Abstract
Wounds represent various significant health concerns for patients and also contribute major costs to healthcare systems. Wound healing comprises of overlapped and various coordinated steps such as homeostasis, inflammation, proliferation, and remodeling. In response to the failure of many strategies in delivering intended results including wound closure, fluid loss control, and exhibiting properties such as durability, targeted delivery, accelerated action, along with histocompatibility, numerous nanotechnological advances have been introduced. To understand the magnitude of wound therapy, this systematic and updated review discussing the effectiveness of nanoemulsions has been undertaken. This review portrays mechanisms associated with wound healing, factors for delayed wound healing, and various technologies utilized to treat wounds effectively. While many strategies are available, nanoemulsions have attracted the tremendous attention of scientists globally for the research in wound therapy due to their long-term thermodynamic stability and bioavailability. Nanoemulsions not only aid in tissue repair, but are also considered as an excellent delivery system for various synthetic and natural actives. Nanotechnology provides several pivotal benefits in wound healing, including improved skin permeation, controlled release, and stimulation of fibroblast cell proliferation. The significant role of nanoemulsions in improved wound healing along with their preparation techniques has also been highlighted with special emphasis on mechanistic insights. This article illustrates recent research advancements for the utilization of nanoemulsions in wound treatment. An adequate literature search has been conducted using the keywords ‘Nanoemulsions in wound healing’, ‘Wound therapy and nanoemulsions’, ‘Herbal actives in wound therapy’, ‘Natural oils and wounds treatment’ etc., from PubMed, Science Direct, and Google Scholar databases. Referred and original publications in the English language accessed till April 2022 has been included, whereas nonEnglish language papers, unpublished data, and nonoriginal papers were excluded from the study.
Keywords: futuristic developments, mechanistic insights, nanoemulsions, nanoemulsions based hydrogels, natural oils, wound healing
Introduction
Highlights
Wounds represent various significant health concerns for patients.
This review portrays mechanisms associated with wound healing.
Nanotechnology provides several pivotal benefits in wound healing.
This review focuses on advancements for the utilization of nanoemulsions in wound treatment.
The skin’s primary function is to act as a defensive barrier against environmental intrusion, including sun radiation and air pollution. When the structural integrity of the skin is compromised, the immune system is impacted, which may lead to morbidity and mortality1. Skin repair and regeneration is a complicated, multifaceted process that involves a highly sophisticated temporal sequence of molecular mechanisms and cellular processes that are triggered by injury and continue sequentially and in harmonious manner to heal the injured tissue2.
A wound is an injury or distortion to physiological structure and functions caused by a simple or significant break in the anatomy of an organ such as the skin, which can spread to nearby tissues and structures including subcutaneous tissue, muscles, tendons, nerves, and arteries, among others3. The microbiota of the skin is intricately related to skin health and illness. The immune response is regulated, and barrier restoration is promoted by interaction between commensal bacteria and various cell types engaged in cutaneous wound healing. This conversation between host cells and the microbiota is disturbed in wounds4. Wounds are generally classified into two types, such as acute injury and chronic injury based on their healing time. Acute injuries heal in a predictable time, followed by structural and functional tissue restoration. While, chronic injuries do not heal within a reasonable time frame, resulting in additional microbial infection complications and difficulty in healing5. In a similar manner, wounds are categorized as either open (cutaneous) or closed (noncutaneous) injuries. An open injury is sometimes defined as a surgical incision (cut) and is further classified as abrasions (superficial injury) and lacerations (frictional force). When an injury is under the skin and not exposed to the air, it is called a closed injury. Closed injuries can be classified as hematomas (blood vessel disruption) and crush injuries (high-pressure) (Fig. 1). Various microorganisms found in wounds including gram-positive, gram-negative, and fungi are described in Table 1.
Figure 1.
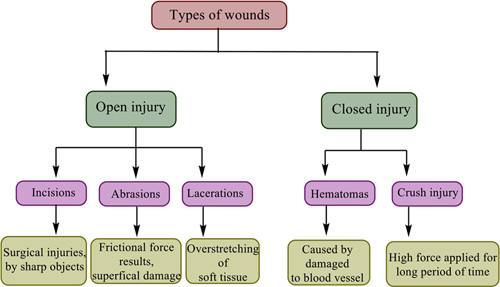
Classification of wounds.
Table 1.
| Group | Species |
|---|---|
| Gram-negative | Acinetobacter, Enterobacter Cloacae, Escherichia Coli, Proteus Mirabilis, Serratia Marcescens, Klebsiella Pneumoniae |
| Gram-positive | Enterococcus Avium, Staphylococcus Aureus, Staphylococcus Haemolyticus, Staphylococcus Schleiferi, Enterococcus Faecalis |
| Fungi | Candida Albicans, Candida Glabrata, Candida Stellatoidea, Candida Tropicalis |
Wound healing and its phases
Wound healing is a multi-step process, which begins with an injury. This injury response is a phylogenetically primal, yet necessary innate host immune response, for tissue integral restoration9. The entire process is usually classified into four stages (Fig. 2) including hemostasis, inflammation, proliferation, and remodeling10,11. Hemostasis begins just after an injury12, causes vessel rupture, exposure of subendothelial collagen to platelets, thereby causing accumulation and activation of the coagulation cascade. Afterwards, neutrophils and then macrophages travel to the site of injury, resulting in the initiation of the inflammation phase, via the production of inflammatory mediators such as interleukin-4 and transforming growth factor (TGF-β). The primary goal of the inflammatory phase is to keep the wound free from becoming infected and persists until all excess bacteria and debris from the wound are removed13,14.
Figure 2.

Mechanism of wound healing.
The proliferative phase, which is characterized by fibroblast migration, extracellular matrix deposition, and the formation of granulation tissue, begins around day 3 and continues for 2–4 weeks after wounding. In this phase, fibroplasia and angiogenesis occur synchronously and in a tightly coordinated manner to form extracellular matrix and granulation tissue15,16. The final step in the proliferation phase is re-epithelialization, which entails numerous steps, viz, the migration of neighboring epidermal keratinocytes into the wound, the proliferation of keratinocytes, the development of neoepithelium into the stratified epidermis, as well as the restoration of an integral basement membrane region that links the epidermis to the underlying dermis. The process of re-epithelialization can only begin once the wound surface is appropriately established with proliferating fibroblasts, new vessels, and collagen matrix17,18. The final phase of wound healing, that is, maturation or remodeling is approximately the same in all wounds and lasts for months or even years19. Collagen III from the newly produced extracellular matrix is eventually replaced by collagen I, which reveals an extra organized lattice structure and improves the tensile strength of the repaired skin20. Matrix metalloproteinases, which are produced by macrophages, neutrophils, and fibroblasts in the wound are accountable for collagen degradation. The underlying connective tissue shrinks, causing the wound borders to become closer together. Capillary growth reduces blood supply and hence metabolic activity, resulting in the production of a developed scar with fewer cells and a high tensile strength21. The key events involved in all four phases are summarized in Fig. 3.
Figure 3.
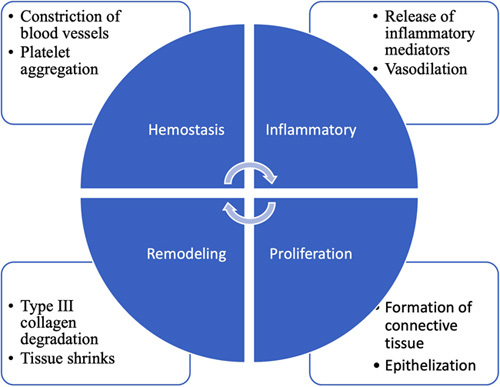
Phases of wound healing and the events involved.
Factors influencing wound healing
There are numerous local and systemic elements that delay wound healing. Examples of local factors include tissue hypoxia22–24, infection25–27, and hydration28–30. Systemic factors include ageing31,32, smoking33–35, stress36,37, diabetes38, and obesity39. To reduce or manage the influence of these factors, different strategies for wound treatment are available like debridement40, negative pressure therapy41, oxygen therapy to provide supplement oxygen42, and gene therapy43. Autografts and allografts are commonly used techniques that effectively generate fascia with full thickness from the donor site of patients or other donators and graft it in the required zone44. The use and administration of growth factors like platelet-derived, fibroblast is considered to be essential for the onset and management of the wound healing process and can also consider as a significant therapeutic substitute for healing wounds45. Aside from the above-described techniques, different strategies like electrical stimulation46, induced pluripotent stem cell47, and low-level laser therapy48 that enhance wound healing activity are also available.
Salient dosage forms for wound healing
Different scientific studies reported important dosage forms like hydrogels49–54, electrospun membranes55–57, wafers58–61, nanoparticles62–64, nanoemulsions65, and nanoemulsion-based hydrogels66–68 used for targeted delivery of drugs against wounds. Nanotechnology-based treatment methods provide an excellent and fascinating opportunity to achieve targeted action towards wounds. Diagnostics and treatment approaches based on nanotechnology provide an overview to target the heterogenicity of the wound healing process. Among the dosage forms listed, nanoemulsion is a promising nanocarrier with huge and potential applications in wound healing69–71 and also offers numerous benefits, viz. enhanced permeability, controlled drug delivery, higher stability, targeted action, and high binding interaction with the lipid layer of the skin. The use of nanoemulsions can improve antibacterial efficacy along with reduced droplet size dispersion causing accelerated wound healing. Moreover, nanoemulsions also have the ability to increase the solubility of drug, resulting in high therapeutic efficacy against wounds. Bacteria and viruses causing wound progression interacts with the lipid layer of nanoemulsions resulting cell wall disruption and subsequently leakage of cellular content. The possible mode of action of nanoemulsions against wound microorganisms is illustrated in Figure 4 72,73. It has been found that the oily phase of nanoemulsions affects their wound healing properties74. Propolis oil containing flavonoids and caffeic acid significantly reduced the inflammatory phase by inhibiting lipoxygenase activity resulting in reduced production of prostaglandins, which further stimulate the immune cells. Moreover, oils can also accelerate debriding activity triggering faster wound healing75. Eucalyptus oil and clove oil significantly enhanced the production of collagen and leucine, respectively, resulting into accelerated wound healing76,77. Different natural oils and their role in wound healing are illustrated in Figure 5.
Figure 4.
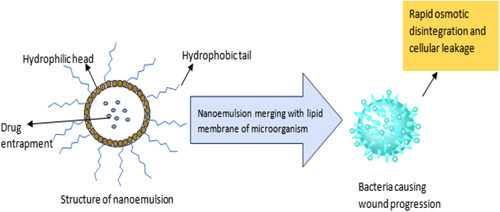
Possible mode of action of nanoemulsions against wound microorganisms.
Figure 5.
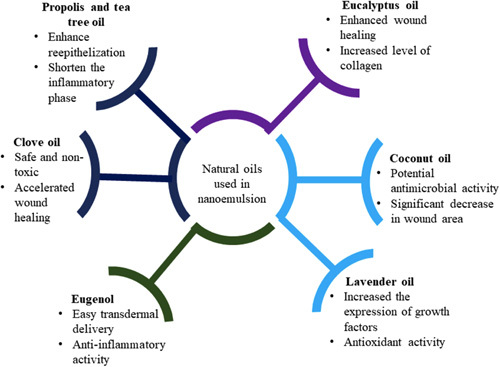
Different natural oils for preparation of nanoemulsion and their significant effect on wound healing.
Advantages of nanoemulsions in wound healing
The emergence of innovative therapies in the field of wound healing highlights the potential role of nanotechnology-based drug delivery systems as a viable approach to promote healing mechanisms at various stages. Nanoemulsions have been affirmed to be an superior choice because of their several properties which resulted in their enlarge utilization in wound therapy. The main benefits of using nanoemulsions as drug delivery carriers in the treatment and management of wound healing include high drug loading capacity, improved drug solubility, and bioavailability, relatively simple preparation and scale-up, controlled drug release, and protection from enzymatic degradation. By lowering surface and interfacial tension and so elevating the system’s overall viscosity and spreadability, the nanoemulsion gives the system thermodynamic stability. Because of its high drug loading capacity, improved difusibility, permeability, and reduced skin irritation, nanoemulgel has several benefits over lipid nanoparticles, microemulsions, or liposomes in transdermal or dental delivery78–80. The numerous benefits of nanoemulsions for enhancing wound healing prospects are also summarized in Table 2.
Table 2.
Important advantages of nanoemulsions in wound therapy.
Preparation methodologies
Various preparation methods of nanoemulsion for wound therapy are broadly classified into two categories as: high energy and low energy approaches. High energy methods concerning with wound healing are further classified as high-pressure homogenization and ultrasonication. Whereas low energy methods include phase inversion and spontaneous emulsification81,82. Some suitable techniques utilized for the nanoemulsion preparations in wound therapy are illustrated in Figure 6.
Figure 6.
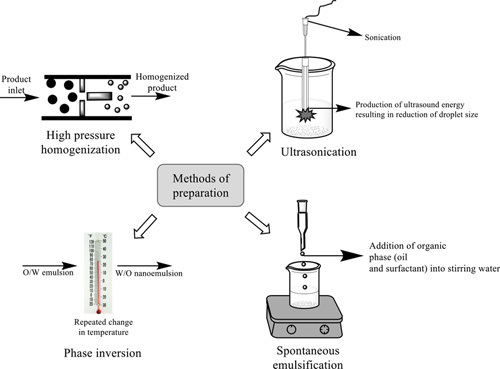
Some important techniques utilized for the preparation of nanoemulsions for wound therapy.
High energy methods
Large disruptive energies are provided using mechanical forces in the high energy approach. The droplet size depends on the equipment, preparation parameters such as temperature and time, as well as the characteristics of the sample. High energy methods are complex and energy-intensive making them expensive, thus, this method is not suitable for thermosensitive products83. This method is divided into the following categories as: high-pressure homogenization and ultrasonication.
High-pressure homogenization
This technique is frequently employed for the development of nanoemulsions using various forces, viz. intense turbulence, cavitations, and hydraulic shear, and the particle size of approximately 1 nm can be produced with this system. It utilizes a high-pressure piston/ homogenizer in which two liquids comprising of surfactants and co-surfactants are pushed under intense pressure (500–5000 psi) through a small orifice to distort the interface between the water and oil to such an extent that the formed droplets are broken up into smaller one. Indeed, the development of nanoemulsions through the high-pressure homogenization method is not impulsive in nature, as it necessitates force input either in the form of chemical or mechanical. Furthermore, if the system’s temperature rises during processing, the preparation may become unstable83,84. This technique has wide applications in wound therapy utilizing herbal actives viz. betulin-enriched extract, oil palm (Elaeis guineensis Jacq.) leaf, etc. Emulsification from this technique leads to a significant reduction in droplet size, which further influence the targeted delivery of drug. Moreover, optimum drug loading and polydispersity index was also observed85,86.
Ultrasonication
Nanoemulsion can be easily fabricated using ultrasonication technique via the agitation of molecules trigged by sound waves that later on break oil droplets through local turbulence. A probe, a metal horn, a generator (which produces electric waves), and a piezoelectric transducer are typically used to produce nanoemulsions using ultrasonication86. In contrast to the high-pressure homogenization method, it requires less energy, easy manipulation, and cost effective. The energy is delivered using sonicator probes, which are sonotrodes. It includes a piezoelectric quartz crystal, which expands and contracts in response to alternating electric energy. When the sonicator’s tip makes contact with the liquid, it causes mechanical vibration and cavitation. Despite its enormous ability, the approach is still limited to laboratory studies and only pertinent for pilot-scale fabrication because of the influence of the wave around the molecules that can affect large-scale production84,87,88. Various synthetic (clindamycin) as well as herbal constituents (eugenol) nanoemulsions have been prepared by this method and showed effective wound healing. A high skin disposition with optimal viscosity was achieved. Moreover, Transmission Electron Microscopy analysis also showed that the nanoemulsions formed had spherical and reduced droplet size75,89.
Low energy methods
Low energy techniques use the characteristics of the surfactant, oil, and water system and also do not require any specific equipment. Because of their low cost and ease of implementation, low energy technologies have prompted a surge in a nanoemulsion study for their development and enormous applications84. Low energy emulsification techniques use the system’s intrinsic chemical energy and only mild agitation to fabricate nanoemulsions, making them more energy-efficient. This method can be classified as phase inversion and spontaneous emulsification.
Phase inversion
The phase invasion technique fabricates nanoemulsion via altering the surfactant’s spontaneous curvature. Under this technique, the utilization of nonionic surfactants alters the system’s temperature, enabling an oil-in-water emulsion (O/W) to transform into a water-in-oil emulsion (W/O) at higher temperatures. For phase inversion emulsification, a critical surfactant concentration is required. Furthermore, the elevation in temperature dehydrates the polymeric chain of surfactant, making it more lipophilic; whereas, it forms a large positive spontaneous curvature at low temperature. Thus, sufficient phase transitions can only be produced by altering the composition at a steady temperature or by changing the temperature at a stable composition84,90. Besides, in some instances, high energy input is usually required to fabricate micrometer ranged droplet sizes via the phase inversion approach, which can be accomplished by high shear stirring or ultrasound generators. This method has wide applications in wound dressings for the delivery of agents like phenytoin and chlorhexidine. Formulation prepared by this technique was found to be stable having high monodispersity. Enhanced drug entrapment efficacy with no sign of precipitation was observed. Moreover, nanoemulsions prepared by this method revealed enhanced wound healing and provided targeted action91.
Spontaneous emulsification
This method is also known as self-emulsification. Droplet formation can be achieved by contacting two immiscible liquid that are not in equilibrium without the need of external energy input. One of the foremost advantages of this technique is that it does not need any particular equipment for the fabrication of nanoemulsion and can be developed effectively at room temperature. However, the presence of solvent and the formation of a less oil-phase are two major drawbacks of the self-emulsification technique. Indeed, this method has wide applications in developing herbal actives loaded nanoemulsions for wound healing. Stable and transparent nanoemulsions were achieved after 30 min of sonication. The droplet size obtained had a large surface area resulting in improved absorption through the skin. Quantitative analysis of nanoemulsions in ultraviolet and visible wavelength showed decreased absorbance with an increase in sonication time65,92,93.
Recent investigational studies on nanoemulsions for wound therapy
Nanoemulsions are considered as rational and impressive dosage form for the effective delivery of medicinal agents at the target region in a safe and efficient manner. Significant research has been done globally on nanoemulsions as carriers of wound healing agents. Nanoemulsion formulations for wound healing have been found to reveal inhibitory and stimulatory effects on inflammatory cytokines and antioxidant enzymes, respectively76. Back et al. fabricated an isoflavone-rich nanoemulsion that increased keratocyte proliferation and migration. A study revealed that nanoemulsions boosted the wound healing process by promoting angiogenesis, reducing lipid oxidation and inflammation, while enhancing re-epithelialization94. Moreover, wounds treated with nanoemulsions displayed a higher level of collagen resulting in faster wound healing95. Eucalyptus oil nanoemulsions prepared by Sugumar et al., showed potent antibacterial activity against Staphylococcus aureus. When tested against animal, nanoemulsion exhibited potential wound healing activity (100% wound closure on day 16th) with no sign of skin irritancy93. Nanoemulsions for wound healing also significantly enhanced the proliferation of fibroblast cells resulting in a reduction in the time of wound closure65. In addition to wound healing effect, insulin loaded nanoemulsions also helped in mitigating diabetes96. Lavender oil nanoemulsions prepared by Kazemi et al., effectively reduced the wound area and significantly increased TGF β1, Type I, and Type II collagen synthesis. Additionally, prepared formulations also displayed the antioxidant potential of superoxide dismutase and glutathione peroxidase resulting in reduced MDA level and lipid peroxidation97. Zain et al., developed nanoemulsions using flavonoid-rich oil palm (Elaeis guineensis Jacq.) leaf extract. Furthermore, wound healing activity and toxicity was evaluated using three different models such as 3T3 fibroblast cells, embryonic and adult Zebrafish. However, the extract and its nanoemulsion were found to be more sensitive towards Zebrafish model. LC50 value of the developed nanoemulsions was observed to be higher than that of unloaded leaf extract. Prepared nanoemulsions displayed accelerated wound healing properties when compared with oil palm leaf extract. Additionally, the inhibitory and stimulatory effects of nanoemulsions on inflammatory cytokines and antioxidant enzymes, respectively, suggested that it has strong potential in wound management86. Farahani et al., prepared a nonfibrous formulation using cellulose acetate and gelatin (CA/GL). Furthermore, a nanoemulsion of Zataria multiflora (antibacterial plant) was also fabricated into a nonfibrous mat. By increasing the CA/GL ratio, improved mechanical strength of the developed formulation was observed. Prepared formulations showed potential activity against E. coli and S. aureus. In vivo evaluation of the nonfibrous formulation showed an accelerated wound healing profile73. Nanoemulsions preparations of different drugs have also been summarized in Tables 3 and 4 as synthetic molecules and natural actives, respectively.
Table 3.
Synthetic molecules loaded nanoemulsions for wound healing.
| Sl. No. | Drug | Methods of preparation | Major additives | Droplet size | Zeta potential | Model used | Salient features | References |
|---|---|---|---|---|---|---|---|---|
| 1. | Clindamycin hydrochloride | Ultrasonication (O/W) | Propolis and tea tree oil. | 19.42±1. 7 nm | −24.5±0.2 mV | Human skin fibroblast. | Improved re-epithelialization, collagen synthesis and potent anti-inflammatory action. | 75 |
| 2. | Levofloxacin | Low energy method | Sesame oil, Tween 80, Tween 85. | 18±5 nm | 0.271 mV | L929 mouse fibroblast cells | Significant reduction in period of epithelialization, wound contraction, and number of inflammatory cells. | 95 |
| 3. | Chlorhexidine acetate | Phase inversion method. | Tween 80, PEG | 6.13 nm | −67.13 mV | MRSA cells | Higher activity towards MRSA. | 72 |
| 4. | Phenytoin | Phase inversion method. | Alkyds and Tween 80 surfactant. | 11.7±0.07 to 13.5±0.13 nm | −7.0±0.04 to −7.9±0.69 mV | Human keratinocytes (HaCaT cells) | Enhanced proliferation of epidermal cells. | 91 |
| 5. | Insulin | – | Oleic acid, Tween 80, PEG 400. | 458±132 nm | −10.2±3.87 mV | Wistar rats | Synergistic effects towards wound healing. | 96 |
| 6. | Simvastatin | Ultrasonication method. | Coconut oil, surfactant, co- surfactant. | 186.0±2.5 nm | 20±1.2 mV | – | 6-fold increased inhibition zone against Staphylococcus aureus, synergistic effects. | 98 |
Table 4.
Natural actives loaded nanoemulsions for wound healing.
| Sl. No | Drug | Methods of preparation | Major additives | Droplet size | Zeta potential | Model used | Salient features | References |
|---|---|---|---|---|---|---|---|---|
| 1. | Betulin-enriched extract and purified spruce balm | High-pressure homogenization | Sunflower oil, Jojoba oil, Triglycerides | 153 to 245 nm | −67.58 to −77.13 mV | Human keratinocytes and fibroblast cells | Enhanced proliferative profile of keratinocytes and fibroblasts. | 85 |
| 2. | Oil palm (Elaeis guineensis Jacq.) leaf | High-pressure homogenization | Palm oil and Gelatin | <100 nm | −24.03 to −34.10 mV | 3T3 fibroblast cells, embryonic and adult zebrafish. | Improved and better wound healing potential. | 86 |
| 3. | Astaxanthin-alpha tocopherol | Spontaneous emulsification method, ultrasonication method. | Span 80, Tween 20, PEG | 189 to 216.6 nm | −20.57 mV | T24, Panc1, CT26, HeLa | Faster healing effect of nanoemulsion and significant antibacterial activity. | 65 |
| 4. | Zataria multiflora essential oil | Ultrasonic homogenization, electrospinning technique. | Cellulose acetate, Glacial acetic acid, Gelatin. | 79. 1 nm | – | L929 mouse fibroblast cells | Promoted the adhesion and proliferation of L929 fibroblast cells significantly. | 73 |
| 5. | Eugenol | Ultrasonication method | Tween 80, Larbasol | 89.98±6.48 nm | −10.05±0.11 mV | Wistar rat hind paw edema mode | High wound healing and easy transdermal delivery. | 89 |
| 6. | Lavender essential oil and liquorice extract. | Spontaneous emulsification. | Tween 80, Tween 20, Glycerin, PEG 400. | – | – | Wistar rats | Potential wound healing effect. | 97 |
| 7. | Tetrahydroxy curcumin derivative. | High-pressure homogenization. | Tween 10 and Lauroglycol 90, Labrafac PG. | 100–300 nm | −30.1 to −31.1 mV | Rat skin | Displayed optimistic results against both gram-positive and negative bacteria. | 99 |
| 8. | Curcumin | – | Labrafac PG, Triacetin, Tween 80, PEG 400. | 84.032±0.023 nm | – | Albino rats | Enhanced skin permeation. | 100 |
| 9. | Isoflavones aglycones | Spontaneous emulsification method. | Daidzein, Glycitein, Genistein, Egg lecithin. | 50 nm | −60 mV | Wistar rats | Increased reepithelization and angiogenesis rate to promote wound healing. | 94 |
| 10. | Alpha tocopherol | Spontaneous emulsification method. | Chitosan and oleic acid. | 220 nm | −56.8±2.1 mV | Human skin cells | Improved alpha tocopherol stability due to encapsulation. | 92 |
| 11. | Eucalyptus oil | Ultrasonic emulsification. | Eucalyptus oil and Tween 80. | 3.8 nm | – | Wistar rats | Complete loss of viability within 15 min. | 93 |
| 12. | Naringenin | Low energy emulsification technique. | PEG 400, Tween 20, Tween 80, Chitosan, Lauroglycol 90 and Capryol 90. | 15.69±0.737 nm | −8.33±3.09 mV | NHT-3T3 mouse cells | Accelerate wound healing. | 101 |
| 13. | Eucalyptus oil | Aqueous phase titration. | Diethylene glycol monoethyl ether, Ethanol, Eucalyptus oil, Polyoxyethylene sorbitan trioleate. | 32.45 nm | −34.25 mV | Rat | Safe, nontoxic and rapid wound healing. | 77 |
| 14. | Clove oil | Spontaneous emulsification method. | Clove oil, Triacetin, Tween 80. | 29.10 nm | −31.40 mV | Rat | Significant proliferation of epithelial cells. | 76 |
Therefore, major and ongoing research efforts have been made around the world towards developing effective nanoemulsions for accelerated wound healing. Treatment of wounds using nanoemulsion technology showed various significant benefits like targeted, effective, and faster wound healing potential. Also, very few patents are available discussing nanoemulsions for wound healing. Therefore, the patent segment still requires significant attention towards the application of nanoemulsions in wound healing. Some patents on nanoemulsions for wound healing are discussed in the subsequent section.
Notably, in 2016, a patent (US 9,259,407 B2) was published describing the composition and therapeutic usage of nanoemulsions in wound treatment, respiratory infection, bacterial infection, and immunogenic composition-related immune response. In addition, the patent also provided information about clinical, industrial, and research applications102. Nanoemulsion’s involvement in the prevention and treatment of burn wounds is also described in the US patent (US 2019 / 0021998 A1) published in 2019. The Patent also detailed the accelerated wound healing activity of nanoemulsions. Moreover, therapeutic uses with clinical, commercial, and scientific applications are also highlighted103. Undoubtedly, plant-based bio-active compounds are decidedly sought-after active ingredients in the cosmeceutical and pharmaceutical industries, because of their bounteous therapeutic potentials, and are widely used in the development of nanotechnology-based drug delivery104. Several patents have been filed worldwide utilizing plant-based bio-active incorporating in nanoemulsion for the treatment of wounds. Kaur and kapoor105, fabricated Hippophae rhamnoide (Sea Buckthorn) seed oil incorporated nanoemulsion gel for wound healing. Similarly, Dubey et al. 106, also filed a patent for topical nanoemulgel containing Moringa oleifera seed oil using a low energy emulsification technique for wound therapy.
To further enhance the wound healing applications, different research groups have also focused on nanoemulsion-based hydrogels resulting in increased viscosity of the formulation, which ultimately improved the retention time of the drug. Different nanoemulsion-based hydrogels formulations for wound healing are summarized in Table 5.
Table 5.
Nanoemulsion based hydrogels preparations for wound healing.
| Sl. No. | Drug | Method | Additives | Droplet size | Zeta potential | Model used | Salien t features | References |
|---|---|---|---|---|---|---|---|---|
| 1. | Thymoquinone | High energy emulsification technique. | Oleic acid, isopropyl myristate, isopropyl alcohol, ethyl oleate, castor oil, sesame oil, PEG 400, tween 20, tween 80. | 40.02±0.83 to 99.66±1.43 nm | −26.7 to −30.6 mV | Wistar rats | Hastens wound healing process. | 107 |
| 2. | Growth Factor (epidermal growth factor (EGF), insulin-like growth factor-I (IGF-I). | Oil-phase titration | Oleic acid, Labrasol, Transcutol. | 127±4.30 nm | −24.29 mV | NH 3T mouse cells | Rapid and prolonged wound healing. | 108 |
| 3. | Beta-glucan | Ultrasonication | Alginate, chitosan, soybean oil, span 80, tween 80. | 200 nm | −38.8 mV | Human dermal fibroblast cells. | Bacterial growth inhibitory activity. | 67 |
| 4. | Atorvastatin | Homogenization | Carboxymethyl cellulose, tween 80 and propylene glycol. | 148 nm | – | Wistar rat | Improved wound healing activity. | 109 |
| 5. | β-caryophyllene | High-speed homogenization | Span 20, tween 20, paraplast. | 284.82±1.438 nm | −35.72±1.103 mV | Rat paw edema and mouse ear edema. | Superior bioadhesiveness. | 66 |
| 6. | Curcumin | Ultrasonication | Carbopol 940, tween 20, tween 40. | 56.25±0.69 nm | −20.26±0.65 mV | Rats | Exhibited thixotropic rheological behavior. | 110 |
| 7. | Tea tree oil | Spontaneous emulsification. | Poly(caprolactone), span 80, tween 80. | 198 nm | −11.5 mV | Wistar rats | Increase the biological activities of oil and protect the skin damage. | 68 |
As a result, many research attempts have been made globally, prompting further efforts towards the development of nanoemulsions based hydrogels for wound healing. It has been found that treatment with the above-mentioned formulations hastens wound healing activity.
Future perspectives and conclusion
Chronic wounds remain a major concern of the healthcare system and affect the livelihood of patients. Various drug delivery strategies for wounds are constantly being updated by several efforts carried out in different formulation avenues such as nanoemulsions, hydrogels, wafers, etc. Nanoemulsion-based wound therapy can overcome various constraints like slow healing, off-targeting, patient incompliance, and frequent dosing. Inspite of significant advantages, many sturdy challenges are still need to be overcome like cost implications, scaling up nanoemulsion production for accelerated wound healing, from the pilot level to the commercial market, etc. More toxicity evaluation on nanoemulsion-based wound healing systems might potentiate further sophisticated development. Efficient tuning of the various techniques and ingredients used in nanoemulsions production may also result in the systems having a better wound healing profile. More comprehensive understanding of the mechanism involved between nanoemulsion and wound healing will definitely allow their optimization and consequently expand enormous application aspects. Moreover, the kinetics of emulsion plays a crucial role in the production of nanoemulsions, which ultimately affect wound healing efficacy. Therefore, more investigational research efforts are required on wound healing strategy. Despite the paucity of research, 3-D bioprinting is considered as one of the most effective approaches in the vistas of skin tissue engineering and can be utilized by surgeons very effectively to develop complex organs. Another intriguing area is self-healing hydrogels, which can be printed, maintain their pre-vascularized microstructure, and function as self-healing scaffolds for wound healing. Future research should also be focused on the bioprinting of skin wound treatments on animal models.
As there are limited research advancements and patents in recent years, which demonstrates the emergence of numerous nanotherapeutics, particularly nanoemulsion systems in wound healing, indicating that much work still needs to be done toward nanotherapeutic measures in the wound healing domain. Even though there is only preliminary levels of progress in research related to the application of nanoemulsion in accordance to wound treatment, further, imperative and powerful evidence at the clinical or human level will be of great succor. Patients with chronic wounds face many challenges due to a limited understanding of wound pathophysiology, which impedes the development of advanced nano-based wound therapies. Several tailored therapies based on phenotype and genotype characteristics, in near future is possible for advanced wound treatment. Nanotechnology approaches will surely be streamlined and individualized treatment plans will also be facilitated from such platforms, resulting in major opportunities for adaptation and invention. Nanotherapeutic interventions are highly anticipated in wound healing given the emergence of various nanotechnologies, particularly multifunctional nanosystems. Nevertheless, there is a significant challenge in obtaining sufficient information on the physicochemical properties and their predicted behavior in the human body. Extensive efforts are also required to develop chronic wound therapies with site-specificity and targeting effectiveness in order to prevent undesired occurrences and interferences that could impair the biological activities of the nanosystems in the human body. Long-term studies are required to provide insight into how nanotechnology-based medicines can be used clinically. We expect that advances in innovative manufacturing and nanosystems development, as well as the knowledge of chronic wounds will lead to the development of efficient nanoemulsion-based wound healing products in the near future. Future products might take the shape of nanoemulsion loaded dressings. This could reduce the clinical-stage failure rate that many of the technologies generally faces, and to achieve quick wound healing, one should be aware of the major difficulties associated with nanoemulsion loaded wound dressings. Currently, several human studies are enrolled utilizing silver nanoparticles, meschencymal stem cell based therapies, collagen/gelatin scaffolds, 3-D printing, and so on, for wound healing. Thus, equal and necessitate development is still required to strengthen the potential of nanoemulsion in clinical wound therapy. As a result, there is also an ongoing need for improved recombinant tools and analytical techniques that will enable the clinical translation of nanoemulsion-based strategies. Although much effort has been made to use nanoemulsion-like formulations in wound healing, there is still much potential for improvement and several problems need to be fixed urgently. Thus, additional clinical studies must be conducted in future research to support the fabrication of nanoemulsions for wound healing. The development of more conceptually inventive design processes for nanoemulsion for efficient application in wound healing is envisaged in the next years. For nanoemulsion development in wound healing applications, numerous factors, including clinical translation should be taken into account. The majority of recently formulated products based on nanotechnology are now quite expensive, which restricts their clinical availability. It seems feasible and advantageous to employ randomized clinical trials to advance research into the efficacy of therapies. Moreover, a lot of research still needs to be done urgently in the area of patents related to wound healing and nanoemulsions. Wound healing both internally or externally is a complex mechanism that requires prompt action within time. Nanoemulsions have a smaller particle size and controlled release action that leads to several encouraging and attractive benefits in wound therapy such as improved skin permeation, controlled release, protection of the drug from the unfavorable environment in the body, and reduced toxicity.
The present review summarized the important wound healing aspects, wound classification, influencing factors and strategies for the improved wound healing. Nanoemulsions loaded with natural oils and their impressive role in wound healing have been described. The mechanism of nanoemulsions in wound treatment has also been clearly illustrated. Moreover, different research advancements of nanoemulsions comprising of synthetic and natural actives that aid in the wound healing process are also explained in the manuscript. Sophisticated developments for nanoemulsion-based hydrogels have also been portrayed for enhanced wound healing profile. These advancements in wound therapy have provided new hopes for patients suffering from severe chronic wounds.
Ethical approval
Not applicable.
Sources of funding
No funding.
Author contributions
J.C.: conceptualization, data curation, writing-original draft preparation, writing- reviewing and editing; H.C.: conceptualization, data curation, writing-original draft preparation, writing- reviewing and editing; R.P.: data curation, writing-original draft preparation, writing- reviewing and editing; N.R.: data curation, writing-original draft preparation, writing- reviewing and editing; K.W.: data curation, writing-original draft preparation, writing-reviewing and editing; S.S.: data curation, writing-original draft preparation, writing-reviewing and editing; P.N.: data curation, writing-original draft preparation, writing-reviewing and editing; M.G.: data curation, writing-original draft preparation, writing-reviewing and editing; I.S.: data curation, writing-original draft preparation, writing-reviewing and editing; H.D.: data curation, writing-original draft preparation, writing-reviewing and editing; T.B.E.: writing-reviewing and editing, visualization, supervision.
Conflicts of interest disclosure
Authors declare that they have no conflicts of interest.
Research registration unique identifying number (UIN)
1. Name of the registry: Not applicable.
2. Unique Identifying number or registration ID: Not applicable.
3. Hyperlink to your specific registration (must be publicly accessible and will be checked): Not applicable.
Guarantor
Talha Bin Emran, Department of Pharmacy, BGC Trust University Bangladesh, Chittagong 4381, Bangladesh. Tel: +880 303 356 193, fax: +880 312 550 224, Cell: +880 181 994 2214. https://orcid.org/0000-0003-3188-2272. Department of Pharmacy, Faculty of Allied Health Sciences, Daffodil International. University, Dhaka 1207, Bangladesh. E-mail: talhabmb@bgctub.ac.bd.
Data availability statement
No specific data collected for the above manuscript.
Provenance and peer review
Not commissioned, internally peer-reviewed.
Acknowledgements
The authors are thankful to their parent institutions.
Footnotes
Sponsorships or competing interests that may be relevant to content are disclosed at the end of this article.
Published online 9 May 2023
Contributor Information
Jatin Chhabra, Email: jatinmph201@kuk.ac.in.
Hitesh Chopra, Email: chopraontheride@gmail.com.
Rakesh Pahwa, Email: rakesh_pahwa2407@yahoo.co.in.
Neha Raina, Email: rainanehaceutics@gmail.com.
Karan Wadhwa, Email: karanwdhw1@gmail.com.
Swati Saini, Email: swatisainimalviya19@gmail.com.
Poonam Negi, Email: poonamgarge@gmail.com.
Madhu Gupta, Email: madhugupta98@gmail.com.
Inderbir Singh, Email: inder.singh@chitkara.edu.in.
Harish Dureja, Email: harishdureja@gmail.com.
Talha Bin Emran, Email: talhabmb@bgctub.ac.bd;talhabmb@gmail.com.
References
- 1.Dreifke MB, Jayasuriya AA, Jayasuriya AC. Current wound healing procedures and potential care. Mater Sci Eng C Mater Biol Appl 2015;48:651–662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Oliveira A, Simões S, Ascenso A, et al. Therapeutic advances in wound healing. J Dermatolog Treat 2022;33:2–22. [DOI] [PubMed] [Google Scholar]
- 3.Boateng J, Catanzano O. Advanced therapeutic dressings for effective wound healing – A review. J Pharm Sci 2015;104:3653–3680. [DOI] [PubMed] [Google Scholar]
- 4.Tomic-Canic M, Burgess JL, O’Neill KE, et al. Skin microbiota and its interplay with wound healing. Am J Clin Dermatol 2020;21:36–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nethi SK, Das S, Patra CR, et al. Recent advances in inorganic nanomaterials for wound-healing applications. Biomater Sci 2019;7:2652–2674. [DOI] [PubMed] [Google Scholar]
- 6.Puca V, Marulli RZ, Grande R, et al. Microbial species isolated from infected wounds and antimicrobial resistance analysis: data emerging from a three-years retrospective study. Antibiotics 2021;10:1162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bessa LJ, Fazii P, Di Giulio M, et al. Bacterial isolates from infected wounds and their antibiotic susceptibility pattern: some remarks about wound infection. Int Wound J 2015;12:47–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dhingra GA, Kaur M, Singh M, et al. Lock stock and barrel of wound healing. Curr Pharm Des 2019;25:4090–4107. [DOI] [PubMed] [Google Scholar]
- 9.Singh MR, Saraf S, Vyas A, et al. Innovative approaches in wound healing: trajectory and advances. Artif Cells Nanomed Biotechnol 2013;41:2–22. [DOI] [PubMed] [Google Scholar]
- 10.Toia F, Rosatti F, Cordova A.Toia F, Rosatti F, Cordova A. Wound healing: physiology and pathology. Textbook of plastic and reconstructive surgery. Springer; 2022:15–25. [Google Scholar]
- 11.Bakshi IS, Chopra H, Sharma M, et al. Bakshi IS, Bala R, Madaan R, Sindhu RK. Herbal bioactives for wound healing application. Herbal bioactives based drug delivery system. Elsevier; 2022:259–282. [Google Scholar]
- 12.Chin JS, Madden L, Chew SY, et al. Drug therapies and delivery mechanisms to treat perturbed skin wound healing. Adv Drug Deliv Rev 2019;149-150:2–18. [DOI] [PubMed] [Google Scholar]
- 13.Kumar V, Abbas AK, Aster JC. Robbins and Cotran, Pathologic Basis of Diseases, 9th ed. Elsevier; 2014:100–110. [Google Scholar]
- 14.Raina N, Pahwa R, Khosla JK, et al. Polycaprolactone-based materials in wound healing applications. Polym Bull 2022;79:7041–7063. [Google Scholar]
- 15.Schreml S, Szeimies RM, Prantl L, et al. Wound healing in the 21st century. J Am Acad Dermatol 2010;63:866–881. [DOI] [PubMed] [Google Scholar]
- 16.Chopra H, Kumar S, Singh I. Strategies and therapies for wound healing: a review. Curr Drug Targets 2022;23:87–98. [DOI] [PubMed] [Google Scholar]
- 17.Öztürk F, Ermertcan AT. Wound healing: a new approach to the topical wound care. Cutan Ocul Toxicol 2011;30:92–99. [DOI] [PubMed] [Google Scholar]
- 18.Pastar I, Stojadinovic O, Yin NC, et al. Epithelialization in wound healing: a comprehensive review. Adv Wound Care 2014;3:445–464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Abazari M, Ghaffari A, Rashidzadeh H, et al. A systematic review on classification, identification, and healing process of burn wound healing. Int J Low Extrem Wounds 2022;21:18–30. [DOI] [PubMed] [Google Scholar]
- 20.Naskar A, Kim KS. Recent advances in nanomaterial-based wound-healing therapeutics. Pharmaceutics 2020;12:499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mir M, Ali MN, Barakullah A, et al. Synthetic polymeric biomaterials for wound healing: a review. Prog Biomater 2018;7:1–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gordillo GM, Sen CK. Revisiting the essential role of oxygen in wound healing. Am J Surg 2003;186:259–263. [DOI] [PubMed] [Google Scholar]
- 23.Gottrup F. Oxygen in wound healing and infection. World J Surg 2004;28:312–315. [DOI] [PubMed] [Google Scholar]
- 24.El-Ashram S, El-Samad LM, Basha AA, et al. Naturally derived targeted therapy for wound healing: beyond classical strategies. Pharmacol Res 2021;170:105749. [DOI] [PubMed] [Google Scholar]
- 25.Jones SG, Edwards R, Thomas DW. Inflammation and wound healing: the role of bacteria in the immuno-regulation of wound healing. Int J Low Extrem Wounds 2004;3:201–208. [DOI] [PubMed] [Google Scholar]
- 26.Rico RM, Ripamonti R, Burns AL, et al. The effect of sepsis on wound healing. J Surg Res 2002;102:193–197. [DOI] [PubMed] [Google Scholar]
- 27.Edwards R, Harding KG. Bacteria and wound healing. Curr Opin Infect Dis 2004;17:91–96. [DOI] [PubMed] [Google Scholar]
- 28.Ousey K, Cutting KF, Rogers AA, et al. The importance of hydration in wound healing: reinvigorating the clinical perspective. J Wound Care 2016;25:124–130. [DOI] [PubMed] [Google Scholar]
- 29.Kannon GA, Garrett AB. Moist wound healing with occlusive wound dressing. Dermatol Surg 1995;21:883–890. [DOI] [PubMed] [Google Scholar]
- 30.Coutts P, Woo KY, Bourque S. Treating patients with painful chronic wounds. Nurs Stand 2008;23:42–46. [DOI] [PubMed] [Google Scholar]
- 31.Gosain A, DiPietro LA. Aging and wound healing. World J Surg 2004;28:321–326. [DOI] [PubMed] [Google Scholar]
- 32.Sadoun E, Reed MJ. Impaired angiogenesis in aging is associated with alterations in vessel density, matrix composition, inflammatory response, and growth factor expression. J Histochem Cytochem 2003;51:1119–1130. [DOI] [PubMed] [Google Scholar]
- 33.Chopra H, Gandhi S, Gautam RK, et al. Bacterial nanocellulose based wound dressings: current and future prospects. Curr Pharm Des 2022;28:570–580. [DOI] [PubMed] [Google Scholar]
- 34.Knuutinen A, Kokkonen N, Risteli J, et al. Smoking affects collagen synthesis and extracellular matrix turnover in human skin. Br J Dermatol 2002;146:588–594. [DOI] [PubMed] [Google Scholar]
- 35.Chan LKW, Withey S, Butler PEM. Smoking and wound healing problems in reduction mammaplasty: is the introduction of urine nicotine testing justified? Ann Plast Surg 2006;56:111–115. [DOI] [PubMed] [Google Scholar]
- 36.Vileikyte L. Stress and wound healing. Clin Dermatol 2007;25:49–55. [DOI] [PubMed] [Google Scholar]
- 37.Christian LM, Graham JE, Padgett DA, et al. Stress and wound healing. Neuroimmuno Modulation 2007;13:337–346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wan R, Weissman JP, Grundman K, et al. Diabetic wound healing: the impact of diabetes on myofibroblast activity and its potential therapeutic treatments. Wound Repair Regen 2021;29:573–581. [DOI] [PubMed] [Google Scholar]
- 39.Shipman AR, Millington GWM. Obesity and the skin. Br J Dermatol 2011;165:743–750. [DOI] [PubMed] [Google Scholar]
- 40.Kamolz LP, Wild T. Wound bed preparation: the impact of debridement and wound cleansing. Wound Med 2013;1:44–50. [Google Scholar]
- 41.Thompson JT, Marks MW. Negative pressure wound therapy. Clin Plast Surg 2007;34:673–684. [DOI] [PubMed] [Google Scholar]
- 42.de Smet GHJ, Kroese LF, Menon AG, et al. Oxygen therapies and their effects on wound healing. Wound Repair Regen 2017;25:591–608. [DOI] [PubMed] [Google Scholar]
- 43.Pang C, Fan KS, Wei L, et al. Gene therapy in wound healing using nanotechnology. Wound Repair Regen 2021;29:225–239. [DOI] [PubMed] [Google Scholar]
- 44.Raina N, Rani R, Pahwa R, et al. Biopolymers and treatment strategies for wound healing: an insight view. Int J Polym Mater Polymer Biomater 2022;71:359–375. [Google Scholar]
- 45.Zarei F, Soleimaninejad M. Role of growth factors and biomaterials in wound healing. Artif Cells Nanomed Biotechnol 2018;46:906–911. [DOI] [PubMed] [Google Scholar]
- 46.Rajendran SB, Challen K, Wright KL, et al. Electrical stimulation to enhance wound healing. J Funct Biomater 2021;12:40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Nakayama C, Fujita Y, Matsumura W, et al. The development of induced pluripotent stem cell-derived mesenchymal stem/stromal cells from normal human and RDEB epidermal keratinocytes. J Dermatol Sci 2018;91:301–310. [DOI] [PubMed] [Google Scholar]
- 48.Ramos FS, Maifrino LBM, Alves S, et al. The effects of transcutaneous low-level laser therapy on the skin healing process: an experimental model. Lasers Med Sci 2018;33:967–976. [DOI] [PubMed] [Google Scholar]
- 49.Farghaly Aly U, Abou-Taleb HA, Abdellatif AA, et al. Formulation and evaluation of simvastatin polymeric nanoparticles loaded in hydrogel for optimum wound healing purpose. Drug Des Devel Ther 2019;13:1567–1580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Elegbede RD, Ilomuanya MO, Sowemimo AA, et al. Effect of fermented and green aspalathus linearis extract loaded hydrogel on surgical wound healing in sprague dawley rats. Wound Med 2020;29:100186. [Google Scholar]
- 51.Alibolandi M, Mohammadi M, Taghdisi SM, et al. Synthesis and preparation of biodegradable hybrid dextran hydrogel incorporated with biodegradable curcumin nanomicelles for full thickness wound healing. Int J Pharm 2017;532:466–477. [DOI] [PubMed] [Google Scholar]
- 52.Liu J, Chen Z, Wang J, et al. Encapsulation of curcumin nanoparticles with MMP9-responsive and Thermos-sensitive hydrogel improves diabetic wound healing. ACS Appl Mater Interfaces 2018;10:16315–16326. [DOI] [PubMed] [Google Scholar]
- 53.Chopra H, Singh I, Kumar S, et al. A comprehensive review on hydrogels. Curr Drug Deliv 2022;19:658–675. [DOI] [PubMed] [Google Scholar]
- 54.Chopra H, Bibi S, Kumar S, et al. Preparation and evaluation of chitosan/PVA based hydrogels films loaded with honey for wound healing application. Gels 2022;8:111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zhu L, Liu X, Du L, et al. Preparation of asiaticoside-loaded coaxially electrospinning nanofibers and their effect on deep partial-thickness burn injury. Biomed Pharmacother 2016;83:33–40. [DOI] [PubMed] [Google Scholar]
- 56.Ganesh M, Aziz AS, Ubaidulla U, et al. Sulfanilamide and silver nanoparticles-loaded polyvinyl alcohol-chitosan composite electrospun nanofibers: synthesis and evaluation on synergism in wound healing. J Ind Eng Chem 2016;39:127–135. [Google Scholar]
- 57.Bayat S, Amiri N, Pishavar E, et al. Bromelain-loaded chitosan nanofibers prepared by electrospinning method for burn wound healing in animal models. Life Sci 2019;229:57–66. [DOI] [PubMed] [Google Scholar]
- 58.Ahmed A, Getti G, Boateng J. Ciprofloxacin-loaded calcium alginate wafers prepared by freeze-drying technique for potential healing of chronic diabetic foot ulcers. Drug Deliv Transl Res 2018;8:1751–1768. [DOI] [PubMed] [Google Scholar]
- 59.Avachat AM, Takudage PJ. Design and characterization of multifaceted lyophilized liposomal wafers with promising wound healing potential. J Liposome Res 2018;28:193–208. [DOI] [PubMed] [Google Scholar]
- 60.Yassin GE, Dawoud MHS, Wasfi R, et al. Comparative lyophilized platelet-rich plasma wafer and powder for wound-healing enhancement: formulation, in vitro and in vivo studies. Drug Dev Ind Pharm 2019;45:1379–1387. [DOI] [PubMed] [Google Scholar]
- 61.Tabassum A, Furtado SC, Bharath S. Development of antimicrobial colloidal silver incorporated lyophilized biopolymer wafers for wound care. Wound Med 2018;21:1–7. [Google Scholar]
- 62.Saddik MS, Alsharif FM, El-Mokhtar MA, et al. Biosynthesis, characterization, and wound-healing activity of phenytoin-loaded copper nanoparticles. AAPS PharmSciTech 2020;21:175. [DOI] [PubMed] [Google Scholar]
- 63.Shahzad A, Khan A, Afzal Z, et al. Formulation development and characterization of cefazolin nanoparticles-loaded cross-linked films of sodium alginate and pectin as wound dressings. Int J Biol Macromol 2019;124:255–269. [DOI] [PubMed] [Google Scholar]
- 64.Küchler S, Wolf NB, Heilmann S, et al. 3D-Wound healing model: influence of morphine and solid lipid nanoparticles. J Biotechnol 2010;148:24–30. [DOI] [PubMed] [Google Scholar]
- 65.Shanmugapriya K, Kim H, Saravana PS, et al. Astaxanthin-alpha tocopherol nanoemulsion formulation by emulsification methods: investigation on anticancer, wound healing, and antibacterial effects. Colloids Surf B Biointerfaces 2018;172:170–179. [DOI] [PubMed] [Google Scholar]
- 66.Parisotto-Peterle J, Bidone J, Lucca LG, et al. Healing activity of hydrogel containing nanoemulsified β-caryophyllene. Eur J Pharm Sci 2020;148:105318. [DOI] [PubMed] [Google Scholar]
- 67.Choi Y, Jang J, Koo HJ, et al. Alginate-chitosan hydrogel patch with beta-glucan nanoemulsion for antibacterial applications. Biotechnol Bioprocess Eng 2021;26:71–77. [Google Scholar]
- 68.Flores FC, de Lima JA, da Silva CR, et al. Hydrogels containing nanocapsules and nanoemulsions of tea tree oil provide antiedematogenic effect and improved skin wound healing. J Nanosci Nanotechnol 2015;15:800–809. [DOI] [PubMed] [Google Scholar]
- 69.Raina N, Pahwa R, Bhattacharya J, et al. Drug delivery strategies and biomedical significance of hydrogels: translational considerations. Pharmaceutics 2022;14:574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Hussain Z, Thu HE, Rawas-Qalaji M, et al. Recent developments and advanced strategies for promoting burn wound healing. J Drug Deliv Sci Technol 2022;68:103092. [Google Scholar]
- 71.Hamdan S, Pastar I, Drakulich S, et al. Nanotechnology-driven therapeutic interventions in wound healing: potential uses and applications. ACS Cent Sci 2017;3:163–175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Song Z, Sun H, Yang Y, et al. Enhanced efficacy and anti-biofilm activity of novel nanoemulsions against skin burn wound multi-drug resistant MRSA infections. Nanomedicine 2016;12:1543–1555. [DOI] [PubMed] [Google Scholar]
- 73.Farahani H, Barati A, Arjomandzadegan M, et al. Nanofibrous cellulose acetate/gelatin wound dressing endowed with antibacterial and healing efficacy using nanoemulsion of Zataria multiflora. Int J Biol Macromol 2020;162:762–773. [DOI] [PubMed] [Google Scholar]
- 74.Nair HB, Sung B, Yadav VR, et al. Delivery of antiinflammatory nutraceuticals by nanoparticles for the prevention and treatment of cancer. Biochem Pharmacol 2010;80:1833–1843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Abdellatif MM, Elakkad YE, Elwakeel AA, et al. Formulation and characterization of propolis and tea tree oil nanoemulsion loaded with clindamycin hydrochloride for wound healing: in-vitro and in-vivo wound healing assessment. Saudi Pharm J 2021;29:1238–1249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Alam P, Ansari MJ, Anwer MK, et al. Wound healing effects of nanoemulsion containing clove essential oil. Artif Cells Nanomed Biotechnol 2017;45:591–597. [DOI] [PubMed] [Google Scholar]
- 77.Alam P, Shakeel F, Anwer MK, et al. Wound healing study of eucalyptus essential oil containing nanoemulsion in rat model. J Oleo Sci 2018;67:957–968. [DOI] [PubMed] [Google Scholar]
- 78.McClements DJ, Jafari SM.Jafari SM, McClements DJ. General aspects of nanoemulsions and their formulation. Nanoemulsions: formulation, applications, and characterization. Academic Press; 2018:3–20. [Google Scholar]
- 79.Gorain B, Pandey M, Leng NH, et al. Advanced drug delivery system containing herbal components for wound healing. Int J Pharm 2022;617:121617. [DOI] [PubMed] [Google Scholar]
- 80.Lo S, Fauzi MB. Current update of collagen nanomaterials fabrication, characterisation and its applications: a review. Pharmaceutics 2021;13:316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Solans C, Solé I. Nano-emulsions: formation by low-energy methods. Curr Opin Colloid Interface Sci 2012;17:246–254. [Google Scholar]
- 82.Qadir A, Faiyazuddin MD, Talib Hussain MDT, et al. Critical steps and energetics involved in a successful development of a stable nanoemulsion. J Mol Liq 2016;214:7–18. [Google Scholar]
- 83.Jasmina H, Džana O, Alisa E, et al. Badnjevic A. Preparation of nanoemulsions by high-energy and low-energy emulsification methods. CMBEBIH 2017 IFMBE Proceedings. Springer; 2017:317–322. [Google Scholar]
- 84.Jaiswal M, Dudhe R, Sharma PK. Nanoemulsion: an advanced mode of drug delivery system. 3 Biotech 2015;5:123–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Vater C, Bosch L, Mitter A, et al. Lecithin-based nanoemulsions of traditional herbal wound healing agents and their effect on human skin cells. Eur J Pharm Biopharm 2022;170:1–9. [DOI] [PubMed] [Google Scholar]
- 86.Zain MSC, Edirisinghe SL, Kim CH, et al. Nanoemulsion of flavonoid enriched oil palm (Elaeis guineensis Jacq.) leaf extract enhances wound healing in zebrafish. Phytomed Plus 2021;1:100124. [Google Scholar]
- 87.Seyed Gharibzahedi MT, Jafari SM. Fabrication of Nanoemulsions by ultrasonication. Nanoemulsions 2018;1:233–285. [Google Scholar]
- 88.Che Marzuki NH, Wahab RA, Abdul Hamid M. An overview of nanoemulsion: concepts of development and cosmeceutical applications. Biotechnol Biotechnol Equip 2019;33:779–797. [Google Scholar]
- 89.Ahmad N, Alam MA, Ahmad FJ, et al. Ultrasonication techniques used for the preparation of novel eugenol-nanoemulsion in the treatment of wounds healings and anti-inflammatory. J Drug Deliv Sci Technol 2018;46:461–473. [Google Scholar]
- 90.Fernandez P, André V, Rieger J, et al. Nano-emulsion formation by emulsion phase inversion. Colloids Surf A Physicochem Eng Aspects 2004;251:53–58. [Google Scholar]
- 91.Teo SY, Yew MY, Lee SY, et al. In vitro evaluation of novel phenytoin-loaded alkyd nanoemulsions designed for application in topical wound healing. J Pharm Sci 2017;106:377–384. [DOI] [PubMed] [Google Scholar]
- 92.Bonferoni MC, Riva F, Invernizzi A, et al. Alpha tocopherol loaded chitosan oleate nanoemulsions for wound healing. Evaluation on cell lines and ex vivo human biopsies, and stabilization in spray dried Trojan microparticles. Eur J Pharm Biopharm 2018;123:31–41. [DOI] [PubMed] [Google Scholar]
- 93.Sugumar S, Ghosh V, Nirmala MJ, et al. Ultrasonic emulsification of eucalyptus oil nanoemulsion: antibacterial activity against Staphylococcus aureus and wound healing activity in Wistar rats. Ultrason Sonochem 2014;21:1044–1049. [DOI] [PubMed] [Google Scholar]
- 94.Back PI, Balestrin LA, Fachel FNS, et al. Hydrogels containing soybean isoflavone aglycones-rich fraction-loaded nanoemulsions for wound healing treatment – In vitro and in vivo studies. Colloids Surf B Biointerfaces 2020;196:111301. [DOI] [PubMed] [Google Scholar]
- 95.Valizadeh A, Shirzad M, Pourmand MR, et al. Levofloxacin nanoemulsion gel has a powerful healing effect on infected wound in streptozotocin-induced diabetic rats. Drug Deliv Transl Res 2021;11:292–304. [DOI] [PubMed] [Google Scholar]
- 96.Chakraborty T, Gupta S, Nair A, et al. Wound healing potential of insulin-loaded nanoemulsion with aloe vera gel in diabetic rats. J Drug Deliv Sci Technol 2021;64:102601. [Google Scholar]
- 97.Kazemi M, Mohammadifar M, Aghadavoud E, et al. Deep skin wound healing potential of lavender essential oil and liquorice extract in a nanoemulsion form: biochemical, histopathological and gene expression evidences. J Tissue Viability 2020;29:116–124. [DOI] [PubMed] [Google Scholar]
- 98.Hosny KM, Alhakamy NA, Sindi AM, et al. Coconut oil nanoemulsion loaded with a statin hypolipidemic drug for management of burns: formulation and in vivo evaluation. Pharmaceutics 2020;12:1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Gotmare VD, Kole SS, Athawale RB. Sustainable approach for development of antimicrobial textile material using nanoemulsion for wound care applications. Fash Text 2018;5:25. [Google Scholar]
- 100.Thomas L, Zakir F, Mirza MA, et al. Development of curcumin loaded chitosan polymer based nanoemulsion gel: in vitro, ex vivo evaluation and in vivo wound healing studies. Int J Biol Macromol 2017;101:569–579. [DOI] [PubMed] [Google Scholar]
- 101.Akrawi SH, Gorain B, Nair AB, et al. Development and optimization of naringenin-loaded chitosan-coated nanoemulsion for topical therapy in wound healing. Pharmaceutics 2020;12:1–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Bake JR, Hemmila MR, Wang SC, et al. inventor; University of Michigan, assignee. Nanoemulsion therapeutic compositions and methods of using the same. US 9259407B2. February 16, 2016.
- 103.Hemmila MR, Baker JR, Dolgachev VA, et al. inventor; University of Michigan, assignee. Topical nanoemulsion therapy for wounds. US 20190021998A1. January 24, 2019.
- 104.Wadhwa K, Kadian V, Puri V, et al. New insights into quercetin nanoformulations for topical delivery. Phytomedicine Plus 2022;2:100257. [Google Scholar]
- 105.Kaur T, Kapoor DN. inventor; Shoolini University, assignee. Development and evaluation of Sea buckthorn (Hippophae rhamnoides l.) seed oil nanoemulsion gel for wound healing. IN201811008389. November 29, 2019.
- 106.Dubey AK, Goyal A, Garg A. inventor; GLA University, assignee. Formulation and evaluation of nano-emulgel containing Moringa oleifera seed oil for wound healing. IN202111054212. December 03, 2021.
- 107.Algahtani MS, Ahmad MZ, Shaikh IA, et al. Thymoquinone loaded topical nanoemulgel for wound healing: formulation design and in-vivo evaluation. Molecules 2021;26:3863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Choi SW, Pangeni R, Park JW. Nanoemulsion-based hydrogel for topical delivery of highly skin-permeable growth factor combinations: preparation and in vitro evaluation. J Nanosci Nanotechnol 2017;17:2363–2369. [DOI] [PubMed] [Google Scholar]
- 109.Morsy MA, Abdel-Latif RG, Nair AB, et al. Preparation and evaluation of atorvastatin-loaded nanoemulgel on wound-healing efficacy. Pharmaceutics 2019;11:609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Algahtani MS, Ahmad MZ, Nourein IH, et al. Preparation and characterization of curcumin nanoemulgel utilizing ultrasonication technique for wound healing: in vitro, ex vivo, and in vivo evaluation. Gels 2021;7:213. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No specific data collected for the above manuscript.


