Key Points
Question
Are prehospital transfer protocols that bypass the nearest local stroke center for direct transport to an endovascular treatment–capable stroke center harmful or beneficial among patients experiencing an intracerebral hemorrhage?
Findings
In this secondary analysis of 302 patients included in the RACECAT randomized clinical trial conducted in Catalonia, Spain, direct transfer to an endovascular treatment–capable stroke center resulted in reduced chances of functional independence at 90 days (adjusted common odds ratio, 0.63) compared with transfer to the nearest local stroke center for patients who received a final diagnosis of intracerebral hemorrhage.
Meaning
These results suggest that transfer protocols based on bypassing local stroke centers may result in worse functional outcomes for patients with intracerebral hemorrhage although this finding requires replication in other settings.
Abstract
Importance
Prehospital transfer protocols are based on rapid access to reperfusion therapies for patients with ischemic stroke. The effect of different protocols among patients receiving a final diagnosis of intracerebral hemorrhage (ICH) is unknown.
Objective
To determine the effect of direct transport to an endovascular treatment (EVT)–capable stroke center vs transport to the nearest local stroke center.
Design, Setting, and Participants
This was a prespecified secondary analysis of RACECAT, a multicenter, population-based, cluster-randomized clinical trial conducted from March 2017 to June 2020 in Catalonia, Spain. Patients were evaluated by a blinded end point assessment. All consecutive patients suspected of experiencing a large vessel occlusion stroke (Rapid Arterial Occlusion Evaluation Scale [RACE] score in the field >4 on a scale of 0 to 9, with lower to higher stroke severity) with final diagnosis of ICH were included. A total of 1401 patients were enrolled in RACECAT with suspicion of large vessel occlusion stroke. The current analysis was conducted in October 2022.
Intervention
Direct transport to an EVT-capable stroke center (n = 137) or to the closest local stroke center (n = 165).
Main Outcomes and Measures
The primary outcome was tested using cumulative ordinal logistic regression to estimate the common odds ratio (OR) and 95% CI of the shift analysis of disability at 90 days as assessed by the modified Rankin Scale (mRS) score (range, 0 [no symptoms] to 6 [death]) in the intention-to-treat population. Secondary outcomes, included 90-day mortality, death or severe functional dependency, early neurological deterioration, early mortality, ICH volume and enlargement, rate of neurosurgical treatment, rate of clinical complications during initial transport, and rate of adverse events until day 5.
Results
Of 1401 patients enrolled, 1099 were excluded from this analysis (32 rejected informed consent, 920 had ischemic stroke, 29 had transient ischemic attack, 12 had subarachnoid hemorrhage, and 106 had stroke mimic). Thus, 302 patients were included (204 [67.5%] men; mean [SD] age 71.7 [12.8] years; and median [IQR] RACE score, 7 [6-8]). For the primary outcome, direct transfer to an EVT-capable stroke center (mean [SD] mRS score, 4.93 [1.38]) resulted in worse functional outcome at 90 days compared with transfer to the nearest local stroke center (mean [SD] mRS score, 4.66 [1.39]; adjusted common OR, 0.63; 95% CI, 0.41-0.96). Direct transfer to an EVT-capable stroke center also suggested potentially higher 90-day mortality compared with transfer to the nearest local stroke center (67 of 137 [48.9%] vs 62 of 165 [37.6%]; adjusted hazard ratio, 1.40; 95% CI, 0.99-1.99). The rates of medical complications during the initial transfer (30 of 137 [22.6%] vs 9 of 165 patients [5.6%]; adjusted OR, 5.29; 95% CI, 2.38-11.73) and in-hospital pneumonia (49 of 137 patients [35.8%] vs 29 of 165 patients [17.6%]; OR, 2.61; 95% CI, 1.53-4.44) were higher in the EVT-capable stroke center group.
Conclusions and Relevance
In this secondary analysis of the RACECAT randomized clinical trial, bypassing the closest stroke center resulted in reduced chances of functional independence at 90 days for patients who received a final diagnosis of ICH.
Trial Registration
ClinicalTrials.gov Identifier: NCT02795962
This prespecified secondary analysis of a randomized clinical trial conducted in Catalonia, Spain, assesses whether direct transport to an endovascular treatment–capable stroke center vs to the nearest local stroke center benefits patients experiencing intracerebral hemorrhage.
Introduction
Intracerebral hemorrhage (ICH) represents approximately 15% of all strokes and is the subtype of stroke with the highest mortality, morbidity, and economic impact.1,2 Unfortunately, no specific treatment has proven to be definitively effective.3,4,5 Early neurological deterioration plays a major role in the devastating clinical course of ICH, mainly due to hematoma enlargement, which affects almost 30% of patients, mostly within the first 3 hours.6,7 High variability in blood pressure, temperature, heart rate, and glycemia are associated with worse ICH outcomes and higher mortality,8,9,10 as well as vomiting, infectious complications, and seizures.11,12,13,14,15 Prompt implementation of intensive care based on anticoagulation reversal, intensive blood pressure lowering, and access to critical care have been found to reduce 30-day mortality.16 Considering the rapid and devastating course of ICH, prehospital transfer protocols may play a major role in the functional outcome and mortality of patients experiencing an ICH.
In the endovascular treatment (EVT) era, prehospital transfer pathways are focused on rapid access to EVT-capable stroke centers17,18 because in patients with large vessel occlusion (LVO) stroke, EVT is associated with better outcomes than treatment with intravenous thrombolysis alone,19 and the benefit of EVT is strongly time dependent.20 However, prehospital transfer protocols should consider not only the potential benefit of rapid EVT access for patients with LVO, but also the effects on all patients with stroke. The effect of regionalized LVO stroke care on patients who receive a final diagnosis of ICH is unknown. Previous studies21 have discussed whether patients with ICH would benefit from higher levels of care22,23,24 where neurocritical care facilities and neurosurgical treatments are available vs earlier temporizing at regional facilities to stabilize vital signs,7,10,11 initiate anticoagulation reversal treatments, and avoid initial long transfers for patients with potential life-threatening associated conditions.15 This prespecified secondary analysis of the RACECAT (Transfer to the Closest Local Stroke Center vs Direct Transfer to Endovascular Stroke Center of Acute Stroke Patients With Suspected Large Vessel Occlusion in the Catalan Territory) randomized clinical trial25 aimed to evaluate the effect of bypassing the nearest local stroke center among patients experiencing ICH.
Methods
Study Design
RACECAT was a population-based, multicenter, open-label, spatial-temporal cluster-randomized clinical trial, with blinded end point assessment, embedded in a mandatory registry of patients with stroke (Codi Ictus Catalunya [CICAT] registry) conducted between March 2017 and June 2020. Two emergency medical services (EMS) routing strategies were compared: the reference intervention (“drip and ship”), which included transfer to the nearest local stroke center; and the experimental intervention (“mothership”), which included transfer to the nearest EVT-enabled stroke center and bypassing the local stroke center. The RACECAT protocol26 and RACECAT trial25 results have been previously published. We performed a predefined secondary prospective analysis of patients who received a final diagnosis of spontaneous ICH included in RACECAT. The study protocol (Supplement 1) was approved by a central ethics committee and the research board at each participating center. All patients or surrogates provided deferred written informed consent. This study followed the Consolidated Standards of Reporting Trials (CONSORT) reporting guideline.
Study Population
The RACECAT eligible population of 1401 participants included (1) functionally independent patients, defined as those with a modified Rankin Scale (mRS) score between 0 and 2 (the mRS assesses disability; scores range from 0 [no symptoms] to 6 [death]; (2) with suspected acute LVO stroke (Rapid Arterial Occlusion Evaluation [RACE] Scale score >4 on a scale of 0 to 9, with lower to higher stroke severity, as identified by EMS at first assistance in the field; (3) located in geographic areas not primarily covered by an EVT-enabled stroke center, covering 3.85 million people; and (4) with an estimated arrival at an EVT-enabled stroke center of less than 7 hours after symptom onset or last time seen well. Patients were excluded if they had an unstable clinical status or diminished level of consciousness requiring emergent life-support care.
For the present study, inclusion criteria were consecutive RACECAT patients with a final diagnosis of spontaneous ICH. Exclusion criteria were secondary ICH, subarachnoid hemorrhage, diagnosis of ischemic stroke, transient ischemic attack, or stroke mimic.
Concomitant Care and Interventions Prohibited During the Trial
In addition to the initial decision about the first transport option, patients received standard clinical care based on current international guidelines for ICH acute management.5,27 Initial transport was performed by ground travel via technically staffed ambulances not capable of administering treatment or placing peripheral venous access. In local stroke centers, all patients were evaluated by an on-site neurologist or telemedicine consultation, depending on the type of stroke center. Secondary transfer to a stroke unit, intensive care unit admission, and neurosurgical treatment were decided following current international guidelines.5,27
Outcomes
The primary outcome was a shift analysis of disability at 90 days, as assessed by the mRS, with scores ranging from 0 (no symptoms) to 6 (death). The primary outcome was centrally evaluated through a structured telephone-based interview28 by 2 certified assessors blinded to group assignment.
Secondary outcomes included (1) 90-day mortality; (2) composite outcome of death or severe functional dependency defined as an mRS score of 5 or 6; (3) early neurological deterioration defined as an increase of 4 or more points in the National Institutes of Health Stroke Scale (NIHSS) score within 24 hours after stroke onset (NIHSS scores range from 0 [no symptoms] to 42 [severe symptoms]); (4) mortality at 24 hours and on the fifth day; (5) baseline ICH volume and substantial hematoma enlargement at 24 to 72 hours; (6) rate of neurosurgical treatment; (7) rate of clinical complications during initial transport, previously predefined as vomiting, orotracheal intubation, seizure, RACE Scale score worsening (increase of ≥1 point), decline in the level of consciousness, and death; and (8) rate of adverse events until day 5, previously prespecified as neurological deterioration (increase of ≥4 points for the NIHSS score), seizure, and pneumonia (defined as suspected respiratory infection requiring antibiotics).
Imaging Evaluation
All cranial computed tomography (CT) images were retrospectively evaluated by 3 evaluators (M.T., C.V., and Y.S.) blinded to group assignment to describe the ICH characteristics.29 We collected the following radiologic variables: hematoma volume, hematoma location (lobar, deep, cerebellum, and brainstem), presence of intraventricular hemorrhage, presence of noncontrast CT (NCCT) markers of hematoma growth (blend sign, island sign, and black hole sign defined in accordance with the hematoma enlargement standards of the International NCCT ICH Study Group for NCCT markers of hematoma enlargement,29 presence of the spot sign30 on CT angiography images, and hematoma enlargement. Hematoma volume was calculated by using the formula (A × B × C)/2, where A is maximum length (in cm), B is width perpendicular to A on the same head CT slice, and C is the number of slices multiplied by the slice thickness. Substantial hematoma enlargement was defined as an increase of 33% or higher or 6 mL or more in hematoma volume between baseline and 24 to 72 hours on available cranial CT images.
Statistical Analysis
Patients were analyzed by their randomization group (intention-to-treat analysis). Baseline characteristics were described and compared between study groups. Continuous variables are displayed as mean and SD or as median and IQR if not normally distributed as tested by the Kolmogorov-Smirnov test. Categorical variables are displayed by their numbers and frequencies. Differences between groups were assessed using a χ2 test (for categorical variables) and the t test or Mann-Whitney U test (for continuous variables with or without normal distribution, respectively).
The primary outcome was tested using cumulative ordinal logistic regression to estimate the common odds ratio (OR) and 95% CI of the shift analysis on the mRS score at 90 days. For the primary analysis and all other outcome analyses, regression models were reported as unadjusted and adjusted by stratifying factors (time slot, territory, and day of the week) and all noncorrelated variables with association P < .10 in the bivariate analysis. The local stroke center transfer group was considered the reference category in all analyses. As a secondary outcome, we evaluated 90-day mortality using a Cox proportional hazards regression model to estimate hazard ratios (HRs) and 95% CIs. The ORs and 95% CIs in the rate of death or severe functional dependency, clinical deterioration at 24 hours, early mortality, proportion of patients receiving neurosurgical treatment, clinical deterioration during initial transport, and adverse events until day 5 are reported. Model estimates are reported unadjusted and adjusted for the same covariates as in the primary analysis. Mean differences and 95% CIs, estimated using a bootstrapping method with 1000 samples, between intervention groups are reported for time metrics, baseline ICH volume, and ICH volume at 24 hours.
For all outcomes, a 2-sided P < .05 or 95% CI excluding 1 was considered statistically significant. Because of the potential for type I errors due to multiple comparisons, findings for analyses of secondary end points should be interpreted as exploratory. Statistical analyses were performed in October 2022 using IBM SPSS, version 23.3 (SPSS Inc).
Results
Baseline Characteristics
Of 1401 patients enrolled in RACECAT, 302 patients (21.6%) received a final diagnosis of spontaneous ICH and were included in the present study (Figure 1). Participants were excluded for secondary ICH (n = 0), subarachnoid hemorrhage (n = 12), and diagnosis of ischemic stroke (n = 920), transient ischemic attack (n = 29), or stroke mimic (n = 106). Included participant mean (SD) age was 71.7 (12.8) years, 204 (67.5%) were men, 98 (32.5%) were women, and the median (IQR) RACE Scale score was 7 (6-8) points. Of 302 patients, 165 (54.6%) were assigned to local stroke center transport and 137 (45.4%) were assigned to direct EVT-capable stroke center transfer. Modification of the initially allocated circuit occurred for 12 patients (4.0%) (1 in the local stroke center group and 11 in the EVT-capable stroke center group), with 9 modifications (75.0%) due to medical reasons (Figure 1). All patients were analyzed according to their assigned group.
Figure 1. Study Population Flowchart.
EVT indicates endovascular treatment; ITT, intention to treat; and SAH, subarachnoid hemorrhage.
Baseline demographic characteristics are given in Table 1. None of the noncorrelated variables showed an association of P < .10 in the bivariate analysis for inclusion in the multivariable regression models. Blood pressure at admission was similar in both groups (systolic, 169 vs 166 mm Hg; diastolic, 91 vs 89 mm Hg). The median (IQR) distance from stroke onset scene to first hospital was 12.6 (3.4-27.2) km for local stroke centers and 88.8 (40.2-110.8) km for EVT-capable stroke centers. The median (IQR) time from symptom onset to first hospital arrival was 94 (67-170) minutes for the local stroke center group and 135 (97-184) minutes for the EVT-capable stroke center group, a mean difference of 46.8 minutes (95% CI, 14.0-80.8 minutes). No differences between groups in time from arrival to CT scan performance were found. After first admission, 37 patients (22.4%) were secondarily transferred from a local stroke center to an EVT-capable stroke center.
Table 1. Participant Characteristics and Workflow Measures.
| Characteristic or measure | Local stroke center (n = 165) | EVT-capable stroke center (n = 137) |
|---|---|---|
| Variables registered at a prehospital level | ||
| Sex, No. (%) | ||
| Male | 105 (63.6) | 99 (72.3) |
| Female | 60 (36.4) | 38 (27.7) |
| Age, mean (SD), y | 70.8 (12.2) | 72.7 (13.4) |
| RACE Scale scorea | ||
| Median (IQR) | 7 (6-8) | 7 (6-7) |
| 8-9 points, No. (%) | 47 (28.5) | 33 (24.1) |
| Located >60 min from EVT-capable stroke center, No. (%) | 73 (45.9) | 71 (54.2) |
| Distance to assigned center, median (IQR), km | 12.6 (3.4-27.2) | 88.8 (40.2-110.8) |
| Time from stroke onset to first hospital arrival <3 h, No. (%) | 126 (76.4) | 100 (73.0) |
| Time from stroke onset to first hospital arrival, median (IQR), min | 94 (67-170) | 135 (97-184) |
| Time from randomization to first hospital arrival, median (IQR), min | 22 (13-35) | 58 (34-86) |
| Time from first hospital arrival to neuroimaging, median (IQR), min | 16 (11-22) | 20 (14-25) |
| Working day (holiday), No. (%) | 61 (37.0) | 41 (29.9) |
| Shift (morning), No. (%) | 89 (53.9) | 76 (55.5) |
| Area (provincial), No. (%) | 82 (49.7) | 76 (55.5) |
| Variables registered at the first center of attention | ||
| Medical history, No. (%) | ||
| Atrial fibrillation | 29 (17.6) | 28 (20.4) |
| Prestroke anticoagulation intake | 25 (15.2) | 25 (18.2) |
| Diabetes | 52 (31.5) | 32 (23.4) |
| Hypertension | 116 (70.3) | 90 (65.7) |
| Dyslipidemia | 83 (50.3) | 56 (40.9) |
| Smoking | 15 (9.1) | 18 (13.1) |
| Alcohol intake | 13 (7.9) | 12 (8.8) |
| Coronary heart disease | 13 (7.9) | 12 (8.8) |
| Peripheral vasculopathy | 2 (1.2) | 4 (2.9) |
| Previous ischemic stroke or transient ischemic attack | 21 (12.7) | 17 (12.4) |
| Prestroke modified Rankin Scale score 0-2, No. (%)b | 160 (97.0) | 131 (95.6) |
| NIHSS score, median (IQR)c | 18 (14-22) | 19 (15-22) |
| Systolic blood pressure at first hospital arrival, median (IQR), mm Hg | 169 (150-196) | 166 (145-190) |
| Diastolic blood pressure at first hospital arrival, median (IQR), mm Hg | 91 (79-110) | 89 (78-101) |
| Glucose level at hospital arrival, median (IQR), mg/dL | 142 (120-181) | 137 (113-170) |
| Wake-up or unknown time from onset, No. (%) | 38 (23.0) | 30 (21.9) |
| Anticoagulation reversal at first center of attention, No. (%) | 17 (68.0) | 12 (48.0) |
Abbreviations: EVT, endovascular treatment; NIHSS, National Institutes of Health Stroke Scale; RACE, Rapid Arterial Occlusion Evaluation.
SI conversion factor: To convert glucose to millimoles per liter, multiply by 0.0555.
RACE Scale scores range from 0 to 9, from lower to higher stroke severity.
Modified Rankin Scale assesses disability (scores range from 0 [no symptoms] to 6 [death]).
The NIHSS assesses stroke severity, with scores ranging from 0 (no symptoms) to 42 (severe symptoms).
Primary Outcome
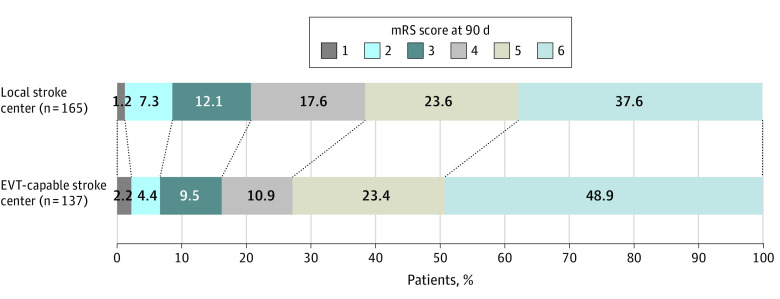
Figure 2 shows the distribution of mRS scores by treatment group at 90 days. For the primary outcome, direct transfer to an EVT-capable stroke center resulted in a reduced chance of functional independence at 90 days, as indicated by the shift in the distribution of the mRS score, compared with transfer to the nearest local stroke center (mean [SD] mRS score, 4.93 [1.38] for direct transfer to an EVT-capable stroke center vs 4.66 [1.39] for transfer to the nearest local stroke center; common OR adjusted by stratifying factors, 0.63; 95% CI, 0.41-0.96).
Figure 2. Distribution of Functional Outcomes at 90 Days.
Distribution of modified Rankin Scale (mRS) scores at 90 days for patients initially transported to a local stroke center vs those directly transported to an endovascular treatment-capable stroke center who were evaluated by telephone interview with blinded investigators. No data on 90-day mRS score follow-up were missing. The mRS scores range from 0 to 6, with 0 indicating no symptoms; 1, no clinically significant disability; 2, slight disability (able to handle own affairs without assistance but unable to carry out all previous activities); 3, moderate disability requiring some help but able to walk unassisted; 4, moderately severe disability (unable to attend to bodily needs and unable to walk); 5, severe disability (requiring constant nursing care and attention); and 6, death. The effect is reported as the common odds ratio (0.63; 95% CI, 0.41-0.96) for better outcome, adjusted by stratifying factors (time slot, territory, and day of week). The local stroke center group was the reference category.
Secondary Outcomes
Secondary outcome analyses are presented in Table 2. The 90-day mortality rate was 62 of 165 (37.6%) in the local stroke center group and 67 of 137 (48.9%) in the EVT-capable stroke center group (adjusted HR, 1.40, 95% CI, 0.99-1.99) (Figure 2 and Figure 3; Table 2). Mortality curves show a divergence between groups that is evident from day 5 after stroke onset (Figure 3). No differences were found between study groups in neurological worsening at 24 hours and mortality at 24 hours and on the fifth day. The cause of death was related to pneumonia in 5 patients (8.0% of deaths) in the local stroke center group and 13 patients (19.4% of deaths) in the EVT-capable stroke center group. The odds of death or severe functional dependency were higher in the EVT-capable stroke center group (adjusted OR [AOR], 1.72; 95% CI, 1.05-2.84). The shift in the distribution of mRS scores after excluding patients who died showed no difference between study groups (AOR, 0.83; 95% CI, 0.47-1.45).
Table 2. Primary and Secondary Outcomes.
| Outcome | Local stroke center (n = 165) | EVT-capable stroke center (n = 137) | Effect variable | Unadjusted value (95% CI) | Adjusted value (95% CI)a |
|---|---|---|---|---|---|
| Primary | |||||
| Modified Rankin Scale score at 90 d, mean (SD) | 4.66 (1.39) | 4.93 (1.38) | Common OR | 0.65 (0.43 to 0.98) | 0.63 (0.41 to 0.96) |
| Secondary | |||||
| Mortality at 90 d, No. (%) | 62 (37.6) | 67 (48.9) | HR | 1.37 (0.97 to 1.94) | 1.40 (0.99 to 1.99) |
| Modified Rankin Scale score at 90 d (excluding deceased patients) | 3.85 (1.16) | 3.91 (1.27) | Common OR | 0.83 (0.48 to 1.45) | 0.83 (0.47 to 1.45) |
| Death or severe functional dependency, No. (%)b | 101 (61.2) | 99 (72.3) | OR | 1.65 (1.01 to 2.69) | 1.72 (1.05 to 2.84) |
| Neurological worsening at 24 h, No. (%)c | 35 (24.5) | 31 (27.4) | OR | 1.17 (0.67 to 2.05) | 1.27 (0.72 to 2.27) |
| Mortality at 24 h, No. (%) | 19 (11.5) | 20 (14.6) | OR | 1.33 (0.68 to 2.62) | 1.38 (0.70 to 2.74) |
| Mortality on fifth day, No. (%) | 36 (22.2) | 40 (30.1) | OR | 1.51 (0.89 to 2.54) | 1.56 (0.92 to 2.66) |
| Baseline ICH volume, median (IQR), mL | 30.2 (10.5 to 59.3) | 27.5 (9.3 to 58.7) | Mean difference | −2.37 (−7.24 to 2.51) | NA |
| Presence of radiologic biomarkers for growth, No. (%)d | 121 (76.1) | 95 (72.5) | OR | 0.83 (0.49 to 1.41) | 0.86 (0.50 to 1.48) |
| ICH volume at 24 h, median (IQR), mLe,f | 25.8 (10.8 to 49.9) | 29.7 (11 to 57.7) | Mean difference | 7.65 (1.06 to 14.25) | NA |
| Hematoma enlargement, No. (%)f,g | 27 (40.3) | 30 (53.6) | OR | 1.71 (0.84 to 3.50) | 1.67 (0.79 to 3.52) |
| Neurosurgical treatment, No. (%) | 6 (3.6) | 12 (8.8) | OR | 2.54 (0.93 to 6.97) | 2.5 (0.91 to 6.88) |
| Complications during the primary transfer, No. (%) | 9 (5.6) | 30 (22.6) | OR | 4.95 (2.26 to 10.86) | 5.29 (2.38 to 11.73) |
| Vomiting, No. (%) | 3 (1.9) | 17 (12.8) | OR | 7.77 (2.22 to 27.12) | NA |
| Epileptic seizures, No. (%) | 0 (0) | 0 (0) | NA | NA | NA |
| Neurological worsening, No. (%)c | 1 (0.6) | 7 (5.3) | OR | 8.94 (1.09 to 73.65) | NA |
| Decrease in level of consciousness, No. (%) | 6 (3.7) | 11 (8.3) | OR | 2.34 (0.84 to 6.52) | NA |
| Intubation, No. (%) | 1 (0.6) | 4 (3) | OR | 4.99 (0.55 to 45.21) | NA |
| Death, No. (%) | 0 (0) | 0 (0) | NA | NA | NA |
| Adverse events until day 5 | 100 (60.6) | 95 (69.3) | OR | 1.47 (0.91 to 2.37) | 1.55 (0.95 to 2.52) |
| Neurological worsening, No. (%)c | 75 (45.5) | 66 (48.2) | OR | 1.12 (0.71 to 1.76) | NA |
| Pneumonia, No. (%) | 29 (17.6) | 49 (35.8) | OR | 2.61 (1.53 to 4.44) | NA |
| Epileptic seizures, No. (%) | 15 (9.1) | 8 (5.8) | OR | 0.62 (0.6 to 1.51) | NA |
Abbreviations: EVT, endovascular treatment; HR, hazard ratio; ICH, intracerebral hemorrhage; NA, not applicable; OR, odds ratio.
Model estimates were adjusted by stratifying factors (time slot, territory, and day of the week).
Death or severe functional dependency was defined as a modified Rankin Scale score of 5 or 6.
Neurological worsening was defined as an increase of 4 or more points in the National Institutes of Health Stroke Scale (NIHSS) score compared with baseline NIHSS score, except for neurological worsening during transfer, which was defined as an increase of 1 or more points for the Rapid Arterial Occlusion Evaluation (RACE) Scale score compared with the baseline RACE Scale score.
Radiologic markers of growth were defined as the presence of black hole, island, blend, or spot signs (eTable 1 in Supplement 2).
The ICH volume was calculated using the formula (A × B × C)/2, where A is the maximum length (in cm), B is the width perpendicular to A on the same head CT slice, and C is the number of slices multiplied by the slice thickness.
A follow-up computed tomographic scan 24 to 72 hours after stroke onset was performed in 53% of all patients.
Hematoma enlargement was defined as an increase in ICH volume of 6 mL or more or 33% compared with the baseline ICH volume.
Figure 3. Kaplan-Meier Analysis for Mortality at 90 Days.
Mortality at 90 days was evaluated using a Cox proportional hazards regression model to estimate hazard ratios and 95% CIs. An adjusted regression model by stratifying factors (time slot, territory, and day of the week) was constructed. The local stroke center group was the reference category. aHR indicates adjusted hazard ratio; EVT, endovascular treatment.
The mean difference in time from stroke onset to neuroimage acquisition was 49 minutes (95% CI, 18-80 minutes) longer in the EVT-capable stroke center group, but no difference in median baseline ICH volumes was found (median [IQR], 30.2 [10.5-59.3] mL for the local stroke center group and 27.5 [9.3-58.7] mL for the EVT-capable stroke center group) (eTable 1 in Supplement 2). The presence of NCCT markers of hematoma enlargement and spot sign at baseline was similar between groups. A follow-up CT scan between 24 and 72 hours after stroke onset was performed in 89 patients (53.9%) taken to local stroke centers and 71 patients (51.8%) taken to EVT-capable stroke centers. The main reasons for not performing follow-up CT scans were early death, limitation of care, and stable clinical status. No significant mean difference in substantial hematoma enlargement was observed (OR, 1.71; 95% CI, 0.84-3.50) (eTable 1 in Supplement 2). Six patients (3.6%) received neurosurgical treatment in the local stroke center group vs 12 patients (8.8%) in the EVT-capable stroke center group.
Primary transfer to an EVT-capable stroke center was associated with a higher rate of complications (9 of 165 [5.6%] vs 30 of 137 [22.6%]; AOR, 5.29; 95% CI, 2.38-11.73). Pneumonia was reported as an adverse event until day 5 after stroke onset in 29 patients (17.6%) in the local stroke center group and in 49 patients (35.8%) in the EVT-capable stroke center group (OR, 2.61; 95% CI, 1.53-4.44).
Considering the results obtained, we performed a sensitivity analysis adjusting regression models by stratifying factors, distance to assigned center, and time from stroke onset to arrival to assess whether the magnitude of the circuit’s effect held (eTable 2 in Supplement 2). Direct transfer to an EVT-capable stroke center was independently associated with reduced chance of functional independence at 90 days (AOR, 0.47; 95% CI, 0.25-0.90), higher mortality (adjusted HR, 1.76; 95% CI, 1.05-2.95), higher odds of death or severe functional dependency (AOR, 2.19; 95% CI, 1.01-4.77) and greater probabilities of adverse events until day 5 (AOR, 2.77; 95% CI, 1.26-6.07).
Discussion
In this prespecified secondary analysis of the RACECAT randomized clinical trial including patients with severe stroke symptoms, direct transport to an EVT-capable stroke center compared with transfer to the nearest local stroke center resulted in worse functional outcomes and higher mortality or severe functional dependency at 90 days among patients who received a final diagnosis of ICH.
The 2022 American Heart Association and American Stroke Association guidelines for the management of patients with spontaneous ICH5 pointed out the need for future research to evaluate whether particular systems of care are specifically beneficial or detrimental to patients with ICH. To the best of our knowledge, the present study provides the first data from a randomized clinical trial about the effect of different prehospital transport protocols among patients experiencing ICH. The RACECAT trial used a RACE Scale31,32 score of higher than 4 points as the best predictor of LVO, as previously defined.33,34 Similarly, recent published guidelines35 recommend prehospital bypassing mothership protocols (ie, transfer to the nearest EVT-capable stroke center, bypassing the local stroke center) to ensure rapid EVT access for patients with severe stroke symptoms in the field (RACE Scale score >4). That patients with ICH, representing 21.5% of the total triaged population, showed worse outcomes and higher rate of complications under the bypassing protocol is an important piece of information that obliges thought about optimal triage and transfer systems. The present study offers data on a population of interest for stroke code organization and may have implications for the design of future prehospital systems of care for acute stroke.
In our study, we found no differences in baseline vital signs and stroke severity between groups on arrival to the first center, despite a mean transport difference of 46.8 minutes. Blood pressure levels at admission, baseline ICH volume, radiologic markers of growth, and hematoma enlargement were similar between groups. Nor were differences found in further neurological worsening or mortality within the first 24 hours. Indeed, mortality curves started to diverge between groups from day 5 after stroke onset. Patients directly transferred to an EVT-capable stroke center had an approximately 5-fold higher chance of experiencing complications during transfer, such as vomiting, and a 2.6-fold higher chance of in-hospital pneumonia. Worse functional outcome observed in the shift analysis was driven by higher mortality among patients directly transferred to an EVT-capable stroke center, which may be caused in part by transfer complications and pneumonia. These results suggest new therapeutic targets to improve ICH outcomes, such as early prevention of vomiting or pneumonia, that potentially could be applied in the prehospital setting.
On the other hand, although a higher percentage of patients treated surgically was observed in the EVT-capable stroke center group, this treatment does not appear to have a significant effect on the prognosis of the patients. Although minimally invasive surgery36,37 may change the paradigm in the future, the only strategy capable of having an effect on outcome at the moment is the application of a “bundle of care”16,38 approach, implementing promptly all evidence-based treatments combined: antithrombotic reversal, blood pressure management, neurosurgery when indicated, and the appropriate level of supportive care. Direct transfer to centers with high capacities favors early access to neurosurgery, and neurocritical care may lead to longer transport times and the delayed implementation of the ICH bundle of care. Our results suggest that for patients experiencing ICH with initial severe stroke symptoms (RACE Scale score >4), transfer to centers with high capacities should be considered in a second stage after first receiving attention in the closest stroke center to guarantee early diagnosis and urgent treatment administration and to ensure the continuum of care.
Prehospital triage algorithms suggest routing patients based on the results of prehospital stroke severity scales. However, our results examining this strategy highlight the need to improve the accuracy of prehospital triage among patients with stroke. Prehospital differential diagnosis between ischemic stroke and ICH should become a priority as an evolution of the present LVO-centered EMS protocols. To this end, future research in prehospital stroke organization should focus on the improvement of prehospital stroke scales, the validation of new simple and reliable prehospital technologies,17 and the implementation of early point-of-care biomarkers.39,40 Moreover, new alternative prehospital strategies that avoid long initial transfers should be explored, such as “drip and drive,”41 in which the patient is transferred to the nearest local stroke center and the neurovascular team travels to provide care in a fully equipped angiography suite locally, and the widespread use of mobile stroke units.42,43,44 However, both alternatives remain a utopian scenario for many regions due to their high cost and low availability. For now, the results of the present study together with the main findings of RACECAT25 suggest that generalized bypass transfer protocols for patients with severe stroke symptoms located in areas not primarily covered by an EVT-capable stroke center should be considered only in specific circumstances.
Strengths and Limitations
This study has several limitations. First, the RACECAT trial population does not represent all patients with ICH because only patients presenting with a RACE Scale score higher than 4 were included. However, previous studies32 demonstrate that more than 70% of patients with ICH present with a RACE score higher than 4 on initial EMS evaluation. Second, the study findings are dependent on the specific characteristics of the Catalonian stroke health care system, in which local stroke center are located in nonurban areas more than 30 minutes from an EVT-capable stroke center (eFigure in Supplement 2). Network characteristics may vary significantly across different regions; thus, the direct application of our findings outside Catalonia, Spain, is limited, especially in urban areas with the coexistence of primary and comprehensive centers. Third, this study may have been underpowered to detect differences in secondary outcomes, as reflected by wide CIs and unstable regression models. Therefore, secondary outcome results should be interpreted as exploratory. Fourth, follow-up neuroimaging was not part of the study protocol. The effect of hematoma enlargement may be underestimated although the rate of follow-up CT was similar between the groups. Fifth, no data about clinical management during hospitalization and follow-up return transfers were collected. Thus, there may be other factors associated with the postacute care of each group that are not reflected in our study. This study has strengths. In particular, a strength of our study is that we provided data on stroke care in a clinical practice, population-based setting.
Conclusions
In this prespecified secondary analysis of a randomized clinical trial, for patients with severe stroke symptoms in areas not primarily covered by an EVT-capable stroke center, bypassing the nearest local stroke center resulted in a reduced chance of functional independence and potentially higher mortality at 90 days for patients who received a final diagnosis of ICH. These findings require replication in other settings.
Trial Protocol
eTable 1. Neuroimaging Characteristics
eTable 2. Primary and Secondary Outcomes (Sensitivity Analysis)
eFigure. Map of the Stroke Network in Catalonia
Nonauthor Collaborators. RACECAT Trial Investigators
Data Sharing Statement
References
- 1.Norrving B, Kissela B. The global burden of stroke and need for a continuum of care. Neurology. 2013;80(3)(suppl 2):S5-S12. doi: 10.1212/WNL.0b013e3182762397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.van Asch CJ, Luitse MJ, Rinkel GJ, van der Tweel I, Algra A, Klijn CJ. Incidence, case fatality, and functional outcome of intracerebral haemorrhage over time, according to age, sex, and ethnic origin: a systematic review and meta-analysis. Lancet Neurol. 2010;9(2):167-176. doi: 10.1016/S1474-4422(09)70340-0 [DOI] [PubMed] [Google Scholar]
- 3.Zahuranec DB, Lisabeth LD, Sánchez BN, et al. Intracerebral hemorrhage mortality is not changing despite declining incidence. Neurology. 2014;82(24):2180-2186. doi: 10.1212/WNL.0000000000000519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Poon MTC, Fonville AF, Al-Shahi Salman R. Long-term prognosis after intracerebral haemorrhage: systematic review and meta-analysis. J Neurol Neurosurg Psychiatry. 2014;85(6):660-667. doi: 10.1136/jnnp-2013-306476 [DOI] [PubMed] [Google Scholar]
- 5.Greenberg SM, Ziai WC, Cordonnier C, et al. 2022 Guideline for the management of patients with spontaneous intracerebral hemorrhage: a guideline from the American Heart Association/American Stroke Association. Stroke. 2022; 53(7):e282-e361. [DOI] [PubMed] [Google Scholar]
- 6.Morotti A, Boulouis G, Dowlatshahi D, et al. Intracerebral haemorrhage expansion: definitions, predictors, and prevention. Lancet Neurol. 2023;22(2):159-171. doi: 10.1016/S1474-4422(22)00338-6 [DOI] [PubMed] [Google Scholar]
- 7.Davis SM, Broderick J, Hennerici M, et al. ; Recombinant Activated Factor VII Intracerebral Hemorrhage Trial Investigators . Hematoma growth is a determinant of mortality and poor outcome after intracerebral hemorrhage. Neurology. 2006;66(8):1175-1181. doi: 10.1212/01.wnl.0000208408.98482.99 [DOI] [PubMed] [Google Scholar]
- 8.Szabo J, Smielewski P, Czosnyka M, et al. Heart rate variability is associated with outcome in spontaneous intracerebral hemorrhage. J Crit Care. 2018;48:85-89. doi: 10.1016/j.jcrc.2018.08.033 [DOI] [PubMed] [Google Scholar]
- 9.Zheng D, Arima H, Sato S, et al. ; INTERACT2 investigators . Low ambient temperature and intracerebral hemorrhage: the INTERACT2 study. PLoS One. 2016;11(2):e0149040. doi: 10.1371/journal.pone.0149040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zheng J, Yu Z, Ma L, et al. Association between blood glucose and functional outcome in intracerebral hemorrhage: a systematic review and meta-analysis. World Neurosurg. 2018;114:e756-e765. doi: 10.1016/j.wneu.2018.03.077 [DOI] [PubMed] [Google Scholar]
- 11.Manning L, Hirakawa Y, Arima H, et al. ; INTERACT2 investigators . Blood pressure variability and outcome after acute intracerebral haemorrhage: a post-hoc analysis of INTERACT2, a randomised controlled trial. Lancet Neurol. 2014;13(4):364-373. doi: 10.1016/S1474-4422(14)70018-3 [DOI] [PubMed] [Google Scholar]
- 12.Shigematsu K, Shimamura O, Nakano H, et al. Vomiting should be a prompt predictor of stroke outcome. Emerg Med J. 2013;30(9):728-731. doi: 10.1136/emermed-2012-201586 [DOI] [PubMed] [Google Scholar]
- 13.Lindner A, Kofler M, Rass V, et al. Early predictors for infectious complications in patients with spontaneous intracerebral hemorrhage and their impact on outcome. Front Neurol. 2019;10(JUL):817. doi: 10.3389/fneur.2019.00817 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Qian C, Löppönen P, Tetri S, et al. Immediate, early and late seizures after primary intracerebral hemorrhage. Epilepsy Res. 2014;108(4):732-739. doi: 10.1016/j.eplepsyres.2014.02.020 [DOI] [PubMed] [Google Scholar]
- 15.Balami JS, Buchan AM. Complications of intracerebral haemorrhage. Lancet Neurol. 2012;11(1):101-118. doi: 10.1016/S1474-4422(11)70264-2 [DOI] [PubMed] [Google Scholar]
- 16.Parry-Jones AR, Sammut-Powell C, Paroutoglou K, et al. An intracerebral hemorrhage care bundle is associated with lower case fatality. Ann Neurol. 2019;86(4):495-503. doi: 10.1002/ana.25546 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ramos A, Guerrero WR, Pérez de la Ossa N. Prehospital stroke triage. Neurology. 2021;97(20)(suppl 2):S25-S33. doi: 10.1212/WNL.0000000000012792 [DOI] [PubMed] [Google Scholar]
- 18.Fassbender K, Walter S, Grunwald IQ, et al. Prehospital stroke management in the thrombectomy era. Lancet Neurol. 2020;19(7):601-610. doi: 10.1016/S1474-4422(20)30102-2 [DOI] [PubMed] [Google Scholar]
- 19.Goyal M, Menon BK, van Zwam WH, et al. ; HERMES collaborators . Endovascular thrombectomy after large-vessel ischaemic stroke: a meta-analysis of individual patient data from five randomised trials. Lancet. 2016;387(10029):1723-1731. doi: 10.1016/S0140-6736(16)00163-X [DOI] [PubMed] [Google Scholar]
- 20.Saver JL, Goyal M, van der Lugt A, et al. ; HERMES collaborators . Time to treatment with endovascular thrombectomy and outcomes from ischemic stroke: a meta-analysis. JAMA. 2016;316(12):1279-1288. doi: 10.1001/jama.2016.13647 [DOI] [PubMed] [Google Scholar]
- 21.Fletcher JJ, Kotagal V, Mammoser A, Peterson M, Morgenstern LB, Burke JF. Cost-effectiveness of transfers to centers with neurological intensive care units after intracerebral hemorrhage. Stroke. 2015;46(1):58-64. doi: 10.1161/STROKEAHA.114.006653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Iihara K, Nishimura K, Kada A, et al. Effects of comprehensive stroke care capabilities on in-hospital mortality of patients with ischemic and hemorrhagic stroke: J-ASPECT study. PLoS One. 2014;9(5):e96819. doi: 10.1371/journal.pone.0096819 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.McKinney JS, Cheng JQ, Rybinnik I, Kostis JB; Myocardial Infarction Data Acquisition System (MIDAS 22) Study Group . Comprehensive stroke centers may be associated with improved survival in hemorrhagic stroke. J Am Heart Assoc. 2015;4(5):1-8. doi: 10.1161/JAHA.114.001448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Naval NS, Carhuapoma JR. Impact of pattern of admission on ICH outcomes. Neurocrit Care. 2010;12(2):149-154. doi: 10.1007/s12028-009-9302-0 [DOI] [PubMed] [Google Scholar]
- 25.Pérez de la Ossa N, Abilleira S, Jovin TG, et al. ; RACECAT Trial Investigators . Effect of direct transportation to thrombectomy-capable center vs local stroke center on neurological outcomes in patients with suspected large-vessel occlusion stroke in nonurban areas: the RACECAT randomized clinical trial. JAMA. 2022;327(18):1782-1794. doi: 10.1001/jama.2022.4404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Abilleira S, Pérez de la Ossa N, Jiménez X, et al. Transfer to the local stroke center versus direct transfer to endovascular center of acute stroke patients with suspected large vessel occlusion in the Catalan Territory (RACECAT): study protocol of a cluster randomized within a cohort trial. Int J Stroke. 2019;14(7):734-744. doi: 10.1177/1747493019852176 [DOI] [PubMed] [Google Scholar]
- 27.Steiner T, Al-Shahi Salman R, Beer R, et al. ; European Stroke Organisation . European Stroke Organisation (ESO) guidelines for the management of spontaneous intracerebral hemorrhage. Int J Stroke. 2014;9(7):840-855. doi: 10.1111/ijs.12309 [DOI] [PubMed] [Google Scholar]
- 28.Saver JL, Filip B, Hamilton S, et al. ; FAST-MAG Investigators and Coordinators . Improving the reliability of stroke disability grading in clinical trials and clinical practice: the Rankin Focused Assessment (RFA). Stroke. 2010;41(5):992-995. doi: 10.1161/STROKEAHA.109.571364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Morotti A, Boulouis G, Dowlatshahi D, et al. ; International NCCT ICH Study Group . Standards for detecting, interpreting, and reporting noncontrast computed tomographic markers of intracerebral hemorrhage expansion. Ann Neurol. 2019;86(4):480-492. doi: 10.1002/ana.25563 [DOI] [PubMed] [Google Scholar]
- 30.Demchuk AM, Dowlatshahi D, Rodriguez-Luna D, et al. ; PREDICT/Sunnybrook ICH CTA study group . Prediction of haematoma growth and outcome in patients with intracerebral haemorrhage using the CT-angiography spot sign (PREDICT): a prospective observational study. Lancet Neurol. 2012;11(4):307-314. doi: 10.1016/S1474-4422(12)70038-8 [DOI] [PubMed] [Google Scholar]
- 31.Pérez de la Ossa N, Carrera D, Gorchs M, et al. Design and validation of a prehospital stroke scale to predict large arterial occlusion: the Rapid Arterial Occlusion Evaluation scale. Stroke. 2014;45(1):87-91. doi: 10.1161/STROKEAHA.113.003071 [DOI] [PubMed] [Google Scholar]
- 32.Carrera D, Gorchs M, Querol M, et al. ; Catalan Stroke Code and Reperfusion Consortium (Cat-SCR) . Revalidation of the RACE scale after its regional implementation in Catalonia: a triage tool for large vessel occlusion. J Neurointerv Surg. 2019;11(8):751-756. doi: 10.1136/neurintsurg-2018-014519 [DOI] [PubMed] [Google Scholar]
- 33.Duvekot MHC, Venema E, Rozeman AD, et al. ; PRESTO investigators . Comparison of eight prehospital stroke scales to detect intracranial large-vessel occlusion in suspected stroke (PRESTO): a prospective observational study. Lancet Neurol. 2021;20(3):213-221. doi: 10.1016/S1474-4422(20)30439-7 [DOI] [PubMed] [Google Scholar]
- 34.Nguyen TTM, van den Wijngaard IR, Bosch J, et al. Comparison of prehospital scales for predicting large anterior vessel occlusion in the ambulance setting. JAMA Neurol. 2021;78(2):157-164. doi: 10.1001/jamaneurol.2020.4418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jauch EC, Schwamm LH, Panagos PD, et al. ; Prehospital Stroke System of Care Consensus Conference . Recommendations for regional stroke destination plans in rural, suburban, and urban communities from the Prehospital Stroke System of Care Consensus Conference: a consensus statement from the American Academy of Neurology, American Heart Association/American Stroke Association, American Society of Neuroradiology, National Association of EMS Physicians, National Association of State EMS Officials, Society of NeuroInterventional Surgery, and Society of Vascular and Interventional Neurology: endorsed by the Neurocritical Care Society. Stroke. 2021;52(5):e133-e152. doi: 10.1161/STROKEAHA.120.033228 [DOI] [PubMed] [Google Scholar]
- 36.Hanley DF, Thompson RE, Rosenblum M, et al. ; MISTIE III Investigators . Efficacy and safety of minimally invasive surgery with thrombolysis in intracerebral haemorrhage evacuation (MISTIE III): a randomised, controlled, open-label, blinded endpoint phase 3 trial. Lancet. 2019;393(10175):1021-1032. doi: 10.1016/S0140-6736(19)30195-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hanley DF, Thompson RE, Muschelli J, et al. ; MISTIE Investigators . Safety and efficacy of minimally invasive surgery plus alteplase in intracerebral haemorrhage evacuation (MISTIE): a randomised, controlled, open-label, phase 2 trial. Lancet Neurol. 2016;15(12):1228-1237. doi: 10.1016/S1474-4422(16)30234-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Parry-Jones AR, Moullaali TJ, Ziai WC. Treatment of intracerebral hemorrhage: from specific interventions to bundles of care. Int J Stroke. 2020;15(9):945-953. doi: 10.1177/1747493020964663 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ramos-Pachón A, López-Cancio E, Bustamante A, et al. D-dimer as predictor of large vessel occlusion in acute ischemic stroke. Stroke. 2021;52(3):852-858. doi: 10.1161/STROKEAHA.120.031657 [DOI] [PubMed] [Google Scholar]
- 40.Bustamante A, Penalba A, Orset C, et al. Blood biomarkers to differentiate ischemic and hemorrhagic strokes. Neurology. 2021;96(15):e1928-e1939. doi: 10.1212/WNL.0000000000011742 [DOI] [PubMed] [Google Scholar]
- 41.Brekenfeld C, Goebell E, Schmidt H, et al. ‘Drip-and-drive’: shipping the neurointerventionalist to provide mechanical thrombectomy in primary stroke centers. J Neurointerv Surg. 2018;10(10):932-936. doi: 10.1136/neurintsurg-2017-013634 [DOI] [PubMed] [Google Scholar]
- 42.Grotta JC, Yamal J-M, Parker SA, et al. Prospective, multicenter, controlled trial of mobile stroke units. N Engl J Med. 2021;385(11):971-981. doi: 10.1056/NEJMoa2103879 [DOI] [PubMed] [Google Scholar]
- 43.Helwig SA, Ragoschke-Schumm A, Schwindling L, et al. Prehospital stroke management optimized by use of clinical scoring vs mobile stroke unit for triage of patients with stroke: a randomized clinical trial. JAMA Neurol. 2019;76(12):1484-1492. doi: 10.1001/jamaneurol.2019.2829 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cooley SR, Zhao H, Campbell BCV, et al. ; Melbourne Mobile Stroke Unit Collaboration . Mobile stroke units facilitate prehospital management of intracerebral hemorrhage. Stroke. 2021;52(10):3163-3166. doi: 10.1161/STROKEAHA.121.034592 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Trial Protocol
eTable 1. Neuroimaging Characteristics
eTable 2. Primary and Secondary Outcomes (Sensitivity Analysis)
eFigure. Map of the Stroke Network in Catalonia
Nonauthor Collaborators. RACECAT Trial Investigators
Data Sharing Statement