Abstract
The nonradiative energy loss (∆Enr) is a critical factor to limit the efficiency of organic solar cells. Generally, strong electron-phonon coupling induced by molecular motion generates fast nonradiative decay and causes high ∆Enr. How to restrict molecular motion and achieve a low ∆Enr is a sticking point. Herein, the free volume ratio (FVR) is proposed as an indicator to evaluate molecular motion, providing new molecular design rationale to suppress nonradiative decay. Theoretical and experimental results indicate proper proliferation of alkyl side-chain can decrease FVR and restrict molecular motion, leading to reduced electron-phonon coupling while maintaining ideal nanomorphology. The reduced FVR and favorable morphology are simultaneously obtained in AQx-6 with pinpoint alkyl chain proliferation, achieving a high PCE of 18.6% with optimized VOC, JSC and FF. Our study discovered aggregation-state regulation is of great importance to the reduction of electron-phonon coupling, which paves the way to high-efficiency OSCs.
Subject terms: Electronic materials, Electronic devices
Reducing non-radiative energy loss is critical to improving power conversion efficiency in organic solar cells. Jiang et al. show that alkyl side-chain engineering in acceptors reduces the free volume ratio, lowering non-radiative decay, to achieve 18.6% efficiency in bulk-heterojunction binary cells.
Introduction
Organic solar cells (OSCs) as a promising photovoltaic technology have been drawing great attention, which presents great potential in flexibility, large-area fabrication, semitransparency and etc1–13. Great progresses have been made in power conversion efficiencies (PCEs) recently, benefitting from subtle molecular design14–27 and device engineering28–39. According to the Shockley-Queisser (SQ) theory, there should be only inevitable radiative energy loss above bandgap in an ideal solar cell40,41, however, a considerable nonradiative energy loss (∆Enr) below bandgap presents in realistic OSCs, which has been recognized as a key issue for higher PCE42,43. Essentially, the energy loss below bandgap originates from the relaxation processes from the excited states (local excited states of individual materials and intermolecular charge-transfer states at donor/acceptor interface) to the ground states accompanied with photon emission and phonon transmission, corresponding to radiative energy loss (∆Er) and ∆Enr, respectively44–47. The competitive relationship between the nonradiative and radiative relaxation processes suggests suppressed nonradiative decay rate (knr) is conducive to improving photoluminescence quantum yield (PLQY) by the equation of 48,49. According to the energy-gap law, nonradiative decay rate knr can be described as 50, where ωM is the maximum and dominant vibrational frequency and ∆E is the excitation energy, indicating that the lower bandgap, the higher nonradiative decay rate and lower PLQY. Recently, Brades et al. confirmed that the higher PLQY of the active-layer material featuring a lower optical bandgap determines the lower limit of ∆Enr41. Therefore, the following question on the way to efficient OSCs with low ∆Enr is how to restrict molecular motions or in other words, suppress electron–phonon coupling, especially in the lower bandgap organic active-layer materials49,51.
Typically, organic semiconductor materials consist of a conjugated backbone and flexible side chains, in which the backbone forms π–π stacking for charge carrier transport, and side chains can not only bring solution processability but also significantly affect the aggregation pattern in solid state52,53. The current state-of-the-art nonfullerene acceptors (NFAs) exhibit a non-linear configuration54, such as Y series with a banana geometry presenting various packing modes, which forms multiple charge transport channels and delivers superior charge transport property24,55. However, these rigid fused backbones inevitably produce voids, named as free volume, between adjacent backbones in aggregation state, which generates the probability for molecular thermal motion, and thus endows NFAs with lower PLQY56. Vandewal et al. demonstrated that intrinsic high-frequency carbon–carbon stretch vibrations are a dominant cause for the nonradiative decay process, a major source for ∆Enr in fullerene-based OSCs57,58. In addition to these high-frequency stretch vibrations, the ∆Enr of NFAs that features an extended (quasi-) planar π-conjugated backbone also is related to out-of-plane bending and in-plane torsion. Since the microscopic molecular thermal motions are difficult to analyze quantitatively, we assume that the free volume ratio (FVR) may be an indicator, allowing a straightforward platform to investigate how to reduce ∆Enr via rational molecular design. Aggregation-induced emission (AIE) mechanism indicates that restriction of intramolecular motions in solid state can slow down the knr but have little influences on the kr59, leading to higher PLQY. Inspired by the AIE science, we expect that flexible alkyl side chains can act as “glue” by lipsoluble supramolecular assembly60,61, and decrease the FVR for restricted molecular motions, leading to an improved PLQY in NFAs and a lower ∆Enr in OSCs.
Our group have been devoted to developing high-efficiency NFAs, and AQx-type acceptors featuring a quinoidal-enhancing quinoxaline moiety were designed and synthesized in 201962, achieving a record certified PCE when it was published63,64. Recently, Wei et al. demonstrated that AQx-type acceptors exhibit much lower reorganization energy than Y665, leading to better charge transport property, longer exciton diffusion length, reduced ∆Eloss and thus allowing superior potential to achieve higher PCEs. Herein, we performed a precise alkyl side-chain modulation on the AQx-type acceptors, which provides a feasible and convenient approach to decrease ∆Enr. In contrast to the most research focus on the symmetrical side-chain modulations22,24,66,67, we selectively proliferated alkyl side chain. Therefore, AQx-2 with symmetrical 2-ethylhexyl, 2,2′-((2Z,2′Z)-((13-(2-ethylhexyl)-14-(2-hexyldecyl)-3,10-diundecyl-13,14-dihydrothieno[2″,3″:4′,5′]thieno[2′,3′:4,5]pyrrolo[3,2-f]thieno-[2″,3″:4′,5′]thieno[2′,3′:4,5]pyrolo-[2,3-h]quinoxaline-2,11-diyl)bis(methaneylylidene))bis(5,6-difluoro-3-oxo-2,3-dihydro-1H-indene-2,1-di-ylidene))dimalononitrile (AQx-6) with asymmetrical 2-ethylhexyl and 2-hexyldecyl and 2,2′-((2Z,2′Z)-((13,14-bis(2-hexyldecyl)-3,10-diundecyl-13,14-dihydrothieno[2″,3″:4′,5′]thieno[2′,3′:4,5]pyrrolo[3,2-f]-thieno[2″,3″:4′,5′]thieno[2′,3′:4,5]pyrrolo[2,3-h]quinoxaline-2,11-diyl)bis(methaneylylidene))bis(5,6-di-fluoro-3-oxo-2,3-dihydro-1H-indene-2,1-diylidene))dimalononitrile (AQx-8) with symmetrical 2-hexyldecyl were designed and synthesized, with regulated alkyl side chains attached on the nitrogen atoms of the pyrrole rings. The theoretical and experimental results demonstrated that the prolonged alkyl side-chain helps to decrease the FVR in AQx-6 and AQx-8, which is beneficial to reduction the electron–phonon coupling, leading to enhanced emission efficiency and reduced ∆Enr. However, nanomorphology that includes phase separation and crystallinity will be affected by the prolonged alkyl side-chain length, making differences in exciton separation, charge transport and recombination processes. Notably, AQx-6-based OSC achieved the subtle balance between suppressed electron–phonon coupling for low ∆Enr and efficient exciton dissociation as well as charge transport for high JSC and FF. Eventually, the AQx-6-based binary OSC delivers a remarkable PCE of 18.6% (certified PCE: 18.4%) with a VOC of 0.892 V, JSC of 26.8 mA cm−2 and FF of 77.8%.
Results
Molecular design and theoretical calculations of AQx-type acceptors
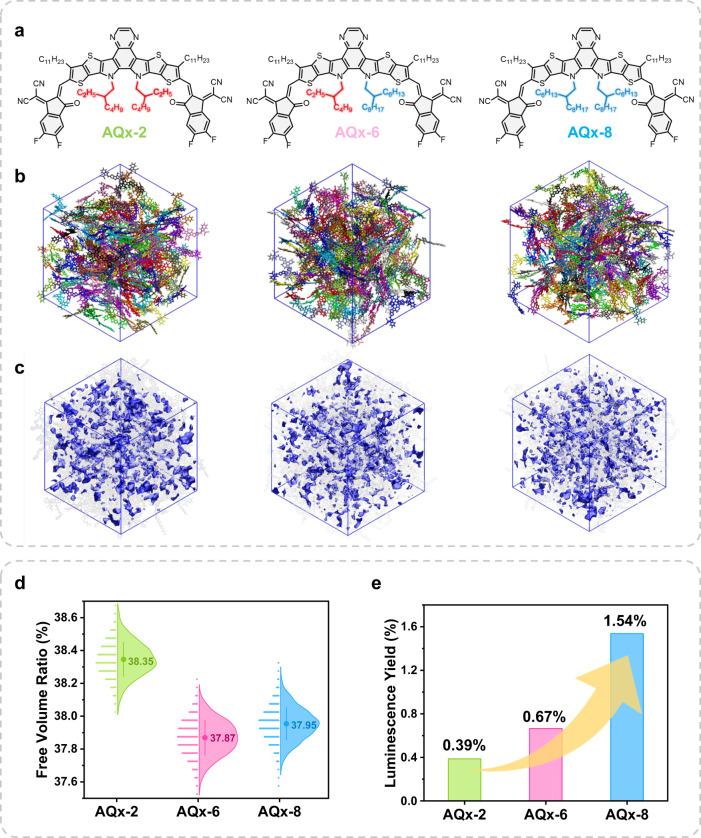
Based on the highly performed NFA AQx-2, AQx-6 and AQx-8 were designed by gradually proliferating 2-ethylhexyl substituents attached on the sp2-hybridized nitrogen atoms in the pyrrole rings with 2-hexyldecyl substituents, the molecular structures of AQx-2, AQx-6 and AQx-8 are shown in Fig. 1a, and the relevant synthetic routes were shown in Supplementary Fig. 1. In order to explore the effect of varied alkyl side chains on molecular motion, the molecular packing structures and FVRs of three acceptors have been investigated by atomistic molecular dynamics simulations (the simulation details see Supplementary information). As shown in Fig. 1b–d, AQx-2 with the shorter alkyl side-chain possesses a FVR of 38.35%, and longer alkyl side chain in AQx-6 and AQx-8 allow decreased FVR values of 37.87% and 37.95%, respectively, which is consistent with what we expected. The reduced FVR allows less space for molecular motion, leading to suppressed nonradiative decay process. The PLQY and time-resolved photoluminescence (TRPL) measurements (Supplementary Fig. 8) were carried out to investigate emission efficiency and exciton lifetime (τ) in three acceptors. As shown in Fig. 1e and Supplementary Table 1, with the increased alkyl side-chain length, PLQY gradually increased from 0.39% (AQx-2) to 0.67% (AQx-6), and then to 1.54% (AQx-8), the higher PLQY contributes to a lower ∆Enr in relevant OSCs68. The τ values for AQx-2, AQx-6 and AQx-8 are 60.99, 104.07 and 103.45 ps, respectively. Based on the equation of , the kr and knr are 6.40 × 107 and 1.63 × 1010, 6.44 × 107 and 9.55 × 109, 1.49 × 108 and 9.52 × 109 s−1 for AQx-2, AQx-6 and AQx-8, respectively. Compared to AQx-2, the knrs get the reduction of an order of magnitude in AQx-6 and AQx-8, which is well consistent with the variation of the FVR. The faster radiative decay process and higher PLQY are observed in AQx-8, which can be attributed to a slightly looser π–π stacking that is caused by long alkyl side-chain, leading to a reduced H-aggregation69. The similar knrs in AQx-6 and AQx-8 agree well with their similar FVRs. Thus, we can conclude that prolonged alkyl side chain helps to suppress nonradiative decay process associated to molecular motion via “adhesive” effect, which is of great importance to reduce Eloss and ultimately realize high performance. Such a conclusion can be confirmed by the experimental Eloss of AQx-based systems and the detailed analysis will be discussed below.
Fig. 1. Chemical structures and theoretical calculation.
a Chemical structures. b Representative snapshots of MD simulated molecular packing morphologies. c Illustration of free volumes. d Free volume ratio. e Luminescence yields of AQx−2, AQx-6 and AQx-8 films.
Photoelectric property
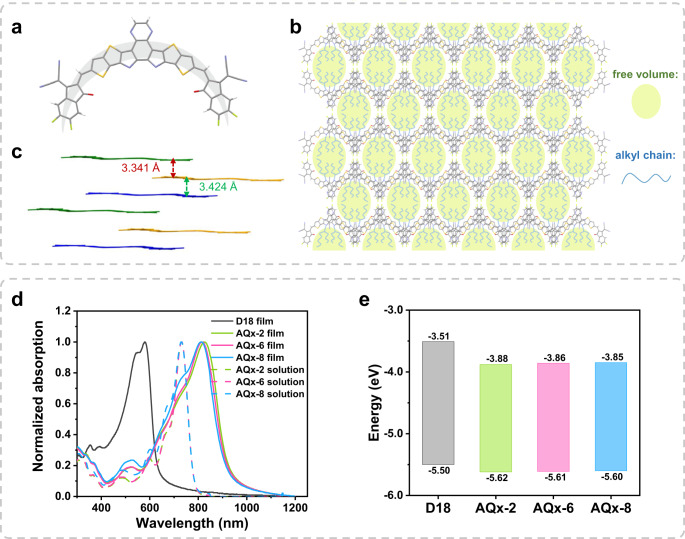
The molecular geometry without alkyl side chain in a single crystal of AQx-2 is exhibited in Fig. 2a and the single crystal was successfully developed through diffusing diethyl ether vapor into chloroform solution. AQx-2 presents a banana-shaped geometry, and such a configuration is inevitable to form free volumes between rigid backbones. As shown in Fig. 2c, the molecular packing in single crystal of AQx-2 presents a dense wave-like molecular arrangement with hexagon-shaped voids. For each molecule, it gives four overlaps with adjacent molecules, in which one type is J-aggregation between the end group and the other is H-aggregation between backbones, with the π–π stacking distances of 3.341 and 3.424 Å (Fig. 2b)70. The compact 2D brick-layer arrangement allows efficient charge transport, contributing to less charge combination and desired FF for high performance in device. The UV–Vis absorption spectra of acceptors in chloroform solution and thin film and donor film are shown in Fig. 2d, in which D1823 as a donor exhibits a complementary absorption with AQx-type acceptors for more photon harvesting. With the similar molecular structure, three acceptors present nearly identical absorption with a maximum absorption peak at 732 nm in diluted chloroform solution, and their absorption spectra in thin film show bathochromic-shift absorption, suggesting strengthened and ordered aggregation in solid state70. The AQx-2, AQx-6 and AQx-8 show maximum absorption peaks at 825, 815 and 812 nm with absorption onset at 941, 936 and 933 nm, corresponding to optical gap of 1.32, 1.32 and 1.33 eV, respectively. The blue-shifted absorption from AQx-2 (825 nm) to AQx-8 (812 nm) can be ascribed to gradually prolonged alkyl side, which induces the reduced π–π stacking in AQx-8 pristine film. D18 film shows the absorption range of 400–650 nm, which is complementary to those of the AQx-type acceptors in a large part of the solar spectrum, benefiting solar-spectrum utilization. The cyclic voltammetry (CV) measurements were performed to measure the energy levels (Supplementary Fig. 11) that includes the highest occupied molecular orbital (HOMO) and the lowest unoccupied molecular orbital (LUMO) energy levels of three acceptors. As shown in Fig. 2e, the HOMO energy levels of AQx-2, AQx-6 and AQx-8 are −5.62, −5.61 and −5.60 eV and the LUMO energy levels are −3.88, −3.86 and −3.85 eV, respectively. There is no obvious difference of HOMO and LUMO energy levels among three acceptors, suggesting fine-tune of alkyl side chain has little impact on energy levels, which is beneficial to investigate the relationship among alkyl side-chain substitution, aggregation structure and nonradiative decay path associated with the electron–phonon coupling. Moreover, as shown in Supplementary Fig. 12, AQx-type acceptors possess good thermal stability (5% weight loss at 345, 347 and 345 °C for AQx-2, AQx-6 and AQx-8, respectively) according to the thermogravimetric analysis (TGA).
Fig. 2. Single-crystal structure and photoelectric properties of AQx-type NFAs.
a The single-crystal molecular configuration of AQx−2 without alkyl chain for clarity (CCDC number: 2161927). b π–π stacking distance. c Molecular packing patterns of AQx-2 in single crystal (The inserted alkyl side chain is schematic diagram). d UV–Vis-NIR spectra. e Energy levels diagram.
Photovoltaic performance
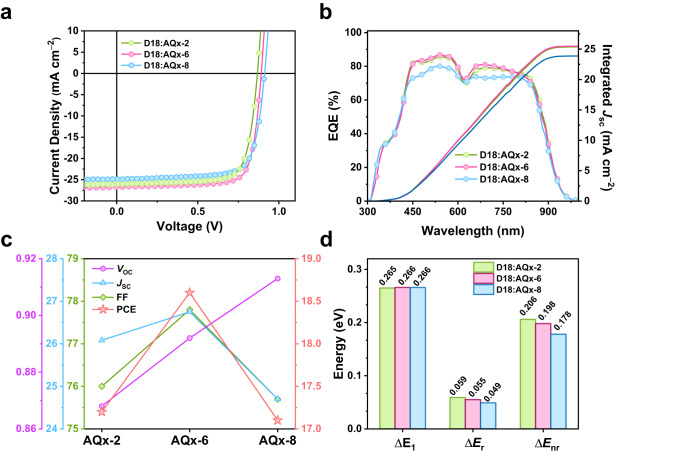
All OSCs based on the conventional device configuration of glass/ITO/PEDOT:PSS/D18:acceptor/PDINN/Ag, in which ITO is indium tin oxide, PEDOT:PSS is poly(3,4-ethylenedioxythiophene):poly(styrenesulfonate) that is used as hole transport layer, PDINN acts as electron transport layer71. Supplementary Tables 2–7 are summarized the detailed device optimization process including the donor:acceptor ratio and thermal annealing temperature. Table 1 gives the detailed photovoltaic parameters of three devices and Fig. 3a shows the corresponding current density-voltage (J-V) curves. The devices based on D18:AQx-2 and D18:AQx-8 show passable PCE of 17.2 and 17.1% with VOC of 0.868 and 0.913 V, JSC of 26.1 and 24.7 mA cm−2, FF of 76.0 and 75.7%, respectively. The AQx-2-based device exhibits the higher JSC (26.1 mA cm−2), while the higher VOC (0.913 V) is observed in AQx-8-based device. As for the AQx-6 with asymmetrical alkyl side chain, the corresponding cells afford an improved VOC of 0.892 V and the highest Jsc of 26.8 mA cm−2 as well as FF of 77.8%, thus a champion efficiency of 18.6% was obtained, which is one of the highest PCE among the reported bulk-heterojunction binary OSCs (Supplementary Fig. 13). Besides, the certification of D18:AQx-6 performance was carried out in National Institute of Metrology (NIM), China, and a certified PCE of 18.4% with a VOC of 0.874 V, a JSC of 26.7 mA cm-2 and an FF of 79.1% was obtained (Supplementary Fig. 14). The external quantum efficiency (EQE) curves are shown in Fig. 3b, and the integrated JSCs from EQE curves are consistent with the JSC values from J–V measurements within 5% error. Noted that despite three acceptors give different absorption edge, AQx-2-, AQx-6- and AQx-8-based devices present almost overlapped EQE curve edges, 944, 941 and 937 nm, which corresponds to the bandgap (Eg) of 1.314, 1.317 and 1.323 eV, respectively. According to the equation of Eloss = Eg – qVoc, where q is element charge, the Elosss are 0.446 eV for D18:AQx-2, 0.425 eV for D18:AQx-6 and 0.410 eV for D18:AQx-8, indicating the prolonged alkyl chain can lower the Eloss and elevate the value of VOC (Fig. 3c). Compared to AQx-2-based OSC, AQx-6-based OSC displays elevated VOC value and obtains highest JSC and FF, which produces the best performance among three devices.
Table 1.
Photovoltaic parameters of D18:AQx-2, D18:AQx-6 and D18:AQx-8 based OSCs under the illumination of AM 1.5 G, 100 mW cm−2
| Acceptors |
Voc (V) |
JSC (mA cm−2) |
JSCcal,b (mA cm−2) |
FF (%) |
PCE (%) |
∆Enrc (eV) |
∆Elossd (eV) |
|---|---|---|---|---|---|---|---|
| AQx-2a |
0.868 (0.869 ± 0.003) |
26.1 (25.8 ± 0.2) |
25.4 |
76.0 (75.6 ± 0.3) |
17.2 (17.0 ± 0.11) |
0.206 | 0.529 |
| AQx-6a |
0.892 (0.890 ± 0.002) |
26.8 (26.7 ± 0.1) |
25.5 |
77.8 (77.1 ± 0.5) |
18.6 (18.3 ± 0.10) |
0.198 | 0.518 |
| AQx-8a |
0.913 (0.909 ± 0.003) |
24.7 (24.9 ± 0.1) |
23.9 |
75.7 (75.0 ± 0.4) |
17.1 (17.0 ± 0.05) |
0.178 | 0.493 |
| AQx-6e | 0.874 | 26.7 | – | 79.1 | 18.4 | – | – |
aAverage values with standard deviation were obtained from 10 individual devices.
bThe calculated JSC values from the EQE curve.
cCalculated using eq. ∆Enr = −(kT/q)In(EQEEL).
dCalculated using eq. ∆Eloss = Eg − qVOC, in which Eg is determined the crossing point of absorption and emission.
eThe certified PCE obtained from National Institute of Metrology (NIM), Chain.
Fig. 3. Photovoltaic performance of OSCs based on AQx-type NFAs.
a Characteristic J–V curves. b EQE curves. c Photovoltaic parameters variation for AQX-based OSCs (VOC: V; JSC: mA cm−2; FF: %; PCE: %.). d Energy loss: ∆E1, ∆Er and ∆Enr.
The exciton generation, dissociation and charge recombination processes were performed to better understanding performance difference in three devices. The dependence of photocurrent density (Jph) on the effective voltage (Veff) was performed to explore exciton dissociation and charge extraction probability. The Jph is equated to Jlight – Jdark, where Jlight and Jdark is current density on illumination and dark condition, respectively. The Veff is defined as V0 – Vapp, where V0 is the voltage when Jph = 0 and Vapp is the applied voltage. The exciton dissociation probability P(E,T) can be calculated with Jph/Jsat (Supplementary Fig. 15). Under the maximum power out condition, P(E,T)s are 98.7, 99.4 and 98.2% for AQx-2-, AQx-6- and AQx-8-based cells and under the short-circuit condition, P(E,T)s were 87.2 90.3 and 85.8% for AQx-2-, AQx-6- and AQx-8-based cells, indicating superior exciton dissociation and charge extraction process existed in AQx-6-based binary cell. In addition, charge recombination behavior in the three OSCs was examined by the dependence of VOC and JSC on light density (Plight). The relationship between VOC and Plight can be described as VOC ∝ (nkT/q)lnPlight, where k, T, q are the Boltzmann constant, absolute temperature and elemental charge, respectively. When n is close to 1, the bimolecular recombination is the dominated mechanism in devices. As displayed in Supplementary Fig. 16, the slope values for AQx-2-, AQx-6- and AQx-8-based devices are 1.05, 1.01 and 1.09 kT/q, respectively, suggesting all devices are subject to bimolecular recombination. The lowest slope value in AQx-6-based OSC means the trap-assisted recombination was well-mitigated. The relationship between JSC and Plight obeys the law of JSC ∝ Plightα, in which α is the exponential factor, and when α = 1 indicates that there is no bimolecular recombination in the devices where all free charge carriers can be completely swept out and then collected by electrodes. As shown in Supplementary Fig. 17, the α values for AQx-2-, AQx-6- and AQx-8-based devices are 0.940, 0.955 and 0.938, respectively. The superior exciton dissociation and charge extraction probability and reduced bimolecular recombination in AQx-6-based OSC contributes to high JSC and FF.
Energy loss analysis
To further in-depth understand the variation of VOC, the detailed energy loss analysis in three AQx-based OSCs was performed. The Eloss can be quantified via SQ theory and is divided into three parts: ∆Eloss = ∆E1 + ∆Er + ∆Enr = (Eg − qVOC,SQ) + (qVOC,SQ − qVOC,rad) + (qVOC,rad − qVOC)72,73, where ∆E1 is inevitable radiative loss above bandgap, ∆Er and ∆Enr are radiative and nonradiative loss below bandgap, VOC,SQ and VOC,rad is the VOC from SQ limit and radiative limit. In generally, ∆E1 is determined by the intrinsic property of materials and relates to their bandgap, the larger bandgap will produce higher ∆E1. The ∆Er relates to absorption edge broadening and it is negligible in state-of-the-art OSCs. The ∆Enr is derived from the difference between the nonideal EQE in realistic OSCs and the ideal EQE from SQ theory (EQESQ = 1 for E > Eg and 0 for E < Eg), indicating the existing of nonradiative decay process. The phonon-mediated nonradiative relaxation from the local excited (LE) or charge-transfer (CT) states to the ground states is affected by the electron–phonon coupling strength that relates to molecular motion, which needs to be suppressed and is the key factor to reach a low ∆Eloss.
The Eg was calculated from the intersection point of relevant emission and external quantum efficiency spectra, as shown in Supplementary Fig. 18 and these for the D18:AQx-2, D18:AQx-6 and D18:AQx-8 based devices are 1.397 eV, 1.410 and 1.406 eV, corresponding to the Elosss are 0.529, 0.518 and 0.493 eV, respectively. The detailed energy loss for the three devices is summarized in Supplementary Table 8. The AQx-2-, AQx-6 and AQx-8-based devices deliver almost identical ∆E1 values of 0.265, 0.266 and 0.266 eV, respectively, deriving from the almost identical bandgap among three blend films. Generally, the ∆Enr was estimated from electroluminescent (EL) measurement (Supplementary Fig. 19), which equals to –(kT/q)In(EQEEL) in value, where k is the Boltzmann constant, T is Kelvin temperature and EQEEL is the external quantum efficiency of electroluminescence47. As exhibited in Fig. 3d, AQx-2-, AQx-6- and AQx-8-based devices exhibit EQEEL of 3.29 × 10−4, 4.05 × 10−4 and 8.65 × 10−4, respectively, corresponding to ∆Enr values of 0.206, 0.198 and 0.178 eV, which is excellently in agreement with the assumption based on theoretical calculation. Considering the ∆Enr may affect by the formation of triplet exciton (T1), transient absorption (TA) in the near-infrared region was carried out to assess the formation of T1 state in AQx-based systems. In the AQx-based blend films, an excited-state absorption (ESA) at ~1450 nm emerged after 100 ps (Supplementary Fig. 21), which can be ascribed to the T1 state of the AQx-type acceptor. The ESA of T1 state emerges less pronouncedly in three AQx-based blend films, suggesting the ∆Enr in AQx systems is not primarily caused by the formation of T1 state but rather controlled by the effect of electron-photon coupling. From AQx-2 to AQx-6 and AQx-8, the increased alkyl side chain length produces decreased FVR and restricted molecular motion with slow knr, which indicates a reduction in electron-photon coupling strength and then allows reduced ∆Enr in OSCs. The diminution of ∆Enr greatly promotes the improvement of VOC, thus causing gradually increased VOC from AQx-2 to AQx-6, and then to AQx-8. The ∆Er that comes from the equation of ∆Er = Eloss - ∆E1 − ∆Enr for AQx-2-, AQx-6- and AQx-8-based cells are 0.059, 0.055 and 0.049 eV, indicating nearly identical radiative recombination loss below bandgap for three devices. Urbach energy of three devices was calculated from highly sensitive EQE (sEQE) throughout exponential fitting, as show in Supplementary Fig. 19, the small Urbach energy of 22~24 meV in AQx-based OSCs suggests low energetic disorder and that has been proved to be of great importance for high performance74. Moreover, the CT energy levels were estimated by fitting reduced EL and EQE curves (Supplementary Fig. 18)75, those for AQx-2-, AQx-6- and AQx-8-based devices are 1.364, 1.379 and 1.372 eV, affording similar ∆ELE-CTs of 0.033, 0.031 and 0.034 eV, respectively. The small ∆ELE-CT values in three devices mean an equally strong LE-CT hybridization and favor to improved emission efficiency, causing suppressed nonradiative decay in CT states via an intensity-borrowing mechanism76. The above results imply that prolonged alkyl side chain will allow low Eloss and thus higher VOC in OSCs as what we speculated from the theoretical calculation, that is the proliferation of alkyl chain length can reduce FVR and restrict molecular motion via suppressed electron–phonon coupling strength, which reveals in-depth mechanism of nonradiative decay process.
Morphology characterization
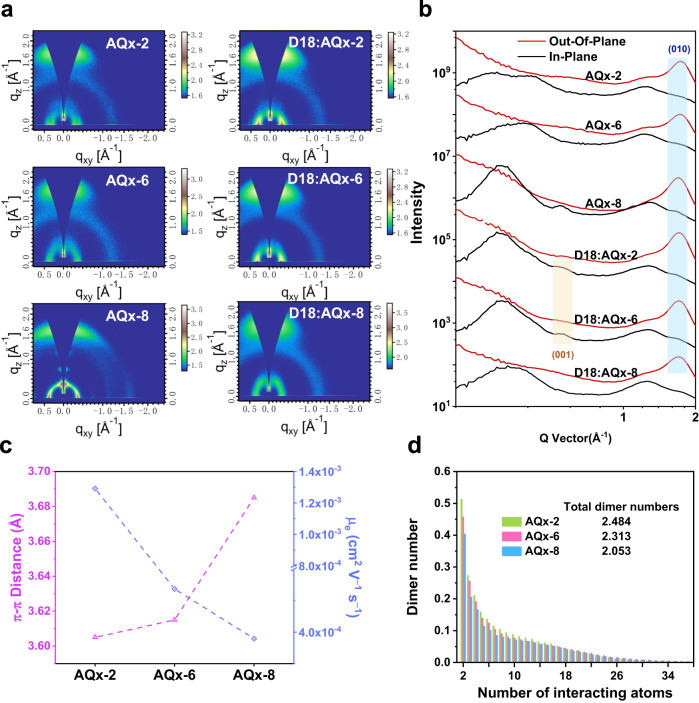
In addition, morphology is a crucial factor for reaching high-performance OSCs and the relevant measurements contribute to understand performance difference in varied devices77. The alteration of alkyl side chain will affect the miscibility between D18 and AQx-type acceptors, making difference in the formation of phase separation. The contact angle measurements of neat donor and acceptor films were performed, the corresponding results are shown in Supplementary Fig. 22 and the relevant data are summarized in Supplementary Table 9. With the increase in alkyl side-chain length, AQx-6 and AQx-8 exhibit reduced surface energies (γ) of 40.35 and 40.61 mN m−1 compared to AQx-2 (42.00 mN m−1). The miscibility between donor and acceptor can be evaluated by the Flory–Huggins interaction parameter (χ), in which χ is defined as . The χ values are 0.11 K for D18:AQx-2, 0.04 K for D18:AQx-6 and 0.05 K for D18:AQx-8. The lower χ vaules in D18:AQx-6 and D18:AQx-8 imply favorable miscibility between D18 and AQx-6/AQx-8 with prolonged alkyl chain length, which is beneficial for the formation of desired phase separation. The surface morphologies of three blend film were investigated by atomic force microscopy (AFM), as shown in Supplementary Fig. 23. The D18:AQx-2 and D18:AQx-6 blend films presented a root-mean-square surface roughness (Rq) of 1.33 and 1.22 nm, respectively, which is probably related to the strong crystallinity of acceptor materials. Whereas the film based on D18:AQx-8 with weaker π–π packing gives a more smooth, uniform surface and a relatively low Rq values of 1.02 nm. In addition, the fibers in D18:AQx-6 based blend film surface help to magnify donor/acceptor interface areas, leading to more efficient exciton dissociation as well as charge transport. Moreover, the resonant soft X-ray scattering (RSoXS), transmission electron microscopy (TEM) and grazing-incidence wide angle X-ray scattering (GIWAXS) measurements were also performed to analyze bulk morphology78. RSoXS is used to investigate the phase separation information in AQx-based blend films. As shown in Supplementary Fig. 24, D18:AQx-2 gives the largest domain size of 30.72 nm, while D18:AQx-6 and D18:AQx-8 exhibit phase separation with domain sizes of 21.55 and 22.10 nm, respectively, which is close to the optimal domain size of 10–20 nm generally accepted for OSCs. Such a result can be further confirmed by the TEM measurements (Supplementary Fig. 23). The GIWAXS results are shown in Fig. 4a, b and the detailed data were summarized in Supplementary Tables 10-11. In the neat film, all three acceptors show preferred face-on orientation, verified by the strong π–π packing (010) peaks in the out-of-plane (OOP) direction and lamellar (100) peaks in the in-plane (IP) direction. Nevertheless, the AQx-8 with the longest alkyl chain exhibits weak (010) π–π packing peaks in the IP direction, suggesting that there is partial edge-on orientation in AQx-8 thin film, which proclaims diverse aggregation behavior in AQx-8 from the other acceptors. From AQx-2 to AQx-8 with the lengthened alkyl chain, the π–π stacking peak (010) gradually shifts to the lower q region, with the specific location at 1.742, 1.737 and 1.704 Å−1, corresponding to the π–π stacking distances of 3.605, 3.615 and 3.685 Å, respectively, and demonstrating that the crystallinity is disturbed by the long alkyl chain, which has also been observed in previous reports22. In Supplementary Fig. 25, D18 exhibits a clear (010) peak in the OOP direction, suggesting the favorable face-on orientation. Moreover, the (001) diffraction peak at ~0.5 Å in the IP direction can be used to access crystallization feature of D1835. Such a peak was observed in the D18:AQx-2 and D18:AQx-6 blend films but disappeared in D18:AQx-8 (Fig. 4b) suggests that D18 can retain a good crystallinity in AQx-2- and AQx-8-based blends, contributing to improved charge transport. For all blend films, the favorable face-on orientation is maintained, which allows the free charge carriers can be efficiently transported to electrodes in the vertical direction. In the OOP direction, the π–π packing distances for AQx-2-, AQx-6- and AQx-8-based blend films are 3.662, 3.666 and 3.664 Å, with relevant crystal coherence lengths (CCLs) are 25.01, 20.11 and 17.66 Å, respectively. The longer CCLs in AQx-2- and AQx-6-based films are conducive to improving charge transport and suppressing charge recombination, while the reduced CCL in AQx-8 may induce more charge recombination. The charge transport property of the three devices can be investigated by the space-charge-limited-current (SCLC) measurement. As shown in Fig. 4c, the electron mobilities (µes) in pristine AQx-2, AQx-6 and AQx-8 are 1.29 × 10−3, 5.37 × 10−4 and 3.79 × 10−4 cm2 V−1 s−1, the higher µe is consistent with the stronger crystallinity of AQx-2 and AQx-6. For the AQx-2-, AQx-6- and AQx-8-based devices, the µes and hole mobilities (µhs) are 4.95 × 10−4, 4.75 × 10−4, 3.66 × 10−4 cm2 V−1 s−1 and 2.54 × 10−4, 3.16 × 10−4, 1.86 × 10−4 cm2 V−1 s−1, respectively, and that corresponds to µe/µh ratios of 1.95, 1.50 and 1.97. The higher charge mobilities in AQx-2- and AQx-6-based blends are in favor of superior charge transport, in comparison to those in AQx-8-based blend, which is consistent with the results of GIWAXS. Besides, the low µe/µh ratio in AQx-6-based blend suggests more balance charge transport, leading to less recombination and the highest FF.
Fig. 4. Morphology investigations.
a GIWAXS 2D patterns for AQx-2, AQx-6 and AQx-8 pristine films and the corresponding blend films. b The corresponding line-cuts of GIWAXS patterns. c π–π distance and electron mobility. d Dimer numbers (per molecule) for AQx-2, AQx-6 and AQx-8 pristine films.
To deeply investigate the influence of alkyl side chain, the calculations on the dimer number for individual rigid backbones were performed, in which dimer number relates to the contact between interacting backbones. The total dimer numbers over 2 indicates good intermolecular connectivity, which is a necessary precondition for efficient charge transport. As presented in Fig. 4d, the total dimer numbers for AQx-2, AQx-6 and AQx-8 film are 2.484, 2.313 and 2.053, respectively, implying the existing of continuous charge transport channels. In addition, the decreasing trend of dimer number with increased alkyl side-chain length in AQx-type acceptors indicates prolonged alkyl side chain will undermine the continuous aggregation of backbones. The π–π packing can be accessed by the theoretical calculations on the distribution of average dimer number (over 8 backbones interacting atoms) for per molecule. As shown in Supplementary Fig. 26, AQx-2, AQx-6 and AQx-8 show the total interactions for π–π stacking of 1.074, 1.036 and 0.973, indicating the high crystallinity exists in AQx-2 and AQx-6, which is in accord with experimental results. The AQx-2 with shortest alkyl chain shows large separation with less D/A interface for exciton dissociation, while the extensively extended alkyl side chain in AQx-8 generates distinct weakened π–π packing, leading to more charge recombination. On the contrary, AQx-6 with subtle prolonged alkyl side chain can form suitable domain size for efficient exciton dissociation and maintain compact π–π packing for the high rate of charge transport. Therefore, there is a delicate balance between low FVR (for reduced nonradiative decay rate) and ideal morphology (desired phase separation for exciton dissociation and strong crystallinity for efficient charge transport), inspiringly, precisely alkyl side-chain modulation can allow a subtle balance and toward higher performance.
Charge dynamics
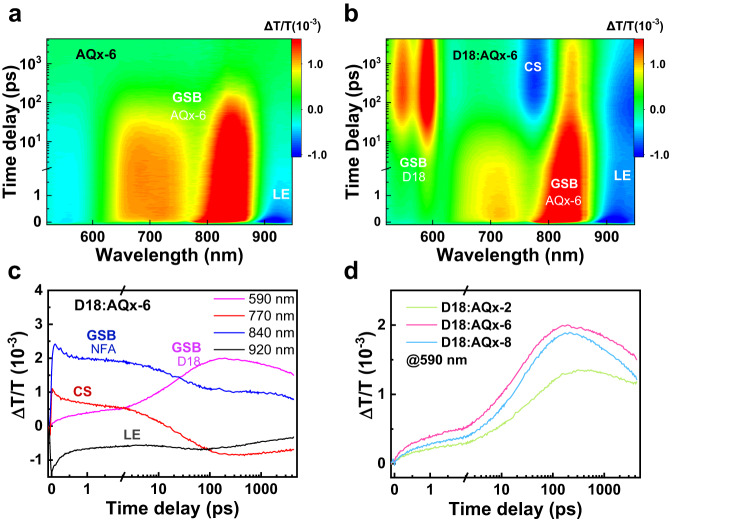
Furthermore, the charge dynamics including exciton transport and charge generation have been in-deep investigated for better understanding of three devices. The TA measurement was carried out to analyze the charge-transfer dynamics in the D/A interface. The 800 nm pump beam was selected to excite NFA in three blends, which can prevent donor from being excited. Figure 5 and Supplementary Figs. 27–28 show the TA results for neat and blend films. The pristine acceptor films exhibit the ground-state bleaching (GSB) peaks at ~730 and ~840 nm and the excited-state absorption (ESA) signal of local excited (LE) state at ~920 nm, which can be observed in blend films. After 10 ps, the ESA signals emerging at ~770 nm, which can be assigned to the charge separation (CS) state in the blend films, indicting the onset of the exciton dissociation process79. The two bleach peaks at ~560 and ~590 nm can be ascribed to the GSB of donor, considering there are on singles in neat acceptor films. Herein, the GSB peak at 590 nm was chosen to analyze the hole transfer kinetics, as shown in Fig. 5d. All three blends give quick emergence of the bleach signal of D18 (Supplementary Fig. 28), indicating an ultrafast hole transfer process from acceptor to donor in three blends. Such phenomenon was also observed in other highly performed OSCs. To get a better understanding, the hole transfer can be evaluated by fitting the GSB peak of donor at 590 nm with a double exponential function, thus came: D18:AQx-2 (τ1 = 2.94 ± 0.12 ps, τ2 = 49.23 ± 0.83 ps), D18:AQx-6 (τ1 = 2.24 ± 0.08 ps, τ2 = 27.40 ± 0.42 ps) and D18:AQx-8 (τ1 = 2.24 ± 0.10 ps, τ2 = 31.71 ± 0.45 ps), respectively, where τ1 is related to exciton dissociation rate at the D/A interface and τ2 represents the time for photo-induced exciton diffuse to D/A interface80. The slower exciton diffusion rate in D18:AQx-2 may be related to its large domain size, while D18:AQx-6 and D18:AQx-8 possess refined phase separation, which can contribute the exciton diffusion from intradomain into D/A interface at higher rate. The photoluminescence (PL) quenching measurements were carried out to study the charge-transfer efficiency (Supplementary Fig. 29). All of three blend films exhibit highly efficient electron transfer efficiency due to the sufficient driving force from LUMO energy offset. When NFA was selected excited, the AQx-2-, AQx-6-, and AQx-8-based bends display the hole transfer efficiency of 94.3, 94.4 and 91.0%, respectively. These results show that D18:AQx-6-based OSC delivers highest exciton dissociation and charge-transfer efficiency than those in AQx-2- and AQx-8-based OSCs, contributing to the formation of superior JSC and FF81,82.
Fig. 5. Transient absorption.
TA spectra of AQx-6 neat film (a) and D18:AQx-6 blend film (b). c TA traces of GSB signal for D18: 590 nm, the CS signal for D18:AQx-6 blend: 770 nm, the GSB (840 nm) and LE signals (920 nm) of AQx-type acceptor. d Kinetic traces of D18:AQx-2, D18:AQx-6 and D18:AQx-8 at indicated wavelength (590 nm).
Discussion
In this work, we demonstrated that efficient OSCs with reduced ∆Enr can be enabled by suppressed electron–phonon coupling, and proposed that the FVR can act as the indicator to evaluate molecular motion in solid state associated with nonradiative decay process. We developed a series of AQx-type acceptors, AQx-2, AQx-6 and AQx-8, with gradually extended alkyl side-chain length based on rational molecular design. Theoretical and experimental results confirmed that prolonged alkyl side chain contributes to decreased FVR in aggregation states. The restricted molecular motion in limited free volume provides suppressed nonradiative decay rate and then offers reduced ∆Enr in OSCs. Energy loss analysis suggests that AQx-6 and AQx-8 possess lower ∆Enr and elevated VOC values, compared to that in AQx-2 with the shortest alkyl side chain. Whereas the variation of alkyl side-chain length also affects the nanomorphology in blend films. For one thing, the increased alkyl side-chain length in AQx-type acceptors induces improved miscibility between NFA and donor, leading the formation of small phase separation that provides efficient exciton dissociation. For another, the long alkyl chain will affect the ordering π–π stacking in film, causing decreased mobilities and increased charge recombination. Inspiringly, the asymmetrical alkyl side chain in AQx-6 endows it with good miscibility with D18 to from suitable separation and compact π–π packing, which is of great importance for efficient charge generation and less charge recombination. As a result, the reduced ∆Enr and favorable nanomorphology for efficient exciton dissociation and charge transport are simultaneously obtained in AQx-6 via precisely unilateral proliferation of alkyl side chain. Ultimately, the OSCs based on AQx-6 achieved a champion PCE of 18.6% (certified PCE: 18.4%), which is one of the highest PCEs among bulk-heterojunction binary OSCs. Tuning hybridization of LE and CT states via modulating energy level, reducing reorganization energy or adjusting electrostatic potential distribution that minishes ∆Enr has been proved to be effective strategies, which is ascribed to the restrained nonradiative decay process41,48,58,83–87. This work generates a new direction that decreases electron–phonon coupling associated with molecular motion to slow down knr, contributing to enlightening research to construct low ∆Enr via a rational design strategy, which is of great significance to the further development of OSCs.
Methods
Materials
D18 was purchased was purchased from Derthon Optoelectronic Materials Science Technology (Shenzhen, China). AQx-2 was synthesized according to reported literature63. The synthesis route of AQx-6 is shown in Scheme S1, AQx-8 was synthesized according to AQx-2.
Theoretical calculation
Molecular dynamics simulations were conducted with GROMACS-4.6.7 software package. All DFT calculations were experimented with the Gaussian 16 codes. Setting of Force Field Parameters: for both the solute AQx-2, AQx-6, AQx-8 and the solvent chloroform force fields, their atom types, intramolecular and intermolecular interaction parameters were provided in the general AMBER force field (GAFF). Their atomic charges were calibrated using restrained electrostatic potential (RESP) obtained by density functional theory (DFT) calculations in vacuum at ωb97xd/cc-pvtz level. The solute’s torsional potentials between their central D and A units were fitted with DFT calculated potential energy, which used B3LYP-D3(BJ) functional with dispersion correction of DFT-D3 and a BJ damping under 6–31G** basis set. MD Simulation Protocol: The protocol consists of an NPT ensemble equilibrium molecular dynamics simulation for 50 ns of a solvated AQx and a quasi-equilibrium molecular dynamics approach to model the solvent evaporation and thin-film formation process of AQx. Both of the simulations proceeded under 300 K, 1 bar to align with experiment environments. The quasi-equilibrated system was a mixture of 60,000 chloroform and 200 conformations of AQx which was extracted during the NPT equilibrated simulation. The system was first equilibrated for 10 ns and then evaporated 100 random solvents for each 100 ps. When the thin film formed, it was annealed to 500 K and quenched back to 300 K. This annealing process is repeated until the system reached equilibrium. The protocol was followed for AQx-2, AQx-6 and AQx-8.
Single-crystal growth
Single crystal of AQx-2 was grown by the liquid diffusion method at room temperature. An appropriate amount of diethyl ether is transferred to a concentrated chloroform solution, which will form crystals over time. Single-crystal diffraction data was collected by single-crystal X-ray diffractometer (XtaLAB PRO 007HF(Mo)). The X-ray crystallographic coordinates for structures reported of AQx-2 has been deposited at the Cambridge Crystallographic Data Centre (CCDC), under deposition numbers 2161927.
Device fabrication
The device with a conventional structure: ITO/PEDOT:PSS/D:A/PDINN/Ag. The substrates were sequentially cleaned by detergent, water, acetone, ethyl alcohol for 30 min each step. The PEDOT:PSS (30 nm) was spin-coated on pre-cleaned ITO substrates after ITO substrates were treated with 30 min oxygen plasma. Then the substrates were heated at 150 °C for 20 min under air condition. The substrates were then transferred into a nitrogen-filled glove box. The weight ratio of D18:acceptor is 1:1.2 and apply chloroform as solvent. The D18:acceptor blend solution with a total concentration of 9.5 mg/mL was heated at 100 °C till completely dissolved. Then the blend solution was spin-coated on PEDOT:PSS by 2000 rpm, the thickness of the active layer is 110 nm. And the D18:accepter was heated at 80 °C for 10 min. The PDINN in ethyl alcohol (1 mg/ml) was spin-coated on the active layer by 3000 rpm. The Ag (100 nm) was deposed onto the substrates by vacuum evaporation. Shadow masks were used to define the OSC active area (0.04 cm2) of the devices.
Device characterization
The current density–voltage (J–V) characteristics of unencapsulated photovoltaic devices were measured under N2 using a Keithley 2400 source meter. The illumination of AM 1.5 G (100 mW cm-2) was achieved by a XES-70S1 solar simulator (SAN-EI Electric Co., Ltd, AAA grade, 70 mm × 70 mm photo-beam size). The external quantum efficiency (EQE) was performed using certified incident photon to current conversion efficiency equipment from Enlitech (Taiwan).
SCLC mobility measurement
Space-charge-limited currents were tested in hole-only devices with a structure of ITO/PEDOT:PSS/active layer/Au and electron-only devices with a configuration of ITO/ZnO/active layer/PDINN/Ag. The devices were prepared following the same procedure described in the experimental section for photovoltaic devices, except for the metal electrode. The hole and electron mobilities were calculated as follows:
| 1 |
Where J is the current density, μ0 is the zero-field mobility, ε0 is the permittivity of free space, εr is the relative permittivity of the material, V is the effective voltage, and L is the thickness of the active layer.
Morphology characterization
Atomic force microscopy (AFM) images of the thin films were obtained on a NanoscopeIIIa AFM (Digital Instruments) operating platform in tapping mode. The samples were prepared by spinning coated the active layers on the PEDOT:PSS layer.
Transmission electron microscopy (TEM) observation was performed on JEOL 2200FS at 80 kV accelerating voltage. The samples for electron microscopy were prepared by dissolving the PEDOT:PSS layer using water and transferring the floating active layer to the TEM grids. Grazing-incidence wide angle X-ray scattering (GIWAXS)was performed at the National Center for Nanoscience and Technology, China (NCNST). Thin film samples were spin-casted on to PEDOT:PSS covered SiO2 wafers.
Energy loss analysis
Highly sensitive EQE was measured using an integrated system (PECT-600, Enlitech), where the photocurrent was amplified and modulated by a lock-in instrument. EQEEL measurements were performed by applying external voltage/current sources through the devices (ELCT-3010, Enlitech). All the devices were prepared for EQEEL measurements according to the optimal device fabrication conditions.
Spectroscopic measurements
Transient Absorption (TA) Spectroscopy measurements were performed on an ultrafast TA spectrometer (Harpia-TA, Light Conversion). An 1030 nm pulse with a repetition rate of 54 kHz was generated by a Yb:KGW laser. Then the pulse was separated into two parts by a beam splitter. One part was coupled into an optical parametric amplifier to generate the pump pulses at various wavelength. The other part was focused onto a sapphire plate to generate supercontinuum white light as the probe beams. The time delay between pump and probe was controlled by a motorized optical delay line with a maximum delay time of 3.6 ns. The pump pulse is chopped by a mechanical chopper and then focused on to the mounted sample with probe beams. The probe beam was collimated and focused into a spectrometer with CCD sensor. The photoluminescence (PL) and photoluminescence quantum yield (PLQY) measurements were performed in Analytical Instrumentation Center of Peking University. The PL spectra were recorded with lifetime and steady state spectrometer (FLS980). The PLQY were detected with lifetime and steady state spectrometer (FLS980), which was coupled with OXFORD microstat. The time-resolution photoluminescence (TRPL) measurement was performed by Femtosecond Ultrafast Spectroscopy (Astrella-Opera solo).
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Supplementary information
Source data
Acknowledgements
This work was supported by the National Key R&D Program of China (2019YFA0705900 (X.Z.) and 2017YFA0204701 (X.Z.)), the National Natural Science Foundation of China (52225305 (X.Z.), 22175187 (X.Z.), 22171273 (S.X.), U2032112 (W.Z.), 21905163 (F.L.) and 22173108 (Y.Y.)), the Open Funding Project of State Key Laboratory of Organic-Inorganic Composites (oic-202201002 (X.Z.)), the International Partnership Program of Chinese Academy of Sciences (027GJHZ2022036GC (X.Z.)) and the Youth Innovation Promotion Association CAS (No. 2020031 (S.X.)). The authors thank Yanni Ouyang and Prof. Chunfeng Zhang for their help with transient absorption measurements, and Jinqiu Xu, Jingming Xin and Prof. Wei Ma for their help with Resonant soft X-ray scattering measurements.
Author contributions
X.Z. conceived and supervised the project. Y.J., Y.L. and F.L. contributed equally to this work. S.X. synthesized the AQx-2, AQx-6 and AQx-8; S.L. synthesized the AQx-6; Y.J. and F.L. fabricated, optimized the devices and performed the device characterizations, which were supervised by X.Z.; Y.L. carried out theoretical calculation, which were supervised by Y.Y.; W.S. performed transient absorption, which were supervised by W.Z.; W.W. performed the EL and FTPS-EQE experiments, which were supervised by J.H; W.L draw the figures. Y.J., Y.L., F.L., S.X., Y.Y. and X.Z. discussed the results and substantially contributed to the preparation of the manuscript.
Peer review
Peer review information
Nature Communications thanks Xiaotao Hao, Tao Wang and the other, anonymous, reviewers for their contribution to the peer review of this work. A peer review file is available.
Data availability
The authors declare that the data supporting the findings of this study are available within the paper and its supplementary information files. The single-crystal data in this study have been deposited at the Cambridge Crystallographic Data Centre (CCDC), under deposition numbers 2161927. The single-crystal data can be obtained free of charge from the Cambridge Crystallographic Data Centre via www.ccdc.cam.ac.uk/data_request/cif. Source data are provided with this paper.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Yuanyuan Jiang, Yixin Li, Feng Liu.
Contributor Information
Shengjie Xu, Email: xushengjie@iccas.ac.cn.
Yuanping Yi, Email: ypyi@iccas.ac.cn.
Xiaozhang Zhu, Email: xzzhu@iccas.ac.cn.
Supplementary information
The online version contains supplementary material available at 10.1038/s41467-023-40806-9.
References
- 1.Li G, et al. High-efficiency solution processable polymer photovoltaic cells by self-organization of polymer blends. Nat. Mater. 2005;4:864–868. [Google Scholar]
- 2.Uhrich C, et al. Organic thin-film photovoltaic cells based on oligothiophenes with reduced bandgap. Adv. Funct. Mater. 2007;17:2991–2999. [Google Scholar]
- 3.Yan C, et al. Non-fullerene acceptors for organic solar cells. Nat. Rev. Mater. 2018;3:18003. [Google Scholar]
- 4.Hou J, Inganas O, Friend RH, Gao F. Organic solar cells based on non-fullerene acceptors. Nat. Mater. 2018;17:119–128. doi: 10.1038/nmat5063. [DOI] [PubMed] [Google Scholar]
- 5.Tan L, et al. Highly efficient flexible polymer solar cells with robust mechanical stability. Adv. Sci. 2019;6:1801180. doi: 10.1002/advs.201801180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sun L, et al. Flexible all-solution-processed organic solar cells with high-performance nonfullerene active layers. Adv. Mater. 2020;32:1907840. doi: 10.1002/adma.201907840. [DOI] [PubMed] [Google Scholar]
- 7.Shen, Y. F. et al. In-situ absorption characterization guided slot-die-coated high-performance large-area flexible organic solar cells and modules. Adv. Mater. 35, 2209030 (2023). [DOI] [PubMed]
- 8.Sun R, et al. High-speed sequential deposition of photoactive layers for organic solar cell manufacturing. Nat. Energy. 2022;7:1087–1099. [Google Scholar]
- 9.Liu, J. et al. Regulation of polymer configurations enables green solvent-processed large-area binary all-polymer solar cells with breakthrough performance and high efficiency stretchability factor. Adv. Mater. 35, 2208008 (2023). [DOI] [PubMed]
- 10.Han Y, et al. 12.42% Monolithic 25.42 cm(2) flexible organic solar cells enabled by an amorphous ito-modified metal grid electrode. Adv. Mater. 2022;34:2110276. doi: 10.1002/adma.202110276. [DOI] [PubMed] [Google Scholar]
- 11.Liu F, et al. Efficient semitransparent solar cells with high nir responsiveness enabled by a small-bandgap electron acceptor. Adv. Mater. 2017;29:1606574. doi: 10.1002/adma.201606574. [DOI] [PubMed] [Google Scholar]
- 12.Liu W, et al. Theory-guided material design enabling high-performance multifunctional semitransparent organic photovoltaics without optical modulations. Adv. Mater. 2022;34:2200337. doi: 10.1002/adma.202200337. [DOI] [PubMed] [Google Scholar]
- 13.Wang D, et al. High-performance see-through power windows. Energy Environ. Sci. 2022;15:2629–2637. [Google Scholar]
- 14.Lin Y, et al. An electron acceptor challenging fullerenes for efficient polymer solar cells. Adv. Mater. 2015;27:1170–1174. doi: 10.1002/adma.201404317. [DOI] [PubMed] [Google Scholar]
- 15.Zhang M, Guo X, Ma W, Ade H, Hou J. A large-bandgap conjugated polymer for versatile photovoltaic applications with high performance. Adv. Mater. 2015;27:4655–4660. doi: 10.1002/adma.201502110. [DOI] [PubMed] [Google Scholar]
- 16.Liu F, et al. A thieno[3,4-b]thiophene-based non-fullerene electron acceptor for high-performance bulk-heterojunction organic solar cells. J. Am. Chem. Soc. 2016;138:15523–15526. doi: 10.1021/jacs.6b08523. [DOI] [PubMed] [Google Scholar]
- 17.Xu S, et al. A twisted thieno[3,4-b]thiophene-based electron acceptor featuring a 14-pi-electron indenoindene core for high-performance organic photovoltaics. Adv. Mater. 2017;29:1704510. doi: 10.1002/adma.201704510. [DOI] [PubMed] [Google Scholar]
- 18.Liu W, et al. Design of a new fused-ring electron acceptor with excellent compatibility to wide-bandgap polymer donors for high-performance organic photovoltaics. Adv. Mater. 2018;30:1800403. doi: 10.1002/adma.201800403. [DOI] [PubMed] [Google Scholar]
- 19.Sun C, et al. A low cost and high performance polymer donor material for polymer solar cells. Nat. Commun. 2018;9:743. doi: 10.1038/s41467-018-03207-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yuan J, et al. Enabling low voltage losses and high photocurrent in fullerene-free organic photovoltaics. Nat. Commun. 2019;10:570. doi: 10.1038/s41467-019-08386-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yuan J, et al. Single-junction organic solar cell with over 15% efficiency using fused-ring acceptor with electron-deficient core. Joule. 2019;3:1140–1151. [Google Scholar]
- 22.Cui Y, et al. Single-junction organic photovoltaic cells with approaching 18% efficiency. Adv. Mater. 2020;32:1908205. doi: 10.1002/adma.201908205. [DOI] [PubMed] [Google Scholar]
- 23.Liu Q, et al. 18% Efficiency organic solar cells. Sci. Bull. 2020;65:272–275. doi: 10.1016/j.scib.2020.01.001. [DOI] [PubMed] [Google Scholar]
- 24.Li C, et al. Non-fullerene acceptors with branched side chains and improved molecular packing to exceed 18% efficiency in organic solar cells. Nat. Energy. 2021;6:605–613. [Google Scholar]
- 25.Zhu X, et al. Design of all-fused-ring electron acceptors with high thermal, chemical, and photochemical stability for organic photovoltaics. CCS Chem. 2021;3:1070–1080. [Google Scholar]
- 26.Chen H, et al. Central unit fluorination of non-fullerene acceptors enables highly efficient organic solar cells with over 18 % efficiency. Angew. Chem. Int. Ed. 2022;61:202209580. doi: 10.1002/anie.202209580. [DOI] [PubMed] [Google Scholar]
- 27.Ma Y, et al. 17% Efficiency for linear-shaped ADA-type nonfullerene acceptors enabled by 3D reticulated molecular packing. Nano Energy. 2023;107:108116. [Google Scholar]
- 28.Zhou Z, et al. High-efficiency small-molecule ternary solar cells with a hierarchical morphology enabled by synergizing fullerene and non-fullerene acceptors. Nat. Energy. 2018;3:952–959. [Google Scholar]
- 29.Sun R, et al. Single-junction organic solar cells with 19.17% efficiency enabled by introducing one asymmetric guest acceptor. Adv. Mater. 2022;34:2110147. doi: 10.1002/adma.202110147. [DOI] [PubMed] [Google Scholar]
- 30.Chong K, et al. Realizing 19.05% efficiency polymer solar cells by progressively improving charge extraction and suppressing charge recombination. Adv. Mater. 2022;34:2109516. doi: 10.1002/adma.202109516. [DOI] [PubMed] [Google Scholar]
- 31.Gao J, et al. Over 19.2% efficiency of organic solar cells enabled by precisely tuning the charge transfer state via donor alloy strategy. Adv. Sci. 2022;9:2203606. doi: 10.1002/advs.202203606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Han, C. et al. Over 19% efficiency organic solar cells by regulating multidimensional intermolecular interactions. Adv. Mater. 35, 2208986 (2023). [DOI] [PubMed]
- 33.He C, et al. Manipulating the D:A interfacial energetics and intermolecular packing for 19.2% efficiency organic photovoltaics. Energy Environ. Sci. 2022;15:2537–2544. [Google Scholar]
- 34.Jiang K, et al. Suppressed recombination loss in organic photovoltaics adopting a planar–mixed heterojunction architecture. Nat. Energy. 2022;7:1076–1086. [Google Scholar]
- 35.Zhu L, et al. Single-junction organic solar cells with over 19% efficiency enabled by a refined double-fibril network morphology. Nat. Mater. 2022;21:656–663. doi: 10.1038/s41563-022-01244-y. [DOI] [PubMed] [Google Scholar]
- 36.Gao H, et al. Self-aggregated light-trapping nanodots for highly efficient organic solar cells. Small. 2022;18:2205128. doi: 10.1002/smll.202205128. [DOI] [PubMed] [Google Scholar]
- 37.Zhan L, et al. Multi-phase morphology with enhanced carrier lifetime via quaternary strategy enables high-efficiency thick-film and large-area organic photovoltaics. Adv. Mater. 2022;34:2206269. doi: 10.1002/adma.202206269. [DOI] [PubMed] [Google Scholar]
- 38.Li, D. et al. Fibrillization of non-fullerene acceptors enables 19% efficiency pseudo-bulk heterojunction organic solar cells. Adv. Mater. 35, 2208211 (2023). [DOI] [PubMed]
- 39.Wang J, et al. An asymmetric wide-bandgap acceptor simultaneously enabling highly efficient single-junction and tandem organic solar cells. Energy Environ. Sci. 2022;15:1585–1593. [Google Scholar]
- 40.Yao, J. et al. Quantifying losses in open-circuit voltage in solution-processable solar cells. Phys. Rev. Appl. 4, 014020 (2015).
- 41.Chen X-K, et al. A unified description of non-radiative voltage losses in organic solar cells. Nat. Energy. 2021;6:799–806. [Google Scholar]
- 42.Vandewal K, Tvingstedt K, Gadisa A, Inganas O, Manca JV. On the origin of the open-circuit voltage of polymer-fullerene solar cells. Nat. Mater. 2009;8:904–909. doi: 10.1038/nmat2548. [DOI] [PubMed] [Google Scholar]
- 43.Gillett AJ, et al. The role of charge recombination to triplet excitons in organic solar cells. Nature. 2021;597:666–671. doi: 10.1038/s41586-021-03840-5. [DOI] [PubMed] [Google Scholar]
- 44.Perebeinos V, Avouris P. Phonon and electronic nonradiative decay mechanisms of excitons in carbon nanotubes. Phys. Rev. Lett. 2008;101:057401. doi: 10.1103/PhysRevLett.101.057401. [DOI] [PubMed] [Google Scholar]
- 45.Vandewal K, et al. Efficient charge generation by relaxed charge-transfer states at organic interfaces. Nat. Mater. 2014;13:63–68. doi: 10.1038/nmat3807. [DOI] [PubMed] [Google Scholar]
- 46.Azzouzi M, et al. Nonradiative energy losses in bulk-heterojunction organic photovoltaics. Phys. Rev. X. 2018;8:031055. [Google Scholar]
- 47.Ullbrich S, et al. Emissive and charge-generating donor-acceptor interfaces for organic optoelectronics with low voltage losses. Nat. Mater. 2019;18:459–464. doi: 10.1038/s41563-019-0324-5. [DOI] [PubMed] [Google Scholar]
- 48.Qian D, et al. Design rules for minimizing voltage losses in high-efficiency organic solar cells. Nat. Mater. 2018;17:703–709. doi: 10.1038/s41563-018-0128-z. [DOI] [PubMed] [Google Scholar]
- 49.Zuo L, et al. Dilution effect for highly efficient multiple-component organic solar cells. Nat. Nanotechnol. 2022;17:53–60. doi: 10.1038/s41565-021-01011-1. [DOI] [PubMed] [Google Scholar]
- 50.Wilson JS, et al. The energy gap law for triplet states in pt-containing conjugated polymers and monomers. J. Am. Chem. Soc. 2001;123:9412–9417. doi: 10.1021/ja010986s. [DOI] [PubMed] [Google Scholar]
- 51.Gong X, et al. Electron-phonon interaction in efficient perovskite blue emitters. Nat. Mater. 2018;17:550–556. doi: 10.1038/s41563-018-0081-x. [DOI] [PubMed] [Google Scholar]
- 52.Lei T, Wang J-Y, Pei J. Roles of flexible chains in organic semiconducting materials. Chem. Mater. 2013;26:594–603. [Google Scholar]
- 53.Yue Q, Liu W, Zhu X. n-Type molecular photovoltaic materials: design strategies and device applications. J. Am. Chem. Soc. 2020;142:11613–11628. doi: 10.1021/jacs.0c04084. [DOI] [PubMed] [Google Scholar]
- 54.Wang J, Xue P, Jiang Y, Huo Y, Zhan X. The principles, design and applications of fused-ring electron acceptors. Nat. Rev. Chem. 2022;6:614–634. doi: 10.1038/s41570-022-00409-2. [DOI] [PubMed] [Google Scholar]
- 55.He C, et al. Asymmetric electron acceptor enables highly luminescent organic solar cells with certified efficiency over 18. Nat. Commun. 2022;13:2598. doi: 10.1038/s41467-022-30225-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zhao Z, et al. Highly efficient photothermal nanoagent achieved by harvesting energy via excited-state intramolecular motion within nanoparticles. Nat. Commun. 2019;10:768. doi: 10.1038/s41467-019-08722-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Benduhn J, et al. Intrinsic non-radiative voltage losses in fullerene-based organic solar cells. Nat. Energy. 2017;2:17053. [Google Scholar]
- 58.Chen XK, Coropceanu V, Bredas JL. Assessing the nature of the charge-transfer electronic states in organic solar cells. Nat. Commun. 2018;9:5295. doi: 10.1038/s41467-018-07707-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Liu J, et al. Through-space interaction of tetraphenylethylene: what, where, and how. J. Am. Chem. Soc. 2022;144:7901–7910. doi: 10.1021/jacs.2c02381. [DOI] [PubMed] [Google Scholar]
- 60.Hong EY, Wong HL, Yam VW. From spherical to leaf-like morphologies: tunable supramolecular assembly of alkynylgold(I) complexes through variations of the alkyl chain length. Chem. 2015;21:5732-–5735. doi: 10.1002/chem.201500078. [DOI] [PubMed] [Google Scholar]
- 61.Giese M, Albrecht M. Alkyl-alkyl interactions in the periphery of supramolecular entities: from the evaluation of weak forces to applications. Chempluschem. 2020;85:715–724. doi: 10.1002/cplu.202000077. [DOI] [PubMed] [Google Scholar]
- 62.Liu W, Zhang J, Xu S, Zhu X. Efficient organic solar cells achieved at a low energy loss. Sci. Bull. 2019;64:1144–1147. doi: 10.1016/j.scib.2019.07.005. [DOI] [PubMed] [Google Scholar]
- 63.Zhou Z, et al. Subtle molecular tailoring induces significant morphology optimization enabling over 16% efficiency organic solar cells with efficient charge generation. Adv. Mater. 2020;32:1906324. doi: 10.1002/adma.201906324. [DOI] [PubMed] [Google Scholar]
- 64.Liu F, et al. Organic solar cells with 18% efficiency enabled by an alloy acceptor: a two-in-one strategy. Adv. Mater. 2021;33:2100830. doi: 10.1002/adma.202100830. [DOI] [PubMed] [Google Scholar]
- 65.Shi Y, et al. Small reorganization energy acceptors enable low energy losses in non-fullerene organic solar cells. Nat. Commun. 2022;13:3256. doi: 10.1038/s41467-022-30927-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Jiang K, et al. Alkyl chain tuning of small molecule acceptors for efficient organic solar cells. Joule. 2019;3:3020–3033. [Google Scholar]
- 67.Ma Y, et al. Efficient organic solar cells from molecular orientation control of M-series acceptors. Joule. 2021;5:197–209. [Google Scholar]
- 68.Lu H, et al. High‐efficiency organic solar cells with reduced nonradiative voltage loss enabled by a highly emissive narrow bandgap fused ring acceptor. Adv. Funct. Mater. 2021;32:2107756. [Google Scholar]
- 69.Zhang H, et al. Aggregate science: from structures to properties. Adv. Mater. 2020;32:2001457. doi: 10.1002/adma.202001457. [DOI] [PubMed] [Google Scholar]
- 70.Lai H, et al. Trifluoromethylation enables a 3D interpenetrated low-band-gap acceptor for efficient organic solar cells. Joule. 2020;4:688–700. [Google Scholar]
- 71.Yao J, et al. Cathode engineering with perylene-diimide interlayer enabling over 17% efficiency single-junction organic solar cells. Nat. Commun. 2020;11:2726. doi: 10.1038/s41467-020-16509-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Shockley W, Queisser HJ. Detailed balance limit of efficiency of p‐n junction solar cells. J. Appl. Phys. 1961;32:510–519. [Google Scholar]
- 73.Rau U, Blank B, Müller TCM, Kirchartz T. Efficiency potential of photovoltaic materials and devices unveiled by detailed-balance analysis. Phys. Rev. Appl. 2017;7:044016. [Google Scholar]
- 74.Liu S, et al. High-efficiency organic solar cells with low non-radiative recombination loss and low energetic disorder. Nat. Photonics. 2020;14:300–305. [Google Scholar]
- 75.Vandewal K, Tvingstedt K, Gadisa A, Inganas O, Manca JV. Relating the open-circuit voltage to interface molecular properties of donor:acceptor bulk heterojunction solar cells. Phys. Rev. B. 2010;81:125204. [Google Scholar]
- 76.He D, Zhao F, Wang C, Lin Y. Non‐radiative recombination energy losses in non‐fullerene organic solar cells. Adv. Funct. Mater. 2022;32:2111855. [Google Scholar]
- 77.Wang Z, et al. The coupling and competition of crystallization and phase separation, correlating thermodynamics and kinetics in OPV morphology and performances. Nat. Commun. 2021;12:332. doi: 10.1038/s41467-020-20515-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Xia X, et al. Uncovering the out-of-plane nanomorphology of organic photovoltaic bulk heterojunction by GTSAXS. Nat. Commun. 2021;12:6226. doi: 10.1038/s41467-021-26510-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Wang R, et al. Charge separation from an intra-moiety intermediate state in the high-performance PM6:Y6 organic photovoltaic blend. J. Am. Chem. Soc. 2020;142:12751–12759. doi: 10.1021/jacs.0c04890. [DOI] [PubMed] [Google Scholar]
- 80.Wan J, et al. All-green solvent-processed planar heterojunction organic solar cells with outstanding power conversion efficiency of 16% Adv. Funct. Mater. 2021;32:2107567. [Google Scholar]
- 81.Zhang X, et al. On the understanding of energy loss and device fill factor trade-offs in non-fullerene organic solar cells with varied energy levels. Nano Energy. 2020;75:105032. [Google Scholar]
- 82.Li S, et al. Highly efficient fullerene-free organic solar cells operate at near zero highest occupied molecular orbital offsets. J. Am. Chem. Soc. 2019;141:3073–3082. doi: 10.1021/jacs.8b12126. [DOI] [PubMed] [Google Scholar]
- 83.Liu J, et al. Fast charge separation in a non-fullerene organic solar cell with a small driving force. Nat. Energy. 2016;1:16089. [Google Scholar]
- 84.Cui Y, et al. Over 16% efficiency organic photovoltaic cells enabled by a chlorinated acceptor with increased open-circuit voltages. Nat. Commun. 2019;10:2515. doi: 10.1038/s41467-019-10351-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Xu Y, et al. Tuning the hybridization of local exciton and charge-transfer states in highly efficient organic photovoltaic cells. Angew. Chem. Int. Ed. 2020;59:9004–9010. doi: 10.1002/anie.201915030. [DOI] [PubMed] [Google Scholar]
- 86.An N, et al. Solution-processed organic solar cells with high open-circuit voltage of 1.3 V and low non-radiative voltage loss of 0.16 V. Adv. Mater. 2020;32:2002122. doi: 10.1002/adma.202002122. [DOI] [PubMed] [Google Scholar]
- 87.Karuthedath S, et al. Intrinsic efficiency limits in low-bandgap non-fullerene acceptor organic solar cells. Nat. Mater. 2021;20:378–384. doi: 10.1038/s41563-020-00835-x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The authors declare that the data supporting the findings of this study are available within the paper and its supplementary information files. The single-crystal data in this study have been deposited at the Cambridge Crystallographic Data Centre (CCDC), under deposition numbers 2161927. The single-crystal data can be obtained free of charge from the Cambridge Crystallographic Data Centre via www.ccdc.cam.ac.uk/data_request/cif. Source data are provided with this paper.