Abstract
Patients with inherited hypercoagulopathies such as protein-S deficiency commonly present with venous thrombosis. However, there are rare cases of arterial thrombosis. We describe a rare case of a diffuse left anterior descending and left ventricular thrombus in a young patient with protein-S deficiency complicated with mid cerebral artery occlusion. (Level of Difficulty: Intermediate.)
Key Words: hypercoagulopathy, myocardial infarction, protein-S deficiency, thrombus, tirofiban
Central Illustration
Intracoronary thrombus is the main pathophysiological mechanism of acute myocardial infarction as a result of atherosclerotic plaque erosion or rupture. In rare cases, intracoronary thrombus may occur in patients with healthy coronary arteries as a manifestation of coronary embolism or another hypercoagulative state. However, hypercoagulation, especially protein-S deficiency, is usually correlated with venous thromboembolism. We report an acute coronary syndrome case of diffuse left anterior descending (LAD) and left ventricular (LV) thrombus in a young patient with protein-S deficiency.1
Learning Objectives
-
•
To understand that arterial thrombosis can be a rare first diagnosis manifestation of protein-S deficiency.
-
•
To consider intracoronary thrombus aspiration as a potential approach in patients with high thrombotic burden, combined with systematic antithrombotic therapy aiming to coronary flow restoration.
-
•
To thoroughly investigate the underlying cause of an arterial thromboembolic event, in particular when concerning younger patients.
History of Presentation
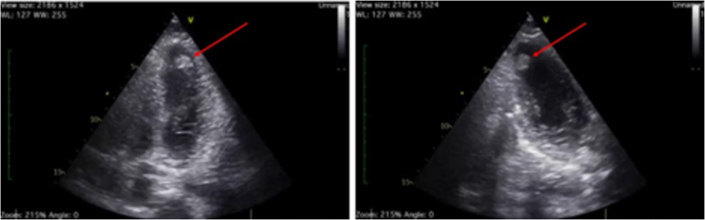
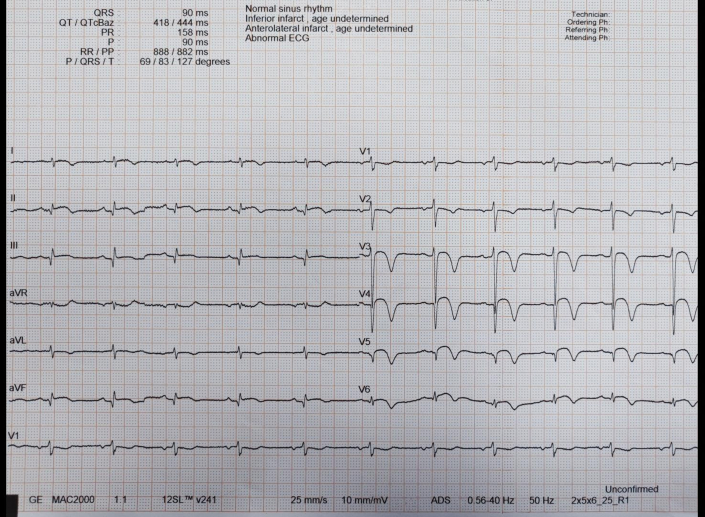
A 24-year-old black African man presented to our hospital with ongoing chest pain of 12 hours onset. On admission, his blood pressure was 130/70 mm Hg and his heart rate was 100 beats/min. His electrocardiogram revealed anterior ST-segment elevation (Figure 1). He was initially treated with 500 mg acetylsalicylic acid (ASA) and 180 mg ticagrelor and was transferred to the catheterization laboratory for an emergency coronary angiogram. Point-of-care ultrasonography (POCUS) revealed akinesia of the apical segments with severely reduced left ventricular (LV) function (ejection fraction 30%) and a large thrombus in the apical inferolateral wall (Figure 2, Video 1).
Figure 1.
Electrocardiogram at Baseline
ST-segment elevation.
Figure 2.
Point-of-Care Transthoracic Echocardiogram Images
4- and 2-chamber views indicate left ventricular apical thrombus (red arrows).
Medical History
He had no known medical history and no cardiovascular risk factors of note.
Differential Diagnosis
Myocardial infarction, pericarditis, pulmonary embolism, and herpes zoster were included in the differential diagnosis on the basis of the patient’s symptoms.
Investigations
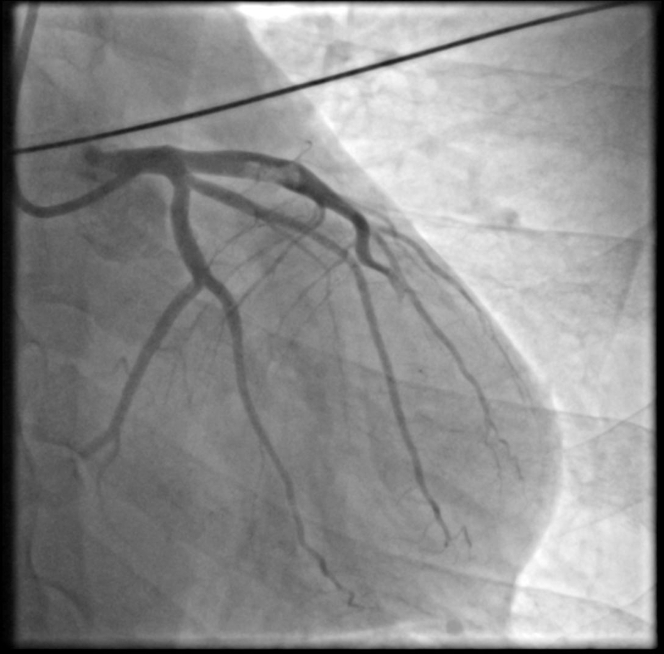
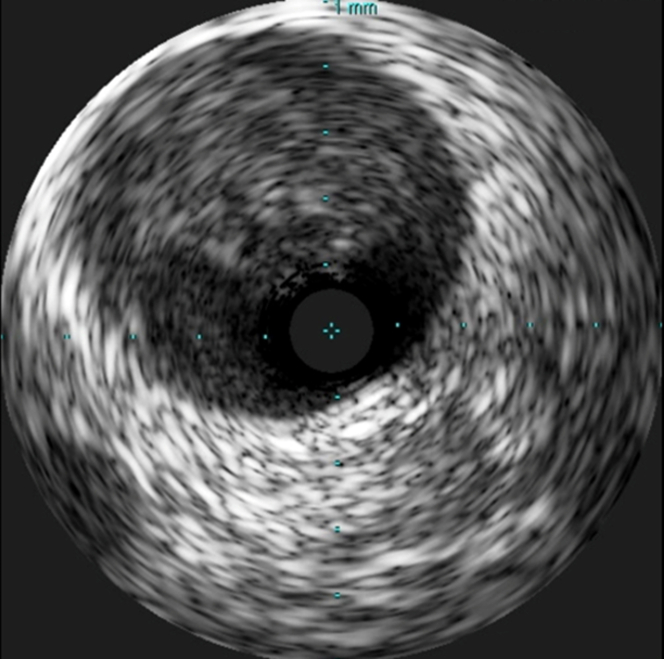
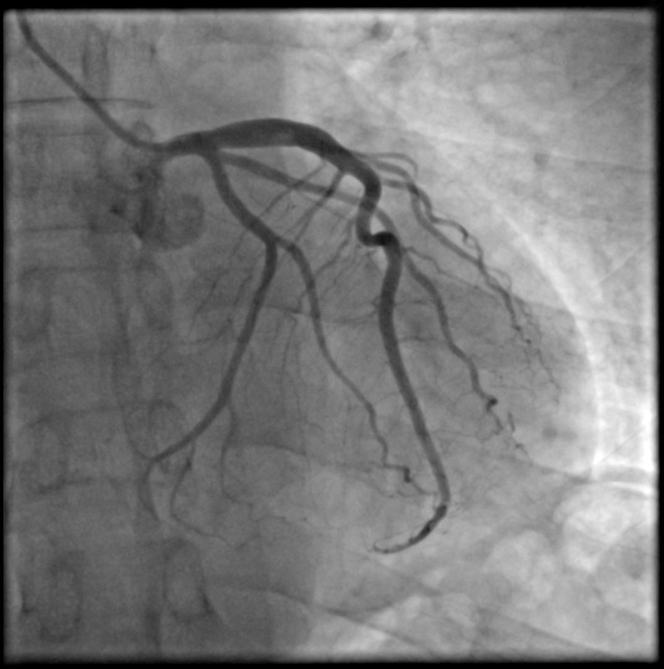
A coronary angiogram revealed a large intracoronary thrombus burden in the proximal LAD with thrombotic total occlusion in the mid segment of the vessel (Figure 3, Video 2). A floppy wire was easily advanced to the distal vessel without restoration of peripheral flow. Intravascular ultrasonography confirmed the presence of a large intracoronary thrombus burden in the proximal segment; however, no endothelial destruction was detected (Figure 4, Video 3).
Figure 3.
Emergency Angiogram
Figure 4.
Intravascular Ultrasound
The differential diagnosis based on the angiography results included coronary artery dissection, coronary calcification, and no-reflow phenomenon.
Management
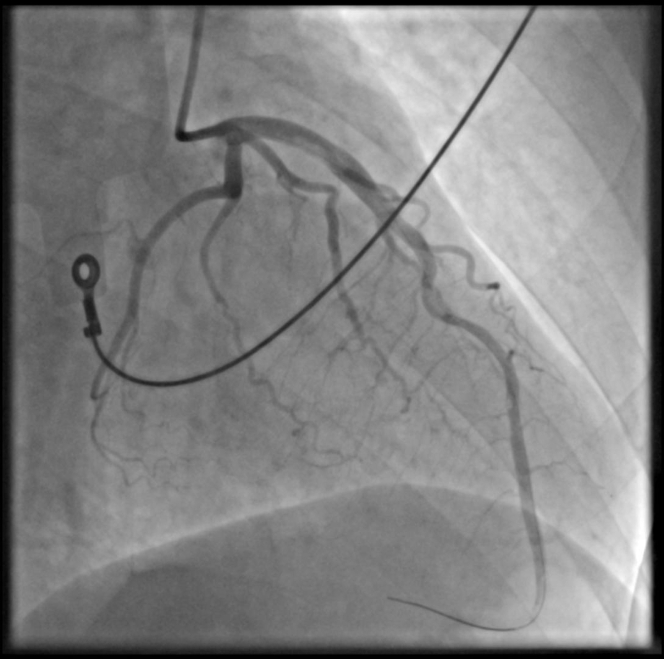
After thrombus was revealed in the LAD, intravenous administration of tirofiban (IIb/IIIa) was immediately initiated at a loading dose, followed by maintenance administration. Multiple consecutive thrombus aspirations were performed with an Export Advance catheter (Medtronic) throughout the length of the vessel without any substantial improvement of the distal flow. Distal thrombus aspiration through a Finecross (Terumo) microcatheter mildly improved the peripheral flow (TIMI flow grade 2) and confirmed the intraluminal position of the guidewire with injection of contrast material (Figure 5, Video 4). The patient was transferred to the coronary care unit and continued to receive tirofiban for 18 hours followed by low-molecular-weight heparin for the next 48 hours. He remained in hemodynamically stable condition, no arrythmia was detected, and he experienced remission of his symptoms. His peak high-sensitivity troponin level was >50,000 pg/mL on day 1 after presentation. A repeated angiogram on day 2 showed restoration of TIMI flow grade 3 across the vessel, and no thrombus was detected (Figure 6, Video 5).
Figure 5.
Final Result
Minimal flow restoration.
Figure 6.
Repeated Angiogram Day 2
Further investigation of the thrombus with a bubble test on a transthoracic echocardiogram (TTE) did not reveal a right-to-left shunt excluding a thromboembolic event through a potential patent foramen ovale. A thrombophilia screening test revealed protein-S deficiency.
The patient was discharged on day 7 to receive acenocoumarol, clopidogrel, aspirin (for a week), and optimal heart failure treatment (Figure 7). A follow-up TTE was advised, as well as clinical assessment 40 days later.
Figure 7.
Electrocardiogram at Discharge
However, 20 days later he was readmitted in a local stroke unit because of a heavy ischemic cerebrovascular accident (CVA) of the mid cerebral artery, presumably originating from the LV thrombus, which was still detectable on TTE.
Discussion
We report a rare case of a first-diagnosis protein-S deficiency in a young patient, manifested with multiple arterial thrombi including LAD (anterior STEMI), left ventricle, and mid cerebral artery. Atherosclerotic plaque rupture or erosion is the main cause of acute coronary syndromes.1 However, myocardial infarction may occur in patients with angiographically normal epicardial coronary arteries. This clinical scenario more frequently includes younger patients (aged <50 years) with no coronary medical history and no cardiovascular risk factors.2,3 Important causative mechanisms include hypercoagulable states, coronary embolism, coronary endothelial dysfunction, aortic dissection, coronary trauma, or spasm and imbalance between oxygen demand and supply.2,3 Thrombus aspiration may be considered as a bail-out approach in the presence of high thrombotic burden.4 Systematic antithrombotic therapy is the appropriate treatment, leading to flow restoration in the majority of these cases.3 Intracoronary imaging with intravascular ultrasonography or optical coherence tomography is of paramount importance in order to investigate the underlying causative mechanism and exclude any endothelial pathologic changes. Percutaneous coronary intervention with stent implantation for similar cases requires cautious consideration. Alternative diagnoses should also be considered, including right-to-left shunt (embolic causes) and hypercoagulopathies.
Hypercoagulopathy can be classified as hereditary, acquired, or mixed. Hereditary hypercoagulability includes antithrombin deficiency; protein-C and protein-S deficiency; factor V Leiden gene mutation; prothrombin 20210A gene mutation; elevated factor VIII, IX, or XI; and hyperhomocysteinemia.5 Protein-S is a cofactor for activated protein-C; 60% of its total amount is bound with a complement component (C4b) while 40% remains free in blood circulation.6 Three 3 types of protein-S deficiency have been identified. Type I is associated with reduced total protein synthesis; in type II both total and free protein-S levels are within normal range but with reduced activity; and type III is characterized by low levels of free protein-S and reduced activity.6 The patient in our case received a diagnosis of type II protein-S deficiency with reduced protein activity. Venous thromboembolism and deep vein thrombosis constitute the most common clinical manifestation of protein-S deficiency.
The relationship between protein-S deficiency and risk of myocardial infarction has not been thoroughly investigated in the literature. There are documented cases of arterial thrombosis including myocardial infarction.7,8 However, larger studies have not shown a strong association between protein-S deficiency and risk of myocardial infarction.9 In a study of young patients presenting with myocardial infarction, the frequency of protein-S deficiency did not differ between case subjects and control subjects.10 By contrast, a British analysis showed that healthy middle-aged men who experienced coronary heart disease had higher plasma levels of free protein-S during follow-up than did those who did not experience coronary heart disease. However, this association disappeared after adjustment for cardiovascular risk factors.11
We present an extremely rare case of mid cerebral arterial thrombosis a few days after an initial acute coronary syndrome (manifested by LV/LAD thrombus) occurred in a patient with protein-S deficiency while taking dual antithrombotic therapy (including acenocoumarol within therapeutic range). The withdrawal of aspirin on day 7 after the acute coronary syndrome could have been a predisposing factor for CVA; however, clopidogrel and acenocoumarol combination treatment was considered a reasonable approach in a young patient with normal coronary vessel walls and no coronary stent implantation. Treatment of thrombosis in patients with protein-S deficiency is similar to that for the general population including vitamin K antagonist and bridging therapy with heparin or low-molecular-weight heparin.6 Patients with protein-S deficiency should also follow a thromboprophylaxis prescription in high-risk situations, such as surgery, trauma, immobilization, pregnancy, or puerperium.12
In recent years, doubts have emerged about whether information obtained in thrombophilia screening is sufficiently relevant to define risk stratification or change clinical decision making.13 As demonstrated in this case report, thrombophilia defects including protein-S deficiency should also make hematology physicians aware of systematic arterial thrombosis. By the same token, this knowledge should lead to larger-scale thrombophilia screening in young patients presenting with arterial thromboembolic events as part of a complete workup. Finally, with regard to antithrombotic treatment, if the relationship of protein-S deficiency and arterial ischemic events is firmly established, the prophylactic role of antiplatelet therapy may prove more beneficial for these patients with hematologic conditions than warfarin.
Follow-Up
After the CVA event, the patient underwent intubation and sadly died after a long hospitalization period as a result of severe heart failure complicated by consecutive lower respiratory tract infections during respiratory mechanical support.
Conclusions
Intracoronary thrombus formation without vessel endothelium destruction can be a common manifestation of an acute coronary syndrome. The present clinical case emphasizes the need for a thorough investigation of any underlying causative cause of arterial thrombus, including inherited hypercoagulable states, such as protein-S deficiency. Despite the fact that venous thromboembolism is the most common manifestation of protein-S deficiency, arterial thrombosis can be a rare complication and potentially should be aggressively treated.
Funding Support and Author Disclosures
The authors have reported that they have no relationships relevant to the contents of this paper to disclose.
Footnotes
The authors attest they are in compliance with human studies committees and animal welfare regulations of the authors’ institutions and Food and Drug Administration guidelines, including patient consent where appropriate. For more information, visit the Author Center.
Appendix
For supplemental videos, please see the online version of this paper.
Appendix
Point of Care Ultrasound
Severe LV dysfunction with apical akinesia, and large thrombus in the apical inferolateral wall.
Emergency Angiogram
Proximal LAD thrombus and distal thrombotic occlusion.
Intravascular Ultrasound
Intact endothelial surface.
Final Angiographic Result
Minimal flow restoration.
Repeated Angiogram Day 2
Restoration of TIMI III flow across the vessel with no thrombus detected.
References
- 1.Soare A.M., Popa C. Deficiencies of proteins C, S and antithrombin and activated protein C resistance: their involvement in the occurrence of arterial thromboses. J Med Life. 2010;3:412–415. [PMC free article] [PubMed] [Google Scholar]
- 2.Chandrasekaran B., Kurbaan A.S. Myocardial infarction with angiographically normal coronary arteries. J R Soc Med. 2002;95:398–400. doi: 10.1258/jrsm.95.8.398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gupta A, Tun AM, Gupta K, Tuma F. Protein S deficiency. 2022 Dec 5. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2023 Jan. PMID: 31335064
- 4.Ibanez B., James S., Agewall S., et al. 2017 ESC Guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation: the task force for the management of acute myocardial infarction in patients presenting with ST-segment elevation of the European Society of Cardiology (ESC) Eur Heart J. 2018;7(39):119–177. doi: 10.1093/eurheartj/ehx393. [DOI] [PubMed] [Google Scholar]
- 5.Sayin M.R., Akpinar I., Karabag T., Aydin M., Dogan S.M., Cil C. Left main coronary artery thrombus resulting from combined protein C and S deficiency. Int Med. 2012;51:3041–3044. doi: 10.2169/internalmedicine.51.8341. [DOI] [PubMed] [Google Scholar]
- 6.Anderson J.A.M., Weitz J.I. Hypercoagulable states. Crit Care Clin. 2011;27:933–952. doi: 10.1016/j.ccc.2011.09.007. [DOI] [PubMed] [Google Scholar]
- 7.Boekholdt S., Kramer M. Arterial thrombosis and the role of thrombophilia. Semin Thromb Hemost. 2007;33:588–596. doi: 10.1055/s-2007-985755. [DOI] [PubMed] [Google Scholar]
- 8.Beattie S., Norton M., Doll D. Coronary thrombosis associated with inherited protein-S deficiency: a case report. Heart Lung. 1997;26:76–79. doi: 10.1016/s0147-9563(97)90012-1. [DOI] [PubMed] [Google Scholar]
- 9.Segev A., Ellis M.H., Segev F., et al. High prevalence of thrombophilia among young patients with myocardial infarction and few conventional risk factors. Int J Cardiol. 2005;98:421–424. doi: 10.1016/j.ijcard.2003.10.057. [DOI] [PubMed] [Google Scholar]
- 10.Rallidis L.S., Belesi C.I., Maniouudaki H.S., et al. Myocardial infarction under the age of 36: prevalence of thrombophilic disorders. Thromb Haemost. 2003;90:272–278. doi: 10.1160/TH02-11-0286. [DOI] [PubMed] [Google Scholar]
- 11.Rudnicka A.R., Miller G.J., Nelson T., Doray D., Comp P.C. An association between plasma free protein-S concentration and risk of coronary heart disease in middle-aged men. Thromb Res. 2001;101:1–11. doi: 10.1016/s0049-3848(00)00379-0. [DOI] [PubMed] [Google Scholar]
- 12.ten Kate M.K., van der Meer J. Protein S deficiency: a clinical perspective. Haemophilia. 2008;14(6):1222–1228. doi: 10.1111/j.1365-2516.2008.01775.x. [DOI] [PubMed] [Google Scholar]
- 13.Middeldorp S., Prins M.H., Buller H.R. No indication for thrombophilia screening in patients with idiopathic venous thromboembolism and their relatives. Ned Tijdschr Geneeskd. 2001;145:1047–1051. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Point of Care Ultrasound
Severe LV dysfunction with apical akinesia, and large thrombus in the apical inferolateral wall.
Emergency Angiogram
Proximal LAD thrombus and distal thrombotic occlusion.
Intravascular Ultrasound
Intact endothelial surface.
Final Angiographic Result
Minimal flow restoration.
Repeated Angiogram Day 2
Restoration of TIMI III flow across the vessel with no thrombus detected.