Abstract
Although the clinical efficacies of third-generation epidermal growth factor receptor (EGFR)-tyrosine kinase inhibitors (TKIs) such as osimertinib in the treatment of non-small cell lung cancer (NSCLC) with EGFR-activating mutations are promising, drug-acquired resistance inevitably occurs whether they are used as first-line or second-line treatment. Therefore, managing the acquired resistance to third-generation EGFR-TKIs is crucial in the clinic for improving patient survival. Great efforts have been made to develop potentially effective strategies or regimens for the treatment of EGFR-mutant NSCLC patients after relapse following these TKIs therapies with the hope that patients will continue to benefit from treatment through overcoming acquired resistance. Although this approach, which aims to overcome drug-acquired resistance, is necessary and important, it is a passive practice. Taking preventive action early before disease progression to manage the unavoidable development of acquired resistance offers an equally important and efficient approach. We strongly believe that early preventive interventions using effective and tolerable combination regimens that interfere with the process of developing acquired resistance may substantially improve the outcomes of EGFR-mutant NSCLC treatment with third-generation EGFR-TKIs. Thus, this review focuses on discussing the scientific rationale and mechanism-driven strategies for delaying and even preventing the emergence of acquired resistance to third-generation EGFR-TKIs, particularly osimertinib.
Keywords: Lung cancer, Third-generation epidermal growth factor receptor-tyrosine kinase inhibitors (EGFR-TKIs), Osimertinib, Acquired resistance, EGFR mutations
Introduction
Lung cancer consists of over 80% non-small cell lung cancer (NSCLC) and has remained the leading cause of cancer deaths in the United States1 and worldwide.2 Due to advances in early diagnosis and various targeted therapies including immunotherapy, there has recently been an encouraging improvement in the 5-year survival rate of lung cancer, which is now over 20% after decades of effort.1 One important and promising type of targeted therapy is the treatment of patients with EGFR-mutant (EGFRm) NSCLC using epidermal growth factor receptor (EGFR)-tyrosine kinase inhibitors (TKIs). Over the past two decades, EGFR-TKIs have progressed rapidly from first-generation (e.g., gefitinib and erlotinib) to second-generation (e.g., afatinib) and currently mutation-selective third-generation (e.g., osimertinib/AZD9291) agents, which have achieved remarkable successes in the clinic and contributed significantly to the improved patient survival rate.
Both first- and second-generation EGFR-TKIs have proven effective in the treatment of advanced lung cancers harboring EGFR-activating mutations including L858R and 19Del. The third-generation EGFR-TKIs such as osimertinib (AZD9291) and aumolertinib (formerly almonertinib; HS-10296) were developed to target both T790M, the major mechanism for acquired resistance to first- and second-generation EGFR-TKIs, and other common EGFR-activating mutations with limited activity against wild-type (WT) EGFR, thus being mutation-selective EGFR-TKIs.3 Osimertinib is now an approved drug for the second-line treatment of NSCLC with resistance to first-generation TKI due to T790M mutation and also a standard first-line option for advanced NSCLC with activating EGFR mutations due to its promising efficacy based on overall survival (OS) and progression-free survival (PFS).4,5 Aumolertinib was recently approved in China for the treatment of NSCLC patients harboring EGFR T790M mutation who have relapsed to other EGFR-TKI therapies based on the promising outcomes of the open-label phase II APOLLO study.6,7 Aumolertinib treatment resulted in an objective response rate of 68.9% and a disease control rate of 93.4% in patients with recurrent NSCLC carrying EGFR T790M mutations. The median duration of response was 15.1 months. The median PFS was 12.4 months. Aumolertinib led to an objective response rate of 61.5% in patients with central nervous system (CNS) metastasis with a median CNS duration of response of 12.5 months.6
Despite these advances, acquired resistance to these third-generation EGFR-TKIs unavoidably occurs no matter whether they are used as a first-line or second-line treatment.8 Therefore, managing the acquired resistance to osimertinib and other third-generation EGFR-TKIs is crucial in the clinic for improving patient survival. Accordingly, great efforts have been made in the field to develop potentially effective strategies or regimens for the treatment of EGFRm NSCLC patients after relapse to osimertinib or other third-generation EGFR-TKIs with the hope that patients will continue to benefit from treatment through overcoming the acquired resistance. Although this approach, which aims to overcome acquired drug resistance, is necessary and important, it is a passive practice in reality. Taking preventive action early, before disease progression, to manage the unavoidable development of acquired resistance may provide an even more important and efficient approach as we have proposed.9,10 We strongly believe that early preventive interventions using effective and tolerable combination regimens that interfere with the process of developing acquired drug resistance may substantially improve the outcomes of EGFRm NSCLC treatment with osimertinib or other third-generation EGFR-TKIs. While there are many good review articles on the topic of overcoming acquired resistance to osimertinib, this review will primarily focus on discussing the scientific rationale/feasibility and possible mechanism-driven strategies for early preventive intervention to delay and even prevent the emergence of acquired resistance to third-generation EGFR-TKIs, particularly osimertinib.
Potential mechanisms for the emergence of acquired resistance to EGFR-TKIs, particularly third-generation EGFR-TKIs
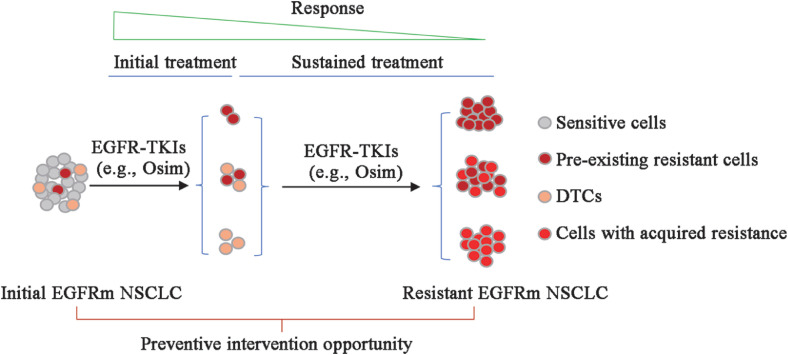
Like other targeted or genotype-directed therapies, EGFR-targeted therapy rarely achieves clinical cure due to the inability to eliminate all tumor cells. The surviving residual tumor cells, subject to sustained drug treatment, can serve as sources for the emergence of acquired drug resistance. In general, resistance may arise from the selection and expansion of pre-existing resistant clones and/or from so-called dormant drug-tolerant persistent cells (DTCs). DTCs can often survive the early phase of drug exposure, have a reversible feature of drug insensitivity upon drug removal, are typically slow cycling or dormant, and do not harbor classical drug resistance driver alterations.11,12 During treatment with an EGFR-TKI, these dormant DTCs can acquire resistance through genetic mutational or non-mutational mechanisms [Fig. 1].11, 12, 13, 14, 15
Fig. 1.
A schema for the emergence of acquired resistance to EGFR-TKIs including third-generation EGFR-TKIs. The emergence of acquired resistance to EGFR-TKIs such as osimertinib (Osim) primarily arises from the expansion of pre-existing resistant clones and/or dormant DTCs survived from initial treatment. During sustained treatment with an EGFR-TKI, these dormant DTCs can acquire resistance through genetic mutational or non-mutational mechanisms. Thus, preventive interventions aiming to prolong response duration or delay the emergence of acquired resistance should focus on maximally eradicating pre-existing resistant clones and DTCs, particularly during the early phase of treatment. DTCs: Drug-tolerant persistent cells; EGFRm: Epidermal growth factor receptor-mutant; EGFR-TKIs: Epidermal growth factor receptor-tyrosine kinase inhibitors; NSCLC: Non-small cell lung cancer; Osim: Osimertinib.
Mechanisms of resistance caused by genetic evolution or mutation include EGFR- or target-dependent (acquisition of a resistant mutation in EGFR such as T790M or C797S) and -independent (acquisition of genetic alterations in other oncogenes such as amplification of MET or HER2 or mutation of Ras or B-Raf) mechanisms: the former causes receptor conformational change that prevents an EGFR-TKI from binding to EGFR and the latter can activate certain oncogenic signaling pathways (e.g., mitogen-activated extracellular signal-regulated kinase [MEK]/extracellular signal-regulated kinase [ERK] and Yes-associated protein [YAP]) to bypass or counteract the suppressive effects of EGFR-TKIs or change histology of tumors such as transformation of NSCLC to small cell lung cancer or squamous carcinoma.3,16, 17, 18 Resistance mechanisms caused by genetic alterations (EGFR-dependent and EGFR-independent) are well-documented in many studies. However, the non-mutational mechanisms, which may account for the majority of resistant cases, particularly caused by third-generation EGFR-TKIs, are poorly understood and may involve heterogeneous mechanisms.
Potential rationale-based preventive intervention strategies to delay or prevent the emergence of acquired resistance to third-generation EGFR-TKIs
Based on the origins of cells that potentially develop acquired resistance to third-generation EGFR-TKIs as discussed above, any interventions that can remove or eliminate pre-existing resistant clones and/or DTCs may have high potential to achieve the goal of delaying or preventing the emergence of acquired resistance to third-generation EGFR-TKIs [Fig. 1].
Targeting Ras/Raf/MEK/ERK signaling
Ras/Raf/MEK/ERK signaling represents a well-established oncogenic pathway that regulates many biological processes including proliferation, survival, differentiation, and apoptosis.19,20 It is also one of the well-known downstream signaling pathways of EGFR. Suppression of this signaling pathway is critical for osimertinib to exert its promising therapeutic effect via the potent induction of apoptosis in sensitive EGFRm NSCLC cells via modulation of the levels of B-cell lymphoma-2 (Bcl-2) interacting mediator of cell death (Bim) and myeloid cell leukemia-1 (Mcl-1)21 [Fig. 2A]. However, the aberrant reactivation of the Ras/Raf/MEK/ERK signaling pathway caused by sustained treatment with a third-generation EGFR-TKI has been well documented to be associated with the emergence of acquired resistance to third-generation EGFR-TKIs based on both preclinical and clinical studies10 [Fig. 2B]. Accordingly, targeting this signaling pathway should be an effective way to delay or prevent the emergence of acquired resistance to third-generation EGFR-TKIs.
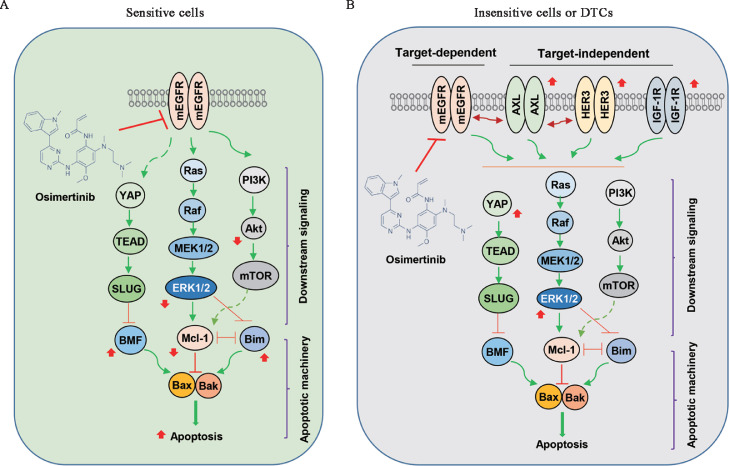
Fig. 2.
Schemas for potential mechanisms accounting for the response of EGFRm NSCLC to EGFR-TKI such as osimertinib. (A) Sensitive cells to EGFR-TKI treatment (e.g., osimertinib) can be rapidly killed by the induction of apoptosis due to suppression of MEK/ERK, YAP/TEAD, and PI3K/Akt/mTOR signaling and subsequent elevation of Bim and BMF and reduction of Mcl-1. (B) In the insensitive cells or DTCs, TKI treatment (e.g., osimertinib) may activate target-independent bypass survival mechanisms via activating other membrane receptors such as AXL, HER3, and IGF-1R, leading to reactivation of survival pathways such as MEK/ERK, YAP/TEAD, and PI3K/Akt/mTOR and eventual resistance to apoptosis. Red arrows are changes caused by an EGFR-TKI such as osimertinib. AXL: AXL receptor tyrosine kinase; Bax: Bcl-2-associated X protein; Bak: Bcl-2 homologous antagonist/killer; Bim: Bcl-2 interacting mediator of cell death; BMF: Bcl-2-modifying factor; DTCs: Drug-tolerant persistent cells; EGFRm: EGFR-mutant; EGFR-TKI: Epidermal growth factor receptor-tyrosine kinase inhibitor (TKI); ERK: Extracellular signal-regulated kinase; HER3: Human epidermal growth factor receptor 3; IGF-1R: Insulin-like growth factor 1 receptor; Mcl-1: Myeloid cell leukemia-1; MEK: Mitogen-activated extracellular signal-regulated kinase; mEGFR: Mutant EGFR; mTOR: Mammalian target of rapamycin; NSCLC: Non-small cell lung cancer; PI3K: Phosphatidylinositide 3-kinase; SLUG: A zinc finger transcriptional repressor; TEAD: Transcriptional enhanced associate domain; YAP: Yes-associated protein.
Because of the finding that sustained treatment of EGFRm NSCLC cells with WZ4002, an early version of third-generation EGFR-TKI, induced ERK1/2 reactivation, WZ4002 was combined with the MEK inhibitor, trametinib. This combination effectively prevented ERK1/2 reactivation, enhanced induction of apoptosis, and delayed the development of acquired resistance to WZ4002 in sensitive EGFRm NSCLC cell and animal models that can eventually develop acquired resistance through both T790M-dependent and -independent mechanisms.22 Similarly, osimertinib in combination with selumetinib, a different MEK inhibitor, was also shown to have activity in delaying or preventing EGFRm NSCLC cells from developing acquired resistance to osimertinib, albeit only evaluated in cell line systems.23 In another study that evaluated the efficacy and safety of osimertinib and selumetinib combination as a first-line therapy in preclinical xenografts harboring EGFR-activating mutations, the combination was shown to increase the response rate (up to 90%), reduce progression and prolong median PFS in comparison with osimertinib single-agent treatment. Importantly, all mice with clinical complete response or partial response maintained these responses for the study period (52 weeks) without relevant toxicities.24
In our own study, we also showed that osimertinib had a reduced effect on the suppression of MEK/ERK signaling and even induced rebound ERK1/2 activation in sensitive EGFRm NSCLC cells.25 The combination of osimertinib with trametinib or the ERK inhibitor, ulixertinib (VRT-752271 or BVD-523), very effectively killed several pre-existing clones that were intrinsically resistant to osimertinib and derived from PC-9 cells via enhanced induction of apoptosis, indicating that the combinations have the capacity to eliminate the pre-existing osimertinib-resistant clones. Both concurrent and intermittent (two weeks of osimertinib followed by two weeks of combination with trametinib or four weeks of osimertinib followed by two weeks of combination with trametinib) application of trametinib with osimertinib substantially delayed the emergence of osimertinib-acquired resistance both in vitro and in vivo.25 Encouragingly, some mice had undetectable tumors in each combination treatment group with a collective cure rate of 27.8% (5 of 18 mice in total),25 suggesting a clinically meaningful benefit since some patients may achieve long-term remission post-treatment with the combinations.
Intriguingly, osimertinib combined with either a MEK or ERK inhibitor did not really have augmented effects in decreasing the survival of NSCLC cell lines carrying WT EGFR,25 suggesting that these combinations may not be toxic to cells or tissues (e.g., normal tissues) harboring normal or WT EGFR. This finding is important given the potential concern that the combination of osimertinib and trametinib, or other similar agents, may accordingly enhance toxicity while augmenting therapeutic efficacy, particularly for prolonged applications since these inhibitors vertically target the same MEK/ERK signaling pathway. These findings suggest that osimertinib combined with either MEK or ERK inhibition may not have accordingly increased toxicity while exerting promising effects in abrogating osimertinib-acquired resistance. Indeed, in the study of delaying the emergence of acquired resistance, the combination of osimertinib with trametinib given concurrently to mice for >3 months was well-tolerated without apparent toxicity.25 Currently, there is an ongoing phase 2 clinical trial for studying the efficacy of osimertinib in combination with selumetinib in EGFR-inhibitor naïve advanced EGFRm NSCLC (NCT03392246; Table 1).
Table 1.
Ongoing preventative intervention clinical trials for osimertinib combined with other agents.
| Clinical trial title | Additional target | Trial registration ID |
|---|---|---|
| A phase 2 study of osimertinib in combination with selumetinib in EGFR-inhibitor naïve advanced EGFR mutant lung cancer | MEK | NCT03392246 |
| Phase 1b/2 safety, pharmacokinetic, and efficacy study of G1T38 in combination with osimertinib in patients with EGFR mutation-positive metastatic NSCLC | CDK4/6 | NCT03455829 |
| Phase 1 study of combination dacomitinib and osimertinib for patients with metastatic EGFR mutant lung cancers | HER family | NCT03810807 |
| A phase Ib study to evaluate the safety and efficacy of osimertinib in combination with ipilimumab in patients with EGFR mutated NSCLC tumors | CTLA-4 | NCT04141644 |
| Prospective observational trial to evaluate the efficacy of the combination of osimertinib and aspirin in patients with disease progression to 1st generation EGFR-TKI due to acquisition of EGFR T790M | COXs | NCT03543683 |
| A multi-center, one-arm, phase II trial of anlotinib combined with osimertinib as the second-line treatment in stage IIIb–IV NSCLC with confirmed EGFRm and T790M | VEGFR, FGFR, PDGFR and c-kit | NCT04029350 |
| Phase I/II study of dasatinib and osimertinib (AZD9291) in patients with advanced NSCLC with EGFR mutations | Src | NCT02954523 |
| Phase I/II study exploring the safety and efficacy of combining APL-101 with frontline osimertinib in patients with EGFR-mutated metastatic NSCLC | c-Met | NCT04743505 |
| Safety and efficacy of osimertinib combined with anlotinib in EGFRm+, treatment-naïve IIIb/IV NSCLC patients: a prospective, single arm, phase Ib/IIa study | VEGFR, FGFR, PDGFR and c-kit | NCT04770688 |
| A phase 2 randomized study of osimertinib versus osimertinib plus chemotherapy for patients with metastatic EGFR-mutant lung cancers that have detectable EGFR-mutant cfDNA in plasma after initiation of osimertinib | DNA | NCT04410796 |
| Additional chemotherapy for EGFRm patients with the continued presence of plasma ctDNA EGFRm at week 3 after start of osimertinib 1st-line treatment (PACE-LUNG) | DNA | NCT05281406 |
These trials will be done in EGFRm patients receiving no osimertinib treatment or initial osimertinib treatment without disease progression. The information was obtained from https://www.clinicaltrials.gov/ct2/home. CDK4/6: Cyclin-dependent kinases 4 and 6; cfDNA: Circulating free DNA; ctDNA: Circulating tumor DNA; COXs: Cyclooxygenases; CTLA-4: Cytotoxic T-lymphocyte associated protein 4; EGFR: Epidermal growth factor receptor; EGFR-TKI: EGFR-tyrosine kinase inhibitor; EGFRm: EGFR-mutant; FGFR: Fibroblast growth factor receptor; HER: Human epidermal growth factor receptor; MEK: Mitogen-activated extracellular signal-regulated kinase; NSCLC: Non-small cell lung cancer; PDGFR: Platelet-derived growth factor receptor; VEGFR: Vascular endothelial growth factor receptor.
Targeting YAP/transcriptional enhanced associate domain (TEAD) signaling
The TEAD transcription factor is best known for the transcriptional output of the Hippo signaling pathway and has been implicated in processes such as development, cell growth and proliferation, tissue homeostasis, and regeneration. In the context of Hippo signaling, TEAD functions as a nuclear DNA-binding protein and is passively activated by YAP and TAZ, transcription coactivators downstream of the Hippo pathway.26 Several previous studies have demonstrated the involvement of YAP/TEAD activation in mediating acquired resistance to EGFR-TKIs including osimertinib.27, 28, 29, 30, 31, 32
Although targeting MEK/ERK signaling is a promising strategy in managing acquired resistance to osimertinib and other third-generation EGFR-TKIs as discussed above, resistance still occurs to this combination treatment as recently demonstrated.15 It was suggested that sustained treatment with a single-agent EGFR-TKI such as osimertinib can lead to both ERK1/2 reactivation and YAP activation [Fig. 2B], whereas, upon combined EGFR/MEK inhibition, activation of YAP becomes the dominant survival mechanism in EGFRm NSCLC cells, leading to the development of resistance.15 The activated YAP/TEAD promotes EGFRm NSCLC cells to enter a senescence-like dormant state or drug-tolerant state via suppression of apoptosis by engaging the epithelial-to-mesenchymal transition (EMT) transcription factor, SLUG, to directly repress pro-apoptotic Bcl-2-modifying factor (BMF)15 [Fig. 2B].
It was also suggested that, although the dormant state resulting from YAP/TEAD activation shared several characteristics with cellular senescence, it is different from treatment-induced senescence caused by DNA-damaging agents. EGFRm NSCLC cells seem to only reversibly adopt the senescence program upon EGFR/MEK inhibition to tolerate the lethal drug treatment and revert back to the normal steady state upon drug withdrawal. Consequently, the senescent-like population can serve as a reservoir of dormant cells that are later, upon acquisition of additional resistance mechanisms, capable of re-establishing the tumor and driving clinically observed drug resistance.15
Accordingly, pharmacological co-inhibition of YAP and TEAD, or genetic deletion of YAP1, depleted dormant cells by enhancing EGFR/MEK inhibition-induced apoptosis.15 Thus, it suggests a necessity to co-target EGFR, MEK, and YAP/TEAD to enhance the initial treatment efficacy in EGFRm NSCLC (e.g., induction of apoptosis) and limit the establishment of the dormant state or reduce residual disease in order to achieve prolonged treatment responses or long-term remissions in cancer patients.15 This will be an exciting strategy if it can be validated in clinical trials.
Targeting AXL signaling
AXL is a member of the TAM (TYRO3, AXL, MER) family of receptor tyrosine kinases with the high-affinity ligand growth arrest-specific protein 6 (GAS6). AXL activation in cancer cells stimulates cell survival, increases migratory and invasive potential, and confers drug resistance.33,34 It was reported that AXL overexpression occurs more frequently in lung adenocarcinomas that harbor EGFR-activating mutations, compared with NSCLC that has WT EGFR,35 and its high expression is associated with a low response rate to EGFR-TKI.36 Moreover, AXL was reported to promote EGFR-induced signaling by binding to EGFR and other members of the human epidermal growth factor receptor (HER) family including MET and platelet-derived growth factor receptor (PDGFR), resulting in acquired resistance to EGFR-TKIs in breast cancer cells.37 In some EGFRm NSCLC cell lines with acquired resistance, upregulation of AXL was detected and thus claimed as one of the mechanisms of acquired resistance to osimertinib and a therapeutic target for overcoming acquired resistance to osimertinib.38,39 Furthermore, like ERK1/2 and YAP as discussed above, osimertinib also induces rapid AXL activation (increase in phosphorylation) in sensitive EGFRm NSCLC cells during the initial treatment that contributes to the emergence of acquired resistance to osimertinib. Activated AXL was associated with EGFR and HER3 in maintaining cell survival and inducing the emergence of cells tolerant to osimertinib36 [Fig. 2B].
Based on this rationale, it is likely that targeting AXL signaling may improve the therapeutic efficacy of osimertinib by abrogating the emergence of acquired resistance. Indeed, continuous co-treatment of a few sensitive EGFRm NSCLC cell lines with the combination of osimertinib and the AXL inhibitor, NPS1034, prevented the emergence of DTCs in vitro. In vivo, NPS1034 alone had no effect on suppressing tumor growth, but when added during either the initial or tolerant phases of osimertinib treatment of sensitive EGFRm NSCLC xenograft tumors, it reduced tumor size and delayed tumor re-growth compared to osimertinib alone.36 The combination treatment was safe since no apparent adverse events, including weight loss, were observed during over 10 weeks of treatment.36 In another study with DS-1205b, a novel selective inhibitor of AXL kinase, delayed onset of resistance to osimertinib was also achieved when used in combination with osimertinib in an HCC827 EGFRm NSCLC xenograft model.40 Similarly, another novel AXL inhibitor, ONO-7475, effectively suppressed the emergence and maintenance of DTCs to osimertinib in vitro and markedly delayed tumor regrowth compared with osimertinib alone in vivo.41
Targeting insulin-like growth factor 1 receptor (IGF-1R) signaling
The insulin-like growth factor 1 (IGF-1) and its receptor, IGF-1R, have been widely studied for their involvement in cancer and are considered potential cancer therapeutic targets.42,43 IGF-1R is a member of the insulin receptor subclass of tyrosine kinase membrane receptors and shares structural homology with the insulin receptor. Following binding to IGF-1, IGF-1R undergoes a conformational change leading to receptor activation, which then signals through adapter proteins insulin receptor substrate (IRS) 1 and 2 to activate downstream targets including the phosphatidylinositide 3-kinase (PI3K)/Akt/mammalian target of rapamycin (mTOR) and Ras/Raf/mitogen-activated protein kinase (MAPK) pathways, exerting its functions in the positive regulation of cell cycle progression, cell proliferation, and cell survival.44 Beyond the canonical pathway, IGF-1R can signal in the absence of the ligand, in the absence of kinase activity, and cross-talk to other receptors and other pathways such as EGFR signaling.43
Early generation EGFR-TKIs were shown to induce DTCs through increasing IGF-1R phosphorylation associated with insulin like growth factor binding protein 3 (IGFBP3) overexpression via stimulating the expression of lysine (K) demethylase 5A (KDM5A), a histone demethylase.13 In another study with the third-generation EGFR-TKI, WZ4002, EGFRm NSCLC cells developed resistance through activation of IGF-1R signaling due to the downregulation of IGFBP3.45 Moreover, a recent study has demonstrated that tolerance of EGFRm NSCLC cells, particularly those with low AXL expression, to osimertinib was induced by increased IGF-1R protein expression caused by the induction of its transcription factor forkhead box protein A1 (FOXA1). IGF-1R then maintained an association with EGFR and adaptor proteins, including Gab1 and IRS1, in the presence of osimertinib and restored survival signaling46 [Fig. 2B].
Based on these findings, the blockade of IGF-1R activation caused by EGFR inhibition represents another logical strategy to manage the emergence of acquired resistance to osimertinib and other third-generation EFR-TKIs. As expected, it has been shown that, in AXL-low-expressing EGFRm NSCLC xenograft and patient-derived xenograft models, transient IGF-1R inhibition combined with continuous osimertinib treatment could eradicate tumors and prevent the regrowth of tumors even after the cessation of osimertinib, suggesting again that optimal inhibition of tolerant signals combined with osimertinib may dramatically improve the outcome of EGFRm NSCLC treatment.46 This study chose the transient combination of the IGF-1R inhibitor with osimertinib to avoid potentially increased toxicities due to sustained inhibition of IGF-1R because IGF-1R plays an indispensable role in homeostasis, and its continuous inhibition may cause various adverse reactions including poor control of blood glucose level.46
Targeting HER3 signaling
HER3 is a member of the HER family and its aberrant expression is associated with poor prognosis in different cancers, including NSCLC.47, 48, 49 HER3 lacks a kinase domain and thus cannot be auto-phosphorylated; however, it can be trans-phosphorylated by coupling with other receptor tyrosine kinases such as EGFR, HER2, or MET.47,50 HER3 has been connected to EGFR-TKI resistance in EGFRm NSCLC. Hepatocyte growth factor receptor (HGFR) or MET amplification has been demonstrated to induce resistance to EGFR-TKIs in NSCLC by driving HER3-dependent activation of the PI3K/Akt pathway.51 A recent study has shown augmented HER3 expression in EGFRm tumors with acquired EGFR-TKI resistance compared with paired pretreatment samples.52 Osimertinib increased HER3 protein levels in multiple EGFRm NSCLC cell lines [Fig. 2B] while repressing Akt phosphorylation.52,53 These findings suggest the rationale for co-targeting HER3 to improve the therapeutic efficacy of osimertinib and other third-generation EGFR-TKIs.
It was shown that the HER3 neutralizing antibody, mAb33, when combined with osimertinib and cetuximab (an EGFR neutralizing antibody) prevented osimertinib-induced upregulation of HER3, likely due to the ability of mAb33 to stimulate endocytosis and intracellular degradation of HER3. Moreover, the combination enhanced the induction of apoptosis and induced complete regression of both cell line xenograft and patient-derived xenograft (PDX) models without relapse or with delayed relapse.54 Another recent study showed that the combination of osimertinib with HER3-DXd, an antibody-drug conjugate (ADC) comprised of HER3-targeting antibody linked to a topoisomerase I inhibitor currently in clinical development, and particularly osimertinib pretreatment followed by HER3-DXd co-treatment, effectively inhibited the growth of EGFRm NSCLC xenografts and PDXs and prevented tumor regrowth.53
Targeting other survival mechanisms
In principle, any intervention that can suppress the activated survival signaling needed for the survival and regrowth of DTCs and/or pre-existing resistant clones caused by treatment may have the potential to delay or prevent the emergence of acquired resistance to osimertinib. Activated Cdc42-associated kinase 1 (ACK1), also called tyrosine kinase non-receptor 2 (TNK2), is a non-receptor tyrosine kinase with an oncogenic function involved in multiple malignancies including lung cancer and has emerged as a potential cancer therapeutic target.55 We found that osimertinib combined with the inhibition of ACK1 with the novel ACK1 inhibitor, (R)-9b, or with gene knockdown, enhanced the effects of osimertinib on decreasing the survival of EGFRm NSCLC cell lines with augmented induction of apoptosis. In both in vitro and in vivo long-term resistance delay assays, the combination of (R)-9b and osimertinib clearly delayed the emergence of osimertinib resistance.56
Receptor-like tyrosine kinase (RYK) is an atypical member of the family of growth factor receptor protein tyrosine kinases and functions as a transmembrane receptor for the Wnt family of secreted protein ligands. RYK is a WNT-binding receptor tyrosine kinase (RTK) and can activate WNT signaling pathways, leading to the activation of several molecules including -catenin.57 It was recently shown that increased RYK expression was observed in osimertinib-induced DTCs from different EGFRm NSCLC cell lines and small interfering RNA (siRNA)-mediated RYK knockdown inhibited the growth of DTCs.58 Thus, it is speculated that targeting RYK may have the potential to delay the emergence of acquired resistance to osimertinib and other third-generation EGFR-TKIs, although this has yet to be tested.
In a study performing an unbiased high-throughput siRNA screening to identify proteins that mediate drug tolerance of EGFRm NSCLC to EGFR-targeted therapy, the deubiquitinase, ubiquitin specific peptidase 13 (USP13), was identified. USP13 could selectively stabilize mutant EGFR in a peptidase-independent manner by counteracting the action of members of the Cbl family of E3 ubiquitin ligases. Targeting USP13 increased the sensitivity of EGFRm NSCLC cells to EGFR inhibition with small molecules in vitro and in vivo, suggesting that USP13 is a strong mutant EGFR-specific co-target that could improve the treatment efficacy of EGFR-targeted therapies.59 Although this study used the second-generation pan-HER/EGFR-TKI, afatinib, we assume that this should also be true for osimertinib and other third-generation EGFR-TKIs. Thus, targeting USP13 may also have the potential in delaying the emergence of acquired resistance to osimertinib and other third-generation EGFR-TKIs if it is involved in osimertinib-induced drug tolerance.
Autophagy is in general considered a survival mechanism favoring cancer cells.60,61 Osimertinib has been shown to induce autophagy in EGFRm NSCLC cells.62, 63, 64, 65 Inhibition of osimertinib-induced autophagy enhances cytotoxicity of osimertinib against EGFRm NSCLC cells.63, 64, 65 Since enhanced autophagy is associated with osimertinib resistance, likely through induction of stem cell-like properties,63 it is logically proposed that targeting autophagy in combination with osimertinib to maximally eradicate stem cell-like resistant cells should also be a valid strategy for delaying and even preventing the emergence of acquired resistance to osimertinib.
Aspirin, a non-steroidal anti‐inflammatory drug widely used as an antiplatelet agent to prevent myocardial infarction and stroke, was shown to synergistically enhance the antitumor activity of osimertinib in osimertinib-resistant NSCLC cells by promoting Bim-dependent apoptosis.66 In the clinic, concurrent use of aspirin with osimertinib is associated with improved survival in patients with advanced EGFRm NSCLC,67 suggesting the potential for aspirin and likely similar agents in delaying the emergence of acquired resistance to osimertinib. The validation trial is now ongoing (NCT03543683; Table 1). Hence, further study on the biology of the combination in delaying the emergence of osimertinib-acquired resistance is thus warranted.
Directly targeting intrinsic apoptotic pathway
The major feature of both pre-existing resistant clones and dormant DTCs is their ability to evade apoptosis for survival.14,15 Both the MEK/ERK and YAP/TEAD survival signaling pathways play critical roles in the negative regulation of apoptosis by reducing the levels of pro-apoptotic proteins Bim and BMF and increasing the levels of Mcl-115,21 [Fig. 2]. Targeting either or both of the survival signaling pathways as discussed above is effective in eliminating pre-existing resistant clones and dormant DTCs via enhanced induction of Bim- or BMF-dependent apoptosis. Hence, any means to maximally eliminate pre-existing resistant clones and/or dormant DTCs during the initial treatment with third-generation EGFR-TKIs will be helpful for prolonging remission or for delaying or even preventing relapse.
It is well known that there are two major apoptotic pathways that can initiate eventual apoptotic death: the death receptor-mediated extrinsic apoptotic pathway and the mitochondria-dependent intrinsic apoptotic pathway.68,69 Since the intrinsic apoptotic pathway is tightly regulated positively and negatively by a group of Bcl-2 family members such as Bim, Bcl-2-associated X protein (Bax), Bcl-2, and Mcl-1, great efforts have been made to develop small molecule inhibitors or activators toward Bcl-2 family members as potential cancer therapeutic agents for the past decades, some of which have progressed to approved drugs in the clinic such as navitoclax (ABT263).70, 71, 72 Modulation of Bim, BMF, Bcl-1, or Mcl-1 eventually activates Bax- or Bcl-2 homologous antagonist/killer (Bak)-mediated apoptosis.72 Given that Bim, BMF, and Mcl-1 are critical proteins in mediating apoptosis induced by third-generation EGFR-TKIs such as osimertinib [Fig. 2A], it is reasonable to speculate that directly targeting the intrinsic apoptotic pathway via available Mcl-1 inhibitors, Bcl-2 inhibitors, or Bax activators to enhance induction of apoptosis of resistant EGFRm NSCLC cells, particularly pre-existing resistant clones and dormant DTCs, may be an effective strategy in delaying the emergence of acquired resistance. Our recent work has shown that direct activation of apoptosis via Mcl-1 inhibition, Bax activation, or both together, effectively induced apoptosis of different osimertinib-resistant EGFRm NSCLC cell lines and suppressed the growth of osimertinib-resistant tumors in vivo.73 Similarly, we speculate that these strategies may also be effective in delaying or even preventing the emergence of acquired resistance to osimertinib and other third-generation EGFR-TKIs. Effort toward this direction is underway.
Targeting activation of endogenous immunity
Programmed death-ligand 1 (PD-L1) expression on cancer cells is a critical mechanism contributing to immunosuppression and immune escape through the inactivation of T cells via interacting with program death-1 (PD-1) on T cells.74 The findings of downregulation of PD-L1 in EGFRm NSCLC cells by osimertinib and other EGFR-TKIs as demonstrated by us and others75, 76, 77, 78 suggest that osimertinib and other EGFR-TKIs may have an indirect effect on eliminating EGFRm NSCLC cells in vivo through activating endogenous immune defense mechanism. This is supported by other studies with EGFRm NSCLCs showing that EGFR-TKI initial treatment can trigger a transient immunostimulatory effect despite the immunosuppressive phenotype observed following longer-term treatment.79,80 Interestingly, there is a rebound PD-L1 elevation in several osimertinib-resistant cell lines and in the majority of EGFRm tissues collected after relapse to EGFR-TKI treatment when compared with their corresponding baseline expression.78,80,81 This may be a potential mechanism for the late stage of the immunosuppressive phenotype observed in previous studies,79,80 contributing to the emergence of acquired resistance to EGFR-TKIs including osimertinib. Hence, any means or strategies such as immunotherapy that can activate endogenous immunity, in theory, will be helpful for eliminating pre-existing resistant clones and/or DTCs when combined with osimertinib to achieve the goal of delaying or even preventing the emergence of acquired resistance or disease progression. Currently, there is an ongoing phase 1b clinical trial that evaluates the safety and efficacy of osimertinib in combination with ipilimumab, which is a CTLA-4 antibody, in patients with EGFRm NSCLC tumors (NCT04141644; Table 1), indicating the effort toward this direction.
Clinical preventive intervention trials aiming for delaying or preventing disease progression
While most clinical trials aim for overcoming acquired resistance to osimertinib in patients who progressed on osimertinib treatment, there are some ongoing clinical trials that are designed for delaying or preventing disease progression in EGFRm patients who are either osimertinib treatment naïve or are in the early stage of treatment with osimertinib without disease progression [Table 1]. The drugs that are used in combination with osimertinib in these trials are either targeted agents such as CDK4/6 inhibitors (e.g., G1T38), immune checkpoint inhibitors (e.g., ipilimumab), multiple tyrosine kinase inhibitors (e.g., dasatinib or anlotinib), chemotherapeutic agents, or others (e.g., aspirin).
Summary and perspectives
It has been proposed that enhancing the initial efficacy of targeted therapies could ultimately lead to prolonged treatment responses in cancer patients.15 Since the emergence of acquired resistance to osimertinib and other third-generation EGFR-TKIs is unavoidable, it appears critical to take action early to “prevent trouble before it happens” before disease progression. Therefore, any measures or strategies that could enhance initial drug-induced apoptosis to maximally reduce residual disease may lead to improved treatment outcomes. In theory, relapse would be prevented if we could effectively or completely eradicate pre-existing resistant clones and/or DTCs during the initial treatment.
Hence, we strongly believe that early preventive intervention using effective and tolerable combination regimens may substantially improve the outcomes of EGFRm NSCLC treatment with osimertinib or other third-generation EGFR-TKIs. Preclinical studies have suggested some potentially promising strategies such as co-targeting the MEK/ERK or YAP/TEAD pathways, or both, to delay or prevent the emergence of acquired resistance to osimertinib and other third-generation EGFR-TKIs, as discussed above. Clinical trials are thus urgently needed to validate these preclinical findings. One major obstacle to the combinational strategy is the potentially increased toxicity and side effects. With further optimized combination schedules, particularly intermittent ones as we recently demonstrated,25 safe and effective combination regimens are likely to be developed. We believe that these approaches give optimism that we can further improve the therapeutic efficacies of osimertinib and third-generation EGFR-TKIs by delaying and even preventing the emergence of acquired resistance.
Effective preventive intervention strategies should be established on what we have learned about the molecular mechanisms underlying the emergence of acquired resistance. Thus, fully understanding the biology that supports the survival and regrowth of DTCs and pre-existing resistant clones will be crucial for us to develop efficacious and rationale-based therapeutic interventions that can eliminate the original cell sources of developing acquired resistance to osimertinib and other third-generation EGFR-TKIs. Expanding the research space in this direction is critical.
Acknowledgments
Acknowledgments
I am thankful to Dr. Anthea Hammond in our department for editing the manuscript. Some of the work done in my lab was supported by the NIH/NCI R01 CA223220, R01 CA245386, UG1 CA233259 awards and Emory University Winship Cancer Institute lung cancer pilot funds. S-Y. S. is a Georgia Research Alliance Distinguished Cancer Scientist.
Conflicts of interest
None.
Edited by: Peifang Wei
References
- 1.Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer statistics, 2022. CA Cancer J Clin. 2022;72:7–33. doi: 10.3322/caac.21708. [DOI] [PubMed] [Google Scholar]
- 2.Sung H, Ferlay J, Siegel RL, et al. Global cancer statistics 2020: Globocan estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71:209–249. doi: 10.3322/caac.21660. [DOI] [PubMed] [Google Scholar]
- 3.Tang ZH, Lu JJ. Osimertinib resistance in non-small cell lung cancer: mechanisms and therapeutic strategies. Cancer Lett. 2018;420:242–246. doi: 10.1016/j.canlet.2018.02.004. [DOI] [PubMed] [Google Scholar]
- 4.Ramalingam SS, Vansteenkiste J, Planchard D, et al. Overall survival with osimertinib in untreated, EGFR-mutated advanced NSCLC. N Engl J Med. 2020;382:41–50. doi: 10.1056/NEJMoa1913662. [DOI] [PubMed] [Google Scholar]
- 5.Soria JC, Ohe Y, Vansteenkiste J, Reungwetwattana T, Chewaskulyong B, Lee KH, et al. Osimertinib in untreated EGFR-mutated advanced non-small-cell lung cancer. N Engl J Med. 2018;378:113–125. doi: 10.1056/NEJMoa1713137. [DOI] [PubMed] [Google Scholar]
- 6.Lu S, Wang Q, Zhang G, et al. Efficacy of aumolertinib (HS-10296) in patients with advanced EGFR T790M+ NSCLC: updated post-national medical products administration approval results from the apollo registrational trial. J Thorac Oncol. 2022;17:411–422. doi: 10.1016/j.jtho.2021.10.024. [DOI] [PubMed] [Google Scholar]
- 7.Romero D. Aumolertinib is effective in NSCLC. Nat Rev Clin Oncol. 2022;19:6. doi: 10.1038/s41571-021-00586-x. [DOI] [PubMed] [Google Scholar]
- 8.Schmid S, Li JJN, Leighl NB. Mechanisms of osimertinib resistance and emerging treatment options. Lung Cancer. 2020;147:123–129. doi: 10.1016/j.lungcan.2020.07.014. [DOI] [PubMed] [Google Scholar]
- 9.Ma G, Sun SY. Taking action early to manage emergence of acquired resistance to osimertinib or other third generation EGFR inhibitors. Oncoscience. 2021;8:101–102. doi: 10.18632/oncoscience.544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yu D, Zhao W, Vallega KA, Sun SY. Managing acquired resistance to third-generation EGFR tyrosine kinase inhibitors through co-targeting MEK/ERK signaling. Lung Cancer. 2021;12:1–10. doi: 10.2147/LCTT.S293902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Suda K, Mitsudomi T. Drug tolerance to EGFR tyrosine kinase inhibitors in lung cancers with EGFR mutations. Cells. 2021;10:1590. doi: 10.3390/cells10071590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cabanos HF, Hata AN. Emerging insights into targeted therapy-tolerant persister cells in cancer. Cancers. 2021;13:2666. doi: 10.3390/cancers13112666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sharma SV, Lee DY, Li B, et al. A chromatin-mediated reversible drug-tolerant state in cancer cell subpopulations. Cell. 2010;141:69–80. doi: 10.1016/j.cell.2010.02.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hata AN, Niederst MJ, Archibald HL, et al. Tumor cells can follow distinct evolutionary paths to become resistant to epidermal growth factor receptor inhibition. Nat Med. 2016;22:262–269. doi: 10.1038/nm.4040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kurppa KJ, Liu Y, To C, et al. Treatment-induced tumor dormancy through YAP-mediated transcriptional reprogramming of the apoptotic pathway. Cancer Cell. 2020;37:104–122. doi: 10.1016/j.ccell.2019.12.006. .e12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Le X, Puri S, Negrao MV, et al. Landscape of EGFR-dependent and -independent resistance mechanisms to osimertinib and continuation therapy beyond progression in EGFR-mutant NSCLC. Clin Cancer Res. 2018;24:6195–6203. doi: 10.1158/1078-0432.CCR-18-1542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Piper-Vallillo AJ, Sequist LV, Piotrowska Z. Emerging treatment paradigms for EGFR-mutant lung cancers progressing on osimertinib: a review. J Clin Oncol. 2020 doi: 10.1200/JCO.19.03123. [DOI] [PubMed] [Google Scholar]
- 18.Murtuza A, Bulbul A, Shen JP, et al. Novel third-generation EGFR tyrosine kinase inhibitors and strategies to overcome therapeutic resistance in lung cancer. Cancer Res. 2019;79:689–698. doi: 10.1158/0008-5472.CAN-18-1281. [DOI] [PubMed] [Google Scholar]
- 19.Friday BB, Adjei AA. Advances in targeting the Ras/Raf/MEK/Erk mitogen-activated protein kinase cascade with MEK inhibitors for cancer therapy. Clin Cancer Res. 2008;14:342–346. doi: 10.1158/1078-0432.CCR-07-4790. [DOI] [PubMed] [Google Scholar]
- 20.Roberts PJ, Der CJ. Targeting the Raf-MEK-ERK mitogen-activated protein kinase cascade for the treatment of cancer. Oncogene. 2007;26:3291–3310. doi: 10.1038/sj.onc.1210422. [DOI] [PubMed] [Google Scholar]
- 21.Shi P, Oh YT, Deng L, et al. Overcoming acquired resistance to AZD9291, a third-generation EGFR inhibitor, through modulation of MEK/ERK-dependent Bim and Mcl-1 degradation. Clin Cancer Res. 2017;23:6567–6579. doi: 10.1158/1078-0432.CCR-17-1574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tricker EM, Xu C, Uddin S, et al. Combined EGFR/MEK inhibition prevents the emergence of resistance in EGFR mutant lung cancer. Cancer Discov. 2015;5:960–971. doi: 10.1158/2159-8290.CD-15-0063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Eberlein CA, Stetson D, Markovets AA, et al. Acquired resistance to the mutant-selective EGFR inhibitor AZD9291 is associated with increased dependence on RAS signaling in preclinical models. Cancer Res. 2015;75:2489–2500. doi: 10.1158/0008-5472.CAN-14-3167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Della Corte CM, Ciaramella V, Cardone C, et al. Antitumor efficacy of dual blockade of EGFR signaling by osimertinib in combination with selumetinib or cetuximab in activated EGFR human NCLC tumor models. J Thorac Oncol. 2018;13:810–820. doi: 10.1016/j.jtho.2018.02.025. [DOI] [PubMed] [Google Scholar]
- 25.Gu J, Yao W, Shi P, et al. MEK or ERK inhibition effectively abrogates emergence of acquired osimertinib resistance in the treatment of EGFR-mutant lung cancers. Cancer. 2020;126:3788–3799. doi: 10.1002/cncr.32996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lin KC, Park HW, Guan KL. Regulation of the hippo pathway transcription factor TEAD. Trends Biochem Sci. 2017;42:862–872. doi: 10.1016/j.tibs.2017.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ghiso E, Migliore C, Ciciriello V, et al. YAP-dependent AXL overexpression mediates resistance to EGFR inhibitors in NSCLC. Neoplasia. 2017;19:1012–1021. doi: 10.1016/j.neo.2017.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lee BS, Park DI, Lee DH, et al. Hippo effector YAP directly regulates the expression of PD-L1 transcripts in EGFR-TKI-resistant lung adenocarcinoma. Biochem Biophys Res Commun. 2017;491:493–499. doi: 10.1016/j.bbrc.2017.07.007. [DOI] [PubMed] [Google Scholar]
- 29.Chaib I, Karachaliou N, Pilotto S, et al. Co-activation of STAT3 and YES-associated protein 1 (YAP1) pathway in EGFR-mutant NSCLC. J Natl Cancer Inst. 2017;109:djx014. doi: 10.1093/jnci/djx014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.McGowan M, Kleinberg L, Halvorsen AR, Helland Å, Brustugun OT. NSCLC depend upon YAP expression and nuclear localization after acquiring resistance to EGFR inhibitors. Genes Cancer. 2017;8:497–504. doi: 10.18632/genesandcancer.136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rong X, Liang Y, Han Q, et al. Molecular mechanisms of tyrosine kinase inhibitor resistance induced by membranous/cytoplasmic/nuclear translocation of epidermal growth factor receptor. J Thorac Oncol. 2019;14:1766–1783. doi: 10.1016/j.jtho.2019.06.014. [DOI] [PubMed] [Google Scholar]
- 32.Dent P, Booth L, Poklepovic A, et al. Osimertinib-resistant NSCLC cells activate ERBB2 and YAP/TAZ and are killed by neratinib. Biochem Pharmacol. 2021;190 doi: 10.1016/j.bcp.2021.114642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Auyez A, Sayan AE, Kriajevska M, Tulchinsky E. AXL receptor in cancer metastasis and drug resistance: when normal functions go askew. Cancers. 2021;13:4864. doi: 10.3390/cancers13194864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhu C, Wei Y, Wei X. AXL receptor tyrosine kinase as a promising anti-cancer approach: functions, molecular mechanisms and clinical applications. Mol Cancer. 2019;18:153. doi: 10.1186/s12943-019-1090-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wu Z, Bai F, Fan L. Coexpression of receptor tyrosine kinase AXL and EGFR in human primary lung adenocarcinomas. Hum Pathol. 2015;46:1935–1944. doi: 10.1016/j.humpath.2015.08.014. [DOI] [PubMed] [Google Scholar]
- 36.Taniguchi H, Yamada T, Wang R, et al. AXL confers intrinsic resistance to osimertinib and advances the emergence of tolerant cells. Nat Commun. 2019;10:259. doi: 10.1038/s41467-018-08074-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Meyer AS, Miller MA, Gertler FB, Lauffenburger DA. The receptor AXL diversifies EGFR signaling and limits the response to EGFR-targeted inhibitors in triple-negative breast cancer cells. Sci Signal. 2013;6:ra66. doi: 10.1126/scisignal.2004155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Namba K, Shien K, Takahashi Y, et al. Activation of AXL as a preclinical acquired resistance mechanism against osimertinib treatment in EGFR-mutant non-small cell lung cancer cells. Mol Cancer Res. 2019;17:499–507. doi: 10.1158/1541-7786.MCR-18-0628. [DOI] [PubMed] [Google Scholar]
- 39.Kim D, Bach DH, Fan YH, et al. AXL degradation in combination with EGFR-TKI can delay and overcome acquired resistance in human non-small cell lung cancer cells. Cell Death Dis. 2019;10:361. doi: 10.1038/s41419-019-1601-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jimbo T, Hatanaka M, Komatsu T, et al. DS-1205b, a novel selective inhibitor of AXL kinase, blocks resistance to EGFR-tyrosine kinase inhibitors in a non-small cell lung cancer xenograft model. Oncotarget. 2019;10:5152–5167. doi: 10.18632/oncotarget.27114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Okura N, Nishioka N, Yamada T, et al. ONO-7475, a novel AXL inhibitor, suppresses the adaptive resistance to initial EGFR-TKI treatment in egfr-mutated non-small cell lung cancer. Clin Cancer Res. 2020;26:2244–2256. doi: 10.1158/1078-0432.CCR-19-2321. [DOI] [PubMed] [Google Scholar]
- 42.Zhang Y, Gao C, Cao F, et al. Pan-cancer analysis of IGF-1 and IGF-1R as potential prognostic biomarkers and immunotherapy targets. Front Oncol. 2021;11 doi: 10.3389/fonc.2021.755341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Crudden C, Girnita A, Girnita L. Targeting the IGF-1R: the tale of the tortoise and the hare. Front Endocrinol. 2015;6:64. doi: 10.3389/fendo.2015.00064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pillai RN, Ramalingam SS. Inhibition of insulin-like growth factor receptor: end of a targeted therapy? Transl Lung Cancer Res. 2013;2:14–22. doi: 10.3978/j.issn.2218-6751.2012.11.05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cortot AB, Repellin CE, Shimamura T, et al. Resistance to irreversible EGF receptor tyrosine kinase inhibitors through a multistep mechanism involving the IGF1R pathway. Cancer Res. 2013;73:834–843. doi: 10.1158/0008-5472.CAN-12-2066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wang R, Yamada T, Kita K, et al. Transient IGF-1R inhibition combined with osimertinib eradicates AXL-low expressing EGFR mutated lung cancer. Nature Commun. 2020;11:4607. doi: 10.1038/s41467-020-18442-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Haikala HM, Jänne PA. Thirty years of HER3: from basic biology to therapeutic interventions. Clin Cancer Res. 2021;27:3528–3539. doi: 10.1158/1078-0432.CCR-20-4465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Del Re M, Cucchiara F, Petrini I, et al. erbB in NSCLC as a molecular target: current evidences and future directions. ESMO Open. 2020;5 doi: 10.1136/esmoopen-2020-000724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kumar R, George B, Campbell MR, et al. HER family in cancer progression: from discovery to 2020 and beyond. Adv Cancer Res. 2020;147:109–160. doi: 10.1016/bs.acr.2020.04.001. [DOI] [PubMed] [Google Scholar]
- 50.Hafeez U, Parslow AC, Gan HK, Scott AM. New insights into ErbB3 function and therapeutic targeting in cancer. Expert Rev Anticancer Ther. 2020;20:1057–1074. doi: 10.1080/14737140.2020.1829485. [DOI] [PubMed] [Google Scholar]
- 51.Engelman JA, Zejnullahu K, Mitsudomi T, et al. MET amplification leads to gefitinib resistance in lung cancer by activating ERBB3 signaling. Science. 2007;316:1039–1043. doi: 10.1126/science.1141478. [DOI] [PubMed] [Google Scholar]
- 52.Yonesaka K, Tanizaki J, Maenishi O, et al. HER3 augmentation via blockade of EGFR/AKT signaling enhances anticancer activity of HER3-targeting patritumab deruxtecan in EGFR-mutated non-small cell lung cancer. Clin Cancer Res. 2022;28:390–403. doi: 10.1158/1078-0432.CCR-21-3359. [DOI] [PubMed] [Google Scholar]
- 53.Haikala HM, Lopez T, Köhlerr J, et al. EGFR inhibition enhances the cellular uptake and antitumor-activity of the HER3 antibody-drug conjugate HER3-DXd. Cancer Res. 2022;82:130–141. doi: 10.1158/0008-5472.CAN-21-2426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Romaniello D, Marrocco I, Belugali Nataraj N, et al. Targeting HER3, a catalytically defective receptor tyrosine kinase, prevents resistance of lung cancer to a third-generation EGFR kinase inhibitor. Cancers. 2020;12:2394. doi: 10.3390/cancers12092394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Mahajan K, Mahajan NP. ACK1/TNK2 tyrosine kinase: molecular signaling and evolving role in cancers. Oncogene. 2015;34:4162–4167. doi: 10.1038/onc.2014.350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Gu J, Qian L, Zhang G, et al. Inhibition of ACK1 delays and overcomes acquired resistance of EGFR mutant NSCLC cells to the third generation EGFR inhibitor, osimertinib. Lung Cancer. 2020;150:26–35. doi: 10.1016/j.lungcan.2020.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Roy JP, Halford MM, Stacker SA. The biochemistry, signalling and disease relevance of RYK and other WNT-binding receptor tyrosine kinases. Growth Factors. 2018;36:15–40. doi: 10.1080/08977194.2018.1472089. [DOI] [PubMed] [Google Scholar]
- 58.Ohara S, Suda K, Fujino T, et al. Dose-dependence in acquisition of drug tolerant phenotype and high RYK expression as a mechanism of osimertinib tolerance in lung cancer. Lung Cancer. 2021;154:84–91. doi: 10.1016/j.lungcan.2021.02.017. [DOI] [PubMed] [Google Scholar]
- 59.Giron P, Eggermont C, Noeparast A, et al. Targeting USP13-mediated drug tolerance increases the efficacy of EGFR inhibition of mutant EGFR in non-small cell lung cancer. Int J Cancer. 2020;148:2579–2593. doi: 10.1002/ijc.33404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ariosa AR, Lahiri V, Lei Y, et al. A perspective on the role of autophagy in cancer. Biochim Biophys Acta Mol Basis Dis. 2021;1867 doi: 10.1016/j.bbadis.2021.166262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Janku F, McConkey DJ, Hong DS, Kurzrock R. Autophagy as a target for anticancer therapy. Nat Rev Clin Oncol. 2011;8:528–539. doi: 10.1038/nrclinonc.2011.71. [DOI] [PubMed] [Google Scholar]
- 62.Tang ZH, Cao WX, Su MX, Chen X, Lu JJ. Osimertinib induces autophagy and apoptosis via reactive oxygen species generation in non-small cell lung cancer cells. Toxicol Appl Pharmacol. 2017;321:18–26. doi: 10.1016/j.taap.2017.02.017. [DOI] [PubMed] [Google Scholar]
- 63.Li L, Wang Y, Jiao L, et al. Protective autophagy decreases osimertinib cytotoxicity through regulation of stem cell-like properties in lung cancer. Cancer Lett. 2019;452:191–202. doi: 10.1016/j.canlet.2019.03.027. [DOI] [PubMed] [Google Scholar]
- 64.Chen H, Lin C, Lu C, et al. Metformin-sensitized NSCLC cells to osimertinib via AMPK-dependent autophagy inhibition. Clin Respir J. 2019;13:781–790. doi: 10.1111/crj.13091. [DOI] [PubMed] [Google Scholar]
- 65.Chen H, Lu C, Lin C, et al. VPS34 suppression reverses osimertinib resistance via simultaneously inhibiting glycolysis and autophagy. Carcinogenesis. 2021;42:880–890. doi: 10.1093/carcin/bgab030. [DOI] [PubMed] [Google Scholar]
- 66.Han R, Hao S, Lu C, et al. Aspirin sensitizes osimertinib-resistant NSCLC cells in vitro and in vivo via Bim-dependent apoptosis induction. Mol Oncol. 2020;14:1152–1169. doi: 10.1002/1878-0261.12682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Liu X, Hong L, Nilsson M, et al. Concurrent use of aspirin with osimertinib is associated with improved survival in advanced EGFR-mutant non-small cell lung cancer. Lung Cancer. 2020;149:33–40. doi: 10.1016/j.lungcan.2020.08.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Cotter TG. Apoptosis and cancer: the genesis of a research field. Nat Rev Cancer. 2009;9:501–507. doi: 10.1038/nrc2663. [DOI] [PubMed] [Google Scholar]
- 69.Hengartner MO. The biochemistry of apoptosis. Nature. 2000;407:770–776. doi: 10.1038/35037710. [DOI] [PubMed] [Google Scholar]
- 70.Fesik SW. Promoting apoptosis as a strategy for cancer drug discovery. Nat Rev Cancer. 2005;5:876–885. doi: 10.1038/nrc1736. [DOI] [PubMed] [Google Scholar]
- 71.Hafezi S, Rahmani M. Targeting BCL-2 in cancer: advances, challenges, and perspectives. Cancers. 2021;13:1292. doi: 10.3390/cancers13061292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Jeng PS, Inoue-Yamauchi A, Hsieh JJ, Cheng EH. BH3-dependent and independent activation of BAX and BAK in mitochondrial apoptosis. Curr Opin Physiol. 2018;3:71–81. doi: 10.1016/j.cophys.2018.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Ma G, Deng Y, Qian L, et al. Overcoming acquired resistance to third-generation EGFR inhibitors by targeting activation of intrinsic apoptotic pathway through Mcl-1 inhibition, Bax activation, or both. Oncogene. 2022;41:1691–1700. doi: 10.1038/s41388-022-02200-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Guan J, Lim KS, Mekhail T, Chang CC. Programmed death ligand-1 (PD-L1) expression in the programmed death receptor-1 (PD-1)/PD-L1 blockade: a key player against various cancers. Arch Pathol Lab Med. 2017;141:851–861. doi: 10.5858/arpa.2016-0361-RA. [DOI] [PubMed] [Google Scholar]
- 75.Zhang N, Zeng Y, Du W, et al. The EGFR pathway is involved in the regulation of PD-L1 expression via the IL-6/JAK/STAT3 signaling pathway in EGFR-mutated non-small cell lung cancer. Int J Oncol. 2016;49:1360–1368. doi: 10.3892/ijo.2016.3632. [DOI] [PubMed] [Google Scholar]
- 76.Lin K, Cheng J, Yang T, Li Y, Zhu B. EGFR-TKI down-regulates PD-L1 in EGFR mutant NSCLC through inhibiting NF-kappaB. Biochem Biophys Res Commun. 2015;463:95–101. doi: 10.1016/j.bbrc.2015.05.030. [DOI] [PubMed] [Google Scholar]
- 77.Jiang XM, Xu YL, Huang MY, et al. Osimertinib (AZD9291) decreases programmed death ligand-1 in EGFR-mutated non-small cell lung cancer cells. Acta Pharmacol Sin. 2017;38:1512–1520. doi: 10.1038/aps.2017.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Qian G, Guo J, Vallega KA, et al. Membrane-associated RING-CH 8 functions as a novel PD-L1 E3 ligase to mediate PD-L1 degradation induced by EGFR inhibitors. Mol Cancer Res. 2021;19:1622–1634. doi: 10.1158/1541-7786.MCR-21-0147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Dominguez C, Tsang KY, Palena C. Short-term EGFR blockade enhances immune-mediated cytotoxicity of EGFR mutant lung cancer cells: rationale for combination therapies. Cell Death Dis. 2016;7:e2380. doi: 10.1038/cddis.2016.297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Maynard A, McCoach CE, Rotow JK, et al. Therapy-induced evolution of human lung cancer revealed by single-cell RNA sequencing. Cell. 2020;182:1232–1251.e22. doi: 10.1016/j.cell.2020.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Isomoto K, Haratani K, Hayashi H, et al. Impact of EGFR-TKI treatment on the tumor immune microenvironment in EGFR mutation-positive non-small cell lung cancer. Clin Cancer Res. 2020;26:2037–2046. doi: 10.1158/1078-0432.CCR-19-2027. [DOI] [PubMed] [Google Scholar]