Key words: Functional constipation, Prebiotics, Microbiota, Dietary fibre
Abstract
Functional constipation is a significant health issue impacting the lives of an estimated 14 % of the global population. Non-pharmaceutical treatment advice for cases with no underlying medical conditions focuses on exercise, hydration and an increase in dietary fibre intake. An alteration in the composition of the gut microbiota is thought to play a role in constipation. Prebiotics are non-digestible food ingredients that selectively stimulate the growth of a limited number of bacteria in the colon with a benefit for host health. Various types of dietary fibre, though not all, can act as a prebiotic. Short-chain fatty acids produced by these microbes play a critical role as signalling molecules in a range of metabolic and physiological processes including laxation, although details are unclear. Prebiotics have a history of safe use in the food industry spanning several decades and are increasingly used as supplements to alleviate constipation. Most scientific research on the effects of prebiotics and gut microbiota has focussed on inflammatory bowel disease rather than functional constipation. Very few clinical studies evaluated the efficacy of prebiotics in the management of constipation and their effect on the microbiota, with highly variable designs and conflicting results. Despite this, broad health claims are made by manufacturers of prebiotic supplements. This narrative review provides an overview of the literature on the interaction of prebiotics with the gut microbiota and their potential clinical role in the alleviation of functional constipation.
Functional constipation is the most common gastrointestinal disorder affecting about 14 % of the global population and it negatively impacts the quality of life of those affected(1). In addition, there is a substantial cost to health care systems and further out of pocket costs are incurred by those suffering from the condition. In the UK, costs of £168 million to the National Health Service have been reported by the Bowel Interest Group for just 1 year (2018–2019), with more than 175 000 patient days spent in hospital and numbers are increasing(2). Functional constipation is defined as infrequent bowel motions fewer than three times per week over a 3-month period with feelings of incomplete evacuation and excessive straining, without an underlying disease(3). Non-pharmacological treatment advice from health care authorities such as the British Nutrition Foundation includes increasing fluid intake, physical activity and increasing dietary fibre consumption to a recommended daily intake of 30 g for adults(4).
The importance of the gut microbiota for digestive health has been increasingly recognised in recent years and considerable research efforts and funding have been invested to elucidate the details(5). An association has been made between the composition and function of the gastrointestinal microbiota in inflammatory bowel disease, coeliac disease, cancer, major depressive disorder and a range of extraintestinal disorders(6,7). Less is known about an association between the gut microbiota and functional constipation(8). Diet greatly influences the composition of these microbial communities and particular research attention has focussed on the effects of fermentable fibres that humans are unable to digest(8). Various species of bacteria have been identified as utilising this fibre as fuel to produce metabolites that contribute to the energy balance of the host and confer colonic and extraintestinal health benefits such as production of B vitamins(9). These fibres known as ‘prebiotics’ have also been in use for several decades as ingredients in manufactured food products as well as in nutritional supplements. However, the mechanisms of how they exert an effect on the microbial environment and the host have not been fully elucidated(9).
This review seeks to summarise the literature pertinent to the topic of functional constipation and the microbiota and outlines key research gaps that require focussed scientific inquiry, namely, the intersect between prebiotics, the gut microbiota and the effect on constipation.
Constipation
Functional constipation (Table 1) is a type of functional gastrointestinal disorder that is characterised by a combination of motility disturbance, visceral hypersensitivity, altered gut microbiota and altered central nervous system processing(10). The causes for constipation are multifactorial and still under investigation. They are broadly characterised as primary idiopathic with normal transit, slow transit or evacuation disorder and secondary constipation which includes medication side-effects, obstruction, metabolic, neurological, psychiatric or systemic causes(10). In clinical practice, the side effects of some medications can result in alterations to bowel movements including constipation. Medications associated with altered bowel movements include non-steroidal anti-inflammatory drugs, calcium channel blockers, opiates, anti-depressants, anticholinergic agents, diuretics, antacids and chemotherapy agents(11).
Table 1.
Terms and definitions of functional constipation, prebiotics and dietary fibre
| Terms and definitions | |
|---|---|
| Functional constipation | Functional constipation, normal and slow transit constipation, chronic or chronic idiopathic constipation are used interchangeably(14,69). Here the definition according to the ROME foundation’s expert committee of gastroenterologists is used, with the Rome IV criteria being current at the time of writing(3). A person must have experienced at least two of the following symptoms for the previous 3 months to be diagnosed with functional constipation: Fewer than three spontaneous bowel movements per week; straining for more than 25 % of defecation attempts; lumpy or hard stools for at least 25 % of defecation attempts; sensation of anorectal obstruction or blockage for at least 25 % of defecation attempts; sensation of incomplete defecation for at least 25 % of defecation attempts; manual manoeuvring required to defecate for at least 25 % of defecation attempts. In addition, loose stools should rarely be present without laxatives and there should be no diagnosis of irritable bowel syndrome (IBS). |
| Prebiotics | In 2016, the International Scientific Association for Probiotics and Prebiotics defined prebiotics as substances which are selectively used by microbes conferring a demonstrated health benefit to the host(70). They are mainly non-digestible oligosaccharides fructans, galactans and inulin(71). They may include polyphenols and conjugated fatty acids. Candidate prebiotics include pectins, arabinoxylan, resistant starches, whole grains, cellulose, mannose, maltose, polydextrose, lactulose and β-glucans(71). |
| Dietary fibre | Natural foods contain a mix of soluble and insoluble fibres. Soluble fibres can be viscous (psyllium) or non-viscous (guar gum), and in addition fermentable (guar gum, pectins, inulin) or non-fermentable (cellulose, lignin) with overlapping categories(72). Only fermentable fibre has an effect on microbiota but not necessarily an effect on laxation(70). |
The negative impact on a person’s quality of life of this ailment is comparable to other chronic conditions such as dermatitis, chronic allergies, depression, diabetes or musculoskeletal conditions such as arthritis or osteoporosis, and the mental health effects are considered to be more severe than the physical components(12). Adults and children have reported substantial limitations to their daily activities and only feel comfortable at rest. These negative impacts on quality of life extend to caregivers of children with constipation(12).
For the patient, understanding the characteristics of a normal stool plays an important role in patient education and for monitoring and managing the treatment of functional constipation. The Bristol Stool Form Chart (Fig. 1) has been developed to describe stool consistency in an identifiable way and it has been validated to reliably indicate intestinal transit time(13). It is broadly used in clinical practice and research for stool classification not only for irritable bowel syndrome (IBS) but also for functional constipation(14–16). Type 1 is classified as severe constipation, type 2 as mild constipation, types 3 and 4 are normal, types 5 to 7 may indicate diarrhoea or urgency. This visual scale can be a valuable tool for integration into self-care and monitoring.
Fig. 1.
Bristol Stool Chart (Cabot Health, http://cdn.intechopen.com/pdfs-wm/46082.pdf). Commonly used classification tool for stools according to consistency.
Standard management recommendations for constipation by health care authorities include exercising, adequate hydration and dietary changes with an increase of fibre intake(17). Despite general agreement about these treatment recommendations, high quality clinical studies evaluating the efficacy of increased fibre intake in the management of functional constipation are lacking(18). Due to the heterogeneity of definitions used for functional constipation, the use of the term dietary fibre without differentiation between non-fermentable fibre and prebiotics (Table 1), and the different types of cohorts studied, outcomes cannot easily be directly compared or generalised. Several meta-analyses of randomised controlled trials and Rome Foundation reports evaluating fermentable or non-fermentable fibre reported some benefits in the treatment of functional constipation, and they reported side effects such as increased flatulence(19,20). Criticism of available studies includes concerns about selection bias, functional constipation being poorly defined or with large differences between studies and large placebo responses with differing endpoints(21).
Microbial composition and constipation
The causes of functional constipation are multifactorial, with dysbiosis of the gut microbiota considered to be one contributing factor(20). Other factors may include low fluid intake, lack of dietary fibre, excess caffeine or alcohol intake, endocrine, neurological or psychological issues(22). Differences in faecal microbial species and abundances have been reported for people with constipation compared to those with normal gut function, but data are limited and findings are very inconsistent between studies(20).
For instance, two single point-in-time studies comparing the microbiota of healthy and constipated participants reported quite different results. Investigating the composition of the faecal microbiota, Mancabelli et al. (23) conducted a profiling analysis targeting the V3 region of the 16S rRNA gene in sixty-eight participants affected by constipation compared to seventy-nine healthy participants. Low counts of faecal Bacteroides, Roseburia and Coprococcus were found but species diversity in general was greater in constipated participants. Considering that an age-specific development and shift of the gut microbiota with substantial differences between children, adults and elderly have been observed, and the ages in the study ranged from 4 to 93 years, it has been suggested that subgroup analyses may have provided more specific information(24). Shotgun sequencing was used to elucidate metabolic pathways in a subset of five constipated and five healthy participants. A high abundance of genes involved in methane production was identified in the constipated, whilst in healthy participants genes involved in carbohydrate and fatty acid metabolism were identified in greater abundance. The authors concluded their findings indicate an association between functional constipation and alterations of key microbial metabolic pathways, although they alert that the results from this small sample size require validation by larger studies.
In contrast, Parthasarathy et al. (25) compared faecal and mucosal microbiota samples of twenty-five healthy and twenty-five constipated females investigating different regions of the 16S rRNA gene (V3–V5 regions), finding characteristic differences between the microbial composition of the two specimen types. Unlike in Mancabelli et al. (2017), methane production was not associated with constipation. In faecal samples, the authors found the same taxa in both groups of participants, with differences only in abundance but not in species richness. It also has to be considered that this group of twenty-five constipated females consisted of thirteen with functional constipation, six with IBS-Constipation and six with moderate to very severe symptoms of mixed IBS, which can be expected to influence outcomes.
Prebiotics
Definition of a prebiotic
The definition of what constitutes a prebiotic had evolved since its inception in 1995 when it was restricted to non-digestible food ingredients that selectively stimulate the growth of a selected number of bacteria in the colon thought to be a benefit for host health, which limited it to fructo-oligosaccharides(26). Other carbohydrates, such as resistant starches, pectin, gums and gluco-oligosaccharides were certainly recognised to be food for microorganisms, but they lacked specificity and they were excluded because of their potential to promote the growth of pathogens.
In the revised definition, a prebiotic selectively stimulates the growth of bacteria that have a ‘favourable’ metabolomic profile in the gastrointestinal tract(27). Consequently, a wide range of plant components are considered to be prebiotics, such as inulin-type fructans, fructo-oligosaccharides and lactulose, which have been shown to increase colonies of microbial genera that are recognised to be health promoting, e.g. Lactobacilli and Bifidobacteria(27). Prebiotics occur naturally in many fruits, vegetables and algae – it has been estimated that some 36 000 plant species contain fructo-oligosaccharides and other polysaccharides(28). They are found in common foods such as asparagus, garlic, onion, wheat, honey, banana, barley, tomato, milk, peas and beans(28). While prebiotics encountered in natural foodstuffs can be classified under dietary fibre, not all dietary fibre is prebiotic; fibre that is not fermentable by microbes is not considered to be prebiotic. Knowledge about prebiotic action in increasing colonic populations of beneficial commensal bacteria has been extrapolated to the assumption that combining probiotics with prebiotics into a synbiotic would confer an increased benefit(29). A range of synbiotics is commercially available, for example Lactobacillus spp. and inulin, or Lactobacillus, Streptococcus and Bifidobacterium spp. and fructo-oligosaccharides.
The narrow definition of a prebiotic continues to be debated, with critics arguing that it is still unclear which microbes are relevant to human diseases, and although dysbiosis accompanies many diseases, the questions about causal relationships are largely unanswered(30). Therefore, microbial selectivity as a criterion becomes questionable since there is no consensus about what constitutes a healthy microbiota. Molecular studies have shown that no single carbohydrate is likely to be fermented by only a selective group of microbes and none is fermented by all(30). Cross-feeding, where metabolites from one bacterial strain create a niche for another, also contributes to greater diversity which is considered an indicator of good health(31). Diet, lifestyle and genotype of the host have been shown to create an environment where microbes normally considered to be beneficial can become detrimental, bringing into question the over-simplistic notion of ‘good v. bad’. Thus, knowledge of functional effects of the gastrointestinal microbiota and prebiotics is needed to support the concept of a ‘prebiotic effect’(30).
The gut microbiota and prebiotics
The gut microbiota, with an estimated 3 million genes, is 150 times larger than the human genome and contributes to such an extent to our metabolic capacity that it is considered by many as fulfilling the functions of another organ(32,33). The microbes produce enzymes that can ferment prebiotics, which humans otherwise have no capacity to metabolise. The effect of this fermentation is of interest since it provides a major energy source for the host and it produces metabolites that interact with the enteric nervous system, influencing motility(34). In regards to constipation, prebiotics are a common plant-based food supplement employed to restore homeostasis of a dysbiotic gut(8). Prebiotic fermentation and resulting metabolites, including short-chain fatty acids (SCFA), have far-reaching physiological consequences. SCFA play an important role as signalling molecules and their regulatory function for local, intermediary and peripheral metabolism has gained research interest since the discovery of receptors across a wide range of cells and tissue types around the body(35). In the distal and innervated colon, SCFA appear to have a regulatory function on motility and defecation reflexes, and different prebiotics have different effects on microbial abundances(34,35).
In addition, the individual microbial composition of each person is of great importance since bacterial species vary in their capacity to produce SCFA from different types of fibre. The optimal amount of SCFA has not been established, and most are absorbed and used by enterocytes or enter the circulation, so amounts measured in faeces may not necessarily reflect SCFA production rates(36). SCFA play a role in the immune response via regulating differentiation and activation of immune cells and through regulation of inflammatory cytokines they act as key mediators of inflammation(35). SCFA also modulate T-cell differentiation and inhibit inflammatory IFN-γ-producing cells. This role is considered by some as the key molecular link between diet, microbiota and health(35). As discussed, the fermentation of prebiotics is a complex process and depending on the individual microbial composition of each person and the type of fibre, SCFA production rates may vary considerably between individuals(37).
Microbiota–gut–brain axis
Historically, functional gastrointestinal disorders have been investigated in terms of interactions between the enteric neuromuscular system, neurotransmitters and the brain, which gave rise to the term ‘gut-brain axis’(38). More recent research identified a regulatory function of the brain on functional intestinal cells via neural and hormonal pathways(39). At the same time, the influence of commensal bacteria as well as pathogens on the brain has been established, leading to the extension of the model now named the ‘microbiota–gut–brain axis’(40). The brain and microbiota are thought to communicate via the immune system, the vagus nerve, tryptophan metabolism and the enteric nervous system, through microbial metabolites such as SCFA, branched-chain amino acids and peptidoglycans. Metabolites of the gut microbiota, in particular the neurotransmitter serotonin produced by Clostridium spp. and Escherichia coli, have been shown to exert a modulatory effect on peristalsis and motility through interactions with the enteric nervous system(41). In addition, ‘cross-talk’ between bile acids and the microbiota regulates motility: bile acids themselves affect the composition of the microbiota whilst deconjugated bile acids are used as signalling molecules by the microbiota(42) (Fig. 2). Research showed that serotonin can also influence microbial composition of the gut: relative abundances of receptive species, such as Turicibacter are increased(43). This, in turn modulates functional capabilities with increases in steroid and lipid metabolism, but details require further investigation.
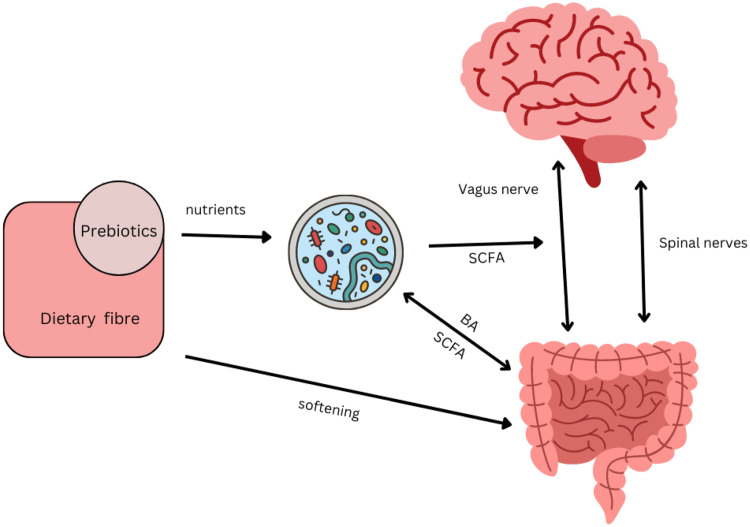
Fig. 2.
Interacting pathways between microbiota, prebiotics, dietary fibre, gut and brain affecting constipation. Several pathway impact on gut motility. There is bidirectional communication between gut and brain via vagal and spinal nerves, with serotonin as the main neurotransmitter(34). SCFA produced by the microbiota can directly stimulate nerves cells in the gut, or indirectly stimulate enteroendocrine cells to produce serotonin and consequently trigger peristaltic reflexes(34). SCFA can also directly stimulate receptors on the vagus nerve(41). Secondary bile acids (BA) produced by the microbiota can affect motility, while BA themselves affect the composition of the microbiota(42). The microbial composition of the gut is influenced by the enteric nervous system through immunological defence secretions, permeability or motility(41). Transit time influences the composition of the microbiota through exposure to water and nutrients. Prebiotics such as galacto-oligosaccharides can increase SCFA-producing Bifidobacterium spp. and increase motility in constipated individuals(51). Non-fermentable dietary fibre such as coarse wheat bran and psyllium husks soften stools making them easier to pass by mechanisms that do not directly involve the microbiota(68) but affects the environment in which they reside.
Studies evaluating clinical and microbial effects of prebiotics in people with constipation
A limited number of clinical trials have investigated the efficacy of prebiotics for alleviating constipation and the concomitant effects on the microbiota. The comparison of studies and the evaluation of prebiotic efficacy is seriously hampered by a number of issues plaguing the field. Only some studies have used people with symptoms of constipation(44–51) (Table 2), with differing criteria of functional constipation ranging from undefined or vague(23,44,46,47,50), Rome I, III or IV(25,45,51), or combinations with IBS-C criteria(49), while others tested healthy people(52–59). The number of participants varies greatly and very small studies lack the power for generalisable results(60,61). There are large differences in age groups of study participants between studies, with some sampling from a very wide range(23,44). The products/formulations and dosages vary considerably between studies, from 1 g/d(52) to 20 g/d(45) in studies on inulin, or single ingredient studies(46) through to formulations that include multiple ingredients such as inulin, lactitol and aloe vera(48), or without listing ingredients at all(50). Finally, every study differs in the methodology used for microbial analysis(23,44–47,49,51) and often a restricted range of microbes were investigated, for example, five particular species(47) or only Lactobacillus spp.(44) or Bifidobacterium spp.(46). This leads to an equivocal overall picture of the efficacy of prebiotics, which is particularly lamentable since functional constipation is of such a high prevalence in the general population. Nonetheless, the trials that have been reported do provide a wealth of information.
Table 2.
Summary of clinical trials in participants with functional constipation that evaluated the effect of prebiotic treatment on bowel frequency and composition of the gut microbiota
| Authors | Title | Product, dosage, duration, participants | Study type | Outcome |
|---|---|---|---|---|
| Takahashi et al. 1994(44) | Influence of partially hydrolysed guar gum on constipation in women | PHGG 11 g/d 9 weeks Fifteen women (18–48 years) with constipation |
Open label, 3 weeks no treatment, 3 weeks PHGG, 3 weeks no treatment |
Increase in stool frequency to 4·4 motions/week; doubling of Lactobacillus spp. during intervention |
| Bouhnik et al. 2004(45) | Prospective, randomised, parallel-group trial to evaluate the effects of lactulose and polyethylene glycol-4000 on colonic flora in chronic idiopathic constipation | Lactulose or polyethylene glycol (PEG) 20 g/d 4 weeks Sixty-five adults (mean age 58 years) with constipation |
Open label, randomised, parallel groups, n 33 lactulose, n 32 PEG; 20 g/d for 1 week, then individual variation according to efficacy and tolerance 10–30 g/d |
Increase in bowel motions to 7·8 motions/week in lactulose and 6·8/week in PEG group; increase in Bifidobacterium spp. in lactulose group, decrease in SCFA production in PEG group |
| Marteau et al. 2011(46) | Effects of chicory inulin in constipated elderly people: a double-blind controlled trial | Inulin 15 g/d 4 weeks Fifty adults (51–62 years) with constipation |
Double-blind, randomised, parallel groups, n 25 inulin, n 25 maltodextrin | Reduced defecation difficulties; increase in Bifidobacterium spp. and total bacterial count with inulin |
| Linetzky Waitzberg et al. 2012(47) | Microbiota benefits after inulin and partially hydrolised guar gum supplementation – a randomised clinical trial in constipated women | Inulin/PHGG blend 15 g/d 3 weeks Thirty-two women (36–40 years) with constipation |
Double-blind, randomised, parallel groups, n 14 inulin/PHGG blend, n 18 maltodextrin | Increase in stool frequency to 3·9 motions/week with inulin/PHGG and 3·2 with maltodextrin; Clostridium spp. count reduced with inulin/PHGG blend; no difference in SCFA production |
| Chu et al. 2019(48) | Prebiotic UG1601 mitigates constipation-related events in association with gut microbiota: A randomised placebo-controlled intervention study | inulin, lactitol, aloe vera mix 13 g/d 4 weeks forty adults with constipation (21–51 years) |
Double-blind, randomised, parallel groups, n 20 prebiotic, n 20 maltodextrin | Increase of bowel motions in both groups to 4·1/week; Roseburia increased and Lachnospiraceae decreased in prebiotic group; no difference in SCFA concentration between groups |
| Jalanka et al. 2019(49) | The effect of psyllium husk on intestinal microbiota in constipated patients and healthy controls | Psyllium 21 g/d 18 d, Sixteen adults with constipation (mean age 41 years ± 15·7 years) were compared to a separate trial involving nine healthy adults (26 years ± 4·1 years) |
Double-blind, randomised crossover, 6 d high dose, 6 d maltodextrin with 1 week washout between. |
Improvement in bowel function; significant changes in microbiota; increased Lachnospira, Faecalibacterium, Phascolarctobacterium, Veillonella, Sutterella and decreased Coriobacteria and Christensenella |
| Stachowska et al. 2022(50) | Improvement of bowel movements among people with a sedentary lifestyle after prebiotic snack supply - preliminary study | Unspecified prebiotic soluble fibre in snack bar high dose 13·9 g/d or low dose 10 g/d 14 d, twenty adults with constipation (mean age 40·6 years ± 5·4 years) |
Double-blind, randomised, parallel groups, n 10 high dose, n 10 low dose | Increase of bowel motions in both groups to 6/weeks; increase in SCFA production in both groups |
| Schoemaker et al. 2022(51) | Prebiotic galacto-oligosaccharides impact stool frequency and fecal microbiota in self-reported constipated adults: A randomised clinical trial | Galacto-oligosaccharide low dose 5·5 g/d or high dose 11 g/d; 3 weeks 132 adults with constipation (25–52 years) |
Double-blind, randomised, parallel groups, n 45 low dose, n 44 high dose, n 43 maltodextrin | Significantly higher increase in bowel motions with high dose for sub-group with low stool frequency at baseline; Higher Bifidobacterium and Anaerostipes hadrus in high dose group |
PHGG, partially hydrolysed guar gum.
Bifidobacterium is a genus that is commonly associated with a healthy gut and is often highlighted by studies investigating an association between gut function and microbiota. In separate trials, faecal Bifidobacteria increased with lactulose(45) and inulin(46) consumption in participants afflicted by functional constipation, and this was accompanied by improved bowel function. Increases in Bifidobacteria and stool frequency were also reported in trials with healthy participants who took lactulose(52), while for inulin Bifidobacteria increased but these healthy participants did not experience changes in stool frequency(58). Bifidobacteria also increased in healthy people, again without a change in stool frequency, with the use of arabinogalactans(56,57) or galacto-oligosaccharides(54,55). Schoemaker et al. (2022) also found that galacto-oligosaccharides increased Bifidobacterium spp. but highlighted the importance of working with participants actually suffering with constipation, as they found a significant increase in stool frequency only on sub-group analysis of participants with a particularly low frequency of bowel motions at the start of the trial(51).
In contrast, no difference in Bifidobacterium spp. was found between two groups of thirty females with constipation who either used a blend of inulin and partially hydrolysed guar gum or maltodextrin for 21 d(47). Both groups had increases in bowel motions, with no significant difference between groups. A decrease in pathogenic Clostridium spp. in the prebiotic group and an increase thereof in the maltodextrin group was observed(47). Similarly, no changes in Bifidobacteria were found in participants taking psyllium husk, whether healthy or constipated, and both groups experienced improved bowel function(49). The authors analysed the V4–V5 regions of the 16S rRNA gene and found that there was a significant effect of the prebiotic on the composition of the microbiota in both groups of participants, although more pronounced in the constipated participants. Here, a significant increase in SCFA producers Lachnospira, Faecalibacterium and Roseburia was identified. These studies show that although Bifidobacteria are generally considered to be beneficial, an increase thereof is not necessarily correlated with an increase in stool frequency.
In health research, increasingly sophisticated bioinformatic technology is employed to predict bacterial genera associated with various illnesses including constipation(62). Machine learning technology is a discipline of computer science where computers are programmed to be able to recognise patterns from data following mathematical rules and statistical assumptions to enable the development of predictive models from a dataset. In a meta-analysis of five research cohorts with 3056 faecal amplicon sequence data, Chen et al. (62) employed systematic machine learning technology to identify potential biomarkers for constipation. The model they constructed enabled the identification of fifteen key genera as possible biomarkers with the most significant being Serratia, Dorea, Agathobacter, Hungatella and Aeromonas. In addition, the taxonomic analyses by Chen et al. (62) also found greater species diversity and richness in the constipated group. Interestingly, the commonly investigated genera Bifidobacterium and Lactobacillus did not feature on their list.
The Rome Foundation stated that quantitative and qualitative changes in mucosal and faecal microbiota have been encountered in functionally constipated patients, with greater changes in IBS compared to those affected by functional constipation(10). The researchers recognised equivocal results reported from several studies and recommended larger clinical trials with clearer target definitions and a closer cooperation between experienced clinical researchers and microbial ecologists, highlighting the importance of interdisciplinary research contributions to trial design and interpretation.
In summary, researchers agree that prebiotic products affect the microbiota but not all act in the same way in every person. There is some data on clinical utility for the use of prebiotics in constipation, but it is inconclusive. Apart from the necessity for large-scale clinical trials, the mechanisms of action of prebiotics need further elucidation. The microbial composition typically encountered in individuals with functional constipation also requires further investigation; although there is some evidence that alterations to the composition of the gut microbiota may contribute to the symptoms(10,18,23), constipation creates a habitat that favours microbes able to proliferate in an environment with longer exposures to water and nutrients(63) and large cohort studies have demonstrated that diet and transit time influence composition of the gut microbiota(64,65). Consequently, determining whether changes in the microbiota have any role in cause or are simply an effect of constipation is exceptionally complex to determine.
Challenges with assessing the gastrointestinal microbiota composition
Despite considerable advances in research, there is still no well-established definition of what constitutes a normal healthy gastrointestinal microbiota(66). Concerted research efforts, including the two major collaborative studies, MetaHIT and Human Microbiome Project, have identified the most dominant bacterial communities of the human gut microbiota to genus or species levels(5,67). This does provide us with an inventory list of ‘who is there’, but what the functions of these bacterial genes encode for remains largely unknown. While a core gastrointestinal microbiota is thought to exist, large site-specific intra- and inter-individual variations have been observed(66). Critics of the concept of a common ‘core of species’ constituting a healthy microbiota would like to see it replaced with that of a healthy ‘functional core’(66). The healthy gut microbiota needs to be able to maintain stability, resist major change induced by diet, pathogens or drugs, and be able to recuperate after having been impacted upon by stressors or perturbations(66). Since a stable, balanced microbiota is considered the cornerstone for a healthy gut, a maladaptive state of imbalance characterises an unhealthy one. This has been described as a state of dysbiosis, which can be broadly described as any change to the composition of resident commensal communities relative to the community found in healthy individuals. Functional constipation may be viewed as a consequence of dysbiosis(66).
Summary
Prebiotics are widely used in clinical practice and more so in the food industry but overall, there is a paucity of evidence from high-quality clinical trials regarding the quality, efficacy and safety of prebiotic formulations used in the management of constipation. Heterogenous study designs and variations in prebiotic types have contributed to a lack of generalisability and impeded the potential for direct application in clinical practice in terms of a diagnostic or therapeutic use. Despite these limitations, the overall body of research indicates a potential role for a range of prebiotic types in the management of constipation. Larger prospective studies with longer intervention periods and multiple timepoints are needed to elucidate the association between changes in the microbiota with gut motility in individuals with constipation. Multiple timepoints of sampling would contribute to understanding the extent of the effects of diet on the composition of the microbiota. In addition, cross-over studies could provide valuable insights into individual responses to changes in dietary fibre intake. Clinical trials designed as complete feeding studies with single ingredient modifications would greatly improve our understanding of the potential benefits of prebiotics and contribute to understanding whether an alleviation of constipation is associated with changes in the microbiota towards that of healthy controls.
Acknowledgements
R Erhardt is supported by a Postgraduate Research Scholarship from The University of Queensland.
E. R. contributed to the conception, design and interpretation of the research. All authors contributed to the drafting of the manuscript. All authors critically revised the manuscript, agree to be fully accountable for ensuring the integrity and accuracy of the work and read and approved the final manuscript.
There are no conflicts of interest.
References
- 1. Suares NC & Ford AC (2011) Prevalence of, and risk factors for, chronic idiopathic constipation in the community: systematic review and meta-analysis. Am J Gastroenterol 106, 1582–1591. [DOI] [PubMed] [Google Scholar]
- 2. Bowel Interest Group (2020) Cost of Constipation Report. https://bowelinterestgroup.co.uk/resources/cost-of-constipation-report-2020/ (accessed June 2022).
- 3. The Rome Foundation (2016) Rome IV Criteria. https://theromefoundation.org/rome-iv/rome-iv-criteria/ (accessed April 2022).
- 4. British Nutrition Foundation (2022) The Science of Fibre. https://www.nutrition.org.uk/healthy-sustainable-diets/starchy-foods-sugar-and-fibre/fibre/?level=Health%20professional (accessed May 2022).
- 5. Human Microbiome Project Consortium (2012) Structure, function and diversity of the healthy human microbiome. Nature 486, 207–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Gilbert JA, Blaser MJ, Caporaso JG, et al. (2018) Current understanding of the human microbiome. Nat Med 24, 392–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Harnett J, Myers SP & Rolfe M (2017) Significantly higher faecal counts of the yeasts candida and saccharomyces identified in people with coeliac disease. Gut Pathog 9, 26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Quigley E (2019) Prebiotics and probiotics in digestive health. Clin Gastroenterol Hepatol 17, 333–344. [DOI] [PubMed] [Google Scholar]
- 9. Oliphant K & Allen-Vercoe E (2019) Macronutrient metabolism by the human gut microbiome: major fermentation by-products and their impact on host health. Microbiome 7, 91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Simren M, Barbara G, Flint HJ, et al. (2013) Intestinal microbiota in functional bowel disorders: a Rome foundation report. Gut 62, 159–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Andrews C & Storr M (2011) The pathophysiology of chronic constipation. Can J Gastroenterol 25, 16–21. [PMC free article] [PubMed] [Google Scholar]
- 12. Belsey J, Greenfield S, Candy D, et al. (2010) Systematic review: impact of constipation on quality of life in adults and children. Aliment Pharmacol Ther 31, 938–949. [DOI] [PubMed] [Google Scholar]
- 13. Lewis SJ & Heaton KW (1997) Stool form scale as a useful guide to intestinal transit time. Scand J Gastroenterol 32, 920–924. [DOI] [PubMed] [Google Scholar]
- 14. Black CJ & Ford AC (2018) Chronic idiopathic constipation in adults: epidemiology, pathophysiology, diagnosis and clinical management. Med J Aust 209, 86–91. [DOI] [PubMed] [Google Scholar]
- 15. Continence Foundation of Australia (2016) Bristol Stool Chart https://www.continence.org.au/bristol-stool-chart (accessed September 2022).
- 16. Sujatha B, Velayutham DR, Deivamani N, et al. (2015) Normal bowel pattern in children and dietary and other precipitating factors in functional constipation. J Clin Diagn Res 9, SC12–SC15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Department of Health Victoria (2014) Constipation. https://www.betterhealth.vic.gov.au/health/conditionsandtreatments/constipation (accessed September 2022).
- 18. Quigley EM (2011) The enteric microbiota in the pathogenesis and management of constipation. Best Pract Res Clin Gastroenterol 25, 119–126. [DOI] [PubMed] [Google Scholar]
- 19. Eswaran S, Muir J & Chey WD (2013) Fiber and functional gastrointestinal disorders. Am J Gastroenterol 108, 718–727. [DOI] [PubMed] [Google Scholar]
- 20. Christodoulides SD, Fragkos E, Farmer KC, et al. (2016) Systematic review with meta-analysis: effect of fibre supplementation on chronic idiopathic constipation in adults. Aliment Pharmacol Ther 44, 103–116. [DOI] [PubMed] [Google Scholar]
- 21. Nee J, Sugarman M, Ballou S, et al. (2019) Placebo response in chronic idiopathic constipation: a systematic review and meta-analysis. Am J Gastroenterol 114, 1838–1846. [DOI] [PubMed] [Google Scholar]
- 22. Diaz S, Bittar K, Magda D, et al. (2022) Constipation. Florida: StatPerls Publishing. [Google Scholar]
- 23. Mancabelli L, Milani C, Lugli GA, et al. (2017) Unveiling the gut microbiota composition and functionality associated with constipation through metagenomic analyses. Sci Rep 7, 9879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Dominguez-Bello MG, Godoy-Vitorino F, Knight R, et al. (2019) Role of the microbiome in human development. Gut 68, 1108–1114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Parthasarathy G, Chen J, Chen X, et al. (2016) Relationship between microbiota of the colonic mucosa vs feces and symptoms, colonic transit, and methane production in female patients with chronic constipation. Gastroenterology 150, 367–379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Gibson G & Roberfroid M (1995) Dietary modulation of the human colonic microbiota: introducing the concept of prebiotics. J Nutr 125, 1401–1412. [DOI] [PubMed] [Google Scholar]
- 27. Roberfroid M, Gibson GR, Hoyles L, et al. (2010) Prebiotic effects: metabolic and health benefits. Br J Nutr 104, S1–S63. [DOI] [PubMed] [Google Scholar]
- 28. Davani-Davari D, Negahdaripour M, Karimzadeh I, et al. (2019) Prebiotics: definition, types, sources, mechanisms, and clinical applications. Foods 8, 92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Markowiak P & Slizewska K (2017) Effects of probiotics, prebiotics, and synbiotics on human health. Nutrients 9, 1021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Bindels LB, Delzenne NM, Cani PD, et al. (2015) Towards a more comprehensive concept for prebiotics. Nat Rev Gastroenterol Hepatol 12, 303–310. [DOI] [PubMed] [Google Scholar]
- 31. Belenguer A, Duncan SH, Calder AG, et al. (2006) Two routes of metabolic cross-feeding between bifidobacterium adolescentis and butyrate-producing anaerobes from the human gut. Appl Environ Microbiol 72, 3593–3599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Rowland I, Gibson G, Heinken A, et al. (2018) Gut microbiota functions: metabolism of nutrients and other food components. Eur J Nutr 57, 1–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Clarke G, Stilling RM, Kennedy PJ, et al. (2014) Minireview: gut microbiota: the neglected endocrine organ. Mol Endocrinol 28, 1221–1238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Gershon MD & Margolis KG (2021) The gut, its microbiome, and the brain: connections and communications. J Clin Invest 131, e143768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Morrison DJ & Preston T (2016) Formation of short chain fatty acids by the gut microbiota and their impact on human metabolism. Gut Microbes 7, 189–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Sakata T (2019) Pitfalls in short-chain fatty acid research: a methodological review. Anim Sci J 90, 3–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Baxter N, Schmidt A, Venkataraman A, et al. (2019) Dynamics of human gut microbiota and short-chain fatty acids in response to dietary interventions with three fermentable fibers. mBio 10, 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Mayer EA (2011) Gut feelings: the emerging biology of gut-brain communication. Nat Rev Neurosci 12, 453–466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Carabotti (2015) The gut-brain axis: interactions between enteric microbiota, central and enteric nervous systems. Ann Gastroenterol 28, 203–209. [PMC free article] [PubMed] [Google Scholar]
- 40. Dinan TG & Cryan JF (2017) The microbiome-gut-brain axis in health and disease. Gastroenterol Clin North Am 46, 77–89. [DOI] [PubMed] [Google Scholar]
- 41. Margolis KG, Cryan JF & Mayer EA (2021) The microbiota-gut-brain axis: from motility to mood. Gastroenterology 160, 1486–1501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Wahlstrom A, Sayin SI, Marschall HU, et al. (2016) Intestinal crosstalk between bile acids and microbiota and its impact on host metabolism. Cell Metab 24, 41–50. [DOI] [PubMed] [Google Scholar]
- 43. Fung TC, Vuong HE, Luna CDG, et al. (2019) Intestinal serotonin and fluoxetine exposure modulate bacterial colonization in the gut. Nat Microbiol 4, 2064–2073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Takahashi H, Wako N, Okubo T, et al. (1994) Influence of partially hydrolyzed guar gum on constipation in women. J Nutr Sci Vitaminol 40, 251–259. [DOI] [PubMed] [Google Scholar]
- 45. Bouhnik Y, Neut C, Raskine L, et al. (2004) Prospective, randomized, parallel-group trial to evaluate the effects of lactulose and polyethylene glycol-4000 on colonic flora in chronic idiopathic constipation. Aliment Pharmacol Ther 19, 889–899. [DOI] [PubMed] [Google Scholar]
- 46. Marteau P, Jacobs H, Cazaubiel M, et al. (2011) Effects of chicory inulin in constipated elderly people: a double-blind controlled trial. Int J Food Sci Nutr 62, 164–170. [DOI] [PubMed] [Google Scholar]
- 47. Linetzky Waitzberg D, Alves Pereira C, Logullo L, et al. (2012) Microbiota benefits after inulin and partially hydrolized guar gum supplementation – a randomized clinical trial in constipated women. Nutr Hosp 27, 123–129. [DOI] [PubMed] [Google Scholar]
- 48. Chu JR, Kang SY, Kim SE, et al. (2019) Prebiotic UG1601 mitigates constipation-related events in association with gut microbiota: a randomized placebo-controlled intervention study. World J Gastroenterol 25, 6129–6144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Jalanka J, Major G, Murray K, et al. (2019) The effect of psyllium husk on intestinal microbiota in constipated patients and healthy controls. Int J Mol Sci 20, 433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Stachowska E, Maciejewska D, Palma J, et al. (2022) Improvement of bowel movements among people with a sedentary lifestyle after prebiotic snack supply – preliminary study. Prz Gastroenterol 17, 73–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Schoemaker MH, Hageman JHJ, Ten Haaf D, et al. (2022) Prebiotic galacto-oligosaccharides impact stool frequency and fecal microbiota in self-reported constipated adults: a randomized clinical trial. Nutrients 14, nu14020309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Sakai Y, Seki N, Hamano H, et al. (2019) A study of the prebiotic effect of lactulose at low dosages in healthy Japanese women. Biosci Microbiota Food Health 38, 69–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Healey G, Murphy R, Butts C, et al. (2018) Habitual dietary fibre intake influences gut microbiota response to an inulin-type fructan prebiotic: a randomised, double-blind, placebo-controlled, cross-over, human intervention study. Br J Nutr 119, 176–189. [DOI] [PubMed] [Google Scholar]
- 54. Walton GE, van den Heuvel EG, Kosters MH, et al. (2012) A randomised crossover study investigating the effects of galacto-oligosaccharides on the faecal microbiota in men and women over 50 years of age. Br J Nutr 107, 1466–1475. [DOI] [PubMed] [Google Scholar]
- 55. Vulevic J, Drakoularakou A, Yaqoob P, et al. (2008) Modulation of the fecal microflora profile and immune function by a novel trans–galactooligosaccharide mixture (B-GOS) in healthy elderly volunteers. Am J Clin Nutr 88, 1438–1446. [DOI] [PubMed] [Google Scholar]
- 56. Chen O, Sudakaran S, Blonquist T, et al. (2021) Effect of arabinogalactan on the gut microbiome: a randomized, double-blind, placebo-controlled, crossover trial in healthy adults. Nutr 90, 111273. [DOI] [PubMed] [Google Scholar]
- 57. Calame W, Weseler AR, Viebke C, et al. (2008) Gum arabic establishes prebiotic functionality in healthy human volunteers in a dose-dependent manner. Br J Nutr 100, 1269–1275. [DOI] [PubMed] [Google Scholar]
- 58. Azpiroz F, Molne L, Mendez S, et al. (2017) Effect of chicory-derived inulin on abdominal sensations and bowel motor function. J Clin Gastroenterol 51, 619–625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Slavin J & Feirtag J (2011) Chicory inulin does not increase stool weight or speed up intestinal transit time in healthy male subjects. Food Funct 2, 72–77. [DOI] [PubMed] [Google Scholar]
- 60. Takahashi H, Yang SH, Hayashi C, et al. (1993) Effect of partially hydrolyzed guar gum on fecal output in human volunteers. Nutr Res 13, 649–657. [Google Scholar]
- 61. Okubo T, Ishihara N, Takahashi H, et al. (1994) Effects of partially hydrolyzed guar gum intake on human intestinal microflora and its metabolism. Biosci Biotechnol Biochem 58, 1364–1369. [Google Scholar]
- 62. Chen Y, Wu T, Lu W, et al. (2021) Predicting the role of the human gut microbiome in constipation using machine-learning methods: a meta-analysis. Microorganisms 9, 2149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Vandeputte D, Falony G, Vieira-Silva S, et al. (2016) Stool consistency is strongly associated with gut microbiota richness and composition, enterotypes and bacterial growth rates. Gut 65, 57–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Zhernakova A, Kurilshikov A, Bonder M, et al. (2016) Population-based metagenomics analysis reveals markers for gut microbiome composition and diversity. Science 352, 565–571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Gacesa R, Kurilshikov A, Vich Vila A, et al. (2022) Environmental factors shaping the gut microbiome in a Dutch population. Nature 604, 732–739. [DOI] [PubMed] [Google Scholar]
- 66. Lloyd-Price J, Abu-Ali G & Huttenhower C (2016) The healthy human microbiome. Genome Med 8, 51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Qin J, Li R, Raes J, et al. (2010) A human gut microbial gene catalogue established by metagenomic sequencing. Nature 464, 59–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. McRorie JW Jr & McKeown NM (2017) Understanding the physics of functional fibers in the gastrointestinal tract: an evidence-based approach to resolving enduring misconceptions about insoluble and soluble fiber. J Acad Nutr Diet 117, 251–264. [DOI] [PubMed] [Google Scholar]
- 69. Quigley E & Spiller R (2016) Constipation and the microbiome: lumen v. mucosa! Gastroenterology 150, 300–303. [DOI] [PubMed] [Google Scholar]
- 70. Gibson GR, Hutkins R, Sanders ME, et al. (2017) Expert consensus document: the International Scientific Association for Probiotics and Prebiotics (ISAPP) consensus statement on the definition and scope of prebiotics. Nat Rev Gastroenterol Hepatol 14, 491–502. [DOI] [PubMed] [Google Scholar]
- 71. Scott KP, Grimaldi R, Cunningham M, et al. (2020) Developments in understanding and applying prebiotics in research and practice-an ISAPP conference paper. J Appl Microbiol 128, 934–949. [DOI] [PubMed] [Google Scholar]
- 72. Slavin J (2013) Fiber and prebiotics: mechanisms and health benefits. Nutrients 5, 1417–1435. [DOI] [PMC free article] [PubMed] [Google Scholar]