Abstract
Transcatheter structural heart intervention (TSHI) has gained popularity over the past decade as a means of cardiac intervention in patients with prohibitive surgical risks. Following the exponential rise in cases and devices developed over the period, there has been increased focus on developing the role of “structural imagers” amongst cardiologists. This review, as part of a growing initiative to develop the field of interventional echocardiography, aims to highlight the role of echocardiography in myriad TSHIs available within Asia. We first discuss the various echocardiography-based imaging modalities, including 3-dimensional echocardiography, fusion imaging, and intracardiac echocardiography. We then highlight a selected list of structural interventions available in the region—a combination of established interventions alongside novel approaches—describing key anatomic and pathologic characteristics related to the relevant structural heart diseases, before delving into various aspects of echocardiography imaging for each TSHI.
Key Words: echocardiography, interventional echocardiography, review, transcatheter structural intervention
Central Illustration
Highlights
-
•
Transcatheter structural heart intervention services have seen an exponential rise across the Asia-Pacific region.
-
•
The need for growing expertise in interventional echocardiography complements the rise in such interventions.
-
•
Developing interventional echocardiography requires a structured curriculum, accessibility to novel echocardiographic modalities and regional networking.
There has been an exponential rise transcatheter structural heart intervention (TSHI) being performed worldwide.1, 2, 3 Data from the UK-TAVI (Transcatheter Aortic Valve Implantation) trial demonstrated an almost 10-fold increase in the number of annual cases from 2007 to 2016 being performed in the UK, with numbers estimated to rise further over the coming years.2,3 Interests in the field of TSHI has since spread across the globe, including in Asia, with many regional registries having been developed and guidelines being published as a result.4, 5, 6, 7
Unlike open and thoracoscopic cardiac surgery, direct visualization of the cardiac anatomy is not possible during TSHI. Therefore, it is entirely dependent on imaging techniques available in the catheterization laboratory for procedural guidance to achieve optimal procedural success, and to avoid complications. The ability to appreciate cardiac structure in 3-dimensional (3D) space and to guide the manipulation of catheters and devices is key to safe and successful TSHI. Following increasing interest in developing TSHI services regionally, the role of interventional echocardiography equally needs to be developed. This includes advocating for a separate, stand-alone subspecialty and driving for more educational endeavors among cardiologist and fellows-in-training interested in the field.8
The aim of this review is to illustrate the role of procedural echocardiographic imaging in TSHI currently available in the Asia-Pacific region. We first discuss various imaging techniques available within the region, including 3D multiplanar imaging, fusion imaging, and intracardiac echocardiography (ICE). This is followed by discussions on the relevant anatomy and pathophysiology of individual cardiac structures and disease processes with known TSHIs available regionally, highlighting the roles of echocardiography periprocedurally (Table 1). Because structural heart disease and their interventional devices have a distinct landscape different from in the Western world, this review aims to encompass diseases that are prevalent or unique in Asia, and devices and techniques invented and developed in this part of the world.
Table 1.
Summary of Periprocedural Echocardiographic Views, Probe Position, and Maneuvers for Structural Heart Intervention
| Procedure | Echocardiography View | Probe Position and Maneuvers | Periprocedural Function of Views |
|---|---|---|---|
| Transseptal puncture | 2D ME bicaval | 80°-100° | Superior-inferior orientation |
| 2D ME short-axis | 30°-45° | Anterior-posterior orientation | |
| Multiplane and 3D-MPR | 80°-100° 30°-45° |
3D orientation of atrial septum | |
| 2D ME 4-chamber | 0°-15° | Alternative view for anterior-posterior orientation | |
| ASD closure | 2D ME bicaval | 80°-100° Withdrawing probe will show superior defects and rim. Advancing probe with anteflexion will show inferior rim. |
|
| 2D ME short-axis | 30°-45° |
|
|
| 2D ME 4-chamber | 0°-15° |
|
|
| 3D imaging (Live 3D, 3D Zoom, or Full Volume) | 3D view from RA | Allows for visualization of all rims | |
| 2D multiplane using ME bicaval and short-axis | 80°-100° 30°-45° |
|
|
| Mitral valve TEER | Transseptal puncture | See Transseptal Puncture, above | Lower punctures are used for lateral lesions, and higher ones for medial lesions. |
| 2D ME bicommissural | 60°-90° | “Index” view: medial and lateral device location and precise localization of the area of interest | |
| 2D ME long-axis | 110°-135° | “Grasping” view: shows the shaft of the delivery system, and if it approaches from too much of an anterior position (ie, “aorta-hugger” position), needing remedial maneuvers | |
| 3D en-face | 3D surgical view (LA perspective) | Assessment of perpendicularity | |
| 2D multiplane imaging (simultaneous ME bicommissural view and long-axis view) | 60°-90° 110°-135° |
Review of clip being pulled back toward the MV (with arms fully extended), leaflets being grasped by the grippers, clip closure, and drawing in of the leaflets | |
| 3D en-face | 3D surgical view | Assessment of tissue bridge, nature of the dual-orifice anatomy, and position and volume of regurgitant jets | |
| Mitral VIV | Transseptal puncture | See Transseptal Puncture, above | A postero-inferior puncture site preferred, with a <3 cm height |
| 2D ME bicommissural | 60°-90° |
|
|
| 2D ME long-axis | 110°-135° | ||
| 2D multiplane imaging (ME bicommissural view and long-axis view) | |||
| 3D en-face | 3D surgical view (LA perspective) | ||
| PTMC | Transseptal puncture | See Transseptal Puncture, above | See Transseptal Puncture, above |
| 2D TG short-axis | 0 -30° Anteflexion from midventricular level reveals LV base and 2D en-face MV |
|
|
| 3D en-face from LA perspective (Live 3D, 3D Zoom, or Full Volume Mode) | 3D surgical view |
|
|
| Tricuspid valve TEER | 2D ME bicaval | 80°-100° |
|
| 2D ME 4-chamber | 0°-15° | ||
| 3D overhead perspective of RA | |||
| 2D TG multiplane imaging (both short-axis and long-axis) | 30°-50° 110°-135° |
Steering of guiding catheter toward the TV plane | |
| 2D multiplane of ME inflow-outflow and 4-chamber | 60°-80° 0°-15° |
Axial alignment of CDS | |
| 2D TG long-axis | 110°-135° | ||
| 2D TG short-axis | 30°-50° | Clip arm orientation, to ensure perpendicular to the line of coaptation between anterior and septal leaflets | |
| 3D-MPR en-face view | |||
| 2D TG multiplane imaging (both short-axis and long-axis) | 30°-50° 110°-135° |
Leaflet grasping | |
| 2D multiplane of ME inflow-outflow and four-chamber | 60°-80° 0°-15° |
||
| 2D TG short-axis | 30°-50° | Leaflet insertion | |
| 2D ME four-chamber | 0°-15° | ||
| 2D ME short-axis | 30°-45° | ||
| 2D TTE apical 4-chamber | – | Additional view for monitoring of leaflet grasping and confirmation of leaflet insertion | |
| Tricuspid annuloplasty device | 2D/3D ME bicaval | 70°-110° with clockwise/counter-clockwise rotation of the probe from the ME bicaval view |
|
| 3D TV en-face view (3D work plane) | 3D TV en-face |
|
|
| Multiplane imaging using ME RV inflow–outflow view as the primary view, (multiplane work plane) | 60°-80 ° | ||
| 3D TV en-face view, 3D work plane) | 3D TV en-face |
|
|
| 2D and 3D imaging, with color Doppler | Multiple views including 3D-MPR and TG short-axis (30°-50°) | Assessment of valve function and residual regurgitation | |
| 2D multiplane imaging using ME RV inflow–outflow view as the primary view | 3D TV en-face view |
|
|
| 3D TV en-face view | 60°-80° | ||
| Transcatheter heterotopic valve implantation | 2D ME bicaval (with simultaneous multiplane imaging for prosthesis apposition) | 80°-100° Probe withdrawal and 110°-120° angulation reveals more of the SVC |
|
| 2D LE bicaval | 80°-100° Advancing probe reveals more of the IVC |
IVC and RA junction | |
| 2D TG basal short-axis (ie, IVC and HV view) | 30°-60° |
|
|
| TAVR | 2D ME 5-chamber | 0°-10° |
|
| 2D ME long-axis | 120°-140° | ||
| 2D ME long-axis | 120°-140° |
|
|
| 3D ME long-axis | 30°-45° | ||
| 2D multiplane imaging (ME long- and short-axis) | |||
| 2D ME 5-chamber | 0°-10° |
|
|
| 2D ME long-axis | 120°-140° | ||
| 2D ME short-axis | 30°-45° | ||
| PVL closure | 2D ME with multiplane imaging | Multiple planes depending on location of leaks |
|
| 3D (Live 3D, 3D Zoom, or Full Volume Mode) | |||
| 3D-MPR | |||
| LAAO | Transseptal puncture | See Transseptal Puncture, above | See Transseptal Puncture, above |
| 2D ME “sweep” (0°-135°) | Advancement, anteflexion and lateral flexion are used to optimize imaging. Single-plane and multiplane imaging should be performed at 0°, 45°, 90°, and 135°. |
|
|
| 2D multiplane | |||
| 3D en-face | 3D reconstruction using 3D-MPR and transparency imaging |
|
|
| 3D overhead perspective of LAA | – | Navigation within the LAA and LAA engagement by wires | |
| 3D en-face view of LAA | |||
| Live 3D-MPR | – | LAA sizing (with corresponding fluoroscopic views) | |
| 2D ME multiplane views of LAA | 0°-60° (corresponding to fluoroscopic RAO-cranial view) 120°-135° (corresponding to fluoroscopic RAO-caudal view) |
|
|
| Live 3D-MPR | – | Device position after release and for residual leak | |
| PIMSRA | 2D TTE in various apical and parasternal windows | – |
|
| Myocardial contrast echocardiography | – |
|
2D = 2-dimensional; 3D = 3-dimensional; 3D-MPR = 3-dimensional multiplanar reconstruction; ASD = atrial septal defect; AV = atrioventricular; CDS = clip delivery system; HV = hepatic vein; IVC = inferior vena cava; LA = left atrium; LAA = left atrial appendage; LAAO = left atrial appendage occluder; LE = lower-esophageal; LV = left ventricle; LVOT = left ventricular outflow obstruction; ME = midesophageal; MV = mitral valve; PIMSRA = percutaneous intramyocardial septal radiofrequency; PTMC = percutaneous transvenous mitral commissurotomy; PVL = paravalvular leak; PW = pulsed-wave; RA = right atrium; RAO = right anterior oblique; RPA = right pulmonary artery; RV = right ventricle; SVC = superior vena cava; TA = tricuspid annulus; TAVR= transcatheter aortic valve replacement; TEE = transesophageal echocardiography; TEER = transcatheter edge-to-edge repair; TG = transgastric; TTE = transthoracic echocardiography; TV = tricuspid valve; VIV = valve-in-valve.
Echocardiographic Modalities in TSHI
Transesophageal echocardiography (TEE) is an essential tool in the cardiologist’s armamentarium when performing TSHI.9, 10, 11 An integral part of structural imaging includes the integration of 3D imaging. Novel 3D techniques have since been developed, including multiplane (with unlimited plane combinations), live 3D-multiplanar reconstruction (3D-MPR), and photorealistic imaging, to better assist in TSHI.12 Multiplane imaging allows for simultaneous views of separate planes with an unlimited combination of tilting and rotation between them, allowing for thorough interrogation of cardiac structures. Live 3D-MPR allows for real-time 2-dimensional (2D) visualization of structures in multiple views. This allows for minimization of errors of parallax and visualization of otherwise impossible views through conventional 2D imaging, while circumventing the need for alteration of views and probe position during imaging. Photorealistic imaging, including transillumination imaging (TI), uses a virtual freely mobile light source to simulate light-tissue interactions, leading to photorealistic images with shadowing. TI enhances the sense of depth and space, allowing images to appear more realistic and helps detect subtle structures and pathologies. Both 3D-MPR and TI can superimpose color Doppler onto anatomic data, to refine visualization of regurgitation jets or shunt flows.
Fusion imaging has also taken precedence following the rise in popularity of transcatheter intervention.13, 14, 15 From having to operate in 2 separate coordinated spaces, there is now appreciation for close integration of information obtained through various imaging modalities, aligning them in both time and space (ie, co-registration). Overlay of TEE images onto fluoroscopic projections can provide improved visual guidance for the interventionalists and facilitates communication between imagers and interventionalists during procedures. Co-registration allows images to be automatically updated, and overlay can occur in real-time while fluoroscopy is made active and as the C-arm moves through concurrent tracking of the TEE probe (Figure 1). This technology will continue to evolve in the field of TSHI because it has the novel ability to harness the strengths of both modalities to improve the safety and efficacy of transcatheter procedures.
Figure 1.
Echocardiography-Fluoroscopy Fusion Imaging
(A) Biplane transesophageal echocardiography (TEE) showing the mitral valve (MV) in the left ventricular outflow (purple) and bicommissural (green) planes. After co-registration, the TEE data set is aligned in the echocardiographic-fluoroscopy fused images. (B) The purple and green sectors of the corresponding 2-dimensional TEE views. (C) The MV within the boundaries of the 3-dimensional pyramidal data set is aligned in the anterior-posterior fluoroscopic projection. The red arrow denotes the wire in the ventricle. (D) Another example shows the ideal transseptal puncture site being determined by biplane TEE in the bicaval and short-axis views. (E) Placement of fiducial marker (red circle) will be displayed on fluoroscopy. (F) In addition, superimposing the color Doppler TEE data set of the MV during a transcatheter edge-to-edge repair to indicate origin of the regurgitation jet can be performed. LA = left atrium; RA = right atrium; SVC = superior vena cava.
ICE has also shown promise in TSHI, including in left atrial appendage occlusion (LAAO), transcatheter aortic valve replacement (TAVR), and mitral valve (MV) and tricuspid valve (TV) interventions.16, 17, 18, 19 Catheter oscillation and imaging variability can pose a challenge, especially in a hyperdynamic heart, leading to a steep operator learning curve. Other limitations include lack of far-field imaging and 3D capability at present, risk of venous vasculature injury and arrhythmias during manipulation, and ongoing struggles with financial reimbursement for the imaging modality. Furthermore, there continues to be a lack in consensus on standardizing procedure protocols, although individual protocol by institutions do exist in the literature. Recently, however, a Chinese expert consensus paper has been published to address the issues surrounding standardization of use of ICE, which has been a great move forward.20
Competency in Interventional Echocardiography
The American Society of Echocardiography has recently released a recommendation paper on the subject which may be adopted for those interested in progressing within the field.21 Clear distinctions are made between conventional echocardiography competencies and those related to interventional echocardiography, because imaging is performed and interpreted in real time, is dependent on nonconventional views with 3D reconstruction being pivotal, and requires effective communication with other members of the TSHI team. The paper highlights institutional requirements, entry requirements for training, and prerequisite competencies via various routes (ie, cardiology or anesthesiology). There is currently no similar structured curriculum being developed within the Asia-Pacific region, although we hope that this may change in the near future. We also recognize that developing skills in interventional echocardiography will require not only having a structured curriculum to assure competency, but also balancing accessibility to novel echocardiographic modalities and strong networking among the echocardiography community within the region (Central Illustration).
Central Illustration.
Developing Interventional Echocardiography in Asia
Development in interventional echocardiography requires having a structured curriculum to ensure competency in the field, access to novel echocardiographic modalities, and close networking within the region.
Transseptal Puncture and Atrial Septum Defect Closure
Atrial septal anatomy
The interatrial septum (IAS) separates the left (LA) and right atrium (RA). The fossa ovalis (FO), making up approximately 20% of the IAS, is considered the “true” septum and is the only area that can be crossed safely without risk of entering into extracardiac space (Figure 2).22 The IAS itself has 3 components: septum primum, septum secundum, and septum of the atrioventricular canal. Atrial septal defects (ASDs) are the third most common congenital heart disease and can be classified based on their anatomic location.23,24 Secundum ASDs are the most common form, and transcatheter closure is now the preferred approach for most secundum ASDs.24, 25, 26, 27
Figure 2.
Imaging of the Interatrial Septum (IAS)
(A) Three-dimensional TEE shows the IAS seen from the right atrium. The red dashed line corresponds to the bicaval view and the blue dashed line to the short-axis view. (B) Biplane TEE demonstrated tenting of the IAS (white arrows) seen from bicaval view (left) and short-axis (right) planes. Ant = anterior; AoV = aortic valve; IVC = inferior vena cava; Post = posterior; other abbreviations as in Figure 1.
Transseptal puncture
Transseptal puncture (TSP) is commonly performed in various TSHIs, such as percutaneous transvenous mitral commissurotomy (PTMC), MV transcatheter edge-to-edge repair (TEER), and LAAO.25, 26, 27 TEE is essential in the pre-procedural assessment of IAS anatomy and morphology. Furthermore, TEE helps guide puncturing toward the FO while avoiding injury toward surrounding structures. The presence or absence of other key structures can be reviewed simultaneously, including patent foramen ovale, ASDs, Eustachian valve, Chiari network, pericardial effusion, and thrombi. Primary views are as highlighted in Table 1. 3D imaging through multiplane and 3D-MPR often allow for better orientation. “Tenting” of the catheter tip onto the FO marks a potential site for entry into the LA (Figure 2). Postprocedurally, TEE allows for evaluation of residual shunt flow, pericardial effusion, or new thrombi formation within chambers that may require intervention.
ASD closure
Preprocedural transthoracic echocardiography (TTE) offers information about the type of defect and hemodynamic consequences due to shunting, along with other associated congenital cardiac defects.27, 28, 29 However, TEE remains essential in detailed assessment of the defect because bone and lung shadowing are avoided and, owing to its proximity to the LA, it allows for remarkable images of atrial structures and the IAS.9, 10, 11 Preprocedurally, it is important to recognize the morphology of ASD and its relationship to surrounding structures, such as the superior vena cava (SVC), inferior vena cava (IVC), pulmonary veins, MV, TV, and coronary sinus. 3D TEE allows “en-face” display of the defect and allows appreciation of the spatial relationship with these structures (Figure 3). Multiplanar imaging also allows for accurate measurements of the ASD dimensions to ensure that an appropriately sized occluder device is selected, which is important because it is often not circular in shape.
Figure 3.
Normal Structure of IAS
Three-dimensional (3D) TEE showing (A to C) right and (D) left septal perspectives of the IAS: (A) 3D spatial relation of the IAS to adjacent structures can be assessed in great detail; (B) transillumination imaging with virtual light source placed in the LA behind the IAS allows appreciation of the thinness, location, and limbus of the fossa ovalis (FO); (C) photorealistic transparency (“glass”) rendering of the IAS; and (D) transillumination of the FO from the left septal perspective with virtual light source in the RA. AV = atrioventricular; CS = coronary sinus; TV = tricuspid valve; other abbreviations as in Figures 1 and 2.
Information on the defect should be determined, including the type of ASD, shunt direction, size, location, shape, and number of defects visible. Interrogation of the ASD rims also is essential. Sequential interrogation of the rims should be performed beginning at the midesophageal short-axis window, with stepwise increase from 0° to 180° (Figure 4). Patients with inadequate rims (<5 mm) especially in the postero-inferior rim, may have higher risk of procedural failure or device embolization. Periprocedurally, TEE is needed during ASD sizing and device deployment, ensuring a parallel position of the device disc and IAS. A “push-and-pull” maneuver may be performed to ensure device stability, which can be demonstrated on TEE. TEE is also crucial to assess the final position of the device holding the IAS (by identifying atrial septal tissue between the device discs) and for any significant residual shunt before release.
Figure 4.
Interrogation of the ASD Rims
(A) ASD en face from 3D TEE view, looking in from the RA. Colored lines represent the degree of views correlated with the respective TEE 2-dimensional views. (B) corresponds to the blue line in A and shows the posterior rim and the aortic rim (midesophagus, at 0°-45°). (C) corresponds to the green line in A and shows the IVC and aortic rim (midesophagus, at 45°-90°). (D) corresponds to the red line in A and shows the IVC and SVC rim (midesophagus, at 100°-130°). (E) corresponds to the purple line in A and shows the AV valve and the posterior rim (midesophagus, at 150°-180°). Abbreviations as in Figures 1, 2, and 3.
ICE provides an additional means of intraprocedural imaging during ASD closure.30 Following fluoroscopy-guided transducer insertion into the mid-RA, ICE imaging begins with a “neutral” view (ie, the catheter parallels the spine and the transducer faces the TV). The transducer is then rotated to face the IAS (septal view, which allows visualization of the septum, ASD, coronary sinus, and pulmonary veins) before further advancing toward the SVC (long-axis view, in which the SVC and RA relationship can be viewed). Manipulation at this level allows for excellent visualization of ASD rims. “Stop-flow” diameter of defects can be measured during balloon sizing and throughout deployment of the closure device.
MV Intervention
MV anatomy
The MV has 2 leaflets − anterior (AML) and posterior (PML) mitral leaflets. The PML is divided into scallops (P1, P2, and P3). The AML is arbitrarily divided into 3 opposing segments (A1, A2, and A3). Chordal support attaches principally to the commissural ends of the valve leaflets, from the posterior-medial and anterior-lateral papillary muscles.31,32 There are key landmarks for the MV, allowing orientation when imaging (Supplemental Figure 1). Mitral regurgitation (MR) can be divided into primary (degenerative) or secondary in origin, the latter being the most common.33,34 For mitral stenosis, the most common etiology in Asia remains rheumatic heart disease.35
Intervention for MR
Inspired by the Alfieri stitch technique, MV TEER with the use of the Mitraclip (Abbott) was developed as a less invasive alternative for significant MR in those with prohibitive surgical risk.5, 6, 7,18,25,36, 37, 38 Despite initial challenges in Asian countries, the technique has gained traction over the past decade.5, 6, 7,36 Key pre- and periprocedural views for assessment include the midesophageal bicommissural and long-axis views (Table 1). Multiplane imaging allows simultaneous display of both views. TEE guidance during TSP is similar to that described earlier, although there are some specific considerations regarding puncture height (Figure 5). Steering, grasping and clip closure, and leaflet insertion measurements should always be performed using multi-planar imaging (Figures 6 and 7, Supplemental Figure 2).
Figure 5.

Transseptal Puncture Height
A lower puncture is needed when treating lateral lesions, because often the clip will continue to gain height above the valve as it is passes further. Conversely, a higher puncture is used when treating a medial lesion, because the clip will have to “dive down” immediately after transseptal puncture. P1, P2, and P3 = scallops of the posterior mitral leaflets.
Figure 6.
Views for Clip Steering and Perpendicularity
(A) The bicommissural “index” view allows assessment of medial and lateral device location, as well as precise localization of the area of interest. When the shaft of the delivery system approaches from too much of an anterior position (ie, an “aorta-hugger” position), as seen in (B) the long-axis “grasping” and (C) 3-dimensional en-face views, remedial maneuvers are frequently required (yellow dashed arrow).
Figure 7.
Views for Clip Grasping and Closure
(A) Biplane imaging of the clip as it is pulled back toward the mitral valve with arms fully extended and the leaflets have been grasped by the grippers. (B) The clip is then closed, and the leaflets are drawn into the mechanism. (C) Color-flow Doppler is used to assess for residual regurgitation. (D) Three-dimensional en-face imaging is used to assess the tissue bridge, the nature of the dual-orifice anatomy, and the position and volume of regurgitant jets.
Transcatheter mitral valve-in-valve
As the rates of bioprosthetic valve implantation continue to rise over the years, so do the rates of degenerative bioprosthetic prosthesis (DBP). For those with surgically prohibitive risks, transcatheter mitral valve-in-valve provides an alternative means to manage DBP. With the use of a balloon-expandable transcatheter heart valve (THV), the transapical approach has largely superseded the transseptal owing to better recovery and complication rates.39 Preprocedural multidetector computed tomography (MDCT) is pivotal for procedural planning and guidance, specifically in annular sizing and predicting the hypothetical neo–left ventricular outflow tract (LVOT) area.40 TSP is required when approaching from the RA, ideally favoring a more posterior-inferior location. This is followed by advancement and deployment of the THV under both fluoroscopy and TEE guidance (Figure 8 and Table 1). Postprocedural monitoring normally includes for presence of LVOT obstruction (which may require surgical bailout or alcohol septal ablation).
Figure 8.
Transcatheter Mitral Valve-in-Valve
(A) Transseptal puncture performed with X-plane imaging. (B) X-Plane imaging using simultaneous midesophageal bicommissural and long-axis views used to guide prosthesis delivery and deployment, and to check leaflet mobility after deployment. (C) Three-dimensional reconstruction with color Doppler is used to assess for paravalvular leaks.
Intervention for mitral stenosis
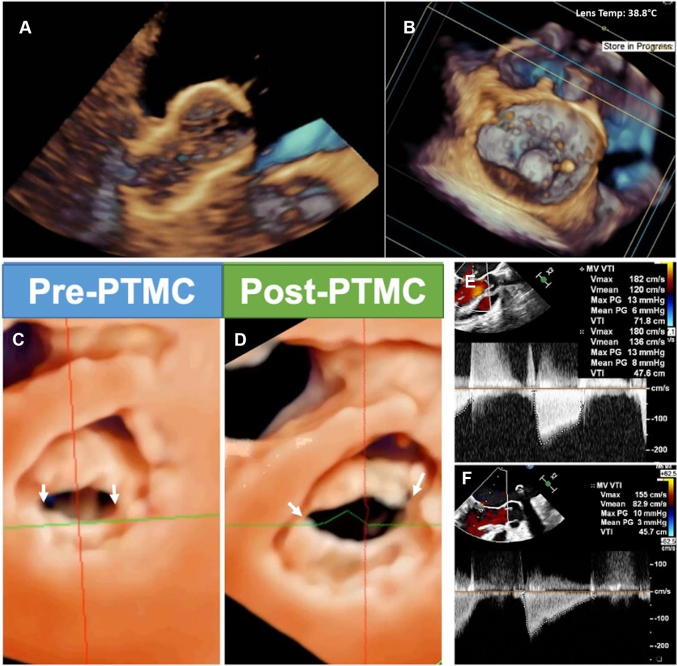
PTMC is often the preferred initial intervention in rheumatic mitral stenosis (MS).37,38 Conventionally, TTE allows for diagnosis and severity grading of MS, to determine suitability for PTMC (Supplemental Figure 3). Various scoring systems exist, including Wilkin’s, Cormier, and Nobuyoshi scores (Supplemental Tables 1 to 3).41, 42, 43 Key components in ensuring successful PTMC include degree of leaflet pliability, degree of degeneration, and calcification of the commissures, subvalvular apparatus, annulus, and leaflets. TSP is a crucial step in PTMC. Following successful puncture, both fluoroscopy and TEE are used to guide an uninflated Inoue balloon catheter pass the stenotic MV (Figure 9). Color Doppler is used to evaluate for the presence and severity of any new MR that may manifest after balloon inflation. A short-axis view of the MV by 2D TEE or a 3D “en-face” view may be used to illustrate the degree of commissural splitting and MV area through planimetry measurement. The mean pressure gradient is recalculated, and reinflation can be performed if suboptimal results are seen. Procedural success is defined as having achieved a valve area of >1.5 cm2 with no more than +2 MR.37,38,44 Complications such as pericardial effusion, tamponade, and thrombi formation may occur after PTMC, and TEE plays an important role in their early recognition.
Figure 9.
Echocardiographic Guidance of Percutaneous Transluminal Mitral Commissurotomy (PTMC)
3D TEE guides the alignment and orientation of the balloon catheter and further monitors the position of the inflated balloon. These pictures show the inflated Inoue balloon via (A) real-time 3D and (B) en-face views. (C, D) Left ventricular perspective 3D imaging showing splitting of both commissures (arrows) after successful PTMC, with increase in valve area and reduction of transvalvular pressure gradient on Doppler assessment (E, F). Abbreviations as in Figures 1 and 3.
TV Intervention
TV anatomy
The TV complex consists of a fibrous tricuspid annulus, typically 3 leaflets, 2 discrete papillary muscles, chordae tendineae, and the myocardium of the RA and right ventricle (RV).45, 46, 47 Approximately 90% of clinically significant tricuspid regurgitation (TR) cases are considered to be “functional” or secondary TR, commonly due to right atrial and/or ventricular remodeling, as well as to pulmonary hypertension.48, 49, 50
Transcatheter TV intervention
Currently, TSHI of the TV capitalizes on TEER devices (ie, TriClip [Abbott]), annuloplasty systems (ie, K-Clip [Huihe Healthcare]), and transcatheter TV implantation.48, 49, 50, 51, 52, 53 Specific imaging considerations for tricuspid TEER include: 1) assessment of tricuspid annular dilation and shape; 2) leaflet length, morphology, and presence of leaflet tethering; 3) coaptation gap location and width; 4) RV chamber size, function, and systolic pressure; and 5) presence and location of RA and RV structures (ie, Chiari network, Eustachian valve; trabeculae, papillary muscles, and moderator band).25,26,45,49 It has been shown that coaptation depth <10 mm, coaptation gap <7.2 mm (ideally <4.0 mm), leaflet length >10 mm, and a central or anterior-septal TR jet direction, with no pacemaker leads in situ or minimal interaction between leads and leaflets predict better success after TEER, whereas greater TR tenting and effective regurgitant orifice area may predict otherwise.25,26 A small and restricted septal leaflet is a common anatomic exclusion for TEER.
TEE remains a crucial imaging tool for TEER. However, TTE may provide good anatomic imaging owing to the proximity of TV to the chest wall and often complements TEE imaging (Figure 10).48,49 Midesophageal bicaval (80°-100°) and 4-chamber (0°-15°) views are used to visualize the guide and clip delivery system entering the RA. Other key views periprocedurally include the transgastric long-axis (with simultaneous multiplane imaging), transgastric short-axis (with 3D “en-face” reconstruction), and midesophageal RV inflow-outflow, 4-chamber, and short-axis views—each with its own role (Table 1). Multiple views are essential to guide leaflet grasping, and sometimes TTE is needed if the image quality via TEE is inadequate. The insertion of leaflets must be confirmed, with the formation of a tissue bridge and clip stability being assessed before eventual deployment. A mean TV gradient <3 mm Hg, after clipping, is generally acceptable.25,26,45,49
Figure 10.
Transcatheter Edge-to-Edge Repair of the Tricuspid Valve
(A) Guide catheter and catheter delivery system (CDS) introduction. (B) Steering of the CDS (arrows) toward the tricuspid anteroseptal commissure using an en face view. (C) Multiplane imaging showing positioning of the CDS. (D) Multiplanar reconstruction with the long axes (red and green) aligned to the CDS. (E, F) Clip arm orientation adjusted perpendicular to the line of coaptation between the anterior and septal leaflet. (G, H, I) Leaflet grasping is monitored, and leaflet insertion confirmed with the use of multiple views. 4Ch = four-chamber; AL = anterior leaflet; LAX = long axis; LE = low-esophageal; MPR = multiplanar reconstruction; PL = posterior leaflet; SAX = short axis; SL = septal leaflet; TG = transgastric; TTE = transthoracic echocardiography; other abbreviations as in Figures 1, 2, and 3.
The transcatheter annuloplasty system consists of a 2-armed clip (with a sheath and delivery system) that clamps onto the annulus together with a cockscrew-shaped anchor to ultimately achieve annular plication and reduction (Figure 11).53, 54, 55 Preprocedural MDCT is important to assess the distance between the right coronary artery (RCA) and the tricuspid annulus (TA), ensuring distance of at least 4 mm to minimize the risk of RCA injury during implantation. The system is introduced percutaneously via the right internal jugular vein with fluoroscopy and echocardiography guidance. The device is steered toward the posterior TA and is anchored to the TA through a corkscrew-like anchor (Table 1). The anchored clip is then opened (oriented along the annular circumference) and the anchor is withdrawn to fold the TA tissue into the clip, before subsequently being closed to achieve annular and TR reduction. Coronary angiography confirms the patency of the RCA before the device is released.
Figure 11.
Tricuspid Valve Transcatheter Annuloplasty Procedure
(A, B) Preprocedural TTE demonstrating tricuspid regurgitation (TR) on computed tomographic imaging. (C) X-Plane imaging using right ventricular inflow-outflow showing torrential regurgitation. (D) Through the guiding catheter, the K-Clip delivery catheter (KCR) is introduced into the RA and steered toward the tricuspid annulus (TA). (E) Fluoroscopy showing the anchor of the device to the posterior TA. (F) Retraction of the anchor, pulling annular tissue into the device, which then closes to fold the annulus within. (G) Annular and TR reduction immediately after implantation. (H) Coronary angiogram confirming right coronary patency after clipping. (I) Sustained TR reduction at 3-month follow-up. An = anchor; At = annular tissue; GC = guiding catheter; RCA = right coronary artery; other abbreviations as in Figures 1 and 10.
TricValve (P&F Products & Features) involves 2 self-expandable prosthetic valves being deployed in the SVC and IVC, anchored at the cavo-atrial inflow.25,26 It does not involve direct intervention of the TV, but instead works by reducing caval backflow, which reduces systemic venous congestion.25,26,47,48,53, 54, 55 Preprocedural assessment is essentially like that for TV TEER, although assessment of TR etiology and severity and TV annulus dimensions are of less importance following little direct intervention on the native valve. Preprocedural MDCT is mandatory to assess the diameter and area of the: 1) SVC at various levels; 2) IVC at the RA-IVC intersection; and 3) IVC at 5 cm below the IVC-RA transition, near the hepatic vein (HV). Distances between structures are also calculated to assess for eligibility, while ensuring a <35-mm prosthesis landing zone.25,26,49,50 The prosthesis is positioned at the height of the diaphragm with the skirt visible just above the hepatic vein inflow, with a 5 mm safety margin to avoid too low or high a valve position that could lead to HV obstruction or paravalvular regurgitation.25,26,49,50 Periprocedural TEE is best viewed using the mid- and lower-esophageal 2D views of the IVC-RA junction and transgastric views of the IVC and HV, both with color Doppler assistance (Figure 12, Table 1).
Figure 12.
Transcatheter Heterotopic Valve Implantation of the Tricuspid Valve
Following fluoroscopic evidence of the desired SVC position (marked using wires and catheters within the right pulmonary artery [RPA] and subclavian and brachiocephalic veins), (A) the guiding wires and catheters (red arrow), and device delivery system (yellow arrow) is advanced into the SVC, where (B) the upper portion of the prosthesis (blue arrow) is positioned above the crossing of the RPA catheter and SVC on simultaneous fluoroscopy. Through TEE, this can be visualized best using the 2-dimensional bicaval window, with 3-dimensional reconstruction if desired. (C) The SVC prosthesis (blue arrows) is fully deployed, and its final positions checked on (D) TEE and (E) fluoroscopy, before being retrieved back along with the RPA catheter. This is followed by (F) IVC prosthesis (white arrows) advancement, positioning, and deployment, before a final check on (G) TEE and (H) fluoroscopy. Abbreviations as in Figures 1, 2, and 10.
Aortic Valve Intervention
Aortic valve anatomy
The aortic valve is trifoliate.56,57 The leaflets are attached throughout the length of the root and take the form of a 3-pronged coronet, with hinges from the supporting ventricular structures forming the crown-like ring. The top of the crown is marked by the sinotubular junction. Coronary artery ostia usually arise from 2 anterior sinuses of Valsalva, positioned below the sinotubular junction, which are important to identify because too low an ostium (or too high a prosthesis position) may affect coronary arterial flow during prosthesis implantation.
TAVR
TAVR is an established therapeutic option for the treatment of severe aortic stenosis.2,37,38 Preprocedural assessment remains key to ensure appropriate indication for TAVR. TTE is crucial in establishing the diagnosis and to further evaluate equivocal cases, including those of low-flow, low-gradient aortic stenosis or with gradient mismatch, through additional tools such as dobutamine stress echocardiography or Pedof probes.25,26,37,38 Aortic root morphology assessment and aneurysm detection is also important preprocedurally.37,38 Coronary ostium height is often assessed with the use of MDCT, although 3D TTE and TEE may provide accurate sizing in selected cases.9, 10, 11,25,26,37,38 Recent studies have also shown that 3D TEE, preprocedurally and periprocedurally, is both accurate and useful in assessing the aortic annulus in cases where contrast-enhanced MDCT is difficult to perform.25,26,57,58
Limitations associated with TEE use periprocedurally include the need for general anesthesia and interruption of fluoroscopic views by the TEE probe. Nevertheless, TEE remains beneficial in TAVR, as highlighted in Table 1.25,26,56 The position of the retrograde wire in the left ventricle can be easily confirmed with the use of TEE via standard midesophageal planes at 0°-135° with clear visualization of the left ventricular apex (Figure 13). Furthermore, TEE ensures accurate positioning of the prosthesis during balloon dilation, alongside fluoroscopy, allowing clear visualization of wires and catheters, with minimal to no contrast needed. TEE is also useful during prosthesis deployment, where it provides a real-time depiction of aortic valve calcifications behavior during implantation, as shown in Figure 13. TEE remains the most useful post-procedurally in screening for success and complications. TEE is used to determine the position and shape of the prosthesis as well as leaflet mobility. Patients are also evaluated for signs of any complications, including periaortic root hematoma, intra-aortic dissection flaps, or new-onset pericardial effusion. Evaluations of aortic regurgitation mechanisms (ie, transvalvular or paravalvular [PVL] leak) and severity postprocedurally are important to determine the need for further intervention.57,59
Figure 13.
TEE in Transcatheter Aortic Valve Implantation
(A) Midesophageal plane at 135°, confirming that the wire is located around the left ventricular apex, along the ventricular septum, and not entangled with the MV. This confirmation is useful in preventing complications such as ventricular perforation or MV injury. If the wire entrains the MV, it may affect the balloon’s position and stability, as well as hemodynamics, by causing mitral regurgitation. (B) A case before balloon inflation and (C) after subnominal inflation by 4 mL. During implantation, calcification of the aortic valve can be seen pushing through the sinus of Valsalva and protruding into the LA. Known as the “Mt. Fuji” sign, this is a useful endpoint guide to avoid potential aortic root rupture. Abbreviations as in Figure 1.
Percutaneous Closure of PVL
Mechanism of PVL
PVL occurs when there are gaps between the native annulus of a surgically or percutaneously implanted prosthesis. PVL is usually caused by suture line disruption due to tissue friability, annular calcification, or endocarditis. According to current valvular heart disease guidelines, percutaneous closure of PVL is reasonable (Class IIa) in patients with symptomatic PVL who have prohibitive surgical risk and with suitable anatomy.37,38 The Paravalvular Leak Academic Research Consortium proposed a 5-class scheme for PVL severity for purposes of reporting (Supplemental Tables 4 and 5).60, 61, 62
Transcatheter PVL closure
For preprocedural planning of percutaneous PVL closure, it is critical to assess the number, anatomy, and exact location of the defects. Regarding the sizing of the PVL, it is key to determine both the radial and circumferential extent of the defect, including its orientation in relation to the sewing ring, prosthetic occluders, or leaflets and subvalvular structures. Care is taken to select the most representative frame with optimal resolution using 3D-Zoom, followed by cine confirmation on 2D/3D color Doppler across the gap, to exclude imaging artifact. Color Doppler is essential for assessment of residual PVL to determine if the result is adequate or if the device needs to be repositioned or changed to a different size. Absence of flow convergence, a change in MV inflow pattern from a high E-wave dominant to an A-wave dominant pattern and normalization of pulmonary vein flow pattern indicate significant abolishment of MR. Similarly, absence of diastolic flow reversal in the descending aorta indicates significant reduction in aortic prosthesis PVL. Standardization of nomenclature and anatomy depiction of PVL is essential for effective communication between imagers and interventionalists.33,52 A case example is depicted in Figures 14 and 15.
Figure 14.
MV Prosthesis on TEE
(A) The aortic valve (AV) is anteriorly located (12 o’clock), the IAS is medially located (3 o’clock), and the left atrial appendage (LAA) is laterally located (9 o’clock). The MV is then divided into 8 quadrants that serve as a common nomenclature to describe paravalvular leaks (PVLs) among the interventionalists and imagers. Multiplanar 2-dimensional views, including the (B) midesophageal 135° long-axis and (C) 45° bicommissural views, permit localization of the 2 PVLs: a larger PVL is located anterolateral (10 o’clock position), and a smaller PVL is located anteromedial (1 o’clock position). Both leaks are clearly visible on the (D) surgical en-face view, with overlapping color Doppler and “Trueview”. Abbreviations as in Figures 1 and 2.
Figure 15.
PVL Closure
(A) Under direct 3D TEE guidance, the anterolateral mitral PVL is crossed with a wire (blue arrow). (B) After deployment of an occluder device, color Doppler shows stable placement of the device with trace mitral regurgitation at this site. (C) The mean transmitral gradient is 3 mm Hg, similar to baseline. (D) 3D en-face TEE view confirms the final stable position of the occluder device (asterisk) with no impingement of the prosthetic leaflet motion. (E) A smaller anteromedial PVL is closed with the use of a 12-mm Vascular Plug II (red arrow). (F) This is accompanied by an increase in the mean transmitral gradient to 5 mm Hg. (G) Simultaneous deployment of 2 smaller devices with verification of 2 catheters (blue arrows) crossing the anteromedial PVL on live 3D to ensure correct canalization of the PVL gap. (H) Final position of the 3 deployed occluders (asterisks) with trace residual leak. Abbreviations as in Figures 1, 3, and 14.
Left Atrial Appendage Intervention
LAA anatomy
The left atrial appendage (LAA) is a thin-walled blind-ended pouch originating from the anterolateral part of the LA. The LAA can be divided into the ostium, the neck, and the distal lobe (Figure 16).63 Atrial fibrillation is a known etiology of systemic embolization. Approximately 90% of LA thrombi reside within the LAA of patients with nonvalvular atrial fibrillation.63,64 TEE is the best imaging modality to assess for LAA thrombi.65,66 The transverse diameter 10 mm below the ostium is called the landing zone.
Figure 16.
TEE of the LAA
The LAA segments are illustrated in (A) 2-dimensional TEE view 45° and (B) 3-dimensional TEE 135° photorealistic “glass” view. The ostium is the opening of LA, the neck is located between the ostial region and lobe demarcated by the left circumflex coronary artery (LCx), and the lobe is situated distal to the neck in adjacent to the pulmonary artery (PA). (C) The LAA cauliflower morphology projected by 3-dimensional (3D) TEE photorealistic imaging shares great similarity with (D) contrast angiography at anterior oblique 30°/caudal 30°. LUPV = left upper pulmonary vein; other abbreviations as in Figures 1 and 14.
LAAO
Periprocedural guidance is often performed with the use of both fluoroscopy and TEE. After TSP, real-time 2D/3D TEE is used to direct wires, catheters, and the LAAO device toward the LAA. An ideal location to “park” the wires is within the left superior pulmonary vein (ie, safest zone to perform exchange of catheters for device deployment sheaths).65, 66, 67 This is followed by device sizing assessment to ensure device stability and sufficient sealing of the LAA orifice. The selection of a suitable LAAO device depends on meticulous measurement of landing zone diameters (at end-systole) where the device should be about 3-5 mm larger than the landing zone dimensions to ensure secure implantation. Oversizing may lead to device embolization, tissue perforation, and cardiac tamponade. Undersizing may lead to device migration and peridevice leakage. The “landing zone,” that is, the transverse diameter 10 mm below the ostium, requires multiplanar imaging for accurate assessment (Figure 17, Table 1).67, 68, 69, 70, 71 LAA ostium and implantation depth measurements are also taken. Too deep an implantation may expose an uncovered lobe, whereas too superficial may cause device dislodgement. Post-procedural assessment includes for pericardial effusion, device embolization, thrombi formation, and peridevice leaks.68,69,71
Figure 17.
Echocardiographic Measurements of the Landing Zone
(A) On 2-dimensional (2D) TEE, landing zone diameter (red lines) measurements should start from inferior part of the ostium (yellow lines) at the level of LCx to an area 1 cm distal to the ligament of Marshall. The LAA depth (green lines) is measured from the level of landing zone to the distal lobe. For most cases, the maximum landing zone is measured from 2D imaging of 90°-135°, but this is subject to anatomic variation. (B) The state-of-art measurement as shown allows an unbiased and reproducible assessment of the LAA landing zone that is noninferior to cardiac computed tomography. Abbreviations as in Figures 1, 14, and 16.
Intervention for Hypertrophic Cardiomyopathy
Pathophysiology of hypertrophic cardiomyopathy
Hypertrophic cardiomyopathy is a common autosomal dominant inherited disease, defined histologically by the presence of myocyte disarray and hypertrophy along with myocardial fibrosis.72 Prevalence ranges from 1:500 to 1:200. Dynamic outflow obstruction, secondary to basal septal hypertrophy and systolic anterior motion of the MV leaflets, is present in up to two-thirds of hypertrophic cardiomyopathy patients, who have poorer prognosis and may benefit from septal reduction therapy.
Percutaneous intramyocardial septal radiofrequency ablation
Percutaneous intramyocardial septal radiofrequency ablation, is a novel, safety-proven, and minimally invasive procedure of the interventricular septum (IVS) done under general anesthesia.73,74 Studies have shown that it is effective in relieving outflow obstruction, reducing clinical symptoms, and improving cardiac function, with acceptable rates of adverse events and complications.75,76 However, dedicated randomized controlled clinical trials are needed to validate the procedure in more patients. By emitting high-frequency alternating current from the tip of a radio-frequency needle introduced into the IVS, this may induce irreversible coagulative necrosis and regional vascular coagulation, leading to IVS reduction and obstruction relief. The procedure is as described in Figure 18 and Table 1.
Figure 18.
Echocardiography in the Percutaneous Intramyocardial Septal Radiofrequency Ablation
(A) Preoperative assessment is done to confirm left ventricular wall hypertrophy and outflow tract obstruction. (B) Transapical needle insertion is performed under 2-dimensional and color Doppler transthoracic echocardiography guidance to avoid vascular injuries, followed by (C, D) ablation of the anterior and posterior septum. (E) Myocardial contrast echocardiography is performed immediately to evaluate adequacy of ablation, followed by (F) assessment for septal reduction and obstruction relief.
Conclusions
Structural heart disease remains a health care burden in Asia that has been severely neglected. Fortunately, the push to develop various transcatheter and percutaneous approaches to manage these diseases opens up a new door in improving care in patients who would otherwise not receive treatment after exclusion from surgical intervention. With the advent of newer approaches in managing structural heart disease, complementary multimodality imaging techniques need to be better developed as well. The role of echocardiography during the periprocedural period in TSHI remains invaluable as it provides imaging in real time with unparalleled temporal resolution and spatial orientation compared with other modalities. This concise review has barely scratched the surface of things to come in the realm of structural interventions. The field of interventional echocardiography has never been more exciting!
Funding Support and Author Disclosures
The authors have reported that they have no relationships relevant to the contents of this paper to disclose.
Footnotes
The authors attest they are in compliance with human studies committees and animal welfare regulations of the authors’ institutions and Food and Drug Administration guidelines, including patient consent where appropriate. For more information, visit the Author Center.
Appendix
For supplemental tables and figures, please see the online version of this paper.
Appendix
References
- 1.Hamid N., Ewe S.H. Interventional echocardiography: current role and progress. Proc Singap Healthc. 2015;24(1):4–15. [Google Scholar]
- 2.Ludman P.F., Moat N., de Belder M.A., et al. Transcatheter aortic valve implantation in the United Kingdom: temporal trends, predictors of outcome, and 6-year follow-up: a report from the UK Transcatheter Aortic Valve Implantation (TAVI) registry, 2007 to 2012. Circulation. 2015;131(13):1181–1190. doi: 10.1161/CIRCULATIONAHA.114.013947. [DOI] [PubMed] [Google Scholar]
- 3.Durko A.P., Osnabrugge R.L., Van Mieghem N.M., et al. Annual number of candidates for transcatheter aortic valve implantation per country: current estimates and future projections. Eur Heart J. 2018;39(28):2635–2642. doi: 10.1093/eurheartj/ehy107. [DOI] [PubMed] [Google Scholar]
- 4.Lee C.H., Inohara T., Hayashida K., et al. Transcatheter aortic valve replacement in Asia: present status and future perspectives. J Am Coll Cardiol Asia. 2021;1(3):279–293. doi: 10.1016/j.jacasi.2021.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wong N., Yeo K.K. MitraClip in Asia—current adoption and regional data. Circ Rep. 2019;1(10):397–400. doi: 10.1253/circrep.CR-19-0074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tay E., Muda N., Yap J., et al. The MitraClip Asia-Pacific registry: Differences in outcomes between functional and degenerative mitral regurgitation. Catheter Cardiovasc Interv. 2016;87(7):e275–e281. doi: 10.1002/ccd.26289. [DOI] [PubMed] [Google Scholar]
- 7.Yeo K.K., Tan J.W.C., Muller D.W., et al. Asian Pacific Society of Cardiology consensus recommendations on the use of MitraClip for mitral regurgitation. Eur Cardiol. 2021;16:e25. doi: 10.15420/ecr.2021.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hahn R.T., Mahmood F., Kodali S., et al. Core competencies in echocardiography for imaging structural heart disease interventions: an expert consensus statement. J Am Coll Cardiol Img. 2019;12(12):2560–2570. doi: 10.1016/j.jcmg.2019.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hahn R.T., Abraham T., Adams M.S., et al. Guidelines for performing a comprehensive transesophageal echocardiographic examination: recommendations from the American Society of Echocardiography and the Society of Cardiovascular Anesthesiologists. J Am Soc Echocardiogr. 2013;26(9):921–964. doi: 10.1016/j.echo.2013.07.009. [DOI] [PubMed] [Google Scholar]
- 10.Zamorano J.L., Badano L.P., Bruce C., et al. EAE/ASE recommendations for the use of echocardiography in new transcatheter interventions for valvular heart disease. Eur Heart J. 2011;32(17):2189–2214. doi: 10.1093/eurheartj/ehr259. [DOI] [PubMed] [Google Scholar]
- 11.Hahn R.T., Saric M., Faletra F.F., et al. Recommended standards for the performance of transesophageal echocardiographic screening for structural heart intervention: from the American Society of Echocardiography. J Am Soc Echocardiogr. 2022;35(1):1–76. doi: 10.1016/j.echo.2021.07.006. [DOI] [PubMed] [Google Scholar]
- 12.Fan Y., Chan J.S.K., Lee A.P.-W. Advances in procedural echocardiographic imaging in transcatheter edge-to-edge repair for mitral regurgitation. Front Cardiovasc Med. 2022;9 doi: 10.3389/fcvm.2022.864341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gao G., Penney G., Ma Y., et al. Registration of 3D trans-esophageal echocardiography to X-ray fluoroscopy using image-based probe tracking. Med Image Anal. 2012;16(1):38–49. doi: 10.1016/j.media.2011.05.003. [DOI] [PubMed] [Google Scholar]
- 14.Barreiro-Perez M., Estevez-Loureiro R., Puga L., et al. Real-time echocardiography-fluoroscopy fusion imaging with automated 3D heart segmentation during transcatheter structural heart interventions. J Am Coll Cardiol Intv. 2022;15(13):e155–e158. doi: 10.1016/j.jcin.2022.04.004. [DOI] [PubMed] [Google Scholar]
- 15.Afzal S., Veulemans V., Piayda K., et al. Real-time echocardiographic-fluoroscopic fusion imaging for transcatheter edge-to-edge mitral valve repair. J Am Soc Echocardiogr. 2020;33(5):635–636. doi: 10.1016/j.echo.2020.01.018. [DOI] [PubMed] [Google Scholar]
- 16.Hijazi Z.M., Shivkumar K., Sahn D.J. Intracardiac echocardiography during interventional and electrophysiological cardiac catheterization. Circulation. 2009;119(4):587–596. doi: 10.1161/CIRCULATIONAHA.107.753046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Goya M., Frame D., Gache L., et al. The use of intracardiac echocardiography catheters in endocardial ablation of cardiac arrhythmia: meta-analysis of efficiency, effectiveness, and safety outcomes. J Cardiovasc Electrophysiol. 2020;31(3):664–673. doi: 10.1111/jce.14367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Alkhouli M., Hijazi Z.M., Holmes D.R., Jr., et al. Intracardiac echocardiography in structural heart disease interventions. J Am Coll Cardiol Intv. 2018;11(21):2133–2147. doi: 10.1016/j.jcin.2018.06.056. [DOI] [PubMed] [Google Scholar]
- 19.Bartel T., Bonaros N., Müller L., et al. Intracardiac echocardiography: a new guiding tool for transcatheter aortic valve replacement. J Am Soc Echocardiogr. 2011;24(9):966–975. doi: 10.1016/j.echo.2011.04.009. [DOI] [PubMed] [Google Scholar]
- 20.Jingquan Z., Deyong L., Huimin C., et al. Intracardiac echocardiography Chinese expert consensus. Front Cardiovasc Med. 2022;9 doi: 10.3389/fcvm.2022.1012731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Little S.H., Rigolin V.H., Garcia-Sayan E., et al. Recommendations for special competency in echocardiographic guidance of structural heart disease interventions: from the American Society of Echocardiography. J Am Soc Echocardiogr. 2023;36(4):350–365. doi: 10.1016/j.echo.2023.01.014. [DOI] [PubMed] [Google Scholar]
- 22.Naqvi N., McCarthy K.P., Ho S.Y. Anatomy of the atrial septum and interatrial communications. J Thorac Dis. 2018;10(Suppl 24):S2837–S2847. doi: 10.21037/jtd.2018.02.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.McCarthy K., Ho S., Anderson R. Defining the morphologic phenotypes of atrial septal defects and interatrial communications. Images Paediatr Cardiol. 2003;5(2):1–24. [PMC free article] [PubMed] [Google Scholar]
- 24.Turner M.E., Bouhout I., Petit C.J., et al. Transcatheter closure of atrial and ventricular septal defects: JACC focus seminar. J Am Coll Cardiol. 2022;79(22):2247–2258. doi: 10.1016/j.jacc.2021.08.082. [DOI] [PubMed] [Google Scholar]
- 25.Patrianakos A.P., Zacharaki A.A., Skalidis E.I., et al. The growing role of echocardiography in interventional cardiology: the present and the future. Hellenic J Cardiol. 2017;58(1):17–31. doi: 10.1016/j.hjc.2017.01.008. [DOI] [PubMed] [Google Scholar]
- 26.Silvestry F.E., Kerber R.E., Brook M.M., et al. Echocardiography-guided interventions. J Am Soc Echocardiogr. 2009;22(3):213–231. doi: 10.1016/j.echo.2008.12.013. [DOI] [PubMed] [Google Scholar]
- 27.Silvestry F.E., Cohen M.S., Armsby L.B., et al. Guidelines for the echocardiographic assessment of atrial septal defect and patent foramen ovale: from the American Society of Echocardiography and Society for Cardiac Angiography and Interventions. J Am Soc Echocardiogr. 2015;28(8):910–958. doi: 10.1016/j.echo.2015.05.015. [DOI] [PubMed] [Google Scholar]
- 28.Rana B.S. Echocardiography guidance of atrial septal defect closure. J Thorac Dis. 2018;10(suppl 24):S2899–S2908. doi: 10.21037/jtd.2018.07.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Roberson D.A., Cui W., Patel D., et al. Three-dimensional transesophageal echocardiography of atrial septal defect: a qualitative and quantitative anatomic study. J Am Soc Echocardiogr. 2011;24(6):600–610. doi: 10.1016/j.echo.2011.02.008. [DOI] [PubMed] [Google Scholar]
- 30.Hijazi Z.M., Shivkumar K., Sahn D.J. Intracardiac echocardiography during interventional and electrophysiological cardiac catheterization. Circulation. 2009;119(4):587–596. doi: 10.1161/CIRCULATIONAHA.107.753046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.McCarthy K.P., Ring L., Rana B.S. Anatomy of the mitral valve: understanding the mitral valve complex in mitral regurgitation. Eur J Echocardiogr. 2010;11(10):i3–i9. doi: 10.1093/ejechocard/jeq153. [DOI] [PubMed] [Google Scholar]
- 32.Dal-Bianco J.P., Levine R.A. Anatomy of the mitral valve apparatus: role of 2D and 3D echocardiography. Cardiol Clin. 2013;31(2):151–164. doi: 10.1016/j.ccl.2013.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Adams D.H., Rosenhek R., FalkA Degenerative mitral valve regurgitation: best practice revolution. Eur Heart J. 2010;31(16):1958–1966. doi: 10.1093/eurheartj/ehq222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Anyanwu A.C., Adams D.H. Etiologic classification of degenerative mitral valve disease: Barlow’s disease and fibroelastic deficiency. Semin Thorac Cardiovasc Surg. 2007;19(2):90–96. doi: 10.1053/j.semtcvs.2007.04.002. [DOI] [PubMed] [Google Scholar]
- 35.Ntusi N.B., Mayosi B.M. Epidemiology of heart failure in sub-Saharan Africa. Expert Rev Cardiovasc Ther. 2009;7(2):169–180. doi: 10.1586/14779072.7.2.169. [DOI] [PubMed] [Google Scholar]
- 36.Yeo K.K., Yap J., Yamen E., et al. Percutaneous mitral valve repair with the MitraClip: early results from the MitraClip Asia-Pacific Registry (MARS) EuroIntervention. 2014;10(5):620–625. doi: 10.4244/EIJV10I5A107. [DOI] [PubMed] [Google Scholar]
- 37.Otto C.M., Nishimura R.A., Bonow R.O., et al. 2020 ACC/AHA guideline for the management of patients with valvular heart disease. J Am Coll Cardiol. 2021;77(4):e25–e197. doi: 10.1016/j.jacc.2020.11.018. [DOI] [PubMed] [Google Scholar]
- 38.Vahanian A., Beyersdorf F., Praz F., et al. 2021 ESC/EACTS guidelines for the management of valvular heart disease. Eur Heart J. 2022;43(7):561–632. doi: 10.1093/eurheartj/ehab395. [DOI] [PubMed] [Google Scholar]
- 39.Mack M., Carroll J.D., Thourani V., et al. Transcatheter mitral valve therapy in the United States: a report from the STS-ACC TVT registry. J Am Coll Cardiol. 2021;78(23):2326–2353. doi: 10.1016/j.jacc.2021.07.058. [DOI] [PubMed] [Google Scholar]
- 40.Yoon S.H., Bleiziffer S., Latib A., et al. Predictors of left ventricular outflow tract obstruction after transcatheter mitral valve replacement. J Am Coll Cardiol Intv. 2019;12(2):182–193. doi: 10.1016/j.jcin.2018.12.001. [DOI] [PubMed] [Google Scholar]
- 41.Wilkins G.T., Weyman A.E., Abascal V.M., et al. Percutaneous balloon dilatation of the mitral valve: an analysis of echocardiographic variables related to outcome and the mechanism of dilatation. Br Heart J. 1988;60(4):299–308. doi: 10.1136/hrt.60.4.299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Iung B., Cormier B., Ducimetie're P., et al. Immediate results of percutaneous mitral commissurotomy. a predictive model on a series of 1514 patients. Circulation. 1996;94(9):2124–2130. doi: 10.1161/01.cir.94.9.2124. [DOI] [PubMed] [Google Scholar]
- 43.Nobuyoshi M., Hamasaki N., Kimura T., et al. Indications, Complications, and short-term clinical outcome of percutaneous transvenous mitral commissurotomy. Circulation. 1989;80(4):782–792. doi: 10.1161/01.cir.80.4.782. [DOI] [PubMed] [Google Scholar]
- 44.Pandian N.G., Kim J.K., Arias-Godinez J.A., et al. Recommendations for the use of echocardiography in the evaluation of rheumatic heart disease: a report from the American Society of Echocardiography. J Am Soc Echocardiogr. 2023;36(1):3–28. doi: 10.1016/j.echo.2022.10.009. [DOI] [PubMed] [Google Scholar]
- 45.Mangieri A., Montalto C., Pagnesi M., et al. Mechanism and implications of the tricuspid regurgitation: from the pathophysiology to the current and future therapeutic options. Circ Cardiovasc Interv. 2017;10(7) doi: 10.1161/CIRCINTERVENTIONS.117.005043. [DOI] [PubMed] [Google Scholar]
- 46.Schlossbauer S.A., Faletra F.F., Paiocchi V.L., et al. Multimodality imaging of the anatomy of tricuspid valve. J Cardiovasc Dev Dis. 2021;8(9):107. doi: 10.3390/jcdd8090107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Taramasso M., Gavazzoni M., Pozzoli A., et al. Tricuspid regurgitation: predicting the need for intervention, procedural success, and recurrence of disease. J Am Coll Cardiol Img. 2019;12(4):605–621. doi: 10.1016/j.jcmg.2018.11.034. [DOI] [PubMed] [Google Scholar]
- 48.Sade L.E., Muraru D., Marsan N.A., et al. How to assess severe tricuspid regurgitation by echocardiography? Eur Heart J Cardiovasc Imaging. 2022;23(10):1273–1276. doi: 10.1093/ehjci/jeac015. [DOI] [PubMed] [Google Scholar]
- 49.Hahn R.T. State-of-the-art review of echocardiographic imaging in the evaluation and treatment of functional tricuspid regurgitation. Circ Cardiovasc Imaging. 2016;9(12) doi: 10.1161/CIRCIMAGING.116.005332. [DOI] [PubMed] [Google Scholar]
- 50.Zaidi A., Oxborough D., Augustine D.X., et al. Echocardiographic assessment of the tricuspid and pulmonary valves: a practical guideline from the British Society of Echocardiography. Echo Res Pract. 2020;7(4):G95–G122. doi: 10.1530/ERP-20-0033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Simard T.J., Eleid M.F. Transcatheter tricuspid valve intervention: current perspective. US Cardiol Rev. 2021;15:e12. [Google Scholar]
- 52.Curio J., Demir O.M., Pagnesi M., et al. Update on the current landscape of transcatheter options for tricuspid regurgitation treatment. Interv Cardiol. 2019;14(2):54–61. doi: 10.15420/icr.2019.5.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Muntané-Carol G., Alperi A., Faroux L., et al. Transcatheter tricuspid valve intervention: coaptation devices. Front Cardiovasc Med. 2020;7:139. doi: 10.3389/fcvm.2020.00139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Praz F., Muraru D., Kreidel F., et al. Transcatheter treatment for tricuspid valve disease. EuroIntervention. 2021;17(10):791–808. doi: 10.4244/EIJ-D-21-00695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Liu Y., Li W., Zhou D., et al. Real-time monitoring and step-by-step guidance for transcatheter tricuspid annuloplasty using transesophageal echocardiography. J Cardiovasc Dev Dis. 2022;9(12):415. doi: 10.3390/jcdd9120415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Piazza N., de Jaegere P., Schultz C., et al. Anatomy of the aortic valvar complex and its implications for transcatheter implantation of the aortic valve. Circ Cardiovasc Interv. 2008;1(1):74–81. doi: 10.1161/CIRCINTERVENTIONS.108.780858. [DOI] [PubMed] [Google Scholar]
- 57.Willson A.B., Webb J.G., Labounty T.M., et al. 3-Dimensional aortic annular assessment by multidetector computed tomography predicts moderate or severe paravalvular regurgitation after transcatheter aortic valve replacement: a multicenter retrospective analysis. J Am Coll Cardiol. 2012;59(14):1287–1294. doi: 10.1016/j.jacc.2011.12.015. [DOI] [PubMed] [Google Scholar]
- 58.Rong L.Q., Hameed I., Salemi A., et al. Three-dimensional transesophageal echocardiography for transcatheter aortic valve replacement sizing: a systematic review and meta-analysis. J Am Heart Assoc. 2019;8(19) doi: 10.1161/JAHA.119.013463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sinning J.M., Hammerstingl C., Vasa-Nicotera M., et al. Aortic regurgitation index defines severity of peri-prosthetic regurgitation and predicts outcome in patients after transcatheter aortic valve implantation. J Am Coll Cardiol. 2012;59(13):1134–1141. doi: 10.1016/j.jacc.2011.11.048. [DOI] [PubMed] [Google Scholar]
- 60.Ruiz C.E., Hahn R.T., Berrebi A. Clinical trial principles and endpoint definitions for paravalvular leaks in surgical prosthesis: an expert statement. J Am Coll Cardiol. 2017;69(16):2067–2087. doi: 10.1016/j.jacc.2017.02.038. [DOI] [PubMed] [Google Scholar]
- 61.Lancellotti P., Pibarot P., Chambers J., et al. Recommendations for the imaging assessment of prosthetic heart valves: a report from the European Association of Cardiovascular Imaging endorsed by the Chinese Society of Echocardiography, the Inter-American Society of Echocardiography, and the Brazilian Department of Cardiovascular Imaging. Eur Heart J Cardiovasc Imaging. 2016;17(6):589–590. doi: 10.1093/ehjci/jew025. [DOI] [PubMed] [Google Scholar]
- 62.Eleid M.F., Cabalka A.K., Malouf J.F., et al. Techniques and outcomes for the treatment of paravalvular leak. Circ Cardiovasc Interv. 2015;8(8) doi: 10.1161/CIRCINTERVENTIONS.115.001945. [DOI] [PubMed] [Google Scholar]
- 63.Agmon Y., Khandheria B.K., Gentil F., et al. Echocardiographic assessment of the left atrial appendage. J Am Coll Cardiol. 1999;34(7):1867–1877. doi: 10.1016/s0735-1097(99)00472-6. [DOI] [PubMed] [Google Scholar]
- 64.Shirani J., Alaeddini J. Structural remodeling of the left atrial appendage in patients with chronic non-valvular atrial fibrillation: Implications for thrombus formation, systemic embolism, and assessment by transesophageal echocardiography. Cardiovasc Pathol. 2000;9(2):95–101. doi: 10.1016/s1054-8807(00)00030-2. [DOI] [PubMed] [Google Scholar]
- 65.Chan A.K., Kannam J.P., Douglas P.S., et al. Multiplane transesophageal echocardiographic assessment of left atrial appendage anatomy and function. Am J Cardiol. 1995;76(7):528–530. doi: 10.1016/s0002-9149(99)80147-7. [DOI] [PubMed] [Google Scholar]
- 66.Nucifora G., Faletra F., Regoli F., et al. Evaluation of the left atrial appendage with real-time 3-dimensional transesophageal echocardiography: implications for catheter-based left atrial appendage closure. Circ Cardiovasc Imaging. 2011;4(5):514–523. doi: 10.1161/CIRCIMAGING.111.963892. [DOI] [PubMed] [Google Scholar]
- 67.Anwar A.M., Nosir Y.F., Ajam A., et al. Central role of real-time three-dimensional echocardiography in the assessment of intracardiac thrombi. Int J Cardiovasc Imaging. 2010;26(5):519–526. doi: 10.1007/s10554-010-9593-4. [DOI] [PubMed] [Google Scholar]
- 68.Mraz T., Neuzil P., Mandysova E., et al. Role of echocardiography in percutaneous occlusion of the left atrial appendage. Echocardiography. 2007;24(4):401–404. doi: 10.1111/j.1540-8175.2007.00404.x. [DOI] [PubMed] [Google Scholar]
- 69.Perk G., Biner S., Kronzon I., et al. Catheter-based left atrial appendage occlusion procedure: role of echocardiography. Eur Heart J Cardiovasc Imaging. 2012;13(2):132–138. doi: 10.1093/ejechocard/jer158. [DOI] [PubMed] [Google Scholar]
- 70.Stollberger C., Schneider B., Finsterer J., et al. Serious complications from dislocation of a Watchman left atrial appendage occluder. J Cardiovasc Electrophysiol. 2007;18(8):880–881. doi: 10.1111/j.1540-8167.2007.00784.x. [DOI] [PubMed] [Google Scholar]
- 71.Reddy V.Y., Holmes D., Doshi S.K., et al. Safety of percutaneous left atrial appendage closure: results from the Watchman Left Atrial Appendage System for Embolic Protection in Patients With AF (PROTECT AF) clinical trial and the continued access registry. Circulation. 2011;123(4):417–424. doi: 10.1161/CIRCULATIONAHA.110.976449. [DOI] [PubMed] [Google Scholar]
- 72.Ommen S.R., Mital S., Burke M.A., et al. 2020 AHA/ACC guideline for the diagnosis and treatment of patients with hypertrophic cardiomyopathy: a report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. J Am Coll Cardiol. 2020;76(25):e159–e240. doi: 10.1016/j.jacc.2020.08.045. [DOI] [PubMed] [Google Scholar]
- 73.Liu L., Liu B., Li J., et al. Percutaneous intramyocardial septal radiofrequency ablation of hypertrophic obstructive cardiomyopathy: a novel minimally invasive treatment for reduction of outflow tract obstruction. EuroIntervention. 2018;13(18):e2112–e2113. doi: 10.4244/EIJ-D-17-00657. [DOI] [PubMed] [Google Scholar]
- 74.Liu F., Fu J., Hsi D., et al. Percutaneous intramyocardial septal radiofrequency ablation for interventricular septal reduction: an ovine model with 1-year outcomes. Cardiology. 2020;145(1):53–62. doi: 10.1159/000502973. [DOI] [PubMed] [Google Scholar]
- 75.Liu L., Li J., Zuo L., et al. Percutaneous intramyocardial septal radiofrequency ablation for hypertrophic obstructive cardiomyopathy. J Am Coll Cardiol. 2018;72(16):1898–1909. doi: 10.1016/j.jacc.2018.07.080. [DOI] [PubMed] [Google Scholar]
- 76.Zhou M., Ta S., Hahn R.T., et al. Percutaneous intramyocardial septal radiofrequency ablation in patients with drug-refractory hypertrophic obstructive cardiomyopathy. JAMA Cardiol. 2022;7(5):529–538. doi: 10.1001/jamacardio.2022.0259. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.