival benefit is generated compared to R1 resection for advanced tumor stages, regardless the histological subtypes.
The reasons for these striking results can be various. First, patients with positive IOC and extended resection had more frequently advanced tumor disease and required a technically more challenging procedure (Table 1). The choice of the surgical approach is most importantly determined by the tumor location respective to the proximal stomach and esophagus5. Tumors infiltrating these regions in our cohort was managed via gastrectomy with transhiatal extension. The operative challenge can be high due to the reduced exposure, resulting in a more frequent need for IOC in proximal tumors.
Indeed, most of the IOCs in our study were conducted for proximal gastric and GEJ tumors and/or in patients undergoing resection with transhiatal extension. By far the most IOCs in our study were performed at the oral resection margin rather than the distal margin. Several prior studies have already shown that proximal tumor location is associated with poorer survival in gastric cancer13–15. Among patients receiving curative intent neoadjuvant chemotherapy, Felismino et al. reported that a larger proportion of patients with GEJ tumor location could not proceed to surgery compared with other gastric tumor location. Even when analyzing the resected patients only, they could still show a significant prognostic difference. This might underline the more aggressive nature of the proximal tumor disease16. Thus, not all patients benefit from perioperative chemotherapy in today’s multimodal treatment of gastric cancer. In some cases, systematic chemotherapy is ineffective in achieving tumor regression, instead large tumor manifestations with advanced locoregional lymphatic metastasis develop under treatment. To achieve a complete R0 resection in these situations, extended resections consecutively become necessary. This may be a possible explanation for the fact that R1 converted to R0 after extension of the initial resection does not affect the patients’ prognosis at all. Only a small number of patients within this retrospective analysis had metastases (80/679 patients). All patients were preoperatively staged as M0 and underwent treatment with curative intent. Palliative resections were not planned. However, in 11.8% of patients, a single liver metastasis or distant lymph node metastasis was found intraoperatively and was resected simultaneously. This practice of proceeding with surgery when a local metastasis is incidentally found was consistent with the recommendations of the German S3 guideline for gastric cancer8. In addition, for oligometastatic gastric cancer, this procedure can also be performed in a planned manner in selected cases according to the ESMO guidelines for this tumor entity17,18. Therefore, we performed the statistical workflow once again excluding all patients without M0 status. Both, univariate and multivariate analysis confirmed the results of the overall cohort showing that those effects observed were not depending on the occurrence of distant metastases in those cohorts.
Beyond, it must be emphasized that comparing the prognostic impact of positive margins in proximal versus distal gastric cancers has methodological limitations. The aspect of remaining R1 is surely only one factor that needs to be consider in this context. As mentioned before, depending on the tumor localization, these tumors have substantially different characteristics including divergent genetic features19 resulting in a survival disadvantage for patients with proximal gastric tumors20–22 independent from the results of IOC. However, a recent meta-analysis performed demonstrated that 3- and 5-year OS of proximal and distal gastric cancer patients after gastrectomy did not differ significantly in western countries21.
Another explanation can be sought in the extent of lymphadenectomy, which can be limited in a transabdominal approach, in case oral margins are positive in IOC and resection must be extended into the mediastinum23. A limited lymphadenectomy can result in residual tumor disease.
Additionally, patients with an initially positive IOC possibly also had a more aggressive tumor biology in the first place. This might be why these patients had poorer survival even after conversion to R0 compared to R0 without extended resection. One of the molecular subgroups are tumors exhibiting chromosomal instability24, that are more frequently present in GEJ and cardia tumor locations, which may also affect prognosis.
In our current study, locally advanced nodal metastases occurred almost twice as often as in patients who initially had tumor-positive resection margins and underwent extension of oncologic resection. There was no difference in lymphatic status between patients with converted R0 and those who remained R1 (Fig. 2). This might be another reason for the fact that the patients’ prognosis did not improve after extended resection due to IOC. Indeed, in advanced tumor stages, other factors such as pT-, pN-, or M-status seem to outweigh the effects of the secondary R0 resection achieved in these patients. The multivariate analysis supports this suggestion, as low pT-, pN-, and M-status as well as R-status in general (but not converted R0) were independent prognostic variables for a favorable prognosis. Our data indicates that the converted R0 and R1 groups are similar in many baseline characteristics (see Fig. 2). We performed additional propensity score matching analysis of the converted R0 and R1 cohorts (matched for the following parameters: sex, pT category, pN category, M-status, tumor location, and type of surgery), which also confirmed these results. Postsurgical prognosis was comparable in both cohorts (P=0.790). The reason why the two groups differ in terms of surgical technique and surgical reconstruction is more likely due to the more aggressive surgical approach used in the converted R0 patients to achieve secondary tumor freedom. Therefore, these patients were more likely to undergo transhiatal resection or colonic interposition.
The rate of IOC in midbody and distal gastric tumors in our study was fairly low and the prognostic benefit of extended resection after positive IOC cannot be specifically assessed for these tumor locations. 28.6% of the IOCs in midbody tumors were positive, which is a much greater proportion than that in GEJ and proximal stomach tumors (16.7%). The impact of IOC and the extension of resection in these location remains unclear.
Considering all tumor locations included in this study, IOC was performed in 35.6% of cases, which is similar to previous reports. IOC via frozen section evaluation has a high diagnostic accuracy7,9. McAuliffe et al. 9 reported a prevalence of 1.7% false negative results. We encountered a rate of 4.1%. In the light of our results, though, one can assert that the false negative results did not cause drawbacks for the patients, as the extended resection failed to show any significant survival benefit. This is supported by survival analyses, where disease-specific survival was similar between patients with false negative IOC result and those with true positive IOC result9. In contrast to these colleagues, we included OS in the current analysis, knowing that it would be more practical to also focus on disease-specific survival and that this limits our findings. However, as a large tertiary center for upper gastrointestinal tumors, patients were referred to our department from all over the country. Subsequently, follow-up data were collected at regular tumor follow-up visits, by direct contact with the patient via mail or telephone, by contact with the general practitioner, or by consultation of the central civil registries of the authorities. Unfortunately, we were not always able to determine the exact reasons for a patient’s death, especially as a result of the tumor disease.
We encountered an R0 resection rate of 91.9% among patients undergoing curative intent surgery, which is in line with other. The rate of tumor-free resection margins in IOC samples was 84.3%. The rate of final R0; however, was lower than in cases without IOC (84.3 vs. 93.1%). Possibly, procedures without an IOC were less challenging than those requiring one. This might have an impact on our results.
Out of 10 patients featuring final R1 result in spite of extended resection, three (30%) had tumor infiltration at the circumferential margin. Thus, after positive IOC, before proceeding with extended resection, an IOC on circumferential resection margin should be considered. The associated costs with additional IOC should be weighed against the technical and medical burden of extended resection with failed margin conversion.
Operative trauma due to extended resection may also limit survival. Although our data failed to show a significant difference in 30-day mortality, length of hospital stay, and postoperative complications between patients with and without extended resection; the statistical significance may be reached in a future study with a larger number of patients.
Finally, this raises the question of whether intraoperative control of resection margins is generally necessary in view of the extended surgery performed to achieve tumor-free margins, particularly in advanced stages of gastric or GEJ adenocarcinoma. At least our results show that in this context a significant extension of the surgical intervention, for example, esophagogastrectomy and reconstruction by colonic interposition, has to be considered very critically.
Although the total patient cohort is large, the number of converted patients is relatively small, which might have prohibited some statistics from reaching significance. Among converted patients, only two cases had a tumor infiltration depth of under (y)pT3, which made it impossible to draw consequences on prognostic value of IOC and extended resection in early-stage cancer. Moreover, additional research is needed to compare further aspects like quality of life.
Future prospective, randomized-controlled trials are required. Based on our results, extended resection after a positive IOC for advanced gastric or GEJ adenocarcinoma does not result in improved long-term patient survival.
Ethics statement
All procedures were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1964 and later versions. Informed consent to be included in the study, or the equivalent, for publication, and any accompanying images was obtained from all patients. The study was approved by the local ethics committee (application number: 19-1480). A copy of the written consent is available for review by the Editor-in-Chief of this journal on request.
Funding
There was no funding for the current study.
Author contribution
P.S.P., A.B., A.P., H.A.: study concept; P.S.P., A.B., A.P., H.A.: study design; P.S.P., A.B., A.P., H.A., A.Q., A.H.H., S.P.M., M.D.: data acquisition; H.A., A.B., A.P., A.B.: quality control of data and algorithms; P.S.P., A.B., A.P., H.A., C.M., S.C., M.H., S.P.M., A.Q., C.J.B., A.H.H., C.C.: data analysis and interpretation; H.A., A.B., A.P., M.H.: statistical analysis; P.S.P., A.B., A.P., H.A., C.M.: manuscript preparation; P.S.P., A.B., A.P., H.A., C.C., C.M., S.P.M.: manuscript editing; P.S.P., A.B., A.P., H.A., C.M., S.C., C.C., M.D., M.H., S.P.M., A.Q., C.J.B., A.H.H.: manuscript review.
Conflicts of interest disclosure
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Research registration unique identifying number (UIN)
Name of the registry: ClinicalTrials.gov.
Unique Identifying number or registration ID: NCT05663450.
Hyperlink to your specific registration (must be publicly accessible and will be checked): https://clinicaltrials.gov/ct2/show/NCT05663450?term=NCT05663450&draw=2&rank=1.
Guarantor
Prof. Dr Hakan Alakus, MD; Department of General, Visceral, Cancer and Transplantation Surgery, Faculty of Medicine, University Hospital of Cologne, Kerpenerstr 62, 50937 Cologne, Germany. Tel: +49 221 478 4803, fax: +49 221 478 6258. E-mail: hakan.alakus@uk-koeln.de.
Data statement
The datasets analyzed during this current study are available from Prof. Hakan Alakus upon reasonable request.
Provenance and peer review
Not commissioned, externally peer-reviewed.
Registered to the ClinicalTrials.gov database.
STROCSS criteria
This work has been reported in line with the STROCSS criteria25.
Footnotes
Patrick S. Plum, Atakan G. Barutcu, and Aylin Pamuk contributed equally in this work.
Sponsorships or competing interests that may be relevant to content are disclosed at the end of this article.
Published online 23 May 2023
Contributor Information
Patrick S. Plum, Email: patrick.plum@t-online.de.
Atakan G. Barutcu, Email: atakanbarutcu@gmail.com.
Aylin Pamuk, Email: apamuk@smail.uni-koeln.de.
Christoph Mallmann, Email: christoph.mallmann@uk-koeln.de.
Seung-Hun Chon, Email: seung-hun.chon@uk-koeln.de.
Costanza Chiapponi, Email: costanza.chiapponi@uk-koeln.de.
Martin Dübbers, Email: martin.duebbers@uk-koeln.de.
Martin Hellmich, Email: martin.hellmich@uni-koeln.de.
Stefan P. Moenig, Email: stefan.moenig@hcuge.ch.
Alexander Quaas, Email: alexander.quaas@uk-koeln.de.
Arnulf H. Hoelscher, Email: A.Hoelscher@contilia.de.
Christiane J. Bruns, Email: christiane.bruns@uk-koeln.de.
Hakan Alakus, Email: hakan.alakus@uk-koeln.de.
References
- 1.Petryszyn P, Chapelle N, Matysiak-Budnik T. Gastric cancer: where are we heading? Dig Dis. 2020;38:280–285. [DOI] [PubMed] [Google Scholar]
- 2.Jung JO, Nienhüser H, Schleussner N, et al. Oligometastatic gastroesophageal adenocarcinoma: molecular pathophysiology and current therapeutic approach. Int J Mol Sci [Internet] 2020;21:e2020004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Poorolajal J, Moradi L, Mohammadi Y, et al. Risk factors for stomach cancer: a systematic review and meta-analysis. Epidemiol Health. 2020;42:e2020004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.GBD 2017 Stomach Cancer Collaborators. The global, regional, and national burden of stomach cancer in 195 countries, 1990-2017: a systematic analysis for the Global Burden of Disease study 2017. Lancet Gastroenterol Hepatol [Internet] 2020;5:42–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shin D, Park SS. Clinical importance and surgical decision-making regarding proximal resection margin for gastric cancer. World J Gastrointest Oncol [Internet] 2013;5:4–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mariette C, Castel B, Balon JM, et al. Extent of oesophageal resection for adenocarcinoma of the oesophagogastric junction. Eur J Surg Oncol [Internet] 2003;29:588–593. [DOI] [PubMed] [Google Scholar]
- 7.Spicer J, Benay C, Lee L, et al. Diagnostic accuracy and utility of intraoperative microscopic margin analysis of gastric and esophageal adenocarcinoma. Ann Surg Oncol [Internet] 2014;21:2580–2586. [DOI] [PubMed] [Google Scholar]
- 8.Moehler M, Al-Batran SE, Andus T, et al. S3-Leitlinie Magenkarzinom – Diagnostik und Therapie der Adenokarzinome des Magens und des ösophagogastralen Übergangs. Z Gastroenterol [Internet] 2019;57:1517–1632. [DOI] [PubMed] [Google Scholar]
- 9.McAuliffe JC, Tang LH, Kamrani K, et al. Prevalence of false-negative results of intraoperative consultation on surgical margins during resection of gastric and gastroesophageal adenocarcinoma. JAMA Surg [Internet] 2019;154:126–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Al-Batran SE, Homann N, Pauligk C, et al. Perioperative chemotherapy with fluorouracil plus leucovorin, oxaliplatin, and docetaxel versus fluorouracil or capecitabine plus cisplatin and epirubicin for locally advanced, resectable gastric or gastro-oesophageal junction adenocarcinoma (FLOT4): a randomised, phase 2/3 trial. Lancet [Internet] 2019;393:1948–1957. [DOI] [PubMed] [Google Scholar]
- 11.Cunningham D, Allum WH, Stenning SP, et al. Perioperative chemotherapy versus surgery alone for resectable gastroesophageal cancer. N Engl J Med [Internet] 2006;355:11–20. [DOI] [PubMed] [Google Scholar]
- 12.Chan WH, Wong WK, Khin LW, et al. Significance of a positive oesophageal margin in stomach cancer. Aust N Z J Surg [Internet] 2000;70:700–703. [DOI] [PubMed] [Google Scholar]
- 13.Yu X, Hu F, Li C, et al. Clinicopathologic characteristics and prognosis of proximal and distal gastric cancer. Onco Targets Ther [Internet] 2018;11:1037–1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Petrelli F, Ghidini M, Barni S, et al. Prognostic role of primary tumor location in non-metastatic gastric cancer: a systematic review and meta-analysis of 50 studies. Ann Surg Oncol [Internet] 2017;24:2655–2668. [DOI] [PubMed] [Google Scholar]
- 15.Harrison LE, Karpeh MS, Brennan MF. Proximal gastric cancers resected via a transabdominal-only approach. Results and comparisons to distal adenocarcinoma of the stomach. Ann Surg [Internet] 1997;225:678–683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Felismino TC, de Oliveira ACF, Alves ACF, et al. Primary tumor location is a predictor of poor prognosis in patients with locally advanced esophagogastric cancer treated with perioperative chemotherapy. J Gastrointest Cancer [Internet] 2020;51:484–490. [DOI] [PubMed] [Google Scholar]
- 17.Lordick F, Carneiro F, Cascinu S, et al. Gastric cancer: ESMO clinical practice guideline for diagnosis, treatment and follow-up. Ann Oncol [Internet] 2022;33:1005–1020. [DOI] [PubMed] [Google Scholar]
- 18.Smyth EC, Verheij M, Allum W, et al. Gastric cancer: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol [Internet] 2016;27(suppl 5):v38–v49. [DOI] [PubMed] [Google Scholar]
- 19.Zhao Q, Chen K, Tong W, et al. Gastric cancer in proximal site exerts poorer survival outcome with divergent genetic features than distal site. Comput Biol Chem [Internet] 2020;88:107360. [DOI] [PubMed] [Google Scholar]
- 20.Wang X, Liu F, Li Y, et al. Comparison on clinicopathological features, treatments and prognosis between proximal gastric cancer and distal gastric cancer: a national cancer data base analysis. J Cancer [Internet] 2019;10:3145–3153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Xue J, Yang H, Huang S, et al. Comparison of the overall survival of proximal and distal gastric cancer after gastrectomy: a systematic review and meta-analysis. World J Surg Oncol [Internet] 2021;19:17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhang Y, Zhang PS, Rong ZY, et al. One stomach, two subtypes of carcinoma—the differences between distal and proximal gastric cancer. Gastroenterol Rep (Oxf) [Internet] 2021;9:489–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mitchell KG, Ikoma N, Nelson DB, et al. Mediastinal Nodal Involvement After Neoadjuvant Chemoradiation for Siewert II/III Adenocarcinoma. Ann Thorac Surg [Internet] 2019;108:845–851. [DOI] [PubMed] [Google Scholar]
- 24.Cancer Genome Atlas Research Network. Comprehensive molecular characterization of gastric adenocarcinoma. Nature [Internet] 2014;513:202–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mathew G, Agha R, Albrecht J, et al. STROCSS 2021: strengthening the reporting of cohort, cross-sectional and case-control studies in surgery. Int J Surg [Internet] 2021;96:106165. [DOI] [PubMed] [Google Scholar]


