Abstract
The Na+-dependent phosphate cotransporter-2A (NPT2A, SLC34A1) is a primary regulator of extracellular phosphate homeostasis. Its most prominent structural element is a carboxy-terminal PDZ ligand that binds Na+/H+ Exchanger Regulatory Factor-1 (NHERF1, SLC9A3R1). NHERF1, a multidomain PDZ protein, establishes NPT2A membrane localization and is required for hormone-inhibitable phosphate transport. NPT2A also possesses an uncharacterized internal PDZ ligand. Two recent clinical reports describe congenital hypophosphatemia in children harboring Arg495His or Arg495Cys variants within the internal PDZ motif. The wild-type internal 494TRL496 PDZ ligand binds NHERF1 PDZ2, which we consider a regulatory domain. Ablating the internal PDZ ligand with a 494AAA496 substitution blocked hormone-inhibitable phosphate transport. Complementary approaches, including CRISPR/Cas9 technology, site-directed mutagenesis, confocal microscopy, and modeling, showed that NPT2A Arg495His or Arg495Cys variants do not support PTH or FGF23 action on phosphate transport. Coimmunoprecipitation experiments indicate that both variants bind NHERF1 similarly to WT NPT2A. However, in contrast with WT NPT2A, NPT2A Arg495His, or Arg495Cys variants remain at the apical membrane and are not internalized in response to PTH. We predict that Cys or His substitution of the charged Arg495 changes the electrostatics, preventing phosphorylation of the upstream Thr494, interfering with phosphate uptake in response to hormone action, and inhibiting NPT2A trafficking. We advance a model wherein the carboxy-terminal PDZ ligand defines apical localization NPT2A, while the internal PDZ ligand is essential for hormone-triggered phosphate transport.
Introduction
NPT2A (SLC34A1), Na+-dependent phosphate cotransporter-2A, is the primary regulator of renal phosphate absorption and serum phosphate homeostasis. Disordered phosphate metabolism associated with chronic kidney disease and frank resistance to hormone action contributes to exceptionally high mortality rates, especially among the elderly and impoverished [1–3].
The most prominent NPT2A structural element is its carboxy-terminal TRL639 (Thr−Arg−Leu 639) PDZ ligand, a Class I PDZ binding site (-Thr/Ser−2-X−1-Φ0, where X at position −1 is permissive and Φ at position 0 is any hydrophobic residue) (Figure 1). Through its carboxy-terminal TRL639 sequence, NPT2A binds Na+/H+ Exchanger Regulatory Factor-1 (NHERF1, SLC9A3R1) (Figure 1), a multidomain PDZ protein, that determines NPT2A apical localization [4–8].
Figure 1. Schematic representation of NPT2A bound NHERF1 at the apical membrane.

The NPT2A carboxy-terminal (CT-TRL) and internal 494TRL496 PDZ ligands bind NHERF1 PDZ1 and PDZ2, respectively. PTH-triggered phosphorylation of Thr494 in the internal 494TRL496 PDZ motif of NPT2A is necessary for hormone-inhibitable phosphate transport.
NHERF1 assembles multiprotein complexes through its two PDZ domains and an ezrin-binding domain (EBD) (Figure 1). Ser/Thr−2 and Φ0 contribute primarily to binding affinity. The carboxy-terminal NPT2A PDZ ligand binds NHERF1 PDZ1 [9,10]. NHERF1 scaffolds relevant kinases [8,11] and phosphatases [12] and controls the association and disassembly of the NHERF1:NPT2A complex, endocytosis, and cessation of phosphate transport. Mice lacking NHERF1 [13–15] and humans harboring NHERF1 mutations [16,17] exhibit frank phosphaturia even in the presence of normal-to-high Parathyroid hormone (PTH) and Fibroblast Growth Factor 23 (FGF23) levels. PTH and FGF23, despite different membrane receptors and distinct signaling pathways, exert their shared phosphaturic action by dissociating NPT2A from NHERF1, sequestering NPT2A, thereby reducing the abundance of NPT2A at apical cell membranes and augmenting phosphate excretion [18–20].
Remarkably, in addition to its well-characterized canonical carboxy-terminal PDZ ligand, NPT2A possesses an uncharacterized internal 494Thr−Arg495−Leu496 PDZ sequence (Figure 1) in the intracellular loop between transmembrane domains 6 and 7. Two recent clinical reports describe inherited autosomal dominant hypophosphatemia in children harboring Arg495His or Arg495Cys variants within this internal PDZ motif [21,22]. The presence of these mutations within a PDZ ligand was unappreciated. The role of this cryptic internal PDZ binding motif in phosphate regulation is unknown.
This study uses complementary approaches, including CRISPR/Cas9 technology, to generate an informative, hormone-inhibitable cell line, site-directed mutagenesis, confocal microscopy, and modeling to characterize the wild-type and disease-associated NPT2A internal 494TRL496 PDZ motif and evaluate its participation in FGF23 and PTH-triggered phosphate regulation. We show that NPT2A Arg495Cys or Arg495His variants bind NHERF1 but do not support FGF23 or PTH action on phosphate transport. In contrast with wild-type NPT2A, Arg495Cys, or Arg495His NPT2A variants remain at the apical membrane and are not endocytosed in response to PTH. We predict that Cys or His substitution of the charged Arg495 changes the electrostatics, preventing phosphorylation of the upstream Thr494, thus interfering with phosphate uptake and inhibiting NPT2A trafficking in response to hormone action. Consistent with this scheme, ablating the internal PDZ ligand blocked hormone-inhibitable phosphate transport.
We advance a conceptual model where the NPT2A carboxy-terminal PDZ ligand binds NHERF1 PDZ1 and serves as a scaffold for tethering required kinases and phosphatases with apically localized NPT2A. We propose that the internal NPT2A PDZ motif is directly involved and necessary for hormone-inhibitable phosphate transport via association with NHERF1 PDZ2 (Figure 1).
Results
The internal NPT2A 494TRL496 PDZ motif determines hormone-inhibitable phosphate transport
We first interrogated whether the internal NPT2A 494TRL496 PDZ motif interacts with NHERF1. For these experiments, we developed CRISPR/Cas9 Npt2a knockout opossum kidney (OK) cells, where the native Npt2a transporter was eradicated. Deleting endogenous Npt2a allowed the introduction of wild-type human NPT2A or other designed structurally informative constructs. CRISPR/Cas9 Npt2a knockout OK cells were transfected with HA-GFP-NPT2A, where the carboxy-terminal -TRL639 (hereafter CT-TRL) PDZ ligand was replaced with a triple Ala sequence (hereafter CT-AAA)1. This modification prevents binding between NPT2A CT-TRL and NHERF1 PDZ1 domain [9,10]. We used WT FLAG-NHERF1 or NHERF1 constructs harboring carboxylate-binding loop GYGF to GAGA modifications in PDZ1 (P1), PDZ2 (P2), or both PDZ domains (P1P2) that disrupt the formation of the canonical interactions between PDZ domains and carboxy-termini of the target ligands [9,11,23].
Coimmunoprecipitation data show that the NPT2A CT-AAA mutant associates with NHERF1 P1, which possesses an intact PDZ2, but not with P2 (Intact PDZ1) or P1P2, indicating that the NPT2A internal 494TRL496 PDZ motif binds NHERF1 PDZ2 (Figure 2A,B). Figure 2C schematically illustrates the interaction between the NPT2A CT-AAA mutant and NHERF1 P1, P2, and P1P2 constructs.
Figure 2. Immunoprecipitation of NPT2A with NHERF1.

(A) NPT2A with triple Ala replacement at the carboxy-terminal PDZ ligand (CT-AAA) immunoprecipitated (IP) with NHERF1 P1 (Intact PDZ2) but not with P2 (Intact PDZ1) or P1P2, indicating the NPT2A internal 494TRL496 PDZ ligand binds NHERF1 PDZ2. CRISPR/Cas9 Npt2a knockout OK cells were transfected with HA-GFP-NPT2A CT-AAA and FLAG-NHERF1 (WT, P1, P2, P1P2). NPT2A was immunoprecipitated using agarose-conjugated monoclonal anti-HA beads. NHERF1 was immunoblotted using a polyclonal anti-FLAG antibody. NPT2A was immunoblotted using a polyclonal anti-HA antibody. Immunoblots for IP are depicted in the top two panels. Lysates controls are in the bottom panels. Molecular weight markers (kDa) on the right of blots A representative experiment is depicted. (B) Quantification of NHERF1 immunoprecipitated with NPT2A. Blots were scanned and quantified using Image J. The amount of mutant NHERF1 immunoprecipitating with NPT2A was normalized to the amount of WT-NHERF1, defined as 100%. n = 3, **** P < 0.0001. (C) Schematic representation of immunoprecipitation of NPT2A CT-AAA with NHERF1 constructs presented in (A). NPT2A CT-AAA binds the P1 construct with the modified PDZ1 core-binding motif (GYGF/GAGA) via NHERF1 PDZ2 and the internal NPT2A 494TRL496 PDZ ligand. NPT2A CT-AAA does not associate with the P2 construct with the modified PDZ2 core-binding motif (GYGF/GAGA) or the P1P2 construct, where both PDZ1 (GYGF/GAGA) and PDZ2 (GYGF/GAGA) are mutated.
Then, we inquired whether the lack of the internal NPT2A 494TRL496 PDZ motif affects hormone-inhibitable phosphate transport. FGF23- or PTH-inhibitable phosphate uptake was measured in CRISPR/Cas9 Npt2a knockout OK cells transfected with NPT2A with the triple Ala replacement at the internal 494AAA496 or CT-AAA PDZ motif and compared with WT NPT2A. Unexpectedly, ablating the internal PDZ motif prevented FGF23- or PTH-inhibitable phosphate uptake compared with WT NPT2A but not basal phosphate transport (Figure 3). In contrast, the NPT2A CT-AAA variant reduced resting phosphate transport (Figure 3). Consistent with the phosphate uptake experiments, the NPT2A 494AAA496 mutant was immunoprecipitated with NHERF1, whereas NPT2A CT-AAA did not (Figure 4A). Figure 4B displays the quantification of NPT2A 494AAA496, immunoprecipitation with NHERF1 comparable to WT NPT2A. The spatial localization of NPT2A variants was analyzed by confocal fluorescence microscopy. WT NPT2A, NPT2A 494AAA496, and NHERF1 are expressed at the apical membrane under resting conditions in the absence of PTH (Figure 5A,B). WT NPT2A and NPT2A 494AAA496 colocalize broadly with NHERF1 but not with NPT2A CT-AAA (Figure 5C). Upon PTH treatment, WT NPT2A internalized extensively in the presence of NHERF1 (Figure 5A), whereas NPT2A 494AAA496 remained localized at the apical membrane (Figure 5B). NPT2A CT-AAA was essentially unchanged (Figure 5C). Colocalization of NPT2A variants with NHERF1 was quantified as described in Materials and Methods and presented in Figure 5D. Together, our experimental data demonstrate that the internal NPT2A 494TRL496 PDZ motif is required for FGF23- and PTH-triggered dissociation of NPT2A from NHERF1, while the NPT2A CT-TRL PDZ ligand determines membrane localization of NPT2A.
Figure 3. Triple alanine replacement in NPT2A internal or carboxy-terminal PDZ motif blocks hormone-inhibitable phosphate uptake.
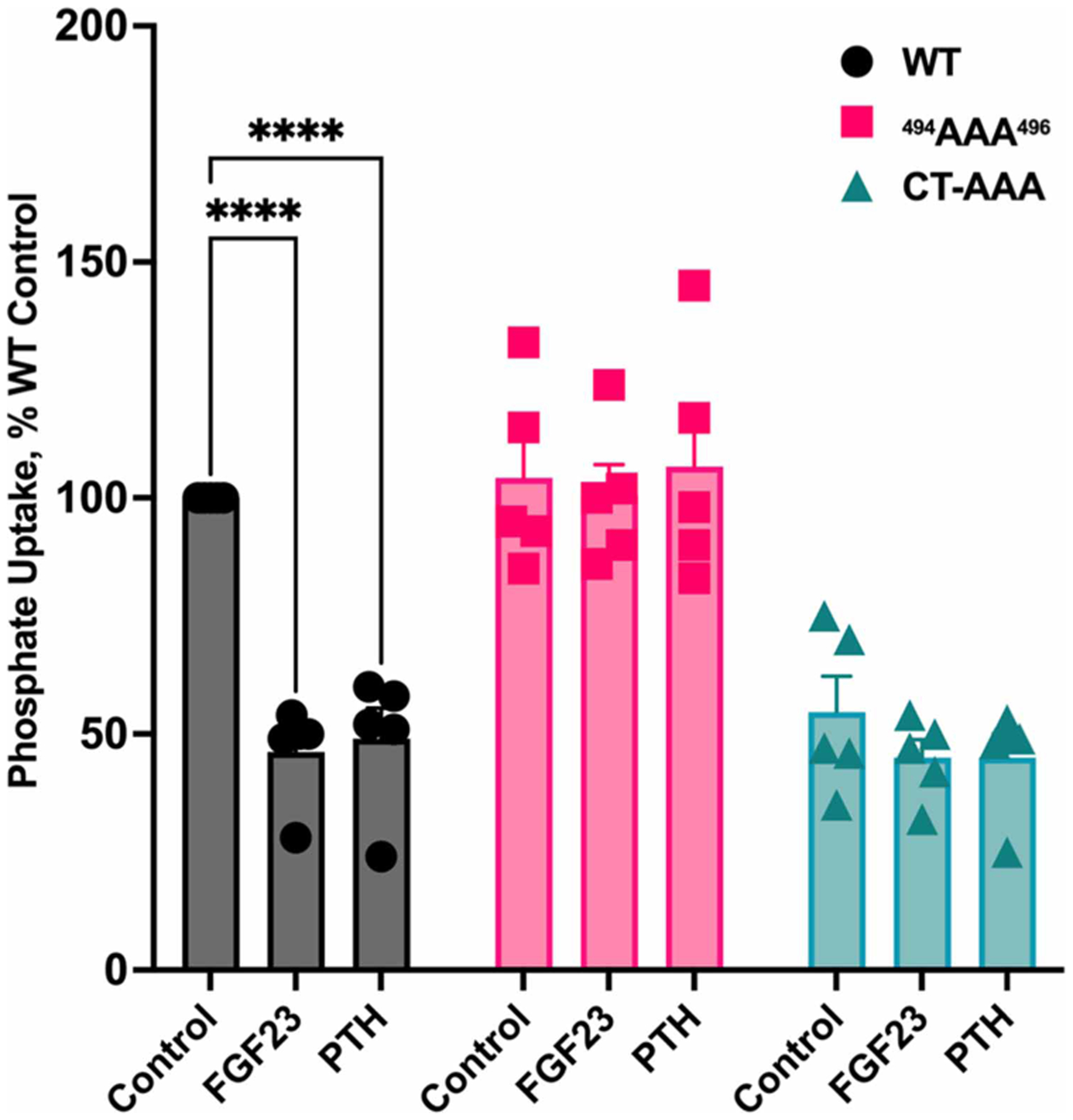
NPT2A 494AAA496 and CT-AAA PDZ ligand mutants do not support FGF23- or PTH-inhibitable phosphate uptake. CRISPR/Cas9 Npt2a knockout OK cells were transfected with the indicated NPT2A mutants. Phosphate uptake was assayed in the presence or absence of FGF23 or PTH, as indicated. n = 5, **** P < 0.0001 PTH or FGF23 vs control.
Figure 4. Effect of triple alanine replacement in NPT2A on the binding with NHERF1.

(A) A representative experiment depicting NPT2A immunoprecipitating with NHERF1. The NPT2A 494AAA496 mutant immunoprecipitated with NHERF1, whereas NPT2A CT-AAA does not. NHERF1 was immunoprecipitated using agarose-conjugated monoclonal anti-FLAG beads. NPT2A was immunoblotted using a polyclonal anti-HA antibody. NHERF1 was immunoblotted using a polyclonal anti-FLAG antibody. Immunoblots for IP are shown in the top two panels. Lysates controls are in the bottom panels. Molecular weight markers (kDa) are to the right of the blots. (B) Quantification of NPT2A immunoprecipitated with NHERF1. CRISPR/Cas9 Npt2a knockout OK cells were transfected with HA-GFP-NPT2A WT, 494AAA496 or CT-AAA and FLAG-NHERF1. The amount of mutant NPT2A immunoprecipitating with NHERF1 was normalized to the amount of WT-NPT2A, defined as 100%. n = 3, **** P < 0.0001 CT-AAA vs. WT.
Figure 5. Confocal fluorescence localization of NPT2A (green) with NHERF1 (red) in CRISPR/Cas9 Npt2a knockout OK cells.
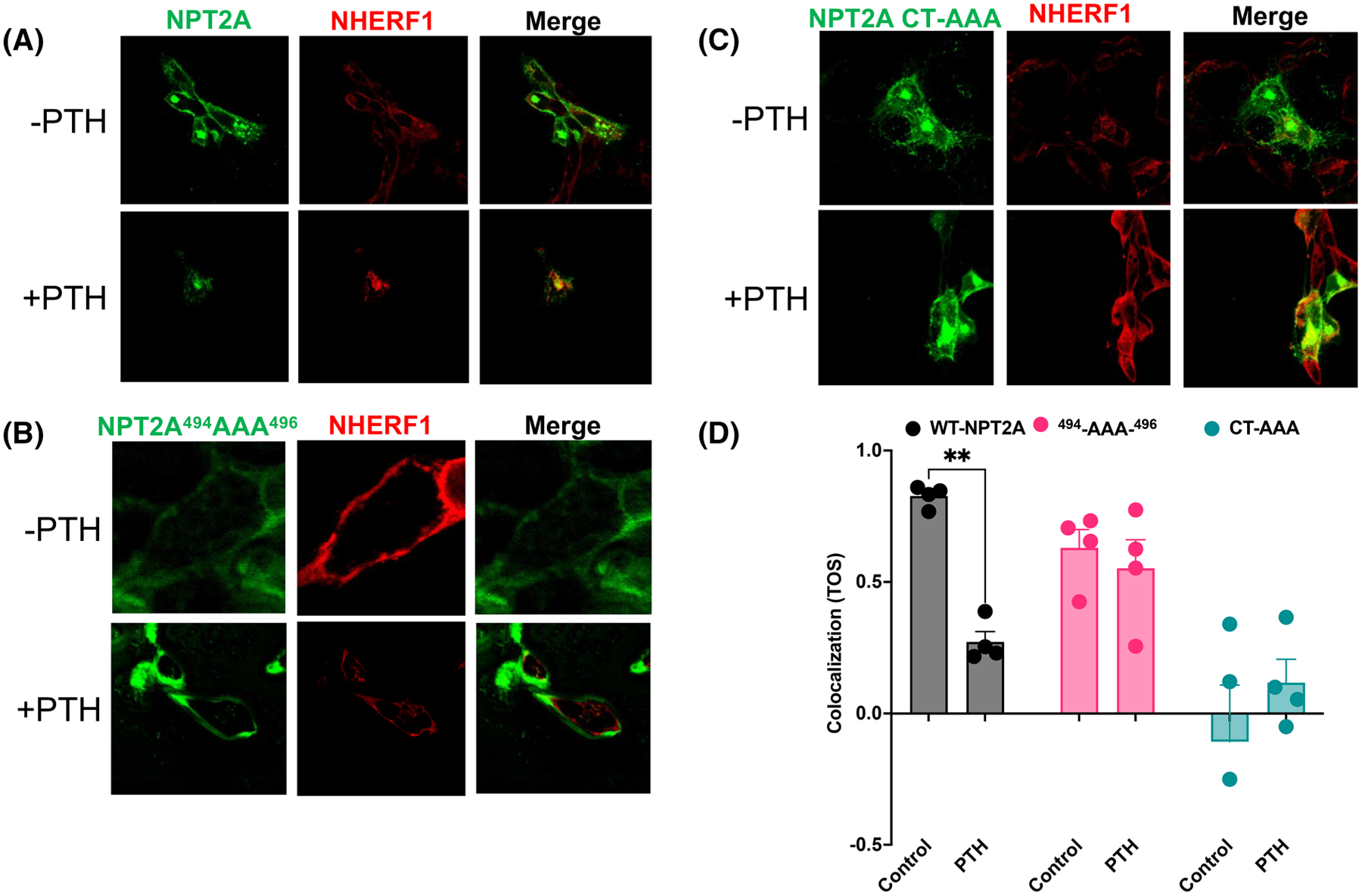
CRISPR/Cas9 Npt2a knockout OK cells were transfected with HA-GFP-NPT2A (WT or the indicated mutants). (A) Under resting conditions without PTH (upper row), HA-GFP-NPT2A or (B) HA-GFP-NPT2A 494AAA496 and endogenous NHERF1 are expressed at the apical membrane. Merged images on the right indicate extensive colocalization for WT NPT2A or NPT2A 494AAA496 with NHERF1 but not for (C) NPT2A CT-AAA. Upon addition of PTH (bottom row), (A) WT NPT2A internalized in the presence of NHERF1, but (B) NPT2A 494AAA496 and (C) NPT2A CT-AAA were essentially unchanged. (D) Colocalization of HA-GFP-NPT2A and endogenous NHERF1 was quantified using Image J. The calculated threshold overlap score (TOS) between +1 and −1 indicates colocalization between NPT2A variants and NHERF1 or its absence, respectively. n = 4.
Disease-associated mutations in the NPT2A internal PDZ motif abolish hormone-inhibitable phosphate transport
Mutation of the highly conserved Arg495 in the internal NPT2A 494TRL496 motif to Cys or His is associated with congenital elevated renal phosphate excretion and hypophosphatemia in children [21,22]. These observations suggest a more precise role for the cryptic internal PDZ motif than revealed by the experimental triple AAA construct described above. We, therefore, introduced NPT2A variants, where Arg495 was replaced by Cys or His, i.e. the reported human mutations. Phosphate uptake was measured in CRISPR/Cas9 Npt2a knockout OK cells transfected with the NPT2A Arg495Cys or Arg495His variant. Notably, FGF23- and PTH-inhibitable phosphate uptake was abolished by the disease-associated NPT2A variants (Figure 6). Coimmunoprecipitation experiments revealed that both NPT2A Arg495Cys and Arg495His variants associate with NHERF1 under resting conditions. However, compared with WT NPT2A, neither Arg495Cys nor Arg495His dissociates from NHERF1 upon challenge with PTH (Figure 7A), the standard biological action that causes cessation of phosphate transport. Concurrently, as demonstrated by confocal fluorescence microscopy, NPT2A Arg495Cys, and Arg495His variants colocalized with NHERF1 at apical cell membranes like WT NPT2A (Figure 7B–D [left panel]). Consistent with the functional results, NPT2A Arg495Cys and Arg495His variants do not internalize in response to PTH but remain at the apical membrane (Figure 7C,D [right panel]). Quantified NPT2A:NHERF1 complex colocalization illustrates that in contrast with WT NPT2A, the disease variants do not dissociate upon challenge with PTH (Figure 7E).
Figure 6. Disease-associated mutations in the NPT2A internal PDZ ligand block FGF23- or PTH- inhibitable phosphate transport.
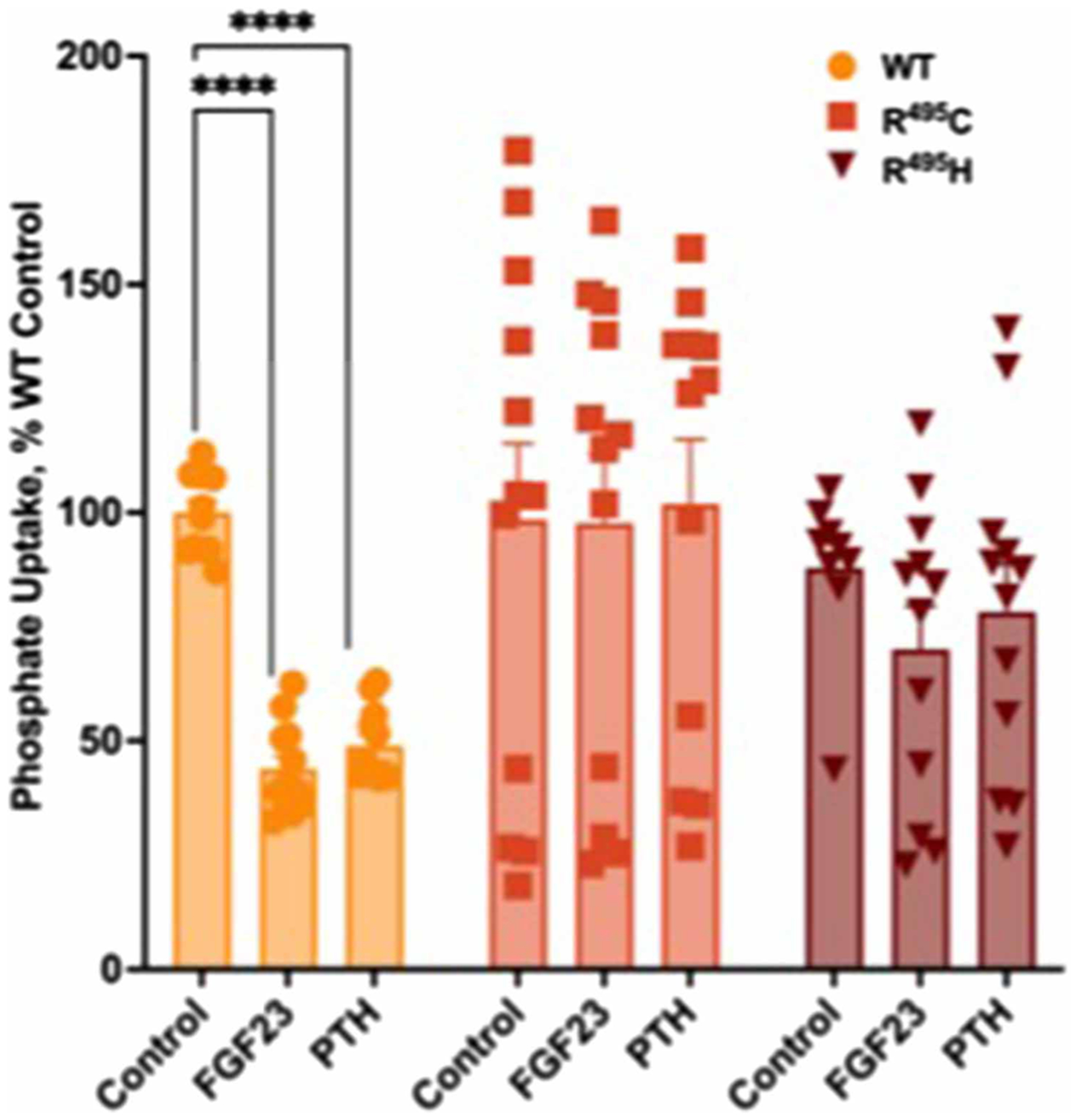
Arg495 (R495) in the 494TRL496 motif was substituted by Cys (R495 C) or His (R495 H). CRISPR/Cas9 Npt2a knockout OK cells were transfected with the indicated NPT2A variants. Phosphate uptake was assayed in the presence or absence of FGF23 or PTH, as indicated. n = 12, **** P < 0.0001 PTH or FGF23 vs. control.
Figure 7. Disease-associated mutations in the NPT2A internal PDZ ligand do not interfere with NPT2A–NHERF1 association or NPT2A–NHERF1 colocalization.

(A) HA-GFP-NPT2A R495C or R495H do not dissociate from NHERF1 in response to PTH in OK cells compared with WT NPT2A. OK cells were transfected with HA-GFP-NPT2A (WT, R495C, or R495H) and FLAG-NHERF1. (B) Confocal fluorescence colocalization of WT NPT2A, (C) NPT2A R495C, or (D) NPT2A R495H (green) with FLAG-NHERF1 (red) in OK cells. (E) NPT2A:NHERF1 colocalization was quantified. We calculated the Threshold Overlap Score (TOS) as a measure for colocalization for each NPT2A mutant using confocal microscopy images and Image J. n = 4, **** P < 0.0001 PTH vs. control.
Class I PDZ domains, such as those in NHERF1, favor ligands containing Thr or Ser at position −2 relative to the carboxy-terminus, position 0 [24]. Phospho-resistant modifications of Ser/Thr at residue −2 of class I PDZ-recognition motifs impair the binding [25,26]. Thr494, located at position −2 of the internal NPT2A 494TRL494 PDZ motif (T−2R−1L0), is a specific determinant of a canonical PDZ-ligand interaction. We hypothesized that replacing Arg495 by Cys or His of the internal NPT2A 494TRL496 motif impairs the phosphorylation of Thr494. To test this idea, HA-GFP-NPT2A Arg495Cys or Arg495His variants were treated with PTH in transfected cells and immunoblotted with a custom anti-phospho-494TRL496 antibody (Figure 8A) and for total NPT2A (Figure 8B). Representative immunoblots (Figure 8A,B) and quantification (Figure 8C) clearly show that PTH promotes phosphorylation of Thr494 in WT NPT2A but not in NPT2A Arg495Cys or Arg495His variants.
Figure 8. Replacement of Arg495 by Cys or His in NPT2A internal TRL motif prevents phosphorylation of the upstream Thr494 compared with WT.

OK cells were transfected with HA-GFP-NPT2A WT, Arg495Cys, or Arg495His variants. Representative immunoblots of HA-GFP-NPT2A Arg495Cys or Arg495His variants treated with PTH and immunoblotted for (A) anti-p494TRL496 antibody or (B) total NPT2A. Molecular weight markers (kDa) are shown on the left of the blots. (C) Quantification of immunoblot analysis. n = 3. **** P < 0.0001 PTH vs. control.
Next, we inquired whether Thr494 is involved in hormone-inhibitable phosphate transport. Phosphate uptake was measured in CRISPR/Cas9 Npt2a knockout OK cells transfected with an NPT2A construct, where Thr494 was replaced conservatively by Cys (Thr494Cys), and the cells were treated with FGF23 or PTH. Notably, the NPT2A Thr494 Cys mutant impaired the inhibitory response to FGF23 and PTH (Figure 9A) but did not affect resting phosphate uptake. We then turned to the interaction of the NPT2A Thr494Cys construct with NHERF1. Representative immunoblots presented in Figure 9B demonstrate that HA-NPT2A Thr494Cys binds FLAG-NHERF1. Notably, the replacement of Thr by Cys prevented the dissociation of HA-NPT2A from FLAG-NHERF1 upon treatment with PTH (Figure 9B). Then CRISPR/Cas9 Npt2a knockout OK cells were transfected with HA-GFP-NPT2A Thr494Cys or WT NPT2A, treated with PTH, and immunoblotted for Rb-anti-494pTRL496 antibody, or Rb-anti-HA. As expected, NPT2A Thr494Cys does not phosphorylate in response to PTH (Figure 10). It is enigmatic how Arg495 mutation affects phosphorylation of the vicinal Thr494. We speculated that the binding of NPT2A and NHERF1 PDZ2 is necessary for the phosphorylation of Thr494 and that the Arg495 mutation interferes with Thr494 phosphorylation. OKH cells were transfected with WT-HA-GFP-NPT2A and FLAG-NHERF1 or FLAG-NHERF1 P2 (Intact PDZ1), treated with PTH and immunoblotted for Rb-anti-494pTRL496 antibody, Rb-anti-HA, or Rb-anti-FLAG (Figure 11). Phosphorylation of Thr494 was detected in cells transfected with WT-NHERF1 but not NHERF1 P2, illustrating the requirement for NHERF1 PDZ2 (Figure 11).
Figure 9. Thr494/Cys replacement in the internal NPT2A PDZ ligand blocks hormone-inhibitable phosphate uptake.

(A) NPT2A 494CRL mutant is refractory to PTH and FGF23 action on phosphate uptake. CRISPR/Cas9 Npt2a knockout OK cells were transfected with the indicated NPT2A mutant and treated with FGF23 or PTH. n = 8. *** P < 0.001 FGF23 vs control; **** P < 0.0001 PTH vs control; * P < 0.1 FGF23 vs PTH. (B) Thr494/Cys substitution supports the binding of HA-NPT2A and FLAG-NHERF1 but prevents the dissociation of HA-NPT2A from FLAG-NHERF1 in the presence of PTH compared with WT-HA-NPT2A as assessed by representative immunoblot. Molecular weight markers (kDa) on the right of blots. n = 4.
Figure 10. PTH does not promote the phosphorylation of NPT2A Thr494Cys.

Representative immunoblot of HA-GFP-NPT2A (WT) or NPT2A Thr494Cys (CRL) expression in transfected CRISPR/Cas9 Npt2a knockout OK cells treated with PTH and immunoblotted with Rb-anti-494pTRL496 antibody (top panel), or total NPT2A (bottom panel). Molecular weight markers (kDa) are shown on the left of the blots. n = 3.
Figure 11. PTH-induced phosphorylation of NPT2A Thr494 requires NHERF1.

Representative immunoblots that assess phosphorylation of NPT2A Thr494 in OKH cells transfected with WT HA-GFP-NPT2A and FLAG-WT NHERF or FLAG-NHERF1 P2 treated with PTH and immunoblotted using a specific Rb-anti-p494TRL496 antibody (top panel), Rb-anti-HA (middle panel), or Rb-anti-FLAG (bottom panel). Molecular weight markers (kDa) are shown on the left of the blots. n = 3.
Molecular modeling of the internal NPT2A 494TRL496 PDZ motif
The structure of NPT2A has not been solved. Therefore, we applied AlphaFold2 (AF2) [27] to generate a 3D computational structure of NPT2A. To explore residue-residue interactions specific for the NPT2A internal 494TRL496 PDZ motif coordinates of residues 310–536 were used to establish a model for MD simulation. As assigned elsewhere, these coordinates correspond to the transmembrane helices 3 to 7 (TM3–TM7) [28]. All-atom explicit solvent MD simulations (200 ns) were performed using AMBER16 [29]. Simulation details are provided in the Materials and Methods. The structure resulting from the MD simulation is similar to that predicted by AF2. The RMSD values calculated for the backbone atoms relative to the AF2 structure along the equilibration and production simulations remain stable for α-helices (not presented here). In contrast, a loop connecting TM5b and TM6a (residues 453–482) changes its conformation along the MD simulation. The amino-terminal flanking region (residues 507–536, segment of TM7) is mobile. These regions AF2 may predict with low confidence compared with α-helices [30]. A representative snapshot from the MD simulation demonstrates that the sidechains of Thr494 and Leu496 are oriented to the solvent. In contrast, the sidechain of Arg495 turns opposite toward the core of NPT2A (Figure 12). Calculation of hydrogen bonds (cpptraj AMBER16) [29] along the production simulation suggests that Arg495 (TM6) forms a salt bridge with Glu438 (TM5a) (Figure 12). We computationally replaced Arg495 with Cys or His and conducted additional MD simulations (100 ns). The equilibration and production simulations were performed as described for WT NPT2A. MD simulations predict that the replacement of Arg495 by Cys or His does not induce conformational changes but rather abrogates the electrostatic interaction with Glu438. The side chain of Thr494 or Leu496 does not change its orientation and remains solvent-exposed. The computational study supports our experimental finding, thus indicating that AF2 can reliably assess the accuracy of NPT2A structure from amino acid sequences and, in combination with MD simulation, is extremely useful.
Figure 12. A computational model of NPT2A (395–536 aa) from MD simulation.

A representative snapshot showing the orientation of the NPT2A internal 494TRL494 PDZ motif. Thr494 and Leu496 are solvent-exposed, whereas Arg495 is buried within the pocket created by TM5a, TM6a, and TM6b. A predicted salt bridge between Arg495 (TM6b) and Glu438 (TM5a) is presented as a dotted black line. The TM helices in the MD model were assigned as published elsewhere [28].
Discussion
The Na+-dependent phosphate cotransporter-2A (NPT2A, SLC34A1) is the principal regulator of phosphate homeostasis. Its most notable structural element is its carboxy-terminal PDZ ligand that binds Na+/H+ Exchanger Regulatory Factor-1 (NHERF1, SLC9A3R1), a multidomain PDZ protein that determines apical NPT2A localization, is required for phosphate transport. NPT2A also harbors an undescribed internal identical PDZ motif 494TRL496. The objectives of the present work were to determine what, if any, biological function the internal PDZ motif fulfills. An important clue suggesting a potentially important role emerged from two case reports describing hypophosphatemia in children with inherited mutations within the internal PDZ motif, though it was not initially appreciated. A third goal was to resolve the biochemical riddle of how Arg mutation prevents obligate, hormone-triggered phosphorylation of the adjacent Thr.
To characterize the two PDZ motifs, we generated NPT2A mutants where CT-TRL or 494TRL496 was replaced with AAA. We show here, using immunoblot and immunoprecipitation analysis and confocal fluorescence imaging, that CT-AAA disrupts the interaction with NHERF1 (Figure 4) and, concurrently, decreases the distribution of NPT2A at the apical membrane and renders NPT2A refractory to PTH (Figure 5C). We conclude that the NPT2A CT-TRL establishes the apical membrane colocalization of NPT2A with NHERF1 that precedes and is a required component for hormone-inhibitable phosphate transport.
Coimmunoprecipitation experiments indicated that the internal NPT2A 494TRL496 PDZ motif binds NHERF1 PDZ2 (Figure 2), which we consider a regulatory domain. In contrast with CT-TRL, ablation of the internal NPT2A 494TRL496 motif does not change NPT2A apical localization (Figure 5B) or the interaction with NHERF1 (Figure 4). Intriguingly, NPT2A 494AAA496 blocks the inhibition of phosphate uptake by FGF23 or PTH but does not affect resting conditions (Figure 3). The 494TRL496 motif is, therefore, an essential regulator of hormone-triggered phosphate transport.
Two recent clinical reports describe hereditary hypophosphatemia in children harboring Arg495His or Arg495Cys variants within the internal NPT2A PDZ motif [21,22]. It was not appreciated that these disease-causing mutations were in a canonical, if cryptic, PDZ motif. We show here that the two variants, NPT2A Arg495Cys and Arg495His, block inhibition of phosphate uptake in response to FGF23 or PTH (Figure 6) while fully able to complex with NHERF1 (Figure 7A). In agreement with immunoblot analysis, NPT2A Arg495His or Arg495Cys fail to internalize in response to PTH and remain at apical cell membranes (Figure 7C,D). The data raise the question of why mutating Arg495 to Cys or His [21,22] at position −1 of the PDZ motif, which is considered permissive [24], blocks phosphate transport (Figure 6). In the absence of NPT2A structural information, we used an AF2 predictor to generate a computational model to resolve this enigma. Further MD simulations show that Thr494 and Leu496 are solvent-exposed and may be involved in the interactions with NHERF1. In contrast, Arg495 forms a salt bridge with Glu438, thereby connecting TM6 and TM5a (Figure 12). We speculate that Thr494 (position −2) and Leu496 (position 0) are PDZ ligand-binding determinants involved in the interaction with NHERF1 PDZ2, whereas Arg495 (permissive position −1) is essential for post-translational modification. The MD simulation predicts that the substitution of Arg495 by Cys or His disrupts the salt bridge between Arg495 and Glu438 and changes the electrostatics that may prevent phosphorylation of the upstream Thr494. We experimentally generated an NPT2A construct to probe this idea, where Cys replaced Thr494. We assumed that the similarity between the side chain of Cys (SH) and Thr (OH) would permit Class I PDZ-ligand interaction [24] and not impede the association with NHERF1. At the same time, Cys cannot be phosphorylated compared with Thr and would prevent PTH action. This was the case. Indeed, the NPT2A Thr494Cys variant binds NHERF1 (Figure 9B), is not phosphorylated (Figure 10), and does not support PTH-inhibitable phosphate uptake (Figure 9A). By employing a custom anti-494pTRL496 antibody, we confirmed the phosphorylation of Thr494 after PTH treatment (Figure 11). Of note, phosphorylation of NPT2A Thr494 was detected only in cells transfected with NHERF1. Furthermore, phosphorylation of Thr494 was not detected in the absence of NHERF1 PDZ2 (P2 construct) (Figure 11), supporting our contention that the NPT2A internal PDZ ligand binds to NHERF1 PDZ2. We speculate that the association between the internal NPT2A 494TRL496 PDZ motif and NHERF1 PDZ2 is necessary for PTH-induced phosphorylation of the Thr494. Additional studies will further characterize this phenomenon.
The case reports that served as the basis for the present inquiry described conspicuous hypophosphatemia in two affected proposition (3.7 mg/dl [21], 4.3 mg/dl [22]; normal range, 4.8–8.2 mg/dl [31]. The described patients harbored the Arg495His/Cys variants in the NPT2A internal PDZ motif. These substitutions prevent the dissociation of NPT2A from NHERF1 upon hormone treatment, blocking NPT2A internalization with ensuing urinary phosphate excretion. Remarkably, mice with targeted Npt2a inactivation at weaning likewise display reduced serum phosphate accompanied by a complex bone phenotype with retarded secondary ossification. However, with increasing age the bone phenotype spontaneously reverted without recovery of serum phosphate levels [32]. The affected patient in case #1 displayed skeletal irregularities at 2 months of age but no signs of rickets or skeletal dysplasia at 3 years [21]. Based on the findings described here, we would expect continued basal phosphate transport, whereas hormone-dependent regulation would be reduced or negligible.
In summary, we identify and characterize the NPT2A internal 494TRL496 PDZ motif as a novel, heretofore undescribed determinant of hormone-inhibitable phosphate transport. We propose an advanced model where both NPT2A PDZ binding motifs play distinct roles in basal and hormone-inhibitable phosphate regulation: the carboxy-terminal PDZ ligand defines the apical localization of NPT2A through the interaction with NHERF1. The internal PDZ motif is an essential determinant of hormone-triggered action on phosphate transport and NPT2A trafficking.
Materials and methods
Peptides
Human [Nle8,18,Tyr34]PTH(1–34) was purchased from Bachem (H9110). Recombinant human R179Q-FGF23 (25–251) (referred to henceforth as FGF23), which is resistant to furin cleavage and inactivation, was obtained from R&D Systems (2604-FG-025).
Antibodies
Antibodies Rabbit polyclonal anti-NHERF1 (ab3452) and monoclonal anti-NHERF1 (Abcam ab31111) were purchased from Abcam. M2-mouse monoclonal anti-HA-agarose (A2095) and rabbit polyclonal anti-FLAG antibodies (F7425) were purchased from Sigma. Rabbit polyclonal anti-HA (sc-805) was purchased from Santa Cruz. Horseradish Peroxidase-conjugated Goat Anti-Rabbit (Dako P044801–2) and Rabbit Anti-Mouse (Dako P044701–5) secondary antibodies were purchased from Agilent. Primary antibodies were used at a 1: 1000 dilution for immunoblots; secondary antibodies were employed at a 1: 5000 dilution. The agarose-conjugated anti-HA was used at a 1: 50 dilution for immunoprecipitation experiments.
Cell culture
A new CRISPR/Cas9 Npt2a knockout OK cell line was developed to explore human NPT2A structural determinants involved in binding with NHERF1 and hormone-inhibitable phosphate transport. FGF23- and PTH-inhibitable phosphate uptake in these cells was rescued with human HA-GFP-NPT2A or mutant variants (494AAA496, CT-AAA, Arg495 Cys, Arg495His, and 494CRL496).
Opossum kidney (parental OK or NHERF1-deficient OKH) cells [9] were grown in DMEM/Ham’s F-12 50: 50 medium (Mediatech, 10–090-CV) supplemented with 10% FBS and 1% pen/strep. Cells were transfected with the indicated plasmids using FuGENE 6 (Promega) or Lipofectamine 3000 (Invitrogen) according to the manufacturer’s instructions. Stable cells expressing FLAG-NHERF1 or FLAG-NHERF1 constructs (P1, P2, or P1P2) were prepared by screening with puromycin or G418.
Preparation of endogenous NHERF1 and NHERF1 constructs
WT or NHERF1 P1, P2, or P1P2 constructs tagged with FLAG epitope were transfected using Lipofectamine 3000 (Invitrogen) as described previously [11].
Immunoprecipitation
OK cells were transfected with HA-GFP-WT-NPT2A (WT) or HA-GFP-NPT2A-494CRL496 (494CRL496) and FLAG-NHERF1. After 48 h post-transfection, cells were serum-starved overnight and then treated for 2 h in the presence of 100 nM PTH. Lysates were prepared, and NHERF1 was immunoprecipitated with Ms-anti-FLAG agarose (Sigma). NPT2A was immunoblotted with Rb-anti-FLAG (Sigma). NHERF1 was immunoblotted using Rb-anti-HA (Santa Cruz).
A custom anti-phospho NPT2A Thr494 (494pThr) antibody (10343 GenScript) generated against the NPT2A 485ILLWYPVPCT494RLPI498 sequence was applied to verify phosphorylation of Thr494 as before [12]. CRISPR/Cas9 Npt2a knockout OK cells were transfected with HA-GFP-NPT2A (WT) or NPT2A with the triple AAA replacement (494AAA496) for 24 h, and serum-starved overnight. The next day, cells were treated with 100 nM PTH for 2 min. Lysates were prepared in the presence of phosphatase inhibitors. Cells were immunoblotted for 494pTRL or total NPT2A (Novus). Primary antibody (1: 1000) or Goat anti-rabbit-HRP secondary antibody (1: 5000) were applied for 10 min.
Phosphate uptake
CRISPR/Cas9 Npt2a knockout OK cells transfected with HA-GFP-NPT2A or NPT2A mutant variants were seeded on 12-well plates. When the cells reached confluence (2–3 days after passaging), they were treated with 100 nM PTH(1–34) or FGF23 in cell culture media. After 2 h, the hormone-supplemented media was aspirated, and the wells were washed three times with 1 ml of Na-replete wash buffer (140 mM NaCl, 4.8 mM KCl, 1.2 mM MgSO4, 0.1 mM KH2PO4, 10 mM HEPES, pH 7.4). The cells were incubated with 1 μCi of 32P orthophosphate (PerkinElmer, NEX053) in 1 ml of Na-replete wash buffer for 10 min. Phosphate uptake was terminated by placing the plate on ice and rinsing the cells three times in Na-free wash buffer (140 mM N-methyl-d-glucamine (NMDG), 4.8 mM KCl, 1.2 mM MgSO4, 0.1 mM KH2PO4, 10 mM HEPES, pH 7.4). The cells in each well were extracted using 500 μl of 1% Triton X100 (Sigma Cat X100) in water overnight at 4°C. A 250 μl aliquot was processed for analysis on a Beckmann Coulter LS6500 liquid scintillation counter. Data were normalized, where 100% was defined as the CPM of phosphate uptake under control conditions.
Confocal microscopy
Confocal fluorescence imaging was performed as described previously with some modifications [9]. Briefly, CRISPR/Cas9 Npt2a knockout OK cells or OK cells, as indicated, were seeded on collagen-coated coverslips. Twenty-four hours later, the cells were transiently transfected with HA-GFP-NPT2A (WT or mutant constructs, as shown in Figures 5A–C and 7B–D), and FLAG-NHERF1. Forty-eight post-transfected, the cells were serum-starved overnight and treated with Vehicle (control) or 100 nM PTH for 2 h, fixed in 4% paraformaldehyde, and stained with Rb-anti-FLAG (Sigma) primary antibody (1: 50) and Goat anti-rabbit-Alexa546 (Invitrogen) secondary (1: 1000) antibody. Coverslips were mounted for immunofluorescence microscopy using ProLong™ Gold Antifade Mountant with DAPI (Thermofisher P36941) and analyzed using an Olympus FluoView 1000 microscope with a 63× oil immersion objective. Colocalization analyses for HA-GFP-NPT2A variants and FLAG-NHERF1 were performed with ImageJ. Confocal images were quantified using the EzColocalization plugin for ImageJ and calculating the Threshold Overlap Score (TOS) [33]. TOS ranges from +1 (perfect correlation) to −1 (perfect but negative correlation), with 0 denoting the absence of a relationship. Confocal fluorescence imaging was also performed for NPT2A with the disease-associated Arg495Cys and Arg495His NPT2A variants as described above.
System setup and molecular dynamics simulation
AF2 [27] was applied to create a 3D model of full-length NPT2A. Because of computational limitations, the model for MD simulation includes TM4-TM7 (residues 310–536). Files for simulation were prepared using the Leap module of AMBER16 [29]. The WT NPT2A model was solvated with TIP3P water molecules [34] in a periodically replicated box and neutralized with Cl− ions. The structure was relaxed by 100 cycles of steepest descent followed by 400 cycles of conjugate gradient energy minimization procedure using the AMBER16 pmemd module [29] with harmonic restraints (force constant −0.5 kcal/mol/Å2) applied to all protein atoms. A 1 ns MD simulation run was performed under the NPT ensemble [constant number of particles (N), pressure (P), and temperature (T)] to reach the correct density of liquid water (~1 g/ml). During this equilibration, harmonic restraints were applied to all residues and methodically lowered from ks = 10 kcal/mol/Å2 to 0.1 kcal/mol/Å2. The simulation was continued under the NVT ensemble [constant number of particles (N), volume (V), and temperature (T)] at 300 K with harmonic restrains ks = 0.1 kcal/mol/Å2 applied to the amino-terminal and carboxy-terminal backbone atoms to prevent diffusion of the protein from the simulation box. Langevin coupling algorithm was applied. The energy minimization and MD simulation were carried out using the AMBER16 package with the AMBER force field (ff99SB). Since the starting structure was not experimentally resolved conformation, we performed a long equilibration protocol (50 ns). A production simulation was run for ~150 ns with the temperature at 300 K and constant volume with the same harmonic restraints as in the equilibration phase. Coordinates were saved every 2 ps for later analyses. The NPT2A Arg495Cys or Arg495His model was built by replacing Arg495 with Cys or His, respectively, using the Leap module of AMBER16 [29]. Except for the length, the simulation details, equilibration, and production simulations were set up as described for WT NPT2A. After solvation and ionization, each system has ~32 920 atoms. The equilibration and production simulations of wild-type and mutant systems were monitored by calculating the root-mean-square deviation (RMSD) (cpptraj of AMBER16) [29] of the backbone atoms from their initial positions (not presented here). The hydrogen bond analysis was performed using the ccptraj module of AMBER16 [29].
Statistical analysis
Results were analyzed using Prism 9 software. Data represent the mean ± SD or SEM calculated on at least three independent experiments. Each figure’s legend indicates the exact number of experiments performed and used for statistical analysis. P-values were calculated using one-way ANOVA with the Brown–Forsythe test, two-way ANOVA with Šidák’s or Dunnett’s multiple comparison test, or three-way ANOVA with Turkey’s multiple comparison procedure. P-values were depicted by * P < 0.05, ** P < 0.01, *** P < 0.001, and **** P < 0.0001. P-values <0.05 were considered statistically significant.
Acknowledgements
We thank Dr. Qiangmin Zhang for preparing the NPT2A constructs used here.
Funding
NIH grant R01DK105811 supported this work.
Abbreviations
- AF2
AlphaFold2
- FGF23
Fibroblast growth factor 23
- MD simulation
molecular dynamics simulation
- NHERF1
Na+/H+ exchanger regulatory factor-1
- NPT2A
Na+-dependent phosphate cotransporter
- OK(H)
opossum kidney cells
- PTH
parathyroid hormone
- RMSD
root-mean-square deviation
- TM
transmembrane domain
- TOS
threshold overlap score
Footnotes
CRediT Author Contribution
Tatyana Mamonova: Conceptualization, Formal analysis, Investigation, Writing — original draft, Writing — review and editing. W. Bruce Sneddon: Resources, Investigation, Visualization, Methodology, Writing — original draft, Writing — review and editing. Peter A. Friedman: Conceptualization, Formal analysis, Supervision, Funding acquisition, Visualization, Writing — original draft, Project administration, Writing — review and editing.
NPT2A with experimentally altered carboxy-terminal or internal PDZ ligands are denoted ‘mutants’, whereas naturally occurring genetic modifications are designated ‘variants’.
Competing Interests
The authors declare that there are no competing interests associated with the manuscript.
Data Availability
The authors agree to make any materials, data, and associated protocols available upon request.
References
- 1.Drueke TB (2010) Klotho, FGF23, and FGF receptors in chronic kidney disease: a yin-yang situation? Kidney Int. 78, 1057–1060 10.1038/ki.2010.339 [DOI] [PubMed] [Google Scholar]
- 2.Gutierrez OM (2015) Contextual poverty, nutrition, and chronic kidney disease. Adv. Chronic Kidney Dis 22, 31–38 10.1053/j.ackd.2014.05.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cannata-Andia JB, Martin-Carro B, Martin-Virgala J, Rodriguez-Carrio J, Bande-Fernandez JJ, Alonso-Montes C et al. (2021) Chronic kidney disease-mineral and bone disorders: pathogenesis and management. Calcif. Tissue Int 108, 410–422 10.1007/s00223-020-00777-1 [DOI] [PubMed] [Google Scholar]
- 4.Weinman EJ and Lederer ED (2012) PTH-mediated inhibition of the renal transport of phosphate. Exp. Cell Res 318, 1027–1032 10.1016/j.yexcr.2012.02.037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Murer H and Biber J (2010) Phosphate transport in the kidney. J. Nephrol 23, S145–S151 [PubMed] [Google Scholar]
- 6.Yamashita T, Konishi M, Miyake A, Inui K and Itoh N (2002) Fibroblast growth factor (FGF)-23 inhibits renal phosphate reabsorption by activation of the mitogen-activated protein kinase pathway. J. Biol. Chem 277, 28265–28270 10.1074/jbc.M202527200 [DOI] [PubMed] [Google Scholar]
- 7.Hollenstein K, de Graaf C, Bortolato A, Wang MW, Marshall FH and Stevens RC (2014) Insights into the structure of class B GPCRs. Trends Pharmacol. Sci 35, 12–22 10.1016/j.tips.2013.11.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.McGarvey JC, Xiao K, Bowman SL, Mamonova T, Zhang Q, Bisello A et al. (2016) Actin-Sorting Nexin 27 (SNX27)-retromer complex mediates rapid parathyroid hormone receptor recycling. J. Biol. Chem 291, 10986–11002 10.1074/jbc.M115.697045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang B, Means CK, Yang Y, Mamonova T, Bisello A, Altschuler DL et al. (2012) Ezrin-anchored protein kinase A coordinates phosphorylation-dependent disassembly of a NHERF1 ternary complex to regulate hormone-sensitive phosphate transport. J. Biol. Chem 287, 24148–24163 10.1074/jbc.M112.369405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mamonova T, Zhang Q, Khajeh JA, Bu Z, Bisello A and Friedman PA (2015) Canonical and noncanonical sites determine NPT2A binding selectivity to NHERF1 PDZ1. PLoS ONE 10, e0129554 10.1371/journal.pone.0129554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vistrup-Parry M, Sneddon WB, Bach S, Stromgaard K, Friedman PA and Mamonova T (2021) Multisite NHERF1 phosphorylation controls GRK6A regulation of hormone-sensitive phosphate transport. J. Biol. Chem 296, 100473 10.1016/j.jbc.2021.100473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhang Q, Xiao K, Paredas JM, Mamonova T, Sneddon WB, Liu H et al. (2019) Parathyroid hormone initiates dynamic NHERF1 phosphorylation cycling and conformational changes that regulate NPT2A-dependent phosphate transport. J. Biol. Chem 294, 4546–4571 10.1074/jbc.RA119.007421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shenolikar S, Voltz JW, Minkoff CM, Wade JB and Weinman EJ (2002) Targeted disruption of the mouse NHERF-1 gene promotes internalization of proximal tubule sodium- phosphate cotransporter type IIa and renal phosphate wasting. Proc. Natl Acad. Sci. U.S.A 99, 11470–11475 10.1073/pnas.162232699 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Morales FC, Takahashi Y, Kreimann EL and Georgescu MM (2004) Ezrin-radixin-moesin (ERM)-binding phosphoprotein 50 organizes ERM proteins at the apical membrane of polarized epithelia. Proc. Natl Acad. Sci. U.S.A 101, 17705–17710 10.1073/pnas.0407974101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Weinman EJ, Mohanlal V, Stoycheff N, Wang F, Steplock D, Shenolikar S et al. (2006) Longitudinal study of urinary excretion of phosphate, calcium, and uric acid in mutant NHERF-1 null mice. Am. J. Physiol. Renal Physiol 290, F838–F843 10.1152/ajprenal.00374.2005 [DOI] [PubMed] [Google Scholar]
- 16.Karim Z, Gerard B, Bakouh N, Alili R, Leroy C, Beck L et al. (2008) NHERF1 mutations and responsiveness of renal parathyroid hormone. N. Engl. J. Med 359, 1128–1135 10.1056/NEJMoa0802836 [DOI] [PubMed] [Google Scholar]
- 17.Courbebaisse M, Leroy C, Bakouh N, Salaun C, Beck L, Grandchamp B et al. (2012) A new human NHERF1 mutation decreases renal phosphate transporter NPT2a expression by a PTH-independent mechanism. PLoS ONE 7, e34764 10.1371/journal.pone.0034764 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mahon MJ (2009) The parathyroid hormone 1 receptor directly binds to the FERM domain of ezrin, an interaction that supports apical receptor localization and signaling in LLC-PK1 cells. Mol. Endocrinol 23, 1691–1701 10.1210/me.2009-0164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cunningham R,X,E, Steplock D, Shenolikar S and Weinman EJ (2005) Defective PTH regulation of sodium-dependent phosphate transport in NHERF-1−/− renal proximal tubule cells and wild-type cells adapted to low-phosphate media. Am. J. Physiol. Renal Physiol 289, F933–F938 10.1152/ajprenal.00005.2005 [DOI] [PubMed] [Google Scholar]
- 20.Hernando N, Deliot N, Gisler SM, Lederer E, Weinman EJ, Biber J et al. (2002) PDZ-domain interactions and apical expression of type IIa Na/Pi cotransporters. Proc. Natl Acad. Sci. U.S.A 99, 11957–11962 10.1073/pnas.182412699 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rajagopal A, Braslavsky D, Lu JT, Kleppe S, Clement F, Cassinelli H et al. (2014) Exome sequencing identifies a novel homozygous mutation in the phosphate transporter SLC34A1 in hypophosphatemia and nephrocalcinosis. J. Clin. Endocrinol. Metab 99, E2451–E2456 10.1210/jc.2014-1517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kang SJ, Lee R and Kim HS (2019) Infantile hypercalcemia with novel compound heterozygous mutation in SLC34A1 encoding renal sodium-phosphate cotransporter 2a: a case report. Ann. Pediatr. Endocrinol. Metab 24, 64–67 10.6065/apem.2019.24.1.64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Karthikeyan S, Leung T and Ladias JAA (2001) Structural basis of the Na+/H+ exchanger regulatory factor PDZ1 interaction with the carboxyl-terminal region of the cystic fibrosis transmembrane conductance regulator. J. Biol. Chem 276, 19683–19686 10.1074/jbc.C100154200 [DOI] [PubMed] [Google Scholar]
- 24.Ernst A, Appleton BA, Ivarsson Y, Zhang Y, Gfeller D, Wiesmann C et al. (2014) A structural portrait of the PDZ domain family. J. Mol. Biol 426, 3509–3519 10.1016/j.jmb.2014.08.012 [DOI] [PubMed] [Google Scholar]
- 25.Chan AS, Clairfeuille T, Landao-Bassonga E, Kinna G, Ng PY, Loo LS et al. (2016) Sorting nexin 27 couples PTHR trafficking to retromer for signal regulation in osteoblasts during bone growth. Mol. Biol. Cell 27, 1367–1382 10.1091/mbc.E15-12-0851 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mamonova T, Zhang Q, Chandra M, Collins BM, Sarfo E, Bu Z et al. (2017) Origins of PDZ binding specificity. A computational and experimental study using NHERF1 and the parathyroid hormone receptor. Biochemistry 56, 2584–2593 10.1021/acs.biochem.7b00078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jumper J, Evans R, Pritzel A, Green T, Figurnov M, Ronneberger O et al. (2021) Highly accurate protein structure prediction with AlphaFold. Nature 596, 583–589 10.1038/s41586-021-03819-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fenollar-Ferrer C, Forster IC, Patti M, Knoepfel T, Werner A and Forrest LR (2015) Identification of the first sodium binding site of the phosphate cotransporter NaPi-IIa (SLC34A1). Biophys. J 108, 2465–2480 10.1016/j.bpj.2015.03.054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Case DA, Betz RM, Cerutti DS, Cheatham TE III, Darden TA, Duke RE. et al. (2016) AMBER 2016
- 30.Fowler NJ and Williamson MP (2022) The accuracy of protein structures in solution determined by AlphaFold and NMR. Structure 30, 925–933. e922 10.1016/j.str.2022.04.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ruppe MD (1993) X-Linked Hypophosphatemia. In GeneReviews (Adam MP, Mirzaa GM, Pagon RA, Wallace SE, Bean LJH, Gripp KW and Amemiya A, eds), Seattle, WA: [PubMed] [Google Scholar]
- 32.Beck L, Karaplis AC, Amizuka N, Hewson AS, Ozawa H and Tenenhouse HS (1998) Targeted inactivation of Npt2 in mice leads to severe renal phosphate wasting, hypercalciuria, and skeletal abnormalities. Proc. Natl Acad. Sci. U.S.A 95, 5372–5377 10.1073/pnas.95.9.5372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Stauffer W, Sheng H and Lim HN (2018) Ezcolocalization:an ImageJ plugin for visualizing and measuring colocalization in cells and organisms. Sci. Rep 8, 15764 10.1038/s41598-018-33592-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jorgensen WL, Chandrasekhar J, Madura JD, Impey RW and Klein ML (1983) Comparison of simple potential functions for simulating liquid water. J. Chem. Phys 79, 926–935 10.1063/1.445869 [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The authors agree to make any materials, data, and associated protocols available upon request.


