Abstract
The prevalence of acute vasodilator response (AVR) to inhaled nitric oxide (iNO) during right heart catheterization (RHC) is 12% in idiopathic pulmonary arterial hypertension (IPAH). AVR, however, is reportedly lower in other disease‐associated pulmonary arterial hypertension (PAH), such as connective tissue disease (CTD). The prevalence of AVR in patients on PAH therapy (prevalent cases) is unknown. We sought to determine AVR prevalence in Group 1 PH in the PVDOMICS cohort of incident and prevalent patients undergoing RHC. AVR was measured in response to 100% O2 and O2 plus iNO, with positivity defined as (1) decrease in mean pulmonary artery pressure (mPAP) by ≥10 mmHg to a value ≤40 mmHg, with no change or an increase in cardiac output (definition 1); or (2) decrease in mPAP by ≥12% and pulmonary vascular resistance by ≥30% (definition 2). AVR rates and cumulative survival were compared between incident and prevalent patients. In 338 mainly prevalent (86%) patients, positive AVR to O2‐only was <2%, and 5.1% to 16.9%, based on definition 1 and 2 criteria, respectively; following O2 + iNO. IPAH AVR prevalence (4.1%–18.7%) was similar to prior reports. AVR positivity was 7.7% to 15.4% in mostly CTD‐PAH prevalent cases, and 2.6% to 11.8% in other PAH groups. Survival was 89% in AVR responders versus 77% in nonresponders from PAH diagnosis, and 91% versus 86% from PVDOMICS enrollment (log‐rank test p = 0.04 and p = 0.05, respectively). In conclusion, AVR in IPAH patients is similar to prior studies. AVR in non‐IPAH patients was higher than previously reported. The relationship between PAH therapy, AVR response, and survival warrants further investigation.
Keywords: pulmonary arterial hypertension, survival, vasoreactivity
INTRODUCTION
Pulmonary arterial hypertension (PAH) remains a disease with significant morbidity and mortality despite a plethora of modern medications which have improved general outcomes. 1 PAH is characterized by severe remodeling of the small pulmonary arteries and a progressive increase in pulmonary vascular resistance (PVR) leading to right ventricular failure and death if left untreated. 2 , 3 , 4
Although remodeling of the pulmonary vessels is the main pathological finding in PAH, vasoconstriction plays an important role in the disease pathophysiology, particularly in patients who display vasoreactivity. 5 Prior studies have demonstrated that patients with idiopathic pulmonary arterial hypertension (IPAH) and a positive acute vasodilator response (AVR) during hemodynamic assessment by right heart catheterization (RHC) had dramatically improved outcomes, with near‐normal survival, when treated with long‐term calcium‐channel blockers (CCB) compared with patients with no acute response. 6 , 7 Therefore, the goal of AVR testing in incident patients is to identify PAH patients who will respond favorably to long‐term CCB therapy. 8 However, less than 10% of IPAH patients have a long‐term response to CCB. 9 Moreover, in other subgroups of PAH, the proportion of AVR responders among incident and prevalent patients may differ, and, even in the presence of AVR, initiation of CCB may be deleterious in some associated conditions. 10 , 11 Furthermore, the prevalence of AVR responsiveness in these other forms of PAH, and the clinical implications and overall outcomes, are essentially unknown.
In the Pulmonary Vascular Disease Phenomics Study, 12 AVR was assessed with 100% oxygen (O2) alone and O2 plus inhaled nitric oxide (O2 + iNO) in all World Symposium on Pulmonary Hypertension (WSPH) groups. AVR was performed systematically in all patients with elevated mean pulmonary artery pressure (mPAP > 25 mmHg) regardless of the etiology or hemodynamic findings. This protocol‐driven assessment of AVR provided an opportunity to examine vasoreactivity systematically in both incident and prevalent group 1 subjects, as well as, in the non‐IPAH population.
In this study, we sought to test the hypothesis that AVR would differ between IPAH and non‐IPAH in WSPH Group 1 PH, and between treatment‐naive (incident) and treated (prevalent) patients, with potential disparities in cumulative survival across groups. Additionally, two different AVR criteria were compared to assess variability in AVR positivity.
PATIENTS AND METHODS
Patient population
Group 1 PH patients enrolled in PVDOMICS underwent RHC for baseline measurements. Details of the PVDOMICS methodology and core adjudication of hemodynamic measurements have been previously reported. 12 The diagnosis of PAH was established by a mPAP greater than 25 mmHg at rest, a pulmonary capillary wedge pressure (PCWP) ≤ 15 mmHg, and a PVR ≥ 3 Wood units according to the definition in effect at the time of the study initiation. 13 Within group 1 PH, we limited our analysis to IPAH, familial PAH (FPAH), drug induced PAH (DI‐PAH), HIV‐associated PAH (HIV‐PAH), portopulmonary hypertension associated PAH (PoPH‐PAH), congenital heart disease associated PAH (CHD‐PAH), PAH due to pulmonary veno‐occlusive disease (PVOD‐PAH), PAH due to pulmonary capillary hemangiomatosis (PCH‐PAH), and PAH due to connective tissue diseases (CTD‐PAH). Other causes of group 1 PH were excluded due to a low number of subjects. Patients' characteristics at enrollment, including, demographic, clinical, and laboratory data, as well hemodynamic and pulmonary function assessments, have been previously fully described 12 and were retrieved from the PVDOMICS registry.
AVR was measured in response to 100% inhaled O2 and 100% O2 plus inhaled nitric oxide (iNO) 40 ppm (O2 + iNO). At baseline and 5 min following each intervention, the following hemodynamic parameters were measured, including right atrial pressure (RAP), mPAP, pulmonary artery wedge pressure (PAWP), and cardiac output (CO) by thermodilution, with calculation of PVR, cardiac index (CI), and systemic vascular resistance (SVR). Rates of AVR to 100% O2 and O2 + iNO were compared between incident and prevalent patients in each PAH subgroup.
Vasoreactivity definition
We utilized two different AVR criteria, based on prior published studies, and compared clinical outcomes based on each definition, as follows:
Statistical methods
Group comparisons were made using the χ 2 and Fisher exact tests as appropriate for categorical variables, and analysis of variance test was used for continuous variables. Patients were grouped as IPAH/FPAH, CTD‐PAH, and other‐PAH (including DI‐PAH, HIV‐PAH, PoPH‐PAH, CHD‐PAH, PVOD‐PAH, and PCH‐PAH). Missing data was considered missing not completely at random, and complete‐case analyses were initially performed, followed by additional sensitivity analyses excluding variables with significant missingness (>5%). Mortality was assessed using the Kaplan–Meier method. Comparisons between groups were performed by log‐rank test. Computations were completed using the Stata statistical software (version 17.0; Stata). A p value < 0.05 was considered statistically significant.
RESULTS
Study population
Three‐hundred and thirty‐eight patients with group 1 PH were enrolled in PVDOMICS. For simplicity, three larger subgroups were assessed and compared, including IPAH/FPAH with a total of 171 patients (145 with IPAH and 46 with FPAH), CTD‐PAH with 91 patients (including 42 systemic sclerosis [SSc], 15 systemic lupus erythematosus [SLE], 16 mixed CTD [MCTD], 10 Sjogren's disease, 7 rheumatoid arthritis, and 1 anti‐synthetase syndrome), and other‐PAH with 76 patients [which included DI‐PAH (15), HIV‐PAH (7), PoPH‐PAH (13), CHD‐PAH (31), PVOD‐PAH (6), and PCH‐PAH (3)]. Baseline demographics, clinical, echocardiographic, and hemodynamic parameters for the three specified PAH subgroups are shown in Table 1. Compared with IPAH/FPAH, patients with CTD‐PAH were older (59.6 ± 12.2 vs. 50.9 ± 14.6 years; p < 0.001) and had a higher proportion of females (81% vs. 70%; p = 0.12). Overall, there was a lower proportion of African Americans (11%) compared with Whites (76%). The cohorts predominantly included prevalent patients (86%), with a similar distribution across subgroups (89% vs. 79% vs. 88%, p = 0.07), and a median time from diagnosis of 3.2 years. WHO FC, assessed during study enrollment, showed an overall high proportion of patients with functional limitation (WHO FC II: 41%, III: 42%, IV: 5%).
Table 1.
Patient characteristics.
| All, n = 338 | IPAH/FPAH, n = 171 | CTD‐PAH, n = 91 | Other‐PAH, n = 76a | p Value | |
|---|---|---|---|---|---|
| Age at enrollment, years, mean | 52.6 ± 13.4 | 50.9 ± 14.6 | 59.6 ± 12.2 | 47.9 ± 13.3 | <0.001 |
| Female, n (%) | 249 (73.7) | 119 (69.6) | 74 (81.3) | 56 (73.7) | 0.12 |
| Race, White/AA/Others, n | 257/37/44 | 137/14/20 | 64/16/11 | 56/7/13 | 0.16 |
| Prevalent/incident | 291 (86)/47 (14) | 152 (89)/19 (11) | 72 (79)/19 (21) | 67 (88)/9 (12) | 0.07 |
| Years since PH diagnosis, median (min, max) | 3.2 (0.0, 35.9) | 3.7 (0.0, 35.9) | 2.5 (0.0, 21.0) | 4.0 (0.0, 27.2) | 0.19 |
| WHO FC at enrollment (I/II/III/IV), n | 43/137/141/17 | 32/76/58/5 | 6/32/46/7 | 6/28/37/5 | <0.001 |
| Vitals signs at enrollment | |||||
| Systolic BP, mmHg, mean | 114 ± 16 | 114 ± 15 | 117 ± 18 | 113 ± 16 | 0.27 |
| Diastolic BP, mmHg, mean | 69 ± 10 | 68 ± 10 | 69 ± 11 | 69 ± 10 | 0.77 |
| Heart rate, bpm, mean | 76 ± 13 | 77 ± 12 | 77 ± 13 | 75 ± 14 | 0.72 |
| Respiratory rate, rpm, mean | 18 ± 4 | 17 ± 2 | 19 ± 6 | 18 ± 3 | 0.06 |
| BMI, kg/m2, mean | 29.1 ± 7.5 | 30.0 ± 7.3 | 27.8 ± 7.1 | 28.5 ± 8.2 | 0.04 |
| Laboratory data at enrollment | |||||
| Creatinine, mg/dL, mean | 0.9 ± 0.4 | 1.0 ± 0.6 | 1.0 ± 0.5 | 1.0 ± 0.4 | 0.93 |
| eGFR, mL/min/1.73 m2, mean | 80.6 ± 24.4 | 82.5 ± 23.8 | 75.4 ± 25.0 | 82.2 ± 24.4 | 0.08 |
| Na, mEq/mL, mean | 139.4 ± 2.8 | 139.6 ± 2.7 | 139.5 ± 2.8 | 139.1 ± 3.1 | 0.47 |
| Bilirubin total, mg/dL, mean | 0.7 ± 0.6 | 0.7 ± 0.5 | 0.6 ± 0.3 | 1.0 ± 0.9 | <0.001 |
| Hemoglobin, g/dL, mean | 13.7 ± 2.1 | 13.9 ± 1.9 | 12.9 ± 1.9 | 14.3 ± 2.5 | <0.001 |
| ProBNP, pg/mL, median [P25, P75] | 258 [97, 997] | 185 [83, 785] | 602 [179, 2252] | 180 [91, 857] | <0.001 |
| Troponin, ng/mL, median (min, max) | 0.01 (0.009, 0.15) | 0.009 (0.009, 0.15) | 0.009 (0.009, 0.03) | 0.009 (0.009, 0.118) | 0.59 |
| Pulmonary function at enrollment | |||||
| 6MWD, m, median (min, max) | 405 (38, 732) | 420 (38, 732) | 364 (122, 615) | 394 (107, 615) | <0.001 |
| FEV1 percentage, mean | 78.1 ± 18.1 | 80.4 ± 17.7 | 74.7 ± 18.1 | 76.6 ± 18.7 | 0.05 |
| FVC percentage, mean | 84.4 ± 17.1 | 87.3 ± 15.2 | 78.9 ± 17.8 | 83.8 ± 19.2 | <0.001 |
| FEV1/FVC percentage, mean | 92.0 ± 10.0 | 91.2 ± 9.5 | 94.5 ± 10.7 | 91.1 ± 9.9 | 0.03 |
| TLC percentage, mean | 89.2 ± 15.9 | 92.9 ± 13.4 | 80.7 ± 14.7 | 91.0 ± 18.9 | <0.001 |
| DLCO percentage, mean | 57.4 ± 20.1 | 63.6 ± 19.0 | 41.9 ± 17.8 | 60.7 ± 19.5 | <0.001 |
| Therapies | |||||
| Diuretic, n (%) | 216 (63.9) | 113 (66.1) | 60 (65.9) | 43 (56.6) | 0.31 |
| PDE5 inhibitor, n (%) | 225 (66.6) | 117 (68.4) | 59 (64.8) | 49 (64.5) | 0.76 |
| ERA, n (%) | 189 (55.9) | 99 (57.9) | 45 (49.5) | 45 (59.2) | 0.34 |
| Prostacyclin, n (%) | 147 (43.5) | 85 (49.7) | 29 (31.9) | 33 (43.4) | 0.02 |
| SGC, n (%) | 17 (5.1) | 8 (4.7) | 3 (3.3) | 6 (7.9) | 0.38 |
| CCB, n (%) | 16 (4.8) | 11 (6.4) | 3 (3.3) | 2 (2.6) | 0.32 |
| 0/1/2/>2 PH drugs, n | 55/62/135/86 | 26/25/68/52 | 19/19/39/14 | 10/18/28/20 | 0.11 |
| Echocardiographic data | |||||
| RV dilation (normal/mild/moderate/severe), n | 66/101/89/70 | 34/55/42/35 | 22/18/30/15 | 10/28/17/20 | 0.07 |
| TR (none/mild/moderate/severe), n | 87/145/75/17 | 53/72/32/7 | 18/34/27/6 | 16/39/16/4 | 0.14 |
| RVSP, mmHg, median [P25, P75] | 64.7 [47.7, 84.3] | 61.6 [44.9, 80.3] | 65.3 [49.3, 77.8] | 69.9 [57.2, 90.8] | 0.05 |
| TAPSE, cm, median [P25, P75] | 1.8 [1.5, 2.2] | 1.9 [1.6, 2.2] | 1.8 [1.5, 2.2] | 1.8 [1.5, 2.0] | 0.91 |
| Pericardial effusion, n (%) | 135 (41.7) | 65 (40.0) | 51 (60.0) | 19 (25.7) | <0.001 |
| Hemodynamics at rest | |||||
| RAP, mmHg, mean | 7 ± 5 | 7 ± 4 | 7 ± 5 | 8 ± 6 | 0.67 |
| mPAP, mmHg, mean | 44 ± 15 | 43 ± 15 | 42 ± 13 | 42 ± 13 | 0.81 |
| PCWP, mmHg, mean | 11 ± 5 | 11 ± 5 | 10 ± 5 | 11 ± 6 | 0.34 |
| CO, L/min, median (min, max) | 5.0 (1.96, 13.7) | 5.2 (2.53, 11.73) | 4.7 (2.37, 13.7) | 5.0 (1.96, 9.05) | 0.18 |
| CI, L/min/m2, mean | 2.79 ± 0.88 | 2.79 ± 0.86 | 2.77 ± 0.97 | 2.79 ± 0.83 | 0.97 |
| PVR, Wood units, median [P25, P75] | 6.2 (0.5, 33.7) | 6.1 (0.5, 19.2) | 6.2 (1.2, 31.7) | 6.7 (2.0, 33.7) | 0.09 |
| SVR, mL/beat, mean | 71.7 ± 26.6 | 75.0 ± 26.6 | 66.7 ± 27.2 | 70.2 ± 24.7 | 0.04 |
| Mortality, n (%) | 42 (12.4) | 17 (9.9) | 17 (18.7) | 8 (10.5) | 0.10 |
Note: Statistics presented as mean ± SD, median [P25, P75], median (min, max) or N (%).
Abbreviations: 6MWD, 6 minute walk distance; AVR, acute vasodilator response; BMI, body mass index; BP, blood pressure; CCB, calcium‐channel blockers; CI, cardiac index; CO, cardiac output; DLCO, diffusion capacity of the lung to carbon monoxide; eGFR, estimated glomerular filtration rate; ERA, endothelin receptor antagonist; FEV1, force expiratory volume in 1 second; FVC, force vital capacity; IPAH, idiopathic pulmonary arterial hypertension; mPAP, mean pulmonary artery pressure; PAH, pulmonary arterial hypertension; PCWP, pulmonary capillary wedge pressure; PDE5, phosphodiesterase‐5; PVR, pulmonary vascular resistance; RAP, right atrial pressure; RV, right ventricle; RVSP, Right Ventricular Systolic Pressure; SCG, soluble guanylate cyclase; SVR, systemic vascular resistance; TAPSE, tricuspid annular plane systolic excursion; TLC, total lung capacity; TR, tricuspid regurgitation.
Other‐PAH includes: DI‐PAH, HIV‐PAH, PH‐PAH, CHD‐PAH, PVOD‐PAH, PCH‐PAH.
At enrollment, systolic blood pressure, diastolic blood pressure, heart rate, respiratory rate, BMI, creatinine, and estimated glomerular filtration rate, were not significantly different across groups. Patients with other‐PAH had the highest mean levels of total bilirubin (1.0 mg/dL ± 0.9; p < 0.001) and hemoglobin (14.3 g/dL ± 2.5; p < 0.001). CTD‐PAH patients displayed the highest levels of proBNP (602 pg/mL, interquartile range: 179–2552, p < 0.001). Regarding PFTs, patients with PAH/FPAH had statistically significant higher mean values across all parameters.
Regarding therapy, the majority of patients were already receiving either two or more PAH‐specific medications (135 vs. 86, respectively). The most commonly used medications included phosphodiesterase‐5 inhibitors (PDE5‐i) (66.6%), followed by endothelin receptor antagonists (55.9%), prostanoids (43.5%), soluble guanylate cyclase (SGC) stimulators (5.1%), and CCB (4.8%). Patients not receiving targeted therapy (55) included participants with a recent PAH diagnosis. Furthermore, 62 patients were clinically stable on monotherapy.
Acute vasodilator testing
Among all participants, 316 underwent AVR with 100% O2 only, and 318 had a combined test with 100% O2 + iNO. Response to O2 and O2 + iNO using AVR definition 1 is shown in Table 2. Overall, O2 alone had little effect on cardiopulmonary hemodynamics across the different groups (four responders), with most of the patients being prevalent (three responders). However, O2 + iNO resulted in a higher number of responders (n = 16), including seven IPAH/FPAH, seven CTD‐PAH, and two other‐PAH patients. CTD‐PAH included five SSc, one MCTD, and one SLE patients. Furthermore, the majority of responders were prevalent participants (n = 12).
Table 2.
AVR evaluation based on Definition 1, O2 versus O2 + iNO responders.
| Patients (N) | 100% O2 (% responders) | O2 + iNO (% responders) | |
|---|---|---|---|
| Group 1 PH | 338 | 4/316 (1.3) | 16/318 (5.1) |
| IPAH/FPAH | 171 | 2 (1.2) | 7 (4.1) |
| Prevalent | 152 | 1 | 5 |
| Incident | 19 | 1 | 2 |
| CTD‐PAH | 91 | 1 (1.1) | 7 (7.7) |
| Prevalent | 72 | 1 | 6 |
| Incident | 19 | 0 | 1 |
| Other‐PAHa | 76 | 1 (1.3) | 2 (2.6) |
| Prevalent | 67 | 1 | 1 |
| Incident | 9 | 0 | 1 |
Abbreviations: CTD, connective tissue disease; FPAH, familial pulmonary arterial hypertension; iNO, inhaled nitric oxide; IPAH, idiopathic pulmonary arterial hypertension; PAH, pulmonary arterial hypertension.
Other‐PAH includes: DI‐PAH, HIV‐PAH, PH‐PAH, CHD‐PAH, PVOD‐PAH, PCH‐PAH.
An increased number of responders to O2 and O2 + iNO was observed in the cohort when using AVR definition 2, as shown in Table 3. Overall, O2 had a similar effect on cardiopulmonary hemodynamics across the different groups compared to definition 1 (6 vs. 4 responders), with most patients being prevalent (4 responders). However, O2 + iNO resulted in a substantially higher number of responders (n = 54 vs. n = 16 for definition 1), including 31 IPAH/FPAH, 14 CTD‐PAH, and 9 other‐PAH patients. CTD‐PAH included nine SSc, two MCTD, two SLE, and one Sjogren's patients. Furthermore, most responders were prevalent participants (n = 48), similar to what was found with definition 1.
Table 3.
AVR evaluation based on Definition 2, O2 versus O2 + iNO responders.
| Patients (N) | 100% O2 (% responders) | O2 + iNO (% responders) | |
|---|---|---|---|
| Group 1 PH | 338 | 6/316 (1.9) | 54/318 (16.9) |
| IPAH/FPAH | 171 | 4 (2.3) | 31 (18.7) |
| Prevalent | 152 | 4 | 28 |
| Incident | 19 | 0 | 3 |
| CTD‐PAH | 91 | 1 (1.1) | 14 (15.4) |
| Prevalent | 72 | 0 | 12 |
| Incident | 19 | 1 | 2 |
| Other‐PAHa | 76 | 1 (1.3) | 9 (11.8) |
| Prevalent | 67 | 1 | 8 |
| Incident | 9 | 0 | 1 |
Abbreviations: CTD, connective tissue disease; FPAH, familial pulmonary arterial hypertension; iNO, inhaled nitric oxide; IPAH, idiopathic pulmonary arterial hypertension; PAH, pulmonary arterial hypertension.
Other‐PAH includes: DI‐PAH, HIV‐PAH, PH‐PAH, CHD‐PAH, PVOD‐PAH, PCH‐PAH.
Demographic, clinical, laboratory, pulmonary function, echocardiographic, and hemodynamic variables were compared between responders and nonresponders using definition 2 given the larger number of AVR positivity (Table 4). There were no significant demographic or clinical differences between AVR and non‐AVR responders, except that the latter group had worse functional impairment (as assessed by functional class), a tendency for higher right atrial pressure (by RHC), a significantly lower tricuspid annular plane systolic excursion (TAPSE), and tendency for more severe tricuspid regurgitation and right ventricle (RV) dilation (by echocardiography).
Table 4.
Patient characteristics among AVR responders versus AVR nonresponders.
| AVR responders, n = 60 | AVR nonresponders, n = 278 | p Value | |
|---|---|---|---|
| Age at enrollment, years, mean | 50.6 ± 13.2 | 52.9 ± 14.6 | 0.25 |
| Female, n (%) | 45 (75) | 204 (74) | 0.79 |
| Race, White/AA/Othersa, n | 46/5/9 | 211/32/35 | 0.50 |
| Prevalent/incident | 53 (88)/7 (12) | 238 (85)/40 (15) | 0.58 |
| Years since PH diagnosis, median (min, max) | 4.9 (0.0, 25.2) | 2.8 (0.0, 35.8) | 0.13 |
| WHO FC at enrollment (I/II/III/IV), n | 13/28/18/1 | 31/108/123/16 | 0.04 |
| Vitals signs at enrollment | |||
| Systolic BP, mmHg, mean | 112 ± 14 | 115 ± 17 | 0.40 |
| Diastolic BP, mmHg, mean | 67 ± 10 | 69 ± 10 | 0.33 |
| Heart rate, bpm, mean | 74 ± 13 | 77 ± 13 | 0.22 |
| Respiratory rate, rpm, mean | 19 ± 6 | 18 ± 3 | 0.03 |
| BMI, kg/m2, mean | 29.7 ± 6.9 | 28.9 ± 7.6 | 0.47 |
| Laboratory data at enrollment | |||
| Creatinine, mg/dL, mean | 1.0 ± 0.5 | 1.0 ± 0.5 | 0.78 |
| eGFR, ml/min/1.73 m2, mean | 81.8 ± 26.4 | 80.3 ± 23.9 | 0.67 |
| Na, mEq/mL, mean | 139.3 ± 2.7 | 139.5 ± 2.8 | 0.66 |
| Bilirubin total, mg/dL, mean | 0.6 ± 0.4 | 0.8 ± 0.6 | 0.12 |
| Hemoglobin, g/dL, mean | 13.7 ± 1.7 | 13.7 ± 2.2 | 0.84 |
| ProBNP, pg/mL, median [P25, P75] | 129 [72, 500] | 286 [105, 1076] | 0.13 |
| Troponin, ng/mL, median (min, max) | 0.009 (0.009, 0.029) | 0.009 (0.009, 0.155) | 0.42 |
| Pulmonary function at enrollment | |||
| 6MWD, m, median (min, max) | 408 (95, 713) | 399 (37, 731) | 0.34 |
| FEV1 percentage, mean | 75.9 ± 18.4 | 78.5 ± 18.1 | 0.33 |
| FVC percentage, mean | 83.2 ± 17.4 | 84.6 ± 17.1 | 0.56 |
| FEV1/FVC percentage, mean | 90.8 ± 10.9 | 92.3 ± 9.8 | 0.32 |
| TLC percentage, mean | 87.9 ± 16.6 | 89.5 ± 15.8 | 0.52 |
| DLCO percentage, mean | 56.5 ± 22.5 | 57.5 ± 20.6 | 0.73 |
| Therapies | |||
| Diuretic, n (%) | 42 (70.0) | 174 (62.6) | 0.27 |
| PDE5 inhibitor, n (%) | 44 (73.3) | 181 (65.1) | 0.22 |
| ERA, n (%) | 31 (51.7) | 158 (56.8) | 0.46 |
| Prostacyclin, n (%) | 27 (45.0) | 120 (43.2) | 0.79 |
| SGC, n (%) | 1 (1.7) | 16 (5.8) | 0.18 |
| CCB, n (%) | 5 (8.3) | 11 (4.0) | 0.14 |
| 0/1/2/>2 PH drugs, n | 5/17/24/14 | 50/45/111/72 | 0.07 |
| Echocardiographic data | |||
| RV dilation (normal/mild/moderate/severe), n | 16/22/15/7 | 62/79/74/63 | 0.20 |
| TR (none/mild/moderate/severe), n | 23/26/10/1 | 78/119/65/16 | 0.16 |
| RVSP, mmHg, median [P25, P75] | 67.2 [47.2, 85.2] | 64.5 [48.2, 84.0] | 0.79 |
| TAPSE, cm, median [P25, P75] | 2.1 [1.7, 2.3] | 1.8 [1.6, 2.2] | 0.02 |
| Pericardial effusion, n (%) | 17 (28.3) | 118 (42.4) | 0.03 |
| Hemodynamics at rest | |||
| RAP, mmHg, mean | 6 ± 4 | 8 ± 2 | 0.07 |
| mPAP, mmHg, mean | 43 ± 14 | 44 ± 15 | 0.60 |
| PCWP, mmHg, mean | 10 ± 4 | 11 ± 3 | 0.08 |
| CO, L/min, median (min, max) | 5.1 (2.40, 10.2) | 4.9 (1.96, 13.6) | 0.95 |
| CI, L/min/m2, mean | 2.71 ± 0.73 | 2.80 ± 0.91 | 0.46 |
| PVR, Wood units, median [P25, P75] | 6.1 [4.5, 8.7] | 6.2 [3.8, 9.4] | 0.98 |
| SVR, mL/beat, mean | 74.3 ± 23.6 | 71.2 ± 27.1 | 0.40 |
| Mortality, n (%) | 5 (8.3) | 37 (13.3) | 0.02 |
Note: Statistics are presented as mean ± SD, median [P25, P75], median (min, max) or N (column %).
Abbreviations: 6MWD, 6 minute walk distance; AVR, acute vasodilator response; BMI, body mass index; BP, blood pressure; CCB, calcium‐channel blockers; CI, cardiac index; CO, cardiac output; DLCO, diffusion capacity of the lung to carbon monoxide; eGFR, estimated glomerular filtration rate; ERA, endothelin receptor antagonist; FEV1, force expiratory volume in 1 second; FVC, force vital capacity; IPAH, idiopathic pulmonary arterial hypertension; mPAP, mean pulmonary artery pressure; PAH, pulmonary arterial hypertension; PCWP, pulmonary capillary wedge pressure; PDE5, phosphodiesterase‐5; PVR, pulmonary vascular resistance; RAP, right atrial pressure; RV, right ventricle; RVSP, Right Ventricular Systolic Pressure; SCG, soluble guanylate cyclase; SVR, systemic vascular resistance; TAPSE, tricuspid annular plane systolic excursion; TLC, total lung capacity; TR, tricuspid regurgitation.
Other‐PAH includes: DI‐PAH, HIV‐PAH, PH‐PAH, CHD‐PAH, PVOD‐PAH, PCH‐PAH.
Survival
The cohort's mortality was analyzed in two different manners: survival from PAH diagnosis (censored at 15 years) and survival after enrollment to PVDOMICS with RHC and AVR evaluation (censored at 3 years). Patients with positive AVR to 100% O2 and O2 + iNO, based on definition 2, were pooled together for the analysis (n = 60). Only patients who underwent AVR test were included (n = 318). Patients who received a heart and/or lung transplant were excluded. We present data on the two largest subgroups, IPAH/FPAH and CTD‐PAH. Other‐PAH had no statistically significant differences in survival.
Cumulative survival for IPAH/FPAH versus non‐IPAH is shown in Figure 1. Median survival for both cohorts, from PAH diagnosis and after PVDOMICS enrollment, was above 50%. However, survival was significantly higher, 82% versus 76% from PAH diagnosis, and 95% versus 80% from PVDOMICS enrollment, among IPAH/FPAH versus non‐IPAH patients, respectively (log‐rank test p = 0.03 and p = 0.04, correspondingly).
Figure 1.
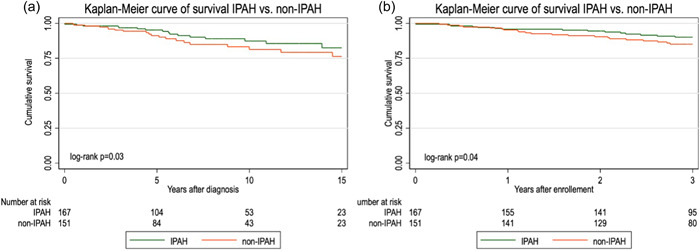
Kaplan–Meier curve of survival after PAH diagnosis (a) and PVDOMICS enrollment (b) in IPAH versus non‐IPAH patients. IPAH, idiopathic pulmonary arterial hypertension; PAH, pulmonary arterial hypertension.
AVR‐responders had a 89% survival versus 77% for nonresponders from PAH diagnosis, and 91% versus 86% from PVDOMICS enrollment (log‐rank test p = 0.04 and p = 0.05, respectively), Figure 2. Subgroup analysis showed a nonsignificant trend for improved survival among AVR responders (p = 0.09 and p = 0.15, diagnosis and enrollment, respectively) compared with non‐AVR responders in IPAH, but no difference in CTD‐PAH (Figure 3).
Figure 2.
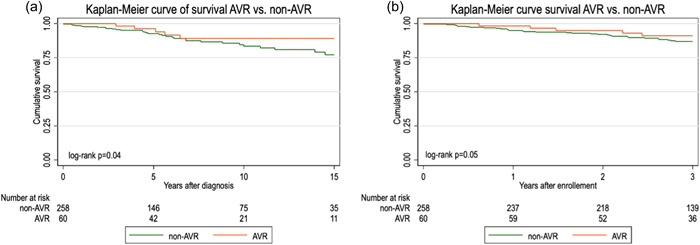
Kaplan‐Meier curve of survival after PAH diagnosis (a) and PVDOMICS enrollment (b) in AVR‐responders versus non‐AVR responders' patients. PAH, pulmonary arterial hypertension.
Figure 3.
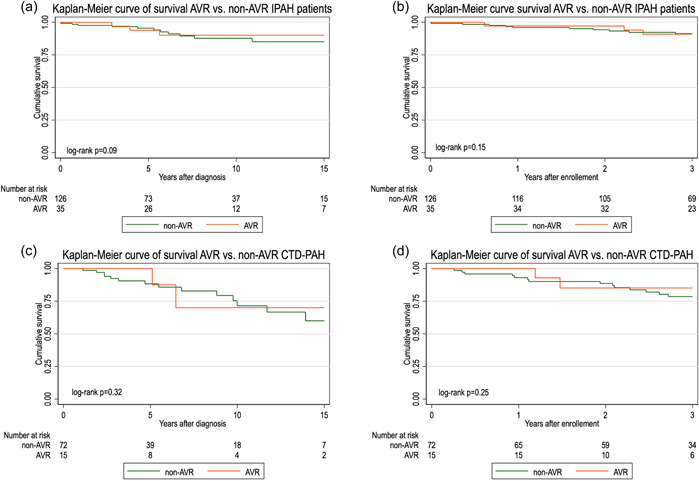
Kaplan–Meier curve of survival after PAH diagnosis and PVDOMICS enrollment in AVR‐responders versus non‐AVR responders' IPAH patients (a and b, respectively), and AVR‐responders versus non‐AVR responders' CTD‐PAH patients (c and d, respectively). IPAH, idiopathic pulmonary arterial hypertension.
DISCUSSION
Very few studies have reported vasoreactivity testing and long‐term outcomes in patients with PH other than IPAH, usually with very limited sample sizes. To our knowledge, this is one of the largest studies reporting on other PAH subgroups. AVR testing has been routinely performed, and recommended, when diagnosing and treating patients with IPAH. 8 , 15 The American College of Chest Physicians 16 and the most recent European Society of Cardiology and European Respiratory Society guidelines for the diagnosis and treatment of PH, highlights the importance to classify patients as “non‐responders” versus “responders” 8 when diagnosing IPAH, given the survival and long‐term benefit in response to CCB therapy. 7 , 9 There is an expanded recommendation in the guidelines for AVR testing to include patients with FPAH, DI‐PAH, and CHD‐PAH with initial systemic‐to‐pulmonary shunting. 8 Unfortunately, there is still a weak recommendation against routinely testing patients with other subgroups within PAH given conflicting findings in regards of CCB response and/or clinical benefits. 17
Based on these guidelines, a decrease in mPAP by ≥10 mmHg to a value ≤ 40 mmHg, with no change or an increase in cardiac output, definition 1 in our study, has been recognized as a positive AVR response. 9 , 13 It is reported, based on this definition, that less than 10% of IPAH, FPAH, or DI‐PAH patients are found to be responders, 9 , 18 which is similar to the findings in our study (4.1%).
However, prior publications have suggested that less strict criteria for AVR, such as in definition 2 of our study, would capture more patients that could benefit from specific therapies and may have better long‐term outcomes. Malhotra and colleagues examined the relationship of changes in PVR and mPAP in response to combined iNO and O2 challenge, to clinical outcomes in patients with PAH. They demonstrated that patients with ≥30% reduction in PVR had a 53% relative reduction in mortality (hazard ratio [HR]: 0.47, 95% confidence interval [CI]: 0.23–0.99, p = 0.047), and those with ≥12% reduction in mPAP had a 55% relative reduction in mortality (HR: 0.45, 95% CI: 0.22–0.96, p = 0.038). Also, for every 10% reduction in baseline PVR, there was a reduction in age‐adjusted mortality by a ratio of 0.82 (95% CI: 0.69–0.98, p = 0.025), while for every 10% reduction in baseline mPAP there was a reduction in mortality by a factor of 0.60 (95% CI: 0.43–0.83, p = 0.002). 14 Applying these criteria to our study population, AVR response was 18.7% among patients with IPAH/FPAH.
Oxygen challenges are typically utilized in patients with CHD or other subgroups with associated resting hypoxemia, a main mechanism of pulmonary vasoconstriction in this group, while they are not routinely performed in other forms of PH. Both AVR definitions revealed only 4 and 6 patients, respectively, with a positive AVR to 100% O2 alone. The remainder of responders with either definition were largely related to iNO.
To date, there are only few studies on CTD‐PAH that have evaluated the prognostic value of AVR response. Malhotra et al. reported retrospective data on 80 group 1 PH patients. Among them, 23 (29%) had a CTD‐PAH diagnosis, with 11 exhibiting a PVR vasodilator response (48%) and 14 having a mPAP response (61%) after AVR testing with combined O2 and iNO. Furthermore, PVR responsiveness was associated with a HR of 0.11 (95% CI: 0.01–0.97, p = 0.047) and mPAP responsiveness with a HR of 0.24 (95% CI: 0.05–1.23, p = 0.09). 14 Hernandez‐Oropeza and colleagues presented data on 25 CTD‐PAH patients. The most frequent rheumatologic diagnosis was SLE (16 patients). About 28% (7 patients) were responders and the remainder were categorized as nonresponders. Nonetheless, the latter group had a tendency for a shorter time to clinical worsening compared with responders (17.8 vs. 41.1 months, respectively, p = 0.052). 19
One of the largest, and most relevant registries, published retrospective findings in 663 consecutive non‐IPAH patients in 2010. This study included 168 CTD‐PAH, 153 PoPH‐PAH, 127 DI‐PAH, 124 HIV‐PAH, 50 CHD‐PAH, and 41 PVOD/PCH‐PAH patients. Positive AVR was defined as >20% decrease in mPAP and PVR. AVR response was observed in 13.4% of DI‐PAH, 12.2% of PVOD/PCH‐PAH, 10.1% of CTD‐PAH, 1.6% of HIV‐PAH, 1.3% of PoPH‐PAH, and was absent in CHD‐PAH. A long‐term response to CCB was reported in 9.4% of DI‐PAH patients but was rare in HIV, PoPH, and CTD‐PAH (1.6, 0.7, and 0.6%, respectively) and absent in PVOD/PCH‐PAH. 18
Given the diverse data and criteria used to define AVR, but with consistent and significant findings in IPAH, we focused our attention on the non‐IPAH population within the PVDOMICS cohort. The AVR rate among CTD‐PAH was 7.7% and 15.4%, for definition 1 and 2, respectively; with most cases being prevalent, which was slightly higher than what was reported by the French registry (10.1%). 18 This response was limited to SSc, MCTD and SLE patients, with SSc being the most common diagnosis. Also, AVR rate among other‐PAH patients was 2.6% to 11.8%, which is a relevant finding based on a broad array of rates published in prior reports, 18 , 20 with most patients being prevalent and having a diagnosis of CHD‐PAH and DI‐PAH.
Survival from diagnosis and from PVDOMICS enrollment is worse for CTD‐PAH compared to IPAH, consistent with multiple previous publications. 2 , 4 , 8 The present study reveals that PAH prevalent patients who display AVR response, as defined herein, present with less functional impairment and more preserved RV function (lower RAP, less RV dilation and better TAPSE) and have superior survival, from diagnosis and from PVDOMICS enrollment, compared with nonresponders. Subgroup analysis (IPAH/FPAH, CTD‐PAH, other‐PAH), however, showed a nonsignificant trend for improved survival among AVR responders. This could be related to lack of power due to small subgroup numbers in our study and would need to be tested in larger prospective cohorts.
Regarding the relevance of a positive AVR in non‐IPAH patients, prior studies have highlighted the concern that “response” is not necessarily predictive of a long‐term therapeutic benefit, and acute testing might not be useful. As previously reported, initiation of CCB therapy in PVOD‐PAH patients with AVR response has led to clinical deterioration, mostly due to severe pulmonary edema. 10 , 11 , 20 , 21 It has been demonstrated that occlusive venopathy may occur in severe PAH associated with different conditions and is relatively frequent in CTD‐ PAH, particularly in SSc. 22 , 23 In addition, most of these CTD‐PAH patients experience a rapid clinical and hemodynamic deterioration in the first months following initiation of CCB. The absence of long‐term response in CTD‐PAH may be at least partly related to the frequent venous or capillary involvement observed in these conditions. 18
Although initiation of CCB therapy in non‐IPAH responders is currently not recommended, there is a clear trend of increased survival among AVR responders across all group 1 PH patients. Whether the latter is related to long‐term PAH therapy affecting pulmonary vasomotor response and/or vascular remodeling in these patients would warrant further studies.
Some limitations of this study must be noted. First, although PVDOMICS continues to collect prospective clinical data in a more limited group of patients, this was a cross‐sectional study with no inference whatsoever to long‐term response to vasodilator therapy. Second, the analysis is limited by a small number of patients included in some subgroups (incident patients, non‐IPAH/non‐CTD‐PAH). Importantly, we cannot conclude whether AVR was acquired over time in response to PAH therapy or was present at diagnosis for patients who displayed AVR at the time of PVDOMICS enrollment since complete AVR testing data at the time of diagnosis was not available. Furthermore, prospective data validation is required before incorporation into clinical practice.
CONCLUSION
In summary, the overall prevalence of AVR in IPAH patients is similar to data published in prior retrospective studies. Non‐IPAH AVR response in this mostly prevalent cohort was higher than previously reported for incident patients. A less stringent AVR definition may prove to be a useful indicator for a subgroup of patients with more favorable outcomes in response to PAH therapy. Whether AVR response at follow‐up and possible survival advantage are related to long‐term PAH therapy affecting pulmonary vasomotor response and/or vascular remodeling in these patients would warrant further investigation.
AUTHOR CONTRIBUTIONS
All authors made a substantial contribution to the concept or design of the work and the acquisition, analysis, or interpretation of data. All authors participated in the drafting of the article, revised it critically for important intellectual content and gave the final approval of the version to be published. Each author has participated sufficiently in the work to take public responsibility for appropriate portions of the content.
CONFLICT OF INTEREST STATEMENT
The authors declare no conflict of interest.
ETHICS STATEMENT
The PVDOMICS study was approved by local institutional review boards and all patients provided written informed consent. An independent OSMB oversaw study conduct.
ACKNOWLEDGMENTS
The authors gratefully acknowledge the patients who participated in this study. This study was supported by the following grants, 1F32HL159917‐01A1, U01 HL125175, U01 HL125218, U01 HL125205, U01 HL125212, U01 HL125208, U01 HL125215, U01 HL125177, and funding from the Pulmonary Hypertension Association.
Naranjo M, Rosenzweig EB, Hemnes AR, Jacob M, Desai A, Hill NS, Larive AB, Finet JE, Leopold J, Horn E, Frantz R, Rischard F, Erzurum S, Beck G, Mathai SC, Hassoun PM. Frequency of acute vasodilator response (AVR) in incident and prevalent patients with pulmonary arterial hypertension: results from the pulmonary vascular disease phenomics study. Pulm Circ. 2023;13:e12281. 10.1002/pul2.12281
REFERENCES
- 1. Galiè N, Channick RN, Frantz RP, Grünig E, Jing ZC, Moiseeva O, Preston IR, Pulido T, Safdar Z, Tamura Y, McLaughlin VV. Risk stratification and medical therapy of pulmonary arterial hypertension. Eur Respir J. 2019;53(1):1801889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Hassan HJ, Naranjo M, Ayoub N, Housten T, Hsu S, Balasubramanian A, Simpson CE, Damico RL, Mathai SC, Kolb TM, Hassoun PM. Improved survival for patients with systemic sclerosis‐associated pulmonary arterial hypertension: the Johns Hopkins Registry. Am J Respir Crit Care Med. 2022;207(3):312–322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Humbert M, Sitbon O, Simonneau G. Treatment of pulmonary arterial hypertension. N Engl J Med. 2004;351(14):1425–1436. [DOI] [PubMed] [Google Scholar]
- 4. Naranjo M, Mercurio V, Hassan H, Alturaif N, Cuomo A, Attanasio U, Diab N, Sahetya SK, Mukherjee M, Hsu S, Balasubramanian A, Simpson CE, Damico R, Kolb TM, Mathai SC, Hassoun PM. Causes and outcomes of ICU hospitalisations in patients with pulmonary arterial hypertension. ERJ Open Res. 2022;8(2):00002‐2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Morrell NW, Adnot S, Archer SL, Dupuis J, Lloyd Jones P, MacLean MR, McMurtry IF, Stenmark KR, Thistlethwaite PA, Weissmann N, Yuan JXJ, Weir EK. Cellular and molecular basis of pulmonary arterial hypertension. JACC. 2009;54(1 Suppl):S20–S31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Rich S, Kaufmann E. High dose titration of calcium channel blocking agents for primary pulmonary hypertension: guidelines for short‐term drug testing. JACC. 1991;18(5):1323–1327. [DOI] [PubMed] [Google Scholar]
- 7. Rich S, Kaufmann E, Levy PS. The effect of high doses of calcium‐channel blockers on survival in primary pulmonary hypertension. N Engl J Med. 1992;327(2):76–81. [DOI] [PubMed] [Google Scholar]
- 8. Humbert M, Kovacs G, Hoeper MM, Badagliacca R, Berger RMF, Brida M, Carlsen J, Coats AJS, Escribano‐Subias P, Ferrari P, Ferreira DS, Ghofrani HA, Giannakoulas G, Kiely DG, Mayer E, Meszaros G, Nagavci B, Olsson KM, Pepke‐Zaba J, Quint JK, Rådegran G, Simonneau G, Sitbon O, Tonia T, Toshner M, Vachiery JL, Vonk Noordegraaf A, Delcroix M, Rosenkranz S, ESC/ERS Scientific Document Group . 2022 ESC/ERS guidelines for the diagnosis and treatment of pulmonary hypertension. Eur Respir J. 2023;61(1):2200879. 10.1183/13993003.00879-2022 [DOI] [PubMed] [Google Scholar]
- 9. Sitbon O, Humbert M, Jaïs X, Ioos V, Hamid AM, Provencher S, Garcia G, Parent F, Hervé P, Simonneau G. Long‐term response to calcium channel blockers in idiopathic pulmonary arterial hypertension. Circulation. 2005;111(23):3105–3111. [DOI] [PubMed] [Google Scholar]
- 10. Montani D, Achouh L, Dorfmüller P, Le Pavec J, Sztrymf B, Tchérakian C, Rabiller A, Haque R, Sitbon O, Jaïs X, Dartevelle P, Maître S, Capron F, Musset D, Simonneau G, Humbert M. Pulmonary veno‐occlusive disease: clinical, functional, radiologic, and hemodynamic characteristics and outcome of 24 cases confirmed by histology. Medicine. 2008;87(4):220–233. [DOI] [PubMed] [Google Scholar]
- 11. Montani D, Price LC, Dorfmuller P, Achouh L, Jais X, Yaici A, Sitbon O, Musset D, Simonneau G, Humbert M. Pulmonary veno‐occlusive disease. Eur Respir J. 2009;33(1):189–200. [DOI] [PubMed] [Google Scholar]
- 12. Hemnes AR, Beck GJ, Newman JH, Abidov A, Aldred MA, Barnard J, Berman Rosenzweig E, Borlaug BA, Chung WK, Comhair SAA, Erzurum SC, Frantz RP, Gray MP, Grunig G, Hassoun PM, Hill NS, Horn EM, Hu B, Lempel JK, Maron BA, Mathai SC, Olman MA, Rischard FP, Systrom DM, Tang WHW, Waxman AB, Xiao L, Yuan JXJ, Leopold JA. PVDOMICS: a multi‐center study to improve understanding of pulmonary vascular disease through phenomics. Circ Res. 2017;121(10):1136–1139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Simonneau G, Montani D, Celermajer DS, Denton CP, Gatzoulis MA, Krowka M, Williams PG, Souza R. Haemodynamic definitions and updated clinical classification of pulmonary hypertension. Eur Respir J. 2019;53(1):1801913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Malhotra R, Hess D, Lewis GD, Bloch KD, Waxman AB, Semigran MJ. Vasoreactivity to inhaled nitric oxide with oxygen predicts long‐term survival in pulmonary arterial hypertension. Pulm Circ. 2011;1(2):250–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Galiè N, Humbert M, Vachiery JL, Gibbs S, Lang I, Torbicki A, Simonneau G, Peacock A, Vonk Noordegraaf A, Beghetti M, Ghofrani A, Gomez Sanchez MA, Hansmann G, Klepetko W, Lancellotti P, Matucci M, McDonagh T, Pierard LA, Trindade PT, Zompatori M, Hoeper M. 2015 ESC/ERS guidelines for the diagnosis and treatment of pulmonary hypertension: the joint task force for the diagnosis and treatment of pulmonary hypertension of the european society of cardiology (ESC) and the european respiratory society (ERS): endorsed by: association for european paediatric and congenital cardiology (AEPC), international society for heart and lung transplantation (ISHLT). Eur Respir J. 2015;46(4):903–975. [DOI] [PubMed] [Google Scholar]
- 16. Klinger JR, Elliott CG, Levine DJ, Bossone E, Duvall L, Fagan K, Frantsve‐Hawley J, Kawut SM, Ryan JJ, Rosenzweig EB, Sederstrom N, Steen VD, Badesch DB. Therapy for pulmonary arterial hypertension in adults. Chest. 2019;155(3):565–586. [DOI] [PubMed] [Google Scholar]
- 17. Mehra MR, Canter CE, Hannan MM, Semigran MJ, Uber PA, Baran DA, Danziger‐Isakov L, Kirklin JK, Kirk R, Kushwaha SS, Lund LH, Potena L, Ross HJ, Taylor DO, Verschuuren EAM, Zuckermann A. The 2016 international society for heart lung transplantation listing criteria for heart transplantation: a 10‐year update. J Heart Lung Transplant. 2016;35(1):1–23. [DOI] [PubMed] [Google Scholar]
- 18. Montani D, Savale L, Natali D, Jais X, Herve P, Garcia G, Humbert M, Simonneau G, Sitbon O. Long‐term response to calcium‐channel blockers in non‐idiopathic pulmonary arterial hypertension. Eur Heart J. 2010;31(15):1898–1907. [DOI] [PubMed] [Google Scholar]
- 19. Hernandez‐Oropeza JL, Rodríguez‐Reyna TS, Carrillo‐Perez DL, Rodríguez‐Andoney JdJ, Narváez‐David R, Salado‐Morales Y, Rivero‐Sigarroa E, Domínguez‐Cherit G, Pulido‐Zamudio T. Pulmonary vasoreactivity and phenotypes in pulmonary arterial hypertension associated to connective tissue diseases. Rev Invest Clin. 2018;70(2):82–87. [DOI] [PubMed] [Google Scholar]
- 20. Humbert M, Sitbon O, Chaouat A, Bertocchi M, Habib G, Gressin V, Yaici A, Weitzenblum E, Cordier JF, Chabot F, Dromer C, Pison C, Reynaud‐Gaubert M, Haloun A, Laurent M, Hachulla E, Simonneau G. Pulmonary arterial hypertension in France: results from a national registry. Am J Respir Crit Care Med. 2006;173(9):1023–1030. [DOI] [PubMed] [Google Scholar]
- 21. Montani D, Kemp K, Dorfmuller P, Sitbon O, Simonneau G, Humbert M. Idiopathic pulmonary arterial hypertension and pulmonary veno‐occlusive disease: similarities and differences. Semin Respir Crit Care Med. 2009;30(4):411–420. [DOI] [PubMed] [Google Scholar]
- 22. Dorfmüller P, Humbert M, Perros F, Sanchez O, Simonneau G, Müller KM, Capron F. Fibrous remodeling of the pulmonary venous system in pulmonary arterial hypertension associated with connective tissue diseases. Hum Pathol. 2007;38(6):893–902. [DOI] [PubMed] [Google Scholar]
- 23. Johnson SR, Patsios D, Hwang DM, Granton JT. Pulmonary veno‐occlusive disease and scleroderma associated pulmonary hypertension. J Rheumatol. 2006;33(11):2347–2350. [PubMed] [Google Scholar]


