Abstract
Introduction:
Specific multimorbidity combinations, in particular those including arthritis, stroke, and cognitive impairment, have been associated with high burden of activities of daily living (ADL)-instrumental activities of daily living (IADL) disability in older adults. The biologic underpinnings of these associations are still unclear.
Methods:
Observational longitudinal study using data from the Health and Retirement Study (N = 8,618, mean age = 74 years, 58% female, 25% non-white) and negative binomial regression models stratified by sex to evaluate the role of inflammatory and glycemic biomarkers (high-sensitivity C-reactive protein (hs-CRP) and HbA1c) in the association between specific multimorbidity combinations (grouped around one of eight index diseases: arthritis, cancer, cognitive impairment, diabetes, heart disease, hypertension, lung disease, and stroke; assessed between 2006 and 2014) and prospective ADL-IADL disability (2 years later, 2008–2016). Results were adjusted for sociodemographic characteristics, body mass index, number of coexisting diseases, and baseline ADL-IADL score.
Results:
Multimorbidity combinations indexed by arthritis (IRR = 1.1, 95% CI = 1.01–1.20), diabetes (IRR = 1.19, 95% CI = 1.09–1.30), and cognitive impairment (IRR = 1.11, 95% CI = 1.01–1.23) among men and diabetes-indexed multimorbidity combinations (IRR = 1.07, 95% CI = 1.01–1.14) among women were associated with higher ADL-IADL scores at increasing levels of HbA1c. Across higher levels of hs-CRP, multimorbidity combinations indexed by arthritis (IRR = 1.06, 95% CI = 1.02–1.11), hypertension (IRR = 1.06, 95% CI = 1.02–1.11), heart disease (IRR = 1.06, 95% CI = 1.01–1.12), and lung disease (IRR = 1.14, 95% CI = 1.07–1.23) were associated with higher ADL-IADL scores among women, while there were no significant associations among men.
Conclusion:
The findings suggest potential for anti-inflammatory management among older women and optimal glycemic control among older men with these particular multimorbidity combinations as focus for therapeutic/preventive options for maintaining functional health.
Keywords: Multimorbidity combinations, Disability, Inflammation, Glycemic status, Biomarkers
Introduction
Functional disability is highly prevalent among older adults in the USA [1, 2], resulting in diminished quality of life, loss of independent living and subsequent institutionalization, substantial medical expenditures, and increased mortality [3–5]. The presence of certain chronic diseases – cardiovascular disease, cancer, diabetes, and chronic respiratory disease, in particular [6] – has been associated with substantial difficulties in performing activities required for independent living. Further, the co-occurrence of chronic diseases (i.e., multimorbidity) is an important contributor to the process of disablement, above and beyond the contribution of each individual disease. The available evidence indicates that a greater number of diseases in the multimorbidity combination predict a higher burden of functional limitations and a faster decline in functional status [7] and also that specific combinations of diseases carry a disproportionate disabling potential [8, 9]. Recognizing the problem, both the U.S. Department of Health and Human Services and the World Health Organization have identified the prevalence of multimorbidity and associated consequences as a key healthcare concern in the USA and globally and recognized the need for additional research to close the gaps in knowledge on the biological basis for medical therapies to improve the care of older adults with multimorbidity [10, 11].
The scarcity of knowledge on the pathophysiology underlying the association between multimorbidity, and more so specific multimorbidity combinations, and functional impairment, is one such gap. We propose to assess two biological processes – systemic chronic inflammation and glucose homeostasis – that are not disease specific, but rather common to many of the chronic diseases prevalent in multimorbidity combinations, as possible links between specific multimorbidity combinations and prospective functional disability.
Prior studies have shown that chronic inflammation, a senescence process associated with many of the diseases frequently found in multimorbidity states [12], is also associated with overall multimorbidity [13, 14] and with prevalent and incident functional disability [15, 16], separately and as an intermediary in their association [17]. However, none of the studies, to our knowledge, have investigated specific multimorbidity combinations, and the existing findings have constrained generalizability to the overall U.S. older adult population due to limited age and/or geographic inclusion criteria.
Diabetes, characterized by impaired glucose metabolism, is highly prevalent among older adults with multimorbidity, and a vast majority of individuals with diabetes have at least one other chronic condition [18, 19]. Diabetes has been robustly linked to poor functional status, especially in the presence of comorbidities [20, 21]. Glucose dysregulation, as indicated by glycemic markers such as HbA1c in the prediabetic (5.7–6.4%) and diabetic (6.5% and above) ranges, is a strong predictor of initiation and progression of functional limitations in older adults with multimorbidity [22, 23]. Interestingly, recent studies have described a J-shaped rather than threshold (i.e., above the 5.7% cutoff) relationship between HbA1c and various health outcomes, such that very low levels of HbA1c – suggestive of disease- or age-related catabolic or malnourished states – among individuals without diabetes are also associated with an increased incidence of functional disability [24]. However, the relationship between the full spectrum of HbA1c values, including values across the normal range, and functional disability in individuals with multimorbidity has not been fully investigated.
To start to address these gaps, this study aimed to evaluate the role of systemic chronic inflammation (assessed by high-sensitivity C-reactive protein levels) and glycemic levels (assessed by HbA1c levels across the full spectrum) in the association between specific multimorbidity combinations and the burden of functional limitations among older adults from the Health and Retirement Study. A better understanding of the biological basis for the multimorbidity-disability link is needed as an initial step toward improving clinical care, self-management recommendations, and public health interventions aimed at reducing the burden of functional disability among older adults with multimorbidity.
Materials and Methods
Data
This is an observational longitudinal study using data from the Health and Retirement Study (HRS), a nationally representative survey of noninstitutionalized middle- and older-aged adults, which explores the changes in health and financial status that occur as individuals transition into retirement and then in later life. HRS started in 1992 by surveying respondents aged 51 years and over and their partners every 2 years from the time of their entry into the study until death or institutionalization. In 2006, HRS began collecting biomarker information as part of an enhanced face-to-face interview. A random one half of 2006 HRS households were preselected for this enhanced face-to-face interview, and the other half was selected in 2008. Then, in 4-year intervals, biomarker collection was repeated; for instance, the first half (initially evaluated in 2006) was interviewed again in 2010 and 2014, and the second half (initially evaluated in 2008) was again assessed in 2012. New cohort households were randomly assigned to one of the two biomarker samples. This study used waves from 2006 to 2014, which are the most currently available in final release. Full descriptions of the biomarker modules have been previously published and are available at https://hrs.isr.umich.edu/documentation/data-descriptions.
Study Population
A total of 20,193 HRS respondents participated in blood-based biomarker collection from 2006 to 2014. Of these respondents, 18,487 participated and answered the activities of daily living (ADL)-instrumental activities of daily living (IADL) questions at the following wave; 18,483 had complete case chronic disease responses; 10,436 were aged 65 years or older; and 8,618 respondents had multimorbidity at the time of the first biomarker assessment. This resulted in an analytic sample of 8,618 respondents, comprised of 3,594 males and 5,024 females. All the participants were self-responders, since proxy participants were not eligible for the biomarkers module. As described in the Data section, biomarker collection occurred every 4 years starting in 2006, and we allowed respondents to be represented more than once in our analytic sample if they participated in more than one biomarker collection; approximately 7% were represented for three waves, 31% were represented for two waves, and 62% were represented for one wave. The selection flow for this analytic sample, at the respondent level and with regard to the number of observations making up this analytic sample, is shown in online supplementary Figure 1 (for all online suppl. material, see www.karger.com/doi/10.1159/000528648).
Variables
Functional Status Assessment
Functional status was assessed prospectively, at the regular HRS wave subsequent to the biomarker and multimorbidity assessment waves (i.e., ~2 years later), as follows: Activities of Daily Living. As part of the HRS interview process, Nagi items (e.g., walking several blocks, climbing stairs, and pushing a heavy object) were used to determine if respondents should be routed to ADL questions. If respondents reported no difficulties with Nagi items, they were not asked about ADLs. If respondents reported one Nagi item difficulty but no difficulty dressing, they were not asked any additional ADL questions. We defined such respondents as having no ADL limitations. The remaining respondents were asked all six ADL questions (dressing, walking across a room, eating, bathing, toileting, and transferring from bed) and were defined as having a specific ADL limitation if (1) the respondent reported difficulty with the ADL due to a health of memory problem or (2) the respondent had help with the ADL. The ADL score was calculated as sum of reported ADL difficulties and ranged from 0 to 6 (higher score indicates higher ADL limitations).
Instrumental Activities of Daily Living.
All respondents were asked about difficulty with five IADLs (meal preparation, grocery shopping, using a telephone, taking medication, and managing money). We defined a specific IADL limitation as (1) difficulty performing the IADL due to a health or memory problem, (2) not performing the IADL due to a health or memory problem, or (3) receiving help perform the IADL. The IADL score was calculated as sum of reported IADL difficulties and ranged from 0 to 5 (higher score indicates higher IADL limitations).
ADL-IADL Score.
The main outcome variable in this study was the combined ADL-IADL score, created by sum counting the number of ADL and IADL difficulties for each respondent who had at least one non-missing ADL or IADL response (range 0–11; higher number indicates higher ADL-IADL limitations). This approach has been previously described and used in studies of disability in older adults [25].
Biomarkers: CRP and HbA1c
Dried blood spots (DBS) were collected, from which high-sensitivity C-reactive protein (CRP henceforth) and glycosylated hemoglobin (HbA1c) were extracted and quantified as detailed elsewhere (https://hrs.isr.umich.edu/documentation/data-descriptions). In following with the HRS recommendation, we used the NHANES equivalent values, which were constructed and made available by HRS. In brief, NHANES equivalent variables were constructed by assuming the distribution of DBS assays was similar to that in NHANES; values of both assays were determined at each percentile; and then the DBS assay values were transformed into the NHANES scale, after adjusting for any between-laboratory differences.
Disease-Indexed Multimorbidity Combinations
Multimorbidity was defined as the presence of two or more co-occurring chronic conditions from among a total of eight chronic conditions available in HRS: seven self-reported chronic somatic diseases and cognitive impairment. Each self-reported somatic disease was prompted by “has a doctor ever told you that you have...” and included heart disease (myocardial infarction, coronary heart disease, angina, congestive heart failure, or other heart problems), hypertension (i.e., high blood pressure), stroke (but not TIA), diabetes, arthritis, lung disease (chronic bronchitis or emphysema but excluding asthma), and cancer (any malignant tumors with the exception of skin cancer). Depressive symptoms were not included in the calculation of multimorbidity because they are potentially reversible and thus not chronic per se. We defined respondents as having the chronic disease if they reported having been diagnosed with the disease prior to or in the interview year. Cognitive function was assessed using cognition measures from the core HRS interview and classified according to Langa-Weir’s derived classifications [26], which map onto a 27-point scale including (1) immediate and delayed 10-noun free recall test to measure memory (0–20 points); (2) a serial sevens subtraction test to measure working memory (0–5 points); and (3) a counting backward test to measure speed of mental processing (0–2 points). Respondents with a score of ≤11 were classified as experiencing cognitive impairment [26].
For this study, we assessed the association between multimorbidity and ADL-IADL score at the subsequent wave, at increasing levels of CRP and HBA1c, separately for respondents with multimorbidity combinations that are indexed (grouped) by each of the aforementioned conditions, for instance, separately for respondents whose multimorbidity combination includes heart disease (i.e., indexed by heart disease) or stroke (i.e., indexed by stroke). This approach is detailed below in the Statistical Analysis section.
Covariates
Covariates were selected a priori, informed by existing knowledge [27] and by the social stratification of aging and health framework [28], which proposes that ascribed and attained social factors, such as age, sex, race/ethnicity, education, and other health risks, lead to inequalities in health outcomes in later life. Sociodemographic covariates included age, sex (male/female), education (number of school years completed), race/ethnicity, and body mass index (BMI). Race/ethnicity was defined using the two following questions: (1) “Do you consider yourself Hispanic or Latino?” and (2) “Do you consider yourself primarily white or Caucasian, black or African American, American Indian, Asian, or something else?” Due to insufficient numbers of American Indian, Asian, or other race/ethnicities, we created three mutually exclusive groups: non-Hispanic white (white), non-Hispanic black (black), and Hispanic. BMI was calculated according to the established formula (BMI = weight [pounds] × 703/height2 [inches]) using respondents’ most recent self-reported height and self-reported weight at each interview.
Statistical Analysis
Descriptive characteristics of the study population were summarized using frequencies and percentages for categorical variables and means and standard deviations or medians and interquartile ranges (IQR) for continuous variables. We identified 213 unique multimorbidity combinations of two or more chronic conditions; of these, approximately 12% were observed in one respondent, and approximately half were observed in 14 or fewer respondents. To include infrequent combinations which would otherwise be underpowered for analysis, all combinations were categorized around each disease as an index disease. For example, all multimorbidity combinations including heart disease (i.e., heart disease along with at least one other chronic condition) are grouped in a single multimorbidity category. This approach has been previously used in multimorbidity research [29] and generated 8 non-mutually exclusive multimorbidity categories that make use of all the available data and chronic disease combinations.
To assess the association between various disease-indexed multimorbidity combinations and prospective ADL-IADL score (i.e., at a subsequent wave) in participants with different levels of CRP and HbA1c, we built a series of negative binomial regression models. We examined various distributions to determine which statistical model would best fit our data, and we selected a negative binomial distribution, allowing us to estimate the count of ADL-IADL limitations as well as account for overdispersion. Additionally, we used cluster robust standard errors to account for the correlation of repeated measurements within an individual, as a respondent may have participated in biomarker collection up to 3 times over our study period (see Data and Study Population sections for more detail). The analyses were stratified by sex because of previously described sex differences [30] in ADL and IADL difficulties (validated in our sample and shown in online suppl. Table 1). For each disease-indexed multimorbidity combination and each biomarker, the following models were created: (1) unadjusted; (2) demographics adjusted (age, education, race/ethnicity, and BMI); (3) function/demographics adjusted (demographics + baseline ADL-IADL score); and (4) fully adjusted (demographics + baseline ADL-IADL score + count of chronic conditions). HbA1c was included as a continuous variable. As CRP was right skewed, we log-transformed CRP values to improve model fit. The model coefficients are the log of the ratio of expected ADL-IADL counts. Exponentiated coefficients and 95% confidence intervals are reported. The interpretation of the exponentiated coefficients, for example, for multimorbidity combinations indexed by heart disease, is as follows: “multimorbidity combinations indexed by heart disease are associated with an ADL-IADL score that increases by a factor of X.XX for each unit increase in HbA1c level.” and “multimorbidity combinations indexed by heart disease are associated with an ADL-IADL score that increases by a factor of X.XX for every doubling in CRP level.”
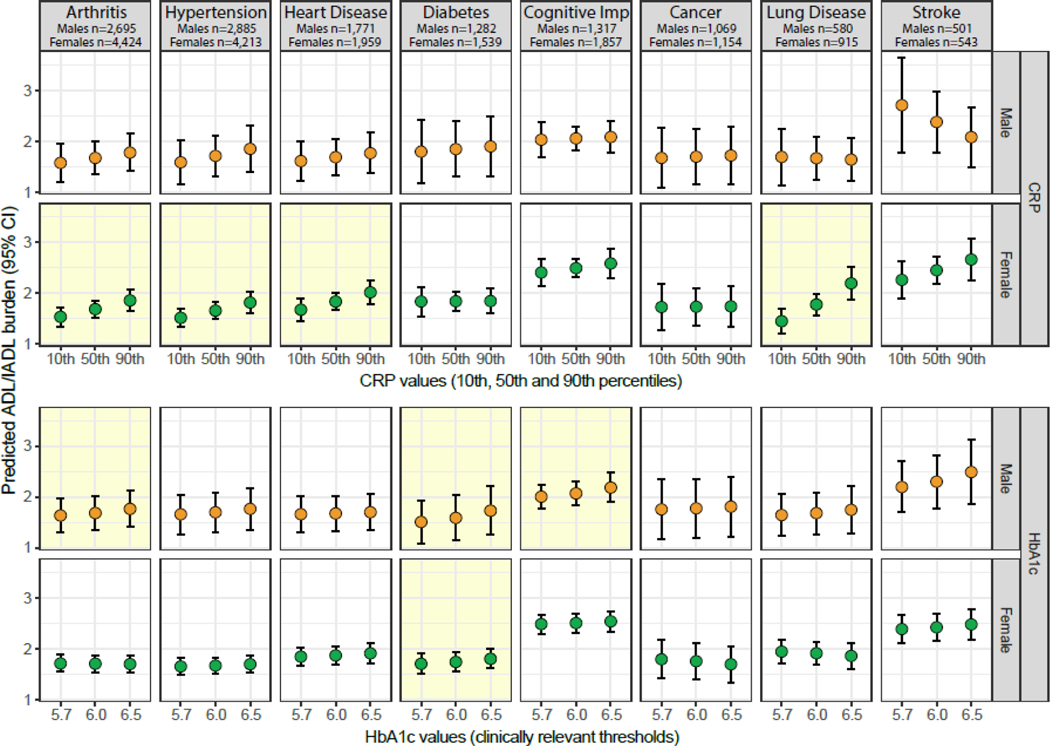
To further describe the associations estimated by our final models, we utilized Stata’s post-estimate command, margins, to calculate the average predicted ADL-IADL score at three HbA1c values (prediabetes cutoff = 5.7%, sample mean = 6.0%, and diabetes cutoff = 6.5%) and at the 10th, 50th, and 90th percentiles for CRP; these average predicted values are presented visually in Figure 1.
Fig. 1.
Predicted ADL-IADL score according to CRP and HbA1c levels, stratified by sex. Shaded boxes indicate statistically significant associations at the p < 0.05 level. The predicted ADL-IADL score (prospective, i.e., at wave subsequent to the wave at which biomarkers/multimorbidity are assessed) is calculated at three HbA1c values: prediabetes cutoff = 5.7%, sample mean = 6.0%, and diabetes cutoff = 6.5% and at the 10th, 50th, and 90th percentiles for CRP. CRP, high-sensitivity C-reactive protein; HbA1c, glycosylated hemoglobin A1c, ADL, activities of daily living; IADL, instrumental activities of daily living.
Models included a standard error computation unit, stratum, and respondent-level weights to account for the complex survey design of HRS. All analyses were conducted in STATA/SE 15 (StataCorp, College Station, TX), while figures were generated in RStudio, version 1.1.456.
Results
This study included 8,618 participants aged 65 years and older (mean age = 74 years), of whom 58% were women and 25% were non-white. In the full sample, arthritis (80.5%) and hypertension (80%) were the most prevalent chronic conditions, while lung disease and stroke were the least prevalent conditions (15% and 10%, respectively). The mean ADL-IADL score at the first observation was 0.6 (SD = 1.5) and was higher among women (mean = 0.7, SD = 1.6) than among men (mean = 0.5, SD = 1.3). The mean baseline HbA1c was 6.0% (SD = 1.0; median [IQR] = 5.8 [5.4–6.3]), and the mean CRP was 4.3 mg/L (SD = 8.3; median [IQR] = 2.0 [0.9–4.5]). The detailed descriptive characteristics, for the full sample and stratified by sex, are presented in Table 1, and the distribution of ADL-IADL scores, separately by sex, is shown in online supplementary Figure 2.
Table 1.
Descriptive characteristics of the study population, overall and stratified by sex.
| Total (N=8,618) | Male (N=3,594) | Female (N=5,024) | |
|---|---|---|---|
|
| |||
| Age at 1st observation (years), mean(SD) | 73.7 (6.9) | 73.6 (6.6) | 73.8 (7.1) |
| Sex (female), n(%) | 5,024 (58.3%) | --- | --- |
| Race/ethnicity, n(%) | |||
| Non-Hispanic White | 6,471 (75.1%) | 2,768 (77.0%) | 3,703 (73.7%) |
| Non-Hispanic Black | 1,232 (14.3%) | 447 (12.4%) | 785 (15.6%) |
| Hispanic | 766 (8.9%) | 311 (8.7%) | 455 (9.1%) |
| Other | 148 (1.7%) | 67 (1.9%) | 81 (1.6%) |
| Education (years), mean(SD) | 12.0 (11.0–14.0) | 12.0 (11.0–15.0) | 12.0 (11.0–14.0) |
| BMI at first observation, mean(SD) | 28.5 (5.7) | 28.6 (4.9) | 28.5 (6.3) |
| Chronic conditions at 1st observation, n(%) | |||
| Arthritis | 6,940 (80.5%) | 2,613 (72.7%) | 4,327 (86.1%) |
| Hypertension | 6,885 (79.9%) | 2,788 (77.6%) | 4,097 (81.5%) |
| Heart disease | 3,303 (38.3%) | 1,601 (44.5%) | 1,702 (33.9%) |
| Diabetes | 2,524 (29.3%) | 1,159 (32.2%) | 1,365 (27.2%) |
| Cognitive impairment | 2,490 (28.9%) | 1,038 (28.9%) | 1,452 (28.9%) |
| Cancer | 1,992 (23.1%) | 957 (26.6%) | 1,035 (20.6%) |
| Lung disease | 1,308 (15.2%) | 511 (14.2%) | 797 (15.9%) |
| Stroke | 844 (9.8%) | 416 (11.6%) | 428 (8.5%) |
| Number of chronic conditions at 1st observation, n(%) | |||
| 2 | 3,408 (39.5%) | 1,354 (37.7%) | 2,054 (40.9%) |
| 3 | 2,686 (31.2%) | 1,144 (31.8%) | 1,542 (30.7%) |
| 4 | 1,561 (18.1%) | 683 (19.0%) | 878 (17.5%) |
| 5 | 670 (7.8%) | 288 (8.0%) | 382 (7.6%) |
| 6 | 238 (2.8%) | 107 (3.0%) | 131 (2.6%) |
| 7 | 50 (0.6%) | 15 (0.4%) | 35 (0.7%) |
| 8 | 5 (0.1%) | 3 (0.1%) | 2 (0.0%) |
| ADL-IADL score at 1st observation, mean(SD) | 0.6 (1.5) | 0.5 (1.3) | 0.7 (1.6) |
| ADL-IADL score at follow-up, mean(SD) | 1.0 (2.0) | 0.8 (1.9) | 1.1 (2.1) |
| HbA1c (%), mean(SD) | 6.0 (1.0) | 6.0 (1.0) | 6.0 (0.9) |
| HbA1c, categorical, n(%) | |||
| Normal (<5.7%) | 3,956 (47.0%) | 1,615 (46.1%) | 2,341 (47.6%) |
| Pre-diabetes (5.7 to <6.5%) | 2,974 (35.3%) | 1,204 (34.3%) | 1,770 (36.0%) |
| Diabetes (≥6.5%) | 1,490 (17.7%) | 688 (19.6%) | 802 (16.3%) |
| CRP (mg/L), mean(SD) | 4.3 (8.3) | 4.1 (9.3) | 4.5 (7.4) |
| CRP (mg/L), median (IQR) | 2.0 (0.9–4.5) | 1.7 (0.8–3.8) | 2.2 (1.0–4.9) |
| CRP, categorical, n(%) | |||
| Normal (≤3mg/L)* | 5,154 (62.7%) | 2,290 (67.0%) | 2,864 (59.6%) |
| High (>3mg/L) | 3,066 (37.3%) | 1,127 (33.0%) | 1,939 (40.4%) |
Abbreviations: BMI, body-mass index; ADL, activities of daily living; IADL, instrumental activities of daily living; CRP, high sensitivity C-reactive protein; HbA1c, glycosylated hemoglobin A1c; SD, standard deviation.
Pearson TA, Mensah GA, Alexander RW, Anderson JL, Cannon RO 3rd, Criqui M, et al. Markers of inflammation and cardiovascular disease: application to clinical and public health practice: A statement for healthcare professionals from the Centers for Disease Control and Prevention and the American Heart Association. Circulation. 2003;107(3):499–511. pmid:12551878.
Disease-Indexed Multimorbidity Combinations and Burden of Multimorbidity
We identified 213 unique multimorbidity combinations further grouped into 8 disease-indexed multimorbidity groups (Table 2; list ranked according to the prevalence of the index disease). The burden of multimorbidity (i.e., the number of diseases in the combination) was highest for the stroke-indexed group (mean = 4.4 diseases per combination) and lowest for the arthritis- and hypertension-indexed groups (mean = 3.3 diseases per combination). The percentage of combinations which included diabetes among the 7 non-diabetes-indexed groups ranged from 27.5% in the cancer-indexed group to 35.5% in the stroke-indexed group.
Table 2.
Mean number of diseases and frequency of diabetes in each disease-indexed multimorbidity group.
| Multimorbidity group | Number of diseases, mean (SD) | Combinations incl. diabetes, % |
|---|---|---|
|
| ||
| Arthritis | 3.3 (1.2) | 29.5% |
| Hypertension | 3.3 (1.2) | 32.7% |
| Heart disease | 3.8 (1.2) | 33.5% |
| Diabetes | 3.9 (1.2) | --- |
| Cognitive impairment | 3.8 (1.3) | 32.6% |
| Cancer | 3.7 (1.3) | 27.5% |
| Lung disease | 4.1 (1.3) | 28.0% |
| Stroke | 4.4 (1.3) | 35.5% |
Abbreviation: SD, standard deviation.
Association between Multimorbidity Combinations, Biomarkers, and Prospective ADL-IADL Score
Table 3 reports the results from unadjusted and adjusted negative binomial regression models, separately for male and female participants. Among male participants, after adjustment for age, education, race/ethnicity, body mass index, and baseline ADL-IADL score, all except the lung disease-indexed combinations showed a significantly higher prospective ADL-IADL score for every one-unit increase in HbA1c. After further adjustment for the count of diseases in the combinations, only the groups indexed by arthritis (IRR = 1.1, 95% CI = 1.01–1.20), diabetes (IRR = 1.19, 95% CI = 1.09–1.30), and cognitive impairment (IRR = 1.11, 95% CI = 1.01–1.23) maintained a significant association with higher prospective ADL-IADL score with increasing levels of HbA1c. These coefficients indicate that, among male participants, the ADL-IADL score is expected to increase by 10% for combinations including arthritis, 19% for combinations including diabetes, and 11% for combinations including cognitive impairment for each one-unit increase in HbA1c. However, none of the 8 multimorbidity groups showed a significant increase in the ADL-IADL score with increasing levels of CRP in either intermediary or fully adjusted models.
Table 3.
Negative binominal regression models of prospective ADL-IADL score for each multimorbidity group, according to CRP/ HbA1c levels and stratified by sex.
| Prospective ADL-IADL Score | |||||
|---|---|---|---|---|---|
|
| |||||
| Biomarker | Index disease multimorbidity group | Unadjusted IRR (95% CI) | Model 1 IRR (95% CI) 1 | Model 2 IRR (95% CI) 2 | Model 3 IRR (95% CI) 3 |
|
| |||||
| Male (N=3,594) | |||||
|
| |||||
| CRP | Arthritis | 1.16 (1.09–1.23) *** | 1.12 (1.04–1.21) ** | 1.05 (0.98–1.12) | 1.04 (0.97–1.11) |
| Hypertension | 1.15 (1.08–1.23) *** | 1.12 (1.03–1.21) ** | 1.05 (0.98–1.13) | 1.05 (0.98–1.13) | |
| Heart disease | 1.17 (1.09–1.26) *** | 1.13 (1.05–1.22) ** | 1.05 (0.98–1.11) | 1.03 (0.97–1.10) | |
| Diabetes | 1.14 (1.03–1.25) ** | 1.14 (1.01–1.28) * | 1.04 (0.94–1.14) | 1.02 (0.93–1.11) | |
| Cognitive impairment | 1.08 (1.00–1.16) * | 1.07 (0.99–1.16) | 1.02 (0.95–1.10) | 1.01 (0.94–1.08) | |
| Cancer | 1.14 (1.07–1.23) ** | 1.10 (1.00–1.21) | 1.01 (0.93–1.09) | 1.01 (0.94–1.09) | |
| Lung disease | 1.02 (0.91–1.14) | 0.98 (0.87–1.10) | 0.99 (0.89–1.10) | 0.99 (0.90–1.10) | |
| Stroke | 1.08 (0.97–1.21) | 0.99 (0.87–1.12) | 0.92 (0.81–1.04) | 0.92 (0.81–1.04) | |
|
| |||||
| HbA1c | Arthritis | 1.15 (1.07–1.24) *** | 1.15 (1.04–1.28) ** | 1.17 (1.07–1.27) *** | 1.10 (1.01–1.20) * |
| Hypertension | 1.13 (1.04–1.22) ** | 1.14 (1.02–1.27) * | 1.15 (1.05–1.25) ** | 1.08 (0.99–1.18) | |
| Heart disease | 1.11 (1.02–1.21) * | 1.12 (1.01–1.24) * | 1.09 (1.01–1.17) * | 1.03 (0.95–1.11) | |
| Diabetes | 1.08 (0.98–1.19) | 1.15 (1.02–1.30) * | 1.19 (1.09–1.30) *** | 1.19 (1.09–1.30) *** | |
| Cognitive impairment | 1.08 (0.99–1.18) | 1.05 (0.96–1.15) | 1.14 (1.04–1.25) ** | 1.11 (1.01–1.23) * | |
| Cancer | 1.21 (1.06–1.38) ** | 1.20 (1.04–1.39) * | 1.12 (1.02–1.24) * | 1.04 (0.93–1.16) | |
| Lung disease | 1.10 (0.96–1.27) | 1.06 (0.91–1.22) | 1.15 (0.97–1.36) | 1.08 (0.90–1.29) | |
| Stroke | 1.15 (1.02–1.30) * | 1.17 (0.99–1.38) | 1.20 (1.02–1.43) * | 1.17 (0.98–1.40) | |
|
| |||||
| Female (N=5,024) | |||||
|
| |||||
| CRP | Arthritis | 1.08 (1.03–1.13) ** | 1.13 (1.07–1.19) *** | 1.07 (1.03–1.12) ** | 1.06 (1.02–1.11) ** |
| Hypertension | 1.08 (1.03–1.13) ** | 1.12 (1.06–1.19) *** | 1.07 (1.02–1.12) ** | 1.06 (1.02–1.11) ** | |
| Heart disease | 1.10 (1.04–1.17) ** | 1.13 (1.06–1.20) *** | 1.06 (1.01–1.12) * | 1.06 (1.01–1.12) * | |
| Diabetes | 1.03 (0.96–1.10) | 1.04 (0.97–1.12) | 1.01 (0.95–1.09) | 1.00 (0.94–1.07) | |
| Cognitive impairment | 1.05 (0.99–1.10) | 1.09 (1.02–1.15) ** | 1.02 (0.97–1.08) | 1.02 (0.97–1.08) | |
| Cancer | 1.05 (0.96–1.15) | 1.08 (0.98–1.20) | 1.00 (0.93–1.08) | 1.00 (0.93–1.08) | |
| Lung disease | 1.14 (1.04–1.25) ** | 1.20 (1.09–1.33) *** | 1.13 (1.05–1.21) ** | 1.14 (1.07–1.23) *** | |
| Stroke | 1.01 (0.92–1.11) | 1.03 (0.93–1.14) | 1.06 (0.98–1.14) | 1.05 (0.98–1.14) | |
|
| |||||
| HbA1c | Arthritis | 1.08 (1.02–1.14) * | 1.05 (0.98–1.12) | 1.07 (1.00–1.13) * | 0.99 (0.93–1.06) |
| Hypertension | 1.09 (1.03–1.15) ** | 1.07 (1.01–1.14) * | 1.11 (1.05–1.17) *** | 1.03 (0.98–1.09) | |
| Heart disease | 1.08 (1.02–1.16) * | 1.07 (0.99–1.15) | 1.11 (1.03–1.19) ** | 1.04 (0.97–1.13) | |
| Diabetes | 1.02 (0.95–1.08) | 1.03 (0.96–1.10) | 1.07 (1.01–1.15) * | 1.07 (1.01–1.14) * | |
| Cognitive impairment | 1.01 (0.95–1.07) | 1.02 (0.96–1.08) | 1.06 (0.99–1.12) | 1.03 (0.96–1.09) | |
| Cancer | 1.03 (0.92–1.16) | 1.04 (0.91–1.18) | 1.05 (0.95–1.17) | 0.93 (0.83–1.04) | |
| Lung disease | 1.09 (0.97–1.22) | 1.04 (0.92–1.18) | 1.02 (0.90–1.15) | 0.95 (0.83–1.08) | |
| Stroke | 1.05 (0.96–1.14) | 1.04 (0.96–1.13) | 1.09 (0.99–1.20) | 1.05 (0.95–1.16) | |
Abbreviations: CI, confidence intervals; CRP, high sensitivity C-reactive protein; HbA1c, hemoglobin A1c; IRR, incidence rate ratio
0.05>p-value≥0.01;
0.01>p-value≥0.001;
p-value<0.001
Model 1 is adjusted for age, education, race/ethnicity, and BMI.
Model 2 is additionally adjusted for baseline ADL-IADL score.
Model 3 is additionally adjusted for the count of chronic diseases.
IRR interpretation for HbA1c: for a specific multimorbidity group, for every unit increase in HbA1c, the prospective ADL-IADL score increases or decreases by a factor of X.
IRR interpretation for CRP: for a specific multimorbidity group, for every doubling of CRP, the prospective ADL-IADL score increases or decreases by a factor of X.
Among female participants, higher HbA1c levels were associated with a higher prospective ADL-IADL score only for multimorbidity combinations including diabetes (IRR = 1.07, 95% CI = 1.01–1.14, in the fully adjusted model). On the other hand, the fully adjusted model indicated that multimorbidity combinations indexed by arthritis (IRR = 1.06, 95% CI = 1.02–1.11), hypertension (IRR = 1.06, 95% CI = 1.02–1.11), heart disease (IRR = 1.06, 95% CI = 1.01–1.12), and lung disease (IRR = 1.14, 95% CI = 1.07–1.23) were associated with higher ADL-IADL scores with each doubling in CRP levels. These coefficients correspond to a 6% higher disability score for combinations including arthritis, hypertension, and heart disease and a 14% higher disability score for combinations including lung disease for every doubling of CRP blood levels. Figure 1 shows the predicted ADL-IADL score for each disease-indexed multimorbidity group, separately for CRP (for the 10th, 50th, and 90th percentiles) and HbA1c (for clinically relevant values: prediabetes cutoff = 5.7%, sample mean = 6.0%, and diabetes cutoff = 6.5%).
Discussion
The present study examined the role of two biomarkers, indicative of inflammation (high-sensitivity CRP) and impaired glucose metabolism (HbA1c), potentially underlying the association between specific disease-indexed multimorbidity combinations and prospective disability in a nationally representative cohort of adults aged 65 years and older in the USA. Our results indicate that higher levels of CRP are associated with higher prospective disability burden among women with multimorbidity including arthritis, hypertension, heart disease, or lung disease, while higher levels of HbA1c are associated with higher prospective disability burden (i.e., ADL-IADL score) among men with multimorbidity that includes arthritis, diabetes, or cognitive impairment.
As the general population continues to age and accumulate chronic morbidity, a better understanding of the factors that influence functional health, and in particular those factors that are amenable to therapeutic and/or lifestyle modification, will be important for ensuring the functional well-being of older persons. Chronic disease multimorbidity has been linked with higher burdens of disability [2, 31, 32], although the biological mechanisms underlying the general relationship between multimorbidity and disability still warrant investigation. Insight into the possible biological mechanisms is important for the design of preventive and/or therapeutic approaches aimed at optimizing the functional health of older persons with multimorbidity. The treatable nature of inflammation and hyperglycemia further underscores the significance of these findings.
The contribution of this study to the current understanding of the biological substrate of the association of multimorbidity disability is enhanced by the following: first, the biomarkers were evaluated as continuous variables, and thus the results may be interpreted as a dose-dependent increase in the burden of ADL-IADL with higher levels of CRP and HbA1c within particular multimorbidity combinations, including within the normal range of respective values (e.g., below the 6.5% threshold for diabetes diagnosis). Second, the analyses captured multimorbidity prior to the reports of ADL-IADL deficits to provide an indication of time sequencing between the main independent variable (multimorbidity combinations) and outcome (ADL-IADL score) and accounted for multiple biomarker measurements per participant to allow for changes in inflammatory and glycemic levels over time and limit the possible contribution of acute or transitory states (in particular for CRP). Third, our analyses comprehensively considered all the combinations of diseases found in this sample, with and without diabetes (except for the diabetes-indexed group). We considered the possibility that certain disease-indexed groups may include a disproportionately high percentage of participants with diabetes and thus may explain the association with HbA1c levels. However, additional analyses (Table 2) showed that the percentage of participants with diabetes was rather similar across disease-indexed groups (except the diabetes-indexed group), thus lending support to our approach. This approach aligns with a multitude of findings showing that diabetes frequently co-occurs with other chronic diseases among older adults [9, 33] and allowed us to evaluate even those combinations that represented only a small number of participants, yet which may be found in clinical practice and thus may hold clinical value.
Prior investigations into the role of inflammation in the relationship between chronic diseases and functional disability have considered multimorbidity as a general condition delineated by the number of diseases an individual has and found that a higher number of diseases was linked to higher levels of inflammation and, in turn, higher levels of ADL and/or IADL disability and lower physical performance and suggested that these results hold irrespective of the specific conditions involved [16, 17, 34]. In light of more recent findings showing that certain combinations of diseases hold particularly high disability potential [8, 9, 21], this study considered specific groupings of chronic diseases (i.e., each grouping defined by a common index disease) and found that, after adjustment for the number of diseases in the combination, inflammation was associated with a higher prospective ADL-IADL score among women but not among men, and only for selected groupings of chronic diseases, in particular those headed by arthritis, hypertension, heart disease, and lung disease. There is substantial evidence in support of an association between inflammation and these four chronic conditions. For example, inflammation was shown to play a central role in the pathogenesis of osteoarthritis by regulating the joint anabolic and catabolic processes through involvement in the prostacyclin and nitric oxide pathways [35, 36], to function as a key mechanism in endothelial dysfunction and arterial damage, and to link these risk factors to vascular disease, arterial stiffness, and excess blood pressure [37], as well as coronary heart disease and other cardiovascular pathology [38, 39], and elevated levels of inflammatory markers were found in patients with chronic obstructive lung disease [40]. However, the incremental contribution of this study lies in its finding that the association between inflammation and functional limitations extends beyond individual diseases to particular multimorbidity combinations which include these diseases. This is an important finding given that multimorbidity combinations including arthritis, hypertension, and/or heart disease are among the most prevalent multimorbidity profiles among US adults aged 65 years and older [9].
The role of inflammatory mechanisms in the association between multimorbidity and ADL-IADL limitations is potentially explained by the harmful effect of chronic inflammation on muscle mass and muscle strength, which in turn, reduces the ability of older persons to perform daily activities requiring muscle involvement [16]. This harmful effect may be partially due to the catabolic influence of inflammatory cytokines on muscle, which results in loss of myofibrillar protein and muscle atrophy [41], in particular among women [42]. Many other studies have documented higher circulating levels of inflammatory markers [43, 44], such as CRP and IL-6, and a higher prevalence of ADL-IADL functional limitations among older women compared to men [30]. Additionally, inflammation appears to partially mediate the association between sex and ADL-IADL impairments [17]. The higher muscle mass and strength in men compared to women may buffer the harmful effect of inflammation and may account for the lack of a relationship between multimorbidity and ADL-IADL impairment in the presence of increasing levels of CRP observed among men in our study. Although our a priori research aim did not include investigating sex differences in these associations, our results contribute to a more nuanced understanding of the mechanisms of sex differences in functional disability among persons with multimorbidity and suggest that multimorbidity combinations that include arthritis, hypertension, heart disease, and lung disease may represent potential targets for anti-inflammatory interventions, as a means to reduce the excess burden of functional limitations in older women.
We also found that the levels of HbA1c, an indicator of glucose homeostasis during the 2–3 months prior to the assessment, intervene in the association between multimorbidity and prospective ADL-IADL limitations among men and women with diabetes multimorbidity, as well as among men with multimorbidity that includes either arthritis or cognitive impairment. Extensive evidence supports the utility of HbA1c not only as a marker of diabetes control but also as a prognostic marker among individuals with and without diabetes for several of the chronic conditions considered in our study, such as cardiovascular conditions [45, 46], stroke [47, 48], and cognitive impairment and dementia [49, 50]. Additionally, HbA1c levels appear to be associated with the presence and severity of arthritic pain and thus with impediments in completing the tasks required for daily activities in persons with diabetes [51, 52], although whether this association holds in persons without diabetes is still unclear [53].
Our findings add to the existing research on the association between multimorbidity, high glycemic states, and functional health by extending the investigation into the intermediate (i.e., prediabetic) and normoglycemic domains. Older persons with poorly controlled diabetes, as indicated by high HbA1c, have 2–3 times greater odds of developing ADL-IADL limitations, especially in the context of existing comorbidities [54, 55]. Further, less well-controlled diabetes (due to a multitude of psychosocial and behavioral factors) and higher levels of HbA1c appear to partially explain the larger number of functional deficits observed in older women (vs. older men) with diabetes [56]. Only a handful of studies have investigated whether a gradient in HbA1c within the nondiabetic range (i.e., below 6.5%) is also associated with a gradient in functional deficits. A cross-sectional study [22] found a higher prevalence of functional limitations, including ADL, IADL, and Nagi tasks, in persons with HbA1c in the prediabetic range (i.e., 5.7–6.4%) compared with those in the normal range (<5.7%), while a recent longitudinal study [57] found a faster accumulation of ADL-IADL deficits among persons with intermediate, prediabetic HbA1c levels. Our findings showing that each one-unit increase across the entire spectrum of HbA1c values available in our sample is associated with a corresponding increase in ADL-IADL deficits of 10% and 11% among men with multimorbidity combinations indexed by arthritis and cognitive impairment, respectively, suggest a plausible opportunity for preservation of functional status and prevention of disability in prediabetic and possibly even normoglycemic older men with certain types of multimorbidity. More research is needed to better understand the association between glycemic markers in the normal and prediabetic range and functional disability within other combinations of chronic conditions and in relation to the well-established sex differences in functional disability, to support improvements in clinical care, self-management, and public health aimed at maintaining functional health of older persons with multimorbidity.
Across the eight disease-indexed multimorbidity combinations, the predicted increase in ADL-IADL score with increasing levels of CRP and HbA1c for men and women ranged from 10% to 19%. The interpretation of the clinical significance of these values varies according to the ADL-IADL starting point and would be improved with longer observation periods. Prior research indicates that an increase of roughly 0.5 ADL or IADL score represents a minimally important change [58]. However, patients do not experience and/or report less than full unit ADL or IADL changes, which may develop over periods longer than the 2-year gap between multimorbidity assessment and ADL-IADL reports in our study. Investigations with longer follow-up are needed to better understand how the changes predicted in our analyses translate in the clinical context.
It should be noted that several other disease combinations – those indexed by hypertension, heart disease, cancer, and stroke among men and by arthritis, hypertension, and heart disease among women – were susceptible to a significantly greater number of ADL-IADL deficits at higher levels of HbA1c in the intermediary model adjusted for demographics, BMI, and baseline ADL-IADL index (Table 3). These effects were rendered not significant upon adjustment for multimorbidity count/burden, raising the possibility that HbA1c may still be a meaningful mechanism to consider in persons with less complex multimorbidity profiles (i.e., with fewer diseases in combination). This possibility is supported by earlier findings showing a diminished cardiovascular benefit from good glycemic control among patients with high comorbidity but not among those with fewer chronic conditions [59]. Future research should investigate more refined multimorbidity profiles to tease out possible differences in the contribution of specific combinations of diseases across the range of morbidity burden (i.e., low vs. high number of co-occurring diseases).
Our study draws from several strengths. First, the prospective design provides a clear time sequencing between the biomarkers’ assessment and the disability outcome. Second, the grouping of all the multimorbidity combinations around index diseases allows us to evaluate all the multimorbidity profiles as they occur in this representative sample of older adults without overlooking the underpowered profiles, an improvement over prior approaches which use pre-specified combinations of diseases or only combinations sufficiently powered for statistical analysis. Third, the use of multiple available measurements of CRP and HbA1c minimizes the likelihood of acute, transitory, or spurious assessments of the inflammatory and glycemic status of the participants. Fourth, the HRS provides a long, ongoing, and robust set of longitudinal data that largely generalize to the U.S. population of middle aged and older adults.
Several limitations should also be noted. First, chronic disease diagnoses were self-reported and may be subject to differential reporting between men and women. However, earlier reports have documented a reasonable concordance between participants’ reports of physician-diagnosed diseases and diagnoses extracted from administrative claims or electronic medical records for both men and women [60, 61]. Similarly, weight and height used in the calculation of BMI were self-reported; prior research documented only small differences between self-reported and objectively measured weight and height in the HRS sample [60]. Second, we used the most updated, full data on the participants evaluated in the HRS Biomarkers module, but we acknowledge that our findings are susceptible to underrepresentation of participants with heavy multimorbidity burden, who may have died, dropped out, or be subject to proxy representation. Third, the number of diseases assessed in HRS is rather limited, though they represent the most prevalent chronic diseases among older US adults. Also, the broad diagnostic categories did not allow a more granular differentiation between conditions involving the same organ/system (e.g., coronary heart disease vs. congestive heart failure). Fourth, the non-mutually exclusive multimorbidity combinations limit the immediate clinical applicability of these findings for treatment of specific patients, but they may suggest shared pathophysiological mechanisms between individual diseases and may guide prevention and optimal management for groups or individuals suffering from a given index disease who develop successive conditions [29]. Future studies may consider extending this research by evaluating a broader and more discriminating array of prevalent chronic conditions, as well as a wider array of potential confounders (e.g., smoking, diet, physical activity), using data from medical records or other validation options.
Conclusion
The results showed that higher levels of CRP are associated with higher prospective disability burden among women with multimorbidity including arthritis, hypertension, heart disease, and lung disease, while higher levels of HbA1c are associated with higher prospective disability burden among men with multimorbidity that includes arthritis, diabetes, and cognitive impairment. These findings have important clinical and research implications. Clinical practice should take into account the potential for anti-inflammatory management among women and optimal glycemic control among men with these particular multimorbidity combinations as it considers therapeutic and preventive options for maintaining and improving functional health of older adults. More research is needed before concrete clinical recommendations can be made, but emphasis should be placed on identifying and treating individuals with the predisposing multimorbidity combinations noted above and with indications of chronic inflammation or suboptimal glycemic status. Further investigations should explore the biological mechanisms underlying other multimorbidity profiles, as well as the prospective, longitudinal association between inflammatory/glycemic states and the progression of disability among older adults with multimorbidity.
Data Availability Statement
Publicly available datasets were used in this study. These can be found at https://hrsdata.isr.umich.edu/data-products/. Further data inquiries and requests should be addressed to the corresponding author.
Supplementary Material
Funding Sources
The study was funded by the National Institute on Aging/National Institutes of Health (R01AG055681 to Dr. Quiñones). Additional funding was provided by the National Institute on Aging/National Institutes of Health (AG024824 from the University of Michigan – Claude D. Pepper Older Americans Independence Center to Dr. Botoseneanu); UL1TR000433 from the Michigan Institute for Clinical and Health Research (to Dr. Botoseneanu); U049539 from the University of Michigan (to Dr. Botoseneanu).
Footnotes
Statement of Ethics
This study protocol was reviewed and approved by the Oregon Health & Science University Institutional Review Board, approval number 17034. The study has been granted an exemption from requiring written informed consent by the Oregon Health & Science University Institutional Review Board, approval number 17034.
Conflict of Interest Statement
The authors are not aware of potential conflicts of interest.
References
- 1.QuickStats. Percentage of adults with activity limitations, by age group and type of limitation — national health interview survey,† United States, 2014. MMWR Morb Mortal Wkly Rep. 2016;65. [DOI] [PubMed] [Google Scholar]
- 2.Jindai K, Nielson CM, Vorderstrasse BA, Quinones AR. Multimorbidity and functional limitations among adults 65 or older, NHANES 2005–2012. Prev Chronic Dis. 2016;13:E151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Millán-Calenti JC, Tubío J, Pita-Fernández S, Gonzalez-Abraldes I, Lorenzo T, Fernandez-Arruty T, et al. Prevalence of functional disability in activities of daily living (ADL), instrumental activities of daily living (IADL) and associated factors, as predictors of morbidity and mortality. Arch Gerontol Geriatr. 2010;50(3):306–10. [DOI] [PubMed] [Google Scholar]
- 4.Khavjou OA, Anderson WL, Honeycutt AA, Bates LG, Razzaghi H, Hollis ND, et al. National health care expenditures associated with disability. Med Care. 2020;58(9):826–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.McGrath R, Al Snih S, Markides K, Hackney K, Bailey R, Peterson M. The burden of functional disabilities for middle-aged and older adults in the United States. J Nutr Health Aging. 2019;23(2):172–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lisy K, Campbell JM, Tufanaru C, Moola S, Lockwood C. The prevalence of disability among people with cancer, cardiovascular disease, chronic respiratory disease and/or diabetes: a systematic review. Int J Evid Based Healthc. 2018;16(3):154–66. [DOI] [PubMed] [Google Scholar]
- 7.Ryan A, Wallace E, O’Hara P, Smith SM. Multimorbidity and functional decline in community-dwelling adults: a systematic review. Health Qual Life Outcomes. 2015; 13(1):168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Marengoni A, Akugizibwe R, Vetrano DL, Roso-Llorach A, Onder G, Welmer AK, et al. Patterns of multimorbidity and risk of disability in community-dwelling older persons. Aging Clin Exp Res. 2021;33(2):457–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Quiñones AR, Markwardt S, Botoseneanu A. Multimorbidity combinations and disability in older adults. J Gerontol A Biol Sci Med Sci. 2016;71(6):823–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Health (ASH). AS for. HHS initiative on multiple chronic conditions. HHS.gov. Published March 11, 2009. https://www.hhs.gov/ash/about-ash/multiple-chronic-conditions/index.html (accessed September 12, 2020).
- 11.World Health Organization. Medication Safety in Polypharmacy: Technical Report. World Health Organization; 2019. https://apps.who.int/iris/handle/10665/325454 (accessed 9 September, 2021). [Google Scholar]
- 12.Friedman E, Shorey C. Inflammation in multimorbidity and disability: an integrative review. Health Psychol. 2019;38(9):791–801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Marengoni A, Roso-Llorach A, Vetrano DL, Fernandez-Bertolin S, Guisado-Clavero M, Violan C, et al. Patterns of multimorbidity in a population-based cohort of older people: sociodemographic, lifestyle, clinical, and functional differences. J Gerontol A Biol Sci Med Sci. 2020;75(4):798–805. [DOI] [PubMed] [Google Scholar]
- 14.Calderón-Larrañaga A, Vetrano DL, Ferrucci L, Mercer SW, Marengoni A, Onder G, et al. Multimorbidity and functional impairment: bidirectional interplay, synergistic effects and common pathways. J Intern Med. 2019; 285(3):255–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Martin-Ruiz C, Jagger C, Kingston A, Collerton J, Catt M, Davies K, et al. Assessment of a large panel of candidate biomarkers of ageing in the Newcastle 85+ study. Mech Ageing Dev. 2011;132(10):496–502. [DOI] [PubMed] [Google Scholar]
- 16.Brinkley TE, Leng X, Miller ME, Kitzman DW, Pahor M, Berry MJ, et al. Chronic inflammation is associated with low physical function in older adults across multiple comorbidities. J Gerontol A Biol Sci Med Sci. 2009;64(4):455–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Friedman EM, Mroczek DK, Christ SL. Multimorbidity, inflammation, and disability: a longitudinal mediational analysis. Ther Adv Chronic Dis. 2019;10:2040622318806848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.NCD Countdown 2030 collaborators; Stevens GA, Mathers CD. NCD Countdown 2030: worldwide trends in non-communicable disease mortality and progress towards Sustainable Development Goal target 3.4. Lancet. 2018;392(10152):1072–88. [DOI] [PubMed] [Google Scholar]
- 19.Magnan EM, Bolt DM, Greenlee RT, Fink J, Smith MA. Stratifying patients with diabetes into clinically relevant groups by combination of chronic conditions to identify gaps in quality of care. Health Serv Res. 2018;53(1): 450–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Beavers KM, Leng I, Rapp SR, Miller ME, Houston DK, Marsh AP, et al. Effects of longitudinal glucose exposure on cognitive and physical function: results from the action for health in diabetes movement and memory study. J Am Geriatr Soc. 2017;65(1):137–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Quiñones AR, Markwardt S, Botoseneanu A. Diabetes-multimorbidity combinations and disability among middle-aged and older adults. J Gen Intern Med. 2019;34(6):944–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lee PG, Cigolle CT, Ha J, Min L, Murphy SL, Blaum CS, et al. Physical function limitations among middle-aged and older adults with prediabetes: one exercise prescription may not fit all. Diabetes Care. 2013;36(10):3076–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bruce DG, Davis WA, Davis TME. Longitudinal predictors of reduced mobility and physical disability in patients with type 2 diabetes: the fremantle diabetes study. Diabetes Care. 2005;28(10):2441–7. [DOI] [PubMed] [Google Scholar]
- 24.Kim KI, Kim S, Kim KW, Jang HC, Kim CH, Chin HJ. Low hemoglobin A1C increases the risk of disability in community-dwelling older non-diabetics adults. J Nutr Health Aging. 2016;20(3):341–6. [DOI] [PubMed] [Google Scholar]
- 25.Spector WD, Fleishman JA. Combining activities of daily living with instrumental activities of daily living to measure functional disability. J Gerontol B Psychol Sci Soc Sci. 1998;53(1):S46–57. [DOI] [PubMed] [Google Scholar]
- 26.Langa KM, Weir DR, Kabeto M, Sonnega A. Langa-weir classification of cognitive function. 1995. 10. [Google Scholar]
- 27.Hernan MA, Hernandez-Diaz S, Werler MM, Mitchell AA. Causal knowledge as a pre-requisite for confounding evaluation: an application to birth defects epidemiology. Am J Epidemiol. 2002;155(2):176–84. [DOI] [PubMed] [Google Scholar]
- 28.House JS, Lantz PM, Herd P. Continuity and change in the social stratification of aging and health over the life course: evidence from a nationally representative longitudinal study from 1986 to 2001/2002 (Americans’ Changing Lives Study). J Gerontol B Psychol Sci Soc Sci. 2005;60 Spec No 2(Special Issue 2):S15–26. [DOI] [PubMed] [Google Scholar]
- 29.Prados-Torres A, Calderón-Larrañaga A, Hancco-Saavedra J, Poblador-Plou B, van den Akker M. Multimorbidity patterns: a systematic review. J Clin Epidemiol. 2014; 67(3):254–66. [DOI] [PubMed] [Google Scholar]
- 30.Leveille SG, Resnick HE, Balfour J. Gender differences in disability: evidence and underlying reasons. Aging. 2000;12(2):106–12. [DOI] [PubMed] [Google Scholar]
- 31.Jacob ME, Ni P, Driver J, Leritz E, Leveille SG, Jette AM, et al. Burden and patterns of multimorbidity: impact on disablement in older adults. Am J Phys Med Rehabil. 2020; 99(5):359–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Calderón-Larrañaga A, Santoni G, Wang HX, Welmer AK, Rizzuto D, Vetrano DL, et al. Rapidly developing multimorbidity and disability in older adults: does social background matter? J Intern Med. 2018;283(5):489–99. [DOI] [PubMed] [Google Scholar]
- 33.King DE, Xiang J, Pilkerton CS. Multimorbidity trends in United States adults, 1988–2014. J Am Board Fam Med. 2018; 31(4):503–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Friedman EM, Ryff CD. Living well with medical comorbidities: a biopsychosocial perspective. J Gerontol B Psychol Sci Soc Sci. 2012;67(5):535–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chow YY, Chin KY. The role of inflammation in the pathogenesis of osteoarthritis. Mediators Inflamm. 2020;2020:8293921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sokolove J, Lepus CM. Role of inflammation in the pathogenesis of osteoarthritis: latest findings and interpretations. Ther Adv Musculoskelet Dis. 2013;5(2):77–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Guzik TJ, Touyz RM. Oxidative stress, inflammation, and vascular aging in hypertension. Hypertension. 2017;70(4):660–7. [DOI] [PubMed] [Google Scholar]
- 38.Kaptoge S, Seshasai SRK, Gao P, Freitag DF, Butterworth AS, Borglykke A, et al. Inflammatory cytokines and risk of coronary heart disease: new prospective study and updated meta-analysis. Eur Heart J. 2014;35(9): 578–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kritchevsky SB, Cesari M, Pahor M. Inflammatory markers and cardiovascular health in older adults. Cardiovasc Res. 2005; 66(2):265–75. [DOI] [PubMed] [Google Scholar]
- 40.Su B, Liu T, Fan H, Chen F, Ding H, Wu Z, et al. Inflammatory markers and the risk of chronic obstructive pulmonary disease: a systematic review and meta-analysis. PLoS One. 2016;11(4):e0150586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Schaap LA, Pluijm SMF, Deeg DJH, Harris TB, Kritchevsky SB, Newman AB, et al. Higher inflammatory marker levels in older persons: associations with 5-year change in muscle mass and muscle strength. J Gerontol A Biol Sci Med Sci. 2009;64(11):1183–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wåhlin-Larsson B, Carnac G, Kadi F. The influence of systemic inflammation on skeletal muscle in physically active elderly women. AGE. 2014;36(5):9718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Khera A, McGuire DK, Murphy SA, Stanek HG, Das SR, Vongpatanasin W, et al. Race and gender differences in C-reactive protein levels. J Am Coll Cardiol. 2005;46(3): 464–9. [DOI] [PubMed] [Google Scholar]
- 44.Friedman EM, Herd P. Income, education, and inflammation: differential associations in a national probability sample (the MIDUS study). Psychosom Med. 2010; 72(3):290–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cavero-Redondo I, Peleteiro B, Álvarez-Bueno C, Rodriguez-Artalejo F, Martínez-Vizcaíno V. Glycated haemoglobin A1c as a risk factor of cardiovascular outcomes and all-cause mortality in diabetic and non-diabetic populations: a systematic review and meta-analysis. BMJ Open. 2017;7(7): e015949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Au Yeung SL, Luo S, Schooling CM. The impact of glycated hemoglobin (HbA1c) on cardiovascular disease risk: a mendelian randomization study using UK biobank. Diabetes Care. 2018;41(9):1991–7. [DOI] [PubMed] [Google Scholar]
- 47.Wu S, Wang C, Jia Q, Liu G, Hoff K, Wang X, et al. HbA1c is associated with increased all-cause mortality in the first year after acute ischemic stroke. Neurol Res. 2014;36(5): 444–52. [DOI] [PubMed] [Google Scholar]
- 48.Mitsios JP, Ekinci EI, Mitsios GP, Churilov L, Thijs V. Relationship between glycated hemoglobin and stroke risk: a systematic review and meta analysis. J Am Heart Assoc. 2018; 7(11):e007858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Garfield V, Farmaki AE, Eastwood SV, Mathur R, Rentsch CT, Bhaskaran K, et al. HbA1c and brain health across the entire glycaemic spectrum. Diabetes Obes Metab. 2021;23(5):1140–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ravona-Springer R, Moshier E, Schmeidler J, Godbold J, Akrivos J, Rapp M, et al. Changes in glycemic control are associated with changes in cognition in non-diabetic elderly. J Alzheimers Dis. 2012;30(2):299–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Alenazi AM, Obaidat SM, Alshehri MM, Alothman S, Gray C, Rucker J, et al. Type 2 diabetes affects joint pain severity in people with localized osteoarthritis: a retrospective study. Pain Med. 2020;21(5):1025–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Charen DA, Solomon D, Zubizarreta N, Poeran J, Colvin AC. Examining the association of knee pain with modifiable cardiometabolic risk factors. Arthritis Care Res. 2021;73(12):1777–83. [DOI] [PubMed] [Google Scholar]
- 53.Garessus EDG, de Mutsert R, Visser AW, Rosendaal FR, Kloppenburg M. No association between impaired glucose metabolism and osteoarthritis. Osteoarthritis Cartilage. 2016;24(9):1541–7. [DOI] [PubMed] [Google Scholar]
- 54.Kalyani RR, Saudek CD, Brancati FL, Selvin E. Association of diabetes, comorbidities, and A1C with functional disability in older adults: results from the national health and nutrition examination survey (NHANES), 1999–2006. Diabetes Care. 2010;33(5):1055–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Yau CK, Eng C, Cenzer IS, Boscardin WJ, Rice-Trumble K, Lee SJ. Glycosylated hemoglobin and functional decline in community-dwelling nursing home–eligible elderly adults with diabetes mellitus. J Am Geriatr Soc. 2012;60(7):1215–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Chiu CJ, Wray LA. Gender differences in functional limitations in adults living with type 2 diabetes: biobehavioral and psychosocial mediators. Ann Behav Med. 2011; 41(1):71–82. [DOI] [PubMed] [Google Scholar]
- 57.Mutambudzi M, Díaz-Venegas C, Menon S. Association between baseline glycemic markers (HbA1c) and 8-year trajectories of functional disability. J Gerontol A Biol Sci Med Sci. 2019;74(11):1828–34. [DOI] [PubMed] [Google Scholar]
- 58.Suijker JJ, van Rijn M, ter Riet G, Moll van Charante EP, de Rooij SE, Buurman BM. Minimal important change and minimal detectable change in activities of daily living in community-living older people. J Nutr Health Aging. 2017;21(2):165–72. [DOI] [PubMed] [Google Scholar]
- 59.Greenfield S, Billimek J, Pellegrini F, Franciosi M, De Berardis G, Nicolucci A, et al. Comorbidity affects the relationship between glycemic control and cardiovascular outcomes in diabetes: a cohort study. Ann Intern Med. 2009;151(12):854–60. [DOI] [PubMed] [Google Scholar]
- 60.Weir D. Elastic powers: the integration of biomarkers into the health and retirement study. In: Weinstein M, Vaupel JW, Wachter KW, editors. Biosocial surveys. Committee on advances in collecting and utilizing biological indicators and genetic information in social science surveys. National Academies Press; 2008. p. 78–95. [Google Scholar]
- 61.Simpson CF, Boyd CM, Carlson MC, Griswold ME, Guralnik JM, Fried LP. Agreement between self-report of disease diagnoses and medical record validation in disabled older women: factors that modify agreement. J Am Geriatr Soc. 2004;52(1):123–7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Publicly available datasets were used in this study. These can be found at https://hrsdata.isr.umich.edu/data-products/. Further data inquiries and requests should be addressed to the corresponding author.