Abstract
Introduction
Vitiligo is an autoimmune disorder resulting in skin depigmentation, with limited approved treatment options. This study evaluated medication utilization and treatment patterns among patients in the first year following vitiligo diagnosis.
Methods
This retrospective analysis of claims data from the Merative® MarketScan Research Databases included patients aged ≥ 12 years newly diagnosed with vitiligo. Patients were identified between October 1, 2016, and April 30, 2021, and had ≥ 12 months of continuous enrollment pre- and post-vitiligo diagnosis. Medication use, treatment line of therapy, time to and number of medication claims, and length of therapy were reported in the 12 months post-vitiligo diagnosis. Results are reported separately for treatment initiators post-vitiligo diagnosis, patients with moderate-to-severe vitiligo, and adolescents (aged 12–17 years).
Results
A total of 19,335 patients were included in the analysis, with half (N = 9648, 49.9%) not receiving any treatment during the 12-month follow-up. Switching was minimal among treatment initiators (N = 5845) in the 12 months post-vitiligo diagnosis, with the most frequent first-line treatments being high-potency topical corticosteroids (25.4%), oral corticosteroids (23.1%), and topical calcineurin inhibitors (TCI, 14.7%). Adolescents initiating treatment (N = 486) most frequently received TCI (30.9%) as first-line therapy. Patients with moderate-to-severe vitiligo (N = 3462) were very likely to receive treatment during follow-up, with only 1.5% not receiving treatment. Among patients with no vitiligo treatment prior to diagnosis, time to first medication claim ranged from 51.9 days (standard deviation [SD], 84.0) for TCI to 178.6 days (SD 116.0) for systemic immunosuppressants; mean total days supplied ranged from 14.4 days (SD 27.1) for oral corticosteroids to 121.0 (SD 114.0) for immunosuppressants.
Conclusion
In this real-world study, a high proportion of patients did not receive any treatment. Among those receiving treatment, most were unlikely to switch or use a combination of treatments within the first year of vitiligo diagnosis.
Keywords: Vitiligo, Medication utilization, Treatment patterns, Real-world evidence
Key Summary Points
| Why carry out this study? |
| Vitiligo is a severely undertreated disease with only one approved treatment for repigmentation (ruxolitinib); other treatment options have limitations, such as poor efficacy and side effects associated with long-term use |
| There is an absence of evidence on treatment utilization or treatment patterns for patients with vitiligo |
| What was learned from the study? |
| In this real-world study, a high proportion of patients newly diagnosed with vitiligo did not receive any treatment |
| Among those receiving treatment, corticosteroids (topical and oral) and topical calcineurin inhibitors were the most used first-line treatment but varied by age |
| Patients were unlikely to switch or use a combination of treatments within the first year of vitiligo diagnosis |
Introduction
Vitiligo is an autoimmune skin disorder characterized by loss of melanocytes resulting in patches of depigmentation, with onset often occurring during childhood or adolescence [1, 2]. Triggers of melanocyte death are multifactorial, including genetic predisposition, oxidative stress, and immunological mechanisms [3]. Vitiligo can be classified as nonsegmental, which is the most common subtype, or as segmental vitiligo, which affects a localized area of the body [4]. Prevalence is estimated to be up to 1.3% in the USA [5].
Although vitiligo does not cause pain and rarely causes pruritis, patients do experience reduced quality of life compared with the general population [5–7]. Disease burden also includes psychosocial effects such as stigmatization, sleep disturbance, relationship difficulties, and impaired social interactions, which have been reported at a higher prevalence than in other skin diseases such as acne or atopic dermatitis but less than with psoriasis [8].
Vitiligo is a severely undertreated disease; ruxolitinib was recently approved by the US Food and Drug Administration (FDA), making it the only approved treatment for vitiligo repigmentation [9, 10]. However, a variety of treatments can be used, including monotherapy and combinations of topical treatments, phototherapy, and off-label immunosuppressants [1]. It is widely acknowledged that vitiligo is not a cosmetic disease, although the use of cosmetics is indicated to improve patient’s quality of life in the absence of approved efficacious treatments [5, 11]. Treatment aims include slowing progression, stabilizing depigmentation, and stimulating and maintaining repigmentation [4].
Vitiligo is a difficult-to-treat disease; current treatments have limitations such as poor efficacy and side effects associated with long-term use [12]. Some treatments have limitations such as multiple topical applications being required per day, or having a lag before a response is seen [13]. A recent questionnaire has also highlighted patients’ desire for new and improved treatments, with 50% reporting they were not satisfied with current therapies [14]. The treatment landscape for vitiligo is evolving, with emerging therapies targeting Janus kinase (JAK) signaling pathways and antioxidant or repigmenting mechanisms [1].
There is an absence of evidence on treatment utilization or treatment patterns for patients with vitiligo. The objective of this study was to evaluate treatment patterns among patients with vitiligo undergoing selected vitiligo-related therapies (corticosteroid treatment, topical tacrolimus treatment, ultraviolet light therapy, photochemotherapy, laser therapy, excimer laser therapy, and epidermal skin graft) in the first year of vitiligo diagnosis.
Methods
Study Design and Participants
This study was a retrospective cohort analysis utilizing data from the Merative® MarketScan Research Databases (Ann Arbor, Michigan) that contain patient-level information on medical and pharmacy claims data in the US.
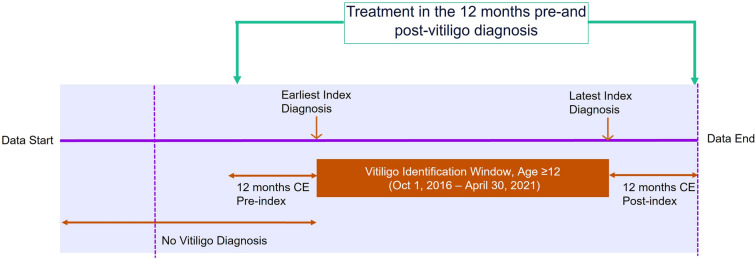
Eligible patients were aged ≥ 12 years with a new diagnosis of vitiligo (International Classification of Diseases [ICD] 10 CM L80.x; vitiligo of eyelids, H02.73-; vitiligo of vulva, N90.89) on any part of the body on a nondiagnostic medical claim between October 1, 2016, and April 30, 2021. The date of the first diagnosis will be considered the index date (Fig. 1). Patients also had ≥ 12 months of continuous enrollment pre- and post-index date. Patients were excluded who had a diagnosis of bullous disorders (ICD-10-CM; L10.x-L14.x), dermatitis or eczema (ICD-10-CM; L20.x-L30.x), papulosquamous disorders (ICD-10-CM; L40.x-L45.x), disorders of skin and appendages (ICD-10-CM; L60.x-L75.x), or other disorders of pigmentation (ICD-10-CM; L80.x-L99.x). Patients were also ineligible who had a diagnosis of vitiligo or evidence of vitiligo-related treatment during the 12-month baseline period.
Fig. 1.
Study design. CE continuous enrollment
Outcomes
This study reported medication utilization and treatment patterns, including vitiligo medication lines of therapy (treatment sequence analysis of first- and second-line treatment; rates of treatment switching), time to first medication claim, overall number of claims for first medication, and length of therapy (number of treated days) among patients with vitiligo in the 12 months post-vitiligo diagnosis.
Treatments included in this analysis were: topical corticosteroids; oral corticosteroids (oral mini pulse therapy) including prednisone, dexamethasone, and methylprednisolone; short-acting injectable corticosteroids including hydrocortisone; medium-acting injectable corticosteroids, including prednisone, methylprednisolone, and triamcinolone; long-acting injectable corticosteroids, including betamethasone and dexamethasone; topical calcineurin inhibitors (TCI); systemic immunosuppressants, including azathioprine, cyclosporine, methotrexate and mycophenolate mofetil; depigmentation therapy; and phototherapy.
Statistical Analyses
Descriptive analyses were conducted for all baseline and treatment outcome variables. Number and percentage of patients were calculated for dichotomous variables and means and standard deviation (SD) for continuous variables.
The analysis set included all patients with a vitiligo diagnosis who received treatment in the first 12 months following the initial vitiligo diagnosis. Results are reported separately for patients who did not receive any vitiligo-related treatments prior to vitiligo diagnosis (treatment initiators post-vitiligo diagnosis). Among patients who were treatment initiators post-vitiligo diagnosis, subanalyses were conducted for patients with moderate-to-severe vitiligo. Moderate-to-severe vitiligo was defined as de novo use of one or more systemic corticosteroid and/or phototherapy, and/or three or more dermatology visits for vitiligo diagnoses in the 12 months post-index date period. Furthermore, first-line treatment trends are reported by age of treatment initiators post-vitiligo diagnosis, reporting results for adolescents (aged 12–17 years) and adults (aged 18–64 years) separately.
Compliance with Ethics Guidelines
Through a paid contract with Merative®, authors were granted permission to use and access the database. Data provided for the study were retrospective de-identified claims data, which were Health Insurance Portability and Accountability Act (HIPAA) compliant and did not require Ethics Committee or Institutional Review Board approval, notification, or exemption.
Results
Study Population
A total of 19,335 patients were included in the analysis, with a mean age of 41.6 years (SD 14.0), and 82.2% were female (Table 1). The most common comorbidities included gastrointestinal disease (28.2%), cardiovascular disease (26.7%), and hypertension (19.8%; Table 1). Similar demographics were reported for the 13,449 (69.6%) patients who did not receive any vitiligo-related treatments prior to vitiligo diagnosis (mean age 40.7 years, SD 14.2; 80.4% female; Table 1) and for the 3462 patients who had moderate-to-severe vitiligo and received treatment (mean age 41.7 years, SD 13.8; 76.3% female; Table 1). Of the patients with moderate-to-severe vitiligo, 2669 patients initiated treatment in the 12 months post-vitiligo diagnosis. The proportion of adolescents across the cohort was 6% (N = 1158) and was similar among treatment initiators post-vitiligo diagnosis and those with moderate-to-severe vitiligo (Table 1).
Table 1.
Baseline characteristics
| Overall (N = 19,335) | No vitiligo treatment prior to diagnosis (N = 13,449) | Moderate-to-severe vitiligo (N = 3462) | |
|---|---|---|---|
| Age (years), mean ± SD | 41.6 ± 14.0 | 40.7 ± 14.2 | 41.7 ± 13.8 |
| 12–17 years, n (%) | 1158 (6.0) | 934 (6.9) | 211 (6.1) |
| 18–64 years, n (%) | 18,177 (94.0) | 12,515 (93.1) | 3251 (93.9) |
| Female, n (%) | 15,884 (82.2) | 10,812 (80.4) | 2643 (76.3) |
| Moderate-to-severe vitiligo, n (%)a | 3462 (17.9) | 2715 (20.2) | 3462 (100.0) |
| Baseline comorbidities, n (%) | |||
| Gastrointestinal disease | 5462 (28.2) | 3226 (24.0) | 1046 (30.2) |
| Cardiovascular disease | 5168 (26.7) | 3092 (23.0) | 955 (27.6) |
| Hypertension | 3830 (19.8) | 2296 (17.1) | 688 (19.9) |
| Anxiety | 3397 (17.6) | 2089 (15.5) | 616 (17.8) |
| Obesity | 2861 (14.8%) | 1703 (12.7) | 571 (16.5) |
| Depression | 2241 (11.6) | 1369 (10.2) | 427 (12.3) |
| Allergic rhinitis | 2162 (11.2) | 1075 (8.0) | 424 (12.3) |
| Sleep disorder | 1788 (9.3) | 1020 (7.6) | 371 (10.7) |
| Chronic pulmonary disease | 1905 (9.8) | 837 (6.2) | 349 (10.1) |
| Asthma | 1408 (7.3) | 635 (4.7) | 276 (8.0) |
| Autoimmune disorders | 1028 (5.3) | 399 (3.0) | 186 (5.4) |
| Conjunctivitis | 869 (4.5) | 480 (3.6) | 171 (4.9) |
| Chronic rhinosinusitis | 828 (4.3) | 337 (2.5) | 130 (3.8) |
| ADHD | 564 (2.9) | 374 (2.8) | 126 (3.6) |
| Atopic dermatitis | 390 (2.0) | 100 (0.7) | 81 (2.3) |
| Rheumatoid arthritis | 319 (1.7) | 95 (0.7) | 51 (1.5) |
| Psoriasis | 302 (1.6) | 79 (0.6) | 75 (2.2) |
| Inflammatory bowel disease | 237 (1.2) | 103 (0.8) | 48 (1.4) |
| Self harm and suicide | 187 (1.0) | 84 (0.6) | 25 (0.7) |
| Alopecia areata | 56 (0.3) | 24 (0.2) | 16 (0.5) |
| Psoriatic arthritis | 21 (0.1) | 7 (0.1) | 5 (0.1) |
ADHD attention-deficit/hyperactivity disorder, SD standard deviation
aModerate-to-severe vitiligo: patients initiating treatment post-vitiligo diagnosis as use of ≥ 1 systemic corticosteroid and/or phototherapy, and/or 3 or more dermatology visits for vitiligo diagnoses in the first 12 months post-index date
All Vitiligo Patients (N = 19,335)
Almost half (N = 9648, 49.9%) of patients did not receive any treatment during the 12-month follow-up. The most common therapies were oral corticosteroids (N = 4063, 21.0%), high-potency topical corticosteroids (HTCS; N = 3908, 20.2%), and TCI (N = 2725, 14.1%; Table 2). The longest time to first medication claim was 153.3 days (SD 108.3) for oral corticosteroids (Table 2). Patients taking immunosuppressants had more than double the mean number of claims (4.4, SD 3.7) and a six-fold higher mean number of days treated (152.2, SD 110.9) than other therapies (Table 2). Phototherapy was used by a low proportion of patients (N = 751, 3.9%).
Table 2.
Medication use for patients with vitiligo following diagnosis: 12-month follow-up (N = 19,335)
| Oral CS (N = 4063) | HTCS (N = 3908) | MTCS (N = 2029) | LTCS (N = 407) | TCI (N = 2725) | Immunosuppressants (N = 430) | |
|---|---|---|---|---|---|---|
| Number of medication claims | 1.9 (2.0) | 1.6 (1.3) | 1.4 (1.0) | 1.3 (0.9) | 1.7 (1.4) | 4.4 (3.7) |
| Time to first medication claim (days) | 153.3 (108.3) | 72.8 (100.3) | 91.2 (108.8) | 93.7 (114.0) | 56.8 (89.2) | 111.6 (107.7) |
| Total days supplied | 26.3 (62.0) | 37.9 (36.6) | 33.8 (29.4) | 30.0 (26.8) | 44.8 (39.9) | 185.8 (149.6) |
| Number of treated days | 23.2 (48.8) | 35.8 (32.4) | 32.4 (26.7) | 28.9 (25.2) | 42.9 (36.0) | 152.2 (110.9) |
Data are mean (SD)
N = 9648 (49.9%) patients did not receive any treatment during the 12-month follow-up
CS corticosteroid, HTCS high-potency CS, LTCS low-potency CS, MTCS medium-potency CS, SD standard deviation, TCI topical calcineurin inhibitors
Patients Without Vitiligo-Related Treatment Prior to Diagnosis (N = 13,449)
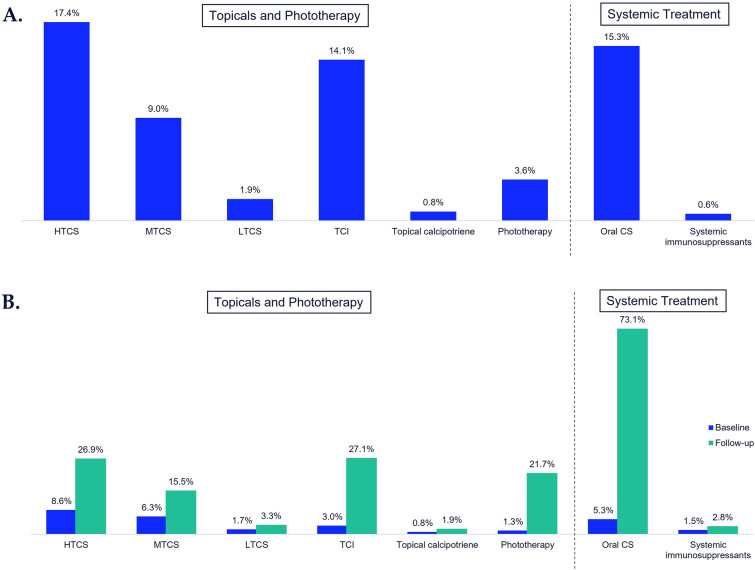
More than half (56.5%, N = 7599) of patients who did not receive vitiligo-related treatment prior to vitiligo diagnosis also did not receive treatment during the 12-month follow-up post-vitiligo diagnosis. Of those who did initiate vitiligo-related treatment (N = 5845), HTCS were the most frequently used treatment at 17.4% (N = 2343), followed by oral corticosteroids (15.3%, N = 2053) and TCI (14.1%, N = 1899; Fig. 2a).
Fig. 2.
Percentage of treatment use in the 12 months post-vitiligo diagnosis. A Patients with no vitiligo treatment prior to diagnosis (N = 13,449)a. B Patients with moderate-to-severe vitiligo (N = 3462)b; a56.5% received no treatment during follow-up; b1.5% received no treatment during follow-up. Surgery (skin grafts) < 0.1%; dermabrasion and monobenzone 0%; Total number sums to > 100% as some patients can have multiple treatments. Moderate-to-severe vitiligo: patients initiating treatment post-vitiligo diagnosis as use of ≥ 1 systemic corticosteroid and/or phototherapy, and/or 3 or more dermatology visits for vitiligo diagnoses in the first 12 months post-index date. CS corticosteroid, HTCS high-potency topical CS, MTCS medium-potency topical CS, LTCS low-potency topical CS, TCI topical calcineurin inhibitors
Time to first medication claim ranged from 51.9 days (SD 84.0) for TCI to 178.6 days (SD 116.0) for systemic immunosuppressants; mean total days supplied ranged from 14.4 days (SD 27.1) for oral corticosteroids to 121.0 (SD 114.0) for immunosuppressants (Table 3). Consistent with the total vitiligo population, a low proportion of treatment initiators post-vitiligo diagnosis received phototherapy (N = 480, 3.6%).
Table 3.
Medication use for patients with no vitiligo treatment prior to diagnosis, 12-month follow-up (N = 13,449)
| Oral CS (N = 2053) | HTCS (N = 2343) | MTCS (N = 1206) | LTCS (N = 252) | TCI (N = 1899) | Immunosuppressants (N = 85) | |
|---|---|---|---|---|---|---|
| Number of medication claims | 1.5 (1.3) | 1.5 (1.1) | 1.4 (1.0) | 1.2 (0.7) | 1.6 (1.3) | 3.3 (2.7) |
| Time to first medication claim (days) | 165.2 (108.5) | 67.4 (98.9) | 91.2 (108.8) | 82.8 (112.0) | 51.9 (84.0) | 178.6 (116.0) |
| Total days supplied | 14.4 (27.1) | 34.7 (31.8) | 33.8 (29.4) | 29.5 (25.4) | 43.5 (38.5) | 121.0 (114.0) |
| Number of treated days | 13.7 (24.4) | 33.1 (29.1) | 32.4 (26.7) | 28.4 (24.1) | 42.0 (35.1) | 95.3 (88.3) |
Data are mean (SD)
CS corticosteroid, HTCS high-potency CS, LTCS low-potency CS, MTCS medium-potency CS, SD standard deviation, TCI topical calcineurin inhibitors
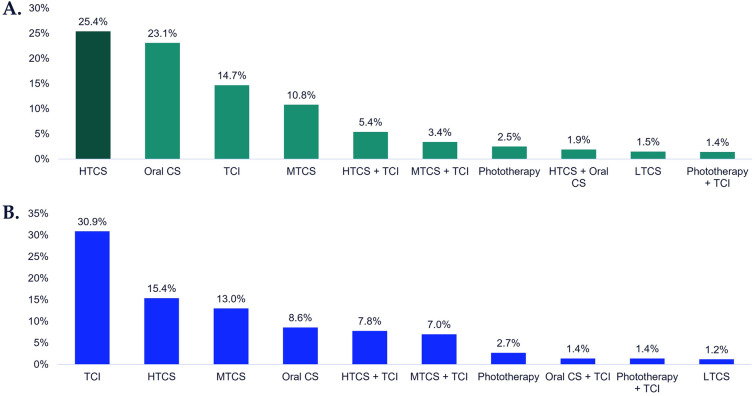
Treatment sequence analysis of patients initiating treatment post-vitiligo diagnosis (N = 5845) showed that switching was minimal in the 12-month follow-up. The most frequent first-line treatments were HTCS (25.4%), oral corticosteroids (23.1%), and TCI (14.7%; Fig. 3a).
Fig. 3.
Most frequent first-line treatments among patients initiating treatment post-vitiligo diagnosis, 12-month follow-up. A All ages (N = 5845). B Among adolescent patients (N = 486). Patients initiating treatment are defined as those who did not receive any vitiligo-related treatments prior to diagnosis of vitiligo. Adolescents defined as those aged 12–17 years. CS corticosteroid, HTCS high-potency topical CS, MTCS medium-potency topical CS, LTCS low-potency topical CS, TCI topical calcineurin inhibitors
For adolescent patients who were treatment initiators post-vitiligo diagnosis (N = 486), first-line therapy was most frequently TCI (30.9%), HTCS (15.4%), and medium-potency topical corticosteroids (13.0%) in the 12 months post-vitiligo diagnosis (Fig. 3b).
Moderate-to-Severe Vitiligo Patients (N = 3462)
Of the 3462 patients with moderate-to-severe vitiligo, the majority received oral corticosteroids (73.1%) and only 1.5% received no treatment during follow-up (Fig. 2b).
Time to first medication claim ranged from 55.6 days (SD 86.1) for TCIs to 161.5 days (SD 108.4) for oral corticosteroids (Table 4). Mean total days of medication supplied ranged from 16.5 days (SD 33.5) for oral corticosteroids to 156.5 days (SD 148.6) for immunosuppressants (Table 4).
Table 4.
Medication use for patients with moderate-to-severe vitiligo, 12-month follow-up (N = 3462)
| Oral CS (N = 2530) | HTCS (N = 932) | MTCS (N = 538) | LTCS (N = 113) | TCI (N = 939) | Immunosuppressants (N = 98) | |
|---|---|---|---|---|---|---|
| Number of medication claims | 1.6 (1.4) | 1.8 (1.5) | 1.5 (1.0) | 1.4 (1.3) | 1.9 (1.7) | 3.6 (3.2) |
| Time to first medication claim (days) | 161.5 (108.4) | 72.0 (97.8) | 92.1 (109.7) | 87.4 (112.2) | 55.6 (86.1) | 149.6 (115.7) |
| Total days supplied | 16.5 (33.5) | 42.2 (42.8) | 37.1 (33.9) | 35.8 (38.4) | 52.7 (50.0) | 156.5 (148.6) |
| Number of treated days | 15.3 (27.8) | 39.9 (35.7) | 35.3 (30.9) | 33.9 (36.1) | 50.0 (43.9) | 119.5 (104.4) |
Moderate-to-severe vitiligo: patients initiating treatment post-vitiligo diagnosis as use of ≥ 1 systemic corticosteroid and/or phototherapy, and/or 3 or more dermatology visits for vitiligo diagnoses in the first 12 months post-index date
Data are mean (SD)
CS corticosteroid, HTCS high-potency topical CS, LTCS low-potency topical CS, MTCS medium-potency topical CS, SD standard deviation, TCI topical calcineurin inhibitors
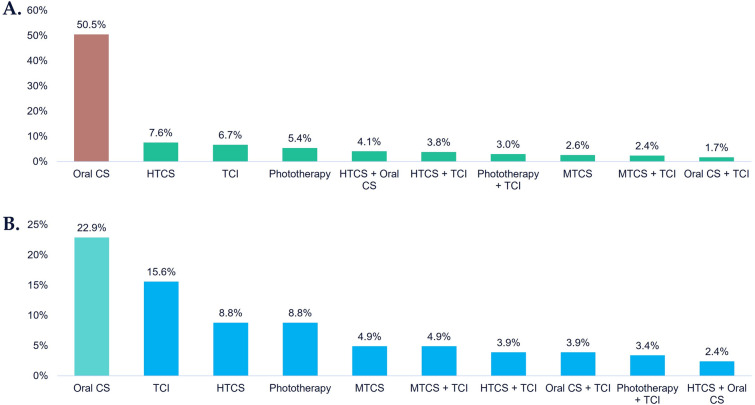
Treatment sequence analysis shows that most patients with moderate-to-severe vitiligo who were treatment initiators post-vitiligo diagnosis had one line of treatment during follow-up, with oral corticosteroids being the most frequent first-line treatment (50.5%, Fig. 4a). Most patients did not change therapy within the follow-up period, but of those who did, 10.0% took oral corticosteroids (data not shown).
Fig. 4.
Most frequent first-line treatments among patients with moderate-to-severe vitiligo diagnosis, 12-month follow-up. A Treatment initiators; all ages (N = 2669). B Among adolescent patients (N = 205). Moderate-to-severe vitiligo: patients initiating treatment post-vitiligo diagnosis as use of ≥ 1 systemic corticosteroid and/or phototherapy, and/or 3 or more dermatology visits for vitiligo diagnoses in the first 12 months post-index date. Treatment initiators are defined as those who did not receive any vitiligo-related treatments prior to diagnosis of vitiligo. Adolescents defined as those aged 12–17 years. CS corticosteroid, HTCS high-potency topical CS, MTCS medium-potency topical CS, LTCS low-potency topical CS, TCI topical calcineurin inhibitors
Analysis by age shows that among patients with moderate-to-severe vitiligo, adolescents (N = 205) were also unlikely to switch treatment during the 12-month follow-up. First-line treatment during follow-up was most commonly oral corticosteroids (22.9%) and TCI (15.6%; Fig. 4b).
Discussion
In this real-world study, more than half of patients did not receive any treatment during the first 12 months following their vitiligo diagnosis, signifying that this is a severely undertreated population. It may also reflect a high use of cosmetic or over-the-counter treatments which were not captured within this claims database. Treatment switch rates and combinations of treatments were low for patients receiving treatment for vitiligo; a high proportion of patients only received a single first-line therapy during follow-up.
HTCS and oral corticosteroids were the most common first-line treatments for patients initiating treatment post-vitiligo diagnosis, but this varied by age. This is consistent with consensus guidance recommending first-line treatment with topical corticosteroids or TCIs [4]. The most common first-line therapy for adolescents initiating treatment was TCI, which is consistent with previously reported studies [2].
Overall, half of all patients with vitiligo in this analysis did not receive treatment in the 12-month follow-up period. Unfortunately, reasons for not initiating treatment are not captured in claims data, and, to date, there are no studies assessing why patients may not initiate treatment. However, there are many factors that may impact treatment decisions, such as perception of disease burden and treatment efficacy. For example, among a group of Turkish patients with vitiligo, 80% reported that their disease did not impact their relationships and 35% reported that it did not have a major impact on their life [15]; such perceptions of disease burden may prevent patients seeking treatment. There are currently no definitive guidelines for the treatment of vitiligo, and current treatment options vary in terms of efficacy and safety. This, coupled with a lack of provider knowledge on treatment of vitiligo, may also greatly impact how physicians approach vitiligo management. Access to treatment, as well as insurance coverage, is likely to affect treatment-seeking behaviors. For example, although phototherapy has shown efficacy in patients with vitiligo, it is a very time-consuming treatment (recommended 3 treatments/week) that often takes several months for improvement [16, 17]. Moreover, it has been shown that vitiligo lesions on the trunk and legs, for example, are less responsive to treatments; thus, it is possible that a perceived lack of efficacy may impact treatment rates [18]. Access to treatment may also be a deciding factor. Indeed, a recent study showed that, across 17 insurers with regional or national coverage in the US, over half did not cover phototherapy, and coverage for topical therapies varied by location [19]. The most common reasons for coverage denials were the idea that vitiligo treatment was an unnecessary cosmetic treatment or that therapies being prescribed were not FDA approved for vitiligo [19]. Altogether, there may be many barriers for patients with vitiligo regarding initiating treatment, and further research is needed to better understand what drives treatment decisions in a real-world setting.
In this study, however, patients with moderate-to-severe vitiligo were very likely to receive treatment within the follow-up time, with only 1.5% not receiving treatment. Among patients receiving treatment, almost half had first-line oral corticosteroids. The definition of moderate-to-severe vitiligo used for this study was empirical and not clinically driven but was used as a proxy in the absence of established disease activity measures; moderate-to-severe vitiligo was defined as de novo use of ≥ 1 systemic corticosteroid and/or phototherapy, and/or three or more dermatology visits for vitiligo diagnoses in the 12 months post-index date period. Physicians can classify vitiligo patients according to disease features, including the body surface area impacted (e.g., Vitiligo Extent Score) [20], facial involvement, or disease status being active versus stable [21]. However, none of these measures are captured in claims databases; therefore, proxies based on utilization were used as a reasonable alternative.
Surgery and dermabrasion use were minimal, which may be due to a preference for conservative treatments in the first year or because such procedures may not be covered by insurance. The number of phototherapy claims, including total number of sessions per patients, were very low, suggesting that, among the few patients receiving this treatment, persistence with phototherapy treatment was poor. Interestingly, there is evidence that 6–12-month duration of phototherapy may be required to demonstrate a treatment response on highly visible areas such as the face and neck for patients with vitiligo [16]. However, the long time to treatment response likely impacted patients’ persistence with this treatment option, suggesting use in combination with other vitiligo treatments would be beneficial.
Given that this study has identified such an undertreated population, this highlights a potential unmet need, especially given the health-related quality-of-life detriments experienced by patients [1]. It has been reported that patients receiving treatment, including prescription or over-the-counter therapies or cosmetics, have higher quality-of-life scores compared to before treatment [1]. Disease burden among vitiligo patients has been shown to be associated with female sex and age < 30 years (particularly adolescents) [8]. In addition, higher quality of life, as measured by the Vitiligo Quality of Life Index, has been associated with the extent of and location of disease, with more visible lesions (e.g., head) rated as being of more concern among patients, contributing to the stigma of the disease [5]. This corresponds with commonly reported patient coping strategies of camouflage and lesion concealment by wearing clothing to cover affected areas [5].
Treatment decisions depend on extent stability of disease and size of body surface affected with many off-label options. Questionnaire results further support the preferences of patients with vitiligo who report their need for new and improved treatments, including 87% who were willing to take part in clinical trials and 68% responding that they would be willing to receive weekly injections if the treatment was effective [14]. Advances in the field may provide patients with vitiligo more treatment options in the future, including topical and oral JAK inhibitors as well as the recently approved topical ruxolitinib [9, 12]. Key developmental research areas are targeted immunotherapy, melanocyte regeneration, and reduced microenvironmental oxidative stress with the aim of achieving long-term disease stability without recurrences and repigmentation to match depigmented areas [12].
This study is limited by the completeness of the claims database to fully capture vitiligo-related treatments and symptoms. It is incomplete in terms of over-the-counter medications and other self-management techniques. As a result, this analysis is limited in its ability to fully control for differences in vitiligo severity that may influence treatment choices and outcomes. Study findings may not be generalizable to all individuals in the US population and experimental and investigational therapies are not captured in the claims database because insurance companies do not reimburse them. In addition, results may be prone to bias due to non-random selection into the treatment group and representative of the commercially insured population only rather than the overall population.
This is the first study to look at treatment patterns of patients with vitiligo in the US, including subanalyses by disease severity and age. An additional strength is that this study uses data from a large, socioeconomically diverse commercial insurance database to describe observed treatment patterns associated with the diagnosis of vitiligo.
Conclusion
This study highlights that, within the first year of being diagnosed with vitiligo, a high proportion of patients do not receive any treatment, and among those receiving treatment, most are unlikely to switch or use a combination of treatments. To help better understand the needs of patients with vitiligo, future studies need to examine which demographic and clinical characteristics are associated with not receiving treatments for vitiligo.
Acknowledgements
We thank the participants of the study.
Author Contributions
Substantial contributions to conception and design: David Rosmarin and Ahmed M. Soliman. Interpretation of data: David Rosmarin, Ahmed M. Soliman and Chao Li. Involved in drafting or revising critically for important intellectual content: David Rosmarin, Ahmed M. Soliman and Chao Li. All authors approved the final version of the article, including the authorship list.
Funding
This work, as well as the journal’s Rapid Service Fee, was funded by AbbVie. AbbVie sponsored the study; contributed to the design; participated in collection, analysis, and interpretation of data; and in writing, reviewing and approval of the final version. All authors had access to the data results, and participated in the development, review, and approval of this abstract. No honoraria or payments were made for authorship.
Medical Writing and Editorial Assistance
Medical writing assistance was provided by Sarah Hodgkinson PhD, of Fishawack Facilitate Ltd, part of Fishawack Health, and was funded by AbbVie Inc., North Chicago, IL.
Data Availability
The data that support the findings of this study are available from Merative® MarketScan® Research databases (Ann Arbor, MI). Restrictions apply to the availability of these data, which were used under license for this study. Data are available from the corresponding author upon request with the permission from Merative® MarketScan® Research.
Ethical Approval
Through a paid contract with Merative®, authors were granted permission to use and access the database. Data provided for the study were de-identified and Health Insurance Portability and Accountability Act compliant, therefore review board approval was not required.
Conflict of Interest
David Rosmarin: David Rosmarin has received honoraria as a consultant for AbbVie, Abcuro, AltruBio, Arena, Boehringer-Ingelheim, Bristol Meyers Squibb, Celgene, Concert, CSL Behring, Dermavant, Dermira, Incyte, Janssen, Kyowa Kirin, Lilly, Novartis, Pfizer, Regeneron, Sanofi, Sun Pharmaceuticals, UCB, VielaBio; has received research support from AbbVie, Amgen, Bristol Meyers Squibb, Celgene, Dermira, Galderma, Incyte, Janssen, Lilly, Merck, Novartis, Pfizer, and Regeneron Pharmaceuticals Inc; and has served as a paid speaker for AbbVie, Amgen, Celgene, Incyte, Janssen, Lilly, Novartis, Pfizer, Regeneron Pharmaceuticals Inc., and Sanofi. Ahmed M. Soliman, and Chao Li: are employees of AbbVie and may own AbbVie stock and/or AbbVie patents.
Footnotes
The original online version of this article was revised: Middle name of author Ahmed M. Soliman updated.
Change history
9/2/2023
A Correction to this paper has been published: 10.1007/s13555-023-01020-z
References
- 1.Bergqvist C, Ezzedine K. Vitiligo: a review. Dermatology. 2020;236(6):571–592. doi: 10.1159/000506103. [DOI] [PubMed] [Google Scholar]
- 2.de Menezes AF, Oliveira de Carvalho F, Barreto RS, de Santana Silva B, Shanmugam S, Gurgel RQ, et al. Pharmacologic treatment of vitiligo in children and adolescents: a systematic review. Pediatr Dermatol. 2017;34(1):13–24. doi: 10.1111/pde.13024. [DOI] [PubMed] [Google Scholar]
- 3.Chen J, Li S, Li C. Mechanisms of melanocyte death in vitiligo. Med Res Rev. 2021;41(2):1138–1166. doi: 10.1002/med.21754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Taieb A, Alomar A, Böhm M, Dell'anna ML, De Pase A, Eleftheriadou V, et al. Guidelines for the management of vitiligo: the European Dermatology Forum consensus. Br J Dermatol. 2013;168(1):5–19. doi: 10.1111/j.1365-2133.2012.11197.x. [DOI] [PubMed] [Google Scholar]
- 5.Bibeau K, Pandya AG, Ezzedine K, Jones H, Gao J, Lindley A, et al. Vitiligo prevalence and quality of life among adults in Europe, Japan and the USA. J Eur Acad Dermatol Venereol. 2022;36(10):1831–1844. doi: 10.1111/jdv.18257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Morrison B, Burden-Teh E, Batchelor JM, Mead E, Grindlay D, Ratib S. Quality of life in people with vitiligo: a systematic review and meta-analysis. Br J Dermatol. 2017;177(6):e338–e339. doi: 10.1111/bjd.15933. [DOI] [PubMed] [Google Scholar]
- 7.Hedayat K, Karbakhsh M, Ghiasi M, Goodarzi A, Fakour Y, Akbari Z, et al. Quality of life in patients with vitiligo: a cross-sectional study based on Vitiligo Quality of Life index (VitiQoL) Health Qual Life Outcomes. 2016;14:86. doi: 10.1186/s12955-016-0490-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ezzedine K, Eleftheriadou V, Jones H, Bibeau K, Kuo FI, Sturm D, et al. Psychosocial effects of vitiligo: a systematic literature review. Am J Clin Dermatol. 2021;22(6):757–774. doi: 10.1007/s40257-021-00631-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rosmarin D, Pandya AG, Lebwohl M, Grimes P, Hamzavi I, Gottlieb AB, et al. Ruxolitinib cream for treatment of vitiligo: a randomised, controlled, phase 2 trial. Lancet. 2020;396(10244):110–120. doi: 10.1016/S0140-6736(20)30609-7. [DOI] [PubMed] [Google Scholar]
- 10.FDA. FDA approves topical treatment addressing repigmentation in vitiligo in patients aged 12 and older 2022. https://www.fda.gov/drugs/news-events-human-drugs/fda-approves-topical-treatment-addressing-repigmentation-vitiligo-patients-aged-12-and-older. Accessed 12 June 2023
- 11.Ezzedine K, Sheth V, Rodrigues M, Eleftheriadou V, Harris JE, Hamzavi IH, et al. Vitiligo is not a cosmetic disease. J Am Acad Dermatol. 2015;73(5):883–885. doi: 10.1016/j.jaad.2015.07.039. [DOI] [PubMed] [Google Scholar]
- 12.Karagaiah P, Valle Y, Sigova J, Zerbinati N, Vojvodic P, Parsad D, et al. Emerging drugs for the treatment of vitiligo. Expert Opin Emerg Drugs. 2020;25(1):7–24. doi: 10.1080/14728214.2020.1712358. [DOI] [PubMed] [Google Scholar]
- 13.Frisoli ML, Essien K, Harris JE. Vitiligo: Mechanisms of pathogenesis and treatment. Annu Rev Immunol. 2020;38:621–648. doi: 10.1146/annurev-immunol-100919-023531. [DOI] [PubMed] [Google Scholar]
- 14.Narayan VS, Uitentuis SE, Luiten RM, Bekkenk MW, Wolkerstorfer A. Patients' perspective on current treatments and demand for novel treatments in vitiligo. J Eur Acad Dermatol Venereol. 2021;35(3):744–748. doi: 10.1111/jdv.16927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Topal IO, Duman H, Goncu OE, Durmuscan M, Gungor S, Ulkumen PK. Knowledge, beliefs, and perceptions of Turkish vitiligo patients regarding their condition. An Bras Dermatol. 2016;91(6):770–775. doi: 10.1590/abd1806-4841.20165060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bae JM, Jung HM, Hong BY, Lee JH, Choi WJ, Lee JH, et al. Phototherapy for vitiligo: a systematic review and meta-analysis. JAMA Dermatol. 2017;153(7):666–674. doi: 10.1001/jamadermatol.2017.0002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zubair R, Hamzavi IH. Phototherapy for vitiligo. Dermatol Clin. 2020;38(1):55–62. doi: 10.1016/j.det.2019.08.005. [DOI] [PubMed] [Google Scholar]
- 18.Anbar TS, Westerhof W, Abdel-Rahman AT, El-Khayyat MA. Evaluation of the effects of NB-UVB in both segmental and non-segmental vitiligo affecting different body sites. Photodermatol Photoimmunol Photomed. 2006;22(3):157–163. doi: 10.1111/j.1600-0781.2006.00222.x. [DOI] [PubMed] [Google Scholar]
- 19.Blundell A, Sachar M, Gabel CK, Bercovitch LG. The scope of health insurance coverage of vitiligo treatments in the United States: Implications for health care outcomes and disparities in children of color. Pediatr Dermatol. 2021;38(Suppl 2):79–85. doi: 10.1111/pde.14714. [DOI] [PubMed] [Google Scholar]
- 20.van Geel N, Lommerts J, Bekkenk M, Wolkerstorfer A, Prinsen CAC, Eleftheriadou V, et al. Development and validation of the Vitiligo Extent Score (VES): an International Collaborative initiative. J Invest Dermatol. 2016;136(5):978–984. doi: 10.1016/j.jid.2015.12.040. [DOI] [PubMed] [Google Scholar]
- 21.Wang Y, Li S, Li C. Clinical features, immunopathogenesis, and therapeutic strategies in vitiligo. Clin Rev Allergy Immunol. 2021;61(3):299–323. doi: 10.1007/s12016-021-08868-z. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from Merative® MarketScan® Research databases (Ann Arbor, MI). Restrictions apply to the availability of these data, which were used under license for this study. Data are available from the corresponding author upon request with the permission from Merative® MarketScan® Research.