Abstract
Introduction:
Adolescence is an important phase in brain maturation, specifically it is a time during which weak synapses are pruned and neural pathways are strengthened. Adolescence is also a time of experimentation with drugs, including cannabis, which may have detrimental effects on the developing nervous system. The cannabinoid type 1 receptor (CB1) is an important modulator of neurotransmitter release and plays a central role in neural development. Neurotrophic factors such as brain-derived neurotrophic factor (BDNF) and its receptor, tropomyosin receptor kinase B (TrkB), are also critical during development for axon guidance and synapse specification.
Objective:
The objective of this study was to examine the effects of the phytocannabinoids, Δ9-tetrahydrocannabinol (THC) and cannabidiol (CBD), on the expression of BDNF, its receptor TrkB, and other synaptic markers in the adolescent mouse hippocampus.
Materials and Methods:
Mice of both sexes were injected daily from P28 to P49 with 3 mg/kg THC, CBD, or a combination of THC/CBD. Brains were harvested on P50, and the dorsal and ventral hippocampi were analyzed for levels of BDNF, TrkB, and several synaptic markers using quantitative polymerase chain reaction, western blotting, and image analyses.
Results:
THC treatment statistically significantly reduced transcript levels of BDNF in adolescent female (BDNF I) and male (BDNF I, II, IV, VI, and IX) hippocampi. These changes were prevented when CBD was co-administered with THC. CBD by itself statistically significantly increased expression of some transcripts (BDNF II, VI, and IX for females, BDNF VI for males). No statistically significant changes were observed in protein expression for BDNF, TrkB, phospho-TrkB, phospho-CREB (cAMP response element-binding protein), and the synaptic markers, vesicular GABA transporter, vesicular glutamate transporter, synaptobrevin, and postsynaptic density protein 95. However, CB1 receptors were statistically significantly reduced in the ventral hippocampus with THC treatment.
Conclusions:
This study found changes in BDNF mRNA expression within the hippocampus of adolescent mice exposed to THC and CBD. THC represses transcript expression for some BDNF variants, and this effect is rescued when CBD is co-administered. These effects were seen in both males and females, but sex differences were observed in specific BDNF isoforms. While a statistically significant reduction in CB1 receptor protein in the ventral dentate gyrus was seen, no other changes in protein levels were observed.
Keywords: cannabinoid type 1 receptor, CB1, brain-derived neurotrophic factor, BDNF, tropomyosin receptor kinase B, TrkB, phytocannabinoids
Introduction
Cannabis has been consumed by humans for thousands of years with its prevalence modulated by political and societal climates. Δ9-Tetrahydrocannabinol (THC) is the major psychoactive phytocannabinoid of cannabis, and cannabidiol (CBD) is the main nonpsychoactive phytocannabinoid. Modern plant breeding has resulted in cannabis strains with high-THC and low-CBD content.1 The negative connotations previously associated with cannabis usage are currently waning in the United States, especially in young adults,2 who now consume cannabis at a higher level than cigarettes.3 With the shift in society's perspective and rate of consumption comes the imperative to understand the molecular alterations occurring within the brain of cannabis-consuming adolescents.
Adolescence is typically characterized by physiological changes that occur before adulthood, including changes in synaptic plasticity and white matter.4–7 Previous research suggests that cannabis consumption during this vulnerable developmental phase can cause persistent adverse effects. Long-term deficits in memory and changes in hippocampal function have been reported in chronic teenage smokers.8–10 Other work suggests deficits in the formation and recall of working memory.11,12
Chronic cannabis use in adolescence correlates with an increased risk for psychiatric disorders, including schizophrenia and anxiety/depressive disorders.13 THC has also been shown to disrupt molecular and behavioral maturation in the central nervous system (CNS) of adolescent rats.14 Characterizing molecular changes within the adolescent brain from phytocannabinoid exposure may illuminate mechanisms behind neurological dysfunction.
The endocannabinoid system (ECS) plays an important role in neurodevelopment and synaptic plasticity. It is centered around two key cannabinoid receptors, cannabinoid type 1 receptor (CB1) and cannabinoid type 2 receptor (CB2).
CB1 is a G protein-coupled receptor (GPCR) that is highly expressed in the CNS; however, there is a discrepancy in binding affinity and receptor distribution across species, with human CB1 having a 50-fold higher THC-binding affinity compared with rodent.15 CB1 has been shown to modulate the release of neurotransmitters in developing and mature glutamatergic and GABAergic neurons.16 It is highly expressed in important brain regions for high level cognition, including the hippocampus, and is activated by THC to produce its characteristic psychoactive effect. The ECS has been shown to play a role in eating disorders, neurodegenerative disorders, anxiety, depression, pain, liver disease, osteoporosis, cancer, and intestinal inflammation.17 The ECS also plays several key roles in pre- and postnatal brain development.18
The CB2 is closely related to CB1. Early work suggested that CB1 was expressed primarily in the CNS and CB2 in the periphery. CB2 is highly expressed within immune tissues such as the spleen and tonsil. While the expression of CB2 in the brain is low compared with CB1, and a complete understanding of the cell types expressing CB2 remains unresolved, recent evidence suggests that this receptor is expressed in the subventricular zone,19 the hippocampus,20 as well as other brain regions.20,21 The CB2 receptor is expressed in the subgranular zone of the dentate gyrus and plays a role in adult neurogenesis and differentiation.20 Thus, the effects of THC and/or CBD acting at CB2 receptors may affect adolescent neurogenesis and the molecules associated with it.
While the mechanistic and behavioral activity of THC has been the subject of many studies, many questions remain on its impact on the developing adolescent brain. The ECS regulates synaptogenesis and target selection, and exogenous endocannabinoids such as THC can interfere with these processes.22 CB1 receptors (CB1Rs) have also been shown to transactivate extracellular signal-regulated kinases 1 and 2, which play an important role in neuronal development.23,24 Finally, CB1 activity modulates TrkB-dependent interneuron migration during corticogenesis, demonstrating that CB1 affects brain-derived neurotrophic factor (BDNF) signaling.25
Mechanisms of CBD signaling are much less well understood. Recent studies suggest that it is a negative allosteric modulator for CB126–28 and CB2.28 Other studies implicate CBD activity at GPR55,29,30 5HT1a,30,31 Trp channels,30 and peroxisome proliferator-activated receptors,30 among others. There is much interest in CBD as a possible therapeutic agent.32
Neurotrophic factors drive neuronal development and synaptic plasticity.33,34 BDNF is a homodimeric neurotrophin that plays a key role in both processes.35 BDNF is active in the mesolimbic dopamine pathway and has been implicated in learning and memory, as well as neuropsychiatric disease.36 BDNF binds to the tropomyosin receptor kinase B (also known as tyrosine receptor kinase B or TrkB). TrkB regulates the Ras-PI3K-Akt pathway to control cell growth and survival as well as the GRB2-Ras-MAPK pathway to control neuronal differentiation.37,38 Activation of TrkB promotes phospholipase Cγ pathways to regulate synaptic plasticity.39 TrkB is also involved in transcriptional regulation via interactions with p21 (Cip21).40
There are 9 known exons and 11 known splice variants for BDNF, with exon 9 being the common coding region. Exons I, IIB, IIC, IV, and VI are the major splice variants.41 High levels of BDNF have been reported in the hippocampus,42 with exons IV and VI constitutively expressed in all principal hippocampal neurons.41
The BDNF/TrkB and endocannabinoid systems are linked. Treatment of CB1R+ interneurons with the CB1R agonist, anandamide (AEA) caused heterocomplex formation between CB1 and TrkB receptors.25 This work showed that endocannabinoids can transactivate TrkB to stimulate migration and neurite development of CCK+ interneurons. However, prolonged exposure inhibited the BDNF-induced extension and branching of interneurons.25 Recent reports suggest that treatment with phytocannabinoids alters BDNF expression levels in rodents.43
In this work, we follow up these observations by focusing on changes to BDNF and TrkB expression in the hippocampus of adolescent mice treated with phytocannabinoids. We find that treatment during this important period evokes differential expression of BDNF within the dorsal hippocampus.
Materials and Methods
Subjects
CD1 mice (n=84) were caged with same-sex littermates under a 12/12-h light cycle. Food and water were available ad libitum. All procedures were approved by the Indiana University Institutional Animal Care and Use Committee.
Drug treatments
THC, CBD, or THC+CBD (1:1) were dissolved in 100% ethanol (6 mg/mL). Before dosing, drugs were mixed into an injection solution (5% phytocannabinoid, 5% Kolliphor in 0.9% sodium chloride) to a final concentration of 0.3 mg/mL. Mice received daily intraperitoneal injections of 3 mg/kg from P28 to P49.
Tissue preparation
Brains were harvested and immediately flash-frozen in 2-methylbutane. Tissue was stored at −80°C. Frozen hippocampi were dissected as previously described.44
Quantitative polymerase chain reaction
Primer sequences for BDNF splice variants were from Fukuchi et al.45 Primers for TrkB were designed using NCBI primer BLAST (www.ncbi.nlm.nih.gov/tools/primer-blast), ordered from Integrated DNA Technologies (Coralville, IA), and are as follows:
TrkB-S 5′ CGT CAC TTC GCC AGC AGT AG 3′
TrkB-AS 5′ GGT AGC AGG ACA GTG CCG 3′
RNA was processed for reverse transcription-quantitative polymerase chain reaction (RTqPCR) (TRIzol [cat no. 15596026; Thermo Fisher], RevertAid [no. K1691; Thermo Fisher Scientific], SYBR green [no. 60082, Brilliant II; Agilent]) as per the manufacturer's instructions and analyzed using the ddCt method.
Protein analysis
Hippocampi were homogenized in RIPA buffer (50 mM Tris, pH 8.0, 150 mM NaCl, 1% Tx-100, 0.1% SDS, 0.5% CHAPS, 1×HALT [no. 78440; Thermo Fisher Scientific]) and centrifuged. Supernatants were run on a 4–12% NuPage gel (no. NP0323BOX; Thermo Fisher Scientific) and transferred to nitrocellulose. Protein was quantitated (no. 926-11011, Revert; Li-Cor), and blots were incubated with primary antibodies (see Table 1) overnight at 4°C. They were then washed and incubated in secondary antibody for 1 h at room temperature. Finally, blots were scanned on a Li-Cor Odyssey near-IR imager. Band intensities were calculated using the ImageJ software and normalized to total protein loaded.
Table 1.
Antibodies Used in This Study
| Antibody | Immunogen | Supplier | Catalog no. | Host, monoclonal vs. polyclonal | Dilution used |
|---|---|---|---|---|---|
| Immunohistochemistry | |||||
| BDNF | Synthetic human BDNF; amino acids 151–247 | Bioss, Inc., Boston, MA | bs-4989R | Rabbit pAb | 1:1,000 |
| P-TrkB | Linear synthetic rat TrkB terminus phosphorylated at Tyr816 | EMD Millipore Corp., Burlington, MA | ABN1381 | Rabbit pAb | 1:1,000 |
| CREB | Synthetic human CREB-1; amino terminus | Cell Signaling Technologies, Danvers, MA | 9197-S | Rabbit mAb | 1:2,000 |
| P-CREB | Synthetic human CREB phosphorylated at Ser133 | Cell Signaling Technologies, Danvers, MA | 9198-S | Rabbit mAb | 1:1,000 |
| Parvalbumin | Frog muscle parvalbumin | Sigma, St. Louis, MO | P3088 | Mouse mAb | 1:2,000 |
| CB1-L15 (rat sequence; amino acids 460–473) | Purified recombinant rat CB1; amino acids 460–473 | Mackie Lab59 | Guinea pig pAb | 1:2,000 | |
| Western assays | |||||
| BDNF | Synthetic human BDNF; center region | GeneTex, Irvine, CA | GTX132621 | Rabbit pAb | 1:1,000 |
| BDNF | Synthetic human BDNF; amino acids 151–247 | Bioss, Inc., Boston, MA | bs-4989R | Rabbit pAb | 1:500 |
| TrkB | Purified recombinant mouse TrkB; amino acids 32–429 | R&D Systems, Minneapolis, MN | AF1494 | Goat pAb | 1:2,000 |
| CREB | Synthetic human CREB-1; amino terminus | Cell Signaling Technologies, Danvers, MA | 9197-S | Rabbit mAb | 1:2,000 |
| VGAT | Synthetic peptide of rat VGAT; amino acids 75–87 | Synaptic Systems, Goettingen, Germany | 131011 | Mouse mAb | 1:2,000 |
| Synaptobrevin 2 | Synthetic peptide of rat synaptobrevin 2; amino acids 2–17 | Synaptic Systems, Goettingen, Germany | 104204 | Guinea pig pAb | 1:2,000 |
| PSD95 | Purified recombinant rat postsynaptic density 95 kDa | GeneTex, Irvine, CA | GTX80682 | Mouse mAb | 1:1,000 |
| VGlut2 | Purified recombinant rat VGAT; amino acids 2–115 | Synaptic Systems, Goettingen, Germany | 131004 | Guinea pig pAb | 1:1,000 |
BDNF, brain-derived neurotrophic factor; CB1, cannabinoid type 1 receptor; CREB, cAMP response element-binding protein; TrkB, tropomyosin receptor kinase B; VGAT, vesicular GABA transporter; VGlut2, vesicular glutamate transporter 2.
Immunohistochemistry
To characterize changes within the adolescent hippocampus with THC treatment, the dentate gyrus was analyzed. This area contains a high level of BDNF and is also a region of neurogenesis. Mice were perfused with 4% paraformaldehyde (PFA). Brains were incubated overnight in 4% PFA at 4°C and then embedded in 30% sucrose for 48 h at 4°C. They were then frozen in isopentane on dry ice and stored at −80°C. Frozen brains were cut at 40 μm and were blocked with 5% normal donkey serum and 0.3% Triton X-100 for 1 h and were then incubated with primary antibody for 48 h at 4°C (see Table 1). They were then incubated in Alexa Fluor secondary antibodies diluted 1:500 in blocking buffer overnight.
Sections were mounted on slides using Fluoromount-G containing the nuclear stain 4′,6-diamidino-2-phenylindole (catalog no. 0100-20; SouthernBiotech). Images were collected at 0.5 μm steps on a Nikon A1 confocal microscope (Northern Lights Imaging Center, Indiana University) using a 20× lens. Camera and device settings were held constant across samples. Images were processed using ImageJ. Background was subtracted, and a maximum intensity z projection of five continuous images was thresholded and integrated intensity was measured.
Statistical analyses
Data manipulations and calculations were performed using Excel (Microsoft, Redmond, WA). Graphing and statistical analyses were done on Prism 7 software (GraphPad Software, Inc., La Jolla, CA). Groups were compared as described in figures.
Results
BDNF transcript expression in the brain
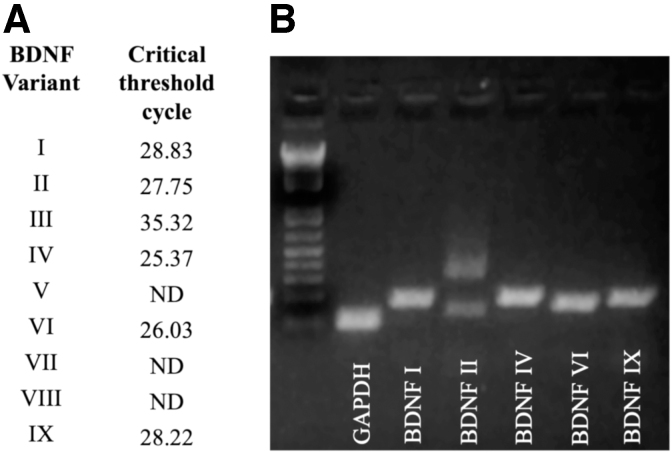
The expression of BDNF variants previously reported45 was confirmed in cerebellum and dorsal hippocampus (Fig. 1A, B). The transcripts most prominently expressed in both tissues were BDNF I, II, IV, VI, and IX. A band for BDNF III was observed near the limit of detection. BDNF V, VII, and VIII were not detected in cerebellum (Fig. 1A). A similar pattern of expression was observed for dorsal hippocampus (Fig. 1B). Amplicon sizes correspond with those expected for the respective BDNF variants.
FIG. 1.
Detection of BDNF variants in mouse brain. (A) Critical threshold was exceeded for BDNF variants I, II, III, IV, VI, and IX. No signal was detected for BDNF V, VII, or VIII (ND). (B) Variants with the highest expression levels were analyzed on a 3% agarose gel. Bands corresponding to predicted sizes were observed (BDNF II displays three bands as expected). BDNF, brain-derived neurotrophic factor; ND, not detected.
Phytocannabinoid-induced changes in BDNF/TrkB transcript levels in the dorsal hippocampus
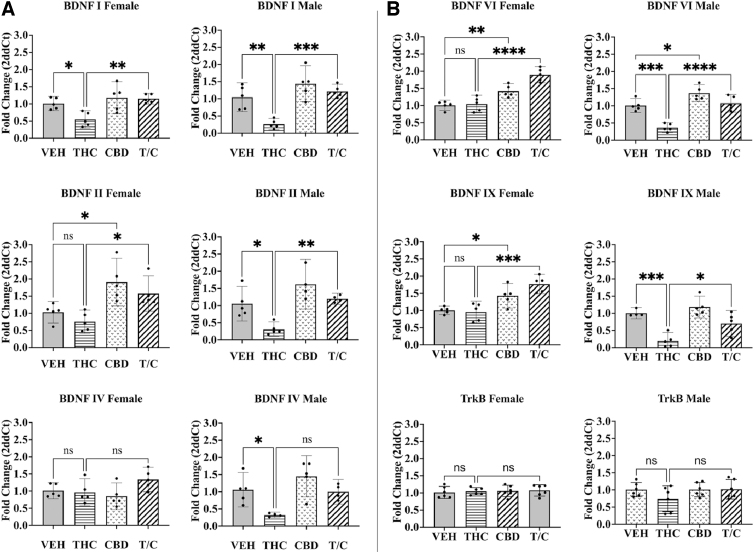
We assessed the effects of adolescent exposure to phytocannabinoids on BDNF transcripts using RT-qPCR. In males, THC exposure statistically significantly decreased BDNF transcript expression in the dorsal hippocampus (n=4–5, p<0.05–0.001; Fig. 2A, B, right). This was true for all variants tested. A similar result was observed in females for BDNF I (n=5, p<0.05; Fig. 2A, top left), but not for the other transcripts tested. Interestingly, these reductions were not observed when CBD was co-administered. CBD treatment alone statistically significantly increased expression of some variants, including BDNF II, VI, and IX in females (n=5, p<0.05–0.01; Fig. 2A, B, left) and BDNF VI in males (n=5, p<0.05; Fig. 2B, top right). Treatment with phytocannabinoids did not significantly change TrkB transcript expression (n=6; Fig. 2B, bottom).
FIG. 2.
Real-time quantitative polymerase chain reaction was used to determine transcript expression for BDNF variants and the TrkB receptor in females (left) and males (right). (A) BDNF variants I, II, and IV. (B) BDNF VI, IX, and the TrkB receptor. A one-way ANOVA with multiple comparisons and Tukey's post hoc test were used to determine differences with a p-value set to 0.05 (*<0.05, **<0.01, ***<0.001, ****<0.0001; ns). The Grubbs test was used to remove single outliers, n=4–6. Error bars represent 95% confidence interval. ANOVA, analysis of variance; CBD, cannabidiol; ns, not significant; T/C, treatment with both THC and CBD; THC, tetrahydrocannabinol; TrkB, tropomyosin receptor kinase B.
Does phytocannabinoid treatment affect expression of BDNF/TrkB protein in the hippocampus?
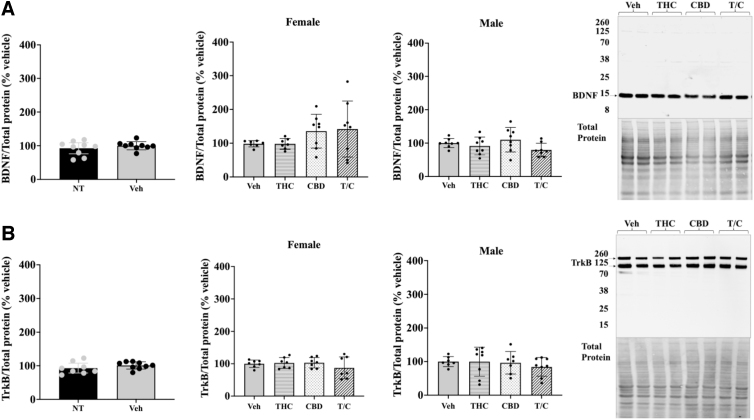
Given that phytocannabinoid treatment altered expression of key BDNF transcripts, we examined BDNF and TrkB protein levels to determine whether changes were detectable. We first assessed if our injection protocol induced changes. No statistically significant differences in protein levels for BDNF or TrkB were apparent for vehicle-treated versus naive mice (n=8–9; Fig. 3, left). Similarly, none of the phytocannabinoid treatments affected protein levels of either BDNF or TrkB (n=7–8; Fig. 3, middle graphs). The pro-BDNF to mature BDNF ratio was also unchanged (data not shown).
FIG. 3.
Quantitation of BDNF and TrkB receptor protein in the hippocampus using western blot analysis. (A) BDNF analysis. (B) TrkB receptor western analysis. Comparison between NT and vehicle-treated mice for BDNF and TrkB receptor (female and male data combined; left). Protein expression of treated female and male mice relative to vehicle (middle graphs). Representative western blots and corresponding total protein images for BDNF and TrkB (right). T/C stands for THC and CBD. A one-way ANOVA with multiple comparisons and Tukey's post hoc test were used to determine differences with a p-value set to 0.05 for statistical significance. No differences were observed between groups, n=7–8. Error bars represent 95% confidence interval. NT, nontreated.
These observations suggest that there are no global changes in BDNF or TrkB protein expression in the hippocampus with moderate THC/CBD exposure during the adolescent period.
A closer look
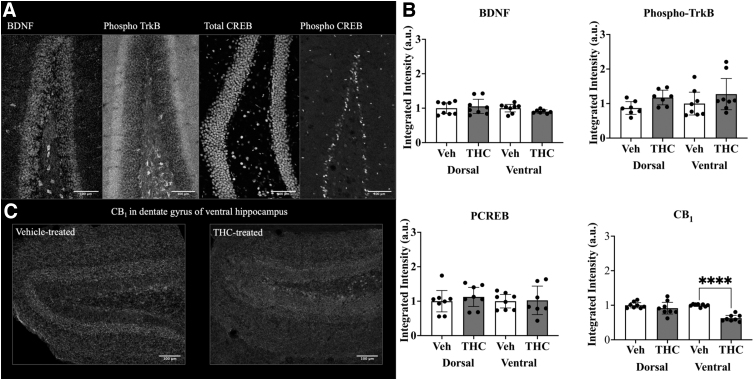
Using immunohistochemistry, we examined the dentate gyrus in males (Fig. 4). This was done for both dorsal and ventral regions. Again, no statistically significant differences were detected in BDNF with THC treatment (n=8; Fig. 4B). Markers for BDNF activity, including phosphorylated TrkB (Tyr816) and cAMP response element-binding protein (CREB) (Ser133), also displayed no change. A statistically significant reduction of the CB1 receptor was observed in the ventral dentate gyrus (n=8, p<0.0001; Fig. 4B, bottom right, and Fig. 4C).
FIG. 4.
Quantitation of BDNF, phospho-TrkB, phospho-CREB, and CB1 using immunohistochemistry. (A) Representative images of dentate gyrus probed for expression of selected molecules: BDNF (left), phosphorylated TrkB (Tyr816, middle left), total CREB (middle right), phospho-CREB (Ser133, right). (B) Protein expression was measured within the dentate gyrus of dorsal and ventral hippocampi of treated male mice. (C) Representative images of CB1 receptor expression in the ventral hippocampus of mice treated with vehicle (left) versus THC (right). A one-way ANOVA with multiple comparisons and Tukey's post hoc test were used to determine differences, ****p<0.0001. Each graph represents two dentate gyrus/mouse, four mice total, n=8. Error bars represent 95% confidence interval. CB1, cannabinoid type 1 receptor; CREB, cAMP response element-binding protein.
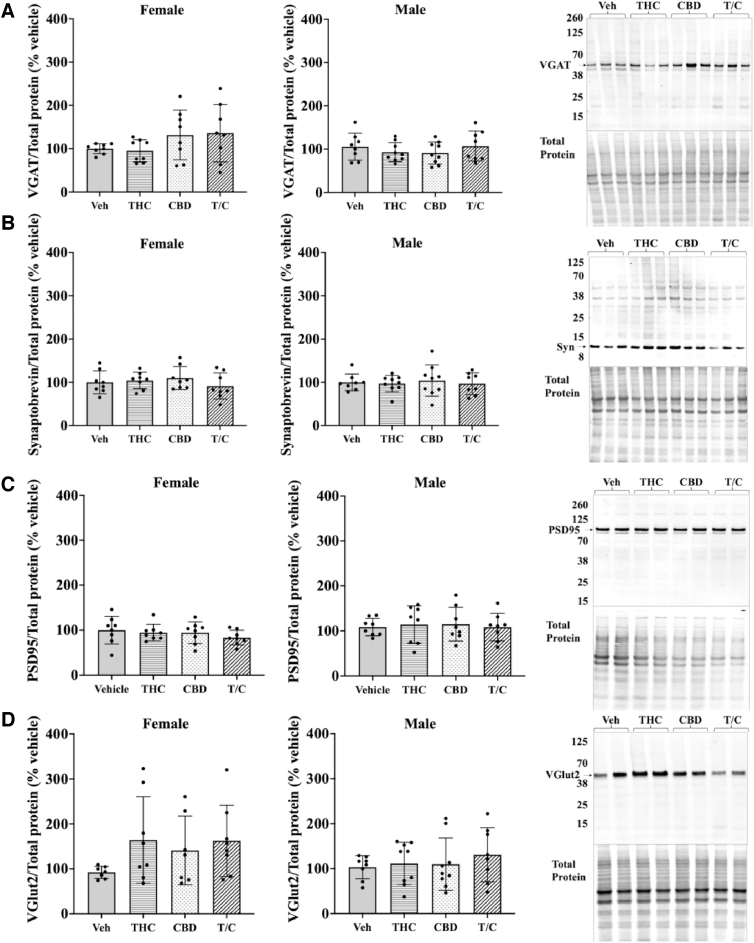
Phytocannabinoid treatment did not affect levels of several synaptic markers in the dorsal hippocampus of adolescent mice
To grossly determine the effects of adolescent phytocannabinoid treatment on synapses, we used western blotting of synaptic markers (n=7–9; Fig. 5). Markers examined included synaptobrevin as a measure of all synaptic structures, vesicular GABA transporter (VGAT) (vesicular inhibitory amino acid transporter or Slc32a1) for inhibitory synapses, postsynaptic density protein 95 for all excitatory synapses, and vesicular glutamate transporter 2 (VGlut2 or Slc17a6) to identify subcortical glutamatergic synapses. As shown in Figure 5A–D, hippocampal expression of these markers at the protein level was unchanged following the various phytocannabinoid treatments.
FIG. 5.
Measurement of changes in synaptic markers with phytocannabinoid treatments. (A–D) Expression levels in hippocampi of treated females (left) and males (middle), with representative western blots on the right. (A) Comparison of VGAT expression across treatment groups. (B) Expression levels for Syn, a calcium-sensing protein in the presynapse. (C) PSD95 expression across treatment groups. (D) Comparison of VGlut2 across treatment groups. A one-way ANOVA with multiple comparisons and Tukey's post hoc test was used in analyses; n=7–9. Error bars represent 95% confidence interval. VGAT, vesicular GABA transporter; PSD95, postsynaptic density protein 95; Syn, synaptobrevin; VGlut2, vesicular glutamate transporter 2.
Discussion
The consumption of cannabis has increased in recent years following its legalization in many U.S. states.2,3 As a result, it has become increasingly important to understand molecular changes that may occur when individuals are exposed to cannabis during important developmental periods. In this work, we have focused on changes in hippocampal expression of a critical neurotrophin, BDNF and its receptor, TrkB, following phytocannabinoid exposure. Activation of TrkB by BDNF is associated with neuronal proliferation, differentiation, and survival. Cannabis-induced changes in BDNF/TrkB expression, especially during developmental periods, may affect neural circuitry and brain function throughout life.
In the present study, mice were exposed to 3 mg/kg THC, CBD, or THC+CBD (1:1). This dose of THC has been shown to induce working memory deficits in rodents, which have long been associated with reduced BDNF levels.46,47 This dosing also results in blood plasma levels similar to human chronic users,48 although it should be noted that THC affinity for mouse CB1 is lower than that for the human receptor. Mice were dosed from P28 to P49 to capture early and mid-adolescence in mice before their transition to adulthood (P49–60).6 This age correlates with the time point in humans that many adolescents first begin using cannabis.49,50
The present work suggests that BDNF mRNA expression in the mouse hippocampus changes following exposure to phytocannabinoids during the adolescent period. These changes are subtle and appear more robustly in adolescent males. Specifically, transcript expression of BDNF I, II, IV, VI, and IX in the dorsal hippocampus of male adolescent mice exposed to THC is reduced (Fig. 2A, B, right). This effect is prevented when CBD is co-administered. This profile is also seen for BDNF I in females (Fig. 2A, left). Surprisingly, CBD by itself increased BDNF II VI, and IX in females, and BDNF VI in males (Fig. 2A, B).
The expression levels of specific transcript variants may indicate which cells or neural circuits are affected by drug treatment. For instance, while BDNF IV and VI are constitutively expressed in rat hippocampus principal neurons, they can be localized to different dendritic compartments depending on neural activity.41,51 In a study by Chiaruttini et al., using in situ hybridization, BDNF IV was restricted to the cell soma and proximal dendrites, appearing only in the stratum pyramidalis in CA1 and CA3 and in the stratum granularis in the dentate gyrus. BDNF VI was expressed throughout the hippocampus and was localized primarily in the soma. This changed following kainite- or pilocarpine-induced epileptic seizures where the BDNF VI signal extended to distal dendrites,41 suggesting that this variant can be upregulated in distal processes during intense synaptic activity. Other work has shown that silencing BDNF IV in cultured hippocampal neurons results in a reduction of proximal dendrites, whereas silencing BDNF VI alters distal dendrite morphology.51 From this, it can be hypothesized that the changes in BDNF transcript expression with THC administration may reflect changes in both proximal dendrite number, as well as distal dendrite morphology in the hippocampus. However, this could not be confirmed with the techniques used in this study.
The changes in BDNF expression with CBD alone and combined with THC provide insight into the potential neurobiological effects of CBD on the dorsal hippocampus. As CBD is a negative allosteric modulator at CB1,26,27 it is possible that CBD reduces CB1 signaling in glutamatergic neurons within the hippocampus, leading to heightened glutamate release. The increase in BDNF could be caused by a CBD-induced increase in synaptic activity. We observed no changes in protein with moderate phytocannabinoid treatment; however, acute treatment of 10 mg/kg CBD has been shown to increase BDNF protein in the hippocampus of adult rats under stress.43 This CBD effect may originate from any combination of the 11 BDNF transcripts found in mice. While BDNF IV and VI are highly expressed in the adolescent mouse hippocampus, all other transcripts tested are present.52 It is also possible that the expression of variants not examined here are significantly altered with CBD exposure during the adolescent period.
CB1 and BDNF/TrkB have important roles in neurodevelopment. CB1 is one of the most abundant GPCRs in the brain, is highly expressed in growth cones, and plays a key role in neurite growth, pathfinding, and retraction.53 Alteration of CB1 signaling during important developmental periods may therefore lead to profound downstream changes. Other groups have reported changes in BDNF mRNA levels with acute54 and chronic55,56 THC exposure. However, these studies involved older male animals. A study with a treatment protocol similar to this work injected mice starting at P56 with 3 mg/kg THC and detected no significant change in BDNF using RT-qPCR.57 They observed a THC-induced increase in BDNF promoter activity using the same protocol in 1-year-old mice, supporting the notion that the molecular response to phytocannabinoids changes as the brain matures. While BDNF/TrkB are important molecules needed for the production and maintenance of synapses, no changes to synaptic markers such as VGAT and VGlut2 were observed (Fig. 5).
To further assess the neurological alterations following adolescent endocannabinoid exposure, we analyzed dorsal and ventral regions of the dentate gyrus for protein expression (Fig. 4). BDNF and phosphorylated TrkB showed no differences in intensity between vehicle and THC-treated mice (Fig. 4B). No changes were observed in phosphorylated CREB. Interestingly, we found a statistically significant decrease in CB1 receptor density in the ventral dentate gyrus with THC treatment (Fig. 4B); however, this decrease was not paralleled in the dorsal region. The decrease in CB1 receptor density following THC treatment could be attributed to hyperactivity and receptor internalization.
There are likely different neurological changes between the sexes when it comes to cannabis use. We detected differences in the expression of BDNF II, IV, VI, and IX in females versus males treated with THC, CBD, or THC/CBD (Fig. 2, left vs. right). Sex differences with exogenous cannabinoid exposure have been demonstrated in several behavioral studies looking at locomotion and object recognition.57,58 In this work,57,58 female mice show increased locomotion and impaired object recognition, indicating that females may be more responsive to the effects of THC within these paradigms. These findings highlight the importance of investigating the neurological mechanisms underlying sex differences with exogenous cannabinoid exposure.
The BDNF/TrkB pathway is important for the growth and maintenance of neurons. This study has found statistically significant changes in BDNF mRNA expression in the hippocampus of adolescent mice exposed to THC and CBD with some sex differences also noted. The direct links between the cannabinoid and neurotrophin pathways are unclear. Higher resolution studies are needed to identify changes to BDNF and TrkB expression within specific brain regions as well as alterations in neural connectivity.
Abbreviations Used
- ANOVA
analysis of variance
- BDNF
brain-derived neurotrophic factor
- CB1
cannabinoid type 1 receptor
- CB2
cannabinoid type 2 receptor
- CBD
cannabidiol
- CNS
central nervous system
- CREB
cAMP response element-binding protein
- ECS
endocannabinoid system
- GPCR
G protein-coupled receptor
- ND
not detected
- ns
not significant
- NT
nontreated
- PFA
paraformaldehyde
- PSD95
postsynaptic density protein 95
- RTqPCR
reverse transcription quantitative polymerase chain reaction
- Syn
synaptobrevin
- THC
tetrahydrocannabinol
- TrkB
tropomyosin receptor kinase B
- VGAT
vesicular GABA transporter
- VGlut2
vesicular glutamate transporter 2
Author Disclosure Statement
No competing financial interests exist.
Funding Information
This work was supported by the National Institutes of Health, DA043982 and DA046196.
Cite this article as: Winstone J, Shafique H, Clemmer ME, Mackie K, Wager-Miller J (2023) Effects of tetrahydrocannabinol and cannabidiol on brain-derived neurotrophic factor and tropomyosin receptor kinase B expression in the adolescent hippocampus, Cannabis and Cannabinoid Research 8:4, 612–622, DOI: 10.1089/can.2021.0025.
References
- 1. ElSohly MA, Mehmedic Z, Foster S, et al. Changes in cannabis potency over the last 2 decades (1995–2014): analysis of current data in the United States. Biol Psychiatry. 2016;79:613–619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Miech R, Johnston L, O'Malley PM. Prevalence and attitudes regarding marijuana use among adolescents over the past decade. Pediatrics. 2017;140:e20170982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Chadwick B, Miller M, Hurd Y. Cannabis use during adolescent development: susceptibility to psychiatric illness. Front Psychiatry. 2013;4:129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Moore EM, Linsenbardt DN, Melón LC, et al. Ontogenetic differences in adolescent and adult C57BL/6J and DBA/2J mice: anxiety-like, locomotor, and consummatory behaviors. Dev Psychobiol. 2011;53:141–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Laviola G, Macrì S, Morley-Fletcher S, et al. Risk-taking behavior in adolescent mice: psychobiological determinants and early epigenetic influence. Neurosci Biobehav Rev. 2003;27:19–31. [DOI] [PubMed] [Google Scholar]
- 6. Brust V, Schindler PM, Lewejohann L. Lifetime development of behavioural phenotype in the house mouse (Mus musculus). Front Zool. 2015;12:S17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Sowell ER, Thompson PM, Tessner KD, et al. Mapping continued brain growth and gray matter density reduction in dorsal frontal cortex: inverse relationships during postadolescent brain maturation. J Neurosci. 2001;21:8819–8829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Jacobsen LK, Mencl WE, Westerveld M, et al. Impact of cannabis use on brain function in adolescents. Ann N Y Acad Sci. 2004;1021:384–390. [DOI] [PubMed] [Google Scholar]
- 9. Solowij N, Jones KA, Rozman ME, et al. Verbal learning and memory in adolescent cannabis users, alcohol users and non-users. Psychopharmacology. 2011;216:131–144. [DOI] [PubMed] [Google Scholar]
- 10. Jacobsen LK, Pugh KR, Constable RT, et al. Functional correlates of verbal memory deficits emerging during nicotine withdrawal in abstinent adolescent cannabis users. Biol Psychiatry. 2007;61:31–40. [DOI] [PubMed] [Google Scholar]
- 11. Chen H-T, Mackie K. Adolescent Δ9-tetrahydrocannabinol exposure selectively impairs working memory but not several other mPFC-mediated behaviors. Front Psychiatry. 2020;11:576214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Moore NL, Greenleaf AL, Acheson SK, et al. Role of cannabinoid receptor type 1 desensitization in greater tetrahydrocannabinol impairment of memory in adolescent rats. J Pharmacol Exp Ther. 2010;335:294–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Moore THM, Zammit S, Lingford-Hughes A, et al. Cannabis use and risk of psychotic or affective mental health outcomes: a systematic review. Lancet. 2007;370:319–328. [DOI] [PubMed] [Google Scholar]
- 14. Rubino TaP D. Long lasting consequences of cannabis exposure in adolescence. Mol Cell Endocrinol. 2008;286:S108-13. [DOI] [PubMed] [Google Scholar]
- 15. McPartland JM, Glass M, Pertwee RG. Meta-analysis of cannabinoid ligand binding affinity and receptor distribution: interspecies differences. Br J Pharmacol. 2007;152:583–593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Patzke C, Dai J, Brockmann MM, et al. Cannabinoid receptor activation acutely increases synaptic vesicle numbers by activating synapsins in human synapses. Mol Psychiatry. 2021;26:6253–6268. [DOI] [PubMed] [Google Scholar]
- 17. Di Marzo V, Bifurcó M, and De Petrocellis L. The endocannabinoid system and its therapeutic exploitation. Nat Rev Drug Discov. 2004;3:771–784. [DOI] [PubMed] [Google Scholar]
- 18. Fride E. Multiple roles for the endocannabinoid system during the earliest stages of life: pre- and postnatal development. J Neuroendocrinol. 2008:75–81. [DOI] [PubMed] [Google Scholar]
- 19. Ferreira FF, Ribeiro FF, Rodrigues RS, et al. Brain-derived neurotrophic factor (BDNF) role in cannabinoid-mediated neurogenesis. Front Cell Neurosci. 2018;12:441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Visvanathar R, Papanikolaou M, Nôga DA, et al. Hippocampal Cb2 receptors: an untold story. Rev Neurosci. 2021;33(4):413–426; DOI: 10.1515/revneuro-2021-0109. [DOI] [PubMed] [Google Scholar]
- 21. Jordan CJ, Xi Z-X. Progress in brain cannabinoid CB2 receptor research: from genes to behavior. Neurosci Biobehav Rev. 2019;98:208–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Berghuis P, Rajnicek AM, Morozov YM, et al. Hardwiring the brain: endocannabinoids shape neuronal connectivity. Science. 2007;316:1212–1216. [DOI] [PubMed] [Google Scholar]
- 23. Bouaboula M, Poinot-Chazel C, Bourrié B, et al. Activation of mitogen-activated protein kinases by stimulation of the central cannabinoid receptor CB1. Biochem J. 1995;312 (Pt 2):637–641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Dalton GD, Howlett AC. Cannabinoid CB1 receptors transactivate multiple receptor tyrosine kinases and regulate serine/threonine kinases to activate ERK in neuronal cells. Br J Pharmacol. 2012;165:2497–2511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Berghuis P, Dobszay MB, Wang X, et al. Endocannabinoids regulate interneuron migration and morphogenesis by transactivating the TrkB receptor. Proc Natl Acad Sci U S A. 2005;102:19115–19120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Laprairie RB, Bagher AM, Kelly MEM, et al. Cannabidiol is a negative allosteric modulator of the cannabinoid CB1 receptor. Br J Pharmacol. 2015;172:4790–4805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Straiker A, Dvorakova M, Zimmowitch A, et al. Cannabidiol inhibits endocannabinoid signaling in autaptic hippocampal neurons. Mol Pharmacol. 2018;94:743–748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Pertwee RG. The diverse CB1 and CB2 receptor pharmacology of three plant cannabinoids: delta9-tetrahydrocannabinol, cannabidiol and delta9-tetrahydrocannabivarin. Br J Pharmacol. 2008;153:199–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Schoenbaum G, Stalnaker TA, Shaham Y. A role for BDNF in cocaine reward and relapse. Nat Neurosci. 2007;10:935–936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Morales P, Hurst DP, Reggio PH. Molecular targets of the phytocannabinoids: a complex picture. In: Kinghorn AD, Falk H, Gibbons S, Kobayashi J, eds. Phytocannabinoids: Unraveling the Complex Chemistry and Pharmacology of Cannabis sativa. Springer International Publishing: Cham, 2017:pp. 103–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Linge R, Jiménez-Sánchez L, Campa L, et al. Cannabidiol induces rapid-acting antidepressant-like effects and enhances cortical 5-HT/glutamate neurotransmission: role of 5-HT1A receptors. Neuropharmacology. 2016;103:16–26. [DOI] [PubMed] [Google Scholar]
- 32. Campos AC, Fogaça MV, Sonego AB, et al. Cannabidiol, neuroprotection and neuropsychiatric disorders. Pharmacol Res. 2016;112:119–127. [DOI] [PubMed] [Google Scholar]
- 33. Lo DC. Neurotrophic factors and synaptic plasticity. Neuron. 1995;15:979–981. [DOI] [PubMed] [Google Scholar]
- 34. Davies AM. Role of neurotrophic factors in development. Trends Genetics. 1988;4:139–143. [DOI] [PubMed] [Google Scholar]
- 35. Li X, Wolf ME. Multiple faces of BDNF in cocaine addiction. Behav Brain Res. 2015;279:240–254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Bolaños CA, Nestler EJ. Neurotrophic mechanisms in drug addiction. Neuromolecular Med. 2004;5:69–83. [DOI] [PubMed] [Google Scholar]
- 37. Cowley S, Paterson H, Kemp P, et al. Activation of MAP kinase kinase is necessary and sufficient for PC12 differentiation and for transformation of NIH 3T3 cells. Cell. 1994;77:841–852. [DOI] [PubMed] [Google Scholar]
- 38. Meakin SOM, JI, Gryz EA, et al. The signaling adapter FRS-2 competes with Shc for binding to the nerve growth factor receptor TrkA. A model for discriminating proliferation and differentiation. J Biol Chem. 1999;274:9861–9870. [DOI] [PubMed] [Google Scholar]
- 39. Huang EJ, Reichardt LF. Trk receptors: roles in neuronal signal transduction. Annu Rev Biochem. 2003;72:609–642. [DOI] [PubMed] [Google Scholar]
- 40. Liu Y, Encinas M, Comella JX, et al. Basic helix-loop-helix proteins bind to TrkB and p21Cip1 promoters linking differentiation and cell cycle arrest in neuroblastoma cells. Mol Cell Biol. 2004;24:2662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Chiaruttini C, Sonego M, Baj G, et al. BDNF mRNA splice variants display activity-dependent targeting to distinct hippocampal laminae. Mol Cell Neurosci. 2008;37:11–19. [DOI] [PubMed] [Google Scholar]
- 42. Conner JM, Lauterborn JC, Yan Q, et al. Distribution of brain-derived neurotrophic factor (BDNF) protein and mRNA in the normal adult rat CNS: evidence for anterograde axonal transport. J Neurosci. 1997;17:2295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Sales AJ, Fogaca MV, Sartim AG, et al. Cannabidiol induces rapid and sustained antidepressant-like effects through increased BDNF signaling and synaptogenesis in the prefrontal cortex. Mol Neurobiol. 2019;56:1070–1081. [DOI] [PubMed] [Google Scholar]
- 44. Wager-Miller J, Murphy Green M, Shafique H, et al. Collection of frozen rodent brain regions for downstream analyses. JoVE. 2020:e60474. [DOI] [PubMed] [Google Scholar]
- 45. Fukuchi M, Izumi H, Mori H, et al. Visualizing changes in brain-derived neurotrophic factor (BDNF) expression using bioluminescence imaging in living mice. Sci Rep. 2017;7:4949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Panlilio LV, Ferré S, Yasar S, et al. Combined effects of THC and caffeine on working memory in rats. Br J Pharmacol. 2012;165:2529–2538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Pan S, Feng W, Li Y, et al. The microRNA-195—BDNF pathway and cognitive deficits in schizophrenia patients with minimal antipsychotic medication exposure. Transl Psychiatry. 2021;11:117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Murphy M, Mills S, Winstone J, et al. Chronic adolescent δ9-tetrahydrocannabinol treatment of male mice leads to long-term cognitive and behavioral dysfunction, which are prevented by concurrent cannabidiol treatment. Cannabis Cannabinoid Res. 2017;2:235–246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Addiction NIoD. Monitoring the Future Survey: High School and Youth Trends. 2020. Available at https://www.drugabuse.gov/drug-topics/related-topics/trends-statistics/infographics/monitoring-future-2020-survey-results (last accessed May 12, 2022).
- 50. Bogin B. Chapter 20—human growth and development. In: Muehlenbein MP, ed. Basics in Human Evolution. Academic Press: Boston, 2015, pp. 285–293. [Google Scholar]
- 51. Chiaruttini C, Vicario A, Li Z, et al. Dendritic trafficking of BDNF mRNA is mediated by translin and blocked by the G196A (Val66Met) mutation. Proc Natl Acad Sci U S A. 2009;106:16481–16486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Aid T, Kazantseva A, Piirsoo M, et al. Mouse and rat BDNF gene structure and expression revisited. J Neurosci Res. 2007;85:525–535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Roland AB, Ricobaraza A, Carrel D, et al. Cannabinoid-induced actomyosin contractility shapes neuronal morphology and growth. Elife. 2014;3:e03159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Derkinderen P, Valjent E, Toutant M, et al. Regulation of extracellular signal-regulated kinase by cannabinoids in hippocampus. J Neurosci. 2003;23:2371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Bilkei-Gorzo A, Albayram O, Draffehn A, et al. A chronic low dose of Δ9-tetrahydrocannabinol (THC) restores cognitive function in old mice. Nat Med. 2017;23:782. [DOI] [PubMed] [Google Scholar]
- 56. Butovsky E, Juknat A, Goncharov I, et al. In vivo up-regulation of brain-derived neurotrophic factor in specific brain areas by chronic exposure to Δ9-tetrahydrocannabinol. J Neurochem. 2005;93:802–811. [DOI] [PubMed] [Google Scholar]
- 57. Wiley JL. Sex-dependent effects of Δ9-tetrahydrocannabinol on locomotor activity in mice. Neurosci Lett. 2003;352:77–80. [DOI] [PubMed] [Google Scholar]
- 58. Kasten CR, Zhang Y, Boehm SL, 2nd. Acute cannabinoids produce robust anxiety-like and locomotor effects in mice, but long-term consequences are age- and sex-dependent. Front Behav Neurosci. 2019;13:32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Bodor AL, Katona I, Nyiri G, et al. Endocannabinoid signaling in rat somatosensory cortex: laminar differences and involvement of specific interneuron types. J Neurosci. 2005;25:6845–6856. [DOI] [PMC free article] [PubMed] [Google Scholar]