Abstract
Pseudoexfoliation syndrome (PES) is one of the most common causes of open-angle glaucoma, with a higher risk of vision loss, a higher maximum and mean intraocular pressure (IOP) at diagnosis, and a wider range of IOP fluctuation compared to primary open-angle glaucoma. Patients with this syndrome have a ten-fold higher risk of developing glaucoma than the normal population. A definite diagnosis can be made by the observation of pseudoexfoliation material (PEM) on the anterior lens surface, ciliary processes, zonules, and iris. PEM deposits on the zonules may explain the clinically observed zonular weakness and lens subluxation or dislocation. An increased incidence of cataract development is also associated with PES. There is growing evidence for systemic associations of PES with peripheral, cardiovascular, and cerebrovascular system diseases, Alzheimer’s disease, hearing loss, and increased plasma homocysteine levels. Indications for surgery are markedly more common in patients with pseudoexfoliation glaucoma than primary open-angle glaucoma. The goal of this article is to review the latest perspectives on the clinical features, therapy, and systemic associations of this clinically and biologically challenging disease.
Keywords: Pseudoexfoliation, pseudoexfoliation syndrome, pseudoexfoliation glaucoma
Introduction
Pseudoexfoliation syndrome (PES) is the most common identified cause of open-angle glaucoma. Pseudoexfoliation is an independent risk factor for open-angle glaucoma in every country around the world. It is important to distinguish pseudoexfoliation glaucoma (PEG) from primary open-angle glaucoma (POAG). PEG is a severe type of glaucoma with a higher risk of vision loss, higher maximum and mean intraocular pressure (IOP) at diagnosis, and wider range of IOP fluctuation compared to POAG.1
PEG may cause increased outflow resistance as a result of progressive accumulation of pseudoexfoliation material (PEM) in the trabecular meshwork, which contributes to alteration of retrobulbar blood flow and optic nerve microvascular blood flow, as well as elastosis of the lamina cribrosa1.
A definite diagnosis can be made by observing PEM on the anterior lens surface. These deposits can be found initially on the ciliary processes, zonules, and iris. Deposits of pseudoexfoliation on the zonules may explain the clinically observed zonular weakness and lens subluxation or dislocation. An increased incidence of cataract development is also associated with PES1.
PES may be an important marker for cardiovascular and cerebrovascular diseases.2 There is growing evidence for systemic associations of PES with peripheral, cardiovascular, and cerebrovascular system diseases, Alzheimer’s disease, hearing loss, and increased plasma homocysteine levels.3,4
Clinical experiences show that controlling IOP is more difficult in PEG than in POAG. PEG patients with high IOP and advanced damage may benefit from a combination of anti-glaucoma medications as initial therapy. The indications for surgery are markedly more common in patients with PEG than in POAG.
The purpose of this article is to review current perspectives on the clinical features, therapy, and systemic associations of this clinically and biologically challenging disease.
Background
Pseudoexfoliation is a late-onset, stress-induced elastotic disorder that causes an aberrant extracellular fibrillar matrix material to accumulate in ocular tissues. PEG is glaucomatous optic neuropathy and IOP elevation associated with PES.
Pseudoexfoliation was first described by Lindberg in 1917 as grayish flecks on the pupillary border accompanied by chronic glaucoma.5 The source of this material was proposed by Vogt,6 who termed it “senile exfoliation” and suggested an origin from the lens. In 1954, the term “pseudoexfoliation” was suggested by Dvorak-Theobald7 to distinguish the disease from the exfoliation seen in glassblowers.
Epidemiology
There has been increasing interest in epidemiological studies focused on PES.16 According to these studies, this condition may be seen in between 10% and 20% of people in the general population above the age of 60. An estimated 70 million people may have PES worldwide. This syndrome occurs more frequently in Finland, Scandinavia, Greece, and Türkiye than in other countries. The differences in the rates are attributed to genetic and environmental or unknown factors. The worldwide prevalence of PES is increasing. Many patients with this condition could be undiagnosed.
PES is age-dependent, and the prevalence increases with age. Pseudoexfoliation affects both sexes, but there are conflicting results concerning its sex distribution. Some studies have found equal numbers among men and women.8 Others have found a greater prevalence in females, while other investigators have found a greater prevalence in males.9,10,11
PES is one of the most prevalent causes of open-angle glaucoma, and patients with this syndrome have a ten-fold higher risk of developing glaucoma than the normal population. Pseudoexfoliation is an independent risk factor for open-angle glaucoma in every country, although reported prevalence rates vary. Population studies have suggested an incidence between 20% and 60% of all open-angle glaucoma. This syndrome is not only a cause of glaucoma but also a risk factor for glaucoma progression.
PES is often a bilateral, asymmetric disease. Both eyes may be involved histopathologically, while the clinical presentation may be seen in only one eye.12,13,14
As a result, PES and glaucoma are regarded as public health problems in older age.
Genetics
PEG is a complex genetic disease. Both genetic and non-genetic variables have a role in the etiopathogenesis.15,16,17 The lysyl oxidase like 1 (LOXL1) gene has been discovered as a significant genetic risk factor for both PES and PEG.18 Lysyl oxidases are essential for the synthesis and stability of elastic fibers. Increasing evidence suggests that LOXL1 is markedly dysregulated depending on the stage of fibrosis. In the early phases of pseudoexfoliation, LOXL1 is involved in the synthesis and aggregation of pseudoexfoliation fiber deposits, and in the advanced stages may affect elastin metabolism. As a result, it has been suggested that pseudoexfoliation is a type of elastosis caused by a high amount of elastic microfibrillar materials like fibrillin.19 Other candidate genes have also been reported. CACNA1A, POMP, and SEMA6A variants have been linked to extracellular matrix metabolism, ubiquitin-proteasome system, calcium signaling, and lipid biosynthesis in pseudoexfoliation pathogenesis, increasing the disease risk.20
Other non-genetic factors related to pseudoexfoliation, including oxidative stress and low-grade inflammation, can influence the expression of LOXL1.16,17 A detailed study of the gene maps of this complicated disease, as well as the functional effects and molecular mechanisms of these loci, will shed light on the disease’s pathophysiology.
Clinical Manifestations
It is important to emphasize that PES is a significant ocular problem. Most patients with pseudoexfoliation are asymptomatic.
Older patients should be carefully examined for diagnosis of pseudoexfoliation by biomicroscopy. Pupillary dilation is necessary to detect deposits on the lens surface. Classic signs of PES include fluffy, white deposits at the anterior lens surface and pupillary margins.
Pseudoexfoliation may also appear on the zonular fibers, ciliary processes, corneal endothelium, trabecular meshwork, intraocular lens, and anterior vitreous face in cases of aphakia.13,21,22
The bush-like fibrillar PEM may be observed in light microscopy, and the electron microscopic presentation of PEM in ocular and extraocular tissues has been demonstrated by Schlötzer-Schrehardt.23,24
Ultrasound-biomicroscopy studies on morphological changes in the anterior segment of eyes with PES revealed zonular weakness, thickened lens, narrow anterior chamber, and occludable angles.25,26 Similar morphological changes in affected and fellow eyes were observed in another study comparing the involved and non-involved fellow eyes of PES patients.27 A study using anterior-segment optical coherence tomography on patients with unilateral PES found that eyes with PES had a narrower anterior chamber angle, reduced angle widening during pupil motions, and greater iridolenticular contact and iris curve compared to healthy subjects’ eyes.28 Furthermore, non-involved fellow eyes presented similar characteristics to some extent.27
Intraocular pressure
Eyes with PES were shown to have higher IOP than non-involved fellow eyes.11 This difference is approximately 2 mmHg. Diurnal IOP fluctuation is also greater in patients with PES than in non-pseudoexfoliation subjects.29
IOP may increase after pharmacologic dilation. Eyes with pseudoexfoliation should be measured after dilation, particularly due to significant pigment release.30 Initial IOP is the strongest risk factor for developing PEG.31,32
Tear film
PEM is associated with reduction in tear secretion and tear film stability.33,34,35 A study demonstrated that tear osmolarity in both eyes of clinically unilateral PES patients is higher compared to normal subjects.36
Schirmer and tear film break-up time test results were decreased in PES and PEG groups compared to healthy controls.36 Additionally, unilateral pseudoexfoliation was found to be associated with significant loss of meibomian gland area and higher meiboscores in both eyes.37
Cornea
Small, fluffy, white pseudoexfoliation deposits may be observed on the corneal endothelium in patients with pseudoexfoliation (Figure 1) together with some pigment deposition on the central corneal endothelium (Figure 2).
Figure 1.
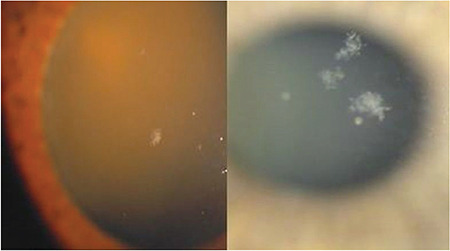
Pseudoexfoliation material deposition on the corneal endothelial surface in pseudoexfoliation syndrome
Figure 2.
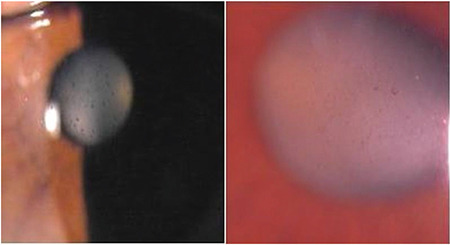
Pigmentary deposits on the corneal endothelial surface in pseudoexfoliation
Corneal endothelial cells may show changes in number and morphology. Patients with pseudoexfoliation have reduced basal epithelial and endothelial cell densities.21,38 The damaged corneal endothelium in eyes with pseudoexfoliation can result in endothelial decompensation.1,24,39 Endothelial cell polymegathism and pleomorphism in pseudoexfoliation keratopathy with glaucoma is more frequent than with cataract.40 Corneal sensitivity is markedly lower in eyes with pseudoexfoliation and is correlated with the decreased basal epithelial cell and subbasal nerve densities.41 Corneal thickness varies.
Iris
Deposits of PEM on the pupillary border and stroma and muscle tissues of the iris are among the changes seen anterior to the lens (Figure 3). Pseudoexfoliation is associated with pigment loss from the pigment epithelium over the iris sphincter, loss of pupillary ruff, and transillumination defect of the pupillary border.22 The iris appears to be more rigid and often dilates poorly. Iris blood vessels may become damaged and obliterated, leading to hypoperfusion of the iris. In the late stages of pseudoexfoliation, vessel wall cells may be totally destroyed.1 Reduced oxygen in the anterior chamber is an important consequence of iris vasculopathy and chronic blood-aqueous barrier breakdown in PES. Posterior synechia may develop and contribute to insufficient pupil dilation (Figure 4).24
Figure 3.

White deposits or flakes and “moth-eaten” pattern on the pupillary margin
Figure 4.
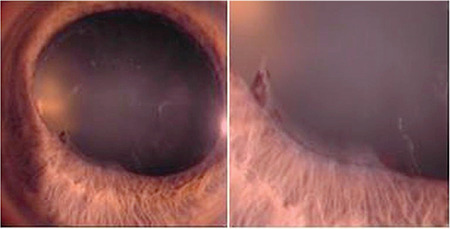
Pigment loss from the peripupillary pigment epithelium of the iris and synechiae between the pupillary border of the iris and anterior lens surface
Lens
PES can be diagnosed by the observation of deposits of white material on the anterior lens surface. The epicapsular deposition appears as a homogenous diffuse ground-glass or matte film on the lens surface. As the epicapsular layer thickens, focal defects occur in the mid-peripheral zone. The classic appearance consists of a central disk, peripheral zone, and clear intermediate area. Eventually, pseudoexfoliation deposition with various appearances can be observed on the anterior lens surface (Figure 5). PEM can also be found on the surface of an implanted posterior chamber intraocular lens and the hyaloid face.1,24,39
Figure 5.
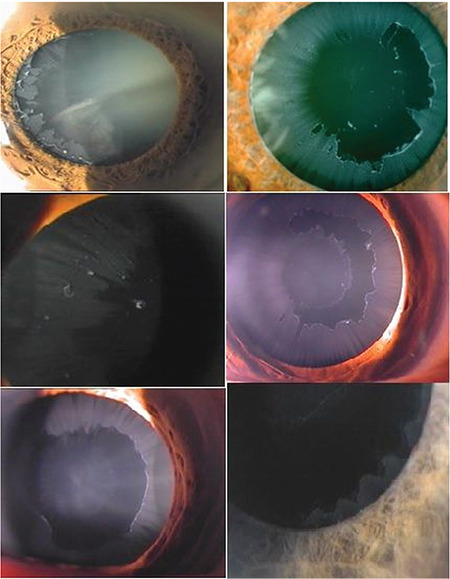
Pseudoexfoliation deposition on the anterior lens surface has a variable presentation. The classic appearance consists of a central disk, peripheral zone, and clear intermediate area separating the two areas
Anterior chamber angle
The defining gonioscopic feature of PES is increased trabecular meshwork pigmentation, which often manifests as patchy involvement.42 The pigmentation is more prominent inferiorly. It is not as dense as that seen in pigmentary glaucoma (Figure 6). Small dust-like white pseudoexfoliation deposits may be observed at the angle.
Figure 6.

Increased trabecular pigmentation may be seen in the anterior chamber angle during gonioscopy, usually on Schwalbe’s line
In patients with pseudoexfoliation, gonioscopically determined angle pigmentation correlates more significantly with a higher presenting IOP than with the quantity of PEM on the anterior lens capsule.42 The involved eye may have a narrower angle than the non-involved fellow eye.28
Zonules
Small dots and flakes of pseudoexfoliation deposits can be found earliest on the ciliary processes and zonules. Deposits on zonules may explain the clinically observed zonular weakness and lens subluxation or dislocation (Figure 7).39 Deposition of PEM on the zonules can be determined by high-resolution ultrasound biomicroscopic examination.
Figure 7.
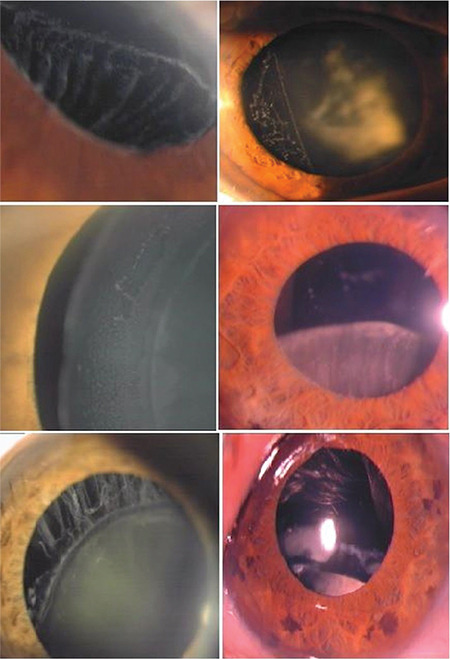
Patients with pseudoexfoliation can develop zonular weakness and lens subluxation or luxation caused by the progressive accumulation of pseudoexfoliation material
Ocular Associations
Cataract
Progressive opacification of the lens is associated with PES (Figure 8).43 Nuclear sclerosis is the most frequently seen cataract with pseudoexfoliation. Cataract development can be explained by a setting of ocular ischemia, elevated growth factor levels, or reduced ascorbic acid levels in the aqueous humor.24,44
Figure 8.
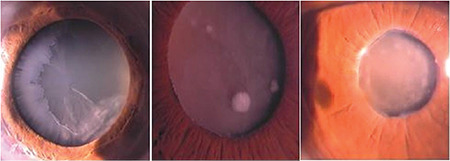
Cataract development is associated with pseudoexfoliation syndrome
Cataract surgery in these patients predisposes to intraoperative and postoperative complications including posterior capsule rupture, zonular rupture, vitreous loss, increased postoperative inflammation, anterior capsule contraction, need for increased secondary intraocular lens implantation, and increased posterior capsule opacification.45,46,47 However, recent advancements in cataract surgery techniques and instruments have substantially enhanced operative handling of patients with pseudoexfoliation. Final success rates for PES patients undergoing cataract surgery may even be comparable to those for non-PES patients with current preoperative, intraoperative, and postoperative techniques.48,49
It is important that a careful preoperative clinical examination be performed after pupillary dilation. Cataract surgeons have to use more complicated instruments due to zonular weakness, inadequate pupil dilation, and blood-aqueous barrier breakdown.48,50
Phacoemulsification provides several advantages. Advanced surgical techniques can decrease the earlier complication rates associated with cataract surgery in PES.51,52 Pupil dilation techniques and devices can be used to expand the pupil. A capsular tension ring may be useful in cases of significant zonular weakness.47 A large-optic intraocular lens is recommended to compensate for the possibility of intraocular lens decentration. Postoperative complications are more common and include early postoperative IOP elevation, prolonged postoperative inflammation, posterior synechiae, and macular edema.50 Foveal thickness in patients with PEG may be increased after uneventful phacoemulsification.52
Pseudoexfoliation and zonular laxity at the time of surgery are associated with late intraocular lens dislocation and anterior capsule contraction (phimosis) (Figure 9).55,56
Figure 9.
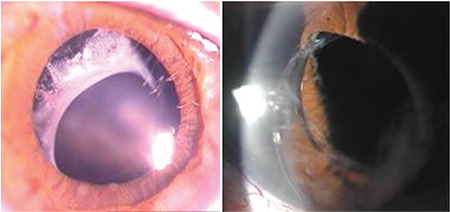
Capsule contraction syndrome and late anterior intraocular lens dislocation in a patient with pseudoexfoliation
PEM may be found on the anterior vitreous face and the anterior surface of the intraocular lens years after cataract extraction. Rarely, a pattern of radial striations on the anterior surface of the intraocular lens may resemble the classical pattern on the crystalline lens (Figure 10).
Figure 10.
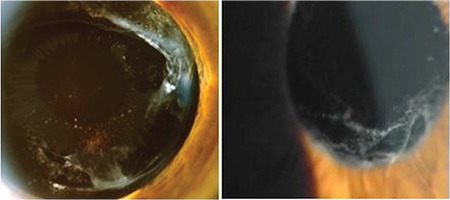
Following cataract surgery, pseudoexfoliation material deposition can be seen on the anterior vitreous face and intraocular lens
Retina
PES without glaucoma may be associated with a thinner retina nerve fiber layer thickness compared to that of age-matched control subjects and non-involved fellow eyes.57,58 Central retinal vein occlusion may be more prevalent in patients with PEG.59
Recently, studies using optical coherence tomography angiography demonstrated that decreased peripapillary and macular vascular density was found in pseudoexfoliation patients relative to the control group, suggesting that a vascular component, including optic nerve hypoperfusion, may be involved in the etiopathogenesis of pseudoexfoliation.60,61,62
The prevalence of epiretinal membrane was reported to be significantly higher in patients with PEG (19.0%) than in age-matched healthy controls (2.4%) and patients with primary open-angle glaucoma (4.1%).63 Furthermore, more eyes with PEG had epiretinal membrane deterioration than eyes without glaucomatous alteration.64 Both incomplete and complete posterior vitreous detachment were also more frequent in eyes with pseudoexfoliation than in fellow eyes or control eyes.65
Associated Systemic Findings
PES appears to be a systemic process. Pseudoexfoliation fibers were found in autopsy tissue specimens of skin, heart, lungs, liver, kidney, and cerebral meninges in addition to the intraocular tissues.23,66
Cardiovascular and cerebrovascular associations reported in PES include a history of angina pectoris, systemic hypertension, stroke, asymptomatic myocardial dysfunction, impaired systemic endothelial functions, transient ischemic attacks, Alzheimer’s disease, and neurosensorial hearing loss.2,4,67,68,69,70,71,72,73,74,75,76
PES may be a manifestation of systemic vascular disease. Middle cerebral artery blood flow velocities appear to be diminished.77 A higher prevalence of silent ischemic brain lesions and white matter abnormalities were found by magnetic resonance imaging and diffusion tensor imaging.78,79
Several studies have found a relation between pseudoexfoliation and hyperhomocysteinemia which may help to explain the elevated risk of vascular diseases in PES patients.80,81,82,83
Increased serum antiphospholipid antibodies, a risk factor for cardiovascular and cerebrovascular disease, are more frequently seen in patients with pseudoexfoliation and glaucoma than in healthy controls and patients with POAG.3 However, other studies found no correlation with cardiovascular and cerebrovascular diseases.84,85
Pseudoexfoliation Glaucoma
PES is the most important risk factor for the development of secondary open-angle glaucoma. Approximately 30% to 50% of patients with pseudoexfoliation develop glaucoma.13 IOP levels and degree of pupil dilation can be important factors for developing glaucoma. At the time of diagnosis, 10-25% of patients with pseudoexfoliation have either glaucoma or elevated IOP.13,86 Patients with PES should be followed up regularly and at short intervals with glaucoma screening tests or therapeutic interventions when appropriate.
Differences between pseudoexfoliation and primary open-angle glaucoma
In the past, the generally accepted clinical signs of PEG were identical to those of POAG. Now, it is important to distinguish PEG from POAG. All patients with glaucoma should be carefully examined for the clinical signs of pseudoexfoliation after pupillary dilation. PEG is clinically differentiated from POAG by the following features:
1. IOP at diagnosis is higher in PEG than in POAG.
2. IOP in PEG may rise above 50 mmHg without acute angle-closure glaucoma.
3. IOP fluctuations are wider in PEG than in POAG. A single IOP measurement is not sufficient for assessing IOP levels.
4. PEG patients may have unilateral or bilateral involvement, but asymmetric involvement is a typical feature.
5. PEG presents later than POAG.
6. PEG has more severe mean visual field defects and optic nerve head cupping than POAG.
7. PEG has more serious progression than POAG.
8. PEG is associated with a higher risk of blindness.
9. Glaucomatous damage in pseudoexfoliation patients is more related to IOP than in POAG patients.
10. PEG is more difficult to treat than POAG.
11. The IOP reduction with medical therapy is higher in PEG than in POAG.
12. Cataract formation and complications are more serious in patients with pseudoexfoliation.13,50,87
Pathogenesis of pseudoexfoliation glaucoma
In recent decades, many clinical findings have contributed to our understanding of the pathomechanisms underlying PEG. Increased outflow resistance is related to the progressive accumulation of PEM in the trabecular meshwork and Schlemm’s canal cells. Subsequent degenerative changes in Schlemm’s canal and juxtacanalicular tissues are causes of elevated IOP.24
The additional pathogenetic factor contributing to pressure elevation is melanin dispersion.14,24
IOP-independent factors may contribute to glaucomatous damage as well. Reported IOP-independent factors include impaired ocular and retrobulbar blood flow velocities and increased accumulation of elastic fibers in the lamina cribrosa.88,89,90
Types of glaucoma
IOP may rise over 50 mmHg despite a wide open angle. Angle-closure glaucoma may be associated with PES (Figure 11). It is a relatively rare entity. Chronic or acute angle-closure glaucoma may occur. Pseudoexfoliation is known to cause zonular weakness, anterior lens subluxation or dislocation, posterior synechia and increased iris rigidity, and occludable angles.13,91
Figure 11.
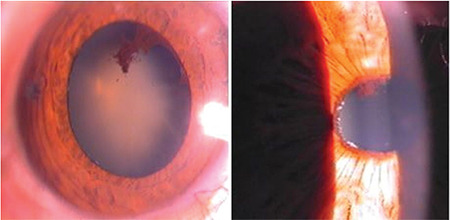
Angle-closure glaucoma in patients with pseudoexfoliation
In addition, neovascular glaucoma may develop after central retinal vein occlusion with PEG (Figure 12).
Figure 12.
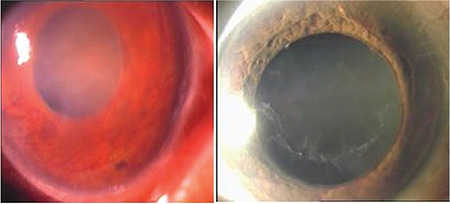
Neovascular glaucoma and pseudoexfoliation
Prognosis
The time of conversion from PES to glaucoma may take years. The risk of developing glaucoma is cumulative and IOP is an important risk factor. PEG has a more severe clinical course and worse prognosis than POAG. Patients should be monitored at regular intervals.
Treatment of PEG
Patients with PES and no evidence of glaucoma are generally not treated, but they should be followed every six months. IOP measurements should be taken at different times of the day to determine diurnal fluctuation.
The general principles of treatment are not different from POAG but medical treatment failure in PEG is more common than in POAG patients. A general approach would be the use of medical treatment first, laser therapy second, and surgical treatment third.
Medical therapy
Patients with PEG often have a poorer response to medical therapy than patients with POAG. Adequate treatment requires a target IOP of 17 mmHg or lower to prevent or slow progressive damage.93
Prostaglandin analogs are increasingly used as the first choice of monotherapy because they are applied once daily, reduce IOP through improved outflow, and have a very low rate of systemic side effects. Travoprost and bimatoprost may provide better IOP reduction than latanoprost.94,95
It is often difficult to achieve target IOP with monotherapy. To prevent further glaucomatous progression, a fixed combination may be necessary as initial therapy. In a comparison of travoprost, latanoprost, and dorzolamide/timolol fixed combination, IOP lowering ranged from 8 to 11 mmHg.96 Diurnal IOPs may be comparable with dorzolamide/timolol fixed combination and brimonidine/timolol fixed combination as first-line therapy.97 Travoprost/timolol fixed combination may provide greater reduction compared with latanoprost/timolol fixed combination.98 Bimatoprost/timolol fixed combination may provide greater reduction (10.2 mmHg) compared with bimatoprost alone (8.1 mmHg).99 Poor medication compliance is common in a large proportion of PEG patients under treatment and can lead to severe deterioration of vision. Failure of optimal medical therapy occurs earlier than in open-angle glaucoma patients.
Laser therapy
Selective laser trabeculoplasty can be effective.100 However, lower energy settings are required due to the increased pigmentation. Additionally, the effectiveness of treatment was shown to diminish over time, and after 18 months it was still effective in only 64% of patients.101
Surgery
If medication and laser therapy fail to control the progression of glaucoma, surgery may be performed with comparable success rates to POAG. Because patients with PEG have higher IOP at diagnosis, they tend to undergo glaucoma filtering surgery more frequently than patients with POAG.14
Trabeculectomy with mitomycin-C has better IOP control than successful maximal medical therapy in advanced disease.94,102 Postoperative inflammatory responses, fibrinous reactions, and posterior synechia formation are higher in PEG patients.
Combined cataract extraction and trabeculectomy are performed more frequently for managing patients with visually significant cataract and PEG than POAG. Furthermore, it has been shown that phacotrabeculectomy has similar success rates as trabeculectomy and can be as safe and effective as trabeculectomy in the long-term follow-up of both PEG and POAG patients.103 Non-penetrating glaucoma surgery may avoid some of the complications associated with trabeculectomy. IOP reduction may be less than with perforating surgery.104,105,106
Recently, minimally invasive glaucoma surgery offers results in patients with mild to moderate glaucoma with the advantage of a safe risk profile.107,108 Studies have also shown that gonioscopy-assisted transluminal trabeculotomy provides effective IOP reduction in PEG.109,110
Conclusions
PES and glaucoma are public health problems in older age worldwide. In recent years, the expansion in knowledge of the epidemiology, pathogenesis, and genetics of PES and glaucoma has provided important insights for understanding this disease and future treatment.
Pseudoexfoliation is an age-dependent and stress-induced extracellular fibrotic matrix disorder in which the oxidative-antioxidative balance is disturbed. LOXL1 is involved in the pathogenesis. Patients with PES have a higher risk of cardiovascular and cerebrovascular diseases.
The presence of pseudoexfoliation is an important risk factor for glaucoma and cataract. It is necessary to distinguish PEG from POAG. Medical management is more difficult and surgery is required more frequently. Early diagnosis, appropriate treatment and more frequent examinations appear to prevent the progression of visual field and vision loss.
In the future, new research can increase our understanding of the epidemiology, pathogenesis, genetics, and classifications of this disease. New therapeutic approaches will be considered for its management.
Footnotes
Ethics
Authorship Contributions
Concept: N.Y., Design: N.Y., Data Collection or Processing: N.Y., B.Y.T., Analysis or Interpretation: N.Y., B.Y.T., Literature Search: N.Y., B.Y.T., Writing: N.Y., B.Y.T.
Conflict of Interest: No conflict of interest was declared by the authors.
Financial Disclosure: The authors declared that this study received no financial support.
References
- 1.Ritch R, Schlötzer-Schrehardt U. Exfoliation syndrome. Surv Ophthalmol. 2001;45:265–315. doi: 10.1016/s0039-6257(00)00196-x. [DOI] [PubMed] [Google Scholar]
- 2.Mitchell P, Wang JJ, Smith W. Association of pseudoexfoliation syndrome with increased vascular risk. Am J Ophthalmol. 1997;124:685–687. doi: 10.1016/s0002-9394(14)70908-0. [DOI] [PubMed] [Google Scholar]
- 3.Altintas O, Yuksel N, Sonmez GT, Ozkan B, Altintas L, Caliskan Ş, Caglar Y. Serum antiphospholipid antibody levels in pseudoexfoliation. J Glaucoma. 2012;21:326–330. doi: 10.1097/IJG.0b013e31821206cd. [DOI] [PubMed] [Google Scholar]
- 4.Praveen MR, Shah SK, Vasavada AR, Diwan RP, Shah SM, Zumkhawala BR, Thomas R. Pseudoexfoliation as a risk factor for peripheral vascular disease: a case-control study. Eye (Lond). 2011;25:174–179. doi: 10.1038/eye.2010.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lindberg JG. Clinical investigations on depigmentation of the pupillary border and translucency of the iris in cases of senile cataract and in normal eyes in elderly persons. Acta Ophthalmol Suppl (1985). 1989;190:1–96. [PubMed] [Google Scholar]
- 6.Vogt A. Ein neues Spaltlampenbild des Pupillargebietes: Hellblauer Pupillarsaumfilz mit Hautchenbildung aus der Linsenvorderkapsel. Kin Monatsbl Atigenheilkd. 1925;75:1–12. [Google Scholar]
- 7.Dvorak-Theobald G. Pseudo-exfoliation of the lens capsule: relation to true exfoliation of the lens capsule as reported in the literature and role in the production of glaucoma capsulocuticulare. Am J Ophthalmol. 1954;37:1–12. [PubMed] [Google Scholar]
- 8.Cumurcu T, Kilic R, Yologlu S. The frequency of pseudoexfoliation syndrome in the middle Black Sea region of Turkey. Eur J Ophthalmol. 2020;20:1007–1011. doi: 10.1177/112067211002000621. [DOI] [PubMed] [Google Scholar]
- 9.Forsius H. Exfoliation syndrome in various ethnic populations. Acta Ophthalmol Suppl (1985). 1988;184:71–85. doi: 10.1111/j.1755-3768.1988.tb02633.x. [DOI] [PubMed] [Google Scholar]
- 10.Ringvold A, Blika S, Elsås T, Guldahl J, Brevik T, Hesstvedt P, Johnsen H, Hoff K, Høisen H, Kjørsvik S, et al. The Middle-Norway eye-screening study. I. Epidemiology of the pseudo-exfoliation syndrome. Acta Ophthalmol (Copenh). 1988;66:652–658. doi: 10.1111/j.1755-3768.1988.tb04056.x. [DOI] [PubMed] [Google Scholar]
- 11.Anastasopoulos E, Topouzis F, Wilson MR, Harris A, Pappas T, Yu F, Koskosas A, Founti P, Coleman AL. Characteristics of pseudoexfoliation in the Thessaloniki Eye Study. J Glaucoma. 2011;20:160–166. doi: 10.1097/IJG.0b013e3181d9d8bd. [DOI] [PubMed] [Google Scholar]
- 12.Mitchell P, Wang JJ, Hourihan F. The relationship between glaucoma and pseudoexfoliation: the Blue Mountains Eye Study. Arch Ophthalmol. 1999;117:1319–1324. doi: 10.1001/archopht.117.10.1319. [DOI] [PubMed] [Google Scholar]
- 13.Ritch R. Exfoliation syndrome-the most common identifiable cause of openangle glaucoma. J Glaucoma. 1994;3:176–177. [PubMed] [Google Scholar]
- 14.Vesti E, Kivelä T. Exfoliation syndrome and exfoliation glaucoma. Prog Retin Eye Res. 2000;19:345–368. doi: 10.1016/s1350-9462(99)00019-1. [DOI] [PubMed] [Google Scholar]
- 15.Koliakos GG, Konstas AG, Schlötzer-Schrehardt U, Bufidis T, Georgiadis N, Ringvold A. Ascorbic acid concentration is reduced in the aqueous humor of patients with exfoliation syndrome. Am J Ophthalmol. 2002;134:879–883. doi: 10.1016/s0002-9394(02)01797-x. [DOI] [PubMed] [Google Scholar]
- 16.Schlötzer-Schrehardt U. Molecular pathology of pseudoexfoliation syndrome/glaucoma--new insights from LOXL1 gene associations. Exp Eye Res. 2009;88:776–785. doi: 10.1016/j.exer.2008.08.012. [DOI] [PubMed] [Google Scholar]
- 17.Schlötzer-Schrehardt U. Genetics and genomics of pseudoexfoliation syndrome/glaucoma. Middle East Afr J Ophthalmol. 2011;18:30–36. doi: 10.4103/0974-9233.75882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Thorleifsson G, Magnusson KP, Sulem P, Walters GB, Gudbjartsson DF, Stefansson H, Jonsson T, Jonasdottir A, Jonasdottir A, Stefansdottir G, Masson G, Hardarson GA, Petursson H, Arnarsson A, Motallebipour M, Wallerman O, Wadelius C, Gulcher JR, Thorsteinsdottir U, Kong A, Jonasson F, Stefansson K. Common sequence variants in the LOXL1 gene confer susceptibility to exfoliation glaucoma. Science. 2007;317:1397–1400. doi: 10.1126/science.1146554. [DOI] [PubMed] [Google Scholar]
- 19.Ritch R, Schlötzer-Schrehardt U. Exfoliation (pseudoexfoliation) syndrome: toward a new understanding. Proceedings of the First International Think Tank. Acta Ophthalmol Scand. 2001;79:213–217. doi: 10.1034/j.1600-0420.2001.079002213.x. [DOI] [PubMed] [Google Scholar]
- 20.Schlötzer-Schrehardt U, Khor CC. Pseudoexfoliation syndrome and glaucoma: from genes to disease mechanisms. Curr Opin Ophthalmol. 2021;32:118–128. doi: 10.1097/ICU.0000000000000736. [DOI] [PubMed] [Google Scholar]
- 21.Naumann GO, Schlötzer-Schrehardt U. Keratopathy in pseudoexfoliation syndrome as a cause of corneal endothelial decompensation: a clinicopathologic study. Ophthalmology. 2000;107:1111–1124. doi: 10.1016/s0161-6420(00)00087-7. [DOI] [PubMed] [Google Scholar]
- 22.Prince AM, Ritch R. Clinical signs of the pseudoexfoliation syndrome. Ophthalmology. 1986;93:803–807. doi: 10.1016/s0161-6420(86)33664-9. [DOI] [PubMed] [Google Scholar]
- 23.Schlötzer-Schrehardt UM, Koca MR, Naumann GO, Volkholz H. Pseudoexfoliation syndrome. Ocular manifestation of a systemic disorder? Arch Ophthalmol. 1992;110:1752–1756. doi: 10.1001/archopht.1992.01080240092038. [DOI] [PubMed] [Google Scholar]
- 24.Schlötzer-Schrehardt U, Naumann GO. Ocular and systemic pseudoexfoliation syndrome. Am J Ophthalmol. 2006;141:921–937. doi: 10.1016/j.ajo.2006.01.047. [DOI] [PubMed] [Google Scholar]
- 25.Ritch R, Vessani RM, Tran HV, Ishikawa H, Tello C, Liebmann JM. Ultrasound biomicroscopic assessment of zonular appearance in exfoliation syndrome. Acta Ophthalmol Scand. 2007;85:495–499. doi: 10.1111/j.1600-0420.2007.00961.x. [DOI] [PubMed] [Google Scholar]
- 26.Guo S, Gewirtz M, Thaker R, Reed M. Characterizing pseudoexfoliation syndrome through the use of ultrasound biomicroscopy. J Cataract Refract Surg. 2006;32:614–617. doi: 10.1016/j.jcrs.2006.01.015. [DOI] [PubMed] [Google Scholar]
- 27.Sbeity Z, Dorairaj SK, Reddy S, Tello C, Liebmann JM, Ritch R. Ultrasound biomicroscopy of zonular anatomy in clinically unilateral exfoliation syndrome. Acta Ophthalmol. 2008;86:565–568. doi: 10.1111/j.1600-0420.2007.01105.x. [DOI] [PubMed] [Google Scholar]
- 28.Zheng X, Sakai H, Goto T, Namiguchi K, Mizoue S, Shiraishi A, Sawaguchi S, Ohashi Y. Anterior segment optical coherence tomography analysis of clinically unilateral pseudoexfoliation syndrome: evidence of bilateral involvement and morphologic factors related to asymmetry. Invest Ophthalmol Vis Sci. 2011;52:5679–5684. doi: 10.1167/iovs.11-7274. [DOI] [PubMed] [Google Scholar]
- 29.Altintas O, Yuksel N, Karabas VL, Caglar Y. Diurnal intraocular pressure variation in pseudoexfoliation syndrome. Eur J Ophthalmol. 2004;14:495–500. doi: 10.5301/EJO.2008.5057. [DOI] [PubMed] [Google Scholar]
- 30.Shihadeh WA, Ritch R, Scharf B, Liebmann JM. Delayed intraocular pressure elevation after pupillary dilation in exfoliation syndrome. Acta Ophthalmol. 2011;89:560–562. doi: 10.1111/j.1755-3768.2009.01744.x. [DOI] [PubMed] [Google Scholar]
- 31.Jeng SM, Karger RA, Hodge DO, Burke JP, Johnson DH, Good MS. The risk of glaucoma in pseudoexfoliation syndrome. J Glaucoma. 2007;16:117–121. doi: 10.1097/01.ijg.0000243470.13343.8b. [DOI] [PubMed] [Google Scholar]
- 32.Topouzis F, Wilson MR, Harris A, Founti P, Yu F, Anastasopoulos E, Pappas T, Koskosas A, Salonikiou A, Coleman AL. Risk factors for primary open-angle glaucoma and pseudoexfoliative glaucoma in the Thessaloniki eye study. Am J Ophthalmol. 2011;152:219–228.e1. doi: 10.1016/j.ajo.2011.01.032. [DOI] [PubMed] [Google Scholar]
- 33.Ritch R. Exfoliation syndrome. Curr Opin Ophthalmol. 2001;12:124–130. doi: 10.1097/00055735-200104000-00008. [DOI] [PubMed] [Google Scholar]
- 34.Kozobolis VP, Christodoulakis EV, Naoumidi II, Siganos CS, Detorakis ET, Pallikaris LG. Study of conjunctival goblet cell morphology and tear film stability in pseudoexfoliation syndrome. Graefes Arch Clin Exp Ophthalmol. 2004;242:478–483. doi: 10.1007/s00417-004-0865-3. [DOI] [PubMed] [Google Scholar]
- 35.Akdemir MO, Kirgiz A, Ayar O, Kaldirim H, Mert M, Cabuk KS, Taskapili M. The Effect of Pseudoexfoliation and Pseudoexfoliation Induced Dry Eye on Central Corneal Thickness. Curr Eye Res. 2016;41:305–310. doi: 10.3109/02713683.2015.1030505. [DOI] [PubMed] [Google Scholar]
- 36.Öncel BA, Pinarci E, Akova YA. Tear osmolarity in unilateral pseudoexfoliation syndrome. Clin Exp Optom. 2012;95:506–509. doi: 10.1111/j.1444-0938.2011.00683.x. [DOI] [PubMed] [Google Scholar]
- 37.Sahin Atik S, Altın Ekin M. The role of Meibomian glands on the development of dry eye disease in patients with unilateral pseudoexfoliation. J Fr Ophtalmol. 2022;45:915–920. doi: 10.1016/j.jfo.2022.03.004. [DOI] [PubMed] [Google Scholar]
- 38.Inoue K, Okugawa K, Oshika T, Amano S. Morphological study of corneal endothelium and corneal thickness in pseudoexfoliation syndrome. Jpn J Ophthalmol. 2003;47:235–239. doi: 10.1016/s0021-5155(03)00022-4. [DOI] [PubMed] [Google Scholar]
- 39.Naumann GO, Schlötzer-Schrehardt U, Küchle M. Pseudoexfoliation syndrome for the comprehensive ophthalmologist. Intraocular and systemic manifestations. Ophthalmology. 1998;105:951–968. doi: 10.1016/S0161-6420(98)96020-1. [DOI] [PubMed] [Google Scholar]
- 40.Wali UK, Bialasiewicz AA, Rizvi SG, Al-Belushi H. In vivo morphometry of corneal endothelial cells in pseudoexfoliation keratopathy with glaucoma and cataract. Ophthalmic Res. 2009;41:175–179. doi: 10.1159/000210831. [DOI] [PubMed] [Google Scholar]
- 41.Zheng X, Shiraishi A, Okuma S, Mizoue S, Goto T, Kawasaki S, Uno T, Miyoshi T, Ruggeri A, Ohashi Y. In vivo confocal microscopic evidence of keratopathy in patients with pseudoexfoliation syndrome. Invest Ophthalmol Vis Sci. 2011;52:1755–1761. doi: 10.1167/iovs.10-6098. [DOI] [PubMed] [Google Scholar]
- 42.Shuba L, Nicolela MT, Rafuse PE. Correlation of capsular pseudoexfoliation material and iridocorneal angle pigment with the severity of pseudoexfoliation glaucoma. J Glaucoma. 2007;16:94–97. doi: 10.1097/01.ijg.0000212283.70422.b0. [DOI] [PubMed] [Google Scholar]
- 43.Puska P, Tarkkanen A. Exfoliation syndrome as a risk factor for cataract development: five-year follow-up of lens opacities in exfoliation syndrome. J Cataract Refract Surg. 2001;27:1992–1998. doi: 10.1016/s0886-3350(01)00972-5. [DOI] [PubMed] [Google Scholar]
- 44.Sekeroglu MA, Bozkurt B, Irkec M, Ustunel S, Orhan M, Saracbasi O. Systemic associations and prevalence of exfoliation syndrome in patients scheduled for cataract surgery. Eur J Ophthalmol. 2008;18:551–555. doi: 10.1177/112067210801800408. [DOI] [PubMed] [Google Scholar]
- 45.Drolsum L, Ringvold A, Nicolaissen B. Cataract and glaucoma surgery in pseudoexfoliation syndrome: a review. Acta Ophthalmol Scand. 2007;85:810–821. doi: 10.1111/j.1600-0420.2007.00903.x. [DOI] [PubMed] [Google Scholar]
- 46.Subasi S, Yuksel N, Karabas VL, Yilmaz Tugan B. Late in-the-bag spontaneous IOL dislocation: risk factors and surgical outcomes. Int J Ophthalmol. 2019;12:954–960. doi: 10.18240/ijo.2019.06.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kristianslund O, Råen M, Østern AE, Drolsum L. Late in-the-bag intraocular lens dislocation: a randomized clinical trial comparing lens repositioning and lens exchange. Ophthalmology. 2017;124:151–159. doi: 10.1016/j.ophtha.2016.10.024. [DOI] [PubMed] [Google Scholar]
- 48.Shingleton BJ, Nguyen BK, Eagan EF, Nagao K, O’Donoghue MW. Outcomes of phacoemulsification in fellow eyes of patients with unilateral pseudoexfoliation: single-surgeon series. J Cataract Refract Surg. 2008;34:274–279. doi: 10.1016/j.jcrs.2007.09.040. [DOI] [PubMed] [Google Scholar]
- 49.Akinci A, Batman C, Zilelioglu O. Phacoemulsification in pseudoex-foliation syndrome. Ophthalmologica. 2008;222:112–116. doi: 10.1159/000112628. [DOI] [PubMed] [Google Scholar]
- 50.Shingleton BJ, Crandall AS, Ahmed II. Pseudoexfoliation and the cataract surgeon: preoperative, intraoperative, and postoperative issues related to intraocular pressure, cataract, and intraocular lenses. J Cataract Refract Surg. 2009;35:1101–1120. doi: 10.1016/j.jcrs.2009.03.011. [DOI] [PubMed] [Google Scholar]
- 51.Hyams M, Mathalone N, Herskovitz M, Hod Y, Israeli D, Geyer O. Intraoperative complications of phacoemulsification in eyes with and without pseudoexfoliation. J Cataract Refract Surg. 2005;31:1002–1005. doi: 10.1016/j.jcrs.2004.09.051. [DOI] [PubMed] [Google Scholar]
- 52.Nagashima RJ. Decreased incidence of capsule complications and vitreous loss during phacoemulsification in eyes with pseudoexfoliation syndrome. J Cataract Refract Surg. 2004;30:127–131. doi: 10.1016/S0886-3350(03)00465-6. [DOI] [PubMed] [Google Scholar]
- 53.Bayraktar S, Altan T, Küçüksümer Y, Yilmaz OF. Capsular tension ring implantation after capsulorhexis in phacoemulsification of cataracts associated with pseudoexfoliation syndrome. Intraoperative complications and early postoperative findings. J Cataract Refract Surg. 2001;27:1620–1628. doi: 10.1016/s0886-3350(01)00965-8. [DOI] [PubMed] [Google Scholar]
- 54.Yüksel N, Doğu B, Karabaş VL, Cağlar Y. Foveal thickness after phacoemulsification in patients with pseudoexfoliation syndrome, pseudoexfoliation glaucoma, or primary open-angle glaucoma. J Cataract Refract Surg. 2008;34:1953–1957. doi: 10.1016/j.jcrs.2008.07.016. [DOI] [PubMed] [Google Scholar]
- 55.Gimbel HV, Condon GP, Kohnen T, Olson RJ, Halkiadakis I. Late in-thebag intraocular lens dislocation: incidence, prevention, and management. J Cataract Refract Surg. 2005;31:2193–2204. doi: 10.1016/j.jcrs.2005.06.053. [DOI] [PubMed] [Google Scholar]
- 56.Pueringer SL, Hodge DO, Erie JC. Risk of late intraocular lens dislocation after cataract surgery, 1980-2009: a population-based study. Am J Ophthalmol. 2011;152:618–623. doi: 10.1016/j.ajo.2011.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kozobolis VP, Glynatsis M, Labiris G, Katsanos A, Fanariotis M, Koukoula S, Alvanos S, Toufexis G. Retinal nerve fiber layer thickness in patients with exfoliation, exfoliative glaucoma, and primary open angle glaucoma. Eur J Ophthalmol. 2010;20:142–148. doi: 10.1177/112067211002000120. [DOI] [PubMed] [Google Scholar]
- 58.Yüksel N, Altintaş O, Celik M, Ozkan B, Cağlar Y. Analysis of retinal nerve fiber layer thickness in patients with pseudoexfoliation syndrome using optical coherence tomography. Ophthalmologica. 2007;221:299–304. doi: 10.1159/000104759. [DOI] [PubMed] [Google Scholar]
- 59.Cursiefen C, Hammer T, Küchle M, Naumann GO, Schlötzer-Schrehardt U. Pseudoexfoliation syndrome in eyes with ischemic central retinal vein occlusion. A histopathologic and electron microscopic study. Acta Ophthalmol Scand. 2001;79:476–478. doi: 10.1034/j.1600-0420.2001.790509.x. [DOI] [PubMed] [Google Scholar]
- 60.Köse HC, Tekeli O. Optical coherence tomography angiography of the peripapillary region and macula in normal, primary open angle glaucoma, pseudoexfoliation glaucoma and ocular hypertension eyes. Int J Ophthalmol. 2020;13:744–754. doi: 10.18240/ijo.2020.05.08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Subasi S, Yuksel N, Basaran E, Pirhan D. Comparison of vessel density in macular and peripapillary regions between primary open-angle glaucoma and pseudoexfoliation glaucoma using OCTA. Int Ophthalmol. 2021;41:173–184. doi: 10.1007/s10792-020-01564-5. [DOI] [PubMed] [Google Scholar]
- 62.Cornelius A, Pilger D, Riechardt A, Reitemeyer E, Rübsam A, Winterhalter S, Maier AB. Macular, papillary and peripapillary perfusion densities measured with optical coherence tomography angiography in primary open angle glaucoma and pseudoexfoliation glaucoma. Graefes Arch Clin Exp Ophthalmol. 2022;260:957–965. doi: 10.1007/s00417-021-05321-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lee JY, Sung KR, Kim YJ. Comparison of the Prevalence and Clinical Characteristics of Epiretinal Membrane in Pseudoexfoliation and Primary Open-angle Glaucoma. J Glaucoma. 2021;30:859–865. doi: 10.1097/IJG.0000000000001851. [DOI] [PubMed] [Google Scholar]
- 64.Lee JY, Sung KR, Kim YJ. Association of Epiretinal Membrane With Pseudoexfoliation Glaucoma and Long-term Factors Affecting Visual Function. J Glaucoma. 2022;31:595–601. doi: 10.1097/IJG.0000000000002024. [DOI] [PubMed] [Google Scholar]
- 65.Adıyeke SK, Kutlu N, Özen K, Doran MA, Demirbaş K, Ture G, Talay E. Is pseudoexfoliation syndrome associated with vitreoretinal interface abnormalities? Graefes Arch Clin Exp Ophthalmol. 2022;260:431–437. doi: 10.1007/s00417-021-05373-z. [DOI] [PubMed] [Google Scholar]
- 66.Streeten BW, Li ZY, Wallace RN, Eagle RC Jr, Keshgegian AA. Pseudoexfoliative fibrillopathy in visceral organs of a patient with pseudoexfoliation syndrome. Arch Ophthalmol. 1992;110:1757–1762. doi: 10.1001/archopht.1992.01080240097039. [DOI] [PubMed] [Google Scholar]
- 67.Andrikopoulos GK, Mela EK, Georgakopoulos CD, Papadopoulos GE, Damelou AN, Alexopoulos DK, Gartaganis SP. Pseudoexfoliation syndrome prevalence in Greek patients with cataract and its association to glaucoma and coronary artery disease. Eye (Lond). 2009;23:442–447. doi: 10.1038/sj.eye.6702992. [DOI] [PubMed] [Google Scholar]
- 68.Demir N, Ulus T, Yucel OE, Kumral ET, Singar E, Tanboga HI. Assessment of myocardial ischaemia using tissue Doppler imaging in pseudoexfoliation syndrome. Eye (Lond). 2011;25:1177–1180. doi: 10.1038/eye.2011.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Atalar PT, Atalar E, Kilic H, Abbasoglu OE, Ozer N, Aksöyek S, Ovünç K, Ozmen F, Gürsel E. Impaired systemic endothelial function in patients with pseudoexfoliation syndrome. Int Heart J. 2006;47:77–84. doi: 10.1536/ihj.47.77. [DOI] [PubMed] [Google Scholar]
- 70.Bourouki E, Oikonomou E, Moschos M, Siasos G, Siasou G, Gouliopoulos N, Deftereos S, Miliou A, Zacharia E, Tousoulis D. Pseudoexfoliative Glaucoma, Endothelial Dysfunction, and Arterial Stiffness: The Role of Circulating Apoptotic Endothelial Microparticles. J Glaucoma. 2019;28:749–755. doi: 10.1097/IJG.0000000000001303. [DOI] [PubMed] [Google Scholar]
- 71.Repo LP, Suhonen MT, Teräsvirta ME, Koivisto KJ. Color Doppler imaging of the ophthalmic artery blood flow spectra of patients who have had a transient ischemic attack. Correlations with generalized iris transluminance and pseudoexfoliation syndrome. Ophthalmology. 1995;102:1199–1205. doi: 10.1016/s0161-6420(95)30890-1. [DOI] [PubMed] [Google Scholar]
- 72.Linnér E, Popovic V, Gottfries CG, Jonsson M, Sjögren M, Wallin A. The exfoliation syndrome in cognitive impairment of cerebrovascular or Alzheimer’s type. Acta Ophthalmol Scand. 2001;79:283–285. doi: 10.1034/j.1600-0420.2001.790314.x. [DOI] [PubMed] [Google Scholar]
- 73.Cumurcu T, Dorak F, Cumurcu BE, Erbay LG, Ozsoy E. Is there any relation between pseudoexfoliation syndrome and Alzheimer’s type dementia? Semin Ophthalmol. 2013;28:224–229. doi: 10.3109/08820538.2013.793726. [DOI] [PubMed] [Google Scholar]
- 74.Aydoğan Ozkan B, Yüksel N, Keskin G, Altintaş O, Karabaş VL, Cağlar Y, Almaç A. Homocysteine levels in plasma and sensorineural hearing loss in patients with pseudoexfoliation syndrome. Eur J Ophthalmol. 2006;16:542–547. doi: 10.1177/112067210601600407. [DOI] [PubMed] [Google Scholar]
- 75.Cahill M, Early A, Stack S, Blayney AW, Eustace P. Pseudoexfoliation and sensorineural hearing loss. Eye (Lond). 2002;16:261–266. doi: 10.1038/sj.eye.6700011. [DOI] [PubMed] [Google Scholar]
- 76.Singham NV, Zahari M, Peyman M, Prepageran N, Subrayan V. Association between Ocular Pseudoexfoliation and Sensorineural Hearing Loss. J Ophthalmol. 2014;2014:825936. doi: 10.1155/2014/825936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Yüksel N, Anik Y, Kiliç A, Karabaş V, Demirci A, Cağlar Y. Cerebrovascular blood flow velocities in pseudoexfoliation. Graefes Arch Clin Exp Ophthalmol. 2006;244:316–321. doi: 10.1007/s00417-004-0942-7. [DOI] [PubMed] [Google Scholar]
- 78.Yüksel N, Anik Y, Altintaş O, Onur I, Cağlar Y, Demirci A. Magnetic resonance imaging of the brain in patients with pseudoexfoliation syndrome and glaucoma. Ophthalmologica. 2006;220:125–130. doi: 10.1159/000090578. [DOI] [PubMed] [Google Scholar]
- 79.Zikou AK, Kitsos G, Astrakas LG, Xydis VG, Spiliopoulos K, Bagli E, Argyropoulou MI. Pseudoexfoliation syndrome without glaucoma: White matter abnormalities detected by conventional MRI and diffusion tensor imaging. Eur J Radiol. 2018;99:82–87. doi: 10.1016/j.ejrad.2017.12.019. [DOI] [PubMed] [Google Scholar]
- 80.Altintaş O, Maral H, Yüksel N, Karabaş VL, Dillioğlugil MO, Cağlar Y. Homocysteine and nitric oxide levels in plasma of patients with pseudoexfoliation syndrome, pseudoexfoliation glaucoma, and primary open-angle glaucoma. Graefes Arch Clin Exp Ophthalmol. 2005;243:677–683. doi: 10.1007/s00417-004-1097-2. [DOI] [PubMed] [Google Scholar]
- 81.Bleich S, Roedl J, Von Ahsen N, Schlötzer-Schrehardt U, Reulbach U, Beck G, Kruse FE, Naumann GO, Kornhuber J, Jünemann AG. Elevated homocysteine levels in aqueous humor of patients with pseudoexfoliation glaucoma. Am J Ophthalmol. 2004;138:162–164. doi: 10.1016/j.ajo.2004.02.027. [DOI] [PubMed] [Google Scholar]
- 82.Roedl JB, Bleich S, Reulbach U, Rejdak R, Kornhuber J, Kruse FE, Schlötzer-Schrehardt U, Jünemann AG. Homocysteine in tear fluid of patients with pseudoexfoliation glaucoma. J Glaucoma. 2007;16:234–239. doi: 10.1097/IJG.0b013e31802d6942. [DOI] [PubMed] [Google Scholar]
- 83.Vessani RM, Ritch R, Liebmann JM, Jofe M. Plasma homocysteine is elevated in patients with exfoliation syndrome. Am J Ophthalmol. 2003;136:41–46. doi: 10.1016/s0002-9394(03)00077-1. [DOI] [PubMed] [Google Scholar]
- 84.Ritland JS, Egge K, Lydersen S, Juul R, Semb SO. Exfoliative glaucoma and primary open-angle glaucoma: associations with death causes and comorbidity. Acta Ophthalmol Scand. 2004;82:401–404. doi: 10.1111/j.1395-3907.2004.00297.x. [DOI] [PubMed] [Google Scholar]
- 85.Shrum KR, Hattenhauer MG, Hodge D. Cardiovascular and cerebrovascular mortality associated with ocular pseudoexfoliation. Am J Ophthalmol. 2000;129:83–86. doi: 10.1016/s0002-9394(99)00255-x. [DOI] [PubMed] [Google Scholar]
- 86.Kozart DM, Yanoff M. Intraocular pressure status in 100 consecutive patients with exfoliation syndrome. Ophthalmology. 1982;89:214–218. doi: 10.1016/s0161-6420(82)34804-6. [DOI] [PubMed] [Google Scholar]
- 87.Konstas AG, Mantziris DA, Stewart WC. Diurnal intraocular pressure in untreated exfoliation and primary open-angle glaucoma. Arch Ophthalmol. 1997;115:182–185. doi: 10.1001/archopht.1997.01100150184006. [DOI] [PubMed] [Google Scholar]
- 88.Yüksel N, Karabaş VL, Arslan A, Demirci A, Cağlar Y. Ocular hemodynamics in pseudoexfoliation syndrome and pseudoexfoliation glaucoma. Ophthalmology. 2001;108:1043–1049. doi: 10.1016/s0161-6420(01)00572-3. [DOI] [PubMed] [Google Scholar]
- 89.Yüksel N, Karabaş VL, Demirci A, Arslan A, Altintaş O, Cağlar Y. Comparison of blood flow velocities of the extraocular vessels in patients with pseudoexfoliation or primary open-angle glaucoma. Ophthalmologica. 2001;215:424–429. doi: 10.1159/000050902. [DOI] [PubMed] [Google Scholar]
- 90.Netland PA, Ye H, Streeten BW, Hernandez MR. Elastosis of the lamina cribrosa in pseudoexfoliation syndrome with glaucoma. Ophthalmology. 1995;102:878–886. doi: 10.1016/s0161-6420(95)30939-6. [DOI] [PubMed] [Google Scholar]
- 91.Ritch R, Schlötzer-Schrehardt U, Konstas AG. Why is glaucoma associated with exfoliation syndrome? Prog Retin Eye Res. 2003;22:253–275. doi: 10.1016/s1350-9462(02)00014-9. [DOI] [PubMed] [Google Scholar]
- 92.Ritch R. Perspective on exfoliation syndrome. J Glaucoma. 2001;10(5 Suppl 1):S33–S35. doi: 10.1097/00061198-200110001-00013. [DOI] [PubMed] [Google Scholar]
- 93.Konstas AG, Hollo G, Astakhov YS, Teus MA, Akopov EL, Jenkins JN, Stewart WC. Factors associated with long-term progression or stability in exfoliation glaucoma. Arch Ophthalmol. 2004;122:29–33. doi: 10.1001/archopht.122.1.29. [DOI] [PubMed] [Google Scholar]
- 94.Konstas AG, Koliakos GG, Karabatsas CH, Liakos P, Schlötzer-Schrehardt U, Georgiadis N, Ritch R. Latanoprost therapy reduces the levels of TGF beta 1 and gelatinases in the aqueous humour of patients with exfoliative glaucoma. Exp Eye Res. 2006;82:319–322. doi: 10.1016/j.exer.2005.07.004. [DOI] [PubMed] [Google Scholar]
- 95.Konstas AG, Kozobolis VP, Katsimpris IE, Boboridis K, Koukoula S, Jenkins JN, Stewart WC. Efficacy and safety of latanoprost versus travoprost in exfoliative glaucoma patients. Ophthalmology. 2007;114:653–657. doi: 10.1016/j.ophtha.2006.07.064. [DOI] [PubMed] [Google Scholar]
- 96.Parmaksiz S, Yuksel N, Karabas VL, Ozkan B, Demirci G, Caglar Y. A comparison of travoprost, latanoprost, and the fixed combination of dorzolamide and timolol in patients with pseudoexfoliation glaucoma. Eur J Ophthalmol. 2006;16:73–80. [PubMed] [Google Scholar]
- 97.Yüksel N, Gök M, Altıntaş O, Cağlar Y. Diurnal intraocular pressure efficacy of the timolol-brimonidine fixed combination and the timolol-dorzolamide fixed combination as a first choice therapy in patients with pseudoexfoliation glaucoma. Curr Eye Res. 2011;36:804–808. doi: 10.3109/02713683.2011.584651. [DOI] [PubMed] [Google Scholar]
- 98.Konstas AG, Mikropoulos DG, Embeslidis TA, Dimopoulos AT, Papanastasiou A, Haidich AB, Stewart WC. 24-h Intraocular pressure control with evening-dosed travoprost/timolol, compared with latanoprost/timolol, fixed combinations in exfoliative glaucoma. Eye (Lond). 2010;24:1606–1613. doi: 10.1038/eye.2010.100. [DOI] [PubMed] [Google Scholar]
- 99.Konstas AG, Holló G, Mikropoulos D, Tsironi S, Haidich AB, Embeslidis T, Georgiadou I, Irkec M, Melamed S. Twenty-four-hour intraocular pressure control with bimatoprost and the bimatoprost/timolol fixed combination administered in the morning, or evening in exfoliative glaucoma. Br J Ophthalmol. 2010;94:209–213. doi: 10.1136/bjo.2008.155317. [DOI] [PubMed] [Google Scholar]
- 100.Goldenfeld M, Geyer O, Segev E, Kaplan-Messas A, Melamed S. Selective laser trabeculoplasty in uncontrolled pseudoexfoliation glaucoma. Ophthalmic Surg Lasers Imaging. 2011;42:390–393. doi: 10.3928/15428877-20110630-01. [DOI] [PubMed] [Google Scholar]
- 101.Gracner T. Intraocular pressure response of capsular glaucoma and primary open-angle glaucoma to selective Nd:YAG laser trabeculoplasty: a prospective, comparative clinical trial. Eur J Ophthalmol. 2002;12:287–292. doi: 10.1177/112067210201200406. [DOI] [PubMed] [Google Scholar]
- 102.Konstas AG, Topouzis F, Leliopoulou O, Pappas T, Georgiadis N, Jenkins JN, Stewart WC. 24-hour intraocular pressure control with maximum medical therapy compared with surgery in patients with advanced open-angle glaucoma. Ophthalmology. 2006;113:761–5.e1. doi: 10.1016/j.ophtha.2006.01.029. [DOI] [PubMed] [Google Scholar]
- 103.Yilmaz Tugan B, Yuksel N, Kesim E, Subasi S. Comparison of long-term results of trabeculectomy and phacotrabeculectomy in patients with pseudoexfoliation glaucoma and primary open-angle glaucoma: a single-center study. Int Ophthalmol. 2022;42:1737–1747. doi: 10.1007/s10792-021-02169-2. [DOI] [PubMed] [Google Scholar]
- 104.Drolsum L. Deep sclerectomy in patients with capsular glaucoma. Acta Ophthalmol Scand. 2003;81:567–572. doi: 10.1111/j.1395-3907.2003.00186.x. [DOI] [PubMed] [Google Scholar]
- 105.Drolsum L. Longterm follow-up after deep sclerectomy in patients with pseudoexfoliative glaucoma. Acta Ophthalmol Scand. 2006;84:502–506. doi: 10.1111/j.1600-0420.2006.00674.x. [DOI] [PubMed] [Google Scholar]
- 106.Rekonen P, Kannisto T, Puustjärvi T, Teräsvirta M, Uusitalo H. Deep sclerectomy for the treatment of exfoliation and primary open-angle glaucoma. Acta Ophthalmol Scand. 2006;84:507–511. doi: 10.1111/j.1600-0420.2006.00654.x. [DOI] [PubMed] [Google Scholar]
- 107.Subaşı S, Yüksel N, Özer F, Yılmaz Tugan B, Pirhan D. A Retrospective Analysis of Safety and Efficacy of XEN 45 Microstent Combined Cataract Surgery in Open-Angle Glaucoma over 24 Months. Turk J Ophthalmol. 2021;51:139–145. doi: 10.4274/tjo.galenos.2020.47629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Rauchegger T, Angermann R, Willeit P, Schmid E, Teuchner B. Two-year outcomes of minimally invasive XEN Gel Stent implantation in primary open-angle and pseudoexfoliation glaucoma. Acta Ophthalmol. 2021;99:369–375. doi: 10.1111/aos.14627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Sharkawi E, Lindegger DJ, Artes PH, Lehmann-Clarke L, El Wardani M, Misteli M, Pasquier J, Guarnieri A. Outcomes of gonioscopy-assisted transluminal trabeculotomy in pseudoexfoliative glaucoma: 24-month follow-up. Br J Ophthalmol. 2021;105:977–982. doi: 10.1136/bjophthalmol-2020-315954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Aktas Z, Ozdemir Zeydanli E, Uysal BS, Yigiter A. Outcomes of Prolene Gonioscopy Assisted Transluminal Trabeculotomy in Primary Open Angle Glaucoma and Pseudoexfoliation Glaucoma: A Comparative Study. J Glaucoma. 2022;31:751–756. doi: 10.1097/IJG.0000000000002063. [DOI] [PubMed] [Google Scholar]


