Abstract
Vesicle trafficking is an essential cellular process upon which many physiological processes of eukaryotic cells rely. It is usually the ‘language’ of communication among the components of the endomembrane system within a cell, between cells and between a cell and its external environment. Generally, cells have the potential to internalize membrane-bound vesicles from external sources by endocytosis. Plants constantly interact with both mutualistic and pathogenic microbes. A large part of this interaction involves the exchange of transport vesicles between the plant cells and the microbes. Usually, in a pathogenic interaction, the pathogen releases vesicles containing bioactive molecules that can modulate the host immunity when absorbed by the host cells. In response to this attack, the host cells similarly mobilize some vesicles containing pathogenesis-related compounds to the pathogen infection site to destroy the pathogen, prevent it from penetrating the host cell or annul its influence. In fact, vesicle trafficking is involved in nearly all the strategies of phytopathogen attack subsequent plant immune responses. However, this field of plant-pathogen interaction is still at its infancy when narrowed down to plant-fungal pathogen interaction in relation to exchange of transport vesicles. Herein, we summarized some recent and novel findings unveiling the involvement of transport vesicles as a crosstalk in plant-fungal phytopathogen interaction, discussed their significance and identified some knowledge gaps to direct future research in the field. The roles of vesicles trafficking in the development of both organisms are also established.
Keywords: Endosomes, Extracellular vesicles, Plant-pathogen interaction, Phytopathogens, Vesicles trafficking
Introduction
Being sessile, plants come in contact with many different microorganisms most of which establish mutualistic relationship with them while a few are pathogenic. Generally, plants lack specific immune cells; upon encounter with a microbe, a plant cell reprograms its physiologic processes to either defend itself or prepare for the establishment of a mutualistic relationship as the case may be (Ruano and Scheuring 2020). Although, it is not yet clearly understood how plants distinguish mutualistic from pathogenic microbes, it is known that pathogenic fungi have ergosterols on their cell surfaces, while mutualistic fungi have sterols instead (Jordá and Puig 2020). Plants associate with mutualistic bacteria or fungi. Association with mutualistic bacteria such as Rhizobia is achieved through symbiosome as an interfacial surface, surrounded by peribacteriod membrane (PBM). Periarbuscular membrane (PAM) however forms the interfacial surface for interaction with mutualistic fungi (mycorrhizal hypha) (Kanazawa and Ueda 2017). On the other hand, pathogenic microbes (fungi and most oomycetes) usually form haustoria which are surrounded by host-generated membrane called extrahaustorial membrane (EHM) that limits pathogen proliferation within the host cell (Berkey et al. 2017). Pathogenic bacteria (and some oomycetes) often use their secretion systems to ‘inject’ effector substances into the host cells. Figure 1 summarizes the various microorganisms that interact with plants.
Fig. 1.
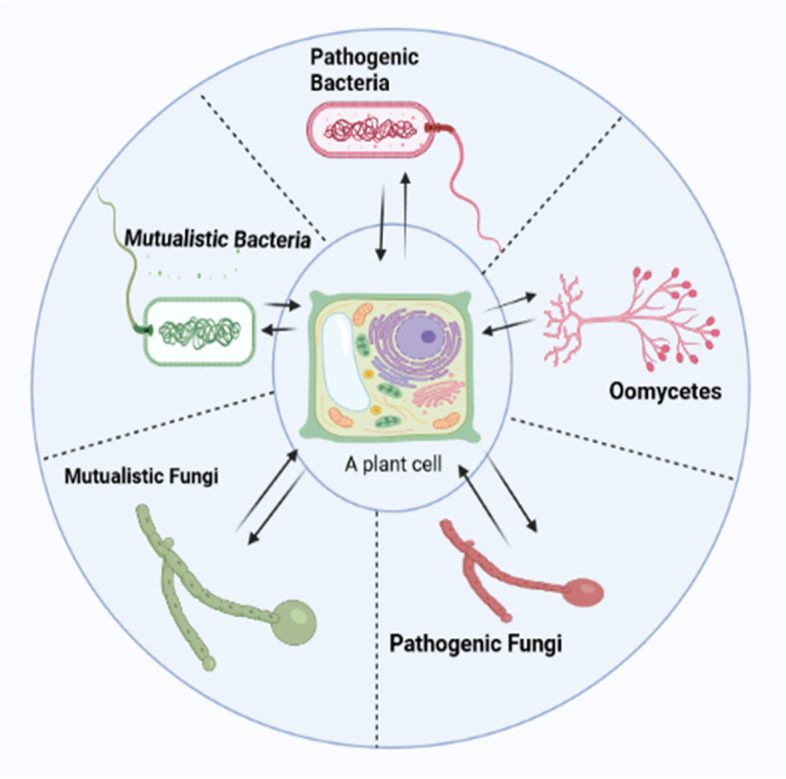
The various microorganisms that interact with plant cells. The interaction can be mutualistic or pathogenic. Pathogenic organisms are shown in red while mutualistic ones are shown in green
Plants generally possess two levels of basal immunity: pattern-triggered immunity (PTI) and effector-triggered immunity (ETI). In PTI, when a pathogen comes in contact with the host plant, a given molecular signature on the pathogen known as pathogen associated molecular pattern (PAMP) is identified by the host plasma membrane-localized receptor proteins called pattern recognition receptors (PRRs) via interaction between the two (Chang et al. 2022). The interaction of a PAMP with a PRR causes phosphorylation (activation) of the latter and it consequently gets internalized for downstream signaling and subsequent degradation in the vacuole to avoid over-activation of the immune response (Couto and Zipfel 2016). PTI is a very effective defense line against many phytopathogens. However, there are some adapted pathogens that release certain effector proteins that block the operation of the PTI, rendering the plant susceptible to the pathogens as the effectors hijack the host cell functions to favor the pathogens’ colonization, and at the same time maintain the viability of the host cells (Toruño et al. 2016). To take care of these effectors, plants evolved the ETI line of innate immunity which involves the recognition and handling of pathogen effectors using intracellular family of protein receptors called nucleotide-binding leucine-rich repeat (NB-LRR) receptors (Gu et al. 2017; Park et al. 2018). This is a very strong response that can trigger programed cell death (PCD) of the infected cells. Plant innate immunity, whether PTI or ETI, generally depends on vesicle/membrane trafficking.
In plants, endomembrane system principally consists of the endoplasmic reticulum (ER), Golgi complex, trans-Golgi network (TGN), endosomes and vacuoles. Effective communication between these organelles and with the plasma membrane (PM) is very necessary for the continuity of cellular activities in both healthy and diseased conditions, and this is achieved through vesicle trafficking. This trafficking occurs for biosynthetic, recycling, degradative and/or defense purposes. It is central in maintaining physiologic homeostasis, signaling, growth and development of eukaryotic cells. It is also very important for the continuity of secretory and endocytic pathways. In plant cells, there are different vesicles trafficking routes including ER-TGN, ER-vacuole, TGN-endosomes, endosomes-vacuoles, TGN-PM, and many more. When a plant cell recognizes the presence of a microbial species (pathogenic or beneficial) around it, it reprograms its molecule homeostasis and endomembrane system in preparation for that encounter. This brings about changes in molecular architecture of the cell, including changes in lipid composition and the symmetry of the plasma membrane. Intracellular vesicle trafficking is also reshaped for appropriate responses. In fact, the entire process of host cell reprogramming heavily depends on vesicle trafficking. A number of previous studies demonstrated the significance of vesicle trafficking in plants during plant–microbe interaction. However, the link between vesicle trafficking in plants and that in phytopathogens during plant-pathogen interaction is not clearly documented. In the present review, we bring together some important findings to demonstrate how phytopathogens make use of their endogenous transport vesicles to promote virulence and manipulate host immunity and how the host plants use same to counteract, disarm and incapacitate the invading pathogens.
Diversity of transport vesicles
Transport vesicles are membrane-bound, small sphere-like intracellular structures containing some selected cargo molecules to be delivered to a particular destination within or outside a eukaryotic cell for a given purpose. Such vesicles could be as small as 40 nm and as large as 10 µm in diameter (Vagner et al. 2019). Transport vesicles can be divided into intracellular and extracellular vesicles (EVs). Usually, intracellular vesicles are generated by endocytosis (e.g. endosomes) and the target destination is within the cell. However, internally generated vesicles that are delivered within the cell (such as ER-Golgi vesicles) are also considered as intracellular vesicles. Extracellular vesicles are generated from within the cell and delivered outside the cell. They are further divided into two based on their sites of biogenesis: exosomes and ectosomes (or microvesicles). The exosomes are smaller with a maximum diameter of 100 nm and are generated from the endosomal system and internalized into the lumen of late endosomes as intraluminal vesicles (ILVs), hence the late endosomes harboring the ILVs are called multivesicular bodies (MVBs). The MVBs are transported to the PM for subsequent release of the ILVs to the extracellular space by exocytosis (Abels and Breakefield 2016; U Stotz et al. 2022). However, MVBs may also deliver their contents to the vacuoles for degradation. What determine the specific pathway for MVB transportation still remain unclear. Ectosomes are generated at the PM following outward folding of the PM, enclosing the cargos, and subsequent budding off of the vesicles to the extracellular environment. They are more than 100 nm in diameter and emerge mainly from the PM for delivery to the extracellular environment (Fig. 2). The ER serves as the major source of vesicles for trafficking purposes. ER-Golgi vesicles are often coated with coatamer protein II (COPII) whereas the reverse transport (Golgi-ER) involves COPI-coated vesicles (Béthune and Wieland 2018; Robinson 2020). An important feature of COPII coated vesicles is that they are rich in lysophospholipids (Melero et al. 2018). Although the factors that determine cargo selectivity and correct targeting of vesicles still remain in the dark, TGN vesicles containing cargos that are destined for vacuolar degradation are clathrin-coated (Dahhan et al. 2022). Secreted proteins from the ER are packaged in certain vesicles at the TGN for subsequent shuttling to the PM. However, there are still some knowledge gaps on the TGN-PM trafficking route. Usually, there is balance between inward and outward vesicle secretion; in plants, however, outward secretion is most often associated with countering pathogen attack. It remains unproven how large extracellular vesicles traverse through the tight and rigid pores of fungal cell walls, though it was hypothesized that the lipid bilayers, being compressible, squeeze themselves through the pores (Casadevall et al. 2009).
Fig. 2.
Some notable vesicle trafficking pathways in plant cells. Cargos from external sources are internalized in vesicles and integrated in a multivesicular body (MVB) for sorting and subsequent channeling to their appropriate destinations. After their synthesis at the endoplasmic reticulum (ER), proteins are packaged in transport vesicles and delivered to the Golgi complex. Cargos in the Golgi can be delivered to the MVB for further transport processes. Cytoplasmic cargos can be packaged in plasma membrane-derived vesicles and released to the extracellular space as ectosomes
Vesicles biogenesis and trafficking events
The biogenesis of transport vesicles begins with donor membrane curvature, usually induced by BAR domain-containing proteins (Abubakar et al. 2017). Cargo recruitment and orchestration then begin in the curved membrane, and these are regulated by a number of proteins/protein complexes including phox homology domain containing proteins, SNX4/41/42, clathrin, retromer complex, and some SNARE proteins (Abubakar et al. 2017, 2021). However, the factors that determine cargo selection are still under investigation, although it is known that prior to ILV formation, the endosomal membrane becomes enriched with tetraspanins, especially CD9 and CD63 (U Stotz et al. 2022). This is accompanied by recruitment of endosomal sorting complexes required for transport (ESCRT) to the endosomal membrane for ILVs formation (Abels and Breakefield 2016). Generally, ESCRT has four different subcomplexes. ESCRT-0 is involved in cargo clustering, ESCRT-I and ESCRT-II function in bud formation while ESCRT-III is for vesicle formation/scission (Hanson et al. 2009; Oliveira et al. 2013). After exerting their functions, the various components of the ESCRT complexes dissociate and are recycled by some accessory proteins (Hanson et al. 2009). Following ILVs formation, MVBs are subsequently transported to the PM for fusion and release of the ILVs. There are many subcellular factors that mediate these processes at different levels. For instance, Rab-GTPases mediate intracellular vesicle trafficking at different stages, including vesicle budding, mobility on cytoskeleton track as well as fusion with the PM (Spang 2004). SNARE proteins are also indispensable for the fusion of vesicles with the PM.
AP2 complex is important for generation of vesicles and cargo selection (Xu et al. 2019). The additional role of this protein complex in the pathogenicity and polarized growth of Fusarium graminearum, a biotrophic phytopathogenic fungus that causes Fusarium head blight (FBH) of wheat and some cereals, further suggests a link between vesicle trafficking and virulence/development of phytopathogens (Zhang et al. 2019). Vesicle budding is mediated by ARF/AR1 (Wang et al. 2016). During plant-pathogen interaction, almost all vesicle transports involve RAB GTPases, including transport to infection sites and in effector binding (Spang 2004; Tripathy et al. 2021). The small GTPase Arf6 is a well-known regulator of vesicle trafficking during endocytosis (Zhu et al. 2016). BIGs serve as guanine-nucleotide exchange factors for ARF proteins, and vesicle trafficking requires the functions of BIG3 and BIG5 for the physiologic functions and development of Arabidopsis (Suo et al. 2021; Xue et al. 2019).
Nearly all intracellular vesicle transports involve motor proteins along the cytoskeletal tracks. There are many transport machineries that are involved in vesicle transport to different destinations but the factors that determine the choice of these machineries for a given vesicle are not very clear. Docking and tethering at the target membrane are regulated by RAB GTPases and tethering factors while SNARE proteins ensure the fusion of the vesicles with the target membrane (Wang et al. 2016;). In Verticillium dahliae (a vascular wilt pathogen), the SNARE proteins VdSso1 and VdSec22 regulate vesicle-mediated cargo delivery at the target membrane (J., Wang et al. 2018a, b). In F. graminearum, SNARE proteins regulate not only vesicle fusion but also the fungal developmental/virulence processes like deoxynivalenol (DON, a mycotoxin) production (Li et al. 2019). In addition to SNARE proteins, RAB GTPases were also shown to be involved in vesicle fusion. MoRab5A and MoRab5B regulate general endocytosis and the fusion of vesicles with the vacuole in Magnaporthe oryzae (Yang et al. 2017). FgSnc1-mediated vesicle fusion with the PM is regulated by the Rab8 GTPase activating protein FgMsb3 (Zheng et al. 2021a, b). The recycling of FgSnc1 for effective vesicle fusion is ensured by Snx41-Snx4 dimer in F. graminearum (Zheng et al. 2018a, b). Due to their intimate association with membranes, flippases also serve as key regulators of vesicle fusion, and blockage of membrane trafficking due to deletion of these enzymes also results in attenuated DON biosynthesis and consequently loss of virulence in F. graminearum (Oliveira et al. 2013; Yun et al. 2020). MoVps17, a subunit of the retromer complex, has also been shown to be indispensable for vesicle budding, fusion and endocytosis in M. oryzae (Wu et al. 2021; Zheng et al. 2017).
Vesicles trafficking and development/virulence of phytopathogenic fungi
Vesicles trafficking is critically important for the growth, development as well as virulence of phytopathogenic fungi (Shoji et al. 2014). Some phytopathogic fungi have been shown to use transport vesicles to deliver growth factors to the fungal apex, necessary for their polarized growths. For example, the polarized growth and virulence of F. graminearum were observed to be impaired after blocking vesicle secretion to the PM by deletion of FgSnc1, a t-SNARE protein that coordinates the fusion of secreted vesicles with the PM (Qiu et al. 2019; Zheng et al. 2018a, b). Interfering with intracellular vesicle transport also negatively affects conidiation, conidial morphology, asexual reproduction and production of DON mycotoxin in F. graminearum (Adnan et al. 2020; Yang et al. 2020; Zheng et al. 2021a, b). Autophagy is an important physiologic process that regulates the intracellular homeostasis of many cytoplasmic components. Operation of autophagy requires the integrated functions of autophagic genes like ATG9. The general autophagy and pathogenicity of F. graminearum is largely dependent on effective vesicle-mediated trafficking of FgAtg9 to phagophore assembly site (Zheng et al. 2018a, b). Ede1p induce site initiation for endocytosis of vesicles in yeast, and this protein is important not only for autophagy but also virulence of F. graminearum (Han et al. 2020). The sorting and transport of cargo molecules depends largely on phox homology (PX) domain-containing proteins. Through their vesicles trafficking functions, these proteins control the growth, reactive oxygen species (ROS) production and virulence of F. graminearum (Lou et al. 2021). There are growing number of evidence demonstrating the requisite of autophagy and vesicle trafficking to F. graminearum growth and pathogenicity (Li et al. 2017; Wu et al. 2023; Yuan et al. 2022).
In the rice blast fungus M. oryzae, conidiation and polarized growth have been shown to be directly linked to endocytosis via ADP-ribosylation factor 6 (Arf6)-mediated vesicle trafficking (Zhu et al. 2016). Also, the Arf-GAP (GTPase activating protein) MoGlo3 regulates COPI-mediated trafficking, endocytosis, stress tolerance, pathogenicity and development of the blast fungus (Zhang et al. 2017). A recent study established that the absence of MoSwa2, a protein that plays a key role in regulating the secretory pathway by uncoating of COPII in M. oryzae, perturbs M. oryzae virulence, suggesting a link between vesicle trafficking and pathogenesis of the hemibiotrophic fungus (Liu et al. 2021). Moreover, the ER-derived vesicle (ERV) proteins MoErv14 and MoErv29 were shown to be indispensable for vesicle-mediated secretion of many proteins, and are also critical for the development and virulence of M. oryzae (Qian et al. 2022, 2023). Generally, the active growth sites of fungal pathogens receive plethora of vesicles containing different cargos for different functions. The pathogenesis of phytopathogens depends on vesicle-mediated extracellular delivery of virulence factors, which is regulated by a number of vesicle trafficking regulators such as the Qc-SNARE protein MoSyn8 and protein phosphatase 1/Vps45 (Bryant and James 2003; Qi et al. 2016). Fungal pathogens also internalize EVs coming either from their hosts or from other fungi and the cargos of such vesicles could modulate their growths and virulence. Depending on their contents, EVs can serve protective and/or pathogenic functions. However, the mechanism of EV cargo selection for a particular function remains unknown. Pathogenic fungi and oomycetes also secrete small RNA-containing vesicles to infection sites for delivery into the host to shut down the host immune responses via RNA interference (U Stotz et al. 2022).
Emerging evidences suggest that pathogenic fungal EVs are determinants of their virulence as a number of bioactive molecules that relate to the fungal virulence and metabolism have been identified as cargos in such EVs (Bleackley et al. 2019b; Herkert et al. 2019). However, very little is known so far about the link between EVs released by phytopathogenic fungi and the fungal virulence. Be that as it may, some phytopathogenic fungi have been shown to release virulence responsive EVs (Bleackley et al. 2019a). Phytopathogenic fungi generally release ILVs containing many different cargos during interactions with their host plants. M. oryzae and Phytophthora infestans were independently demonstrated to deliver effector molecules to their hosts using unconventional secretion pathway (Liu et al. 2021; S., Wang et al. 2018a, b). This pathway largely depends on vesicle transport from biotrophic interfacial complex (BIC)/haustorium formed by the two fungi, respectively, to their hosts.
EVs may contain not only protein cargos but also some other molecules like carbohydrates, lipids and nucleic acids such as DNA, RNA or even non-coding RNA, and these cargos are protected from proteases and nucleases by the vesicle membrane after release into the extracellular space Bleackley et al. 2019a). Although not proven in plants/plan fungal pathogens, animal exosomes could contain mRNAs that are translated into proteins after delivery to a target cell/organism (Colombo et al. 2014). During plant-fungal pathogen interaction, both the host plants and the fungal pathogen exchange vesicles containing cargos that modulate each other’s gene expression, suppress immunity, inhibit important pathways (e.g. vesicle trafficking pathway) or exert antimicrobial activity, as the case may be (Wang and Dean 2020). Phytopathogenic fungi package and release virulence factors in EVs for delivery to infection sites to manipulate their hosts and establish successful infection. Effector substances from phytopathogenic fungi and oomycetes are released through their haustoria during interaction with the hosts. The oomycete Phytophthora infestans uses the haustoria to release the effector protein AVRblb2 into its hosts to block the trafficking of the host C14 papain-like cysteine protease to the apoplast (Wang et al. 2017). Some effector molecules block the host vesicle trafficking pathways in favor of the pathogens (Fig. 3) and most effector proteins from phytopathogens directly or indirectly target and suppress the host plant’s PTI (Hetmann and Kowalczyk 2019). Vesicle-mediated recycling of PEN1 between the PM and the cytoplasm of plants is indispensable for their basal immunity. The fungal pathogen Golovinomyces orontii targets and blocks the PEN1 recycling pathway for effective proliferation in Arabidopsis (Kim et al. 2016). Effectors from some phytopathogenic fungi are restricted by certain specialized plant-derived membranes other than the EHM, such as extra-invasive hyphal membrane (EIHM) due to infection by fungi belonging to the order Colletotrichum (Shimada et al. 2019). The sunflower pathogen Sclerotinia sclerotiorum was shown to take up EVs from the host plant, and this negatively affects the fungal invasive growth in the host (Regente et al. 2017).
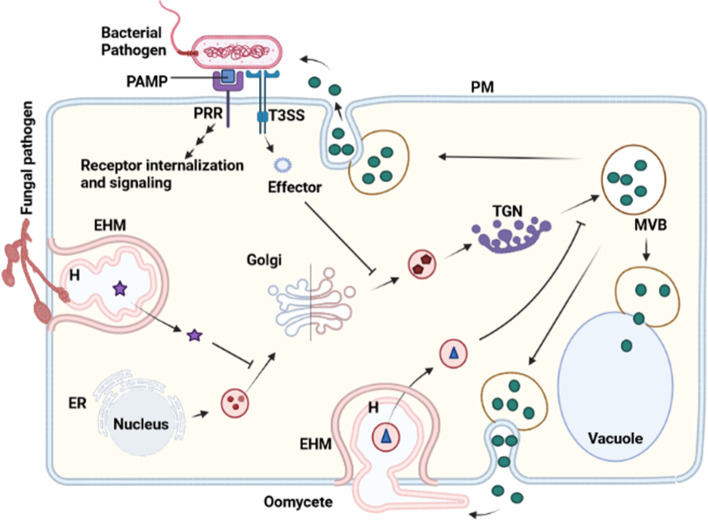
Fig. 3.
Vesicle trafficking-dependent plant immune responses and their suppression by phytopathogens during interaction. Upon identification of bacterial PAMPs, PRRs are internalized to trigger downstream signaling which leads to immune responses by the host. The host counterattacks include the synthesis, packaging and release of antimicrobials to the infection site. The pathogen releases effector substances which block some vesicle trafficking pathways to render the host susceptible and prevent counter-responses. Oomycetes and fungal pathogens also release virulence factors through the haustoria for same purposes. PRR: Pattern recognition receptor, PAMP: Pathogen associated molecular pattern, H: haustoria, EHM: Extra haustorial membrane, ER: Endoplasmic reticulum, TGN: Trans-Golgi network, MVB: Multivesicular body, T3SS: Type 3 secretion system, PM: Plasma membrane
Roles of vesicles trafficking in plant development and immunity against fungal pathogens
The vesicle trafficking pathways in plants play many important roles including, but not limited to, prevention of pathogen entry, cell–cell communication, growth and development, endomembrane system reprogramming, delivery of important biomolecules to targeted sites (Suharta et al. 2021). The ER play a key role in ensuring a continuous vesicle trafficking process, especially that of the secretory pathway. Most important membrane proteins, such as the PRRs, are synthesized in the ER, packaged in vesicles at the Golgi and transported to the PM where they exert their biological functions, suggesting a link between vesicle trafficking and pathogen detection (Ruano and Scheuring 2020; Underwood 2016). Following endocytosis, these PM-localized proteins are internalized in endocytic vesicles and delivered to the endosomes where cargos are sorted and channeled to other destinations. Internalization of PRRs due to pathogen attack triggers signal transduction that leads to immune responses (Gu et al. 2017). The internalized PM proteins are sorted and returned back to the PM through MVBs. After activation and subsequent internalization of FLS2, a well-studied plant PRR that detects flg22 (a notable bacterial PAMP) and promotes plant immunity against bacterial pathogens, the protein is recycled back to the PM via MVB-mediated trafficking. The sorting and trafficking of these vesicles are mediated by different motor proteins such as the retromer complex, dynamins and kinesins (Abubakar et al. 2017; Heucken and Ivanov 2018; Paez Valencia et al. 2016). However, how these vesicles selected by the different molecular motors in different trafficking pathways are still unclear. Also, how transport vesicles are targeted to a particular PM domain during secretion awaits future research.
Plant cells also release EVs into the extracellular space for specific purposes (Bhandari and Brandizzi 2020). This ensures timely and effective responses to invading pathogens. Almost all steps of plant immune responses to pathogen attack involve targeted vesicle transport, including pathogen recognition, secretion of antimicrobials, ROS generation, and so on (Gu et al. 2017; Ruano and Scheuring 2020). Plants synthesize pathogenesis-related proteins, lipids, nucleic acids and other metabolites, which are packaged in transport vesicles and delivered to pathogen infection sites for antimicrobial activity (Gu et al. 2017; Tsatsaronis et al. 2018). Arabidopsis release EVs containing sRNA at Botrytis cinerea infection sites, which, when taken up, target and silence the fungal pathogenesis-related genes (Cai et al. 2018). Generally, under pathogen attack, plants release EVs into the extracellular space as part of their immune responses (U Stotz et al. 2022). In fact, plant EVs are known to contain bioactive compounds against pathogenic fungi (Boevink 2017; Regente et al. 2017). Sunflower releases cell wall degrading enzymes against B. cinerea (U Stotz et al. 2022). Arabidopsis delivers biotic and abiotic tress-response proteins in EVs to extracellular environment (Rutter and Innes 2017). PEN1/SYP121 and PEN3/PDR8 were observed to accumulate at infection sites following attempted penetration by powdery mildew fungi (Pakuła et al. 2023; Wu et al. 2020). Tetraspanin-rich vesicles were also shown to concentrate at infection sites; tetraspanins are important for membrane trafficking and signaling in plants (Cai et al. 2018; Reimann et al. 2017).
Oomycetes and fungal phytopathogens form haustoria through which they release effector substances to subvert the plant immune responses whereas bacterial pathogens use their secretion systems to release the virulence factors (Fig. 3). To restrict pathogen influence, plants surrounds the hautorial membrane with EHM the formation of which requires transport vesicles delivery (to the haustorial neck) rather than PM invagination (Berkey et al. 2017; U Stotz et al. 2022). Arabidopsis ARA6 (RAB5 homologue) product was also shown to localize to the EHM; some host proteins are however selectively excluded on this defense barrier ( Inada et al. 2016). Vesicle-Associated Membrane Proteins (VAMPs) are very crucial in plants’ counter responses to fungal pathogens. The post-invasive resistance protein RPW8.2 is delivered to the EHM from TGN in VAMP722-positive vesicles and the EHM localization of this protein enhances Arabidopsis resistance to powdery mildew pathogen (Park et al. 2018). Furthermore, VAMP721-dependent delivery of cell wall modification enzymes in Arabidopsis is critical in preventing pathogen penetration at the EHM (Uemura et al. 2019). Similarly, in rice, VAMP714-positive membranes were shown to localize to M. oryzae infection sites, suggesting a role in blast resistance (Sugano et al. 2016).
Conclusion and prospects
Plants interact with different microorganisms and the interactions can be mutualistic or pathogenic. This review focuses on the exchange of cargo-containing vesicles between plants and phytopathogenic fungi. Findings from previous studies clearly demonstrated that both plants and their invading fungal pathogens utilize vesicle trafficking for different purposes. Both organisms need vesicle trafficking for their normal physiologic activities. Additionally, the pathogenesis of fungal pathogens largely depends on the delivery of pathogenesis-related molecules packed in membrane-bound vesicles. Most of these pathogens lose their pathogenicity when their vesicle trafficking pathways are perturbed. In other words, the pathogens package virulence factors in transport vesicles and release them to the hosts for successful colonization. Similarly, hosts also use their vesicle trafficking pathways to defend themselves against the pathogen attacks. They do not only neutralize the virulence substances from the pathogens but also block the pathogens’ vesicle trafficking pathways. However, a number of knowledge gaps in our understanding of the use of transport vesicles in plant-fungal pathogen interactions still exists. What are the factors that determine cargo selection for a kind of vesicle? What determines the specific pathway for MVB transportation? Can EVs be released for plants/pathogenic fungi detection or are they only released post detection? These and many related questions await future investigations. Understanding these will provide new horizon in plant-fungus warfare and plant disease management.
Acknowledgements
We thank all the funding bodies as well as the authors whose published works have been used for the review, their works are well acknowledged by means of citations in the manuscript.
Authors’ contributions
ZW and WZ identified the review area, acquired funding and critically revised the manuscript; YSA conducted the review and wrote the first draft of the manuscript; IZS designed the figures and revised the manuscript; AA revised and reorganized the contents.
Funding
This work was supported by the National Natural Science Foundation of China (32122071, 32272481, 31772106), and the Natural Science Foundation of Fujian Province (2021J06015).
Availability of data and materials
All the data supporting the claims contained in this manuscript are provided in the submission and can be shared publicly after acceptance of the manuscript for publication by Stress Biology.
Declarations
Ethics approval and consent to participate
Not Applicable.
Consent for publication
All the authors have given their consent for publication of this manuscript by Stress Biology, if accepted.
Competing interests
Zonghua Wang is a member of the Editorial Board, but was not involved in the journal's review of, or any decisions related to, this manuscript.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Zonghua Wang, Email: wangzh@fafu.edu.cn.
Wenhui Zheng, Email: wenhuiz@fafu.edu.cn.
References
- Abels ER, Breakefield XO. Introduction to Extracellular Vesicles: Biogenesis, RNA Cargo Selection, Content, Release, and Uptake. Cell Mol Neurobiol. 2016;36:301–312. doi: 10.1007/s10571-016-0366-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abubakar YS, Qiu H, Fang W, Zheng H, Lu G, Zhou J, et al. FgRab5 and FgRab7 are essential for endosomes biogenesis and non-redundantly recruit the retromer complex to the endosomes in Fusarium graminearum. Stress Biology. 2021;1:17. doi: 10.1007/s44154-021-00020-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abubakar YS, Zheng W, Olsson S, Zhou J. Updated Insight into the Physiological and Pathological Roles of the Retromer Complex. Int J Mol Sci. 2017;18:E1601. doi: 10.3390/ijms18081601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adnan M, Fang W, Sun P, Zheng Y, Abubakar YS, Zhang J, et al. R-SNARE FgSec22 is essential for growth, pathogenicity and DON production of Fusarium graminearum. Curr Genet. 2020;66:421–435. doi: 10.1007/s00294-019-01037-y. [DOI] [PubMed] [Google Scholar]
- Berkey R, Zhang Y, Ma X, King H, Zhang Q, Wang W, et al. Homologues of the RPW8 Resistance Protein Are Localized to the Extrahaustorial Membrane that Is Likely Synthesized De Novo. Plant Physiol. 2017;173:600–613. doi: 10.1104/pp.16.01539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Béthune J, Wieland FT. Assembly of COPI and COPII Vesicular Coat Proteins on Membranes. Annu Rev Biophys. 2018;47:63–83. doi: 10.1146/annurev-biophys-070317-033259. [DOI] [PubMed] [Google Scholar]
- Bhandari DD, Brandizzi F. Plant endomembranes and cytoskeleton: moving targets in immunity. Curr Opin Plant Biol. 2020;58:8–16. doi: 10.1016/j.pbi.2020.09.003. [DOI] [PubMed] [Google Scholar]
- Bleackley, M.R., Dawson, C.S. & Anderson, M.A. (2019a) Fungal Extracellular Vesicles with a Focus on Proteomic Analysis. Proteomics, 19, e1800232. 10.1002/pmic.201800232. [DOI] [PubMed]
- Bleackley, M.R., Samuel, M., Garcia-Ceron, D., McKenna, J.A., Lowe, R.G.T., Pathan, M., et al. (2019b) Extracellular Vesicles From the Cotton Pathogen Fusarium oxysporum f. sp. vasinfectum Induce a Phytotoxic Response in Plants. Frontiers in Plant Science, 10, 1610. 10.3389/fpls.2019.01610. [DOI] [PMC free article] [PubMed]
- Boevink PC. Exchanging missives and missiles: the roles of extracellular vesicles in plant-pathogen interactions. J Exp Bot. 2017;68:5411–5414. doi: 10.1093/jxb/erx369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryant NJ, James DE. The Sec1p/Munc18 (SM) protein, Vps45p, cycles on and off membranes during vesicle transport. J Cell Biol. 2003;161:691–696. doi: 10.1083/jcb.200212078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai, Q., Qiao, L., Wang, M., He, B., Lin, F.-M., Palmquist, J., et al. (2018) Plants send small RNAs in extracellular vesicles to fungal pathogen to silence virulence genes. Science (New York, N.Y.), 360, 1126–1129. 10.1126/science.aar4142. [DOI] [PMC free article] [PubMed]
- Casadevall A, Nosanchuk JD, Williamson P, Rodrigues ML. Vesicular transport across the fungal cell wall. Trends Microbiol. 2009;17:158–162. doi: 10.1016/j.tim.2008.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang M, Chen H, Liu F, Fu ZQ. PTI and ETI: convergent pathways with diverse elicitors. Trends Plant Sci. 2022;27:113–115. doi: 10.1016/j.tplants.2021.11.013. [DOI] [PubMed] [Google Scholar]
- Colombo M, Raposo G, Théry C. Biogenesis, secretion, and intercellular interactions of exosomes and other extracellular vesicles. Annu Rev Cell Dev Biol. 2014;30:255–289. doi: 10.1146/annurev-cellbio-101512-122326. [DOI] [PubMed] [Google Scholar]
- Couto D, Zipfel C. Regulation of pattern recognition receptor signalling in plants. Nat Rev Immunol. 2016;16:537–552. doi: 10.1038/nri.2016.77. [DOI] [PubMed] [Google Scholar]
- Dahhan DA, Reynolds GD, Cárdenas JJ, Eeckhout D, Johnson A, Yperman K, et al. Proteomic characterization of isolated Arabidopsis clathrin-coated vesicles reveals evolutionarily conserved and plant-specific components. Plant Cell. 2022;34:2150–2173. doi: 10.1093/plcell/koac071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu Y, Zavaliev R, Dong X. Membrane Trafficking in Plant Immunity. Mol Plant. 2017;10:1026–1034. doi: 10.1016/j.molp.2017.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han, X., Chen, L., Li, W., Zhang, Li, Zhang, Liyuan, Zou, S., et al. (2020) Endocytic FgEde1 regulates virulence and autophagy in Fusarium graminearum. Fungal genetics and biology: FG & B, 141, 103400. 10.1016/j.fgb.2020.103400. [DOI] [PubMed]
- Hanson PI, Shim S, Merrill SA. Cell biology of the ESCRT machinery. Curr Opin Cell Biol. 2009;21:568–574. doi: 10.1016/j.ceb.2009.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herkert PF, Amatuzzi RF, Alves LR, Rodrigues ML. Extracellular Vesicles as Vehicles for the Delivery of Biologically Active Fungal Molecules. Curr Protein Pept Sci. 2019;20:1027–1036. doi: 10.2174/1389203720666190529124055. [DOI] [PubMed] [Google Scholar]
- Hetmann, A. & Kowalczyk, S. (2019) [Suppression of PAMP-triggered immunity (PTI) by effector proteins synthesized by phytopathogens and delivered into cells of infected plant]. Postepy Biochemii, 65, 58–71. 10.18388/pb.2019_257. [DOI] [PubMed]
- Heucken, N. & Ivanov, R. (2018) The retromer, sorting nexins and the plant endomembrane protein trafficking. Journal of Cell Science, 131, jcs203695. 10.1242/jcs.203695. [DOI] [PubMed]
- Inada N, Betsuyaku S, Shimada TL, Ebine K, Ito E, Kutsuna N, et al. Modulation of Plant RAB GTPase-Mediated Membrane Trafficking Pathway at the Interface Between Plants and Obligate Biotrophic Pathogens. Plant Cell Physiol. 2016;57:1854–1864. doi: 10.1093/pcp/pcw107. [DOI] [PubMed] [Google Scholar]
- Jordá T, Puig S. Regulation of Ergosterol Biosynthesis in Saccharomyces cerevisiae. Genes. 2020;11:795. doi: 10.3390/genes11070795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanazawa T, Ueda T. Exocytic trafficking pathways in plants: why and how they are redirected. New Phytol. 2017;215:952–957. doi: 10.1111/nph.14613. [DOI] [PubMed] [Google Scholar]
- Kim H, Kwon H, Kim S, Kim MK, Botella MA, Yun HS, et al. Synaptotagmin 1 Negatively Controls the Two Distinct Immune Secretory Pathways to Powdery Mildew Fungi in Arabidopsis. Plant Cell Physiol. 2016;57:1133–1141. doi: 10.1093/pcp/pcw061. [DOI] [PubMed] [Google Scholar]
- Li, B., Dong, X., Zhao, R., Kou, R., Zheng, X. & Zhang, H. (2019) The t-SNARE protein FgPep12, associated with FgVam7, is essential for ascospore discharge and plant infection by trafficking Ca2+ ATPase FgNeo1 between Golgi and endosome/vacuole in Fusarium graminearum. PLoS pathogens, 15, e1007754. 10.1371/journal.ppat.1007754. [DOI] [PMC free article] [PubMed]
- Li B, Liu L, Li Y, Dong X, Zhang H, Chen H, et al. The FgVps39-FgVam7-FgSso1 Complex Mediates Vesicle Trafficking and Is Important for the Development and Virulence of Fusarium graminearum. Molecular Plant-Microbe Interactions: MPMI. 2017;30:410–422. doi: 10.1094/MPMI-11-16-0242-R. [DOI] [PubMed] [Google Scholar]
- Liu M, Hu J, Zhang A, Dai Y, Chen W, He Y, et al. Auxilin-like protein MoSwa2 promotes effector secretion and virulence as a clathrin uncoating factor in the rice blast fungus Magnaporthe oryzae. New Phytol. 2021;230:720–736. doi: 10.1111/nph.17181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lou, Y., Zhang, J., Wang, G., Fang, W., Wang, S., Abubakar, Y.S., et al. (2021) Genome-Wide Characterization of PX Domain-Containing Proteins Involved in Membrane Trafficking-Dependent Growth and Pathogenicity of Fusarium graminearum. mBio, 12, e0232421. 10.1128/mBio.02324-21. [DOI] [PMC free article] [PubMed]
- Melero A, Chiaruttini N, Karashima T, Riezman I, Funato K, Barlowe C, et al. Lysophospholipids Facilitate COPII Vesicle Formation. Current Biology: CB. 2018;28:1950–1958.e6. doi: 10.1016/j.cub.2018.04.076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliveira DL, Rizzo J, Joffe LS, Godinho RMC, Rodrigues ML. Where do they come from and where do they go: candidates for regulating extracellular vesicle formation in fungi. Int J Mol Sci. 2013;14:9581–9603. doi: 10.3390/ijms14059581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paez Valencia J, Goodman K, Otegui MS. Endocytosis and Endosomal Trafficking in Plants. Annu Rev Plant Biol. 2016;67:309–335. doi: 10.1146/annurev-arplant-043015-112242. [DOI] [PubMed] [Google Scholar]
- Pakuła K, Sequeiros-Borja C, Biała-Leonhard W, Pawela A, Banasiak J, Bailly A, et al. Restriction of access to the central cavity is a major contributor to substrate selectivity in plant ABCG transporters. Cellular and Molecular Life Sciences: CMLS. 2023;80:105. doi: 10.1007/s00018-023-04751-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park E, Nedo A, Caplan JL, Dinesh-Kumar SP. Plant-microbe interactions: organelles and the cytoskeleton in action. New Phytol. 2018;217:1012–1028. doi: 10.1111/nph.14959. [DOI] [PubMed] [Google Scholar]
- Qi Z, Liu M, Dong Y, Zhu Q, Li L, Li B, et al. The syntaxin protein (MoSyn8) mediates intracellular trafficking to regulate conidiogenesis and pathogenicity of rice blast fungus. New Phytol. 2016;209:1655–1667. doi: 10.1111/nph.13710. [DOI] [PubMed] [Google Scholar]
- Qian B, Su X, Ye Z, Liu X, Liu M, Shen D, et al. MoErv29 promotes apoplastic effector secretion contributing to virulence of the rice blast fungus Magnaporthe oryzae. New Phytol. 2022;233:1289–1302. doi: 10.1111/nph.17851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qian, B., Su, X., Ye, Z., Liu, X., Liu, M., Zhang, H., et al. (2023) MoErv14 mediates the intracellular transport of cell membrane receptors to govern the appressorial formation and pathogenicity of Magnaporthe oryzae. PLoS pathogens, 19, e1011251. 10.1371/journal.ppat.1011251. [DOI] [PMC free article] [PubMed]
- Qiu H, Zhao X, Fang W, Wu H, Abubakar YS, Lu G, et al. Spatiotemporal nature of Fusarium graminearum-wheat coleoptile interactions. Phytopathology Research. 2019;1:26. doi: 10.1186/s42483-019-0033-7. [DOI] [Google Scholar]
- Regente M, Pinedo M, San Clemente H, Balliau T, Jamet E, Canal L. Plant extracellular vesicles are incorporated by a fungal pathogen and inhibit its growth. J Exp Bot. 2017;68:5485–5495. doi: 10.1093/jxb/erx355. [DOI] [PubMed] [Google Scholar]
- Reimann R, Kost B, Dettmer J. TETRASPANINs in Plants. Front Plant Sci. 2017;8:545. doi: 10.3389/fpls.2017.00545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson DG. Plant Golgi ultrastructure. J Microsc. 2020;280:111–121. doi: 10.1111/jmi.12899. [DOI] [PubMed] [Google Scholar]
- Ruano G, Scheuring D. Plant Cells under Attack: Unconventional Endomembrane Trafficking during Plant Defense. Plants (basel, Switzerland) 2020;9:E389. doi: 10.3390/plants9030389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutter BD, Innes RW. Extracellular Vesicles Isolated from the Leaf Apoplast Carry Stress-Response Proteins. Plant Physiol. 2017;173:728–741. doi: 10.1104/pp.16.01253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimada TL, Betsuyaku S, Inada N, Ebine K, Fujimoto M, Uemura T, et al. Enrichment of Phosphatidylinositol 4,5-Bisphosphate in the Extra-Invasive Hyphal Membrane Promotes Colletotrichum Infection of Arabidopsis thaliana. Plant Cell Physiol. 2019;60:1514–1524. doi: 10.1093/pcp/pcz058. [DOI] [PubMed] [Google Scholar]
- Shoji J, Kikuma T, Kitamoto K. Vesicle trafficking, organelle functions, and unconventional secretion in fungal physiology and pathogenicity. Curr Opin Microbiol. 2014;20:1–9. doi: 10.1016/j.mib.2014.03.002. [DOI] [PubMed] [Google Scholar]
- Spang A. Vesicle transport: a close collaboration of Rabs and effectors. Current Biology: CB. 2004;14:R33–34. doi: 10.1016/j.cub.2003.12.021. [DOI] [PubMed] [Google Scholar]
- Sugano S, Hayashi N, Kawagoe Y, Mochizuki S, Inoue H, Mori M, et al. Rice OsVAMP714, a membrane-trafficking protein localized to the chloroplast and vacuolar membrane, is involved in resistance to rice blast disease. Plant Mol Biol. 2016;91:81–95. doi: 10.1007/s11103-016-0444-0. [DOI] [PubMed] [Google Scholar]
- Suharta S, Barlian A, Hidajah AC, Notobroto HB, Ana ID, Indariani S, et al. Plant-derived exosome-like nanoparticles: A concise review on its extraction methods, content, bioactivities, and potential as functional food ingredient. J Food Sci. 2021;86:2838–2850. doi: 10.1111/1750-3841.15787. [DOI] [PubMed] [Google Scholar]
- Suo Y, Hu F, Zhu H, Li D, Qi R, Huang J, et al. BIG3 and BIG5 Redundantly Mediate Vesicle Trafficking in Arabidopsis. Biomolecules. 2021;11:732. doi: 10.3390/biom11050732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toruño TY, Stergiopoulos I, Coaker G. Plant-Pathogen Effectors: Cellular Probes Interfering with Plant Defenses in Spatial and Temporal Manners. Annu Rev Phytopathol. 2016;54:419–441. doi: 10.1146/annurev-phyto-080615-100204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tripathy MK, Deswal R, Sopory SK. Plant RABs: Role in Development and in Abiotic and Biotic Stress Responses. Curr Genomics. 2021;22:26–40. doi: 10.2174/1389202922666210114102743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsatsaronis JA, Franch-Arroyo S, Resch U, Charpentier E. Extracellular Vesicle RNA: A Universal Mediator of Microbial Communication? Trends Microbiol. 2018;26:401–410. doi: 10.1016/j.tim.2018.02.009. [DOI] [PubMed] [Google Scholar]
- U Stotz, H., Brotherton, D. & Inal, J. (2022) Communication is key: extracellular vesicles as mediators of infection and defence during host-microbe interactions in animals and plants. FEMS microbiology reviews, 46, fuab044. 10.1093/femsre/fuab044. [DOI] [PMC free article] [PubMed]
- Uemura T, Nakano RT, Takagi J, Wang Y, Kramer K, Finkemeier I, et al. A Golgi-Released Subpopulation of the Trans-Golgi Network Mediates Protein Secretion in Arabidopsis. Plant Physiol. 2019;179:519–532. doi: 10.1104/pp.18.01228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Underwood W. Contributions of host cellular trafficking and organization to the outcomes of plant-pathogen interactions. Semin Cell Dev Biol. 2016;56:163–173. doi: 10.1016/j.semcdb.2016.05.016. [DOI] [PubMed] [Google Scholar]
- Vagner, T., Chin, A., Mariscal, J., Bannykh, S., Engman, D.M. & Di Vizio, D. (2019) Protein Composition Reflects Extracellular Vesicle Heterogeneity. Proteomics, 19, e1800167. 10.1002/pmic.201800167. [DOI] [PMC free article] [PubMed]
- Wang J, Tian L, Zhang D-D, Short DPG, Zhou L, Song S-S, et al. SNARE-Encoding Genes VdSec22 and VdSso1 Mediate Protein Secretion Required for Full Virulence in Verticillium dahliae. Molecular Plant-Microbe Interactions: MPMI. 2018;31:651–664. doi: 10.1094/MPMI-12-17-0289-R. [DOI] [PubMed] [Google Scholar]
- Wang M, Dean RA. Movement of small RNAs in and between plants and fungi. Mol Plant Pathol. 2020;21:589–601. doi: 10.1111/mpp.12911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S, Boevink PC, Welsh L, Zhang R, Whisson SC, Birch PRJ. Delivery of cytoplasmic and apoplastic effectors from Phytophthora infestans haustoria by distinct secretion pathways. New Phytol. 2017;216:205–215. doi: 10.1111/nph.14696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, S., Welsh, L., Thorpe, P., Whisson, S.C., Boevink, P.C. & Birch, P.R.J. (2018b) The Phytophthora infestans Haustorium Is a Site for Secretion of Diverse Classes of Infection-Associated Proteins. mBio, 9, e01216–18. 10.1128/mBio.01216-18. [DOI] [PMC free article] [PubMed]
- Wang W-M, Liu P-Q, Xu Y-J, Xiao S. Protein trafficking during plant innate immunity. J Integr Plant Biol. 2016;58:284–298. doi: 10.1111/jipb.12426. [DOI] [PubMed] [Google Scholar]
- Wu C, Chen H, Yuan M, Zhang M, Abubakar YS, Chen X, et al. FgAP1σ Is Critical for Vegetative Growth, Conidiation, Virulence, and DON Biosynthesis in Fusarium graminearum. Journal of Fungi (basel, Switzerland) 2023;9:145. doi: 10.3390/jof9020145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu C, Lin Y, Zheng H, Abubakar YS, Peng M, Li J, et al. The retromer CSC subcomplex is recruited by MoYpt7 and sequentially sorted by MoVps17 for effective conidiation and pathogenicity of the rice blast fungus. Mol Plant Pathol. 2021;22:284–298. doi: 10.1111/mpp.13029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu H, Zhang W, Schuster M, Moch M, Windoffer R, Steinberg G, et al. Alloxan Disintegrates the Plant Cytoskeleton and Suppresses mlo-Mediated Powdery Mildew Resistance. Plant Cell Physiol. 2020;61:505–518. doi: 10.1093/pcp/pcz216. [DOI] [PubMed] [Google Scholar]
- Xu L, Wang M, Tang G, Ma Z, Shao W. The endocytic cargo adaptor complex is required for cell-wall integrity via interacting with the sensor FgWsc2B in Fusarium graminearum. Curr Genet. 2019;65:1071–1080. doi: 10.1007/s00294-019-00961-3. [DOI] [PubMed] [Google Scholar]
- Xue S, Zou J, Liu Y, Wang M, Zhang C, Le J. Involvement of BIG5 and BIG3 in BRI1 Trafficking Reveals Diverse Functions of BIG-subfamily ARF-GEFs in Plant Growth and Gravitropism. Int J Mol Sci. 2019;20:E2339. doi: 10.3390/ijms20092339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang C, Li J, Chen X, Zhang X, Liao D, Yun Y, et al. FgVps9, a Rab5 GEF, Is Critical for DON Biosynthesis and Pathogenicity in Fusarium graminearum. Front Microbiol. 2020;11:1714. doi: 10.3389/fmicb.2020.01714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang CD, Dang X, Zheng HW, Chen XF, Lin XL, Zhang DM, et al. Two Rab5 Homologs Are Essential for the Development and Pathogenicity of the Rice Blast Fungus Magnaporthe oryzae. Front Plant Sci. 2017;8:620. doi: 10.3389/fpls.2017.00620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan Y, Zhang M, Li J, Yang C, Abubakar YS, Chen X, et al. The Small GTPase FgRab1 Plays Indispensable Roles in the Vegetative Growth, Vesicle Fusion, Autophagy and Pathogenicity of Fusarium graminearum. Int J Mol Sci. 2022;23:895. doi: 10.3390/ijms23020895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yun Y, Guo P, Zhang J, You H, Guo P, Deng H, et al. Flippases play specific but distinct roles in the development, pathogenicity, and secondary metabolism of Fusarium graminearum. Mol Plant Pathol. 2020;21:1307–1321. doi: 10.1111/mpp.12985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang S, Liu X, Li L, Yu R, He J, Zhang H, et al. The ArfGAP protein MoGlo3 regulates the development and pathogenicity of Magnaporthe oryzae. Environ Microbiol. 2017;19:3982–3996. doi: 10.1111/1462-2920.13798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, J., Yun, Y., Lou, Y., Abubakar, Y.S., Guo, P., Wang, S., et al. (2019) FgAP-2 complex is essential for pathogenicity and polarised growth and regulates the apical localisation of membrane lipid flippases in Fusarium graminearum. Cellular Microbiology, 21, e13041. 10.1111/cmi.13041. [DOI] [PubMed]
- Zheng H, Guo Z, Xi Y, Yuan M, Lin Y, Wu C, et al. Sorting nexin (MoVps17) is required for fungal development and plant infection by regulating endosome dynamics in the rice blast fungus. Environ Microbiol. 2017;19:4301–4317. doi: 10.1111/1462-2920.13896. [DOI] [PubMed] [Google Scholar]
- Zheng H, Li L, Yu Z, Yuan Y, Zheng Q, Xie Q, et al. FgSpa2 recruits FgMsb3, a Rab8 GAP, to the polarisome to regulate polarized trafficking, growth and pathogenicity in Fusarium graminearum. New Phytol. 2021;229:1665–1683. doi: 10.1111/nph.16935. [DOI] [PubMed] [Google Scholar]
- Zheng, H., Miao, P., Lin, X., Li, L., Wu, C., Chen, X., et al. (2018a) Small GTPase Rab7-mediated FgAtg9 trafficking is essential for autophagy-dependent development and pathogenicity in Fusarium graminearum. PLoS genetics, 14, e1007546. 10.1371/journal.pgen.1007546. [DOI] [PMC free article] [PubMed]
- Zheng, Q., Yu, Z., Yuan, Y., Sun, D., Abubakar, Y.S., Zhou, J., et al. (2021b) The GTPase-Activating Protein FgGyp1 Is Important for Vegetative Growth, Conidiation, and Virulence and Negatively Regulates DON Biosynthesis in Fusarium graminearium. Frontiers in Microbiology, 12, 621519. 10.3389/fmicb.2021.621519. [DOI] [PMC free article] [PubMed]
- Zheng W, Lin Y, Fang W, Zhao X, Lou Y, Wang G, et al. The endosomal recycling of FgSnc1 by FgSnx41-FgSnx4 heterodimer is essential for polarized growth and pathogenicity in Fusarium graminearum. New Phytol. 2018;219:654–671. doi: 10.1111/nph.15178. [DOI] [PubMed] [Google Scholar]
- Zhu, X., Zhou, T., Chen, L., Zheng, S., Chen, S., Zhang, D., et al. (2016) Arf6 controls endocytosis and polarity during asexual development of Magnaporthe oryzae. FEMS microbiology letters, 363, fnw248. 10.1093/femsle/fnw248. [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All the data supporting the claims contained in this manuscript are provided in the submission and can be shared publicly after acceptance of the manuscript for publication by Stress Biology.