Abstract
Tendon injuries disrupt the transmission of forces from muscle to bone, leading to chronic pain, disability, and a large socioeconomic burden. Tendon injuries are prevalent; there are over 300,000 tendon repair procedures a year in the United States to address acute trauma or chronic tendinopathy. Successful restoration of function after tendon injury remains challenging clinically. Despite improvements in surgical and physical therapy techniques, the high complication rate of tendon repair procedures motivates the use of therapeutic interventions to augment healing. While many biological and tissue engineering approaches have attempted to promote scarless tendon healing, there is currently no standard clinical treatment to improve tendon healing. Moreover, the limited efficacy of systemic delivery of several promising therapeutic candidates highlights the need for tendon-specific drug delivery approaches to facilitate translation. This review article will synthesize the current state-of-the-art methods that have been used for tendon-targeted delivery through both systemic and local treatments, highlight emerging technologies used for tissue-specific drug delivery in other tissue systems, and outline future challenges and opportunities to enhance tendon healing through targeted drug delivery.
Impact statement
Tendon injuries heal in a scar-mediated manner, which lead to numerous complications. However, there is currently no pharmacological approach to augment the tendon healing process. This can be attributed to the lack of targeting modalities within the tendon to which drug carriers can be directed, as well as the lack of effective therapeutic molecules. In this review, we identify promising approaches that are currently used in other tissues, which can be leveraged to promote effective, tendon-targeted drug delivery and provide our perspective on the key considerations for tendon-targeted drug delivery, and the challenges and opportunities that exist in this space.
Keywords: drug delivery, biomaterials, nanoparticles, tendon injury
Introduction
Tendons are dense, fibrous connective tissues primarily composed of Type I collagen that transmit mechanical forces from muscles to the bone.1 While there is diversity in tendons, such as size, location, and structure, there are several conserved means through which tendon homeostasis is disrupted. More specifically, tendons are susceptible to tendonitis, tendinopathy, age-related degeneration, sprains, and acute rupture or traumatic injury.2–6 Nearly half of all musculoskeletal injuries reported in the United States involve tendons and ligaments, and these disorders lead to many complications and challenges.3 The most common complications that occur with tendon injuries include re-rupture due to an insufficient initial healing response, excess postoperative scarring, joint contracture, and infections.1,2,4,7–12
Collectively, these complications result in a lengthy rehabilitation process and unsatisfactory healing outcomes that substantially impair the restoration of quality of life and impact patients' ability to meet their recreational, occupational, and physical function goals.11 Moreover, as the human population ages and life expectancy increases, it is estimated that 25% of all adults will suffer a tendon-related injury due to a combination of disrupted tendon homeostasis and an impaired healing response after injury, which will further burden the health care system financially, and thus increase the need for therapeutic interventions.5,7,10,11,13
Current approaches to treat tendon disorders and their associated complications can be broadly classified as either operative or nonoperative (conservative) treatments. Operative treatments include surgery with or without a tendon transplant, usually followed by lengthy periods of rehabilitation and nonsteroidal anti-inflammatory drugs (NSAIDs) to manage pain.14 Alternatively, conservative treatment methods such as ultrasound, laser, and shock wave therapy are also effective in managing pain. However, growing evidence from randomized controlled trials and observational studies suggests that these conservative treatments are only beneficial when used in combination with other interventions like exercise, which increases loading and helps to stimulate healing.15,16
In addition, surgical and conservative treatment approaches have also incorporated adjuvant approaches, including biomaterials such as chitosan, poly(lactic acid) (PLA), and hyaluronic acid as mechanisms to deliver therapeutics to resolve inflammation, promote matrix remodeling, and increase mechanical integrity. However, to date, there is no consensus on standard biomaterial treatment that restores preinjury tendon structure and function. In particular, the lack of effective therapeutic strategies to improve tendon healing can be attributed to the paucity of fundamental cellular and molecular understanding of tendon cell biology and the mechanisms underlying tendon pathophysiology.
This gap in knowledge limits the identification of possible targets that can be leveraged for improved tendon healing and function. Collectively, the successful therapeutic approach(es) to facilitate tendon healing requires a complete understanding of the anatomy, and molecular basis of healing of tendons in different anatomical locations and their potential complications and outcomes,5 to facilitate the successful restoration of function, minimize disability, and reduce the risk of complications.
In this review, we discuss tendon-targeted and systemic approaches to deliver drugs to the tendon to promote healing and the advantages and disadvantages associated with each method. In addition, we provide our perspective on targeting approaches that can be leveraged in the tendon to control drug delivery. Since most studies employing nanotechnology in the tendon use mid-substance injuries as experimental models compared to other injury models like tendon-bone injuries, we have chosen to primarily focus this review on acute mid-substance injuries. While we do not explicitly discuss tendon-bone injuries due to the unique challenges and opportunities associated with this tissue interface, we provide a few examples of how nanotechnology can be used in this setting in our comprehensive tables. We also briefly discuss other tendon injuries, including tendonitis and degeneration, within our comprehensive tables and future perspectives section.
Systemic Versus Local Drug Delivery
Drugs can be delivered to the tendon by two major approaches: systemically or locally. Local delivery refers to using biomaterials, synthetic or natural, to deliver a minimal amount of drug locally to the affected tissue. Local delivery can be classified into noninjectable/preformed solid implants (preformed hydrogels, films, sponges, stents, and fibers) and injectable implants (in situ cross-linkable hydrogels and nanoparticles/microparticles).17 Alternatively, systemic delivery refers to a route of delivery directly into the bloodstream to reach all areas of the body. It can be achieved through intravenous injection, intramuscular injection, subcutaneous injection, and oral delivery.18
Both systemic and local delivery approaches have unique context-dependent strengths and weaknesses that must be considered in designing an efficient drug delivery system. For example, the properties of the drug (e.g., size, surface modality, etc.), the desired therapeutic window, and the location of the injury all need to be considered. To leverage therapeutic efficacy, while reducing side effects such as cytotoxicity, drugs can be administered directly to the diseased site (i.e., local delivery) or delivered systemically based on the considerations. In the tendon, localized drug delivery mechanisms have dominated the field due to the ease of administration and the lack of tendon-targeted drug delivery methods for systemic treatments.19,20
Local drug delivery modalities include natural and synthetic scaffolds, fibers, sponges, and stents.12 The primary advantage of local delivery consists of the immediate supply of a therapeutic to the injury site, while minimizing off-target tissue exposure. Furthermore, biomaterials used to deliver drugs locally can also be engineered to provide relevant cues to dictate specific aspects of cell function. However, these local delivery approaches have pitfalls. For example, it can be difficult to control the degradation rate of biomaterials at the injury site, which can cause an increased inflammatory response, impact the metabolic activity and extracellular matrix (ECM) deposition capacity of tenocytes, and cause cytotoxicity.12,21
Furthermore, drugs and growth factors delivered locally with biomaterials are typically released and cleared rapidly due to suboptimal loading capacity and release kinetics. In addition, inadequate binding affinity (e.g., how tightly a drug binds to the scaffold) is a crucial consideration as the binding affinity must be low enough to ensure the release of the drug, but high enough to prevent drug dumping and toxicity.22 Furthermore, using biomaterials to deliver drug depots to the tendon ensures site-specific drug delivery; however, this approach requires invasive procedures, which may disrupt the physiological healing response.12,20,22–26 Even with the intraoperative application, embedding foreign materials at the repair site may cause additional complications, such as sustained inflammation.26 In addition, local injection of drugs directly into the tendon may reactivate the inflammatory response and can result in drug dumping, leading to rapid local clearance and off-target effects.9,27,28
Systemic drug delivery approaches such as nanotechnology have been employed to combat the disadvantages of local drug delivery. Nanotechnology enables the controlled delivery of drug payloads, and this approach has primarily been used to target immune and reparative responses after injury.29 Due to their small size and surface properties, nanoparticles (NPs) can reach all tissues, which can be disadvantageous if drugs are distributed to unintended tissues.30 However, nanotechnology modalities are attractive because they can be designed to have high specificity, ensure the stability of the loaded drug, have high loading capacity, and, perhaps most importantly, be biocompatible.31,32
Biomaterials for Local Drug Delivery
Coated sutures
Acute tendon injuries are common and often require surgical intervention. However, postoperative healing is often associated with an increased risk of re-tear, especially during early postoperative mobilization protocols.33 High re-tear rates are attributed to the weakened tendon tissue around the suture, which allows the suture to cut through the tissue after exposure to tensile stresses. To overcome these complications, exogenous cross-linking agents, growth factors, and cells can be applied to the surgical site to augment healing and increase the mechanical integrity and prevent repair failure.33–41
While the primary purpose of sutures is to reduce gap formation and promote mechanical stability after tendon rupture, sutures can also be leveraged as drug carriers to reduce re-tear rates and/or minimize scar tissue formation. For example, Pasternak et al investigated the effects of doxycycline-coated sutures on the suture-holding capacity of repaired rat Achilles tendon (AT).33 This study used a 3–0 polybutylester monofilament suture treated with radiofrequency plasma and coated with doxycycline, a matrix metalloproteinase (MMP) inhibitor, to repair an AT transection. The doxycycline-coated sutures inhibited degradative processes associated with surgical re-tears, thereby improving the suture-holding capacity.33
Moreover, Chamberlain et al used BMP-12-releasing sutures to repair AT ruptures and demonstrated reduced adhesion formation relative to tendons that were fixed with untreated mineral sutures.34 Several other studies have reported the beneficial effects of sutures as drug carriers on tendon healing (Table 1). Coated sutures are advantageous as drug vehicles because they are close to the tendon and therefore do not require biomaterial drug vehicles. However, a significant disadvantage is that they can only be used to deliver extremely small amounts of the drug (nanograms—micrograms) because only a few centimeters of the suture is typically required for surgical repair. Drug-laden sutures also have no sustained release characteristics, which limits therapeutic efficacy.
Table 1.
Summary of Coated Sutures in Tendon Healing
| Drug or growth factors delivered | Suture material | Suture size | Tendon | Reference |
|---|---|---|---|---|
| bFGF VEGFA |
Ethilon/PDS II | 5–0 | FDL | 39 |
| Myostatin | Ethibond Excel braided polyester | 4–0 | AT | 37 |
| Doxycycline | Polybutylester monofilament | 3–0 | Digital flexor tendon | 31 |
| BMP-12 | Vicryl Polyglactin 910 | 6–0 | AT | 32 |
| rhPDGF-BB | Vicryl | 4–0 | AT | 33 |
| Butyric acid | Polypropylene | 0–0 | AT | 36 |
| bFGF | Monofilament nylon | 4–0 | Flexor digitorum fibularis tendon | 35 |
| EDC | Braided poly-bend suture | 4–0 | Flexor digitorum profundus | 38 |
AT, Achilles tendon; bFGF, basic fibroblast growth factor; EDC, 1-ethyle-3-(3-dimethylaminopropyl) carbodiimide; FDL, flexor digitorum longus; PDS, polydioxanone; rhPDGF-BB, recombinant human platelet-derived growth factor-BB; VEFGA, vascular endothelial growth factor A.
Furthermore, since the sutures used for tendon repair are usually only administered intraoperatively, the drugs loaded onto the sutures are only available during the early phases of tendon healing. They therefore have limited ability to modulate later stages of the healing process. Finally, sutures are suboptimal carriers for growth factors, given the rapid degradation of growth factors by proteases in the healing environment. Hence, more recent studies are focused on using nanocarriers/plasmid complex-coated sutures to facilitate sustained release.41
Polymeric scaffolds
To date, numerous biopolymers have been assessed for tendon repair, with variable degrees of efficacy.12,19,20,22–26,36,42–48 Advancements in polymer chemistry and biotechnology have increased the availability of technologies to fabricate hierarchical three-dimensional scaffolds. The scaffolds can be customized to closely imitate native tendon architectural features, while enabling localized and sustained delivery of therapeutics. These scaffolds come in various forms, including electrospun fibers, composite hydrogels, and cell sheets (Fig. 1).12,22,24,42 Although polymeric scaffolds can be designed and applied in various forms, material compositions can be broadly classified as either synthetic or natural polymers.1,20,49
FIG. 1.
Approaches to promote tendon healing. Current approaches to promote tendon healing include tissue engineering approaches mediated by use biomaterials such as scaffolds and NPs. Scaffolds, including (A) electrospun fibers, (B) coated sutures, (C) injectable hydrogels, and (D) regenerative implants, can be used to deliver drugs locally to injured sites, while NPs can be employed for systemic delivery approaches. NPs, nanoparticles. Color images are available online.
Synthetic polymers allow a high degree of customization, which facilitates broad biological application. For example, synthetic polymer scaffolds such as PLA, poly(glycolic acid) (PGA), poly-ɛ-caprolactone (PCL), and poly(lactic-co-glycolic acid) (PLGA) are commonly used in preclinical models of tendon injury as they can provide a high degree of mechanical stabilization.48,50,51 However, synthetic polymers have several disadvantages. Most synthetic polymers have very low biodegradability and must therefore be eliminated through renal excretion. As such, the molecular weight of polymers is a key consideration to ensure successful excretion.22,46,49,52,53 Because of this, polymer scaffolds are primarily used for the repair of minor injuries that require relatively small scaffolds. For example, a controlled-release scaffold for the delivery of Ibuprofen was developed in a previous study by Hu et al, which revealed that electrospun fibers loaded with Ibuprofen were effective in minimizing adhesion formation in a chicken flexor tendon model.
Specifically, poly(lactic acid) (PLA) fibers synthesized by a co-solvent method were loaded with mesoporous silica nanoparticles (MMS) containing Ibuprofen and electrospun to form composite fibrous membranes. Following transection and repair of the flexor digitorum profundus tendon, ibuprofen-loaded fibrous membranes were wrapped around the repair site. Histological analysis of harvested tendons 4 weeks postinjury revealed that the PLA-MMS-Ibuprofen fibrous membranes were effective in reducing tissue adhesion and inflammation. Although no significant improvement in mechanical properties was observed, the PLLA-MMS-Ibuprofen membrane demonstrated controlled release of the loaded drug over a long period of time (8 weeks).54
Natural polymers (e.g., chitosan, chondroitin sulfate, alginate, and hyaluronic acid) are broadly used to treat tendon injuries because they provide important biochemical cues and facilitate cell adhesion.55 Natural polymers also overcome many challenges of synthetic polymers due to uncontrolled cell-dictated degradation. However, natural polymers lack the mechanical stability of synthetic polymers, and often lack the desired topographical properties, matrix stiffness, alignment, and fiber pore size required to influence the proper function of immune cells and tendon cells, including enabling these cells to contract and migrate as needed to phagocytose debris and dead cells, as well as deposit and remodel the matrix.12,22
Furthermore, natural scaffolds depend on basic diffusion and desorption to release drug depots.56 As such, natural scaffolds result in an initial, uncontrolled burst release of the loaded drug, which may be followed by a first-order release depending on the material properties of the scaffold. This burst release exposes cells to high levels of drug soon after polymer delivery, which may be undesirable for proper healing, and can lead to high cytotoxicity.
Therefore, further studies are needed to identify the properties required for these engineered scaffolds to serve as optimal drug carriers, to promote tendon healing.57 To investigate the potential of a small molecule drug delivered in a controlled-release manner, a hydrogel-based scaffold comprising hyaluronic acid was applied in a chicken flexor tendon model to deliver rhynchophylline (Rhy). Rhy is a small molecule drug which can act by downregulating TGF-β1 to reverse tenocyte dysfunction, and has been proven effective in minimizing abdominal adhesions. Biomechanical analysis of harvested tendons 6 weeks postinjury revealed that Rhy-loaded hydrogels inhibited tendon adhesion formation and improved gliding compared to the control group.58 This study established the benefit of using PEG diacrylate as a cross-linker to enable sustained, controlled release of small molecule drugs from hydrogels.
In summary, polymeric scaffolds have several benefits including extended bioavailability to increase the duration of drug action, minimizing the frequency of drug administration, and subsequently reducing possible drug side effects. In addition, polymeric scaffolds improve drug utilization by minimizing premature drug degradation and loss and require lower drug doses due to the benefits of local administration12,20,22,23,26 (Table 2). However, a major pitfall of polymeric scaffolds for localized drug delivery is the necessary surgical intervention to either administer or remove the scaffold when needed, which may lead to further complications such as excessive scarring, and can lengthen the recovery and rehabilitation process.12 In addition, maximizing drug utilization is a key consideration for polymeric scaffold design.
Table 2.
Summary of Drug-Loaded Polymeric Scaffolds in Tendon Healing
| Drug or growth factors delivered | Mode of administration | Tendon | Reference |
|---|---|---|---|
| GDF-7 | Quadrol-hexamethylene diisocyanate-methacrylic anhydride polyurethane polymer | Rotator cuff | 66 |
| Ibuprofen | PLGA microspheres within sintered poly (ɛ-caprolactone) electrospun scaffold | Rotator cuff | 47 |
| Ibuprofen | PLGA | Rotator cuff | 67 |
| PDGF-BB | DegraPol® polyester urethane | AT | 68 |
| PDGF-BB | Fibrin/HBDS | Flexor digitorum profundus | 69 |
| PDGF-BB | Fibrin/ HBDS PLGA nanofiber | Flexor digitorum profundus | 44 |
| bFGF | Fibrin/HBDS | Flexor digitorum profundus | 70 |
| rhPDGF-BB | Collagen 1 carrier (BioBlanket) | Rotator cuff | 71 |
| PDGF | Microneedle patch | AT | 72 |
| PDGF-BB | DegraPol tube | AT | 73 |
| bFGF | Dextran glassy nanoparticles electrospun PPLA copolymer fiber | AT | 43 |
| bFGF | PLGA fibrous membranes | Rotator cuff | 74 |
| TGF-β1 | Absorbable alginate scaffold | Rotator cuff | 75 |
| Parathyroid hormone | Alginate scaffold | Rotator cuff | 76 |
| Sprifermin | Sodium alginate hydrogel | Rotator cuff | 77 |
| TGF-β3 or GDF-7 | Cross-linking gelatin (Col-Tgel) | Rotator cuff | 78 |
| Mitomycin-C | Drug-loaded hyaluronan hydrosols encapsulated in PLLA fibrous membranes | AT and FDL | 79 |
GDF-7, growth and differentiation factor 7; HBDS, heparin-based delivery system; PDGF-BB, platelet-derived growth factor-BB; PLGA, poly(lactic-co-glycolic) acid; PPLA, poly-l-lactic acid; TGF-β1, transforming growth factor β1.
More specifically, polymeric scaffolds must allow uniform drug distribution throughout the entire scaffold, permit drug release at a pre-established rate, possess sufficiently low drug binding affinity to ensure drug release at physiological conditions (i.e., pH, temperature), and must possess stable chemical structure, biological activity, and physical dimension over an extended duration. However, the design and manufacturing of polymeric scaffolds that meet these criteria represent a key technical hurdle.22,59 Some of these hurdles include reproducibility (scale-up), sterilizability, and physicochemical stability of drug during scaffold manufacture and processing.59 Studies in other tissues such as the bone and heart have addressed some of these challenges. In bone tissue engineering, approaches to sustain drug release has been an area of intense interest. These include incorporating mechanisms to decouple the drug release mechanism from the intrinsic degradation kinetics of the scaffold to promote longer drug retention.60
Furthermore, mechanisms to modify the degradability or electrochemistry of polymers by incorporating counter-ion charged agents and alternating the rows of charged polymers to extend the span of drug release have been investigated.60,61 As an approach to ensure drug stability during manufacturing, as well as promote sustained drug release, other studies have encapsulated drugs in nanospheres/microspheres embedded in hydrogels. This mechanism is attractive because the encapsulation system can be made from a different material than the scaffold, thus allowing several possible combinations of polymers to promote effective scaffold synthesis.62–64 Furthermore, encapsulating the drug in a nanocarrier/microcarrier establishes the presence of a second physical hindrance to minimize premature drug loss.65
Tissue-Targeted Systemic Treatment: Current Approaches and Opportunities
Systemic delivery refers to direct drug delivery to the bloodstream, resulting in broad release to most areas of the body. These routes include intravenous injection, intramuscular injection, subcutaneous injection, and oral delivery.80 Despite the relative success of systemic delivery, it is a challenge to avoid burst release at the time of administration followed by subsequent drops to subtherapeutic drug levels, and difficulty controlling drug levels over extended periods of time.81 Recently, new strategies have been developed that enable precise control over multiple properties to enhance treatment performance, including improved tissue-specific delivery.62,82,83 Therefore, the advantages of current systemic drug delivery approaches over local delivery include the ability to deliver a drug more accurately with less frequent dosing, the ability to engineer carriers that enable reduced variability in systemic drug concentration, and reduced toxicity.49
NPs for systemic drug delivery
NPs have been employed in systemic drug delivery approaches because of their high tunability. NPs can be engineered with specific properties that are required for effective drug delivery,29,31,32,84,85 which lower possible side effects of the drug payload in nontarget organs and tissues. NPs can also be designed to control drug release mechanisms and kinetics once at the target site through physiological or chemical triggers.53 Hence, NP-based drug delivery technology can overcome many limitations of traditional delivery, including issues related to biodistribution and intracellular trafficking, can effectively prolong circulation time of drug payloads to increase efficacy, enhance the solubility and stability of drugs, and promote transport across membranes.29,31,32,86
Although the field of targeted drug delivery systems has seen significant growth within the last decade, only a few studies have attempted to use these approaches to enhance tendon healing (Table 3). For example, a collagen hybrid peptide (CHP) was used to modify the surface of PLGA NPs, which were used to deliver rapamycin to injured tendon sites by subcutaneous injection.86 The targeting of denatured collagen chains facilitated accurate positioning and integration of the PLGA into the injured tendon rather than adjacent healthy tendon and resulted in sustained release of rapamycin and inhibition of endochondral ossification.86 The PLGA NPs were synthesized using oil/water emulsion-solvent evaporation technique and were subsequently conjugated to the CHP by covalent bonding with glutaraldehyde as a cross-linker.86
Table 3.
Summary of Drug-Loaded Nanotechnology in Tendon Healing
| Drug or growth factors delivered | Material | Mode of administration | Tendon | Reference |
|---|---|---|---|---|
| COX-1 and COX-2 siRNA | Nanoparticle gel | Local administration during repair surgery | Flexor digitorum profundus | 89 |
| Celecoxib | PPZ nanoparticle hydrogel system | Local injection one week after Achilles tendinitis defect | AT | |
| TGF-β3 | PLGA-b-PEG NPs loaded in chitosan films | Local administration of chitosan film during repair surgery | AT | 90 |
| Lactoferrin | LF/Hep-PLGA NPs | Local injection one week after Achilles tendinitis defect | AT | 91 |
| Rapamycin | PLGA NPs functionalized with a CHP | Subcutaneous injection one week after Achilles tendinitis defect | AT | 86 |
| COX-1 and COX-2 engineered miRNA | PLGA NPs embedded in thiol-modified hyaluronic acid cross-linked with PEG-diacrylate hydrogel | Local administration during repair surgery | Flexor digitorum profundus | 92 |
| Curcumin | Gold nanorods loaded into polymeric nanomicelles composed of PLGA-b-PEG copolymer | Local injection into proximal and distal tendons during repair surgery | AT | 93 |
| PDGF | Mesoporous silica nanoparticles | Local injection into tendon during repair model | AT | 94 |
| microRNA29a | Lipid nanoparticles loaded into PLA-PEG copolymer (PELA) core-shell nanofibrous membranes | Local administration during repair model | AT | 95 |
| Smad3-siRNA | GelMA microspheres encapsulated with NPs entrapped within hyaluronic acid hydrogel | Local administration during repair model | AT | 96 |
| TGF-β1 miRNA | Polydopamine nanoparticles combined with gelatin, hyaluronic acid, and sodium alginate and simultaneously bioprinted with PCL | Scaffolds locally sutured during repair model | Flexor digitorum profundus | 97 |
| Serpine1 siRNA | DMAEMA NPs | Subcutaneous injection after repair model | Digital flexor tendon | 88 |
| IGF1 mimetic protein | Clay microparticles encapsulated in alginate hydrogel matrix | Local administration during repair model | AT | 98 |
| bFGF and VEGFA gene | PLGA NPs | Local injection during repair model | Flexor digitorum profundus | 99 |
| TGF-β1 miRNA | PLGA nanospheres | Local injection into tendon during repair model | Flexor digitorum profundus | 100 |
| Diclophenac diethylammonium | Gold NP gel | Local administration in combination with phonophoresis | AT | 101 |
| pNFCMV-luc (pDNA) | Mesoporous silica NPs | Local injection into AT | AT | 102 |
CHP, collagen hybrid peptide; COX, cyclooxygenase; DMAEMA, dimethylaminoethyl methacrylate; GelMA, gelatin-methacryloyl; LF/Hep-PLGA NPs, lactoferrin-immobilized, heparin-anchored, poly(lactic-co-glycolic acid) nanoparticles; NPs, nanoparticles; PEG, poly(ethylene glycol); PPZ, poly (organophosphazene).
Collectively, this study demonstrated the potential of CHP-PLGA NPs as an effective drug delivery system with targeted binding, sustained-release ability, and retention of drug bioactivity to treat tendon disease. However, while PLGA has been shown to produce acidic byproducts, which can result in inflammation, 86 no study was conducted to investigate the inflammatory response of cells in the tendon to these NPs, which will be an important consideration for future studies. In another study, siRNAs complexed with cationic NPs facilitated intracellular delivery of Serpine1 silencing siRNAs.87 Diblock copolymer NP with cationic corona blocks of dimethylaminoethyl methacrylate (DMAEMA) and pH-responsive hydrophobic core blocks of DMAEMA, butyl methacrylate, and propylacrylic acid (PAA) were synthesized through reversible addition-fragmentation transfer.
While the cationic corona blocks of DMAEMA enabled complexing to, and protection of the siRNA, the pH-responsive hydrophobic core blocks enabled endosomal escape after protonation, ensuring cytocompatibility and effective gene silencing. Following flexor tendon injury, NP-siRNASerpine1 labeled with Cy-3 dye was subcutaneously injected into the laceration site. Live animal imaging showed that, although NP-siRNASerpine1 was localized to the injury site, it was rapidly cleared after 24 h. Nevertheless, Serpine1 gene expression in the treated tissues was significantly reduced compared to controls, resulting in reduced MMP activity.87 Hence, this study demonstrates the potential of polymeric NPs as siRNA carriers for controlled-release drug delivery in the tendon.
To leverage the beneficial properties of NP drug delivery systems, while overcoming limitations of polymeric scaffolds as drug carriers, other studies have incorporated NPs into porous scaffolds to overcome issues relating to drug loading and release kinetics (Table 3). For example, Liu et al, used electrospun fibrous membranes loaded with basic fibroblast growth factor (bFGF) to prevent peritendinous adhesions in the AT. In this study, preformulated dextran glassy NPs were loaded with bFGF and then electrospun into a poly-L-lactic acid (PLLA) copolymer fiber as a mechanism to secure the bioactivity of bFGF in a sustained manner.
Following complete transection and repair of the AT, the electrospun bFGF/dextran glassy nanoparticles (DGNs)-PLLA fibrous membrane was wrapped around the repaired tendon. Histological and biomechanical assessments of the injured tendon revealed that the bFGF/DGNs-PLLA membrane was effective in minimizing adhesion formation and exhibited better tendon healing compared to control groups, thus establishing a promising, controlled-release mechanism to deliver therapeutics to the tendon.43
Altogether, most attempts to improve tendon healing using NP delivery systems involve either injecting the drug directly into the tendon or incorporating the NPs within a scaffold (Table 3). Although these mechanisms are effective, they can result in high toxicity due to rapid clearance; hence, more effective tendon targeting strategies need to be developed to reduce these undesired occurrences. In the next section, perspectives will be offered on potential strategies to promote systemic targeting of the tendon.
Future Perspectives and Benefits of Tissue-Targeted Systemic Therapies
NPs are attractive drug delivery mechanisms due to their high tunability and sustained delivery properties. However, NPs have low tissue specificity, and must, therefore, be delivered in large amounts to ensure maximum drug utilization at target sites, which can lead to adverse side effects, including increased collateral damage to other tissues like the liver and brain.84,102 Alternatively, systemic delivery of drugs or drug-loaded polymers functionalized with tissue-specific targeting mechanisms (i.e., tissue-targeted systemic drug delivery) provides a noninvasive approach for localized therapeutic delivery.64,103 Moreover, tissue-targeted systemic drug delivery does not require intraoperative administration and facilitates the treatment of conditions that may not require surgical intervention, such as tendonitis and age-related tendon degeneration. Despite these advantages, there is a lack of tendon-targeting systemic drug delivery methods, which is primarily attributed to the lack of an identified targeting mechanism within the tendon to which the NPs can be directed.104
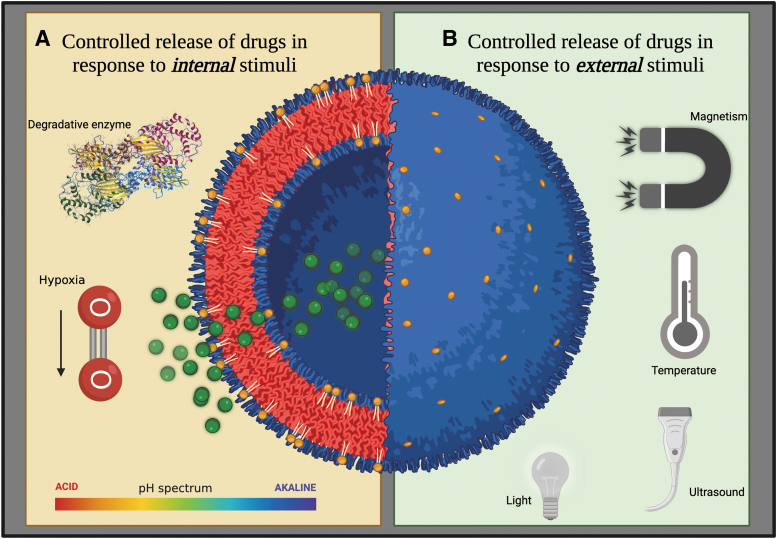
Opportunities for targeting the tendon include exploiting stimuli in the healing milieu such as pH, redox potential, lysosomal enzymes, and glucose, or to external stimuli such as temperature, light impulses, magnetic field, or ultrasound, or to a combination of both internal and external stimuli (dual response/multiresponse NPs) (Fig. 2).85,105–140 To leverage stimuli-responsive NPs, it is critical to have an in-depth understanding of the tendon healing process to identify specific stimuli that can be employed, and the specific phases of healing in which they can be used.5,7,9,13,27,28,141,142
FIG. 2.
Stimuli-responsive NPs for controlled drug delivery. (A) Characteristics of the tendon healing environment, including acidic pH levels, presence of degradative enzymes such as lysosomal acid phosphatase, and hypoxia, can be capitalized to act as stimuli to trigger drug release from NPs only when they reach the tendon. (B) Application of an external stimuli such as magnetic field, temperature, ultrasound, and light to trigger drug release from nanoparticles can allow precise temporal, spatial, and dose control over drug release through a remote device. Hence, the NPs protect the drug from organs that are not the drug target, thus reducing off-target effects. Color images are available online.
Mechanisms to target the tendon during the inflammatory phase of tendon healing: Hypoxia
Following injury, the tendon immediately progresses through a short inflammatory phase, which lasts for a few days.1 During this period, there is an increase in vascular permeability, correlated with an increase in expression of growth factors such as vascular endothelial growth factor (VEGF) and bFGF.9 There is also an influx of inflammatory cells, including neutrophils, monocytes, and erythrocytes, which stimulate and regulate expression of inflammatory cytokines and growth factors including IL-6, IL1-β, IGF-1, TGF-β, and PDGF.1,9,141 The expression of these various cytokines and growth factors promotes chemotaxis and proliferation of macrophages and resident tendon fibroblasts.7,13,27
As in other tissues, hypoxia has been identified as an inducing factor of angiogenesis and an essential factor to obtain physiological healing during tendon injury.143–146 Various studies have shown that angiogenesis results from the activation of hypoxia-inducible factor, which modulates hypoxic effects and eventually leads to upregulation of several genes, including VEGF.143–147
Furthermore, hypoxia has been shown to promote the expression of various proinflammatory cytokines like IL–1β, IL-6, and IL-10.143,144 A study by Sivakumar et al also demonstrated the role of hypoxia in promoting and maintaining inflammatory responses in rheumatoid tendon disease by neovascularization.145 These studies establish the presence and role of hypoxia in tendon disease and present a targeting opportunity for systemic delivery. Specifically, internal stimuli-responsive NPs that respond to hypoxia/redox can be employed to ensure self-controlled drug release because of the presence of glutathione (GSH) in the hypoxic environment. GSH is a reducing agent that can cleave redox-responsive bonds such as nitroimidazole, azo, and disulfide bonds on polymers (Fig. 3a).148
FIG. 3.
The tendon healing cascades associated enzymatic, biological, and physical changes, which can be leveraged for targeted drug delivery. (A) During the inflammatory phase of healing, various internal stimuli like hypoxia lead to the expression of glutathione. Stimuli-responsive nanoparticles containing drugs can be delivered to the healing environment containing these stimuli (pH and redox) to facilitate drug release to modulate healing. (B) During the proliferative healing phase, binding peptides with affinity for specific growth factors like VEGF can be tethered to nanocarriers and used as a targeting opportunity to deliver drugs. (C) During the remodeling phase of healing, collagen can be leveraged as a binding target to deliver peptide-functionalized drug-loaded nanoparticles with high affinity for collagen. Alternatively, MMPs in the microenvironment can be leveraged to cleave liable linkers between a drug and a carrier. Throughout the healing phase, enzymes like hexokinase, lysosomal acid phosphatase, and alkaline phosphatase are present in the microenvironment and cause acidic pH levels, and can therefore be used to facilitate drug release from stimuli-responsive NPs. MMP, matrix metalloproteinase; VEGF, vascular endothelial growth factor. Color images are available online.
For example, Cheng et al developed redox-sensitive drug release poly(ethylene glycol)–poly(acrylic acid)–poly(N-isopropylacrylamide) (PEG-PAA-PNIPAAM) triblock copolymers through carbodiimide chemistry. Although the polymersomes were robust in physiological conditions, they rapidly dissociated upon uptake by MCF-7 breast cancer cells, resulting in the release of exogenous proteins due to redox-triggered de-cross-linking and disruption of the polymersome, thus enhancing drug accumulation at the tumor site, while reducing premature drug release.149
In another study by Dai et al, highly packed interlayer-cross-linked micelle (HP-ICM) comprising a PEG corona and a disulfide-cross-linked interlayer was synthesized and loaded with doxorubicin. When the reducing agents, GSH or dithiothreitol, were added to the micelle solution, the HP-ICM micelles disassembled, indicating that the disulfide bonds had been broken, with the polymer chains completely dissolved as detected by dynamic light scattering and transmission electron microscopy.115 In summary, current literature reports that the occurrence of hypoxia after tendon injury induces angiogenesis, promotes and maintains inflammatory responses, and modulates Hedgehog signaling, resulting in cell differentiation, proliferation, and vascularization.143–146,148,150,151
Furthermore, previous studies report increased levels of GSH due to hypoxia and oxidative stress within the tendon after injury.150,152,153 However, since these redox-responsive NPs have not yet been leveraged for use in the tendon, it cannot yet be determined whether the levels of GSH in the tendon are sufficient for localized drug delivery after systemic treatment or not. Hence, further studies are needed to investigate the peak GSH levels and durations in the tendon to ensure more effective therapies.
Mechanisms to target the proliferative phase of tendon healing: Collagen
During the proliferative phase of healing, there is an expansion of macrophages and recruitment of various Scx+/S100a4+ fibroblast populations, which start to proliferate and create a matrix primarily composed of collagen type 3.1,5,7,13,43 This process is mediated by the expression of a variety of growth factors involved in cell proliferation, collagen synthesis, ECM composition, and cell–matrix interactions. These growth factors include IGF-1, PDGF, TGF-β, bFGF, BMP-12, BMP-13, BMP-14, GDF-5, GDF-6, and GDF-7.1
Depending on the organism, injury type, specific tendon, and treatment regimen, the proliferative stage can last a few days to weeks.13 During this phase, the denatured collagen can be employed as a binding target to deliver drugs systemically. A collagen-binding domain peptide derived from Clostridium histolyticum can be used as a synthetic extension of a peptide-functionalized drug-loaded polymer.154 This collagen-binding domain peptide specifically binds to the undertwisted triple-helical regions of collagen, with binding affinities in the micromolar range. It has been shown to preferentially bind bone and skin within 12 h of delivery and successfully localize drug payloads to sites of injury.154–156 Alternatively, a collagen mimetic peptide that specifically targets denaturing collagen can also be employed during this phase (Fig. 3b).86,157,159
Mechanisms to target the remodeling phase of tendon healing: MMPs
The final stage of tendon healing is the remodeling phase, which lasts from weeks to months and is characterized by the termination of cell proliferation and decreased vascularity. During this phase, there is high turnover of collagen and ongoing ECM remodeling. Furthermore, there is an increase in collagen type I synthesis, mediated by expression of various growth factors such as TGF-β, BMP-12, and BMP-14.1,52 During this phase, MMPs (e.g., MMP2, MMP9, MMP13) are secreted as inactive proteins and are activated when cleaved by extracellular proteinases, leading to upregulation of these MMPs.
Several MMPs are involved in collagen degradation,159–161 and this function can be leveraged for systemic drug delivery. Specifically, peptides for MMP2 (TLTYTWS/CTTHWGFTLC) and MMP9 (CRRHWGFEFC) have been identified through phage display.161 Hence, binding peptides that have an affinity for these targets can be synthesized and conjugated to drug-loaded polymeric NPs for drug delivery. Alternatively, MMP cleavage can be used to cleave labile linkers between a drug and a carrier. These MMP-sensitive linkers can be introduced to polymeric drug carriers to ensure selective release at the injury site (Fig. 3c).
Mechanisms to target the tendon at all healing phases: Enzymatic changes and binding peptides
Drastic enzymatic changes occur during the inflammatory, proliferative, and remodeling phases of tendon healing, which can be leveraged to deliver enzyme-sensitive NPs.162 During the first 12–72 h following tenotomy, glycolysis and release of citric acid cycle enzymes occur immediately throughout the tendon, with the most significant changes observed in the levels of lysosomal enzymes.52,163
Lysosomal acid phosphatase (LAP) enzymes are generally responsible for hydrolyzing organic phosphates at acidic pH,164 and are expressed in monocyte/phagocyte lineage cells. Within 12 h of injury, LAP activity is significantly upregulated. However, peak activity does not typically occur until the beginning of the proliferative phase, followed by a decline during the remodeling phase of healing.165 In addition, the expression and activity of several other enzymes change drastically during healing. They can therefore be employed for tendon-targeted systemic delivery, including alkaline phosphatase, glucose-6-phosphate dehydrogenase, and hexokinase (Fig. 3).164,165 These enzymes can trigger the release of drugs from polymeric nanocarriers synthesized with enzyme-sensitive bonds to ensure effective intracellular drug delivery.
Specifically, enzyme-responsive NPs can either be designed to have their exterior scaffold contain enzyme-sensitive materials that disintegrate when in contact with enzymes or can have the drug payload conjugated to the nanocarrier through electrostatic interactions or enzymatically biodegradable bonds (e.g., ester).62,64,82,83,103,166,167 Enzyme-responsive NPs have been used in various applications, including cancer therapy,168 arthritis,166 myocardial infarction,169 diabetes,170 and bacterial infections.171 They may therefore be used as a reliable drug delivery system in tendon diseases.
To further fine-tune drug release and increase the therapeutic efficiency of drug-loaded NPs, sophisticated polymeric NPs that are responsive to more than one stimulus, such as pH/redox and enzyme/redox, and a combination of internal and external stimuli, such as temperature/enzyme and temperature/pH/redox, have been pursued. These dual stimuli and multistimuli responsive NPs offer extraordinary control over drug release and facilitate NP synthesis and drug loading under mild conditions in tissues such as the heart, lung, bone, muscle, and brain.20,102,105,108–116,118,119,121,122,133,137,140,150,172–175 Alternatively, high-affinity peptides and aptamers can be designed to specifically bind target proteins, molecules, or cells in the tendon. Through phage display, studies have identified peptides that bind to tartrate-resistant acid phosphatase,176 LAP,177 collagen,154 and VEGF.161 These binding peptides can be incorporated onto drug-loaded NPs and delivered systemically to the tendon.
Translational potential of systemically delivered tendon-targeted NPs
Recent advances in surgical repair techniques and suture material have improved healing outcomes of tendon injuries.178–181 However, successful tendon repair remains challenging due to the high potential for gap formation, repair failure, and adhesion formation. Moreover, adhesion formation, particularly in the flexor tendon, often necessitates tenolysis, or scar removal surgery, in an attempt to restore function.5,10,11,28,180,181 Hence, the primary goal of these procedures is to provide sufficient tension between the two tendon ends, minimize bulkiness, and provide as much range of motion as possible during and after the procedure.28,178,180 To successfully achieve all these outcomes, sutures can be coated with nanomaterials encapsulating drug payloads, especially for use in areas like Zone 2 of the flexor tendons, which are relatively anatomically constrained.
Currently, core sutures used for Zone 2 repairs range between 0.7 and 1 cm in length at minimum.178,180 Thus, NP-coated sutures can therefore be used to deliver a moderate amount of growth factors,182 gene therapy,41 and small molecules like mitomycin-C,79 a chemotherapy agent with the ability to reduce postoperative scarring,79 in a sustained delivery manner to strengthen the tendon ends and minimize adhesion formation, while minimizing bulk at the repair site. Furthermore, composite scaffold implants containing drug-loaded NPs can be applied in load-bearing tendons such as the AT after suture repair to provide mechanical strength, while promoting regenerative tendon healing.
Chronic pathological changes to the tendon can result from overuse and/or age-related tendon degeneration, and subsequently alter the mechanical and material properties of the tendon, negatively impact quality of life, and increase the potential for acute tendon rupture. Since these chronic pathologies do not require surgical intervention, it is impractical to use scaffold implants that require invasive procedures.
Furthermore, direct injection of NSAIDs such as Ibuprofen, corticosteroids, or other biologics (e.g., platelet-rich plasma) has not improved functional or material outcomes in clinical studies of rotator cuff pathology and tendinopathies.16,25,183–186 Furthermore, these injections can have deleterious effects on the tendon (e.g., decreased mechanical and material properties), increase the risk of infection and inflammation, increase the chances of needing a revision surgery, and require multiple treatments.25,184–186 Alternatively, nanotechnology can be used systemically to safely deliver biologics intravenously to facilitate controlled release and increased circulation time of drug payloads, which can subsequently improve material quality and functional outcomes of the tendon without the risks associated with classic treatment approaches.
Although tendon healing using nanotechnology has not yet reached clinical trial stages, investigations using NP-mediated drug delivery approaches to promote healing of acute tendon injuries,29,41,86,90,94,100,182 tendonitis,29,90,93,98 and bone-to-tendon injuries47,66,71,74–76,78 are emerging.
Conclusions
Despite the severity and frequency of complications associated with tendon injuries, there is no pharmacological approach to promote tendon regeneration. Although current approaches have focused on using scaffolds as carriers to deliver growth factors and therapeutics, these approaches can only be applied intraoperatively before knowing how healing will progress, thus limiting the potential for individualized care. Moreover, several complications associated with intraoperative procedures can arise, including exacerbation of tissue fibrosis. Hence, there is a need to develop personalized and customizable approaches that promote high specificity targeting of the healing tendon. NPs offer precise control over drug loading and release kinetics and are therefore an attractive translational platform that can deliver therapeutics without invasive procedures.
Moreover, advances in nanotechnology for tendon regeneration can be combined with stimuli-responsive polymers tailored for individual tendon injuries and the stage of healing that needs to be modulated. In this review, we have discussed various targeting opportunities that can be leveraged at different phases of recovery, based on the unique microenvironment that characterizes the tendon-healing cascade. By optimizing the targeting specificity of NP delivery systems, the outcomes of precision medicine therapeutics can be enhanced to improve patient outcomes, thereby reducing complications associated with tendon injuries, including disability, risk of reinjury, and increased socioeconomic burden.
Authors' Contributions
Conceptualization (A.E.L. and E.A.-S.), writing (E.A.-S.), and editing (A.E.L., E.A.-S., and D.S.W.B.). All authors have read and approved the final version of this article.
Disclosure Statement
No competing financial interests exist.
Funding Information
This work was supported, in part, by NIH/NIAMS R21 AR081063 (A.E.L. and D.S.W.B.), and The University of Rochester Clinical and Translational Science Institute Trainee Pilot Award UL1TR002001 from the NIH (E.A.-S.).
References
- 1. Andarawis-Puri N, Flatow EL, Soslowsky LJ. Tendon basic science: Development, repair, regeneration, and healing. J Orthop Res 2015;33(6):780–784; doi: 10.1002/jor.22869 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Aicale R, Oliviero A, Maffulli N. Management of Achilles and patellar tendinopathy: What we know, what we can do. J Foot Ankle Res 2020;13(1):59; doi: 10.1186/s13047-020-00418-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Clark ST, Zhu M, Gamble GD, et al. Epidemiology of tendon and ligament injuries in Aotearoa/New Zealand between 2010 and 2016. Inj Epidemiol 2020;7(1):5; doi: 10.1186/s40621-020-0231-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Järvinen TA, Kannus P, Maffulli N, et al. Achilles tendon disorders: Etiology and epidemiology. Foot Ankle Clin 2005;10(2):255–266; doi: 10.1016/j.fcl.2005.01.013 [DOI] [PubMed] [Google Scholar]
- 5. Klifto CS, Capo JT, Sapienza A, et al. Flexor tendon injuries. J Am Acad Orthop Surg 2018;26(2):e26–e35; doi: 10.5435/jaaos-d-16-00316 [DOI] [PubMed] [Google Scholar]
- 6. Raikin SM, Garras DN, Krapchev PV. Achilles tendon injuries in a United States population. Foot Ankle Int 2013;34(4):475–480; doi: 10.1177/1071100713477621 [DOI] [PubMed] [Google Scholar]
- 7. Müller SA, Todorov A, Heisterbach PE, et al. Tendon healing: An overview of physiology, biology, and pathology of tendon healing and systematic review of state of the art in tendon bioengineering. Knee Surg Sports Traumatol Arthrosc 2015;23(7):2097–2105; doi: 10.1007/s00167-013-2680-z [DOI] [PubMed] [Google Scholar]
- 8. Nichols AEC, Oh I, Loiselle AE. Effects of type II diabetes mellitus on tendon homeostasis and healing. J Orthop Res 2020;38(1):13–22; doi: 10.1002/jor.24388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. D'Addona A, Maffulli N, Formisano S, et al. Inflammation in tendinopathy. Surgeon 2017;15(5):297–302; doi: 10.1016/j.surge.2017.04.004 [DOI] [PubMed] [Google Scholar]
- 10. de Jong JP, Nguyen JT, Sonnema AJ, et al. The incidence of acute traumatic tendon injuries in the hand and wrist: A 10-year population-based study. Clin Orthop Surg 2014;6(2):196–202; doi: 10.4055/cios.2014.6.2.196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Gojowy D, Homann HH, Schreier D. [Flexor tendon injuries of the hand]. Chirurg 2020;91(10):895–902; doi: 10.1007/s00104-020-01235-2 [DOI] [PubMed] [Google Scholar]
- 12. Hou J, Yang R, Vuong I, et al. Biomaterials strategies to balance inflammation and tenogenesis for tendon repair. Acta Biomater 2021;130:1–16; doi: 10.1016/j.actbio.2021.05.043 [DOI] [PubMed] [Google Scholar]
- 13. Nichols AEC, Best KT, Loiselle AE. The cellular basis of fibrotic tendon healing: Challenges and opportunities. Transl Res 2019;209:156–168; doi: 10.1016/j.trsl.2019.02.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Weinfeld SB. Achilles tendon disorders. Med Clin North Am 2014;98(2):331–338; doi: 10.1016/j.mcna.2013.11.005 [DOI] [PubMed] [Google Scholar]
- 15. Al-Siyabi Z, Karam M, Al-Hajri E, et al. Extracorporeal shockwave therapy versus ultrasound therapy for plantar fasciitis: A systematic review and meta-analysis. Cureus 2022;14(1):e20871; doi: 10.7759/cureus.20871 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Burton I. Combined extracorporeal shockwave therapy and exercise for the treatment of tendinopathy: A narrative review. Sports Med Health Sci 2022;4(1):8–17; doi: 10.1016/j.smhs.2021.11.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Chen Y-C, Gad SF, Chobisa D, et al. Local drug delivery systems for inflammatory diseases: Status quo, challenges, and opportunities. J Control Release 2021;330:438–460; doi: 10.1016/j.jconrel.2020.12.025 [DOI] [PubMed] [Google Scholar]
- 18. Campbell S, Smeets N.. Drug delivery: Localized and systemic therapeutic strategies with polymer systems. In: Functional Polymers. (Jafar Mazumder MA, Sheardown H, Al-Ahmed A, eds.) Springer International Publishing: Cham; 2019; pp. 1–56. [Google Scholar]
- 19. Lomas AJ, Ryan CN, Sorushanova A, et al. The past, present and future in scaffold-based tendon treatments. Adv Drug Deliv Rev 2015;84:257–277; doi: 10.1016/j.addr.2014.11.022 [DOI] [PubMed] [Google Scholar]
- 20. Paredes JJ, Andarawis-Puri N. Therapeutics for tendon regeneration: A multidisciplinary review of tendon research for improved healing. Ann N Y Acad Sci 2016;1383(1):125–138; doi: 10.1111/nyas.13228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Jacob S, Nair AB, Shah J, et al. Emerging role of hydrogels in drug delivery systems, tissue engineering and wound management. Pharmaceutics 2021;13(3); doi: 10.3390/pharmaceutics13030357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Garg T, Singh O, Arora S, et al. Scaffold: A novel carrier for cell and drug delivery. Crit Rev Ther Drug Carrier Syst 2012;29(1):1–63; doi: 10.1615/critrevtherdrugcarriersyst.v29.i1.10 [DOI] [PubMed] [Google Scholar]
- 23. Chen E, Yang L, Ye C, et al. An asymmetric chitosan scaffold for tendon tissue engineering: In vitro and in vivo evaluation with rat tendon stem/progenitor cells. Acta Biomater 2018;73:377–387; doi: 10.1016/j.actbio.2018.04.027 [DOI] [PubMed] [Google Scholar]
- 24. Lei T, Zhang T, Ju W, et al. Biomimetic strategies for tendon/ligament-to-bone interface regeneration. Bioact Mater 2021;6(8):2491–2510; doi: 10.1016/j.bioactmat.2021.01.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Prabhath A, Vernekar VN, Sanchez E, et al. Growth factor delivery strategies for rotator cuff repair and regeneration. Int J Pharm 2018;544(2):358–371; doi: 10.1016/j.ijpharm.2018.01.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Rodrigues MT, Reis RL, Gomes ME. Engineering tendon and ligament tissues: Present developments towards successful clinical products. J Tissue Eng Regen Med 2013;7(9):673–686; doi: 10.1002/term.1459 [DOI] [PubMed] [Google Scholar]
- 27. Sharma P, Maffulli N. Biology of tendon injury: Healing, modeling and remodeling. J Musculoskelet Neuronal Interact 2006;6(2):181–190. [PubMed] [Google Scholar]
- 28. Thomopoulos S, Parks WC, Rifkin DB, et al. Mechanisms of tendon injury and repair. J Orthop Res 2015;33(6):832–839; doi: 10.1002/jor.22806 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Parchi PD, Vittorio O, Andreani L, et al. Nanoparticles for tendon healing and regeneration: Literature review. Front Aging Neurosci 2016;8:202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Bao J, Zhang Q, Duan T, et al. The fate of nanoparticles in vivo and the strategy of designing stealth nanoparticle for drug delivery. Curr Drug Targets 2021;22(8):922–946; doi: 10.2174/1389450122666210118105122 [DOI] [PubMed] [Google Scholar]
- 31. Sun T, Zhang YS, Pang B, et al. Engineered nanoparticles for drug delivery in cancer therapy. Angew Chem Int Ed Engl 2014;53(46):12320–12364; doi: 10.1002/anie.201403036 [DOI] [PubMed] [Google Scholar]
- 32. Wilczewska AZ, Niemirowicz K, Markiewicz KH, et al. Nanoparticles as drug delivery systems. Pharmacol Rep 2012;64(5):1020–1037; doi: 10.1016/s1734-1140(12)70901-5 [DOI] [PubMed] [Google Scholar]
- 33. Pasternak B, Missios A, Askendal A, et al. Doxycycline-coated sutures improve the suture-holding capacity of the rat Achilles tendon. Acta Orthop 2007;78(5):680–686; doi: 10.1080/17453670710014392 [DOI] [PubMed] [Google Scholar]
- 34. Chamberlain CS, Lee JS, Leiferman EM, et al. Effects of BMP-12-releasing sutures on Achilles tendon healing. Tissue Eng Part A 2015;21(5–6):916–927; doi: 10.1089/ten.TEA.2014.0001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Cummings SH, Grande DA, Hee CK, et al. Effect of recombinant human platelet-derived growth factor-BB-coated sutures on Achilles tendon healing in a rat model: A histological and biomechanical study. J Tissue Eng 2012;3(1):2041731412453577; doi: 10.1177/2041731412453577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Evrova O, Houska J, Welti M, et al. Bioactive, elastic, and biodegradable emulsion electrospun degrapol tube delivering PDGF-BB for tendon rupture repair. Macromol Biosci 2016;16(7):1048–1063; doi: 10.1002/mabi.201500455 [DOI] [PubMed] [Google Scholar]
- 37. Hamada Y, Katoh S, Hibino N, et al. Effects of monofilament nylon coated with basic fibroblast growth factor on endogenous intrasynovial flexor tendon healing. J Hand Surg Am 2006;31(4):530–540; doi: 10.1016/j.jhsa.2005.12.003 [DOI] [PubMed] [Google Scholar]
- 38. Leek BT, Tasto JP, Tibor LM, et al. Augmentation of tendon healing with butyric acid-impregnated sutures: Biomechanical evaluation in a rabbit model. Am J Sports Med 2012;40(8):1762–1771; doi: 10.1177/0363546512450691 [DOI] [PubMed] [Google Scholar]
- 39. Muraoka K, Le W, Behn AW, et al. The effect of growth differentiation factor 8 (myostatin) on bone marrow-derived stem cell-coated bioactive sutures in a rabbit tendon repair model. Hand (N Y) 2020;15(2):264–270; doi: 10.1177/1558944718792708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Thoreson AR, Hiwatari R, An KN, et al. The effect of 1-ethyl-3-(3-dimethylaminopropyl) carbodiimide suture coating on tendon repair strength and cell viability in a canine model. J Hand Surg Am 2015;40(10):1986–1991; doi: 10.1016/j.jhsa.2015.06.117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Zhou YL, Yang QQ, Yan YY, et al. Gene-loaded nanoparticle-coated sutures provide effective gene delivery to enhance tendon healing. Mol Ther 2019;27(9):1534–1546; doi: 10.1016/j.ymthe.2019.05.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. TK Hiong Teh, JCHong Goh, Toh SL. Controlled bioactive molecules delivery strategies for tendon and ligament tissue engineering using polymeric nanofibers. Curr Pharm Des 2015;21(15):1991–2005; doi: 10.2174/1381612821666150302153030 [DOI] [PubMed] [Google Scholar]
- 43. Liu S, Qin M, Hu C, et al. Tendon healing and anti-adhesion properties of electrospun fibrous membranes containing bFGF loaded nanoparticles. Biomaterials 2013;34(19):4690–4701; doi: 10.1016/j.biomaterials.2013.03.026 [DOI] [PubMed] [Google Scholar]
- 44. Manning CN, Schwartz AG, Liu W, et al. Controlled delivery of mesenchymal stem cells and growth factors using a nanofiber scaffold for tendon repair. Acta Biomater 2013;9(6):6905–6914; doi: 10.1016/j.actbio.2013.02.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Stace ET, Nagra NS, Tiberwel S, et al. The use of electrospun scaffolds in musculoskeletal tissue engineering: A focus on tendon and the rotator cuff. Curr Stem Cell Res Ther 2018;13(8):619–631; doi: 10.2174/1574888x13666180129105707 [DOI] [PubMed] [Google Scholar]
- 46. Stratton S, Shelke NB, Hoshino K, et al. Bioactive polymeric scaffolds for tissue engineering. Bioact Mater 2016;1(2):93–108; doi: 10.1016/j.bioactmat.2016.11.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Taylor BL, Kim DH, Huegel J, et al. Localized delivery of ibuprofen via a bilayer delivery system (BiLDS) for supraspinatus tendon healing in a rat model. J Orthop Res 2020;38(11):2339–2349; doi: 10.1002/jor.24670 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Wu S, Zhou R, Zhou F, et al. Electrospun thymosin Beta-4 loaded PLGA/PLA nanofiber/microfiber hybrid yarns for tendon tissue engineering application. Mater Sci Eng C Mater Biol Appl 2020;106:110268; doi: 10.1016/j.msec.2019.110268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Robinson DH, Mauger JW. Drug delivery systems. Am J Hosp Pharm 1991;48(10 Suppl 1):S14–S23 [PubMed] [Google Scholar]
- 50. Liu S, Zhao J, Ruan H, et al. Antibacterial and anti-adhesion effects of the silver nanoparticles-loaded poly (L-lactide) fibrous membrane. Mater Sci Eng C 2013;33(3):1176–1182 [DOI] [PubMed] [Google Scholar]
- 51. Araque-Monrós MC, García-Cruz DM, Escobar-Ivirico JL, et al. Regenerative and resorbable PLA/HA hybrid construct for tendon/ligament tissue engineering. Ann Biomed Eng 2020;48(2):757–767; doi: 10.1007/s10439-019-02403-0 [DOI] [PubMed] [Google Scholar]
- 52. Carlstedt CA. Mechanical and chemical factors in tendon healing. Effects of indomethacin and surgery in the rabbit. Acta Orthop Scand Suppl 1987;224:1–75; doi: 10.3109/17453678709154163 [DOI] [PubMed] [Google Scholar]
- 53. Coelho JF, Ferreira PC, Alves P, et al. Drug delivery systems: Advanced technologies potentially applicable in personalized treatments. Epma j 2010;1(1):164–209; doi: 10.1007/s13167-010-0001-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Hu C, Liu S, Zhang Y, et al. Long-term drug release from electrospun fibers for in vivo inflammation prevention in the prevention of peritendinous adhesions. Acta Biomater 2013;9(7):7381–7388; doi: 10.1016/j.actbio.2013.03.040 [DOI] [PubMed] [Google Scholar]
- 55. Rafiei P, Haddadi A. Docetaxel-loaded PLGA and PLGA-PEG nanoparticles for intravenous application: Pharmacokinetics and biodistribution profile. Int J Nanomed 2017;12:935–947; doi: 10.2147/ijn.S121881 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Liu C-F, Aschbacher-Smith L, Barthelery NJ, et al. What we should know before using tissue engineering techniques to repair injured tendons: A developmental biology perspective. Tissue Eng B Rev 2011;17(3):165–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Riley G. The pathogenesis of tendinopathy. A molecular perspective. Rheumatology 2004;43(2):131–142. [DOI] [PubMed] [Google Scholar]
- 58. Yang QQ, Zhang L, Ju F, et al. Sustained-release hydrogel-based rhynchophylline delivery system improved injured tendon repair. Colloids Surf B Biointerfaces 2021;205:111876; doi: 10.1016/j.colsurfb.2021.111876 [DOI] [PubMed] [Google Scholar]
- 59. Mouriño V. Chapter 23 - Scaffolds with drug delivery capability. In: Tissue Engineering Using Ceramics and Polymers (3rd ed.). (Boccaccini AR, Ma PX, Liverani L. eds.) Woodhead Publishing: 2022; pp. 817–840. [Google Scholar]
- 60. Cook AD, Hrkach JS, Gao NN, et al. Characterization and development of RGD-peptide-modified poly(lactic acid-co-lysine) as an interactive, resorbable biomaterial. J Biomed Mater Res 1997;35(4):513–523; doi: [DOI] [PubMed] [Google Scholar]
- 61. Ma PX. Biomimetic materials for tissue engineering. Adv Drug Deliv Rev 2008;60(2):184–198; doi: 10.1016/j.addr.2007.08.041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Newman MR, Benoit DSW. In vivo translation of peptide-targeted drug delivery systems discovered by phage display. Bioconjug Chem 2018;29(7):2161–2169; doi: 10.1021/acs.bioconjchem.8b00285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Wang Y, Malcolm DW, Benoit DSW. Controlled and sustained delivery of siRNA/NPs from hydrogels expedites bone fracture healing. Biomaterials 2017;139:127–138; doi: 10.1016/j.biomaterials.2017.06.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Wang Y, Newman MR, M Ackun-Farmmer, et al. Fracture-targeted delivery of β-catenin agonists via peptide-functionalized nanoparticles augments fracture healing. ACS Nano 2017;11(9):9445–9458; doi: 10.1021/acsnano.7b05103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Chaisri W, Ghassemi AH, Hennink WE, et al. Enhanced gentamicin loading and release of PLGA and PLHMGA microspheres by varying the formulation parameters. Colloids Surf B Biointerfaces 2011;84(2):508–514; doi: 10.1016/j.colsurfb.2011.02.006 [DOI] [PubMed] [Google Scholar]
- 66. Wang D, Zhang X, Ng KW, et al. Growth and differentiation factor-7 immobilized, mechanically strong quadrol-hexamethylene diisocyanate-methacrylic anhydride polyurethane polymer for tendon repair and regeneration. Acta Biomater 2022;154:108–122; doi: 10.1016/j.actbio.2022.10.029 [DOI] [PubMed] [Google Scholar]
- 67. Riggin CN, Qu F, Kim DH, et al. Electrospun PLGA Nanofiber scaffolds release ibuprofen faster and degrade slower after in vivo implantation. Ann Biomed Eng 2017;45(10):2348–2359; doi: 10.1007/s10439-017-1876-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Evrova O, Bürgisser GM, Ebnöther C, et al. Elastic and surgeon friendly electrospun tubes delivering PDGF-BB positively impact tendon rupture healing in a rabbit Achilles tendon model. Biomaterials 2020;232:119722; doi: 10.1016/j.biomaterials.2019.119722 [DOI] [PubMed] [Google Scholar]
- 69. Thomopoulos S, Das R, Silva MJ, et al. Enhanced flexor tendon healing through controlled delivery of PDGF-BB. J Orthop Res 2009;27(9):1209–1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Thomopoulos S, Kim HM, Das R, et al. The effects of exogenous basic fibroblast growth factor on intrasynovial flexor tendon healing in a canine model. J Bone Joint Surg Am 2010;92(13):2285–2293; doi: 10.2106/jbjs.I.01601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Kovacevic D, Gulotta LV, Ying L, et al. rhPDGF-BB promotes early healing in a rat rotator cuff repair model. Clin Orthop Relat Res 2015;473(5):1644–1654; doi: 10.1007/s11999-014-4020-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Liu X, Li Y, Wang S, et al. PDGF-loaded microneedles promote tendon healing through p38/cyclin D1 pathway mediated angiogenesis. Mater Today Bio 2022;16:100428; doi: 10.1016/j.mtbio.2022.100428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Bürgisser GM, Evrova O, Heuberger DM, et al. Electrospun tube reduces adhesion in rabbit Achilles tendon 12weeks post-surgery without PAR-2 overexpression. Sci Rep 2021;11(1):23293; doi: 10.1038/s41598-021-02780-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Zhao S, Zhao J, Dong S, et al. Biological augmentation of rotator cuff repair using bFGF-loaded electrospun poly(lactide-co-glycolide) fibrous membranes. Int J Nanomed 2014;9:2373–2385; doi: 10.2147/ijn.S59536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Yoon JP, Lee CH, Jung JW, et al. Sustained delivery of transforming growth factor β1 by use of absorbable alginate scaffold enhances rotator cuff healing in a rabbit model. Am J Sports Med 2018;46(6):1441–1450; doi: 10.1177/0363546518757759 [DOI] [PubMed] [Google Scholar]
- 76. Yoon JP, Chung SW, Jung JW, et al. Is a local administration of parathyroid hormone effective to tendon-to-bone healing in a rat rotator cuff repair model? J Orthop Res 2020;38(1):82–91; doi: 10.1002/jor.24452 [DOI] [PubMed] [Google Scholar]
- 77. Zhou Z, Song W, Zhang G, et al. The recombinant human fibroblast growth factor-18 (sprifermin) improves tendon-to-bone healing by promoting chondrogenesis in a rat rotator cuff repair model. J Shoulder Elbow Surg 2022;31(8):1617–1627; doi: 10.1016/j.jse.2022.01.137 [DOI] [PubMed] [Google Scholar]
- 78. Han B, Jones IA, Yang Z, et al. Repair of rotator cuff tendon defects in aged rats using a growth factor injectable gel scaffold. Arthroscopy 2020;36(3):629–637; doi: 10.1016/j.arthro.2019.09.015 [DOI] [PubMed] [Google Scholar]
- 79. Zhao X, Jiang S, Liu S, et al. Optimization of intrinsic and extrinsic tendon healing through controllable water-soluble mitomycin-C release from electrospun fibers by mediating adhesion-related gene expression. Biomaterials 2015;61:61–74; doi: 10.1016/j.biomaterials.2015.05.012 [DOI] [PubMed] [Google Scholar]
- 80. Stover TC, Sharma A, Robertson GP, et al. Systemic delivery of liposomal short-chain ceramide limits solid tumor growth in murine models of breast adenocarcinoma. Clin Cancer Res 2005;11(9):3465–3474; doi: 10.1158/1078-0432.CCR-04-1770 [DOI] [PubMed] [Google Scholar]
- 81. Patra JK, Das G, Fraceto LF, et al. Nano based drug delivery systems: Recent developments and future prospects. J Nanobiotechnol 2018;16(1):71; doi: 10.1186/s12951-018-0392-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Chandrasiri I, Liu Y, Adjei-Sowah E, et al. Reproducible and controlled peptide functionalization of polymeric nanoparticles. Front Biomater Sci 2022;1; doi: 10..3389/fbiom.2022.1003172 [Google Scholar]
- 83. Sims KR Jr., He B, Koo H, et al. Electrostatic interactions enable nanoparticle delivery of the flavonoid myricetin. ACS Omega 2020;5(22):12649–12659; doi: 10.1021/acsomega.9b04101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Fan Y, Marioli M, Zhang K. Analytical characterization of liposomes and other lipid nanoparticles for drug delivery. J Pharm Biomed Anal 2021;192:113642; doi: 10.1016/j.jpba.2020.113642 [DOI] [PubMed] [Google Scholar]
- 85. Lo C-L, Lin K-M, Hsiue G-H. Preparation and characterization of intelligent core-shell nanoparticles based on poly (D, L-lactide)-g-poly (N-isopropyl acrylamide-co-methacrylic acid). J Control Release 2005;104(3):477–488. [DOI] [PubMed] [Google Scholar]
- 86. Chen Y, Shen W, Tang C, et al. Targeted pathological collagen delivery of sustained-release rapamycin to prevent heterotopic ossification. Sci Adv 2020;6(18):eaay9526; doi: 10.1126/sciadv.aay9526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Freeberg MAT, Farhat YM, Easa A, et al. Serpine1 knockdown enhances MMP activity after Flexor tendon injury in mice: Implications for adhesions therapy. Sci Rep 2018;8(1):5810; doi: 10.1038/s41598-018-24144-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Yang QQ, Chen J, Zhou YL, et al. The influence of a nanoparticle gel loaded with siRNA-cyclooxygenase on flexor tendon healing: An in vivo animal study. J Hand Surg Eur Vol 2022;47(10):1064–1070; doi: 10.1177/17531934221109709 [DOI] [PubMed] [Google Scholar]
- 89. Cetik RM, S Yabanoglu Ciftci, Arica B, et al. Evaluation of the effects of transforming growth factor-beta 3 (TGF-β3) loaded nanoparticles on healing in a rat achilles tendon injury model. Am J Sports Med 2022;50(4):1066–1077; doi: 10.1177/03635465211073148 [DOI] [PubMed] [Google Scholar]
- 90. Choi HJ, Choi S, Kim JG, et al. Enhanced tendon restoration effects of anti-inflammatory, lactoferrin-immobilized, heparin-polymeric nanoparticles in an Achilles tendinitis rat model. Carbohydr Polym 2020;241:116284; doi: 10.1016/j.carbpol.2020.116284 [DOI] [PubMed] [Google Scholar]
- 91. Zhou YL, Yang QQ, Yan YY, et al. Localized delivery of miRNAs targets cyclooxygenases and reduces flexor tendon adhesions. Acta Biomater 2018;70:237–248; doi: 10.1016/j.actbio.2018.01.047 [DOI] [PubMed] [Google Scholar]
- 92. Zhang W, Li X, M Comes Franchini, et al. Controlled release of curcumin from curcumin-loaded nanomicelles to prevent peritendinous adhesion during Achilles tendon healing in rats. Int J Nanomedicine 2016;11:2873–2881; doi: 10.2147/ijn.S103867 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Suwalski A, Dabboue H, Delalande A, et al. Accelerated Achilles tendon healing by PDGF gene delivery with mesoporous silica nanoparticles. Biomaterials 2010;31(19):5237–5245; doi: 10.1016/j.biomaterials.2010.02.077 [DOI] [PubMed] [Google Scholar]
- 94. Chen W, Chen Y, Ren Y, et al. Lipid nanoparticle-assisted miR29a delivery based on core-shell nanofibers improves tendon healing by cross-regulation of the immune response and matrix remodeling. Biomaterials 2022;291:121888; doi: 10.1016/j.biomaterials.2022.121888 [DOI] [PubMed] [Google Scholar]
- 95. Cai C, Zhang X, Li Y, et al. Self-healing hydrogel embodied with macrophage-regulation and responsive-gene-silencing properties for synergistic prevention of peritendinous adhesion. Adv Mater 2022;34(5):e2106564; doi: 10.1002/adma.202106564 [DOI] [PubMed] [Google Scholar]
- 96. Wu G, Sun B, Zhao C, et al. Three-dimensional tendon scaffold loaded with TGF-β1 gene silencing plasmid prevents tendon adhesion and promotes tendon repair. ACS Biomater Sci Eng 2021;7(12):5739–5748; doi: 10.1021/acsbiomaterials.1c00747 [DOI] [PubMed] [Google Scholar]
- 97. Li J, Weber E, Guth-Gundel S, et al. Tough composite hydrogels with high loading and local release of biological drugs. Adv Healthc Mater 2018;7(9):e1701393; doi: 10.1002/adhm.201701393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Yang QQ, Shao YX, Zhang LZ, et al. Therapeutic strategies for flexor tendon healing by nanoparticle-mediated co-delivery of bFGF and VEGFA genes. Colloids Surf B Biointerfaces 2018;164:165–176; doi: 10.1016/j.colsurfb.2018.01.031 [DOI] [PubMed] [Google Scholar]
- 99. Zhou Y, Zhu C, Wu YF, et al. Effective modulation of transforming growth factor-β1 expression through engineered microRNA-based plasmid-loaded nanospheres. Cytotherapy 2015;17(3):320–329; doi: 10.1016/j.jcyt.2014.09.004 [DOI] [PubMed] [Google Scholar]
- 100. Dohnert MB, Ferreira GK, Silveira PC, et al. Inflammatory cytokines content in Achilles tendinopathy after phonophoresis treatment combined with gold nanoparticles and diclophenac diethylammonium in rats. Inflammation 2015;38(3):1044–1049; doi: 10.1007/s10753-014-0069-x [DOI] [PubMed] [Google Scholar]
- 101. Brevet D, Hocine O, Delalande A, et al. Improved gene transfer with histidine-functionalized mesoporous silica nanoparticles. Int J Pharm 2014;471(1):197–205; doi: 10.1016/j.ijpharm.2014.05.020 [DOI] [PubMed] [Google Scholar]
- 102. Luo Y, Yang H, Zhou YF, et al. Dual and multi-targeted nanoparticles for site-specific brain drug delivery. J Control Release 2020;317:195–215; doi: 10.1016/j.jconrel.2019.11.037 [DOI] [PubMed] [Google Scholar]
- 103. Sims KR, Maceren JP, Liu Y, et al. Dual antibacterial drug-loaded nanoparticles synergistically improve treatment of Streptococcus mutans biofilms. Acta Biomater 2020;115:418–431; doi: 10.1016/j.actbio.2020.08.032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Cheng R, Meng F, Deng C, et al. Dual and multi-stimuli responsive polymeric nanoparticles for programmed site-specific drug delivery. Biomaterials 2013;34(14):3647–3657; doi: 10.1016/j.biomaterials.2013.01.084 [DOI] [PubMed] [Google Scholar]
- 105. Chen Y-C, Liao L-C, Lu P-L, et al. The accumulation of dual pH and temperature responsive micelles in tumors. Biomaterials 2012;33(18):4576–4588. [DOI] [PubMed] [Google Scholar]
- 106. Li W, Li J, Gao J, et al. The fine-tuning of thermosensitive and degradable polymer micelles for enhancing intracellular uptake and drug release in tumors. Biomaterials 2011;32(15):3832–3844. [DOI] [PubMed] [Google Scholar]
- 107. Xing Z, Wang C, Yan J, et al. Dual stimuli responsive hollow nanogels with IPN structure for temperature controlling drug loading and pH triggering drug release. Soft Matter 2011;7(18):7992–7997. [Google Scholar]
- 108. Shen Y, Ma X, Zhang B, et al. Degradable dual pH-and temperature-responsive photoluminescent dendrimers. Chem Eur J 2011;17(19):5319–5326. [DOI] [PubMed] [Google Scholar]
- 109. Cui W, Lu X, Cui K, et al. Dual-responsive controlled drug delivery based on ionically assembled nanoparticles. Langmuir 2012;28(25):9413–9420. [DOI] [PubMed] [Google Scholar]
- 110. Chiang W-H, Ho VT, Huang W-C, et al. Dual stimuli-responsive polymeric hollow nanogels designed as carriers for intracellular triggered drug release. Langmuir 2012;28(42):15056–15064. [DOI] [PubMed] [Google Scholar]
- 111. Zhang J, Wu L, Meng F, et al. pH and reduction dual-bioresponsive polymersomes for efficient intracellular protein delivery. Langmuir 2012;28(4):2056–2065. [DOI] [PubMed] [Google Scholar]
- 112. RB KC, Thapa B, Xu P. pH and redox dual responsive nanoparticle for nuclear targeted drug delivery. Mol Pharm 2012;9(9):2719–2729. [DOI] [PubMed] [Google Scholar]
- 113. Chen J, Qiu X, Ouyang J, et al. pH and reduction dual-sensitive copolymeric micelles for intracellular doxorubicin delivery. Biomacromolecules 2011;12(10):3601–3611. [DOI] [PubMed] [Google Scholar]
- 114. Pan Y-J, Chen Y-Y, Wang D-R, et al. Redox/pH dual stimuli-responsive biodegradable nanohydrogels with varying responses to dithiothreitol and glutathione for controlled drug release. Biomaterials 2012;33(27):6570–6579. [DOI] [PubMed] [Google Scholar]
- 115. Dai J, Lin S, Cheng D, et al. Interlayer-crosslinked micelle with partially hydrated core showing reduction and pH dual sensitivity for pinpointed intracellular drug release. Angew Chem Int Ed Engl 2011;50(40):9404–9408. [DOI] [PubMed] [Google Scholar]
- 116. Chen W, Zhong P, Meng F, et al. Redox and pH-responsive degradable micelles for dually activated intracellular anticancer drug release. J Control Release 2013;169(3):171–179. [DOI] [PubMed] [Google Scholar]
- 117. Shu S, Zhang X, Wu Z, et al. Gradient cross-linked biodegradable polyelectrolyte nanocapsules for intracellular protein drug delivery. Biomaterials 2010;31(23):6039–6049. [DOI] [PubMed] [Google Scholar]
- 118. Liang K, Such GK, Zhu Z, et al. Charge-shifting click capsules with dual-responsive cargo release mechanisms. Adv Mater 2011;23(36):H273–H277. [DOI] [PubMed] [Google Scholar]
- 119. Hu X, Li H, Luo S, et al. Thiol and pH dual-responsive dynamic covalent shell cross-linked micelles for triggered release of chemotherapeutic drugs. Polym Chem 2013;4(3):695–706. [Google Scholar]
- 120. Jia Z, Wong L, Davis TP, et al. One-pot conversion of RAFT-generated multifunctional block copolymers of HPMA to doxorubicin conjugated acid-and reductant-sensitive crosslinked micelles. Biomacromolecules 2008;9(11):3106–3113. [DOI] [PubMed] [Google Scholar]
- 121. Wei C, Guo J, Wang C. Dual stimuli-responsive polymeric micelles exhibiting “AND” logic gate for controlled release of adriamycin. Macromol Rapid Commun 2011;32(5):451–455. [DOI] [PubMed] [Google Scholar]
- 122. Yoon S, Kim WJ, Yoo HS. Dual-responsive breakdown of nanostructures with high doxorubicin payload for apoptotic anticancer therapy. Small 2013;9(2):284–293. [DOI] [PubMed] [Google Scholar]
- 123. Mahmoud EA, Sankaranarayanan J, Morachis JM, et al. Inflammation responsive logic gate nanoparticles for the delivery of proteins. Bioconjug Chem 2011;22(7):1416–1421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Barick KC, Singh S, Jadhav NV, et al. pH-responsive peptide mimic shell cross-linked magnetic nanocarriers for combination therapy. Adv Funct Mater 2012;22(23):4975–4984. [Google Scholar]
- 125. Zhao Z, Huang D, Yin Z, et al. Magnetite nanoparticles as smart carriers to manipulate the cytotoxicity of anticancer drugs: Magnetic control and pH-responsive release. J Mater Chem 2012;22(31):15717–15725. [Google Scholar]
- 126. Guo M, Yan Y, Zhang H, et al. Magnetic and pH-responsive nanocarriers with multilayer core–shell architecture for anticancer drug delivery. J Mater Chem 2008;18(42):5104–5112. [Google Scholar]
- 127. Guo M, Yan Y, Liu X, et al. Multilayer nanoparticles with a magnetite core and a polycation inner shell as pH-responsive carriers for drug delivery. Nanoscale 2010;2(3):434–441. [DOI] [PubMed] [Google Scholar]
- 128. Yu S, Wu G, Gu X, et al. Magnetic and pH-sensitive nanoparticles for antitumor drug delivery. Colloids Surf B Biointerfaces 2013;103:15–22. [DOI] [PubMed] [Google Scholar]
- 129. Curcio A, Marotta R, Riedinger A, et al. Magnetic pH-responsive nanogels as multifunctional delivery tools for small interfering RNA (siRNA) molecules and iron oxide nanoparticles (IONPs). Chem Commun 2012;48(18):2400–2402. [DOI] [PubMed] [Google Scholar]
- 130. Gan Q, Lu X, Yuan Y, et al. A magnetic, reversible pH-responsive nanogated ensemble based on Fe3O4 nanoparticles-capped mesoporous silica. Biomaterials 2011;32(7):1932–1942. [DOI] [PubMed] [Google Scholar]
- 131. Gan Q, Lu X, Dong W, et al. Endosomal pH-activatable magnetic nanoparticle-capped mesoporous silica for intracellular controlled release. J Mater Chem 2012;22(31):15960–15968. [Google Scholar]
- 132. Xu H, Meng F, Zhong Z. Reversibly crosslinked temperature-responsive nano-sized polymersomes: Synthesis and triggered drug release. J Mater Chem 2009;19(24):4183–4190. [Google Scholar]
- 133. Du J-Z, Du X-J, Mao C-Q, et al. Tailor-made dual pH-sensitive polymer–doxorubicin nanoparticles for efficient anticancer drug delivery. J Am Chem Soc 2011;133(44):17560–17563. [DOI] [PubMed] [Google Scholar]
- 134. Sankaranarayanan J, Mahmoud EA, Kim G, et al. Multiresponse strategies to modulate burst degradation and release from nanoparticles. ACS Nano 2010;4(10):5930–5936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Li Y, Xiao W, Xiao K, et al. Well-defined, reversible boronate crosslinked nanocarriers for targeted drug delivery in response to acidic pH values and cis-diols. Angew Chem 2012;124(12):2918–2923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Liu T-Y, Hu S-H, Liu K-H, et al. Instantaneous drug delivery of magnetic/thermally sensitive nanospheres by a high-frequency magnetic field. Langmuir 2008;24(23):13306–13311. [DOI] [PubMed] [Google Scholar]
- 137. Chen C, Geng J, Pu F, et al. Polyvalent nucleic Acid/Mesoporous silica nanoparticle conjugates: Dual stimuli-responsive vehicles for intracellular drug delivery. Angew Chem Int Ed Engl 2011;50(4):882–886. [DOI] [PubMed] [Google Scholar]
- 138. Soppimath KS, Tan DW, Yang YY. pH-triggered thermally responsive polymer core–shell nanoparticles for drug delivery. Adv Mater 2005;17(3):318–323. [Google Scholar]
- 139. Soppimath KS, Liu LH, Seow WY, et al. Multifunctional core/shell nanoparticles self-assembled from pH-induced thermosensitive polymers for targeted intracellular anticancer drug delivery. Adv Funct Mater 2007;17(3):355–362. [Google Scholar]
- 140. Zhang L, Guo R, Yang M, et al. Thermo and pH dual-responsive nanoparticles for anti-cancer drug delivery. Adv Mater 2007;19(19):2988–2992. [Google Scholar]
- 141. Hoffmann A, Gross G. Tendon and ligament engineering: From cell biology to in vivo application. Regen Med 2006;1(4):563–574; doi: 10.2217/17460751.1.4.563 [DOI] [PubMed] [Google Scholar]
- 142. O'Brien M. Functional anatomy and physiology of tendons. Clin Sports Med 1992;11(3):505–520. [PubMed] [Google Scholar]
- 143. Millar NL, Reilly JH, Kerr SC, et al. Hypoxia: A critical regulator of early human tendinopathy. Ann Rheum Dis 2012;71(2):302–310; doi: 10.1136/ard.2011.154229 [DOI] [PubMed] [Google Scholar]
- 144. Rhatomy S, Utomo DN, Prakoeswa CRS, et al. Ligament/tendon culture under hypoxic conditions: A systematic review. Adv Pharm Bull 2021;11(4):595–600; doi: 10.34172/apb.2021.069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145. Sivakumar B, Kang N, Taylor P, et al. The role of hypoxia in rheumatoid tendon disease. Arthritis Res Ther 2005;7(1):P7; doi: 10.1186/ar1528 [DOI] [Google Scholar]
- 146. Zhao J, Zhang P, Qin L, et al. Hypoxia is essential for bone-tendon junction healing: The molecular biological evidence. Int Orthop 2011;35(6):925–928; doi: 10.1007/s00264-010-1157-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147. Järvinen TA. Neovascularisation in tendinopathy: From eradication to stabilisation? Br J Sports Med 2020;54(1):1–2; doi: 10.1136/bjsports-2019-100608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148. Zeng Y, Ma J, Zhan Y, et al. Hypoxia-activated prodrugs and redox-responsive nanocarriers. Int J Nanomedicine 2018;13:6551–6574; doi: 10.2147/ijn.S173431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149. Cheng R, Meng F, Ma S, et al. Reduction and temperature dual-responsive crosslinked polymersomes for targeted intracellular protein delivery. J Mater Chem 2011;21(47):19013–19020; doi: 10.1039/C1JM13536H [DOI] [Google Scholar]
- 150. Banstola A, Poudel K, Pathak S, et al. Hypoxia-mediated ROS amplification triggers mitochondria-mediated apoptotic cell death via PD-L1/ROS-responsive, dual-targeted, drug-laden thioketal nanoparticles. ACS Appl Mater Interfaces 2021;13(19):22955–22969; doi: 10.1021/acsami.1c03594 [DOI] [PubMed] [Google Scholar]
- 151. Wang K, Cheng L, He B, et al. Hypoxia inducible factor-1α mediates the mechanism of the Hedgehog pathway in tendinopathy repair by Asperosaponin VI. Regen Ther 2022;21:511–518; doi: 10.1016/j.reth.2022.10.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152. Lui PPY, Zhang X, Yao S, et al. Roles of oxidative stress in acute tendon injury and degenerative tendinopathy-A target for intervention. Int J Mol Sci 2022;23(7); doi: 10.3390/ijms23073571 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153. Meng J, Yu P, Tong J, et al. Hydrogen treatment reduces tendon adhesion and inflammatory response. J Cell Biochem 2018; doi: 10.1002/jcb.27441 [DOI] [PubMed] [Google Scholar]
- 154. Ponnapakkam T, Katikaneni R, Sakon J, et al. Treating osteoporosis by targeting parathyroid hormone to bone. Drug Discov Today 2014;19(3):204–208; doi: 10.1016/j.drudis.2013.07.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155. Ponnapakkam T, Katikaneni R, Miller E, et al. Monthly administration of a novel PTH-collagen binding domain fusion protein is anabolic in mice. Calcif Tissue Int 2011;88(6):511–520; doi: 10.1007/s00223-011-9485-1 [DOI] [PubMed] [Google Scholar]
- 156. Ponnapakkam T, Katikaneni R, Suda H, et al. A single injection of the anabolic bone agent, parathyroid hormone-collagen binding domain (PTH-CBD), results in sustained increases in bone mineral density for up to 12months in normal female mice. Calcif Tissue Int 2012;91(3):196–203; doi: 10.1007/s00223-012-9626-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157. San BH, Li Y, Tarbet EB, et al. Nanoparticle assembly and gelatin binding mediated by triple helical collagen mimetic peptide. ACS Appl Mater Interfaces 2016;8(31):19907–19915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158. Li Y, Foss CA, Summerfield DD, et al. Targeting collagen strands by photo-triggered triple-helix hybridization. Proc Natl Acad Sci U S A 2012;109(37):14767–14772; doi: 10.1073/pnas.1209721109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159. Millar NL, Murrell GA, McInnes IB. Inflammatory mechanisms in tendinopathy - towards translation. Nat Rev Rheumatol 2017;13(2):110–122; doi: 10.1038/nrrheum.2016.213 [DOI] [PubMed] [Google Scholar]
- 160. Tsuzaki M, Guyton G, Garrett W, et al. IL-1 beta induces COX2, MMP-1, -3 and -13, ADAMTS-4, IL-1 beta and IL-6 in human tendon cells. J Orthop Res 2003;21(2):256–264; doi: 10.1016/s0736-0266(02)00141-9 [DOI] [PubMed] [Google Scholar]
- 161. D'Onofrio N, Caraglia M, Grimaldi A, et al. Vascular-homing peptides for targeted drug delivery and molecular imaging: Meeting the clinical challenges. Biochim Biophys Acta 2014;1846(1):1–12; doi: 10.1016/j.bbcan.2014.03.004 [DOI] [PubMed] [Google Scholar]
- 162. Kjaer M. Role of extracellular matrix in adaptation of tendon and skeletal muscle to mechanical loading. Physiol Rev 2004;84(2):649–698; doi: 10.1152/physrev.00031.2003 [DOI] [PubMed] [Google Scholar]
- 163. Xu Y, Murrell GA. The basic science of tendinopathy. Clin Orthop Relat Res 2008;466(7):1528–1538; doi: 10.1007/s11999-008-0286-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164. Ashtari N, Jiao X, Rahimi-Balaei M, et al. Lysosomal acid phosphatase biosynthesis and dysfunction: A mini review focused on lysosomal enzyme dysfunction in brain. Curr Mol Med 2016;16(5):439–446; doi: 10.2174/1566524016666160429115834 [DOI] [PubMed] [Google Scholar]
- 165. Stromberg BV, Wood FM, Simmons DJ. Enzymatic changes in the healing rat achilles tendon. J Surg Res 1977;23(2):132–140; doi: 10.1016/0022-4804(77)90201-3 [DOI] [PubMed] [Google Scholar]
- 166. Zhang M, Hu W, Cai C, et al. Advanced application of stimuli-responsive drug delivery system for inflammatory arthritis treatment. Mater Today Bio 2022;14:100223; doi: 10.1016/j.mtbio.2022.100223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167. Van Hove A, Cui Z, Benoit DSW. Stimuli-responsive polymer delivery systems. In: Engineering Polymer Systems for Improved Drug Delivery (Bader RA, Putnam DA. eds.) Wiley: 2013; pp. 375–427. [Google Scholar]
- 168. Li D, Zhang R, Liu G, et al. Redox-responsive self-assembled nanoparticles for cancer therapy. Adv Healthc Mater 2020;9(20):e2000605; doi: 10.1002/adhm.202000605 [DOI] [PubMed] [Google Scholar]
- 169. Nguyen MM, Carlini AS, Chien MP, et al. Enzyme-responsive nanoparticles for targeted accumulation and prolonged retention in heart tissue after myocardial infarction. Adv Mater 2015;27(37):5547–5552; doi: 10.1002/adma.201502003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170. Jana BA, Shinde U, Wadhwani A. Synthetic enzyme-based nanoparticles act as smart catalyst for glucose responsive release of insulin. J Biotechnol 2020;324:1–6; doi: 10.1016/j.jbiotec.2020.09.023 [DOI] [PubMed] [Google Scholar]
- 171. Zuo YM, Yan X, Xue J, et al. Enzyme-responsive Ag nanoparticle assemblies in targeting antibacterial against methicillin-resistant Staphylococcus aureus. ACS Appl Mater Interfaces 2020;12(4):4333–4342; doi: 10.1021/acsami.9b22001 [DOI] [PubMed] [Google Scholar]
- 172. Tashjian RZ. Epidemiology, natural history, and indications for treatment of rotator cuff tears. Clin Sports Med 2012;31(4):589–604; doi: 10.1016/j.csm.2012.07.001 [DOI] [PubMed] [Google Scholar]
- 173. Banik B, Surnar B, Askins BW, et al. dual-targeted synthetic nanoparticles for cardiovascular diseases. ACS Appl Mater Interfaces 2020;12(6):6852–6862; doi: 10.1021/acsami.9b19036 [DOI] [PubMed] [Google Scholar]
- 174. Iyer R, Ramachandramoorthy H, Nguyen T, et al. Lung cancer targeted chemoradiotherapy via dual-stimuli responsive biodegradable core-shell nanoparticles. Pharmaceutics 2022;14(8); doi: 10.3390/pharmaceutics14081525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175. Zhang T, Ouyang H, Liu S, et al. pH/Thermosensitive dual-responsive hydrogel based sequential delivery for site-specific acute limb ischemia treatment. J Mater Chem B 2022;10(38):7836–7846; doi: 10.1039/d2tb00474g [DOI] [PubMed] [Google Scholar]
- 176. Sheu TJ, Schwarz EM, RJO'Keefe, et al. Use of a phage display technique to identify potential osteoblast binding sites within osteoclast lacunae. J Bone Miner Res 2002;17(5):915–922; doi: 10.1359/jbmr.2002.17.5.915 [DOI] [PubMed] [Google Scholar]
- 177. Pohlmann R, Krentler C, Schmidt B, et al. Human lysosomal acid phosphatase: Cloning, expression and chromosomal assignment. Embo J 1988;7(8):2343–2350; doi: 10.1002/j.1460-2075.1988.tb03078.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178. Polykandriotis E, Ruppe F, Niederkorn M, et al. Polytetrafluoroethylene (PTFE) suture vs fiberwire and polypropylene in flexor tendon repair. Arch Orthop Trauma Surg 2021;141(9):1609–1614; doi: 10.1007/s00402-021-03899-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179. Scheibel MT, Habermeyer P. A modified Mason-Allen technique for rotator cuff repair using suture anchors. Arthroscopy 2003;19(3):330–333; doi: 10.1053/jars.2003.50079 [DOI] [PubMed] [Google Scholar]
- 180. Tang JB. New Developments are improving flexor tendon repair. Plast Reconstr Surg 2018;141(6):1427–1437; doi: 10.1097/prs.0000000000004416 [DOI] [PubMed] [Google Scholar]
- 181. Tang JB, Lalonde D, Harhaus L, et al. Flexor tendon repair: Recent changes and current methods. J Hand Surg Eur Vol 2022;47(1):31–39; doi: 10.1177/17531934211053757 [DOI] [PubMed] [Google Scholar]
- 182. Zhou YL, Yang QQ, Zhang L, et al. Nanoparticle-coated sutures providing sustained growth factor delivery to improve the healing strength of injured tendons. Acta Biomater 2021;124:301–314; doi: 10.1016/j.actbio.2021.01.008 [DOI] [PubMed] [Google Scholar]
- 183. Isaac C, Gharaibeh B, Witt M, et al. Biologic approaches to enhance rotator cuff healing after injury. J Shoulder Elbow Surg 2012;21(2):181–190; doi: 10.1016/j.jse.2011.10.004 [DOI] [PubMed] [Google Scholar]
- 184. Johnson AJ, Bradsell H, Frank RM. Use of Injections and Biologics for the Nonoperative Treatment of Rotator Cuff Pathology. Clin Sports Med 2023;42(1):53–68; doi: 10.1016/j.csm.2022.08.002 [DOI] [PubMed] [Google Scholar]
- 185. O'Dowd A. Update on the use of platelet-rich plasma injections in the management of musculoskeletal injuries: A systematic review of studies from 2014 to 2021. Orthop J Sports Med 2022;10(12):23259671221140888; doi: 10.1177/23259671221140888 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186. Montgomery SR, Petrigliano FA, Gamradt SC. Failed rotator cuff surgery, evaluation and decision making. Clin Sports Med 2012;31(4):693–712; doi: 10.1016/j.csm.2012.07.006 [DOI] [PubMed] [Google Scholar]