Graphical abstract

Keywords: Cardiac tumors, Hemangioma, Echocardiography, Right atrium, Pathology
Highlights
-
•
Most primary cardiac tumors are benign with a broad differential diagnosis.
-
•
Imaging helps characterize tumors, but pathology is required for final diagnosis.
-
•
Cardiac hemangiomas are rare and often misdiagnosed, requiring biopsy.
Introduction
Although rare, cardiac masses can be associated with a high degree of morbidity and mortality. Given the wide differential diagnosis, an accurate identification of a mass is therefore critical for optimal management. Noninvasive multimodality imaging is often sufficient to establish the diagnosis, prognosis, and management of a mass. This case, however, demonstrates the limitations of imaging alone to accurately distinguish between benign and malignant tumors and highlights the contribution histopathology provides to reach an accurate diagnosis.
Case Presentation
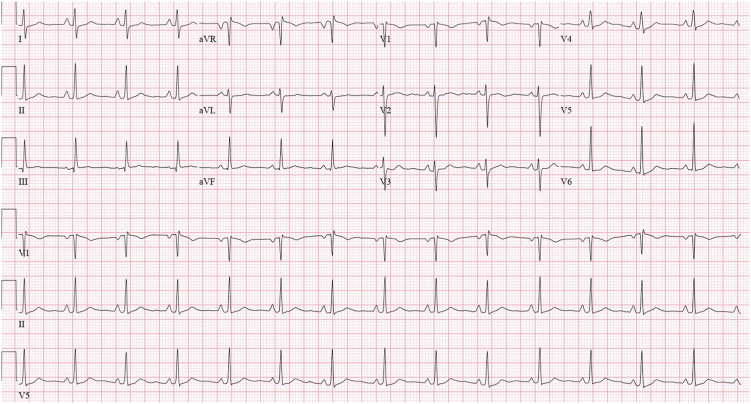
A 23-year-old woman (G2P0202) with no medical history presented to the emergency department 5 days after a normal spontaneous vaginal delivery reporting chest pain, headaches, and vision changes and was found to have markedly elevated blood pressure. Given concern for preeclampsia with severe features and/or pulmonary embolism, the patient was admitted to the hospital and started on a magnesium drip and intravenous medications for blood pressure control. Notable laboratory studies included elevated brain natriuretic peptide (1,168 pg/mL) and mildly elevated troponin I (0.07 ng/mL). Otherwise, the complete metabolic panel was normal, including normal aminotransferase levels. Electrocardiography showed normal sinus rhythm (Figure 1). Contrast-enhanced computed tomography (CT) of the chest reported to be performed as a pulmonary arterial embolism protocol excluded pulmonary embolism but revealed a large solid mass (6.4 × 6.5 × 6.6 cm) in the right atrium with reported central contrast enhancement versus calcification (Figure 2). Subsequent transesophageal echocardiography reportedly confirmed the presence of a large mass occupying the entire right atrium and impinging on the tricuspid valve, although these images were not available to us for review. Given these findings, the patient was transferred to a hospital with cardiac surgery for planned excision of the mass.
Figure 1.
The patient’s electrocardiogram on admission, showing normal sinus rhythm.
Figure 2.
Contrast-enhanced CT of the chest reported to be performed as a pulmonary arterial embolism protocol was obtained to rule out pulmonary embolism. It shows a large solid mass in the right atrium (arrows) in the (A) axial, (B) coronal, and (C) sagittal planes.
At the second hospital, the patient underwent a median sternotomy, mediastinal exploration, and biopsy of the mass, but surgical excision was aborted given inability to obtain clear margins. Pathology from intraoperative frozen section of the mass was suggestive of a “spindle-cell sarcoma.” The patient was then transferred to our institution for further management with consideration of repeat attempt at surgical excision versus heart transplantation evaluation.
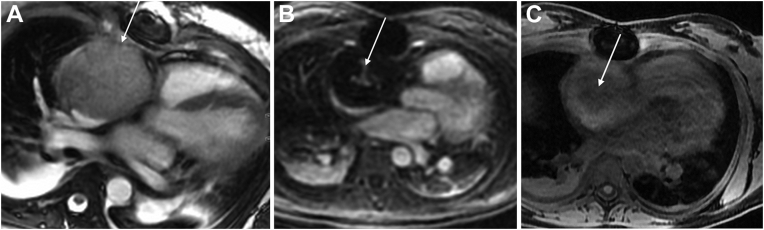
Upon arrival to our cardiac intensive care unit, the patient was extubated and weaned off antihypertensive medications. Her vital signs remained stable. Notably, physical examination demonstrated normal heart sounds without murmur or gallop, no peripheral edema, no pulmonary rales, no jugular venous distention, no engorged facial veins, and no facial or arm swelling. Transthoracic echocardiography (TTE) demonstrated a large echodense mass measuring 7.0 × 5.2 cm in the right atrium, normal biventricular systolic function, and no obstruction of the tricuspid valve (Figure 3; Videos 1 and 2). Cardiovascular magnetic resonance imaging (CMR) was notable for a large mass centered in the right atrioventricular groove, extending into the right atrium, along with an enlarged cardiophrenic lymph node (Figure 4; Video 3). The mass was noted to be heterogenous on T1-weighted imaging, predominantly hyperintense on T2-weighted imaging, and with avid central enhancement on both perfusion and early postcontrast T1-weighted imaging, highlighting the hypervascularity of the lesion. On T1-weighted noncontrast fat saturation imaging, the mass demonstrated an intermediate signal with no fat. Furthermore, the mass was noted to be abutting and inseparable from the inferior aspect of the superior vena cava, with associated compression. Taken together, these findings were concerning for an angiosarcoma. The patient was discharged pending final pathology review of the biopsy, with a plan for expedited outpatient workup, including outpatient CT and attenuation-corrected positron emission tomography (PET) for initial staging of the tumor, primarily to identify extracardiac metastases given concern for malignancy and follow-up with oncology.
Figure 3.
(A) Two-dimensional TTE, apical four-chamber view, systolic frame, demonstrating a large mass in the right atrium (arrows). (B) Continuous-wave Doppler shows a 2.0 mm Hg mean gradient across the tricuspid valve and normal right ventricular systolic pressure. LV, Left ventricle; RV, right ventricle.
Figure 4.
CMR images of the mass. (A) Four-chamber bright-blood image demonstrating a large mass (arrow) occupying the right atrium. (B) Perfusion image showing enhancement in the center of the mass due to vascularity (arrow). (C) On T1-weighted noncontrast fat saturation imaging, the mass (arrow) demonstrates an intermediate signal with no fat.
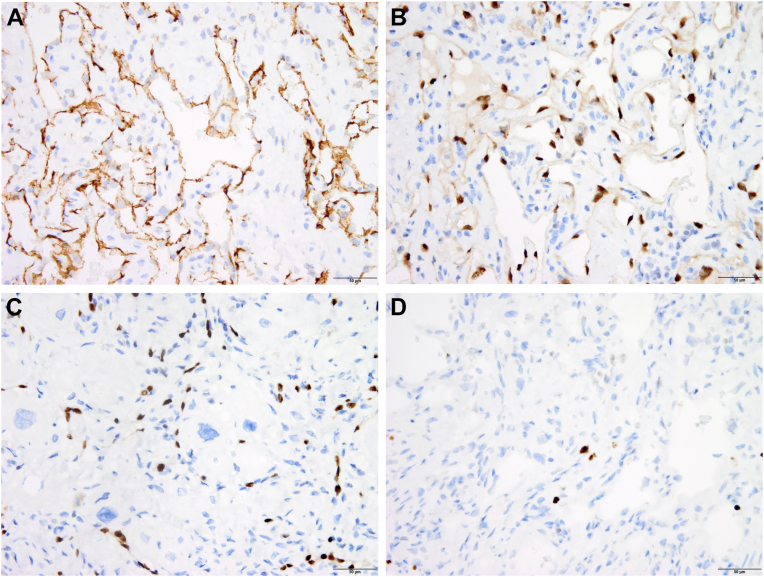
PET/CT showed only mild fluorodeoxyglucose uptake, predominantly at the periphery of the mass, with a maximum standardized uptake value of 3.1 (Figure 5), as well as mild heterogenous fluorodeoxyglucose uptake in the anterior mediastinum, likely related to postsurgical inflammatory changes in the setting of recent surgery and biopsy due to leukocytic infiltration in the granulation tissue during wound healing. Of note, prior studies have shown that a maximum standardized uptake value cutoff of about 3.5 has good diagnostic performance for differentiating malignant from benign tumors, in which the latter has lower uptake.1,2 The mild maximum standardized uptake value of 3.1 of this patient’s mass was therefore more consistent with a benign cardiac tumor. Permanent tissue sections were obtained from the outside hospital for review and demonstrated the tumor to be composed of well-formed vascular spaces surrounded by fibrous tissue and extending into the underlying myocardium. The lumina of the vascular neoplasm were lined by flat, bland, and uniform endothelial cells (Figure 6). The surrounding cardiomyocytes with irregular, hyperchromatic nuclei showed no immunoreactivity for rhabdomyoblastic markers, suggesting degenerative atypia over neoplastic transformation. Despite the infiltrative nature of the neoplasm, histologic findings were therefore compatible with a benign hemangioma. The endothelial origin of the neoplasm was confirmed with immunoperoxidase staining for the endothelial cell markers ERG, CD31, and CD34 (Figure 7) and absence of markers of rhabdomyoma and lymphangioma. Fewer than 1% of the neoplastic cells were positive for the proliferation marker Ki67, confirming the benign nature of the tumor (Figure 7).
Figure 5.
Whole-body fluorodeoxyglucose PET/CT fusion images of the mass (arrows) in (A) axial and (B) coronal views, with mild fluorodeoxyglucose uptake by the mass, which is predominantly at the periphery (maximum standardized uptake value, 3.1).
Figure 6.
Histologic sections of the right atrial mass show closely packed, well-formed vascular spaces containing red blood cells (A, at lower magnification). Capillaries are lined by bland and uniform endothelial cells (B, at higher magnification). Vascular spaces infiltrate underlying myocardium (C). Hematoxylin and eosin stain.
Figure 7.
Immunohistochemistry findings from the excised mass. The cells lining the vascular spaces are positive (brown color) for the markers CD31 (A) and ERG (B), confirming their endothelial origin. At high magnification, ERG-positive cells are seen infiltrating underlying myocardial cells (C). Very few cells react (in brown color) for the proliferation marker Ki67 (D).
Given the benign nature of the lesion, the oncology team determined no role for systemic chemotherapy. However, given its large size and potential to cause significant hemodynamic compromise, the patient ultimately underwent repeat sternotomy for excision of the mass. The mass was found to be tightly adherent to the atrial wall but only loosely adherent to the atrioventricular groove. Complete resection was achieved but left a large defect of the right atrial free wall that was repaired with a bovine pericardial patch. Histopathologic analysis confirmed the diagnosis of infiltrative cardiac hemangioma, with no neoplastic tissue evident on the resection margins. The patient recovered well after surgery, without any significant complications and was discharged home on postoperative day 4. Six months later, CT of the chest showed no evidence of recurrence. Further follow-up will include periodic echocardiography.
Discussion
This report presents the case of a 23-year-old woman admitted to the hospital 5 days postpartum with chest pain who was found to have a large right atrial mass. The differential diagnosis of cardiac masses is wide and includes benign tumors, malignant tumors (primary or, much more frequently, metastatic), and non-neoplastic lesions (including thrombi, vegetations, calcifications, and artifacts). Every cardiac mass should be evaluated in its clinical context, with particular attention to the patient’s age, sex, cardiac rhythm, presence of valvular disease or prosthetic valves, and history of cardiomyopathy or malignancies. The location of the mass (e.g., chamber of the heart, valvular attachment), mobility, size, morphology and echogenicity can offer clues to further characterize the nature of a cardiac mass.
Despite remarkable advances in imaging techniques, characterizing cardiac masses noninvasively remains challenging. TTE is the preferred initial imaging modality because of its wide availability and expertise, low cost, and lack of radiation. The location, size, mobility, and hemodynamic impact of a mass can be easily determined, as well as the presence of a pericardial effusion. The use of ultrasound-enhancing agents can help differentiate malignant masses, which demonstrate hyperenhancement compared with the adjacent myocardium, from mural thrombi that do not enhance at all. Transesophageal echocardiography provides increased spatial resolution and can therefore identify smaller lesions compared with TTE; it is particularly useful to identify valvular involvement and in patients with poor acoustic windows on TTE. Cardiac CT provides cross-sectional imaging with high spatial resolution and allows evaluation of the surrounding chest and pulmonary structures.
CMR is widely recognized as the preferred imaging modality in the diagnosis of cardiac masses. Because of its high temporal and spatial resolution, it allows excellent visualization of a mass location, dimensions, extension to surrounding structures, and tissue infiltration.3 Signal characteristics such as calcification, fatty infiltration, and vascularity can offer important information about the chemical microenvironment of the mass, further aiding accurate diagnosis.4 Despite these advantages, it can still be difficult to exclude malignancy on the basis of imaging alone.5 Several features on CMR, including hyperenhancement and vascularity, have been shown to be suggestive of malignant tumors (e.g., angiosarcoma); however, this can be misleading in cases of benign masses that are highly vascular, such as hemangiomas or masses with an extensive vascular supply such as paragangliomas.6
These limitations are well illustrated in this case. In fact, even though CMR was suggestive of a cardiac angiosarcoma, which is the most common primary cardiac malignancy,7 our patient was ultimately diagnosed with a cardiac hemangioma through the analysis of permanent tissue sections and the use of several specific immunohistochemical markers. As we demonstrated, histopathology and immunohistochemistry remain the gold standards for diagnosing cardiac masses.3 Another major limitation demonstrated by our case is that imaging is often incomplete, limited in quality, or inaccessible to direct review when obtained at other institutions. For example, one lost opportunity occurred by not using an ultrasound-enhancing agent during TTE, which could have further characterized the mass as described earlier. Obtaining a non-contrast-enhanced computed tomographic image would have allowed us to distinguish contrast from calcification. The late gadolinium enhanced images were motion degraded, which in real-world clinical practice is a common occurrence that limits the utility of imaging. The inability to review transesophageal echocardiographic images from the outside hospital likely led to unnecessary repeat imaging and delays in the patient’s care.
Cardiac hemangiomas are exceedingly rare; they represent 2.8% of primary cardiac tumors8 and can occur in patients of all ages. In a review of 200 cases compiled from different institutions, the most common location of cardiac hemangiomas was the right atrium (26.2%), as in our case, followed by the left ventricle (23.1%); notably, most cases were misdiagnosed before pathologic diagnosis.9 Likewise, in an analysis of cardiac tumors in children by Beroukhim et al.,5 half of the 10 right atrial masses were hemangiomas. Common presenting symptoms depend on tumor size, location, and involvement of surrounding structures but usually include dyspnea, palpitations, chest discomfort, or clinical signs of heart failure. In this patient’s case, the hemangioma could have been the cause of her presenting symptom of chest pain because of the activation of atrial nociceptors or demand ischemia from the highly vascularized lesion, although this remains speculative. Our patient endorsed chest pain at follow-up, although it was thought to be sternal pain from her operation site. Despite their benign histologic features, these tumors can have significant morbidity and occasional mortality because of hemodynamically significant obstruction, cardiac tamponade, ventricular arrythmias, or heart block.10 Patients with resectable tumors usually have a good prognosis, but close follow-up with periodic echocardiography is recommended given reports of recurrence after resection.11
Conclusion
This case demonstrates that despite a noninvasive multimodality imaging approach involving TTE, transesophageal echocardiography, CMR, and PET/CT, only an invasive surgical approach was able to confirm the tissue diagnosis of this cardiac mass. Although imaging can assist in the anatomic characterization, surgical planning, and follow-up of intracardiac tumors, imaging alone is not always sufficient to diagnose a rare benign primary cardiac tumor, and an accurate histopathologic and immunohistochemical diagnosis is key to appropriate further management. Although rare, it is imperative to consider hemangiomas in the differential diagnosis of primary cardiac tumors, as they are often misdiagnosed but are benign and can be definitively treated with surgical excision.
Ethics Statement
The authors declare that the work described has been carried out in accordance with The Code of Ethics of the World Medical Association (Declaration of Helsinki) for experiments involving humans.
Consent Statement
Complete written informed consent was obtained from the patient (or appropriate parent, guardian, or power of attorney) for the publication of this study and accompanying images.
Funding Statement
The authors declare that this research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Disclosure Statement
The authors report no conflict of interest.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.case.2023.04.006.
Supplementary Data
Apical four-chamber view of TTE revealing a large solid mass in the right atrium.
Apical four-chamber view of TTE with color flow Doppler showing minimal tricuspid regurgitation.
CMR perfusion imaging demonstrates avid central enhancement, consistent with a hypervascular lesion.
References
- 1.Rahbar K., Seifarth H., Schäfers M., Stegger L., Hoffmeier A., Spieker T., et al. Differentiation of malignant and benign cardiac tumors using 18F-FDG PET/CT. J Nucl Med. 2012;53:856–863. doi: 10.2967/jnumed.111.095364. [DOI] [PubMed] [Google Scholar]
- 2.Meng J., Zhao H., Liu Y., Chen D., Hacker M., Wei Y., et al. Assessment of cardiac tumors by (18)F-FDG PET/CT imaging: histological correlation and clinical outcomes. J Nucl Cardiol. 2021;28:2233–2243. doi: 10.1007/s12350-019-02022-1. [DOI] [PubMed] [Google Scholar]
- 3.Tyebally S., Chen D., Bhattacharyya S., Mughrabi A., Hussain Z., Manisty C., et al. Cardiac tumors: JACC CardioOncology state-of-the-art review. JACC CardioOncol. 2020;2:293–311. doi: 10.1016/j.jaccao.2020.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gupta R., Meghrajani V., Desai R., Gupta N. Primary malignant cardiac tumors: a rare disease with an adventurous journey. J Am Heart Assoc. 2020;9 doi: 10.1161/JAHA.120.016032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Beroukhim R.S., Prakash A., Buechel E.R., Cava J.R., Dorfman A.L., Festa P., et al. Characterization of cardiac tumors in children by cardiovascular magnetic resonance imaging: a multicenter experience. J Am Coll Cardiol. 2011;58:1044–1054. doi: 10.1016/j.jacc.2011.05.027. [DOI] [PubMed] [Google Scholar]
- 6.Mousavi N., Cheezum M.K., Aghayev A., Padera R., Vita T., Steigner M., et al. Assessment of cardiac masses by cardiac magnetic resonance imaging: histological correlation and clinical outcomes. J Am Heart Assoc. 2019;8 doi: 10.1161/JAHA.117.007829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Motwani M., Kidambi A., Herzog B.A., Uddin A., Greenwood J.P., Plein S. MR imaging of cardiac tumors and masses: a review of methods and clinical applications. Radiology. 2013;268:26–43. doi: 10.1148/radiol.13121239. [DOI] [PubMed] [Google Scholar]
- 8.McAllister H.A., Jr. Primary tumors and cysts of the heart and pericardium. Curr Probl Cardiol. 1979;4:1–51. doi: 10.1016/0146-2806(79)90008-2. [DOI] [PubMed] [Google Scholar]
- 9.Li W., Teng P., Xu H., Ma L., Ni Y. Cardiac hemangioma: a comprehensive analysis of 200 cases. Ann Thorac Surg. 2015;99:2246–2252. doi: 10.1016/j.athoracsur.2015.02.064. [DOI] [PubMed] [Google Scholar]
- 10.Cina S.J., Smialek J.E., Burke A.P., Virmani R., Hutchins G.M. Primary cardiac tumors causing sudden death: a review of the literature. Am J Forensic Med Pathol. 1996;17:271–281. doi: 10.1097/00000433-199612000-00001. [DOI] [PubMed] [Google Scholar]
- 11.Colli A., Budillon A.M., DeCicco G., Agostinelli A., Nicolini F., Tzialtas D., et al. Recurrence of a right ventricular hemangioma. J Thorac Cardiovasc Surg. 2003;126:881–883. doi: 10.1016/s0022-5223(03)00720-7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Apical four-chamber view of TTE revealing a large solid mass in the right atrium.
Apical four-chamber view of TTE with color flow Doppler showing minimal tricuspid regurgitation.
CMR perfusion imaging demonstrates avid central enhancement, consistent with a hypervascular lesion.